Shadow of a Pandemic: Persistence of Prenatal SARS-CoV-2 Antibodies in Newborn Blood Spots
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Maternal-Newborn Linkage
2.3. Laboratory Testing
2.4. Cohorts
2.5. Statistics
3. Results
3.1. CalREDIE Mother–Infant Linkage
3.2. Screening Performance
3.2.1. Intrinsic ADAP Performance
3.2.2. Relative Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAP | Antibody Detection by Agglutination-PCR assay |
| CalREDIE | California Reportable Disease Information Exchange |
| CBP | California Biobank Program |
| DBS | Dried blood spots |
| GDSP | Genetic Disease Screening Program |
| NBS | Newborn screening |
| PNS | Prenatal screening |
| PPV | Positive Predictive Value |
| RT-PCR | reverse-transcriptase PCR |
References
- Ellington, S.; Strid, P.; Tong, V.T.; Woodworth, K.; Galang, R.R.; Zambrano, L.D.; Nahabedian, J.; Anderson, K.; Gilboa, S.M. Characteristics of Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, 22 January–7 June 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 769–775. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F., 3rd; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, 22 January–3 October 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1641–1647. [Google Scholar] [CrossRef]
- Delahoy, M.J.; Whitaker, M.; O’Halloran, A.; Chai, S.J.; Kirley, P.D.; Alden, N.; Kawasaki, B.; Meek, J.; Yousey-Hindes, K.; Anderson, E.J.; et al. Characteristics and Maternal and Birth Outcomes of Hospitalized Pregnant Women with Laboratory-Confirmed COVID-19—COVID-NET, 13 States, 1 March–22 August 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1347–1354. [Google Scholar] [CrossRef]
- Purwono, A.; Agustin, H.; Lisnawati, Y.; Faisal, H.K.P. Respiratory perspective of COVID-19 in pregnancy. J. Infect. Dev. Ctries. 2023, 17, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Flaherman, V.J.; Afshar, Y.; Boscardin, J.; Keller, R.L.; Mardy, A.; Prahl, M.K.; Phillips, C.; Asiodu, I.V.; Berghella, W.V.; Chambers, B.D.; et al. Infant Outcomes Following Maternal Infection with SARS-CoV-2: First Report from the PRIORITY Study. Clin. Infect. Dis. 2020, 73, e2810–e2813. [Google Scholar] [CrossRef]
- Panagiotakopoulos, L.; Myers, T.R.; Gee, J.; Lipkind, H.S.; Kharbanda, E.O.; Ryan, D.S.; Williams, J.T.B.; Naleway, A.L.; Klein, N.P.; Hambidge, S.J.; et al. SARS-CoV-2 Infection Among Hospitalized Pregnant Women: Reasons for Admission and Pregnancy Characteristics—Eight U.S. Health Care Centers, 1 March–30 May 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1355–1359. [Google Scholar] [CrossRef]
- Nethery, E.; Hutcheon, J.A.; Kotaska, A.; Law, M.R.; Janssen, P. Weight gain in pregnancy and infant birthweight after the onset of the COVID-19 pandemic: An interrupted time series analysis. Am. J. Clin. Nutr. 2023, 117, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Kontovazainitis, C.G.; Katsaras, G.N.; Gialamprinou, D.; Mitsiakos, G. COVID-19 vaccination and pregnancy: A systematic review of maternal and neonatal outcomes. J. Perinat. Med. 2023, 1–17. [Google Scholar] [CrossRef]
- Neto, E.C.; Rubin, R.; Schulte, J.; Giugliani, R. Newborn screening for congenital infectious diseases. Emerg. Infect. Dis. 2004, 10, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.; Cohn, J.; Roberts, T.; Camp, J.; Chauffour, J.; Gummadi, N.; Ishizaki, A.; Nagarathnam, A.; Tuaillon, E.; van de Perre, P.; et al. Diagnostic accuracy of serological diagnosis of hepatitis C and B using dried blood spot samples (DBS): Two systematic reviews and meta-analyses. BMC Infect. Dis. 2017, 17 (Suppl. S1), 700. [Google Scholar] [CrossRef] [PubMed]
- Greenman, J.; Roberts, T.; Cohn, J.; Messac, L. Dried blood spot in the genotyping, quantification and storage of HCV RNA: A systematic literature review. J. Viral Hepat. 2015, 22, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Xu, C.; Fan, J.; Tang, Y.; Deng, Q.; Zhang, W.; Long, X. Antibodies in Infants Born to Mothers With COVID-19 Pneumonia. JAMA 2020, 323, 1848–1849. [Google Scholar] [CrossRef]
- Dong, L.; Tian, J.; He, S.; Zhu, C.; Wang, J.; Liu, C.; Yang, J. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA 2020, 323, 1846–1848. [Google Scholar] [CrossRef] [PubMed]
- Edlow, A.G.; Li, J.Z.; Collier, A.Y.; Atyeo, C.; James, K.E.; Boatin, A.A.; Gray, K.J.; Bordt, E.A.; Shook, L.L.; Yonker, L.M.; et al. Assessment of Maternal and Neonatal SARS-CoV-2 Viral Load, Transplacental Antibody Transfer, and Placental Pathology in Pregnancies During the COVID-19 Pandemic. JAMA Netw. Open 2020, 3, e2030455. [Google Scholar] [CrossRef]
- Cosma, S.; Carosso, A.R.; Corcione, S.; Cusato, J.; Borella, F.; Antonucci, M.; Marozio, L.; Revelli, A.; Preti, M.; Ghisetti, V.; et al. Longitudinal analysis of antibody response following SARS-CoV-2 infection in pregnancy: From the first trimester to delivery. J. Reprod. Immunol. 2021, 144, 103285. [Google Scholar] [CrossRef]
- la Cour Freiesleben, N.; Egerup, P.; Hviid, K.V.R.; Severinsen, E.R.; Kolte, A.M.; Westergaard, D.; Fich Olsen, L.; Praetorius, L.; Zedeler, A.; Christiansen, A.H.; et al. SARS-CoV-2 in first trimester pregnancy: A cohort study. Hum. Reprod. 2021, 36, 40–47. [Google Scholar] [CrossRef]
- Cosma, S.; Borella, F.; Carosso, A.; Sciarrone, A.; Cusato, J.; Corcione, S.; Mengozzi, G.; Preti, M.; Katsaros, D.; Di Perri, G.; et al. The “scar” of a pandemic: Cumulative incidence of COVID-19 during the first trimester of pregnancy. J. Med. Virol. 2021, 93, 537–540. [Google Scholar] [CrossRef]
- Liu, F.; Nguyen, M.; Vijayakumar, P.; Kaplan, A.; Meir, A.; Dai, Y.; Wang, E.; Walsh, H.; Ring, A.M.; Omer, S.B.; et al. Newborn Dried Blood Spots for Serologic Surveys of COVID-19. Pediatr. Infect. Dis. J. 2020, 39, e454–e456. [Google Scholar] [CrossRef]
- Matteson, J.; Sciortino, S.; Feuchtbaum, L.; Bishop, T.; Olney, R.S.; Tang, H. Adrenoleukodystrophy Newborn Screening in California Since 2016: Programmatic Outcomes and Follow-Up. Int. J. Neonatal Screen. 2021, 7, 22. [Google Scholar] [CrossRef]
- Tsai, C.T.; Robinson, P.V.; Cortez, F.J.; Elma, M.L.B.; Seftel, D.; Pourmandi, N.; Pandori, M.W.; Bertozzi, C.R. Antibody detection by agglutination-PCR (ADAP) enables early diagnosis of HIV infection by oral fluid analysis. Proc. Natl. Acad. Sci. USA 2018, 115, 1250–1255. [Google Scholar] [CrossRef]
- Tsai, C.T.; Robinson, P.V.; Spencer, C.A.; Bertozzi, C.R. Ultrasensitive Antibody Detection by Agglutination-PCR (ADAP). ACS Cent. Sci. 2016, 2, 139–147. [Google Scholar] [CrossRef]
- Karp, D.G.; Danh, K.; Espinoza, N.F.; Seftel, D.; Robinson, P.V.; Tsai, C.T. A serological assay to detect SARS-CoV-2 antibodies in at-home collected finger-prick dried blood spots. Sci. Rep. 2020, 10, 20188. [Google Scholar] [CrossRef] [PubMed]
- Karp, D.G.; Cuda, D.; Tandel, D.; Danh, K.; Robinson, P.V.; Seftel, D.; Tian, H.; Pandori, M.; Miller, K.W.P.; Tsai, C.T. Sensitive and Specific Detection of SARS-CoV-2 Antibodies Using a High-Throughput, Fully Automated Liquid-Handling Robotic System. SLAS Technol. 2020, 25, 545–552. [Google Scholar] [CrossRef]
- Kish, L. Survey Sampling; John Wiley & Sons: New York, NY, USA, 1965. [Google Scholar]
- Xu, X.; Blanton, L.; Elal, A.I.A.; Alabi, N.; Barnes, J.; Biggerstaff, M.; Brammer, L.; Budd, A.P.; Burns, E.; Cummings, C.N.; et al. Update: Influenza Activity in the United States during the 2018–19 Season and Composition of the 2019–20 Influenza Vaccine. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 544–551. [Google Scholar] [CrossRef]
- Rosebrock, A.P. DNA Cross-Reactivity of the CDC-Specified SARS-CoV-2 Specimen Control Leads to Potential for False Negatives and Underreporting of Viral Infection. Clin. Chem. 2021, 67, 435–437. [Google Scholar] [CrossRef]
- Ng, K.W.; Faulkner, N.; Cornish, G.H.; Rosa, A.; Harvey, R.; Hussain, S.; Ulferts, R.; Earl, C.; Wrobel, A.G.; Benton, D.J.; et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020, 370, 1339–1343. [Google Scholar] [CrossRef]
- Gong, W. Jeffreys Interval for One-Sample Proportion with SAS/STAT Software. SAS Proceedings 15: Support.sas.com; 2015. Available online: https://support.sas.com/resources/papers/proceedings15/3020-2015.pdf (accessed on 16 June 2023).
- Woodworth, G.G. Biostatistics: A Bayesian Introduction; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Pambabay-Calero, J.J.; Bauz-Olvera, S.A.; Nieto-Librero, A.B.; Galindo-Villardón, M.P.; Sánchez-García, A.B. A Tutorial for Meta-Analysis of Diagnostic Tests for Low-Prevalence Diseases: Bayesian Models and Software. Methodology 2020, 16, 258–277. [Google Scholar] [CrossRef]
- Rogan, W.J.; Gladen, B. Estimating prevalence from the results of a screening test. Am. J. Epidemiol. 1978, 107, 71–76. [Google Scholar] [CrossRef]
- Reiczigel, J.; Foldi, J.; Ozsvari, L. Exact confidence limits for prevalence of a disease with an imperfect diagnostic test. Epidemiol. Infect. 2010, 138, 1674–1678. [Google Scholar] [CrossRef] [PubMed]
- CDC. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html (accessed on 16 June 2023).
- Flor, M.; Weiss, M.; Selhorst, T.; Muller-Graf, C.; Greiner, M. Comparison of Bayesian and frequentist methods for prevalence estimation under misclassification. BMC Public Health 2020, 20, 1135. [Google Scholar] [CrossRef]
- Amini, F.; Auma, E.; Hsia, Y.; Bilton, S.; Hall, T.; Ramkhelawon, L.; Heath, P.T.; Le Doare, K. Reliability of dried blood spot (DBS) cards in antibody measurement: A systematic review. PLoS ONE 2021, 16, e0248218. [Google Scholar] [CrossRef]
- Lampasona, V.; Pittman, D.L.; Williams, A.J.; Achenbach, P.; Schlosser, M.; Akolkar, B.; Winter, W.E.; Participating, L. Islet Autoantibody Standardization Program 2018 Workshop: Interlaboratory Comparison of Glutamic Acid Decarboxylase Autoantibody Assay Performance. Clin. Chem. 2019, 65, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Feng, Y.; Mo, X.; Zheng, P.; Wang, Q.; Li, P.; Peng, P.; Liu, X.; Chen, Z.; Huang, H.; et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 940–948. [Google Scholar] [CrossRef]
- CDPH. Tracking COVID-19 in California. Available online: https://covid19.ca.gov/state-dashboard/#county-statewide (accessed on 16 June 2023).
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Laing, E.D.; Pena DaMata, J.; Pohida, K.; Tso, M.S.; Samuels, E.C.; Epsi, N.J.; Dorjbal, B.; Lake, C.; Richard, S.A.; et al. Durability of SARS-CoV-2-Specific T-Cell Responses at 12 Months Postinfection. J. Infect. Dis. 2021, 224, 2010–2019. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obs. Gynecol. 2021, 225, 303.e301–303.e317. [Google Scholar] [CrossRef] [PubMed]
- Burbelo, P.D.; Riedo, F.X.; Morishima, C.; Rawlings, S.; Smith, D.; Das, S.; Strich, J.R.; Chertow, D.S.; Davey, R.T., Jr.; Cohen, J.I. Sensitivity in Detection of Antibodies to Nucleocapsid and Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus 2 in Patients with Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, 206–213. [Google Scholar] [CrossRef]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef]
- Bowen, J.E.; Park, Y.J.; Stewart, C.; Brown, J.T.; Sharkey, W.K.; Walls, A.C.; Joshi, A.; Sprouse, K.R.; McCallum, M.; Tortorici, M.A.; et al. SARS-CoV-2 spike conformation determines plasma neutralizing activity elicited by a wide panel of human vaccines. Sci. Immunol. 2022, 7, eadf1421. [Google Scholar] [CrossRef] [PubMed]
- Musa, S.S.; Bello, U.M.; Zhao, S.; Abdullahi, Z.U.; Lawan, M.A.; He, D. Vertical Transmission of SARS-CoV-2: A Systematic Review of Systematic Reviews. Viruses 2021, 13, 1877. [Google Scholar] [CrossRef] [PubMed]
- CDPH. COVID-19 Age, Race and Ethnicity Data: California Department of Public Health; 2023. Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/COVID-19/Race-Ethnicity.aspx (accessed on 16 June 2023).
- Ferrara, A.; Hedderson, M.M.; Zhu, Y.; Avalos, L.A.; Kuzniewicz, M.W.; Myers, L.C.; Ngo, A.L.; Gunderson, E.P.; Ritchie, J.L.; Quesenberry, C.P.; et al. Perinatal Complications in Individuals in California With or Without SARS-CoV-2 Infection During Pregnancy. JAMA Intern. Med. 2022, 182, 503–512. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.; Murphy, E.A.; Sukhu, A.C.; Yee, J.; Singh, S.; Eng, D.; Zhao, Z.; Riley, L.E.; Yang, Y.J. Antibody Response to Coronavirus Disease 2019 (COVID-19) Messenger RNA Vaccination in Pregnant Women and Transplacental Passage Into Cord Blood. Obstet. Gynecol. 2021, 138, 278–280. [Google Scholar] [CrossRef] [PubMed]
| All Cohorts | Relative Performance Analysis b | |||
|---|---|---|---|---|
| Cohorts | Newborn Blood Spots Tested | Twin Pairs | Unique Pregnancies a | CalREDIE Mother–Newborn Matches |
| Pre-pandemic | 50 | 0 | 50 | 0 |
| October 2020 | 1248 | 10 | 1238 | 131 |
| December 2020 | 1167 | 11 | 1156 | 105 |
| March 2021 | 498 | 2 | 496 | 76 |
| Total | 2963 | 23 | 2940 | 312 |
| N | Antibody Detected | Prevalence (95% CI) a | Prevalence Ratio (95% CI) b | ||
|---|---|---|---|---|---|
| Total | 2890 | 453 | 15.7% (14.3%, 17.0%) | ||
| Cohort | October 2020 | 1238 | 147 | 11.9% (10.1%, 13.7%) | 0.9 (0.7, 1.1) |
| December 2020 | 1156 | 141 | 12.2% (10.3%, 14.1%) | 1.0 ref | |
| March 2021 | 496 | 165 | 33.3% (29.1%, 37.4%) | 3.4 (2.7, 4.2) *** | |
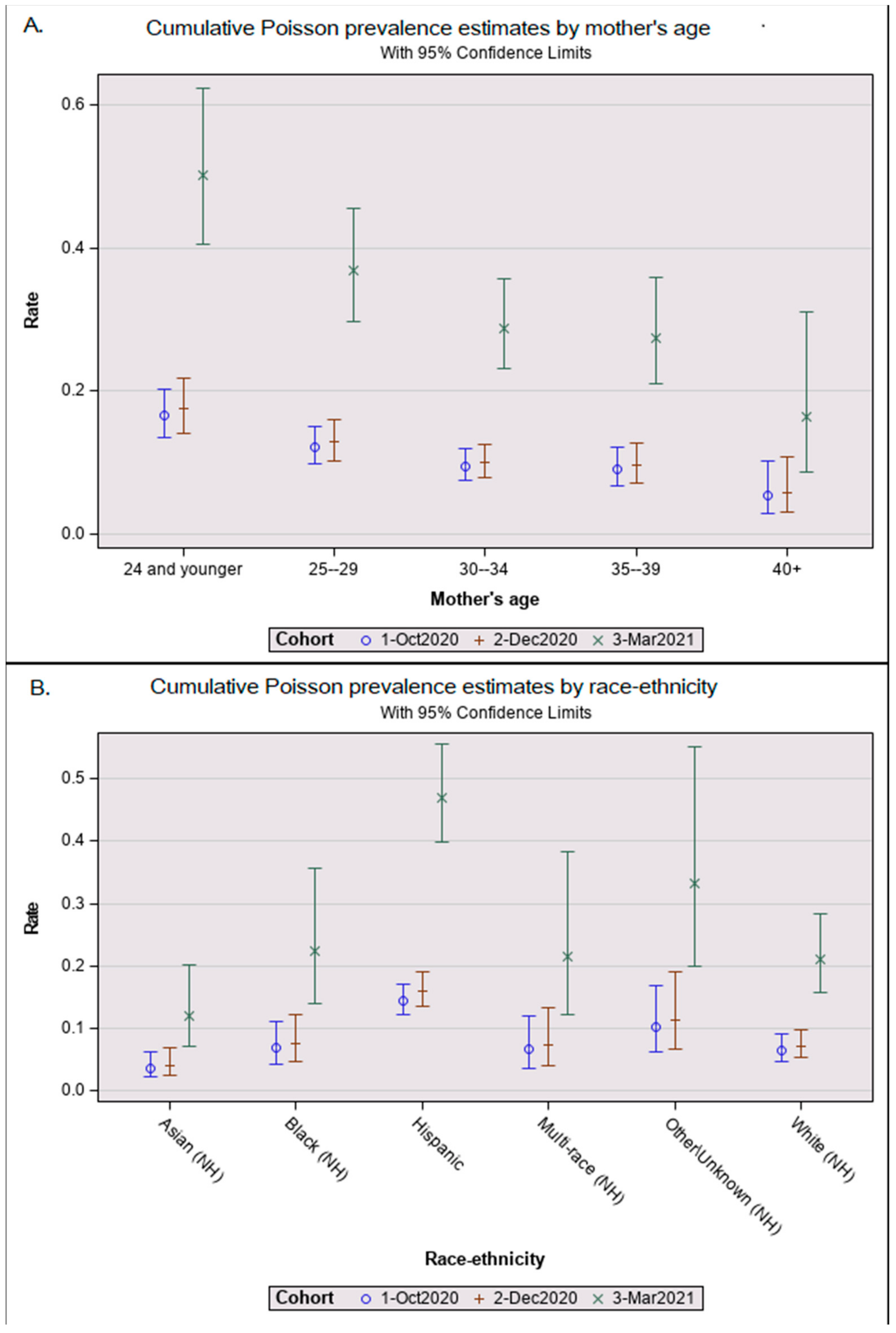
| Race-ethnicity | Hispanic | 1737 | 342 | 19.7% (17.8%, 21.6%) | 2.1 (1.5, 2.8) *** |
| Asian (NH) | 270 | 15 | 5.6% (2.8%, 8.3%) | 0.6 (0.3, 1.1) | |
| Black (NH) | 204 | 19 | 9.3% (5.3%, 13.3%) | 1.0 (0.6, 1.7) | |
| Multi-race (NH) | 108 | 12 | 11.1% (5.2%, 17.0%) | 1.0 (0.6, 1.9) | |
| Other (NH) | 115 | 16 | 13.9% (7.6%, 20.2%) | 1.6 (0.9, 2.7) | |
| White (NH) | 456 | 49 | 10.7% (7.9%, 13.6%) | 1.0 ref | |
| Maternal age (years) c | 15–24 | 662 | 139 | 21.0% (17.9%, 24.1%) | 2.6 (1.4, 5.0) ** |
| 25–29 | 800 | 130 | 16.3% (13.7%, 18.8%) | 2.0 (1.1, 3.9) * | |
| 30–34 | 826 | 111 | 13.4% (11.1%, 15.8%) | 1.8 (0.9, 3.4) | |
| 35–39 | 471 | 63 | 13.4% (10.3%, 16.4%) | 1.7 (0.9, 3.4) | |
| 40+ | 130 | 10 | 7.7% (3.1%, 12.3%) | 1.0 ref |
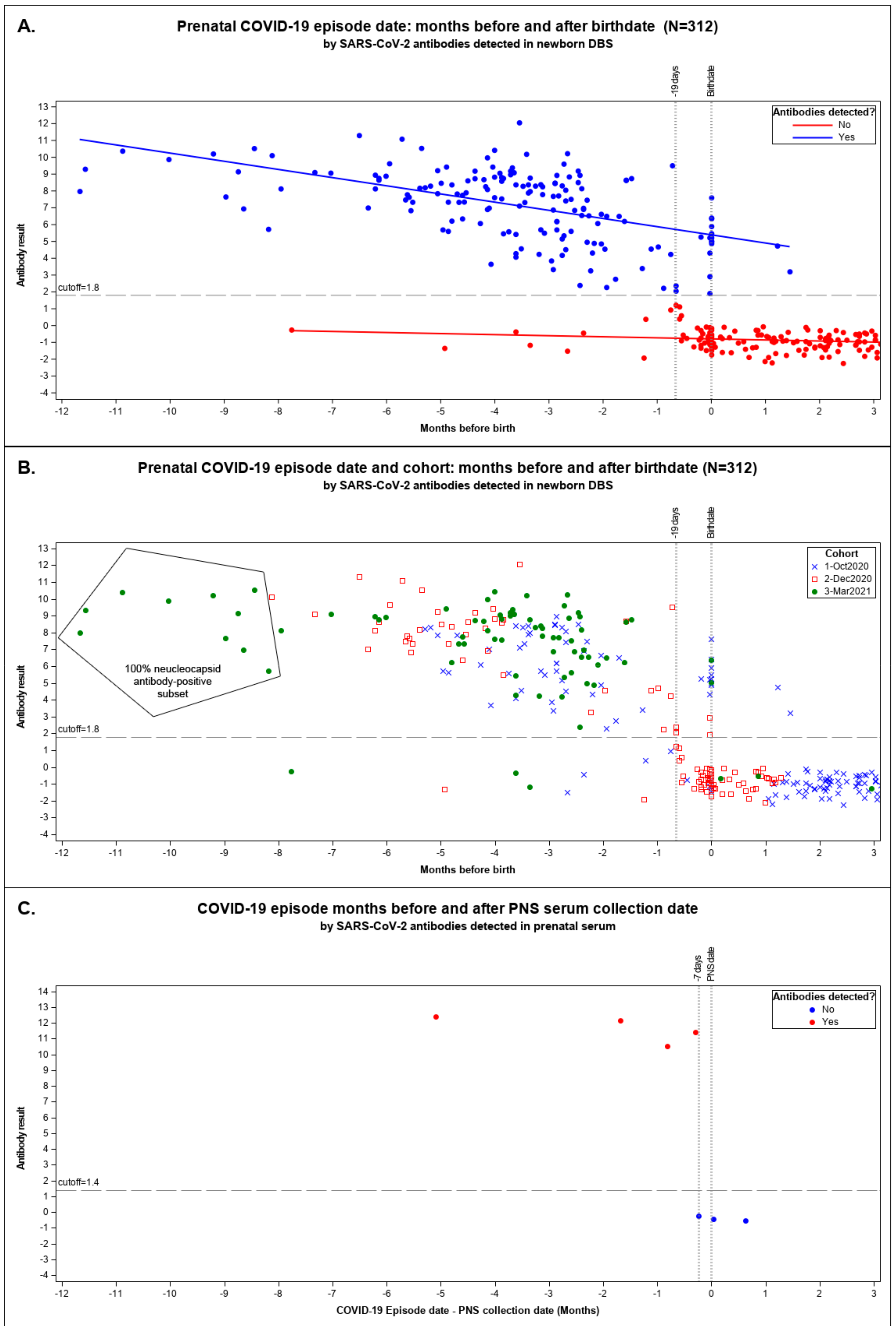
| Sensitivity | Specificity | PPV | |||||
|---|---|---|---|---|---|---|---|
| Cohort | N | Observed a | Estimated b (95% CI) | Observed a | Estimated b (95% CI) | Observed a | Estimated b (95% CI) |
| Combined c | 312 | 76.6% | 86.3% (79.3%, 91.0%) | 98.6% | 98.1% (94.8%, 99.0%) | 98.7% | 98.5% (94.6%, 99.6%) |
| October 2020 | 131 | 85.9% | 86.1% (75.8%, 92.5%) | 97.0% | 98.1% (94.3%, 99.4%) | 96.5% | 97.7% (83.6%, 99.7%) |
| December 2020 | 105 | 54.4% | 55.7% (44.8%, 66.2%) | 100.0% | 98.5% (94.9%, 99.6%) | 100.0% | 99.1% (96.7%, 99.7%) |
| March 2021 | 76 | 95.8% | 94.8% (87.8%, 97.9%) | 100.0% | 97.8% (94.2%, 99.2%) | 100.0% | 98.8% (95.1%, 99.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciortino, S.; Graham, S.; Fillman, T.; Kandasamy, H.; Cooley, R.; Hanson, C.; Eckert, V.; Tang, H.; Yang, J.; Seftel, D.; et al. Shadow of a Pandemic: Persistence of Prenatal SARS-CoV-2 Antibodies in Newborn Blood Spots. Int. J. Neonatal Screen. 2023, 9, 43. https://doi.org/10.3390/ijns9030043
Sciortino S, Graham S, Fillman T, Kandasamy H, Cooley R, Hanson C, Eckert V, Tang H, Yang J, Seftel D, et al. Shadow of a Pandemic: Persistence of Prenatal SARS-CoV-2 Antibodies in Newborn Blood Spots. International Journal of Neonatal Screening. 2023; 9(3):43. https://doi.org/10.3390/ijns9030043
Chicago/Turabian StyleSciortino, Stanley, Steve Graham, Toki Fillman, Hari Kandasamy, Robin Cooley, Carl Hanson, Valorie Eckert, Hao Tang, Juan Yang, David Seftel, and et al. 2023. "Shadow of a Pandemic: Persistence of Prenatal SARS-CoV-2 Antibodies in Newborn Blood Spots" International Journal of Neonatal Screening 9, no. 3: 43. https://doi.org/10.3390/ijns9030043
APA StyleSciortino, S., Graham, S., Fillman, T., Kandasamy, H., Cooley, R., Hanson, C., Eckert, V., Tang, H., Yang, J., Seftel, D., Tsai, C.-t., & Robinson, P. (2023). Shadow of a Pandemic: Persistence of Prenatal SARS-CoV-2 Antibodies in Newborn Blood Spots. International Journal of Neonatal Screening, 9(3), 43. https://doi.org/10.3390/ijns9030043






