Perforated Small Intestine in a Patient with T-Cell Lymphoma; A Rare Cause of Peritonitis
Abstract
Introduction
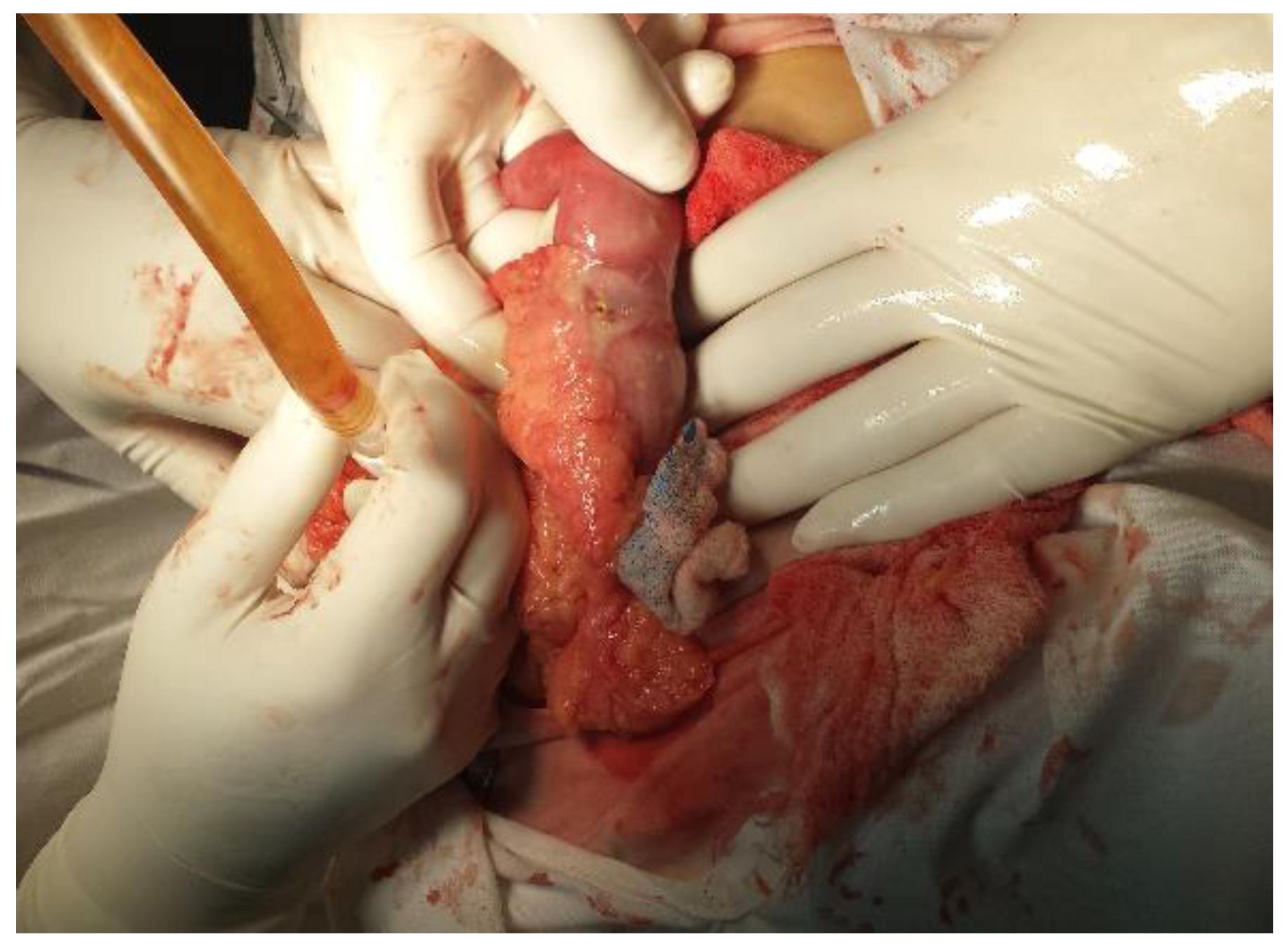
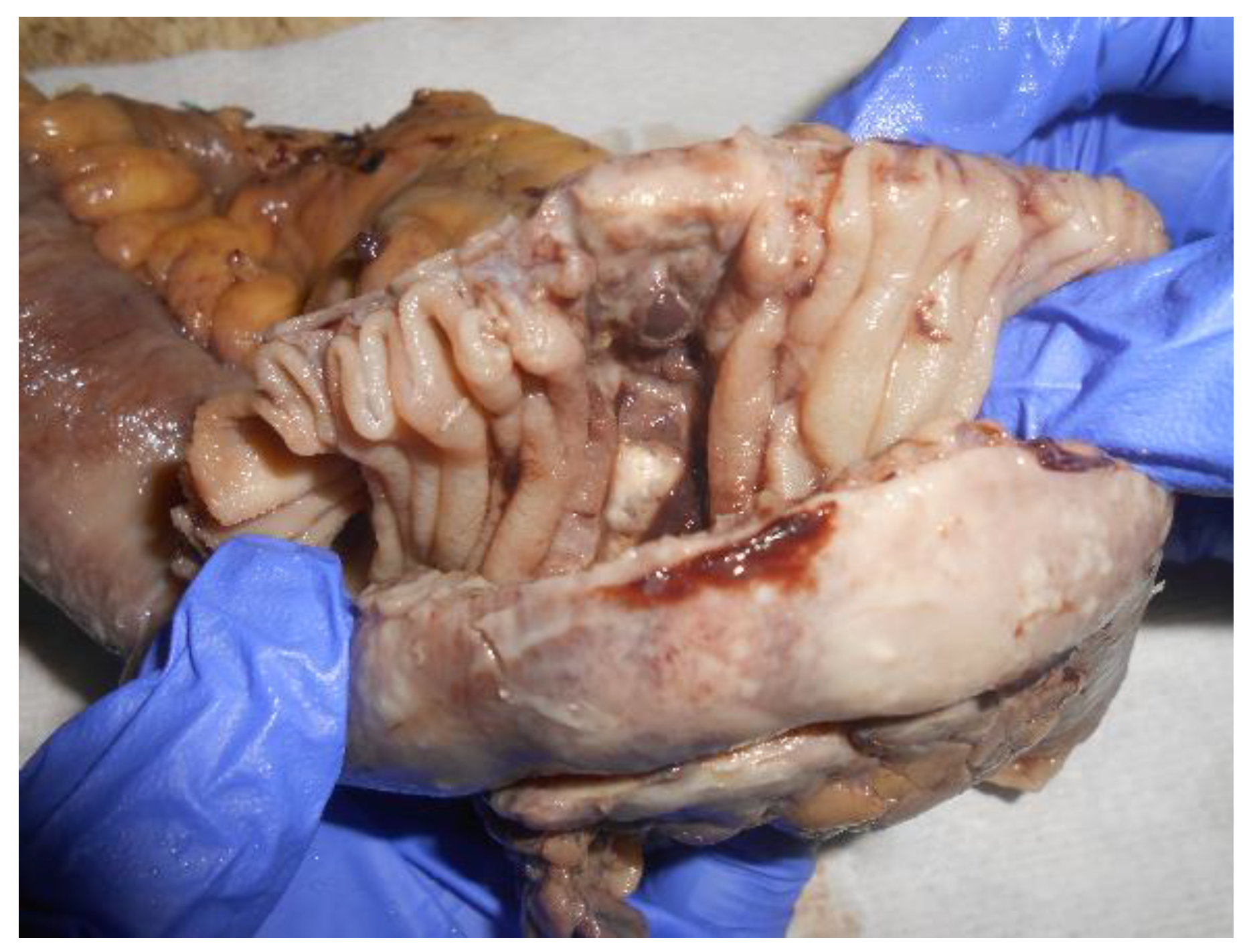
Case report
Discussion
Conclusions
References
- Eustache, J.M.; Kreis, D.J. Typhoid perforation of the small intestine. Arch Surg 1983, 118, 1269–71. [Google Scholar] [PubMed]
- Khanna, A.K.; Misra, M.K. Typhoid perforation of the gut. Postgrad Med J 1984, 60, 523–5. [Google Scholar]
- Leijonmarck, C.E.; Fenyo, G.; Raf, L. Nontraumatic perforation of the small intestine. ActaChirScand 1984, 150, 405–411. [Google Scholar]
- Kimchi, N.A.; Broide, E.; Shapiro, M.; Scapa, E. Non-traumatic perforation of the small intestine. Report of 13 cases and review of the literature. Hepato-Gastroenterology 2002, 49, 1017–22. [Google Scholar] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J Clin. 2015, 65, 5–29. [Google Scholar] [PubMed]
- Bilimoria, K.Y.; Bentrem, D.J.; Wayne, J.D.; Ko, C.Y.; Bennett, C.L. ; TalamontiMS Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009, 249, 63–71. [Google Scholar]
- Anderson, T.; Chabner, B.A.; Young, R.C.; Berard, C.W.; Garvin, A.J.; Simon, R.M.; DeVita, V.T., Jr. Malignant lymphoma. 1. The histology and staging of 473 patients at the National Cancer Institute. Cancer 1982, 50, 2699–707. [Google Scholar]
- Abbott, S.; Nikolousis, E.; Badger, I. Intestinal lymphoma--a review of the management of emergency presentations to the general surgeon. Int J Colorectal Dis. 2015, 30, 151–7. [Google Scholar]
- Gurney, K.A.; Cartwright, R.A.; Gilman, E.A. Descriptive epidemiology of gastrointestinal non-Hodgkin's lymphoma in a population- based registry. Br J Cancer 1999, 79, 1929–34. [Google Scholar]
- Ghimire, P.; Wu, G.Y.; Zhu, L. Primary gastrointestinal lymphoma. World Journal of Gastroenterology 2011, 17, 697–707. [Google Scholar]
- Mori, M.; Kobayashi, Y.; Maeshima, A.M.; Gotoda, T.; Oda, I.; Kagami, Y.; Bennett, S.; Nomoto, J.; Azuma, T.; Yokoyama, H.; Maruyama, D.; Kim, S.W.; Watanabe, T.; Matsuno, Y.; Tobinai, K. The indolent course and high incidence of t(14;18) in primary duodenal follicular lymphoma. Ann Oncol. 2010, 21, 1500–5. [Google Scholar] [PubMed]
- Ruskoné-Fourmestraux, A.; Rambaud, J.C. Gastrointestinal lymphoma: prevention and treatment of early lesions. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 337–354. [Google Scholar]
- Stolte, M.; Bayerdorffer, E.; Morgner, A.; Alpen, B.; Wundisch, T.; Thiede, C.; Neubauer, A. Helicobacter and gastric MALT lymphoma. Gut. 2002, 50, 19–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herrmann, R.; Panahon, A.M.; Barcos, M.P.; Walsh, D.; Stutzman, L. Gastrointestinal involvement in non-Hodgkin's lymphoma. Cancer 1980, 46, 215–22. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.; Montalbán, C. Primary diffuse large B cell lymphoma of the stomach. Crit Rev Oncol Hematol. 2007, 63, 65–71. [Google Scholar]
- Koch, P.; del Valle, F.; Berdel, W.E.; Willich, N.A.; Reers, B.; Hiddemann, W.; Grothaus-Pinke, B.; Reinartz, G.; Brockmann, J.; Temmesfeld, A.; Schmitz, R.; Rübe, C.; Probst, A.; Jaenke, G.; Bodenstein, H.; Junker, A.; Pott, C.; Schultze, J.; Heinecke, A.; Parwaresch, R.; Tiemann, M. Primary gastrointestinal non-Hodgkin’s lymphoma: I. anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol. 2001, 19, 3861–73. [Google Scholar] [CrossRef]
- Dionigi, G.; Annoni, M.; Rovera, F.; Boni, L.; Villa, F.; Castano, P.; Bianchi, V.; Dionigi, R. Primary colorectal lymphomas: review of the literature. Surg Oncol. 2007, 16, S169–71. [Google Scholar] [CrossRef]
- Anderson, T.; Chabner, B.A.; Young, R.C.; Berard, C.W.; Garvin, A.J.; Simon, R.M.; DeVita, V.T., Jr. Malignant lymphoma. 1. The histology and staging of 473 patients at the National Cancer Institute. Cancer 1982, 50, 2699–707. [Google Scholar]
- Pennazio, M. Small-intestinal pathology on capsule endoscopy: spectrum of vascular lesions. Endoscopy 2005, 37, 864–9. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nakamura, S.; Esaki, M.; Yada, S.; Moriyama, T.; Yanai, S.; Hirahashi, M.; Yao, T.; Iida, M. Double-Balloon Endoscopy Depicts Diminutive Small Bowel Lesions in Gastrointestinal Lymphom. Dig Dis Sci. 2010, 55, 158–165. [Google Scholar]
- Dawson, I.M.; Cornes, J.S.; Morson, B.C. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961, 49, 80–89. [Google Scholar]
- Cooper, M.J.; Williamson, R.C. Enteric adenoma and adenocarcinoma. World J Surg. 1985, 9, 914–20. [Google Scholar]
- Sweetenham, J.W.; Mead, G.M.; Wright, D.H.; McKendrick, J.J.; Jones, D.H.; Williams, C.J.; Whitehouse, J.M. Involvement of the ileocaecal region by non-Hodgkin's lymphoma in adults: clinical features and results of treatment. Br J Cancer. 1989, 60, 366–9. [Google Scholar]
- Ruskoné-Fourmestraux, A.; Dragosics, B.; Morgner, A.; Wotherspoon, A.; de Jong, D. Paris staging system for primary gastrointestinal lymphomas. Gut. 2003, 52, 912–913. [Google Scholar] [PubMed]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011, 117, 5019–5032. [Google Scholar]
- Nakamura, S.; Matsumoto, T. Gastrointestinal lymphoma: Recent advances in diagnosis and treatment. Digestion 2013, 87, 182–188. [Google Scholar] [PubMed]
- Fischbach, W.; Goebeler-Kolve, M.E.; Dragosics, B.; Greiner, A.; Stolte, M. Long term outcome of patients with gastric marginal zone B cell lymphoma of mucosa associated lymphoid tissue (MALT) following exclusive Helicobacter pylori eradication therapy: experience from a large prospective series. Gut. 2004, 53, 34–7. [Google Scholar]
- Hansen, P.B.; Vogt, K.C.; Skov, R.L.; Pedersen- Bjergaard, U.; Jacobsen, M.; Ralfkiaer, E. Primary gastrointestinal non-Hodgkin's lymphoma in adults: a population-based clinical and histopathologic study. J Intern Med. 1998, 244, 71–8. [Google Scholar] [PubMed]
- Khosla, D.; Kumar, R.; Kapoor, R.; Kumar, N.; BeraA, *!!! REPLACE !!!*; Sharma, S.C. A retrospective analysis of clinicopathological characteristics, treatment, and outcome of 27 patients of primary intestinal lymphomas. J Gastrointest Canc. 2013, 44, 417–421. [Google Scholar]
- Dickson, B.C.; Serra, S.; Chetty, R. Primary gastrointestinal tract lymphoma: diagnosis and management of common neoplasms. Expert Rev Anticancer Ther. 2006, 6, 1609–1628. [Google Scholar]
- Ghimire, P.; Wu, G.Y.; Zhu, L. Primary gastrointestinal lymphoma. World Journal of Gastroenterology 2011, 17, 697–707. [Google Scholar]
- Chen, J.H.; Ho, C.L.; Chen, Y.C.; Chao, T.Y.; Kao, W.Y. Clinicopathological analysis and prognostic factors of 11 patients with primary non-Hodgkin lymphoma of the small intestine in a single institute. Oncol Lett. 2014, 8, 876–880. [Google Scholar]
- Ibrahim, E.M.; Ezzat, A.A.; El-Weshi, A.N.; Martin, J.M.; Khafaga, Y.M.; Al Rabih, A.; AjarimDS, *!!! REPLACE !!!*; Al- Foudeh, M.O.; Zucca, E. Primary intestinal diffuse large B-cell non-Hodgkinslymphoma: clinical features, management, and prognosis of 66 patients. Ann Oncol. 2001, 12, 53–58. [Google Scholar]
- Vaidya, R.; Habermann, T.M.; Donohue, J.H.; Ristow, K.M.; Maurer, M.J.; Macon, W.R.; Colgan, J.P.; Inwards, D.J.; Ansell, S.M.; Porrata, L.F.; Micallef, I.N.; Johnston, P.B.; Markovic, S.N.; Thompson, C.A.; Nowakowski, G.S. ; WitzigTE Bowel perforation in intestinal lymphoma: incidence and clinical features. Ann Oncol 2013, 24, 2439–2443. [Google Scholar]
- Vaidya, R.; Habermann, T.M.; Donohue, J.H.; Ristow, K.M.; Maurer, M.J.; Macon, W.R.; Colgan, J.P.; Inwards, D.J.; Ansell, S.M.; Porrata, L.F.; Micallef, I.N.; Johnston, P.B.; Markovic, S.N.; Thompson, C.A.; Nowakowski, G.S.; Witzig, T.E. Bowel perforation in intestinal lymphoma: incidence and clinical features. Ann Oncol. 2013, 24, 2439–2443. [Google Scholar]
- Li, M.; Zhang, S.; Gu, F.; Xiao, W.; Yao, J.; Chao, K.; Chen, M.; Li, J.; Zhong, B. Clinicopathological characteristics and prognostic factors of primary gastrointestinal lymphoma: a 22-year experience from South China. Int J Clin ExpPathol. 2014, 7, 2718–2728. [Google Scholar]
© 2016 by the authors. 2016 Petrişor Banu, Vlad D. Constantin, Florian Popa, Mihaela F. Nistor. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banu, P.; Constantin, V.D.; Popa, F.; Nistor, M.F. Perforated Small Intestine in a Patient with T-Cell Lymphoma; A Rare Cause of Peritonitis. J. Mind Med. Sci. 2016, 3, 88-98. https://doi.org/10.22543/2392-7674.1029
Banu P, Constantin VD, Popa F, Nistor MF. Perforated Small Intestine in a Patient with T-Cell Lymphoma; A Rare Cause of Peritonitis. Journal of Mind and Medical Sciences. 2016; 3(1):88-98. https://doi.org/10.22543/2392-7674.1029
Chicago/Turabian StyleBanu, Petrişor, Vlad D. Constantin, Florian Popa, and Mihaela F. Nistor. 2016. "Perforated Small Intestine in a Patient with T-Cell Lymphoma; A Rare Cause of Peritonitis" Journal of Mind and Medical Sciences 3, no. 1: 88-98. https://doi.org/10.22543/2392-7674.1029
APA StyleBanu, P., Constantin, V. D., Popa, F., & Nistor, M. F. (2016). Perforated Small Intestine in a Patient with T-Cell Lymphoma; A Rare Cause of Peritonitis. Journal of Mind and Medical Sciences, 3(1), 88-98. https://doi.org/10.22543/2392-7674.1029


