The Short-Term Effects of an Exercise Protocol Incorporating Blood Flow Restriction and Body Cooling in Healthy Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instrument
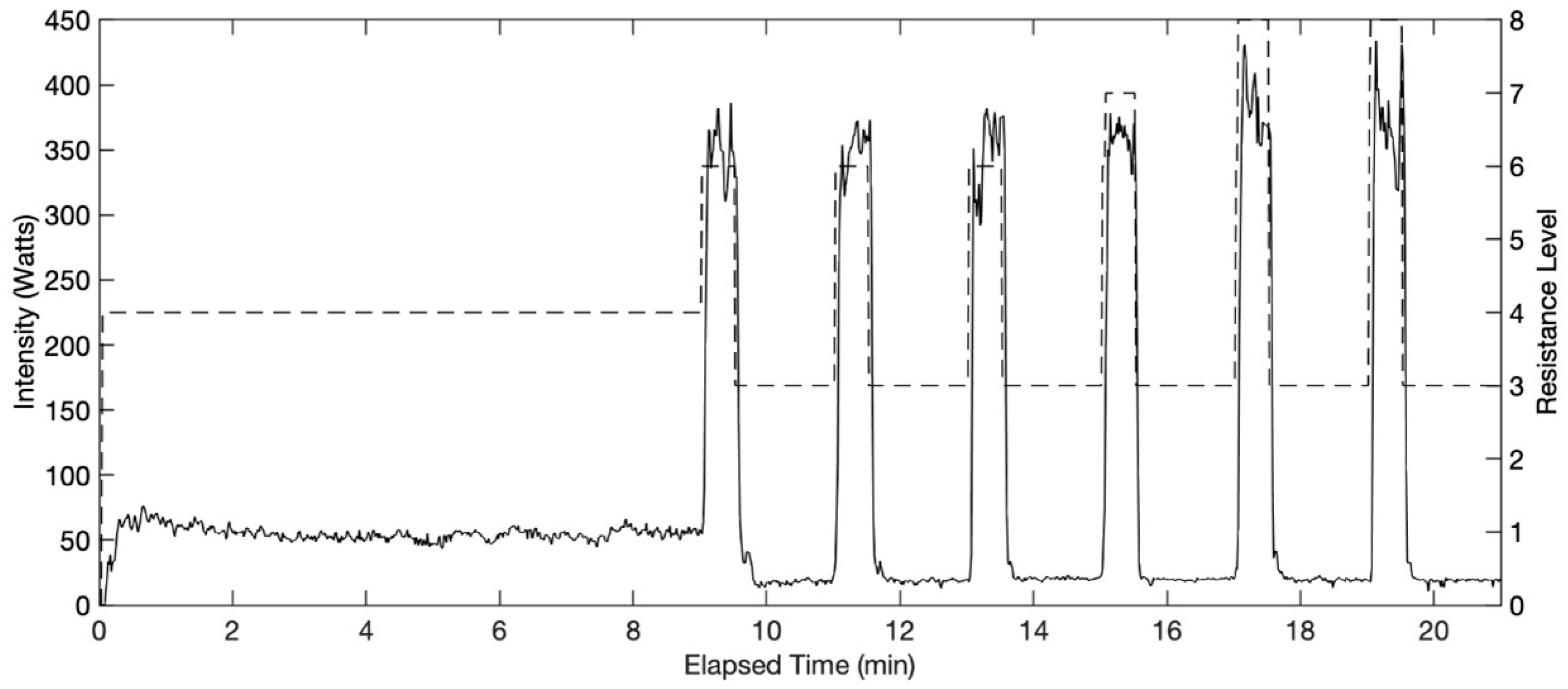
2.3. Exercise Protocol
2.4. Data Analysis
3. Results
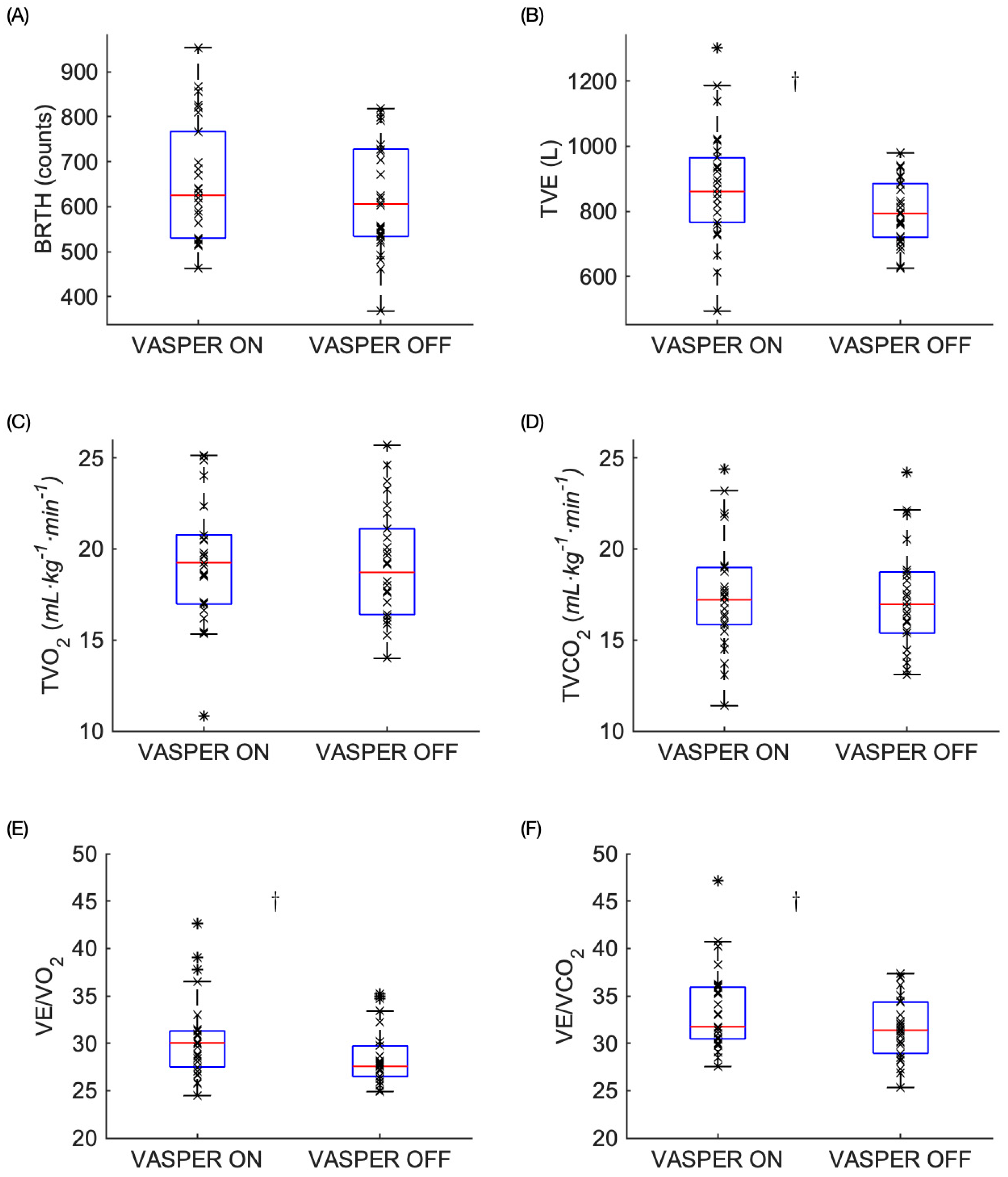
3.1. Physiological Responses to EX
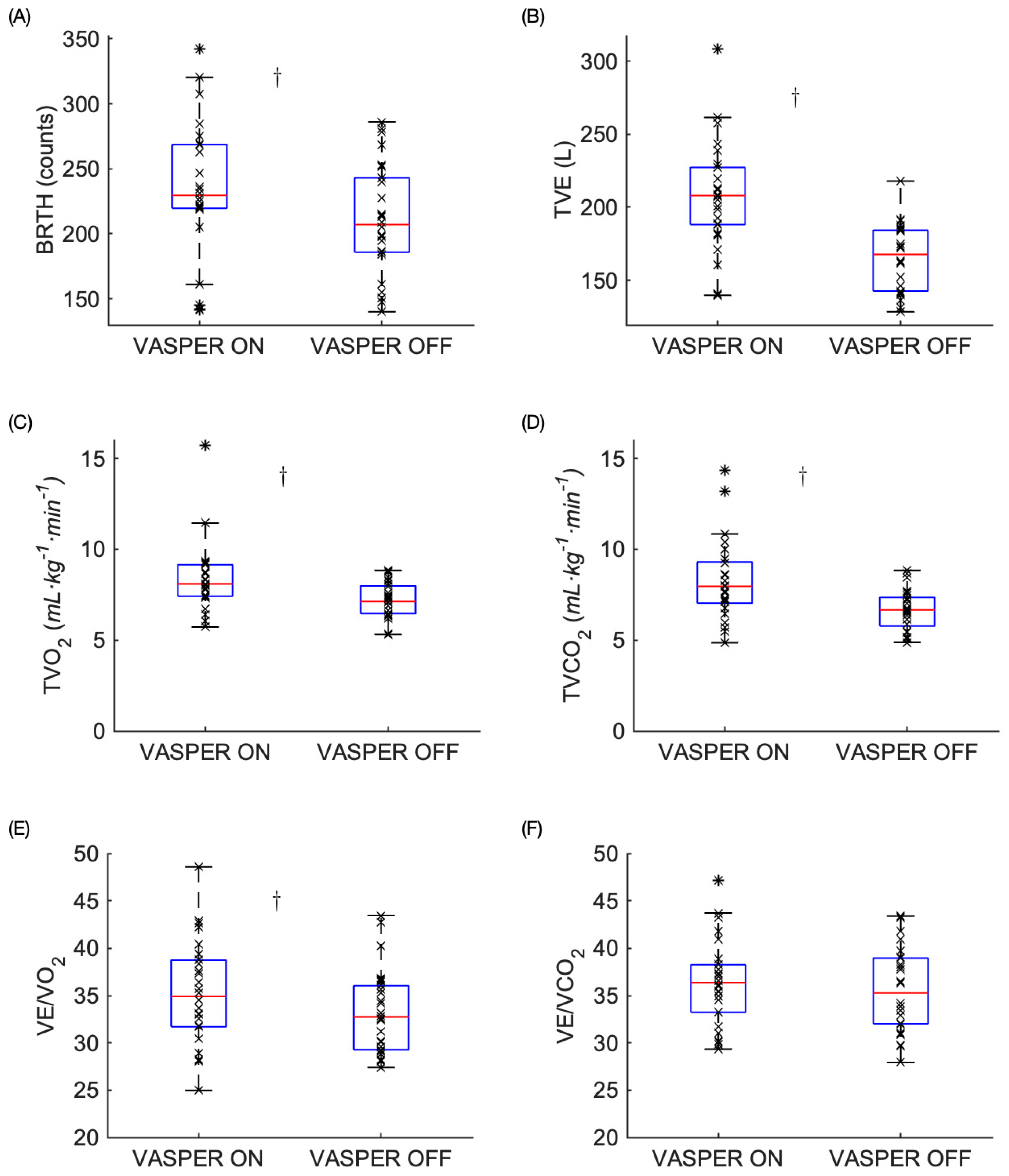
3.2. Physiological Responses Post-EX
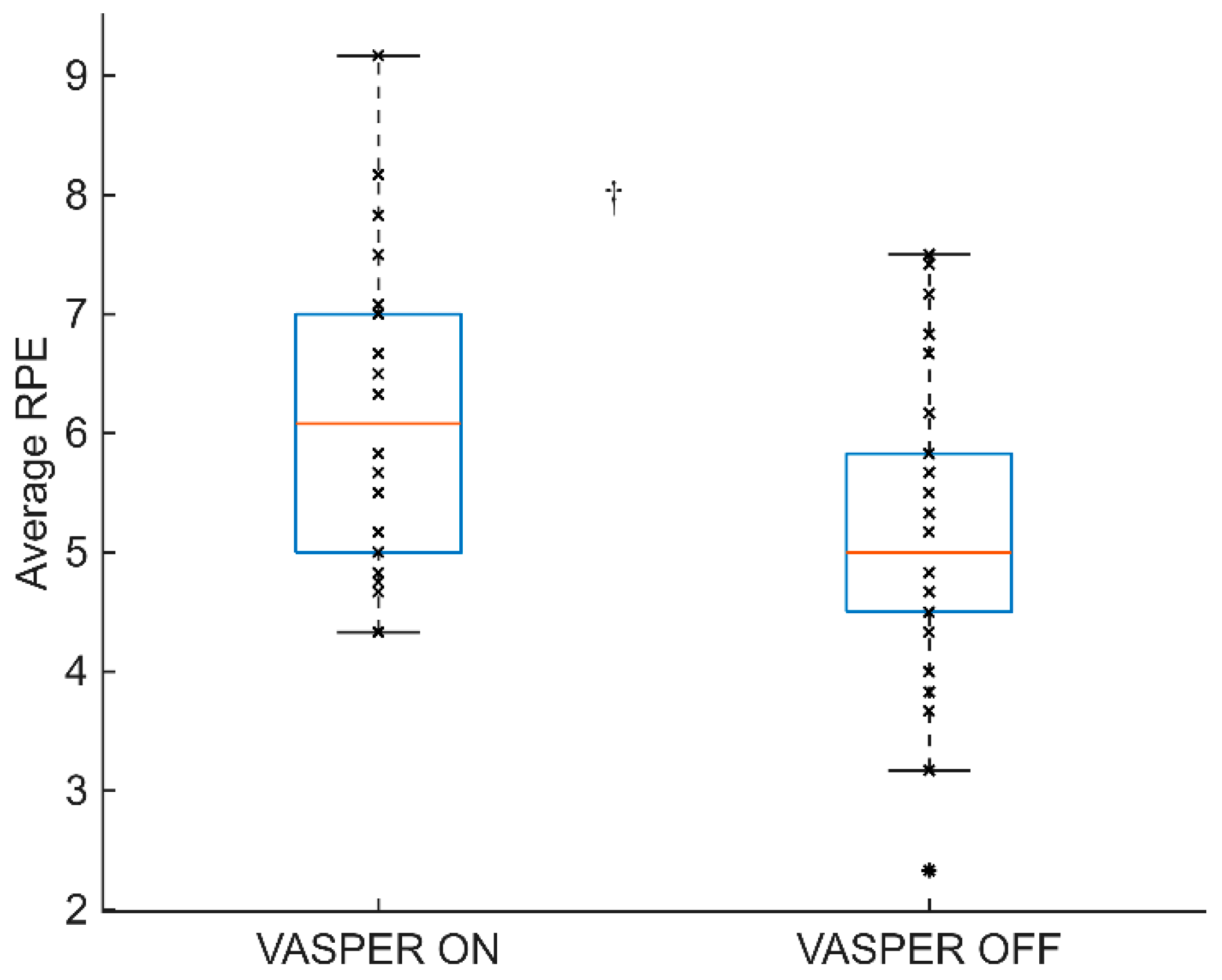
3.3. RPE During EX
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilber, C.G.; Leddy, J.J.; Bezherano, I.; Bromley, L.; Edwards, A.E.; Willer, B.S.; Haider, M.N. Rehabilitation of Concussion and Persistent Postconcussive Symptoms. Semin. Neurol. 2021, 41, 124–131. [Google Scholar] [CrossRef]
- Abe, T.; Kearns, C.F.; Sato, Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J. Appl. Physiol. 2006, 100, 1460–1466. [Google Scholar] [CrossRef]
- Karabulut, M.; Perez, G. Neuromuscular response to varying pressures created by tightness of restriction cuff. J. Electromyogr. Kinesiol. 2013, 23, 1494–1498. [Google Scholar] [CrossRef]
- Lauver, J.D.; Cayot, T.E.; Rotarius, T.; Scheuermann, B.W. The effect of eccentric exercise with blood flow restriction on neuromuscular activation, microvascular oxygenation, and the repeated bout effect. Eur. J. Appl. Physiol. 2017, 117, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Kim, D.; Fahs, C.A.; Thiebaud, R.S.; Abe, T.; Larson, R.D.; Bemben, D.A.; Bemben, M.G. Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle Nerve 2015, 51, 713–721. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Wilson, J.M.; Marín, P.J.; Zourdos, M.C.; Bemben, M.G. Low intensity blood flow restriction training: A meta-analysis. Eur. J. Appl. Physiol. 2011, 112, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Slysz, J.; Stultz, J.; Burr, J.F. The efficacy of blood flow restricted exercise: A systematic review & meta-analysis. J. Sci. Med. Sport 2016, 19, 669–675. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Fukuda, T.; Iida, H.; Imuta, H.; Sato, Y.; Yamasoba, T.; Nakajima, T. Effects of low-intensity, elastic band resistance exercise combined with blood flow restriction on muscle activation. Scand. J. Med. Sci. Sports 2012, 24, 55–61. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.K.; Choi, H.M.; Kim, H.G.; Beekley, M.D.; Nho, H. Increase in maximal oxygen uptake following 2-week walk training with blood flow occlusion in athletes. Eur. J. Appl. Physiol. 2010, 109, 591–600. [Google Scholar] [CrossRef]
- Bennett, H.; Slattery, F. Effects of Blood Flow Restriction Training on Aerobic Capacity and Performance: A Systematic Review. J. Strength Cond. Res. 2019, 33, 572–583. [Google Scholar] [CrossRef]
- Bayati, M.; Farzad, B.; Gharakhanlou, R.; Agha-Alinejad, H. A practical model of low-volume high-intensity interval training induces performance and metabolic adaptations that resemble ‘all-out’ sprint interval training. J. Sports Sci. 2011, 10, 571–576. [Google Scholar]
- Burgomaster, K.A.; Heigenhauser, G.J.F.; Gibala, M.J. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J. Appl. Physiol. 2006, 100, 2041–2047. [Google Scholar] [CrossRef]
- Pimenta, F.C.; Montrezol, F.T.; Dourado, V.Z.; da Silva, L.F.M.; Borba, G.A.; Vieira, W.d.O.; Medeiros, A. High-intensity interval exercise promotes post-exercise hypotension of greater magnitude compared to moderate-intensity continuous exercise. Eur. J. Appl. Physiol. 2019, 119, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Safdar, A.; Wilkin, G.P.; Tarnopolsky, M.A.; Gibala, M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J. Physiol. 2010, 588, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Mok, E.; Suga, T.; Sugimoto, T.; Tomoo, K.; Dora, K.; Takada, S.; Hashimoto, T.; Isaka, T. Negative effects of blood flow restriction on perceptual responses to walking in healthy young adults: A pilot study. Heliyon 2020, 6, e04745. [Google Scholar] [CrossRef]
- Douzi, W.; Dugué, B.; Theurot, D.; Vinches, L.; Hallé, S.; Dupuy, O. Cooling During Exercise May Induce Benefits Linked to Improved Brain Perfusion. Int. J. Sports Med. 2020, 42, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Eijsvogels, T.M.H.; Bongers, C.C.W.G.; Veltmeijer, M.T.W.; Moen, M.H.; Hopman, M. Cooling during Exercise in Temperate Conditions: Impact on Performance and Thermoregulation. Int. J. Sports Med. 2014, 35, 840–846. [Google Scholar] [CrossRef]
- Hausswirth, C.; Duffield, R.; Pournot, H.; Bieuzen, F.; Louis, J.; Brisswalter, J.; Castagna, O. Postexercise cooling interventions and the effects on exercise-induced heat stress in a temperate environment. Appl. Physiol. Nutr. Metab. 2012, 37, 965–975. [Google Scholar] [CrossRef]
- Hohenauer, E.; Taeymans, J.; Baeyens, J.-P.; Clarys, P.; Clijsen, R. The Effect of Post-Exercise Cryotherapy on Recovery Characteristics: A Systematic Review and Meta-Analysis. PLOS ONE 2015, 10, e0139028. [Google Scholar] [CrossRef]
- Geng, X.; Rao, Z.; Zhang, J.; Huang, P.; Qu, C.; Wu, D.; Wei, Q.; Liu, S.; Zhuang, X.; Zhao, J. Combined Cold Exposure and Exercise Improves NAFLD: Mechanistic Insights. Med. Sci. Sports Exerc. 2025, 57, 1988–2000. [Google Scholar] [CrossRef]
- Conceição, M.S.; Gáspari, A.F.; Ramkrapes, A.P.B.; Junior, E.M.M.; Bertuzzi, R.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Anaerobic metabolism induces greater total energy expenditure during exercise with blood flow restriction. PLOS ONE 2018, 13, e0194776. [Google Scholar] [CrossRef] [PubMed]
- Corvino, R.B.; Rossiter, H.B.; Loch, T.; Martins, J.C.; Caputo, F. Physiological responses to interval endurance exercise at different levels of blood flow restriction. Eur. J. Appl. Physiol. 2016, 117, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Nassis, G.P.; Gu, Z.; Zou, Y.; Wang, X.; Li, Y. Acute physiological and perceptual responses to moderate intensity cycling with different levels of blood flow restriction. Biol. Sport 2021, 38, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, G.V.; Vaz, J.R.; Teixeira, M.S.; Grácio, T.; Pezarat-Correia, P. Metabolic cost of locomotion during treadmill walking with blood flow restriction. Clin. Physiol. Funct. Imaging 2013, 34, 308–316. [Google Scholar] [CrossRef]
- Wu, Y.-N.; Stark, C.; Gravel, J.; White, M.; Avery, J.; Enis, T.; Cantu, R.C. Effects of Interval-Training Exercise on People Who Have Had Persistent Post-Concussive Symptoms for Less Than One Year: A Pilot Study. J. Neurotrauma 2021, 38, 573–581. [Google Scholar] [CrossRef]
- Doubt, T.J. Physiology of Exercise in the Cold. Sports Med. 1991, 11, 367–381. [Google Scholar] [CrossRef][Green Version]
- Laurentino, G.C.; Ugrinowitsch, C.; Roschel, H.; Aoki, M.S.; Soares, A.G.; Neves, M.; Aihara, A.Y.; Fernandes, A.D.R.C.; Tricoli, V. Strength Training with Blood Flow Restriction Diminishes Myostatin Gene Expression. Med. Sci. Sports Exerc. 2012, 44, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M. ACSM’s Health/Fitness Facility Standards and Guidelines, 5th ed.; Human Kinetics: Champaign, IL, USA, 2019. [Google Scholar]
- da Silva, J.C.G.; Silva, K.F.; Domingos-Gomes, J.R.; Batista, G.R.; Freitas, E.D.d.S.; Torres, V.B.C.; Cirilo-Sousa, M.D.S. Aerobic exercise with blood flow restriction affects mood state in a similar fashion to high intensity interval exercise. Physiol. Behav. 2019, 211, 112677. [Google Scholar] [CrossRef]
- Stevens, C.J.; Taylor, L.; Dascombe, B.J. Cooling During Exercise: An Overlooked Strategy for Enhancing Endurance Performance in the Heat. Sports Med. 2016, 47, 829–841. [Google Scholar] [CrossRef]
- Ruddock, A.; Robbins, B.; Tew, G.; Bourke, L.; Purvis, A. Practical Cooling Strategies During Continuous Exercise in Hot Environments: A Systematic Review and Meta-Analysis. Sports Med. 2016, 47, 517–532. [Google Scholar] [CrossRef]
- Yasuda, T.; Abe, T.; Brechue, W.F.; Iida, H.; Takano, H.; Meguro, K.; Kurano, M.; Fujita, S.; Nakajima, T. Venous blood gas and metabolite response to low-intensity muscle contractions with external limb compression. Metabolism 2010, 59, 1510–1519. [Google Scholar] [CrossRef]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.; Owens, J.; Abe, T.; Nielsen, J.; Libardi, C.A.; Laurentino, G.; et al. Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front. Physiol. 2019, 10, 533. [Google Scholar] [CrossRef]
- Gagnon, D.D.; Hancock, C.; McCue, A.; Beckett-Brown, N.; Gagnon, J.; Williams, L.; Marsh, D.; Munten, S. Muscle cooling modulates tissue oxidative and biochemical responses but not energy metabolism during exercise. Eur. J. Appl. Physiol. 2020, 120, 1761–1775. [Google Scholar] [CrossRef]
- Bond, B.; Hind, S.; Williams, C.A.; Barker, A.R. The Acute Effect of Exercise Intensity on Vascular Function in Adolescents. Med. Sci. Sports Exerc. 2015, 47, 2628–2635. [Google Scholar] [CrossRef]
- Harris, N.K.; Dulson, D.K.; Logan, G.R.; Warbrick, I.B.; Merien, F.L.; Lubans, D.R. Acute Responses to Resistance and High-Intensity Interval Training in Early Adolescents. J. Strength Cond. Res. 2017, 31, 1177–1186. [Google Scholar] [CrossRef]
- Wood, K.M.; Olive, B.; LaValle, K.; Thompson, H.; Greer, K.; Astorino, T.A. Dissimilar Physiological and Perceptual Responses Between Sprint Interval Training and High-Intensity Interval Training. J. Strength Cond. Res. 2016, 30, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Engel, F.; Härtel, S.; Strahler, J.; Wagner, M.O.; Bös, K.; Sperlich, B. Hormonal, Metabolic, and Cardiorespiratory Responses of Young and Adult Athletes to a Single Session of High-Intensity Cycle Exercise. Pediatr. Exerc. Sci. 2014, 26, 485–494. [Google Scholar] [CrossRef]
- Børsheim, E.; Bahr, R. Effect of Exercise Intensity, Duration and Mode on Post-Exercise Oxygen Consumption. Sports Med. 2003, 33, 1037–1060. [Google Scholar] [CrossRef]
- Bahr, R.; Sejersted, O.M. Effect of intensity of exercise on excess postexercise O2 consumption. Metabolism 1991, 40, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Howley, E.T. Exercise Physiology: Theory and Application to Fitness and Performanc, 10th ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Reis, J.F.; Fatela, P.; Mendonca, G.V.; Vaz, J.R.; Valamatos, M.J.; Infante, J.; Mil-Homens, P.; Alves, F.B. Tissue Oxygenation in Response to Different Relative Levels of Blood-Flow Restricted Exercise. Front. Physiol. 2019, 10, 407. [Google Scholar] [CrossRef]
- Mendonca, G.V.; Vaz, J.R.; Pezarat-Correia, P.; Fernhall, B. Effects of Walking with Blood Flow Restriction on Excess Post-exercise Oxygen Consumption. Int. J. Sports Med. 2015, 36, e11–e18. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.G.; Neto, E.A.P.; Pfeiffer, P.A.S.; Neto, G.R.; Rodrigues, A.S.; Bemben, M.G.; Patterson, S.D.; Batista, G.R.; Cirilo-Sousa, M.S. Acute and Chronic Responses of Aerobic Exercise With Blood Flow Restriction: A Systematic Review. Front. Physiol. 2019, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, M.J. Unpacking the mitochondrial bioenergetics of blood flow restricted resistance exercise. J. Physiol. 2019, 598, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Osawa, M.; Koga, S.; Li, K.; Sakaue, H.; Sengoku, Y.; Takagi, H. Effects of muscle cooling on kinetics of pulmonary oxygen uptake and muscle deoxygenation at the onset of exercise. Physiol. Rep. 2018, 6, e13910. [Google Scholar] [CrossRef]

| Male (n = 19) | Female (n = 7) | Total (n = 26) | |
|---|---|---|---|
| Age (years) | 20.4 ± 2.8 | 19.7 ± 0.7 | 20.2 ± 2.4 |
| Height (cm) | 175.8 ± 9.3 | 162.2 ± 5.2 | 173.9 ± 0.1 |
| Weight (kg) | 77.2 ± 8.8 | 56.3 ± 4.9 | 71.5 ± 11.8 |
| BMI (kg/m2) | 25.0 ± 2.6 | 21.5 ± 3.1 | 24.1 ± 3.1 |
| p Value | Rank | Adjusted α | BH Significance | |
|---|---|---|---|---|
| EX | ||||
| BRTH | 0.057 | 10 | 0.038 | no |
| TVE | 0.011 | 7.5 | 0.029 | yes |
| TVO2 | 0.493 | 13 | 0.050 | no |
| TVCO2 | 0.492 | 12 | 0.046 | no |
| VE/O2 | 0.005 | 5 | 0.019 | yes |
| VE/CO2 | 0.011 | 7.5 | 0.029 | yes |
| RPE | 0.001 | 2.5 | 0.010 | yes |
| Post-EX | ||||
| BRTH | 0.009 | 6 | 0.023 | yes |
| TVE | 0.001 | 2.5 | 0.010 | yes |
| TVO2 | 0.001 | 2.5 | 0.010 | yes |
| TVCO2 | 0.001 | 2.5 | 0.010 | yes |
| VE/O2 | 0.022 | 9 | 0.035 | yes |
| VE/CO2 | 0.282 | 11 | 0.042 | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanwicks, A.J.; Pang, P.C.; Allgood, H.C.; Kim, Y.; Wu, Y.-N. The Short-Term Effects of an Exercise Protocol Incorporating Blood Flow Restriction and Body Cooling in Healthy Young Adults. Methods Protoc. 2025, 8, 135. https://doi.org/10.3390/mps8060135
Stanwicks AJ, Pang PC, Allgood HC, Kim Y, Wu Y-N. The Short-Term Effects of an Exercise Protocol Incorporating Blood Flow Restriction and Body Cooling in Healthy Young Adults. Methods and Protocols. 2025; 8(6):135. https://doi.org/10.3390/mps8060135
Chicago/Turabian StyleStanwicks, Andrew J., Patrick C. Pang, Hannah C. Allgood, Yuho Kim, and Yi-Ning Wu. 2025. "The Short-Term Effects of an Exercise Protocol Incorporating Blood Flow Restriction and Body Cooling in Healthy Young Adults" Methods and Protocols 8, no. 6: 135. https://doi.org/10.3390/mps8060135
APA StyleStanwicks, A. J., Pang, P. C., Allgood, H. C., Kim, Y., & Wu, Y.-N. (2025). The Short-Term Effects of an Exercise Protocol Incorporating Blood Flow Restriction and Body Cooling in Healthy Young Adults. Methods and Protocols, 8(6), 135. https://doi.org/10.3390/mps8060135








