Role of Hydrogen-Rich Water on Growth Performance and Liver Antioxidant Capacity of Mandarin Fish (Siniperca chuatsi)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design and Breeding Management
2.3. Sample Collection
2.4. Index Detection of Samples
2.5. Muscle Growth, Brain Feeding and Liver Antioxidant Related Gene mRNA Relative Expression
2.6. Data Statistics and Analysis
- Survival rate (SR, %) = (final fish number/initial fish number) × 100 [17]
- Weight gain rate (WGR, %) = 100 × (final weight − initial weight)/initial weight [18]
- Feed conversion ratio (FCR) = dry feed consumed/(final weight − initial weight) [17]
- Specific growth rate (SGR, %/d) = 100 × (Ln final weight − Ln initial weight)/trial days [19]
- Feed rate (FR, g/fish) = 100 × dry feed consumed/[feeding days × (final fish weight + initial fish weight)/2] [20]
3. Results
3.1. Effect of Growth Performance of Mandarin Fish
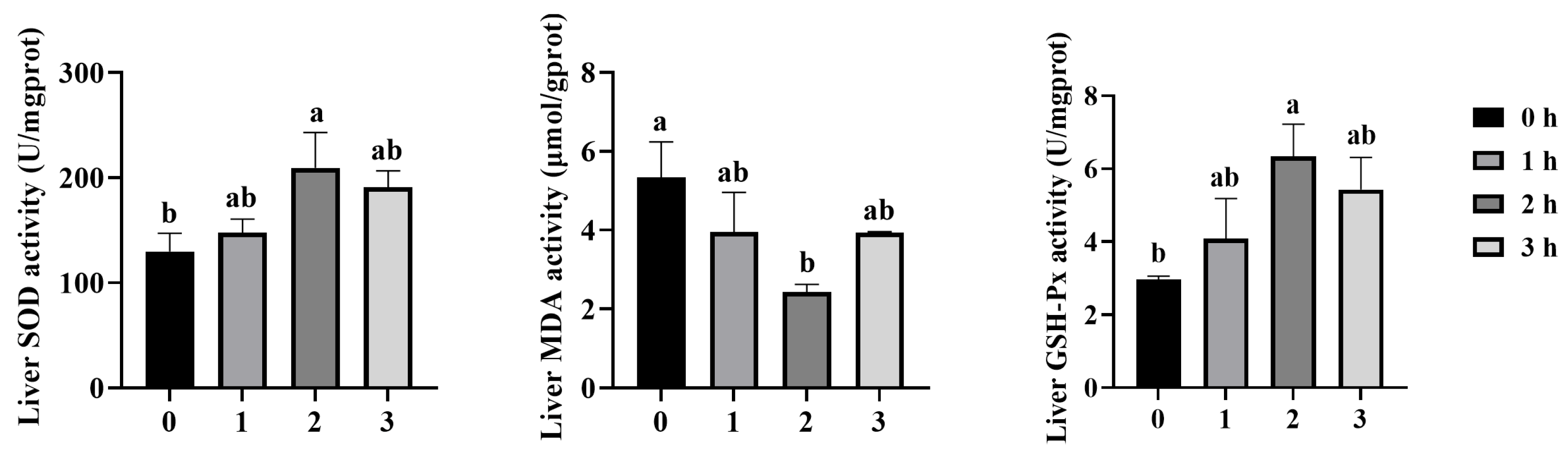
3.2. Effect of Antioxidant Enzyme Activity in Liver of Mandarin Fish
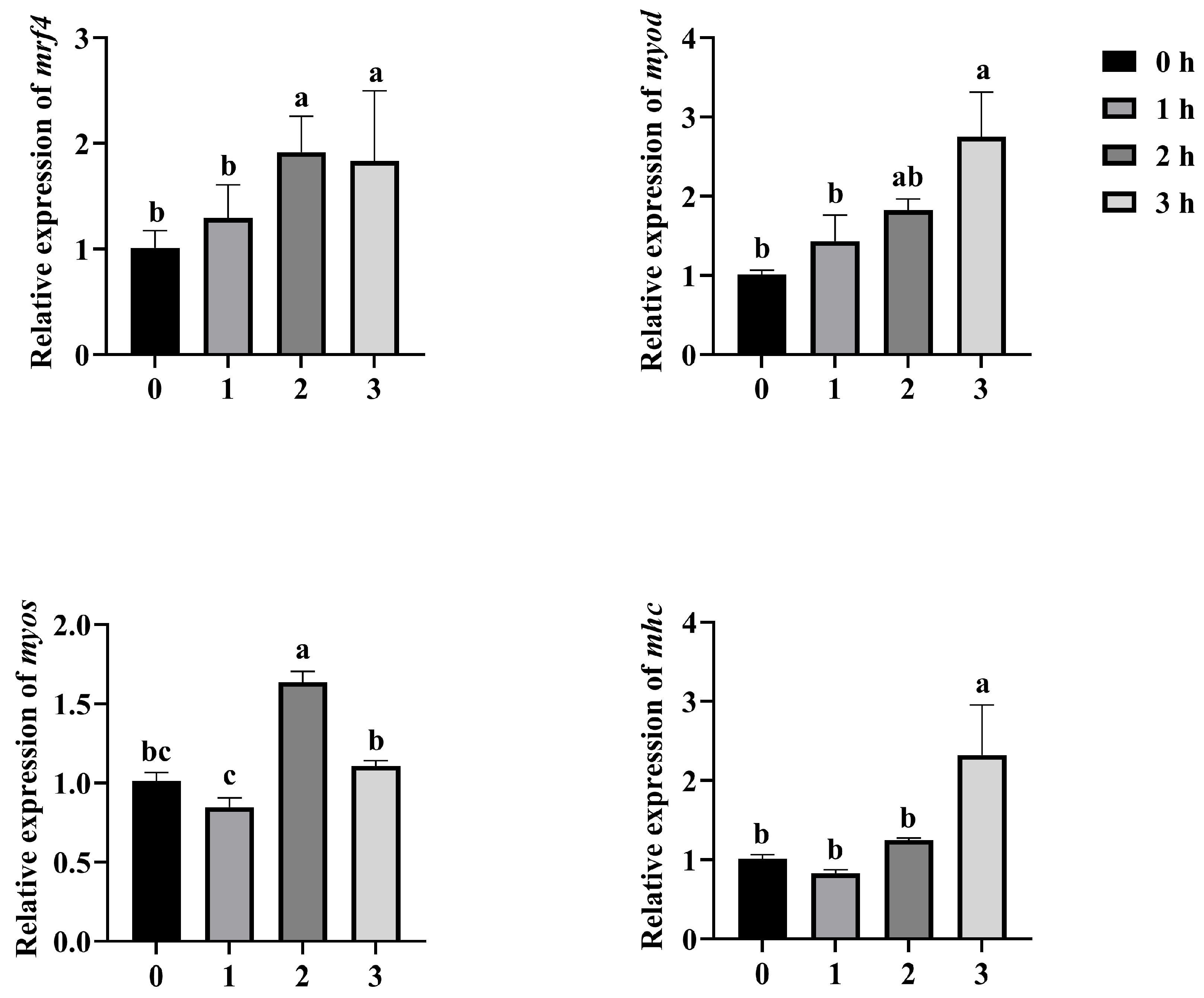
3.3. Effect of Muscle Growth Gene Expression in Mandarin Fish
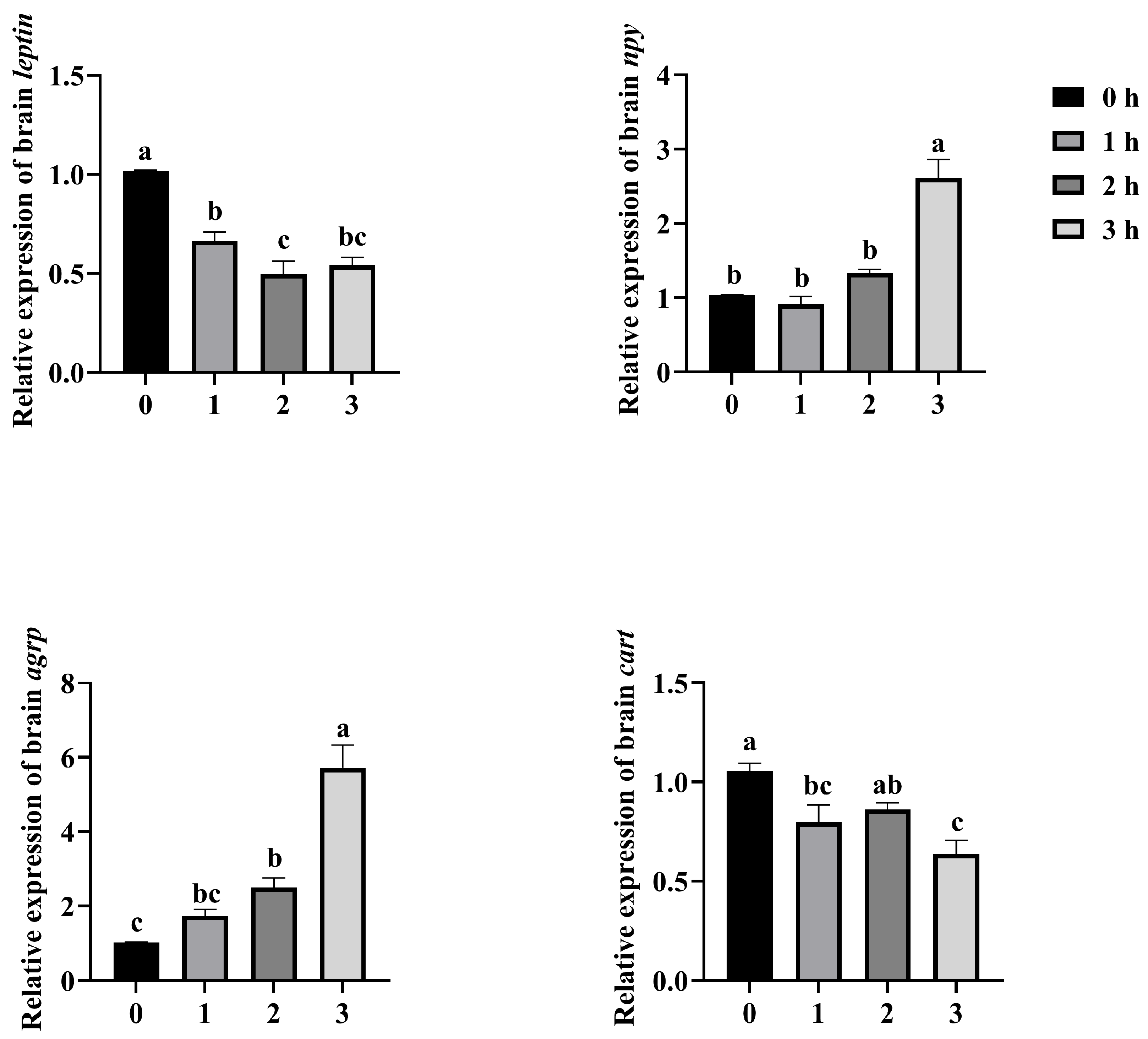
3.4. Effects on the Expression of Appetite-Related Genes in the Brain of Mandarin Fish
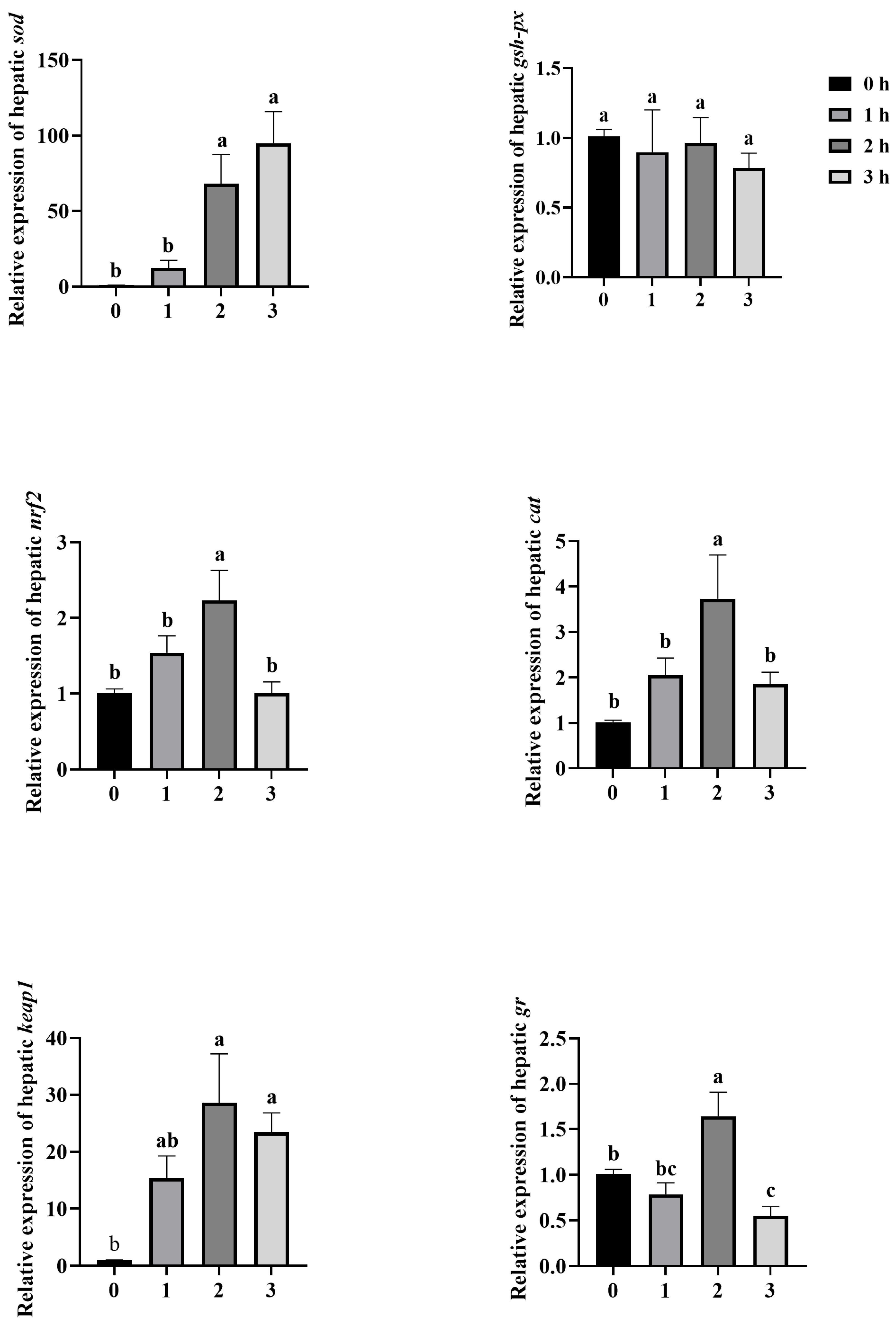
3.5. Effect of Antioxidant Gene Expression in Liver of Mandarin Fish
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Full Name | Abbreviations | Full Name |
| HRW | Hydrogen-rich water | npy | neuropeptide Y |
| SOD | superoxide dismutase | agrp | agouti related neuropeptide |
| GSH-Px | glutathione peroxidase | cart | cocaine and amphetamine-regulated transcript-like |
| MDA | malondialdehyde | sod | superoxide dismutase |
| mrf4 | myogenic regulatory factor 4 | gsh-px | glutathione peroxidase |
| myod | myogenic differentiation | nrf2 | nuclear factor erythroid factor 2 |
| myos | myostatin | cat | catalase |
| mhc | myosin heavy chain | keap1 | ECH-associated protein 1b |
| leptin | leptin-like | gr | glutathione reductase |
References
- Huang, D.; Liang, X.; Yuan, X.; Cai, W.; Li, A.; He, S.; Xue, M. Effects of γ-aminobutyric acid on food intake and appetite in mandarin fish Siniperca chuatsi. Acta Hydrobiol. Sin. 2017, 41, 1312–1317. [Google Scholar]
- LeBaron, T.W.; Sharpe, R.; Pyatakovich, F.A.; Artamonov, M.Y. Hydrogen: From stars to fuel to medicine. In Molecular Hydrogen in Health and Disease; Springer: Cham, Switzerland, 2024; Volume 27, pp. 1–20. [Google Scholar]
- Zhou, Q.; Li, H.; Zhang, Y.; Zhao, Y.; Wang, C.; Liu, C. Hydrogen-Rich Water to Enhance Exercise Performance: A Review of Effects and Mechanisms. Metabolites 2024, 14, 537. [Google Scholar] [CrossRef]
- Sim, M.; Kim, C.S.; Shon, W.J.; Lee, Y.K.; Choi, E.Y.; Shin, D.M. Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: A randomized, double-blind, controlled trial. Sci. Rep. 2020, 10, 12130. [Google Scholar] [CrossRef]
- Dhillon, G.; Buddhavarapu, V.; Grewal, H.; Sharma, P.; Verma, R.K.; Munjal, R.; Devadoss, R.; Kashyap, R. Hydrogen Water: Extra Healthy or a Hoax?—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 973. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Liu, Y.H.; Wang, S.; Du, H.M.; Shen, W.B. Hydrogen agronomy: Research progress and prospects. J. Zhejiang Univ.-Sci. B 2020, 21, 841–855. [Google Scholar] [CrossRef]
- Ostendarp, M.; de Breuyn, M.; El-Khaled, Y.C.; Garcias-Bonet, N.; Carvalho, S.; Peixoto, R.S.; Wild, C. Temperature-dependent responses of the hard corals Acropora sp. and Pocillopora verrucosa to molecular hydrogen. PLoS ONE 2025, 20, e0308894. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, H.; Chen, S.; Lin, Y.; Peng, J.; Hu, J.; Wang, Y. The effects of different concentrations of hydrogen-rich water on the growth performance, digestive ability, antioxidant capacity, glucose metabolism pathway, mTOR signaling pathway, and gut microbiota of largemouth bass (Micropterus salmoides). Fishes 2024, 9, 210. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Wang, W.; Liu, Q.; Li, N.; He, B.; Jiang, Y.; Ma, J. Mn-SOD alleviates methotrexate-related hepatocellular injury via GSK-3β affecting anti-oxidative stress of HO-1 and Drp1. Zhong Nan Da Xue Xue Bao Yi Xue Ban J. Cent. South Univ. Med. Sci. 2022, 47, 1191–1199. [Google Scholar]
- Hu, J.; Huang, L.; Wang, L.; Huang, W.; Lai, M.; Li, X.; Lin, Y.; Sun, Y. Hydrogen administration improves the growth performance of juvenile largemouth bass (Micropterus salmoides) by increasing feed intake, reducing serum lipids, activating mTOR and Nrf2 signaling pathways, and altering the intestinal microbiota. Aquac. Rep. 2023, 33, 101749. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, B.; Meng, F.; Zhou, Z.; Lu, H.; Zhao, H. Impact of molecular hydrogen treatments on the innate immune activity and survival of zebrafish (Danio rerio) challenged with Aeromonas hydrophila. Fish Shellfish Immunol. 2017, 67, 554–560. [Google Scholar] [CrossRef]
- Carnovali, M.; Mariotti, M.; Banfi, G. Molecular hydrogen enhances osteogenesis in Danio rerio embryos. J. Fish Biol. 2021, 98, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cao, Y.; Kohei, F.; Hao, H.; Peng, G.; Cheng, C.; Ye, J. Nano-bubble hydrogen water: An effective therapeutic agent against inflammation related disease caused by viral infection in zebrafish model. Virol. Sin. 2022, 37, 277–283. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, X.; Wu, H.; Gao, J.; Wu, J.; Xiong, Z.; Feng, Z.; Xie, M.; Li, S.; Xie, Z. The effect of short-term artificial feed domestication on the expression of oxidative-stress-related genes and antioxidant capacity in the liver and gill tissues of Mandarin fish (Siniperca chuatsi). Genes 2024, 15, 487. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Huggett, J.F. The Digital MIQE Guidelines Update: Minimum Information for Publication of Quantitative Digital PCR Experiments for 2020. Clin. Chem. 2020, 66, 1012–1029. [Google Scholar] [PubMed]
- Siagian, E.R.; Nugroho, R.A. Growth performance and carcass composition of African catfish, Clarias gariepinus fed different protein and energy levels. Iran. J. Fish. Sci. 2020, 19, 1828–1839. [Google Scholar]
- Jia, R. Natural Antioxidants and Aquatic Animal Health. Antioxidants 2025, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, M. Fish nutrition and feed technology. Fishes 2023, 8, 146. [Google Scholar] [CrossRef]
- Yang, P.; Li, X.; Yao, W.; Li, M.; Wang, Y.; Leng, X. Dietary effect of Clostridium autoethanogenum protein on growth, intestinal histology and flesh lipid metabolism of largemouth bass (Micropterus salmoides) based on metabolomics. Metabolites 2022, 12, 1088. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Li, C.; Gong, T.; Bian, B.; Liao, W. Roles of hydrogen gas in plants: A review. Funct. Plant Biol. 2018, 45, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Esteves de Lima, J.; Relaix, F. Master regulators of skeletal muscle lineage development and pluripotent stem cells differentiation. Cell Regen. 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Ayisi, C.L.; Afriyie, G.; Owusu-Afriyie, G. Modulation of Muscle Growth Related Genes in Fish in Response to Plant Based Ingredients. Genet. Aquat. Org. 2021, 6, 413. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Garrosa, E.; Seco-Calvo, J.; Garrosa, M. Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes 2022, 10, 29. [Google Scholar] [CrossRef]
- Abouel Azm, F.R.; Kong, F.; Wang, X.; Zhu, W.; Yu, H.; Long, X.; Tan, Q. The Interaction of Dried Distillers Grains with Solubles (DDGS) Type and Level on Growth Performance, Health, Texture, and Muscle-Related Gene Expression in Grass Carp (Ctenopharyngodon idellus). Front. Nutr. 2022, 9, 832651. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, S.; Liu, S.; Wang, G.; Lai, H.; Zhao, X.; Bi, S.; Guo, D.; Chen, X.; Yi, H.; et al. Identifying the Related Genes of Muscle Growth and Exploring the Functions by Compensatory Growth in Mandarin Fish (Siniperca chuatsi). Front. Physiol. 2020, 11, 553563. [Google Scholar] [CrossRef]
- Nazari, S.E.; Tarnava, A.; Khalili-Tanha, N.; Darroudi, M.; Khalili-Tanha, G.; Avan, A.; Khazaei, M.; LeBaron, T.W. Therapeutic potential of hydrogen-rich water on muscle atrophy caused by immobilization in a mouse model. Pharmaceuticals 2023, 16, 1436. [Google Scholar] [CrossRef]
- Volkoff, H.; Canosa, L.F.; Unniappan, S.; Cerdá-Reverter, J.M.; Bernier, N.J.; Kelly, S.P.; Peter, R.E. Neuropeptides and the control of food intake in fish. Gen. Comp. Endocrinol. 2005, 142, 3–19. [Google Scholar] [CrossRef]
- Ji, W.; Ping, H.-C.; Wei, K.-J.; Zhang, G.-R.; Shi, Z.-C.; Yang, R.-B.; Zou, G.-W.; Wang, W.-M. Ghrelin, neuropeptide Y (NPY) and cholecystokinin (CCK) in blunt snout bream (Megalobrama amblycephala): cDNA cloning, tissue distribution and mRNA expression changes responding to fasting and refeeding. Gen. Comp. Endocrinol. 2015, 223, 108–119. [Google Scholar] [CrossRef]
- López-Patiño, M.A.; Guijarro, A.I.; Isorna, E.; Delgado, M.J.; Alonso-Bedate, M.; de Pedro, N. Neuropeptide Y has a stimulatory action on feeding behavior in goldfish (Carassius auratus). Eur. J. Pharmacol. 1999, 377, 147–153. [Google Scholar] [CrossRef]
- Carpio, Y.; Acosta, J.; Morales, A.; Herrera, F.; González, L.J.; Estrada, M.P. Cloning, expression and growth promoting action of Red tilapia (Oreochromis sp.) neuropeptide Y. Peptides 2006, 27, 710–718. [Google Scholar] [CrossRef]
- Shiozaki, K.; Kawabe, M.; Karasuyama, K.; Kurachi, T.; Hayashi, A.; Ataka, K.; Iwai, H.; Takeno, H.; Hayasaka, O.; Kotani, T.; et al. Neuropeptide Y deficiency induces anxiety-like behaviours in zebrafish (Danio rerio). Sci. Rep. 2020, 10, 5913. [Google Scholar] [CrossRef]
- Tachibana, T.; Tsutsui, K. Neuropeptide control of feeding behavior in birds and its difference with mammals. Front. Neurosci. 2016, 10, 485. [Google Scholar] [CrossRef]
- Shainer, I.; Michel, M.; Marquart, G.D.; Bhandiwad, A.A.; Zmora, N.; Ben-Moshe Livne, Z.; Zohar, Y.; Hazak, A.; Mazon, Y.; Förster, D.; et al. Agouti-Related Protein 2 Is a New Player in the Teleost Stress Response System. Curr. Biol. 2019, 29, 2009–2019. [Google Scholar] [CrossRef]
- Agulleiro, M.J.; Cortés, R.; Leal, E.; Ríos, D.; Sánchez, E.; Cerdá-Reverter, J.M. Characterization, tissue distribution and regulation by fasting of the agouti family of peptides in the sea bass (Dicentrarchus labrax). Gen. Comp. Endocrinol. 2014, 205, 251–259. [Google Scholar] [CrossRef]
- Cerdá-Reverter, J.M.; Peter, R.E. Endogenous melanocortin antagonist in fish: Structure, brain mapping, and regulation by fasting of the goldfish agouti-related protein gene. Endocrinology 2003, 144, 4552–4561. [Google Scholar] [CrossRef] [PubMed]
- Catteau, A.; Caillon, H.; Barrière, P.; Denis, M.G.; Masson, D.; Fréour, T. Leptin and its potential interest in assisted reproduction cycles. Hum. Reprod. Update 2016, 22, 320–341. [Google Scholar] [CrossRef]
- Tan, H.L.; Yin, L.; Tan, Y.; Ivanov, J.; Plucinska, K.; Ilanges, A.; Herb, B.R.; Wang, P.; Kosse, C.; Cohen, P.; et al. Leptin-activated hypothalamic BNC2 neurons acutely suppress food intake. Nature 2024, 636, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Singh, R.; Rai, U. Trajectory of leptin and leptin receptor in vertebrates: Structure, function and their regulation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2022, 257, 110652. [Google Scholar] [CrossRef] [PubMed]
- Rønnestad, I.; Gomes, A.S.; Murashita, K.; Angotzi, R.; Jönsson, E.; Volkoff, H. Appetite-Controlling Endocrine Systems in Teleosts. Front. Endocrinol. 2017, 8, 73. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Captivating Colors, Crucial Roles: Astaxanthin’s Antioxidant Impact on Fish Oxidative Stress and Reproductive Performance. Animals 2023, 13, 3357. [Google Scholar] [CrossRef]
- Zengin, H. The effects of feeding and starvation on antioxidant defence, fatty acid composition and lipid peroxidation in reared Oncorhynchus mykiss fry. Sci. Rep. 2021, 11, 16716. [Google Scholar] [CrossRef]
- Lu, Y.; Li, C.F.; Ping, N.N.; Sun, Y.Y.; Wang, Z.; Zhao, G.X.; Yuan, S.H.; Zibrila, A.I.; Soong, L.; Liu, J.J. Hydrogen-rich water alleviates cyclosporine A-induced nephrotoxicity via the Keap1/Nrf2 signaling pathway. J. Biochem. Mol. Toxicol. 2020, 34, e22467. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Liu, L.; Li, S.; Zhang, Z.; Zhang, R.; Zhou, Y.; Liu, F. Effect of hydrogen-rich water on the Nrf2/ARE signaling pathway in rats with myocardial ischemia-reperfusion injury. J. Bioenerg. Biomembr. 2019, 51, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jia, X.; Zheng, F.; Wang, Y.; Fan, Y.; Zhang, H.; Dang, Z.; Wang, L. SUMOylation Inhibitors Exert a Protective Effect on Oxidative Damage in Retinal Pigment Epithelial Cells Through the Keap1/Nrf2/ARE Signaling Pathway. Curr. Mol. Med. 2025, 24, 1180–1190. [Google Scholar] [CrossRef]
- Li, E.; Wang, Y.; Li, Q.; Li, L.; Wei, L. Protective effects of Sal B on oxidative stress-induced aging by regulating the Keap1/Nrf2 signaling pathway in zebrafish. Molecules 2021, 26, 5239. [Google Scholar] [CrossRef]
- Oh, Y.S.; Jun, H.S. Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling. Int. J. Mol. Sci. 2017, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Teng, H.; Zhang, K.Y.; Skalicka-Woźniak, K.; Georgiev, M.I.; Xiao, J. Agrimonolide and Desmethylagrimonolide Induced HO-1 Expression in HepG2 Cells through Nrf2-Transduction and p38 Inactivation. Front. Pharmacol. 2016, 7, 513. [Google Scholar] [CrossRef] [PubMed]

| Genes | Primer Sequence | Accession No. |
|---|---|---|
| β-actin | F:CAATGAATGAAGCGGATGAGG R:CCGACCAAGCCAGACAAAAG | FJ436084.1 |
| mrf4 | F:GCAGACGCTGGATGAACAGGAG R:GCGGTGGAATGGTCAGCAGAG | JX682564.1 |
| myod | F:CCAAACGGCGGTCTGAAGAGC R:GCTGCTGTTGTCGGTGGAGATC | XM_044193465.1 |
| myos | F:AAGGATGTGGCTATGGAGGAGGAC R:TGGACGATGGACTCGGGTTCAG | JF896453.1 |
| mhc | F:CCAGATTCAGGAAGGCTCAGCATG R:CGGCTCTTGGCTCGCATCTTG | XM_044181018.1 |
| leptin | F:CTGCCAGTGGAAGTAGTGAAGATGA R:ACCCGTCAGCGAAGAGATGTCA | XM_044194787.1 |
| npy | F:CGCTGCTGACATTCTGACCTCTAC R:CAACATGCCCTCCCGTACTTTACTT | XM_044172804.1 |
| agrp | F:CCTACCTGTTGACTCAGTGGAGGAT R:GATGGCGTTAAAGAAGCGGCAGTA | XM_044195210.1 |
| cart | F:TCCTCCTCTTCCTCCTCCTCTTCC R:TCTGCGGCTGGCTCTGGTTT | XM_044204893.1 |
| sod | F:CACGCTCCCTGACCTGACA | XM_044168059.1 |
| R:GGAGGGCAACCTGTGCTG | ||
| gpx | F:GCCCATCCCCTGTTTGTG | XM_044172415.1 |
| R:AACTTCCTGCTGTAACGCTTG | ||
| nrf2 | F:ACGAAAGCGAAAGCTCCTCA | MT270449.1 |
| R:GCTCTCTTCCAGAATGGCGT | ||
| cat | F:GCGTTTGGCTACTTTGAGGT | XM_044194118.1 |
| R:CACAGTGGAGAAGCGGACA | ||
| keap1 | F:GTGGCAACCCAGGAGGAG | XM_044189604.1 |
| R:GGGAATGGCAACGGACA | ||
| gr | F:CAGGCATCCTTTCCACCC | XM_044204920.1 |
| R:TCCAGTCCTCTGTCCGTTTTA |
| Group | 0 h | 1 h | 2 h | 3 h |
|---|---|---|---|---|
| IW (g) | 30.12 ± 0.09 a | 30.57 ± 0.1 a | 30.05 ± 0.08 a | 30.11 ± 1.11 a |
| FW (g) | 57.08 ± 7.05 d | 63.39 ± 5.49 c | 71.23 ± 5.08 b | 76.31 ± 6.48 a |
| WGR (%) | 90.26 ± 33.1 d | 111.3 ± 25.4 c | 137.43 ± 23.9 b | 154.36 ± 30.43 a |
| SGR (%/d) | 1.01 ± 0.01 c | 1.25 ± 0.04 b | 1.48 ± 0.02 a | 1.61 ± 0.09 a |
| FCR | 1.61 ± 0.04 a | 1.29 ± 0.02 b | 1.19 ± 0.14 c | 1.01 ± 0.06 d |
| FR (g/fish) | 44.54 ± 0.38 c | 42.8 ± 1.03 b | 49.57 ± 2.12 a | 47.73 ± 1.46 a |
| SR (%) | 95.66 ± 2.15 a | 98.33 ± 1.92 a | 98.66 ± 1.86 a | 97.33 ± 1.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Huang, J.; Liu, H.; Yang, Y.; Hu, J. Role of Hydrogen-Rich Water on Growth Performance and Liver Antioxidant Capacity of Mandarin Fish (Siniperca chuatsi). Fishes 2025, 10, 581. https://doi.org/10.3390/fishes10110581
Wang H, Huang J, Liu H, Yang Y, Hu J. Role of Hydrogen-Rich Water on Growth Performance and Liver Antioxidant Capacity of Mandarin Fish (Siniperca chuatsi). Fishes. 2025; 10(11):581. https://doi.org/10.3390/fishes10110581
Chicago/Turabian StyleWang, Haolin, Jing Huang, Hua Liu, Ying Yang, and Junru Hu. 2025. "Role of Hydrogen-Rich Water on Growth Performance and Liver Antioxidant Capacity of Mandarin Fish (Siniperca chuatsi)" Fishes 10, no. 11: 581. https://doi.org/10.3390/fishes10110581
APA StyleWang, H., Huang, J., Liu, H., Yang, Y., & Hu, J. (2025). Role of Hydrogen-Rich Water on Growth Performance and Liver Antioxidant Capacity of Mandarin Fish (Siniperca chuatsi). Fishes, 10(11), 581. https://doi.org/10.3390/fishes10110581






