Simple Summary
In pancreatic cancer, the amount and distribution of muscle and fat can influence how well the patient does after surgery and during treatment. Standard measures like body mass index do not reliably predict survival, whereas detailed assessments of body composition using CT scans may provide more accurate prognostic information. Patients with higher levels of visceral fat (fat around abdominal organs) tend to have a higher risk of early cancer recurrence and shorter overall survival, while those with more subcutaneous fat (fat under the skin) and greater muscle density generally have better survival outcomes.
Abstract
Background/Objectives: Pancreatic cancer is among the most aggressive malignancies, with poor survival rates. Emerging evidence suggests that body composition, including skeletal muscle mass and adiposity distribution, plays a crucial role in predicting patient outcomes. However, its impact on survival in pancreatic cancer remains incompletely understood. The aim of this systematic review was to assess the correlation between body composition parameters and survival outcomes in pancreatic cancer patients, focusing on overall survival. Methods: A comprehensive literature search was conducted, including three main components: pancreatic cancer, body composition, and survival outcomes. Results: 23 studies were included in this review. The findings indicate that body composition can serve as a predictor of survival in pancreatic cancer patients, with 21 studies reporting a significant correlation. The most frequently observed predictor, with 11 studies reporting, was not a baseline parameter but rather changes in parameters over time during treatment. However, discrepancies remain regarding the extent of predictive power and the relative importance of individual components. Conclusions: Specific body composition parameters hold potential as prognostic indicators of survival in pancreatic cancer patients. However, further research is necessary to establish consistent patterns and to clarify which parameters are most predictive and under what conditions.
1. Introduction
Pancreatic cancer (PC) is one of the most lethal malignancies worldwide, characterized by poor prognosis and a high mortality rate, with the 5-year survival rate remaining low at around 5% [1]. The late-stage diagnosis, coupled with the tumour’s resistance to treatment, contributes significantly to the poor survival outcomes observed in PC patients. The most common subtype is pancreatic ductal adenocarcinoma (PDAC), which arises from the ductal cells lining the pancreatic ducts. PDAC is known for its aggressive nature and poor prognosis, often presenting at an advanced stage due to subtle early symptoms. Currently, 30–35% of patients present with locally advanced disease and 50–55% with metastatic disease at diagnosis [2]. Between 1990 and 2021, the global number of incident cases increased by 168.7%, and deaths increased by 168.2%, with disability-adjusted life years increasing by 148.5% during this same period [3]. This alarming trend is driven by population aging, increasing prevalence of modifiable risk factors such as obesity, diabetes, and smoking, and early-onset pancreatic cancer becoming more prevalent, particularly among younger women [4].
Cancer patients undergo many imaging examinations during their journey to fight cancer, including most frequently computed tomography (CT) and magnetic resonance imaging (MRI) [5,6,7,8,9]. Computed tomography (CT) is the most effective and widely used imaging modality for assessing body composition in patients with pancreatic cancer, primarily because it is routinely performed for staging and follow-up, and allows for precise quantification of skeletal muscle and adipose tissue compartments, including muscle quality (e.g., myosteatosis) and visceral adiposity [10]. In fact, from these imaging exams, it has been demonstrated that an opportunistic evaluation of many metrics can be performed, to add valuable information on the global status of the patient, including a comprehensive body composition assessment [11,12]. This opportunistic assessment of routinely acquired imaging represents a cost-effective approach to extract prognostic information without requiring additional testing or radiation exposure. CT-based analysis is highly reproducible, can be automated with artificial intelligence, and provides detailed regional information relevant to cancer cachexia and prognosis.
Body composition (BC) refers to the proportion of the main tissue components of the body (fat, muscle and bone). The BC evaluation, based on imaging examinations such as CT, may include quantitative assessment of muscle mass by skeletal muscle area (SMA) and skeletal muscle index (SMI), as well as assessment of fat distribution as subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) [13]. Additionally, muscle quality can be assessed through skeletal muscle radiodensity (SMD) or skeletal muscle attenuation (SMA), with lower values indicating myosteatosis (fat infiltration in muscle) [14]. There is a growing literature showing associations between body composition assessment and prognosis in many cancer subtypes, including ovarian [15], lung [16] and bladder [17] cancer. Unlike body weight or body mass index (BMI), that provide nonspecific measurements, imaging-based BC assessment offers a more detailed understanding of health by distinguishing between lean mass, including muscles and bones, and fat mass. This distinction is crucial, as it influences metabolic functions, physical performance, and overall health. Notably, studies have demonstrated that normal or even elevated BMI may mask significant muscle depletion, a phenomenon known as “hidden sarcopenia” or sarcopenic obesity, which affects prognosis independently of body weight [14].
Indeed, a reduction in lean body mass, also referred to as sarcopenia, is highly prevalent in pancreatic cancer patients, affecting approximately 30–65% of patients at diagnosis depending on the diagnostic criteria used. [18]. The pooled prevalence across studies is 45% when measured using skeletal muscle index, making pancreatic cancer the malignancy with one of the highest rates of sarcopenia [19]. This muscle depletion occurs due to the combined effects of systemic inflammation, malnutrition, and tumor-related metabolic disturbances [20]. Pancreatic cancer-associated cachexia, a multifactorial syndrome encompassing sarcopenia, weight loss, anorexia, and metabolic derangement, affects over 80% of pancreatic cancer patients, the highest rate among all malignancies, contributing to reduced quality of life, decreased tolerance to treatment, and significantly reduced survival. The relationship between changes in BC and survival in PC is therefore of clinical interest, as it may help identify patients at higher risk of poor outcomes and might guide personalized interventions [21]. Recent evidence demonstrates that sarcopenia at diagnosis is an independent prognostic factor, with meta-analyses showing hazard ratios of 1.42 for overall survival and 1.41 for progression-free survival [22]. Furthermore, longitudinal changes in muscle mass during treatment appear equally or more important than baseline measurements, with studies showing that patients experiencing greater muscle loss during chemotherapy have significantly worse outcomes regardless of initial muscle status [23]. Beyond muscle mass, other body composition parameters including visceral adiposity (particularly visceral-to-subcutaneous fat ratio), myosteatosis, and their longitudinal changes have emerged as independent predictors of survival, suggesting that a comprehensive assessment of multiple body composition compartments may provide more nuanced prognostic information than any single parameter [24]. Understanding how BC affects the survival of PC patients could be crucial for improving prognostication, informing patients about treatment strategies, and enhancing their care. This systematic review aims to critically analysing and synthesizing the existing literature, in order to provide a clearer understanding of whether BC measurements extracted from CT scans, can serve as reliable prognostic indicators for PC outcomes. Given the high prevalence of body composition abnormalities in pancreatic cancer, the availability of routine imaging for opportunistic assessment, and the growing evidence for their prognostic significance, integrating body composition analysis into clinical practice may facilitate earlier nutritional interventions, better risk stratification, and personalized treatment planning to ultimately improve patient outcomes.
2. Materials and Methods
2.1. Search Strategy
The research was done in compliance with the PRISMA guidelines (see Supplementary Table S1). The review was registered with the INPLASY with the registration code INPLASY202610026. To ensure comprehensive coverage of relevant literature, the databases PubMed and SCOPUS were screened for studies published between 2014 and 2024, with search strings and terms tailored to each database. Details of the screening progress are available in the Appendix A.
2.2. Selection Criteria
The following inclusion criteria were applied: (1) publications written in English; (2) available full free text and/or open access; (3) adults only. Exclusion criteria were as follows: (1) studies that did not present original data; (2) non-CT-based body composition assessment; (3) focus on other cancer types; (4) animal studies.
2.3. Data Extraction
For each eligible article, information was collected enabling comparisons based on factors such as basic study data, including authors, country of origin, year of publication, and the study design being prospective or retrospective; population characteristics, such as number of patients, sex, age and sarcopenic status; body composition features evaluated were extracted and included: skeletal muscle (SKM), VAT, SAT, muscle mass (MM), SMI, mean muscle attenuation (MMA), total adipose tissue (TAT), intramuscular adipose tissue (IMAT), visceral to muscle ratio (VMR), visceral to subcutaneous adipose tissue area (VSR), subcutaneous fat area (SFA), visceral fat area (VFA), subcutaneous fat area (SFA), total fat mass (TFM), total fat area (TFA), skeletal muscle density (SMD), skeletal muscle radiodensity (SMRD), fatty muscle fraction (FMF), intramuscular fat fraction (IMFF), visceral adipose tissue index (VATI), and subcutaneous adipose tissue index (SATI); association of body composition features and survival. After extraction, we realized that it was difficult to compare features that were named in different ways but indicated the same assessment. For this reason, we secondarily grouped some features to ensure comparisons, as detailed in the results Section (see Results Section 3.3).
2.4. Quality Assessment
The overall quality of the included studies was critically evaluated based on the revised “Quality Assessment of Diagnostic Accuracy Studies” tool (QUADAS-2) [25]. This tool comprises four domains (patient selection, index test, reference standard, and flow and timing) and each domain was assessed in terms of risk of bias, and a graph was constructed appropriately. More in details, in the patient selection domain, it was examined how study participants were chosen, including inclusion/exclusion criteria, recruitment methods (consecutive, random, or convenience sampling), and whether inappropriate exclusions were made, with the aim of assessing whether the patient spectrum matched the intended clinical population and if selection methods could have introduced bias. In the index test domain, we evaluated the conduct and interpretation of the body composition parameters included in the papers, if the parameters were standardized, and whether the test threshold was pre-specified. In the reference standard domain, we evaluated the presence and appropriateness of a reference standard. In the flow and timing domain, we examined the sequence and timing of the index test and reference standard and whether all patients were included in the analysis.
3. Results
3.1. Study Selection Process
The database research resulted in 497 findings. According to the inclusion and exclusion criteria, 23 studies were included in this review (Figure 1).
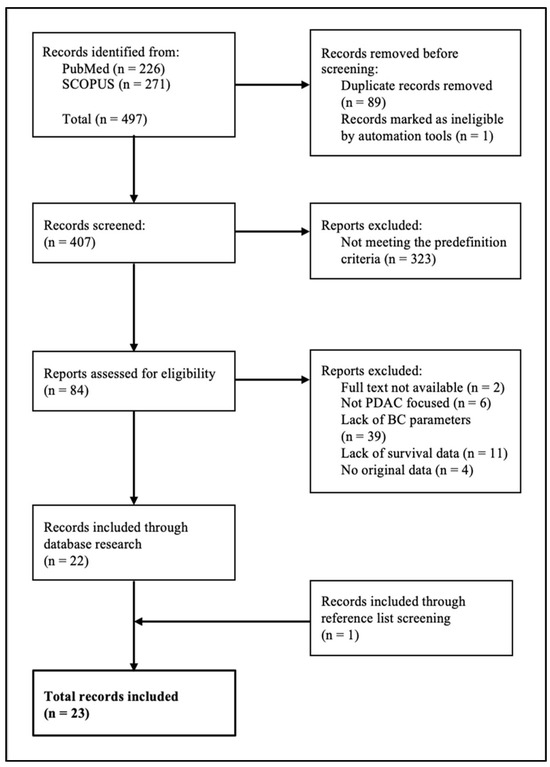
Figure 1.
Flowchart for screening literature.
3.2. Characteristics of Included Studies
All studies [21,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] were conducted in high- and middle-income countries with the majority being retrospective in design (3/23; 13%). Sample size ranged from 53 [34] to 476 patients [39], with only one study including more women than men.
The reported mean age of participants ranged from 59.5 to 74.6 years across the included studies. All studies reported the mean or median BMI of participants, which ranged from 21.0 to 28.7 kg/m2. The percentages of sarcopenic patients were not available in all studies. In those available they ranged from 32.2 [27] to 65.1% [47].
3.3. Technical Aspects and Features Evaluated
All 23 studies used diagnostic CT scans at the L3 vertebral level for body composition analysis. Since the included studies used different definitions and cut off values for their measurements and calculations, as well as for definition of sarcopenia, we grouped some of them to facilitate the comparison among the different body components. More in details: fatty muscle infiltration (FMF) included FMF, IMAC, IMAT, IMATI, IMFA, IMFF; muscle attenuation (MA) included MA, MD, MMA, PMD, RA, SMD, SMRD; muscle mass (MM) included PMA, SKM, SMA; skeletal muscle indices (SMI), usually indicating the skeletal muscle area divided by the square height, included SMI, ASMAH, PMAH, PMTH, TPI; total adiposity (TA) included TA, TAT, TFA, TFM; subcutaneous adipose tissue (SAT) included SAT and SFA; subcutaneous adipose tissue index (SATI), usually indicating the subcutaneous adipose tissue divided by the square height, included SATI and SFI; visceral adipose tissue (VAT) included VAT and VFA; and visceral adipose tissue index (VATI), usually indicating the visceral adipose tissue divided by the square height, included VATI and VFI.
Twenty-one studies reported an association of features accounting for skeletal muscle and adipose tissue with survival (Table 1). 21/23 studies found a significant association between at least one BC parameter and OS. Two studies did not find a significant association between standard BC metrics and survival [27,42], but Damm et al. noted myosteatosis as a significant prognostic factor [27], and Bareiss et al. highlighted the nutritional risk score (NRS) as a predictor of survival [42], thus confirming that the body composition, although evaluated by different metrics, was still important for survival. Eight studies [29,30,31,32,33,41,42,43,44] mentioned a correlation of low baseline SMI (referring to it as sarcopenia) and shorter OS, although some of these findings were limited to specific subgroups based on sex or BMI thresholds. Two studies [36,44] further noticed that the correlation became even stronger if a high VAT was present. Four studies [35,36,46,47] found a correlation between low SMI and shorter overall survival only in the presence of high adipose tissue, indicating that not sarcopenia alone, but sarcopenic obesity, was associated with reduced OS. Visceral adiposity showed mixed effects across studies: while four studies [34,36,39,44] reported shorter OS in patients with high baseline VAT, one study [21] found greater OS associated with high VAT. Subcutaneous adiposity was mentioned by two studies [21,39], both reporting a higher SAT/SATI correlating with greater OS. Eleven studies [21,26,28,33,34,37,40,41,43,45,46] reported that changes in one or more of the body composition parameters during treatment were predictive of OS, with some emphasizing that these dynamic changes were more informative than baseline values alone. These results underline the growing consensus that body composition is a potential meaningful predictor of survival in pancreatic cancer patients. However, it also shows variability in methodology and findings, particularly in the relative importance of each parameter, as well as the timing (and naming) of their measurement, and their monitoring.

Table 1.
Key findings of included studies.
3.4. Quality Assessment Results
The overall quality assessment of the studies is reported in Figure 2.
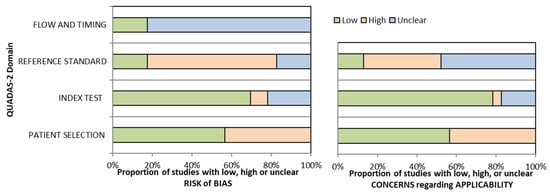
Figure 2.
Overall Quality Assessment of the Studies Included, according to the QUADAS-2 Tool.
4. Discussion
This systematic review included 23 studies that investigated the association between CT-based body composition and overall survival in patients with pancreatic cancer. In this systematic review, we included only CT as imaging method to assess body composition because CT remains the gold standard for body composition analysis in pancreatic cancer due to its precision, accessibility, and ability to assess both quantity and quality of muscle and adipose tissue. Dual-energy X-ray absorptiometry (DXA) is a validated alternative for whole-body composition assessment, offering accurate measurements of fat mass and lean mass in the general population and in cancer patients. However, DXA is less effective than CT for quantifying regional muscle and visceral adipose tissue, and is not interchangeable with CT in cancer populations, including pancreatic cancer survivors, due to systematic biases and limited ability to distinguish tissue compartments affected by cancer or treatment [48]. DXA estimates of visceral adipose tissue show high correlation with CT, but substantial bias exists, especially in patients with prior abdominal surgery or ascites, limiting its clinical utility for this population. Bioelectrical impedance analysis (BIA) is less accurate than both CT and DXA, with significant under- or overestimation of fat-free mass and poor agreement in cancer patients [48]. Magnetic resonance imaging (MRI) offers similar accuracy to CT but is less accessible, not routinely used for body composition in pancreatic cancer patients and more prone to technical issues for comparisons.
As technical aspect of body composition evaluation on CT scanners, the third lumbar vertebra (L3) is the standard anatomical landmark used in the vast majority of studies for body composition analysis and image protocols. This level is chosen because single-slice measurements at L3 correlate strongly with whole-body muscle and adipose tissue volumes [49]. Although images without or with contrast medium may be used for body composition assessment from CT scans, in the specific setting of pancreatic cancer, portal venous phase images at the L3 level are the most commonly analyzed.
Eight studies [29,30,31,32,33,41,42,43,44] mentioned a correlation of sarcopenia and shorter OS, although some of these findings were limited to specific sub-groups based on sex or BMI thresholds. Four studies [35,36,46,47] found a correlation between low SMI and shorter OS only in the presence of high adipose tissue, indicating that more than sarcopenia alone, sarcopenic obesity, was associated with reduced OS. Similar results were demonstrated in patients candidate to surgery for PC [50]. Indeed, some studies found a correlation between sarcopenia and survival, others did not report a correlation, thus resulting in unequivocal conclusions. These conflicting results may be partially explained by differences in treatment intention (curative versus palliative) and disease stage, as demonstrated by recent large-scale meta-analyses showing stronger associations between sarcopenia and survival in patients undergoing curative treatments (hazard ratio [HR] 1.53–1.62) compared to palliative settings [51]. The corresponding meta-analysis confirmed a reduced survival in patients with sarcopenia, whereas a clear correlation with short-term postoperative outcomes was not evident [50]. Accordingly, Liu et al. demonstrated that preoperative sarcopenia was significantly associated with poor prognosis in pancreatic cancer patients following radical surgery, but they underlined that this association would require further validation through prospective studies [52].
Large-scale cohort studies and prospective clinical trials have provided more definitive evidence. A recent meta-analysis of 48 studies involving 9063 patients demonstrated a pooled sarcopenia prevalence of 45% in pancreatic cancer, with significant prognostic impact on both overall survival (pooled adjusted HR 1.39, 95% CI 1.16–1.66) and progression-free survival (pooled adjusted HR 1.31, 95% CI 1.11–1.55) [53].
Importantly, one of the first prospective multicenter randomized trials examining body composition in 90 patients with resectable pancreatic adenocarcinoma receiving neoadjuvant chemotherapy found that visceral adipose tissue area (HR 1.58, 95% CI 1.00–2.51, p = 0.05) was associated with survival, but surprisingly found no significant difference in median overall survival between patients with versus without sarcopenia (23.6 versus 27.9 months, p > 0.05) [54]. This suggests that the prognostic impact of body composition parameters may differ significantly between early-stage resectable disease and advanced disease, highlighting the need for stage-specific and treatment-specific validation of body composition markers.
Visceral adiposity showed mixed effects across studies, with some studies reporting shorter OS in patients with high baseline VAT [34,36,39,44], and one study [21] showing longer OS associated with high VAT. On the other hand, SAT and SATI correlated with longer OS [24,39].
Visceral fat is more metabolically active, secreting higher levels of pro-inflammatory cytokines and adipokines, and is closely associated with systemic inflammation, insulin resistance, and a tumor-promoting microenvironment. Its anatomical proximity to the pancreas facilitates direct crosstalk between adipocytes and cancer cells, promoting invasion, metastasis, and aggressive tumor behavior. Increased visceral fat density, which may reflect fibrosis, inflammation, or cachexia-related adipocyte shrinkage, is associated with shorter survival and higher risk of disease progression in pancreatic cancer. On the other hand, subcutaneous fat is less metabolically active and less involved in inflammatory signaling. Higher subcutaneous fat area is associated with longer survival in PC patients, potentially because it serves as an energy reservoir and is less susceptible to cancer-driven catabolism and cachexia. Patients with greater subcutaneous fat are less likely to experience rapid nutritional decline and may have better tolerance to cancer therapies [39].
Recent large multicenter studies have clarified these contradictory findings by demonstrating that the visceral-to-subcutaneous fat ratio (VSR), rather than absolute visceral fat quantity alone, is a more robust predictor, with high VSR independently predicting both early recurrence (OR 2.30, p = 0.001) and worse overall survival (HR 1.46, p = 0.007) in resectable pancreatic cancer [24]. Additionally, visceral adipose tissue density, rather than volume alone, has emerged as a novel predictive biomarker, with increased VAT density showing strong association with overall survival (p = 0.0019) across all pancreatic cancer subgroups (resectable, unresectable, and metastatic) [55].
The impact of adiposity, including subcutaneous fat and visceral fat, on survival in PC patients appears complex and context-dependent, with mixed findings across studies. Most importantly, many studies emphasized that not only baseline measurements but rather their development after diagnosis and during treatment hold prognostic value.
Large prospective cohort studies have confirmed that longitudinal changes in body composition may be equally or more important than baseline values. In a study of 456 patients with metastatic pancreatic cancer, body composition changes occurred predominantly during the first 2 months after starting chemotherapy, with change in subcutaneous adipose tissue index being significantly associated with overall survival in male patients (HR 0.51, 95% CI 0.35–0.75, p < 0.001), independent of baseline sarcopenia status [43].
Similarly, a study of 105 patients with unresectable pancreatic cancer demonstrated that early changes in skeletal muscle index (ΔSMI) at first follow-up were prognostic for overall survival (HR 1.2, 95% CI 1.08–1.33, p = 0.001) even after adjusting for RECIST response assessment, with patients experiencing lower rates of muscle loss showing median survival of 233 versus 143 days. Therefore, the relationship between baseline and overtime measurements requires more research, possibly in prospective randomized trials [23].
A deeper insight the top quality studies reporting data on correlations between adiposity and survival, shows that Damm et al. [27], including 354 patients and used standardized, sex-adjusted regression formulas derived from a healthy population, demonstrated that myosteatosis was the only independent prognostic factor among body composition parameters. Pecchi et al. [21] analysed both baseline and longitudinal changes in adiposity parameters showing that higher visceral fat index was associated with greater survival rates in Cox regression models.
Bian et al. [44] included 203 patients with advanced pancreatic cancer and used multivariable analyses controlling for performance status and tumor stage. Visceral-to-subcutaneous adipose tissue ratio was identified as an independent risk factor (HR 1.38, p = 0.005), demonstrating the importance of fat distribution over total adiposity.
Therefore, the relationship between baseline and overtime measurements requires more research, possibly in prospective randomized trials.
Furthermore, several studies in this review suggest that a combined effect of sarcopenia and adiposity, commonly referred to as sarcopenic obesity, does exist. Three studies specifically found that sarcopenic obesity was significantly associated with poorer OS compared to either condition alone [35,46,47]. Additionally, two other studies reported that the negative impact of low muscle mass on survival became more pronounced in the presence of high visceral fat, suggesting a synergistic adverse effect [36,44]. Gruber et al. demonstrated in 133 patients with resectable PC that sarcopenic obese patients had significantly poorer overall survival compared to non-sarcopenic obese patients (14 versus 23 months, p = 0.007) and showed higher incidence of major postoperative complications (p < 0.001) [56]. These findings support the idea that evaluating muscle and fat compartments together provides a more accurate prognostic picture than assessing either in isolation. However, the definitions and thresholds for sarcopenic obesity varied among studies, limiting direct comparisons. This underscores the need for standardized criteria in assessing and reporting sarcopenic obesity in PC populations. Furthermore, sarcopenic obesity can be defined itself according to different definitions [57,58,59], but only two [35,38] of the included articles specified the definition of sarcopenic obesity they referred to [57,58].
Eleven studies [21,26,28,33,34,37,40,41,43,45,46] reported that changes in one or more of the BC parameters during treatment were predictive of OS, with some emphasising that these dynamic changes were more informative than baseline values alone. These results emphasize that BC measurements are a potential meaningful predictor of survival in cancer patients. However, it also reveals some variability in methodology and findings, particularly in the relative importance of each parameter, as well as the timing of their measurement and monitoring.
It should also be noted that more than half of the included studies showed high risk of bias or uncertain risk of bias, and several studies also raised concerns regarding applicability, therefore the degree of evidence of the results is downgraded, which means that the true effect of the body composition component may be substantially different from the review’s findings. In practice, this systematic review, with a predominance of high-risk studies may only be able to suggest associations rather than establish causality.
If a significant correlation between BC and survival is unanimously confirmed, BC assessments could be incorporated into routine clinical practice as a prognostic tool. This would allow healthcare providers to identify patients at higher risk of poor outcomes early, leading to more personalised handling. However, this review highlights several critical limitations in current research worldwide. The most significant limitation is the lack of standardized definitions for sarcopenia and adiposity across different populations. A recent comprehensive analysis applying 14 different published sarcopenia cutoff definitions to the same cohort of 179 patients found that prevalence ranged from 8.9% to 69.8%, with only 3 of 14 definitions showing significant association with overall survival [60]. Similarly, a systematic review and meta-analysis demonstrated that sarcopenia prevalence in pancreatic cancer varied from 19% to 57% depending on whether cutoff values were <40 cm2/m2, 40–50 cm2/m2, or >50 cm2/m2 [53]. This substantial variability undermines the comparability of studies and clinical applicability of findings. Furthermore, skeletal muscle mass and strength are significantly influenced by factors such as gender, ethnicity, body size, age, lifestyle, and cultural background, yet most studies have been conducted in high- and middle-income countries with predominantly White populations, restricting the generalizability of findings [61].
An additional major limitation is the predominance of retrospective study designs. While meta-analyses have synthesized data from thousands of patients, the vast majority of included studies are retrospective and observational. Of the 23 studies included in this systematic review, involving 5888 patients, all were retrospective.
The limited number of prospective trials restricts our ability to establish causal relationships and optimal intervention strategies. Moreover, many studies focused on specific subgroups defined by disease stage or treatment type, which limits external validity and the ability to develop universal prognostic models applicable across the full spectrum of pancreatic cancer presentations.
Future research should aim to establish consensus criteria and standardised methods for assessing BC in PC patients, and more in general in cancer patients.
Recent efforts to apply artificial intelligence (AI) and deep learning to body composition analysis offer promising solutions to standardization challenges. Several studies have demonstrated that fully automated AI-based body composition analysis from routine staging CT scans can provide consistent, reproducible measurements with high accuracy (Dice coefficients ≥ 0.92–0.94), eliminating inter-observer variability inherent in manual or semi-automated methods [55].
A multicenter study of 601 pancreatic cancer patients demonstrated that deep learning-derived muscle-to-bone ratio (MBR) and myosteatosis were significantly associated with overall survival (MBR: HR 0.60, p < 0.005; myosteatosis: HR 3.73, p < 0.005) independent of age, sex, and AJCC stage, with the AI analysis automatically processing routine staging CT scans across different institutions [61]. This automated approach enables seamless integration into standard oncologic workflows without additional procedural burden or radiation exposure, transforming opportunistic imaging into actionable clinical data.
Future research directions should focus on several key areas. First, prospective randomized controlled trials are needed to validate body composition cutoffs and evaluate whether interventions targeting sarcopenia and cachexia can improve outcomes. Second, longitudinal studies examining temporal changes in body composition throughout the cancer care continuum—from diagnosis through treatment and survivorship—are essential to understand the dynamic nature of body composition changes and identify critical time windows for intervention. Third, investigation into the biological mechanisms linking body composition alterations to pancreatic cancer outcomes is crucial. Preliminary studies suggest that sarcopenia may worsen prognosis by suppressing local.
This review has some limitations. One is the limited external validity, as some studies focused on subgroups defined by disease stages or specific treatment. In addition, all studies were conducted in high- and middle-income countries, restricting the applicability of findings to populations with similar characteristics and lifestyles. However, the body composition, as well as the nutritional intake in these populations would be anyway not comparable with that of low-income countries. A second limitation is that the studies included in this review were of varied design, including observational studies, cohort studies and others. This diversity in design can lead to heterogeneity in findings, as different methodologies may introduce varying degrees of bias and confounding factors. An additional limitation is that although all studies selected used CT-based body composition assessment, the accuracy of these measurements may vary according to the CT equipment and protocols, as well as to the specific software used for the extraction. Furthermore, along with varying levels of precision, different tools can name the same measure differently, and some studies may emphasize one measure, without a comprehensive assessment of all the measurements available. In order to partially overcome these limitations, we analysed the measurements included in different papers and grouped under the same name those that measured the same (or almost the same) body compartment. This helped us to make consistent comparisons across studies. Another limitation relies on the lack of standardization in definition of sarcopenia and adiposity. Indeed, there is no universal threshold for defining these entities based on imaging, as skeletal muscle mass and strength are significantly influenced by factors such as gender, ethnicity, body size, age, lifestyle, and cultural background leading to inconsistencies in how BC was categorized across studies, thus leading to a limited generalizability to specific populations. To overcome this limitation, in order to compare results of different papers, we grouped the BC definitions in wider categories.
5. Conclusions
In conclusion, body composition parameters show promise as prognostic markers, with longitudinal changes appearing more predictive than single-point measurements. However, future research should prioritize prospective studies employing standardized definitions and validated thresholds in order to help integrate BC assessment into routine practice in pancreatic cancer care.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tomography12010008/s1, Table S1: the PRISMA guidelines for abstract checklist. Ref. [62] is cited in Supplementary Materials.
Author Contributions
Conceptualization, L.S. and S.R.; methodology, L.S. and S.R.; software, L.S. and S.R.; formal analysis, L.S. and S.R.; resources, S.R.; data curation, L.S.; writing—original draft preparation, L.S. and S.R.; writing—review and editing, L.S. and S.R.; visualization, S.R.; supervision, S.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
During the preparation of this systematic review, the authors used ChatGPT (version ChatGPT-5) for the purposes of language improvement. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BC | Body Composition |
| BIA | Bioelectrical impedance analysis |
| BMI | Body Mass Index |
| CT | Computed Tomography |
| DXA | Dual-energy X-Ray Absorbiometry |
| FMF | Fatty Muscle Fraction |
| IMAT | Intramuscular Adipose Tissue |
| IMFF | Intramuscular Fat Fraction |
| L3 | Third lumbar vertebra |
| MA | Muscle Attenuation |
| MM | Muscle Mass |
| MMA | Mean Muscle Attenuation |
| MRI | Magnetic Resonance Imaging |
| OS | Overall Survival |
| PC | Pancreatic Cancer |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| PFS | Progression Free Survival |
| SAT | Subcutaneous Adipose Tissue |
| SATI | Subcutaneous Adipose Tissue Index |
| SKM | Skeletal Muscle |
| SMA | Skeletal Muscle Area |
| SMD | Skeletal Muscle Density |
| SMI | Skeletal Muscle Index |
| SMRD | Skeletal Muscle Radiodensity |
| TA | Total Adiposity |
| TAI | Total Adipose Index |
| TAMA | Total Abdominal Muscle Area |
| TAT | Total Adipose Tissue |
| TFA | Total Fat Area |
| TFM | Total Fat Mass |
| VAT | Visceral Adipose Tissue |
| VATI | Visceral Adipose Tissue Index |
| VMR | Visceral to Muscle Ratio |
| VSR | Visceral to Subcutaneous Adipose Tissue Area |
Appendix A
Screening Details
The last screening for this systematic review was performed on the 17 January 2025 for PubMed and on the 20 January 2025 for Scopus. The used search strings were as follows: [“Pancreatic Neoplasms”[Mesh] OR “pancreatic cancer” OR “pancreatic tumour” OR “pancreatic carcinoma”] AND [[“Body Composition”[Mesh] OR “body composition” OR “muscle mass” OR “sarcopenia” OR “adiposity” OR “fat mass” OR “lean body mass” OR “BMI” OR “body mass index”] AND [“Survival Analysis”[Mesh] OR “survival” OR “prognosis” OR “survival rate” OR “mortality” OR “outcome”]] for PubMed and TITLE-ABS-KEY[“pancreatic cancer”] AND TITLE-ABS-KEY[“body composition” OR “sarcopenia” OR “muscle mass” OR “fat mass” OR “adiposity” OR “BMI”] AND TITLE-ABS-KEY[“outcomes” OR “survival” OR “prognosis” OR “treatment response”].
References
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Kan, C.; Liu, N.; Zhang, K.; Wu, D.; Liang, Y.; Cai, W.; Jing, Q.; Han, F.; Xing, S.; Sun, X. Global, Regional, and National Burden of Pancreatic Cancer, 1990–2019: Results From the Global Burden of Disease Study 2019. Ann. Glob. Health 2023, 89, 33. [Google Scholar] [CrossRef]
- Zottl, J.; Sebesta, C.G.; Tomosel, E.; Sebesta, M.C.; Sebesta, C. Unraveling the Burden of Pancreatic Cancer in the 21st Century: Trends in Incidence, Mortality, Survival, and Key Contributing Factors. Cancers 2025, 17, 1607. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’reilly, E.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- Rizzo, S.; Summers, P.; Raimondi, S.; Belmonte, M.; Maniglio, M.; Landoni, F.; Colombo, N.; Bellomi, M. Diffusion-weighted MR imaging in assessing cervical tumour response to nonsurgical therapy. Radiol. Med. 2011, 116, 766–780. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.M.R.; Kalra, M.K.; Schmidt, B.; Raupach, R.; Maher, M.M.; Blake, M.A.; Saini, S. CT images of abdomen and pelvis: Effect of nonlinear three-dimensional optimized reconstruction algorithm on image quality and lesion characteristics. Radiology 2005, 237, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Genovese, E.; Canì, A.; Rizzo, S.; Angeretti, M.G.; Leonardi, A.; Fugazzola, C. Comparison between MRI with spin-echo echo-planar diffusion-weighted sequence (DWI) and histology in the diagnosis of soft-tissue tumours. Radiol. Med. 2011, 116, 644–656. [Google Scholar] [CrossRef]
- Vogel, A.; Ducreux, M. ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org. ESMO Clinical Practice Guideline interim update on the management of biliary tract cancer. ESMO Open 2025, 10, 104003. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using comput-ed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Pickhardt, P.J. Value-added Opportunistic CT Screening: State of the Art. Radiology 2022, 303, 241–254. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Graffy, P.M.; Perez, A.A.; Lubner, M.G.; Elton, D.C.; Summers, R.M. Opportunistic Screening at Abdominal CT: Use of Automated Body Composition Biomarkers for Added Cardiometabolic Value. Radiographics 2021, 41, 524–542. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Vincenzo, G.; Rizzo, S.; Del Grande, F.; Sconfienza, L.M. An update in musculoskeletal tumors: From quantitative imaging to radiomics. Radiol. Med. 2021, 126, 1095–1105. [Google Scholar] [PubMed]
- Khristenko, E.; Sinitsyn, V.; Rieden, T.; Girod, P.; Kauczor, H.U.; Mayer, P.; Klauss, M.; Lyadov, V. CT-based screening of sarcopenia and its role in cachexia syndrome in pancreatic cancer. PLoS ONE 2024, 19, e0291185. [Google Scholar] [CrossRef]
- Del Grande, M.; Rizzo, S.; Nicolino, G.M.; Colombo, I.; Rossi, L.; Manganaro, L.; Del Grande, F. Computed Tomography-Based Body Composition in Patients With Ovarian Cancer: Association With Chemotoxicity and Prognosis. Front. Oncol. 2021, 11, 718815. [Google Scholar]
- Rizzo, S.; Petrella, F.; Bardoni, C.; Bramati, L.; Cara, A.; Mohamed, S.; Radice, D.; Raia, G.; Del Grande, F.; Spaggiari, L. CT-Derived Body Composition Values and Complications After Pneumonectomy in Lung Cancer Patients: Time for a Sex-Related Analysis? Front. Oncol. 2022, 12, 826058. [Google Scholar] [CrossRef]
- Sanchez, A.; Kissel, S.; Coletta, A.; Scott, J.; Furberg, H. Impact of body size and body composition on bladder cancer outcomes: Risk stratification and opportunity for novel interventions. Urol. Oncol. 2020, 38, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.; Prado, C.M.; Daly, R.M.; Denehy, L.; Edbrooke, L.; Baguley, B.J.; Fraser, S.F.; Khosravi, A.; Abbott, G. Low muscle mass, malnutrition, sarcopenia, and associations with survival in adults with cancer in the UK Biobank cohort. J. Cachexia Sarcopenia Muscle 2023, 14, 1775–1788. [Google Scholar]
- Láinez Ramos-Bossini, A.J.; Gámez Martínez, A.; Luengo Gómez, D.; Valverde-López, F.; Mel-guizo, C.; Prados, J. Prevalence of Sarcopenia Determined by Computed Tomography in Pancreatic Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Cancers 2024, 16, 3356. [Google Scholar] [CrossRef]
- Cabasag, C.J.; Arnold, M.; Rutherford, M.; Bardot, A.; Ferlay, J.; Morgan, E.; Little, A.; De, P.; Dixon, E.; Woods, R.R.; et al. Pancreatic cancer survival by stage and age in seven high-income countries (ICBP SURVMARK-2): A population-based study. Br. J. Cancer 2022, 126, 1774–1782. [Google Scholar] [CrossRef]
- Pecchi, A.; Valoriani, F.; Costantini, R.C.; Squecco, D.; Spallanzani, A.; D’amico, R.; Dominici, M.; Di Benedetto, F.; Torricelli, P.; Menozzi, R. Role of Body Composition in Patients with Resectable Pancreatic Cancer. Nutrients 2024, 16, 1834. [Google Scholar] [CrossRef] [PubMed]
- Thormann, M.; Hinnerichs, M.; Barajas Ordonez, F.; Saalfeld, S.; Perrakis, A.; Croner, R.; Omari, J.; Pech, M.; Zamsheva, M.; Meyer, H.J.; et al. Sarcopenia is an Independent Prog-nostic Factor in Patients With Pancreatic Cancer—A Meta-analysis. Acad. Radiol. 2023, 30, 1552–1561. [Google Scholar] [CrossRef]
- Salinas-Miranda, E.; Deniffel, D.; Dong, X.; Healy, G.M.; Khalvati, F.; O’Kane, G.M.; Knox, J.; Bathe, O.F.; Baracos, V.E.; Gallinger, S.; et al. Prognostic value of early changes in CT-measured body composition in patients receiving chemotherapy for unresectable pan-creatic cancer. Eur. Radiol. 2021, 31, 8662–8670. [Google Scholar] [CrossRef]
- Wu, L.; Nie, T.; Zhi, X.; Ouyang, D.; Zhang, L.; Wu, H.; Li, X.; Wu, H.; Han, P.; Chen, L.; et al. Prognostic value of body composition on early recurrence and long-term survival of re-sectable pancreatic ductal adenocarcinoma. Eur. Radiol. 2025, 1–20. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Nogueras-González, G.M.; Prakash, L.R.; Petzel, M.Q.B.; Parker, N.H.; Ngo-Huang, A.T.; Fogelman, D.; Denbo, J.W.; Garg, N.; Kim, M.P.; et al. Anthropometric Changes in Patients with Pancreatic Cancer Undergoing Preoperative Therapy and Pancreatoduodenectomy. J. Gastrointest. Surg. 2018, 22, 703–712. [Google Scholar] [CrossRef]
- Damm, M.; Efremov, L.; Jalal, M.; Nadeem, N.; Dober, J.; Michl, P.; Wohlgemuth, W.A.; Wadsley, J.; Hopper, A.D.; Krug, S.; et al. Body composition parameters predict survival in pancreatic cancer-A retrospective multicenter analysis. United Eur. Gastroenterol. J. 2023, 11, 998–1009. [Google Scholar]
- Naumann, P.; Eberlein, J.; Farnia, B.; Liermann, J.; Hackert, T.; Debus, J.; Combs, S.E. Cachectic body composition and inflammatory markers portend a poor prognosis in patients with locally advanced pancreatic cancer treated with chemoradiation. Cancers 2019, 11, 1655. [Google Scholar] [CrossRef]
- Ninomiya, G.; Fujii, T.; Yamada, S.; Yabusaki, N.; Suzuki, K.; Iwata, N.; Kanda, M.; Hayashi, M.; Tanaka, C.; Nakayama, G.; et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: A retrospective cohort study. Int. J. Surg. 2017, 39, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Choi, M.H.; Lee, I.S.; Hong, T.H.; Lee, M.A. Clinical significance of skeletal muscle density and sarcopenia in patients with pancreatic cancer undergoing first-line chemotherapy: A retrospective observational study. BMC Cancer 2021, 21, 77. [Google Scholar] [CrossRef]
- Sugimoto, M.; Farnell, M.B.; Nagorney, D.M.; Kendrick, M.L.; Truty, M.J.; Smoot, R.L.; Chari, S.T.; Moynagh, M.R.; Petersen, G.M.; Carter, R.E.; et al. Decreased Skeletal Muscle Volume Is a Predictive Factor for Poorer Survival in Patients Undergoing Surgical Resection for Pancreatic Ductal Adenocarcinoma. J. Gastrointest. Surg. 2018, 22, 831–839. [Google Scholar] [CrossRef]
- Nowak, S.; Kloth, C.; Theis, M.; Marinova, M.; Attenberger, U.I.; Sprinkart, A.M.; Luetkens, J.A. Deep learning–based assessment of CT markers of sarcopenia and myosteatosis for outcome assessment in patients with advanced pancreatic cancer after high-intensity focused ultrasound treatment. Eur. Radiol. 2024, 34, 279–286. [Google Scholar] [CrossRef]
- Suzuki, Y.; Saito, K.; Nakai, Y.; Oyama, H.; Kanai, S.; Suzuki, T.; Sato, T.; Hakuta, R.; Ishigaki, K.; Saito, T.; et al. Early skeletal muscle mass decline is a prognostic factor in patients receiving gemcitabine plus nab-paclitaxel for unresectable pancreatic cancer: A retrospective observational study. Support. Care Cancer 2023, 31, 197. [Google Scholar] [CrossRef] [PubMed]
- Wochner, R.; Clauss, D.; Nattenmüller, J.; Tjaden, C.; Bruckner, T.; Kauczor, H.-U.; Hackert, T.; Wiskemann, J.; Steindorf, K. Impact of progressive resistance training on CT quantified muscle and adipose tissue compartments in pancreatic cancer patients. PLoS ONE 2020, 15, e0242785. [Google Scholar] [CrossRef]
- Balcer, K.; Garnier, J.; Richa, Y.; Bruneel-Zupanc, C.; Piessen, G.; Turrini, O.; Truant, S.; El Amrani, M. Impact on survival of sarcopenia, systemic inflammatory response and anthropometric factors after pancreatectomy for resectable pancreatic adenocarcinoma. World J. Surg. Oncol. 2024, 22, 232. [Google Scholar] [CrossRef]
- Beetz, N.L.; Geisel, D.; Maier, C.; Auer, T.A.; Shnayien, S.; Malinka, T.; Neumann, C.C.M.; Pelzer, U.; Fehrenbach, U. Influence of Baseline CT Body Composition Parameters on Survival in Patients with Pancreatic Adenocarcinoma. J. Clin. Med. 2022, 11, 2356. [Google Scholar] [CrossRef]
- Miki, M.; Lee, L.; Hisano, T.; Sugimoto, R.; Furukawa, M. Loss of adipose tissue or skeletal muscle during first-line gemcitabine/nab-paclitaxel therapy is associated with worse survival after second-line therapy of advanced pancreatic cancer. Asia-Pac. J. Clin. Oncol. 2022, 18, 297–305. [Google Scholar] [CrossRef]
- van Dijk, D.P.J.; Bakens, M.J.A.M.; Coolsen, M.M.E.; Rensen, S.S.; van Dam, R.M.; Bours, M.J.L.; Weijenberg, M.P.; Dejong, C.H.C.; Damink, S.W.M.O. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Gunchick, V.; Brown, E.; Liu, J.; Locasale, J.W.; Philip, P.A.; Wang, S.C.; Su, G.L.; Sahai, V. Morphomics, Survival, and Metabolites in Patients With Metastatic Pancreatic Cancer. JAMA Netw. Open 2024, 7, e2440047. [Google Scholar] [CrossRef] [PubMed]
- Babic, A.; Rosenthal, M.H.; Bamlet, W.R.; Takahashi, N.; Sugimoto, M.; Danai, L.V.; Morales-Oyarvide, V.; Khalaf, N.; Dunne, R.F.; Brais, L.K.; et al. Postdiagnosis Loss of Skeletal Muscle, but Not Adipose Tissue, Is Associated with Shorter Survival of Patients with Advanced Pancreatic Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 2062–2069. [Google Scholar] [CrossRef]
- Choi, M.H.; Yoon, S.B.; Lee, K.; Song, M.; Lee, I.S.; Lee, M.A.; Hong, T.H.; Choi, M. Preoperative sarcopenia and post-operative accelerated muscle loss negatively impact survival after resection of pancreatic cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 326–334. [Google Scholar] [CrossRef]
- Bareiß, S.; Merkel, S.; Krautz, C.; Weber, G.F.; Grützmann, R.; Brunner, M. Prognostic role of nutrition parameters on short- and long-term outcome in patients with primary resectable pancreatic ductal adenocarcinoma. Clin. Nutr. ESPEN 2024, 62, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Jeon, S.K.; Paik, W.H.; Yoon, J.H.; Joo, I.; Lee, J.M.; Lee, S.H. Prognostic value of initial and longitudinal changes in body composition in metastatic pancreatic cancer. J. Cachexia Sarcopenia Muscle 2024, 15, 735–745. [Google Scholar] [CrossRef]
- Bian, X.; Dai, H.; Feng, J.; Ji, H.; Fang, Y.; Jiang, N.; Li, W. Prognostic values of abdominal body compositions on survival in advanced pancreatic cancer. Medicine 2018, 97, e10988. [Google Scholar] [CrossRef] [PubMed]
- Nakano, O.; Kawai, H.; Kobayashi, T.; Kohisa, J.; Ikarashi, S.; Hayashi, K.; Yokoyama, J.; Terai, S. Rapid decline in visceral adipose tissue over 1 month is associated with poor prognosis in patients with unresectable pancreatic cancer. Cancer Med. 2021, 10, 4291–4301. [Google Scholar] [CrossRef]
- Kays, J.K.; Shahda, S.; Stanley, M.; Bell, T.M.; O’Neill, B.H.; Kohli, M.D.; Couch, M.E.; Koniaris, L.G.; Zimmers, T.A. Three cachexia phenotypes and the impact of fat-only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 673–684. [Google Scholar] [CrossRef]
- Pecorelli, N.; Carrara, G.; De Cobelli, F.; Cristel, G.; Damascelli, A.; Balzano, G.; Beretta, L.; Braga, M. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br. J. Surg. 2016, 103, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Tewari, N.; Awad, S.; Macdonald, I.A.; Lobo, D.N. A comparison of three methods to assess body composition. Nutrition 2018, 47, 1–5. [Google Scholar] [CrossRef]
- Caan, B.J.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef]
- Mintziras, I.; Miligkos, M.; Wächter, S.; Manoharan, J.; Maurer, E.; Bartsch, D.K. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis. Int. J. Surg. 2018, 59, 19–26. [Google Scholar] [CrossRef]
- Stretch, C.; Aubin, J.M.; Mickiewicz, B.; Leugner, D.; Al-Manasra, T.; Tobola, E.; Salazar, S.; Suther-land, F.R.; Ball, C.G.; Dixon, E.; et al. Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PLoS ONE 2018, 13, e0196235. [Google Scholar] [CrossRef]
- Liu, C.; An, L.; Zhang, S.; Deng, S.; Wang, N.; Tang, H. Association between preoperative sarcopenia and prognosis of pancreatic cancer after curative-intent surgery: A updated systematic review and meta-analysis. World J. Surg. Oncol. 2024, 22, 38. [Google Scholar] [CrossRef] [PubMed]
- Láinez Ramos-Bossini, A.J.; Gámez Martínez, A.; Luengo Gómez, D.; Valverde-López, F.; Mo-rillo Gil, A.J.; González Flores, E.; Salmerón Ruiz, Á.; Jiménez Gutiérrez, P.M.; Melguizo, C.; Pra-dos, J. Computed Tomography-Based Sarcopenia and Pancreatic Cancer Survival-A Com-prehensive Meta-Analysis Exploring the Influence of Definition Criteria, Prevalence, and Treatment Intention. Cancers 2025, 17, 607. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Boutin, R.D.; Lenchik, L.; Kim, J.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L., 3rd; Guthrie, K.A.; Chiorean, E.G.; Ahmad, S.A.; et al. Body composition meas-urements and clinical outcomes in patients with resectable pancreatic adenocarcinoma-analysis from SWOG S1505. J. Gastrointest. Surg. 2024, 28, 232–235. [Google Scholar] [CrossRef]
- Theis, M.; Hong, W.; Lee, B.; Nowak, S.; Luetkens, J.; Stuckey, S.; Gibbs, P.; Thomson, B.; Michael, M.; Sprinkart, A.M.; et al. Skeletal muscle and visceral fat density are predictive imaging bi-omarkers for overall survival in patients with pancreatic adenocarcinoma: A retrospective multicenter analysis. Surg. Oncol. 2025, 61, 102251. [Google Scholar] [CrossRef]
- Gruber, E.S.; Jomrich, G.; Tamandl, D.; Gnant, M.; Schindl, M.; Sahora, K. Sarcopenia and sar-copenic obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS ONE 2019, 14, e0215915. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity-defnition, etiology and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Surov, A.; Thormann, M.; Wienke, A.; Ricke, J.; Seidensticker, M. Different cutoff values of the skeletal muscle mass and myosteatosis result in different clinical impact on overall sur-vival in oncology. A subanalysis of a clinical trial. J. Cancer Res. Clin. Oncol. 2025, 151, 141. [Google Scholar] [CrossRef]
- Leão Mendes, J.; Ferreira, R.Q.; Mata, I.; Vasco Barreira, J.; Rodrigues, Y.C.; Silva Dias, D.; Capelas, M.L.; Mäkitie, A.; Guerreiro, I.; Pimenta, N.M.; et al. Body Composition Analysis in Meta-static Non-Small-Cell Lung Cancer: Depicting Sarcopenia in Portuguese Tertiary Care. Cancers 2025, 17, 539. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.