Abstract
Starting from a new organic sunscreen synthesized in our laboratory by the condensation of sodium salt of the p-aminosalicylic acid with 2,6 – dichloroacetanilide, efficient cosmetic formulation with broad photoprotective properties was obtained by using the new compound as organic UV filter, metal oxides as inorganic filters, and vegetable extracts, oils and other ingredients with emollient, protective, and moisturizing effect. After showing the lack of toxicity of the new compound, in order to reduce the amount of UV filter used, it was encapsulated into nanostructured lipid carriers (NLCs). The final cream has demonstrated good qualities for skin application, possessing suitable physicochemical characteristics (pH, viscosity) and spreadability. The SPF value of the product is 16, a value considered to be satisfactory.
Introduction
Salicylates are water-insoluble, stable, and safe aromatic compounds used as UVB absorbers [1]. For the present study we used an organic sunscreen synthesized in our laboratory by the condensation of sodium salt of the p- aminosalicylic acid with 2,6 – dichloroacetanilide.
The molecular structure of the obtained compound was established by IR absorption, as represented in Figure 1.
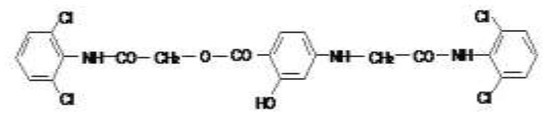
Figure 1.
The molecular structure of the synthesized esteramide.
A study of acute toxicity was developed in order to prove the safety of the product. Acute toxicity studies are designed to determine the dose that will produce either mortality or serious toxicological effects when given once or as multiple administrations. These studies provide an opportunity to determine the effects the compound on morphology, clinical chemistry, or other parameters [2].
After demonstrating the structure and the lack of toxicity of the new compound, it was encapsulated into nanostructured lipid carriers (NLCs) and we prepared a dermocosmetic product under a form of a cream. For this, we have selected, beside the studied esteramide, more active ingredients (inorganic filters, natural products, antioxidants), chosen to ensure a photoprotective and hydrating effect on the skin. In order to reduce the toxicity and the amount of UV filter used in cosmetic formulation [3], the condensation of sodium salt of the p-aminosalicylic acid with 2,6 – dichloroacetanilide was incorporated into nanostructured lipid carriers (NLCs).
Sunscreen creams are presenting a triple action: intense hydration; strong, well-balanced and photostable UVA and UVB protection [4], and synergistic long-lasting anti-free radicals’ protection.
These lipid nanostructures are new colloidal delivery systems developed from oil/ water emulsions type, where the oil phase was replaced by a mixture of solid and liquid lipids. NLCs are submicron particles with spherical shape and average diameters raging of 40-500 nm that are generally used for the delivery of lipophilic compounds. The lipid matrix is formed by physiological lipids that are not toxic [5].
The sea buckthorn seed oil has a remarkable ability to nourish, soothe, and protect the skin from sunburns. It slows the aging process of epidermis by the anti-free radicals’ effect [6,7] and by the regeneration of age-altered connective tissue. It can be successfully used for treating eczema, rash, and pruritus caused by different skin lesions. Lately, grapeseed oil is often used on the skin, which it nourishes, prevents its drying, and helps maintain youth and natural elasticity. It contains two powerful antioxidant ingredient called proanthocyanidin and trans-resveratrol, showing protective effects against UV-induced oxidative stress, by supporting the functionality of endogenous antioxidant systems, prevention of the photodegradation of biological macromolecules (lipids, proteins, DNA), and inhibition of activation of MAPK (mitogen-activated protein kinase) and NF-kB (kappa B nuclear factor) cell signaling pathways [8].
The present study aimed to formulate, prepare, and subsequently evaluate a sunscreen cream (W/O emulsion) assessed for organoleptic, functional, physicochemical, and rheological behavior parameters.
Materials and Methods
The synthesis of esteramide is activated by microwave action [9]. After 1 minute, only 25% of the reaction took place, according to the HPLC checking. That is why, in order to complete it, the reaction time was extended to 6.5 minutes, which is approximately 20 times as short as the time needed in classical conditions.
The 2,6 – dichloroacetanilide was prepared according to the reference material. The sodiumaminosalicylate, a Merck product, was not purified. The obtained compound was characterized in a previous study [3,9].
Within the electronic spectra there are wide absorption bands (from 280 to 320 nm) as result of the extended conjugation of the chromophore on the whole molecule after condensation of raw materials. The new compound presents the potential photoprotective action. The molecular structure of these compounds was established by UV, IR absorption and 1H-NMR spectra [3,9].
The acute toxicity study:
Animals
Ten healthy male NMRI mice weighing 31.5 ± 3 g obtained from the “Cantacuzino” Institute, Bucharest, Romania, were used. The animals were housed in plastic cages in an air-conditioned animal room and fed on granulated food with free access to water. The temperature and relative humidity were continuously monitored using a thermo-hygrometer ranging between 20–22 °C and respectively 35–45%. All procedures were carried out in accordance with the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes and the implementing Law no. 43/2014 on the protection of animals used for scientific purposes.
Experimental design
The esteramide obtained by condensation of sodium salt of the p-aminosalicylic acid with 2,6 – dichloroacetanilide was orally administered to the animals in the dose of 1000 mg/kg bw, as an 10% aqueous suspension. The mice were monitored for 14 days for: lethality, body weight dynamics, social behaviour, and appearance.
Statistical analyses
Statistical analyses were performed with GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com. Results are expressed as mean ± standard deviation. The applied t test has a 90% confidence interval (CI90%). Statistical significance was considered for p < 0.05.
The preparation method of NLCs was the melt emulsification method coupled with high shear homogenization method. The two phases (lipid and aqueous) were separately heated at 85°C while stirring. Then, the lipid phase was gradually added over the aqueous phase. The emulsion was heated under stirring at 85°C for 1 h, and then homogenized by applying 25000 rpm for 10 min (High-shear homogenizer PRO250). To remove the water, the dispersions were frozen for 24 h and then lyophilized using a Christ Alpha 1–2 LD Freeze Dryer for 72 h at −55 °C.
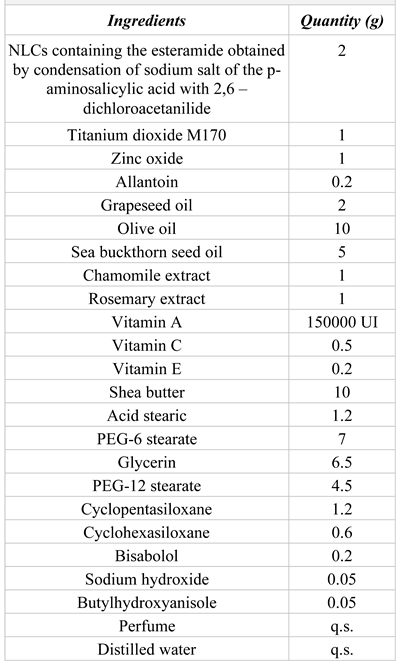
Formulation and obtaining the cosmetic preparation The cream formulation consists in the association between the active ingredients and the adequate excipients, chosen in certain amounts, in order to assure the final product with suitable physicochemical characteristics and an optimal photoprotective efficiency. The formulation is shown in Table I.

Table I.
The formulation of the photoprotective product.
Ingredients:
- -
- one new organic filtering photoprotective substance previously obtained according to the reference method encapsulated in the form of NLCs, in 2% dose;
- -
- two inorganic screen photoprotective substances: titanium dioxide coated with alumina and silicon (titanium dioxide M170) and zinc oxide which acts on the entire UVA and UVB spectrum;
- -
- vegetal oils: grape seed oil (contains important amounts of vitamins E and F, minerals: zinc, copper, selenium, and above all, the procyanidins which are antiaging agents 50 times stronger than vitamin E and C), olive oil (consisting mainly of fatty acids), sea buckthorn seed oil (extracted from Hippophae rhamnoides seeds, is rich in bioactive substances as carotenoids, flavonoids, vitamins A, C, E, B1, B2, K and PP, as well as microelements, essential fatty acids and phytosterols);
- -
- vegetal extracts: Chamomile extract (bisabolol and matricine are wound healing, anti-inflammatory and antiallergic agents), Rosemary extract (antioxidative, antimicrobial, anti-inflammatory and anti-aging properties, inhibits the oxidation of polyunsaturated fatty acids in membranes),
- -
- natural antioxidants: vitamins A, E, and C;
- -
- natural products having a slight photoprotective, a hydrating and emollient actions like allantoin and shea butter [10];
- -
- cyclopentasiloxane and cyclohexasiloxane are silicon- based compounds with emollient and lubricant properties. After their use, the skin remains soft, with a silky texture, due to the fact that it forms a seal or barrier on the skin. This barrier protects against transepidermal water loss. Usually they are used in combination to assure a better function as carrying and wetting agents. They have no known side effects;
- -
- viscosity modifying agents; preservatives, natural perfumed composition.
The lipid components and zinc oxide were melted together on a water bath heated at a temperature of about 40-50ºC. The titanium dioxide was dispersed in the aqueous phase and then heated at a temperature slightly higher than that of the lipophilic phase, and then they were gradually brought together while stirring. The obtained emulsion was cooled to a temperature of 30ºC, a temperature that allowed the incorporation of the esteramide, vitamin A, C, E and perfume.
Quality control of the photoprotective product
The organoleptic control (appearance, color, smell, finesse, adhesion) was performed according to F.R.X guidelines.
The pH determination was carried out in an aqueous phase, which was obtained by shaking 1 g of product into 10 ml heated water.
Spreadability on the skin: The determination was performed using the extensiometric method. This analysis determines the deformation ability of the product when several forces are acting on it, by using Ojeda Arbussa method [11,12,13].
Viscosity: In order to evaluate the structural characteristics of the product, we used Thermo Haake VT550 (ViscoTesterVT550), Thermo Electron (Karlsruhe) GmbH, Germany, a rheometer with the cylinder assembly SV – DIN (0 – 100 range of shear speeds, 10 ml volume). The primary registration and processing of the dates was done by using Haake RheoWin DataManager, Version 4.30.0011, Thermo Electron (Karlsruhe) GmbH, Germany, a compatible specialized software.
For analysis of product flowability, the recorded data has been evaluated by applying the Ostwald de Waele model [14,15].
The determination of SPF: In this study, the SPF was determined in vitro according to the methodology of Diffey-Robson (16), using an UV- VIS Spectrometer V670 apparatus (Jasco) equipped with integrating sphere and adequate software. The method is based on measuring the radiation transmitted by the sample deposited on the substrate (2 mg/cm2), by recording the photocurrent from 5 to 5 nm on 290-400 nm (UVB, UVA). The cosmetic preparation was applied to a synthetic skin (Transpore tape) and subjected to spectrophotometric analysis at six different points. The average recorded values were used to calculate the SPF, according to the equation:
where Eλ is the spectral irradiance of terrestrial sun light under defined conditions; Bλ is the erythema effectiveness; MPFλ is the monochromatic protection factor for each wavelength increment [3].
SPF =Σ ΕλΒλ / Σ (ΕλΒλ)/ΜPFλ
The results registered in the acute toxicity study are presented in Figure 2.
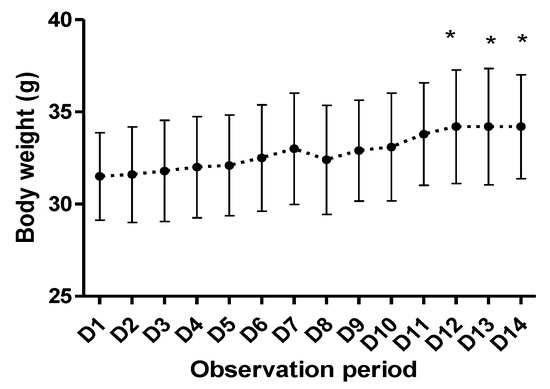
Figure 2.
Body weight evolution of the tested animals (* p < 0.05).
Photoprotective product characteristics
The cosmetic formulation showed a homogeneous appearance, with white to yellowish color and a pleasant smell. These characteristics remained unchanged during 3 months storage, there being no phase separation or any sort of precipitation. The formulation presented a pH of 6.3, immediately after preparation, and remained unchanged throughout the 90 days of testing.

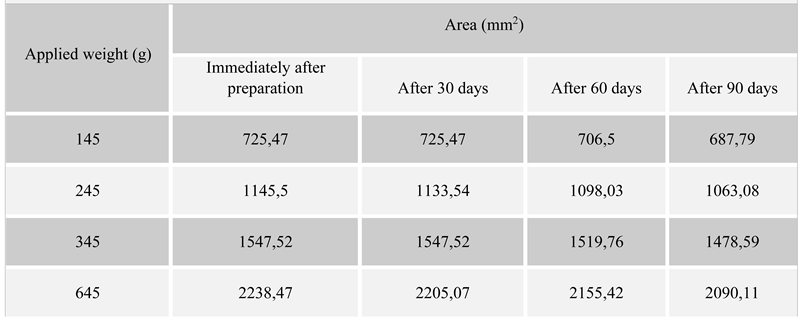
Table II.
Variation of spread area with the applied weight.
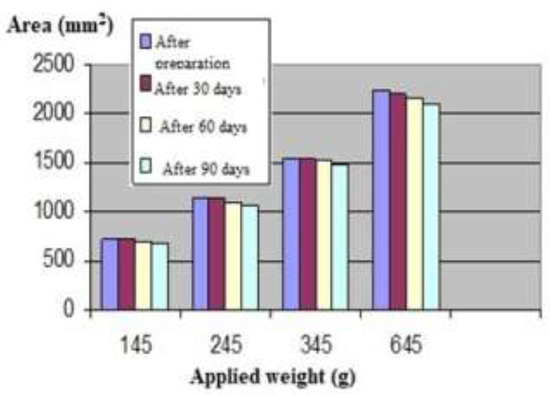
Figure 3.
Extensiometric curve of the cosmetic product.
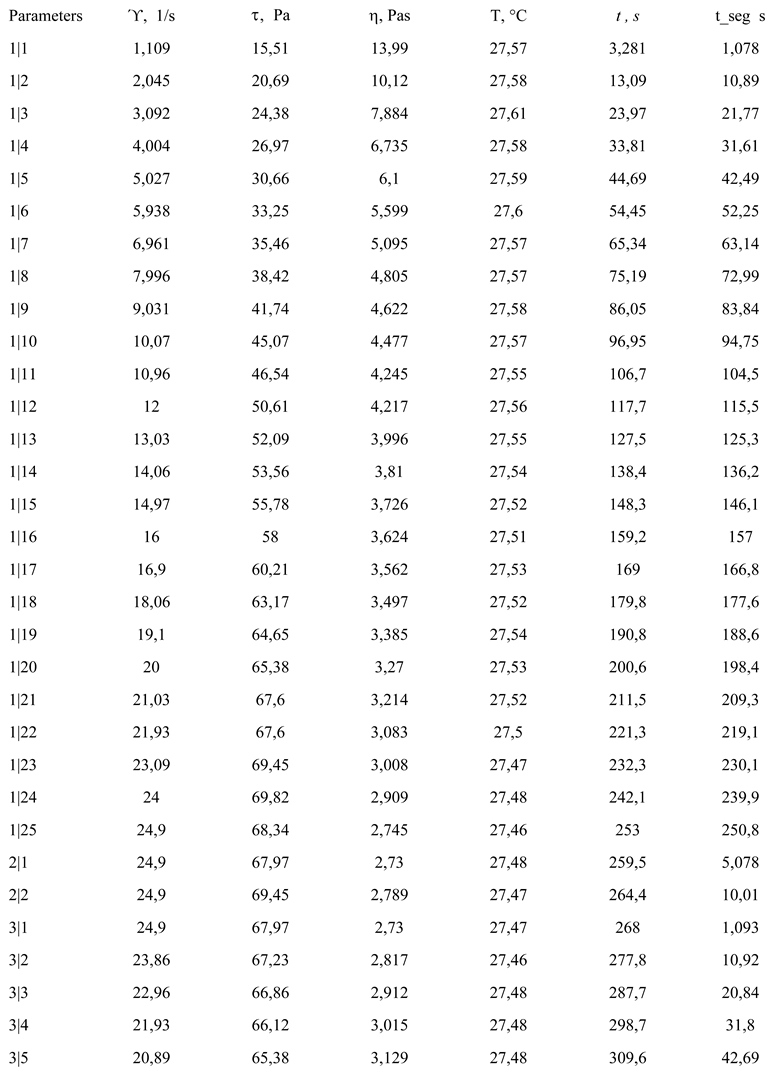
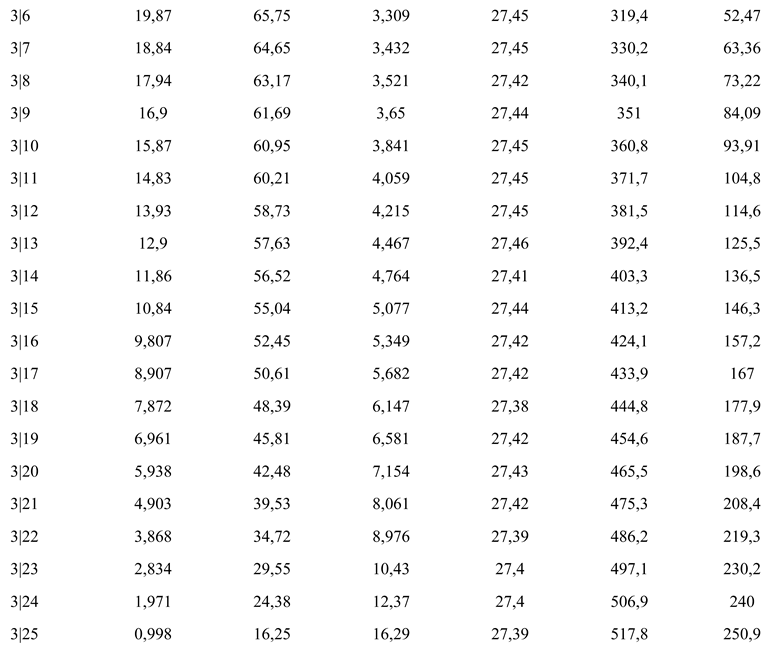
The experimental results obtained for the photoprotective product are presented by variation in shear tension as a function of shear speed and viscosity (Table III and Figure 4).

Table III.
Variation of shear tension and viscosity.
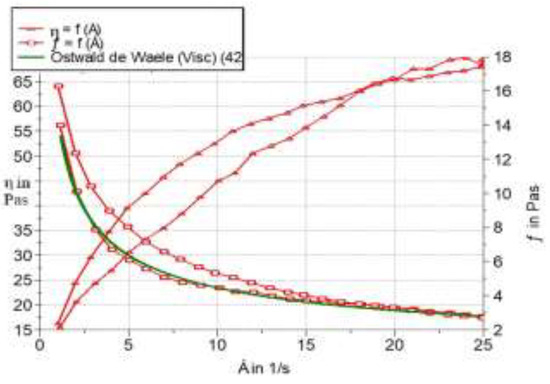
Figure 4.
Variation of viscosity function of shear tension.
The determination of SPF
UV protection factor of the final product was 16, a value considered to be satisfactory, the product being able to offer a good protection.
Discussions
After the animal’s exposure to the studied compound, in the dose of 1000 mg/kg bw, no lethal effect was registered. During the observation period no signs of alterations related to social behavior or appearance were noted. The mice body weight showed a positive trend during the observation period, with statistical significance by the end of the experiment (Figure 2).
The pH values obtained after preparation and during the 90 days of testing show that the product is compatible with the skin pH and does not causes skin irritation when applied [17].
Regarding the spreading characteristics, the surface of display increases with increasing the added weights, showing good plasticity which is maintained during the storage time of 90 days. The formulation has very good spreadability [18,19].
The flowability is an essential element both for the purpose of ensuring compliance, but also to ensure a controlled deformation after administration, favorable for drug release.
The coefficients of correlation between experimental and estimated data, according to the model, present values higher than 0.999. We note the sub-unit values of the flow indices, which confirm the pseudoplastic character of the product [17,20].
The dichloroacetanilide, and also to the other ingredients used in the formulation (titanium dioxide, zinc oxide, grapeseed, sea buckthorn seed oil and olive oil), leading to the conclusion that the selected ingredients had a synergic mechanism of action [18,19,20,21].
Conclusions
The present study started with the synthesis process of a photoprotective product by condensation of sodium salt of the p-aminosalicylic acid with 2,6 – dichloroacetanilide. After establishing its molecular structure, a test for acute toxicity was conducted. At the end of the observation period, no changes in the term of lethality, motor behavior, aggressivity, or aspect (fur, mucus) were registered. The product was encapsulated into nanostructured lipid carriers (NLCs) in order to reduce the amount of UV filter used in cream formulation.
Starting from the lyophilized esteramide-lipid nanostructures, efficient cosmetic formulation with broad photoprotective properties was obtained by using the new compound as organic UV filter, metal oxides as inorganic filters, and vegetable extracts, oils and other ingredients with emollient, protective, and moisturizing effects. The obtained cosmetic product has proven qualities for skin application, possessing suitable physicochemical characteristics (pH, viscosity) and spreadability.
Regarding the cream’s flow behavior, the rheological profile showed it is fluid enough to flow easily, but also will not drip and remains on the application site in the presence of a mechanical stress, inevitable and characteristic for the administration of a topical semi-solid product. The flowability significantly affects the amount of ingredients transferred to the target area of the human body.
The studied product has a pseudoplastic flow behavior, this being confirmed by applying the Ostwald de Waele method.
The SPF value of the product is 16, the final pharmaceutical form showing an adequate protection of the skin from sunburn.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- Bury, D.; Brüning, T.; Koch, H.M. Determination of metabolites of the UV filter 2-ethylhexyl salicylate in human urine by online-SPE-LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2019, 1110–1111, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, R. Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Eds.; Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3rd ed.; Academic press: Cambridge, MA, USA, 2013; pp. 595–618. [Google Scholar]
- Mănescu, I.G.; Badea, G.; Iscrulescu, L.; Iovu, M.; Balaci, T. Incorporation of new benzimidazole compounds into lipid nanostructures in order to obtain photoprotective formulations. Farmacia 2015, 63, 518–525. [Google Scholar]
- Sbora, R.; Budura, E.A.; Niţulescu, G.M.; Balaci, T.; Lupuleasa, D. Preparation and characterization of inclusion complexes formed by avobenzone with β- cyclodextrin, hydroxypropyl-β-cyclodextrin and hydroxypropyl-α-cyclodextrin. Farmacia 2015, 63, 548–555. [Google Scholar]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. ArtiCells Nanomed Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Dărmănescu, D.; Mihele, D.; Dogaru, E.; Cocu, F. Experimental evaluation of some biochemical parameters after administration of fatty acids ethanolamides from extra virgin olive oil in rats. Farmacia 2010, 58, 228–236. [Google Scholar]
- Mititelu, M.; Moroşan, E.; Iosif, M.; Ioniţă, E.I. Analisys of qulity of different types of honey from various sources. Proceedings of The Romanian National Congress of Pharmacy–17th Edition, “21st Century Pharmacy–Between Intelligent Specialization and Social Responsibility”; Filodiritto Editore–Proceedings 2018; pp. 84–87. [Google Scholar]
- Mantena, S.K.; Katiyar, S.K. Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF- kappaB signaling in human epidermal keratinocytes. Free Radic Biol Med. 2006, 40, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, S.R.; Tampa, M.; Paunica, S.; et al. Distribution of post-finasteride syndrome in men with androgenic alopecia. J. Investig. Dermatol. 2015, 135 (Suppl. S2), S40. [Google Scholar]
- Perianu, L.; Khafaf, B.; Nițulescu, G.M.; Iacob, D.; Iovu, M. Microwave-assisted Synthesis of 2-p-Amino- Salicyloxyacetanilides. REV. CHIM. (Bucharest). 2010, 61, 562–564. [Google Scholar]
- Hovaneţ, M.V.; Oprea, E.; Ancuceanu, R.V.; Duţu, L.E.; Budura, E.A.; Şeremet, O.; Ancu, I.; Moroşan, E. Wound healing properties of Ziziphus jujuba mill. Leaves. Rom. Biotechnol. Lett. 2016, 21, 11842–9. [Google Scholar]
- Motofei, I.G.; Rowland, D.L.; Baconi, D.L.; et al. Androgenetic alopecia; drug safety and therapeutic strategies. Expert Opin Drug Saf. 2018, 17, 407–412. [Google Scholar] [CrossRef]
- Dimcevici Poesina, N.; Bălălău, C.; Bârcă, M.; et al. Testicular histopathological changes following sodium fluoride administration in mice. Rom J Morphol Embryol. 2013, 54, 1019–1024. [Google Scholar] [PubMed]
- del Pozo Carrascosa, A.; Suñé Negre, J.M.; Faulí Trillo, C. Diseño de los modelos matemáticos que rigen los fenómenos de extensibilidad de pomadas [Design of mathematical models of ointment extensibility]. Boll Chim Farm. 1987, 126, 330–335. [Google Scholar] [PubMed]
- Popa, E.A.; Popovici, I.; Braha, S.L. Popovici, I., Lupuleasa, D., Eds.; Forme farmaceutice bioadezive, cap. XXIX. In Tehnologie Farmaceutică; 2008; Volume 2, pp. 742–749. [Google Scholar]
- Ostwald, W. Ueber die rechnerische darstellung des strukturgebietes der viskositat. Kolloid-Z. 1929, 47, 176–187. [Google Scholar] [CrossRef]
- Motofei, I.G.; Rowland David LBaconi, D.L.; et al. Therapeutic considerations related to finasteride administration in male androgenic alopecia and benign prostatic hyperplasia. Farmacia 2017, 65, 660–666. [Google Scholar]
- Shapovalov, V.M. On the Applicability of the Ostwald–De Waele Model in Solving Applied Problems. J Eng Phys Thermophy. 2017, 90, 1213–1218. [Google Scholar] [CrossRef]
- Bălălău, C.; Voiculescu, S.; Motofei, I.; Scăunașu, R.V.; Negrei, C. Low dose tamoxifen as treatment of benign breast proliferative lesions. Farmacia 2015, 63, 1028–1036. [Google Scholar]
- Diffey, B.; Robson, J. A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J Soc Cosmet Chem. 1989, 40, 127–133. [Google Scholar]
- Gavriloaia, M.R.; Budura, E.A.; Toma, C.C.; Mitu, M.A.; Karampelas, O.; Arama, C.; Lupuleasa, D. In vitro evaluation of diffusion and rheological profiles for dexamethasone inclusion complexes with β- cyclodextrin or hydroxypropyl β-cyclodextrin. Farmacia 2012, 60, 895–904. [Google Scholar]
© 2020 by the authors. 2020 Teodora Dalila Balaci, Emma Adriana Ozon, Daniela Luiza Baconi, Georgiana Nițulescu, Bruno Velescu, Cristian Bălălău, Ioana Păunică, Cătălina Ancuța Fița