First Record of Urceolaria carmenae n. sp. (Ciliophora, Peritrichia, Mobilida) Infesting the Gills of Octopus bimaculatus Verrill from the Gulf of California, Mexico
Abstract
1. Introduction
2. Materials and Methods
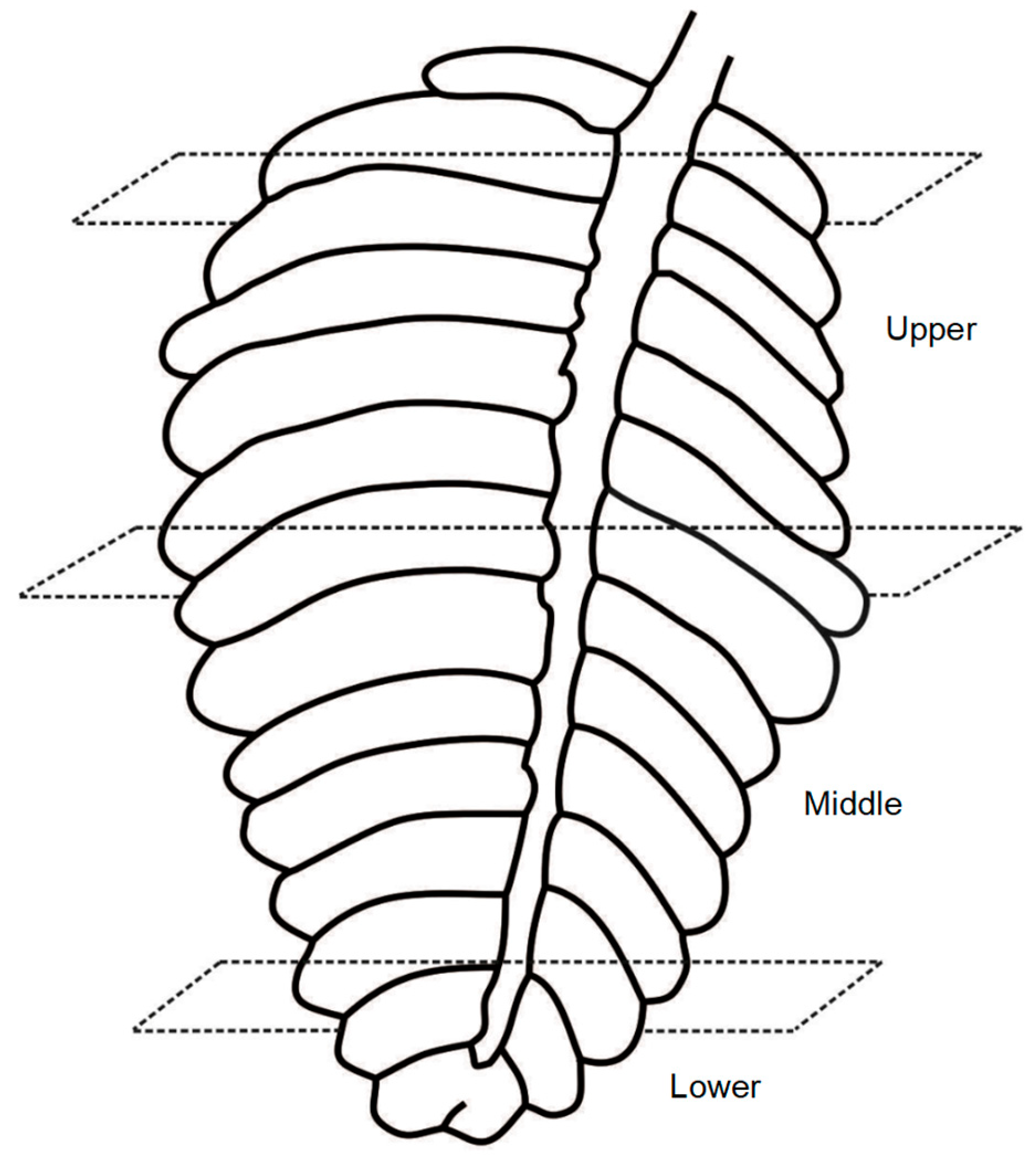
2.1. Sample Collection
2.2. Morphological Characterization
2.3. Molecular Characterization
2.4. Phylogenetic Analyses
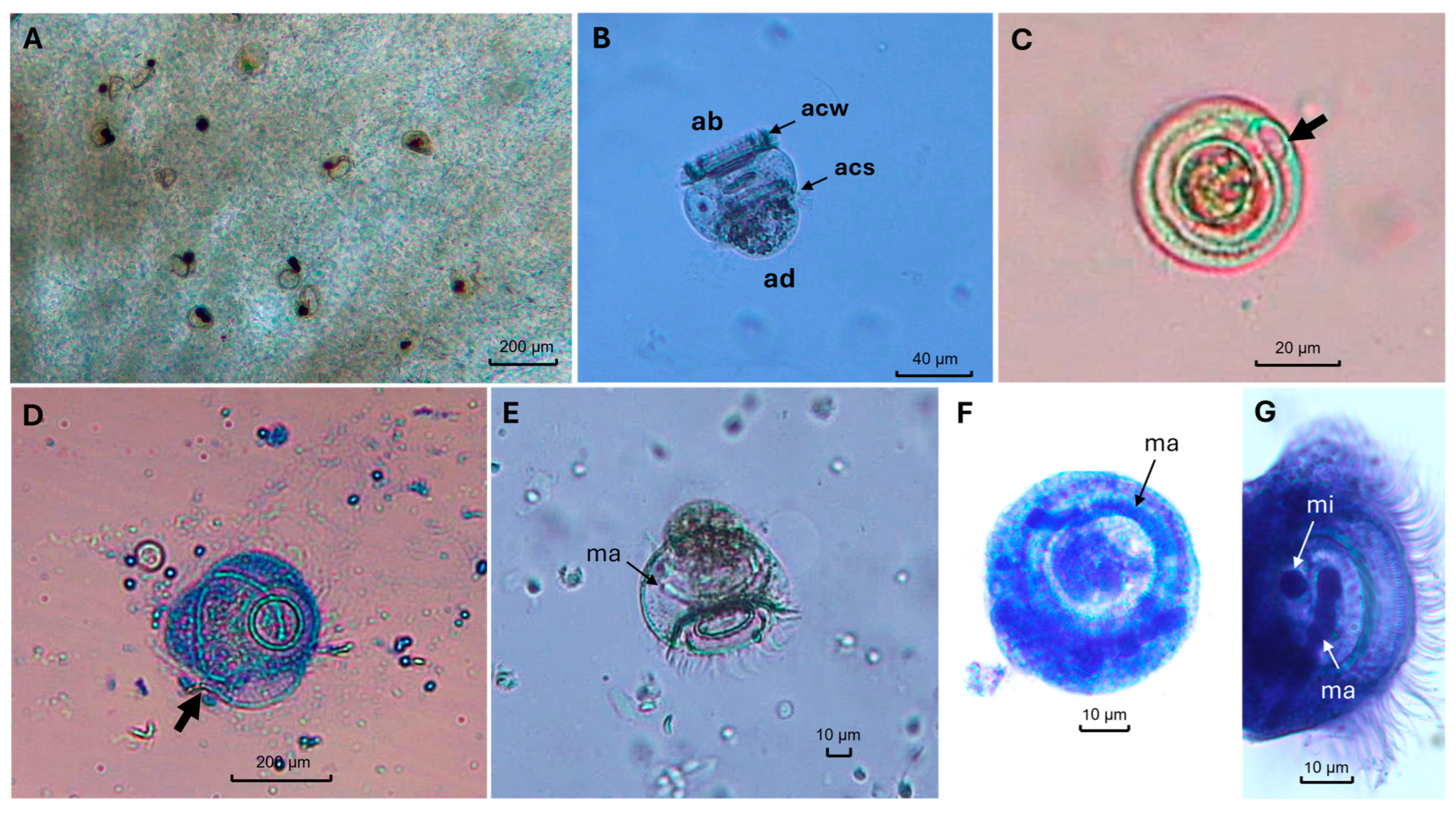
3. Result
4. Discussion
4.1. Morphological Diagnosis and Taxonomic Distinctness
4.2. Phylogenetic Affiliation and Evolutionary Relationships
4.3. Ecological Context and Host-Symbiont Dynamics
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tedesco, P.; Bevilacqua, S.; Fiorito, G.; Terlizzi, A. Global patterns of parasite diversity in cephalopods. Sci. Rep. 2020, 10, 11303. [Google Scholar] [CrossRef]
- Souidenne, D.; Furuya, H. Protist (Ciliates) and related diseases. In Handbook of Pathogens and Diseases in Cephalopods; Gestal, C., Pascual, S., Guerra, Á., Fiorito, G., Vieites, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 153–158. ISBN 978-3-030-11330-8. [Google Scholar]
- Zhan, Z.; Xu, K.; Warren, A.; Gong, Y. Reconsideration of phylogenetic relationships of the subclass Peritrichia (Ciliophora, Oligohymenophorea) based on small subunit ribosomal RNA gene sequences, with the establishment of a new subclass Mobilia Kahl, 1933. J. Eukaryot. Microbiol. 2009, 56, 552–558. [Google Scholar] [CrossRef]
- Lynn, D.H. The ciliate taxa, including families and genera. In The Ciliated Protozoa: Characterization, Classification, and Guide to the Literature; Springer: Dordrecht, The Netherlands, 2008; pp. 339–440. ISBN 978-1-4020-8239-9. [Google Scholar]
- Corliss, J.O. The Ciliated Protozoa: Characterization, Classification and Guide to the Literature, 2nd ed.; Pergamon: Oxford, UK, 2016; ISBN 978-1-4831-5417-6. [Google Scholar]
- Xu, K.; Song, W.; Warren, A. Observations on trichodinid ectoparasites (Ciliophora: Peritricha) from the gills of maricultured molluscs in China, with descriptions of three new species of Trichodina Ehrenberg, 1838. Syst. Parasitol. 2000, 45, 17–24. [Google Scholar] [CrossRef]
- Zhan, Z.; Xu, K.; Dunthorn, M. Evaluating molecular support for and against the monophyly of the Peritrichia and phylogenetic relationships within the Mobilida (Ciliophora, Oligohymenophorea). Zool. Scr. 2013, 42, 213–226. [Google Scholar] [CrossRef]
- Tang, F.; Zhang, Y.; Zhao, Y. Morphological and molecular identification of the new species, Trichodina pseudoheterodentata sp. n. (Ciliophora, Mobilida, Trichodinidae) from the channel catfish, Ictalurus punctatus, in Chongqing China. J. Eukaryot. Microbiol. 2017, 64, 45–55. [Google Scholar] [CrossRef]
- Irwin, N.A.T.; Sabetrasekh, M.; Lynn, D.H. Diversification and phylogenetics of mobilid peritrichs (Ciliophora) with description of Urceolaria parakorschelti sp. nov. Protist 2017, 168, 481–493. [Google Scholar] [CrossRef]
- Van As, L.L.; Basson, L.; Van As, J. Mobiline peritrichs (Ciliophora) collected from the gills of African limpets. Acta Protozool. 2017, 56, 245–254. [Google Scholar] [CrossRef][Green Version]
- Xu, K.; Song, W. Two trichodinid ectoparasites from marine molluscs in the Yellow Sea, Off China, with the description of Trichodina caecellae n. sp. (Protozoa: Ciliophora: Peritrichia). Syst. Parasitol. 2007, 69, 1–11. [Google Scholar] [CrossRef]
- Hirshfield, H. The morphology of Urceolaria karyolobia, sp. nov., Trichodina tegula sp. nov., and Scyphidia ubiquita sp. nov., three new ciliates from Southern California limpets and turbans. J. Morphol. 1949, 85, 1–33. [Google Scholar] [CrossRef]
- Richards, C.S. Urceolaria viridis n. sp., a ciliate (Peritrichida, Mobilina) with elongate symbiotic green algae. J. Protozool. 1971, 18, 410–413. [Google Scholar] [CrossRef]
- Rataj, M.; Vd’ačný, P. Cryptic host-driven speciation of mobilid ciliates epibiotic on freshwater planarians. Mol. Phylogenetics Evol. 2021, 161, 107174. [Google Scholar] [CrossRef]
- Hausmann, K.; Bradbury, P.C. Ciliates: Cells as Organisms; Gustav Fischer Verlag: Stuttgart, Germany, 1996; ISBN 978-1-56081-432-0. [Google Scholar]
- Noble, G.A. Trichodina urechi n. sp., an entozoic ciliate from the echiuroid worm, Urechis caupo. J. Parasitol. 1940, 26, 387–405. [Google Scholar] [CrossRef]
- Haider, G. Monographie der Familie Urceolariidae (Ciliata, Peritricha, Mobilina) mit besonderer berucksichtigung der imsu¨ ddeutschen raum vorkommenden arten. Parasitol. Schriftenr. 1964, 17, 1–251. [Google Scholar]
- Irwin, N.A.T.; Lynn, D.H. Molecular phylogeny of mobilid and sessilid ciliates symbiotic in Eastern Pacific limpets (Mollusca: Patellogastropoda). J. Eukaryot. Microbiol. 2015, 62, 543–552. [Google Scholar] [CrossRef]
- Martinez, G.; Leander, B.S.; Park, E. Morphology and molecular phylogeny of endosymbiotic ciliates (Peritrichia, Mobilida) of marine invertebrates with descriptions of two novel species Urceolaria clepsydra n. sp. and Urceolaria bratalia n. sp. J. Eukaryot. Microbiol. 2025, 72, e70003. [Google Scholar] [CrossRef]
- Zick, K. Urceolaria korschelti sp. n., eine neue marine Urceolariide nebst einem Oberblick fiber die Urceolarinen. Z. Wiss. Zool. 1928, 132, 356–403. [Google Scholar]
- Jereb, P.; Roper, C.F.E.; Norman, M.D.; Finn, J.K. Cephalopods of the world. In An Annotated and Illustrated Catalogue of Cephalopod Species Known to Date: Octopods and Vampire Squids; FAO: Rome, Italy, 2016; Volume 3, ISBN 978-92-5-107989-8. [Google Scholar]
- Domínguez-Contreras, J.F.; Munguia-Vega, A.; Ceballos-Váazquez, B.P.; Arellano-Martínez, M.; García-Rodríguez, F.J.; Culver, M.; Reyes-Bonilla, H. Life histories predict genetic diversity and population structure within three species of octopus targeted by small-scale fisheries in Northwest Mexico. PeerJ 2018, 6, 1–25. [Google Scholar] [CrossRef]
- Díaz-Santana-Iturrios, M.; Salinas-Zavala, C.A.; García-Rodríguez, F.J.; Granados-Amores, J. Taxonomic assessment of species of the genus Octopus from the northeastern Pacific via morphological, molecular and morphometric analyses. PeerJ 2019, 7, e8118. [Google Scholar] [CrossRef]
- DOF Acuerdo por el que se Modifica el Similar por el que se Establece la Veda Temporal y Tallas Mínimas de Captura para la Pesca de las Especies de Pulpo en Bahía de Los Ángeles, Baja California, Publicado El 1 de Junio de 2016, para Considerar como Zona de Veda la Reserva de La Biosfera Bahía de Los Ángeles, Canales de Ballenas y de Salsipuedes, Respecto del Pulpo Café (Octopus bimaculatus) y Pulpo Verde (Octopus hubbsorum). Available online: https://dof.gob.mx/nota_detalle.php?codigo=5503748&fecha=07/11/2017#gsc.tab=0 (accessed on 18 September 2025).
- Colunga-Ramírez, G.E.; Del Rio-Zaragoza, O.B.; Castellanos-Martínez, S. Unusual formation of air bubbles in the arms of the california two-spot octopus, Octopus bimaculatus Verrill, 1883 during handling in captivity. Mar. Biol. 2023, 170, 143. [Google Scholar] [CrossRef]
- Shomrat, T.; Zarrella, I.; Fiorito, G.; Hochner, B. The octopus vertical lobe modulates short-term learning rate and uses LTP to acquire long-term memory. Curr. Biol. 2008, 18, 337–342. [Google Scholar] [CrossRef]
- Fiorito, G.; Affuso, A.; Basil, J.; Cole, A.; De Girolamo, P.; D’Angelo, L.; Dickel, L.; Gestal, C.; Grasso, F.; Kuba, M.; et al. Guidelines for the care and welfare of cephalopods in research –a consensus based on an initiative by CephRes, FELASA and the Boyd group. Lab. Anim. 2015, 49, 1–90. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Klein, B.M. The “Dry” silver method and its proper use. J. Protozoo. 1958, 5, 99–103. [Google Scholar] [CrossRef]
- Culling, C.F.A.; Allison, R.T.; Barr, W.T.; Culling, C.F.A. Cellular Pathology Technique, 4th ed.; Butterworths: London, UK; Boston, MA, USA, 1985; ISBN 978-0-407-72903-2. [Google Scholar]
- Bourland, W.A.; Strüder-Kypke, M.M. Agolohymena aspidocauda nov. gen., nov. spec., a histophagous freshwater tetrahymenid ciliate in the family Deltopylidae (Ciliophora, Hymenostomatia), from Idaho (northwest U.S.A.): Morphology, ontogenesis and molecular phylogeny. Eur. J. Protistol. 2010, 46, 221–242. [Google Scholar] [CrossRef]
- Tang, F.; Zhao, Y.; Warren, A. Phylogenetic analyses of Trichodinids (Ciliophora, Oligohymenophora) inferred from 18S rRNA gene sequence data. Curr. Microbiol. 2012, 30, 306–313. [Google Scholar] [CrossRef][Green Version]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Lom, J.; Dyková, I. Protozoan Parasites of Fishes; Elsevier Science: Amsterdam, The Netherlands, 1992; ISBN 978-0-444-89434-2. [Google Scholar]
- Reynoldson, T.B. The dispersal of Urceolaria mitra (Peritricha) epizoic on flatworms. J. Anim. Ecol. 1951, 20, 123. [Google Scholar] [CrossRef]
- Rataj, M.; Vd’ačný, P. Living morphology and molecular phylogeny of Oligohymenophorean ciliates associated with freshwater turbellarians. Dis. Aquat. Org. 2019, 134, 147–166. [Google Scholar] [CrossRef]
- Xu, H.; Song, W.; Warren, A.; Al-Rasheid, K.A.S.; Al-Farraj, S.A.; Gong, J.; Hu, X. Planktonic protist communities in a semi-enclosed mariculture pond: Structural variation and correlation with environmental conditions. J. Mar. Biol. Assoc. 2008, 88, 1353–1362. [Google Scholar] [CrossRef]
- Martins, M.L.; Cardoso, L.; Marchiori, N.; Benites de Pádua, S. Protozoan infections in farmed fish from Brazil: Diagnosis and pathogenesis. Rev. Bras. Parasitol. Vet. 2015, 24, 1–20. [Google Scholar] [CrossRef]
- Ruiz-Escobar, F.; Colunga-Ramírez, G.E.; Oceguera-Figueroa, A.; Castellanos-Martínez, S. Cochimibdella mexicana n. gen. n. sp. (Hirudinida: Piscicolidae), from Octopus bimaculatus Verrill (Cephalopoda: Octopodidae) in the Gulf of California, Mexico. Syst. Parasitol. 2025, 102, 23. [Google Scholar] [CrossRef]
- Chan-Martin, A.D.J.; Castellanos-Martínez, S.; Aguirre-Macedo, M.L.; Martínez-Aquino, A. Immature trematodes of Lecithochirium sp. (Digenea: Hemiuridae) in the California two-spot octopus (Octopus bimaculatus) from Mexico. Parasitol. Res. 2022, 121, 2651–2660. [Google Scholar] [CrossRef]
- Colunga-Ramírez, G.E.; Martínez-Aquino, A.; Flores-López, C.A.; Gestal, C.; Azevedo, C.; Castellanos-Martínez, S. Aggregata polibraxiona n. sp. (Apicomplexa: Aggregatidae) from Octopus bimaculatus Verrill, 1883 (Mollusca: Cephalopoda) from the Gulf of California, Mexico. Eur. J. Protistol. 2021, 81, 125825. [Google Scholar] [CrossRef]
- McConnaughey, B.H. Mesozoa of the family Dicyemidae from California. Univ. Calif. publ. Zool. 1949, 55, 1–34. [Google Scholar]


| Urceolaria | U. carmenae n. sp. | U. bratalia | U. clepsydra | U. serpularum | U. urechi |
|---|---|---|---|---|---|
| Host | Octopus bimaculatus | Terebratalia transversa | Cucumaria miniata; Eupentacta quinquesemita | Serpula sp. | Urechis caupo |
| Adhesive disc diameter | 36.5 ± 10.7 µm (29.2–74.6) | 29.1 (23.8–35.7) | 68.5 (63–75) | – | – |
| Body shape | Turban | Cylindrical | Flattened conical | – | Turban |
| Body diameter | 44.2 ± 13.2 µm (31.3–88.6) | – | – | 25–40 | 58 (48–75) |
| Body width | 58.9 ± 12.1 µm (43.0–75.3) | 43.37 (32.5–63.2) | 71.65 (60.5–83.5) | – | – |
| Body height | 29.4 ± 7.5 µm (18.0–42.3) | – | – | 15–20 | 40 (24–50) |
| Border membrane width | 1.6 ± 0.4 µm (1–2) | – | – | – | – |
| Central zone diameter | 22.4 ± 4.1 µm (17.2–37.6) | – | – | – | – |
| Country | Mexico (Bahia de Los Angeles, Baja California) | Canada (Quadra Island, British Columbia) | Canada (Sooke, British Columbia) | – | – |
| Plate length | 11.0 ± 0.86 µm (9–12) | – | – | – | – |
| Excircle of plate ring diameter | 28.5 ± 7.1 µm (24.1–54.6) | 15.3 (12.1–19.8) | 48 (45.5–50.5) | – | – |
| Mean intensity | 687 ± 228 (279–1077) cells | – | – | – | – |
| Prevalence | 100% | – | – | – | – |
| Radial pins | 166–169 | – | – | – | – |
| Skeletal plate number | 18–19 | 12–13 | 45(41–49) | – | 18–25 |
| Total body height | 67.0 ± 14.2 µm (43.0–86.1) | 50.2 (40.9–71.8) | 114.7 (90.5–149.2) | – | – |
| Reference | The present study | Martinez et al. (2025) [19] | Martinez et al. (2025) [19] | Haider (1964) [17] | Noble (1940) [16] |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Urceolaria carmenae n. sp. (This study) | 98.8 | 98.8 | 97.1 | 90.9 | 90.5 | 90.3 | 97.3 | 90.6 | |
| 2 | Urceolaria serpularum (JQ663867) | 1.2 | 100.0 | 97.5 | 91.6 | 91.1 | 91.2 | 97.8 | 91.3 | |
| 3 | Urceolaria urechi (FJ499388) | 1.3 | 0.0 | 97.5 | 91.8 | 91.1 | 91.4 | 97.8 | 91.3 | |
| 4 | Urceolaria korschelti (JQ663870) | 2.9 | 2.5 | 2.5 | 92.0 | 91.6 | 91.4 | 97.9 | 91.6 | |
| 5 | Urceolaria korschelti (KY596045) | 9.1 | 8.4 | 8.2 | 8.0 | 97.6 | 91.7 | 91.6 | 96.9 | |
| 6 | Urceolaria parakorschelti (KP698204-6) | 9.5 | 8.9 | 8.9 | 8.4 | 2.4 | 91.8 | 91.4 | 97.8 | |
| 7 | Urceolari mitra (MW759660-2) | 9.7 | 8.8 | 8.6 | 8.6 | 8.3 | 8.2 | 91.0 | 91.4 | |
| 8 | Urceolaria clepsydra (PQ066414) | 2.7 | 2.2 | 2.2 | 2.1 | 8.4 | 8.6 | 9.0 | 91.7 | |
| 9 | Urceolaria bratalia (PQ066416) | 9.4 | 8.7 | 8.7 | 8.4 | 3.1 | 2.2 | 8.6 | 8.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colunga-Ramírez, G.E.; Castellanos-Martínez, S. First Record of Urceolaria carmenae n. sp. (Ciliophora, Peritrichia, Mobilida) Infesting the Gills of Octopus bimaculatus Verrill from the Gulf of California, Mexico. Fishes 2025, 10, 553. https://doi.org/10.3390/fishes10110553
Colunga-Ramírez GE, Castellanos-Martínez S. First Record of Urceolaria carmenae n. sp. (Ciliophora, Peritrichia, Mobilida) Infesting the Gills of Octopus bimaculatus Verrill from the Gulf of California, Mexico. Fishes. 2025; 10(11):553. https://doi.org/10.3390/fishes10110553
Chicago/Turabian StyleColunga-Ramírez, Graciela Esmeralda, and Sheila Castellanos-Martínez. 2025. "First Record of Urceolaria carmenae n. sp. (Ciliophora, Peritrichia, Mobilida) Infesting the Gills of Octopus bimaculatus Verrill from the Gulf of California, Mexico" Fishes 10, no. 11: 553. https://doi.org/10.3390/fishes10110553
APA StyleColunga-Ramírez, G. E., & Castellanos-Martínez, S. (2025). First Record of Urceolaria carmenae n. sp. (Ciliophora, Peritrichia, Mobilida) Infesting the Gills of Octopus bimaculatus Verrill from the Gulf of California, Mexico. Fishes, 10(11), 553. https://doi.org/10.3390/fishes10110553







