Prevalence and Mutation Analysis of Medium-Chain Acyl-CoA Dehydrogenase Deficiency Detected by Newborn Screening in Hefei, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Newborn Screening for MCADD
2.3. Genetic Analysis
2.4. Screening and Diagnostic Criteria
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Genetic Analysis of Newborns with MCADD
3.3. Clinical Follow-Up Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mason, E.; Hindmarch, C.C.; Dunham-Snary, K.J. Medium-chain Acyl-COA dehydrogenase deficiency: Pathogenesis, diagnosis, and treatment. Endocrinol. Diabetes Metab. 2023, 6, e385. [Google Scholar] [CrossRef]
- Wilcken, B.; Haas, M.; Joy, P.; Wiley, V.; Chaplin, M.; Black, C.; Fletcher, J.; McGill, J.; Boneh, A. Outcome of neonatal screening for medium-chain acyl-CoA dehydrogenase deficiency in Australia: A cohort study. Lancet 2007, 369, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Rücklová, K.; Hrubá, E.; Pavlíková, M.; Hanák, P.; Farolfi, M.; Chrastina, P.; Vlášková, H.; Kousal, B.; Smolka, V.; Foltenová, H.; et al. Impact of Newborn Screening and Early Dietary Management on Clinical Outcome of Patients with Long Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency and Medium Chain Acyl-CoA Dehydrogenase Deficiency-A Retrospective Nationwide Study. Nutrients 2021, 13, 2925. [Google Scholar] [CrossRef]
- Dessein, A.-F.; Fontaine, M.; Andresen, B.S.; Gregersen, N.; Brivet, M.; Rabier, D.; Napuri-Gouel, S.; Dobbelaere, D.; Mention-Mulliez, K.; Martin-Ponthieu, A.; et al. A novel mutation of the ACADM gene (c.145C>G) associated with the common c.985A>G mutation on the other ACADM allele causes mild MCAD deficiency: A case report. Orphanet J. Rare Dis. 2010, 5, 26. [Google Scholar] [CrossRef]
- Touw, C.M.L.; A Smit, G.P.; de Vries, M.; de Klerk, J.B.C.; Bosch, A.M.; Visser, G.; Mulder, M.F.; Rubio-Gozalbo, M.; Elvers, B.; E Niezen-Koning, K.; et al. Risk stratification by residual enzyme activity after newborn screening for medium-chain acyl-CoA dehyrogenase deficiency: Data from a cohort study. Orphanet J. Rare Dis. 2012, 7, 30. [Google Scholar] [CrossRef]
- Karaceper, M.D.; Khangura, S.D.; Wilson, K.; Coyle, D.; Brownell, M.; Davies, C.; Dodds, L.; Feigenbaum, A.; Fell, D.B.; Grosse, S.D.; et al. Health services use among children diagnosed with medium-chain acyl-CoA dehydrogenase deficiency through newborn screening: A cohort study in Ontario, Canada. Orphanet J. Rare Dis. 2019, 14, 70. [Google Scholar] [CrossRef]
- Grosse, S.D.; Khoury, M.J.; Greene, C.L.; Crider, K.S.; Pollitt, R.J. The epidemiology of medium chain acyl-CoA dehydrogenase deficiency: An update. Genet Med. 2006, 8, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Andresen, B.S.; Lund, A.M.; Hougaard, D.M.; Christensen, E.; Gahrn, B.; Christensen, M.; Bross, P.; Vested, A.; Simonsen, H.; Skogstrand, K.; et al. MCAD deficiency in Denmark. Mol. Genet. Metab. 2012, 106, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Andresen, B.S.; Lund, A.M.; Hougaard, D.M.; Christensen, E.; Gahrn, B.; Christensen, M.; Bross, P.; Vested, A.; Simonsen, H.; Skogstrand, K.; et al. Neonatal screening for medium chain acyl-CoA deficiency: High incidence in Lower Saxony (northern Germany). Eur. J. Pediatr. 2001, 160, 318–319. [Google Scholar]
- Oerton, J.; Khalid, J.M.; Besley, G.; Dalton, R.N.; Downing, M.; Green, A.; Henderson, M.; Krywawych, S.; Leonard, J.; Andresen, B.S.; et al. Newborn screening for medium chain acyl-CoA dehydrogenase deficiency in England: Prevalence, predictive value and test validity based on 1.5 million screened babies. J. Med. Screen. 2011, 18, 173–181. [Google Scholar] [CrossRef]
- Shibata, N.; Hasegawa, Y.; Yamada, K.; Kobayashi, H.; Purevsuren, J.; Yang, Y.; Dung, V.C.; Khanh, N.N.; Verma, I.C.; Bijarnia-Mahay, S.; et al. Diversity in the incidence and spectrum of organic acidemias, fatty acid oxidation disorders, and amino acid disorders in Asian countries: Selective screening vs. expanded newborn screening. Mol. Genet. Metab. Rep. 2018, 16, 5–10. [Google Scholar] [CrossRef]
- Tong, F.; Jiang, P.P.; Yang, R.L.; Huang, X.L.; Zhou, X.L.; Hong, F.; Shu, Q. Medium-chain acyl-CoA dehydrogenase deficiency: Neonatal screening and follow-uP. Zhongguo Dang Dai Er Ke Za Zhi Chin. J. Contemp. Pediatr. 2019, 21, 52–57. [Google Scholar]
- Chien, Y.H.; Lee, N.C.; Chao, M.C.; Chen, L.C.; Chen, L.H.; Chien, C.C.; Hwu, W.L. Fatty Acid oxidation disorders in a chinese population in taiwan. JIMD Rep. 2013, 11, 165–172. [Google Scholar]
- Sun, Y.; Yu, C.; Lü, J.; Lü, Y.; Li, W. Screening and mutation analysis of medium-chain acyl-CoA dehydrogenase deficiency in newborns. Chin. J. Child Health Care 2019, 27, 4. [Google Scholar]
- Dong, L.; Ji, C.; Xu, J.; Cui, Y. Screening and follow-up results of neonate medium-chain acyl-CoA dehydrogenase deficiency in Zibo, Shandong province. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022, 51, 284–289. [Google Scholar] [CrossRef]
- Hall, P.L.; Wittenauer, A.; Hagar, A. Newborn screening for medium chain acyl-CoA dehydrogenase deficiency: Performance improvement by monitoring a new ratio. Mol. Genet. Metab. 2014, 113, 274–277. [Google Scholar] [CrossRef]
- Khalid, J.M.; Oerton, J.; Besley, G.; Dalton, N.; Downing, M.; Green, A.; Henderson, M.; Krywawych, S.; Wiley, V.; Wilcken, B.; et al. Relationship of octanoylcarnitine concentrations to age at sampling in unaffected newborns screened for medium-chain acyl-CoA dehydrogenase deficiency. Clin. Chem. 2010, 56, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassnan, Z.N.; Imtiaz, F.; Al-Amoudi, M.; Rahbeeni, Z.; Al-Sayed, M.; Al-Owain, M.; Rashed, M.S. Medium-chain acyl-CoA dehydrogenase deficiency in Saudi Arabia: Incidence, genotype, and preventive implications. J. Inherit. Metab. Dis. 2010, 33 (Suppl. S3), S263–S267. [Google Scholar] [CrossRef]
- Shigematsu, Y.; Hirano, S.; Hata, I.; Tanaka, Y.; Sudo, M.; Sakura, N.; Tajima, T.; Yamaguchi, S. Newborn mass screening and selective screening using electrospray tandem mass spectrometry in Japan. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2002, 776, 39–48. [Google Scholar] [CrossRef]
- Ensenauer, R.; Winters, J.L.; Parton, P.A.; Kronn, D.F.; Kim, J.-W.; Matern, D.; Rinaldo, P.; Hahn, S.H. Genotypic differences of MCAD deficiency in the Asian population: Novel genotype and clinical symptoms preceding newborn screening notification. Genet. Med. 2005, 7, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Zhu, B.; Wang, L.; Ji, Y.P.; Wang, X.H.; Wang, X.; Zhang, M.L.; Wang, Y.; Kang, W.G.; Qin, L. Carrier screening and analysis of pathogenic genes for short-, medium-, and very long-chain acyl-CoA dehydrogenase deficiency in newborns from Inner Mongolia. J. Shanxi Med. Univ. 2024, 55, 1343–1346. [Google Scholar]
- Ahrens-Nicklas, R.C.; Pyle, L.C.; Ficicioglu, C. Morbidity and mortality among exclusively breastfed neonates with medium-chain acyl-CoA dehydrogenase deficiency. Genet. Med. 2016, 18, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.W.; Zytkovicz, T.H.; Comeau, A.M.; Strauss, A.W.; Marsden, D.; Shih, V.E.; Eaton, R.B. Spectrum of medium-chain acyl-CoA dehydrogenase deficiency detected by newborn screening. Pediatrics 2008, 121, e1108–e1114. [Google Scholar] [CrossRef]
- Venditti, L.N.; Venditti, C.P.; Berry, G.T.; Kaplan, P.B.; Kaye, E.M.; Glick, H.; Stanley, C.A. Newborn screening by tandem mass spectrometry for medium-chain Acyl-CoA dehydrogenase deficiency: A cost-effectiveness analysis. Pediatrics 2003, 112, 1005–1015. [Google Scholar] [CrossRef]
- Hara, K.; Tajima, G.; Okada, S.; Tsumura, M.; Kagawa, R.; Shirao, K.; Ohno, Y.; Yasunaga, S.; Ohtsubo, M.; Hata, I.; et al. Significance of ACADM mutations identified through newborn screening of MCAD deficiency in Japan. Mol. Genet. Metab. 2016, 118, 9–14. [Google Scholar] [CrossRef] [PubMed]
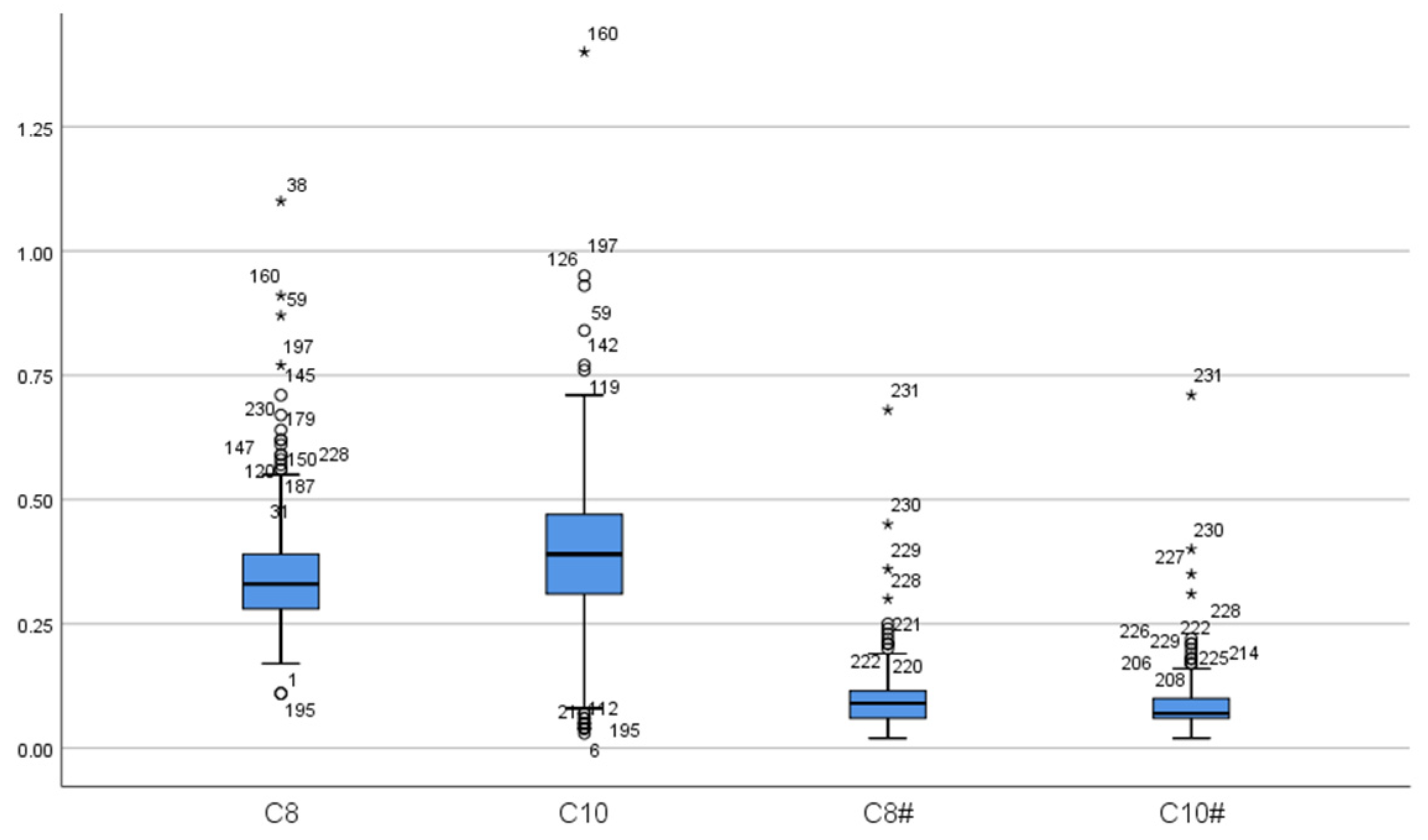
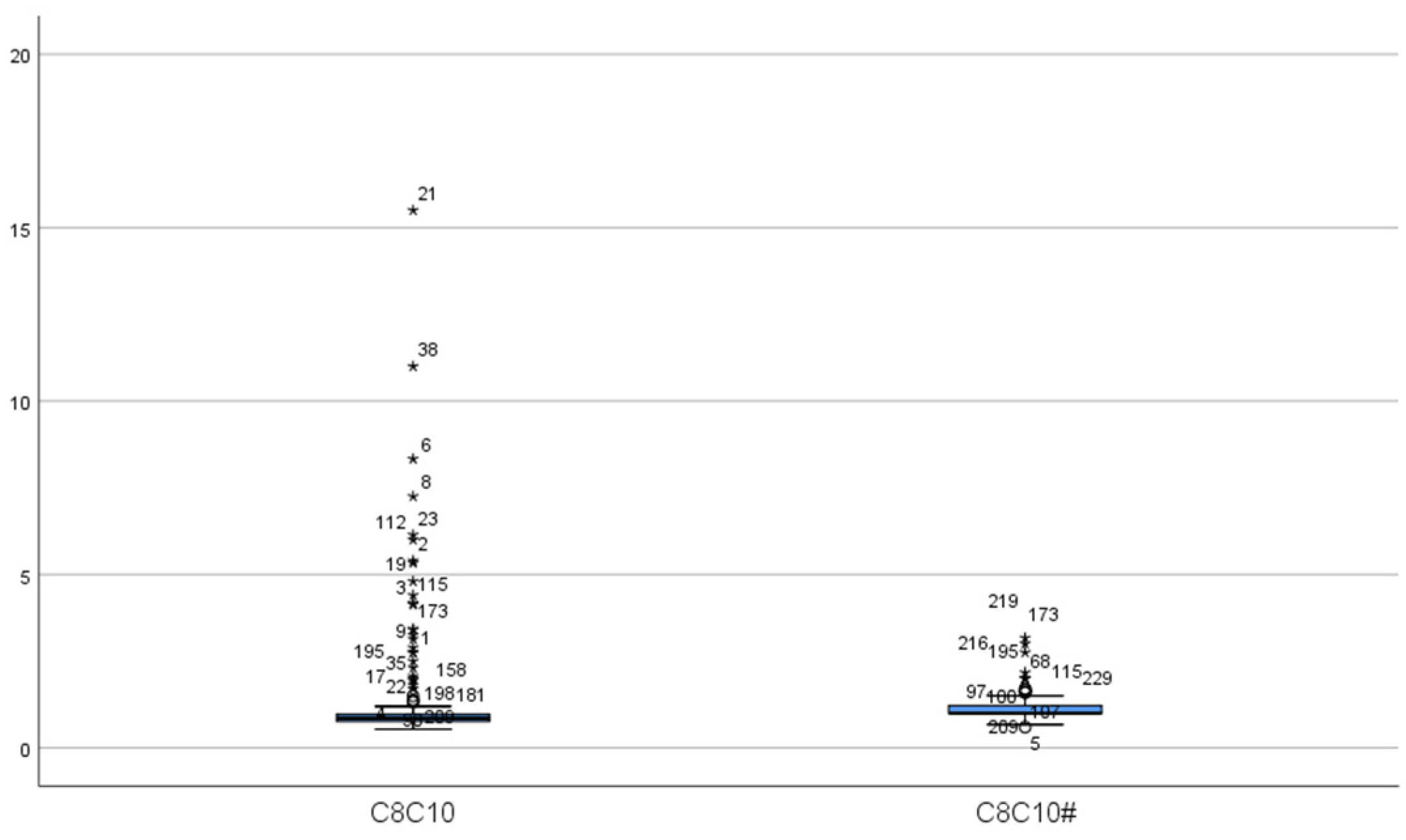
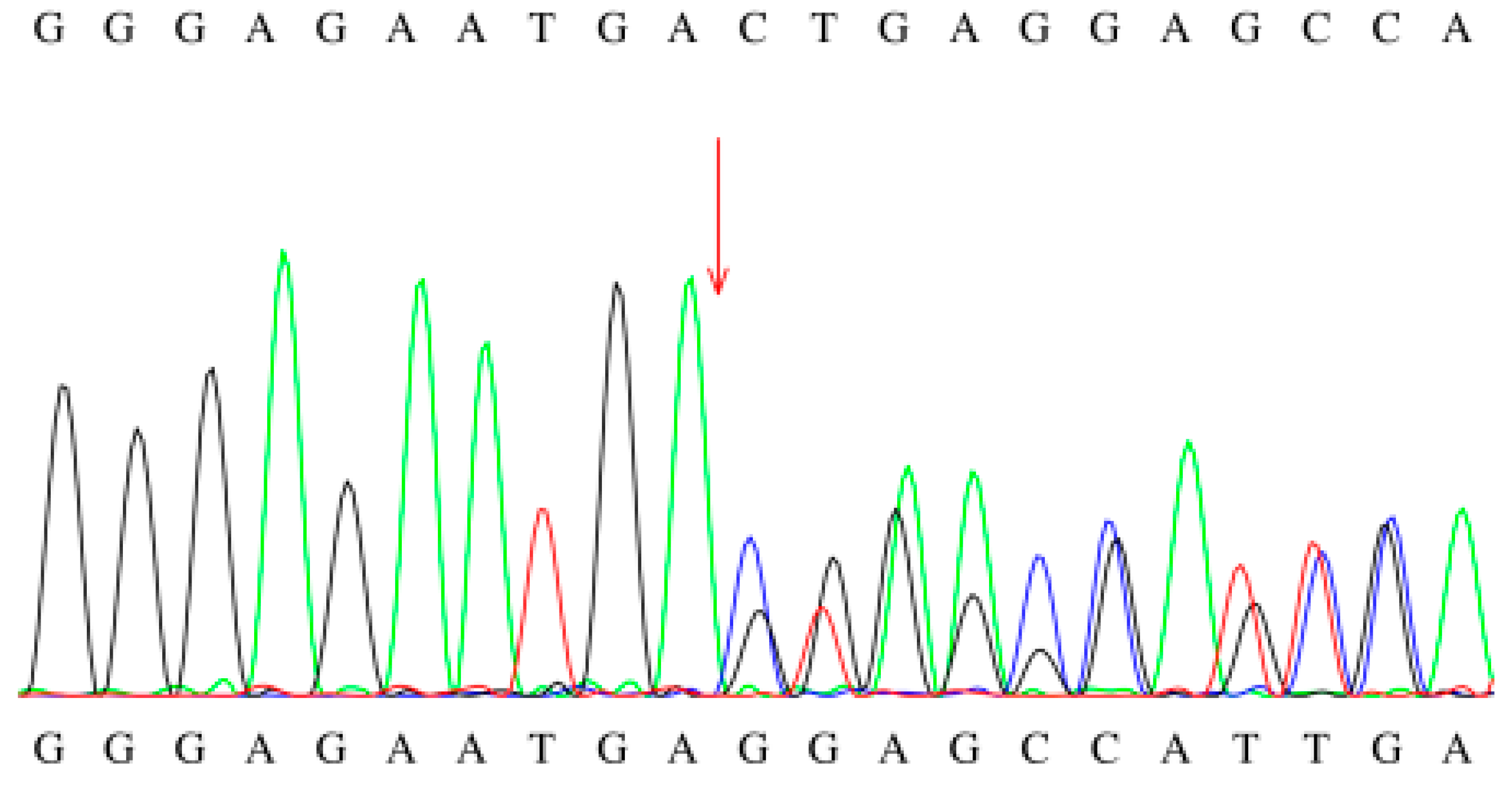

| Case | Gender | Initial Screening | Secondary Screening | C8 Fluctuation Range (μmol/L) * | Other Abnormalities | ||||
|---|---|---|---|---|---|---|---|---|---|
| C0 (μmol/L) | C8 (μmol/L) | C8/C10 | C0 (μmol/L) | C8 (μmol/L) | C8/C10 | ||||
| 1 | Male | 26.89 | 2.7 | 18 | 20.22 | 1.69 | 15.364 | 0.95–3.66 | C0: 8.09 |
| 2 | Male | 33.17 | 11.3 | 17.12 | 24.97 | 2.12 | 15.143 | 0.95–11.3 | C0: 6.57, CK: 219 |
| 3 | Male | 22.72 | 1.47 | 16.33 | 17.46 | 1.64 | 18.222 | 1.17–5.65 | Cardiac ultrasound: mild tricuspid valve regurgitation |
| 4 | Male | 35.01 | 0.64 | 3.05 | 30.81 | 0.64 | 3.765 | 0.09–1.05 | |
| 5 | Female | 35.59 | 5.75 | 10.27 | 19.46 | 0.73 | 10.429 | 0.22–5.75 | C0: 9.94, blood ammonia: 77, sinus arrhythmia |
| 6 | Female | 30.06 | 1.4 | 8.75 | 23.39 | 1.28 | 9.143 | 0.25–1.4 | |
| 7 | Female | 31.08 | 2.44 | 14.35 | 20.06 | 2.8 | 16.471 | 2.44–2.8 | |
| 8 | Female | 36.42 | 1.94 | 5.54 | 26.45 | 1.01 | 5.611 | 0.57–1.94 | |
| 9 | Female | 31.94 | 1.19 | 5.67 | 32.09 | 0.51 | 3.923 | 0.13–1.19 | CK-MB: 48.1 |
| 10 | Female | 22.94 | 5.8 | 14.15 | 26.8 | 1.87 | 12.467 | 1.87–5.8 | |
| 11 | Female | 30.43 | 4.15 | 15.37 | 22.93 | 1.76 | 11.733 | 1.76–4.15 | |
| 12 | Female | 27.24 | 0.32 | 3.56 | 20.19 | 0.25 | 4.167 | 0.25–0.95 | |
| 13 | Male | 19.79 | 5.12 | 6.65 | 15.32 | 1.00 | 9.091 | 0.86–5.12 | |
| 14 | Male | 24.05 | 2.3 | 19.17 | 17.64 | 1.63 | 20.375 | 1.63–2.3 | |
| 15 | Female | 30.86 | 2.77 | 5.43 | 35.9 | 1.09 | 9.083 | 0.92–2.77 | |
| 16 | Female | 27.96 | 3.43 | 15.59 | 29.79 | 2.93 | 14.65 | 1.93–3.43 | |
| C0 (μmol/L) | C6 (μmol/L) | C8 (μmol/L) | C10 (μmol/L) | C10:1 (μmol/L) | C8/C10 | |
|---|---|---|---|---|---|---|
| Initial screening | 29.13 ± 4.94 | 0.63 ± 0.42 | 3.30 ± 2.73 | 0.31 ± 0.21 | 0.41 ± 0.27 | 11.19 ± 5.63 |
| Repeat screening | 23.97 ± 5.93 | 0.40 ± 0.14 | 1.43 ± 0.77 | 0.13 ± 0.04 | 0.27 ± 0.11 | 11.23 ± 5.23 |
| t | 3.708 | 2.205 | 2.908 | 3.324 | 1.917 | 0.079 |
| p | 0.002 | 0.044 | 0.011 | 0.002 | 0.074 | 0.938 |
| Case | Mutation Site 1 | Mutation Site 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Location | Nucleotide Alteration | Amino Acid Alteration | Pathogenic Risk | Source | Location | Nucleotide Alteration | Amino Acid Alteration | Pathogenic Risk | Source | |
| 1 | exon1 | c.1A>G | p.M1V | Likely pathogenic | Mother | exon11 | c.1085G>A | p.G362E | Pathogenic | Father |
| 2 | exon8 | c.668T>C | p.I223T | Variant of uncertain significance | Mother | intron6 | c.468+5G>A# | - | Variant of uncertain significance | Father |
| 3 | exon1 | c.1A>G | p.M1V | Likely pathogenic | Mother | 5′UTR-exon10 | 5′UTR-exon10del | - | Likely pathogenic | Not detected |
| 4 | exon2 | c.91C>T | p.R31C | Variant of uncertain significance | Mother | exon10 | c.854C>G# | p.A285G | Variant of uncertain significance | Father |
| 5 | exon11 | c.1085G>A | p.G362E | Pathogenic | Mother | exon8 | c.616C>T | p.R206C | Pathogenic | Father |
| 6 | exon11 | c.1085G>A | p.G362E | Pathogenic | Mother | exon2 | c.91C>T | p.R31C | Variant of uncertain significance | Father |
| 7 | intron4 | c.286+232C>G | - | Variant of uncertain significance | Mother | exon5 | c.383delG | p.L128Wfs*22 | Pathogenic | Father |
| 8 | exon6 | c.449-452del | p.T150Rfs*4 | Pathogenic | Mother | exon11 | c.1171A>G | p.M391V | Variant of uncertain significance | Father |
| 9 | exon6 | c.428-431 delinsTCTTCTTTTGTT# | p.K143Ifs*10 | Pathogenic | Mother | exon11 | c.982A>G | p.M328V | Pathogenic | Father |
| 10 | exon1-exon10 | exon1-exon10del | - | Pathogenic | Not detected | exon6 | c.449-452del | p.T150Rfs*4 | Pathogenic | Father |
| 11 | exon6 | c.449-452del | p.T150Rfs*4 | Pathogenic | Mother | exon6 | c.449-452del | p.T150Rfs*4 | Pathogenic | Father |
| 12 | exon2 | c.33C>T | p.V11= | Variant of uncertain significance | Mother | exon6 | c.428-431 delinsTCTTCTTTTGTT# | p.K143Ifs*10 | Pathogenic | Father |
| 13 | exon11 | c.1085G>A | p.G362E | Pathogenic | Mother | exon9 | c.718A>G | p.M240V | Likely pathogenic | Father |
| 14 | exon11-exon12 | exon11-exon12del | - | Likely pathogenic | Not detected | exon11 | c.1085G>A | p.G362E | Pathogenic | Father |
| 15 | exon11 | c.1171A>G | p.M391V | Variant of uncertain significance | Mother | exon11-exon12 | exon11-exon12del | - | Likely pathogenic | Not detected |
| 16 | Not detected | exon6 | c.449-452del | p.T150Rfs*4 | Pathogenic | Father | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Ma, Q.; Huang, Y.; Song, W.; Xu, H.; Zhu, P.; Wang, Y. Prevalence and Mutation Analysis of Medium-Chain Acyl-CoA Dehydrogenase Deficiency Detected by Newborn Screening in Hefei, China. Int. J. Neonatal Screen. 2025, 11, 83. https://doi.org/10.3390/ijns11030083
Hu H, Ma Q, Huang Y, Song W, Xu H, Zhu P, Wang Y. Prevalence and Mutation Analysis of Medium-Chain Acyl-CoA Dehydrogenase Deficiency Detected by Newborn Screening in Hefei, China. International Journal of Neonatal Screening. 2025; 11(3):83. https://doi.org/10.3390/ijns11030083
Chicago/Turabian StyleHu, Haili, Qingqing Ma, Yong Huang, Wangsheng Song, Hongyu Xu, Peng Zhu, and Yan Wang. 2025. "Prevalence and Mutation Analysis of Medium-Chain Acyl-CoA Dehydrogenase Deficiency Detected by Newborn Screening in Hefei, China" International Journal of Neonatal Screening 11, no. 3: 83. https://doi.org/10.3390/ijns11030083
APA StyleHu, H., Ma, Q., Huang, Y., Song, W., Xu, H., Zhu, P., & Wang, Y. (2025). Prevalence and Mutation Analysis of Medium-Chain Acyl-CoA Dehydrogenase Deficiency Detected by Newborn Screening in Hefei, China. International Journal of Neonatal Screening, 11(3), 83. https://doi.org/10.3390/ijns11030083






