The Allium cepa Model: A Review of Its Application as a Cytogenetic Tool for Evaluating the Biosafety Potential of Plant Extracts
Abstract
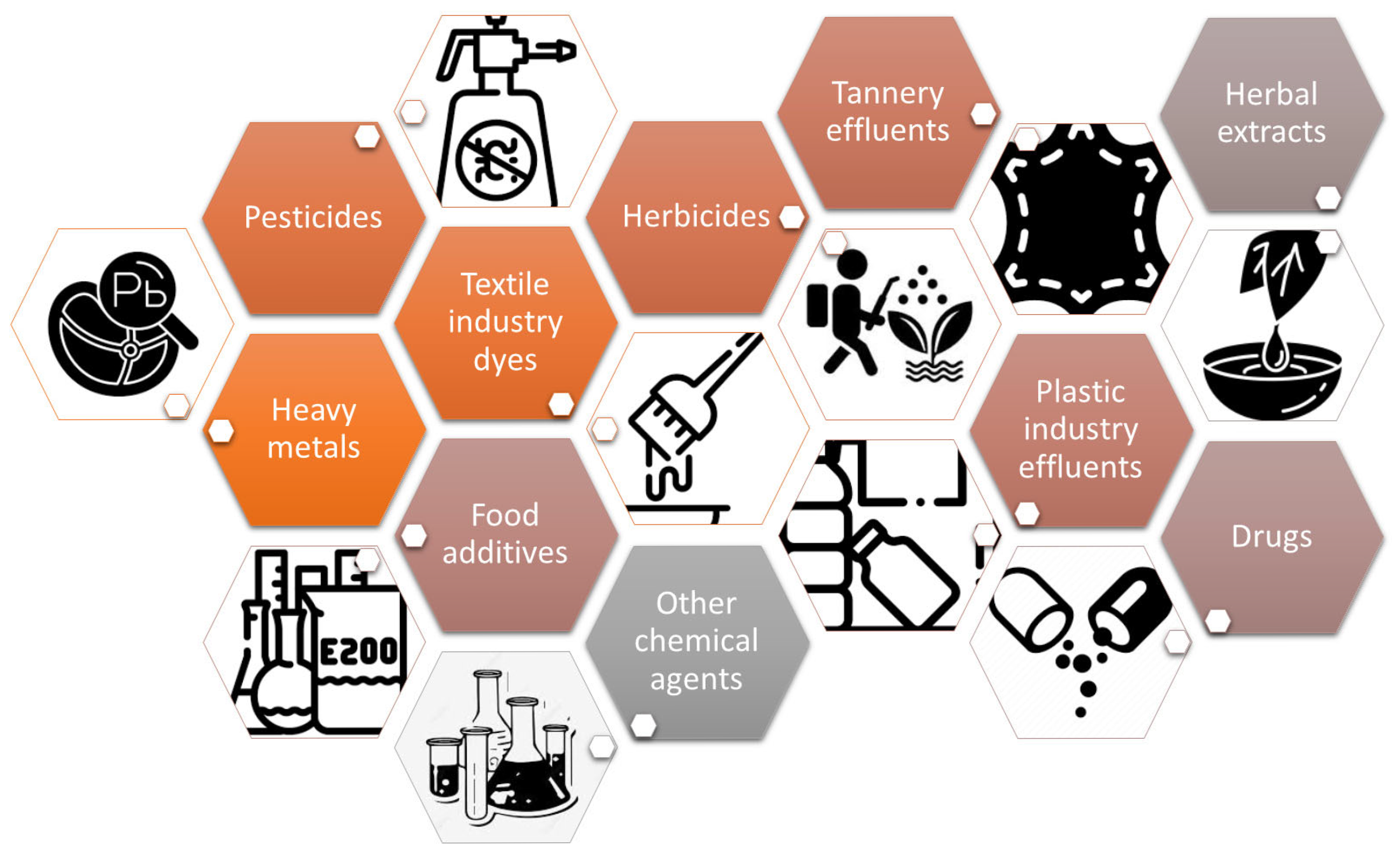
1. Introduction
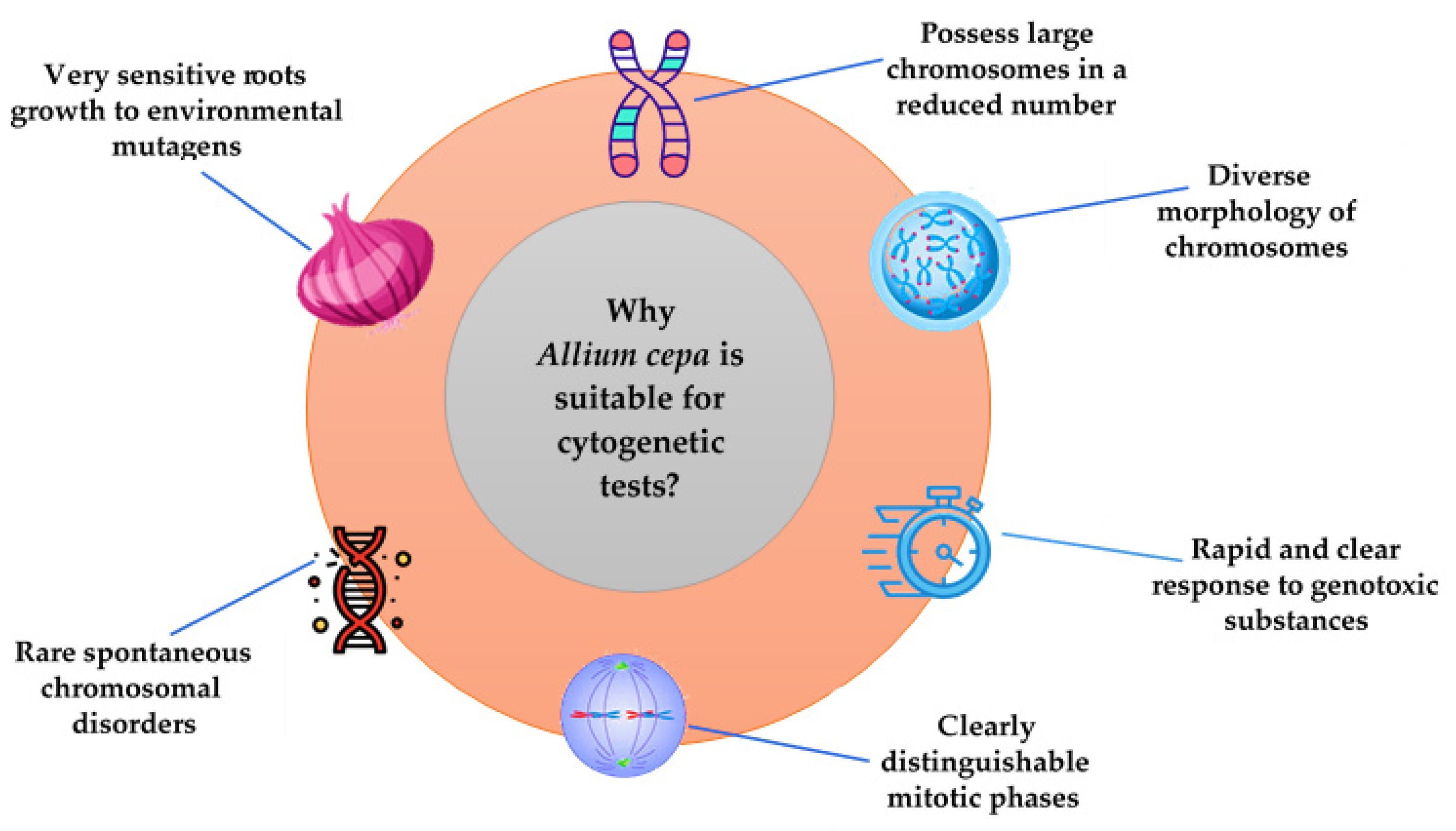
2. The Allium cepa Model: General Considerations
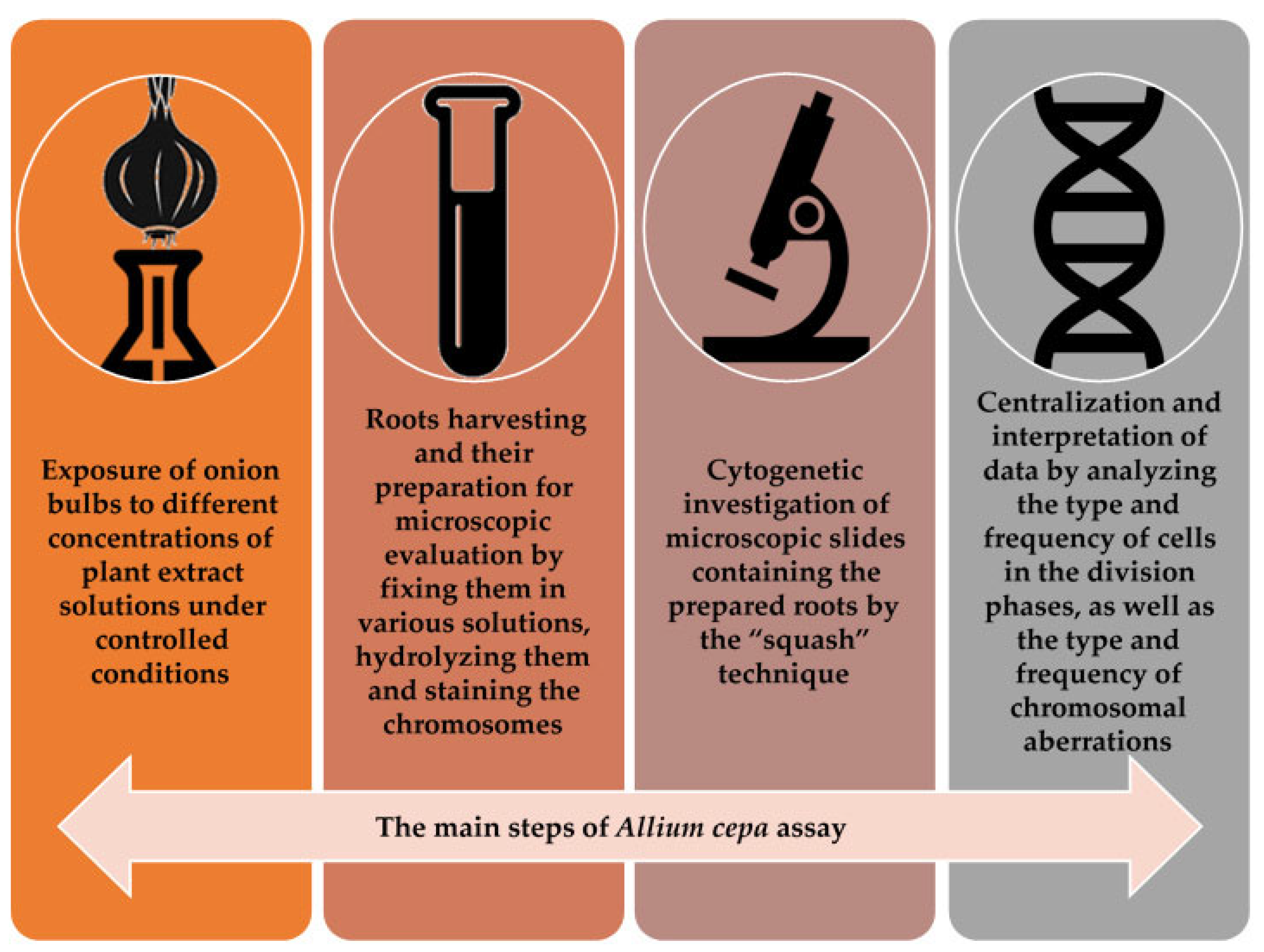
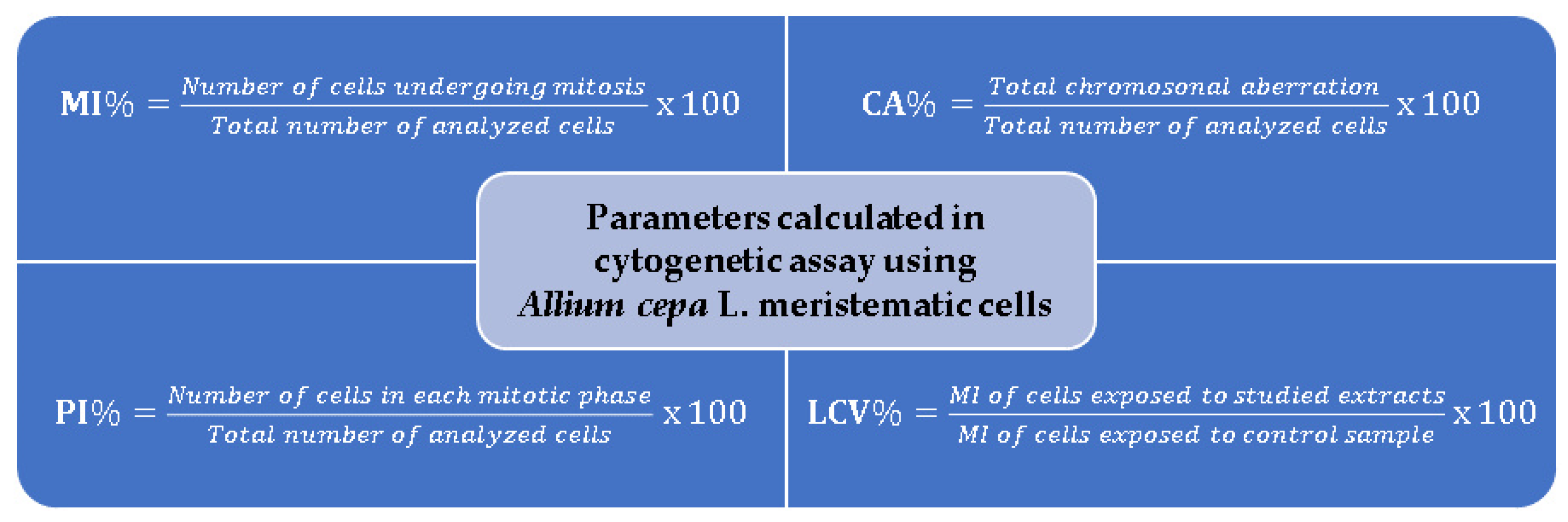
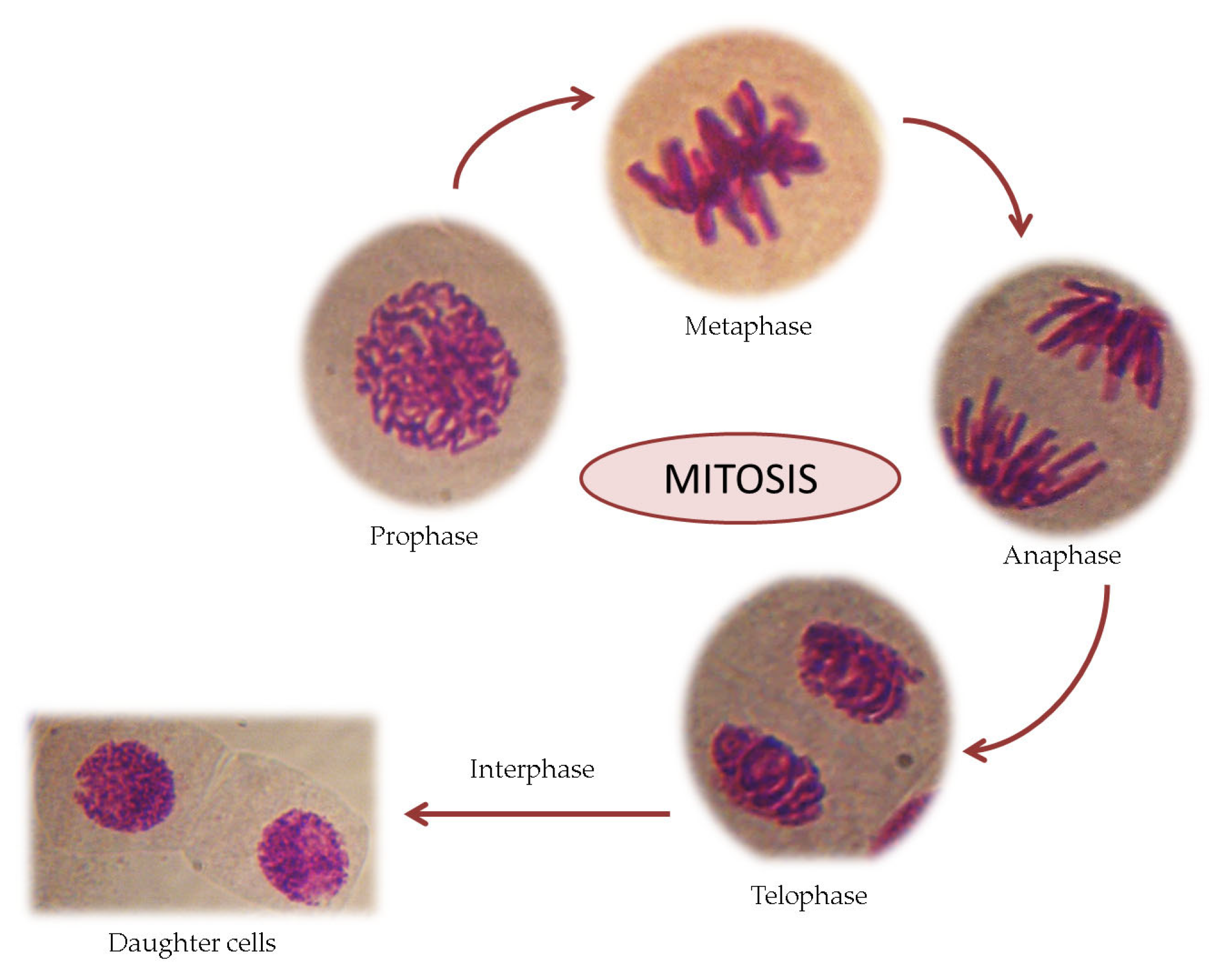
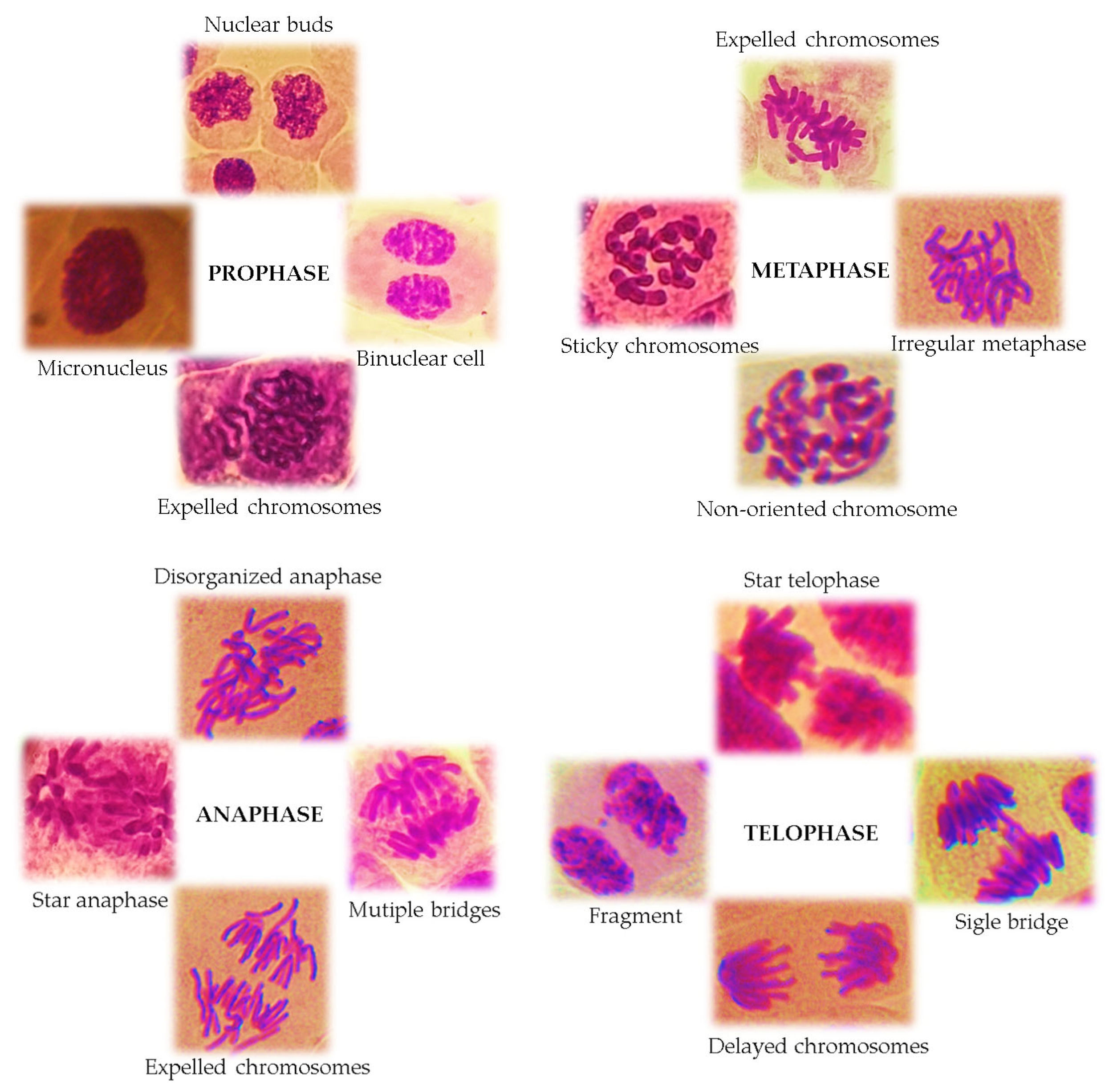
3. Basic Principles of the Allium cepa Test and Protocol
4. Results of the Cytotoxic and Genotoxic Evaluation of Plant Extracts Using the Allium cepa Model: Literature Review
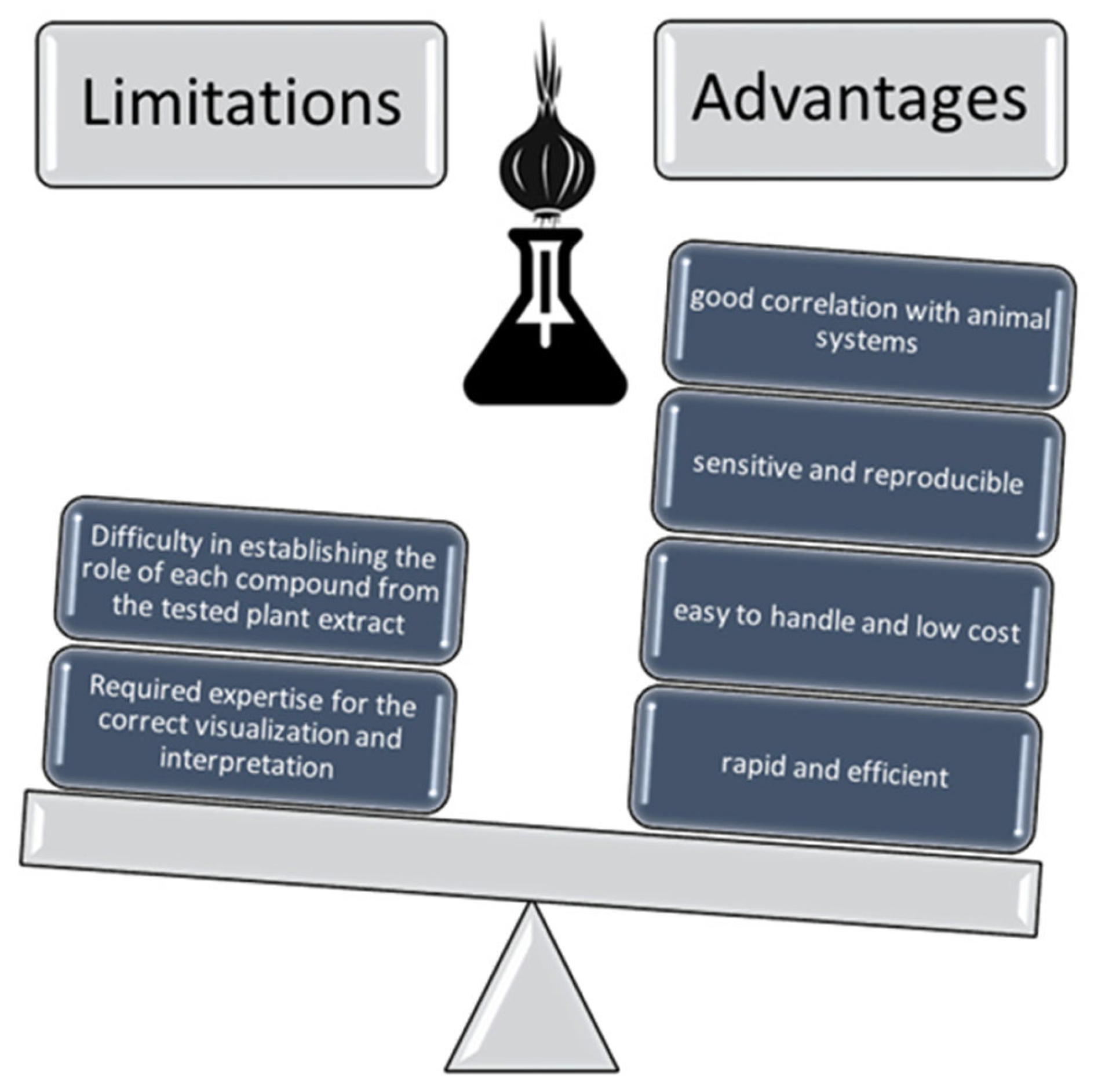
5. Discussion
6. Conclusions
7. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El Allaoui, H.; El Ahmadi, K.; El Abdouni, A.; Dira, I.; El Bastrioui, M.; Bouhrim, M.; Eto, B.; Shahat, A.A.; Herqash, R.N.; Haboubi, K. Trends and Insights in Medicinal Plant Extract Research: A Ten-Year Bibliometric and Visualization Study. Horticulturae 2024, 10, 1163. [Google Scholar] [CrossRef]
- Proestos, C. The Benefits of Plant Extracts for Human Health. Foods 2020, 9, 1653. [Google Scholar] [CrossRef]
- Almasri, R.S.; Bedir, A.S.; Al Raish, S.M. Comprehensive Ethnopharmacological Analysis of Medicinal Plants in the UAE: Lawsonia inermis, Nigella sativa, Ziziphus spina-christi, Allium cepa, Allium sativum, Cymbopogon schoenanthus, Matricaria aurea, Phoenix dactylifera, Portulaca oleracea, Reichardia tingitana, Salvadora persica, Solanum lycopersicum, Trigonella foenum-graecum, Withania somnifera, and Ziziphus lotus. Nutrients 2025, 17, 411. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivastava, P.; Pandey, A.; Khanna, V.K.; Pant, A.B. Phytomedicine: A Potential Alternative Medicine in Controlling Neurological Disorders. In New Look to Phytomedicine; Khan, M.S.S., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; pp. 625–655. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Soni, V.; Raizada, P.; Singh, P.; Cuong, H.N.; Saini, A.; Saini, R.V.; Le, Q.V.; Nadda, A.K.; Le, T.T.; Nguyen, V.H. Sustainable and green trends in using plant extracts for the synthesis of biogenic metal nanoparticles toward environmental and pharmaceutical advances: A review. Environ. Res. 2021, 202, 111622. [Google Scholar] [CrossRef]
- Sarkar, A.; Sarkar, D.; Poddar, K. Plant metabolites as new leads to drug discovery: Approaches and challenges. In Medicinal Plants: Chemistry, Pharmacology, and Therapeutic Applications, 1st ed.; Swamy, M.K., Patra, J.K., Rudramurthy, G.R., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 61–70. [Google Scholar] [CrossRef]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef]
- Anywar, G.; Kakudidi, E.; Byamukama, R.; Mukonzo, J.; Schubert, A.; Oryem-Origa, H.; Jassoy, C. A Review of the Toxicity and Phytochemistry of Medicinal Plant Species Used by Herbalists in Treating People Living With HIV/AIDS in Uganda. Front. Pharmacol. 2021, 12, 615147. [Google Scholar] [CrossRef]
- Hamidi, M.R.; Jovanova, B.; Panovska, T.K. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014, 60, 9–18. [Google Scholar] [CrossRef]
- Ghica, A.; Tănase, M.L.; Niculițe, C.M.; Tocilă, A.; Popescu, L.; Luță, E.A.; Olaru, O.T.; Popovici, V.; Balaci, T.D.; Duțu, L.E.; et al. In Vitro Toxicity Evaluation of Some Plant Extracts and Their Potential Application in Xerosis cutis. Cosmetics 2024, 11, 124. [Google Scholar] [CrossRef]
- Mulaszynska, J.; Juchimiuk, J. Plant genotoxicity: A molecular cytogenetic approach in plant bioassays. Arh. Hig. Rada Toksikol. 2005, 56, 177–184. [Google Scholar] [PubMed]
- Turkez, H.; Arslan, M.E.; Ozdemir, O. Genotoxicity testing: Progress and prospects for the next decade. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1089–1098. [Google Scholar] [CrossRef]
- Morozesk, M.; Bonomo, M.M.; Souza, I.D.C.; Rocha, L.D.; Duarte, I.D.; Martins, I.O.; Dobbss, L.B.; Carneiro, M.T.; Fernandes, M.N.; Matsumoto, S.T. Effects of humic acids from landfill leachate on plants: An integrated approach using chemical, biochemical and cytogenetic analysis. Chemosphere 2017, 184, 309–317. [Google Scholar] [CrossRef]
- Onisan, E.; Sarac, I.; Petolescu, C.; Horablaga, M.N.; Mate, C.; Simina, A.; Camen, D.; Ganea, M.; Ardelean, D.R.; Călugar, L.; et al. Application of the Allium Test in Toxicity Studies of Lead and Copper: A Cytological Perspective. Appl. Sci. 2025, 15, 1491. [Google Scholar] [CrossRef]
- Şuţan, N.A.; Fierăscu, I.; Fierăscu, R.; Ionica, D.; Soare, L.C. Phytochemical analysis and in vitro assessment of Polystichum setiferum extracts for their cytotoxic and antimicrobial activities. Caryologia 2019, 72, 53–61. [Google Scholar] [CrossRef]
- Klein, P.; Chauvey, L.; Kallerhoff, J.; Pinelli, E.; Morard, M.; Silvestre, J. A Tool Derived from the Vicia faba Micronucleus Assay, to Assess Genotoxicity, Cytotoxicity or Biostimulation of Novel Compound used in Agriculture. Agronomy 2021, 11, 321. [Google Scholar] [CrossRef]
- Palm, E.R.; Guidi Nissim, W.; Adamcová, D.; Podlasek, A.; Jakimiuk, A.; Vaverková, M.D. Sinapis alba L. and Triticum aestivum L. as biotest model species for evaluating municipal solid waste leachate toxicity. J. Environ. Manag. 2022, 302, 114012. [Google Scholar] [CrossRef] [PubMed]
- Banti, C.N.; Hadjikakou, S.K. Evaluation of Genotoxicity by Micronucleus Assay In vitro and by Allium cepa test in vivo. Bio-protocol 2019, 9, e3311. [Google Scholar] [CrossRef]
- Santos, C.L.V.; Pourrut, B.; Ferreira de Oliveira, J.M.P. The use of comet assay in plant toxicology: Recent advances. Front. Genet. 2015, 6, 216. [Google Scholar] [CrossRef]
- Tedesco, B.S.; Laughinghouse, H.D., IV. Bioindicator of genotoxicity: The Allium cepa test. In Environmental Contamination; Srivastava, J.K., Ed.; IntechOpen Limited: London, UK, 2012; pp. 137–156. [Google Scholar] [CrossRef]
- Leme, D.M.; Marin-Morales, M.A. Allium cepa test in environmental monitoring: A review on its application. Mutat. Res. 2009, 682, 71–81. [Google Scholar] [CrossRef]
- Ciobanu, D.G. A Review About Phytotoxicity with a Focus on the Allium test. Biostudent 2019, 2, 65–74. Available online: https://cbg.uvt.ro/wp-content/uploads/2021/10/A-review-about-phytotoxicity-with-a-focus-on-the-Allium-testBIOSTUDENT_December2019_Ciobanu_65-74.pdf (accessed on 15 November 2024).
- Nicuță, D.; Grosu, L.; Alexa, I.-C.; Fînaru, A.-L. Sustainable Characterization of Some Extracts of Origanum vulgare L. and Biosafety Evaluation Using Allium cepa Assay. Horticulturae 2024, 10, 504. [Google Scholar] [CrossRef]
- Grosu, L.; Ferenț, E.; Nicuță, D.; Alexa, I.-C. Approach regarding the biosafety evaluation of black and red currant pomace extracts using Allium cepa test. Ovidius Univ. Ann. Chem. 2024, 35, 126–136. [Google Scholar] [CrossRef]
- de Melo, E.C.; da Silva Pinheiro, R.; Costa, B.S.; de Lima, R.M.T.; Dias, A.C.S.; de Jesus Aguiar dos Santos, T.; de Nascimento, M.L.L.B.; de Castro e Sousa, J.M.; Islam, M.T.; de Carvalho Melo Cavalcante, A.A.; et al. Allium cepa as a toxicogenetic investigational tool for plant extracts: A systematic review. Chem. Biodivers. 2024, 21, e202401406. [Google Scholar] [CrossRef] [PubMed]
- Levan, A. The effect of colchicine on root mitoses in Allium. Hereditas 1938, 24, 471–486. [Google Scholar] [CrossRef]
- Grant, W.F. Chromosome aberration assays in allium: A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat. Res. 1982, 99, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.F. The present status of higher plant bioassays for detection of environmental mutagens. Mutat. Res. 1994, 310, 175–185. [Google Scholar] [CrossRef]
- Fiskesjö, G. The Allium test as a standard in environmental monitoring. Hereditas 1985, 102, 99–112. [Google Scholar] [CrossRef]
- Fiskesjö, G. The Allium test—A potential standard protocol of assessment of environmental toxicity. In Environmental Toxicology and Risk Assessment; Gorsuch, J.W., Dwyer, F.J., Ingorsoll, C.G., La Point, T.W., Eds.; American Society for Testing and Materials: Philadelphia, PA, USA, 1993; Volume 2, pp. 331–345. [Google Scholar]
- Rank, J.; Nielsen, M.H. Evaluation of the Allium anaphase-telophase test in relation to genotoxicity screening of industrial wastewater. Mutat. Res. 1994, 312, 17–24. [Google Scholar] [CrossRef]
- Rasgele, P.G. The use of Allium cepa L. assay as bioindicator for the investigation of genotoxic effects of industrial waste water. Arch. Environ. Prot. 2021, 47, 3–8. [Google Scholar] [CrossRef]
- Iqbal, M.; Abbas, M.; Nisar, J.; Nazir, A. Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: A review. Chem. Int. 2019, 5, 1–80. Available online: https://ssrn.com/abstract=3407325 (accessed on 16 April 2025).
- Bonciu, E.; Firbas, P.; Fontanetti, C.S.; Wusheng, J.; Karaismailoğlu, M.C.; Liu, D.; Menicucci, F.; Pesnya, D.S.; Popescu, A.; Romanovsky, A.V.; et al. An evaluation for the standardization of the Allium cepa test as cytotoxicity and genotoxicity assay. Caryologia 2018, 71, 191–209. [Google Scholar] [CrossRef]
- Wijeyaratne, W.M.D.N.; Wadasinghe, L.G.Y.J.G. Allium cepa Bio Assay to Assess the Water and Sediment Cytogenotoxicity in a Tropical Stream Subjected to Multiple Point and Nonpoint Source Pollutants. J. Toxicol. 2019, 2019, 5420124. [Google Scholar] [CrossRef] [PubMed]
- Cauich-Suárez, L.Y.; Sánchez-Sánchez, V.E.; Moreno-Ortiz, G.; Noreña-Barroso, E.; Rodríguez-Fuentes, G. Bioassays with Allium cepa for the Monitoring of Toxicity in the Groundwater of Yucatan, Mexico. Appl. Sci. 2024, 14, 11428. [Google Scholar] [CrossRef]
- Sabeen, M.; Mahmood, Q.; Bhatti, Z.A.; Faridullah; Irshad, M.; Bilal, M.; Hayat, M.T.; Irshad, U.; Akbar, T.A.; Arslan, M.; et al. Allium cepa assay based comparative study of selected vegetables and the chromosomal aberrations due to heavy metal accumulation. Saudi J. Biol. Sci. 2020, 27, 1368–1374. [Google Scholar] [CrossRef]
- Taychew, A.; Kerisew, B. Assessment of Cytotoxicity and Genotoxicity Potential of Effluents from Bahir Dar Tannery Using Allium cepa. Adv. Public Health. 2022, 2022, 5519304. [Google Scholar] [CrossRef]
- Wijeyaratne, W.M.D.N.; Wickramasinghe, P.G.M.U. Chromosomal Abnormalities in Allium cepa Induced by Treated Textile Effluents: Spatial and Temporal Variations. J. Toxicol. 2020, 2020, 8814196. [Google Scholar] [CrossRef]
- Hassan, G.M.; Yassein, A.A.M. Cytogenotoxicity Evaluation of Water Contaminated with Some Textile Azo Dyes Using Rapd Markers and Chromosomal Aberrations of Onion (Allium cepa) Root Cells. Egypt. J. Genet. Cytol. 2014, 43, 39–57. [Google Scholar] [CrossRef]
- Mohammed, J.S.; Mustapha, Y.; Him, M.A.; Danladi, Z.N. Assessment of Cytogenotoxicity of Plastic Industrial Effluent Using Allium cepa Root Tip Cells. Int. J. Cell Biol. 2023, 2023, 5161017. [Google Scholar] [CrossRef]
- Pathiratne, A.; Hemachandra, C.K.; De Silva, N. Efficacy of Allium cepa test system for screening cytotoxicity and genotoxicity of industrial effluents originated from different industrial activities. Environ. Monit. Assess. 2015, 187, 730. [Google Scholar] [CrossRef]
- Datcu, A.-D.; Ciobanu, D.-G.; Boros, B.-V.; Ostafe, V.; Ianovici, N. A new approach for phytotoxicity testing using Allium cepa bulbs. Rom. Biotechnol Lett. 2020, 25, 1488–1494. [Google Scholar] [CrossRef]
- Camilo-Cotrim, C.F.; Bailão, E.F.L.C.; Ondei, L.S.; Carneiro, F.M.; Almeida, L.M. What can the Allium cepa test say about pesticide safety? A review. Environ. Sci. Pollut. Res. 2022, 29, 48088–48104. [Google Scholar] [CrossRef]
- Feretti, D.; Zerbini, I.; Zani, C.; Ceretti, E.; Moretti, M.; Monarca, S. Allium cepa chromosome aberration and micronucleus tests applied to study genotoxicity of extracts from pesticide-treated vegetables and grapes. Food Addit. Contam. 2007, 24, 561–572. [Google Scholar] [CrossRef]
- Kuchy, A.H.; Wani, A.A.; Kamili, A.N. Cytogenetic effects of three commercially formulated pesticides on somatic and germ cells of Allium cepa. Environ. Sci. Pollut. Res. 2016, 23, 6895–6906. [Google Scholar] [CrossRef]
- Bianchi, J.; Fernandes, T.C.C.; Marin-Morales, M.A. Induction of mitotic and chromosomal abnormalities on Allium cepa cells by pesticides imidacloprid and sulfentrazone and the mixture of them. Chemosphere 2016, 144, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Çıldır, D.S.; Liman, R. Cytogenetic and genotoxic assessment in Allium cepa exposed to imazalil fungicide. Environ. Sci. Pollut. Res. 2020, 27, 20335–20343. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Y.A.; Christofoletti, C.A.; Pedro, J.; Bueno, O.C.; Malaspina, O.; Ferreira, R.A.; Fontanetti, C.S. Allium cepa and Tradescantia pallida bioassays to evaluate effects of the insecticide imidacloprid. Chemosphere 2015, 120, 438–442. [Google Scholar] [CrossRef]
- Animasaun, D.A.; Adedibu, P.A.; Afolabi, S.O.; Abdulkareem, K.A.; Ibrahim, S.; Krishnamurthy, R. Hazard assessment and cytogenotoxic effect of different concentrations of mercury chloride sterilant using the Allium cepa assay. Discov. Toxicol. 2024, 1, 2. [Google Scholar] [CrossRef]
- Khalil, A.M.; Salman, W.K.; Al-Qaoud, K.M. Preliminary evaluation of acute cytogenotoxicity of a novel phenylboronic acid derivative; 2- (bromoacetamido) phenylboronic acid using the Allium cepa chromosome aberrations assay. Caryologia 2016, 70, 34–41. [Google Scholar] [CrossRef]
- Das, D.; Mitra, P.K.; Gupta, S. Evaluation of cytotoxicity indiced by the anti-cancerous drugs doxorubicin and erlotinib in Allium cepa assay for eco-safety monitoring. Cytologia 2021, 86, 19–199. [Google Scholar] [CrossRef]
- Alias, C.; Feretti, D.; Viola, G.V.C.; Zerbini, I.; Bisceglie, F.; Pelosi, G.; Zani, C.; Aflatox group. Allium cepa tests: A plant-based tool for the early evaluation of toxicity and genotoxicity of newly synthetized molecules. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2023, 889, 503654. [Google Scholar] [CrossRef]
- Diniz, J.S.; Souza-Silva, G.D.; De Souza, C.R.; De Paula Freitas, L.A.; Souki Parreira, A.L.; Rocha Pena, B.; Mol, M.P.G.; Silveira, M.R. Cytotoxicity, genotoxicity, and mutagenicity of the active pharmaceutical ingredient nevirapine and a nevirapine-based drug on the plant species Allium cepa. Ann. Environ. Sci. Toxicol. 2023, 7, 025–033. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Siam, M.S.H.; Ahamed, M.R.; Roy, U.K.; Hossain, M.I.; Rokonuzzman, M.; Islam, T.; Sharafat, R.; Bappi, M.H.; Mia, M.N.; et al. Toxicity Analysis of Some Frequently Used Food Processing Chemicals Using Allium cepa Biomonitoring System. Biology 2023, 12, 637. [Google Scholar] [CrossRef]
- Rosculete, E.; Olaru, A.L.; Rosculete, C.A.; Bonciu, E. Assessment of cytological effects of food preservative potassium metabisulphite to Allium cepa. Am. J. Plant Sci. 2020, 11, 11–23. [Google Scholar] [CrossRef]
- Tkachuk, N.; Zelena, L. An onion (Allium cepa L.) as a test plant. Biota. Hum. Technol. 2022, 3, 50–59. [Google Scholar] [CrossRef]
- Dragoeva, A.P.; Koleva, V.P.; Nanova, Z.D.; Kaschieva, M.Z. Allelopathy of cold water extracts from Origanum vulgare ssp. vulgare L. J. Agric. Chem. Environ. 2014, 3, 144–150. [Google Scholar] [CrossRef]
- Dragoeva, A.P.; Koleva, V.P.; Nanova, Z.D.; Kaschieva, M.Z.; Yotova, I.R. Allelopathic and cytotoxic activity of Origanum vulgare ssp. vulgare growing wild in Bulgaria. Chem. Bulg. J. Sci. Educ. 2014, 23, 914–924. Available online: https://www.researchgate.net/publication/287350745 (accessed on 20 March 2025).
- Valente, P.M.; Valente, V.M.M.; Silva, M.C.; dos Reis, L.B.; Silva, F.D.; Praça-Fontes, M.M. Phytotoxicity and cytogenotoxicity of Dionaea muscipula Ellis extracts and its major compound against Lactuca sativa and Allium cepa. Biologia 2022, 77, 2975–2988. [Google Scholar] [CrossRef]
- Felicidade, I.; Lima, J.D.; Pesarini, J.R.; Monreal, A.C.; Mantovani, M.S.; Ribeiro, L.R.; Oliveira, R.J. Mutagenic and antimutagenic effects of aqueous extract of rosemary (Rosmarinus officinalis L.) on meristematic cells of Allium cepa. Genet. Mol. Res. 2014, 13, 9986–9996. [Google Scholar] [CrossRef]
- Wierzbicka, M. An improved method of preparing onion bulbs for Allium test. Acta Soc. Bot. Pol. 1987, 56, 43–53. [Google Scholar] [CrossRef]
- Akinboro, A.; Bakare, A.A. Cytotoxic and genotoxic effects of aqueous extracts of five medicinal plants on Allium cepa Linn. J. Ethnopharmacol. 2007, 112, 470–475. [Google Scholar] [CrossRef]
- Aşkin Celik, T.; Aslantürk, O.S. Evaluation of Cytotoxicity and Genotoxicity of Inula viscosa Leaf Extracts with Allium Test. J. Biomed. Biotechnol. 2010, 2010, 189252. [Google Scholar] [CrossRef]
- Chukwujekwu, J.C.; Van Staden, J. Cytotoxic and genotoxic effects of water extract of Distephanus angulifolius on Allium cepa Linn. S. Afr. J. Bot. 2014, 92, 147–150. [Google Scholar] [CrossRef]
- Ihegboro, G.O.; Alhassan, A.J.; Ononamadu, C.J.; Owolarafe, T.A.; Sule, M.S. Evaluation of the biosafety potentials of methanol extracts/fractions of Tapinanthus bangwensis and Moringa oleifera leaves using Allium cepa model. Toxicol. Rep. 2020, 7, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Owolarafe, T.A.; Salawu, K.; Ihegboro, G.O.; Ononamadu, C.J.; Alhassan, A.J.; Wudil, A.M. Investigation of cytotoxicity potential of different extracts of Ziziphus mauritiana (Lam) leaf Allium cepa model. Toxicol. Rep. 2020, 7, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Yuet Ping, K.; Darah, I.; Yusuf, U.K.; Yeng, C.; Sasidharan, S. Genotoxicity of Euphorbia hirta: An Allium cepa Assay. Molecules 2012, 17, 7782–7791. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.W.; de Freitas, J.M.B.; Funk, N.L.; de Oliva Araujo, L.C.; Frescura, V.; do Canto-Dorow, T.S.; da Silva, C.B.; Andriolo, J.L.; Hister, C.; Tedesco, S. Cytogenotoxicity of Basil (Ocimum basilicum ‘Basilicão’) by Allium cepa Test under Saline Stress Induction. Braz. Arch. Biol. Technol. 2024, 67, e24231031. [Google Scholar] [CrossRef]
- Akinboro, A.; Baharudeen, I.; Mohamed, K. Evaluation of cytogenotoxic and antimutagenic potency of water extract of Centella asiatica Linn. using the Allium cepa assay. Int. Food Res. J. 2016, 23, 2449–2452. Available online: http://www.ifrj.upm.edu.my (accessed on 28 March 2025).
- Sabini, M.C.; Cariddi, L.N.; Escobar, F.M.; Bachetti, R.A.; Sutil, S.B.; Contigiani, M.S.; Zanon, S.M.; Sabini, L.I. Evaluation of Cytogenotoxic Effects of Cold Aqueous Extract from Achyrocline satureioides by Allium cepa L test. Nat. Prod. Commun. 2011, 6, 995–998. [Google Scholar] [CrossRef]
- Dey, A.; Hazra, A.K.; Mukherjee, A.; Nandy, S.; Pandey, D.K. Chemotaxonomy of the ethnic antidote Aristolochia indica for aristolochic acid content: Implications of anti-phospholipase activity and genotoxicity study. J. Ethnopharmacol. 2021, 266, 113416. [Google Scholar] [CrossRef]
- Dey, A.; Hazra, A.K.; Nandy, S.; Kaur, P.; Pandey, D.K. Selection of elite germplasms for industrially viable medicinal crop Bacopa monnieri for bacoside A production: An HPTLC-coupled chemotaxonomic study. Ind. Crops Prod. 2020, 158, 112975. [Google Scholar] [CrossRef]
- Şuţan, N.A.; Fierăscu, I.; Fierăscu, R.C.; Manolescu, D.Ş.; Soare, L.C. Comparative analytical characterization and in vitro cytogenotoxic activity evaluation of Asplenium scolopendrium L. leaves and rhizome extracts prior to and after Ag nanoparticles phytosynthesis. Indust. Crops Prod. 2016, 83, 379–386. [Google Scholar] [CrossRef]
- Ouzid, Y.; Kaci-Boudiaf, M.N.; Zeghouini, A.; Madi, A.-O.; Smail-Saadoun, N.; Houali, K. Antimitotic and genotoxic effect of methanolic extracts of leaves of Peganum harmala L. on the meristematic cells of Allium cepa L. Bioagro 2023, 35, 97–104. [Google Scholar] [CrossRef]
- Souza, L.F.B.; Laughinghouse, H.D., IV; Pastori, T.; Tedesco, M.; Kuhn, A.W.; Canto-Dorow, T.S.; Tedesco, S.B. Genotoxic potential of aqueous extracts of Artemisia verlotorum on the cell cycle of Allium cepa. Int. J. Environ. Stud. 2010, 67, 871–877. [Google Scholar] [CrossRef]
- Frescura, V.D.S.; Laughinghouse, H.D., IV; Tedesco, S.B. Antiproliferative effect of the tree and medicinal species Luehea divaricata on the Allium cepa cell cycle. Caryologia 2012, 65, 27–33. [Google Scholar] [CrossRef][Green Version]
- Han, C.; Shao, H.; Zhou, S.; Mei, Y.; Cheng, Z.; Huang, L.; Lv, G. Chemical composition and phytotoxicity of essential oil from invasive plant, Ambrosia artemisiifolia L. Ecotoxicol. Environ. Saf. 2021, 211, 111879. [Google Scholar] [CrossRef]
- Senna Pereira, J.; Aparecida Hister, C.; Ubessi, C.; Bosio Tedesco, S. Genotoxicity, Cytotoxicity and Phenolic Compounds from Aqueous Extracts of Phyllanthus tenellus Roxb. Cultivated Under Different Light Conditions. Pak. J. Biol. Sci. 2022, 25, 575–585. Available online: https://scialert.net/fulltext/?doi=pjbs.2022.575.585 (accessed on 6 July 2025).
- Ubessi, C.; Tedesco, B.S.; da Silva, C.B.; Baldoni, M.; Krysczun, D.K.; Heinzmann, B.M.; Rosa, I.A.; Mori, N.C. Antiproliferative potential and phenolic compounds of infusions and essential oil of chamomile cultivated with homeopathy. J. Ethnopharmacol. 2019, 239, 111907. [Google Scholar] [CrossRef]
- Ikechukwu, E.C.; Agu, P.N.; Olumuji, H.B.; Anagboso, M.O.; Johnny, I.I.; Okokon, J.E.; Ebong, N.O. Evaluation of Genotoxic and Cytotoxic Activities of Three Vegetables (Heinsia crinata, Justicia insularis and Lasianthera africana) Using Allium cepa Test. Asian J. Biochem. Gen. Mol. Biol. 2024, 16, 10–20. [Google Scholar] [CrossRef]
- Okokon, J.E.; Osigwe, C.C.; Uwaeme, U.F.; Johnny, I.I.; Gabriel, J.; Udo, I.J. Evaluation of genotoxic and cytotoxic activities of Solenostemon monostachyus (P. Beauv.) Brig. (Lamiaceae) using Allium cepa test. Niger. J. Pharm. Appl. Sci. Res. 2025, 14, 1–8. [Google Scholar]
- Santana, G.M.; Deus, M.S.M.; Sousa, J.M.C.; Ferreira, P.M.P.; Fernandes, H.B.; Peron, A.P. Antimitotic and antimutagenic action of the Hymenaea stigonocarpa bark on dividing cells. Braz. J. Biol. 2016, 76, 520–525. [Google Scholar] [CrossRef][Green Version]
- Sharma, A.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Chemical profiling, cytotoxicity and phytotoxicity of foliar volatiles of Hyptis suaveolens. Ecotoxicol. Environ. Saf. 2019, 171, 863–870. [Google Scholar] [CrossRef]
- Rossato, L.V.; Tedesco, S.B.; Laughinghouse, H.D., IV; Farias, J.G.; Nicoloso, F.T. Alterations in the mitotic index of Allium cepa induced by infusions of Pluchea sagittalis submitted to three different cultivation systems. An. Acad. Bras. Ciênc. 2010, 82, 857–860. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento Cavalcante, A.; Lima, L.K.F.; Araújo, C.M.; da Silva Santos, F.P.; do Nascimento, M.O.; de Castro E Sousa, J.M.; Rai, M.; Feitosa, C.M. Toxicity, cytotoxicity, mutagenicity and in vitro antioxidant models of 2-oleyl-1,3-dipalmitoyl-glycerol isolated from the hexane extract of Platonia insignis MART seeds. Toxicol. Rep. 2020, 7, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Alabi, O.A.; Atanda, H.C.; Olumurewa, J.A.V. Cytogenotoxicity of the aqueous extract of Parquetina nigrescens leaf using Allium cepa assay. Protoplasma 2022, 259, 1417–1425. [Google Scholar] [CrossRef]
- Akarsha, B.; Krishnakumar, G. Genotoxic and antigenotoxic potential of the Lagenandra toxicaria Dalz. rhizome methanol extract using Allium cepa assay. Asian J. Pharm. Clin. Res. 2021, 14, 82–90. [Google Scholar] [CrossRef]
- Almeida, L.M.; Prado, A.D.; Xavier-Silva, K.R.; Firmino, M.T.; Paula, M.I.; Gomes, P.N.; Paula, J.A.; Bailão, E.F. Cytotoxic effect of Vernonanthura polyanthes leaves aqueous extracts. Braz. J. Biol. 2021, 81, 575–583. [Google Scholar] [CrossRef]
- Mesic, A.; Mahmutović-Dizdarević, I.; Tahirović, E.; Durmišević, I.; Eminovic, I.; Jerković-Mujkić, A.; Bešta-Gajević, R. Evaluation of toxicological and antimicrobial activity of lavender and immortelle essential oils. Drug. Chem. Toxicol. 2021, 44, 190–197. [Google Scholar] [CrossRef]
- Tóth, G.; Háhn, J.; Radó, J.; Szalai, A.D.; Kriszt, B.; Szoboszlay, S. Cytotoxicity and hormonal activity of glyphosate-based herbicides. Environ. Pollut. 2020, 265, 115027. [Google Scholar] [CrossRef]
- Mercado, S.A.S.; Caleño, J.D.Q. Cytotoxic evaluation of glyphosate, using Allium cepa L. as bioindicator. Sci. Total Environ. 2020, 700, 134452. [Google Scholar] [CrossRef]
- Hister, C.A.; Laughinghouse, H.D.; da Silva, C.B.; Dorow, T.S.; Tedesco, S.B. Evaluation of the Antiproliferative Effect of Infusions and Essential Oil of Aloysia gratissima. Pak. J. Biol. Sci. 2009, 12, 1581–1584. [Google Scholar] [CrossRef]
- Grondona, E.; Gatti, G.; López, A.G.; Sánchez, L.R.; Rivero, V.; Pessah, O.; Zunino, M.P.; Ponce, A.A. Bio-efficacy of the Essential Oil of Oregano (Origanum vulgare Lamiaceae. Ssp. Hirtum). Plant Foods Hum. Nutri. 2014, 69, 351–357. [Google Scholar] [CrossRef]
- Sangur, K.; Smith, A.; Tomasoa, M. The mitotic index of Cajanus cajan from Kisar island, in the Southwest of Maluku. Biosaintifika J. Biol. Biol. Educ. 2021, 13, 128–134. [Google Scholar] [CrossRef]
- Matagne, R. Duration of mitotic cycle and patterns of DNA replication in chromosomes of Allium cepa. Caryologia 1968, 21, 209–224. [Google Scholar] [CrossRef]
- Timothy, O.; Idu, M.; Olorunfemi, D.I.; Ovuakporie-Uvo, O. Cytotoxic and genotoxic properties of leaf extract of Icacina trichantha Oliv. S. Afr. J. Bot. 2014, 91, 71–74. [Google Scholar] [CrossRef]
- Bilonozhko, Y.; Shut, T.; Krupodorova, T.; Pirko, N.; Holubchak, O.; Pryvalikhin, S.; Lykholat, O.; Pirko, Y. Impact of aqueous extract of Viscum album on different organisms. Regul. Mech. Biosyst. 2023, 14, 432–438. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, A. Chromosomes and the mitotic cell cycle phase in onion roots. Int. Internal Med. J. 2023, 1, 224–228. [Google Scholar]
- Ria Das, R.; Goswami, S.; Ghosh, P.; Roy, S.; Ray, S. Cytotoxic and Cytostatic Effects of of Crinum asiaticum in Allium cepa Root Apical Meristem Cells. Cytologia 2022, 87, 107–112. [Google Scholar] [CrossRef]
- Yajía, M.E.; Martí, D.A.; Bidau, C.J.; Amat, A.G.; Riglos, A.G.; Silvestroni, A. Genotoxicity evaluation of Allophylus edulis (Camb.) Radlk. (Sapindaceae) aqueous extract. Acta Hortic. 1999, 501, 31–36. [Google Scholar] [CrossRef]
- Peron, A.P.; Mariucci, R.G.; de Almeida, I.V.; Düsman, E.; Manto-vani, M.S.; Vicentini, V.E. Evaluation of the cytotoxicity, mutagenicity and antimutagenicity of a natural antidepressant, Hypericum perforatum L. (St. John’s wort), on vegetal and animal test systems. BMC Complement Altern. Med. 2013, 13, 97. [Google Scholar] [CrossRef]
- Vicentini, V.E.P.; Camparoto, M.L.; Teixeira, R.O.; Mantovani, M.S. Averrhoa carambola L., Syzygium cumini (L.) Skeels and Cissus sicyoides L.: Medicinal herbal tea effects on vegetal and animal test systems. Acta Sci. Agron. 2001, 23, 593–598. [Google Scholar]
- Üstündag, Ü.; Macar, O.; Kalefetoğlu Macar, T.; Yalcin, E.; Cavusoglu, K. Effect of Melissa officinalis L. leaf extract on manganese-induced cyto-genotoxicity on Allium cepa L. Sci. Rep. 2023, 13, 22110. [Google Scholar] [CrossRef]
- Yekeen, T.A.; Azeez, M.A.; Lateef, A.; Asafa, T.B.; Oladipo, I.C.; Badmus, J.A.; Adejumo, S.A.; Ajibola, A.A. Cytogenotoxicity potentials of cocoa pod and bean-mediated green synthesized silver nanoparticles on Allium cepa cells. Caryologia 2017, 70, 366–377. [Google Scholar] [CrossRef]
- Schwarzacher, T.; Liu, Q.; (Pat) Heslop-Harrison, J.S. Plant Cytogenetics: From Chromosomes to Cytogenomics. In Plant Cytogenetics and Cytogenomics. Methods in Molecular Biology; Heitkam, T., Garcia, S., Eds.; Humana: New York, NY, USA, 2003; Volume 2672, pp. 3–21. [Google Scholar] [CrossRef]
- Eren, Y.; Özata, A. Determination of mutagenic and cytotoxic effects of Limonium globuliferum aqueous extracts by Allium, Ames, and MTT tests. Rev. Bras. Farmacogn. 2014, 24, 51–59. [Google Scholar] [CrossRef]
- Neves, C.S.; Gomes, S.S.L.; dos Santos, T.R.; de Almeida, M.M.; de Souza, Y.O.; Garcia, R.M.G.; Otoni, W.C.; Chedier, L.M.; Raposo, N.R.B.; Viccini, L.F.; et al. “Brazilian ginseng” (Pfaffia glomerata Spreng. Pedersen, Amaranthaceae) methanolic extract: Cytogenotoxicity in animal and plant assays. S. Afr. J. Bot. 2016, 106, 174–180. [Google Scholar] [CrossRef]
- Firbas, P.; Amon, T. Chromosome damage studies in the onion plant Allium cepa L. Caryologia 2014, 67, 25–35. [Google Scholar] [CrossRef]
- de Sousa, W.C.; Paz, A.N.T.; Rocha, J.D.; da Conceição, E.C.; de Almeida, L.M.; Chen, L.C.; Borges, L.L.; Bailão, E.F.L.C. In vivo assessment of cyto/genotoxic, antigenotoxic and antifungal potential of Costus spiralis (Jacq.) Roscoe leaves and stems. An. Acad. Bras. Cienc. 2018, 90, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Lubini, G.; Fachinetto, J.M.; Laughinghouse, H.D., IV; Paranhos, J.T.; Silva, A.C.F.; Tedesco, S.B. Extracts affecting mitotic division in root-tip meristematic cells. Biologia 2008, 63, 647–651. [Google Scholar] [CrossRef]
- Bagatini, M.D.; Fachinetto, J.M.; da Silva, A.C.F.; Tedesco, S.B. Cytotoxic effects of infusions (tea) of Solidago microglossa DC. (Asteraceae) on the cell cycle of Allium cepa. Rev. Bras. Pharmacogn. 2009, 19, 632–636. [Google Scholar] [CrossRef]
- Barman, M.; Roy, S.; Ray, S. Colchicine like metaphase and cell cycle delay inducing effects of leaf aqueous extract of Clerodendrum inerme L. Gaertn. in Allium cepa root apical meristem cells. Cytologia 2020, 85, 197–201. [Google Scholar] [CrossRef]
- Pawlowski, A.; Kaltchuk-Santos, E.; Brasil, M.C.; Caramão, E.B.; Zini, C.A.; Soares, G.L.G. Chemical composition of Schinus lentiscifolius March. essential oil and its phytotoxic and cytotoxic effects on lettuce and onion. S. Afr. J. Bot. 2013, 88, 198–203. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Kuttithodi, A.M.; Alfarhan, A.; Rajagopal, R.; Barcelo, D. Chemical Composition, Insecticidal and Mosquito Larvicidal Activities of Allspice (Pimenta dioica) Essential Oil. Molecules 2021, 26, 6698. [Google Scholar] [CrossRef] [PubMed]
- Rathnasamy, S.; Kamaruzaman, B.M.; Sulaiman, S.F.; Akinboro, A. Evaluation of cytotoxic, mutagenic and antimutagenic potential of leaf extracts of three medicinal plants using Allium cepa chromosome assay. Int. Curr. Pharm. J. 2013, 2, 131–140. [Google Scholar] [CrossRef]
- Schreiner, G.E.; Eckert, G.L.; Schuster, M.F.; Baroni, S.; de Pelegrin, C.M.G.; Dartora, N. Cytotoxic and genotoxic effects of aqueous extracts of Aloysia gratissima (Gillies & Hook.) Tronc. using Allium cepa L. assay. Pharmacol. Res. 2024, 2, 100011. [Google Scholar] [CrossRef]
- de Sousa, M.A.A.; Silva, F.S.L.; Orlanda, J.F.F. Genotoxic and antiproliferative effects of Alpinia zerumbet (Zingiberaceae) essential oil in Allium cepa biotest. Ciênc. Nat. 2024, 46, e73445. [Google Scholar] [CrossRef]
- Prajitha, V.; Thoppil, J.E. Genotoxic and antigenotoxic potential of the aqueous leaf extracts of Amaranthus spinosus L. using Allium cepa assay. S. Afr. J. Bot. 2016, 102, 18–25. [Google Scholar] [CrossRef]
- Sultan, A.O.; Çelik, T.A. Genotoxic and Antimutagenic Effects of Capparis spinosa L. on the Allium cepa L. Root Tip Meristem Cells. Caryologia 2009, 62, 114–123. [Google Scholar] [CrossRef]
- Fagodia, S.K.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytotoxicity and cytotoxicity of Citrus aurantiifolia essential oil and its major constituents: Limonene and citral. Ind. Crops Prod. 2017, 108, 708–715. [Google Scholar] [CrossRef]
- Roy, A.; Roy, S. Assessment of Cytotoxic Effects of Aqueous and Methanolic Leaf Extracts of Clerodendrum inerme (L.) Gaertn. and C. viscosum Vent. Using Allium Test. Cytologia 2019, 84, 73–76. [Google Scholar] [CrossRef]
- Annapurna, A.S.; Abhirami, D.; Umesh, T.G. Comparative study of phytochemicals and bioactivities of the leaf extracts of Curcuma amada and Curcuma karnatakensis. S. Afr. J. Bot. 2021, 142, 441–450. [Google Scholar] [CrossRef]
- Silva, D.S.B.S.; Barboza, B.; Garcia, A.C.F.S.; de Oliveira, B.; Estevam, C.S.; Neto, V.A.; Santos, A.L.L.M.; Dias, A.S.; Scher, R.; Pantaleao, S.M. Investigation of protective effects of Erythrina velutina extract against MMS induced damages in the root meristem cells of Allium cepa. Rev. Bras. Farmacogn. 2013, 23, 273–278. [Google Scholar] [CrossRef]
- Raheel, R.M.; Saddiqe, Z.; Iram, M.; Afzal, S. In vitro antimitotic, antiproliferative and antioxidant activity of stem bark extracts of Ficus benghalensis L. S. Afr. J. Bot. 2017, 111, 248–257. [Google Scholar] [CrossRef]
- Shilpa, K.J.; Gopi, K.P.; Muzhapravan, A.; Prabhakaran, K. Evaluation of cytotoxicity potential of Garcinia cambogia (Gaertn.) Desr. male flower extract using Allium cepa model. Adv. Pharm. J. 2023, 8, 136–141. [Google Scholar] [CrossRef]
- Akwu, N.; Naidoo, Y.; Singh, M. Cytogenotoxic and biological evaluation of the aqueous extracts of Grewia lasiocarpa: An Allium cepa assay. S. Afr. J. Bot. 2019, 125, 371–380. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Sousa, T.R.; Arruda, A.S.; Peixoto, N.; Gonçalves, P.J.; Almeida, L.M. Evaluation of cytotoxicity and genotoxicity of Hancornia speciosa latex in Allium cepa root model. Braz. J. Biol. 2016, 76, 245–249. [Google Scholar] [CrossRef]
- Lacerda, L.P.; Malaquias, G.; Peron, A.P. Antiproliferative action of aqueous extracts of Hymenaea stigonocarpa Mart. (Fabaceae) on the cell cycle of Allium cepa L. An. Acad. Bras. Ciênc. 2014, 86, 1147–1150. [Google Scholar] [CrossRef][Green Version]
- da Silva, L.M.; de Sousa Carvalho, F.R.; Martins, L.; de Barros Fernandes, H.; Calou, I.B.F.; Peron, A.P. Antiproliferative effect of the hydroalcoholic extract of Hymenaea stigonocarpa Mart. ex Hayne (Fabaceae, Caesalpinioideae) on the cell cycle of roots of Allium cepa L. Biotemas 2015, 28, 45–49. [Google Scholar] [CrossRef][Green Version]
- Bidau, C.J.; Amat, A.G.; Yajía, M.; Martí, D.A.; Riglos, A.G. Silvestroni: Evaluation of the Genotoxicity of Aqueous Extracts of Ilex paraguariensis St. Hil. (Aquifoliaceae) Using the Allium Test. Cytologia 2004, 69, 109–117. [Google Scholar] [CrossRef][Green Version]
- Ciappina, A.L.; Ferreira, F.A.; Pereira, I.R.; Sousa, T.R.; Matos, F.S.; de Melo-Reis, P.R.; Goncalves, P.J.; Bailão, E.F.L.C.; Almeida, L.M. Toxicity of Jatropha curcas L. latex in Allium cepa test. Biosci. J. 2017, 33, 1295–1304. [Google Scholar] [CrossRef]
- Eckert, G.L.; Smaniotto, T.A.; Dartora, N.; Pelegrin, C.M.G.; Baroni, S. The chemical composition of different leaf extracts of Lantana fucata Lindl. influences its cytotoxic potential: A study using the Allium cepa model. J. Ethnopharmacol. 2022, 289, 115003. [Google Scholar] [CrossRef]
- Barman, A.; Ray, S. Mitotic Index Reduction and Cytotoxic Effects of Leaf Aqueous Extract of Maesa macrophylla (Wall.) A. DC. in Allium cepa Root Tip Cells. Cytologia, 2022; 87, 81–85. [Google Scholar] [CrossRef]
- Camparoto, M.L.; Teixeira, R.O.; Mantovani, M.S.; Vicentini, V.E.P. Effects of Maytenus ilicifolia Mart. and Bauhinia candicans Benth infusions on onion root-tip and rat bone-marrow cells. Genet. Mol. Biol. 2002, 25, 85–89. [Google Scholar] [CrossRef]
- Araújo, M.S.; Santos, S.P.; Barros-Filho, B.A.; Lima, M.M.O.; Leite, A.S. Toxicogenetic potential of Mimosa pigra (Fabaceae) infusion in Allium cepa meristematic cells. Genet. Mol. Res. 2020, 19, gmr18588. [Google Scholar] [CrossRef]
- Raj, G.G.; Varghese, H.S.; Kotagiri, S.; Swamy, B.V.; Swamy, A.; Pathan, R.K. Pathan Anticancer Studies of Aqueous Extract of Roots and Leaves of Pandanus Odoratissimus f.ferreus (Y. Kimura) Hatus: An In Vitro Approach. J. Trad. Complement Med. 2014, 4, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Neves, E.S.B.; Ferreira, P.M.P.; Lima, L.H.; Peron, A.P. Action of Aqueous Extracts of Phyllanthus niruri L. (Euphorbiaceae) leaves on Meristematic Root Cells of Allium cepa L. An. Acad. Bras. Ciênc. 2014, 86, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Macovei, I.; Luca, S.V.; Skalicka-Woźniak, K.; Sacarescu, L.; Pascariu, P.; Ghilan, A.; Doroftei, F.; Ursu, E.L.; Rimbu, C.M.; Horhogea, C.E.; et al. Phyto-Functionalized Silver Nanoparticles Derived from Conifer Bark Extracts and Evaluation of Their Antimicrobial and Cytogenotoxic Effects. Molecules 2022, 27, 217. [Google Scholar] [CrossRef]
- Aşkin Çelik, T.; Aslantürk, O.S. Anti-mitotic and anti-genotoxic effects of Plantago lanceolata aqueous extract on Allium cepa root tip meristem cells. Biologia 2006, 61, 693–697. [Google Scholar] [CrossRef]
- Sheela, A.; Thoppil, J.E. Evaluation of cytotoxicity of the aqueous leaf extract of Pogostemon heyneanus Benth. (Java Patchouli). Biotropia 2017, 24, 28–34. [Google Scholar] [CrossRef]
- Pereira, M.L.; Monteiro, C.N.; Siqueira, C.; Ribeiro, M.S.; Lopes, A.P.; Sousa, R.; Oliveira, M.; Júnior, J.; Martins, F.A.; Almeida, P.M. Evaluation of effects of Poincianella bracteosa (Tul.) L.P. Queiroz leaves in Allium cepa and Mus musculus. Biotech Histochem. 2020, 95, 464–473. [Google Scholar] [CrossRef]
- Teixeira, R.O.; Camparoto, M.L.; Mantovani, M.S.; Vicentini, V.E.P. Assesment of two medicinal plants Psidium guajava L. and Achillea millefolium L., in in vitro and in vivo assays. Genet. Mol. Biol. 2003, 26, 551–555. [Google Scholar] [CrossRef][Green Version]
- Knoll, M.F.; Silva, A.C.F.; Tedesco, S.B.; Canto-Dorow, T.S. Effects of Pterocaulon polystachyum DC. (Asteraceae) on onion (Allium cepa) root-tip cells. Genet. Mol. Biol. 2006, 29, 539–542. [Google Scholar] [CrossRef]
- Alves, S.F.; Gomes, C.M.; de Oliveira, M.G.; de Andrade, W.M.; Moreira, L.C.; Borges, L.L.; da Silva, C.C.; de Souza, G.O.; da Silva, V.B.; Valadares, M.C.; et al. Cytotoxicity, phagocytic activity, and leishmanicidal potential of extract standardized in geranylgeraniol obtained from the fruit of Pterodon emarginatus vogel. Pharmacogn. Mag. 2020, 16, 140–147. [Google Scholar] [CrossRef]
- Viel, A.M.; Silva, L.P.; Martins, G.R.; Urtremari, B.; Sekiya, A.; Dokkedal, A.L.; de Souza, E.B.; Camargo, I.C.; Silva, R.M. Toxicological, genotoxic and antioxidant potential of Pyrostegia venusta. Biosci. J. 2019, 35, 570–585. [Google Scholar] [CrossRef]
- Cardoso, G.H.S.; Dantas, E.B.S.; Sousa, F.R.C.; Peron, A.P. Cytotoxicity of aqueous extracts of Rosmarinus officinalis L. (Labiatae) in plant test system. Braz. J. Biol. 2014, 74, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, A.; Kaltchuk-Santos, E.; Zini, C.A.; Caramão, E.B.; Soares, G.L.G. Essential oils of Schinus terebinthifolius and S. molle (Anacardiaceae): Mitodepressive and aneugenic inducers in onion and lettuce root meristems. S. Afr. J. Bot. 2012, 80, 96–103. [Google Scholar] [CrossRef]
- Oyeyemi, I.T.; Bakare, A.A. Genotoxic and anti-genotoxic effect of aqueous extracts of Spondias mombin L., Nymphea lotus L. and Luffa cylindrica L. on Allium cepa root tip cells. Caryologia 2013, 66, 360–367. [Google Scholar] [CrossRef]
- Tom, A.; Job, J.T.; Rajagopal, R.; Alfarhan, A.; Kim, H.-J.; Kim, Y.O.; Na, S.W.; Narayanankutty, A. Thottea siliquosa (Lam.) Ding Hou leaf methanolic extract inhibits lipopolysaccharide-induced TLR4 activation and cytokine production as well as ethyl methyl sulfonate induced genotoxicity. Physiol. Mol. Plant Pathol. 2022, 117, 101772. [Google Scholar] [CrossRef]
- Issa, M.; Chandel, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K.; Yadav, S.S.; Kumari, A. Appraisal of phytotoxic, cytotoxic and genotoxic potential of essential oil of a medicinal plant Vitex negundo. Ind. Crops Prod. 2020, 145, 112083. [Google Scholar] [CrossRef]

| Plant Species |
Type of Extract/ Plant Organ | Findings | Ref. |
|---|---|---|---|
| Achyrocline satureioides | aqueous extracts/ dried aerial parts |
| [72] |
| Adhatoda vasica Carica papaya Clinacanthus nutans | aqueous and methanolic extracts/ dried powdered leaves |
| [117] |
| Allophylus edulis | aqueous extracts (decoction)/ fresh leaves and young stems |
| [102] |
| Aloysia gratissima | aqueous extracts (infusion) and essential oils/fresh leaves |
| [94] |
| Aloysia gratissima (Gillies & Hook.) Tronc. | aqueous and hydroethanolic extracts/leaves |
| [118] |
| Alpinia zerumbet (Zingiberaceae) | essential oils/ fresh leaves |
| [119] |
| Amaranthus spinosus | aqueous extracts/ dried powdered leaves |
| [120] |
| Ambrosia artemisiifolia L. | essential oils/ dried aerial part |
| [79] |
| Aristolochia indica | aqueous extracts/ dried powdered roots |
| [73] |
| Artemisia verlotorum | aqueous extracts (infusion)/ dried leaves |
| [77] |
| Asplenium scolopendrium L. | ethanolic extracts (prior to and after silver nanoparticles phytosynthesis)/fresh leaves and rhizome |
| [75] |
| Averrhoa carambola L. Cissus sicyoides L. Syzygium cumini (L.) Skeels | aqueous extracts (infusion)/ fresh leaves |
| [104] |
| Azadirachta indica (A. Juss) Carica papaya (Linn.) Cymbopogon citratus (DC Stapf.) Mangifera indica (Linn.) Morinda lucida (Benth.) | aqueous extracts (decoctions or squeezed extracts)/leaves or bark |
| [64] |
| Bacopa monnieri | aqueous extracts/dried powdered plants |
| [74] |
| Capparis spinose L. | aqueous extracts (decoction)/flower buds |
| [121] |
| Centella asiatica Linn. | aqueous extracts (decoction)/ground dried leaves |
| [71] |
| Chamomilla recutita (Asteraceae) | aqueous extracts (infusions) and essential oil/inflorescences |
| [81] |
| Citrus aurantiifolia | essential oils/ fresh leaves |
| [122] |
| Clerodendrum inerme L. Gaertn. | aqueous extracts/ leaves powder |
| [114] |
| Clerodendrum inerme (L.) Gaertn C. viscosum Vent. | aqueous and methanolic extracts/fresh leaves |
| [123] |
| Costus spiralis (Jacq.) Roscoe | aqueous extracts/ dried and powdered leaves and stems |
| [111] |
| Crinum asiaticum | aqueous extracts/ pulverized leaves powder |
| [101] |
| Curcuma amada Curcuma karnatakensis | methanolic extracts/dried leaves |
| [124] |
| Dionaea muscipula Ellis | dichloromethanolic extracts/fresh and dried leaves cultivated in vitro |
| [61] |
| Distephanus angulifolius | aqueous extracts/ dried leaves powdered |
| [66] |
| Erythrina velutina | aqueous extracts/dried and powdered leaves |
| [125] |
| Euphorbia hirta | methanolic extracts/dried plant material |
| [69] |
| Ficus benghalensis L. | crude methanol extract and subsequent fractions/dried stem bark material |
| [126] |
| Garcinia cambogia (Gaertn.) Desr. | extracts in different solvents (water, ammonia, hexane, chloroform)/ fresh male flower powder |
| [127] |
| Grewia lasiocarpa | aqueous extracts/ leaves and stem bark |
| [128] |
| Hancornia speciosa Gomes | latex |
| [129] |
| Heinsia crinata, Justicia insularis Lasianthera africana | ethanolic extracts (maceration)/dried powdered leaves |
| [82] |
| Helichrysum italicum (Roth) G. Don Lavandula angustifolia Mill. (OmniaNatura, B&H) | essential oils (commercial products) |
| [91] |
| Hymenaea stigonocarpa | hydroalcoholic extract/dried and powdered bark |
| [84] |
| Hymenaea stigonocarpa Mart. (Fabaceae) | crude aqueous extracts /rhytidome |
| [130] |
| Hymenaea stigonocarpa Mart. ex Hayne (Fabaceae, Caesalpinioideae) | hydroalcoholic extracts (macerate)/dried and grounded bark |
| [131] |
| Hypericum perforatum L. (St. John’s wort) | aqueous extracts/ dried and grounded leaves |
| [103] |
| Hyptis suaveolens | essential oil/ fresh leaves |
| [85] |
| Icacina trichantha Oliv. | aqueous extracts/ dried leaves |
| [98] |
| Ilex paraguariensis St. Hil. (Aquifoliaceae) | aqueous extracts (infusions and concoctions)/dried leaves and young stems |
| [132] |
| Inula viscosa | aqueous extracts/leaf |
| [65] |
| Jatropha curcas L. | latex |
| [133] |
| Lagenandra toxicaria Dalz. | methanolic extracts/ dried rhizomes |
| [89] |
| Lantana fucata Lindl. | aqueous and hydroalcoholic extracts/dried and grounded leaves |
| [134] |
| Limonium globuliferum | aqueous extracts/dried powdered leaves, stems and roots |
| [108] |
| Luehea divaricata | aqueous extracts (teas and decoctions)/leaves and bark |
| [78] |
| Maesa macrophylla (Wall.) A. DC. | aqueous extract/ fresh ground leaves |
| [135] |
| Maytenus ilicifolia (Mart.) Bauhinia candicans (Benth) | aqueous extracts (infusions)/ fresh leaves |
| [136] |
| Mimosa pigra (Fabaceae) | aqueous and ethanolic extracts/dried and crushed leaves and stems |
| [137] |
| Ocimum basilicum | aqueous extracts and essential oil/leaves and inflorescences |
| [70] |
| Origanum vulgare L. | aqueous extracts (infusion/decoction) and hydroethanolic extracts/dried leaves |
| [24] |
| Origanum vulgare Lamiaceae. Ssp. Hirtum | essential oil/ dried leaves |
| [95] |
| Origanum vulgare ssp. vulgare L. | aqueous extracts (infusions in cold/hot water) |
| [59,60] |
| Pandanus Odoratissimus f.ferreus (Y. Kimura) Hatus | aqueous and methanolic extracts/dried and powdered roots and leaves |
| [138] |
| Parquetina nigrescens | aqueous crude extracts/fresh leaves |
| [88] |
| Peganum harmala L. | methanolic extracts/powdered leaves |
| [76] |
| Pfaffia glomerata Spreng. Pedersen, Amaranthaceae | methanolic extract/ small pieces of dried roots |
| [109] |
| Phyllanthus niruri L. (Euphorbiaceae) | aqueous extracts (infusions)/ dried leaves |
| [139] |
| Phyllanthus tenellus Roxb | aqueous extracts (infusions)/ aerial part |
| [80] |
| Picea abies Pinus nigra | aqueous extracts and aqueous extracts derived silver nanoparticles/bark |
| [140] |
| Pimenta dioica | essential oils/freeze-dried powdered leaves |
| [116] |
| Plantago lanceolata | aqueous extracts/leaves |
| [141] |
| Platonia insignis | hexanic extract (dichloromethane fraction)/seeds |
| [87] |
| Pluchea sagittalis | aqueous extracts (infusions)/ fresh leaves cultivated in different conditions (in vitro, acclimatized growth chamber, and field) |
| [86] |
| Pogostemon heyneanus Benth. (Java Patchouli) | aqueous extract (decoction)/dried powder leaves |
| [142] |
| Poincianella bracteosa (Tul.) L.P. Queiroz | aqueous extracts/dried and powdered leaves |
| [143] |
| Polystichum setiferum (Forssk.) Moore ex Woyn. | methanol and ethanol extracts/frozen leaves and rhizomes |
| [16] |
| Psidium guajava L. Achillea millefolium L. | aqueous extracts (infusions)/ fresh leaves |
| [144] |
| Psychotria myriantha Mull. Arg. and Psychotria leiocarpa Cham. et Schlecht | aqueous extracts (infusions)/ fresh leaves |
| [112] |
| Pterocaulon polystachyum DC. (Asteraceae) | aqueous extracts (infusions)/ fresh young leaves |
| [145] |
| Pterodon emarginatus vogel | crude alcoholic extract standardized in geranylgeraniol/crushed fruits |
| [146] |
| Pyrostegia venusta | ethanolic extracts/ dried flowers |
| [147] |
| Rosmarinus officinalis L. | aqueous extracts/ leaves powder |
| [62] |
| Rosmarinus officinalis L. (Labiatae) | aqueous extracts (decoction followed by infusion)/dried leaves |
| [148] |
| Schinus lentiscifolius March. | essential oils/ air-dried leaves |
| [115] |
| Schinus molle Schinus terebinthifolius (Anacardiaceae) | essential oils/ dried leaves |
| [149] |
| Solenostemon monostachyus (P. Beauv.) Brig. (Lamiaceae) | ethanolic extracts (maceration)/ powdered dried leaves |
| [83] |
| Solidago microglossa DC. (Asteraceae) | aqueous extracts (infusions)/leaves |
| [113] |
| Spondias mombin L., Nymphea lotus L. Luffa cylindrica L. | aqueous extracts (decoction)/leaves and whole plant |
| [150] |
| Tapinanthus bangwensis Moringa oleifera | methanol extracts/ ethylacetate fractions/ acetone fractions/ powdered leaves |
| [67] |
| Thottea siliquosa (Lam.) Ding Hou | methanolic extract/dried and powdered leaves |
| [151] |
| Vernonanthura polyanthes | aqueous extracts (infusion)/dried leaves |
| [90] |
| Viscum album different mistletoe host trees: Abies alba, Acer saccharinum, Malus domestica, Pinus sylvestris | aqueous extracts/ fresh plant |
| [99] |
| Vitex negundo | essential oils/ fresh leaves |
| [152] |
| Ziziphus mauritiana (Lam) | extracts in different solvents (waters/ethanol/ ethylacetate/hexane)/powdered leaves |
| [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicuță, D.; Grosu, L.; Patriciu, O.-I.; Voicu, R.-E.; Alexa, I.-C. The Allium cepa Model: A Review of Its Application as a Cytogenetic Tool for Evaluating the Biosafety Potential of Plant Extracts. Methods Protoc. 2025, 8, 88. https://doi.org/10.3390/mps8040088
Nicuță D, Grosu L, Patriciu O-I, Voicu R-E, Alexa I-C. The Allium cepa Model: A Review of Its Application as a Cytogenetic Tool for Evaluating the Biosafety Potential of Plant Extracts. Methods and Protocols. 2025; 8(4):88. https://doi.org/10.3390/mps8040088
Chicago/Turabian StyleNicuță, Daniela, Luminița Grosu, Oana-Irina Patriciu, Roxana-Elena Voicu, and Irina-Claudia Alexa. 2025. "The Allium cepa Model: A Review of Its Application as a Cytogenetic Tool for Evaluating the Biosafety Potential of Plant Extracts" Methods and Protocols 8, no. 4: 88. https://doi.org/10.3390/mps8040088
APA StyleNicuță, D., Grosu, L., Patriciu, O.-I., Voicu, R.-E., & Alexa, I.-C. (2025). The Allium cepa Model: A Review of Its Application as a Cytogenetic Tool for Evaluating the Biosafety Potential of Plant Extracts. Methods and Protocols, 8(4), 88. https://doi.org/10.3390/mps8040088







