Abstract
The tropical gar (Atractosteus tropicus) has significant ecological, economic, and cultural importance in southeast Mexico, where aquaculture is increasing and fish are frequently exposed to stress. In this sense, feed additives such as sage (Salvia officinalis) strengthen organisms’ growth, immune systems, antioxidant capacities, and digestive capabilities. A 30-day experiment was conducted on larvae to determine the effect of different concentrations of sage essential oil (0%, 0.5%, 1%, 1.5%, and 2% treatments) supplemented in balanced diets. Significant differences (p < 0.05) between 0.5% and 2% sage oil supplement treatments for average weight were found. The highest acid and alkaline proteases, chymotrypsin, leucine aminopeptidase, amylase, and lipase activities were obtained for the sage oil-supplemented treatments. In contrast, trypsin showed the highest activity for treatment 0%, followed by diets with 0.5% and 2% sage oil. Regarding the antioxidant enzymatic activity for GPx, CAT and SOD, the highest activity was obtained in the diet with 1% sage oil, while in PEROx, the highest activity was recorded in the treatment with 0%, 1.5% and 2% S. officinalis supplementation. On the other hand, for relative gene expression, the highest expression was observed in sage-supplemented treatments for the nod, zo-1, zo-2, and occ genes. In contrast, the lowest expression was found in supplemented treatments for the il-10 and muc2 genes. These findings suggest that incorporating sage essential oil into the diets of tropical gar larvae, particularly at concentrations of 1.66% and 1.77%, holds potential for enhancing aquaculture practices for this important species.
Key Contribution:
The supplementation with 1.66% and 1.77% Salvia officinalis essential oil enhances immune system gene expression and antioxidant activity in tropical gar larvae.
1. Introduction
Due to global population growth, alternatives have been sought to ensure the supply of high-quality food to the human population, positioning aquaculture as a key sector in food production [1]. In this sense, balanced feeds are being developed based on digestive physiology to enhance digestion and nutrient absorption [2]. Additionally, these types of balanced feeds for aquaculture can be supplemented with prebiotics, probiotics, postbiotics, symbiotics, functional additives, and phytogenics, which contribute to improved health and performance in aquatic species. These supplements support optimal growth and a stimulated immune system to combat potential opportunistic pathogens [2,3,4]. Functional additives are dietary ingredients that not only fulfill basic nutritional requirements but also provide additional benefits such as improved growth, immune responses and resistance to diseases [5]. Moreover, phytogenic compounds, which are bioactive compounds obtained from plants, are classified into hydroalcohols and essential oils, and have recently been included in balanced feeds at low concentrations, having growth-promoting, antimicrobial, immunostimulant, anti-inflammatory, sedative, and antioxidant properties in several animal species [6]. In relation to the above, various essential oils (Eos) have been identified such as pepper, oregano, cinnamon, mint, and Salvia sp., which have been used in humans and animals with various health benefits [7,8,9,10,11], in addition to enhancing the antioxidant capacity in the organisms that consume them, thus promoting the synthesis and release of enzymes such as glutathione peroxidase (Gpx), which is a selenoenzyme that reduces reactive oxygen species (ROS) and limits their toxicity, catalyzing the reduction of hydrogen peroxide or organic hydroperoxides to water or corresponding alcohols [12]; catalase (CAT), which is a hemoenzyme that acts as an oxidoreductase, where the decomposition of hydrogen peroxide mitigates oxidative stress, resulting from the imbalance in the levels of reactive oxygen species and antioxidants [13]; superoxide dismutase (SOD), which catalyzes the dismutation of superoxide anion into oxygen and hydrogen peroxide, thus protecting cells from oxidative damage caused by reactive oxygen species, so that the inactivation of SOD can induce or aggravate oxidative stress [14]; and peroxidase (PEROx), which catalyzes the oxidation of various compounds by interacting with hydrogen peroxide and related compounds [15].
On the other hand, the essential oil of sage (Salvia officinalis) is a compound rich in steroids, polyphenols, triterpenoids, diterpenoids and sesquiterpenoids, in addition, it has potent antibacterial, antifungal, antioxidant, anticancer, anticholinesterase and anti-inflammatory properties, as well as a great capacity to improve mood and cognitive function [16]. It is obtained through hydrodistillation, steam distillation, enzyme-assisted ensiling, extraction with conventional organic solvents, or near-critical extraction (liquid or supercritical) using CO2 [17]. Thus, the essential oil contains natural biomolecules that remain unchanged during the extraction process. When included in food, they exhibit biostimulant properties that enhance the immune system by activating various components, such as mucins, interleukins, occludins, claudins, and nucleotide-binding oligomerization domain (NOD) receptors [18]. Within the mucins is muc-2, which plays a crucial role in protecting and maintaining intestinal homeostasis; therefore, reduced levels of muc-2 may be associated with various intestinal diseases [19]. From the group of interleukins, there is IL-10, which synthesizes a key protein involved in regulating the inflammatory response. By interacting with various immune cells, it blocks their activation and ability to produce inflammatory substances, thus contributing to maintaining tissue homeostasis [20]. The group of occludins is zo-1 and zo-2, which play an important role in the formation and maintenance of tight junctions between epithelial cells, which seal the intercellular space, forming a barrier that regulates the passage of substances between tissues, protecting the integrity of the organs [21]. In this sense, claudins such as occ, which synthesizes a protein that acts as a barrier between cells by regulating cell permeability, playing an essential role in maintaining vascular homeostasis [22]; Finally, from the group of nucleotide-binding oligomerization domain molecules, there is nod2, which synthesizes a protein that helps detect the presence of foreign or harmful substances, triggering an alarm response that activates the immune system [23].
Based on the above, the benefits of sage oil have been studied in various species, as is the case with a study by Alanazi et al. [24], where they studied the effect of the combination of sage oil and thymoquinone on hyperglycemia, oxidative stress, blood pressure, and lipid profile in rats fed a high-fat diet. They highlighted that the combination of these compounds exhibited hypoglycemic, hypolipidemic, and antioxidant actions, making it a valuable addition to diabetes management. Likewise, research has been carried out to understand the effect of sage oil on some fish species, such as tilapia hybrids (Oreochromis niloticus × Oreochromis aureus) [25], rainbow trout (Oncorhynchus mykiss) [26], sea bream (Sparus aurata) [27], European sea bass (Dicentrarchus labrax) [28] and common carp (Cyprinus carpio) [10], where they found that the addition of the oil improves fat production values by decreasing it, increases somatic growth and feed efficiency, causes a positive effect on oxidative stress, improves systemic immune response and blood indices, balances the intestinal microbiome and increases fertility in breeders.
Instead, tropical gar (Atractosteus tropicus, Gill 1863) is a fish of the Lepisosteidae family, distributed from southeastern Mexico to Central America, including the coasts of Guatemala, El Salvador, Honduras, Nicaragua, and Costa Rica [29]. The gar culture is divided into four main phases: reproduction, larviculture, pre-growing, and growing [30,31]. Adequate feeding and nutrition are essential for maintaining good health and preventing disease in captivity [32]. Culture and production of tropical gar (Atractosteus tropicus), valued for both its aquaculture potential and its cultural significance in southeastern Mexico and Central America, face significant challenges, particularly in its early stages, as the technology for its management is still considered incomplete or in pre-commercial development [33]. Although progress has been made in diverse specific areas, further research is still required on digestive physiology, appropriate stocking densities, and protocols that maximize growth for specific developmental stages to enhance commercial culture [34].
In this context, several studies have been conducted on the tropical gar (Atractosteus tropicus), focusing on its genetics [35,36,37,38,39,40,41], use of additives, prebiotics and probiotics [32,42,43,44,45], digestive physiology [23,46,47] and nutrition and ingredient use [48,49,50,51]. Thus, A. tropicus has been studied to understand the metabolic, biochemical, and molecular processes that enhance the aquaculture technological package in the southeastern region of Mexico. Therefore, it is relevant to evaluate the effect of supplementing Salvia officinalis essential oil in balanced diets for tropical gar (Atractosteus tropicus) larvae on growth, survival, digestive and antioxidant enzyme activity, and expression of immune system and antioxidant genes.
2. Materials and Methods
The Salvia officinalis essential oil used in this study was obtained from Perfumería Roxana, a local establishment located at Avenida José María Pino Suárez 702, Colonia Arboledas, Centro, Villahermosa, Tabasco, Mexico. It was characterized through chromatographic analysis and antimicrobial testing.
2.1. Chromatographic Characterization
To determine the main volatile compounds that are present in sage (Salvia officinalis) essential oil, the relevant analyses were performed at the Center for Research and Assistance in Technology and Design of the State of Jalisco, A.C. (CIATEJ). The compounds were identified by gas chromatography-mass spectrometry (GC-MS) and subsequently quantified by gas chromatography with flame ionization detection (GC-FID) under translated analytical conditions. The tentative identification of the most abundant compounds separated in the sample was performed by comparing the mass spectra of the chromatographic peaks in the total ion chromatogram (TIC) with those of the NIST 14 spectral base, as well as by comparing RIs available in the literature.
2.2. Antimicrobial Activity
The antimicrobial activity of Salvia officinalis essential oil was evaluated using the agar well diffusion and disk diffusion methods against a panel of fish pathogenic microorganisms. The bacterial strains tested included: Aeromonas hydrophila (NCIBM 11343), Aeromonas dhakensis (CAIM 1873), Aeromonas ichthiosmia (CAIM 1876), Staphylococcus arlettae (CAIM 658 and UJAT 002), Vibrio parahaemolyticus (ATCC 17802), Vibrio harveyi (CAIM 1622), Vibrio campbellii (CIBGEN 001), Vibrio diabolicus (CIBGEN 002), and Photobacterium damselae (CAIM 1910). The test organisms were cultured in tryptic soy broth (TSB) at 28 °C for 24 h. Bacterial suspensions were then adjusted to 1 × 108 CFU/mL using a 0.5 McFarland standard. For each assay, 3.5 mL of the adjusted bacterial suspension was homogenized into 31.5 mL of molten Mueller-Hinton agar (cooled to 45–50 °C), and the mixture was immediately poured into sterile square Petri dishes (10 × 10 cm) previously fitted with 12 stainless steel cylinders (6 mm diameter). Each well was filled with 100 µL of S. officinalis essential oil at three concentrations: undiluted, 50%, and 25% (v/v in DMSO). Sterile DMSO: water (50:50, v/v) was used as the negative control, and amikacin (100 µg/mL) served as the positive control. Plates were incubated at 28 °C for 24 h, after which the diameter of the inhibition zones was measured in millimeters using a digital caliper. For the disk diffusion assay, sterile paper disks (6 mm diameter) were impregnated with 10 µL of the same essential oil concentrations (undiluted, 50%, and 25%) and placed onto the surface of the inoculated agar. Plates were incubated under the same conditions, and inhibition zones were measured after 24 h. All assays were performed in triplicate for each strain and concentration. Antimicrobial activity was expressed as the diameter (mm) of the inhibition zones, including the well or disk diameter.
2.3. Sampling and Larviculture
Eleutheroembryos of A. tropicus (2000 individuals) were obtained from a batch of broodstock from the Laboratory of Physiology in Aquatic Resources (LAFIRA) of the Academic Division of Biological Sciences at the Universidad Juárez Autónoma de Tabasco. In accordance with Márquez-Couturier et al. [31] a female (3.4 kg, 91 cm) was induced to spawn by intramuscular application of the LHRHa hormone (Sigma-Aldrich, Taufkirchen, Germany) (30 µg/kg fish). The female and three males (1.6 kg, 35 cm, without hormone induction) were subsequently placed in a circular tank (2000 L) with raffia thread to simulate the natural spawning site. Eleutheroembryos (0.1 ± 0.06 g, three days post fertilization, DPF) were placed in a recirculating system consisting of 18 circular tanks of 70 L, fed by a 0.5 HP water pump (Jacuzzi, JWPA5D-230A, Delavan, WI, USA) and a 1000 L reservoir for solids deposition and a biological filter. Once the yolk was absorbed (three days after hatching, DAH), the bioassay was initiated, which lasted 30 days (from larval to juvenile period), during which biometrics were performed every 15 days to monitor growth. The feeding protocol began with the supply of Artemia nauplii (5 nauplii/mL) from yolk absorption (3 DAH) until 30 DAH. Starting at 7 DAE, the co-feeding process was initiated by mixing the Artemia nauplii with the experimental feeds. Beginning at 10 DAH, the experimental feeds were supplied exclusively, considering 10% of the total biomass and adjusting it periodically as the fish grew five times a day (8:00, 10:30, 13:00, 16:00, and 18:00).
2.4. Experimental Design
The bioassay consisted of five treatments (Salvia officinalis oil supplementation doses, based on previous studies [10,26,27,28] in the feed: 0%, 0.5%, 1%, 1.5%, and 2%. There were three replicates per treatment. One hundred larvae were maintained per 70 L tank under a recirculating system. Water quality parameters were monitored daily (YSI multi parameter model Pro 2030, YSI Inc., Yellow Springs, OH, USA), with a temperature of 26 ± 1 °C, a dissolved oxygen concentration of 4.5 ± 0.1 mg L−1, a pH of 7.3 ± 0.2, and a photoperiod of 12 h of light and 12 h of dark.
2.5. Preparation of Microparticulate Feeds
To prepare the experimental feeds, the meals were sieved, following the procedure described by Sepúlveda-Quiróz et al. [51] the sage essential oil was mixed with the fish oil in each of the previously established experimental proportions and the mixing continued with the aforementioned protocol (Table 1). To verify the protein, lipid, ash, and fiber levels of the diets, chemical proximal analyses were performed at the Faculty of Marine Sciences of the Autonomous University of Baja California, Mexico, using the AOAC method [52]. Additionally, Standard procedures determined the proximate composition of the diets. Crude protein (CP) was quantified through micro-Kjeldahl method with a conversion factor of 6.25.7. For crude lipids (CL), a variant of the Folch method was used, using dichloromethane–methanol (2:1) as the extract solvent. To determine moisture content, samples were dried at 105 °C until constant weight was achieved. Ash content was determined by incinerating the samples at 550 °C for 6 h. Nitrogen-free extract (NFE) was estimated by subtracting the percentages of moisture, crude protein, crude lipid, and ash from 100% of the sample. The gross energy was analyzed by direct combustion in an oxygen bomb calorimeter (PARR Instruments, model 1261).

Table 1.
Composition of experimental diets with different concentrations of Salvia officinalis essential oil.
2.6. Fish Sampling
The primacy of animal welfare over scientific interest and, ethical principles are aligned to Helsinki Declaration for scientific justification and minimization of animal suffering under experimentation in accordance with the 3R principles of this declaration (Replacement, Reduction, and Refinement), the recommendations for the welfare of farmed fish of the Aquatic Animal Health Code by the World Organization for Animal Health [53], the closest national regulatory framework for the use of animals in research NOM-062-ZOO-2000, NOM-033-SAG/ZOO-2014, and the protocol and experiment were approved by the Institutional Commission of Ethics in Research of the Universidad Juárez Autónoma de Tabasco, Mexico (CIEI-2025-073).
At the end of the bioassay, the larvae (5 individuals per tank, N = 15 per treatment) were euthanized with an overdose of anesthetic (clove oil, 0.1 mL/L of water) [51], Each organism was dissected to remove the visceral package, then homogenized in 5 volumes (w/v) of cold distilled water (4 °C) using a biological replicate and an Ultraturax tissue disruptor. The homogenate was centrifuged at 17,709× g for 15 min at 4 °C. The supernatant was recovered and stored at −80 °C until analysis.
2.7. Digestive Enzyme Analysis
To determine digestive enzyme activity, the soluble protein of each multi-enzyme extract was previously calculated using the Bradford technique [52]. The activities of alkaline protease, acid protease, trypsin, chymotrypsin, leucine aminopeptidase, lipase, and amylase were determined from these extracts.
Alkaline protease-like activity was estimated using the Walter method [54] with 0.25% casein as the substrate in a 50 mM Tris/HCl buffer at pH 9. Acid protease (pepsin activity) was measured using the Anson technique [55], in which 0.25% hemoglobin in a 0.1 mM Glycine/HCl buffer, pH 2, was the substrate. The mixtures were incubated at 37 °C, and the reaction was stopped by the addition of 0.5 mL of 10% TCA; the absorbance of the reaction products was measured at 280 nm in a light spectrophotometer. The unit of enzyme activity was defined as 1 μg of tyrosine released per minute, based on the molar extinction coefficient (0.005 mL μg−1 cm−1). Trypsin activity was measured as described by Erlanger et al. [56], at 37 °C and with BAPNA (N-α-benzoyl-DL-arginine p-nitroanilide) as substrate in 50 mM Tris-HCl buffer at pH 8.2 (with 10 mM CaCl2). Chymotrypsin activity was identified using the method of DelMar et al. [57], at 37 °C with SAAPNA (N-succinyl-ala-ala-pro-phe p-nitroanilide) as substrate in 10 mM DMSO and 100 mM Tris/HCl (with 10 mM CaCl2) at pH 7.8. Leucine aminopeptidase activity was determined using leucine p-nitroanilide in 0.1 mM DMSO, with 50 mM sodium phosphate buffer, at pH 7.2, and 37 °C [58]. In the techniques described above, reactions were stopped with 30% acetic acid. The enzyme activity was defined as 1 μg of nitroanilide released per minute, using the molar extinction coefficients of trypsin (8.8), chymotrypsin, and leucine-aminopeptidase (8.2 mL μg−1 cm−1). The amylase activity was determined according to [59] using 2% starch as the substrate in 100 mM citrate-phosphate buffer with 50 mM NaCl, pH 7.5; the reaction product was measured at 600 nm. The lipase activity was determined according to the technique of [60] using 100 mM β-naphthyl acetate as substrate in 50 mM Tris-HCl buffer (pH 7.5) with 100 mM sodium cholate; the reaction was measured at 540 nm. The specific activity of the extracts was expressed using the following equations: (1) Units per mL = (Δabs × final reaction volume (mL)/(MEC × time (min) × extract volume (mL)) and (2) Units per mg of protein = Units per mL/mg of soluble protein, where Δabs represents the absorbance increase at a given wavelength and MEC represents the molar extinction coefficient for the reaction product (mL μg−1 cm−1). All assays were performed in triplicate.
2.8. Antioxidant Enzyme Analysis
For the evaluation of antioxidant enzymes, the previously obtained extracts were used, and measurements of the following enzymes were performed: glutathione peroxidase (GPx) levels were determined kinetically using the glutathione peroxidase cellular activity assay kit (Cat. CGP1, Sigma-Aldrich, St. Louis, MO, USA). Catalase (CAT) enzyme activity was determined using the specific kinetic kit (Cat. 707002, Cayman Chem., Ann Arbor, MI, USA). The superoxide dismutase (SOD) assay kit (Cat. 19160, Sigma-Aldrich Corp., MO, USA) was used to examine the activities in spleen and liver. The assay kit (Cat. T5525, 3,3′, 5,5′-Tetramethylbenzidine, Sigma-Aldrich, MO, USA) was used to determine the peroxidase (PEROx) activity. The antioxidant enzyme activities were normalized to the soluble protein content by the Bradford technique [52]. SOD, GPx, PEROx U/mg protein (Units of enzymatic activity (U) are present for each milligram (mg) of total protein in the sample), and CAT nm/min/mg protein (nanometers per minute per milligram of protein) activities were calculated according to the supplier’s instructions.
2.9. Analysis of Immune System Gene Expression
Total RNA was extracted from visceral tissue using the RNeasy Mini kit (74102, Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Quantification and quality assessment of the RNA were performed by spectrophotometric analysis at 260 and 280 nm (NanoDrop 2000, Thermo Fisher Scientific, Madrid, Spain). RNA integrity of the extracted sample was verified by visualizing the ribosomal RNA subunits (28S and 18S) on a 1.2% agarose gel. cDNA was synthesized via the reverse transcription of RNA in 20 μL per reaction volume with 2 μg of total RNA using SuperScript™ First Strand Synthesis System for RT-PCR (Invitrogen, Waltham, MA, USA) with oligo (dT) (0.5 μg μL−1), random hexamer primers (50 ng μL−1) and 10 mmol L−1 dNTP mix, 10× RT buffer [200 mmol L−1 Tris-HCl (pH = 8.4), 500 mmol L−1 KCl], 25 mmol L−1 MgCl2, 0.1 mol L−1 DTT, RNaseOUT (40 U μL−1) and SuperScript™ II RT, followed by RNase H treatment. The resulting cDNA was diluted 1:20 in DEPC-treated water, and the diluted samples were used for gene expression analysis.
qPCR reactions were performed in triplicate for each individual per treatment using a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Madrid, Spain). The reaction mixture was standardized to 20 μL, with a consistent aliquot of 10 μL of SsoAdvanced™ Universal SYBR ® Green Supermix (Life Technologies, Carlsbad, CA, USA), 0.50 μL of forward primer, 0.50 μL of reverse primer, 2 μL of cDNA tissue sample, and 7 μL of RNase/DNase-free water. The qPCR assay workflow began at 95 °C for 3 min, followed by 40 cycles of 15 s at 95 °C, 30 s at the annealing temperature specific to each primer pair, and a 30 s melting curve step at 72 °C to establish the standard curve for each amplicon. Relative expression was determined via CFX Manager 3.0 software (Bio-Rad, Madrid, Spain), with elongation factor 1 alpha (EF1α) as an endogenous control, while basal samples (control) and sage groups were used to normalize relative quantification within each experimental diet group. All samples were run in triplicate to confirm the absence of genomic DNA contamination, using a negative control reaction in which the RT enzyme was omitted. Additionally, an extra negative control was placed on each plate, containing no cDNA. The primers used in this study (Table 2) were previously designed from the A. tropicus larval transcriptome (BioProject PRJNA395289) and published by Martínez-Burguete et al. [40].

Table 2.
Oligonucleotide design for real-time polymerase chain reaction (qPCR) of immune and antioxidant system genes in A. tropicus larvae.
2.10. Statistical Analysis
To assess growth, enzyme activity, and gene expression, normality (Kolmogorov–Smirnov) and homoscedasticity (Levene) tests were performed, respectively. A one-way analysis of variance (ANOVA) was then performed to determine differences between treatments for each quality criterion, followed by Tukey’s post hoc test. To determine the optimal levels of supplementation of S. officinalis oil, a second-order quadratic model (Y = a + bX + cX2) was applied in relation to the average weight and total length growth values on the inclusion levels. All statistical analyses were performed using GraphPad Prism 10 software (La Jolla, CA, USA) with a significance level of 0.05.
3. Results
3.1. Chromatographic Characterization
Through chromatographic characterization, the predominant volatile compounds present in Salvia officinalis oil, characterized by its bioactive properties, were identified (Table 3). Camphor, 1,8-cineole, α-pinene, D-limonene, and linalyl acetate are notable.

Table 3.
GC/FID analysis was used to quantify the main volatile components in sage essential oil.
3.2. Antimicrobial Activity of S. officinalis Essential Oil
The antimicrobial activity of S. officinalis essential oil against ten fish pathogenic bacteria was evaluated using agar well diffusion and disk diffusion assays at three concentrations: undiluted, 50%, and 25%. In both assays, a dose-dependent effect was observed, with a progressive decrease in inhibition zone diameter as the concentration of the essential oil decreased (Table 4). In the well diffusion assay, the oil exhibited broad-spectrum activity, inhibiting the growth of all bacterial strains tested, regardless of the concentration applied. The highest antimicrobial effect was recorded against Gram-negative bacteria, particularly those of the genus Aeromonas. At the highest concentration, inhibition zones of 20.11 mm, 19.95 mm, and 19.66 mm were observed for A. hydrophila, A. dhakensis, and A. ichthiosmia, respectively (Figure 1). Moderate inhibition was also observed against V. harveyi, V. parahaemolyticum, and V. campbellii, with halos ranging from 18.71 mm to 13.61 mm depending on the dilution. The largest inhibition zone was recorded for Vibrio diabolicus, with a diameter of 22.43 mm. In contrast, the Gram-positive strains of Staphylococcus arlettae (CAIM 658 and UJAT 002) showed the lowest sensitivity, with inhibition zones ranging from 16.68 mm to 12.51 mm.

Table 4.
Inhibition zones (mm) of Salvia officinalis essential oil against fish pathogenic bacteria, evaluated by agar wells and disk diffusion assays at three concentrations.
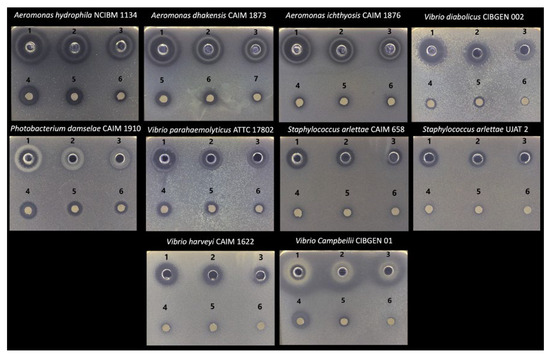
Figure 1.
Antimicrobial activity of Salvia officinalis essential oil against fish pathogens. Zones of inhibition are shown for the agar well diffusion assay (1, 2, and 3 correspond to 100%, 50%, and 25%, respectively) and the disk diffusion assay (4, 5, and 6 correspond to 100%, 50%, and 25%, respectively).
3.3. Growth and Survival
Growth, survival, and feed quality parameters in A. tropicus larvae (Table 5) show significant differences (p < 0.05) only for final average weight (Figure 2A), where larvae fed with S. officinalis oil in their diet showed the greatest significant growth compared to larvae fed the control treatment, particularly with the 0.5% and 2% S. officinalis treatments (0.48 and 0.49 g, respectively). Regarding total length (Figure 2C), no statistically significant differences (p > 0.05) were found between larvae fed sage oil and those fed the control diet. As shown in Figure 2, the quadratic models demonstrated correlation coefficients of 0.43 for average weight and 0.48 for total length. Both models indicated that the optimal dietary supplementation of S. officinalis essential oil in a balanced diet for A. tropicus larvae was found to be 1.66% (Figure 2B) for maximum growth in average weight and 1.77% for maximum growth in total length (Figure 2D). Regarding survival, no significant differences (p > 0.05) were found between the treatments where sage oil was included in the larval diets. However, a pattern emerged in which larvae fed diets supplemented with 0.5% or 2% S. officinalis oil showed the highest values.

Table 5.
Growth variables, percent survival, and food quality indexes of A. tropicus larvae fed different dietary S. officinalis supplementation (Mean ± SD).
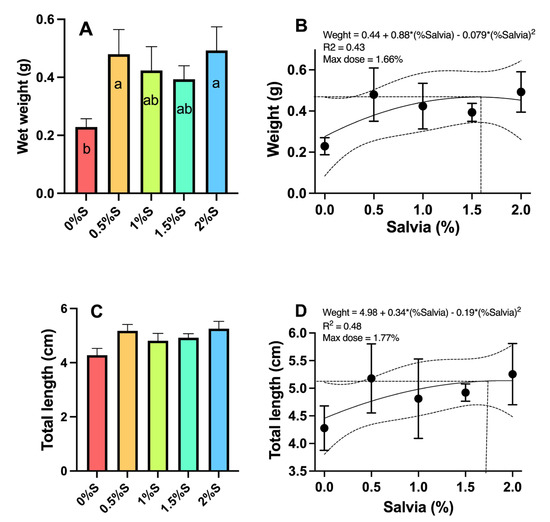
Figure 2.
Growth in (A) weight (g), (B) quadratic model for weight, (C) length (cm), and (D) quadratic model for total length of tropical gar (Atractosteus tropicus) larvae fed diets supplemented with different concentrations (0.5%, 1%, 1.5%, and 2%) of sage (Salvia officinalis) oil and a control diet. Significant differences between diets are indicated by different letters (p < 0.05).
3.4. Digestive Enzymes
Regarding digestive enzyme activity, the activity of acid proteases (Figure 3A) and alkaline proteases (Figure 3B) in larvae fed the 1.5% sage oil diet was found to have statistically significantly higher values (p < 0.05) than in larvae fed the other experimental diets. Chymotrypsin (Figure 3D), leucine aminopeptidase (Figure 3E), and amylase (Figure 3G) activity in larvae fed the diet supplemented with 1% sage oil had statistically higher values (p < 0.05) compared to the other treatments. In the case of lipase activity (Figure 3F), larvae fed diets containing 0.5%, 1%, and 1.5% sage oil showed the highest values statistically (p < 0.05) compared to larvae fed 2% sage oil and those on the control diet. On the other hand, trypsin enzymatic activity (Figure 3C) is statistically higher in larvae fed the control diet (p < 0.05) compared to larvae fed diets supplemented with sage oil.
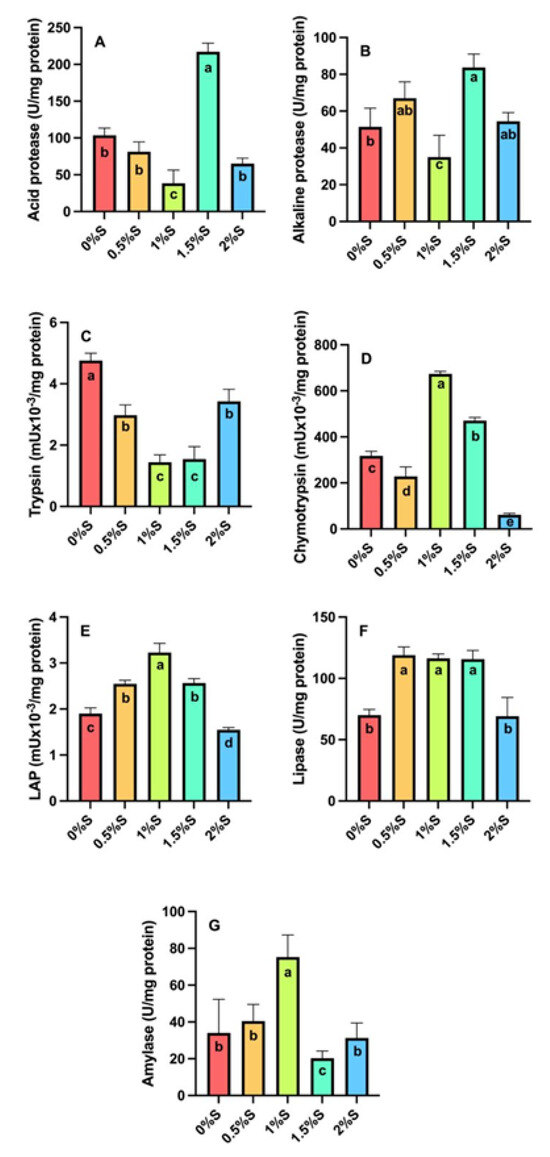
Figure 3.
Digestive enzyme activity of (A) acid proteases, (B) alkaline proteases, (C) trypsin, (D) chymotrypsin, (E) leucine aminopeptidase (LAP), (F) lipases and (G) amylase in tropical gar (A. tropicus) larvae fed diets supplemented with sage oil at different concentrations (0.5%, 1%, 1.5% and 2%) and a control diet. Significant differences between diets are indicated by different letters (p < 0.05).
3.5. Oxidative Enzymes
Significant differences (p < 0.05) were detected in the activities of glutathione peroxidase (Gpx, Figure 4A), catalase (CAT, Figure 4B), and superoxide dismutase (SOD, Figure 4C), with larvae fed 1% sage oil showing higher activity than larvae fed the other treatments. In the case of peroxidase activity (PEROx, Figure 4D), values were statistically higher (p < 0.05) in larvae supplemented with 1.5% and 2% sage oil, and those fed the control diet.
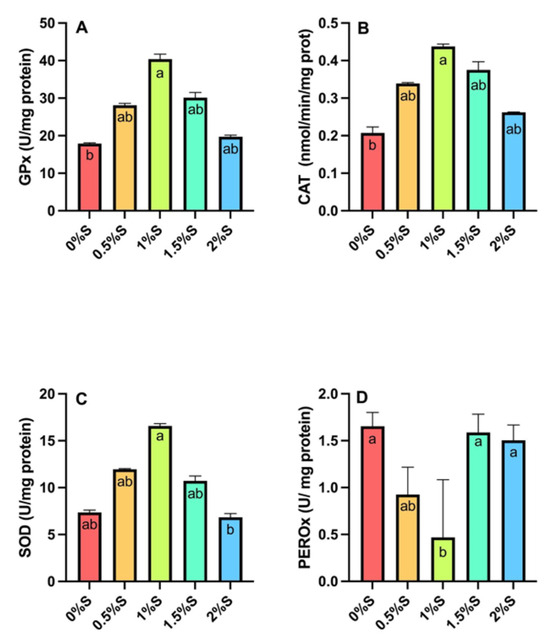
Figure 4.
Antioxidant enzyme activity of (A) Glutathione peroxidase (Gpx), (B) Catalase (CAT), (C) superoxide dismutase (SOD), and (D) peroxidase (PEROx) in tropical gar larvae (A. tropicus) fed different concentrations of sage oil and a control diet. Significant differences between diets are indicated by different letters (p < 0.05).
3.6. Expression of Immune System Genes
Regarding the expression of mucin-2 (muc2, Figure 5A), higher expression (p < 0.05) was detected in larvae fed the control diet and larvae fed 0.5%, 1%, and 2% sage oil compared to larvae fed the diet supplemented with 1.5% sage oil. Regarding the expression of interleukin 10 (il10, Figure 5B), overexpression was seen in larvae fed the control diet and those fed 0.5% sage oil, which was statistically different (p < 0.05) compared to larvae fed the other treatments. On the other hand, the expression of occludins 1 (zo-1, Figure 5C) and occludins 2 (zo-2, Figure 5D) shows an overexpression in larvae fed with the diet supplemented with 2% sage oil, which is statistically different (p < 0.05) compared to larvae fed with the control diet and those fed with the other diets supplemented with sage oil. In the case of occ expression (Figure 5E), a higher expression was detected in the diet with 2% sage oil. Finally, the expression of nod (Figure 5F) showed a higher expression in larvae fed with diets containing 0.5%, 1% and 1.5% sage oil, being significantly different from the control diet and the diet with 2% sage oil.
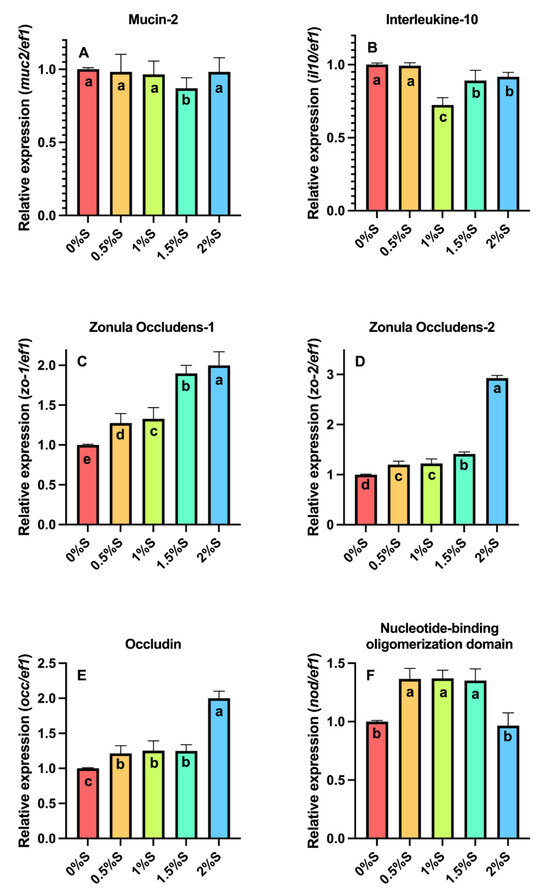
Figure 5.
Relative gene expression of (A) Mucin-2 (muc-2), (B) Interleukine-10 (il-10), (C) Zonula Occludens-1 (zo-1), (D) Zonula Occludens-2 (zo-2), (E) Occludin (occ), and (F) Nucleotide-binding oligomerization domain (nod) in A. tropicus larvae fed diets supplemented with sage (S. officinalis) oil and a control diet. Significant differences between diets are indicated by different letters (p < 0.05).
4. Discussion
According to the results obtained through chromatographic characterization, the essential oil of Salvia officinalis presented a composition rich in bioactive compounds, including camphor, 1,8-cineole, α-pinene, D-limonene, and linalyl acetate. These compounds have been widely reported in the literature as major constituents of EOs from plants such as Cinnamomum camphora, Alpinia uraiensis, Rosmarinus officinalis, Eucalyptus globulus, Pinus sylvestris, and Citrus aurantifolia and are attributed antioxidant, antimicrobial, and anti-inflammatory properties [63,64,65,66].
On the other hand, antimicrobial analysis revealed that sage oil (Salvia officinalis) exhibits a significant inhibitory effect on bacterial growth, specifically against Aeromonas hydrophila, Aeromonas dhakensis, Aeromonas ichthiosmia, Staphylococcus arlettae, Vibrio parahaemolyticus, Vibrio harveyi, Vibrio campbellii, Vibrio diabolicus, and Photobacterium damselae. This can be attributed to the joint action of its bioactive compounds, primarily oxygenated monoterpenes such as 1,8-cineole, α-thujone, β-thujone, and camphor, which have demonstrated a high capacity to disrupt the integrity of bacterial cell membranes [67,68]. These lipophilic compounds penetrate the lipid bilayer, causing leakage of essential metabolites, membrane depolarization, and metabolic collapse; in addition, they have been observed to induce oxidative stress through the generation of reactive oxygen species (ROS), which damage cellular DNA, proteins, and lipids, affecting bacterial viability [69]. In recent studies, sage oil has shown significant zones of inhibition against Gram-positive bacteria, such as Staphylococcus spp., and Gram-negative bacteria, including Yersinia enterocolitica and Listeria monocytogenes, suggesting a broad spectrum of antimicrobial action [70].
In addition to its antimicrobial properties, sage oil (Salvia officinalis) also showed direct benefits in productive performance, such as growth and enzymatic activity, as well as in the expression of genes of the immune and antioxidant system, since it has various bioactive compounds such as steroids, polyphenols, triterpenoids, diterpenoids and sesquiterpenoids that stimulate good health of the organisms that consume it [15]. Fish fed diets supplemented with 0.5% and 2% sage oil showed greater weight growth compared to the control diet, which did not contain S. officinalis oil, which may be related to the polyphenolic compounds in the oil, since they have been reported as growth promoters in aquaculture [71]. In addition, it has been shown that ursolic acid (triterpenoid) stimulates muscle growth through hypertrophy of skeletal muscle fibers [72]. On the other hand, the digestive enzyme activity of acid proteases, alkaline proteases, chymotrypsin, leucine aminopeptidase (LAP), lipase and amylase of organisms fed with diets supplemented with 0.5%, 1% and 1.5% was higher than in the control diet (0% S. officinalis), which may be influenced by the action of sage oil compounds. In the case of 1,8 cineole, it stimulates gastric secretion and could stimulate pancreatic secretion, creating a favorable environment for the activation of these proteolytic enzymes [73,74] influencing the activity of acid proteases, alkaline proteases and chymotrypsin; phenolic diterpenes such as carnosic acid and carnosol have antioxidant properties that stabilize enzymes and protect their structures against oxidative stress, prolonging their activity [75,76] increasing the activity of LAP and lipases; rosmarinic acid modulates intestinal inflammation and improves the bioavailability of nutrients [77,78], which favors the action of enzymes that act in the small intestine such as amylase and lipases; and, in the case of terpenes such as α and β pinene, linalool and salveno, which have choleretic effects and the production of cholagogues, thus stimulating the production of bile and facilitating the digestion of fats and carbohydrates [79,80], influencing the enzymatic activity of the digestive enzymes. In contrast, trypsin activity was higher in the control treatment than in the treatments added with sage oil, which could be because, during the early stages of larval development, chymotrypsin can activate faster or reach higher levels as part of a compensatory mechanism to ensure efficient protein digestion [81], which agrees with results obtained in previous ontogeny investigations with A. tropicus larvae [49] and Cynoscion nebulosus [82], where they found that chymotrypsin exhibits higher activity than trypsin.
On the other hand, the activity of antioxidant enzymes such as glutathione peroxidase (GPx), catalase (CAT) and superoxide dismutase (SOD) increased in fish fed with the diet supplemented with 1% sage essential oil, while the activity of peroxidase (PEROx) was higher in the control diet and in the diets with 1.5% and 2% sage oil; For its part, the essential oil of S. officinalis contains bioactive compounds such as terpenoids, flavonoids and phenols, which have antioxidant properties, which can induce the activity of endogenous antioxidant enzymes such as GPx, CAT and SOD, which are part of the cellular defense system against oxidative stress, which agrees with studies carried out with Oncorhynchus mykiss, Sparus aurata and Cyprinus carpio [10,26,27]. On the other hand, PEROx, being a non-specific exogenous enzyme, is directly competing with the phenolic and terpenoid compounds of sage oil, such as rosmarinic acid, α-thujone, and 1,8-cineole, so these can directly interfere with the oxidation reaction of PEROx since the oxidizing intermediate (such as hydrogen peroxide) is reduced, binding to the peroxidase enzyme and inactivating it [83,84]. Regarding the expression of immune and antioxidant target genes, we found an overexpression of zo-1, zo-2, occ, and nod in fish fed with treatments supplemented with S. officinalis, which could be mainly due to the activation of the Keap1/Nrf2 (Kelch-like ECH-associated protein 1/Nuclear factor erythroid-related factor 2) antioxidant pathway and the inhibition of inflammation mediated by NF-κB (Nuclear factor kappa light chain-enhancer of activated B cells) and the NLRP3 (NOD-like receptor pyrin domain 3) inflammasome. A study in a DSS (dextran sodium sulfate)-induced colitis model in mice [85] demonstrated that carnosic acid blocks the Keap1–Cullin3 interaction, stabilizing Nrf2, which leads to the induction of antioxidant genes such as heme oxygenase-1, glutathione peroxidase 2, and superoxide dismutase 2 (HO-1, GPX 2, SOD 2). These actions were accompanied by epithelial preservation, decreased levels of proinflammatory cytokines, and reduced caspase-1/NLRP3 activation. As a result, an increase in the expression of tight junction proteins (such as zo-1 and occ) was observed, which underlies the improvement in epithelial integrity. Consequently, it is suggested that similar behavior could be observed in zo-2 and nod 1/nod 2, although these were not directly evaluated in the study.
On the contrary, concerning the muc-2 and il10 genes, no significant differences were found between the control treatment (0% sage) and diets supplemented with sage oil. This absence of effect can be explained by the fact that all treatments were maintained under normal physiological conditions, without exposure to stressors, intestinal inflammation, or immunological challenges, which are classic stimuli for the overexpression of these genes. Previous studies have shown that compounds such as carnosic acid and carnosol do not directly stimulate the expression of anti-inflammatory genes such as il10, but act by preventing the activation of proinflammatory pathways (such as NF-κB and NLRP3), which are not activated under basal experimental conditions [86,87]. Therefore, the function of sage oil can be considered more preventive than stimulatory, as it modulates the immune response only in the presence of an inflammatory stimulus. This hypothesis is supported by previous research, which shows that phytogenic compounds have more potent effects on immune and intestinal mucosal genes when applied in conjunction with infectious challenges or oxidative stress conditions [6,88]. Consequently, the similar levels of muc2 and il10 between groups could reflect a shared state of intestinal homeostasis, in which the mucosa does not require additional adjustments from either the immune system or its protective barrier. The results obtained in this research are consistent with previous research carried out with various aquatic organisms, where they found that sage oil (Salvia officinalis) has positive effects on the growth, immune system, and antioxidant capacities of the organisms that consume it [10,25,26,27,28].
Additionally, the application of EOs has been established as a promising and feasible strategy in modern aquaculture, due to their productivity and benefits for animal health and the sustainability of the system. Multiple recent studies have shown that bioactive compounds present in plant oils such as Origanum vulgare, Salvia officinalis, Thymus vulgaris, or Rosmarinus officinalis promote growth, improve feed conversion, and reduce mortality in commercial species such as tilapia, common carp, rainbow trout, and African catfish [26,89,90]. These positive effects are related to the stimulation of digestive enzymes (proteases, lipases, amylases) thanks to components such as cineole, thymol, or carnosic acid, as well as to the modulation of the intestinal microbiota and the integrity of tight junctions (zo-1, occ), which favor greater nutrient absorption [91,92]. Furthermore, EOs act as natural immunostimulants, inducing the expression of genes related to the innate immune response (NOD, IL-1β, TNF-α), and present antimicrobial properties against aquatic pathogens such as Aeromonas hydrophila, Vibrio spp., and Streptococcus iniae, reducing the need for antibiotics [93,94]. These effects significantly reduce antibiotic use, thereby contributing to the control of antimicrobial resistance—a critical problem in intensive aquaculture. Technologically, the encapsulation and emulsification of EOs have improved their stability during feed processing and their controlled release in the digestive tract, increasing their efficacy without compromising feed palatability [95]. However, their optimal application depends on factors such as species, dose, treatment duration, and synergy with other functional ingredients. Therefore, the development of specific protocols for each species and culture stage is recommended. Consequently, we believe that EOs offer a comprehensive solution that not only improves production performance but also contributes to sustainability and biosecurity in 21st-century aquaculture.
5. Conclusions
This study indicated that diets supplemented with 1.66% and 1.77% of sage (S. officinalis) essential oil for tropical gar (A. tropicus) larvae have multibeneficial effects on weight growth, digestive and antioxidant enzyme activity, as well as on the expression of immune and antioxidant system genes, which can be attributed to the synergy of the oil’s bioactive compounds such as terpenes and polyphenols, which act as growth promoters as well as preventive agents and modulators of intestinal and enzymatic health, so we consider that supplementation could strengthen the intestinal barrier and the body’s defense capacity against future pathogenic or stress challenges, contributing to a more sustainable aquaculture by reducing dependence on antibiotics and other additives; however, future research should attempt to replicate the study using a more diverse population and different life stages to increase the generalizability of this findings, and further analytics must be carried out to fully understand the impact on different mechanisms of action to determine the understand the long-term effects.
Author Contributions
Conceptualization, G.G.A.-A., S.D.L.R.-G., C.A.Á.-G. and R.M.-G.; methodology, Y.J.-L., G.M.P.-J., O.M.-M., C.A.S.-Q. and G.G.A.-A.; software, L.D.J.-M., G.M.P.-J., S.D.L.R.-G., C.A.S.-Q. and G.G.A.-A.; validation, Y.J.-L., G.M.P.-J., G.G.A.-A., C.A.S.-Q. and C.A.Á.-G.; formal analysis, Y.J.-L., L.D.J.-M., C.A.S.-Q., G.G.A.-A. and C.A.Á.-G.; investigation, Y.J.-L., L.D.J.-M., G.G.A.-A., C.A.S.-Q., C.A.Á.-G. and R.M.-G.; resources, G.G.A.-A., S.D.L.R.-G. and C.A.Á.-G.; data curation, Y.J.-L., L.D.J.-M. and G.M.P.-J.; writing—original draft preparation, Y.J.-L., G.G.A.-A., C.A.S.-Q., C.A.Á.-G. and R.M.-G.; writing—review and editing, Y.J.-L., G.G.A.-A., C.A.S.-Q., L.D.J.-M., C.A.Á.-G., S.D.L.R.-G. and R.M.-G.; visualization, R.M.-G., C.A.S.-Q., G.G.A.-A. and C.A.Á.-G.; supervision, G.G.A.-A., S.D.L.R.-G. and C.A.Á.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Secretariat of Science, Humanities, Technology, and Innovation (SECIHTI) for financing the Academic Postdoctoral Fellowship for the project entitled “Impact on the immune system and intestinal microbiota of the tropical gar (Atractosteus tropicus) through phytogenic supplementation in balanced feed”. Grant number: CVU 418072.
Institutional Review Board Statement
The animal study protocol was in accordance with the primacy of animal welfare over scientific interest and, ethical principles are aligned to Helsinki Declaration for scientific justification and minimization of animal suffering under experimentation in accordance with the 3R principles of this declaration (Replacement, Reduction, and Refinement), the recommendations for the welfare of farmed fish of the 2024 Aquatic Animal Health Code by the World Organization for Animal Health [54], the closest national regulatory framework for the use of animals in research NOM-062-ZOO-2000, NOM-033-SAG/ZOO-2014, and the protocol and experiment were approved by the Institutional Commission of Ethics in Research of the Universidad Juárez Autónoma de Tabasco, Mexico (Approval Code: CIEI-2025-073; Approval Date: 12 October 2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting this study’s findings are available upon request from the authors.
Acknowledgments
To the Universidad Juárez Autónoma de Tabasco (UJAT), Academic Division of Biological Sciences (DACBiol), for the Sponsor-a-Student scholarship to Yuliana Jiménez León, and for authorizing the facilities of the Aquatic Resources Physiology Laboratory (LAFIRA) for conducting experiments and analyzing data. To Mirna Estarrón Espinosa of the Center for Research and Assistance in Technology and Design of the State of Jalisco, A.C. (CIATEJ), for her invaluable support in the analysis of sage oil compounds. The authors gratefully acknowledge Felix Maldonado Desena for his valuable support in conducting the antimicrobial assays.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. Transformación Azul. Contribución de la Pesca y la Acuicultura al Nuevo Marco Estratégico de la FAO—COPPESAALC-XVIII-7. 2022. Available online: https://openknowledge.fao.org/items/b14f7614-8dbf-42c8-82af-fc71e68bc4ad (accessed on 17 May 2025).
- Manam, V.K. Fish feed nutrition and its management in aquaculture. Int. J. Fish. Aquat. Stud. 2023, 11, 58–61. [Google Scholar] [CrossRef]
- Rohani, M.F.; Islam, S.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: Upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish Immunol. 2022, 120, 569–589. [Google Scholar] [CrossRef]
- Wang, J.; Deng, L.; Chen, M.; Che, Y.; Li, L.; Zhu, L.; Chen, G.; Feng, T. Phytogenic feed additives as natural antibiotic alternatives in animal health and production: A review of the literature of the last decade. Anim. Nutr. 2024, 17, 244–264. [Google Scholar] [CrossRef]
- Onomu, A.J.; Okuthe, G.E. The Role of Functional Feed Additives in Enhancing Aquaculture Sustainability. Fishes 2024, 9, 167. [Google Scholar] [CrossRef]
- Firmino, J.P.; Galindo-Villegas, J.; Reyes-López, F.E.; Gisbert, E. Phytogenic Bioactive Compounds Shape Fish Mucosal Immunity. Front. Immunol. 2021, 12, 695973. [Google Scholar] [CrossRef]
- Farouk, S.M.; Yusuf, M.S.; El Nabtiti, A.A.S.; Abdelrazek, H.M.A. Effect of oregano essential oil supplementation on performance, biochemical, hematological parameters and intestinal histomorphometry of Japanese quail (Coturnix coturnix Japonica). Vet. Res. Forum 2020, 11, 219–227. [Google Scholar] [CrossRef]
- Hurtado, R.; Peltroche, N.; Mauricio, F.; Gallo, W.; Alvítez-Temoche, D.; Vilchez, L.; Mayta-Tovalino, F. Antifungal efficacy of four different concentrations of the essential oil of cinnamomum zeylanicum (Canela) against Candida albicans: An in vitro study. J. Int. Soc. Prev. Community Dent. 2020, 10, 724–730. [Google Scholar] [CrossRef]
- Jeena, K.; Liju, V.B.; Umadevi, N.P.; Kuttan, R. Antioxidant, Anti-inflammatory and Antinociceptive Properties of Black Pepper Essential Oil (Piper nigrum Linn). J. Essent. Oil-Bear. Plants 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Metin, S.; Yigit, N.O.; Didinen, B.I.; Koca, S.B.; Ozmen, O.; Aslankoc, R.; Kara, N. Effects of sage (Salvia officinalis) essential oil on growth, health and antioxidant capacity of common carp (Cyprinus carpio). Vet. Res. Commun. 2024, 48, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint essential oil: Its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Ali, F.; Manzoor, U.; Khan, F.I.; Lai, D.; Khan, M.K.A.; Chandrashekharaiah, K.S.; Singh, L.R.; Dar, T.A. Effect of polyol osmolytes on the structure-function integrity and aggregation propensity of catalase: A comprehensive study based on spectroscopic and molecular dynamic simulation measurements. Int. J. Biol. Macromol. 2022, 209, 198–210. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, H.; Li, T.; Yu, L.; Qi, Y.; Tian, G.; He, F.; Li, X.; Sun, N.; Liu, R. Size-dependent effects of nanoplastics on structure and function of superoxide dismutase. Chemosphere 2022, 309, 136768. [Google Scholar] [CrossRef]
- de Oliveira, F.K.; Santos, L.O.; Buffon, J.G. Mechanism of action, sources, and application of peroxidases. Food Res. Int. 2021, 143, 110266. [Google Scholar] [CrossRef] [PubMed]
- Farruggia, D.; Di Miceli, G.; Licata, M.; Leto, C.; Salamone, F.; Novak, J. Foliar application of various biostimulants produces contrasting response on yield, essential oil and chemical properties of organically grown sage (Salvia officinalis L.). Front. Plant Sci. 2024, 15, 1397489. [Google Scholar] [CrossRef] [PubMed]
- Conde-Hernández, L.A.; Luna-Guevara, M.L.; Luna-Guevara, J.J.; Pérez-Vázquez, J.; Aranda-García, R.J. Mexican sage (Salvia officinalis) extraction using factorial design and its effect on chemical and antibacterial properties. J. Chem. 2021, 2021, 5594278. [Google Scholar] [CrossRef]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int. J. Biol. Macromol. 2020, 164, 884–891. [Google Scholar] [CrossRef]
- Sun, H.; Wu, Y.; Zhang, Y.; Ni, B. IL-10-Producing ILCs: Molecular Mechanisms and Disease Relevance. Front. Immunol. 2021, 12, 650200. [Google Scholar] [CrossRef]
- Qiao, X.; Roth, I.; Féraille, E.; Hasler, U. Different effects of ZO-1, ZO-2 and ZO-3 silencing on kidney collecting duct principal cell proliferation and adhesion. Cell Cycle 2014, 13, 3059–3075. [Google Scholar] [CrossRef]
- Du, Y.; Duan, Y.; Zhang, S. The Role of Occludin in Vascular Endothelial Protection. In Endothelial Dysfunction—A Novel Paradigm; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, X.; Tang, X.; Xing, J.; Chi, H.; Zhan, W. Genome-wide identification, phylogenetic relationships and expression patterns of the NOD-like receptor (NLR) gene family in flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2023, 141, 109083. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, S.M.; Alsaqer, R.A.; Alsaeed, F.I.; Almakhaytah, R.M.; Buwashl, N.T.; Mohamed, M.E.; Younis, N.S. Studying the actions of sage and thymoquinone combination on metabolic syndrome induced by high-fat diet in rats. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2404–2418. [Google Scholar] [CrossRef]
- El-Kholy, K. Effect of marjoram (marjorana hortensis) or sage (Salvia officinalis) additives on growth performance and feed utilization of tilapia hybrid (oreochromis niloticus × oreochromis aureus) monosex fingerlings. J. Anim. Poult. Prod. 2012, 3, 115–126. [Google Scholar] [CrossRef]
- Sönmez, A.Y.; Bilen, S.; Alak, G.; Hisar, O.; Yanık, T.; Biswas, G. Growth performance and antioxidant enzyme activities in rainbow trout (Oncorhynchus mykiss) juveniles fed diets supplemented with sage, mint and thyme oils. Fish Physiol. Biochem. 2015, 41, 165–175. [Google Scholar] [CrossRef]
- Salomón, R.; Firmino, J.P.; Reyes-López, F.E.; Andree, K.B.; González-Silvera, D.; Esteban, M.A.; Tort, L.; Quintela, J.C.; Pinilla-Rosas, J.M.; Vallejos-Vidal, E.; et al. The growth promoting and immunomodulatory effects of a medicinal plant leaf extract obtained from Salvia officinalis and Lippia citriodora in gilthead seabream (Sparus aurata). Aquaculture 2020, 524, 735291. [Google Scholar] [CrossRef]
- Hussein, E.E.; El Basuini, M.F.; Ashry, A.M.; Habiba, M.M.; Teiba, I.I.; El-Rayes, T.K.; Khattab, A.A.A.; El-Hais, A.M.; Shahin, S.A.; El-Ratel, I.T.; et al. Effect of dietary sage (Salvia officinalis L.) on the growth performance, feed efficacy, blood indices, non-specific immunity, and intestinal microbiota of European sea bass (Dicentrarchus labrax). Aquac. Rep. 2023, 28, 101460. [Google Scholar] [CrossRef]
- Daniels, A.; Maiz-Tome, L. Atractosteus tropicus: IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2018. [Google Scholar] [CrossRef]
- Maldonaldo, A.; Cruz, J.; Gómez, D.; López, L.; Fernández, A.; Peña-Marín, E.S.; Álvarez-González, C.A. Cultivo de Pejelagarto (Atractosteus tropicus); Centro del Cambio Global y Sustentabilidad A.C.: Tabasco, Mexico, 2020. [Google Scholar] [CrossRef]
- Márquez-Couturier, G.; Vázquez-Navarrete, C.J.; Contreras Sánchez, W.M.; Álvarez-González, C.A. Acuicultura Tropical Sustentable Una Estrategia para la Producción y Conservación del Pejelagarto (Atractosteus tropicus) en Tabasco, México, 2nd ed.; Universidad Juárez Autónoma de Tabasco: Tabasco, Mexico, 2015. [Google Scholar]
- Arellano-Carrasco, J.G.; Martínez-García, R.; Asiain-Hoyos, A.; Reta-Mendiola, J.L.; Díaz-Rivera, P.; Frías-Gómez, S.A.; Martínez-Burguete, T.; Asencio-Alcudia, G.G.; Jiménez-Martínez, L.D.; Guerrero-Zarate, R.; et al. Effects of Dietary Sodium Propionate on Growth, Digestive Enzyme Activity, and Expression of Immune System Genes in Juveniles of Tropical Gar (Atractosteus tropicus). Aquac. J. 2023, 3, 227–237. [Google Scholar] [CrossRef]
- Gobierno de México. Acuacultura|Pejelagarto. IMIPAS. 2017. Available online: https://www.gob.mx/imipas/acciones-y-programas/acuacultura-pejelagarto (accessed on 28 October 2025).
- Sepúlveda-Quiróz, C.A.; Martínez-García, R.; Asencio-Alcudia, G.G.; Pérez-Jiménez, G.M.; Jiménez-Martínez, L.D.; Alvarez-Villagomez, C.S.; De La Rosa-García, S.; Nuñez-Nogueira, G.; Guerrero-Zárate, R.; Méndez-Marín, O.; et al. Infraestructura y Desarrollo Tecnológico del Cultivo de Peces de agua Dulce y Marinos en Latinoamérica: Pejelagarto (Atractosteus tropicus); Red CYTET Larvaplus; Centro de Investigaciones Biológicas del Noroeste S.C.: La Paz, Mexico, 2025; p. 269. ISBN 978-607-7634-47-8. [Google Scholar]
- Arias-Rodríguez, L.; Páramo-Delgadillo, S.; Contreras-Sánchez, W.M.; Álvarez-González, C.A. Cariotipo del pejelagarto tropical Atractosteus tropicus (Lepisosteiformes: Lepisosteidae) y variación cromosómica en sus larvas y adultos. Rev. Biol. Trop. Int. J. Trop. Biol. 2009, 57, 529–539. [Google Scholar] [CrossRef]
- Del Rió-Portilla, M.A.; Vargas-Peralta, C.E.; Lafarga-De La Cruz, F.; Arias-Rodriguez, L.; Delgado-Vega, R.; Galván-Tirado, C.; Garciá-De-León, F.J. The complete mitochondrial DNA of the tropical gar (Atractosteus tropicus). Mitochondrial DNA 2016, 27, 557–558. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, L.D.; Álvarez-González, C.A.; De la Cruz-Hernández, E.; Tovar-Ramírez, D.; Galaviz, M.A.; Camarillo-Coop, S.; Martínez-García, R.; Concha-Frías, B.; Peña, E. Partial sequence characterization and ontogenetic expression of genes involved in lipid metabolism in the tropical gar (Atractosteus tropicus). Aquac. Res. 2018, 50, 162–172. [Google Scholar] [CrossRef]
- Jesús-De la Cruz Kde, M.; Ávila-Fernández, Á.; Peña-Marín, E.S.; Jiménez-Martínez, L.D.; Tovar-Ramírez, D.; Martínez-García, R.; Guerrero-Zárate, R.; Asencio-Alcudia, G.G.; Alvarez-González, C.A. Trypsin gene expression in adults and larvae of tropical gar Atractosteus tropicus. Fish Physiol. Biochem. 2020, 46, 145–155. [Google Scholar] [CrossRef]
- Jiménez Martínez, L.D.; Morales García, V.; Frías Quintana, C.A.; Castillo Collado Adel, C.; Asencio Alcudia, G.G.; Alvarez Villagomez, C.S.; Peña Marín, E.S.; Concha Frías, B.; Álvarez-González, C.A. Quality evaluation of reference gene expression on different tissues in adults of tropical gar Atractosteus tropicus. Pak. J. Zool. 2021, 54, 1–10. [Google Scholar] [CrossRef]
- Martínez-Burguete, T.; Peña-Marin, E.S.; García-Gasca, A.; Alvarez-González, C.A.; Llera-Herrera, R. Nutrigenomic marker discovery by de novo transcriptomic sequencing during early development of the tropical gar (Atractosteus tropicus). Aquac. Res. 2021, 52, 3829–3842. [Google Scholar] [CrossRef]
- Palacios Mejia, M.; Arias-Rodriguez, L.; Arciniega, M.; Rodríguez, V.; Barraza Sandoval, J.E.; Herrera, N.; Marroquín Mora, D.C.; Ulloa Rojas, J.B.; Márquez Couturier, G.; Voelker, G.; et al. Conservation genetics of the tropical gar (Atractosteus tropicus, Lepisosteidae). Conserv. Genet. 2023, 24, 405–415. [Google Scholar] [CrossRef]
- De La Cruz-Marín, E.; Martínez-García, R.; López-Hernández, J.F.; Méndez-Marín, O.; De la Rosa-García, S.C.; Peña-Marín, E.S.; Tovar-Ramírez, D.; Sepúlveda-Quiroz, C.A.; Pérez-Jiménez, G.M.; Jiménez-Martínez, L.D.; et al. Inulin Supplementation in Diets for Tropical Gar (Atractosteus tropicus) Larvae: Effects on Growth, Survival, and Digestive and Antioxidant Enzyme Activities. Aquac. J. 2023, 3, 43–55. [Google Scholar] [CrossRef]
- Pérez-Jiménez, G.M.; Peña-Marín, E.S.; Maytorena-Verdugo, C.I.; Sepúlveda-Quiroz, C.A.; Jiménez-Martínez, L.D.; De la Rosa-García, S.; Asencio-Alcudia, G.G.; Martínez, R.; Tovar-Ramírez, D.; Galaviz, M.A.; et al. Incorporation of Fructooligosaccharides in Diets Influence Growth Performance, Digestive Enzyme Activity, and Expression of Intestinal Barrier Function Genes in Tropical Gar (Atractosteus tropicus) Larvae. Fishes 2022, 7, 137. [Google Scholar] [CrossRef]
- Hernández-López, I.A.; Tovar-Ramírez, D.; De la Rosa-García, S.; Álvarez-Villagómez, C.S.; Asencio-Alcudia, G.G.; Martínez-Burguete, T.; Galaviz, M.A.; Guerrero-Zárate, R.; Martínez-García, R.; Peña-Marín, E.S.; et al. Dietary live yeast (Debaryomyces hansenii) provides no advantages in tropical gar, atractosteus tropicus (actinopterygii: Lepisosteiformes: Lepisosteidae), juvenile aquaculture. Acta Ichthyol. Piscat. 2021, 51, 311–320. [Google Scholar] [CrossRef]
- Maytorena-Verdugo, C.I.; Peña-Marín, E.S.; Alvarez-Villagómez, C.S.; Pérez-Jiménez, G.M.; Sepúlveda-Quiroz, C.A.; Alvarez-González, C.A. Inclusion of Mannan-Oligosaccharides in Diets for Tropical Gar Atractosteus tropicus Larvae: Effects on Growth, Digestive Enzymes, and Expression of Intestinal Barrier Genes. Fishes 2022, 7, 127. [Google Scholar] [CrossRef]
- Cigarroa-Ruiz, L.A.; Toledo-Solís, F.J.; Frías-Gómez, S.A.; Guerrero-Zárate, R.; Camarillo-Coop, S.; Alvarez-Villagómez, C.S.; Peña-Marín, E.S.; Galaviz, M.A.; Martínez-García, R.; Álvarez-González, C.A. Addition of β-glucans in diets for tropical gar (Atractosteus tropicus) larvae: Effects on growth, digestive enzymes and gene expression of intestinal epithelial integrity and immune system. Fish Physiol. Biochem. 2023, 49, 613–626. [Google Scholar] [CrossRef]
- Pérez-Jiménez, G.M.; Alvarez-Villagomez, C.S.; Martínez-Porchas, M.; Garibay-Valdez, E.; Sepúlveda-Quiroz, C.A.; Méndez-Marín, O.; Martínez-García, R.; Jesús-Contreras, R.; Alvarez-González, C.A.; De la Rosa-García, S.d.C. The Indigenous Probiotic Lactococcus lactis PH3-05 Enhances the Growth, Digestive Physiology, and Gut Microbiota of the Tropical Gar (Atractosteus tropicus) Larvae. Animals 2024, 14, 2663. [Google Scholar] [CrossRef]
- Frías-Quintana, C.; Álvarez-González, C.; Márquez-Couturier, G. Design of microdiets for the larviculture of tropical gar Atractosteus tropicus, Gill 1863. Univ. Cienc. 2010, 26, 265–282. [Google Scholar]
- Frías-Quintana, C.A.; Márquez-Couturier, G.; Alvarez-González, C.A.; Tovar-Ramírez, D.; Nolasco-Soria, H.; Galaviz-Espinosa, M.A.; Martínez-García, R.; Camarillo-Coop, S.; Martínez-Yañez, R.; Gisbert, E. Development of digestive tract and enzyme activities during the early ontogeny of the tropical gar Atractosteus tropicus. Fish Physiol. Biochem. 2015, 41, 1075–1091. [Google Scholar] [CrossRef]
- Guerrero-Zárate, R.; Olvera-Novoa, M.Á.; Jesus-Contreras, R.; Frías-Quintana, C.A.; Martínez-García, R.; Álvarez-González, C.A. Evaluation of protein and lipid ingredients through in vitro digestibility for tropical gar (Atractosteus tropicus) juveniles. Lat. Am. J. Aquat. Res. 2024, 52, 47–58. [Google Scholar] [CrossRef]
- Sepúlveda-Quiroz, C.A.; Pérez-Jiménez, G.M.; Asencio-Alcudia, G.G.; Mendoza-Porras, O.; Jiménez-Martínez, L.D.; Galaviz-Espinoza, M.A.; Tovar-Ramirez, D.; Martinez-Garcia, R.; Alvarez-Villagomez, C.S.; Alvarez-Gonzalez, C.A. Tryptophan Reduces Intracohort Cannibalism Behavior in Tropical Gar (Atractosteus tropicus) Larvae. Fishes 2024, 9, 40. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- World Organization for Animal Health (WOAH). Manual of Diagnostic Tests for Aquatic Animals, 11th ed.; WOAH: Paris, France, 2024. Available online: https://www.woah.org/es/que-hacemos/normas/codigos-y-manuales/ (accessed on 15 October 2025).
- Walter, H. Proteinases: Methods with hemoglobin, casein and azocoll as substrates. In Methods of Enzymatic Analysis; Bergmeyern, H.U., Ed.; Verlag Chemic Weinham: Weinheim, Germany, 1984; Volume V, pp. 270–277. [Google Scholar]
- Anson, M.L. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef]
- Erlanger, B.F.; Kokowsky, N.; Cohen, W. The preparation and properties of two new chromogenic substrates of trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef]
- DelMar, E.G.; Largman, C.; Brodrick, J.W.; Geokas, M.C. A sensitive new substrate for chymotrypsin. Anal. Biochem. 1979, 99, 316–320. [Google Scholar] [CrossRef]
- Maroux, S.; Louvard, D.; Barath, J. The aminopeptidase from hog intestinal brush border. Biochim. Biophys. Acta Enzymol. 1973, 321, 282–295. [Google Scholar] [CrossRef]
- Robyt, J.F.; French, D. Multiple attack hypothesis of α-amylase action: Action of porcine pancreatic, human salivary, and Aspergillus oryzae α-amylases. Arch. Biochem. Biophys. 1967, 122, 8–16. [Google Scholar] [CrossRef]
- Versaw, W.K.; Cuppett, S.L.; Winters, D.D.; Williams, L.E. An Improved Colorimetric Assay for Bacterial Lipase in Nonfat Dry Milk. J. Food Sci. 1989, 54, 1557–1558. [Google Scholar] [CrossRef]
- Sepúlveda-Quiroz, C.A.; Peña-Marín, E.S.; Pérez-Morales, A.; Martínez-García, R.; Alvarez-Villagomez, C.S.; Maytorena-Verdugo, C.I.; Camarillo-Coop, S.; Vissio, P.G.; Sirkin, D.P.; Tovar-Ramírez, D.; et al. Fructooligosaccharide supplementation in diets for tropical gar (Atractosteus tropicus) juvenile: Effects on morphophysiology and intestinal barrier function. Aquac. Res. 2020, 52, 37–50. [Google Scholar] [CrossRef]
- Nieves-Rodríguez, K.N.; Álvarez-González, C.A.; Peña-Marín, E.S.; Vega-Villasante, F.; Martínez-García, R.; Camarillo-Coop, S.; Tovar-Ramírez, D.; Guzmán-Villanueva, L.T.; Andree, K.B.; Gisbert, E. Effect of β-Glucans in Diets on Growth, Survival, Digestive Enzyme Activity, and Immune System and Intestinal Barrier Gene Expression for Tropical Gar (Atractosteus tropicus) Juveniles. Fishes 2018, 3, 27. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Kevrešan, Ž.; Šunić, L.; Stanojević, L.; Milenković, L.; Stanojević, J.; Milenković, A.; Cvetković, D. Chemical Profiling and Antioxidant Activity of Wild and Cultivated Sage (Salvia officinalis L.) Essential Oil. Horticulturae 2023, 9, 624. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.; Park, S.; Park, H. Phytochemistry and Applications of Cinnamomum camphora Essential Oils. Molecules 2022, 27, 2695. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, F.; Liu, T.; Huang, Y.; Chen, Y.; Hsu, F. Analysis of Chemical Composition and Biological Activities of Essential Oils from Different Parts of Alpinia uraiensis Hayata. Molecules 2025, 30, 1515. [Google Scholar] [CrossRef]
- Tadtong, S.; Kamkaen, N.; Watthanachaiyingcharoen, R.; Ruangrungsi, N. Chemical Components of Four Essential Oils in Aromatherapy Recipe. Nat. Prod. Commun. 2015, 10, 1091–1092. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Zhumaliyeva, G.; Zhussupova, A.; Zhusupova, G.E.; Błońska-Sikora, E.; Cerreto, A.; Omirbekova, N.; Zhunusbayeva, Z.; Gemejiyeva, N.; Ramazanova, M.; Wrzosek, M.; et al. Natural Compounds of Salvia L. Genus and Molecular Mechanism of Their Biological Activity. Biomedicines 2023, 11, 3151. [Google Scholar] [CrossRef]
- Assaggaf, H.M.; Mrabti, H.N.; Rajab, B.S.; Attar, A.A.; Alyamani, R.A.; Hamed, M.; Omari, N.E.; Menyiy, N.E.; Hazzoumi, Z.; Benali, T.; et al. Chemical Analysis and Investigation of Biological Effects of Salvia officinalis Essential Oils at Three Phenological Stages. Molecules 2022, 27, 5157. [Google Scholar] [CrossRef]
- Zdolec, N.; Franičević, M.; Klanac, L.; Kavain, I.; Batinić, J.; Zadravec, M.; Pleadin, J.; Čobanov, D.; Kiš, M. Antimicrobial Properties of Basil (Ocimum basilicum L.), Sage (Salvia officinalis L.), Lavender (Lavandula officinalis L.), Immortelle (Helichrysum italicum (Roth) G. Don), and Savory (Satureja montana L.) and Their Application in Hard Cheese Production. Hygiene 2024, 4, 135–145. [Google Scholar] [CrossRef]
- García-Beltrán, J.M.; Esteban, M.Á. Nature-identical compounds as feed additives in aquaculture. Fish Shellfish Immunol. 2022, 123, 409–416. [Google Scholar] [CrossRef]
- Kunkel, S.D.; Elmore, C.J.; Bongers, K.S.; Ebert, S.M.; Fox, D.K.; Dyle, M.C.; Bullard, S.A.; Adams, C.M. Ursolic Acid Increases Skeletal Muscle and Brown Fat and Decreases Diet-Induced Obesity, Glucose Intolerance and Fatty Liver Disease. PLoS ONE 2012, 7, e39332. [Google Scholar] [CrossRef]
- Mirghaed, A.T.; Hoseini, S.M.; Ghelichpour, M. Effects of dietary 1,8-cineole supplementation on physiological, immunological and antioxidant responses to crowding stress in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2018, 81, 182–188. [Google Scholar] [CrossRef]
- Hoch, C.C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Dezfouli, A.B.; Wollenberg, B. 1,8-cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic Acid and Carnosol, Two Major Antioxidants of Rosemary, Act through Different Mechanisms. Plant Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Hou, N.; Li, J.; Liu, M.; Peng, S.; Zhang, Y.; Luo, Y.; Zhao, B.; Wang, S.; et al. Carnosol as a Nrf2 Activator Improves Endothelial Barrier Function Through Antioxidative Mechanisms. Int. J. Mol. Sci. 2019, 20, 880. [Google Scholar] [CrossRef]
- Li, K.; Wu, J.; Xu, S.; Li, X.; Zhang, Y.; Gao, X. Rosmarinic acid alleviates intestinal inflammatory damage and inhibits endoplasmic reticulum stress and smooth muscle contraction abnormalities in intestinal tissues by regulating gut microbiota. Microbiol. Spectr. 2023, 11, e01914-23. [Google Scholar] [CrossRef]
- Gonfa, Y.H.; Tessema, F.B.; Bachheti, A.; Rai, N.; Tadesse, M.G.; Singab, A.N.; Chaubey, K.K.; Bachheti, R.K. Anti-inflammatory activity of phytochemicals from medicinal plants and their nanoparticles: A review. Curr. Res. Biotechnol. 2023, 6, 100152. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Amenotie, A.J.; Ben-Azu, B.; Esuku, D.T.; Chijioke, B.S.; Abo, E.; Ozah, E.O.; Lawrence, E.O.; Efejene, O.I.; Onyeukwu, O.B.; Alabi, B.A.; et al. Sabinene Inhibits Lipopolysaccharide-Induced Memory Decline by Enhancing Cholinergic Function, Decreasing Molybdenum Enzymes, and Suppressing Oxidative Stress and Neuroinflammation. Neurotox. Res. 2025, 43, 26. [Google Scholar] [CrossRef]
- Fu, Z.; Akula, S.; Thorpe, M.; Hellman, L. Marked difference in efficiency of the digestive enzymes pepsin, trypsin, chymotrypsin, and pancreatic elastase to cleave tightly folded proteins. Biol. Chem. 2021, 402, 861–867. [Google Scholar] [CrossRef]
- Arenas-Pardo, M.A.; Gaxiola-Cortés, M.G.; Barreto-Altamirano, A.F.; Del Carmen Paredes-Medina, A.; Palomino-Albarrán, I.G.; Balam-Uc, P.M.; Maldonado-Flores, J.C.; Álvarez-González, C.A. Changes in Digestive Enzyme Activities during Larval Development of Spotted Seatrout (Cynoscion nebulosus). Aquac. Nutr. 2024, 2024, 1309390. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Wink, M.; Setzer, W.N. Radical Scavenging and Antioxidant Activities of Essential Oil Components—An Experimental and Computational Investigation. Nat. Prod. Commun. 2015, 10, 1934578X1501000135. [Google Scholar] [CrossRef]
- Baccouri, B.; Rajhi, I. Potential Antioxidant Activity of Terpenes. In Biochemistry; InTechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Yang, N.; Xia, Z.; Shao, N.; Li, B.; Xue, L.; Peng, Y.; Zhi, F.; Yang, Y. Carnosic acid prevents dextran sulfate sodium-induced acute colitis associated with the regulation of the Keap1/Nrf2 pathway. Sci. Rep. 2017, 7, 11036. [Google Scholar] [CrossRef]
- Lin, G.; Li, N.; Li, D.; Chen, L.; Deng, H.; Wang, S.; Tang, J.; Ouyang, W. Carnosic acid inhibits NLRP3 inflammasome activation by targeting both priming and assembly steps. Int. Immunopharmacol. 2023, 116, 109819. [Google Scholar] [CrossRef]
- Habtemariam, S. Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol. Biomedicines 2023, 11, 545. [Google Scholar] [CrossRef]
- Vieira, S.F.; Ferreira, H.; Neves, N.M. Antioxidant and Anti-Inflammatory Activities of Cytocompatible Salvia officinalis Extracts: A Comparison between Traditional and Soxhlet Extraction. Antioxidants 2020, 9, 1157. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Abdel-Tawwab, M.; Khafaga, A.F.; Dawood, M.A. Dietary origanum essential oil improved antioxidative status, immune-related genes, and resistance of common carp (Cyprinus carpio L.) to Aeromonas hydrophila infection. Fish Shellfish. Immunol. 2020, 104, 1–7. [Google Scholar] [CrossRef]
- Karataş, T.; Korkmaz, F.; Karataş, A.; Yildirim, S. Effects of Rosemary (Rosmarinus officinalis) extract on growth, blood biochemistry, immunity, antioxidant, digestive enzymes and liver histopathology of rainbow trout, Oncorhynchus mykiss. Aquac. Nutr. 2020, 26, 1533–1541. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, J.; Jia, L.; He, P.; Wang, Y.; Zhang, X.; Huang, Y.; Cheng, Q.; Zhang, Z.; Dai, Y.; et al. Oregano essential oil modulates colonic homeostasis and intestinal barrier function in fattening bulls. Front. Microbiol. 2023, 14, 1293160. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Elabd, H.; Mahboub, H.H.; Assayed, M.E.M.; Hamed, H.S.; Elsayyad, A.; Mohamed, E.M.M. The protective efficacy of dual dietary rosemary plus cinnamon mix against lead nitrate-induced immune suppression, genotoxicity, and oxidant/antioxidant status in Nile tilapia fingerlings. Aquac. Int. 2023, 32, 4009–4029. [Google Scholar] [CrossRef]
- Yildirim, A.; Türker, H. Bazı aromatik bitki esansiyel yağlarının patojenik balık bakterilerine karşı antibakteriyel aktivitesi. J. Limnol. Freshw. Fish. Res. 2018, 4, 67–74. [Google Scholar] [CrossRef]
- Terrazas-Pineda, K.A.; Alamilla-Beltrán, L.; Acero-Ortega, C.A.; Damas-Espinoza, J.A.; Calderón-Domínguez, G.; Mora-Escobedo, R.; Vega-Sánchez, V.; Anda, F.R.G. Antimicrobial Activity of Cinnamon, Tea Tree, and Thyme Essential Oils Against Pathogenic Bacteria Isolated from Tilapia (Oreochromis spp.) in Aquaculture Farms. Molecules 2025, 30, 2799. [Google Scholar] [CrossRef]
- Reis, D.R.; Ambrosi, A.; Di Luccio, M. Encapsulated essential oils: A perspective in food preservation. Future Foods 2022, 5, 100126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).