SPECT/CT in the Evaluation of Suspected Skeletal Pathology
Abstract
1. Introduction
2. Oncologic Applications of Bone SPECT/CT
3. Non-Oncologic Applications of Bone SPECT/CT
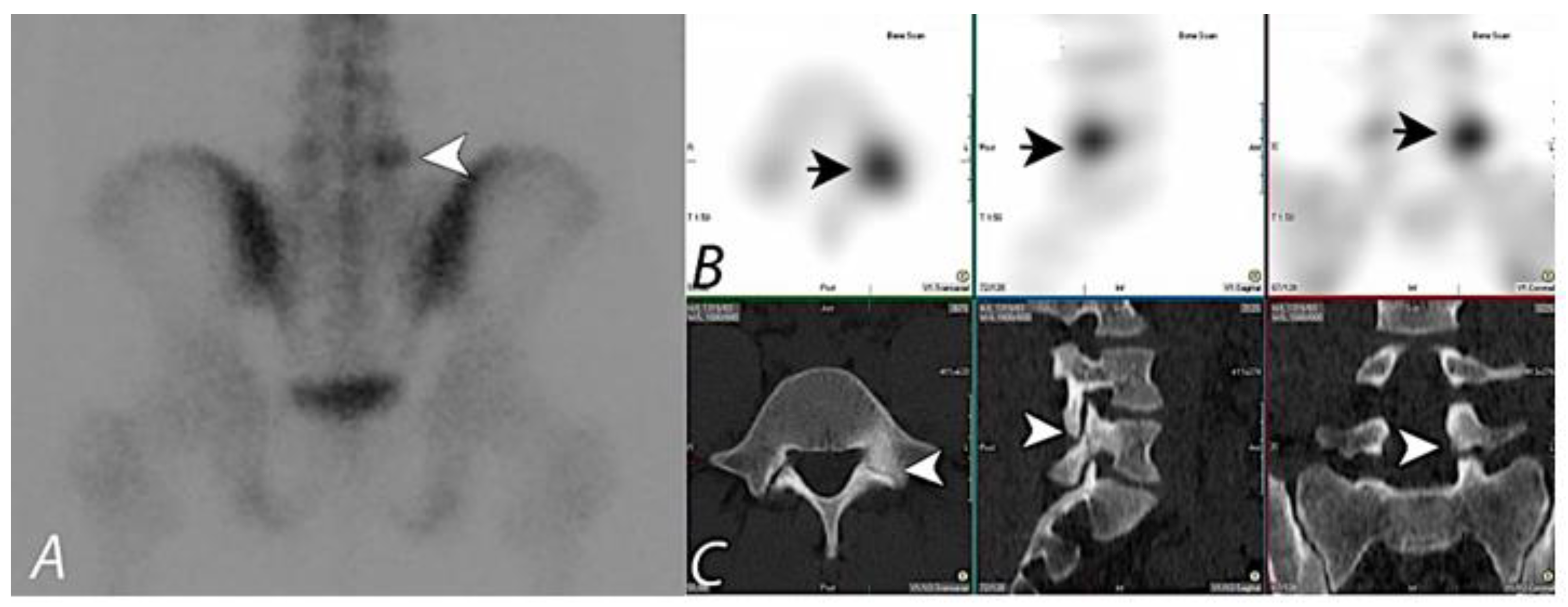
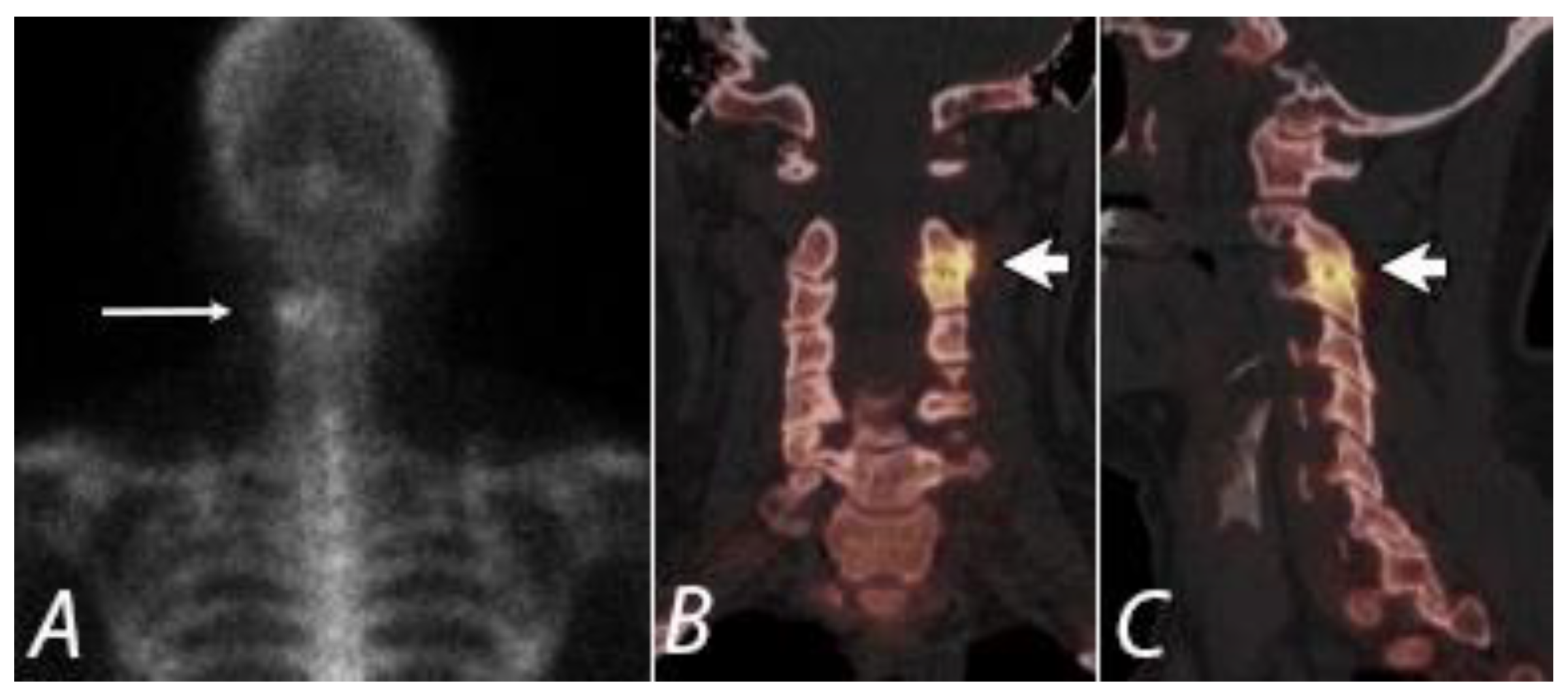
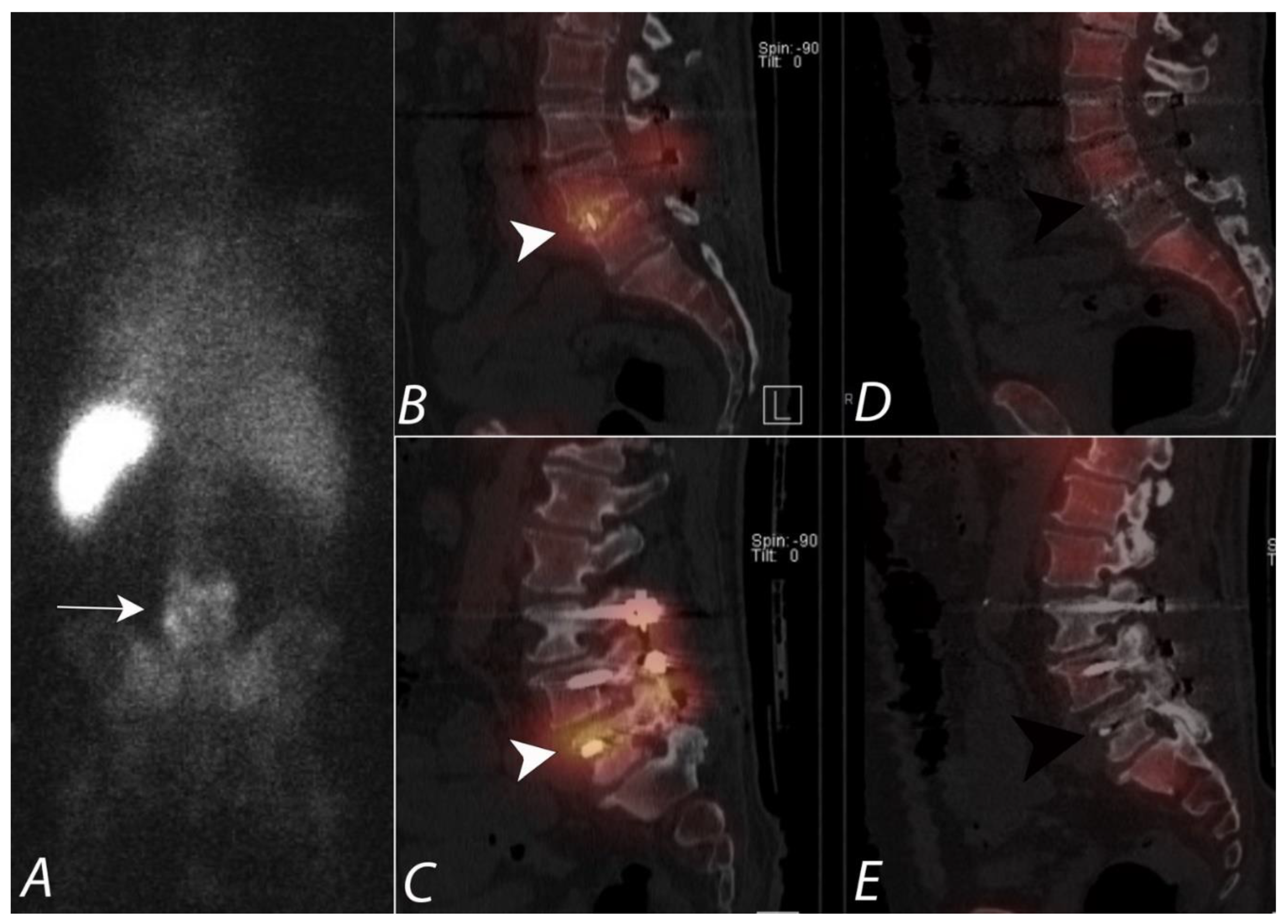
4. Evaluation of Pain of Spinal Origin
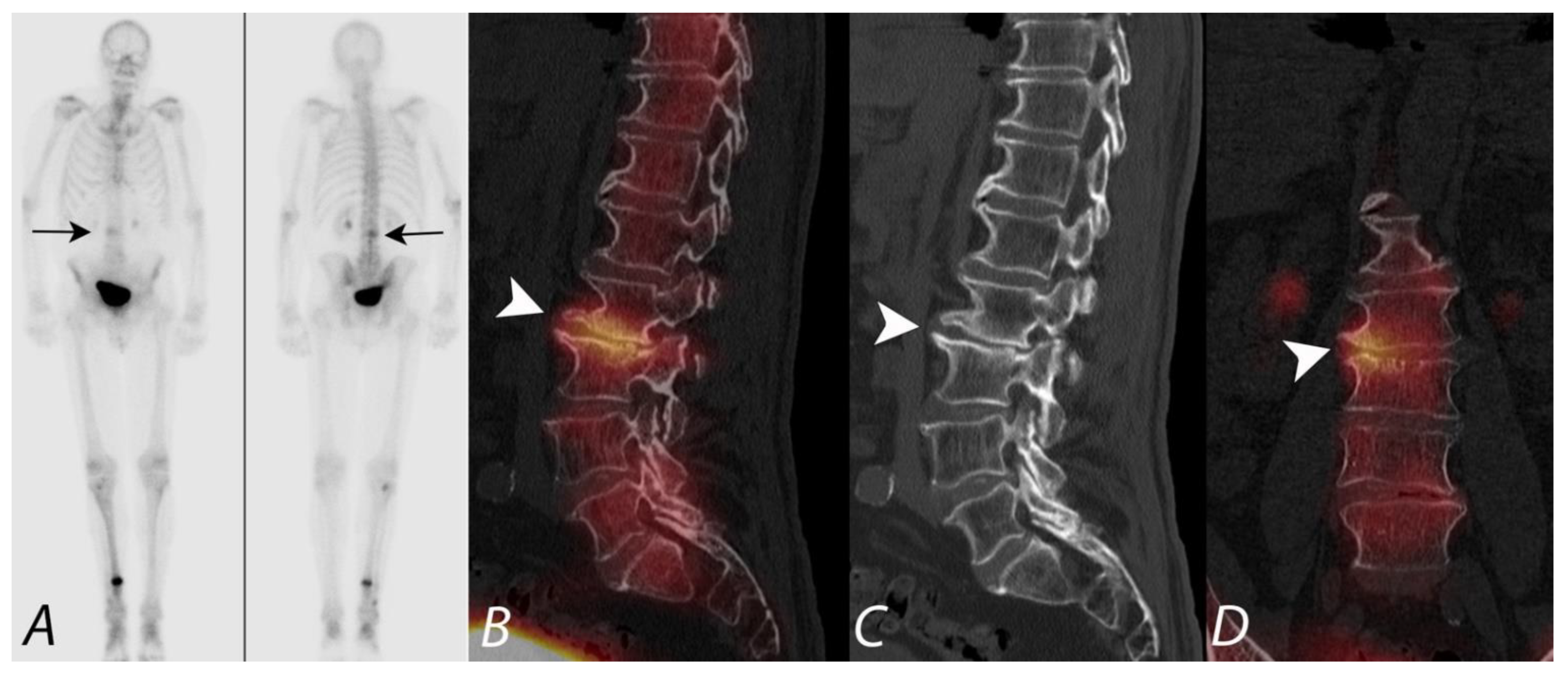
5. Evaluation of Postoperative Spine
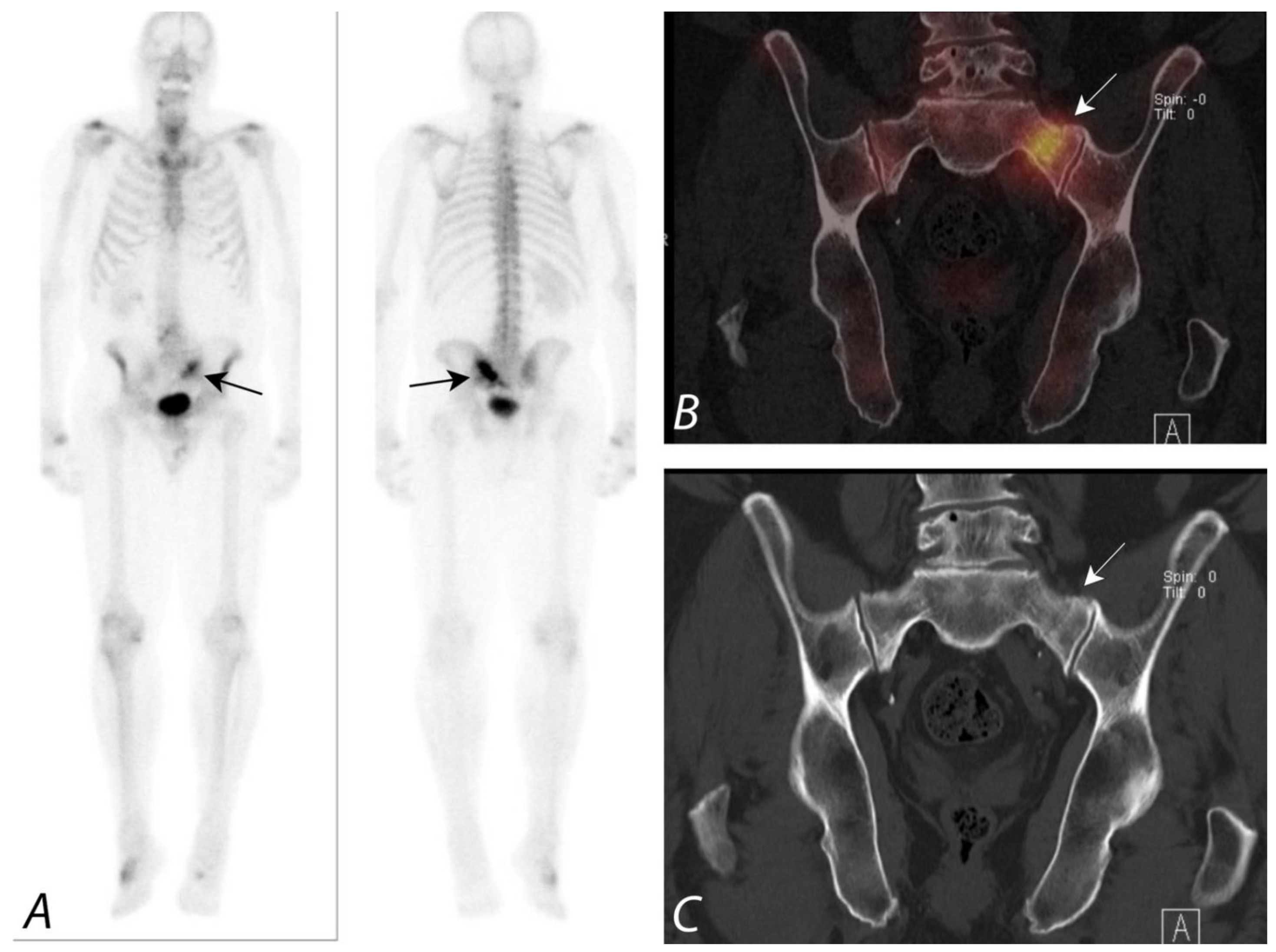
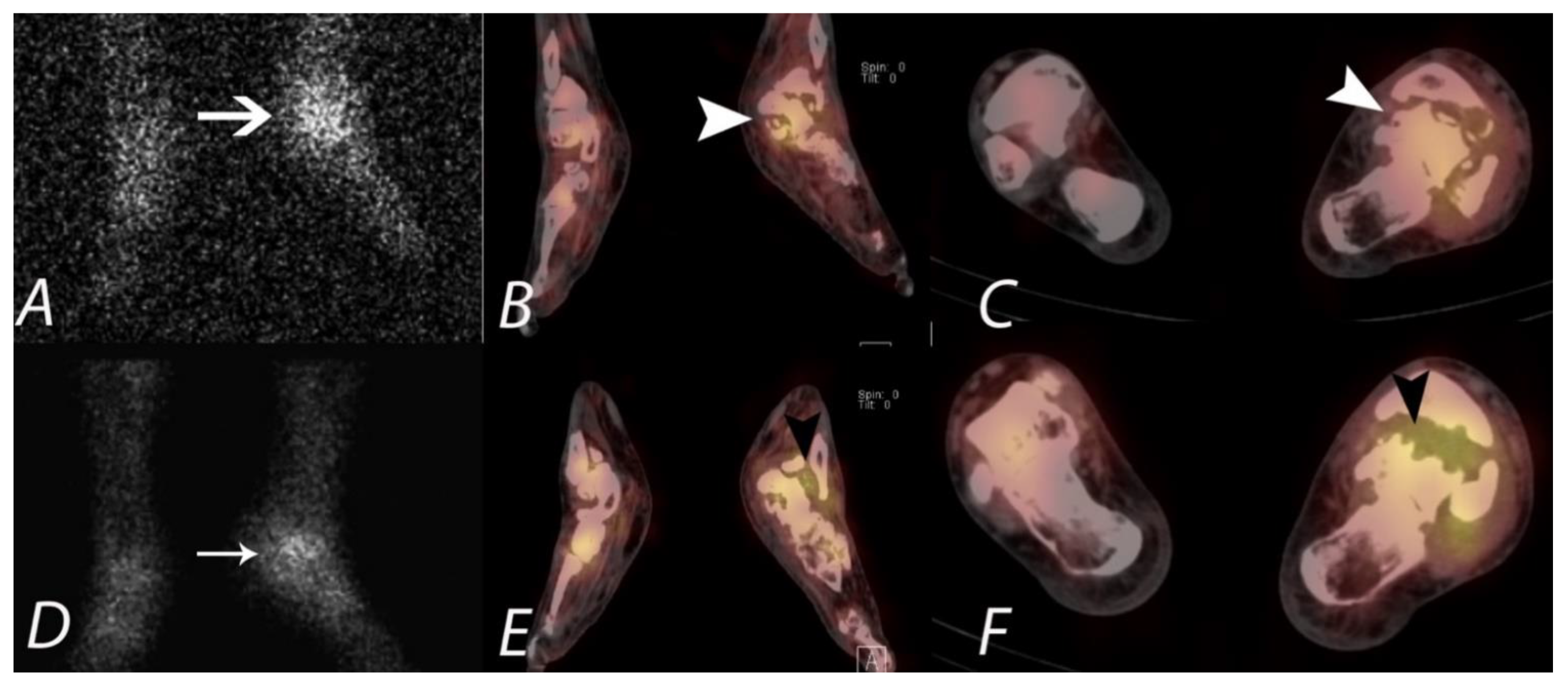
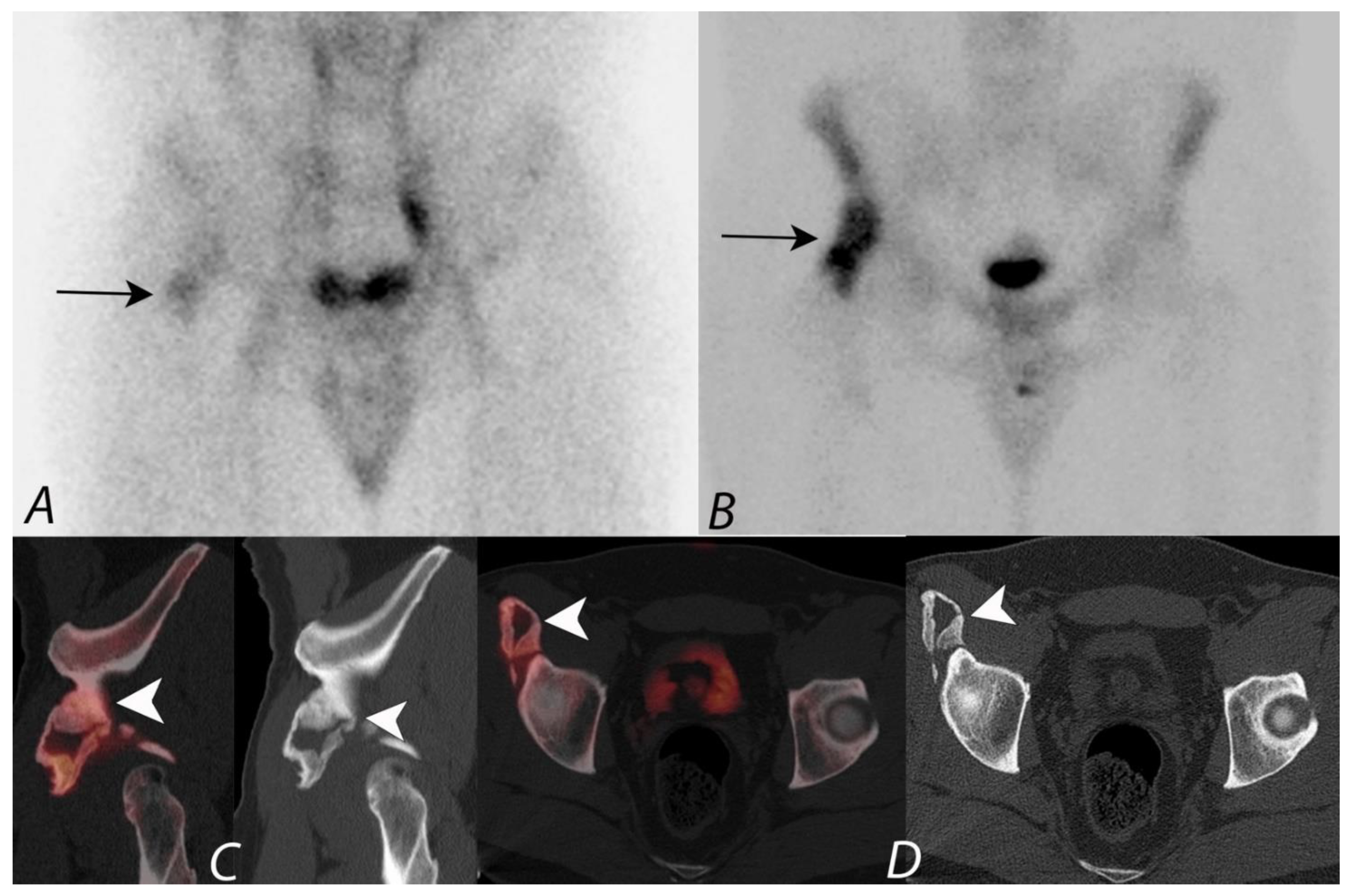
6. Hip Pain
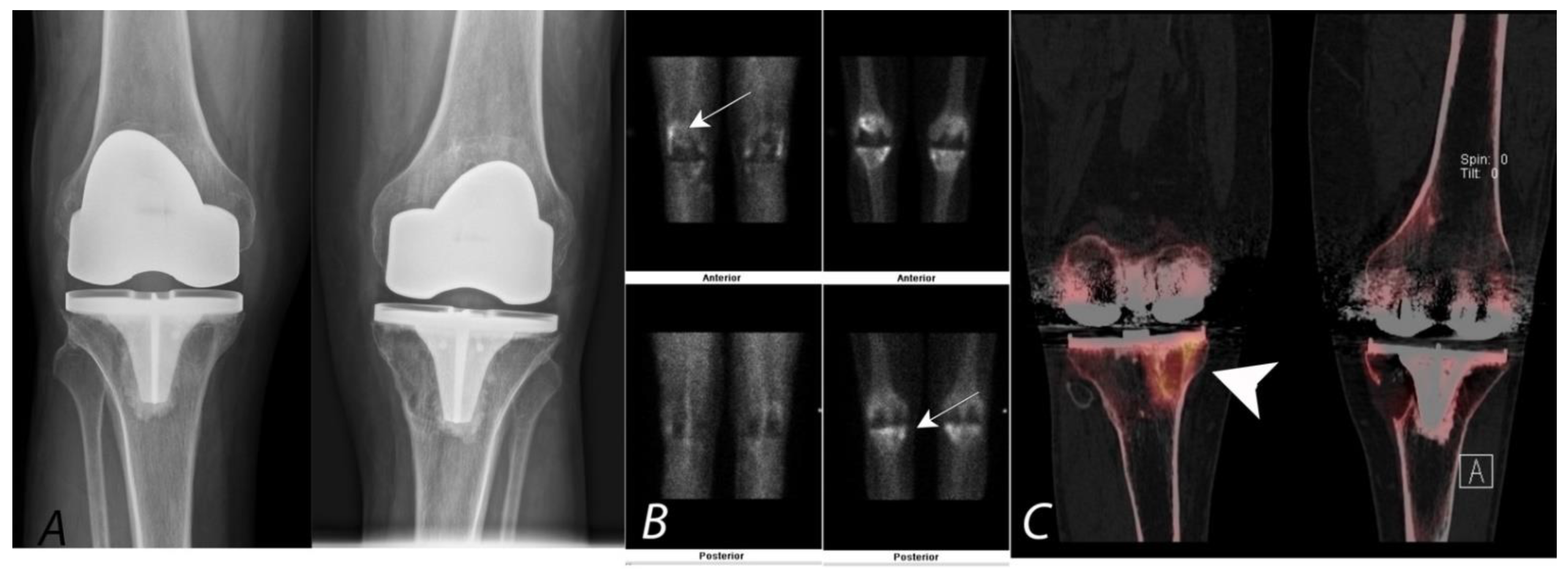
7. Knee Pain
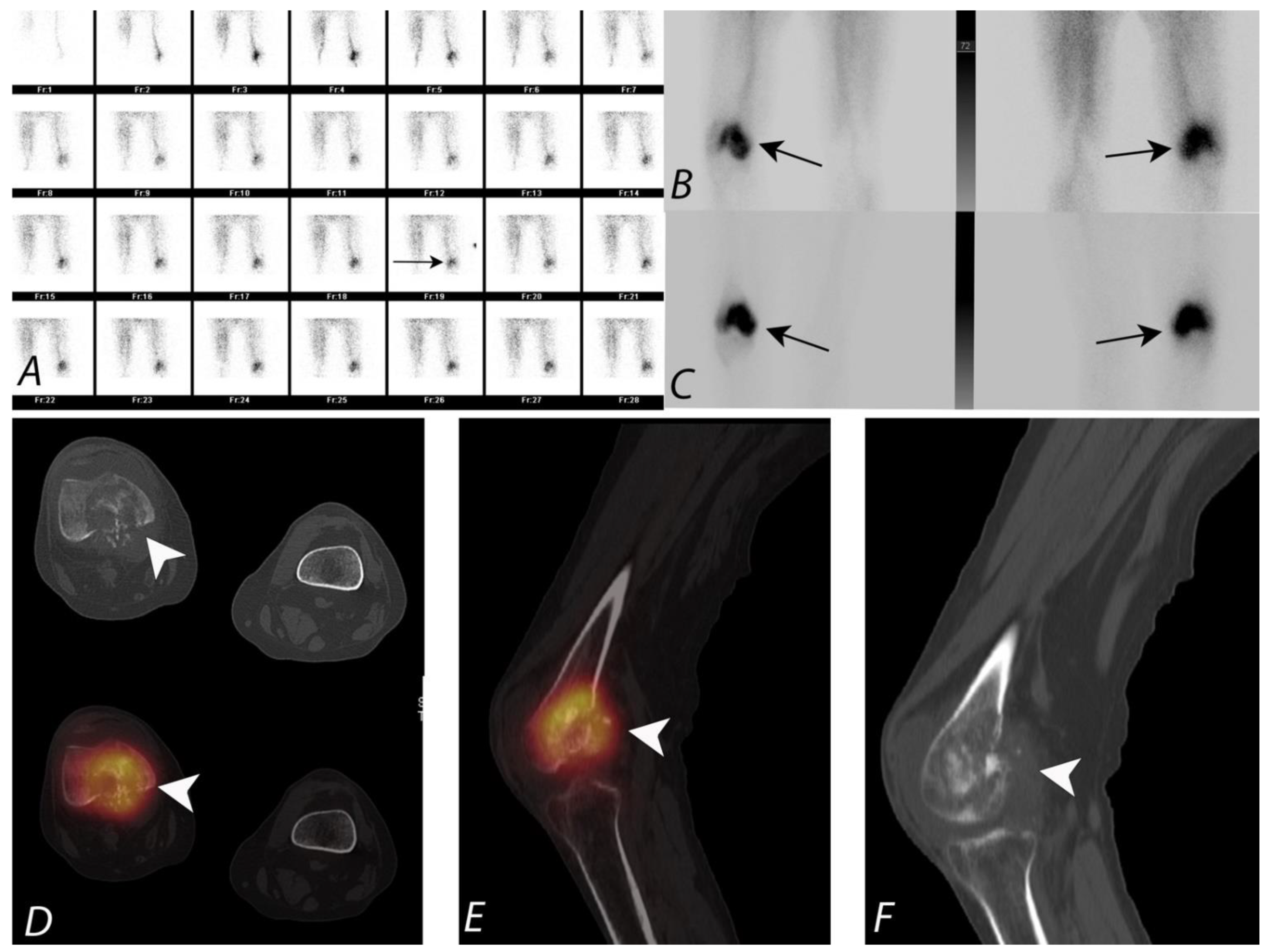
8. SPECT/CT of Extremities
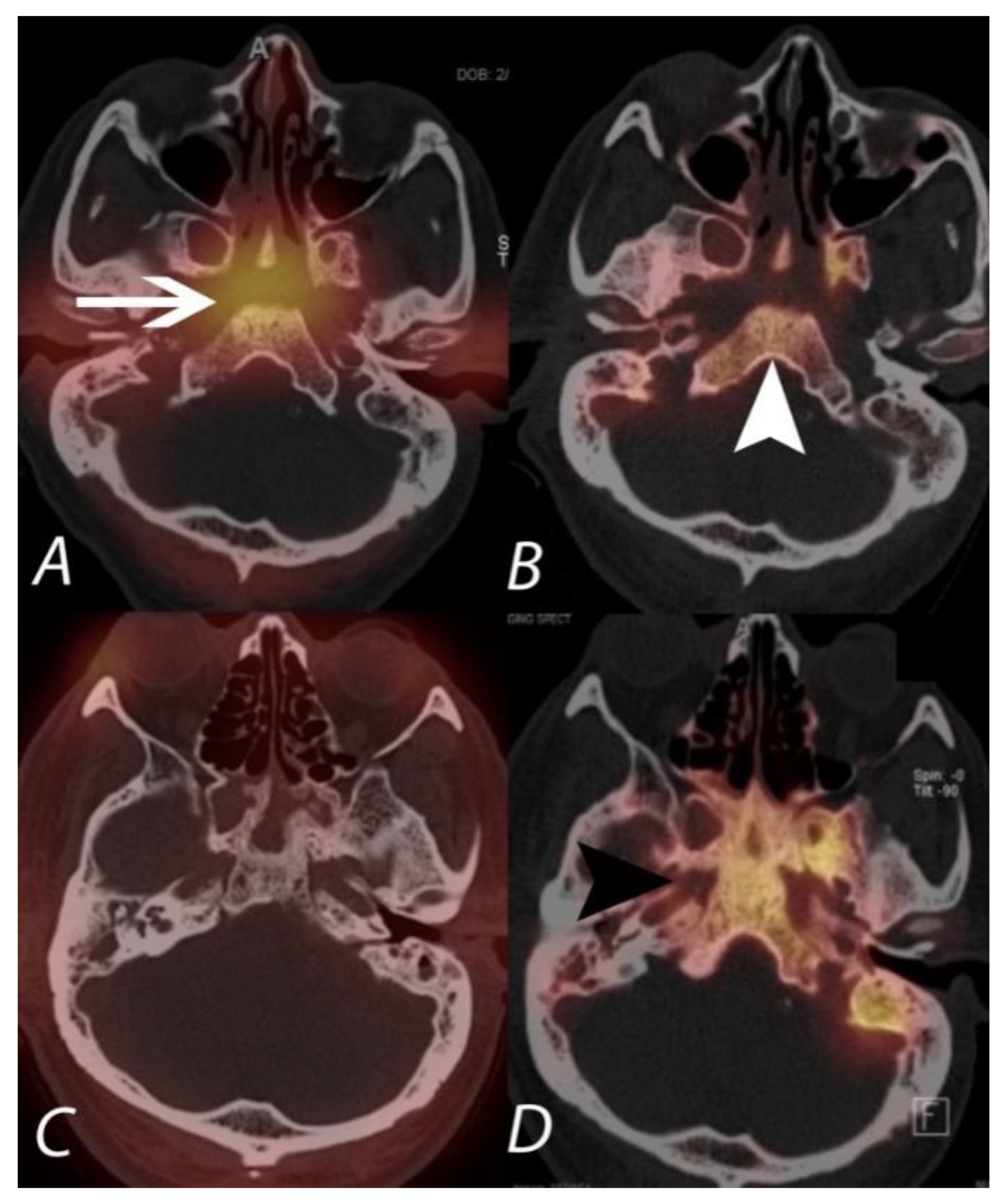
9. Infection and Inflammation
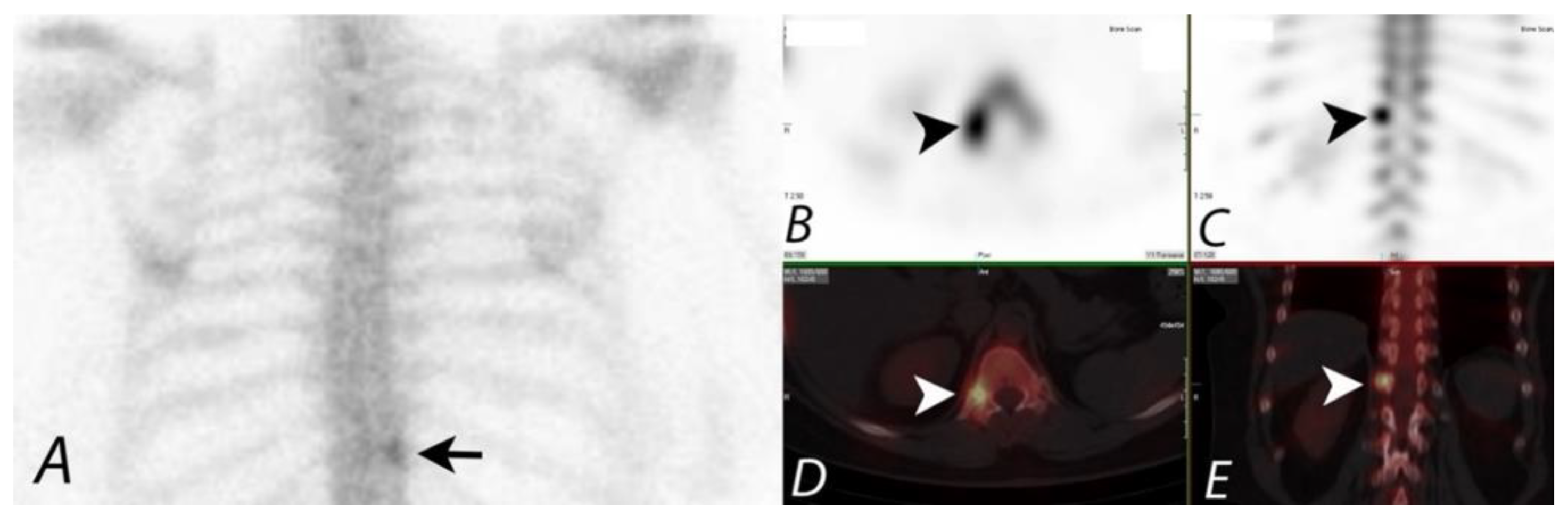
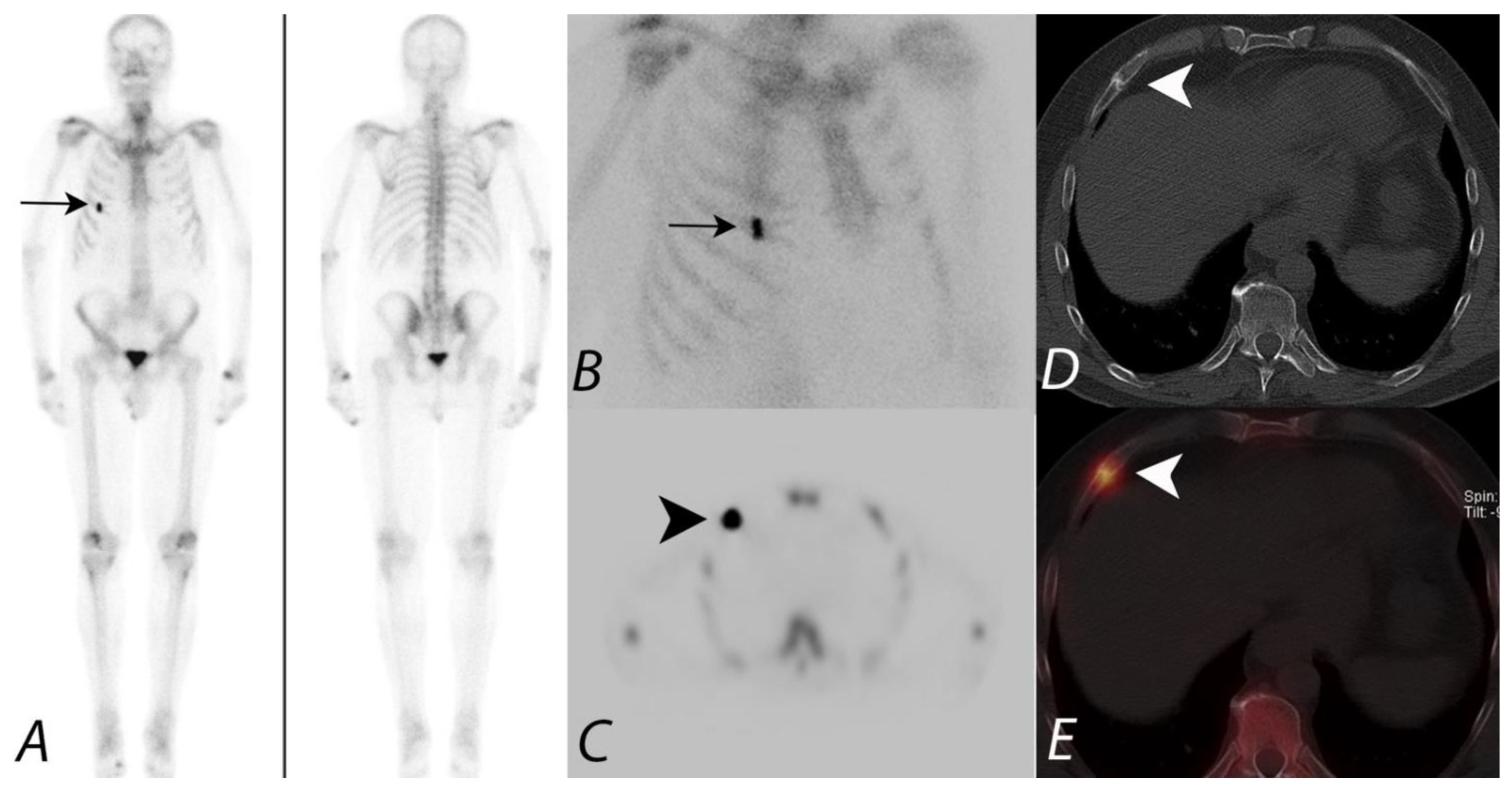
10. Extraosseous Uptake of Tracer on Bone Scans
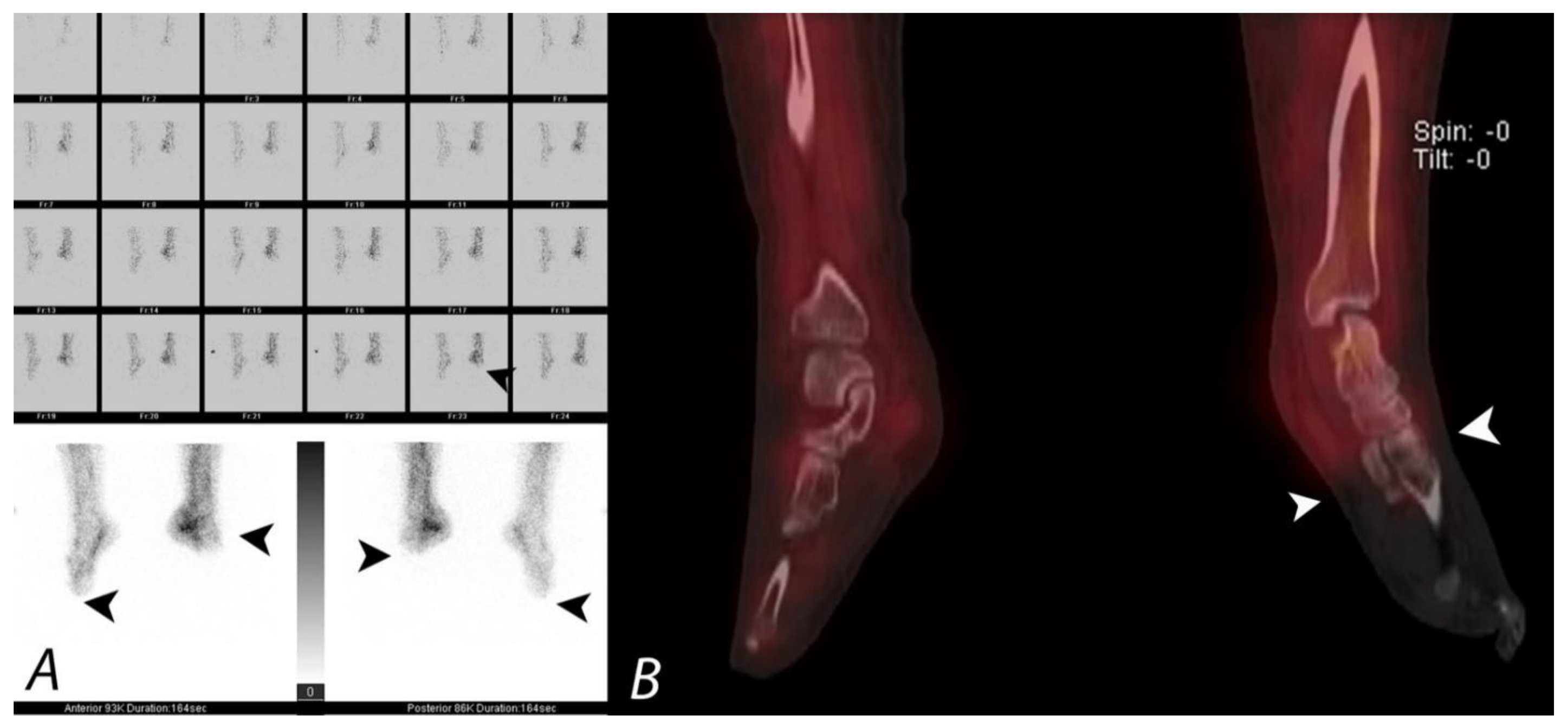
11. Frostbite
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Buckland-Wright, J.C.; Fogelman, I.; Emery, P.; Lynch, J.A. Technetium 99m methylene diphosphonate bone scanning in osteoarthritic hands. Eur. J. Nucl. Med. 1991, 18, 12–16. [Google Scholar] [CrossRef]
- Collier, B.D., Jr.; Krasnow, A.Z. Bone Spect. Semin. Nucl. Med. 1987, 17, 247–266. [Google Scholar] [CrossRef]
- Fogelman, I.; Boyle, I.T. The bone scan in clinical practice. Scott. Med. J. 1980, 25, P45–P49. [Google Scholar] [CrossRef] [PubMed]
- Groshar, D.; Ben-Haim, S.; Jerusalmi, J.; Liberson, A. Lower extremity scintigraphy: The foot and ankle. Semin. Nucl. Med. 1998, 28, 62–77. [Google Scholar] [CrossRef]
- Hain, S.F.; O’doherty, M.J.; Smith, M.A. Functional imaging and the orthopaedic surgeon. J. Bone Jt. Surg. Br. 2002, 84, 315–321. [Google Scholar] [CrossRef]
- Macfarlane, D.G.; Buckland-Wright, J.; Lynch, J.; Fogelman, I. A study of the early and late 99technetium scintigraphic images and their relationship to symptoms in osteoarthritis of the hands. Br. J. Rheumatol. 1993, 32, 977–981. [Google Scholar] [CrossRef]
- Mohamed, A.; Ryan, P.; Lewis, M.; Jarosz, J.M.; Fogelman, I.; Spencer, J.D.; Clarke, S.E. Registration bone scan in the evaluation of wrist pain. J. Hand Surg. Br. 1997, 22, 161–166. [Google Scholar] [CrossRef]
- Minoves, M. Bone and joint sports injuries: The role of bone scintigraphy. Nucl. Med. Commun. 2003, 24, 3–10. [Google Scholar] [CrossRef]
- Ryan, P.J.; Fogelman, I. The bone scan: Where are we now? Semin. Nucl. Med. 1995, 25, 76–91. [Google Scholar] [CrossRef]
- Ryan, P.J.; Fogelman, I. The role of nuclear medicine in orthopaedics. Nucl. Med. Commun. 1994, 15, 341–360. [Google Scholar] [CrossRef]
- Shehab, D.; Elgazzar, A.; Collier, B.D.; Naddaf, S.; Al-Jarallah, K.; Omar, A.; Al-Mutairy, M. Impact of three-phase bone scintigraphy on the diagnosis and treatment of complex regional pain syndrome type I or reflex sympathetic dystrophy. Med. Princ. Pract. 2006, 15, 46–51. [Google Scholar] [CrossRef]
- O’Connor, M.K.; Kemp, B.J. Single-photon emission computed tomography/computed tomography: Basic instrumentation and innovations. Semin. Nucl. Med. 2006, 36, 258–266. [Google Scholar] [CrossRef]
- Pietrzyk, U.; Herholz, K.; Fink, G.; Jacobs, A.; Mielke, R.; Slansky, I.; Würker, M.; Heiss, W.D. An interactive technique for three-dimensional image registration: Validation for PET, SPECT, MRI and CT brain studies. J. Nucl. Med. 1994, 35, 2011–2018. [Google Scholar]
- Förster, G.J.; Laumann, C.; Nickel, O.; Kann, P.; Rieker, O.; Bartenstein, P. Thoracic and abdominal SPECT-CT image fusion without external markers in endocrine carcinomas; The Group of Thyroid Tumoral Pathology of Champagne-Ardenne. J. Nucl. Med. 1997, 38, 1234–1242. [Google Scholar]
- Slomka, P.J. Software approach to merging molecular with anatomic information. J. Nucl. Med. 2004, 45, 36S–45S. [Google Scholar]
- Förster, G.J.; Laumann, C.; Nickel, O.; Kann, P.; Rieker, O.; Bartenstein, P. PET/CT image co-registration in the abdomen with a simple and cost-effective tool. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 32–39. [Google Scholar]
- Metser, U.; Golan, O.; Levine, C.D.; Even-Sapir, E. Tumor lesion detection: When is integrated positron emission tomography/computed tomography more accurate than side-by-side interpretation of positron emission tomography and computed tomography? J. Comput. Assist. Tomogr. 2005, 29, 554–559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hasegawa, B.H.; Wong, K.H.; Iwata, K.; Barber, W.C.; Hwang, A.B.; Sakdinawat, A.E.; Ramaswamy, M.; Price, D.C.; Hawkins, R.A. Dual-modality imaging of cancer with SPECT/CT. Technol. Cancer Res. Treat. 2002, 1, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.W.; Cherry, S.R. Combining anatomy and function: The path to true image fusion. Eur. Radiol. 2001, 11, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.F.; Hasegawa, B.H.; Liew, S.C.; Brown, J.K.; Blankespoor, S.C.; Reilly, S.M.; Gingold, E.L.; Cann, C.E. Description of a prototype emission-transmission computed tomography imaging system. J. Nucl. Med. 1992, 33, 1881–1887. [Google Scholar] [PubMed]
- Bocher, M.; Balan, A.; Krausz, Y.; Shrem, Y.; Lonn, A.; Wilk, M.; Chisin, R. Gamma camera-mounted anatomical X-ray tomography: Technology, system characteristics and first images. Eur. J. Nucl. Med. 2000, 27, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.A.; Sandler, M.P. Image fusion using an integrated, dual-head coincidence camera with X-ray tube-based attenuation maps. J. Nucl. Med. 2000, 41, 1364–1368. [Google Scholar] [PubMed]
- Ljungberg, M.; Pretorius, P.H. SPECT/CT: An update on technological developments and clinical applications. Br. J. Radiol. 2018, 91, 1081. [Google Scholar] [CrossRef]
- Savelli, G.; Maffioli, L.; Maccauro, M.; De Deckere, E.; Bombardieri, E. Bone scintigraphy and the added value of SPECT (single photon emission tomography) in detecting skeletal lesions. Q. J. Nucl. Med. 2001, 45, 27–37. [Google Scholar]
- Even-Sapir, E.; Martin, R.H.; Barnes, D.C.; Pringle, C.R.; Iles, S.E.; Mitchell, M.J. Role of SPECT in differentiating malignant from benign lesions in the lower thoracic and lumbar vertebrae. Radiology 1993, 187, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Even-Sapir, E. Imaging of malignant bone involvement by morphologic, s., and hybrid modalities. J. Nucl. Med. 2005, 46, 1356–1367. [Google Scholar] [PubMed]
- Bushnell, D.L.; Kahn, D.; Huston, B.; Bevering, C.G. Utility of SPECT imaging for determination of vertebral metastases in patients with known primary tumors. Skelet. Radiol. 1995, 24, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Han, L.J.; Au-Yong, T.K.; Tong, W.C.; Chu, K.S.; Szeto, L.T.; Wong, C.P. Comparison of bone single photon emission tomography and planar imaging in the detection of vertebral metastases in patients with back pain. Eur. J. Nucl. Med. 1998, 25, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, S.J.; Ramsingh, P.S. Bone scintigraphy and multimodality imaging in bone neoplasia: Strategies for imaging in the new health climate. Semin. Nucl. Med. 1994, 24, 188–207. [Google Scholar] [CrossRef]
- Krishnamurthy, G.T.; Tubis, M.; Hiss, J.; Blahd, W.H. Distribution pattern of metastatic bone disease. A need for total body skeletal image. JAMA 1977, 237, 2504–2506. [Google Scholar] [CrossRef]
- McNeil, B.J. Value of bone scanning in neoplastic disease. Semin. Nucl. Med. 1984, 14, 277–286. [Google Scholar] [CrossRef]
- Utsunomiya, D.; Shiraishi, S.; Imuta, M.; Tomiguchi, S.; Kawanaka, K.; Morishita, S.; Awai, K.; Yamashita, Y. Added value of SPECT/CT fusion in assessing suspected bone metastasis: Comparison with scintigraphy alone and nonfused scintigraphy and CT. Radiology 2006, 238, 264–271. [Google Scholar] [CrossRef]
- Strobel, K.; Burger, C.; Seifert, B.; Husarik, D.B.; Soyka, J.D.; Hany, T.F. Characterization of focal bone lesions in the axial skeleton: Performance of planar bone scintigraphy compared with SPECT and SPECT fused with CT. AJR Am. J. Roentgenol. 2007, 188, W467–W474. [Google Scholar] [CrossRef]
- Horger, M.; Eschmann, S.M.; Pfannenberg, C.; Vonthein, R.; Besenfelder, H.; Claussen, C.D.; Bares, R. Evaluation of combined transmission and emission tomography for classification of skeletal lesions. AJR Am. J. Roentgenol. 2004, 183, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Römer, W.; Nömayr, A.; Uder, M.; Bautz, W.; Kuwert, T. SPECT-guided CT for evaluating foci of increased bone metabolism classified as indeterminate on SPECT in cancer patients. J. Nucl. Med. 2006, 47, 1102–1106. [Google Scholar] [PubMed]
- Barwick, T.; Gnanasegaran, G.; Rashika, F.; Mohan, H.K. The use of 99mTc-MDP SPECT/CT in the evaluation of indeterminate bone lesions on whole body planar imaging in cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2008, 35 (Suppl. 2), S155. (In abstract) [Google Scholar]
- Helyar, V.; Barwick, T.; Livieratos, L.; Gnanasegaran, G.; Clarke, S.E.; Fogelman, I. The added value of multislice SPECT/CT in patients with equivocal bony metastasis from carcinoma of the prostate. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Even-Sapir, E.; Lerman, H.; Lievshitz, G.; Metser, U. SPECT/multislice low-dose CT: A clinically relevant constituent in the imaging algorithm of nononcologic patients referred for bone scintigraphy. J. Nucl. Med. 2007, 48, 319–324. [Google Scholar] [PubMed]
- Linke, R.; Uder, M.; Forst, R.; Wuest, W. Skeletal SPECT/CT of the peripheral extremities. Am. J. Roentgenol. 2010, 194, 329–335. [Google Scholar] [CrossRef]
- Dagenais, S.; Haldeman, S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008, 8, 8–20. [Google Scholar] [CrossRef]
- Ryan, P.J.; Evans, P.A.; Gibson, T.; Fogelman, I. Chronic low back pain: Comparison of bone SPECT with and radiograph and CT. Radiology 1992, 182, 849–854. [Google Scholar] [CrossRef]
- Dolan, A.L.; Ryan, P.J.; Arden, N.K.; Stratton, R.; Wedley, J.R.; Hamann, W.; Fogelman, I.; Gibson, T. The value of SPECT scans in identifying back pain likely and to benefit from facet joint injection. Br. J. Rheumatol. 1996, 35, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Pneumaticos, S.G.; Hipp, J.A.; Moore, W.H.; Esses, S.I. Low back pain: Prediction of short-term outcome of facet joint injection with bone scintigraphy. Radiology 2006, 238, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Holder, L.E.; Asdourian, P.L.; Links, J.M.; Sexton, C.C. Planar and high-resolution SPECT and high resolution SPECT bone imaging in the diagnosis of facet syndrome. J. Nucl. Med. 1995, 36, 37–44. [Google Scholar] [PubMed]
- Ryan, P.J.; Gibson, T.; Fogel, I. Osteoporosis and chronic back pain: A study with single photon emission computed tomography bone scintigraphy. J. Bone Miner. Res. 1992, 7, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Haid, R.W. Current treatment strategies for the painful lumbar and motion segment: Posterolateral fusion versus interbody fusion. Spine 2005, 30, S33–S43. [Google Scholar] [CrossRef] [PubMed]
- Hayeri, M.R.; Tehranzadeh, J. Diagnostic imaging of spinal fusion and complications. Appl. Radiol. 2009, 38, 14–28. [Google Scholar]
- Lipson, S.J. Spinal-fusion surgery—Advances and concerns. N. Engl. J. Med. 2004, 350, 643–644. [Google Scholar] [CrossRef]
- Al-Riyami, K.; Van den Wyngaert, T.; Bomanji, J. Bone SPECT/CT in the postoperative spine: A focus on spinal fusion. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- Herrera Herrera, I.; Moreno de la Presa, R.; González Gutiérrez, R.; Bárcena Ruiz, E.; García Benassi, J.M. Evaluation of the and postoperative lumbar spine. Radiologia 2013, 55, 12–23. [Google Scholar] [CrossRef]
- Sarrazin, J.L. Imaging of postoperative lumbar spine. J. Radiol. 2003, 84, 241–250. [Google Scholar]
- Sanders, W.P.; Truumees, E. Imaging of the postoperative spine. Semin. Ultrasound CT MRI 2004, 25, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.I.; Mirza, S.K.; Comstock, B.A.; Gray, D.T.; Kreuter, W.; Deyo, R.A. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine 2007, 32, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M.; Capen, D.A. Pseudarthrosis of the Lumbar Spine. J. Am. Acad. Orthop. Surg. 1997, 5, 153–162. [Google Scholar] [CrossRef]
- Rutherford, E.E.; Davies, E.M.; Harley, J.M.; King, L.J. Lumbar spine fusion and stabilization: Hardware, techniques, and imaging appearances. Radiographics 2007, 27, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, M.; Madsen, J.L. The role of bone SPECT/CT in the evaluation of lumbar spinal fusion with metallic fixation devices. Clin. Nucl. Med. 2010, 35, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Young, P.M.; Berquist, T.H.; Bancroft, L.W.; Peterson, J.J. Complications of spinal instrumentation. Radiographics 2007, 27, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, M.; Aggeliki, L.; Papadopoulos, E.C.; Girardi, F.P. Common surgical complications in and degenerative spinal surgery. World J. Orthop. 2013, 4, 62–66. [Google Scholar] [CrossRef]
- Kornblum, M.B.; Fischgrund, J.S.; Herkowitz, H.N.; Abraham, D.A.; Berkower, D.L.; Ditkoff, J.S. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective long-term study comparing fusion and pseudarthrosis. Spine 2004, 29, 726–733. [Google Scholar] [CrossRef]
- Etminan, M.; Girardi, F.P.; Khan, S.N.; Cammisa, F.P., Jr. Revision strategies for lumbar pseudarthrosis. Orthop. Clin. N. Am. 2004, 33, 381–392. [Google Scholar] [CrossRef]
- Gates, G.F. SPECT bone scanning of the spine. Semin. Nucl. Med. 1998, 28, 78–94. [Google Scholar] [CrossRef]
- Iseda, T.; Nakano, S.; Suzuki, Y.; Miyahara, D.; Uchinokura, S.; Moriyama, T.; Sameshima, T.; Goya, T.; Wakisaka, S. Radiographic and scintigraphic courses of union in and cervical interbody fusion: Hydroxyapatite grafts versus iliac bone autografts. J. Nucl. Med. 2000, 41, 1642–1645. [Google Scholar] [PubMed]
- Sumer, J.; Schmidt, D.; Ritt, P.; Lell, M.; Forst, R.; Kuwert, T.; Richter, R. SPECT/CT in patients with lower back pain after and lumbar fusion surgery. Nucl. Med. Commun. 2013, 34, 964–970. [Google Scholar] [CrossRef]
- Rager, O.; Schaller, K.; Payer, M.; Tchernin, D.; Ratib, O.; Tessitore, E. SPECT/CT in differentiation of pseudarthrosis from and other causes of back pain in lumbar spinal fusion. Clin. Nucl. Med. 2012, 37, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Littenberg, B.; Siegel, A.; Tosteson, A.N.; Mead, T. Clinical efficacy of SPECT bone imaging for low and back pain. J. Nucl. Med. 1995, 36, 1707–1713. [Google Scholar] [PubMed]
- Americal College of Radiology. Clinical Condition: Low back pain. ACR Appropriateness criteria 2015. Available online: https://acsearch.acr.org/docs/69483/Narrative (accessed on 7 January 2018).
- Smith, J.S.; Shaffrey, C.I.; Ames, C.P.; Demakakos, J.; Fu, K.M.; Keshavarzi, S.; Li, C.M.; Deviren, V.; Schwab, F.J.; Lafage, V.; et al. International Spine Study Group. Assessment of symptomatic rod fracture after, posterior instrumented fusion for adult spinal deformity. Neurosurgery 2012, 71, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Sandén, B.; Olerud, C.; Petrén-Mallmin, M.; Johansson, C.; Larsson, S. The significance of radiolucent zones and surrounding pedicle screws. J. Bone Jt. Surg. Br. 2004, 86, 457–461. [Google Scholar] [CrossRef]
- Saha, S.; Burke, C.; Desai, A.; Vijayanathan, S.; Gnanasegaran, G. SPECT-CT: Applications in musculoskeletal radiology. Br. J. Radiol. 2013, 86, 201–205. [Google Scholar] [CrossRef]
- Hudyana, H.; Maes, A.; Vandenberghe, T.; Fidlers, L.; Sathekge, M.; Nicolai, D.; Van de Wiele, C. Accuracy of bone SPECT/CT for identifying and hardware loosening in patients who underwent lumbar fusion with pedicle screws. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 349–354. [Google Scholar] [CrossRef]
- Mulholland, N.J.; Mohan, H.K.; Vijayanathan, S.; Clarke, S.E.; Fogelman, I. Recognition of the femoroacetabular impingement syndrome on MDP SPECT/CT. Clin. Nucl. Med. 2008, 33, 125–127. [Google Scholar] [CrossRef]
- Beall, D.P.; Sweet, C.F.; Martin, H.D.; Lastine, C.L.; Grayson, D.E.; Ly, J.Q.; Fish, J.R. Imaging findings of femoroacetabular impingement and syndrome. Skelet. Radiol. 2005, 34, 691–701. [Google Scholar] [CrossRef]
- Kassarjian, A.; Palmer, W.E. Femoroacetabular impingement. Eur. J. Radiol. 2007, 63, 29–35. [Google Scholar] [CrossRef]
- Bredella, M.A.; Stoller, D.W. MR imaging of femoroacetabular impingement. MRI Clin. N. Am. 2005, 13, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.N.; Sundaram, M. Atypical Fractures Following Bisphosphonate Therapy. Semin. Musculoskelet. Radiol. 2016, 20, 376–381. [Google Scholar] [PubMed]
- Luk, W.H.; Yang, M.K. Diagnostic value of SPECT versus SPECT/CT in femoral avascular necrosis: Preliminary results. Nucl. Med. Commun. 2010, 31, 958–961. [Google Scholar] [CrossRef]
- Wylde, V.; Learmonth, I.D.; Dieppe, P. Persistent pain after joint replacement: Prevalence, sensory qualities, and postoperative determinants. Pain 2011, 152, 566–572. [Google Scholar] [CrossRef]
- Erlenwein, J.; Falla, D.; Przemeck, M.; Pfingsten, M.; Budde, S.; Quintel, M.; Petzke, F. Clinical relevance of persistent postoperative pain after total hip replacement—A prospective observational cohort study. J. Pain Res. 2017, 10, 2183–2193. [Google Scholar] [CrossRef]
- Dobrindt, O.; Amthauer, H.; Krueger, A.; Ruf, J.; Wissel, H.; Grosser, O.S.; Seidensticker, M.; Lohmann, C.H. Hybrid SPECT/CT for the assessment of a painful hip after uncemented total hip arthroplasty. BMC Med. Imaging 2015, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.J.; Kärrholm, J.; Hailer, N.P. Total Hip Arthroplasty in 6690 Patients with Inflammatory Arthritis: Effect of Medical Comorbidities and Age on Early Mortality. J. Rheumatol. 2016, 43, 1320–1327. [Google Scholar] [CrossRef]
- Williamson, B.R.; Wang, G.W.; Miller, C.W.; Teates, C.D.; Bray, S.T. Radionuclide bone imaging as a means of differentiating loosening and infection in patients with a painful total hip prosthesis. Radiology 1979, 133, 723–725. [Google Scholar] [CrossRef]
- Williams, F.; Park, W.M.; O’Connor, B.T.; Morris, V. Gallium-67 scanning in the painful total hip replacement. Clin. Radiol. 1981, 32, 431–439. [Google Scholar] [CrossRef]
- Strobel, K.; Steurer-Dober, I.; Da Silva, A.J.; Huellner, M.W.; Del Sol Perez Lago, M.; Bodmer, E.; von Wartburg, U.; Veit-Haibach, P.; Tornquist, K.; Hug, U. Feasibility and preliminary results of SPECT/CT arthrgraphy of the wrist in comparison with MR arthrography in patients with suspected ulnocarpal impaction. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 548–555. [Google Scholar] [CrossRef]
- Tam, H.H.; Rahman, F.; Weller, A.; Ejindu, V.; Parthipun, A. SPECT-CT in total hip arthroplasty. Clin. Radiol. 2014, 69, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Huellner, M.W.; Strobel, K. Clinical applications of SPECT/CT in imaging the extremities. Eur. J. Nucl. Med. Commun. 2014, 41 (Suppl. 1), S50–S58. [Google Scholar] [CrossRef] [PubMed]
- Shehab, D.; Collier, B.D. Heterotopic ossification. J. Nucl. Med. 2002, 43, 346–353. [Google Scholar] [PubMed]
- Hayter, C.L.; Shah, P.; Koch, K.M.; Miller, T.T.; Potter, H.G. MRI after arthroplasty: Comparison of MAVRIC and conventional fast spin-echo techniques. AJR Am. J. Roentgenol. 2011, 197, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.J.; Chauduri, R.; Bingham, J.; Fogelman, I. A comparison of MRI and bone SPET in the diagnosis and of knee pathology. Nucl. Med. Commun. 1996, 17, 125–131. [Google Scholar] [CrossRef]
- Collier, B.D.; Johnson, R.P.; Carrera, G.F.; Isitman, A.T.; Veluvolu, P.; Knobel, J.; Hellman, R.S.; Barthelemy, C.R. Chronic knee pain assessed by SPECT: Comparison and with other modalities. Radiology 1985, 157, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.J.; Ryan, P.J.; Clarke, S.E.; Fogelman, I. SPECT bone scintigraphy of anterior cruciate ligament and injury. J. Nucl. Med. 1996, 37, 1353–1356. [Google Scholar]
- Fernando, R.A.; Panchadhar, S.; Barwick, T. Initial experience of SPECT/CT in patients with problematic painful knee. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, S379. (In abstract) [Google Scholar]
- Hirschmann, M.T.; Rasch, H. Clinical value of SPECT/CT in the painful total knee arthroplasty (TKA): A prospective study in a consecutive series of 100 TKA. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1869–1882. [Google Scholar] [CrossRef] [PubMed]
- Abele, J.T.; Russell, G.; Masson, E.C.; Flemming, J.P. The accuracy of single photon emission computed tomography/computed tomography Arthrography in evaluating aseptic loosening of hip and knee prostheses. J. Arthroplast. 2015, 30, 1647–1651. [Google Scholar] [CrossRef]
- Mohan, H.K.; Gnanasegaran, G.; Vijayanathan, S.; Fogelman, I. SPECT/CT in imaging foot and ankle pathology-the demise of other coregistration techniques. Semin. Nucl. Med. 2011, 40, 41–51. [Google Scholar] [CrossRef]
- Leumann, A.; Valderrabano, V.; Plaass, C.; Rasch, H.; Studler, U.; Hintermann, B.; Pagenstert, G.I. A novel imaging method for osteochondral lesions of the talus—Comparison of SPECT/CT with MRI. Am. J. Sports Med. 2011, 39, 1095–1101. [Google Scholar]
- Wiewioski, M.; Rasch, H.; Jacob, A.L.; Valderrabano, V. Pain in osteochondral lesions. Foot Ankle Spec. 2011, 4, 92–99. [Google Scholar] [CrossRef]
- Breunung, N.; Fernando, R.; Gnanasegaran, G.; Vijayanathan, S.; Hosahalli, M.; Fogelman, I. Additional benefit of SPECT/CT in investigating heel pain. Clin. Nucl. Med. 2008, 33, 705–706. [Google Scholar] [CrossRef]
- Langroudi, B.; Gnanasegaran, G. SPECT-CT in the assessment of bony foot pathology. J. Nucl. Med. 2007, 48, 122–124. [Google Scholar]
- Medicare Coverage Database. Available online: https://www.cms.gov/medicare-coverage-database/search-results.aspx?keyword=FDG&keywordType=starts&areaId=all&docType=NCA,CAL,NCD,MEDCAC,TA,MCD,6,3,5,1,F,P&contractOption=all&sortBy=relevance (accessed on 13 July 2021).
- Arıcan, P.; Şefizade, R.; Naldöken, S. Diagnostic Value of Bone SPECT/CT in Patients with Suspected Osteomyelitis. Mol. Imaging Radionucl. Ther. 2019, 28, 89–95. [Google Scholar] [CrossRef]
- Horger, M.; Eschmann, S.M.; Pfannenberg, C.; Storek, D.; Vonthein, R.; Claussen, C.D.; Bares, R. The value of SPECT/CT in chronic osteomyelitis. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shalom, R.; Guralnik, L.; Keidar, Z.; Engel, A.; Nitecki, S.; Israel, O. SPECT/CT using Ga67 and In111-labeled leukocyte scintigraphy for diagnosis of infection. J. Nucl. Med. 2006, 47, 587–594. [Google Scholar] [PubMed]
- Filippi, L.; Schillaci, O. Usefulness of hybrid SPECT/CT in Tc99m-HMPAO-labeled leukocytescintigraphy for bone and joint infections. J. Nucl. Med. 2006, 47, 1908–1913. [Google Scholar]
- Newman, L.G.; Waller, J.; Palestro, C.J.; Schwartz, M.; Klein, M.J.; Hermann, G.; Harrington, E.; Harrington, M.; Roman, S.H.; Stagnaro-Green, A. Unsuspected osteomyelitis in diabetic foot ulcers: Diagnosis and monitoring by leukocyte scanning with In-111 oxyquinoline. JAMA 1991, 266, 1246–1251. [Google Scholar] [CrossRef]
- Palestro, C.J.; Patel, M.; Freeman, S.J.; Harrington, W.N.; Tomas, M.B.; Marwin, S.E. Marrow versus infection in the Charcot joint: Indium-111 leukocyte and technetium-99m sulfur colloid scintigraphy. J. Nucl. Med. 1998, 39, 346–350. [Google Scholar]
- Palestro, C.J.; Tronco, G.G.; Tomas, M.B.; Rini, J.N. Combined labeled leukocyte and technetium 99m sulfur colloid bone marrow imaging for diagnosing musculoskeletal infection. Radiographics 2006, 26, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, P.B.; Khayam-Bashi, H. The association of Ga-67 and lactoferrin. J. Nucl. Med. 1977, 18, 713–717. [Google Scholar] [PubMed]
- Schauwecker, D.S.; Mock, B.H.; Burt, R.W.; Kernick, C.B.; Ruoff, A.C., 3rd; Sinn, H.J.; Wellman, H.N. Evaluation of complicating osteomyelitis with Tc-99m MDP, In-111 granulocytes, and Ga-67 citrate. J. Nucl. Med. 1984, 25, 849–853. [Google Scholar]
- Noyek, A.M.; Greyson, N.M.D.; Wortzman, G.; Jazrawy, H.; Freeman, J.L.; Blair, R.L.; Chapnik, J.S. The clinical significance of radionuclide bone and gallium scanning in osteomyelitis of the head and neck. Laryngoscope 1984, 94 (Suppl. 34), 1–21. [Google Scholar] [CrossRef] [PubMed]
- Peller, P.J.; Kransdorf, M.J. Extraosseous Tc-99m MDP uptake: A pathophysiologic approach. Radiographics 1993, 13, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Gentili, A.; Bellon, E.M. Nonosseous accumulation of bone-seeking radiopharmaceuticals. Radiographics 1990, 10, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Piert, M. Dynamic bone imaging with Tc99m-labeled diphosphonates and F18-NaF: Mechanisms and applications. J. Nucl. Med. 2013, 54, 590–599. [Google Scholar] [CrossRef]
- Zuckier, L.S. Nonurologic uptake on bone scintigraphy: Atlas and analysis. Semin. Nucl. Med. 2010, 40, 242–256. [Google Scholar] [CrossRef]
- Love, C.; Tomas, M.B.; Kalapparambath, T.P.; Palestro, C.J. Radionuclide bone imaging: An illustrative review. Radiographics 2003, 23, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, R.; Naswa, N.; Sharma, P.; Karunanithi, S.; Nazar, A.H.; Das, K.J.; Bal, C.; Malhotra, A.; Kumar, R. SPECT-CT for characterization of extraosseous uptake of Tc99m-methylene diphosphonate on bone scintigraphy. Diagn. Interv. Radiol. 2013, 19, 405–410. [Google Scholar] [PubMed]
- Tuncel, M.; Mahmoudian, B. Gamut: Soft tissue uptake of bone radiopharmaceuticals. Semin. Nucl. Med. 2003, 33, 334–337. [Google Scholar]
- Loutfi, I.; Mohammed, A.M. Non-osseous abnormalities on bone scans. J. Nucl. Med. Technol. 2003, 31, 149–153. [Google Scholar]
- Deryk, S.; Vanhove, C. Imaging characteristics of heterotopic mesenteric ossification on FDG PET and Tc-99m bone SPECT. Clin. Nucl. Med. 2008, 33, 496–499. [Google Scholar] [CrossRef]
- Lin, Y.; Kao, C.H.; Tsai, S.C. Easy interpretation of heterotopic ossification demonstrated on bone SPECT/CT. Clin. Nucl. Med. 2014, 39, 62–63. [Google Scholar] [CrossRef]
- Shawgi, M. Heterotopic ossification of the hips in a patient with Guillain Barre syndrome demonstrated on SPECT/CT. Clin. Nucl. Med. 2012, 37, e253–e254. [Google Scholar] [CrossRef]
- Millet, J.D.; Brown, R.K.; Levi, B.; Kraft, C.T.; Jacobson, J.A.; Gross, M.D.; Wong, K.K. Frostbite: Spectrum of imaging findings and guidelines for management. Radiographics 2016, 36, 2154–2169. [Google Scholar] [CrossRef]
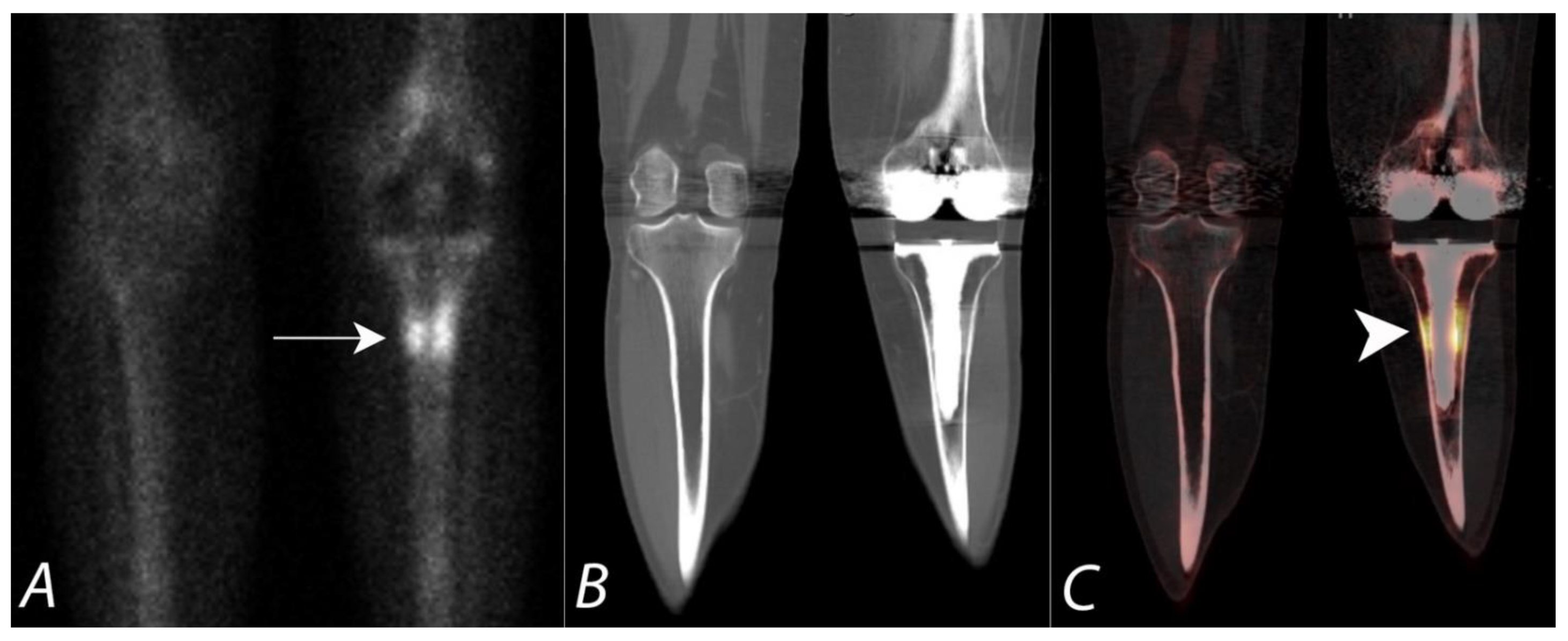
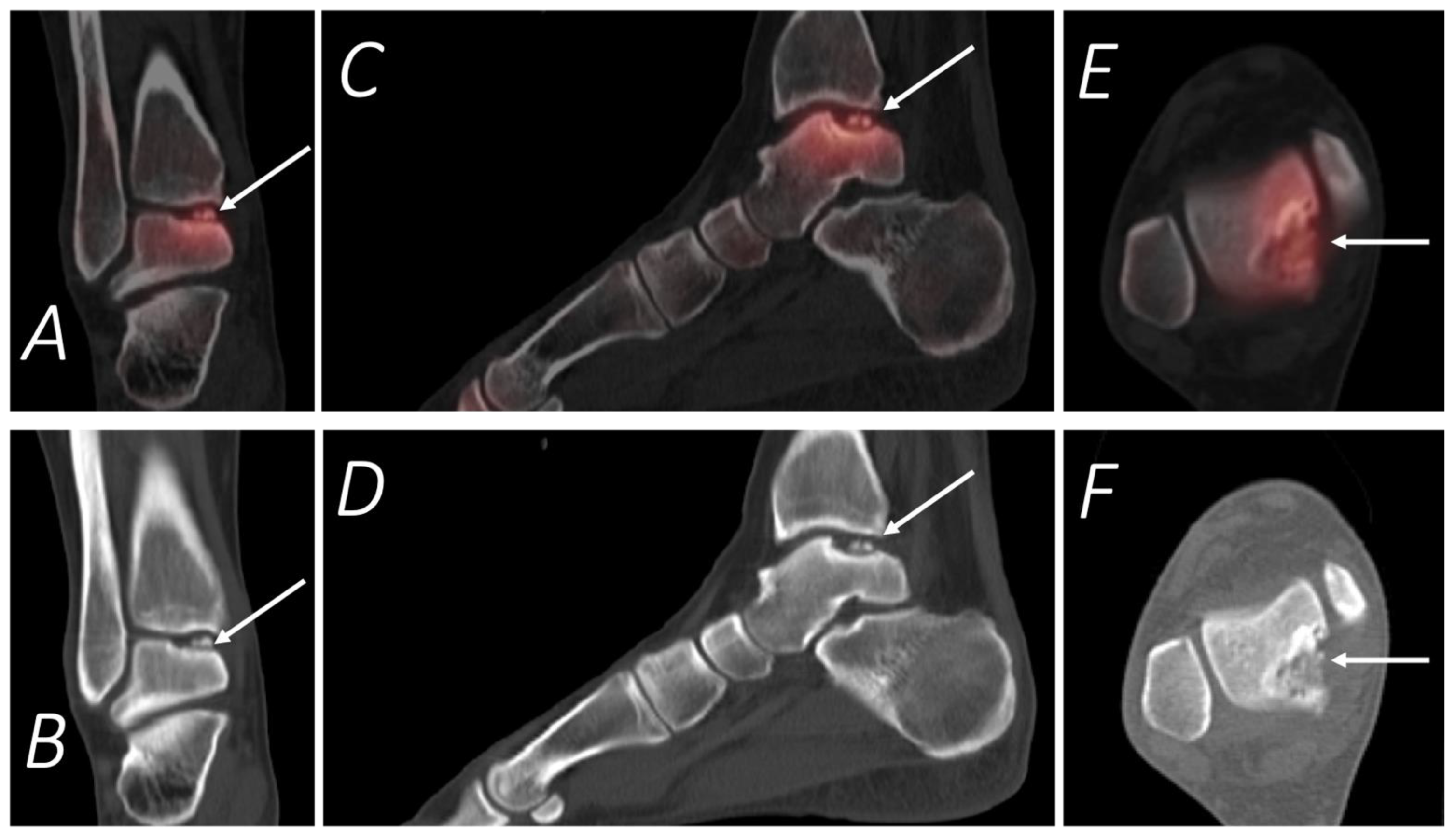
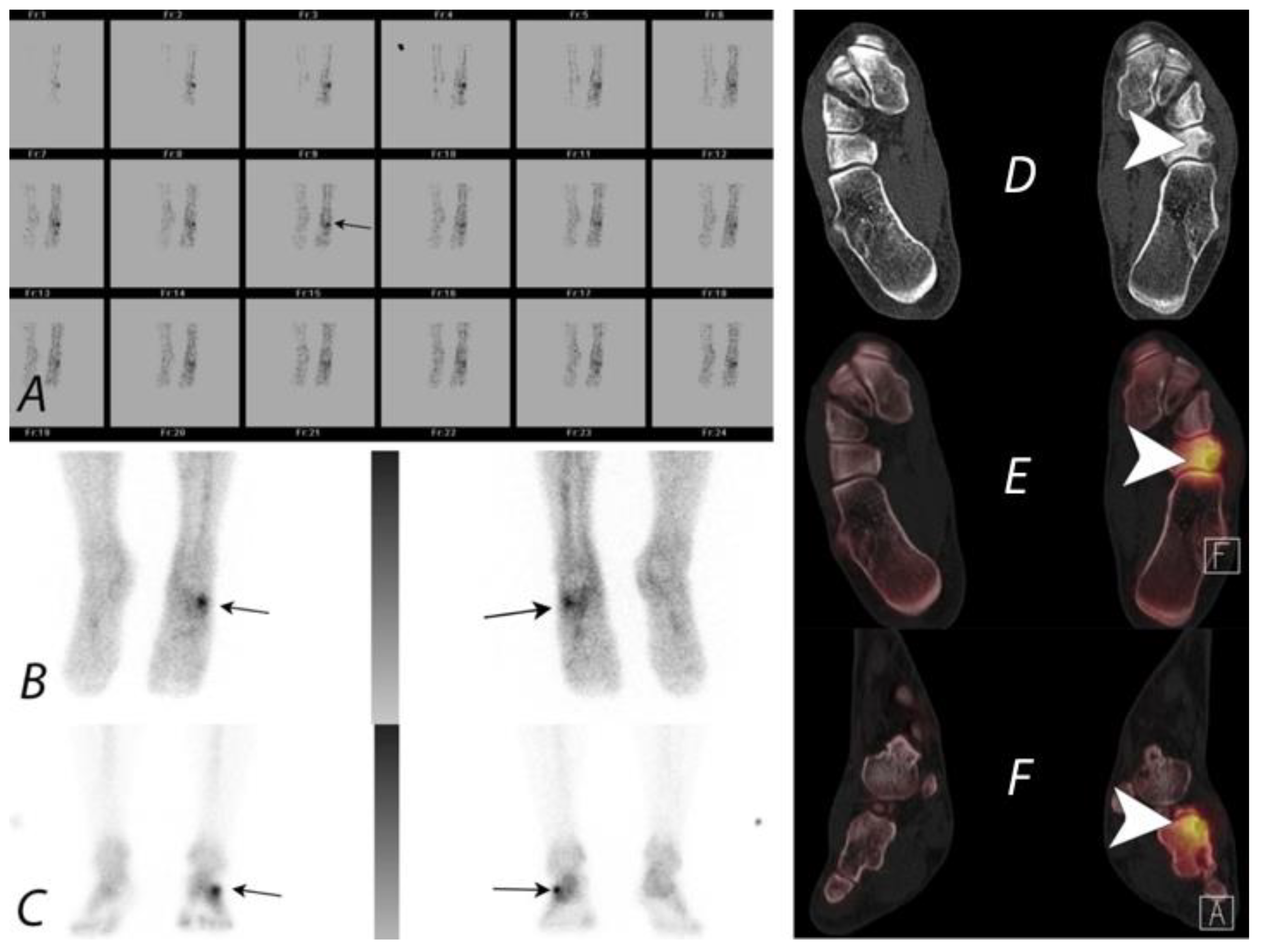
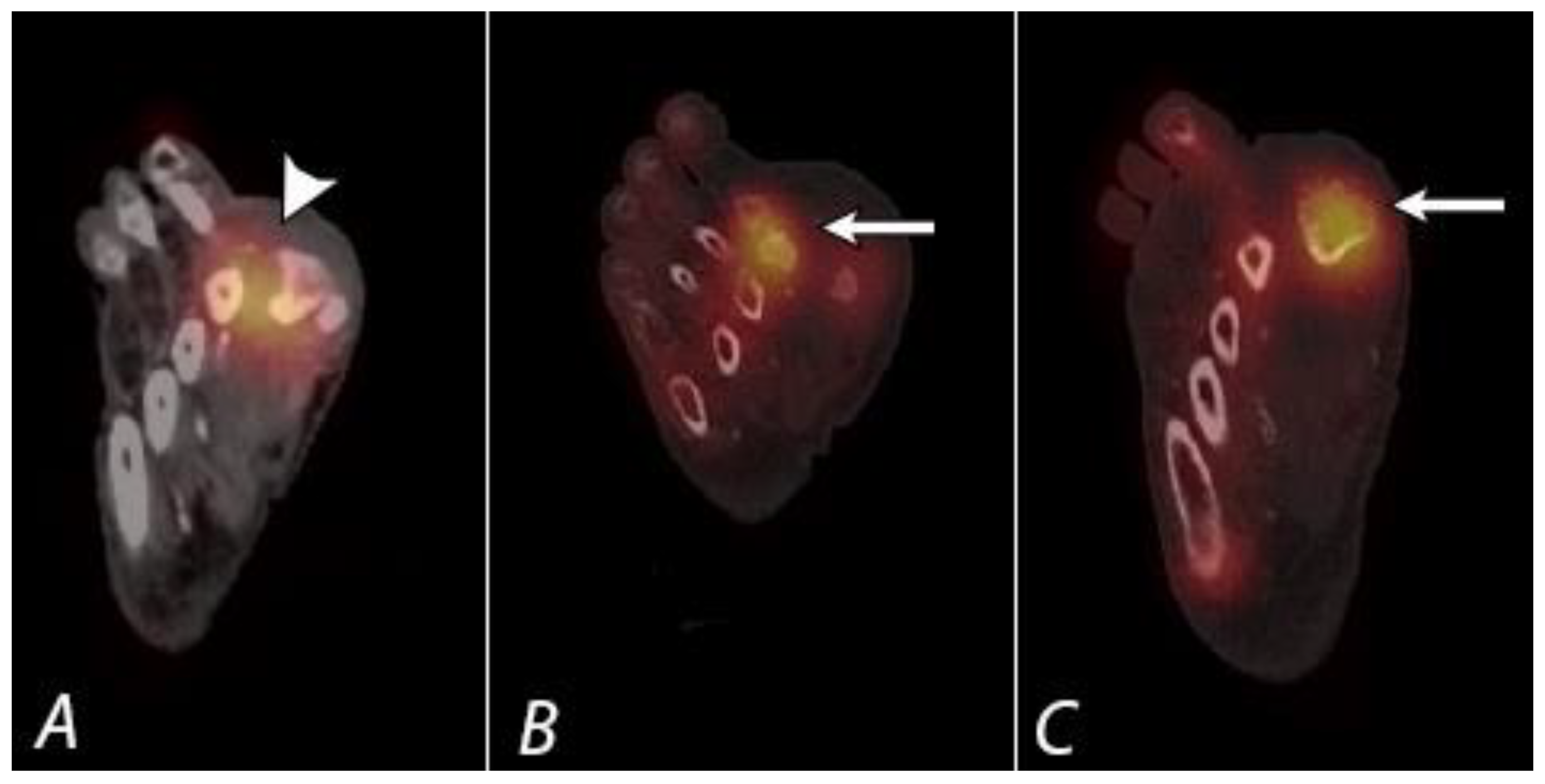
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koppula, B.R.; Morton, K.A.; Al-Dulaimi, R.; Fine, G.C.; Damme, N.M.; Brown, R.K.J. SPECT/CT in the Evaluation of Suspected Skeletal Pathology. Tomography 2021, 7, 581-605. https://doi.org/10.3390/tomography7040050
Koppula BR, Morton KA, Al-Dulaimi R, Fine GC, Damme NM, Brown RKJ. SPECT/CT in the Evaluation of Suspected Skeletal Pathology. Tomography. 2021; 7(4):581-605. https://doi.org/10.3390/tomography7040050
Chicago/Turabian StyleKoppula, Bhasker Rao, Kathryn A. Morton, Ragheed Al-Dulaimi, Gabriel C. Fine, Nikolas M. Damme, and Richard K. J. Brown. 2021. "SPECT/CT in the Evaluation of Suspected Skeletal Pathology" Tomography 7, no. 4: 581-605. https://doi.org/10.3390/tomography7040050
APA StyleKoppula, B. R., Morton, K. A., Al-Dulaimi, R., Fine, G. C., Damme, N. M., & Brown, R. K. J. (2021). SPECT/CT in the Evaluation of Suspected Skeletal Pathology. Tomography, 7(4), 581-605. https://doi.org/10.3390/tomography7040050




