The Impact of Cystic Fibrosis Algorithm Changes: A Case Study of Challenges and Strategies
Abstract
1. Introduction
2. Methods
2.1. Solving Phase 1 Problems
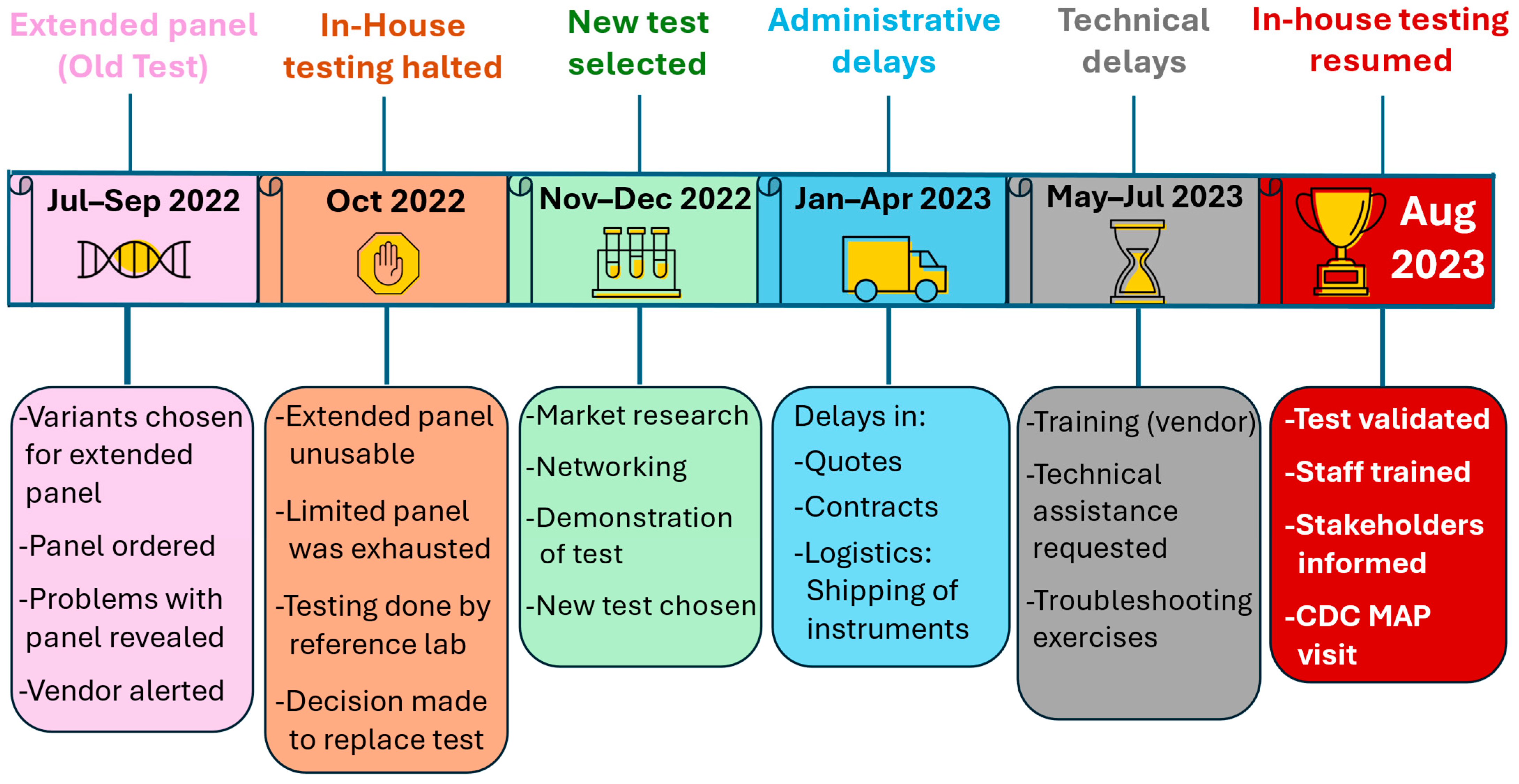
2.1.1. Deciding to Change Our CFTR DNA Test
2.1.2. Reference Labs
- Enactment of existing memoranda of understanding (MOUs)/contracts and the establishment of new agreements. This involved the legal teams of both entities to determine the contract’s length (e.g., 1 year), renewal date and the duration of testing.
- Agreement on the price of testing per sample and whether repeat testing (to confirm detected variants) was an additional charge.
- Obtained information on the reference lab’s assay and variant panel. After the first reference lab, we ensured that subsequent labs used the same test/panel to minimize the impact on our patient reports, database and standard operating procedures (SOPs).
- Decided on the sample type to be sent (whole blood spots vs. punches).
- Ascertained the reference lab’s testing schedule, agreed on a preferred carrier and created a compatible shipping schedule (amended during holidays).
- Organized the secure delivery of reports and the return of residual blood spots to SHL.
- Labs exchanged the email addresses of staff involved in testing to ensure redundancy in the receipt of samples, results and correspondence.
2.1.3. INSP Preparations
- It should be noted that only in-house CFTR DNA testing was halted at SHL, and no other tests were affected. Also, downstream NBS processes remained fully functional and unaltered (follow-up, GC and medical consultations, confirmatory testing, diagnostic and therapeutic approaches, etc.).
- SHL updated its laboratory information management system (LIMS) to reflect the variants on the reference lab’s panel (performed only once because the 3 reference labs ran the same test/panel).
- We amended patient reports to indicate that CFTR DNA analysis was performed at a reference lab (address and Clinical Laboratory Improvement Amendments [CLIA] number were included).
- Shipping SOPs were revised, and shipping fees were calculated.
- SHL and follow-up SOPs were updated to reflect the new variants, and staff were trained accordingly.
2.1.4. Notifying Clients About Reference Labs (See Table 1)
- The initial communication was sent in October 2022, to the state coordinators of AK, IA, ND and SD, seeking approval for their samples to be tested by a non-SHL lab, as required contractually.
- The second major communication was to birthing facilities in January 2023. Owing to the increased turnaround time (TAT) for CFTR DNA results, the timeliness of patient reporting was delayed, resulting in a significant increase in call volume. To address this, SHL crafted a letter explaining the delays, that accompanied reports.
- The last major communication was in Aug 2023 to inform clients of the resumption of SHL’s in-house CFTR testing.
- In addition to these communiqués, CFTR assay challenges were routinesly discussed among the four states at our monthly ‘Quad state’ meeting.
| Contents of Communication | Recipient(s) |
|---|---|
| A concise and straightforward explanation of the problem and that pausing in-house testing was a necessary but temporary measure to ensure reliable test results | All Clients |
| Reassurance that SHL would implement a stable, robust and reliable alternative | All Clients |
| A request by SHL for approval to send samples to reference lab(s) | AK, IA, ND and SD state partners |
| Information on the use of a reference lab to perform CFTR DNA testing | All Clients |
| A list of variants on the reference lab’s panel | AK, IA, ND and SD state partners |
| An explanation of the decreased timeliness of patient reports awaiting CFTR DNA results | Birthing Facilities |
| Assurance that critical result reporting remained unaffected | Birthing Facilities |
| Explanation of SHL’s ongoing efforts to resolve issues | All Clients |
| Resumption of in-house testing and SHL’s appreciation of clients’ patience and understanding | AK, IA, ND and SD state partners |
2.2. Solving Phase 2 Problems: Implementing the New Test
2.2.1. SHL Identified Three Main Attributes Required for the New CFTR DNA Test
2.2.2. Networking and Market Research
- This began in Oct 2022 at the Association of Public Health Laboratories (APHL) [11] NBS symposium and allowed SHL staff to meet with vendors and state labs with expertise in CFTR DNA analysis. This exercise yielded three viable options. After follow-up meetings with the respective vendors and examining the feedback from various state labs, only one vendor met our requirements, so a demonstration of the test at an experienced state lab was conducted in Nov 2022.
- In Dec 2022, SHL officially selected a 39-variant CFTR DNA test from vendor #2, with patented technology that utilizes PCR and flow cytometry.
- SHL relayed the urgency of test implementation to vendor #2. Both parties agreed on a feasible completion deadline (end of February 2023) and developed a plan of action for the following: (1) quotes and contracts, (2) instrument procurement and service contracts, (3) purchase of auxiliary equipment and consumables, (4) reconfiguration of lab space, (5) instrument installation, (6) test validation, (7) staff training. SHL also requested that these documents be given priority by University of Iowa departments (legal, accounting, procurement services) for expedited document reviews.
2.2.3. Resolving the Issues Resulting from Delayed Test Implementation
- Despite our extensive planning, test implementation was not completed within the expected timeframe due to administrative (quotes and contracts), and logistical (instrument procurement/shipment) delays and technical issues.
- The administrative issues required a great deal of effort and communication with vendor #2 to be resolved. Once the equipment was installed, training of SHL staff by vendor #2 started. However, we experienced technical problems while trying to validate the test. Vendor #2 advised the lab on troubleshooting exercises which did not solve the problem. The lab then requested technical assistance from the U.S. Centers for Disease Control and Prevention (CDC), National Centers for Environmental Health, Newborn Screening and Molecular Biology Branch [12] and other public health labs.
3. Results
3.1. Effective Communication with Clients and Vendors
3.2. SHL Utilized In-House and External Technical Expertise to Resolve CFTR Assay Issues
3.3. Staff Initiative and Collaboration with the NBS Community Helped Resume In-House Testing
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pappas, K.B. Newborn Screening. Pediatr. Clin. N. Am. 2023, 70, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Gaviglio, A.; McKasson, S.; Singh, S.; Ojodu, J. Infants with Congenital Diseases Identified through Newborn Screening—United States, 2018–2020. Int. J. Neonatal Screen. 2023, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Recommended Uniform Screening Panel|HRSA. Available online: https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp (accessed on 19 June 2025).
- Intro to CF|Cystic Fibrosis Foundation. Available online: https://www.cff.org/intro-cf (accessed on 19 June 2025).
- Mall, M.A.; Burgel, P.-R.; Castellani, C.; Davies, J.C.; Salathe, M.; Taylor-Cousar, J.L. Cystic Fibrosis. Nat. Rev. Dis. Primer 2024, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Massie, J.; Sontag, M.; Southern, K.W. Newborn Screening for Cystic Fibrosis. Lancet Respir. Med. 2016, 4, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Travert, G.; Heeley, M.; Heeley, A. History of Newborn Screening for Cystic Fibrosis—The Early Years. Int. J. Neonatal Screen. 2020, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Iowa Newborn Screening Program|Health & Human Services. Available online: https://hhs.iowa.gov/programs-and-services/family-health/congenital-inherited-disorders/iowa-newborn-screening-program (accessed on 19 June 2025).
- State Hygienic Laboratory|The University of Iowa. Available online: https://shl.uiowa.edu/ (accessed on 19 June 2025).
- Stead Family Department of Pediatrics|Carver College of Medicine|The University of Iowa. Available online: https://pediatrics.medicine.uiowa.edu/ (accessed on 19 June 2025).
- APHL. Available online: http://www.aphl.org (accessed on 19 June 2025).
- CDC. Newborn Screening Home. Newborn Screening. Available online: https://www.cdc.gov/newborn-screening/index.html (accessed on 19 June 2025).
- Gallego Romero, I.; Ober, C. CFTR Mutations and Reproductive Outcomes in a Population Isolate. Hum. Genet. 2008, 122, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Pique, L.; Graham, S.; Pearl, M.; Kharrazi, M.; Schrijver, I. Cystic Fibrosis Newborn Screening Programs: Implications of the CFTR Variant Spectrum in Nonwhite Patients. Genet. Med. 2017, 19, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Rehani, M.R.; Marcus, M.S.; Harris, A.B.; Farrell, P.M.; Ren, C.L. Variation in Cystic Fibrosis Newborn Screening Algorithms in the United States. Pediatr. Pulmonol. 2023, 58, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Rock, M.J.; Baker, M.; Antos, N.; Farrell, P.M. Refinement of Newborn Screening for Cystic Fibrosis with Next Generation Sequencing. Pediatr. Pulmonol. 2023, 58, 778–787. [Google Scholar] [CrossRef] [PubMed]
- CF NBS Advisory: Michigan Department of Health and Human Services. Available online: https://www.michigan.gov/-/media/Project/Websites/mdhhs/Folder3/Folder10/Folder2/Folder110/Folder1/Folder210/CF_Advisory_to_Lab_Submitters_4_18_16.pdf?rev=7d77c88bce804c228c50e099bd509ed3 (accessed on 18 August 2025).
- Olney, R.S.; Bonham, J.R.; Schielen, P.C.J.I.; Slavin, D.; Ojodu, J. 2023 APHL/ISNS Newborn Screening Symposium. Int. J. Neonatal Screen. 2023, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Jeanne, M.; Chung, W.K. DNA Sequencing in Newborn Screening: Opportunities, Challenges, and Future Directions. Clin. Chem. 2025, 71, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Koval-Burt, C.; Kay, D.M.; Suchy, S.F.; Begtrup, A.; Langley, K.G.; Hernan, R.; Amendola, L.M.; Boyd, B.M.; Bradley, J.; et al. Expanded Newborn Screening Using Genome Sequencing for Early Actionable Conditions. JAMA 2025, 333, 232–240. [Google Scholar] [CrossRef] [PubMed]
- McGarry, M.E.; Raraigh, K.S.; Farrell, P.; Shropshire, F.; Padding, K.; White, C.; Dorley, M.C.; Hicks, S.; Ren, C.L.; Tullis, K.; et al. Cystic Fibrosis Newborn Screening: A Systematic Review-Driven Consensus Guideline from the United States Cystic Fibrosis Foundation. Int. J. Neonatal Screen. 2025, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Dwight, M.; Faro, A. It Takes All of Us: How the Cystic Fibrosis Foundation Is Supporting States in Advancing Cystic Fibrosis Newborn Screening. Int. J. Neonatal Screen. 2025, 11, 39. [Google Scholar] [CrossRef] [PubMed]

| Test Characteristics | Old Test (Custom Microfluidic Plates) | New Test (39-Variant Panel) |
|---|---|---|
| # Variants | Limited 25-variant panel (the defunct 42-variant expanded panel failed validation and was never used for patient testing) | Provided suitable detection of CF-causing variants in all 4 states |
| Vendor and Technology | Vendor #1—Single nucleotide polymorphism genotyping test | Vendor #2—Patented technology utilizing PCR and flow cytometry |
| Customization | Custom panel allowed greater flexibility in choosing variant panel | Variant panel is set by manufacturer |
| Physical footprint of test equipment/workspace | Workspace and instrument spaces were shared with other molecular tests | Needed larger footprint for test workflow, requiring lab reconfiguration |
| Cost of test | Relatively inexpensive | Almost double the cost of old test |
| FDA approved test | No (LDT) | Yes |
| Test runtime | ~4 h | Longer test (~6 h) |
| Reliability and Robustness of assay | Physical anomalies on assay plates and lot-to-lot performance variability observed | Robust, reliable and used by many US NBS state labs |
| Test type: qualitative/quantitative | Qualitative results, some manual formatting of data analysis software necessary | Qualitative results, data analysis software formatted by the manufacturer |
| Automation/ease of use | Mostly automated | Less automated: numerous manual pipetting and mixing steps |
| Training | Training is relatively simple | Training can be prolonged (depending on technical skill) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alleyne, J.; Coursey, K.; Noble Piper, K.; Cass, C.; Pentella, M. The Impact of Cystic Fibrosis Algorithm Changes: A Case Study of Challenges and Strategies. Int. J. Neonatal Screen. 2025, 11, 82. https://doi.org/10.3390/ijns11030082
Alleyne J, Coursey K, Noble Piper K, Cass C, Pentella M. The Impact of Cystic Fibrosis Algorithm Changes: A Case Study of Challenges and Strategies. International Journal of Neonatal Screening. 2025; 11(3):82. https://doi.org/10.3390/ijns11030082
Chicago/Turabian StyleAlleyne, Jerusalem, Kenneth Coursey, Kimberly Noble Piper, Cynthia Cass, and Michael Pentella. 2025. "The Impact of Cystic Fibrosis Algorithm Changes: A Case Study of Challenges and Strategies" International Journal of Neonatal Screening 11, no. 3: 82. https://doi.org/10.3390/ijns11030082
APA StyleAlleyne, J., Coursey, K., Noble Piper, K., Cass, C., & Pentella, M. (2025). The Impact of Cystic Fibrosis Algorithm Changes: A Case Study of Challenges and Strategies. International Journal of Neonatal Screening, 11(3), 82. https://doi.org/10.3390/ijns11030082






