Abstract
Chronic varicose veins disease is widespread worldwide (up to 60% in the adult population), with a significant impact on patients’ quality of life and a high cost to society, both due to treatment and the limitation of physical activity imposed by the disease. This article presents a retrospective study on a number of 14 patients treated in Arestetic Clinic in Galati, for a period of one year. The patients included in the study presented reticular and varicose veins up to 6 mm in diameter and under 14 mm on the saphenous vein. Patients completed the Aberdeen Varicose Disease Questionnaire (excluding the first part of the questionnaire) and the Venous Clinical Severity Score was used by the physician. Doppler ultrasound was used for preoperative evaluation and postoperative monitoring. Polidocanol was administered in concentrations of 0.5% and 2%, with the patients being monitored one year after treatment. All patients were declared satisfied with the results and 1 year after treatment no recurrences were found. Polidocanol sclerotherapy is a safe, easy-to-administer, low-cost treatment with no healing time and can be administered multiple times if needed, with minimal risks of complications. Aesthetic results are good, with an important symptom remission rate and high patient satisfaction. Complications were rare and minor (represented by local hyperpigmentation and hardening of the injected path), and the recurrence rate is comparable to surgical treatment.
Introduction
The chronic varicose vein disease is widely spread across the world, with significant impact on the quality of a patients’ life and with a high cost for society, on one hand because of the treatment and on the other hand because the patients need to take time off from their daily activities. Chronic venous disease is characterized by dilatation of the subcutaneous veins of the lower limbs. Varicose veins are determined by the saphenous-femoral valve failure, which causes the appearance of reflux on the large saphenous vein and its collaterals but can also affect the small saphenous vein [1].
Varicose vein disease has a high incidence among the adult population, in a percentage between 20% and 60% [2]. In Brazil, chronic venous disease ranks no. 14 for temporary absenteeism and no. 32 for permanent disability and public funding assistance [3]; in USA the incidence is between 15-28% among women and about 6% among men [4].
Due to the considerations above, solutions were constantly sought for a more efficient therapeutic solution and with lower costs for patient, society, and health systems. Sclerotherapy seems to fit both desires, being an easy therapy, with low cost, no recovery time and with results that can be compared with other forms of treatment. Sclerotherapy ca be used as a single method of treatment or it can be associated with other procedures as laser or radiofrequency [5].
Materials and Methods
A number of 14 patients, treated in Arestetic Clinic Galati, were analyzed for a period of one year. Inclusion criteria in the study: patients with reticular veins and hydrostatic varices caused by chronic primary venous insufficiency due to reflux in superficial veins, with a diameter below 6 mm and large saphenous vein below 1.4 cm in diameter. Exclusion criteria for the study: patients with active ulcers and people with associated diseases that can influence the evolution and prognosis of varicose disease: peripheral arterial disease, known allergic history of polidocanol, diabetes mellitus, heart failure, respiratory failure, non-controlled hypertension, hypothyroidism, pregnancy, lactation, pulmonary hypertension, deep vein thrombosis (DVT), known hypercoagulability or thrombophilia, asthma, migraine.
Patients completed the Aberdeen questionnaire for varicose disease (AVVQ), apart from the first point (https://www.vasculab.it/obchiva/forms/QoL/AVVQ_form_temp.pdf).
The Venous Clinical Severity Score (VCSS) was used by the physician (Table 1).

Table 1.
Venous Clinical Severity Score (VCSS).
Prior to the start of the treatment, the following anatomical segments were subjected to Doppler ultrasound: the femoral vein, the popliteal vein, the sapheno-femoral junction, the sapheno-popliteal junction, the perforating veins and the small saphenous vein.
Prior the injection, the pelvic limb subject to treatment was raised to an angle of 45° for 15 min. The sapheno-femoral junction was compressed during the injection and about 10 min after the injection. After the treatment, the patient remained for about 20 min in clinostatism. No local anesthesia was applied. After the procedure was done, class 2 compression stockings (22-23 mmHg) were applied for 14 days. The sessions were repeated after 4 weeks. Between 1 and 3 treatment sessions were performed. After treatment completion Doppler ultrasound was repeated at 6 and 12 months. At 1 year, a new Venous Clinical Severity Score (VCSS) was completed again, and the results were evaluated. As sclerosing agents, it was used 0.5% and 2% polidocanol (Aethoxysklerol).
Results
The age of the patients included in the study was between 31 and 62 years, the average age was 49.5 years. The gender disposition was: 13 women and 1 man, with a female/male ration of 13/1. A number of 12 women included in the study had one or more births in their case history.
Other associated factors were obesity (4 persons—28.5%), smoking (5 persons—35.7%). Prolonged orthostatism was associated to 9 persons (64.2%) (Figure 1). Family history of varicose vein disease was present in 12 of the 14 patients (85%).

Figure 1.
Associated factors.
According to the number of sessions, 9 persons had taken only one session, 4 persons had taken 2 sessions and just 1 patient has taken 3 sessions. The amount of sclerosing agent injected was between 1 and 3 vials, depending on the number of blood vessels which needed treatment, their length and diameter.
No relapses were observed after 1 year from the completion of the treatment. Localized hyperpigmentation was found in 2 patients (14.2%). In one patient, we could observe improvement in about 2 months, as for the second patient, the hyperpigmentation was still visible 1 year after the procedure.
The patients were evaluated using the VCSS score and the link between favoring factors, age and gender, is presented in the following table (Table 3).

Table 3.
The evaluation of the patients in according with the VCSS score.
Compared statistical analysis of VCSS before and 1 year after treatment is shown in Figure 2 and Table 4, as presented below.
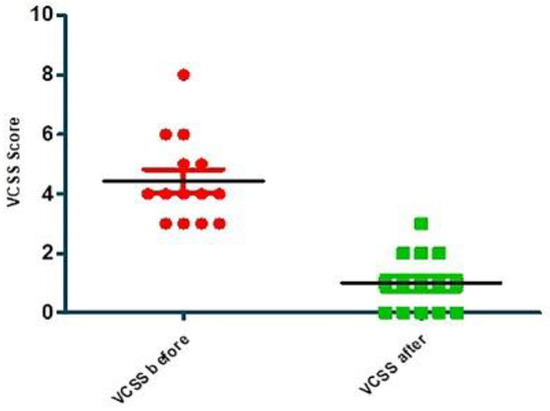
Figure 2.
Compared statistical analysis of VCSS before and 1 year after treatment.

Table 4.
Compared statistical analysis of VCSS before and 1 year after treatment
Aberdeen score in all patients was very good, even though it is a subjective score, based on patients’ reports. The results presented improvement of the symptoms and even remissions. Medication was reduced or unnecessary anymore. Only 3 patients out of 8 were still on medication and needed to take oral treatment for another 3 months after the procedure.
Discussions
In 1995, Cabrera reported that he was using polidocanol mixed with physiological gases (oxygen and carbon dioxide) in treating the great saphenous vein failure. He used echoed injection in the saphenous trunks [6]. Later, Cavezzi reported in another study, that he obtained good results in 93% out of 194 patients treated only with polidocanol [7]. This technique became widely spread across southern Europe, Australia, New Zeeland, South America and USA, polidocanol being injected directly in the varicose veins or used as polidocanol foam (Figure 3) [8].
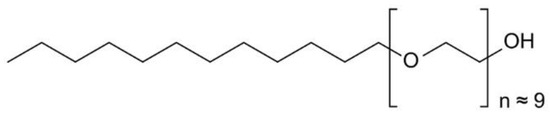
Figure 3.
Chemical formula of polidocanol.
In the United Kingdom recently was reported a series of 60 patients in which compared the surgical treatment and sclerotherapy using polidocanol combined with saphenous-femoral ligature, the results were comparative but with a significant difference in costs and recovery time [9].
Nowadays, varicose disease represents a very common pathology, especially in the women population. It affects a quarter of women and implies significant costs for both society and health systems. If we take into account only the varicose ulcers, about 0.5% of persons with varicose disease develop this complication, with medium costs. For example, in the USA approximately 10.500 dollars are spent every year on one patient only [10]. Even more, in patients included in advanced studies of chronic venous disease, the rates of developing varicose ulcers can grow over 25%; the prevalence in USA varies between 500.000 and 2 million persons [11]. Another study indicates that the management of chronic ulcers of the lower limbs represents up to 1% of all healthcare costs in the western world [12,13,14]. Besides the use of external content, topical dermatological treatment of ulcers is needed. It involves the use of various chemical debridements, granulation stimulation of the epidermis and antiseptics. The treatment of the problematic skin often affected by ulcerous eczema and limb eczema as a result of the vein failure, implies using topical dermatocorticoids for determined periods that may have local implications—atrophy, addiction, contact eczema that can generate additional cost [15,16]. In the context of ever-increasing costs, solutions for the best cost/efficiency ratio have always been required. There are also viable topical treatments for the control of venous ulcers at low costs. An example is the use of Mikulicz ointment even in long periods of time for complicated ulcers [17]. Also, Chronic varicose disease and mental health, including anxiety and depression, are interconnected through various mechanisms. The pain, discomfort, and physical limitations associated with varicose disease can lead to emotional distress, contributing to the development or exacerbation of anxiety and depression symptoms. Additionally, body image concerns, sleep disturbances, chronic stress, and shared risk factors may further contribute to the complex relationship between these physical and mental health conditions [18,19].
The main methods for the treatment of varicose veins are represented by: laser treatments, sclerotherapy and surgical management.
Laser treatments can be applied externally transcutaneously or endovenously. The most used laser with external application, is the NdYag laser of 1064 nm. However, the disadvantage of this type of laser consists in the fact that it can treat vessels up to 4 mm diameter. The approach of the great saphenous vein is possible using endovenous lasers, either by echo-guided puncture or by surgical approach and photocoagulation of the terminal portion of the great saphenous vein. This type of laser has different wavelengths, but the most frequent length used is 1064 nm, which is necessary for larger vessels. For smaller veins, which are below the level of ablation or localized in the calf, it is necessary to use a transcutaneous laser or sclerotherapy. The limitations of this laser are represented by the diameter of the saphene vein and the degree of reflux from the level of the saphene arch. The anesthesia is minimal, it does not require a recovery period and surgery is not mandatory, represent great advantages for this method of treatment. But they have the cost disadvantage, both for the laser device and for consumables and maintenance; there is also a risk of skin burns in transcutaneous lasers or hyperpigmentations, or nerve damage during treatment with endovenous laser. Surgical treatment is indicated for cases where all other procedures cannot be applied. As any surgical treatment, it involves a period of recovery, general anesthesia, or spinal anesthesia. Possible complications after the procedure such as hematomas, infections, bleeding or keloid or hypertrophic scars are expected. It also involves hospitalization costs and high costs for the health system. From the same point of view, sclerotherapy is a cheap procedure, easy to perform, requires very little equipment and can be repeated several times to obtain the desired results. Because the procedure is very quick and painless, it can be performed under local anesthesia or without anesthesia at all. After the procedure, the patient has the great advantage of continuing his daily activities. In 2013 polidocanol was first accredited in the USA for the treatment of varicose veins, later becoming more widespread [20].
The present study included patients with reticular veins and hydrostatic varices (Figure 4A,B, Figure 5A,B and Figure 6A,B).
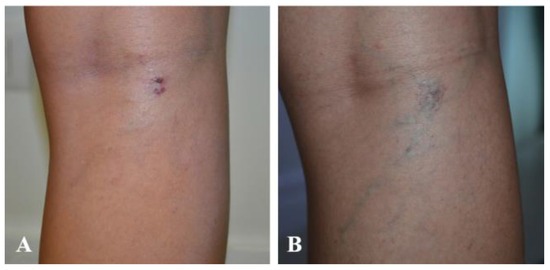
Figure 4.
(A) Patient 1—Before treatment; (B) Patient 1—0.5% polidocanol, 1 vial, 1 session—After treatment.
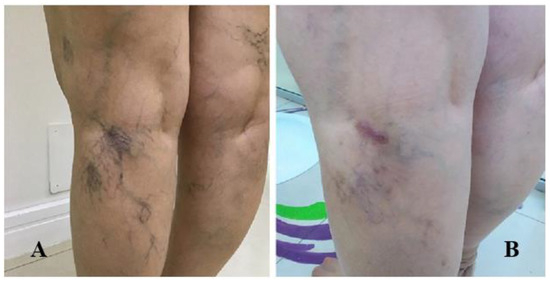
Figure 5.
(A) Patient 2—Before treatment; (B) Patient 2—2% polidocanol, (1 vial), 0.5% (2 vials), 1 session (one month after treatment).
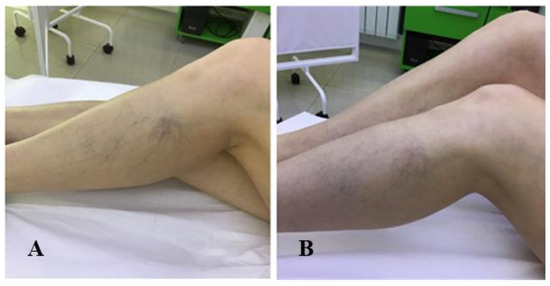
Figure 6.
(A) Patient 3—Before treatment; (B) Patient 3—0.5% polidocanol, 1 vial, 1 session (one month after treatment).
According to the definition of clinical, etiological, anatomical and pathological classification (CEAP), the reticular veins are included in class C1 and are mainly associated with aesthetic claims. The reticular veins have a diameter not greater than 4 mm and are straight, bluish and are located in the subcutaneous tissue of the lower limbs. Stage C2 defines varicose veins. The other stages define more or less complications of varicose disease. They have the same complex etiology of all types of primary venous insufficiency, such as hormonal problems, family history and prolonged orthostatism [21]. However, despite its common occurrence, the condition remains poorly understood [22].
The most common treatments performed nowadays for varicose disease are laser ablation (31%), radiofrequency ablation (20%), sclerotherapy (11%) and classical surgery. The lowest costs are recorded for sclerotherapy combined with laser ablation or radiofrequency [1].
Complications reported by recent studies show comparative data between surgical and sclerotherapy treatment, respectively 14.2% for surgery and 13% for sclerotherapy, all of which represent minor complications; complications after surgical treatment consist of infections in the place of incisions, lymphorrhea, ecchymosis, neuropraxia along the large saphenous pathway and cellulite. For sclerotherapy the complications are transient dyspnea and skin pigmentation [23]. The recurrence rate is comparative between these two procedures, so there is no clear argument in favor of surgical treatment and also, the healing rate of varicose ulcers is similar between sclerotherapy and the classic surgical treatment of varicose veins [23].
Different substances have been used over time for sclerotherapy, such as hypertonic glucose, but the best results have been recorded with polidocanol, being the preferred substance today [24].
Also, regarding the durability of the results, treatment with polidocanol offers long-term results, both aesthetically and in terms of symptom remission [25,26].
In this study we chose to use the Aberdeen questionnaire for patients, being considered by the majority of specialists as the most eloquent in this regard [27]. Likewise, we have used the Venous Clinical Severity Score (VCSS) which in our point of view is the most eloquent for monitoring results; VCSS was developed from elements of the CEAP classification (clinical grade, etiology, anatomy, pathophysiology), which is the worldwide standard for describing the clinical features of chronic venous disease, as over time the clinical experience has shown CEAP score deficiencies. So, American Venous Forum committee on outcomes assessment developed the Venous Clinical Severity Score in 2000. There are three components of this scoring system, the Venous Disability Score, the Venous Segmental Disease Score, and the Venous Clinical Severity Score (VCSS). The VCSS score is continuously analyzed and updated according to the needs and the changes of the pathology [28].
Comparing our results with other studies performed on classical surgery, we found that the results are comparatively the same, but with far fewer complications, much lower costs and very important, we believe without recovery time for patients as well as without pain. Compared to other studies reported on polidocanol, the results obtained by us are on average the same as the results obtained by other clinicians. We also found that all patients were very pleased with the results both for the improvement of the symptomatology and aesthetically. We believe that this treatment is as effective as other treatments applied for this pathology, that it is an easy technique, which does not require special equipment, repetitive, with minimal discomfort for the patient, much cheaper and much easier accepted by patients. But at the same time, we believe that it is very important to set correctly the indications and limits of this technique like a special care for local fibrotic skin changes, previous skin alterations on the site like scleroderma, lichen sclerosus or other immunological conditions sometimes triggered or aggravated by Koebner phenomenon [29,30,31].
Polidocanol is used not only in the treatment of venectasia or varicose veins. For example, it is used as a spray in combination with menthol and minerals having a refreshing role and can be used to relieve irritations, in dermatitis, psoriasis or after various procedures involving skin irritation [32,33,34]. Also, we can consider that polidocanol is the election treatment of chronic venous disease as trichloroacetic acid is for chemical peeling [35] and phosphatidylcholine is for chemical destruction of adipose tissue [36].
Conclusions
The treatment of reticular veins and varicose veins with polidocanol is effective and safe, comparable to classical surgery or laser ablation. The advantages of using this technique consist of lower costs, fewer complications, it can be easily repeated, does not require special equipment and does not require recovery time. We definitely consider it a technique of the future, with many benefits, but mandatory before this kind of treatment, are investigations such as Doppler ultrasound and performing a thorough family history of the patient in order to correctly establish the indications, but especially the contraindications.
Author Contributions
V.A.: L.-A.M., A.L.T. and I.-R.A. contributed to the conceptualization; A.P.S., C.C., V.A., I.-R.A. were major contributors in methodology and software. V.A., L.-A.M. and I.-R.A. contributed to the conception, writing, review, and editing. All authors read and approved the final manuscript to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work.
Institutional Review Board Statement
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
References
- Carlton, R.; Mallick, R.; Campbell, C.; Raju, A.; O’Donnell, T.; Eaddy, M. Evaluating the Expected Costs and Budget Impact of Interventional Therapies for the Treatment of Chronic Venous Disease. Am Health Drug Benefits. 2015, 8, 366–374. [Google Scholar] [CrossRef] [PubMed]
- van Eekeren, R.R.; Boersma, D.; Konijn, V.; de Vries, J.P.; Reijnen, M.M. Postoperative pain and early quality of life after radiofrequency ablation and mechanochemical endovenous ablation of incompetent great saphenous veins. J Vasc Surg. 2013, 57, 445–450. [Google Scholar] [CrossRef]
- Nicolaides, A.N.; Allegra, C.; Bergan, J.; et al. Management of chronic venous disorders of the lower limbs: Guidelines according to scientific evidence. Int Angiol. 2008, 27, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Jamosmos, M.; Fronek, A.; et al. Chronic venous disease in an ethnically diverse population: The San Diego Population Study. Am J Epidemiol. 2003, 158, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.H.; Bjoern, L.; Lawaetz, M.; Blemings, A.; Lawaetz, B.; Eklof, B. Randomized trial comparing endovenous laser ablation of the great saphenous vein with high ligation and stripping in patients with varicose veins: Short-term results. J Vasc Surg. 2007, 46, 308–315. [Google Scholar] [CrossRef]
- Cabrera, J.; Cabrera, J.; et al. Treatment of Varicose Long Saphenous Veins with Sclerosant in Microfoam Form: Long-Term Outcomes. Phlebology. 2000, 15, 19–23. [Google Scholar] [CrossRef]
- Cavezzi, A.; Frullini, A.; Ricci, S.; Tessari, L. Treatment of Varicose Veins by Foam Sclerotherapy: Two Clinical Series. Phlebology. 2002, 17, 13–18. [Google Scholar] [CrossRef]
- Breu, F.X.; Guggenbichler, S. European Consensus Meeting on Foam Sclerotherapy, April, 4-6, 2003, Tegernsee, Germany. Dermatol Surg. 2004, 30, 709–717. [Google Scholar] [CrossRef]
- Bountouroglou, D.G.; Azzam, M.; Kakkos, S.K.; et al. Ultrasound-guided foam sclerotherapy combined with sapheno-femoral ligation compared to surgical treatment of varicose veins: Early results of a randomised controlled trial. Eur J Vasc Endovasc Surg. 2006, 31, 93–100. [Google Scholar] [CrossRef]
- Purwins, S.; Herberger, K.; Debus, E.S.; et al. Cost-of-illness of chronic leg ulcers in Germany. Int Wound J. 2010, 7, 97–102. [Google Scholar] [CrossRef]
- Margolis, D.J.; Bilker, W.; Santanna, J.; Baumgarten, M. Venous leg ulcer: Incidence and prevalence in the elderly. J Am Acad Dermatol. 2002, 46, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Nelzèn, O. Leg Ulcers: Economic Aspects. Phlebology. 2000, 15, 110–114. [Google Scholar] [CrossRef]
- Paunică-Panea, G.; Teodorescu, S.; Preda, A.; Gligor, L.E.; Silaghi, A.; Constantin, V.D. Chronic wound management; surgical therapy and complementary nursing with Manuka honey. J Mind Med Sci. 2023, 10, 139–147. [Google Scholar] [CrossRef]
- Babtan, A.M.; Ilea, A.; Feurdean, C.N.; et al. Biostimulation with low-level laser therapy and its effects on soft and hard tissue regeneration. Literature review. J Mind Med Sci. 2022, 9, 28–37. [Google Scholar] [CrossRef]
- Tatu, A.L.; Ionescu, M.A.; Nwabudike, L.C. Contact Allergy to Topical Mometasone Furoate Confirmed by Rechallenge and Patch Test. Am J Ther. 2018, 25, e497–e498. [Google Scholar] [CrossRef]
- Paius, C.T.; Constantin, V.D.; Carap, A.; Tarus, A.; Tinica, G. Revascularization impact: Quality of life enhancement in chronic limb-threatening ischemia. J Mind Med Sci. 2023, 10, 321–329. [Google Scholar] [CrossRef]
- Nwabudike, L.C.; Tatu, A.L. Magistral Prescription With Silver Nitrate and Peru Balsam in Difficult-to-Heal Diabetic Foot Ulcers. Am J Ther. 2018, 25, e679–e680. [Google Scholar] [CrossRef]
- Droahnă, A.R.; Moroianu, L.A.; Pietrosel, V.A.; Bica, C.I.; Salmen, T.; Curis, C.; Merlo, E.M.; Stoica, R.A.; Moroianu, M. Anxio-depressive disorders in a pandemic context: A comparative analysis: Year 2019 versus 2020. J Mind Med Sci. 2023, 10, 156–162. [Google Scholar] [CrossRef]
- Moroianu, M.; Moroianu, L.A.; Ciubara, A.; Matei, N.M. Persistent Depressive Disorder: The Clinical Approach of the Patient Associating Depression and Dental Pathology—Case Report and Clinical Considerations. BRAIN. Broad Research in Artificial Intelligence and Neuroscience. 2022, 13, 229–238. [Google Scholar] [CrossRef]
- BTG. FDA approves Varithena (polidocanol injectable foam) for the treatment of patients with varicose veins. Press release. November 26, 2013. https://www.prnewswire.com/news-releases/btg-plc-fda-approves-varithena-polidocanol-injectable-foam-for-the-treatment-of-patients-with-varicose-veins-233441701.html.
- Peterson, J.D.; Goldman, M.P.; Weiss, R.A.; et al. Treatment of reticular and telangiectatic leg veins: Double-blind, prospective comparative trial of polidocanol and hypertonic saline. Dermatol Surg. 2012, 38, 1322–1330. [Google Scholar] [CrossRef]
- Benigni, J.P.; Bihari, I.; Rabe, E.; et al. Venous symptoms in C0 and C1 patients: UIP consensus document. Int Angiol. 2013, 32, 261–265. [Google Scholar] [PubMed]
- Campos, W., Jr.; Torres, I.O.; da Silva, E.S.; Casella, I.B.; Puech-Leão, P. A prospective randomized study comparing polidocanol foam sclerotherapy with surgical treatment of patients with primary chronic venous insufficiency and ulcer. Ann Vasc Surg. 2015, 29, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Bertanha, M.; Sobreira, M.L.; Pinheiro Lúcio Filho, C.E.; et al. Polidocanol versus hypertonic glucose for sclerotherapy treatment of reticular veins of the lower limbs: Study protocol for a randomized controlled trial. Trials. 2014, 15, 497, Published 2014 Dec 19. [Google Scholar] [CrossRef]
- Todd, K.L., 3rd; Wright, D.I.; VANISH-2 Investigator Group. Durability of treatment effect with polidocanol endovenous microfoam on varicose vein symptoms and appearance (VANISH-2). J Vasc Surg Venous Lymphat Disord. 2015, 3, 258–264.e1. [Google Scholar] [CrossRef]
- Fometescu, S.G.; Costache, M.; Coveney, A.; Oprescu, S.M.; Serban, D.; Savlovschi, C. Peritoneal fibrinolytic activity and adhesiogenesis. Chirurgia (Bucur). 2013, 108, 331–340. [Google Scholar]
- Staniszewska, A.; Tambyraja, A.; Afolabi, E.; Bachoo, P.; Brittenden, J. The Aberdeen varicose vein questionnaire, patient factors and referral for treatment. Eur J Vasc Endovasc Surg. 2013, 46, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.A.; Rabe, E.; McLafferty, R.B.; et al. Revision of the venous clinical severity score: Venous outcomes consensus statement: Special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg. 2010, 52, 1387–1396. [Google Scholar] [CrossRef]
- Bobeica, C.; Niculet, E.; Craescu, M.; et al. Immunologic and nonimmunologic sclerodermal skin conditions—review. Front Immunol. 2023, 14, 1180221. [Google Scholar] [CrossRef]
- Tatu, A.L.; Ionescu, M.A. Multiple autoimmune syndrome type III-thyroiditis, vitiligo and alopecia areata. Acta Endocrinol (Buchar) 2017, 13, 124–125. [Google Scholar] [CrossRef]
- Happle, R.; Kluger, N. Koebner’s sheep in Wolf’s clothing: Does the isotopic response exist as a distinct phenomenon? J Eur Acad Dermatol Venereol. 2018, 32, 542–543. [Google Scholar] [CrossRef]
- Ardeleanu, V.; Dobre, M.; Georgescu, E.M. Deep Facial Wrinkle Treatment Outcome After First Injection of Reticulated Hyaluronic Acid. Rev. Chim. 2015, 66, 2129–2131, http://bch.ro/pdfRC/ARDELEANU%2012%2015.pdf. [Google Scholar]
- Ardeleanu, V.; Toma, A.; Pafili, K.; et al. Current Pharmacological Treatment of Painful Diabetic Neuropathy: A Narrative Review. Medicina (Kaunas). 2020, 56, 25, Published 2020 Jan 9. [Google Scholar] [CrossRef] [PubMed]
- Ardeleanu, V.; Sabina Radaschin, D.; Tatu, A.L. Excimer laser for psoriasis treatment: A case report and short review. Exp Ther Med. 2020, 20, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Jecan, R.C.; Nicolau, A.; Florescu, I.P.; et al. Use of trichloroacetic acid in treating facial hyperpigmentation. Mater Plast. 2017, 54, 88–90, https://revmaterialeplastice.ro/pdf/20%20JECAN%20R%20C%201%2017.pdf. [Google Scholar] [CrossRef]
- Hexsel, D.; Serra, M.; Mazzuco, R.; Dal’Forno, T.; Zechmeister, D. Phosphatidylcholine in the treatment of localized fat. J Drugs Dermatol. 2003, 2, 511–518. [Google Scholar]
© 2024 by the authors. 2024 Valeriu Ardeleanu, Lavinia-Alexandra Moroianu, Cecilia Curis, Alin Laurentiu Tatu, Iasmina-Raisa Ardeleanu.