Histological and Molecular Characterisation of Gonadal Phenotypes Confirms Diandry in a Protogynous Wrasse †
Abstract
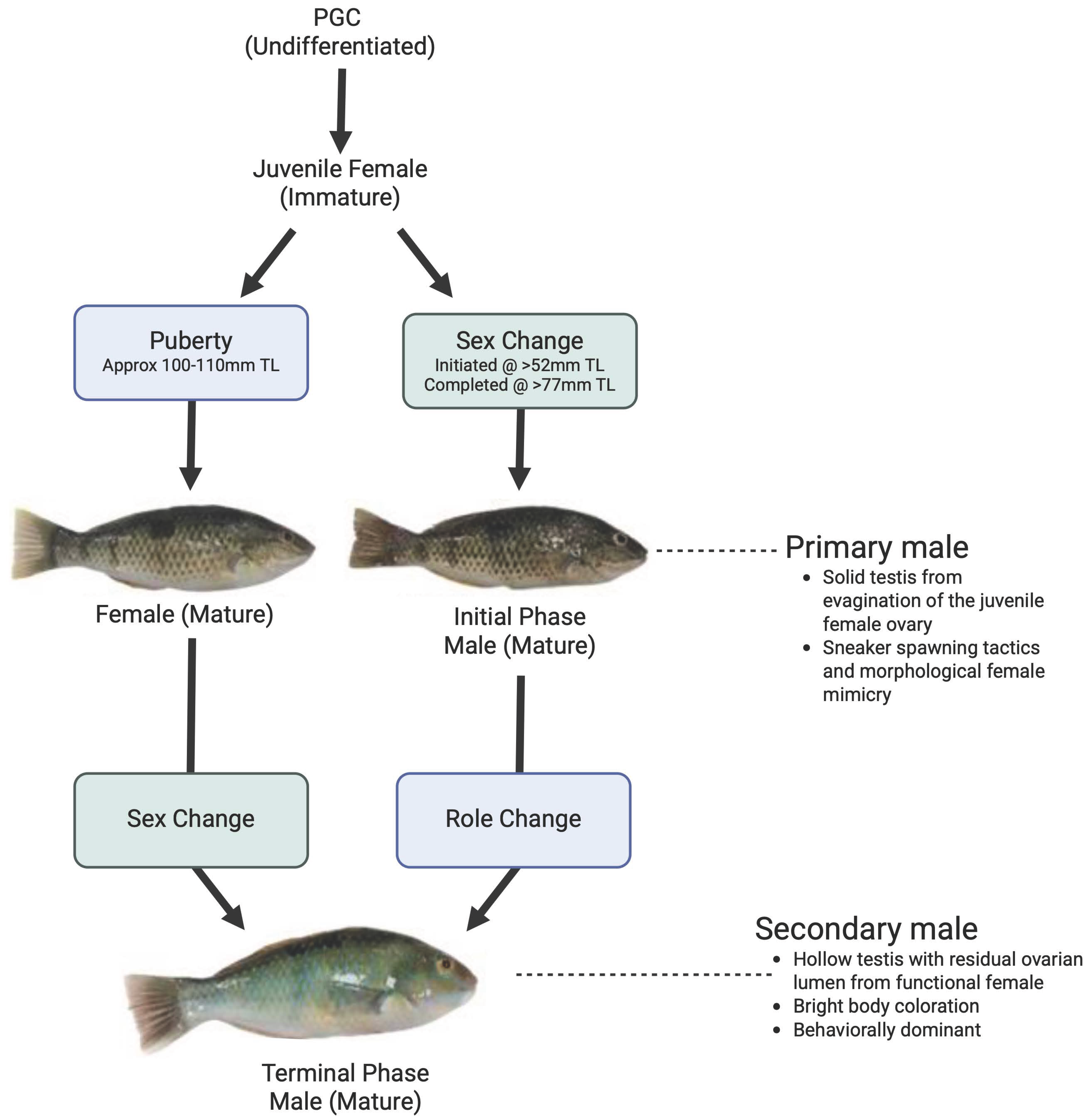
1. Introduction
2. Materials and Methods
2.1. Fish Husbandry
2.2. Gonadal Dissection and Histology
2.3. Quantitative PCR
3. Results
3.1. Gonadal Histology
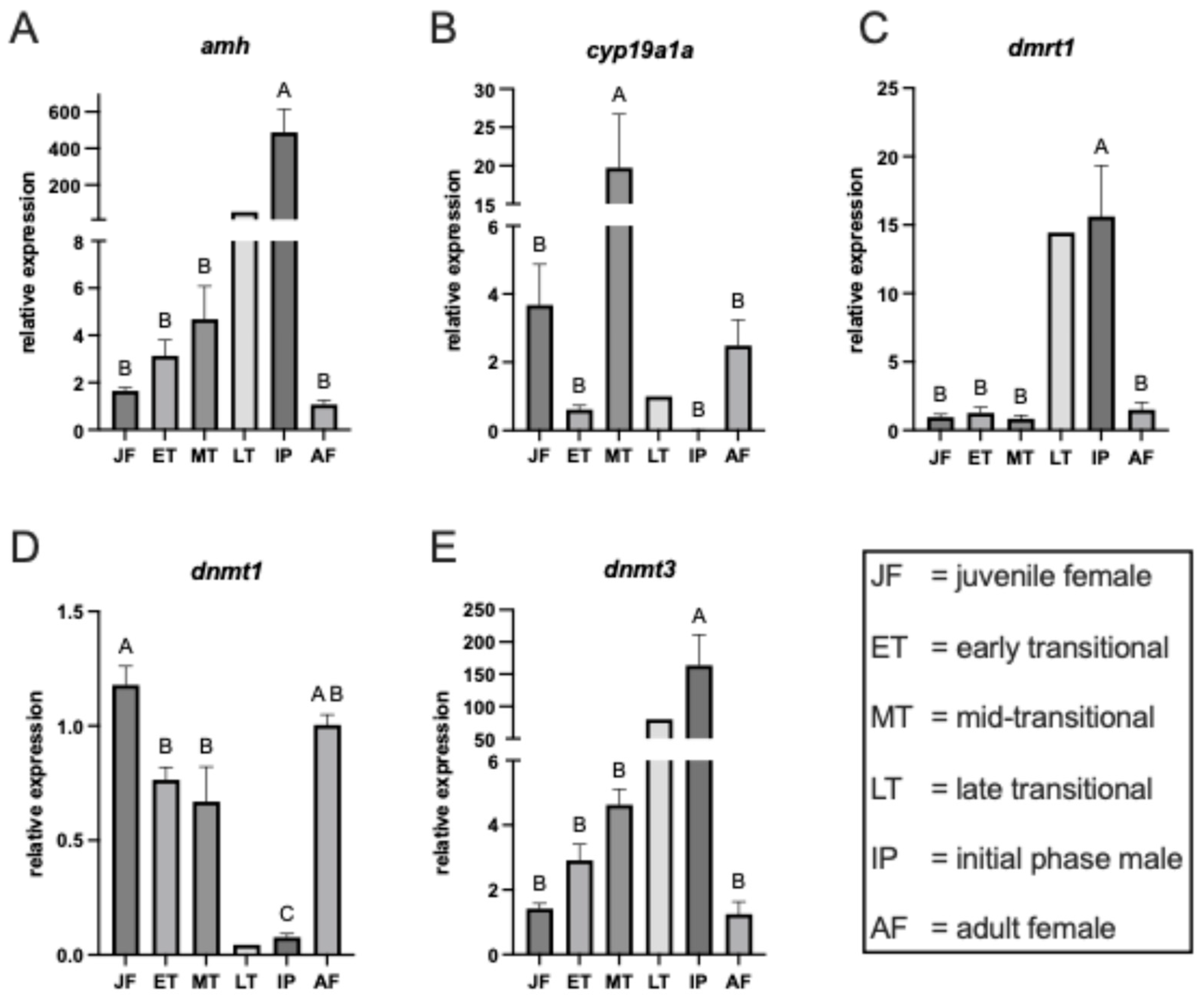
3.2. Gene Expression Profiles
3.2.1. Anti-Mullerian Hormone
3.2.2. Gonadal Aromatase
3.2.3. Doublesex and mab-3 Related Transcription Factor 1
3.2.4. DNA Methyltransferase 1
3.2.5. DNA Methyltransferase 3a
4. Discussion
Author Contributions
Funding
Institutional Board Review Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMH | Anti-Mullerian hormone |
| AtO | Atretic oocyte |
| Cyp19a1a | Gonadal aromatase |
| Dmrt1 | Doublesex- and mab-3 related transcription factor |
| Dnmt1 | DNA methyltransferase 1 |
| Dnmt3a | DNA methyltransferase 3a |
| EGr | Eosinic granulocytes |
| Gon | Gonial cells |
| Oog | Oogonia |
| PvO | Previtellogenic oocyte |
| Spc | Spermatocytes |
| Spg | Spermatogonia |
| Spt | Spermatids |
| StC | Stromal cells |
| Vit | Vitellogenic oocyte |
References
- Yamamoto, T.-O. 3 Sex differentiation. Fish Physiol. 1969, 3, 117–175. [Google Scholar]
- Takahashi, H. Juvenile hermaphroditism in the zebrafish, Brachydanio rerio. Bull. Fac. Fish. Hokkaido Univ. 1977, 28, 57–65. [Google Scholar]
- Takahashi, H.; Shimizu, M. Juvenile Intersexuality in a Cyprinid Fish, the Sumatra Barb. Bull. Fac. Fish. Hokkaido Univ. 1983, 34, 69–78. [Google Scholar]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- De Mitcheson, Y.S.; Liu, M. Functional hermaphroditism in teleosts. Fish Fish. 2008, 9, 1–43. [Google Scholar] [CrossRef]
- Leonard, J.L. Sexual conflict in simultaneous hermaphrodites: Evidence from serranid fishes. Environ. Biol. Fishes 1993, 36, 135–148. [Google Scholar] [CrossRef]
- Warner, R.R.; Robertson, D.R.; Leigh, E.G., Jr. Sex Change and Sexual Selection: The reproductive biology of a labrid fish is used to illuminate a theory of sex change. Science 1975, 190, 633–638. [Google Scholar] [CrossRef]
- Domingos, J.A.; Budd, A.M.; Banh, Q.Q.; Goldsbury, J.A.; Zenger, K.R.; Jerry, D.R. Sex-specific dmrt1 and cyp19a1 methylation and alternative splicing in gonads of the protandrous hermaphrodite barramundi. PLoS ONE 2018, 13, e0204182. [Google Scholar] [CrossRef]
- Todd, E.V.; Liu, H.; Muncaster, S.; Gemmell, N.J. Bending genders: The biology of natural sex change in fish. Sex. Dev. 2016, 10, 223–241. [Google Scholar] [CrossRef]
- Godwin, J. Social determination of sex in reef fishes. Semin. Cell Dev. Biol. 2009, 20, 264–270. [Google Scholar] [CrossRef]
- Warner, R.R. Mating behavior and hermaphroditism in coral reef fishes. Am. Sci. 1984, 72, 128–136. [Google Scholar]
- Charnov, E. Alternative life-histories in protogynous fishes: A general evolutionary theory. Mar. Ecol. Prog. Ser. 1982, 9, 305–307. [Google Scholar] [CrossRef]
- Munday, P.L.; Wilson White, J.; Warner, R.R. A social basis for the development of primary males in a sex-changing fish. Proc. R. Soc. B Biol. Sci. 2006, 273, 2845–2851. [Google Scholar] [CrossRef] [PubMed]
- Cossington, S.; Hesp, S.; Hall, N.; Potter, I. Growth and reproductive biology of the foxfish Bodianus frenchii, a very long-lived and monandric protogynous hermaphroditic labrid. J. Fish. Biol. 2010, 77, 600–626. [Google Scholar] [CrossRef]
- McBride, R.S.; Johnson, M. Sexual development and reproductive seasonality of hogfish (Labridae: Lachnolaimus maximus), an hermaphroditic reef fish. J. Fish. Biol. 2007, 71, 1270–1292. [Google Scholar] [CrossRef]
- Muncaster, S.; Andersson, E.; Kjesbu, O.S.; Taranger, G.L.; Skiftesvik, A.B.; Norberg, B. The reproductive cycle of female Ballan wrasse Labrus bergylta in high latitude, temperate waters. J. Fish. Biol. 2010, 77, 494–511. [Google Scholar] [CrossRef]
- Sadovy, Y.; Shapiro, D.Y. Criteria for the diagnosis of hermaphroditism in fishes. Copeia 1987, 1987, 136–156. [Google Scholar] [CrossRef]
- Kazancıoğlu, E.; Alonzo, S.H. A comparative analysis of sex change in Labridae supports the size advantage hypothesis. Evolution 2010, 64, 2254–2264. [Google Scholar] [CrossRef]
- McCaffrey, K.; Hawkins, M.B.; Godwin, J. Sexual phenotype differences in zic2 mRNA abundance in the preoptic area of a protogynous teleost, Thalassoma bifasciatum. PLoS ONE 2011, 6, e23213. [Google Scholar] [CrossRef]
- Nakamura, M.; Hourigan, T.F.; Yamauchi, K.; Nagahama, Y.; Grau, E.G. Histological and ultrastructural evidence for the role of gonadal steroid hormones in sex change in the protogynous wrasse Thalassoma duperrey. Environ. Biol. Fishes 1989, 24, 117–136. [Google Scholar] [CrossRef]
- Fennessy, S.T.; Sadovy, Y. Reproductive biology of a diandric protogynous hermaphrodite, the serranid Epinephelus andersoni. Mar. Freshw. Res. 2002, 53, 147–158. [Google Scholar] [CrossRef]
- Jones, G. Growth and reproduction in the protogynous hermaphrodite Pseudolabrus celidotus (Pisces: Labridae) in New Zealand. Copeia 1980, 1980, 660–675. [Google Scholar] [CrossRef]
- Thomas, J.T.; Todd, E.V.; Muncaster, S.; Lokman, P.M.; Damsteegt, E.L.; Liu, H.; Soyano, K.; Gléonnec, F.; Lamm, M.S.; Godwin, J.R. Conservation and diversity in expression of candidate genes regulating socially-induced female-male sex change in wrasses. PeerJ 2019, 7, e7032. [Google Scholar] [CrossRef]
- Muncaster, S.; Goikoetxea, A.; Lokman, P.; De Farias e Moraes, C.; Damsteegt, E.; Edgecombe, J.; Gemmell, N.; Todd, E. Genes involved in sex differentiation, epigenetic reprogramming, and cell fate regulate sex change in a wrasse. Rev. Fish Biol. Fish. 2023, 33, 281–294. [Google Scholar] [CrossRef]
- Goikoetxea, A.; Muncaster, S.; Todd, E.; Lokman, P.; Robertson, H.; De Farias e Moraes, C.; Damsteegt, E.; Gemmell, N. A new experimental model for the investigation of sequential hermaphroditism. Sci. Rep. 2021, 11, 22881. [Google Scholar] [CrossRef] [PubMed]
- Kamstra, K.; van der Burg, C.; Quertermous, H.M.; Muncaster, S.; Todd, E.V.; Jasoni, C.L.; Brown, C.; Gemmell, N.J. Neuroanatomy of a sex changing fish: The New Zealand spotty wrasse (Notolabrus celidotus) brain atlas. N. Z. J. Zool. 2024, 51, 228–239. [Google Scholar] [CrossRef]
- Candi, G.; Castriota, L.; Andaloro, F.; Finoia, M.; Marino, G. Reproductive cycle and sex inversion in razor fish, a protogynous labrid in the southern Mediterranean Sea. J. Fish Biol. 2004, 64, 1498–1513. [Google Scholar] [CrossRef]
- Shapiro, D.; Rasotto, M.B. Sex differentiation and gonadal development in the diandric, protogynous wrasse, Thalassoma bifasciatum (Pisces, Labridae). J. Zool. 1993, 230, 231–245. [Google Scholar] [CrossRef]
- Quertermous, H.M.; Kamstra, K.; van der Burg, C.A.; Muncaster, S.; Todd, E.; Jasoni, C.L.; Brown, C.; Gemmell, N. Behavioural and neural correlates of social hierarchy formation in a sex-changing fish. Proc. B 2025, 292, 20242097. [Google Scholar] [CrossRef]
- Taranger, G.L.; Carrillo, M.; Schulz, R.W.; Fontaine, P.; Zanuy, S.; Felip, A.; Weltzien, F.-A.; Dufour, S.; Karlsen, Ø.; Norberg, B. Control of puberty in farmed fish. Gen. Comp. Endocrinol. 2010, 165, 483–515. [Google Scholar] [CrossRef]
- Miura, T.; Higuchi, M.; Ozaki, Y.; Ohta, T.; Miura, C. Progestin is an essential factor for the initiation of the meiosis in spermatogenetic cells of the eel. Proc. Natl. Acad. Sci. USA 2006, 103, 7333–7338. [Google Scholar] [CrossRef]
- Jones, G.; Thompson, S. Social inhibition of maturation in females of the temperate wrasse Pseudolabrus celidotus and a comparison with the blennioid Tripterygion varium. Mar. Biol. 1980, 59, 247–256. [Google Scholar] [CrossRef]
- Todd, E.V.; Ortega-Recalde, O.; Liu, H.; Lamm, M.S.; Rutherford, K.M.; Cross, H.; Black, M.A.; Kardailsky, O.; Marshall Graves, J.A.; Hore, T.A.; et al. Stress, novel sex genes, and epigenetic reprogramming orchestrate socially controlled sex change. Sci. Adv. 2019, 5, eaaw7006. [Google Scholar] [CrossRef] [PubMed]
- Piferrer, F. Epigenetics of sex determination and gonadogenesis. Dev. Dyn. 2013, 242, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-Y.; Tseng, P.-W.; Hwang, J.-S.; Wu, G.-C.; Chang, C.-F. Potential role of DNA methylation of cyp19a1a promoter during sex change in protogynous orange-spotted grouper, Epinephelus coioides. Gen. Comp. Endocrinol. 2021, 311, 113840. [Google Scholar] [CrossRef]
- Asoh, K.; Kasuya, M. Gonadal development and mode of sexuality in a coral-reef damselfish, Dascyllus trimaculatus. J. Zool. 2002, 256, 301–309. [Google Scholar] [CrossRef]
- Robertson, R.D.; Reinboth, R.; Bruce, R.W. Gonochorism, protogynous sex-change and spawning in three sparisomatinine parrotfishes from the western Indian Ocean. Bull. Mar. Sci. 1982, 32, 868–879. [Google Scholar]
- Liu, M.; Sadovy, Y. Early gonadal development and primary males in the protogynous epinepheline, Cephalopholis boenak. J. Fish Biol. 2004, 65, 987–1002. [Google Scholar] [CrossRef]
- Robertson, D.R.; Warner, R.R. Sexual Patterns in the Labroid Fishes of the Western Caribbean, II: The Parrotfishes (Scaridae); Smithsonian Institution Press: Washington, WA, USA, 1978. [Google Scholar]
- Liu, M.; Sadovy, Y. Gonad development during sexual differentiation in hatchery-produced orange-spotted grouper (Epinephelus coioides) and humpback grouper (Cromileptes altivelis) (Pisces: Serranidae, Epinephelinae). Aquaculture 2009, 287, 191–192. [Google Scholar] [CrossRef]
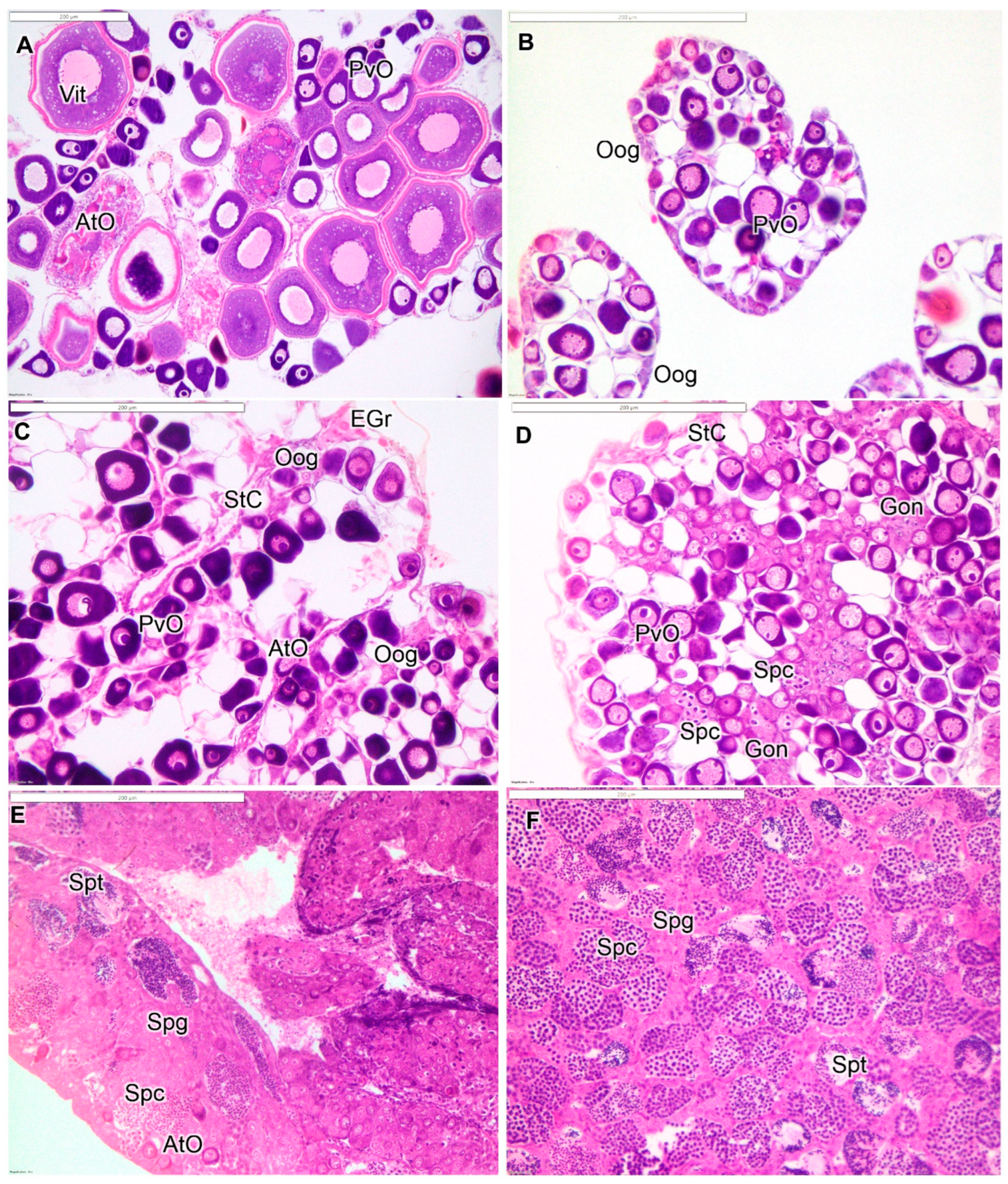
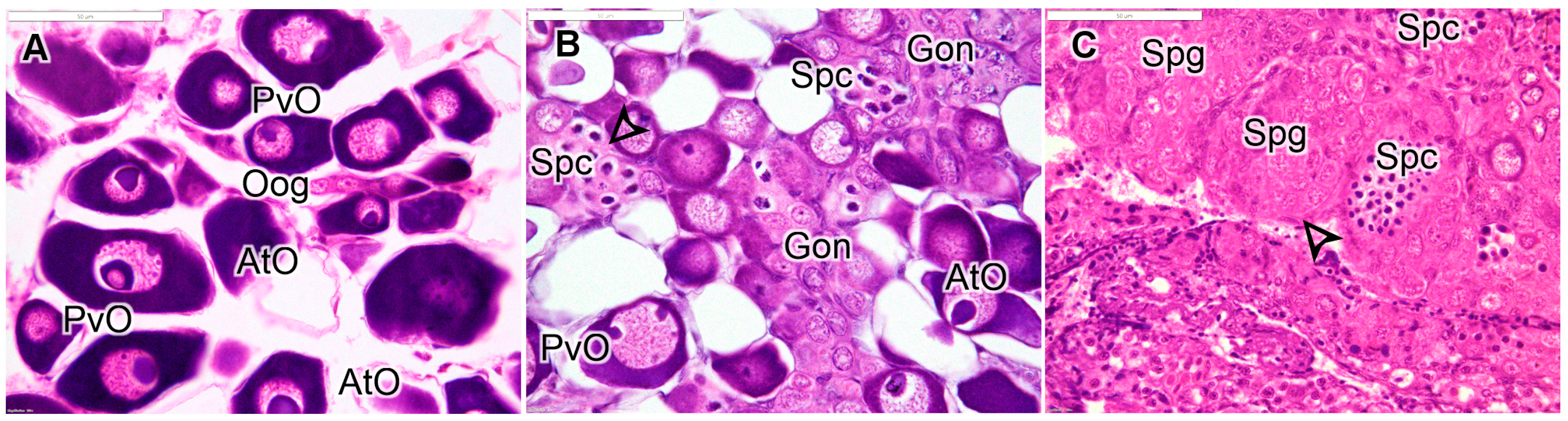
| Stage | Description |
|---|---|
| AF (>110 mm) | Clear ovarian lamellar structure with previtellogenic oocytes of varying sizes. May contain vitellogenic and/or maturing oocytes depending on season. No evidence of male structure. |
| JF (<110 mm) | Ovary dominated by small previtellogenic oocytes, includes oogonial nests in lamellar periphery. |
| ET | Atretic previtellogenic oocytes and nests of gonial cells are common. May include presence of eosinophilic granulocytes and cellular debris. Male germ cells not present. |
| MT | Diminished presence of oocytes and those remaining are mostly atretic. Stromal cells, increased connective tissue, and eosinophilic granulocytes are common. Proliferation of gonial cells is evident, and spermatocytes are present. |
| LT | Number of spermatogenic cysts predominates over oocytes, with some lobular testicular structure becoming evident. Stromal cells are often present. |
| IPM | Clear lobular structure is evident, with many cysts of spermatogenic germ cells. May include more advanced stages of development, such as spermatids and spermatozoa. |
| Gene | Primer | Sequence (5′–3′) | Annealing Temp. (°C) | Tm (°C) | Amplicon Size (bp) | Efficiency | GC (%) |
|---|---|---|---|---|---|---|---|
| cyp19a1a | fw | TGGACACTGTTGTTGGTGAC | 60 | 62.5 | 161 | 0.91 ± 0.03 | 50 |
| rv | AGGTTACTCTAAAGCCCTAGTAGTG | 60 | 62.2 | 44 | |||
| amh | fw | GAAGACGTAAAACAAGATCTGCAC | 60 | 62.2 | 134 | 0.90 ± 0.02 | 42 |
| rv | GGATTACAGGTGAAGGGAAGAG | 60 | 62.6 | 52 | |||
| dmrt1 | fw | ACCCTCACAACTCACAATAACC | 61 | 61.2 | 205 | 0.91 ± 0.02 | 42 |
| rv | AGACCTCCTGGAGAAAAGAG | 61 | 62.1 | 50 | |||
| g6pd | fw | CGAGCTCATGGCAAACCA | 60 | 62.5 | 106 | 0.96 ± 0.02 | 50 |
| rv | GCACAGCTTCAACCTTTTGT | 60 | 60.6 | 50 | |||
| actb2 | fw | CCCACTCACATGAAGATTAAGATCA | 60 | 62.2 | 200 | 0.95 ± 0.03 | 40 |
| rv | AGTGTGTTTTTGGGGGAGG | 60 | 64.4 | 55 | |||
| dnmt1 | fw | TGGCCACCTTTGTCCATTTG | 60 | 58 | 140 | 0.96 ± 0.35 | 50 |
| rv | CGTTGATGGGTCCAAGCTTC | 60 | 59 | 55 | |||
| dnmt3a | fw | GGAGAACAGGCTACACCCAG | 60 | 60 | 209 | 2.48 ± 1.56 | 60 |
| rv | TTCTCCACGCAAACCACAGA | 60 | 58 | 50 |
| Stage of Development | % Total | Mean TL ± SE (mm) | Range TL (mm) |
|---|---|---|---|
| Adult female (AF) | 21.0% (n = 30) | 153.6 ± 31.4 | 112–270 |
| Juvenile female (JF) | 52.5% (n = 75) | 89.3 ± 11.8 | 64–110 |
| Early-transitional (ET) | 14.7% (n = 19) | 75.9 ± 14.6 | 52–107 |
| Mid-transitional (MT) | 3.5% (n = 5) | 70.2 ± 6.0 | 60–72 |
| Late-transitional (LT) | 2.8% (n = 4) | 87.8 ± 14.4 | 72–104 |
| Male (IPM) | 5.6% (n = 8) | 95.5 ± 19.3 | 77–137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamer, T.E.; Robertson, H.A.; Miller, N.; Bird, S.; Kamstra, K.; Muncaster, S. Histological and Molecular Characterisation of Gonadal Phenotypes Confirms Diandry in a Protogynous Wrasse. Fishes 2025, 10, 554. https://doi.org/10.3390/fishes10110554
Hamer TE, Robertson HA, Miller N, Bird S, Kamstra K, Muncaster S. Histological and Molecular Characterisation of Gonadal Phenotypes Confirms Diandry in a Protogynous Wrasse. Fishes. 2025; 10(11):554. https://doi.org/10.3390/fishes10110554
Chicago/Turabian StyleHamer, Tessa E., Holly A. Robertson, Nicole Miller, Steve Bird, Kaj Kamstra, and Simon Muncaster. 2025. "Histological and Molecular Characterisation of Gonadal Phenotypes Confirms Diandry in a Protogynous Wrasse" Fishes 10, no. 11: 554. https://doi.org/10.3390/fishes10110554
APA StyleHamer, T. E., Robertson, H. A., Miller, N., Bird, S., Kamstra, K., & Muncaster, S. (2025). Histological and Molecular Characterisation of Gonadal Phenotypes Confirms Diandry in a Protogynous Wrasse. Fishes, 10(11), 554. https://doi.org/10.3390/fishes10110554








