Abstract
Gut health is essential for optimal growth, immune function, and robustness in aquaculture. This study evaluated the potential of dietary supplementation with micro- and macroalgae to promote intestinal recovery following an insult. Four experimental diets were formulated for gilthead seabream (Sparus aurata) juveniles (176 ± 0.32 g): a control commercial-like diet (CTRL), and the same diet supplemented with either microalgae (Phaeodactylum tricornutum; PHA) or macroalgae (Gracilaria gracilis; GRA) at 2.5%, or a 5% blend of both (50:50; BLEND). To induce an intestinal insult, fish from each dietary group were assisted-fed with gelatine capsules containing soy saponins (CTRL + S, PHA, GRA, BLEND), while control fish received empty capsules (CTRL). After 72 h, CTRL and CTRL + S groups were fed the control diet, while PHA, GRA, and BLEND received their respective algae-supplemented diets. After 20 days, CTRL + S fish had significantly increased mucus cell numbers and submucosal cellular infiltration compared to CTRL fish, indicating intestinal disruption. PHA diet significantly upregulated igm, il10, and gpx. Fish fed GRA displayed a significant increase in mucosal vacuolation. BLEND diet showed synergistic effects, significantly upregulating il1b and pcna and reducing ALP activity. These results highlight the potential of combining micro- and macroalgae compounds to enhance gut recovery and immune activation.
Key Contribution:
Dietary micro- and macroalgae supplementation can promote intestinal recovery in gilthead seabream juveniles following a saponin-induced insult. The algal blend provided the most comprehensive benefits, suggesting synergistic effects that enhanced immune and antioxidant responses, thereby strengthening gut resilience.
1. Introduction
Gut health has a major role in the sustainability and profitability of aquaculture, as it is well-established that fish intestinal homeostasis and permeability have implications for efficient nutrient digestion and absorption [1,2,3]. Some of the more direct implications encompass fish growth performance, feed conversion and health. Aquaculture represents the food industry’s fastest-growing sector [4]. However, as a result of intensive production techniques, the sector is currently facing some challenges to attain higher productivity while accommodating more sustainable concepts [5,6].
Common practices in industrial aquaculture (e.g., transportation, handling, overcrowding) along with environmental stressors, such as abrupt temperature changes, poor water quality, or anti-nutritional factors in feeds, can negatively affect fish health and performance. These conditions impair growth and induce oxidative stress, characterised by excessive production of reactive oxygen species (ROS) [7]. In response, fish activate antioxidant defences, including the upregulation of genes encoding enzymes such as superoxide dismutase and catalase. Prolonged exposure to such stressors can disrupt intestinal homeostasis, compromising epithelial integrity and barrier function, which is often reflected by increased mucus secretion, permeability, and histological alterations [8]. Moreover, stress-induced immune dysregulation may weaken defence capacity or trigger inappropriate inflammatory responses, typically involving altered expression of cytokines such as tumour necrosis factor-alpha (tnfa) and interleukin-1 beta (il1b) [1,3,7,8].
In recent decades, aquaculture feed innovation has focused on functional feeds. Formulations containing specific additives that enhance animal performance and health beyond basic nutritional needs. These feeds are typically administered for short, predetermined periods as prophylactic treatments [9,10]. Increasing attention has been given to the biotherapeutic potential of micro- and macroalgae, whose rich bioactive profiles can modulate gut health, immune function, antioxidant capacity, intestinal morphology, and microbiota composition in fish. However, such effects are influenced by the algal species, dietary inclusion level, and feeding duration [11,12,13].
Among microalgae, Phaeodactylum tricornutum stands out as a valuable functional additive due to its low silica content, organic cell wall, and richness in bioactive compounds such as long-chain polyunsaturated fatty acids (LC-PUFA), fucoxanthin, and glucomannan 1–3, all known to support intestinal and overall fish health [14,15,16]. Dietary inclusion of this diatom at 5% for four weeks in gilthead seabream (Sparus aurata) juveniles enhanced immune-related molecular responses and preserved intestinal architecture [14,15], while lower inclusion levels (1%) also improved immune gene expression after 12 weeks of feeding [16].
Macroalgae from the genus Gracilaria, especially Gracilaria gracilis, have gained attention due to a potential role as a health promoter. In fact, compounds like R-phycoerythrin, arachidonic acid, and polyphenols are found in G. gracilis [17,18]. For instance, oxidative and immune response biomarkers were enhanced and intestine morphology was maintained when European seabass (Dicentrarchus labrax) juveniles were fed a diet containing G. gracilis at 2.5% for 47 days [19]. Gut morphology, growth and bacterial resistance were improved in gilthead seabream juveniles fed a 5% G. gracilis-enriched diet for 52 days [20]. In addition, a 47-day feeding trial with a diet containing G. gracilis at 5% promoted a beneficial shift in gut microbiota composition, suggesting improved microbial balance and intestinal health in European seabass juveniles [17].
Combining micro- and macroalgae has recently gained attention as a promising functional feed strategy. In fact, micro- and macroalgae blends may mitigate the detrimental effects that single algae inclusion has on the fish, such as poor digestibility, reduced intestinal absorption, and gut dysbiosis [21,22]. Feeding juvenile European seabass with a diet containing a blend of 4% of Nannochloropsis oceanica and 4% of G. gracilis for 106 days increased the number of intestinal mucosa cells and enhanced antioxidant capacity in contrast to fish fed a 5% G. gracilis-supplemented diet [21,23]. This same dietary blend also influenced the gut microbiota composition, promoting higher microbial diversity in contrast to diets containing 8% of either G. gracilis or N. oceanica individually, which resulted in lower microbial diversity [22]. Therefore, the incorporation of both macro- and microalgae into the feed resulted in improved fish performance and health, with observed potential synergistic effects.
Currently, one of the aims of fish nutritionists is to explore innovative ingredients that may promote intestinal health and fish robustness [24,25,26]. Gilthead seabream stands as the most cultured fish species in southern Europe. Its production has seen a notable rise in the last few decades due to rising market demand and value. Consequently, this surge has led to the adoption of intensive production methods characterised by high stocking densities, prolonged transportation procedures, and increased handling and grading, thereby fostering stressful rearing conditions in this species. These scenarios contribute to depression of the fish immune system, which can result in poor health status and growth performance, increased disease susceptibility, higher mortalities, and considerable economic losses [1,2,3,4,5,6,25,27]. Considering the importance of intestinal health for fish robustness and aquaculture sustainability, it is essential to explore nutritional strategies that enhance intestinal recovery and resilience. Dietary supplementation with micro- and macroalgae, individually or in combination, could enhance intestinal recovery in gilthead seabream following an induced insult by strengthening the immune system, antioxidant capacity, and intestinal integrity. Therefore, the present study aimed to assess whether a nutritional intervention (diet including one or both micro- and macroalgae, P. tricornutum and G. gracilis, respectively) could help intestinal recovery in gilthead seabream after an insult. Following a 20-day nutritional trial, histological integrity and physiological and gene expression responses were assessed to evaluate the effectiveness of this dietary approach in promoting intestinal recovery.
2. Materials and Methods
2.1. Experimental Diets
Four experimental diets were formulated using practical ingredients (Table 1). A control diet (CTRL) was formulated to be a commercial-like feed for gilthead seabream juveniles. The experimental diets were formulated by supplementing the CTRL diet with microalgae (Phaeodactylum tricornutum; PHA) or macroalgae (Gracilaria gracilis; GRA) at 2.5%, or a blend of micro- and macroalgae at 5% (50:50; BLEND). P. tricornutum was obtained from Allmicroalgae (Pataias, Portugal) and G. gracilis from Alga+ (Ílhavo, Portugal).

Table 1.
Formulation and proximate composition of the experimental diets.
Experimental diets were manufactured at SPAROS Lda. (Olhão, Portugal). Diets (pellet size 3.0 mm) were produced by extrusion using a pilot-scale twin-screw extruder (CLEXTRAL BC45; Clextral, Firminy, France) with a screw diameter of 55.5 mm and temperature ranging from 105 to 110 °C. Upon extrusion, all batches of extruded feeds were dried in a vibrating fluid bed dryer (model DR100; TGC Extrusion, Roullet-Saint-Estèphe, France). Following drying, pellets were allowed to cool at room temperature and subsequently, the oil fraction was added under vacuum coating in a Pegasus vacuum mixer (PG-10VCLAB; DINNISEN, Sevenum, The Netherlands). Throughout the experiment, experimental feeds were stored at room temperature in a cool and aerated storage room. Proximate composition of the diets is shown in Table 1.
2.2. Fish Rearing
Experiments were carried out in compliance with the Guidelines of the European Union Council (Directive 2010/63/EU) and Portuguese (Decreto-Lei n° 113/2013 de 7 de agosto) legislation for the use of laboratory animals. Animal protocols were directed by trained scientists under Group-C licenses from the Direção Geral de Alimentação e Veterinária, Ministério da Agricultura, Florestas e Desenvolvimento Rural, Portugal.
The experiment was conducted at the Ramalhete Station of the Centre of Marine Sciences of Algarve (CCMAR, Faro, Portugal). Fish were adapted to the new conditions in a flow-through system and fed a commercial diet. Gilthead seabream juveniles with a mean body weight of 176 ± 0.32 g were distributed into 15-cylinder 500 L tanks at an initial density of 9.8 kg m−3 (420 fish, 28 fish/tank) under natural photoperiod conditions (end of February to mid-March; 37°0′22.496″, 7°58′2.809″).
2.2.1. Assisted-Feeding to Provoke an Insult
Gut disruption was induced through an assisted-feeding procedure using soy saponins, a glycosylated compound known to impair intestinal integrity in fish and used as a model to mimic the effects of aquaculture-related stressors [2,3]. To study the intestinal recovery, gilthead seabream juveniles were subjected to an assisted-feeding procedure using gelatine capsules containing saponins. Each fish received two gelatine capsules (n = 2; total of 850 mg saponins) filled with soy saponins at 98% purity. Assisted feeding was performed after 24 h of fasting. Fish were anesthetised (300 ppm 2-phenoxyethanol; Sigma-Aldrich, Madrid, Spain) and placed on a dry plastic tray using a fish net. The capsules were carefully inserted through the oesophagus into the stomach using a technique previously tested to avoid injury, with the entire procedure lasting approximately 10 s. Following administration, fish were placed in a 20 L bucket with clean, aerated seawater and monitored for 10 min to detect any capsule regurgitation. Once recovered, fish were returned to their respective tanks. After the procedure, fish were assigned to one of four dietary treatments (CTRL + S, PHA, GRA, or BLEND). To control for potential effects of the assisted feeding technique itself, an internal control group (CTRL) was included, in which fish received two empty gelatine capsules.
2.2.2. Nutritional Trial
After the insult, fish underwent a 72 h fasting period before the start of the feeding trial. This duration was based on preliminary data indicating that 72 h post-oral administration of soy saponins is required to effectively induce disruption of intestinal homeostasis in gilthead seabream, in contrast to shorter periods of 24 or 48 h [28]. Fish from the CTRL and CTRL + S groups were fed the CTRL diet (no algae supplementation), whereas fish in the PHA, GRA, and BLEND groups were fed diets supplemented with microalgae (PHA), macroalgae (GRA), or a combination of both (BLEND), respectively. The experimental diets were randomly assigned to triplicate tanks per treatment. Feeding was carried out by hand to apparent satiation twice daily (10 h 00 and 16 h 00) from Monday to Friday and once daily on Saturdays (10 h 00). Feed distribution and apparent feed intake were recorded daily. Mortality and water quality parameters, including temperature, dissolved oxygen, and salinity, were monitored daily. Throughout the trial, water temperature averaged 15.2 ± 0.9 °C, salinity was 35.8 ± 0.8‰, and dissolved oxygen remained at 94.6 ± 1.8% of saturation.
2.3. Sampling and Analytical Procedures
At the end of the trial, fish were fasted for 24 h and euthanised with a lethal dose of anaesthetic (1500 ppm 2-phenoxyethanol; Sigma-Aldrich). Blood was withdrawn individually from the caudal vein of five fish from each replicate tank (n = 15 per treatment) using heparinised syringes. Blood was centrifuged at 1500× g for 2 min, the collected plasma was frozen in liquid nitrogen and stored at −80 °C for metabolic enzymes analysis. The anterior intestine of three fish per tank (n = 9 per treatment) was carefully dissected. Approximately 1 cm of the anterior intestine of each fish was collected for the analysis of gene expression. Samples were preserved in RNAlater and stored at −80 °C until further analysis. For histology analysis, another 1 cm sample of the anterior intestine was collected (n = 3 per tank, n = 9 per treatment) and fixed in 10% neutral buffered formalin until analysis. The remaining fish from each replicate tank (n = 28) were bulk-weighed and counted after individual sampling for molecular and histological analyses.
2.3.1. Metabolic Enzyme Analysis
The activities of the metabolic enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) and the ratio ALT:AST were determined in plasma. Enzyme activities were assayed at 30 °C using spectrophotometric procedures in a multimode microplate reader (BioTek Synergy Neo2, BioTek Instruments, Inv., Winooski, Vermont, USA). Samples from three fish per replicate tank (n = 9 per treatment) were run in duplicates using commercial kits (SPINREACT, S.A., Barcelona, Spain): ALT (Ref: 1001170), AST (Ref: 1001160), ALP (Ref: 1001130), following the manufacturer’s protocols. Enzyme activity units (IU), defined as micromoles of substrate converted to product per min in standard conditions, were expressed in units per litre of sample (U L−1).
2.3.2. Histology Analysis
Anterior intestine samples fixed in 10% neutral buffered formalin were processed according to standard histological procedures for paraffin embedding of tissues involving a sequence of dehydration of tissues in ethanol, consecutive xylene baths and inclusion in paraffin wax. Tissues were placed in histology blocks, and 4 µm-thick sections were mounted on glass slides and stained using Haematoxylin and Eosin (H&E) for semi-quantitative histological analysis using a light microscope (Nikon Eclipse E200, Nikon Instruments Europe B.V., Amstelveen, The Netherlands). Histological alterations were assessed using a semi-quantitative scoring system designed to ensure reproducibility and consistency across samples. Each parameter was individually evaluated according to its incidence and severity, using a score from 0 to 3 (0: no incidence; 1: mild; 2: moderate; 3: marked). The assessed criteria included cellular infiltration in the mucosa and submucosa, increased mucus cell abundance, apoptotic forms, and mucosal vacuolation. In addition, the presence (scored as 1) or absence (scored as 0) of marked vascular congestion in the submucosa was recorded. The detailed scoring approach allowed for standardized comparison among treatments and enhanced reproducibility of the histological assessment.
2.3.3. Gene Expression Analysis
RNA Extraction and cDNA Synthesis
Total RNA from the anterior intestine was extracted using Tri reagent (Sigma, Madrid, Spain) according to the manufacturer’s instructions. A NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) was used to measure the concentration of total RNA based on absorbance at 260 nm. RNA integrity was determined using agarose gel electrophoresis. cDNA was synthesised from 500 ng of purified RNA using the M-MLV Reverse Transcriptase Kit (Invitrogen, Thermo Fisher Scientific, Porto Salvo, Portugal) following the manufacturer’s instructions. Negative control reactions were carried out in the absence of the enzyme.
Real-Time PCR (RT-PCR)
For their roles in immunological, antioxidant defence, and epithelial permeability and protection, a panel of 10 genes were selected for analysis (Table 2). The selected genes represent key functional categories of intestinal health. Immune response: il1b, tnfa, il10, and igm; antioxidant defence: cat, and gpx; cell proliferation: pcna; barrier integrity: tjp, ocl and muc13. This approach allowed for an integrative evaluation of immune, oxidative, and structural recovery processes. Geneious Prime software (version 2023.1) was used to design the primers. Information on specific primers (GenBank accession numbers, forward and reverse primer sequences, annealing temperatures, and primer efficiency) is listed in Table 2. Real-time quantitative PCR was carried out on a CFX96 Touch Real-Time PCR system (Bio-Rad Laboratories, Hercules, CA, USA) with SsoFast EvaGreen chemistry (Bio-Rad, Hercules, CA, USA). For hot-start polymerase activation, thermal cycling started at 95 °C for 30 s. A total of forty-five PCR cycles were carried out, each consisting of heating at 95 °C for 5 s for denaturing, and for 10 s for annealing and extension at a temperature that varied depending on the primer pair utilised (Table 2). To assess reaction specificity, melting curve analysis was performed with ramping rates of 0.5 °C per 10 s through a temperature range of 55 to 95 °C. All reactions were run in duplicate, with a negative control included in each run. Ct values were determined using CFX MaestroTM Software (Bio-Rad, Hercules, USA). Relative gene expression was determined using the Pfaffl method [29]. The stability of the reference gene elongation factor 1-alpha (ef1a) was validated with RefFinder [30] and utilised for normalisation. Expression levels of target genes were compared across dietary treatments relative to the expression level of ef1a in fish from the CTRL + S treatment, which was arbitrarily assigned a value of 1. Data presented is shown as relative mRNA expression.

Table 2.
Primer sequences and amplification parameters used for real-time PCR analysis.
2.4. Disruption Assessment
A comprehensive histological analysis was performed to assess the intestinal disruption resulting from the soy saponin insult. This evaluation aimed to compare the CTRL group (administration of empty gelatine capsules) with the CTRL + S group (administration of soy saponin-filled gelatine capsules). Histological assessment was carried out using predefined criteria and a standardised scoring system, as detailed in Section 2.3.2, focusing on parameters indicative of intestinal health and tissue disruption. A score of 0 for any given parameter indicated the absence of disruption, whereas scores from 1 to 3 denoted varying degrees of disruption (1: mild; 2: moderate; 3: marked).
2.5. Dietary Recovery Assessment
To investigate the potential for intestinal recovery following the insult, dietary supplementation with micro- and macroalgae was evaluated using the PHA, GRA, and BLEND diets. The assessment included analysis of metabolic enzymes (Section 2.3.1), histological parameters (Section 2.3.2), and gene expression (Section 2.3.3). Results from the algae-supplemented treatments (PHA: microalgae; GRA: macroalgae; BLEND: combination of both) were compared to the CTRL + S group, which received no algae supplementation.
2.6. Statistical and Data Analysis
All data were expressed as mean ± standard deviation (SD). All data were subjected to normality (Shapiro–Wilk test) and homogeneity of variance (Levene’s test) tests. For the Disruption Assessment, the treatments CTRL and CTRL + S were subjected to a t-test analysis to identify whether there were any significant differences between the treatments tested. For the Dietary Recovery Assessment, if the assumptions for analysis were met, a one-way Analysis of Variance (ANOVA) was performed on means to determine if any significant differences occurred between the treatments evaluated (CTRL + S, PHA, GRA, and BLEND), followed by Tukey’s HSD post hoc test when significant effects were found (p < 0.05). When the assumptions for ANOVA failed, a non-parametric Kruskal-Walli’s test was applied, followed by Dunn’s test with Bonferroni correction for pairwise comparisons. All statistical analyses were performed in RStudio (Version: 2023.03.0 + 386).
3. Results
Feeding gilthead seabream juveniles with the experimental diets for 20 days did not significantly affect (p > 0.05) fish weight and weight gain. Fish presented a final weight of 189.51 ± 4.62 g and the weight gain during that period was 7.89 ± 2.73% of initial body weight.
3.1. Disruption Assessment
To validate the efficacy of the induced insult and establish a disrupted baseline for subsequent recovery assessment, histological comparisons were performed between fish that received empty gelatine capsules (CTRL group) and those that received soy saponin-filled capsules (CTRL + S group). Fish in the CTRL + S group exhibited a significant increase (p < 0.05) in the number of mucus cells in the intestinal mucosa, with mild to moderate incidence (Figure 1b), in contrast to the CTRL group, which showed no such alterations (Figure 1a). Additionally, cellular infiltration in the submucosa was significantly elevated (p < 0.05) in the CTRL + S group with mild incidence (Figure 1d), whereas no infiltration was observed in the CTRL group (Figure 1c). No statistically significant differences (p > 0.05) were observed between the two groups for the remaining histological parameters evaluated. These results confirm that the administration of soy saponins effectively triggered mild but measurable intestinal disruption, thereby validating the model for subsequent dietary recovery trials.
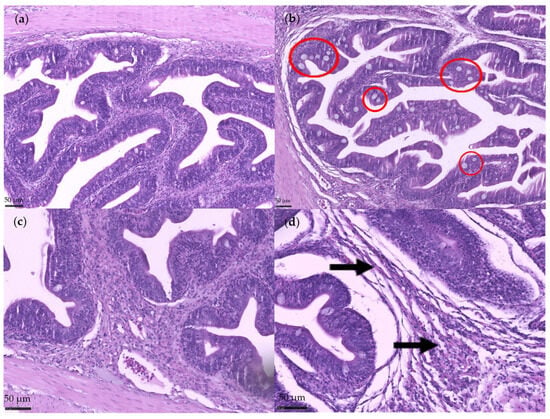
Figure 1.
Histological features of the anterior intestine from gilthead seabream. Mucosa sections showing mucus cells in fish from the CTRL treatment (a) and the CTRL + S (b). Red circles indicate an increased number of mucus cells in the epithelium of fish from the CTRL + S group, whereas no such increase was observed in the CTRL group. Submucosal sections showing cellular infiltrates in fish from the CTRL +S treatment (d) indicated by black arrows, compared to the absence of cellular infiltration in the CTRL group (c).
3.2. Dietary Recovery Assessment
3.2.1. Metabolic Enzymes Analysis
Analysis of metabolic enzyme activities revealed that fish fed the BLEND diet (containing both micro- and macroalgae) exhibited significantly lower (p < 0.05) levels of alkaline phosphatase (ALP) compared to fish from the CTRL + S group (no algae supplementation) and those fed either the PHA or GRA diets (Table 3). In contrast, the activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) did not differ significantly (p > 0.05) among any of the algae-supplemented groups (PHA, GRA, and BLEND) compared to the CTRL + S group. Similarly, the ALT:AST ratio remained unchanged (p > 0.05) across all dietary treatments, suggesting that these markers of hepatic function or damage were not influenced by algae supplementation under the present conditions.

Table 3.
Activities of alkaline phosphate (ALP), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the plasma of gilthead seabream juveniles fed the experimental diets for 20 days.
3.2.2. Histological Analysis
Regarding intestinal histology, fish fed the GRA diet (macroalgae supplementation) showed a significant increase (p < 0.05) in mucosal vacuolation (Figure 2a) compared to fish from the CTRL + S group (Figure 2d). Fish in the PHA group (microalgae diet) presented intermediate levels of vacuolation (Figure 2b), although differences were not statistically significant relative to the CTRL + S group. No significant differences (p > 0.05) in mucosal vacuolation were observed between the BLEND (Figure 2c) and CTRL + S groups. Moreover, no significant changes (p > 0.05) were found in any of the remaining histological parameters among the algae-supplemented groups (PHA, GRA, and BLEND) when compared with the CTRL + S group.
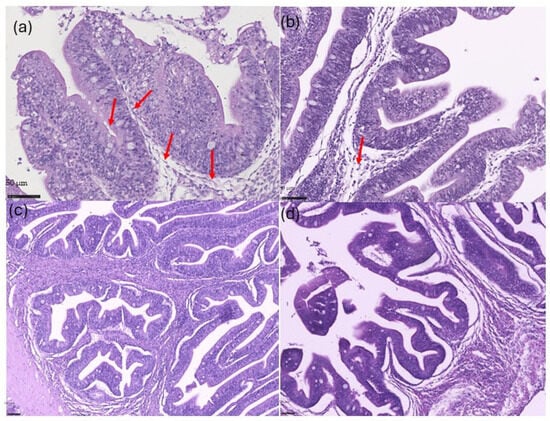
Figure 2.
Representative mucosal sections from the histological assessment of the anterior intestine in gilthead seabream juveniles. (a) Fish fed the diet supplemented with the macroalgae (GRA) showed a significant increase in mucosa vacuolation (red arrows). (b) Microalgae supplementation (PHA) showed intermediate levels of vacuolation (red arrow). (c,d) No presence of vacuoles in the BLEND (c) and the CTRL + S (d) treatments.
3.2.3. Gene Expression Analysis
Concerning the genes related to the immune response, fish fed the PHA diet displayed a significant upregulation of igm and il10 compared with fish from the CTRL + S treatment (p < 0.05). The expression levels of il1b and pcna were significantly upregulated in fish fed the BLEND diet in comparison with fish from the CTRL + S treatment (p < 0.05). Tnfa expression was not significantly different between fish fed micro- and/or macroalgae when compared to CTRL + S fish (p > 0.05) (Figure 3a). Intestinal epithelium permeability and protection assessment, through the expression of tjp, ocl, and muc13, showed no significant dietary effect (p > 0.05) (Figure 3b). Regarding the genes involved in the antioxidant response, a significant upregulation of gpx expression was observed in fish fed the diet supplemented with P. tricornutum (PHA) in comparison with fish from the CTRL + S treatment (p < 0.05) (Figure 3c). The expression levels of cat were not significantly influenced by the treatments (p > 0.05) (Figure 3c).
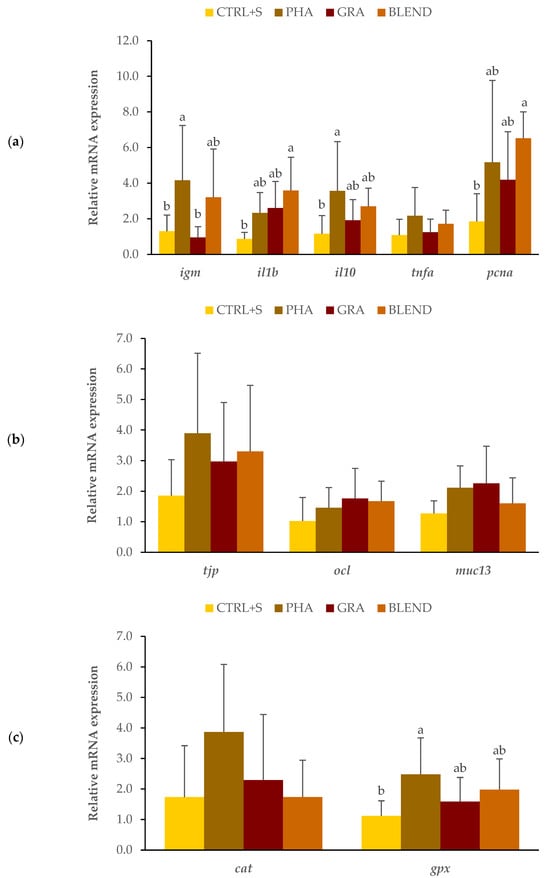
Figure 3.
Relative mRNA expression of genes involved in immunological defense (a): igm: immunoglobulin M, il1b: interleukin-1 beta, il10: interleukin-10, tnfa: tumor necrosis factor-alpha, and pcna: proliferating cell nuclear antigen; epithelial permeability and protection (b): tjp: tight junction protein, ocl: occludin, and muc13: mucin 13; antioxidant defense (c): cat: catalase and gpx: glutathione peroxidase. Values are presented as means ± standard deviation (n = 7–9). Different letters indicate significant differences among treatments (p < 0.05).
4. Discussion
This study aimed to evaluate the effects of three functional diets, based on marine-derived ingredients, specifically a microalga (PHA), a macroalga (GRA), and their combination (BLEND), on the intestinal recovery of gilthead seabream juveniles following an oral insult with soy saponins. Recovery was assessed through histological, physiological and molecular parameters.
4.1. Disruption Assessment
The administration of soy saponins (CTRL + S) led to noticeable changes in the fish intestinal structure. Histological analysis showed an increase in mucus cells in the intestinal mucosa and moderate submucosal inflammatory infiltrates, both indicative of irritation and a compensatory mucosal response. The intestinal mucosal barrier is essential nutrient absorption and defence, and its disruption can compromise gut homeostasis. Soy saponins exacerbate this situation since they disrupt cell membranes due to their amphipathic nature increasing membrane permeability and triggering inflammatory responses [1,3]. This can compromise cellular homeostasis and can stimulate the proliferation of goblet cells, responsible for mucus secretion [31,32,33,34]. The elevated number of mucus cells observed in the CTRL + S fish likely represents a compensatory mechanism of the intestine in response to the irritation caused by the insult. Likewise, the infiltration of immune cells into the submucosa suggests activation of local defence mechanisms to restore homeostasis. No significant alterations were observed in other histological criteria, indicating that the insult induced a targeted, moderate reaction rather than extensive tissue damage.
4.2. Recovery Assessment
The evaluation of biochemical markers indicated that alkaline phosphatase (ALP) activity decreased its levels in fish fed the BLEND diet compared to the CTRL + S. Biochemical analyses, like measuring plasma levels of nonspecific serum enzymes, including ALP, serve as a useful health assessment marker in fish. ALP plays a role in maintaining gut barrier function and intestinal homeostasis. While high levels of serum ALP are associated with bone anomalies, liver dysfunction and inflammation, low levels are correlated with beneficial effects on hepatic and gut function [35,36,37]. Similarly, ALP levels decreased in Nile tilapia (Oreochromis niloticus) fed Schizochytrium sp. at 10% for eight weeks [38] and in Nile tilapia fed supplemented diets with Spirulina platensis and Chlorella vulgaris blends [39]. Likewise, dietary supplementation with the macroalga Sargassum kjellmanianum at 5% reduced ALP levels in yellow catfish (Pelteobagrus fulvidraco) [40] after 28 days. These effects have been attributed to the bioactive compounds present in micro- and macroalgae, including polysaccharides, antioxidants, and polyunsaturated fatty acids, which exhibit anti-inflammatory and hepatoprotective effects [41,42,43]. However, no significant changes in ALP levels were observed in fish fed the PHA or GRA diets alone. These results may be attributed to the relatively short feeding period (20 days) and the inclusion level (2.5%). Therefore, the reduction in ALP levels in fish fed the BLEND diet suggests that this diet displayed beneficial synergetic effects. The combination of both algae may have enhanced the bioavailability and functionality of their bioactive compounds, potentiating a more rapid or pronounced physiological response. The large variability observed in ALP activity may reflect individual differences in metabolic or physiological recovery capacity following the insult.
Regarding the histological analysis, fish fed the GRA diet displayed increased mucosal vacuolation. Vacuoles play a role in maintaining intracellular homeostasis and isolating potentially harmful metabolites. However, excessive or diffuse vacuolation can indicate persistent stress [44,45,46]. The absence of significant vacuolation in fish fed the BLEND diet could indicate a more advanced stage of recovery, whereas GRA fish could still be undergoing adaptive responses. Since vacuolation is a dynamic and potentially transient process, future studies with multiple time points would help clarify the temporal dynamics of vacuolation and better elucidate how these functional feeds support recovery processes in the gut.
Dietary supplementation also modulated immune- and stress-related genes, reflecting different recovery mechanisms. Fish fed the PHA diet exhibited a significant upregulation of igm and il10 compared to CTRL + S group. These results agree with studies reporting igm upregulation in gilthead seabream juveniles fed P. tricornutum at 10% for four weeks and il10 upregulation in fish fed a diet supplemented with P. tricornutum-derived β-glucans at 21 and 37% for eight weeks [14,15,16]. Immunoglobulin M (IgM) is the primary immunoglobulin in fish, involved in systemic immunity, whereas interleukin-10 plays a key role in regulating inflammatory responses [47,48,49]. Despite the shorter feeding duration in the current study, there was still a significant upregulation of igm and il10, suggesting a prompt activation of both immune and anti-inflammatory pathways in the PHA diet. The lack of significant modulation of these markers in the GRA and BLEND groups may indicate that longer dietary exposure is required to exert comparable effects on the adaptive immune response or that it has occurred earlier in the recovery period, thus the molecular machinery has since downregulated transcriptional activity to conserve energy.
The BLEND diet significantly upregulated the expression of il1b, a pro-inflammatory cytokine that triggers leukocyte recruitment and tissue repair [50]. Similar responses were reported in European seabass juveniles fed a diet supplemented with a blend of two microalgae at 2% (Nannochloropsis oceanica and Chlorella vulgaris) and two macroalgae at 2% (G. gracilis and Ulva rigida) for 12 weeks [51]. The observed il1b upregulation suggests that the BLEND diet may have elicited a rapid immune response even within a limited timeframe. This highlights the potential of the algae-based diets to quickly stimulate pro-inflammatory responses, possibly due to their rich content of immunostimulatory compounds. Therefore, the significant upregulation of il1b may indicate a swift and effective immune activation, showing that a short-term supplementation with algae blend can influence intestinal immune responses.
The significant upregulation of pcna expression in fish fed the BLEND diet indicated a diet-induced stimulation of cellular proliferation in the intestinal epithelium. Previous studies reported pcna expression in European seabass juveniles, fed a diet supplemented with 4% of microalgae (Nannochloropsis oceanica, Chlorella vulgaris) and macroalgae (Gracilaria gracilis, Ulva rigida) for 12 weeks [51]. A similar effect was observed after eight weeks with a 2% blend of Nannochloropsis sp., Aurantiochytrium sp., and Gracilaria sp. [22]. Moreover, Reis and co-authors [30] observed a dose-dependent effect of P. tricornutum-derived β-glucans after two weeks of feeding, where an inclusion of 21% upregulated pcna, while a higher level (37%) downregulated it. In the present study, fish fed the PHA diet, which included a lower dose (2.5%) of whole P. tricornutum cells, exhibited intermediate pcna levels. This suggests that the biological response is influenced not only by dosage, but also by the form of administration (whole cell and extract) and the feeding duration. Pcna is involved in DNA replication and repair, supporting cell cycle progression in the intestinal epithelium and reflecting epithelial cell turnover, which is essential for maintaining gut integrity and function [52,53]. The significant upregulation of pcna expression in BLEND fed-fish suggests that the inclusion of micro- and macroalgae may have acted synergistically to stimulate tissue repair mechanisms, supporting intestinal recovery processes. These findings underscore the potential of tailored algal blends to modulate intestinal health through stimulation of epithelial sound cell turnover. The synergistic effects observed in the BLEND group may arise from complementary bioactive interactions among micro- and macroalgal compounds such as polysaccharides, polyphenols, carotenoids, and LC-PUFAs, that jointly enhance antioxidant defences and immune modulation [11,12,13,14,15,16,17,18]. These findings highlight the complexity of dietary interactions and support the combined use of micro- and macroalgae as a multifactorial approach to gut health recovery.
In terms of antioxidant defence, fish fed the PHA diet showed a significant upregulation of gpx expression. Microalgae-derived antioxidants, such as polyphenols, pigments, vitamins, and minerals, can mitigate oxidative stress by activating enzymatic pathways, including GPx [54,55,56]. Similar gpx activation was observed in zebrafish fed P. tricornutum (5%) for 30 days [33]. The observed upregulation of gpx in PHA-fed fish suggests that microalgae may rapidly stimulate antioxidant defense mechanisms, potentially providing early protection against insult-induced oxidative stress. Interestingly, despite the well-documented antioxidant properties of G. gracilis, no significant differences in gpx expression were observed in fish fed the GRA diet. This lack of response may be attributed to the lower bioavailability of antioxidant compounds from macroalgae compared to microalgae, or to the relatively short feeding duration, which might have been insufficient to elicit detectable transcriptional changes.
The expression of tjp, ocl, and muc13 remained unaffected, indicating that dietary effects more focused on immune and antioxidant pathways, rather than in epithelial permeability and protection. The absence of significant changes in tjp, ocl, and muc13 expression suggests that intestinal barrier modulation requires either longer feeding duration or higher inclusion levels. This aligns with studies showing that tight-junction gene responses are species-, dose-, and time-dependent [57,58].
5. Conclusions
This study assessed the efficacy of dietary supplementation with marine-derived micro- and macroalgae in supporting intestinal recovery and homeostasis in gilthead seabream juveniles following an induced intestinal insult. Among the experimental diets, the BLEND diet offered the most comprehensive support for intestinal recovery, suggesting potential synergistic effects between micro- and macroalgal bioactive compounds. This diet was associated with reduced serum ALP activity, indicative of improved gut barrier integrity and liver function. Furthermore, the BLEND diet significantly upregulated il1b and pcna expression, reflecting enhanced immune activation, epithelial cell proliferation and tissue repair. The PHA diet significantly increased the expression of igm, il10, and gpx, highlighting its role in boosting immune function and antioxidant defence mechanisms. In contrast, the GRA diet appeared less effective in promoting intestinal recovery over the short feeding duration assessed in this study. Overall, each algal treatment presented distinct advantages: PHA primarily enhanced antioxidant and immune responses, GRA promoted cellular protection, and the BLEND diet combined these benefits, achieving a more balanced recovery profile. However, the short experimental period (20 days) limited the observation of potential long-term or cumulative effects.
Future research should evaluate longer term experiments to confirm the persistence of the beneficial effects and to explore other inclusion levels on health status, stress resilience, and gut microbiota composition. Additionally, elucidating the mechanisms of action of key bioactive compounds will be essential to fully understand their roles in modulating intestinal recovery, immune competence, and antioxidative status. Collectively, these findings contribute valuable knowledge towards the development of functional feeds aimed at improving fish health and productivity in aquaculture.
Author Contributions
I.G.-G.: formal analysis, investigation, writing—original draft, writing—review and editing, and visualization. C.A.: conceptualization, validation, resources, data curation, writing—review and editing, supervision, project administration, and funding acquisition. R.T.: conceptualization, validation, investigation, formal analysis, and writing—review and editing. A.T.G.: conceptualization, validation, methodology, and writing—review and editing. S.E.: conceptualization, validation, resources, data curation, writing—review and editing, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study has received funding from the European Union’s Horizon 2020 Marie Skłodowska-Curie ITN Program under grant agreement No. 956697 (EATFISH), from Project FICA, co-financed by COMPETE 2020, CRESC Algarve 2020, Portugal 2020 and the European Union through ERDF under reference ALG-01-0247-FEDER-047175, from “Pacto da Bioeconomia Azul” (Project No C644915664-00000026) within the WP5 Algae Vertical, funded by Next Generation EU European Fund and the Portuguese Recovery and Resilience Plan (PRR), under the scope of the incentive line “Agendas for Business and Innovation” through the funding scheme C5—Capitalization and Business Innovation, and from Portuguese national funds from FCT—Foundation for Science and Technology—through contracts UID/04326/2025, UID/PRR/04326/2025 and LA/P/0101/2020 (DOI:10.54499/LA/P/0101/2020).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of CCMAR (protocol code EATFISH, 19 June 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in the study are openly available in Zenodo at Aragão, C., Teodósio, R., & Engrola, S. (2025). Functional Algal Feeds in Aquaculture: Gut Recovery in Gilthead Seabream through Micro- and Macroalgae Supplementation [Data set]. Zenodo. https://doi.org/10.5281/zenodo.17236051.
Acknowledgments
The authors would like to acknowledge technical support by Anna Stryzhak (GreenCoLab) and Rita Colen (CCMAR).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, Z.; Yang, Q.; Liu, H.; Jin, J.; Yang, Y.; Zhu, X.; Han, D.; Zhou, Z.; Xie, S. Potential Functions of the Gut Microbiome and Modulation Strategies for Improving Aquatic Animal Growth. Rev. Aquac. 2025, 17, e12959. [Google Scholar] [CrossRef]
- Kumar, V.; Hossain Md., S.; Ragaza, J.A.; Benito, M.R. The Potential Impacts of Soy Protein on Fish Gut Health. Soybean Hum. Consum. Anim. Feed 2020, 10, 91–115. [Google Scholar] [CrossRef]
- Hardy, R.W. History of fish nutrition. In Nutrition and Physiology of Fish and Shellfish: Feed Regulation, Metabolism, and Digestion; Academic Press: Cambridge, MA, USA, 2025; pp. 3–11. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Papaharisis, L.; Tsironi, T.; Dimitroglou, A.; Taoukis, P.; Pavlidis, M. Stress assessment, quality indicators and shelf life of three aquaculture important marine fish, in relation to harvest practices, water temperature and slaughter method. Aquac. Res. 2019, 50, 2608–2620. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Stress management in aquaculture: A review of dietary interventions. Rev. Aquac. 2021, 13, 2190–2247. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, Y.; Shi, X.; Qiao, D.; Pei, C.; Kong, X. Signalling regulation of reactive oxygen species in fish inflammation. Rev. Aquac. 2024, 16, 1266–1285. [Google Scholar] [CrossRef]
- Egerton, S.; Wan, A.; Murphy, K.; Collins, F.; Ahern, G.; Sugrue, I.; Busca, K.; Egan, F.; Muller, N.; Whooley, J.; et al. Replacing fishmeal with plant protein in Atlantic salmon (Salmo salar) diets by supplementation with fish protein hydrolysate. Sci. Rep. 2020, 10, 4194. [Google Scholar] [CrossRef]
- Onomu, A.J.; Okuthe, G.E. The Role of Functional Feed Additives in Enhancing Aquaculture Sustainability. Fishes 2024, 9, 167. [Google Scholar] [CrossRef]
- Madhulika Meinam, M.; Deepti, M.; Ngasotter, S.; Gupta, S.S.; Varghese, T. Functional Feed Additives in Aquaculture to Improve Food Security. Food Security, Nutrition and Sustainability Through Aquaculture Technologies; Springer Nature: Berlin, Germany, 2025; pp. 375–396. [Google Scholar] [CrossRef]
- Vijayaram, S.; Ringø, E.; Ghafarifarsani, H.; Hoseinifar, S.H.; Ahani, S.; Chou, C.C. Use of Algae in Aquaculture: A Review. Fishes 2024, 9, 63. [Google Scholar] [CrossRef]
- Bakky, M.A.H.; Tran, N.T.; Zhang, Y.; Li, S. Utilization of marine macroalgae-derived sulphated polysaccharides as dynamic nutraceutical components in the feed of aquatic animals: A review. Aquac. Res. 2022, 53, 5787–5808. [Google Scholar] [CrossRef]
- Ringø, E.; Ashour, M.; Ahmed, S.; Sharawy, Z.; Goda, A.; El-Haroun, E.; Ringø, E.; Ashour, M.; Ahmed, S.; Sharawy, Z.; et al. Microalgae and Seaweeds as Feed Additives for Aquatic Animals: Effects on Growth, Immunity, and Disease Resistance. Algae-Science and Applications; IntechOpen: Rijeka, Croatia, 2025. [Google Scholar] [CrossRef]
- Cerezuela, R.; Fumanal, M.; Tapia-Paniagua, S.T.; Meseguer, J.; Morinigo, M.Á.; Esteban, M.Á. Histological alterations and microbial ecology of the intestine in gilthead seabream (Sparus aurata L.) fed dietary probiotics and microalgae. Cell Tissue Res. 2012, 350, 477–489. [Google Scholar] [CrossRef]
- Cerezuela, R.; Guardiola, F.A.; Meseguer, J.; Esteban, M.Á. Enrichment of gilthead seabream (Sparus aurata L.) diet with microalgae: Effects on the immune system. Fish Physiol. Biochem. 2012, 38, 1729–1739. [Google Scholar] [CrossRef]
- Reis, B.; Gonçalves, A.T.; Santos, P.; Sardinha, M.; Conceição, L.E.C.; Serradeiro, R.; Pérez-sánchez, J.; Calduch-giner, J.; Schmid-staiger, U.; Frick, K.; et al. Immune status and hepatic antioxidant capacity of gilthead seabream sparus aurata juveniles fed yeast and microalga derived β-glucans. Mar. Drugs 2021, 19, 653. [Google Scholar] [CrossRef]
- Gonçalves, A.T.; Simões, M.; Costa, C.; Passos, R.; Baptista, T. Modulatory effect of Gracilaria gracilis on European seabass gut microbiota community and its functionality. Sci. Rep. 2022, 12, 14836. [Google Scholar] [CrossRef]
- Biotechnology, B.; Khandwal, D.; Maniar, J.N.; Pandey, A.K.; Gupta, N.K.; Mishra, A. Proximate, physicochemical, bioactive and antiproliferative characteristics of Gracilaria debilis extract reveal its nutraceutical potential. Blue Biotechnol. 2025, 2, 10. [Google Scholar] [CrossRef]
- Peixoto, M.J.; Ferraz, R.; Magnoni, L.J.; Pereira, R.; Gonçalves, J.F.; Calduch-Giner, J.; Pérez-Sánchez, J.; Ozório, R.O.A. Protective effects of seaweed supplemented diet on antioxidant and immune responses in European seabass (Dicentrarchus labrax) subjected to bacterial infection. Sci. Rep. 2019, 9, 16134. [Google Scholar] [CrossRef]
- Passos, R.; Correia, A.P.; Ferreira, I.; Pires, P.; Pires, D.; Gomes, E.; do Carmo, B.; Santos, P.; Simões, M.; Afonso, C.; et al. Effect on health status and pathogen resistance of gilthead seabream (Sparus aurata) fed with diets supplemented with Gracilaria gracilis. Aquaculture 2021, 531, 735888. [Google Scholar] [CrossRef]
- Mota, C.S.C.; Pinto, O.; Sá, T.; Ferreira, M.; Delerue-Matos, C.; Cabrita, A.R.J.; Almeida, A.; Abreu, H.; Silva, J.; Fonseca, A.J.M.; et al. A commercial blend of macroalgae and microalgae promotes digestibility, growth performance, and muscle nutritional value of European seabass (Dicentrarchus labrax L.) juveniles. Front. Nutr. 2023, 10, 1165343. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Abdelhafiz, Y.; Abreu, H.; Silva, J.; Valente, L.M.P.; Kiron, V. Gracilaria gracilis and Nannochloropsis oceanica, singly or in combination, in diets alter the intestinal microbiota of European seabass (Dicentrarchus labrax). Front. Mar. Sci. 2022, 9, 1001942. [Google Scholar] [CrossRef]
- Batista, S.; Pereira, R.; Oliveira, B.; Baião, L.F.; Jessen, F.; Tulli, F.; Messina, M.; Silva, J.L.; Abreu, H.; Valente, L.M.P. Exploring the potential of seaweed Gracilaria gracilis and microalga Nannochloropsis oceanica, single or blended, as natural dietary ingredients for European seabass Dicentrarchus labrax. J. Appl. Phycol. 2020, 32, 2041–2059. [Google Scholar] [CrossRef]
- Carvalho, M.; Montero, D.; Betancor, M.; Kaur, K.; Serradell, A.; Izquierdo, M.; Ginés, R.; Claeyssens, V.; Torrecillas, S. Benefits of dietary krill meal inclusion towards better utilization of nutrients, and response to oxidative stress in gilthead seabream (Sparus aurata) juveniles. Aquaculture 2025, 598, 741957. [Google Scholar] [CrossRef]
- Mendes, R.; Rema, P.; Dias, J.; Gonçalves, A.T.; Teodósio, R.; Engrola, S.; Sánchez-Vázquez, F.J.; Conceição, L.E.C. Socially Acceptable Feed Formulations May Impact the Voluntary Feed Intake and Growth, but Not Robustness of Nile Tilapia (Oreochromis niloticus). Fishes 2024, 9, 361. [Google Scholar] [CrossRef]
- Aragão, C.; Colen, R.; Teodósio, R.; Cabano, M.; Antelo, L.T.; Vázquez, J.A.; Engrola, S. Fish Protein Hydrolysates Mitigate the Adverse Effects of No-Fishmeal Diets in Gilthead Seabream Juveniles. Aquac. Nutr. 2025, 2025, 1352251. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Nazzaro-Alvarez, J.; Jardí-Pons, A.; Reig, L.; Carella, F.; Carrassón, M.; Roque, A. Linking stocking densities and feeding strategies with social and individual stress responses on gilthead seabream (Sparus aurata). Physiol. Behav. 2020, 213, 112723. [Google Scholar] [CrossRef]
- Teodósio, R.; Engrola, S.; Dias, J.; Gonçalves, A.T. Evaluation of Potential Disruptors of Intestinal Homeostasis in Gilthead Seabream Juveniles. In Aquaculture Europe 2022 Abstract Book, Proceedings of Aquaculture Europe 2022, Rimini, Italy, 27–30 September 2022; European Aquaculture Society: Oostende, Belgium, 2022; pp. 477–478. [Google Scholar]
- Pfaffl, M.W. Quantification strategies in real-time PCR. AZ Quant. PCR 2004, 1, 89–113. [Google Scholar]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef]
- Zhang, H.; Ran, C.; Teame, T.; Ding, Q.; Hoseinifar, S.H.; Xie, M.; Zhang, Z.; Yang, Y.; Olsen, R.E.; Gatlin, D.M.; et al. Research progress on gut health of farmers teleost fish: A viewpoint concerning the intestinal mucosal barrier and the impact of its damage. Rev. Fish Biol. Fish. 2020, 30, 569–586. [Google Scholar] [CrossRef]
- Couto, A.; Kortner, T.M.; Penn, M.; Østby, G.; Bakke, A.M.; Krogdahl, A.; Oliva-Teles, A. Saponins and phytosterols in diets for European sea bass (Dicentrarchus labrax) juveniles: Effects on growth, intestinal morphology and physiology. Aquac. Nutr. 2015, 21, 180–193. [Google Scholar] [CrossRef]
- Hernandez, A.I.; dos Santos Azevedo, R.; Reyes, M.E.; Figueiredo, B.X.; dos Santos Flores, I.; Anni, L.S.A.; Silveira, T.; Tesser, M.B.; Jardim, R.D.; Junior, A.S.V.; et al. Impact of dietary supplementation with Synechococcus elongatus PCC 7942 expressing a β-glucosidase from Amazonian soil on the physiology of zebrafish (Danio rerio) fed a soybean meal-rich diet. J. Appl. Phycol. 2025, 37, 2577–2590. [Google Scholar] [CrossRef]
- Wang, X.; Wen, J.; Hou, X.; Wu, H.; Zhou, Q.; Fu, X.; Cui, C.; Lin, S.-M.; Chen, Y.; Luo, L.; et al. Effects of Dietary Soya-Saponins on Growth Performance and Intestinal Health in Juvenile Largemouth Bass (Micropterus Salmoides). Aquac. Rep. 2025, 45, 103142. [Google Scholar] [CrossRef]
- Oliveira, J.; Oliva-Teles, A.; Couto, A. Tracking Biomarkers for the Health and Welfare of Aquaculture Fish. Fishes 2024, 9, 289. [Google Scholar] [CrossRef]
- Santos, G.M.; Ismael, S.; Morais, J.; Araújo, J.R.; Faria, A.; Calhau, C.; Marques, C. Intestinal Alkaline Phosphatase: A Review of This Enzyme Role in the Intestinal Barrier Function. Microorganisms 2022, 10, 746. [Google Scholar] [CrossRef]
- Bacchetta, C.; Cazenave, J.; Mora, C.; Michlig, M.P.; Repetti, M.R.; Rossi, A.S. Non-lethal biomarkers as promising tools for fish health assessment: In situ exposure to bifenthrin as a case study. Aquat. Toxicol. 2024, 276, 107083. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zhang, X.; Zhu, F.; Wen, H.; Dong, L.; Tian, J.; Zhang, J.; Yang, C.; Xiao, J.; Duan, X.; et al. Schizochytrium sp. can improve feed utilization, fillet DHA content, and non-specific immunity of juvenile Nile tilapia (Oreochromis niloticus) fed fish oil free diet. J. Appl. Phycol. 2024, 36, 3341–3352. [Google Scholar] [CrossRef]
- Fadel, A.; Mahmoud, M.A.; Abdelsalam, M.; Eissa, E.S.H.; Sherif, A.H. Aeromonas veronii infection in cultured Oreochromis niloticus: Prevalence, molecular and histopathological characterization correlated to water physicochemical characteristics, with the protective autochthonous probiotic. Aquac. Int. 2025, 33, 298. [Google Scholar] [CrossRef]
- Shi, Q.; Hu, P.; Wen, Z.; Wang, J.; Zou, Y. Ameliorative effects of Sargassum kjellmanianum on hexavalent chromium-induced growth inhibition, immune suppression, and oxidative stress in yellow catfish. J. Appl. Phycol. 2024, 36, 2225–2236. [Google Scholar] [CrossRef]
- de Sousa, A.K.; Araujo, A.S.M.L.; da Silva, T.M.L.; de Sousa de Lima, F.M.; dos Santos Ferreira, J.; de Brito, T.V.; dos Reis Barbosa, A.L. Polysaccharides from macro algae: Anti-inflammatory actions against systemic inflammatory process and in the gastrointestinal tract. J. Appl. Phycol. 2023, 35, 381–395. [Google Scholar] [CrossRef]
- Eldessouki, E.A.A.; Elshopakey, G.E.; Elbahnaswy, S.; Shakweer, M.S.; Abdelwarith, A.A.; Younis, E.M.; Davies, S.J.; Mili, A.; Abd El-Aziz, Y.M.; Abdelnour, S.A.; et al. Influence of astaxanthin-enriched Haematococcus pluvialis microalgae on the growth efficacy, immune response, antioxidant capacity, proinflammatory cytokines, and tissue histomorphology of hybrid red tilapia. Aquac. Int. 2024, 32, 7447–7468. [Google Scholar] [CrossRef]
- Kim, Y.R.; Park, S.Y.; Kim, J.Y. Therapeutic potential of seaweed extracts: In vitro and in vivo studies on alleviating inflammation and enhancing intestinal barrier function. Food Biosci. 2024, 59, 103774. [Google Scholar] [CrossRef]
- Creasey, E.A.; Isberg, R.R. Maintenance of vacuole integrity by bacterial pathogens. Curr. Opin. Microbiol. 2014, 17, 46–52. [Google Scholar] [CrossRef]
- Emilian, O.; Ioan, S.; Irina, P.; Raul, P.; Adriana, C.; Dorin, C.; Ciprian, S. Cytological Applications of the Vacuolization Phenomenon as a Means of Determining Saline Cytotoxicity. Appl. Sci. 2023, 13, 8461. [Google Scholar] [CrossRef]
- Costa, V.; Teixeira, V. Vacuolar ATPase-mediated regulation of neutral lipid dynamics: Insights into lipid droplet homeostasis and stress response mechanisms. Biochim. Et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2024, 1869, 159465. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. The Function of Fish Cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Sakai, M.; Hikima, J.I.; Kono, T. Fish cytokines: Current research and applications. Fish. Sci. 2021, 87, 1–9. [Google Scholar] [CrossRef]
- Salinas, I.; Zhang, Y.A.; Sunyer, J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011, 35, 1346–1365. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Etayo, A.; Hordvik, I. Immunoglobulins in teleosts. Immunogenetics 2021, 73, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Sousa, V.; Oliveira, B.; Canadas-Sousa, A.; Abreu, H.; Dias, J.; Kiron, V.; Valente, L.M.P. An in-depth characterisation of European seabass intestinal segments for assessing the impact of an algae-based functional diet on intestinal health. Sci. Rep. 2023, 13, 11686. [Google Scholar] [CrossRef]
- Maga, G.; Hübscher, U. Proliferating cell nuclear antigen (PCNA): A dancer with many partners. J. Cell Sci. 2003, 116, 3051–3060. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Giari, L.; Lui, A.; Squerzanti, S.; Castaldelli, G.; Shinn, A.P.; Manera, M.; Lorenzoni, M. Proliferative cell nuclear antigen (PCNA) expression in the intestine of Salmo trutta trutta naturally infected with an acanthocephalan. Parasites Vectors 2012, 5, 198. [Google Scholar] [CrossRef]
- Fan, J.; Bao, Q.; Ma, K.; Li, X.; Jia, J.; Wu, H. Antioxidant and innate immunity of Danio rerio against Edwardsiella tarda in response to diets including three kinds of marine microalgae. Algal Res. 2022, 64, 102689. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Ciências 2018, 90, 3403–3414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, H.; Han, D.; Lu, S.; Lv, W.; Guo, K.; Wang, C.; Li, S.; Han, S.; Liu, H. Effects of dietary chrysophyte (Poterioochromonas malhamensis) rich in beta-glucan on the growth performance, intestinal health, lipid metabolism, immune gene expression, and disease resistance against Aeromonas salmonicida in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 2022, 561, 738589. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Ahmadifar, E.; Soltani, M.; Tayefi-Nasrabadi, H.; Mousavi, S.; Naiel, M.A.E. Brown Seaweed (Padina australis) Extract can Promote Performance, Innate Immune Responses, Digestive Enzyme Activities, Intestinal Gene Expression and Resistance against Aeromonas hydrophila in Common Carp (Cyprinus carpio). Animals 2022, 12, 3389. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).