Utility of Dried Blood Spots for the Diagnosis of Congenital Cytomegaloviruses within the First 21 Days of Life in a Single Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. DBS Retrieval and Storage
2.3. CMV DNA Extraction and PCR Testing
2.4. Data Collection
2.5. Data Analysis
2.6. Definitions
- Confirmed cCMV (CcCMV): infant with virological confirmation of CMV infection via urine culture, urine PCR, and blood PCR and/or saliva culture within the first 21 DOLs.
- Not congenital CMV (NcCMV): infant with a negative confirmatory test within the first 21 DOLs.
- cCMV disease classification, as published by Kimberlin and colleagues, with minor changes based on discussions of the International cCMV Recommendations Group [7]:
- Asymptomatic cCMV: no apparent abnormalities to suggest cCMV and normal hearing.
- Asymptomatic cCMV with isolated SNHL: no apparent abnormalities to suggest cCMV, but SNHL present (≥21 decibels)
- Mild symptomatic cCMV: one or two isolated manifestations of cCMV infection that are mild or transient (i.e., mild hepatomegaly or a single measurement of low platelet count).
- Moderate to severe symptomatic cCMV: multiple manifestations attributable to cCMV or Central Nervous System (CNS) involvement such as microcephaly, radiographic abnormalities consistent with CMV CNS disease (ventriculomegaly, intracerebral calcifications, periventricular echogenicity, cortical or cerebellar manifestations), abnormal cerebrospinal fluid (CSF) indices for age, chorioretinitis, SNHL, or the detection of CMV DNA in CSF.
- We categorized SNHL as follows:
- Congenital: infants diagnosed using auditory brainstem response (ABR) in one or both ears within the first month of life or a failed hearing screen with a diagnostic ABR within the first year of life.
- Early onset: a passed hearing screen with an abnormal ABR assessment from ≥1 month to 12 months of life.
- Delayed onset: detected after ≥1 assessments with normal hearing after 12 months of life.
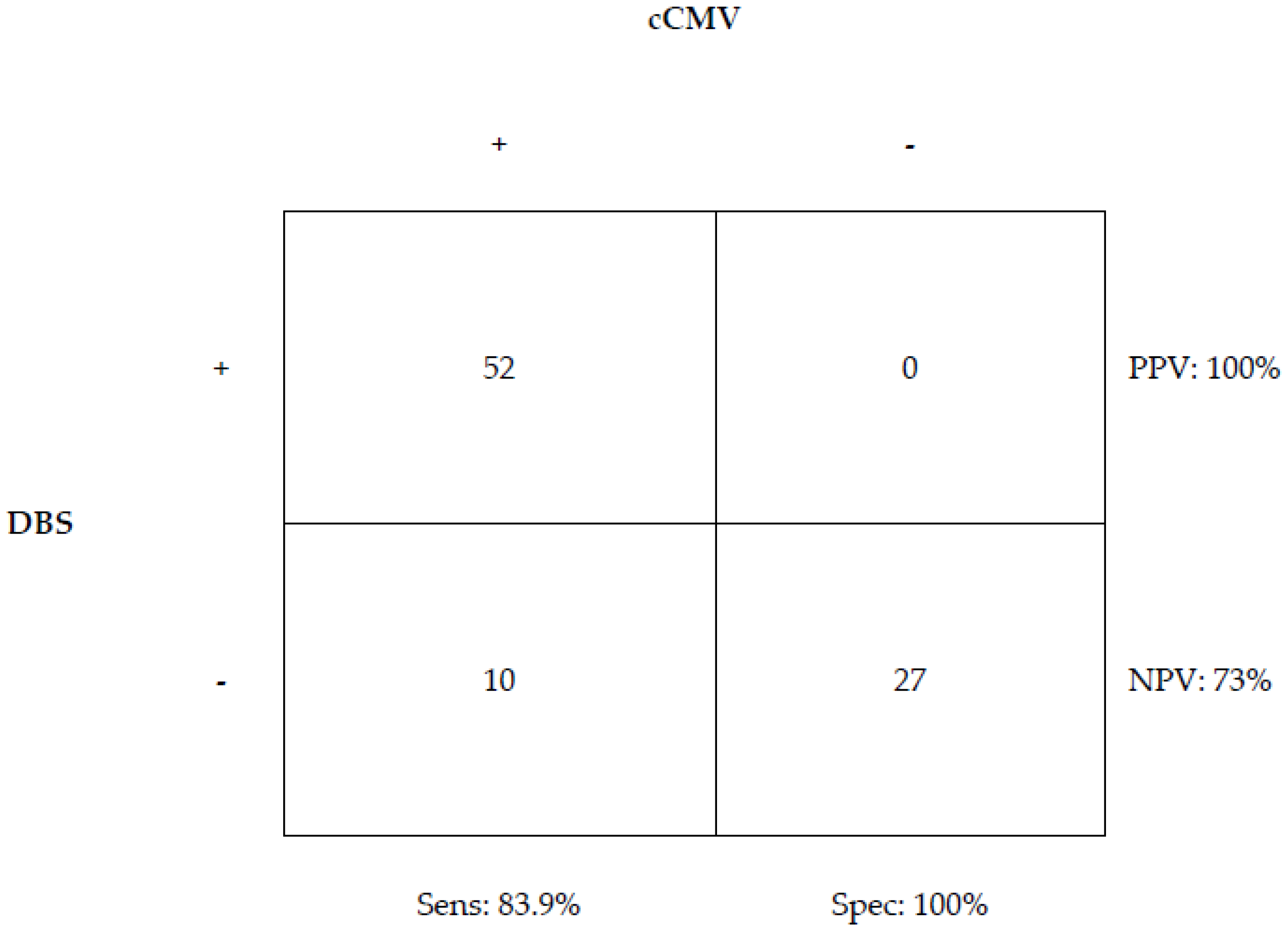
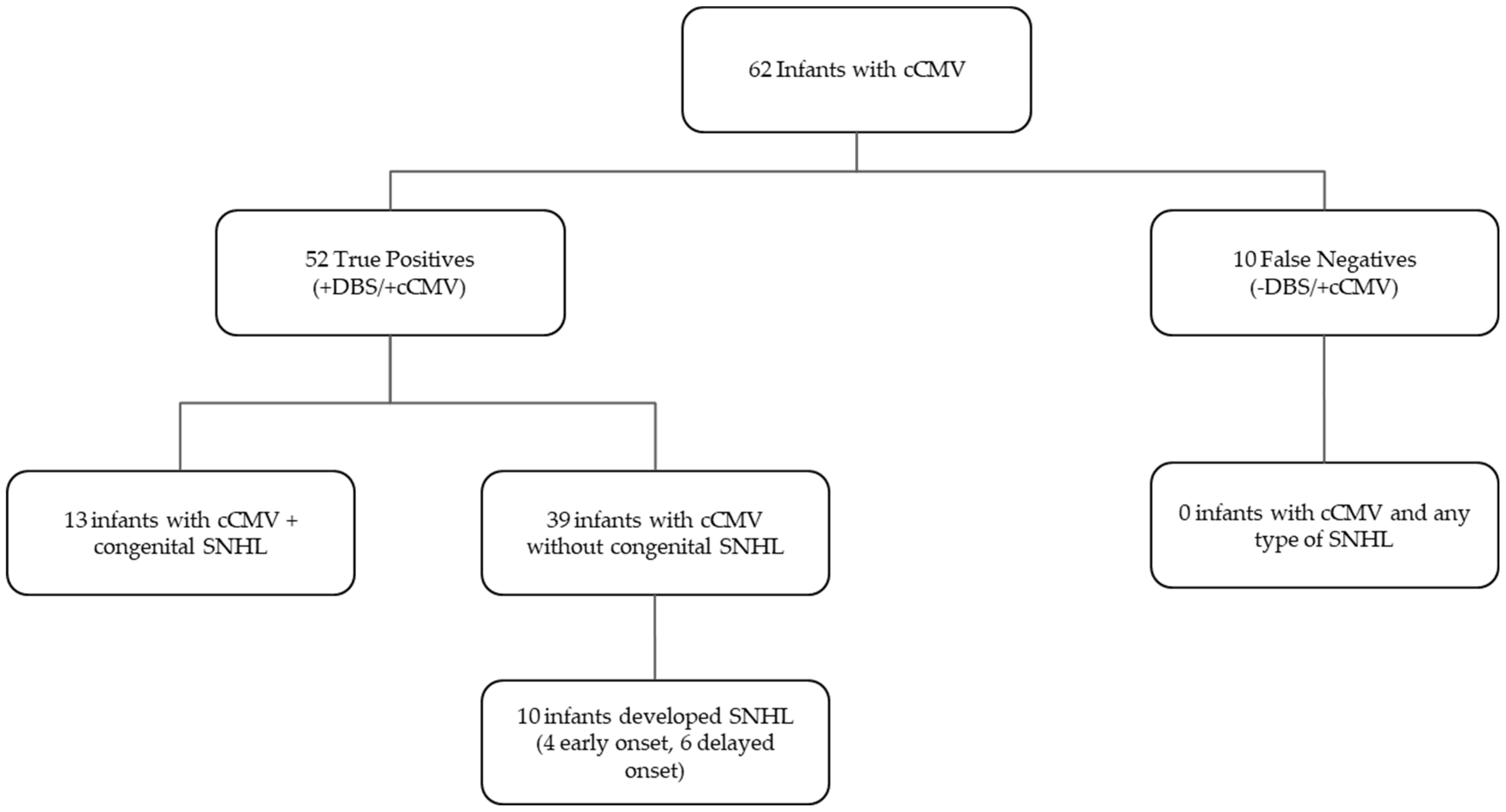
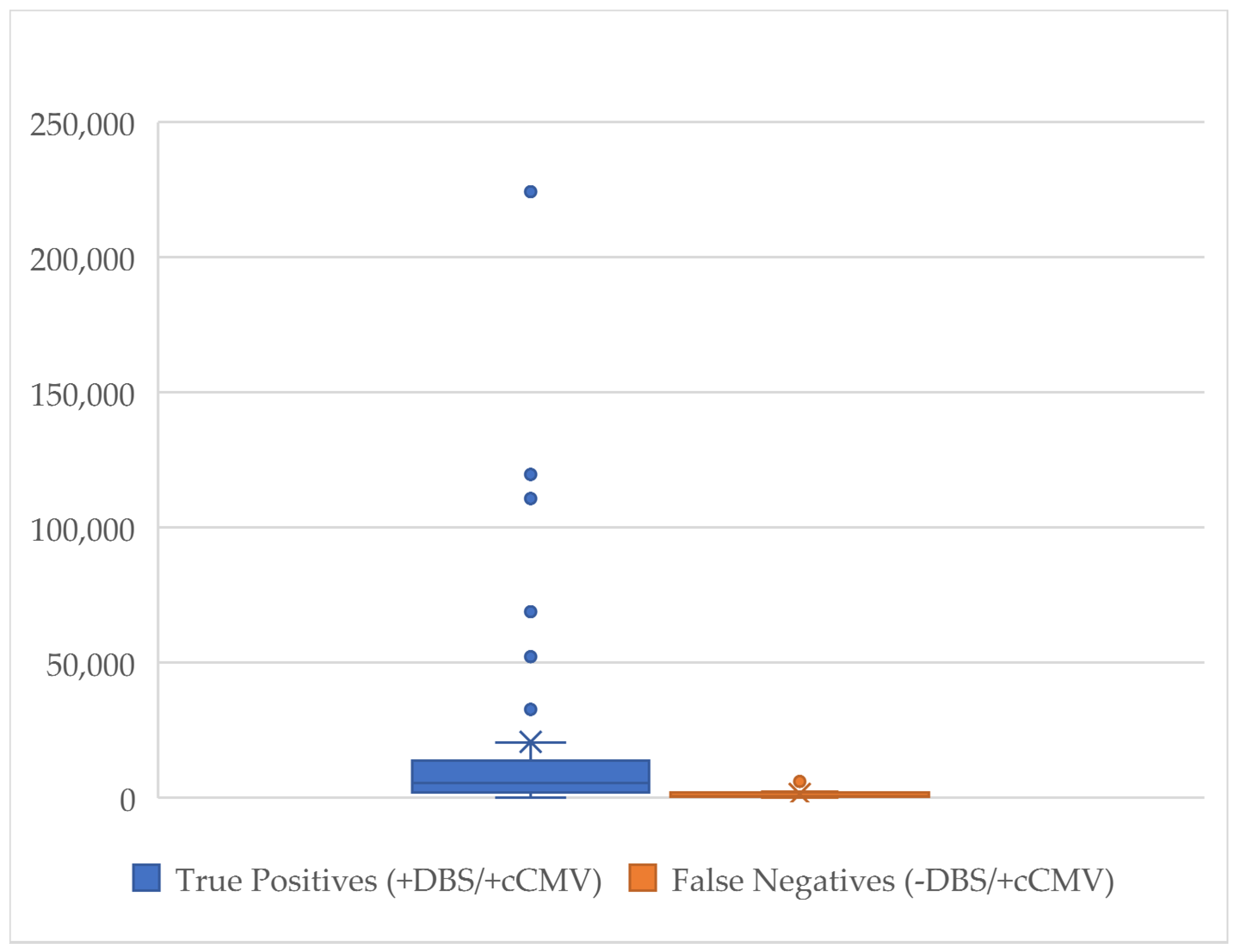
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manicklal, S.; Emery, V.C.; Lazzarotto, T.; Boppana, S.B.; Gupta, R.K. The “silent” global burden of congenital cytomegalovirus. Clin. Microbiol. Rev. 2013, 26, 86–102. [Google Scholar] [CrossRef]
- Boppana, S.B.; Ross, S.A.; Fowler, K.B. Congenital cytomegalovirus infection: Clinical outcome. Clin. Infect. Dis. Off. Publ. Infect Dis. Soc. Am. 2013, 57 (Suppl. S4), S178–S181. [Google Scholar] [CrossRef] [PubMed]
- Lanzieri, T.M.; Chung, W.; Flores, M.; Blum, P.; Caviness, A.C.; Bialek, S.R.; Grosse, S.D.; Miller, J.A.; Demmler-Harrison, G. Congenital Cytomegalovirus Longitudinal Study Group. Hearing Loss in Children with Asymptomatic Congenital Cytomegalovirus. Infect. Pediatr. 2017, 139, e20162610. [Google Scholar] [CrossRef]
- Pesch, M.H.; Schleiss, M.R. Emerging Concepts in Congenital Cytomegalovirus. Pediatrics 2022, 150, e2021055896. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.B.; Pass, R.F.; Britt, W.J.; Stagno, S.; Alford, C.A. Symptomatic congenital cytomegalovirus infection: Neonatal morbidity and mortality. Pediatr. Infect Dis. J. 1992, 11, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Shahar-Nissan, K.; Oikawa Tepperberg, M.; Mendelson, E.; Bilavsky, E. Retrospective identification of congenital cytomegalovirus infection using dried blood samples—Missed opportunities and lessons. J. Clin. Virol. 2022, 152, 105186. [Google Scholar] [CrossRef]
- Rawlinson, W.D.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutré, S.; Gibson, L.; Giles, M.L.; et al. Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef]
- Cannon, M.J.; Griffiths, P.D.; Aston, V.; Rawlinson, W.D. Universal newborn screening for congenital CMV infection: What is the evidence of potential benefit? Rev. Med. Virol. 2014, 24, 291–307. [Google Scholar] [CrossRef]
- Din, E.S.; Brown, C.J.; Grosse, S.D.; Wang, C.; Bialek, S.R.; Ross, D.S.; Cannon, M.J. Attitudes toward newborn screening for cytomegalovirus infection. Pediatrics 2011, 128, e1434–e1442. [Google Scholar] [CrossRef]
- Lee, E.R.; Chan, D.K. Implications of dried blood spot testing for congenital CMV on management of children with hearing loss: A preliminary report. Int. J. Pediatr. Otorhinolaryngol. 2019, 119, 10–14. [Google Scholar] [CrossRef]
- Dollard, S.C.; Dreon, M.; Hernandez-Alvarado, N.; Amin, M.M.; Wong, P.; Lanzieri, T.M.; Osterholm, E.A.; Sidebottom, A.; Rosendahl, S.; McCann, M.T.; et al. Sensitivity of Dried Blood Spot Testing for Detection of Congenital Cytomegalovirus Infection. JAMA Pediatr. 2021, 175, e205441. [Google Scholar] [CrossRef]
- Boppana, S.B.; Ross, S.A.; Novak, Z.; Shimamura, M.; Jr, R.W.T.; Palmer, A.L.; Ahmed, A.; Michaels, M.G.; Sánchez, P.J.; Bernstein, D.I.; et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA 2010, 303, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Leruez-Ville, M.; Vauloup-Fellous, C.; Couderc, S.; Parat, S.; Castel, C.; Avettand-Fenoel, V.; Guilleminot, T.; Grangeot-Keros, L.; Ville, Y.; Grabar, S.; et al. Prospective identification of congenital cytomegalovirus infection in newborns using real-time polymerase chain reaction assays in dried blood spots. Clin. Infect. Dis. 2011, 52, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Barbi, M.; Binda, S.; Primache, V.; Caroppo, S.; Didò, P.; Guidotti, P.; Corbetta, C.; Melotti, D. Cytomegalovirus DNA detection in Guthrie cards: A powerful tool for diagnosing congenital infection. J. Clin. Virol. 2000, 17, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Soetens, O.; Vauloup-Fellous, C.; Foulon, I.; Dubreuil, P.; De Saeger, B.; Grangeot-Keros, L.; Naessens, A. Evaluation of different cytomegalovirus (CMV) DNA PCR protocols for analysis of dried blood spots from consecutive cases of neonates with congenital CMV infections. J. Clin. Microbiol. 2008, 46, 943–946. [Google Scholar] [CrossRef]
- Walter, S.; Atkinson, C.; Sharland, M.; Rice, P.; Raglan, E.; Emery, V.C.; Griffiths, P.D. Congenital cytomegalovirus: Association between dried blood spot viral load and hearing loss. Arch. Dis. Child Fetal. Neonatal. Ed. 2008, 93, F280–F285. [Google Scholar] [CrossRef]
- Forner, G.; Abate, D.; Mengoli, C.; Palù, G.; Gussetti, N. High Cytomegalovirus (CMV) DNAemia Predicts CMV Sequelae in Asymptomatic Congenitally Infected Newborns Born to Women with Primary Infection During Pregnancy. J. Infect. Dis. 2015, 212, 67–71. [Google Scholar] [CrossRef]
- Boppana, S.B.; Fowler, K.B.; Pass, R.F.; Rivera, L.B.; Bradford, R.D.; Lakeman, F.D.; Britt, W.J. Congenital cytomegalovirus infection: Association between virus burden in infancy and hearing loss. J Pediatr. 2005, 146, 817–823. [Google Scholar] [CrossRef]
- Almeida, S.; Gouveia, P.; Jorge, A.; Fortuna, A.; Binda, S.; Barbi, M.; Nascimento, M.S.J.; Paixão, P. Diagnosing congenital cytomegalovirus infecitons using archived dried blood spots: A 15-year observational study, Portugal. J. Clin. Virol. 2023, 165, 105516. [Google Scholar] [CrossRef]
- Demmler Harrison, G.J. Newborn Screening for Congenital Cytomegalovirus Infection…It Is Time. Clin. Infect. Dis. 2020, 70, 1385–1387. [Google Scholar] [CrossRef]
- Gantt, S.; Dionne, F.; Kozak, F.K.; Goshen, O.; Goldfarb, D.M.; Park, A.H.; Fowler, K. Cost-effectiveness of Universal and Targeted Newborn Screening for Congenital Cytomegalovirus Infection. JAMA Pediatr. 2016, 170, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, D.W.; Jester, P.M.; Sánchez, P.J.; Ahmed, A.; Arav-Boger, R.; Michaels, M.G.; Ashouri, N.; Englund, J.A.; Estrada, B.; Jacobs, R.F. Valganciclovir for symptomatic congenital cytomegalovirus disease. N. Engl. J. Med. 2015, 372, 933–943. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | All Patients, n = 89 | ||
|---|---|---|---|
| Gender | Male | 42 | 47% |
| Female | 47 | 53% | |
| Ethnicity | Hispanic | 22 | 25% |
| Non–Hispanic | 66 | 74% | |
| Unknown | 1 | 1% | |
| Race | White | 61 | 69% |
| Asian | 5 | 6% | |
| Black/African-American | 21 | 24% | |
| Other | 0 | 0% | |
| Unknown | 2 | 2% | |
| Gestational age (weeks) | >40 | 3 | 3% |
| 37–40 | 66 | 74% | |
| 34–36 | 12 | 13% | |
| <34 | 8 | 9% | |
| Birth weight (g) | ≥2500 | 52 | 58% |
| 1500–2500 | 28 | 31% | |
| <1500 | 6 | 7% | |
| Unknown | 3 | 3% | |
| Maternal age | ≤20 | 16 | 18% |
| 21–30 | 48 | 54% | |
| 31–40 | 23 | 26% | |
| Unknown | 2 | 2% | |
| History of IUGR | Reported | 33 | 37% |
| Reason for testing | History of maternal infection | 27 | 30% |
| Failed hearing screen | 32 | 36% | |
| Signs and symptoms suggestive of cCMV | 54 | 61% | |
| cCMV classification | Asymtomatic at birth | 11 | 12% |
| Asymptomatic with isolated hearing loss | 4 | 5% | |
| Mild disease | 10 | 11% | |
| Moderate/severe disease | 37 | 42% | |
| Not cCMV | 27 | 30% | |
| Subject | Disease Severity | Hearing Loss? | Long-Term Sequelae | Testing Done for cCMV | CMV qPCR Plasma (IU/mL) |
|---|---|---|---|---|---|
| 38 | M | No | None | BP, UC | <471 |
| 63 | M | No | None | BP, UC | <471 |
| 131 | M/S | No | Learning delays, growth failure | BP, UP | 2400 |
| 162 | A | No | None | BP, UP | 0 |
| 189 | A | No | None | BP, UP | 598 |
| 213 | M | No | None | BP, UP | ND |
| 221 | M | No | None | UP | ND |
| 254 | M/S | No | Hypertonia | BP, UP | <471 |
| 302 * | M/S | No | None | BP, UP | 6040 |
| 323 | M/S | No | None | BP, UP | 1099 |
| Clinical Characteristic | N | DBS Median Viral Load (IU/mL) | p | N | CMV in Blood Median Viral Load (IU/mL) | p |
|---|---|---|---|---|---|---|
| No SNHL | 29 | 2758 (IQR 1479–8759) | 28 | 2405 (IQR 723–5974) | ||
| SNHL present | 22 | 3421 (IQR 859–7292) | 0.662 | 18 | 11,406 (IQR 2863–56,382) | 0.003 |
| Absent vs. congenital SNHL | 12 | 2885 (IQR 1725–7723) | 0.864 | 12 | 15,001 (IQR 2541–100,304) | 0.007 |
| Absent vs. early onset SNHL | 4 | 5575 (IQR 4510–27016) | 0.295 | 2 | 42,490 (IQR not applicable) | 0.020 |
| Absent vs. late onset SNHL | 6 | 824 (IQR 545–8513) | 0.115 | 4 | 5628 (IQR 1237–9558) | 0.425 |
| Absent vs. congenital + early onset SNHL | 16 | 4046 (IQR 2381–7723) | 0.758 | 14 | 19,065 (IQR 2863–79,347) | 0.001 |
| Subject | 1st DBS | DBS qPCR (IU/mL) | 2nd DBS | DBS qPCR (IU/mL) | Traditional Testing | Disease Severity | Hearing Loss Onset | CMV qPCR Plasma (IU/mL) | Antiviral Treatment | Started Prior to Collecting 2nd DBS? |
|---|---|---|---|---|---|---|---|---|---|---|
| 302 * | − | NA | + | 7870 | + | M/S | No | 6040 | Yes | Yes |
| 25 | + | 2543 | − | NA | + | M/S | Congenital | 17,547 | Yes | Yes |
| 59 | + | 689 | − | NA | + | M/S | Congenital | 497 | Yes | Yes |
| 95 | + | 2606 | − | NA | + | M/S | No | 1700 | Yes | Yes |
| 96 | + | 3597 | E | NA | + | M/S | No | 6800 | Yes | Yes |
| 103 | + | 920 | − | NA | + | M/S | No | 13,600 | Yes | Yes |
| 142 | + | 603 | − | NA | + | M/S | Delayed | 7160 | Yes | Unk |
| 205 | + | 766 | − | NA | + | M/S | No | 2767 | Yes | Yes |
| 207 | + | 369 | − | NA | + | M/S | Delayed | 4096 | Yes | No |
| 222 | + | 35,350 | − | NA | + | M/S | No | 2409 | Yes | Yes |
| 241 | + | 1719 | E | NA | + | M/S | No | 5774 | Yes | Yes |
| 242 | + | 1722 | − | NA | + | M/S | No | 1976 | Yes | Unk |
| 311 | + | 846 | − | NA | + | A | No | NA | No | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Valle Penella, A.; Miller, J.; Rochat, R.; Demmler-Harrison, G. Utility of Dried Blood Spots for the Diagnosis of Congenital Cytomegaloviruses within the First 21 Days of Life in a Single Center. Int. J. Neonatal Screen. 2023, 9, 44. https://doi.org/10.3390/ijns9030044
Del Valle Penella A, Miller J, Rochat R, Demmler-Harrison G. Utility of Dried Blood Spots for the Diagnosis of Congenital Cytomegaloviruses within the First 21 Days of Life in a Single Center. International Journal of Neonatal Screening. 2023; 9(3):44. https://doi.org/10.3390/ijns9030044
Chicago/Turabian StyleDel Valle Penella, Ana, Jerry Miller, Ryan Rochat, and Gail Demmler-Harrison. 2023. "Utility of Dried Blood Spots for the Diagnosis of Congenital Cytomegaloviruses within the First 21 Days of Life in a Single Center" International Journal of Neonatal Screening 9, no. 3: 44. https://doi.org/10.3390/ijns9030044
APA StyleDel Valle Penella, A., Miller, J., Rochat, R., & Demmler-Harrison, G. (2023). Utility of Dried Blood Spots for the Diagnosis of Congenital Cytomegaloviruses within the First 21 Days of Life in a Single Center. International Journal of Neonatal Screening, 9(3), 44. https://doi.org/10.3390/ijns9030044





