Catch Losses and Reduction of Bycatch for Jellyfish Using Marine Mammal Bycatch Reduction Devices in Midwater Trawl Gear
Abstract
1. Introduction
2. Materials and Methods
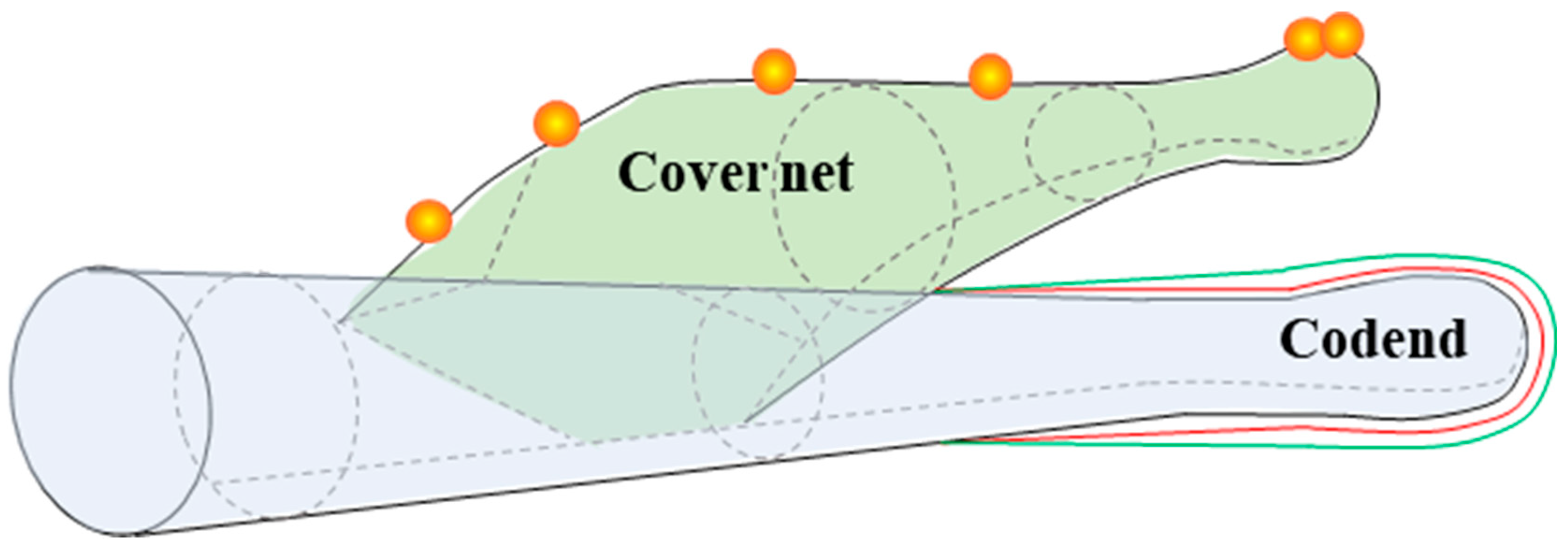
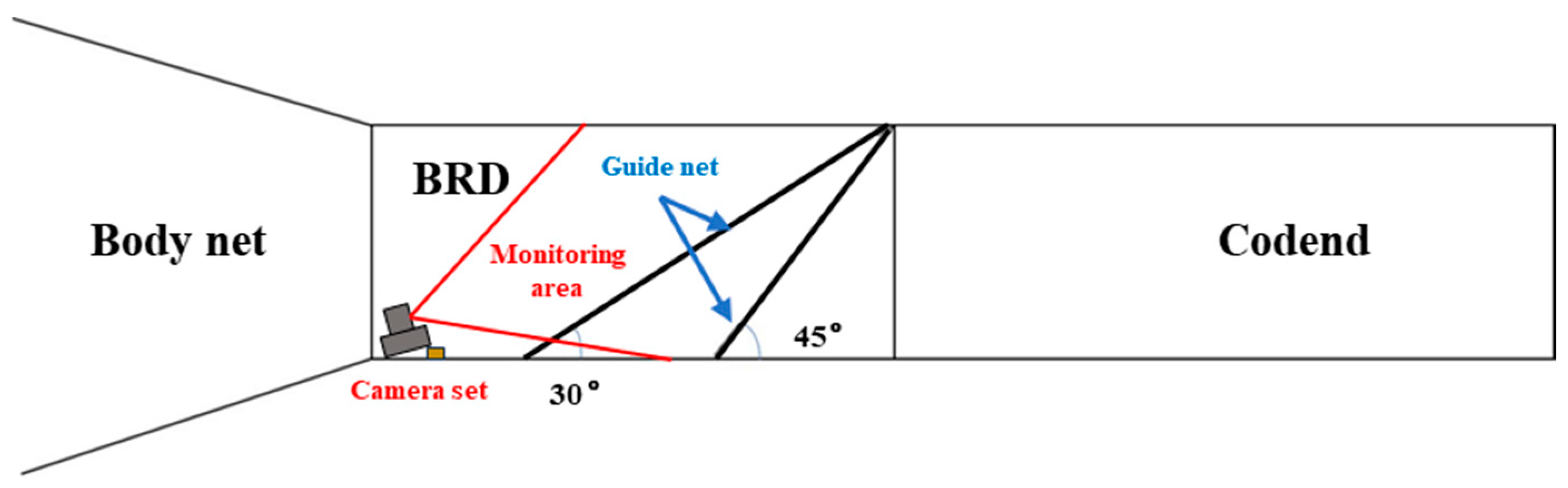
2.1. Test Fishing Gear with Bycatch Reduction Device
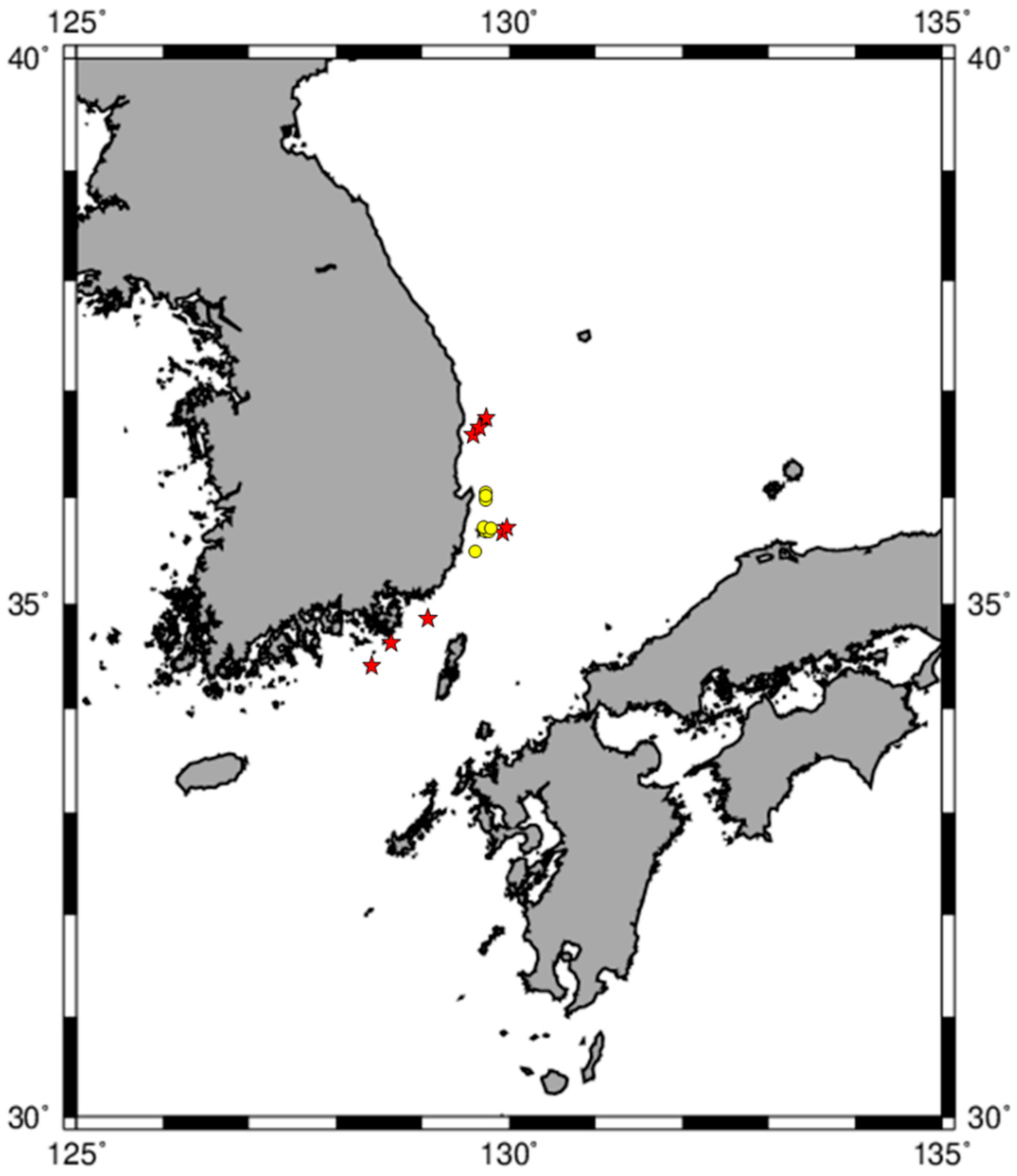
2.2. Fishing Area and Sea Trial
2.3. Camera Observation
2.4. Data Analysis
3. Results
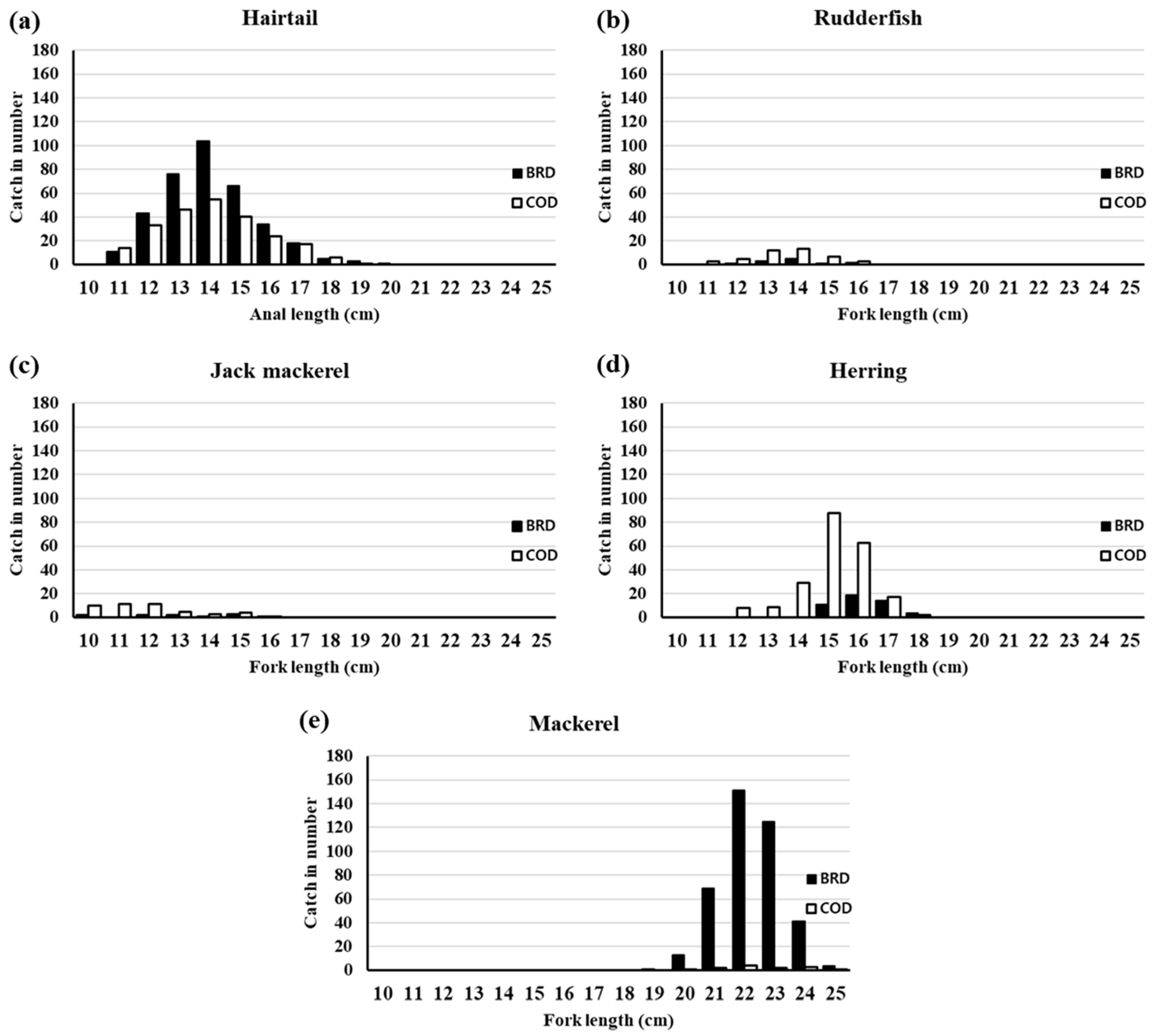
3.1. Body Length Composition
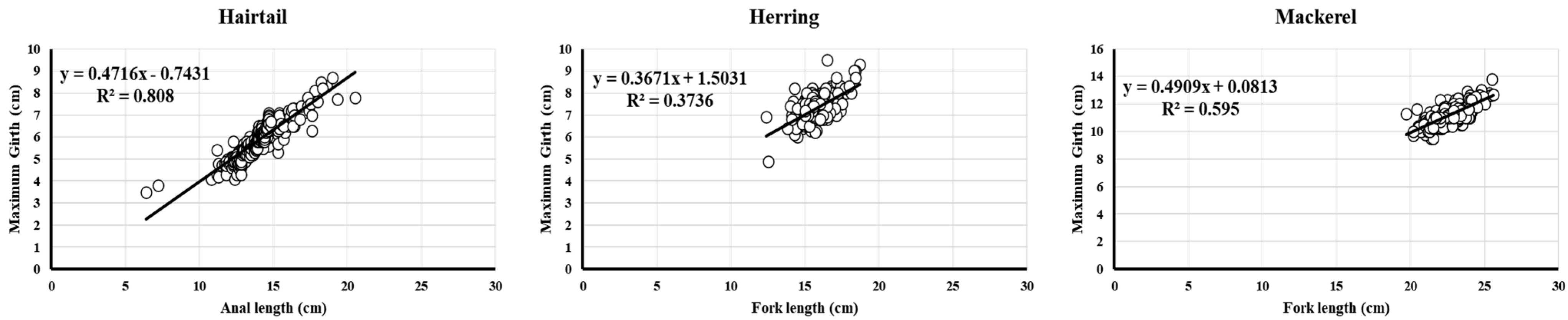
3.2. Relationship Between Body Length and Maximum Girth (And Weight)
- Hairtail: G = 0.4716 × AL + 0.7431 (3) ( = 0.899, < 0.05, = 597, Figure 6)
- Herring: G = 0.3671 × FL + 1.5031 (4) ( = 0.611, < 0.05, = 264, Figure 6)
- Mackerel: G = 0.4909 × FL + 0.0813 (5) ( = 0.771, < 0.05, = 417, Figure 6)
3.3. Bycatch Reduction and Catch Loss Inside the Gear
3.4. Mesh Selectivity of BRD
- 30° BRD: 28.7 cm (sum of haul) and 20.8 cm (average of each haul)
- 45° BRD: 9.2 cm (sum of haul) and 52.3 cm (average of each haul)
- Combined data: 299.0 cm (sum of haul) and 21.4 cm (average of each haul).
- 30° BRD: 16.5 cm (sum of haul) and 16.6 cm (average of each haul)
- 45° BRD: 20.4 cm (sum of haul) and 22.8 cm (average of each haul)
- Combined data: 18.6 cm (sum of haul) and 18.7 cm (average of each haul).
- 30° BRD: 16.7 cm (sum of haul) and 15.6 cm (average of each haul)
- 45° BRD: 16.8 cm (sum of haul) and 16.6 cm (average of each haul)
- Combined data: 16.4 cm (sum of haul) and 16.1 cm (average of each haul).
- Mackerel (30° BRD): 20.0 cm (sum of haul) and 19.9 cm (average of each haul)
- Herring (45° BRD): 17.1 cm (sum of haul) and 17.1 cm (average of each haul).
3.5. Catch Loss Results by BRD and Bycatch Results for Jellyfish by BRD
4. Discussion
4.1. Importance of Caught Fish Species
4.2. Efficiency of the Bycatch Reduction Device
4.2.1. Selectivity Regarding the Bycatch Reduction Device Tilt Angle
4.2.2. Selectivity Regarding the Bycatch Reduction Device Mesh Size
4.2.3. Selectivity Regarding Fish School Behavior and Biological Characteristics
4.3. Catch Losses and Mitigation Measures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavigne, D.M. Marine mammals and fisheries: The role of science in the culling debate. In Fisheries, Tourism, and Management Issues; Mammals, M., Gales, N., Hindell, M., Kirkwood, R., Eds.; CSIRO: Melbourne, Australia, 2003. [Google Scholar]
- Roman, J.; Estes, J.A.; Morissette, L.; Smith, C.; Costa, D.; McCarthy, J.; Nation, J.B.; Nicol, S.; Pershing, A.; Smetacek, V. Whales as marine ecosystem engineers. Front. Ecol. Environ. 2014, 12, 377–385. [Google Scholar] [CrossRef]
- Morissette, L.; Christensen, V.; Pauly, D. Marine mammal impacts in exploited ecosystems: Would large scale culling benefit fisheries? PLoS ONE 2012, 7, e43966. [Google Scholar] [CrossRef]
- Martin, A.H.; Pearson, H.C.; Saba, G.K.; Olsen, E.M. Integral functions of marine vetebrates in the ocean carbon cycle and climate change mitigation. One Earth 2021, 4, 680–693. [Google Scholar] [CrossRef]
- Bering, J.; Gargan, H.; Kuesel, J.; Morrison, M.; Mullaney, C.; Read, A.J.; Roady, S.E.; Rowe, A. Will unilateral action improve the global conservation status of marine mammals? A first analysis of the U.S. Marine Mammal Protection Act’s Import Provisions Rule. Mar. Policy 2022, 135, 104832. [Google Scholar] [CrossRef]
- Jung, J.M.; Park, M.S.; Choi, K.S. A study of the catch losses and mesh selectivity related to the attachment of marine mammal bycatch reduction devices on Midwater trawl gear. Fishes 2024, 9, 391. [Google Scholar] [CrossRef]
- Leeney, R.H.; Berrow, S.; McGrath, D.; O’Brien, J.; Cosgrove, R.; Godley, B.J. Effects of pingers on the behaviour of bottlenose dolphins. J. Mar. Biol. Assoc. UK 2007, 87, 129–133. [Google Scholar] [CrossRef]
- Brotons, J.M.; Munilla, Z.; Grau, A.M.; Rendell, L. Do pingers reduce interactions between bottlenose dolphins and nets around the Balearic Islands? Endang. Species Res. 2008, 5, 301–308. [Google Scholar] [CrossRef]
- Gazo, M.; Gonzalvo, J.; Aguilar, A. Pingers as deterrents of bottlenose dolphins interacting with trammel nets. Fish. Res. 2008, 92, 70–75. [Google Scholar] [CrossRef]
- Buscaino, G.; Buffa, G.; Sarà, G.; Bellante, A.; Tonello, A.J.; Hardt, F.A.S.; Cremer, M.J.; Bonanno, A.; Cuttitta, A.; Mazzola, S. Pinger affects fish catch efficiency and damage to bottom gill nets related to bottlenose dolphins. Fish. Sci. 2009, 75, 537–544. [Google Scholar] [CrossRef]
- Gönener, S.; Özsandikçi, U. Technical measures in order to decrease interactions between dolphins and fishermen: Pingers. J. Aquacult. Eng. Fish. Res. 2017, 3, 151–159. [Google Scholar] [CrossRef]
- Buscaino, G.; Ceraulo, M.; Alonge, G.; Pace, D.S.; Grammauta, R.; Maccarrone, V.; Bonanno, A.; Mazzola, S.; Papale, E. Artisanal fishing, dolphins, and interactive pinger: A study from a passive acoustic perspective. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 2241–2256. [Google Scholar] [CrossRef]
- Schakner, Z.A.; Blumstein, D.T. Behavioral biology of marine mammal deterrents: A review and prospectus. Biol. Conserv. 2013, 167, 380–389. [Google Scholar] [CrossRef]
- Spitz, J.; Richard, E.; Meynier, L.; Pusineri, C.; Ridoux, V. Dietary plasticity of the oceanic striped dolphin, Stenella coeruleoalba, in the neritic waters of the Bay of Biscay. J. Sea Res. 2006, 55, 309–320. [Google Scholar] [CrossRef]
- Jensen, F.H.; Perez, J.M.; Johnson, M.; Soto, N.A.; Madsen, P.T. Calling under pressure: Short-finned pilot whales make social calls during deep foraging dives. Proc. Biol. Sci. 2011, 278, 3017–3025. [Google Scholar] [CrossRef]
- Dewar, W.K.; Bingham, R.J.; Iverson, R.L.; Nowacek, D.P.; Laurent, L.C.; Wiebe, P.H. Does the marine biosphere mix the ocean? J. Mar. Res. 2006, 64, 541–561. [Google Scholar] [CrossRef]
- Lavery, T.J.; Roudnew, B.; Gill, P.; Seymour, J.; Seuront, L.; Johnson, G.; Mitchell, J.G.; Smetacek, V. Iron defecation by sperm whales stimulates carbon export in the Southern Ocean. Proc. Biol. Sci. 2010, 277, 3527–3531. [Google Scholar] [CrossRef]
- Roman, J.; McCarthy, J.J. The whale pump: Marine mammals enhance primary productivity in a coastal basin. PLoS ONE 2010, 5, e13255. [Google Scholar] [CrossRef]
- Smith, L.V.; McMinn, A.; Martin, A.; Nicol, S.; Bowie, A.R.; Lannuzel, D.; van der Merwe, P. Preliminary investigation into the stimulation of phytoplankton photophysiology and growth by whale faeces. J. Exp. Mar. Biol. Ecol. 2013, 446, 1–9. [Google Scholar] [CrossRef]
- Reilly, S.B.; Bannister, J.L.; Best, P.B.; Brown, M.; Brownell, R.L., Jr.; Rudderworth, D.S.; Clapham, P.J.; Cooke, J.; Donovan, G.P.; Urbàn, J.; et al. Megaptera novaeangliae. IUCN red list threat. Species 2008, Version 2013.1. Available online: http://www.iucnredlist.org (accessed on 3 June 2025).
- Yoon, Y.Y.; Jung, S.J.; Yoon, S.C. Characteristics and long-term variation trend of water mass in the coastal part of East Sea, Korea. J. Korean Soc. Mar. Environ. Eng. 2007, 10, 59–65. [Google Scholar]
- Song, H.; Song, Y.S.; Hwang, K.; Sohn, D. Characteristics of changes in species composition with water temperature in set net fishing on the Southern Coast of East Sea. Korean. J. Fish. Aquat. Sci. 2022, 55, 625–637. [Google Scholar] [CrossRef]
- Kang, Y.S. Unusual population explosion of the jellyfish and damage status. J. Korean Prof. Eng. Assoc. 2010, 43, 46–49. [Google Scholar]
- Lee, J.H.; Kim, E.H.; Lee, K.; Park, K.J.; An, Y.R.; Kim, H.W.; Sohn, H.; Choi, S.G. Occurrence and spatial distribution of marine mammals by sighting surveys in Korean waters during 2011–2020. Korean. J. Fish. Aquat. Sci. 2022, 55, 938–945. [Google Scholar] [CrossRef]
- Park, K.J.; An, Y.R.; Kim, Z.G.; Choi, S.G.; Moon, D.Y.; Park, J.E. Abundance estimates of the Minke whale Balaenoptera acutorostrata, in the East Sea, Korea. Korean. J. Fish. Aquat. Sci. 2009, 42, 642–649. [Google Scholar]
- Lee, D.; An, Y.R.; Park, K.J.; Kim, H.W.; Lee, D.; Joo, H.T.; Oh, Y.G.; Kim, S.M.; Kang, C.K.; Lee, S.H. Spatial distribution of common Minke whale (Balaenoptera acutorostrata) as an indication of a biological hotspot in the East Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 91–99. [Google Scholar] [CrossRef]
- Fryer, R.J. A model of between-haul variation in selectivity. ICES J. Mar. Sci. 1991, 48, 281–290. [Google Scholar] [CrossRef]
- Kinacigil, H.T.; Ìlkyaz, A.T.; Akyol, O.; Metin, G.; Çira, E.; Ayaz, A. Growth parameters of red mullet (Mullus barbatus L., 1758) and seasonal cod-end selectivity of traditional bottom trawl nets in Izmir Bay (Aegean Sea). Acta Adriat. 2001, 42, 113–123. [Google Scholar]
- Özbilgin, H.; Tosunoglu, Z.; Tokac, A.; Metin, G. Seasonal variation in the trawl coded selectivity of red mullet (Mullus barbatus). Turk. J. Fish. Aquat. Sci. 2011, 11, 191–198. [Google Scholar] [CrossRef]
- Bahamon, N.; Sardà, F.; Suuronen, P. Improvement of trawl selectivity in the NW Mediterranean demersal fishery by using a 40 mm square mesh codend. Fish. Res. 2006, 81, 15–25. [Google Scholar] [CrossRef]
- Jørgensen, T.; Ingólfsson, Ó.A.; Graham, N.; Isaksen, B. Size selection of cod by rigid grids—Is anything gained compared to diamond mesh codends only? Fish. Res. 2006, 79, 337–348. [Google Scholar] [CrossRef]
- Bak-Jensen, Z.; Herrmann, B.; Santos, J.; Melli, V.; Feekings, J.P.; Stepputtis, D. Fixed mesh constructions are required to reduce variability in codend size selectivity. In Proceedings of the 15th International Workshop on Methods for the Development and Evaluation of Maritime Technologies, Rostock, Germany, 13 September 2022; Menzel-Verlag: Kühlungsborn, Germany, 2023; pp. 61–70. [Google Scholar]
- Brooks, M.E.; Melli, V.; Savina, E.; Santos, J.; Millar, R.; O’Neill, F.G.; Veiga-Malta, T.; Krag, L.A.; Feekings, J.P. Introducing selfisher: Open source software for statistical analyses of fishing gear selectivity. Can. J. Fish. Aquat. Sci. 2022, 79, 1189–1197. [Google Scholar] [CrossRef]
- Millar, R.B.; Walsh, S.J. Analysis of trawl selectivity studies with an application to trouser trawls. Fish. Res. 1992, 13, 205–220. [Google Scholar] [CrossRef]
- Wileman, D.A.; Ferro, R.S.T.; Fonteyne, R.; Millar, R.B. Manual of Methods of Measuring the Selectivity of Towed Fishing Gears; ICES Cooperative Research Reports: Toronto, ON, Canada, 1996; Volume 215, pp. 1–216. [Google Scholar]
- Millar, R.B.; Fryer, R.J. Estimating the size-selection curves of towed gears, traps, nets and hooks. Rev. Fish. Biol. Fish. 1999, 9, 89–116. [Google Scholar] [CrossRef]
- Erzini, K.; Gonçalves, J.M.S.; Bentes, L.; Moutopoulos, D.K.; Casal, J.A.H.; Soriguer, M.C.; Puente, E.; Errazkin, L.A.; Stergiou, K.I. Size selectivity of trammel nets in southern European small-scale fisheries. Fish. Res. 2006, 79, 183–201. [Google Scholar] [CrossRef]
- National Institute of Fisheries Science (NIFS). Catch, Fishing Effort, and Length Distribution Data by Year, Sea Block Number, Fishery, and Species; NIFS: Busan, Republic of Korea, 2020. [Google Scholar]
- National Institute of Fisheries Science (NIFS). In Fishing Trends and Sock Status Assessments for Korean Coast Major Fisheries; NIFS: Busan, Republic of Korea, 2020.
- Nazir, A.; Lin, T.H.; Kuo, T.H.; Shirai, K.; Wang, P.L.; Shiao, J.C. Seasonal distribution and population genetic structure of Psenopsis anomala (Japanese butterfish) inferred from otolith oxygen isotope ratios and mitochondrial DNA. Estuar. Coast. Shelf Sci. 2024, 309, 108974. [Google Scholar] [CrossRef]
- Cho, S.K.; An, H.C.; Shin, J.K.; Yang, Y.S.; Park, C.D.; Lee, J.H. Study on the survival rate of fishes escaped from trawl net. J. Korean Soc. Fish. Ocean Technol. 2006, 42, 69–77. [Google Scholar]
- Park, C.D.; Kim, I.O.; Lee, K.H.; Lee, G.H.; Park, S.W. The performance of a wedge type jellyfish excluder device inserted in a trawl net. J. Korean Soc. Fish. Ocean Technol. 2010, 46, 302–312. [Google Scholar] [CrossRef]
- Cha, B.J.; Roth, R.; Cho, S.K. Model test to understand shape change of BRD (bycatch reduction device) for demersal trawl of Argentina. J. Korean Soc. Fish. Technol. 2015, 51, 312–320. [Google Scholar] [CrossRef]
- Kim, I.O.; An, H.C.; Shin, J.K.; Cha, B.J. The development of basic structure of jellyfish separator system for a trawl net. J. Korean Soc. Fish. Ocean Technol. 2008, 44, 99–111, (in Korean with English abstract). [Google Scholar] [CrossRef]
- Boopendranath, M.R.; Pravin, P. Technologies for responsible fishing-BRDs and TEDs. In Proceedings of the International Symposium on Marine Ecosystems—Challenges and Strategies, Kochi, India, 9–12 February 2009; pp. 9–12. [Google Scholar]
- Lucchetti, A.; Punzo, E.; Virgili, M. Flexible Turtle Excluder Device (TED): An effective tool for Mediterranean coastal multispecies bottom trawl fisheries. Aquat. Living Resour. 2016, 29, 201. [Google Scholar] [CrossRef]
- Vasapollo, C.; Virgili, M.; Petetta, A.; Bargione, G.; Sala, A.; Lucchetti, A. Bottom trawl catch comparison in the Mediterranean Sea: Flexible Turtle Excluder Device (TED) vs traditional gear. PLoS ONE 2019, 14, e0216023. [Google Scholar] [CrossRef]
- Matsushita, Y.; Inoue, Y. Variation of square mesh codend selectivity for walleye pollock Theragra chalcogramma with respect to difference in body shape. Nippon. Suisan Gakkaishi 1997, 63, 23–29. [Google Scholar] [CrossRef]
- Liang, Z.; Horikawa, H.; Tokimura, M.; Tokai, T. Effect of cross-sectional shape of fish body on mesh selectivity of trawl codend. Nippon. Suisan Gakkaishi 1999, 65, 441–447. [Google Scholar] [CrossRef]
- Petrakis, G.; Stergiou, K.I. Size selectivity of diamond and square mesh codends for four commercial Mediterranean fish species. ICES J. Mar. Sci. 1997, 54, 13–23. [Google Scholar] [CrossRef]
- Bak-Jensen, Z.; Herrmann, B.; Santos, J.; Jacques, N.; Melli, V.; Feekings, J.P. Fixed mesh shape reduces variability in codend size selection. Can. J. Fish. Aquat. Sci. 2022, 79, 1820–1829. [Google Scholar] [CrossRef]
- Mashiro, M. Fishery Biology of Ribbon Fish, Trichiurus lepturus; Bay, W.W., Ed.; Kyoto Institute of Oceanic and Fishery Science Special Report: Kyoto, Japan, 1991; Volume 3, pp. 1–74. [Google Scholar]
- Kenji, K. Study on the improvement of the productivity of trawl fishery. J. Tokyo Univ. Fish. 1960, 4, 1–158. [Google Scholar]
- Lee, D.J.; Kang, S.; Choi, K.H.; Jung, K.M. Species composition and seasonal variations of fishes collected by set net in coastal waters of Gijang. Korean J. Fish. Aquat. Sci. 2014, 47, 983–996. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, S.J.; Kim, S.G. A study on the behaviour of fish schools in the process of catch of the purse seine fishing method. J. Korean Soc. Fish. Ocean Technol. 1997, 33, 173–182. [Google Scholar]
- Koo, M.S.; Kim, S.J. Studies on the development of the fishing system of set net in the coast of Jeju Island 3. The model experiment of fyke net for construction improvement. J. Korean Soc. Fish. Ocean Technol. 2004, 40, 37–46, (In Korean with English Abstract). [Google Scholar]
- Madsen, N.; Hansen, K.E.; Moth-Poulsen, T. The kite cover: A new concept for covered codend selectivity studies. Fish. Res. 2001, 49, 219–226. [Google Scholar] [CrossRef]
- Lyle, J.M.; Willcox, S.T. Dolphin and seal interactions with mid-water trawling in the Small Pelagic Fishery, including an assessment of bycatch mitigation strategies. Final. Rep. Proj. 2008, 5. [Google Scholar]
- Baker, B.; Hamilton, S.; McIntosh, R.; Finley, L. Technical Review: Development and Application of Bycatch Mitigation Devices for Marine Mammals in Mid-water Trawl Gear. In Report prepared for the Department of the Environment (on Behalf of the Expert Panel); The Department of the Environment: Canberra, Australia, 2014; p. 12. [Google Scholar]
- Sabu, S.; Boopendranath, M.R. Studies on Soft Bycatch Reduction Devices for Selective Trawling. Ph.D. Thesis, Cochin University of Science & Technology, Cochin, India, 2008. [Google Scholar]



| Haul No. | Date | Start Position of the Haul | Underwater Camera Record | |
|---|---|---|---|---|
| Latitude | Longitude | |||
| #101 | 2 September 2024 | 34°56.058′ N | 129°03.446′ E | - |
| #102 | 3 September 2024 | 34°31.000′ N | 128°24.128′ E | - |
| #103 | 4 September 2024 | 34°46.076′ N | 128°37.967′ E | - |
| #104 | 4 September 2024 | 36°50.784′ N | 129°39.087′ E | - |
| #105 | 4 September 2024 | 35°56.072′ N | 129°40.945′ E | - |
| #106 | 5 September 2024 | 35°58.026′ N | 129°40.785′ E | - |
| #107 | 5 September 2024 | 35°05.693′ N | 129°42.921′ E | - |
| #108 | 6 September 2024 | 36°09.762′ N | 129°43.325′ E | ✓ |
| #109 | 6 September 2024 | 36°16.434′ N | 129°34.745′ E | ✓ |
| #110 | 8 September 2024 | 36°03.900′ N | 129°41.283′ E | ✓ |
| #111 | 9 September 2024 | 36°00.205′ N | 129°41.414′ E | ✓ |
| #112 | 9 September 2024 | 36°00.205′ N | 129°41.706′ E | ✓ |
| #113 | 10 September 2024 | 35°42.863′ N | 129°41.067′ E | ✓ |
| #114 | 10 September 2024 | 35°43.841′ N | 129°43.267′ E | ✓ |
| #115 | 11 September 2024 | 35°43.059′ N | 129°44.584′ E | ✓ |
| #116 | 11 September 2024 | 35°43.577′ N | 129°41.777′ E | ✓ |
| #117 | 12 September 2024 | 35°31.000′ N | 129°34.210′ E | ✓ |
| Common Name | Scientific Name | Number Caught | Weight of Catch |
|---|---|---|---|
| Hairtail | Trichiurus lepturus | 597 | 30.77 kg |
| Rudderfish | Psenopsis anomala | 55 | 4.07 kg |
| Jack mackerel | Trachurus japonicus | 61 | 1.43 kg |
| Mackerel | Scomber japonicus | 417 | 58.25 kg |
| Herring | Clupea pallasii | 264 | 8.42 kg |
| Firefly squid | Watasenia scintillans | 265 | 2.01 kg |
| Pearlsides | Maurolicus muelleri | 1,144,551 | 1230.20 kg |
| Nomura’s jellyfish | Nemopilema nomurai | 47 | 807.47 kg |
| Total | 1,146,257 | 2142.62 kg | |
| Haul No. | Date *1 | Recorded Duration | Remark | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COD *2 | BRD *2 | |||||||||||
| H | R | JM | M | J | H | R | JM | M | J | |||
| #108 | 6 September 2024 | 00 h 59 min 50 s | 1 | - | 2 | - | 1 | - | - | 36 | - | 6 |
| #109 | 6 September 2024 | 00 h 59 min 20 s | 2 | - | 15 | - | 1 | - | - | 5 | - | 13 |
| #110 | 8 September 2024 | 00 h 34 min 19 s | - | 3 | - | - | - | - | 1 | - | - | - |
| #111 | 9 September 2024 | 00 h 59 min 01 s | 4 | 2 | - | - | 1 | 12 | 1 | - | - | 5 |
| #112 | 9 September 2024 | 01 h 00 min 34 s | 28 | - | - | - | - | 39 | - | - | - | - |
| #113 | 10 September 2024 | 00 h 50 min 25 s | - | - | - | 10 | - | - | - | - | 30 | - |
| #114 | 10 September 2024 | 01 h 02 min 22 s | - | - | - | - | 1 | - | - | 1 | - | 5 |
| #115 | 11 September 2024 | 01 h 00 min 44 s | - | - | - | - | - | 10 | 1 | - | - | 2 |
| #116 | 11 September 2024 | 00 h 44 min 17 s | 12 | - | - | - | - | 53 | - | - | - | - |
| #117 | 12 September 2024 | 00 h 27 min 27 s | 10 | - | - | - | - | 11 | - | - | - | 3 |
| Common Name (Angle of BRD) | Estimation Method *1 | Logistic Parameters *2 | Selectivity Curve Parameters *3 | Model Fit | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a | b | L50 | C.I. of L50 | S.R. | C.I. of S.R. | Deviance | df | p-Value | ||
| Hairtail (30°) | Average of each haul | −0.110 (0.841) | 0.005 (0.072) | 20.8 | Error | 416.3 | Error | 0 | 19 | 0.98 |
| Sum of hauls | −0.032 (0.732) | 0.001 (0.052) | 28.7 | - | 1997.2 | - | 0 | 19 | 0.94 | |
| Hairtail (45°) | Average of each haul | −0.161 (0.845) | 0.003 (0.072) | 52.3 | Error | 715.5 | Error | 0 | 19 | 0.97 |
| Sum of hauls | −0.019 (2.82) | 0.002 (0.191) | 9.2 | - | 1046.3 | - | 0 | 19 | 0.99 | |
| Hairtail (total) | Average of each haul | −0.096 (0.843) | 0.004 (0.072) | 21.4 | Error | 491.3 | Error | 0 | 19 | 0.95 |
| Sum of hauls | −0.003 (0.071) | 0.001 (0.005) | 299.0 | - | 21974.9 | - | 0 | 19 | 0.99 | |
| Rudderfish (30°) | Average of each haul | −9.16 (5.26) | 0.55 (0.21) | 16.6 | 15.2–17.9 | 4.0 (2.7) | Error | 0 | 15 | 0.28 |
| Sum of hauls | −11.20 (7.68) | 0.67 (0.53) | 16.5 | - | 3.2 (0.5) | - | 0.1 | 15 | 0.19 | |
| Rudderfish (45°) | Average of each haul | −2.63 (1.55) | 0.12 (0.01) | 22.8 | Error | 19.0 (12.3) | Error | 0 | 15 | 0.42 |
| Sum of hauls | −2.60 (1.77) | 0.11 (0.03) | 20.4 | - | 20.4 (16.3) | - | 0.1 | 15 | 0.75 | |
| Rudderfish (total) | Average of each haul | −4.86 (2.95) | 0.26 (0.13) | 18.7 | Error | 8.4 (4.6) | Error | 0 | 15 | 0.27 |
| Sum of hauls | −4.97 (2.72) | 0.27 (0.16) | 18.6 | - | 8.2 (4.2) | - | 0.1 | 15 | 0.32 | |
| Jack mackerel (30°) | Average of each haul | −11.31 (7.79) | 0.72 (0.31) | 15.6 | 15.0–16.2 | 3.0 (1.6) | 0.1–4.8 | 0 | 15 | >0.05 |
| Sum of hauls | −7.90 (4.47) | 0.47 (0.21) | 16.7 | - | 4.6 (2.1) | - | 0 | 15 | <0.05 | |
| Jack mackerel (45°) | Average of each haul | −3.63. (1.94) | 0.22 (0.16) | 16.6 | 14.9–18.3 | 10.0 (6.63) | Error | 0 | 15 | 0.18 |
| Sum of hauls | −3.37 (1.95) | 0.20 (0.15) | 16.8 | - | 11.0 (7.51) | - | 0 | 15 | 0.19 | |
| Jack mackerel (total) | Average of each haul | −5.95 (3.57) | 0.37 (0.27) | 16.1 | 15.5–16.7 | 5.9 (4.1) | Error | 0 | 15 | >0.05 |
| Sum of hauls | −6.00 (2.02) | 0.37 (0.15) | 16.4 | - | 6.0 (2.5) | - | 0 | 15 | 0.02 | |
| Mackerel (30°) | Average of each haul | −35.97 (26.74) | 1.81 (1.34) | 19.9 | 19.8–20.0 | 2.2 (0.9) | 2.0–2.4 | 0 | 24 | <0.05 |
| Sum of hauls | −35.00 (4.38) | 1.75 (0.21) | 20.0 | - | 2.3 (0.1) | - | 0 | 24 | <0.01 | |
| Herring (45°) | Average of each haul | −20.61 (16.13) | 1.20 (0.84) | 17.1 | 17.0–17.2 | 2.1 (1.3) | 2.0–2.2 | 0 | 17 | 0.12 |
| Sum of hauls | −18.08 (3.11) | 1.05 (0.19) | 17.1 | - | 2.1 (0.4) | - | 0 | 17 | <0.01 | |
| Name | Scientific Name | Catch Loss Rate (%) *1 | |||||
|---|---|---|---|---|---|---|---|
| 30° | 45° | Total (Average) | |||||
| N | W | N | W | N | W | ||
| Hairtail | Trichiurus lepturus | 53 | 52 | 52 | 58 | 53 | 54 |
| Rudderfish | Psenopsis anomala | 18 | 19 | 24 | 26 | 22 | 23 |
| Jack mackerel | Trachurus japonicus | 14 | 16 | 20 | 19 | 18 | 18 |
| Mackerel | Scomber japonicus | 97 | 97 | - | - | 97 | 97 |
| Herring | Clupea pallasii | - | - | 13 | 11 | 13 | 11 |
| Nomura’s jellyfish *2 | Nemopilema nomurai | 93 | 93 | 88 | 89 | 89 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-M.; Kim, H.-Y.; Cha, B.-J.; Kim, S.-J.; Kim, T.-S.; Hyun, G.-C.; Choi, K.-S. Catch Losses and Reduction of Bycatch for Jellyfish Using Marine Mammal Bycatch Reduction Devices in Midwater Trawl Gear. Fishes 2025, 10, 276. https://doi.org/10.3390/fishes10060276
Jung J-M, Kim H-Y, Cha B-J, Kim S-J, Kim T-S, Hyun G-C, Choi K-S. Catch Losses and Reduction of Bycatch for Jellyfish Using Marine Mammal Bycatch Reduction Devices in Midwater Trawl Gear. Fishes. 2025; 10(6):276. https://doi.org/10.3390/fishes10060276
Chicago/Turabian StyleJung, Jung-Mo, Hyun-Young Kim, Bong-Jin Cha, Sung-Jae Kim, Tae-Suk Kim, Gyeong-Cheol Hyun, and Kyu-Suk Choi. 2025. "Catch Losses and Reduction of Bycatch for Jellyfish Using Marine Mammal Bycatch Reduction Devices in Midwater Trawl Gear" Fishes 10, no. 6: 276. https://doi.org/10.3390/fishes10060276
APA StyleJung, J.-M., Kim, H.-Y., Cha, B.-J., Kim, S.-J., Kim, T.-S., Hyun, G.-C., & Choi, K.-S. (2025). Catch Losses and Reduction of Bycatch for Jellyfish Using Marine Mammal Bycatch Reduction Devices in Midwater Trawl Gear. Fishes, 10(6), 276. https://doi.org/10.3390/fishes10060276






