Electron Density and Effective Atomic Number of Normal-Appearing Adult Brain Tissues: Age-Related Changes and Correlation with Myelin Content
Abstract
1. Introduction
2. Materials and Methods
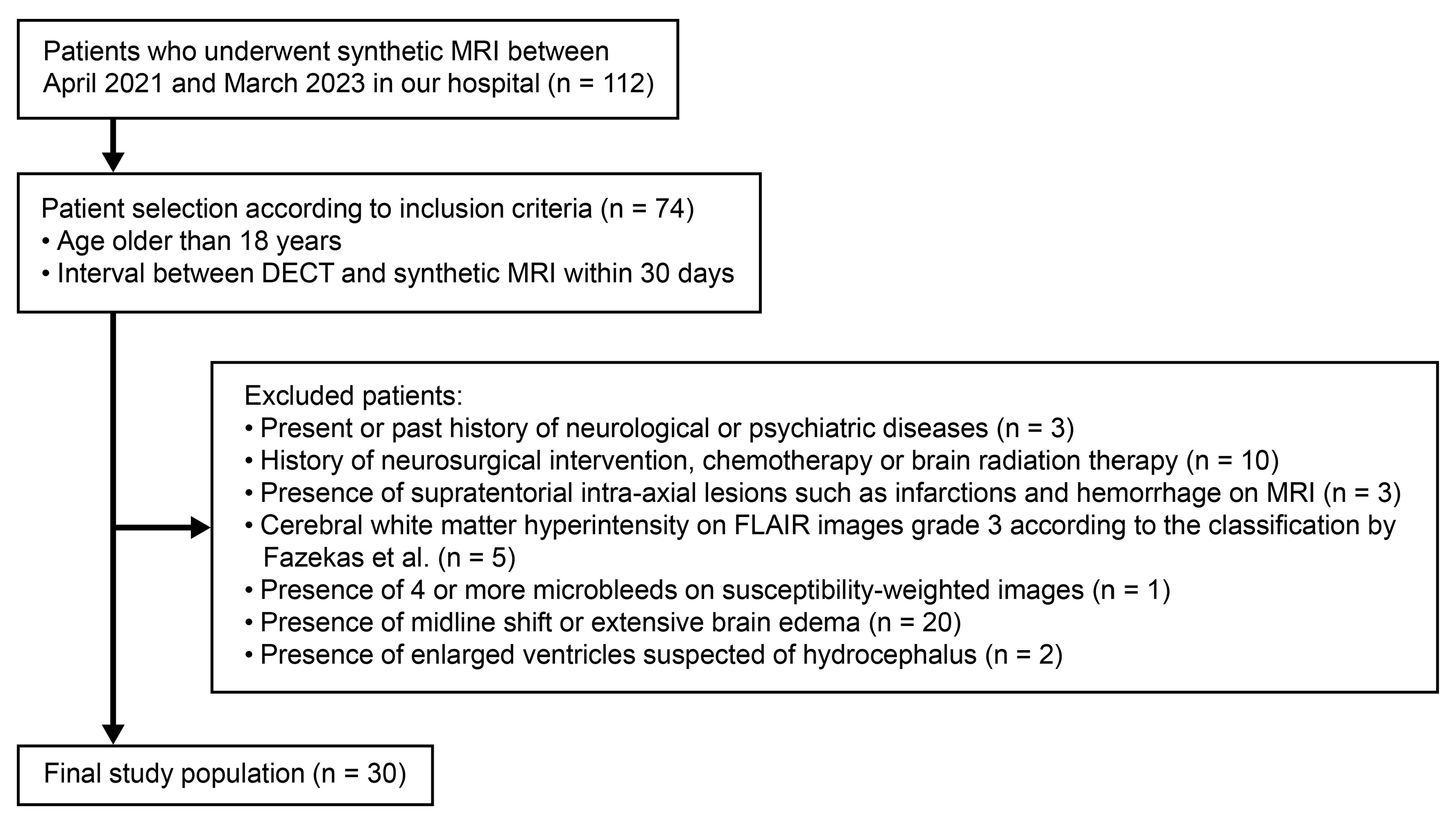
2.1. Study Population
2.2. CT Imaging
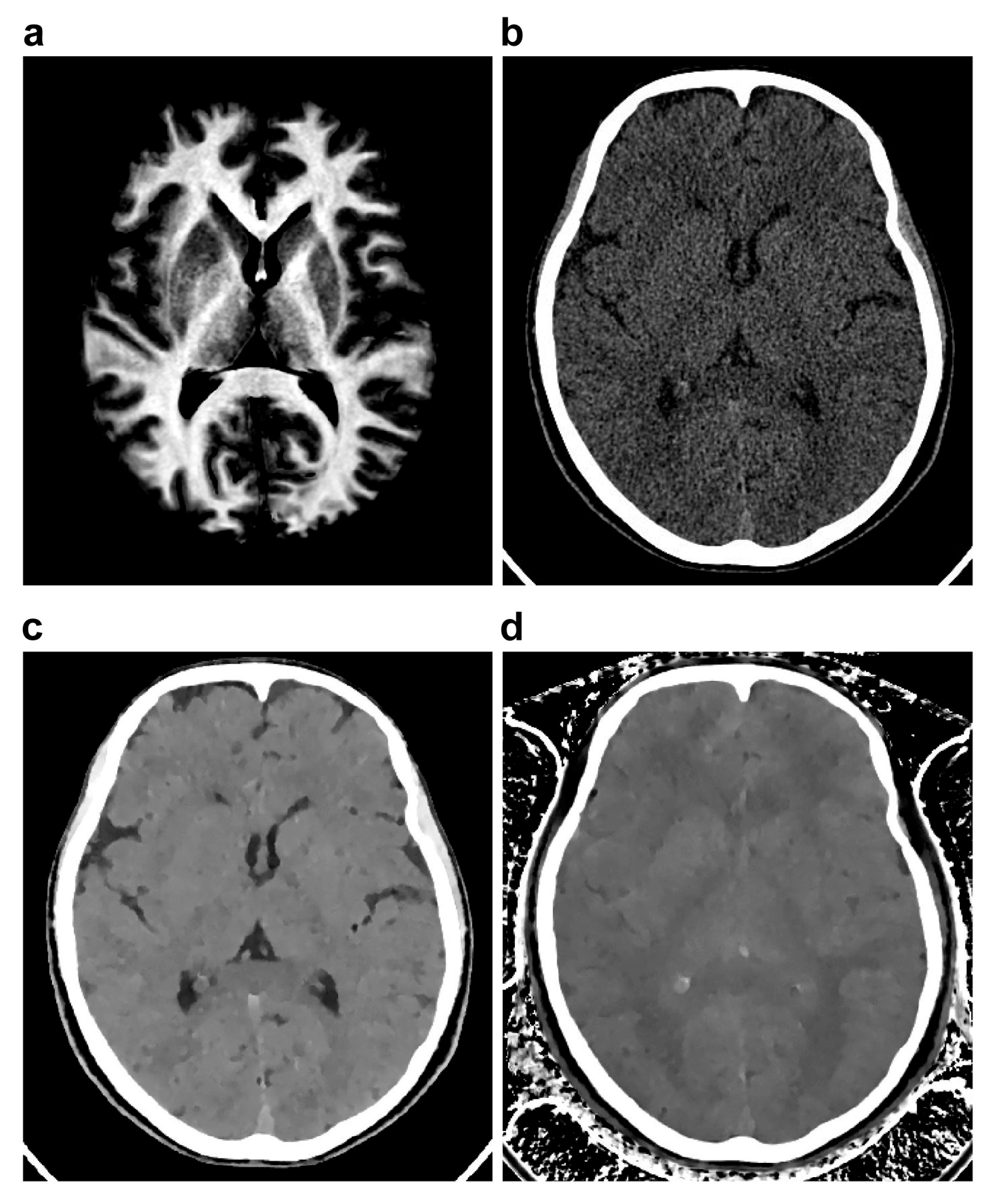
2.3. MR Imaging
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Participants
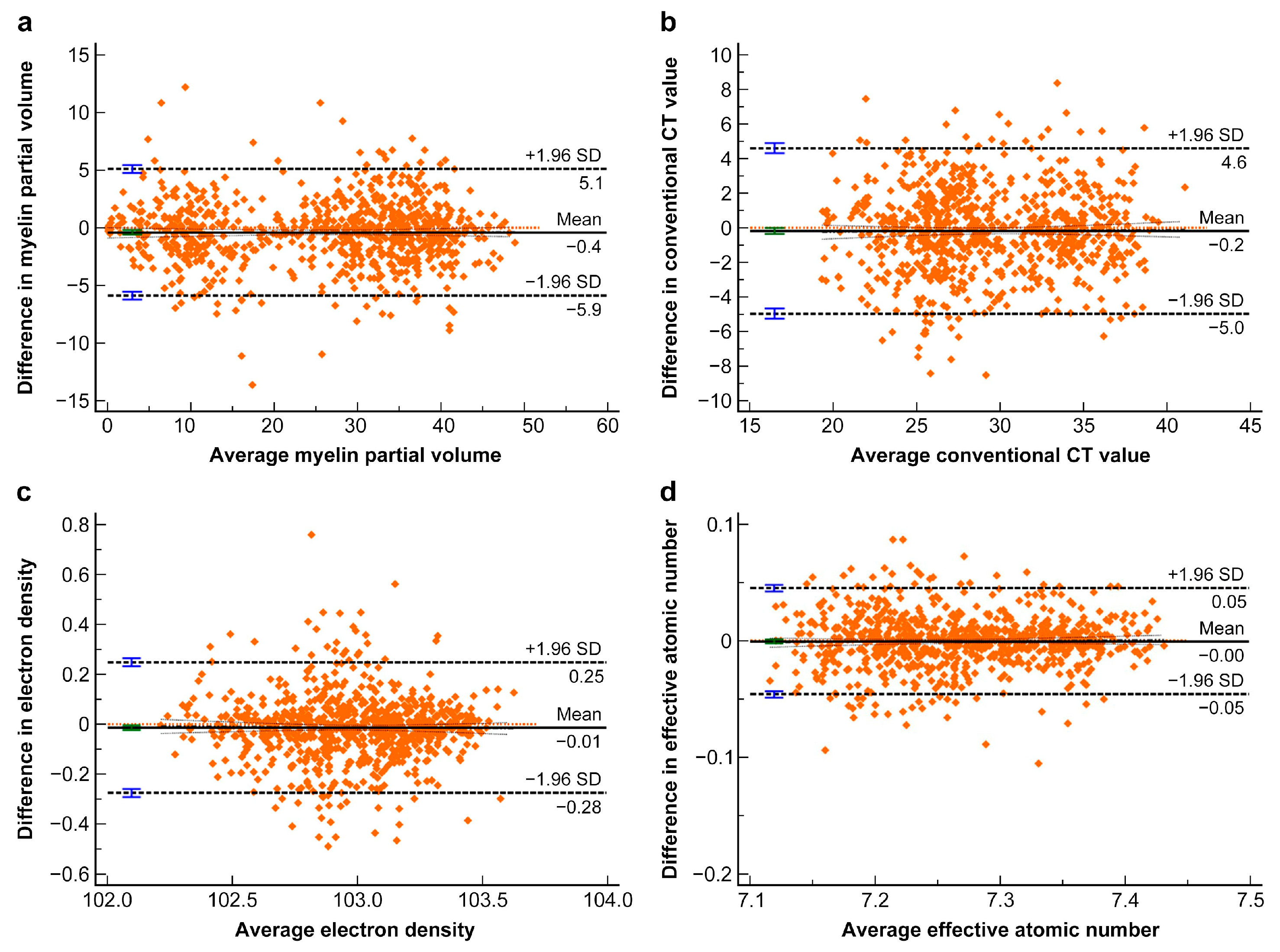
3.2. Interobserver Agreement of the Measurements
3.3. Comparison of Vmy and CT Parameters Between the WM and GM
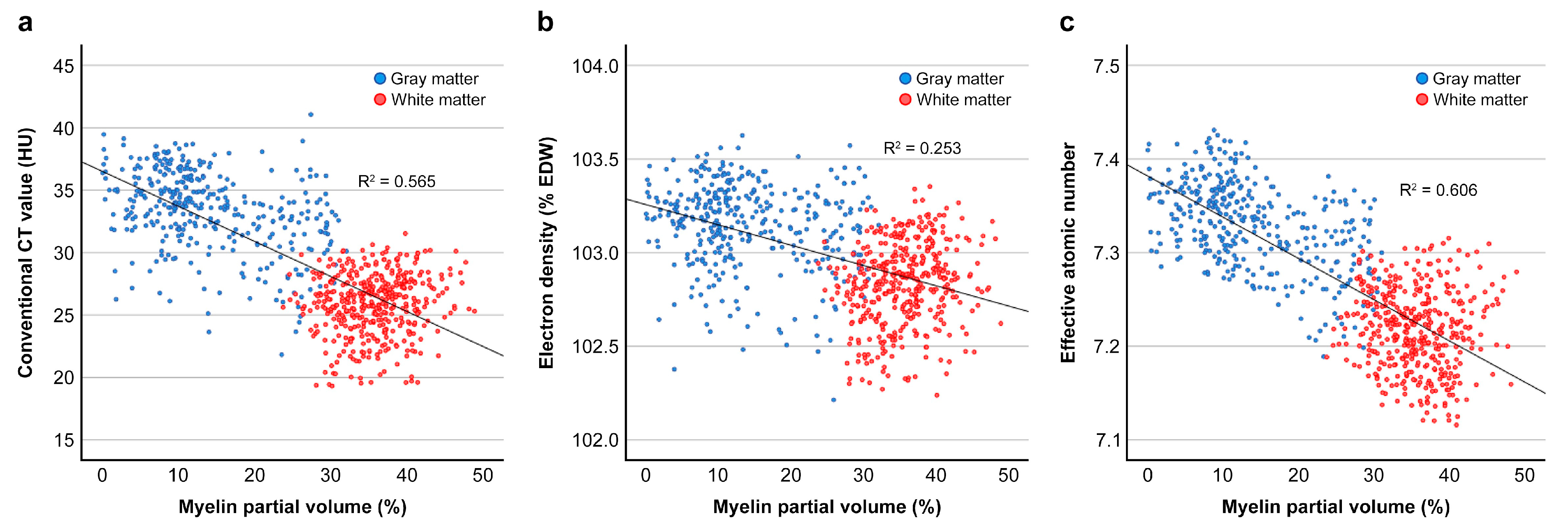
3.4. Correlation Between Vmy and CT Parameters and Regression Analyses
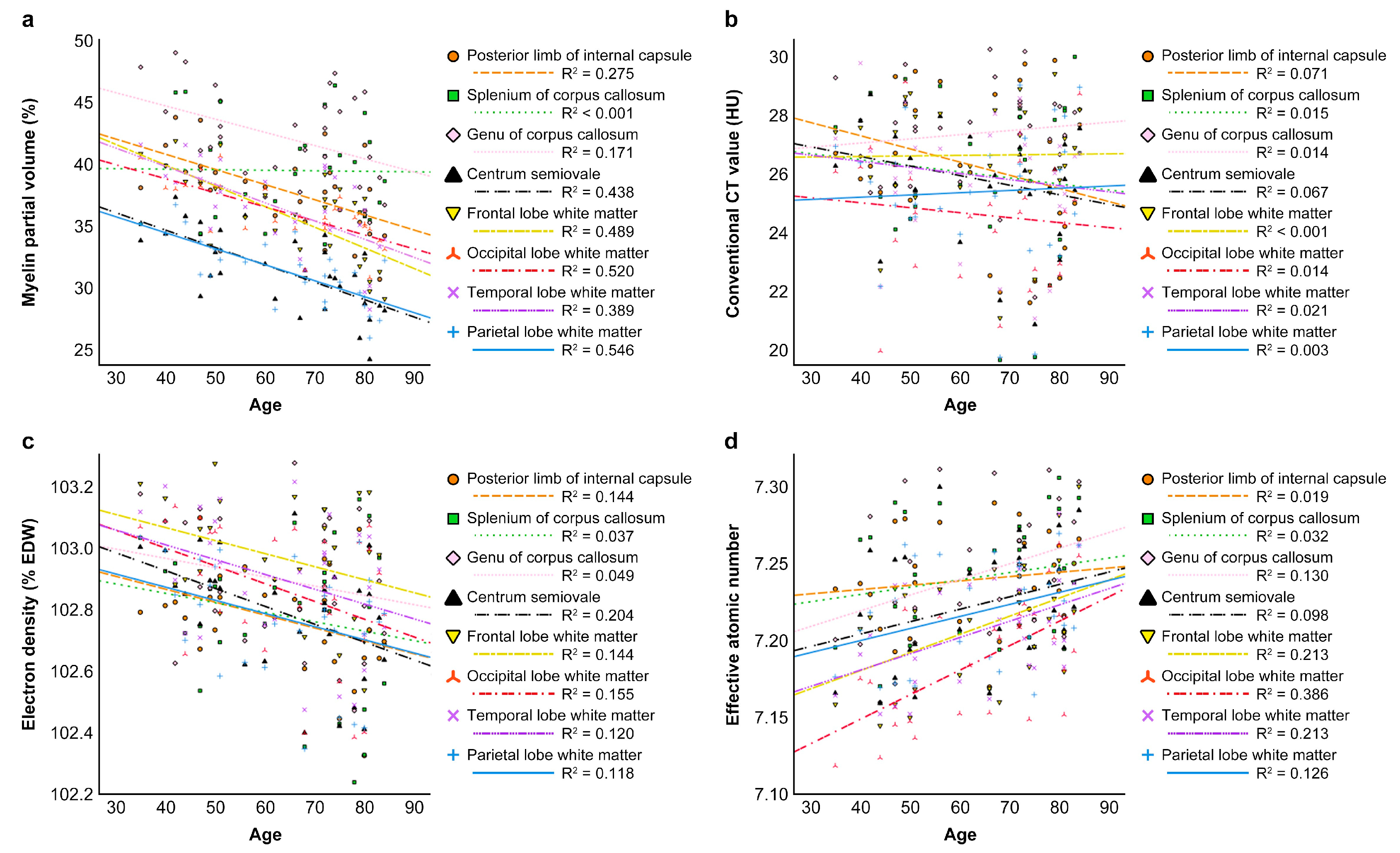
3.5. Correlation Between Patient Age and Vmy or CT Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS | Centrum semiovales |
| CSF | Cerebrospinal fluid |
| CT | Computed tomography |
| CTconv | Conventional CT value |
| CTDIvol | Volume CT dose index |
| DECT | Dual-energy computed tomography |
| DLP | Dose–length product |
| ED | Electron density |
| GC | Genu of the corpus callosum |
| FLAIR | Fluid-attenuated inversion recovery |
| GM | Gray matter |
| ICC | Intraclass correlation coefficient |
| MRI | Magnetic resonance imaging |
| PD | Proton density |
| PLIC | Posterior limb of the internal capsule |
| QRAPMASTER | Quantification of relaxation times and PD by multi-echo acquisition of saturation-recovery using turbo spin-echo readout |
| R2 | Coefficient of determination |
| ROI | Regions of interest |
| SC | Splenium of the corpus callosum |
| SWI | Susceptibility weighted imaging |
| Vmy | Myelin partial volume |
| WM | White matter |
| Zeff | Effective atomic number |
| %EDW | Percent electron density relative to water |
References
- Truwit, C.L.; Barkovich, A.J.; Gean-Marton, A.; Hibri, N.; Norman, D. Loss of the insular ribbon: Another early CT sign of acute middle cerebral artery infarction. Radiology 1990, 176, 801–806. [Google Scholar] [CrossRef]
- Moulin, T.; Cattin, F.; Crépin-Leblond, T.; Tatu, L.; Chavot, D.; Piotin, M.; Viel, J.F.; Rumbach, L.; Bonneville, J.F. Early CT signs in acute middle cerebral artery infarction: Predictive value for subsequent infarct locations and outcome. Neurology 1996, 47, 366–375. [Google Scholar] [CrossRef]
- Brooks, R.A.; Di Chiro, G.; Keller, M.R. Explanation of cerebral white-gray contrast in computed tomography. J. Comput. Assist. Tomogr. 1980, 4, 489–491. [Google Scholar] [CrossRef]
- Nakajima, S.; Ito, H.; Mitsuhashi, T.; Kubo, Y.; Matsui, K.; Tanaka, I.; Fukui, R.; Omori, H.; Nakaoka, T.; Sakura, H.; et al. Clinical application of effective atomic number for classifying non-calcified coronary plaques by dual-energy computed tomography. Atherosclerosis 2017, 261, 138–143. [Google Scholar] [CrossRef]
- Kaichi, Y.; Tatsugami, F.; Nakamura, Y.; Baba, Y.; Iida, M.; Higaki, T.; Kiguchi, M.; Tsushima, S.; Yamasaki, F.; Amatya, V.J.; et al. Improved differentiation between high- and low-grade gliomas by combining dual-energy CT analysis and perfusion CT. Medicine 2018, 97, e11670. [Google Scholar] [CrossRef]
- Nagano, H.; Takumi, K.; Nakajo, M.; Fukukura, Y.; Kumagae, Y.; Jinguji, M.; Tani, A.; Yoshiura, T. Dual-energy CT-derived electron density for diagnosing metastatic mediastinal lymph nodes in non-small cell lung cancer: Comparison with conventional CT and FDG PET/CT findings. Am. J. Roentgenol. 2022, 218, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Shen, S.; Ke, T.; Jiang, J.; Wang, Y.; Xie, X.; Hu, X.; Tang, X.; Han, D.; Chen, J. Clinical value of dual-energy CT for predicting occult metastasis in central neck lymph nodes of papillary thyroid carcinoma. Eur. Radiol. 2024, 34, 16–25. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; Ban, X.; Jiang, Y.; Su, Y.; Yang, L.; Shi, G.; Yang, L.; Han, R.; Duan, X. Evaluation of quantitative dual-energy computed tomography parameters for differentiation of parotid gland tumors. Acad. Radiol. 2024, 31, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Ham, S.; Shim, E.; Kim, B.H.; Kang, W.Y.; Kang, C.H.; Ahn, K.S.; Lee, K.C.; Choi, H. Electron density dual-energy CT can improve the detection of lumbar disc herniation with higher image quality than standard and virtual non-calcium images. Eur. Radiol. 2024, 34, 7334–7346. [Google Scholar] [CrossRef]
- Aizaz, M.; Bierens, J.; Gijbels, M.J.J.; Schreuder, T.H.C.M.L.; van Orshoven, N.P.; Daemen, J.H.C.; Mess, W.H.; Flohr, T.; van Oostenbrugge, R.J.; Postma, A.A.; et al. Differentiation of atherosclerotic carotid plaque components with dual-energy computed tomography. Investig. Radiol. 2025, 60, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A.; Simons, M. Myelin in the central nervous system: Structure, function, and pathology. Physiol. Rev. 2019, 99, 1381–1431. [Google Scholar] [CrossRef]
- Poitelon, Y.; Kopec, A.M.; Belin, S. Myelin fat facts: An overview of lipids and fatty acid metabolism. Cells 2020, 9, 812. [Google Scholar] [CrossRef]
- Natu, V.S.; Gomez, J.; Barnett, M.; Jeska, B.; Kirilina, E.; Jaeger, C.; Zhen, Z.; Cox, S.; Weiner, K.S.; Weiskopf, N.; et al. Apparent thinning of human visual cortex during childhood is associated with myelination. Proc. Natl. Acad. Sci. USA 2019, 116, 20750–20759. [Google Scholar] [CrossRef]
- Schmierer, K.; Tozer, D.J.; Scaravilli, F.; Altmann, D.R.; Barker, G.J.; Tofts, P.S.; Miller, D.H. Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J. Magn. Reson. Imaging 2007, 26, 41–51. [Google Scholar] [CrossRef]
- Alonso-Ortiz, E.; Levesque, I.R.; Pike, G.B. MRI-based myelin water imaging: A technical review. Magn. Reson. Med. 2015, 73, 70–81. [Google Scholar] [CrossRef]
- Warntjes, M.; Engström, M.; Tisell, A.; Lundberg, P. Modeling the presence of myelin and edema in the brain based on multi-parametric quantitative MRI. Front. Neurol. 2016, 7, 16. [Google Scholar] [CrossRef]
- Warntjes, J.B.M.; Persson, A.; Berge, J.; Zech, W. Myelin detection using rapid quantitative MR imaging correlated to macroscopically registered Luxol fast blue-stained brain specimens. Am. J. Neuroradiol. 2017, 38, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Warntjes, M.; Hori, M.; Andica, C.; Nakazawa, M.; Kunishima Kumamaru, K.; Abe, O.; Aoki, S. SyMRI of the brain: Rapid quantification of relaxation rates and proton density, with synthetic MRI, automatic brain segmentation, and myelin measurement. Investig. Radiol. 2017, 52, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Moon, Y.; Han, S.-H.; Kim, H.K.; Moon, W.-J. Myelin loss in white matter hyperintensities and normal-appearing white matter of cognitively impaired patients: A quantitative synthetic magnetic resonance imaging study. Eur. Radiol. 2019, 29, 4914–4921. [Google Scholar] [CrossRef] [PubMed]
- Andica, C.; Hagiwara, A.; Hori, M.; Haruyama, T.; Fujita, S.; Maekawa, T.; Kamagata, K.; Takemura Yoshida, M.; Suzuki, M.; Sugano, H.; et al. Aberrant myelination in patients with Sturge-Weber syndrome analyzed using synthetic quantitative magnetic resonance imaging. Neuroradiology 2019, 61, 1055–1066. [Google Scholar] [CrossRef]
- Parlak, S.; Coban, G.; Gumeler, E.; Karakaya, J.; Soylemezoglu, F.; Tezer, I.; Bilginer, B.; Saygi, S.; Oguz, K.K. Reduced myelin in patients with isolated hippocampal sclerosis as assessed by SyMRI. Neuroradiology 2022, 64, 99–107. [Google Scholar] [CrossRef]
- Ladopoulos, T.; Matusche, B.; Bellenberg, B.; Heuser, F.; Gold, R.; Lukas, C.; Schneider, R. Relaxometry and brain myelin quantification with synthetic MRI in MS subtypes and their associations with spinal cord atrophy. NeuroImage Clin. 2022, 36, 103166. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.-H.; Shapira, N.; Merchant, T.E.; Klahr, P.; Yagil, Y. Accuracy of electron density, effective atomic number, and iodine concentration determination with a dual-layer dual-energy computed tomography system. Med. Phys. 2018, 45, 2486–2497. [Google Scholar] [CrossRef]
- Warntjes, J.B.M.; Dahlqvist Leinhard, O.; West, J.; Lundberg, P. Rapid Magnetic Resonance Quantification on the Brain: Optimization for Clinical Usage. Magn. Reson. Med. 2008, 60, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Anal. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Hünemohr, N.; Paganetti, H.; Greilich, S.; Jäkel, O.; Seco, J. Tissue decomposition from dual energy CT data for MC based dose calculation in particle therapy. Med. Phys. 2014, 41, 061714. [Google Scholar] [CrossRef]
- Woodard, H.Q.; White, D.R. The composition of body tissues. Br. J. Radiol. 1986, 59, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Fujimoto, K.; Kamagata, K.; Murata, S.; Irie, R.; Kaga, H.; Someya, Y.; Andica, C.; Fujita, S.; Kato, S.; et al. Age-related changes in relaxation times, proton density, myelin, and tissue volumes in adult brain analyzed by 2-dimensional quantitative synthetic magnetic resonance imaging. Investig. Radiol. 2021, 56, 163–172. [Google Scholar] [CrossRef]
- Schwartz, M.; Creasey, H.; Grady, C.L.; DeLeo, J.M.; Frederickson, H.A.; Cutler, N.R.; Rapoport, S.I. Computed tomographic analysis of brain morphometrics in 30 healthy men, aged 21 to 81 years. Ann. Neurol. 1985, 17, 146–157. [Google Scholar] [CrossRef]
- Stafford, J.L.; Albert, M.S.; Naeser, M.A.; Sandor, T.; Garvey, A.J. Age-related differences in computed tomographic scan measurements. Arch. Neurol. 1988, 45, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, O.; Sochen, N.; Gur, Y.; Intrator, N.; Assaf, Y. Free water elimination and mapping from diffusion MRI. Magn. Reson. Med. 2009, 62, 717–730. [Google Scholar] [CrossRef] [PubMed]
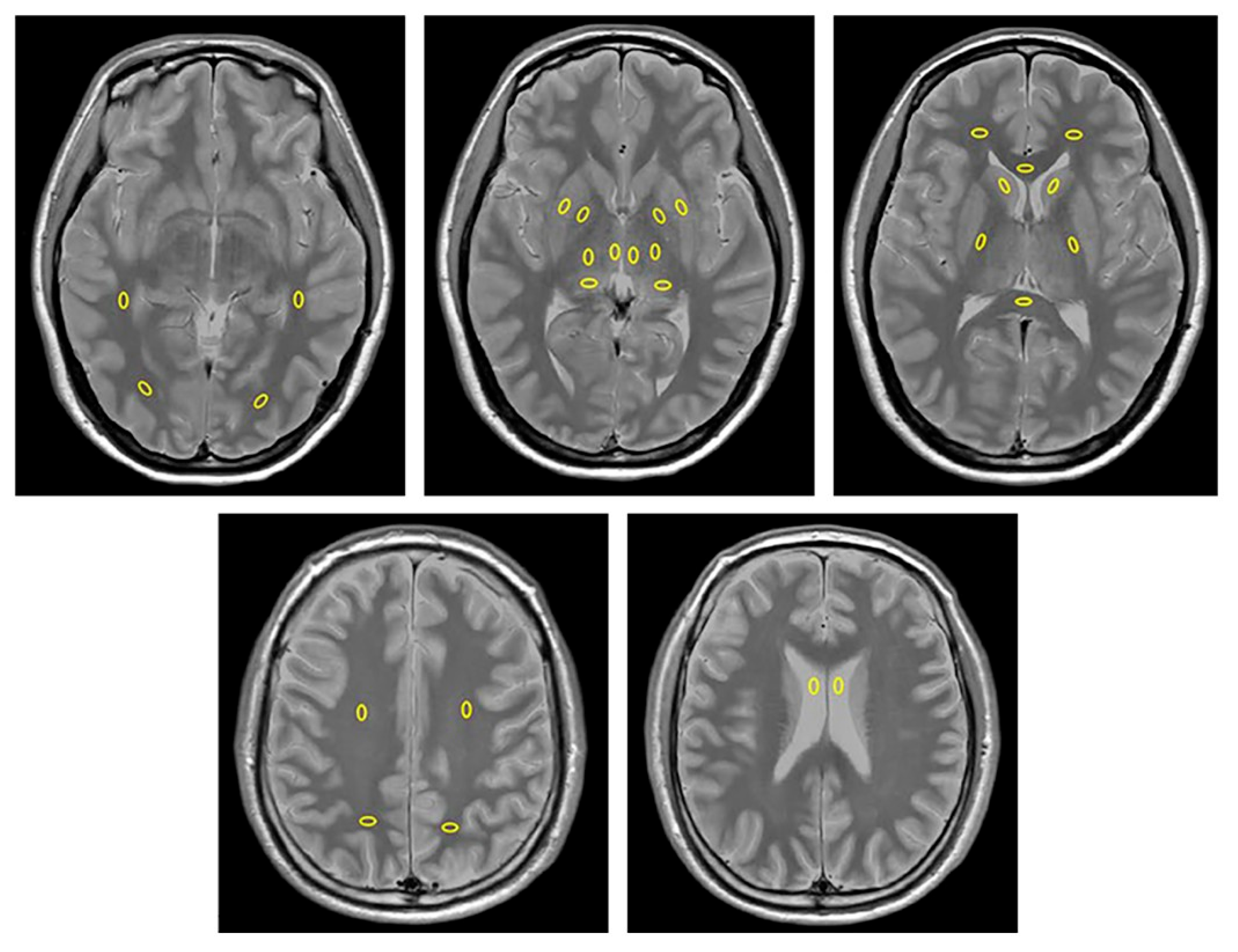
| Parameter | FLAIR | SWI |
|---|---|---|
| Plane | Axial | Axial |
| TR (ms) | 9000 | 31 |
| TE (ms) | 120 | 7.2/13.4/19.6/25.8 |
| TI (ms) | 2700 | - |
| FA (°) (Refocus °) | 180 (120) | 17 |
| Bandwidth (Hz/pixel) | 322 | 255 |
| Number of signal averages | 1 | 4 |
| Turbo factor | 15 | - |
| Acceleration factor | 1.6 | 7 |
| FOV (mm) | 230 × 230 | 230 × 230 |
| Matrix (frequency × phase) | 511 × 511 | 767 × 767 |
| Thickness (mm) | 5 | 1 |
| Slice number | 24 | 150 |
| Acquisition time (s) | 162 | 116 |
| White Matter (14 Regions) | Gray Matter (12 Regions) |
| Right frontal lobe | Right caudate head |
| Left frontal lobe | Left caudate head |
| Right temporal lobe | Right putamen |
| Left temporal lobe | Left putamen |
| Right occipital lobe | Right globus pallidus |
| Left occipital lobe | Left globus pallidus |
| Right parietal lobe | Right medial thalamus |
| Left parietal lobe | Left medial thalamus |
| Right centrum semiovale | Right dorsal thalamus |
| Left centrum semiovale | Left dorsal thalamus |
| Right posterior limb of the internal capsule | Right lateral thalamus |
| Left posterior limb of the internal capsule | Left lateral thalamus |
| Genu of the corpus callosum | |
| Splenium of the corpus callosum | |
| Cerebrospinal fluid (2 regions) | |
| Right lateral ventricle | |
| Left lateral ventricle |
| Characteristic | Value |
|---|---|
| Age | |
| Median | 67.5 years |
| Range | 35–84 years |
| 30–39 | 1 (3%) |
| 40–49 | 6 (20%) |
| 50–59 | 4 (13%) |
| 60–69 | 5 (17%) |
| 70–79 | 8 (27%) |
| 80–89 | 6 (20%) |
| Sex | |
| Female | 22 (73%) |
| Indication of imaging studies | |
| Extra-axial tumor | 23 (77%) |
| Infratentorial lesion | 7 (23%) |
| Fazekas score | |
| Periventricular | |
| 0 | 9 (30%) |
| 1 | 16 (53%) |
| 2 | 5 (17%) |
| Deep white matter | |
| 0 | 17 (57%) |
| 1 | 12 (40%) |
| 2 | 1 (3%) |
| Number of microbleeds | |
| 0 | 25 (83%) |
| 1 | 3 (10%) |
| 2 | 1 (3%) |
| 3 | 1 (3%) |
| Intraclass Correlation Coefficients (95% CI) | Bland–Altman Analysis | |||
|---|---|---|---|---|
| Mean Differences (95% CI) | Lower Limit (95% CI) | Upper Limit (95% CI) | ||
| Myelin partial volume | 0.975 (0.971, 0.979) | −0.398 (−0.595, −0.201) | −5.890 (−6.226, −5.553) | 5.093 (4.756, 5.430) |
| Conventional CT value | 0.873 (0.855, 0.889) | −0.174 (−0.3458, −0.000) | −4.963 (−5.256, −4.670) | 4.615 (4.321, 4.908) |
| Electron density | 0.884 (0.868, 0.899) | −0.013 (−0.023, −0.260) | −0.276 (−0.297, −0.260) | 0.249 (0.233, 0.265) |
| Effective atomic number | 0.948 (0.940, 0.955) | −0.000 (−0.002, 0.001) | −0.046 (−0.049, −0.043) | 0.044 (0.043, 0.048) |
| WM (n = 420) | GM (n = 360) | CSF (n = 60) | p Value for Difference | |||
|---|---|---|---|---|---|---|
| WM vs. GM | GM vs. CSF | CSF vs. WM | ||||
| Myelin partial volume (%) | 35.6 ± 4.7 | 14.0 ± 8.1 | 0.0 ± 0.0 | <0.001 | <0.001 | <0.001 |
| Conventional CT value (HU) | 25.9 ± 2.5 | 33.4 ± 3.2 | 4.1 ± 2.4 | <0.001 | <0.001 | <0.001 |
| Electron density (%EDW) | 102.8 ± 0.2 | 103.2 ± 0.2 | 100.2 ± 0.2 | <0.001 | <0.001 | <0.001 |
| Effective atomic number | 7.2 ± 0.0 | 7.3 ± 0.1 | 7.3 ± 0.1 | <0.001 | 0.742 | <0.001 |
| All (WM + GM) (n = 780) | WM (n = 420) | GM (n = 360) | ||||
|---|---|---|---|---|---|---|
| ρ | p | ρ | p | ρ | p | |
| Conventional CT value | −0.705 | <0.001 | 0.104 | 0.033 | −0.379 | <0.001 |
| Electron density | −0.491 | <0.001 | 0.202 | <0.001 | −0.151 | 0.004 |
| Effective atomic number | −0.756 | <0.001 | −0.098 | 0.044 | −0.478 | <0.001 |
| All (WM + GM) (n = 780) | WM (n = 420) | GM (n = 360) | |||||
|---|---|---|---|---|---|---|---|
| R2 | p | R2 | p | R2 | p | ||
| Simple regression analysis | Conventional CT value | 0.565 | <0.001 | 0.010 | 0.040 | 0.145 | <0.001 |
| Electron density | 0.253 | <0.001 | 0.036 | <0.001 | 0.027 | 0.002 | |
| Effective atomic number | 0.606 | <0.001 | 0.005 | 0.133 | 0.252 | <0.001 | |
| Multiple regression analysis | Electron density + Effective atomic number | 0.675 | <0.001 | 0.036 | <0.001 | 0.290 | <0.001 |
| Myeline Partial Volume | Conventional CT Value | Electron Density | Effective Atomic Number | |||||
|---|---|---|---|---|---|---|---|---|
| ρ | p | ρ | p | ρ | p | ρ | p | |
| Caudate nucleus | −0.324 | 0.081 | 0.020 | 0.915 | −0.119 | 0.532 | 0.184 | 0.331 |
| Putamen | −0.276 | 0.139 | −0.121 | 0.525 | −0.205 | 0.276 | 0.283 | 0.130 |
| Globus Pallidus | −0.646 | <0.001 | 0.137 | 0.471 | −0.166 | 0.380 | 0.275 | 0.142 |
| Lateral thalamus | −0.605 | <0.001 | −0.001 | 0.995 | −0.124 | 0.515 | 0.180 | 0.341 |
| Medial thalamus | −0.378 | 0.040 | 0.047 | 0.807 | −0.303 | 0.103 | 0.198 | 0.295 |
| Dorsal thalamus | −0.334 | 0.071 | 0.308 | 0.098 | 0.034 | 0.858 | 0.211 | 0.264 |
| Posterior limb of internal capsule | −0.552 | 0.002 | −0.263 | 0.160 | −0.413 | 0.023 | 0.149 | 0.433 |
| Splenium of the corpus callosum | 0.008 | 0.965 | −0.009 | 0.964 | −0.106 | 0.579 | 0.177 | 0.348 |
| Genu of the corpus callosum | −0.413 | 0.023 | 0.165 | 0.383 | −0.204 | 0.280 | 0.331 | 0.074 |
| Centrum semiovale | −0.701 | <0.001 | −0.262 | 0.162 | −0.450 | 0.013 | 0.264 | 0.158 |
| Frontal lobe WM | −0.741 | <0.001 | 0.016 | 0.931 | −0.251 | 0.181 | 0.405 | 0.026 |
| Occipital lobe WM | −0.678 | <0.001 | −0.148 | 0.436 | −0.411 | 0.024 | 0.549 | 0.002 |
| Temporal lobe WM | −0.649 | <0.001 | −0.199 | 0.293 | −0.323 | 0.082 | 0.437 | 0.016 |
| Parietal lobe WM | −0.719 | <0.001 | 0.084 | 0.658 | −0.305 | 0.101 | 0.308 | 0.098 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, T.; Nakajo, M.; Gohara, M.; Kamimura, K.; Nakano, T.; Kamizono, J.; Takumi, K.; Ejima, F.; Pahn, G.; Langzam, E.; et al. Electron Density and Effective Atomic Number of Normal-Appearing Adult Brain Tissues: Age-Related Changes and Correlation with Myelin Content. Tomography 2025, 11, 95. https://doi.org/10.3390/tomography11090095
Hasegawa T, Nakajo M, Gohara M, Kamimura K, Nakano T, Kamizono J, Takumi K, Ejima F, Pahn G, Langzam E, et al. Electron Density and Effective Atomic Number of Normal-Appearing Adult Brain Tissues: Age-Related Changes and Correlation with Myelin Content. Tomography. 2025; 11(9):95. https://doi.org/10.3390/tomography11090095
Chicago/Turabian StyleHasegawa, Tomohito, Masanori Nakajo, Misaki Gohara, Kiyohisa Kamimura, Tsubasa Nakano, Junki Kamizono, Koji Takumi, Fumitaka Ejima, Gregor Pahn, Eran Langzam, and et al. 2025. "Electron Density and Effective Atomic Number of Normal-Appearing Adult Brain Tissues: Age-Related Changes and Correlation with Myelin Content" Tomography 11, no. 9: 95. https://doi.org/10.3390/tomography11090095
APA StyleHasegawa, T., Nakajo, M., Gohara, M., Kamimura, K., Nakano, T., Kamizono, J., Takumi, K., Ejima, F., Pahn, G., Langzam, E., Nakanosono, R., Yamagishi, R., Kanzaki, F., & Yoshiura, T. (2025). Electron Density and Effective Atomic Number of Normal-Appearing Adult Brain Tissues: Age-Related Changes and Correlation with Myelin Content. Tomography, 11(9), 95. https://doi.org/10.3390/tomography11090095







