Abstract
Shewanella putrefaciens is a significant bacterial pathogen causing high mortality in farmed largemouth bass (Micropterus salmoides). This study investigated the molecular immune responses in its primary target organs, the liver and spleen, via transcriptomic profiling at 24 h post-infection. We identified 458 significantly differentially expressed genes (DEGs) in the liver and 1405 in the spleen. Gene Ontology enrichment analysis revealed organ-specific immune strategies: the liver response was characterized by type I interferon signaling pathway, whereas the spleen response centered on the regulation of innate immune response. Furthermore, Kyoto Encyclopedia of Genes and Genomes pathway analysis showed that fatty acid metabolism and cytokine-cytokine receptor interaction were significantly enriched in the liver. In contrast, the C-type lectin receptor signaling pathway and cytokine-cytokine receptor interaction were the most prominent in the spleen. Several key DEGs (e.g., stat1a, rsad2, pglyrp5, pglyrp6, acaca, stat2, lepb) associated with immune response, metabolic adaptation, and cellular stress were identified, suggesting a coordinated host mechanism involving pathogen recognition, immunomodulation, and tissue repair. These results provide crucial insights into the immunomodulatory processes in largemouth bass against S. putrefaciens infection.
Key Contribution:
(1) We provide the first dual-organ (liver & spleen) in vivo transcriptomic map of largemouth bass challenged with Shewanella putrefaciens at 24 h, revealing 458 liver DEGs and 1405 spleen DEGs. (2) We uncover organ-specific immune programs: liver responses enriched for type I interferon signaling and metabolic reprogramming (fatty-acid pathways), while spleen responses are dominated by C-type lectin receptor signaling and cytokine–receptor interactions. (3) We identify candidate biomarkers/targets—including pglyrp5/pglyrp6, stat1a/stat2, rsad2, acaca, and lepb—that underpin pathogen recognition, IFN-pathway modulation, and immunometabolic adaptation. (4) We validate RNA-seq trends with qRT-PCR and histopathology, linking molecular signatures to tissue pathology, and provide a resource to guide diagnostics, therapeutics, and selective breeding for disease resistance in aquaculture.
1. Introduction
The largemouth bass (Micropterus salmoides), also known as the California bass, is native to North America and is a popular freshwater game fish in the United States [1]. This carnivorous fish (order Perciformes, family Centrarchidae) was introduced to Chinese aquaculture in the 1980s [2]. In recent years, largemouth bass has become an important aquaculture species in China, with annual production exceeding 800,000 tons in 2022—a 14.3% increase over 2021 [3]. As the bass farming industry rapidly expands, disease outbreaks have become a major constraint on sustainable development. Bacterial diseases pose the most serious threat, and Shewanella putrefaciens is a prime example. S. putrefaciens is a widely distributed Gram-negative opportunistic pathogen that can infect various aquatic animals, including largemouth bass [4]. Infected fish typically exhibit external bleeding, ulceration, and congestion, along with enlargement and necrosis of internal organs—particularly the liver and spleen—ultimately resulting in high mortality rates (outbreaks can cause 30–80% mortality) [5,6]. Notably, pathological studies have shown that the liver and spleen are the primary target organs and are the most severely affected tissues during S. putrefaciens infection [7,8], suggesting that these organs play central roles in host defense and disease pathogenesis.
In fish, the liver is not only the central organ of metabolism but also an important immune defense organ, enriched with macrophages and other immune cells. The liver clears blood-borne pathogens and toxins and is a key site of acute-phase immune responses [9,10]. The spleen is the most important peripheral (secondary) lymphoid organ in teleost fish, serving as a crucial site for antigen filtration, lymphocyte activation, proliferation and differentiation, antibody production, and antigen clearance [11,12]. Given these roles, a simultaneous investigation of S. putrefaciens infection effects on both liver and spleen transcriptomes can illuminate the host’s molecular response networks in two key immune and metabolic organs, providing a unique perspective on host–pathogen interactions.
Although some studies have examined the pathogenicity of S. putrefaciens, the resulting pathological changes in largemouth bass, and a few immune parameters post-infection [13], a comprehensive understanding of genome-wide host transcriptomic regulation after infection remains lacking—particularly regarding organ-specific and coordinated responses in liver versus spleen. High-throughput RNA sequencing (RNA-Seq) offers an unbiased, powerful tool to capture global gene expression dynamics, key pathway activations/suppressions, and inter-organ functional differences during pathogen infection [13,14]. Indeed, transcriptomic approaches have been used in past decades to explore how bacterial infections affect largemouth bass physiology. For example, transcriptome analyses have revealed distinct responses of largemouth bass to early Aeromonas hydrophila infection versus immunization [15], identified histological damage and changes in immune-related and apoptosis genes in bass infected with Nocardia seriolae [16], and demonstrated that Edwardsiella tarda infection induces significant changes in pattern recognition receptor signaling pathways, pro-inflammatory cytokines, phagosome and lysosome activity, adaptive immune genes, and metabolic pathways in bass liver, indicating a strong innate immune reaction [17]. However, to our knowledge, a systematic in vivo transcriptomic analysis of largemouth bass liver and spleen responses to S. putrefaciens infection has not yet been described.
Based on this background, the present study aimed to use RNA-Seq to comprehensively map the liver and spleen transcriptomic profiles of largemouth bass at 24 h post S. putrefaciens infection. We sought to identify infection-induced differentially expressed genes (DEGs); perform Gene Ontology functional annotation and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses to determine significantly enriched biological processes, molecular functions, and signaling pathways; compare the transcriptomic response patterns between liver and spleen to reveal organ-specific functional responses; and pinpoint candidate genes and core pathways that may play key roles in anti-infection immunity or infection-induced pathology.
This study is the first to systematically characterize the dual-organ (liver and spleen) transcriptomic regulatory network of largemouth bass in response to S. putrefaciens infection. Our findings deepen the understanding of antibacterial immune mechanisms in Perciform fish, enriching theoretical knowledge of fish immunology and host–pathogen interactions. The key DEGs and pathways identified may serve as potential biomarkers for disease or targets for drug/vaccine development. Resistance-related genes discovered could provide a theoretical basis and genetic resources for breeding disease-resistant bass strains. Ultimately, these insights offer a scientific foundation for new precise prevention and control strategies for S. putrefaciens disease based on host immune modulation, supporting the healthy and sustainable development of largemouth bass aquaculture.
2. Materials and Methods
2.1. Experimental Animals and Bacterial Strain
The pathogenic bacterium used in this study was Shewanella putrefaciens strain Mu-1, isolated from diseased largemouth bass. Healthy juvenile largemouth bass (body length 10 ± 2 cm, body weight 12 ± 3 g) were obtained from a fish farm in Zhejiang Province, China. Fish were acclimated in aerated tanks prior to experiments.
2.2. Challenge Experiment for LC50 Determination
To determine the median lethal concentration (LC50) of S. putrefaciens for largemouth bass, an immersion challenge was conducted. Before the experiment, fish were housed (10 fish per tank) and maintained without feeding for 2 days, then fed normally for the next 2 days, and finally fasted for 24 h prior to infection. Frozen stocks of S. putrefaciens Mu-1 were thawed and streaked onto solid medium; a single colony was inoculated into 5 mL of liquid medium and cultured at 30 °C with shaking for 24 h. The broth culture was serially diluted (10−5 to 10−9) and plated to estimate the initial bacterial concentration. For the challenge, the culture was diluted to a series of concentrations (the initial undiluted culture and 2×, 5×, 10× dilutions in sterile saline). Fish were injected intraperitoneally with 200 μL of each bacterial suspension (or saline for controls). Each dose group contained 30 fish (3 replicate tanks of 10 fish each). Mortality was monitored daily for 7 days to maintain the accuracy of our LC50 determination. The probit analysis (SPSS 18.0 software) was used to calculate the LC50 on Day 2 post-infection. The estimated 48 h LC50 of S. putrefaciens for largemouth bass was 2.22 × 109 CFU/mL.
All experimental procedures were conducted in accordance with the ethical guidelines of Jiangsu Ocean University, and the protocol was approved by the University’s Animal Care and Use Committee.
2.3. Experimental Infection and Sample Collection
Following LC50 determination, fish were randomly divided into two groups for the main infection experiment: an infected group and a control group. In the infected (challenge) group, 30 bass were each injected intraperitoneally with 200 μL of the S. putrefaciens suspension at the LC50 concentration (approximately 2.22 × 109 CFU/mL). In the control group, 30 bass were injected with 200 μL of sterile physiological saline. Fish were maintained at 24 ± 2 °C, pH 6.8–8.0, with continuous aeration to maintain dissolved oxygen around 5.7 mg/L.
At 24 h post-injection, six surviving fish from each group were humanely euthanized for sample collection. For each group, liver and spleen tissues were collected from three fish and immediately fixed in 10% neutral buffered formalin for histological analysis (Section 2.4). Liver and spleen from the other three fish were snap-frozen in liquid nitrogen and stored at −80 °C for RNA extraction and transcriptomic analysis. Samples were labeled as follows: LICS (liver, injection control saline), LIS (liver, infected with S. putrefaciens), SICS (spleen, injection control saline), and SIS (spleen, infected with S. putrefaciens). All procedures involving animals were performed under the ethical guidelines of Jiangsu Ocean University.
2.4. Histological Examination
For histopathology, tissue samples (liver and spleen) were fixed in 10% formalin for 24 h. Fixed samples were processed using an automated tissue processor with graded ethanol dehydration (50% to 100%), cleared, and embedded in paraffin. Paraffin blocks were sectioned at 4–6 μm thickness using a rotary microtome (Leica Microsystems, Wetzlar, Germany), and sections were mounted on glass slides. Paraffin sections were deparaffinized and rehydrated, then stained with hematoxylin and eosin (H&E) for general histological evaluation. Stained sections were examined under an Olympus CX33 light microscope (Olympus, Tokyo, Japan), and images were captured with a microscope-mounted camera system.
2.5. RNA Extraction and Sequencing
Total RNA was extracted from liver and spleen tissue samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. RNA quality and integrity were assessed by 1% agarose gel electrophoresis (to check for intact 28S and 18S rRNA bands and absence of genomic DNA contamination) and by using a NanoPhotometer spectrophotometer (Implen, Munich, Germany) to measure OD_260/280 and OD_260/230 ratios (ensuring minimal protein or organic contaminant presence). RNA integrity was further evaluated with an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) to obtain the RNA integrity number (RIN) for each sample. Only high-quality RNA (RIN > 7.0, OD_260/280~2.0) was used for library construction. All steps were carried out in an RNase-free environment to prevent RNA degradation.
For each sample, poly(A)+ mRNA was enriched from 1 μg of total RNA using oligo(dT) magnetic beads. The mRNA was fragmented using NEB Fragmentation Buffer under elevated temperature and divalent cations. First-strand cDNA was synthesized from fragmented mRNA using random hexamer primers and reverse transcriptase, followed by second-strand cDNA synthesis. The double-stranded cDNA fragments were end-repaired, A-tailed, and ligated to Illumina sequencing adapters according to the NEB standard library preparation protocol (New England Biolabs, Ipswich, MA, USA) [15]. The ligated products were purified and PCR-amplified to create the final cDNA libraries. Library quality was checked using a Qubit 2.0 fluorometer (initial concentration, diluted to ~1.5 ng/μL) and an Agilent 2100 Bioanalyzer (to confirm proper insert size distribution). Quantitative PCR (Bio-Rad Laboratories, Hercules, CA, USA) was used to ensure the effective library concentration was ≥2 nM, compatible with Illumina sequencing.
Equimolar amounts of qualified libraries from all samples were pooled. The pooled libraries were sequenced on an Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA) with a paired-end 150 bp (PE150) read configuration to generate high-throughput sequencing data.
2.6. Differential Gene Expression and Enrichment Analysis
Raw sequencing reads were subjected to quality control to obtain clean reads for analysis. Adapter sequences, low-quality reads (Phred < 20), and reads containing ploy-N stretches were removed to produce high-quality clean reads. The clean reads for each sample were then aligned to the reference largemouth bass genome (assembly ASM1485139v1) using HISAT2 v2.2.1, a fast and sensitive spliced-read aligner [18]. Alignment results provided the mapping location and orientation of reads on the reference genome. Gene expression levels were quantified (e.g., in fragments per kilobase of transcript per million mapped reads, FPKM).
Differential expression analysis between groups was performed using DESeq2 [19]. To identify genes responsive to S. putrefaciens infection, we performed pairwise differential expression analysis between infected and control groups for each organ. Specifically, the comparison LIS vs. LICS identified liver infection-responsive DEGs, while SIS vs. SICS identified spleen infection-responsive DEGs. Genes with an adjusted p-value (false discovery rate, FDR) < 0.05 and |log_2(fold change)| > 1 were considered significantly differentially expressed. DEGs were compiled for downstream functional analysis.
Functional annotation of DEGs was conducted through Gene Ontology (GO) classification and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment. GO enrichment analysis (biological process, molecular function, cellular component categories) was performed to identify GO terms significantly over-represented among DEGs relative to the genome background (using a hypergeometric test, p < 0.05 considered significant). KEGG pathway enrichment analysis was similarly performed to identify metabolic or signaling pathways significantly enriched with DEGs. These analyses provide insight into the biological processes and pathways most affected by S. putrefaciens infection in each organ.
2.7. qRT-PCR Validation
To validate the RNA-Seq results, 12 DEGs were selected for quantitative real-time PCR (qRT-PCR) analysis. Six genes were chosen from the liver transcriptome (pglyrp6, il13ra2, ptx3a, dhx58, rsad2, irf3) and six from the spleen transcriptome (fkbp7, fkbp11, dcn, il2rb, prlrb, hprt1l). Gene-specific primers were designed using Primer Premier 5 (Premier Biosoft) based on the mRNA sequences (Table 1), and synthesized by Sangon Biotech (Shanghai, China). β-Actin was used as an internal reference gene for normalization.

Table 1.
Primers used for qRT-PCR validation of selected DEGs.
Total RNA from liver and spleen (the same samples used for RNA-Seq) was reverse-transcribed into cDNA using a PrimeScript RT reagent kit (Takara, Shiga, Japan) according to the manufacturer’s instructions. qRT-PCR was performed on a Roche LightCycler 96 system with TB Green Premix Ex Taq II (Tli RNase H Plus, Takara, Shiga, Japan). Each 20 μL PCR reaction contained 2 μL of diluted cDNA, 0.4 μL of each primer (10 μM forward and reverse), 10 μL of 2 × TB Green PCR master mix, and nuclease-free water up to volume. Thermal cycling conditions were: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Melting curve analysis was conducted to confirm single specific amplification.
Each sample was run in triplicate. The comparative 2−ΔΔCt method was used to calculate relative gene expression levels [20]. Expression of each target gene was normalized to β-actin and compared between infected and control groups for each tissue. The qRT-PCR results (fold-change direction and magnitude) were then compared to the RNA-Seq expression data for validation. Pearson correlation analysis was used to assess the consistency between RNA-Seq and qRT-PCR results.
3. Results
3.1. Lethal Dose and Mortality in Infection Challenge
Using the colony-count method, the initial S. putrefaciens culture concentration was estimated at 7.5 × 109 CFU/mL for injection. In the preliminary challenge, fish injected with the highest concentrations showed rapid mortality: by Day 3 post-infection, nearly all fish in the undiluted, 2×, and 5× diluted groups had died (Table 2). Mortality in lower dose groups plateaued by Day 3 with much lower overall rates. The LC50 was therefore determined based on the cumulative 48 h mortality. Probit analysis (Table 3) indicated that the 48 h LC50 for S. putrefaciens injected into largemouth bass was 2.22 × 109 CFU/mL. This dose was used for subsequent infection experiments.

Table 2.
Mortality of largemouth bass at different S. putrefaciens challenge doses over 7 days (mean ± SD, n = 3 tanks per dose).

Table 3.
Probit analysis for LC50 determination: cumulative mortality in the first 2 days post-injection at each dose.
3.2. Histopathological Changes in Liver and Spleen
In control fish, liver histology was normal, with hepatocytes forming radial cords and moderate vacuolation, and clear hepatic sinusoids (Figure 1A–C). In contrast, largemouth bass injected with S. putrefaciens showed notable liver lesions by 24 h post-infection. Hepatocytes exhibited markedly condensed (more eosinophilic) cytoplasm, indicative of cellular stress or degeneration. The liver tissue showed blood congestion in hepatic sinusoids and veins (Figure 1D, arrow d). Occasional foci of dark brown pigment accumulation were observed in infected livers (Figure 1E,F, arrows e, f), which may represent hemosiderin or other pigment deposition associated with tissue damage. Hepatocyte vacuolation appeared reduced compared to controls, likely due to the cytoplasmic condensation.

Figure 1.
Histopathological changes in largemouth bass liver after S. putrefaciens injection. (A–C) Liver of control fish (saline injection), showing hepatocytes arranged in cords (lobular structure) and moderate cytoplasmic vacuolation (arrows a, b, c). (D–F) Liver of infected fish 24 h post-injection, showing condensed hepatocyte cytoplasm and pathological congestion of hepatic blood sinusoids (arrow d), with occasional localized pigment deposition in hepatocytes (arrows e, f).
In the spleen, control and infected fish both showed disorganized splenic architecture typical of teleosts (absence of clear follicular structure and indistinct red/white pulp boundaries). Both groups had scattered melanomacrophage centers (pigmented macrophage aggregates) visible as brown deposits (Figure 2). However, the infected spleens had a slight depletion of red pulp erythrocytes relative to controls, suggesting mild anemia or pooling of blood elsewhere (Figure 2D–F vs. Figure 2A–C). Rare instances of cell necrosis were noted in the spleen of infected fish, reflecting some degree of tissue damage. Overall, the spleen lesions were less pronounced than those in the liver, but the reduction in red pulp cellularity implies a potential impairment of splenic hematopoietic or immune function after infection.
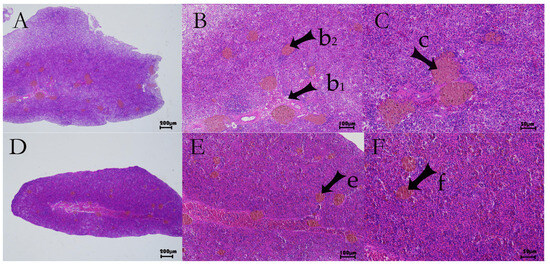
Figure 2.
Histopathological changes in largemouth bass spleen after S. putrefaciens injection. (A–C) Spleen of control fish (saline injection). (D–F) Spleen of infected fish. Melanin-like pigment deposits (brown granules) are present in both groups (arrows b, c). Infected spleens show slightly reduced red pulp erythrocyte density (arrows e, f) and occasional cellular necrosis (indicative of tissue damage).
These histopathological findings offer significant novel insights into the early and organ-specific pathological progression of the infection and also confirm that S. putrefaciens infection causes significant tissue damage in largemouth bass, with particularly severe effects in the liver (parenchymal degeneration and vascular congestion) and detectable, though milder, changes in the spleen.
3.3. Transcriptome Sequencing Data Quality
To investigate the molecular response of bass to S. putrefaciens, we performed transcriptome sequencing on liver and spleen samples from infected and control fish (3 biological replicates per tissue per treatment, 12 libraries total). The Illumina NovaSeq 6000 run generated a total of 680,282,288 raw reads. After quality filtering, an average of >99.99% of reads per sample were retained as high-quality clean reads. On average, 97.73% of clean reads from each library mapped to the M. salmoides reference genome (total mapping rate). The average Q30 quality score (percentage of bases with Phred quality ≥ 30) was 96.56%, indicating high sequencing accuracy. The average GC content of the reads was 46.40%. These metrics demonstrate that the sequencing data were of high quality and sufficient depth for reliable downstream analysis (Table 4).

Table 4.
Summary of transcriptome sequencing data for largemouth bass liver and spleen samples (three biological replicates each for control and infected groups).
3.4. Differential Gene Expression Analysis
Using the thresholds of FDR < 0.05 and |log2(FoldChange)| > 1, a total of 12,547 genes were identified as differentially expressed in at least one of the comparisons related to S. putrefaciens infection.
In the liver (infected group LIS vs. control LICS), S. putrefaciens infection induced 458 DEGs, of which 143 were significantly up-regulated and 315 were down-regulated in infected liver (Figure 3A, Table S1). In the spleen (infected SIS vs. control SICS), 1405 DEGs were identified, with 580 up-regulated and 825 down-regulated in infected spleen (Figure 3B, Table S2). Thus, the spleen showed approximately three times as many DEGs as the liver in response to infection. We also compared the infected liver vs. infected spleen (LIS vs. SIS) to examine organ-specific responses under infection conditions, which yielded 10,684 DEGs (4998 higher in liver, 5686 higher in spleen), reflecting the substantial baseline differences in liver and spleen transcriptomes and their distinct responses to infection.
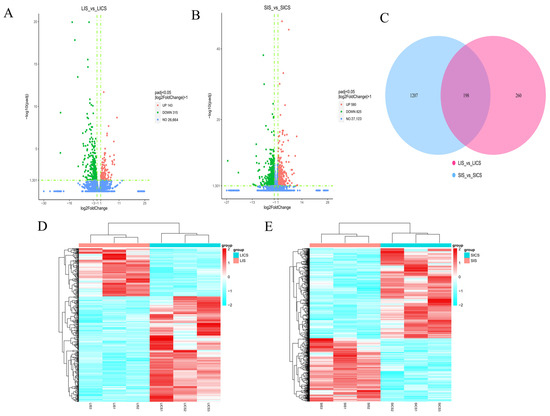
Figure 3.
Differentially expressed genes (DEGs) in largemouth bass liver and spleen in response to S. putrefaciens infection. (A,B) Volcano plots of gene expression changes in (A) liver (LIS vs. LICS) and (B) spleen (SIS vs. SICS). Each point represents a gene; red points indicate significantly up-regulated DEGs and green points indicate significantly down-regulated DEGs (FDR < 0.05, |log2FC| > 1); the horizontal dashed line indicates the statistically significant threshold (padj < 0.05). (C) Venn diagram of DEGs in liver and spleen comparisons, showing the overlap of 198 DEGs common to both tissues and the large number of DEGs unique to spleen. (D,E) Heatmaps of DEGs for liver (D) and spleen (E) samples, illustrating distinct clustering of infected vs. control groups and overall expression patterns.
A Venn diagram of DEGs in the liver and spleen comparisons revealed overlap and specificity (Figure 3C). There were 198 DEGs common to both the liver (LIS vs. LICS) and spleen (SIS vs. SICS) comparisons, indicating a core set of genes commonly responsive to S. putrefaciens infection in both organs. The liver had 260 unique DEGs not found in spleen, whereas the spleen had a much larger organ-specific DEG set of 1207 genes not differentially expressed in liver. This underscores that the spleen mounted a broader transcriptional response and that many responsive genes were organ-specific.
Hierarchical clustering of expression patterns (heatmaps) showed clear segregation of samples by treatment within each tissue (Figure 3D,E). In both liver and spleen, the infected samples clustered together and separately from controls, demonstrating high within-group similarity and distinct differences between infected and control transcriptomes.
3.5. GO Functional Enrichment Analysis
GO enrichment analysis was performed to identify the biological processes, molecular functions, and cellular components significantly associated with the DEGs. In the liver (LIS vs. LICS DEGs), immune-related GO terms were prominently enriched. The two most significant Biological Process (BP) terms were “type I interferon signaling pathway” and “cellular response to type I interferon”, indicating strong activation of interferon-mediated antiviral responses in the liver (Figure 4A). The BP terms with highest enrichment ratios (fold enrichment) were “response to molecule of bacterial origin” and “regulation of innate immune response”, suggesting that liver DEGs are heavily involved in recognizing bacterial components and modulating innate immunity. In the Molecular Function (MF) category, the top two significant terms were “peptidoglycan muralytic activity” and “G protein–coupled receptor binding”, with the latter also being the most enriched MF term overall, reflecting roles in pathogen recognition and signaling. In the Cellular Component (CC) category, the most significant terms were “clathrin-coated endocytic vesicle” and “alveolar lamellar body”, while the most enriched CC term was “vesicle lumen”. These CC enrichments suggest involvement of intracellular vesicular structures, possibly related to endocytosis and secretion, in the liver’s response.
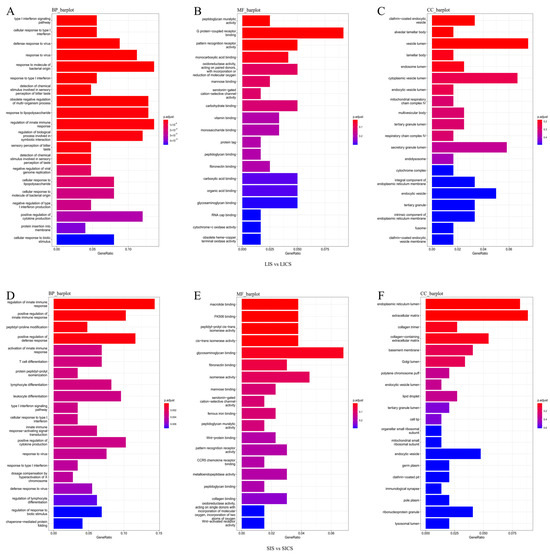
Figure 4.
GO enrichment of DEGs in (A–C) liver and (D–F) spleen following S. putrefaciens infection. Bar charts show the top GO terms in three categories: Biological Process (BP), Molecular Function (MF), and Cellular Component (CC). The length of each bar represents the number of DEGs associated with the term, and the color indicates the significance of enrichment (darker colors = lower FDR). In the liver (A), immune-related processes like type I interferon signaling and innate immune regulation are highly significant. In the spleen (B), terms related to innate immune response regulation are prominent, along with terms indicating changes in the endoplasmic reticulum and extracellular matrix.
In the spleen (SIS vs. SICS DEGs), the GO analysis likewise highlighted immune processes. The top BP terms by significance were “regulation of innate immune response” and “positive regulation of innate immune response”, indicating that spleen DEGs predominantly modulate innate immunity (Figure 4B). The term “regulation of innate immune response” was also the one with the highest enrichment in BP. For MF, the most significant terms were “macrolide binding” and “FK506 binding”; interestingly, the term with highest enrichment was “glycosaminoglycan binding”, pointing to altered interactions with extracellular matrix components or pathogens. In the CC category, the most significant terms were “endoplasmic reticulum lumen” and “extracellular matrix”, with “extracellular matrix” also showing the highest enrichment. This implies spleen DEGs may affect secretory pathways and the extracellular environment, perhaps in the context of tissue remodeling or immune cell migration.
Overall, GO analysis indicates that S. putrefaciens infection triggers a strong innate immune response in both liver and spleen, with the liver showing signatures of interferon signaling and pathogen sensing, and the spleen showing broad regulation of innate immunity and changes in cell–matrix interactions.
3.6. KEGG Pathway Enrichment Analysis
KEGG pathway analysis was used to identify which metabolic or signaling pathways were significantly enriched with DEGs in each tissue. In the liver (LIS vs. LICS), the two most significantly enriched pathways (by statistical significance) were “Fatty acid metabolism” and “Biosynthesis of unsaturated fatty acids” (Figure 5A). This suggests that S. putrefaciens infection substantially impacts hepatic lipid metabolic processes, perhaps reflecting alterations in energy utilization during the immune response. Notably, pathways with the highest enrichment ratios in liver DEGs were “Cytokine–cytokine receptor interaction” and “Phagosome”. Enrichment of the cytokine signaling pathway indicates active immune communication (e.g., cytokine production and receptor expression changes), and enrichment of the phagosome pathway reflects involvement in pathogen uptake and processing by liver phagocytic cells (such as Kupffer cells).
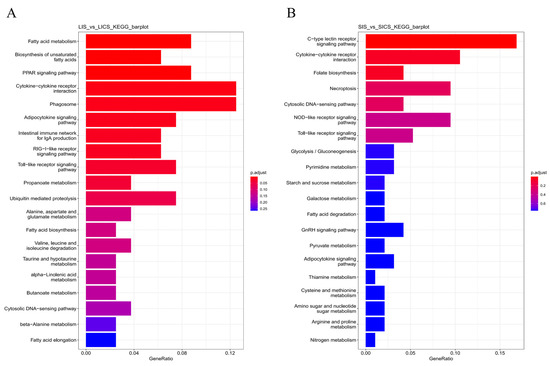
Figure 5.
Top 20 enriched KEGG pathways among DEGs in (A) liver (LIS vs. LICS) and (B) spleen (SIS vs. SICS). The x-axis indicates the Rich Factor (ratio of DEG count to total gene count in each pathway), and point size reflects the number of DEGs in that pathway. Point color denotes the statistical significance (adjusted p-value). In liver (A), metabolic pathways like fatty acid metabolism and unsaturated fatty acid biosynthesis are significantly enriched, alongside immune pathways like cytokine–receptor interactions and phagosome. In spleen (B), immune pathways dominate: C-type lectin receptor signaling and cytokine–cytokine receptor interaction are both highly enriched and significant, indicating a strong immune activation in splenic tissue.
In the spleen (SIS vs. SICS), the top two pathways by significance were the “C-type lectin receptor signaling pathway” and “Cytokine–cytokine receptor interaction” (Figure 5B). C-type lectin receptors are pattern recognition receptors important for detecting pathogens (especially fungi and bacteria) and initiating immune responses, so their signaling pathway being enriched underscores the spleen’s role in pathogen sensing during infection. Additionally, the pathways with the highest enrichment in spleen DEGs were “C-type lectin receptor signaling pathway” and “Cytokine–cytokine receptor interaction” (the same two are both highly significant and highly enriched), highlighting these as dominant response pathways in the spleen. These results indicate that spleen DEGs strongly involve cell-surface immune receptors and cytokine networks, consistent with an activated state of innate immune cells (such as macrophages and dendritic cells) and intercellular immune signaling in the spleen following infection.
In summary, KEGG analysis suggests that S. putrefaciens infection causes metabolic reprogramming in the liver (notably affecting fatty acid metabolism, likely to meet the energetic and biosynthetic demands of immune activation), and robust activation of immune signaling pathways in both liver and spleen. The spleen shows a pronounced enrichment of pattern recognition receptor signaling (C-type lectins) and cytokine interactions, corroborating its central role in orchestrating systemic immune responses to the bacterial infection.
3.7. qRT-PCR Validation of DEG Expression
To verify the RNA-Seq findings, we performed qRT-PCR on 12 selected DEGs (6 from liver, 6 from spleen). The qRT-PCR results corroborated the transcriptomic data for all tested genes, showing consistent direction and similar magnitude of expression changes (Figure 6). In the liver (Figure 6A), pglyrp6, il13ra2, and ptx3a were confirmed to be up-regulated in infected fish relative to controls, while dhx58, rsad2, and irf3 were down-regulated, matching the RNA-Seq trends. In the spleen (Figure 6B), fkbp7, fkbp11, and dcn were validated as up-regulated in infected fish, whereas il2rb, prlrb, and hprt1l were down-regulated, again in agreement with the sequencing results. The expression fold changes measured by qRT-PCR were generally in good quantitative agreement with the RNA-Seq fold changes for each gene.
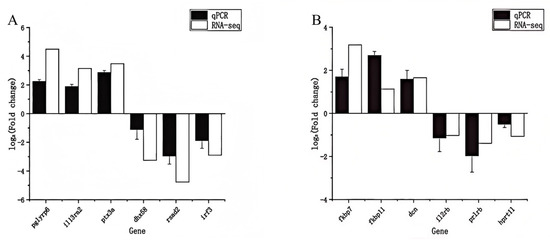
Figure 6.
qRT-PCR validation of selected DEGs identified by RNA-Seq. Expression levels of six genes in (A) liver and (B) spleen from infected vs. control fish were measured by qRT-PCR (black filled bars) and compared to RNA-Seq fold changes (white open bars). Data are presented as mean relative expression (infected/control) ± SD (n = 3). In the liver (A), pglyrp6, il13ra2, and ptx3a showed up-regulation, and dhx58, rsad2, irf3 showed down-regulation, consistent with transcriptome results. In the spleen (B), fkbp7, fkbp11, dcn were up-regulated, and il2rb, prlrb, hprt1l were down-regulated, matching RNA-Seq trends. The high concordance between qRT-PCR and RNA-Seq indicates the reliability of the gene expression data.
Overall, there was a strong positive correlation between the RNA-Seq and qRT-PCR expression data (Pearson r > 0.9, p < 0.01), indicating high reliability of the RNA-Seq analysis. The successful validation of these DEGs—including genes involved in pathogen recognition (pglyrp6, ptx3a), cytokine signaling (il13ra2, il2rb), antiviral response (rsad2, irf3), and other immune or stress responses–supports the robustness of our transcriptomic findings and the biological significance of the observed gene expression changes following S. putrefaciens infection.
4. Discussion
Shewanella putrefaciens poses a severe threat to largemouth bass aquaculture, yet the molecular mechanisms underlying the host’s immune response remain poorly characterized. This study provides the first comprehensive, dual-organ transcriptomic analysis of largemouth bass challenged with S. putrefaciens at 24 h post-infection. We identified 458 and 1405 DEGs in the liver and spleen, respectively, revealing distinct organ-specific immune and metabolic reprogramming. The liver response was characterized by type I interferon signaling and altered fatty acid metabolism, while the spleen mounted a broader innate immune response dominated by C-type lectin receptor signaling and cytokine interactions. Key DEGs, including stat1a, rsad2, pglyrp5, pglyrp6, acaca, stat2, and lepb, were identified as central players in pathogen recognition, immunomodulation, and metabolic adaptation.
The liver transcriptome revealed a pronounced enrichment of type I interferon (IFN-I) signaling, a pathway typically associated with antiviral defense. This suggests a potential crossover in immune recognition between viral and bacterial pathogens in teleosts [21]. However, the concurrent significant downregulation of central mediators of the IFN-I pathway, stat1a and stat2, presents a compelling paradox. This likely indicates an active bacterial strategy to subvert host immunity by suppressing the JAK-STAT signaling cascade, a mechanism noted in other host–pathogen interactions [22]. Beyond interferon biology, the liver exhibited a significant shift in lipid metabolism, with the “Fatty acid metabolism” pathway being highly enriched. The downregulation of acaca, a rate-limiting enzyme in fatty acid synthesis, suggests a metabolic rewiring away from energy storage and towards beta-oxidation to meet the high energy demands of the activated immune response [23]. This establishes the liver as a critical nexus where metabolic and immune processes intersect during infection.
In contrast to the liver, the splenic transcriptome was overwhelmingly dominated by terms related to the regulation of innate immunity. The most significantly enriched KEGG pathway was the “C-type lectin receptor (CLR) signaling pathway.” CLRs are key pattern recognition receptors for detecting bacterial components, and their enrichment underscores the spleen’s primary role in systemic pathogen surveillance and initiation of innate immunity [24]. This robust pathogen-sensing capacity was complemented by a strong enrichment in “Cytokine-cytokine receptor interaction,” indicative of intense intercellular communication among immune cells to orchestrate a coordinated defense. While previous research has detailed the histopathological damage in the spleen during S. putrefaciens infection [13], our data provide the molecular signature driving these pathological changes, linking tissue-level necrosis and erythrocyte depletion to the dysregulation of specific immune signaling pathways.
A core set of immune genes was dysregulated in both organs, highlighting their systemic importance. The peptidoglycan recognition proteins pglyrp5 and pglyrp6 were significantly upregulated. These genes are critical for detecting Gram-negative bacterial cell walls and initiating antibacterial effector responses. Their induction aligns with recent findings in other teleost species, confirming a conserved role for PGRPs in antibacterial defense [25]. Another key finding was the downregulation of rsad2 (viperin) in both tissues. Although viperin is an interferon-stimulated gene with known antiviral functions, its suppression during this bacterial challenge may further reflect successful pathogen disruption of interferon-mediated immunity or a host strategy to resolve potential inflammation. The coordinated suppression of stat1a, stat2, and rsad2 points to a targeted impairment of the IFN-I system by S. putrefaciens, a novel insight into the host–pathogen interaction [21].
The host response extended beyond classical immunity to encompass metabolic and structural adaptations. The upregulation of lepb (leptin B) in the liver suggests a role in linking metabolic status to immune activation, as leptin is known to modulate inflammatory responses in fish [26]. In the spleen, the enrichment of “extracellular matrix” (ECM) components and the upregulation of dcn (decorin) indicate active tissue remodeling [27]. The ECM is not merely a structural scaffold but actively regulates immune cell migration and function [28]. These changes may facilitate immune cell infiltration during the acute phase and initiate repair processes to restore tissue architecture following infection-induced damage.
In summary, our study elucidates a multifaceted host response to S. putrefaciens. The liver engages in interferon signaling and immunometabolic reprogramming, while the spleen acts as the primary hub for pathogen recognition via CLRs and innate immune coordination. However, the pathogen appears to deploy countermeasures, potentially suppressing critical nodes of the IFN-I pathway. The identified key genes and pathways, such as the PGRPs for pathogen recognition and acaca for metabolic adaptation, represent valuable candidates for future applied research. These molecules could serve as potential biomarkers for health monitoring or genetic targets for selective breeding programs aimed at enhancing disease resistance in largemouth bass aquaculture, contributing to the sustainable development of this important industry.
5. Conclusions
In this study, we investigated the effects of Shewanella putrefaciens infection on the liver and spleen of largemouth bass at the transcriptome level. We identified 458 significantly differentially expressed genes (DEGs) between infected and control liver, and 1405 DEGs between infected and control spleen. GO enrichment analysis revealed that in the liver, the most significantly enriched biological processes were related to type I interferon signaling and response, underscoring the importance of immune reactions in that organ; in the spleen, regulation of innate immune response was the most significant process, highlighting the spleen’s immunological role. KEGG pathway analysis showed that fatty acid metabolism and cytokine–cytokine receptor interaction pathways were prominently enriched in the liver, whereas the C-type lectin receptor signaling pathway and cytokine–cytokine receptor interaction dominated in the spleen. Through further screening of DEGs, we identified genes closely associated with immune response, cellular stress, and metabolic regulation—such as stat1a, rsad2, pglyrp5, pglyrp6, acaca, stat2, and lepb. These genes point to multi-level host mechanisms during infection, including pathogen recognition, immune signaling modulation, and metabolic adaptation, and they underscore critical regulatory points in the host’s immune response to S. putrefaciens.
Overall, our findings provide valuable clues for understanding the immune response and related biological processes in largemouth bass during bacterial infection. The results contribute to a better molecular understanding of fish antibacterial immunity and may inform the development of diagnostic markers or genetic selection for disease resistance. The key DEGs and pathways identified in this study could serve as potential targets for novel therapeutic or preventive strategies (such as enhancing specific immune pathways) to control S. putrefaciens infections, thereby supporting the sustainable and healthy growth of the largemouth bass aquaculture industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10110587/s1, Table S1 Differentially expressed genes (DEGs) in largemouth bass liver in response to S. putrefaciens infection; Table S2 Differentially expressed genes (DEGs) in largemouth bass spleen in response to S. putrefaciens infection.
Author Contributions
Writing—original draft, C.Z.; software and investigation, Y.Z. (Yinjin Zhu); writing—review & editing, X.D., Y.S., Y.Z. (Yangsong Zhang) and Y.H.; validation and resources, Y.F.; Funding acquisition and Conceptualization, M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the China Postdoctoral Science Foundation Project (2022M721399); the Priority Academic Program Development of Jiangsu Higher Education Institutions; the Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX25_3853, KYCX25_3855).
Institutional Review Board Statement
This research involves the use of laboratory animals. After review by the Jiangsu Ocean University Institutional Animal Care and Use Committee, it is determined that the animal experimentation protocols and related materials comply with ethical requirements for animal welfare and laboratory animal research (approval code: 2024000012; date: 10 September 2024).
Data Availability Statement
The data presented in this study are openly available in NCBI at https://submit.ncbi.nlm.nih.gov/subs/sra/SUB15769368 (accessed on 10 September 2025), reference number SUB15769368.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Miranda, L.E.; Bettoli, P.W. Largemouth bass natural history. In Largemouth Bass Aquaculture; CABI: Wallingford, UK, 2019; pp. 1–27. [Google Scholar]
- Bai, J.; Li, S. Development of largemouth bass (Micropterus salmoides) culture. In Aquaculture in China: Success Stories and Modern Trends; Gui, J.-F., Tang, Q., Li, Z., Liu, J., De Silva, S.S., Eds.; Wiley-Blackwell: Oxford, UK, 2018; pp. 421–429. [Google Scholar]
- Zhu, T.; Du, J.; Song, H.; Lei, C.; Cen, Y.; Wang, C.; Li, S. Whole genome resequencing reveals the correlation between selection signatures and adaptability of Micropterus salmoides to artificial feed. Sci. Rep. 2024, 14, 30058. [Google Scholar] [CrossRef] [PubMed]
- Paździor, E. Shewanella putrefaciens—A new opportunistic pathogen of freshwater fish. J. Vet. Res. 2016, 60, 429–434. [Google Scholar] [CrossRef]
- Sood, N.; Pradhan, P.K.; Verma, D.K.; Yadav, M.K.; Mishra, R.K.; Kumar, U.; Sood, N.K. Large-scale mortality in cultured tilapia (Oreochromis niloticus) due to infection with Shewanella putrefaciens in India. J. World Aquacult. Soc. 2020, 51, 563–570. [Google Scholar] [CrossRef]
- Lu, S.; Levin, R.E. Shewanella in a tilapia fish farm. J. Fish. Sci. 2010, 4, 159–170. [Google Scholar] [CrossRef]
- Kozińska, A.; Pękala, A. First isolation of Shewanella putrefaciens from freshwater fish—A potential new pathogen of fish. Bull. Eur. Assoc. Fish Pathol. 2004, 24, 189–193. [Google Scholar]
- El-Barbary, M.I. First recording of Shewanella putrefaciens in cultured Oreochromis niloticus and its identification by 16S rRNA in Egypt. Egypt. J. Aquat. Res. 2017, 43, 101–107. [Google Scholar] [CrossRef]
- Crispe, I.N. The liver as a lymphoid organ. Annu. Rev. Immunol. 2009, 27, 147–163. [Google Scholar] [CrossRef]
- Kubes, P.; Jenne, C.N. Immune responses in the liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef]
- Press, C.M.; Evensen, Ø. The morphology of the immune system in teleost fishes. Fish Shellfish Immunol. 1999, 9, 309–318. [Google Scholar] [CrossRef]
- Whyte, S.K. The innate immune response of finfish—A review of current knowledge. Fish Shellfish Immunol. 2007, 23, 1127–1151. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X.; Li, L.; Niu, C.; Pei, C.; Zhu, L.; Kong, X. Identification of Shewanella putrefaciens as a novel pathogen of the largemouth bass (Micropterus salmoides) and histopathological analysis of diseased fish. Front. Cell. Infect. Microbiol. 2022, 12, 1042977. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Y.; Zhang, X.T.; Xia, Y.T.; Zhang, Y.Q.; Wang, B.; Ye, W.W.; Ye, Z.F.; Qian, S.C.; Huang, M.M.; Yang, S. Transcriptome and 16S rRNA analyses revealed differences in the responses of largemouth bass (Micropterus salmoides) to early Aeromonas hydrophila infection and immunization. Aquaculture 2021, 541, 736759. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.L.; Qian, Y.X.; Chen, Q.; He, J.L.; Wang, J.T.; Han, T.; Zhang, X.M.; Deng, Y.T. Integrated analysis of transcriptome and metabolome reveals the regulatory mechanism of largemouth bass (Micropterus salmoides) in response to Nocardia seriolae infection. Fish Shellfish Immunol. 2024, 145, 109322. [Google Scholar] [CrossRef]
- Chen, H.; Feng, C.; Zhang, Q.; Zhang, Q.; Huang, Y.; Chen, X.; Gao, Y.; Gao, H.; Liu, H. Liver transcriptome profiling of the largemouth bass (Micropterus salmoides) suggests host immunoregulatory mechanism in response to Edwardsiella tarda infection. J. Fish Biol. 2025, 106, 239–250. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Gallagher, G.; Baurin, V.V.; Lewis-Antes, A.; Shen, M.; Shah, N.K.; Langer, J.A.; Sheikh, F.; Dickensheets, H.; Donnelly, R.P. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2003, 4, 69–77. [Google Scholar] [CrossRef]
- Schoggins, J.W.; Wilson, S.J.; Panis, M.; Murphy, M.P.; Jones, C.T.; Bieniasz, P.; Rice, C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 2011, 472, 481–485. [Google Scholar] [CrossRef]
- Librán-Pérez, M.; Pereiro, P.; Figueras, A.; Novoa, B. Transcriptome analysis of turbot (Scophthalmus maximus) infected with Aeromonas salmonicida reveals a direct effect on leptin synthesis as a neuroendocrine mediator of inflammation and metabolism regulation. Front. Mar. Sci. 2022, 9, 888115. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, X.; Liu, X.; Wang, Z.; Zhang, D. A unique C-type lectin, Ladderlectin, from large yellow croaker (Larimichthys crocea) is involved in bacterial cell membrane damage. Fish Shellfish Immunol. 2023, 136, 108744. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiong, G.; Luo, M.; Mao, S.; Zhang, R.; Meng, Z.; Li, J.; Liao, X. Peptidoglycan recognition protein PGRP-5 is involved in immune defence and neuro-behavioral disorders in zebrafish embryos. PLoS ONE 2025, 20, e0315714. [Google Scholar] [CrossRef] [PubMed]
- Bardagi, A.A.; Paschoal, C.S.; Favero, G.G.; Moraes-Vieira, P.M.; Mestriner, F.L.A.C.; D’Ávila, H. Leptin’s immune action: A review beyond satiety. Immunol. Investig. 2023, 52, 117–133. [Google Scholar] [CrossRef]
- del Fresno, C.; Soulat, D.; Roth, S.; Blazek, K.; Chalvensole, C.; Ruckerl, D.; Bekker, L.V.; Milota, S.; Sancho, D.; Akira, S.; et al. Interferon-β production via Dectin-1–Syk–IRF5 signaling in dendritic cells is crucial for immunity to Candida albicans. Immunity 2018, 48, 962–975.e7. [Google Scholar]
- Vidal, M.; Santillán-Araneda, M.J.; Rivera, A.; Marshall, S.H.; Ramírez, C. Modulation of immune response and tissue repair mechanisms in the gill filaments of Atlantic salmon (Salmo salar) affected by complex gill disease (CGD) in a marine open sea-cage environment. Fish Shellfish Immunol. 2025, 166, 110605. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).