Impact of Stocking Density on Growth, Feeding Behavior, and Flesh Quality of Largemouth Bass (Micropterus salmoides) in Coupled Aquaponic Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. In Vivo Trial and Recordings
2.3. Water Quality
2.4. In Vivo Recordings on Fish
2.5. Recordings at Fish Slaughtering and Vegetable Harvesting
2.6. Statistical Analysis
3. Results
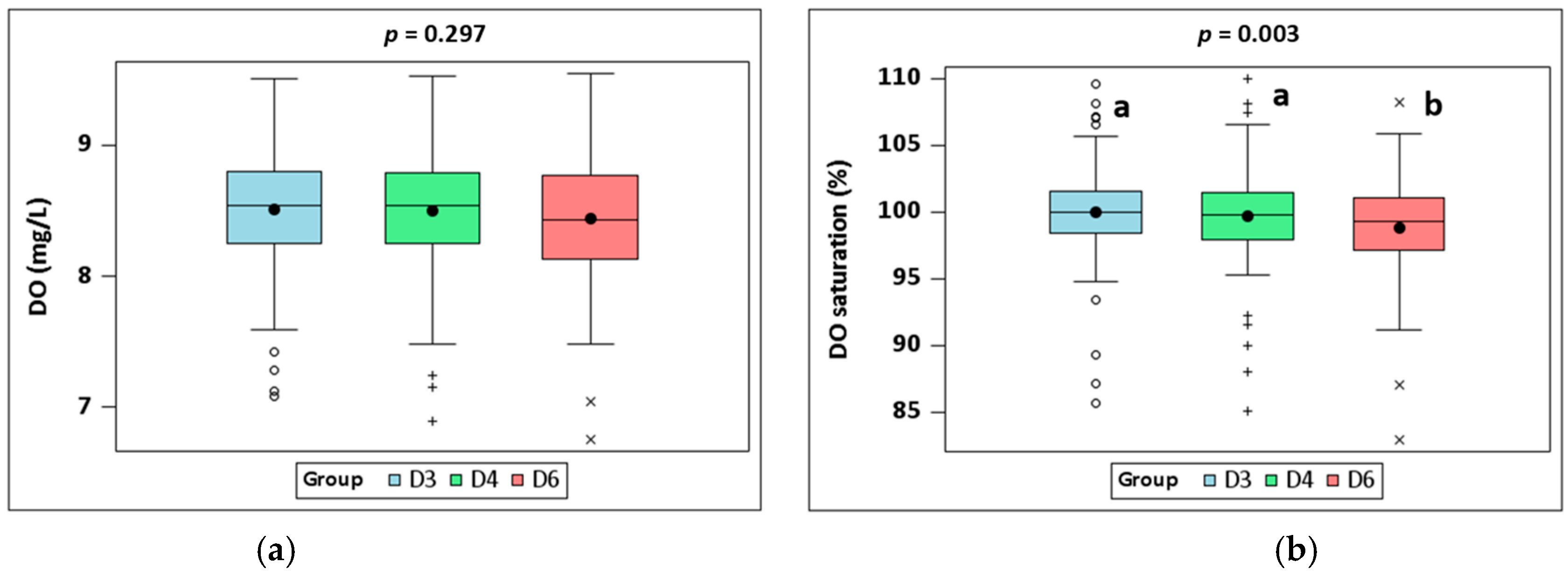
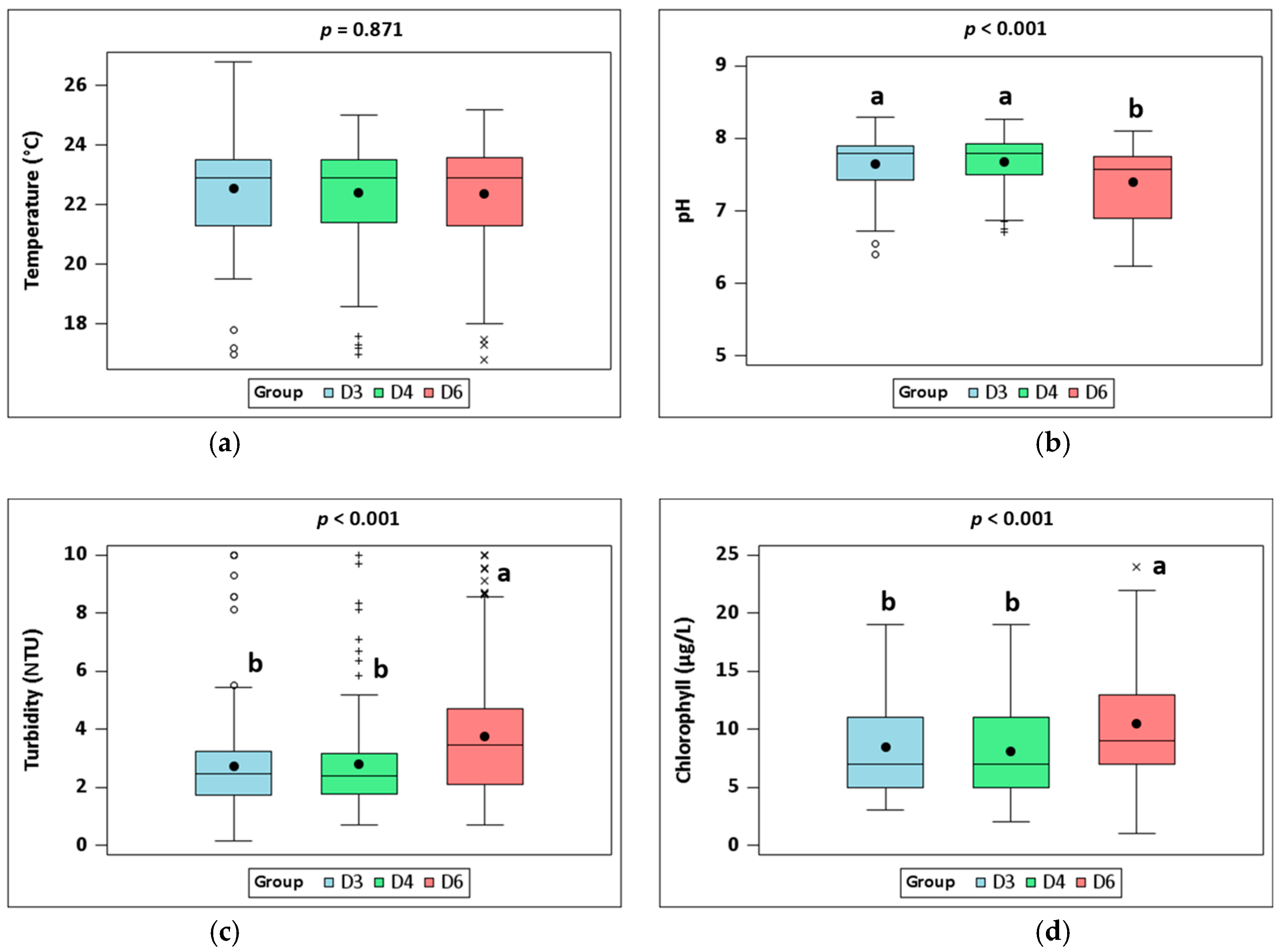
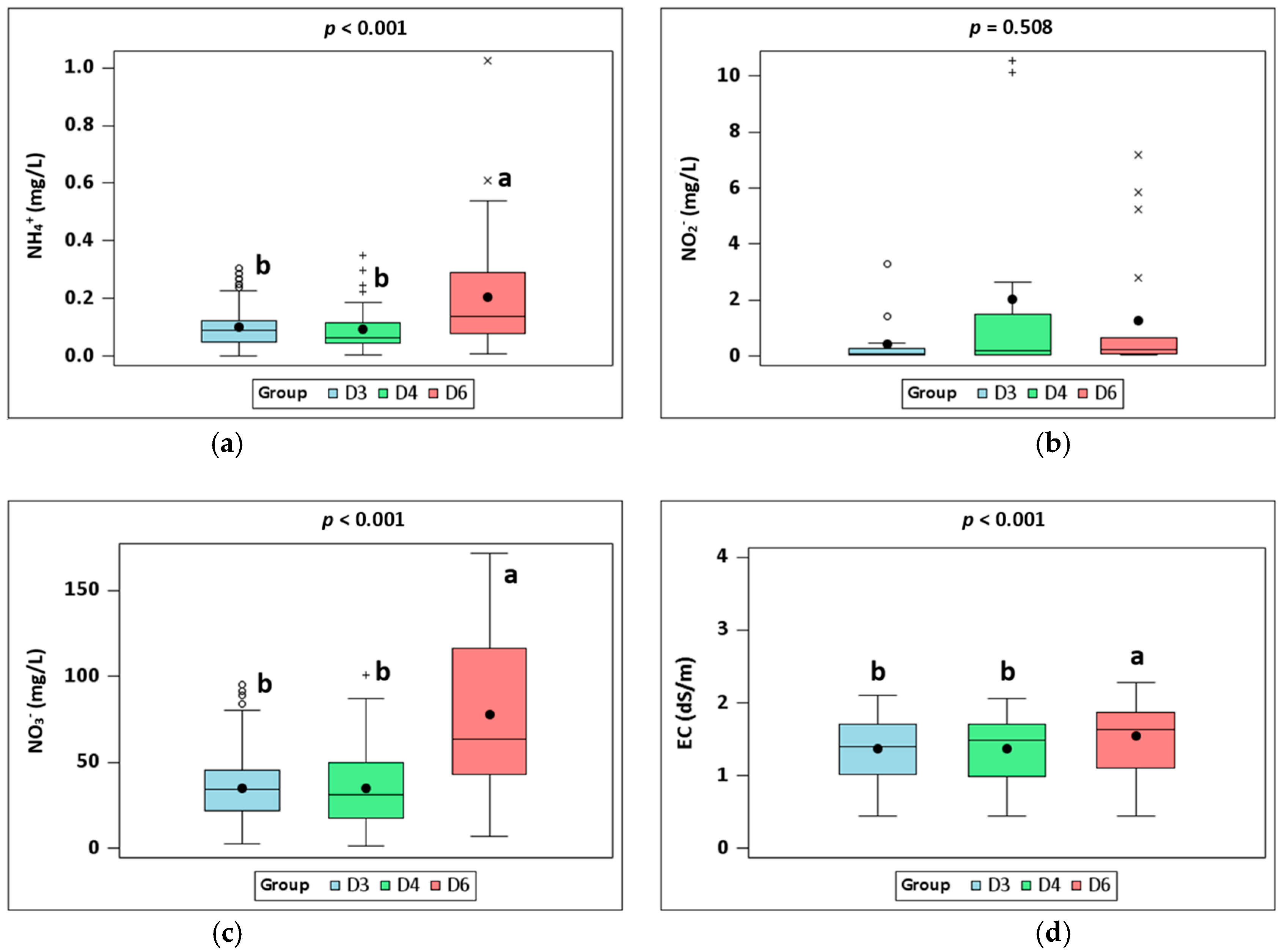
3.1. Water Quality
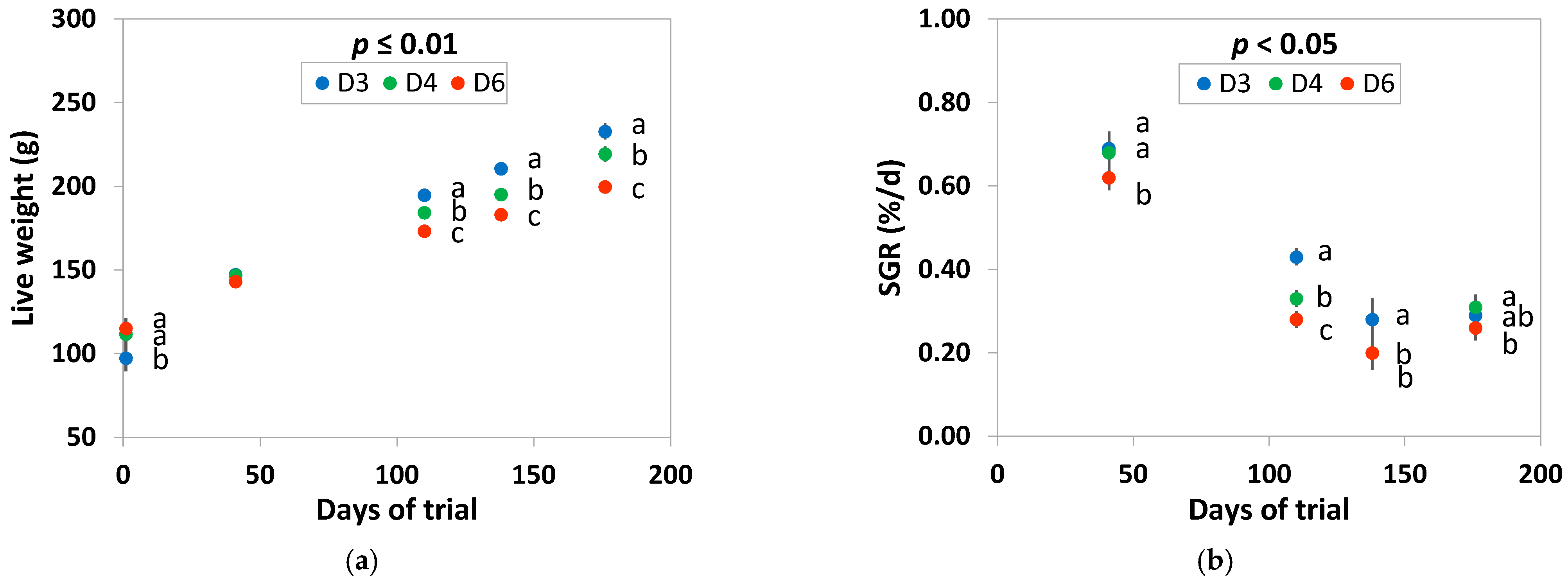
3.2. Survival and Growth Performance of Fish
3.3. Feeding Behavior
3.4. Carcass Characteristics and Filet Quality
3.5. Vegetables Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| DO | Dissolved oxygen |
| EC | Electrical conductivity |
| FCR | Feed conversion ratio |
| HIS | Hepatosomatic index |
| RAS | Least square means |
| RMSE | Root mean square error |
| SAS | Statistical analysis system (software) |
| SGR | Specific Growth Rate |
| VSI | Viscerosomatic Index |
References
- Rakocy, J.E.; Shultz, R.C.; Bailey, D.S.; Thoman, E.S. Aquaponic production of tilapia and basil: Comparing a batch and staggered cropping system. Acta Hortic. 2004, 648, 63–69. [Google Scholar] [CrossRef]
- Atique, F.; Lendholm-Letho, P.; Pirhonen, J. Is Aquaponics beneficial in terms of fish and plant growth and water quality in comparison to separate recirculating aquaculture and hydroponic systems? Water 2022, 14, 1447. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; Zanin, G.; Birolo, M.; Trocino, A.; Sambo, P.; Borin, M.; Xiccato, G. Effect of stocking density of fish on water quality and growth performance of European carp and leafy vegetables in a low-tech aquaponic system. PLoS ONE 2019, 14, e0217561. [Google Scholar] [CrossRef]
- Ni, M.; Li, J.; Chi, M.; Bu, Y.; Ren, Y.; Zhang, M.; Song, Z.; Ding, H. Effects of stocking density on mortality, growth and physiology of juvenile Amur sturgeon (Acipenser schrenckii). Aquac. Res. 2014, 47, 1596–1604. [Google Scholar] [CrossRef]
- Saraiva, J.L.; Rachinas-Lopes, P.; Arechavala-Lopez, P. Finding the “golden stocking density”: A balance between fish welfare and farmers’ perspectives. Front. Vet. Sci. 2022, 9, 2022. [Google Scholar] [CrossRef]
- Yildiz, H.Y.; Robaina, L.; Pirhonen, J.; Mente, E.; Domíniguez, D.; Parisi, G. Fish Welfare in Aquaponic Systems: Its Relation to Water Quality with an Emphasis on Feed and Faeces—A Review. Water 2017, 9, 13. [Google Scholar] [CrossRef]
- Birolo, M.; Bordignon, F.; Trocino, A.; Fasolato, L.; Pascual, A.; Godoy, S.; Nicoletto, C.; Mauceri, C.; Xiccato, G. Effects of stocking density on the growth and flesh quality of rainbow trout (Oncorhynchus mykiss) reared in a low-tech aquaponic system. Aquaculture 2020, 529, 735653. [Google Scholar] [CrossRef]
- Suárez, M.D.; García-Gallego, M.; Trenzado, C.E.; Guil-Guerrero, J.L.; Furné, M.; Domezain, A.; Alba, L.; Sanza, A. Influence of dietary lipids and culture density on rainbow trout (Oncorhynchus mykiss) flesh composition and quality parameter. Aquac. Eng. 2014, 63, 16–24. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, J.; Nie, Z.; Gao, J.; Sun, Y.; Shao, N.; Li, Q.; Hu, J.; Xu, P.; Xu, G. Effects of stocking density on growth, serum parameters, antioxidant status, liver and intestine histology and gene expression of largemouth bass (Micropterus salmoides) farmed in the in-pond raceway system. Aquac. Res. 2020, 51, 5228–5240. [Google Scholar] [CrossRef]
- Yu, P.; Hong, C.; Mingli, L.; Haitao, Z.; Xueyan, W.; Yilin, W.; Yu, S.; Chang, W.; Shi, W.; Chiye, Z.; et al. Current status and application of largemouth bass (Micropterus salmoides) germplasm resources. Reprod. Breed. 2024, 4, 73–82. [Google Scholar] [CrossRef]
- Fischer, H.; Romano, N.; Jones, J.; Howe, J.; Renukdas, N.; Sinha, A.K. Comparing water quality, bacterial composition, and productivity of largemouth bass (Micropterus salmoides) juveniles in a recirculating aquaculture system versus aquaponics, as well as plant growth and mineral composition with or without media. Aquaculture 2021, 538, 736554. [Google Scholar] [CrossRef]
- Hussein, G.H.G.; Chen, M.; Qi, P.-P.; Cui, Q.K.; Yu, Y.; Hu, W.H.; Tian, Y.; Fan, Q.-X.; Gao, Z.X.; Feng, M.-W.; et al. Aquaculture industry development, annual price analysis and out-of-season spawning in largemouth bass Micropterus salmoides. Aquaculture 2020, 519, 734901. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, G.; Nie, Z.; Li, Q.; Shao, N.; Xu, P. Effect of stocking density on growth, serum biochemical parameters, digestive enzymes activity and antioxidant status of largemouth bass, Micropterus salmoides. Pakistan J. Zool. 2019, 51, 1509–1517. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Z.; Pu, D.; Li, P.; Wei, X.; Li, M.; Li, D.; Gao, L.; Zhai, X. Effects of stocking density on intestinal health of juvenile Micropterus salmoides in industrial aquaponics. Fishes 2023, 8, 555. [Google Scholar] [CrossRef]
- Bordignon, F.; Birolo, M.; Trocino, A.; Mauceri, C.; Nicoletto, C.; Pascual, A.; Zanin, G.; Sambo, P.; Borin, M.; Xiccato, G. Farming largemouth bass (Micropterus salmoides) with lettuce (Lactuca sativa) and radicchio (Cichorium intybus) in aquaponics: Effects of stocking density on fish growth and quality, and vegetable production. Acta Fytotechn. Zootech. 2020, 23, 79–87. [Google Scholar] [CrossRef]
- Al-Zahrani, M.S.; Hassanien, H.A.; Alsaade, F.W.; Wahsheh, H.A.M. Effect of stocking density on sustainable growth performance and water quality of nile tilapia-spinach in NFT aquaponic system. Sustainability 2023, 15, 6935. [Google Scholar] [CrossRef]
- Kieffer, J.D.; Colgan, P.W. The role of learning in fish behaviour. J. Fish Biol. 1992, 2, 125–143. [Google Scholar] [CrossRef]
- Benedek, I.; Molnár, T. Size preference of live fish prey in the pellet-consuming pikeperch. Appl. Sci. 2023, 13, 2259. [Google Scholar] [CrossRef]
- Eriksen, M.S.; Færevik, G.; Kittilsen, S.; McCormick, M.I.; Damsgård, B.; Braithwaite, V.A.; Braastad, B.O.; Bakken, M. Stressed mothers—Troubled offspring: A study of behavioural maternal effects in farmed Salmo salar. J. Fish Biol. 2011, 79, 575–586. [Google Scholar] [CrossRef]
- Wankowski, J.M.J.; Thorpe, J.E. Spatial distribution and feeding in Atlantic salmon Salmo salar L. juveniles. J. Fish Biol. 1979, 14, 239–247. [Google Scholar] [CrossRef]
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Strauch, S.M.; Kotzen, B. Coupled aquaponics systems. In Aquaponics Food Productions Systems. Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer: Cham, Switzerland, 2019; pp. 163–199. [Google Scholar]
- Tetreault, J.; Fogle, R.L.; Guerdat, T. Scalable coupled aquaponics design: Lettuce and tilapia production using a parallel unit process approach. Front. Sustain. Food Syst. 2023, 7, 2023. [Google Scholar] [CrossRef]
- Monsees, H.; Kloas, W.; Wuertz, S. Decoupled systems on trial: Eliminating bottlenecks to improve aquaponic processes. PLoS ONE 2017, 12, e0183056. [Google Scholar] [CrossRef]
- Gichana, Z.M.; Liti, D.; Waidbacher, H.; Zollitsch, W.; Drexler, S.; Waikibia, J. Waste management in recirculating aquaculture system through bacteria dissimilation and plant assimilation. Aquac. Int. 2018, 26, 1541–1572. [Google Scholar] [CrossRef]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production: Integrated Fish and Plant Farming; FAO Fisheries and Aquaculture Technical Paper No. 589; FAO: Rome, Italy, 2014. [Google Scholar]
- Jia, R.; Wang, L.; Hou, Y.; Feng, W.; Li, B.; Zhu, J. Effects of stocking density on the growth performance, physiological parameters, redox status and lipid metabolism of Micropterus salmoides in integrated rice-Fish farming systems. Antioxidants 2022, 11, 1215. [Google Scholar] [CrossRef]
- Dara, M.; Carbonara, P.; La Corte, C.; Parrinello, D.; Cammarata, M.; Parisi, M.G. Fish welfare in aquaculture: Physiological and immunological activities for diets, social and spatial stress on mediterranean aqua cultured species. Fishes 2023, 8, 414. [Google Scholar] [CrossRef]
- Li, L.; Shen, Y.; Yang, W.; Xu, X.; Li, J. Effect of different stocking densities on fish growth performance: A meta-analysis. Aquaculture 2021, 544, 737152. [Google Scholar] [CrossRef]
- Li, M.; Yang, L.; Liu, Y.; Ma, H. Long-term crowding stress disrupts intestinal homeostasis in largemouth bass (Micropterus salmoides). Aquaculture 2025, 599, 742171. [Google Scholar] [CrossRef]
- Wang, L.; Jia, S.; Guo, X.; Lu, K.; Zhang, L.; Gong, J.; Guo, X.; Hu, Y.; Cheng, T.; Shang, Q.; et al. Effect of stocking density on growth of largemouth bass (Micropterus salmoides) cultured in containers in a land-based recirculating aquaculture system (C-RAS). Aquac. Res. 2021, 53, 1518–1526. [Google Scholar] [CrossRef]
- Xie, Y.-X.; Yang, X.-M.; Kaneko, G.; Liang, J.-N.; Wen, L.-T.; Li, Y.-J.; Ao, Q.-W.; Huang, L.-M.; Li, P.; Min, W.-W.; et al. Effects of different stocking densities and feeding frequencies on growth, physiological and biochemical indexes, and intestinal microflora of largemouth bass (Micropterus salmoides) under land-based round pond. Aquaculture 2024, 580, 740385. [Google Scholar] [CrossRef]
- Watts, C.; Bright, L.A.; Coyle, S.; Tidwell, J. Evaluation of stocking density during second-year growth of largemouth bass, Micropterus salmoides, raised indoors in a recirculating aquaculture system: Stocking density with largemouth bass raised indoors in RAS. J. World Aquac. Soc. 2016, 47, 538–543. [Google Scholar] [CrossRef]
- Si, N.; Jie, B.; Cheng, G.; Ma, Y.; Dong, L.; Gu, C.; Cheng, M.; Wei, Y.; Yu, K. Effect of stocking density on culture efficiency and physiological indicators of largemouth bass (Micropterus salmoides) under recirculating water conditions in land-based round ponds. Aquac. Res. 2023, 2023, 4391306. [Google Scholar] [CrossRef]
- Ni, M.; Liu, M.; Lou, J.; Mi, G.; Yuan, J.; Gu, Z. Stocking density alters growth performance, serum biochemistry, digestive enzymes, immune response, and muscle quality of largemouth bass (Micropterus salmoides) in in-pond raceway system. Fish Physiol. Biochem. 2021, 47, 1243–1255. [Google Scholar] [CrossRef]
- Ellis, T.; North, B.; Scott, A.P.; Bromage, N.R.; Porter, M.; Gadd, D. The relationships between stocking density and welfare in farmed rainbow trout. J. Fish Biol. 2002, 61, 493–531. [Google Scholar] [CrossRef]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Li, S.; Lian, X.; Chen, N.; Wang, M.; Sang, C. Effects of dietary vitamin E level on growth performance, feed utilization, antioxidant capacity and nonspecific immunity of largemouth bass, Micropterus salmoides. Aquac. Nutr. 2018, 24, 1679–1688. [Google Scholar] [CrossRef]
- Guo, J.-L.; Zhou, Y.-L.; Zhao, H.; Chen, W.-Y.; Chen, Y.-J.; Lin, S.-M. Effect of dietary lipid level on growth, lipid metabolism and oxidative status of largemouth bass, Micropterus salmoides. Aquaculture 2019, 506, 394–400. [Google Scholar] [CrossRef]
- Yuan, J.; Ni, M.; Liu, M.; Wang, H.; Zhang, C.; Mi, G.; Gu, Z. Analysis of the growth performances, muscle quality, blood biochemistry and antioxidant status of Micropterus salmoides farmed in in-pond raceway systems versus usual-pond systems. Aquaculture 2019, 511, 734241. [Google Scholar] [CrossRef]
- Brown, T.G.; Runciman, B.; Pollard, S.; Grant, A.D.A. Biological synopsis of largemouth bass (Micropterus salmoides). Can. Manuscr. Rep. Fish. Aquat. Sci. 2009, 2884, 1–27. [Google Scholar]
- Keretz, K.R.; Dinken, C.P.; Allen, P.J.; Colvin, M.E.; Schramm, H.L. The effect of water temperature, angling time, and dissolved oxygen on the survival of largemouth bass subjected to simulated angling and tournament handling procedures. North Am. J. Fish. Manag. 2018, 38, 606–622. [Google Scholar] [CrossRef]
- Hussain, T.; Verma, A.K.; Tiwari, V.K.; Prakash, C.; Rathore, G.; Shete, A.P.; Nuwansi, K.K.T. Optimizing koi carp, Cyprinus carpio var. koi (Linnaeus, 1758), stocking density and nutrient recycling with spinach in an aquaponic system. J. World Aquacult. Soc. 2014, 45, 652–661. [Google Scholar] [CrossRef]
- Rayhan, M.Z.; Rahman, M.A.; Hossain, M.A.; Akter, T.; Akter, T. Effect of stocking density on growth performance of monosex tilapia (Oreochromis niloticus) with Indian spinach (Basella alba) in a recirculating aquaponic system. Int. J. Environ. Agric. Biotech. 2018, 3, 343–349. [Google Scholar] [CrossRef]
- Ma, H.; Tan, M.; Li, W.; Xu, Q.; Duan, M. Effects of different LED lights colors on the feeding behavior of Chinese longsnout catfish (Leiocassis longirostris). Aquaculture 2026, 612, 743124. [Google Scholar] [CrossRef]
- Huntingford, F.A.; Adams, C.; Braithwaite, V.A.; Kadri, S.; Pottinger, T.G.; Sandoe, P.; Turnbull, J.F. Current issues in fish welfare. J. Fish Biol. 2006, 68, 332–372. [Google Scholar] [CrossRef]
- Martins, C.I.; Galhardo, L.; Noble, C.; Damsgård, B.; Spedicato, M.T.; Zupa, W.; Beauchaud, M.; Kulczykowska, E.; Massabuau, J.C.; Carter, T.; et al. Behavioural indicators of welfare in farmed fish. Fish. Physiol. Biochem. 2012, 38, 17–41. [Google Scholar] [CrossRef]
- Castanheira, M.F.; Conceiçao, L.E.; Millot, S.; Rey, S.; Begout, M.L.; DamsgAard, B.; Martins, C.I. Coping styles in farmed fish: Consequences for aquaculture. Rev. Aquac. 2017, 9, 23–41. [Google Scholar] [CrossRef]
- Kralik, B.; Weisstein, F.; Meyer, J.; Neves, K.; Anderson, D.; Kershaw, J. From water to table: A multidisciplinary approach comparing fish from aquaponics with traditional production methods. Aquaculture 2022, 552, 737953. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Z.; Liang, Z.; Xie, Y.; Su, J.; Luo, Q.; Zhu, J.; Liu, Q.; Han, T.; Wang, A. Effects of dietary fish oil replacement by soybean oil and L-carnitine supplementation on growth performance, fatty acid composition, lipid metabolism and liver health of juvenile largemouth bass, Micropterus salmoides. Aquaculture 2020, 516, 734596. [Google Scholar] [CrossRef]
- Hematyar, N.; Rahimnejad, S.; Waghmare, S.G.; Malinovskyi, O.; Policar, T. Effects of Stocking density and pre-slaughter handling on the fillet quality of largemouth bass (Micropterus salmoides): Implications for fish welfare. Foods 2024, 13, 1477. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; Junge, R.; Schmautz, Z.; Sambo, P.; Borin, M. Hydroponic systems and water management in aquaponics: A review. Ital. J. Agron. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Suhl, J.; Dannehl, D.; Kloas, W.; Baganz, D.; Jobs, S.; Scheibe, G.; Schmidt, U. Advanced aquaponics: Evaluation of intensive tomato production in aquaponics vs. conventional hydroponics. Agr. Water Manag. 2016, 178, 335–344. [Google Scholar] [CrossRef]
- Lennard, W.; Ward, J. A Comparison of plant growth rates between an NFT hydroponic system and an NFT aquaponic system. Horticulturae 2019, 5, 27. [Google Scholar] [CrossRef]
- Lennard, W.A.; Leonard, B.V. A comparison of three different hydroponic sub-systems (gravel bed, floating and nutrient film technique) in an aquaponic test system. Aquac. Int. 2006, 14, 539–550. [Google Scholar] [CrossRef]
- Avgoustaki, D.D.; Xydis, G. How energy innovation in indoor vertical farming can improve food security, sustainability, and food safety? Adv. Food Secur. Sustain. 2020, 5, 1–51. [Google Scholar]
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Goddek, S.; Strauch, S.M.; Vermeulen, T.; Jijakli, H.; Kotzen, B. Towards commercial aquaponics: A review of systems, designs, scales and nomenclature. Aquac. Int. 2019, 27, 1319–1336. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: Aquaponics—Integrating Fish and Plant Culture; SRAC Publication. No. 454; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2006; 16p.


| Feeding Behavior | Description |
|---|---|
| Attack latency (s) | Time elapsed between the entry of the feed (extruded pellet) into the tank and the first successful attack that resulted in ingestion [17,18]. |
| Score (0–3) | Feeding activity score: 0, no response to feed; 1, ingestion only of pellets falling directly in front of the fish without active movement; 2, fish move to capture feed but return to the original position; 3, fish move freely between feed items and ingest all presented pellets [19]. |
| Level (1–3) | Vertical location in the tank where most feed was consumed: 1, surface; 2, mid-water; 3, bottom [19,20]. |
| Attempts (n) | Number of approaches to the feed without ingestion [18]. |
| Attacks (n) | Number of approaches to the feed resulting in ingestion [18]. |
| Duration (s) | Time elapsed between the first and the last successful attack during a meal. |
| Feed intake rate (g/fish/min) | Feed consumption rate per fish, calculated as the ratio of the total feed consumed to the duration of the meal and the number of fish in the tank. |
| Group | RMSE | p-Value | |||
|---|---|---|---|---|---|
| D3 | D4 | D6 | |||
| Tanks, n | 3 | 3 | 3 | ||
| Total length at 176 days (cm) | 25.2 | 24.4 | 24.0 | 0.87 | <0.001 |
| Condition factor at 176 days | 1.44 | 1.49 | 1.44 | 0.11 | 0.067 |
| Daily weight gain from 0 to 176 days (g/day) | 0.69 a | 0.61 b | 0.50 c | 0.08 | <0.001 |
| Specific growth rate from 0 to 176 days (%/day) | 0.43 a | 0.39 b | 0.34 c | 0.04 | <0.001 |
| Feed intake from 0 to 176 days (% biomass/day) | 0.72 a | 0.60 b | 0.61 b | 0.04 | 0.015 |
| Feed conversion ratio from 0 to 176 days | 1.46 a | 1.43 a | 1.76 b | 0.09 | 0.009 |
| Initial biomass (kg/m3) | 3.112 c | 4.094 b | 6.218 a | 0.168 | <0.001 |
| Final biomass (kg/m3) | 5.437 b | 5.968 ab | 8.065 a | 1.032 | 0.045 |
| Biomass growth from 0 to 176 days (kg/m3) | 2.325 | 1.874 | 1.847 | 1.092 | 0.839 |
| Group | p-Value | |||
|---|---|---|---|---|
| D3 | D4 | D6 | ||
| Tanks, n | 3 | 3 | 3 | |
| Attack latency (s) | 0.38 ± 0.13 | 0.60 ± 0.13 | 0.31 ± 0.09 | 0.058 |
| Score | 2.04 ± 0.05 | 1.96 ± 0.09 | 2.15 ± 0.06 | 0.343 |
| Level | 1.83 ± 0.09 | 1.85 ± 0.10 | 1.63 ± 0.08 | 0.493 |
| Attempts (n) | 2.10 ± 0.33 | 2.83 ± 0.40 | 3.31 ± 0.55 | 0.200 |
| Attacks (n) | 47.0 ± 3.6 | 49.7 ± 4.3 | 65.0 ± 5.0 | 0.129 |
| Feeding duration (s) | 47.4 ± 3.3 | 47.2 ± 3.5 | 65.4 ± 4.3 | 0.314 |
| Feed intake rate (g/fish/min) | 2.21 ± 0.16 a | 1.93 ± 0.12 a | 1.23 ± 0.06 b | 0.003 |
| Group | RMSE | p-Value | |||
|---|---|---|---|---|---|
| D3 | D4 | D6 | |||
| Carcass characteristics | |||||
| Carcass yield (%) | 91.2 | 90.4 | 91.3 | 4.73 | 0.649 |
| Hepatosomatic index (%) | 2.38 | 2.42 | 2.21 | 0.51 | 0.074 |
| Viscerosomatic index (%) | 5.00 | 4.87 | 4.97 | 0.89 | 0.808 |
| Filets quality traits | |||||
| Filets yield (%) | 46.6 | 46.8 | 46.0 | 2.05 | 0.320 |
| pH | 6.47 | 6.46 | 6.47 | 0.09 | 0.942 |
| L* | 40.3 | 40.8 | 40.6 | 2.82 | 0.830 |
| a* | −3.31 | −3.64 | −3.66 | 0.482 | 0.058 |
| b* | 1.42 | 1.78 | 1.61 | 1.30 | 0.710 |
| Group | RMSE | p-Value | |||
|---|---|---|---|---|---|
| D3 | D4 | D6 | |||
| Lettuce yield, kg/m2 | 6.126 b | 9.587 a | 9.664 a | 0.969 | 0.007 |
| Sweet basil yield, kg/m2 | 11.077 | 10.636 | 13.106 | 1.167 | 0.085 |
| Swiss chard yield, kg/m2 | 7.489 | 8.404 | 9.776 | 1.783 | 0.353 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birolo, M.; Trabacchin, V.; Sambo, P.; Triolone, S.; Nicoletto, C. Impact of Stocking Density on Growth, Feeding Behavior, and Flesh Quality of Largemouth Bass (Micropterus salmoides) in Coupled Aquaponic Systems. Fishes 2025, 10, 552. https://doi.org/10.3390/fishes10110552
Birolo M, Trabacchin V, Sambo P, Triolone S, Nicoletto C. Impact of Stocking Density on Growth, Feeding Behavior, and Flesh Quality of Largemouth Bass (Micropterus salmoides) in Coupled Aquaponic Systems. Fishes. 2025; 10(11):552. https://doi.org/10.3390/fishes10110552
Chicago/Turabian StyleBirolo, Marco, Veronica Trabacchin, Paolo Sambo, Stefano Triolone, and Carlo Nicoletto. 2025. "Impact of Stocking Density on Growth, Feeding Behavior, and Flesh Quality of Largemouth Bass (Micropterus salmoides) in Coupled Aquaponic Systems" Fishes 10, no. 11: 552. https://doi.org/10.3390/fishes10110552
APA StyleBirolo, M., Trabacchin, V., Sambo, P., Triolone, S., & Nicoletto, C. (2025). Impact of Stocking Density on Growth, Feeding Behavior, and Flesh Quality of Largemouth Bass (Micropterus salmoides) in Coupled Aquaponic Systems. Fishes, 10(11), 552. https://doi.org/10.3390/fishes10110552






