Abstract
Medium and variable vessel vasculitides are a heterogeneous group of rare, immune-mediated vascular disorders that are associated with significant morbidity and mortality. The standard treatment approach involves glucocorticoids and immunosuppressive agents. However, many patients exhibit poor tolerance or respond inadequately to these medications. Recent advances in biologic therapies and Janus Kinase inhibitors (JAKis) offer promising alternatives. This review consolidates current knowledge on the pathogenesis, immunology, and therapeutic efficacy of biologics and JAKis in the management of medium and variable vessel vasculitis. While further research is needed to establish long-term safety and optimize treatment protocols, biologics and JAKis represent emerging therapeutic strategies with the potential to improve outcomes.
1. Introduction to Medium and Variable Vessel Vasculitis
Medium vessel vasculitis (MVV) and variable vessel vasculitis (VVV) refer to a group of autoimmune disorders characterized by inflammation and damage to the blood vessel walls. Medium-sized vessels are defined as those measuring 800 μm or less in diameter, featuring four to eight layers of medial smooth muscle, and possessing an indistinct tunica adventitia. These vessels are primarily located in the subcutis or at the dermal–subcutaneous junction and include the main visceral vessels and their initial branches [1]. MVV includes polyarteritis nodosa (PAN) and Kawasaki disease (KD). VVVs involve arteries and veins of all sizes and include Behçet’s disease (BD), deficiency of ADA2 (DADA2), and Cogan syndrome (CS) (Figure 1).
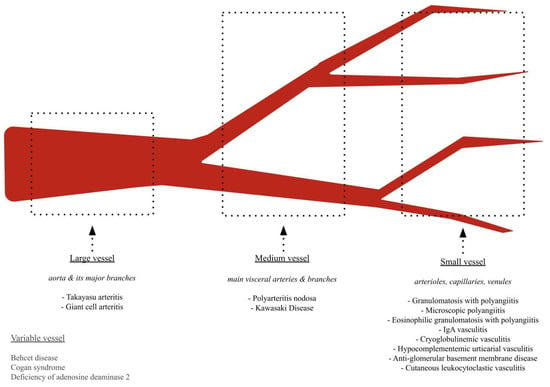
Figure 1.
Overview of Vasculitis.
Advancements in our understanding of the pathogenesis of MVV and VVV have led to the utilization of biologic and Janus Kinase inhibitor (JAKi) therapies. Case reports, case series, and preliminary clinical trials have highlighted their therapeutic potential, with ongoing and future studies aimed at validating their effectiveness and determining how they may complement or potentially supplant existing treatment regimens with traditional immunosuppressants. Importantly, these medications may also reduce prolonged glucocorticoid use [2].
In recent years, literature has been published reviewing the use of biologics and JAKis in large- and small-vessel vasculitis, leading to evidence-based recommendations for the use of tocilizumab (TCZ) in giant cell arteritis (GCA) and rituximab (RTX) for ANCA-associated vasculitis [3,4,5,6]. Yet, no comparable reviews exist for medium and variable vessel vasculitis. This review seeks to build upon existing findings to fill this gap in the literature (Table 1 and Table 2) [7,8].

Table 1.
Level of Evidence for Biologic and Janus Kinase Inhibitor Therapies in PAN, KD, BD, DADA2, and CS.

Table 2.
Overview of Biologic and Janus Kinase Inhibitor Therapies in Medium- and Variable-Vessel Vasculitides.
2. Polyarteritis Nodosa
2.1. Overview
PAN is a necrotizing vasculitis of medium-sized vessels, characterized by the absence of glomerulonephritis and antineutrophil cytoplasmic antibodies (ANCA). The majority of cases are idiopathic. While historically linked to the hepatitis B virus (HBV), the incidence of HBV-associated PAN has markedly decreased in recent years, largely due to widespread vaccination efforts [195,214]. Systemic PAN presents with multisystem involvement, with the skin and peripheral nerves being the most commonly affected. Gastrointestinal tract, kidneys, heart, or central nervous system involvement is associated with increased mortality [215]. Of note, cutaneous PAN is a rare localized variant of PAN that presents without systemic organ involvement and manifests predominantly with cutaneous manifestations, such as livedo racemosa, nodules, and ulcers [216]. Histopathologic confirmation, preferably from skin, muscle, or nerve biopsies, is helpful for diagnosis. When biopsy results are inconclusive or difficult to obtain, microaneurysms observed in a visceral angiography can provide additional diagnostic support [217].
The pathophysiology of PAN is not fully understood but is believed to involve an inflammatory response mediated by various cytokines, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), interferon-alpha (IFN-α), interleukin-2 (IL-2), and interleukin-1 beta (IL-1β) [218,219]. Biologics and JAKis that target these cytokines have shown promising results. The unclear role of B-cells in PAN, with no specific antibodies identified, may explain the mixed evidence for RTX efficacy in treating the disease.
2.2. Treatment
Historically, idiopathic PAN has been treated first-line with high-dose systemic glucocorticosteroids for induction, combined with immunosuppressants like cyclophosphamide (CYC), azathioprine (AZA), methotrexate (MTX), and mycophenolate mofetil (MMF) for maintenance therapy [220,221,222]. HBV-associated PAN is treated primarily with antivirals and with short-term glucocorticoids for acute inflammation; however, broad immunosuppression is typically avoided to prevent the exacerbation of viral replication [220].
Regarding biologics, a 2021 narrative review of 51 articles, involving 58 patients with PAN, found promising results from TNF-α inhibitors (infliximab (IFX), etanercept (ETN), and adalimumab (ADA)) and the IL-6 receptor antagonist TCZ, with mixed results for RTX. IFX successfully treated 83% (25/30) of patients, ETN treated 80% (8/10), ADA treated 50% (2/4), TCZ treated 92% (11/12), and RTX treated 61% (8/13), with four treatment failures and one relapse [21]. A 2022 European retrospective study of 42 PAN patients found TCZ achieved the highest remission rate at 50% (5/10), followed by TNF-α blockers at 40% (6/15), and RTX at 33% (6/18). No response was observed in patients treated with other biologics, including anakinra (n = 4), alemtuzumab (n = 3), IFN-α (n = 2), and abatacept (n = 1) [111]. TCZ has since successfully treated cutaneous PAN (cPAN) with refractory lower-limb ulcers in a 61-year-old Japanese patient, who had not responded to corticosteroids, cyclophosphamide, and endovascular therapy in a 2024 case report [135]. A phase-two, randomized, placebo-controlled clinical trial (NCT05168475) investigating the efficacy of IFX, RTX, and TCZ in treating refractory non-ANCA-associated vasculitis (NAAV), including PAN, is currently underway [38].
There is very limited data on the use of JAKis in PAN, with only seven cases published to date, with six describing the use of JAK1/3 inhibitor tofacitinib (TOF) and one describing the use of JAK1/JAK2 inhibitor baricitinib (BAR). JAKis modulate JAK-STAT signaling pathways, disrupting pro-inflammatory cytokine signaling, including IL-6, IFN-γ, and GM-CSF, which are implicated in PAN vascular inflammation and endothelial dysfunction [221,222]. JAK1 mediates signaling for IL-6, IL-10, IL-11, and type I interferons, while JAK3, when paired with JAK1, transmits signals from cytokines such as IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 [222,223]. A 2016 case report described a 28-year-old man with systemic PAN who was successfully treated with TOF [196]. In 2022, a case was published detailing a 9-year-old girl with PAN who, after remaining steroid-dependent on ETN, achieved disease remission after switching to TOF [224]. In a 2023 case series, four patients with cPAN were successfully treated with TOF monotherapy [192]. In a 2024 case, a 29-year-old woman with cPAN quickly achieved remission with a low dose of BAR and prednisone [201].
3. Kawasaki Disease
3.1. Overview
KD is an acute pediatric vasculitis characterized by fever, conjunctivitis, rash, and lymphadenopathy, with the potential to cause coronary artery aneurysms. KD is theorized to be an abnormal immune response in genetically predisposed individuals to a common environmental trigger, possibly a virus or environmental toxin. Early in the disease, there is necrotizing arteritis of medium-sized vessels, most notably the coronary arteries. Infiltration of vessel walls by monocytes, macrophages, neutrophils, CD8+ T cells, and IgA+ plasma cells damages the intima, media, and adventitia layers of vessel walls. Pro-inflammatory cytokines, namely TNF-α and IL-1β, further promote endothelial cell injury. Serum concentrations of IL-1β and IL-18 are higher in children with KD. Mouse models provide further support for the overactivation of the NLRP3 inflammasome and resultant excessive production of IL-1β. This contributes to both the acute inflammatory response and the persistent chronic inflammation seen in KD. IL-1β is therefore an emerging target for therapeutic intervention by biologic therapies. As KD progresses, vascular damage evolves into subacute or chronic vasculitis and luminal myofibroblast proliferation. The immune infiltrates at this stage consist mainly of CD8+ T cells, IgA+ plasma cells, eosinophils, and macrophages, continuing to secrete inflammatory cytokines that drive the progression of disease. Myofibroblast proliferation leads to progressive luminal narrowing, stenosis, and potential thrombosis of the vasculature [225].
3.2. Treatment
Standard treatment involves early administration of intravenous immunoglobulin (IVIG) and aspirin. For patients with persistent symptoms, repeat IVIG or methylprednisolone may be effective. Among biologics, anakinra and infliximab (IFX) are the most commonly used. Emerging evidence also supports the potential use of ETN, RTX, TCZ, canakinumab (CAN), and abciximab (ABC), though their applications are less well-defined compared to anakinra and IFX.
Anakinra binds to the IL-1 receptor, blocking the action of the pro-inflammatory cytokine IL-1. A comprehensive systematic review performed in March 2020 identified 36 cases of anakinra use. Since then, four additional cases have been described of anakinra used as adjunctive treatment for KD [62,173,177]. A phase-I/IIa dose-escalation study (NCT02179853) of anakinra in 22 children with acute KD and coronary artery aneurysms found that up to 6 weeks of treatment (up to 11 mg/kg/day) was safe, well-tolerated, and effective in reducing coronary artery inflammation. Patients receiving IVIG and anakinra had similar reductions in inflammation markers (IL-6, IL-8, TNF-α) as those treated with IVIG and IFX alone. Both IV and SC anakinra were safe and effective, with simulations suggesting that more frequent IV dosing could sustain drug levels without increasing peak concentrations, highlighting the potential benefits of short-term IV therapy during hospitalization [156]. In a phase-II study (NCT02390596) of 16 IVIG-resistant KD patients, anakinra was well-tolerated and led to a rapid reduction in fever, with 75% (12/16) of patients in the intention-to-treat group and 87.5% (7/8) in the per-protocol group becoming afebrile within 48 h. Additionally, anakinra improved disease activity, normalizing CRP levels by day 30, and showed some efficacy in reducing coronary artery dilation, with 50% (8/16) of patients achieving improved coronary artery Z scores by day 45 [158].
Several clinical trials investigating anakinra for KD are ongoing. Currently, there is an active pilot study (NCT04747847) investigating the ability of anakinra administered with atorvastatin to prevent coronary artery damage in children with early KD-related coronary abnormalities [169]. There is a phase-III trial underway (ANACOMP, NCT04656184) comparing anakinra with IVIG retreatment in KD patients who failed initial IVIG therapy, which is currently recruiting [170]. Planned for the future, there is an interventional trial (NIKITA, NCT06697431) evaluating whether anakinra is non-inferior to IVIG in terms of controlling fever and preventing coronary dilation or aneurysms in the acute phase of KD [171]. These trials will help offer conclusive evidence for anakinra as an effective, safe alternative to traditional therapies for KD, particularly in patients with refractory symptoms or ongoing coronary risks.
The TNF-α inhibitor IFX has been widely explored for IVIG-resistant KD with promising results. There have been 1257 cases published thus far [14]. A multicenter, randomized, prospective trial (NCT00271570) of IFX versus second IVIG infusion in individuals up to 18 years of age with IVIG-resistant KD published in 2008 found that fever resolved within 24 h for 92% (11/12) of subjects treated with IFX, compared to 67% (8/12) of subjects retreated with a second IVIG infusion. There were no infusion reactions, serious adverse events, or significant differences in laboratory variables, fever, or echocardiographic assessments of coronary arteries between treatment groups [35]. In a 2016 study of IVIG-resistant KD patients, 66% (21/32) of patients responded to retreatment with IVIG compared to 91% (10/11) of patients who responded to IFX and did not need additional treatment. There was no significant difference in the occurrence of adverse events or coronary artery lesions [36]. A 2018 phase-three, randomized multicenter trial (NCT01596335) of 31 IVIG-resistant KD patients, with 16 assigned to IFX and 15 to repeat IVIG, found that IFX improved the 48 h defervescence rate and time to defervescence compared to IVIG and was well tolerated, with no significant difference in the occurrence of adverse effects or coronary artery lesions [13]. A randomized, multicenter comparative effectiveness trial (NCT03065244) of IFX versus second IVIG infusion for IVIG-resistant KD published in 2021 found that 77% (40/52) of patients in the IFX group achieved absence of fever recurrence during the first week after discharge, compared to 51% (25/49) in the second IVIG group. The IFX group patients also experienced fewer days of fever, less severe anemia, and fewer serious adverse events [12].
The TNF-α inhibitor ETN has also been explored as a potential treatment for KD. The highest-level evidence comes from a 2018 randomized, double-blind, placebo-controlled study (NCT00841789) of ETN as an adjunct to IVIG in 205 Kawasaki disease (KD) patients, with 100 receiving ETN and 101 receiving a placebo. Subgroup analysis revealed a significant reduction in IVIG resistance in patients older than one year (p = 0.03) and mitigation of coronary artery dilation, particularly in those with baseline abnormalities (p = 0.03), without added safety concerns. A follow-up analysis reported IVIG resistance in 13/100 ETN patients versus 22/101 placebo patients, with coronary artery dilation in 56/100 ETN patients compared to 53/101 placebo patients [86,87,99,100,101,112,124]. A long-term follow-up, however, found that the ETN (n = 22) group had similar long-term outcomes to the placebo group, suggesting that ETN may accelerate the resolution of coronary artery dilation but does not alter long-term disease progression [226].
RTX (n = 4) and TCZ (n = 4) have successfully treated KD in a few published cases [227]. CAN, a monoclonal antibody against IL-1β, shows promise. In 2017, an industry-sponsored, phase-two, multicenter trial (EudraCT 2019-002783-2) was initiated in Europe, enrolling IVIG-resistant and early-diagnosed, treatment-naïve patients with KD for treatment with CAN [173]. However, findings have yet to be reported. Although no findings are available for human subjects, a mouse model of KD treated with an anti-IL-1β antibody showed reduced vasculitis severity [228].
ABC, a chimeric monoclonal antibody that inhibits the platelet glycoprotein IIb/IIIa receptor, may help treat large coronary aneurysms in Kawasaki disease. It has shown greater regression of the aneurysm size when combined with standard therapy, suggesting it promotes vascular remodeling, though further research is needed [68,86,87,99,100,101,112,124,156,158,160,161,162,163,164,165,166,167,169,170,171,173,177,178,179,180,181,182].
4. Behçet’s Disease
4.1. Overview
BD, a variable vessel vasculitis affecting medium, small, and large vessels, is characterized by mucocutaneous ulcers and heterogeneous skin and organ involvement. Th1 cells are the key players in BD via TNF-α and IFN-γ- mediated inflammation [229]. Emerging evidence highlights Th17 cell involvement, with increased IL-17 and IFN-γ production by CD8+ and γδ+ T cells [230]. Studies have found elevated levels of TNF-a, IL-1, sIL-2R, IL-6, IL-8, IL-10, and IL-12 in the serum of patients with Behçet’s disease [231,232]. B cells, especially memory B cells, also play a role. Elevated levels of BAFF, a protein implicated in B-cell function, are present in serum, and BAFF mRNA expression in the skin is increased [233]. Neutrophils in Behçet’s disease exhibit increased activation markers (CD64, CD66b, CD11b, and CD11c), enhanced reactive oxygen species production, and elevated NETosis, alongside significant dysregulation of genes associated with innate immunity, signaling, and chemotaxis. These pathways have led to increased interest in biologics that target these cytokines for the treatment of Behçet’s disease. For instance, phosphodiesterase-4 (PDE4) inhibitors, which suppress neutrophil activation markers and modulate dysregulated immune pathways, have been utilized with success [234]. Similarly, TNF-α inhibitors, ustekinumab (binds p40 of IL-12 and IL-23), anakinra (blocks IL-1, interfering with production of TNF-α and other pro-inflammatory cytokines), and CAN (binds and neutralizes IL-1β, inhibiting the upregulation of IL-6), have shown efficacy.
4.2. Treatment
The highly heterogeneous presentation of BD necessitates a tailored therapeutic approach based on the specific clinical manifestation. Biologic therapies, particularly TNF inhibitors, have shown strong efficacy in managing refractory ocular, neurological, and vascular BD. RTX has demonstrated promise for severe ocular disease, while TCZ and low-dose IL-2 are emerging options for refractory manifestations. Secukinumab and CAN may provide targeted benefits for mucocutaneous and systemic inflammation. These trials underscore the potential of biologics in BD management, although further large-scale studies are needed to establish their long-term efficacy and safety across diverse disease phenotypes. Cluster analyses of BD clinical presentations have identified three disease phenotypes: (1) mucocutaneous and articular, (2) extra-parenchymal neurological and peripheral vascular, and (3) parenchymal neurological and ocular [235].
4.2.1. Mucocutaneous and Articular
Colchicine is first-line for BD, leading to significant improvements in oral and genital ulcers, erythema nodosum, and articular symptoms. Colchicine mitigates inflammation by inhibiting neutrophil chemotaxis and downstream cytokine production, including IL-1β, TNF-α, and IL-6 [236]. In patients intolerant to colchicine, second-line options include AZA, apremilast [237], thalidomide [238], TNF-α inhibitors, and IFN-α inhibitors [239,240,241]. When it comes to biologic agents for mucocutaneous and articular manifestations of BD, TNF-α inhibitors (ETN, ADA, and IFX), ustekinumab, anakinra, secukinumab, and abatacept show the greatest promise. Among JAKis, TOF possesses mixed evidence, while baracitinib (BAR) demonstrates potential.
TNF-α inhibitors have demonstrated significant efficacy in managing Behçet’s disease (BD), particularly for mucocutaneous and intestinal manifestations. In a double-blind, placebo-controlled study involving 40 BD patients, ETN was shown to effectively reduce the frequency of oral ulcers, nodular lesions, and papulopustular lesions [88]. Additionally, a prospective cohort study of 19 patients with intestinal BD found that ETN increased the healing rate of intestinal ulcers and decreased inflammatory markers [102]. For severe refractory mucocutaneous BD, ADA and IFX have proven highly effective. The BEGIN Study (NCT02505568) is a phase three open-label, single-arm multicenter study evaluating IFX use in participants with moderate-to-severe refractory mucocutaneous BD. A total of 33 patients treated with IFX therapy experienced significant decreases in the mean disease activity index for intestinal BD (DAIBD) scores (50.5 ± 36.4 at week 8 and 61.5 ± 38.5 following 24 weeks of maintenance therapy), with 92.3% (25/27) of patients maintaining a clinical response at week 32 [37]. A multicenter randomized controlled trial revealed that 94% of patients treated with ADA achieved remission (defined as the improvement of active manifestations and absence of new episodes of oro-genital ulcers or erythema nodosum), compared to 64% in the IFX group. Both therapies significantly improved quality of life, with a low incidence of adverse events. Notably, ADA demonstrated a faster median response time (42 days) compared to IFX (152 days) [69]. Further supporting their use, a 2023 systematic review and meta-analysis highlighted the efficacy of ADA and IFX in intestinal BD. IFX showed high partial response rates of 82% at 3 months, 71% at 6 months, 65% at 12 months, and 50% at 24 months. Similarly, ADA demonstrated partial response rates of 45% at 6 months, 60% at 12 months, and 40% at 24 months [70].
Ustekinumab, an IL-12/23 inhibitor, was evaluated in a prospective, open-label phase-two study (NCT02648581) involving Behçet’s patients with recurrent oral or genital ulcers refractory to colchicine. The study demonstrated promising short- and long-term efficacy, with 73.3% (11/15) of patients achieving a clinical response within 24 weeks (60% complete response) and 90.9% (10/11) maintaining treatment effects at 52 weeks [147,148]. Significant improvements were also observed in key clinical measures: mean oral ulcers decreased from 2.1 ± 1.3 to 0.3 ± 0.5 (p = 0.0017), oral ulcer pain scores were markedly reduced (p = 0.0005), and both quality of life and disease activity scores improved significantly [148].
A phase-two, open-label, pilot study (NCT01441076) evaluated dose-escalated anakinra (100–300 mg daily) in six patients with refractory oral and genital ulcers with mixed results [157]. Two out of six patients achieved remission in 3–6 months, and five out of six experienced a decrease in the number of ulcers. While the 200 mg dose showed an acceptable safety profile and moderate effectiveness, increasing the dose to 300 mg did not enhance outcomes [157,242]. Although no ocular flares occurred during treatment, two patients with prior eye disease experienced flares shortly after stopping anakinra [243].
The British Association of Dermatologists and British Society for Rheumatology suggests the consideration of secukinumab as a fourth-line option after the failure of conventional systemic therapies (glucocorticosteroids, colchicine, azathioprine, and mycophenolate mofetil) and anti-TNF therapy for patients older than 6 years with Behçet’s disease, specifically for the treatment of mucocutaneous lesions without gastrointestinal and/or ocular involvement. Secukinumab is also suggested for treating arthritis and arthralgias in this population [244].
A multicenter (Italy, Australia) retrospective study of 15 patients with refractory mucosal and articular BD evaluated secukinumab at doses of 150 mg or 300 mg. At 3 months, 66.7% (9/15) of patients had a partial or full response, as measured by the number of new oral ulcers in the 28 days prior to the appointment (compared to baseline). At 6 months, 12 months, 18 months, and 24 months, 86.7% (13/15), 76.9% (10/13), 90.0% (9/10), and 100.0% (8/8) of patients, respectively, exhibited a response. All six patients who began with secukinumab 300 mg/month achieved a complete response by 6 months, while 46.7% (7/15) of patients required increasing the dose to achieve a response [154].
In 2024, a patient undergoing lymphoma treatment developed abdominal pain, intestinal ulceration, and perforation, leading to a diagnosis of intestinal BD. The patient underwent ileostomy and omentoplasty, followed by postoperative treatment with CAN and glucocorticoids. As of two years post-surgery, there has been no recurrence of intestinal BD [175].
Abatacept (ABA), a CTLA-4 agonist, was evaluated in an open-label pilot study (NCT01693640) in mucocutaneous BD. A total of 30 female patients with resistant Behçet’s ulcers (20 patients with oral ulcers, 10 patients with genital ulcers) received weekly injections of 125 mg of ABA over 6 months, though official results have not yet been published [190].
Evidence for TOF for the management of BD-related gastrointestinal manifestations is mixed, with success reported in only three of eight cases described in the literature [193,194,197,198,199]. The precise mechanism underlying TOF’s therapeutic effects in these conditions is not fully understood. Its ability to directly stimulate the JAK-STAT3 pathway may allow it to bypass IL-6 signaling, specifically pathways mediated by TLR4, TLR7, TLR9, and IL-23 [196]. BAR demonstrated a favorable safety profile in refractory intestinal BD (n = 13), with 76.92% (10/13) of patients achieving complete remission of global gastrointestinal symptom scores, and 66.7% (6/9) had mucosal healing on endoscopy [203].
TCZ should be avoided in patients presenting with this phenotype, considering the reported TCZ-induced exacerbation of mucosal ulcers in Behçet’s patients [240].
4.2.2. Extra-Parenchymal Neurological and Vascular
BD can present extra-parenchymal neurological and vascular involvement, including superficial venous thrombosis (SVT), deep vein thrombosis (DVT), cerebral venous sinus thrombosis (CVST), arterial occlusion, and aneurysm formation [235]. The coexistence of central and peripheral vascular involvement is thought to stem from a shared thrombotic pathway driven by neutrophil-induced oxidative inflammation, endothelial dysfunction, tissue factor overexpression, and cytokine-mediated platelet activation (Th1 phenotype) [235,245]. Rapid remission with glucocorticoids remains the cornerstone of treatment, followed by maintenance therapy with traditional immunosuppressants such as AZA, cyclophosphamide (CYC), or cyclosporine (CsA). The role of anticoagulation in BD remains controversial, as thrombosis in BD arises from vessel wall inflammation rather than hypercoagulability [246]. A recent meta-analysis found that combination immunosuppressive therapy with anticoagulation decreased rates of VTE recurrence compared to immunosuppressive therapy alone [247]. However, other studies have found no benefit, and EULAR continues to recommend immunosuppressive agents alone [248].
Evidence supports the use of TNF-α inhibitors like ADA and IFX, TCZ, and BAR for neurological and vascular manifestations of BD. Anti-TNF-α agents, used as standalone therapy or in combination with DMARDs, showed benefit in improving outcomes in severe cases of both arterial and venous BD [249]. A 2024 study described a novel mechanism by which TNF inhibitors mitigated BD vasculitis, targeting the mevalonate pathway in neutrophils, and reducing the production of farnesyl pyrophosphate and subsequent TRPM2-calcium signaling [250]. ADA and IFX are the most widely studied, with a multicenter observational study of 18 patients with major vessel involvement in BD showing that patients treated with ADA/IFX experienced a high rate of vascular remission (89%) and a significantly lower risk of relapse compared to conventional immunosuppressants [242]. Furthermore, a retrospective analysis comparing ADA to DMARDs in 70 patients with BD-related venous thrombosis found that ADA-based regimens were more effective and rapid in improving venous thrombosis and allowed for a significant corticosteroid dose reduction [251,252]. A 2023 retrospective cohort study found that IFX was effective for inducing remission in Behçet’s syndrome patients with vascular involvement, with a 73% remission rate at six months, though relapse and adverse events occurred in some cases [245].
Results from a phase-three, multicenter, randomized controlled trial (NCT03371095) comparing IFX to CYC therapy among 53 patients with severe vascular (n = 37) and neurological (n = 15) BD were recently published, demonstrating superiority of IFX over CYC in achieving a complete response [15]. A total of 81% (22/27) of patients treated with intravenous IFX (5 mg/kg at weeks 0, 2, 6, 12, and 18) achieved a complete response, versus 56% (14/25) of those receiving CYC (0.7 g/m2 intravenously at weeks 0, 4, 8, 12, 16, and 20, with a maximal dose of 1.2 g/infusion), and patients demonstrated decreased CRP levels, fewer adverse effects, and lower rates of relapse [15].
BAR has also shown promise in refractory vascular BD. A pilot study reported that 76.5% (13/17) of patients achieved a complete response at three months, increasing to 88.2% at the last visit. Significant reductions were observed in inflammatory markers (ESR, CRP), disease activity scores (BDCAF, BVAS), and glucocorticoid dosage, with no serious adverse events reported, highlighting BAR as a potential therapeutic option for vascular BD [202].
4.2.3. Parenchymal CNS and Ocular Phenotype
To date, no RCT has determined the optimal therapeutic management of neurological BD [253]. Standard treatment includes high-dose glucocorticosteroids, followed by maintenance with disease-modifying antirheumatic drugs [254,255]. There is growing evidence for the role of biologic agents and JAKis. Specifically, these include TNF-α inhibitors, IL-6, B-cell targeted therapies like TCZ and RTX, secukinumab, CAN, golimumab (GOL), abatacept (ABA), and the JAK inhibitors BAR, UPA, and potentially filgotinib.
TNF-α inhibitors, particularly IFX and ADA, are well-supported by evidence for their effectiveness in neuro-Behçet’s and Behçet’s uveitis (BU). A 2023 systematic review and meta-analysis assessing IFX for neuro-Behçet’s across 21 studies found that 93.7% (60/64) of patients responded positively, defined as a significant improvement in neurological symptoms or stabilization/improvement in neuroimaging findings [73]. Additionally, cumulative analysis over the past 20 years demonstrated an increasing trend in the effectiveness of IFX therapy for neuro-Behçet’s, potentially due to advances in diagnostic accuracy [73]. A 2024 systematic review and meta-analysis of TNF-α-inhibitors for patients with BU found that 85.0% (982/1156) of patients across 12 studies achieved ocular inflammation remission, with 77.4% (895/1156) experiencing an improvement in visual acuity [256]. Subgroup analysis revealed a slight advantage for ADA as compared to IFX in terms of improved remission and drug retention rates, and lower incidence of severe reaction [256].
A prospective, phase two non-comparative interventional study demonstrated intravitreal IFX safety and efficacy amongst 20 patients with refractory BU. Patients who received three consecutive intravitreal injections of infliximab (1 mg/0.05 mL) six weeks apart demonstrated significant improvement in best-corrected visual acuity (BCVA), decreased mean central foveal thickness OCT, and a reduction in mean vitreous haze gradings [72]. Furthermore, no significant ocular adverse inflammatory reactions were observed. ADA has effectively prevented relapses and maintained visual acuity in ocular BD, making it a preferred option for long-term treatment.
Another TNF-α inhibitor, golimumab (GOL), successfully managed BD-related nervous system involvement in a 50-year-old female in 2020, leading to significant improvements in numbness and tremor [126]. Additionally, GOL has been effective for Behçet’s uveitis in published cases (n = 14) [105,106]. However, there have been two cases of myelitis developing in patients receiving GOL treatment for neuro-Behçet’s, thus indicating the importance of long-term monitoring and the necessity of further research [107,108]. A 2017 retrospective study of 17 patients with BD found that GOL was able to control BD manifestations in 94.1% (16/17) of cases [109]. BD Current Activity Form (BDCAF) was significantly improved in patients receiving a combination of GOL and DMARDs than those undergoing GOL monotherapy [109]. Additionally, GOL was effective regardless of the number of previously failed biologic agents, suggesting GOL may be helpful for patients with especially refractory disease [109].
Beyond TNF inhibitors, IL-6 and B-cell targeted therapies also show promise. A 2020 systematic review assessing the effectiveness of IL-6 inhibitor TCZ for the treatment of Behçet’s disease found TCZ was highly effective in managing refractory ocular, neurological, and vascular involvement, as well as reducing glucocorticoid dependence. However, it did cause the worsening of oral/genital ulcers and skin lesions in some cases [125]. A 2022 observational cohort study of 10 patients with refractory arterial Behçet’s lesions receiving 8 mg/kg TCZ infusions every 4 weeks for 24 weeks found TCZ to be a safe and effective treatment option [126]. A total of 90% (9/10) of patients experienced improvement in symptoms (6/10 complete remission, 3/10 partial response), average ESR and CRP values decreased from 50 mm/h and 32.9 mg/dL to 4 mm/h and 2.9 mg/dL, respectively, and the average daily glucocorticoid dose reduced from 54.5 to 8.3 mg/dL [126]. A 2023 multicenter study of 30 patients with BD found that TCZ was effective in 83% (25/30) of patients, with a complete response of 67%, 60%, and 42% in patients with uveitis (18/30), neurological (5/30), and mucocutaneous/articular manifestations (7/30), respectively [126]. TCZ therapy also led to decreased median steroid doses and fewer patients needing concomitant DMARD therapy. However, a 2024 study warned that TCZ could potentially exacerbate vascular manifestations of Behçet’s, with relapses including severe presentations, such as bilateral pulmonary artery aneurysms, pulmonary artery thrombus, and pseudotumor cerebri [126].
Rituximab (RTX), a CD20 monoclonal antibody, demonstrated significant efficacy in severe ocular BD in a completed single-blind, randomized trial (NCT00700297) [122]. Patients showed marked improvements in Total Adjusted Disease Activity Index (TADAI) scores after six months compared to those treated with CYC and AZA. RTX also reduced glucocorticoid dependence and stabilized ocular inflammation, highlighting its potential for refractory cases [122]. RTX has also been shown to be promising in treating refractory neuro-Behçet’s in several case studies. One 49-year-old man presenting with parenchymal subtype Behçet’s with brainstem lesions, and one 46-year-old woman with non-parenchymal meningeal Behçet’s, both experienced the resolution of lesions and clinical symptoms following RTX therapy with low-dose prednisone [257]. Three additional cases of patients with neuro-Behçet’s disease who failed traditional therapy achieved continuous remission in both clinical and neuroimaging parameters, with normal inflammatory markers after low-dose RTX therapy [258,259,260].
Low-dose IL-2 is being explored for Behçet’s disease [185,186,213,261,262,263]. A completed phase II trial (NCT04065672) evaluating low-dose IL-2 in BD patients with vascular and mucocutaneous manifestations demonstrated reduced lesion counts and improved inflammatory markers [185,186]. An open-label, phase-2a basket trial (NCT01988506) of 13 autoimmune diseases included eight patients with Behçet’s disease (total n = 81). On day eight, there was a 1.9-fold increase in the percentage of regulatory T cells and a 2.17-fold increase in the ratio of regulatory T cells to CD25+ effector T cells. For the participants with Behçet’s disease, there was a trend of improvement in the Behçet’s Disease Current Activity Form. At 3 and 6 months, there were significant improvements in Clinical Global Impression activity, severity, and efficacy that persisted, but was less pronounced, two months after treatment discontinuation. At the 6-month time point, over 50% of the participants with Behçet’s disease were classified as treatment responders [187].
Secukinumab, an IL-17 inhibitor, has shown mixed but promising results in treating parenchymal neuro-Behçet’s disease [264]. In a small case series, two patients with refractory neurological symptoms achieved significant improvements. One patient with seizures unresponsive to levetiracetam experienced complete remission and the resolution of brain lesions on MRI after secukinumab therapy (150 mg weekly for one month, then monthly) combined with colchicine and methotrexate [265]. The second patient, with fevers, headaches, diplopia, and brainstem lesions, saw symptom resolution and reduced lesions after four months of treatment (150 mg weekly for one month, then biweekly) [265].
The SHIELD study (NCT00995709), a phase III trial evaluating secukinumab for recurrent Behçet’s uveitis (n = 118), found no significant difference in ocular exacerbation rates compared to a placebo, though secukinumab-treated patients required less immunosuppressive medication and had fewer recurrences. Adverse effects, including the exacerbation of Behçet’s syndrome, uveitis, and folliculitis, led to the discontinuation of seven patients [150].
A multicenter, randomized phase II study (NCT00685399) comparing the safety and efficacy of various IV and SC doses of secukinumab in patients with non-infectious BU found statistical and clinical superiority of the 30 mg/kg IV dose compared with the 300 mg SC dose. Participants treated with high-dose IV secukinumab experienced a faster time to response onset and improved visual acuity and changes in the vitreous haze score than those receiving SC administration [152]. These mixed results highlight the need for further research to clarify the role of secukinumab in Behçet’s disease.
A retrospective study of IL-1 inhibition included 15 BD patients treated with canakinumab. A total of 46.7% (7/15) of patients (1 pediatric, 6 adults) achieved a partial response to canakinumab therapy, while another 46.7% (7/15) of patients achieved a complete response, and 6.6% (1/15) of patients demonstrated no response [154]. CAN was also shown to be effective in the case of a BD patient with refractory bilateral retinal vasculitis, who achieved symptom control after failing multiple therapies, including AZA, cyclophosphamide (CYC), IFX, and ADA [175].
ABA, a CTLA-4 inhibitor, did not achieve clinical remission in a 13-year-old patient with BD-related uveitis in a 2024 study, but it did achieve short-term efficacy in a 47-year-old woman with ocular and skin BD but failed to sustain remission, necessitating a switch to TC [152,266].
JAKis are emerging as potential therapeutic options. BAR is being explored in an open-label, multicenter phase three trial (NCT04088409) as a treatment for pediatric non-infectious uveitis in Behçet’s disease (BD) [204,267,268]. A retrospective case series (n = 2) demonstrated the efficacy of UPA in the treatment of BD-related macular edema, with improvements observed in visual acuity and intraocular inflammation [212]. A prospective study of uveitis, including one patient with BD and anterior uveitis, demonstrated complete disease control, with treatment consisting of UPA 15 mg and AZA 200 mg daily [210,269]. A phase two randomized clinical trial (NCT03207815) found that filgotinib significantly reduced the risk of treatment failure in patients with active non-infectious uveitis (37.5%, 12/32) compared to a placebo (67.6%, 23/34) by week 24. Unfortunately, the study was terminated early for undisclosed business reasons, unrelated to the efficacy or safety of filgotinib [207]. Additionally, a case study demonstrated UPA effectively treated BD symptoms in a 42-year-old woman, leading to significant improvements in oral and genital ulcers, ocular inflammation, and hearing loss [213].
5. Deficiency of ADA2
5.1. Overview
Deficiency of adenosine deaminase 2 (DADA2) is a rare autosomal recessive disease caused by a monogenic loss-of-function (LOF) mutation in the ADA2 gene (previously the CECR1 gene) located on chromosome 22q11.1 [263]. While initially described in 2014 as a vasculopathy of infants and young children clinically resembling PAN, its clinical phenotype has since expanded to involve adults and includes hematologic, immunologic, and autoinflammatory manifestations in addition to vasculitis [270].
ADA2 is a dimeric enzyme produced by myeloid cells responsible for catalyzing the deamination of adenosine and 2′-deoxyadenosine into insosine and deoxyinosine, respectively. By modulating extracellular adenosine levels, ADA2 is crucial in regulating both innate and adaptive immune activity, including promoting T-cell-dependent monocyte differentiation into macrophages, with predominance of the anti-inflammatory M2 phenotype [271]. ADA2 also has a role as a paracrine growth factor promoting endothelial proliferation, angiogenesis, and tissue repair [272].
In DADA2, inactivating biallelic mutations of the ADA2 gene leads to accumulation of extracellular adenosine, initiating a downstream inflammatory cascade characterized by chronic neutrophil activation and dysregulation with neutrophil extracellular trap (NET) formation, macrophage polarization towards the inflammatory M1 subtype with resultant hypersecretion of TNF-α and other inflammatory cytokines, and increased endothelial IFN-β secretion [271]. Newer research describes additional adaptive immunity effects in DADA2 patients, with altered CD40L expression leading to impaired B-cell differentiation and switching with subsequent hypoimmunoglobulinemia [266]. Recent studies have also shown an activation of the IFN–JAK–STAT1 pathway in T cells and monocytes of DADA2 patients, further contributing to M1 macrophage polarization [268].
DADA2 presents with a broad, highly variable spectrum of clinical phenotypes, including systemic vasculopathy, recurrent ischemic and hemorrhagic strokes, livedoid rash, and fevers. While the majority of patients are young children, with 50% of cases presenting before the age of five, and nearly 80% of patients experiencing their first symptom by 18, more adult-onset cases are being recognized [269]. While the pathophysiology remains unclear, systematic reviews indicate potential differences in clinical presentation based on age of onset, with hematological and immunological manifestations predominating in early childhood and cutaneous and vascular symptoms occurring more commonly in adult patients [269]. PAN-like small and medium vessel vasculitis and ischemic stroke remain the hallmark presentations of DADA2, occurring in 76% and 52% of patients, respectively [273]. However, immunological manifestations such as hypoimmunoglobulinemia and lymphopenia are also significant, with low IgM being the most common (30.7%), followed by low IgG (23%) and low IgA (18%) [273].
The complex pathogenesis and variable clinical presentation of DADA2 necessitate a phenotype-driven approach to treatment. For patients with severe inflammation and vasculopathy, tumor necrosis factor (TNF) inhibitors are the mainstay, effectively reducing stroke risk and systemic inflammation [273]. In contrast, those with immunodeficiency or hematologic involvement may require immunoglobulin replacement therapy or hematopoietic stem-cell transplantation (HSCT) in severe cases [274]. Emerging interest in targeting specific molecular pathways has led to exploration of JAK/STAT inhibitors, such as ruxolitinib and TOF, for their potential to modulate the aberrant inflammatory signaling seen in DADA2 [275]. These therapies represent promising avenues for patients with refractory or overlapping disease phenotypes.
5.2. Treatment
There is evidence from published case reports and series of the use of TNF-α-inhibitors in DADA2. A 2021 narrative review identified 76 cases of DADA2 treated with a biologic agent between 2005 and 2020. The strongest evidence was for ETN, with 78% (32/41) of patients achieving complete or partial remission, although ETN was found to be ineffective in halting peripheral vasculopathy progression. Complete or partial remission was also achieved in 61.5% (8/13) of cases treated with IFX and 87.5% (14/16) with ADA. Evidence in favor of biologic agents other than TNF-α-inhibitors is lacking. Only 50% (1/2) of patients treated with canakinumab (CAN) achieved remission, and none responded to GOL (0/1), TCZ (0/4), RTX (0/3), or anakinra (0/4) [21].
Since the 2021 narrative review, additional cases have been reported, further supporting the efficacy of TNF-α inhibitors in DADA2. Complete or partial remission was achieved in six out of seven patients treated with IFX, seven out of eight patients treated with ETN, and six out of six patients treated with ADA [18,19,20,80,81,82,89,90,91,92,93,174].
Although individual outcomes for ETN and ADA were not specified, a 2024 descriptive analysis of 27 DADA2 patients treated with TNF-α-inhibitors (ETN, n = 26; ADA, n = 1) found that 35.7% achieved a complete response, primarily in cases with nervous system or skin involvement, while another 35.7% had a partial response [276].
There are a few cases indicating the limited utility of TCZ and RTX for DADA2. TCZ was trialed but discontinued in the treatment of a 3-year-old boy due to intestinal perforation and in a 5-year-old boy due to severe epigastric pain, headaches, and livedo reticularis on all extremities [277,278]. RTX was one of many medications attempted but found to be ineffective for a woman with a delayed genetic diagnosis of DADA2 [279].
CAN has been used in two pediatric cases with variable success [174,280].
6. Cogan’s Syndrome
6.1. Overview
Cogan’s Syndrome (CS) is a rare VVV that primarily affects the eyes and ears, typically presenting with non-syphilitic interstitial keratitis and Menière-like vestibulo-auditory symptoms [281]. Approximately one-fifth of patients experience vasculitic manifestations, such as arteritis, aortitis, aortic aneurysms, and valvulitis [282]. The exact pathogenesis of CS remains unclear, but it is theorized to be an autoimmune disorder, potentially triggered by viral infections. The basis of this theory stems from the identification of auto-antibodies against corneal and inner ear antigens, endothelial cells, and Cogan peptide, which have been identified in patients with CS [282].
6.2. Treatment
TNF-α inhibitors, RTX, and TOF have been utilized with variable efficacy in CS. A recent 2024 systematic review searching for cases of CS found auditory improvements or complete resolutions in 0/2 individuals with CS treated with ADA, 9/9 individuals treated with IFX, 1/2 individuals treated with RTX, and 3/3 individuals treated with TCZ [283]. We found three additional cases of TCZ used for CS that were not included in that review. These include a 31-year-old with continual hearing impairment despite treatment with dexamethasone, MTX, and IFX, a 49-year-old male with an insufficient response to steroids alone, and a 59-year-old male with an insufficient response to steroids, MTX, AZA, MMF, and CYC; they were all successfully treated with TCZ [128,129,130,131,132,133,284]. In a 2023 case, RTX, TCZ, and ETN were all utilized as part of the treatment regimen for a 35-year-old female with CS, with some clinical improvement. The addition of TOF, however, enabled the remission of tinnitus and joint pain, the normalization of inflammatory markers, and facilitated glucocorticosteroid tapering [195]. There is one case of successful use of TOF for CS [195].
The use of biologics and JAKis in CS is still relatively experimental at this point in time, with IFX demonstrating the greatest efficacy in published cases thus far. Further research and clinical trials are needed to determine their long-term efficacy and safety.
7. Conclusions
Biologic therapies and JAKis hold significant promise for the treatment of medium and variable vessel vasculitis, offering a more targeted approach that can improve both disease induction and maintenance. As these therapies evolve, important questions regarding the integration of biologics and JAKis into the treatment landscape will need to be addressed. Biologics have the potential to improve patient survival rates and enhance our understanding of disease pathogenesis, paving the way for more precise and effective treatments in the future.
Author Contributions
Conceptualization, A.B. and G.C.; writing—original draft preparation, A.B., R.G., C.C. and G.C.; writing—review and editing, A.B., R.G., C.C. and G.C.; supervision, A.B. and G.C.; project administration, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MVV | Medium Vessel Vasculitis |
| VVV | Variable Vessel Vasculitis |
| PAN | Polyarteritis Nodosa |
| KD | Kawasaki Disease |
| BD | Behçet’s Disease |
| DADA2 | Deficiency of Adenosine Deaminase 2 |
| CS | Cogan’s Syndrome |
| JAKi | Janus Kinase Inhibitor |
| TCZ | Tocilizumab |
| GCA | Giant Cell Arteritis |
| RTX | Rituximab |
| ANCA | Antineutrophil Cytoplasmic Antibodies |
| AZA | Azathioprine |
| MTX | Methotrexate |
| MMF | Mycophenolate Mofetil |
| CYC | Cyclophosphamide |
| IFX | Infliximab |
| ETN | Etanercept |
| ADA | Adalimumab |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor-Alpha |
| IFN-α | Interferon-Alpha |
| IL-2 | Interleukin-2 |
| IL-1β | Interleukin-1 Beta |
| NAAV | Non-ANCA-Associated Vasculitis |
| TOF | Tofacitinib |
| BAR | Baricitinib |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| JAK-STAT | Janus Kinase-Signal Transducer and Activator of Transcription |
| IVIG | Intravenous Immunoglobulin |
| CAN | Canakinumab |
| ABC | Abciximab |
| NLRP3 | NLR Family Pyrin Domain Containing 3 |
| ANACOMP | Anakinra vs. IVIG Comparative Trial |
| NIKITA | Non-Inferiority of anakinra vs. IVIG in Kawasaki Disease Treatment Assessment |
| PDE4 | Phosphodiesterase-4 |
| DAIBD | Disease Activity Index for Intestinal Behçet’s Disease |
| BEGIN | Study of Infliximab in Behçet’s Disease |
| SHIELD | Secukinumab in Behçet’s Uveitis Trial |
| GOL | Golimumab |
| UPA | Upadacitinib |
| ABA | Abatacept |
| CTLA-4 | Cytotoxic T-Lymphocyte-Associated Protein 4 |
| TLR4/7/9 | Toll-Like Receptor 4/7/9 |
| BDCAF | Behçet’s Disease Current Activity Form |
| BVAS | Behçet’s Vasculitis Activity Score |
| TADAI | Total Adjusted Disease Activity Index |
| LOF | Loss-of-Function |
| NET | Neutrophil Extracellular Trap |
| HSCT | Hematopoietic Stem Cell Transplantation |
| CRP | C-Reactive Protein |
| ESR | Erythrocyte Sedimentation Rate |
| SC | Subcutaneous |
| IV | Intravenous |
| OCT | Optical Coherence Tomography |
| MRI | Magnetic Resonance Imaging |
References
- Chen, K.R.; Carlson, J.A. Clinical approach to cutaneous vasculitis. Am. J. Clin. Dermatol. 2008, 9, 71–92. [Google Scholar] [CrossRef]
- Tavares, L.C.P.; Caetano, L.V.N.; Ianhez, M. Side effects of chronic systemic glucocorticoid therapy: What dermatologists should know. An. Bras. Dermatol. 2024, 99, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Rathore, U.; Thakare, D.R.; Patro, P.; Agarwal, V.; Sharma, A.; Misra, D.P. A systematic review of clinical and preclinical evidences for Janus kinase inhibitors in large vessel vasculitis. Clin. Rheumatol. 2022, 41, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Silva-Fernández, L.; Loza, E.; Martínez-Taboada, V.M.; Blanco, R.; Rúa-Figueroa, I.; Pego-Reigosa, J.M.; Muñoz-Fernández, S.; Systemic Autoimmune Diseases Study Group of the Spanish Society for Rheumatology (EAS-SER). Biological therapy for systemic vasculitis: A systematic review. Semin. Arthritis Rheum. 2014, 43, 542–557. [Google Scholar] [CrossRef]
- Stone, J.H.; Tuckwell, K.; Dimonaco, S.; Klearman, M.; Aringer, M.; Blockmans, D.; Brouwer, E.; Cid, M.C.; Dasgupta, B.; Rech, J.; et al. Trial of Tocilizumab in Giant-Cell Arteritis. PLoS ONE 2014, 9, e115026. [Google Scholar] [CrossRef]
- McAdoo, S.P.; Medjeral-Thomas, N.R.; Mukhtyar, C.; Biddle, K.; Jade, J.; Wilson-Morkeh, H.; Adikari, M.; Al Yaghchi, C.; Anastasa, Z.; Basu, N.; et al. British Society for Rheumatology guideline on the management of ANCA-associated vasculitis. Ann. Rheum. Dis. 2023, 82, 3–16. [Google Scholar] [CrossRef]
- Araújo Correia, J.; Crespo, J.; Alves, G.; Salvador, F.; Matos-Costa, J.; Delgado Alves, J.; Fortuna, J.; Almeida, I.; Campar, A.; Brandão, M.; et al. Biologic therapy in large and small vessels vasculitis, and Behçet’s disease: Evidence- and practice-based guidance. Autoimmun. Rev. 2023, 22, 103362. [Google Scholar] [CrossRef]
- Serling-Boyd, N.; Wallace, Z.S. Management of primary vasculitides with biologic and novel small molecule medications. Curr. Opin. Rheumatol. 2021, 33, 8–14. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Liu, L.; Gong, X.; Liu, J.; Sun, L.; Liu, X.; Wu, L.; Chen, L.; Wang, L.; et al. Fine Comparison of the Efficacy and Safety Between GB242 and Infliximab in Patients with Rheumatoid Arthritis: A Phase III Study. Rheumatol. Ther. 2022, 9, 175–189. [Google Scholar] [CrossRef]
- Do, N.; Ringold, S.; Brandling-Bennett, H. Cutaneous polyarteritis nodosa in pediatric patients successfully treated with TNF-α inhibitor and methotrexate: Case series and literature review. Pediatr. Dermatol. 2019, 36, 932–935. [Google Scholar] [CrossRef]
- Eleftheriou, D.; Melo, M.; Marks, S.D.; Tullus, K.; Sills, J.; Cleary, G.; Dolezalova, P.; Ozen, S.; Pilkington, C.; Woo, P.; et al. Biologic therapy in primary systemic vasculitis of the young. Rheumatology 2009, 48, 978–986. [Google Scholar] [CrossRef]
- Burns, J.C.; Roberts, S.C.; Tremoulet, A.H.; He, F.; Printz, B.F.; Ashouri, N.; Jain, S.S.; Michalik, D.E.; Sharma, K.; Truong, D.T.; et al. Infliximab versus second intravenous immunoglobulin for treatment of resistant Kawasaki disease in the USA (KIDCARE): A randomised, multicentre comparative effectiveness trial. Lancet Child Adolesc. Health 2021, 5, 852–861, Erratum in Lancet Child Adolesc. Health 2022, 6, e5. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Hara, T.; Kikuchi, M.; Shimizu, H.; Miyamoto, T.; Iwashima, S.; Oonishi, T.; Hashimoto, K.; Kobayashi, N.; Waki, K.; et al. Infliximab versus intravenous immunoglobulin for refractory Kawasaki disease: A phase 3, randomized, open-label, active-controlled, parallel-group, multicenter trial. Sci. Rep. 2018, 8, 1994. [Google Scholar] [CrossRef]
- Wang, L.; He, M.; Wang, W.; Li, S.; Zhao, G. Efficacy and safety of infliximab in the treatment of Kawasaki disease: A systematic review and meta-analysis. Eur. J. Pediatr. 2024, 183, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Induction Therapy with Anti-TNFα vs Cyclophosphamide in Severe Behçet Disease (ITAC). ClinicalTrials.gov. Identifier: NCT03371095. Updated 19 September 2023. Available online: https://clinicaltrials.gov/study/NCT03371095 (accessed on 28 January 2025).
- Izumo, H.; Ishikawa, N.; Kobayashi, Y.; Doi, T.; Okada, S. A Successful Infliximab Treatment of a Pediatric Case of Severe Polyarteritis Nodosa with a Cerebral Infarction and a Decreased Adenosine Deaminase 2 Activity. Cureus 2023, 15, e47952. [Google Scholar] [CrossRef]
- Sha, S.; Xu, B.; Wang, K.; Qiao, C.; Shi, H.; Jiang, J.; Quan, X.; Liu, X. Case report: Successful remission with upadacitinib in a young patient with anti-TNF-refractory intestinal Behçet’s disease. Front. Immunol. 2024, 15, 1483993. [Google Scholar] [CrossRef]
- Ates, M.B.; Karup, S.; Ugurlu, S. Infliximab as a successful treatment option in a case of adenosine deaminase 2 deficiency. Reumatismo 2023, 75, 225–228. [Google Scholar] [CrossRef]
- Simão Raimundo, D.; Cordeiro, A.I.; Parente Freixo, J.; Valente Pinto, M.; Neves, C.; Farela Neves, J. Case Report: Patient with deficiency of ADA2 presenting leukocytoclastic vasculitis and pericarditis during infliximab treatment. Front. Pediatr. 2023, 11, 1200401. [Google Scholar] [CrossRef]
- Parlar, K.; Tahir Turanli, E.; Nuhoglu Kantarci, E.; Hacioglu, A.; Kirectepe Aydin, A.; Yagiz Ayla, A.; Voyvoda, U.; Ozdogan, H.; Ugurlu, S. A case with febrile attacks and vasculopathy associated with ADA2 and MEFV pathogenic variants. Mod. Rheumatol. Case Rep. 2023, 8, 121–124. [Google Scholar] [CrossRef]
- Conticini, E.; Sota, J.; Falsetti, P.; Lamberti, A.; Miracco, C.; Guarnieri, A.; Frediani, B.; Cantarini, L. Biologic drugs in the treatment of polyarteritis nodosa and deficit of adenosine deaminase 2: A narrative review. Autoimmun. Rev. 2021, 20, 102784. [Google Scholar] [CrossRef] [PubMed]
- Al-Bishri, J.; le Riche, N.; Pope, J.E. Refractory polyarteritis nodosa successfully treated with infliximab. J. Rheumatol. 2005, 32, 1371–1373. [Google Scholar]
- Atamyıldız Uçar, S.; Demir, M.; Sözeri, B. Polyarteritis nodosa with life-threatening intracranial aneurysms in a child, and treatment with infliximab. Turk. J. Pediatr. 2024, 66, 801–808. [Google Scholar] [CrossRef]
- Brik, R.; Gepstein, V.; Shahar, E.; Goldsher, D.; Berkovitz, D. Tumor necrosis factor blockade in the management of children with orphan diseases. Clin. Rheumatol. 2007, 26, 1783–1785. [Google Scholar] [CrossRef]
- Campanilho-Marques, R.; Ramos, F.; Canhão, H.; Fonseca, J.E. Remission induced by infliximab in a childhood polyarteritis nodosa refractory to conventional immunosuppression and rituximab. Jt. Bone Spine 2014, 81, 277–278. [Google Scholar] [CrossRef] [PubMed]
- de Kort, S.W.; van Rossum, M.A.; ten Cate, R. Infliximab in a child with therapy-resistant systemic vasculitis. Clin. Rheumatol. 2006, 25, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.; Rosner, I.; Slobodin, G.; Rozenbaum, M.; Kaly, L.; Jiries, N.; Boulman, N.; Awisat, A.; Hussein, H.; Novofastovski, I.; et al. Infliximab for the treatment of refractory polyarteritis nodosa. Clin. Rheumatol. 2019, 38, 2825–2833. [Google Scholar] [CrossRef] [PubMed]
- Lerkvaleekul, B.; Treepongkaruna, S.; Ruangwattanapaisarn, N.; Treesit, T.; Vilaiyuk, S. Recurrent ruptured abdominal aneurysms in polyarteritis nodosa successfully treated with infliximab. Biologics 2019, 13, 111–116. [Google Scholar] [CrossRef]
- Matsuo, S.; Hayashi, K.; Morimoto, E.; Kato, A.; Sada, K.-E.; Watanabe, H.; Takano-Narazaki, M.; Sunahori-Watanabe, K.; Kawabata, T.; Wada, J. The Successful Treatment of Refractory Polyarteritis Nodosa Using Infliximab. Intern. Med. 2017, 56, 1435–1438. [Google Scholar] [CrossRef]
- Teixeira, V.; Oliveira-Ramos, F.; Costa, M. Severe and Refractory Polyarteritis Nodosa Associated with CECR1 Mutation and Dramatic Response to Infliximab in Adulthood. J. Clin. Rheumatol. 2020, 26, e66–e69. [Google Scholar] [CrossRef]
- Tous-Romero, F.; Rodríguez-Almaraz, E.; Rodríguez-Peralto, J.L.; Postigo-Llorente, C. Polyarteritis Nodosa with a Systemic Inflammatory Response Pattern: Effectiveness of anti-TNF. Panarteritis nudosa con patrón de respuesta inflamatoria sistémica: Respuesta a anti-TNF. Actas Dermo-sifiliogr 2017, 108, 787–790. [Google Scholar] [CrossRef]
- Vega Gutierrez, J.; Rodriguez Prieto, M.A.; Garcia Ruiz, J.M. Successful treatment of childhood cutaneous polyarteritis nodosa with infliximab. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 570–571. [Google Scholar] [CrossRef] [PubMed]
- Wahezi, D.M.; Gomes, W.A.; Ilowite, N.T. Cranial nerve involvement with juvenile polyarteritis nodosa: Clinical manifestations and treatment. Pediatrics 2010, 126, e719–e722. [Google Scholar] [CrossRef]
- Wu, K.; Throssell, D. A new treatment for polyarteritis nodosa. Nephrol. Dial. Transplant. 2006, 21, 1710–1712, Erratum in Nephrol. Dial. Transplant. 2006, 21, 2044. [Google Scholar] [CrossRef]
- Burns, J.C.; Best, B.M.; Mejias, A.; Mahony, L.; Fixler, D.E.; Jafri, H.S.; Melish, M.E.; Jackson, M.A.; Asmar, B.I.; Lang, D.J.; et al. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J. Pediatr. 2008, 153, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.; Kim, J.; Hong, Y.M.; Sohn, S. Infliximab as the First Retreatment in Patients with Kawasaki Disease Resistant to Initial Intravenous Immunoglobulin. Pediatr. Infect Dis. J. 2016, 35, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.H.; Kim, H.S.; Han, D.S.; Kim, S.K.; Shin, S.J.; Kim, J.S.; Ye, B.D.; Song, G.A.; Lee, Y.; Kim, Y.; et al. Efficacy and Safety of Infliximab in Intestinal Behçet’s Disease: A Multicenter, Phase 3 Study (BEGIN). Gut Liver 2023, 17, 777–785. [Google Scholar] [CrossRef]
- Cambridge University Hospitals NHS Foundation Trust. Biologics in Refractory Vasculitis (BIOVAS). ClinicalTrials.gov. Identifier: NCT05168475. Available online: https://clinicaltrials.gov/ct2/show/NCT05168475 (accessed on 13 December 2024).
- Saji, T.; Kemmotsu, Y. Infliximab for Kawasaki syndrome. J. Pediatr. 2006, 149, 426. [Google Scholar] [CrossRef]
- Blaisdell, L.L.; Hayman, J.A.; Moran, A.M. Infliximab treatment for pediatric refractory Kawasaki disease. Pediatr. Cardiol. 2011, 32, 1023–1027. [Google Scholar] [CrossRef]
- Girish, M.; Subramaniam, G. Infliximab treatment in refractory Kawasaki syndrome. Indian J. Pediatr. 2008, 75, 521–522. [Google Scholar] [CrossRef]
- Madan, D.; Maheshwari, A.; Mahto, D.; Mendiratta, V.; Sharma, S. Kawasaki Disease with Peripheral and Facial Gangrene: A Case report and review of literature. Trop. Doct. 2022, 52, 449–452. [Google Scholar] [CrossRef]
- Venkatnarayanan, K.; Arora, H.S.; Gupta, A.; Adhikari, K.M. Infliximab as a rescue therapy in the management of refractory typical infantile Kawasaki disease. Med. J. Armed Forces India 2020, 76, 225–228. [Google Scholar] [CrossRef]
- Jimenez-Fernandez, S.G.; Tremoulet, A.H. Infliximab treatment of pancreatitis complicating acute kawasaki disease. Pediatr. Infect Dis. J. 2012, 31, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Oishi, T.; Fujieda, M.; Shiraishi, T.; Ono, M.; Inoue, K.; Takahashi, A.; Ogura, H.; Wakiguchi, H. Infliximab treatment for refractory Kawasaki disease with coronary artery aneurysm. Circ. J. 2008, 72, 850–852, Erratum in Circ. J. 2008, 72, 1212. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Rikitake, Y.; Tsuruda, T.; Kawata, C.; Rikitake, M.; Iwao, K.; Aizawa, A.; Kariya, Y.; Matsuda, M.; Miyauchi, S.; et al. Infliximab as an alternative therapy for refractory adult onset Kawasaki disease: A case report. Medicine 2018, 97, e12720. [Google Scholar] [CrossRef]
- Accomando, S.; Liotta, A.; Maggio, M.C.; Cardella, F.; Corsello, G. Infliximab administration effective in the treatment of refractory Kawasaki disease. Pediatr. Allergy Immunol. 2010, 21, 1091–1092. [Google Scholar] [CrossRef]
- Zulian, F.; Zanon, G.; Martini, G.; Mescoli, G.; Milanesi, O. Efficacy of infliximab in long-lasting refractory Kawasaki disease. Clin. Exp. Rheumatol. 2006, 24, 453. [Google Scholar]
- Weiss, J.E.; Eberhard, B.A.; Chowdhury, D.; Gottlieb, B.S. Infliximab as a novel therapy for refractory Kawasaki disease. J. Rheumatol. 2004, 31, 808–810. [Google Scholar]
- Agarwal, S.; Mulkutkar, S.; Suri, D.; Singh, S.; Gupta, A. Retinal Vasculitis in Kawasaki Disease. Indian J. Pediatr. 2015, 82, 1183–1184. [Google Scholar] [CrossRef] [PubMed]
- Kikuoka, I.; Yamamoto, K.; Kodo, K.; Maeda, J.; Yamagishi, H. Successful and unsuccessful management for indolent Kawasaki disease. Pediatr. Int. 2022, 64, e14865. [Google Scholar] [CrossRef] [PubMed]
- Salguero, J.S.; Durán, D.G.; Peracaula, C.S.; Iznardi, C.R.; Tardío, J.O. Enfermedad de Kawasaki refractaria con aneurismas coronarios tratada con infliximab [Refractory Kawasaki disease with coronary aneurysms treated with infliximab]. An. Pediatr. 2010, 73, 268–271. [Google Scholar] [CrossRef]
- Shirley, D.A.; Stephens, I. Primary treatment of incomplete Kawasaki disease with infliximab and methylprednisolone in a patient with a contraindication to intravenous immune globulin. Pediatr. Infect Dis. J. 2010, 29, 978–979. [Google Scholar] [CrossRef] [PubMed]
- Servel, A.C.; Vincenti, M.; Darras, J.P.; Lalande, M.; Rodière, M.; Filleron, A. Maladie de Kawasaki résistante aux immunoglobulines et compliquée de syndrome d’activation macrophagique [Intravenous immunoglobulin-resistant Kawasaki disease with hemophagocytosis]. Arch. Pediatr. 2012, 19, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Marzan, K.; De Oliveira, E.; Wagner-Lees, S.; Szmuszkovicz, J. Recurrent Kawasaki Disease: A Case Report of Three Separate Episodes at >4-Year Intervals. Children 2018, 5, 155. [Google Scholar] [CrossRef]
- Hoole, T.J.; Athapathu, A.S.; Abeygunawardene, A.D. A Sri Lankan infant with immunoglobulin resistant incomplete Kawasaki disease with a vesicular psoriasiform rash, hypertension and late onset small joint arthritis: A case report. BMC Pediatr. 2022, 22, 444. [Google Scholar] [CrossRef]
- O’connor, M.J.; Saulsbury, F.T. Incomplete and atypical Kawasaki disease in a young infant: Severe, recalcitrant disease responsive to infliximab. Clin. Pediatr. 2007, 46, 345–348. [Google Scholar] [CrossRef]
- Kanda, S.; Fujii, Y.; Hori, S.I.; Ohmachi, T.; Yoshimura, K.; Higasa, K.; Kaneko, K. Combined Single Nucleotide Variants of ORAI1 and BLK in a Child with Refractory Kawasaki Disease. Children 2021, 8, 433. [Google Scholar] [CrossRef]
- Krakowski, A.C.; Kim, S.S.; Burns, J.C. Transient lingual papillitis associated with confirmed herpes simplex virus 1 in a patient with kawasaki disease. Pediatr. Dermatol. 2014, 31, e124–e125. [Google Scholar] [CrossRef]
- Geller, L.; Kellen, R. Tumor necrosis factor antagonist-induced psoriasis in a 3-year-old boy with Kawasaki disease. Dermatol. Online J. 2017, 23, 22. [Google Scholar] [CrossRef]
- Malekzadeh, I.; Ziaee, V.; Sadrosadat, T.; Moardinejad, M.H.; Sayadpour-Zanjani, K. Kawasaki Disease and Peripheral Gangrene in Infancy. Iran. J. Pediatr. 2015, 25, e3309. [Google Scholar] [CrossRef]
- Amorim-Figueiredo, R.; Pereira Lemos, A.; Rito, T.; Conde, M.; Brito, M.J.; Pinto, F. Multiresistant Kawasaki Disease in a Young Infant with Giant Aneurysms Growing Fast. J. Cardiovasc. Dev. Dis. 2024, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.M.; Milner, D.C.; Frisbie, R.; Puente, M.A. Abducens nerve palsy: A rare copresenting sign of incomplete Kawasaki Disease. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2025, 29, 104061. [Google Scholar] [CrossRef]
- Suganuma, E.; Kambe, T.; Sato, S.; Hamamoto, M.; Kawano, Y. A case of Kawasaki disease complicated with retinal vasculitis. Pediatr. Int. 2019, 61, 829–830. [Google Scholar] [CrossRef]
- Vigil-Vázquez, S.; Butragueño-Laiseca, L.; López-González, J.; García-San Prudencio, M.; Rincón-López, E. A Case of Kawasaki Disease Presenting as Severe Myositis. Indian J. Pediatr. 2019, 86, 1066–1067. [Google Scholar] [CrossRef]
- Navidi, N.; Najibi, B.; Dinarvand, N.; Zamani, A.; Fathi, M.R. Atypical Kawasaki disease with giant coronary artery aneurysms in a 2-month-old boy: A case report. J. Med. Case Rep. 2024, 18, 630. [Google Scholar] [CrossRef]
- Lersch, R.; Mandilaras, G.; Schrader, M.; Anselmino, F.; Haas, N.A.; Jakob, A. Have we got the optimal treatment for refractory Kawasaki disease in very young infants? A case report and literature review. Front. Pediatr. 2023, 11, 1210940. [Google Scholar] [CrossRef]
- Sivakumar, K.; Pavithran, S. Extensive coronary aneurysms with thrombosis in resistant Kawasaki disease. Pediatr. Cardiol. 2013, 34, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Talarico, R.; Italiano, N.; Emmi, G.; Piga, M.; Cantarini, L.; Mattioli, I.; Floris, A.; Gentileschi, S.; Di Cianni, F.; Urban, M.L.; et al. Comparative efficacy and safety of infliximab and adalimumab in severe mucocutaneous Behçet’s disease: Results from a multicenter randomized controlled trial. Ann. Rheum. Dis. 2024. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Liu, T.; Han, W.; Bai, X.; Ruan, G.; Lv, H.; Shu, H.; Li, Y.; Li, J.; et al. The efficacy and safety of anti-tumor necrosis factor agents in the treatment of intestinal Behcet’s disease, a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2022, 37, 608–619. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Li, Y.; Li, Z. Effectiveness and safety of anti-tumor necrosis factor-alpha therapy in Behçet’s disease-associated uveitis: A systematic review and meta-analysis. Front. Pharmacol. 2020, 11, 941. [Google Scholar] [CrossRef]
- Hamza, M.M.; Macky, T.A.; Sidky, M.K.; Ragab, G.; Soliman, M.M. INTRAVITREAL INFLIXIMAB IN REFRACTORY UVEITIS IN BEHCET’S DISEASE: A Safety and Efficacy Clinical Study. Retina 2016, 36, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.H.A.; Woldeamanuel, Y.W. The effectiveness of the anti-tumor necrosis factor therapy infliximab in neuro-Behçet’s disease: A systematic review and meta-analysis. J. Int. Med. Res. 2023, 51, 3000605231169895. [Google Scholar] [CrossRef]
- Batu, E.D.; Sener, S.; Cam, V.; Aktay Ayaz, N.; Ozen, S. Treatment with Biologic Drugs in Pediatric Behçet’s Disease: A Comprehensive Analysis of the Published Data. BioDrugs 2023, 37, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Poddighe, D.; Mukusheva, Z.; Dauyey, K.; Assylbekova, M. Adalimumab in the treatment of pediatric Behçet’s disease: Case-based review. Rheumatol. Int. 2019, 39, 1107–1112. [Google Scholar] [CrossRef]
- Maccora, I.; Orsini, S.I.; Gallizzi, R.; Montin, D.; Cattalini, M.; La Torre, F.; Spagnolo, A.; Diomeda, F.; Simonini, G. Uveitis in paediatric Behçet disease: A large multicentric Italian cohort. Ther. Adv. Musculoskelet. Dis. 2024, 16, 1759720X241275822. [Google Scholar] [CrossRef]
- Vena, G.A.; Cassano, N. Drug focus: Adalimumab in the treatment of moderate to severe psoriasis. Biologics 2007, 1, 93–103. [Google Scholar] [PubMed]
- Wang, C.R.; Yang, C.C. Adalimumab therapy in hepatitis B virus-negative polyarteritis nodosa: A case report. Medicine 2018, 97, e11053. [Google Scholar] [CrossRef] [PubMed]
- Al-Balbeesi, A.O.; Alhallaf, R.A.; Alsubeeh, N.A.; Fathaddin, A.A.; Bedaiwi, A.A.; Omair, M.A. Coexistence of Polyarteritis Nodosa of the Vulva and Retina in a Behçet’s Disease Patient: A Case Report. Cureus 2021, 13, e16096. [Google Scholar] [CrossRef]
- Alharthi, A.; Alhashmi Alamer, G.R.; Alqurashi, S.S.; Alsaeedi, E.E.; Alsulami, H. A Unique Case of Deficiency of Adenosine Deaminase 2 Single in a Young Adult Patient. Cureus 2023, 15, e33273. [Google Scholar] [CrossRef]
- Yuxuan, B.; Yan, D. Adenosine deaminase 2 deficiency in a Chinese patient: Report of one novel mutation and literature review. J. Cosmet. Dermatol. 2024, 23, 68–75. [Google Scholar] [CrossRef]
- Oster, C.; Stolte, B.; Asan, L.; Pul, R.; Klebe, S.; Köhrmann, M.; Breuckmann, K.; Rischpler, C.; Deuschl, C.; Dolff, S.; et al. Brainstem Infarction in Immunodeficiency Identified as Adenosine Deaminase 2 Deficiency: Case Report. J. Clin. Immunol. 2023, 43, 1597–1602. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Safety and Efficacy of Adalimumab Therapy for Treatment of Behcet’s Disease-related Uveitis in Sohag University Hospital. Identifier: NCT05683626. Available online: https://clinicaltrials.gov/study/NCT05683626 (accessed on 25 January 2025).
- ClinicalTrials.gov. Comparison of the Efficacy and Eafety of Adalimumab to That of Tocilizumab in Severe Uveitis of Behçet’s Disease. Identifier: NCT05874505. Updated May 25, 2023. Available online: https://clinicaltrials.gov/study/NCT05874505 (accessed on 25 January 2025).
- Tracey, D.; Klareskog, L.; Sasso, E.H.; Salfeld, J.G.; Tak, P.P. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol. Ther. 2008, 117, 244–279. [Google Scholar] [CrossRef]
- Portman, M. A Randomized, Double-Blind, Placebo-Controlled Study of Etanercept in Children with Kawasaki Disease. ClinicalTrials.gov. NCT00841789. Last updated 6 May 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT00841789 (accessed on 27 January 2025).
- Portman, M.A.; Dahdah, N.S.; Slee, A.; Olson, A.K.; Choueiter, N.F.; Soriano, B.D.; Buddhe, S.; Altman, C.A.; for the EATAK Investigators. Etanercept with IVIg for Acute Kawasaki Disease: A Randomized Controlled Trial. Pediatrics 2019, 143, e20183675. [Google Scholar] [CrossRef]
- Melikoglu, M.; Fresko, I.; Mat, C.; Ozyazgan, Y.; Gogus, F.; Yurdakul, S.; Hamuryudan, V.; Yazici, H. Short-term trial of etanercept in Behçet’s disease: A double blind, placebo controlled study. J. Rheumatol. 2005, 32, 98–105. [Google Scholar]
- Krutzke, S.; Horneff, G. Treatment of Two Boys Suffering from Deficiency of Adenosine Deaminase Type 2 (DADA2) with TNF-Inhibitor Etanercept. J. Clin. Rheumatol. 2021, 27, S509–S512. [Google Scholar] [CrossRef]
- Yu, H.; Lin, S.; Li, L.; Li, J.; Chen, Q.; Wu, Y.; Qi, Y.; Wang, W.; Chang, X.; Zhang, J.; et al. Case Report: Novel ADA2 variants cause atypical adenosine deaminase 2 deficiency. Front. Genet. 2025, 15, 1478581. [Google Scholar] [CrossRef]
- Ferriani, M.P.L.; Valera, E.T.; de Sousa, G.R.; Sandrin-Garcia, P.; de Moura, R.R.; Hershfield, M.S.; de Carvalho, L.M. ADA2 deficiency (DADA2) associated with Evans syndrome and a severe ADA2 genotype. Rheumatology 2021, 60, e237–e239. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Adrovic, A.; Barut, K.; Baran, S.; Tahir Turanli, E.; Canpolat, N.; Kizilkilic, O.; Ozkaya, O.; Kasapcopur, O. A 9.5-year-old boy with recurrent neurological manifestations and severe hypertension, treated initially for polyarteritis nodosa, was subsequently diagnosed with adenosine deaminase type 2 deficiency (DADA2) which responded to anti-TNF-α. Paediatr. Int. Child Health 2020, 40, 65–68. [Google Scholar] [CrossRef]
- Al-Ghoul, M.; Yazbak, J.; Rummanneh, I.; Abuhammad, A.; Khalilia, A.H.; Wahdan, A.A.M. Deficiency of adenosine deaminase 2 (DADA2) presented with bilateral renal subcapsular hematoma: A case report and literature review. Ann. Med. Surg. 2024, 86, 6717–6720. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Shimizu, M.; Mizuta, M.; Ikawa, Y.; Yachie, A. Refractory cutaneous polyarteritis nodosa: Successful treatment with etanercept. Pediatr. Int. 2017, 59, 751–752. [Google Scholar] [CrossRef] [PubMed]
- Capuozzo, M.; Ottaiano, A.; Nava, E.; Cascone, S.; Fico, R.; Iaffaioli, R.V.; Cinque, C. Etanercept induces remission of polyarteritis nodosa: A case report. Front. Pharmacol. 2014, 5, 122. [Google Scholar] [CrossRef]
- Valor, L.; Monteagudo, I.; de la Torre, I.; González Fernández, C.; Montoro, M.; López Longo, J.; Carreño, L. Young male patient diagnosed with cutaneous polyarteritis nodosa successfully treated with etanercept. Mod. Rheumatol. 2014, 24, 688–689. [Google Scholar] [CrossRef]
- Zoshima, T.; Matsumura, M.; Suzuki, Y.; Kakuchi, Y.; Mizushima, I.; Fujii, H.; Yamada, K.; Yamagishi, M.; Kawano, M. A case of refractory cutaneous polyarteritis nodosa in a patient with hepatitis B carrier status successfully treated with tumor necrosis factor alpha blockade. Mod. Rheumatol. 2013, 23, 1029–1033. [Google Scholar] [CrossRef]
- Feinstein, J.; Arroyo, R. Successful treatment of childhood onset refractory polyarteritis nodosa with tumor necrosis factor alpha blockade. J. Clin. Rheumatol. 2005, 11, 219–222. [Google Scholar] [CrossRef]
- Marriaga-Núñez, B.; Arellano-Valdez, A.; Paz, J.P.A.; Bonal-Pérez, M.A.; Montaño-Durón, J.G.; Solórzano-Santos, F. Immunoglobulin-resistant Kawasaki disease. Enfermedad de Kawasaki refractaria a tratamiento con inmunoglobulina. Bol. Méd. Hosp. Infant Méx. 2023, 80, 260–264. [Google Scholar] [CrossRef]
- Walser, M.; Hermann, M.; Hufnagel, M.; Haas, N.A.; Fischer, M.; Dalla-Pozza, R.; Jakob, A. Anakinra and Etanercept Combination Treatment in a Child with Severe, Nonresponsive Kawasaki Disease. Pediatr. Infect Dis. J. 2020, 39, e310–e313. [Google Scholar] [CrossRef]
- Peyre, M.; Laroche, C.; Etchecopar, C.; Brosset, P. Place des immunosuppresseurs dans les formes évoluées de maladie de Kawasaki: À propos de deux cas avec atteinte cardiaque sévère [The role of immunosuppressive agents in Kawasaki disease: A discussion of two cases]. Arch. Pediatr. 2013, 20, 748–753. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, C.J.; Wang, R.P.; Wang, L.; Yang, H. Etanercept in the treatment of intestinal Behcet’s disease. Cell Biochem. Biophys. 2014, 69, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, S.; Greenwald, D. Golimumab. mAbs 2009, 1, 422–431. [Google Scholar] [CrossRef]
- Fabiani, C.; Sota, J.; Rigante, D.; Vitale, A.; Emmi, G.; Vannozzi, L.; Franceschini, R.; Bacherini, D.; Frediani, B.; Galeazzi, M.; et al. Rapid and Sustained Efficacy of Golimumab in the Treatment of Multirefractory Uveitis Associated with Behçet’s Disease. Ocul. Immunol. Inflamm. 2019, 27, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Mesquida, M.; Victoria Hernández, M.; Llorenç, V.; Pelegrín, L.; Espinosa, G.; Dick, A.D.; Adán, A. Behçet Disease-associated Uveitis Successfully Treated with Golimumab. Ocul. Immunol. Inflamm. 2012, 21, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Coma, M.; Salom, D.; Díaz-Llopis, M.; López-Prats, M.J.; Calleja, S. Golimumab as rescue therapy for refractory immune-mediated uveitis: A three-center experience. Mediat. Inflamm. 2014, 2014, 717598. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.; Hasui, K.; Suzuki, C.; Nishijima, H.; Tomiyama, M. Isolated myelitis in a patient with Behcet’s disease during golimumab therapy. J. Neuroimmunol. 2021, 354, 577533. [Google Scholar] [CrossRef]
- Kudsi, M.; Shahada, Z.; Haidar, G.; Safiah, M.H.; Khalayli, N. Golimumab therapy-induced isolated myelitis in a Behcet’s disease patient: A case report. Ann. Med. Surg. 2023, 85, 951–954. [Google Scholar] [CrossRef]
- Vitale, A.; Emmi, G.; Lopalco, G.; Fabiani, C.; Gentileschi, S.; Silvestri, E.; Di Scala, G.; Iannone, F.; Frediani, B.; Galeazzi, M.; et al. Long-term efficacy and safety of golimumab in the treatment of multirefractory Behçet’s disease. Clin. Rheumatol. 2017, 36, 2063–2069. [Google Scholar] [CrossRef]
- Cerny, T.; Borisch, B.; Introna, M.; Johnson, P.; Rose, A.L. Mechanism of action of rituximab. Anti-Cancer Drugs 2002, 13 (Suppl. S2), S3–S10. [Google Scholar] [CrossRef]
- Hadjadj, J.; Canzian, A.; Karadag, O.; Contis, A.; Maurier, F.; Sanges, S.; Sartorelli, S.; Denis, L.; de Moreuil, C.; Durel, C.; et al. Use of biologics to treat relapsing and/or refractory polyarteritis nodosa: Data from a European collaborative study. Rheumatology 2022, 62, 341–346. [Google Scholar] [CrossRef]
- Sauvaget, E.; Bonello, B.; David, M.; Chabrol, B.; Dubus, J.C.; Bosdure, E. Resistant Kawasaki disease treated with anti-CD20. J. Pediatr. 2012, 160, 875–876. [Google Scholar] [CrossRef] [PubMed]
- Orsoni, J.G.; Laganà, B.; Rubino, P.; Zavota, L.; Bacciu, S.; Mora, P. Rituximab ameliorated severe hearing loss in Cogan’s syndrome: A case report. Orphanet. J. Rare Dis. 2010, 5, 18. [Google Scholar] [CrossRef]
- Boukhris, I.; Hamdi, M.S.; Hariz, A.; Kesentini, M.; Azzabi, S.; Cherif, E.; Kechaou, I.; Ben Hassine, L. Efficacy of rituximab in refractory polyarteritis nodosa: A case report. Pan Afr. Med. J. 2023, 45, 92. [Google Scholar] [CrossRef]
- Seri, Y.; Shoda, H.; Hanata, N.; Nagafuchi, Y.; Sumitomo, S.; Fujio, K.; Yamamoto, K. A case of refractory polyarteritis nodosa successfully treated with rituximab. Mod. Rheumatol. 2017, 27, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.; Cressend, T.; Duffau, P.; Grenouillet-Delacre, M.; Rouanet-Larivière, M.; Vital, A.; Longy-Boursier, M.; Mercié, P. Rituximab Efficacy during a Refractory Polyarteritis Nodosa Flare. Case Rep. Med. 2009, 2009, 738293. [Google Scholar] [CrossRef] [PubMed]
- Néel, A.; Masseau, A.; Hervier, B.; Bossard, C.; Cacoub, P.; Pagnoux, C.; Hamidou, M. Life-threatening hepatitis C virus-associated polyarteritis nodosa successfully treated by rituximab. J. Clin. Rheumatol. 2011, 17, 439–441. [Google Scholar] [CrossRef]
- Krishnan, S.; Bhakuni, D.S.; Kartik, S. Rituximab in refractory cutaneous polyarteritis. Int. J. Rheum. Dis. 2012, 15, e127. [Google Scholar] [CrossRef]
- Sonomoto, K.; Miyamura, T.; Watanabe, H.; Takahama, S.; Nakamura, M.; Ando, H.; Minami, R.; Yamamoto, M.; Saito, T.; Imayama, S.; et al. A case of polyarteritis nodosa successfully treated by rituximab. Nihon Rinsho Meneki Gakkai Kaishi 2008, 31, 119–123. [Google Scholar] [CrossRef]
- Ohta, R.; Sano, C. Comprehensive Management of Vasculitis and Suspected Polyarteritis Nodosa in an Older Patient. Cureus 2023, 15, e36307. [Google Scholar] [CrossRef]
- da Silva, L.S.; de Campos, K.V.; de Melo, A.K.; de Brito, D.C.; Braz, A.S.; Freire, E.A. Rituximab as an alternative for patients with severe systemic vasculitis refractory to conventional therapy: Report of seven cases and literature review. Rev. Bras. Reumatol. 2015, 55, 531–535. [Google Scholar] [CrossRef]
- Davatchi, F.; Shams, H.; Rezaipoor, M.; Sadeghi-Abdollahi, B.; Shahram, F.; Nadji, A.; Chams-Davatchi, C.; Akhlaghi, M.; Faezi, T.; Naderi, N. Rituximab in intractable ocular lesions of Behcet’s disease; randomized single-blind control study (pilot study). Int. J. Rheum. Dis. 2010, 13, 246–252. [Google Scholar] [CrossRef]
- Kaegi, C.; Wuest, B.; Schreiner, J.; Steiner, U.C.; Vultaggio, A.; Matucci, A.; Crowley, C.; Boyman, O.; Pan, W.; Li, X.; et al. Efficacy and safety of rituximab in the treatment of autoimmune diseases: A systematic review and meta-analysis. Front. Immunol. 2019, 10, 1990. [Google Scholar] [CrossRef]
- Ling, J.; Xie, F.; Zhou, Q.; Ouyang, Q.; Li, L.; Zhao, W.; Liu, X. Case Series on the Efficacy and Safety of Tocilizumab in IVIG-Resistant Kawasaki Disease: A Retrospective Analysis of Five Patients. J. Inflamm. Res. 2024, 17, 10991–10998. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Kaneko, Y.; Takeuchi, T. Effectiveness of tocilizumab in Behcet’s disease: A systematic literature review. Semin. Arthritis Rheum. 2020, 50, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Liu, T.; Liu, Y.; Zhang, X.; Zhou, Y.; Su, Y. Efficacy and safety of tocilizumab in Behçet’s syndrome with refractory arterial lesions: A single-centre observational cohort study in China. Rheumatology 2022, 61, 2923–2930. [Google Scholar] [CrossRef] [PubMed]
- Khitri, M.Y.; Bartoli, A.; Maalouf, G.; Deroux, A.; Salvarani, C.; Emmi, G.; Karadag, O.; Espinosa, G.; Leclercq, M.; Simonini, G.; et al. Tocilizumab in Behçet Disease: A Multicenter Study of 30 Patients. J. Rheumatol. 2023, 50, 916–923. [Google Scholar] [CrossRef]
- Kougkas, N.; Bertsias, G.; Stratoudaki, R.; Avgoustidis, N. Successful treatment of Cogan’s syndrome with tocilizumab. Scand. J. Rheumatol. 2021, 50, 330–331. [Google Scholar] [CrossRef]
- Higashida-Konishi, M.; Akiyama, M.; Tabata, H.; Hama, S.; Oshige, T.; Izumi, K.; Oshima, H.; Okano, Y. Atypical Cogan’s Syndrome with Large-vessel Vasculitis Successfully Treated with Tocilizumab. Intern. Med. 2023, 62, 3413–3417. [Google Scholar] [CrossRef]
- Hara, K.; Umeda, M.; Segawa, K.; Akagi, M.; Endo, Y.; Koga, T.; Kawashiri, S.; Ichinose, K.; Nakamura, H.; Maeda, T.; et al. Atypical Cogan’s Syndrome Mimicking Giant Cell Arteritis Successfully Treated with Early Administration of Tocilizumab. Intern. Med. 2022, 61, 1265–1270. [Google Scholar] [CrossRef]
- Shibuya, M.; Fujio, K.; Morita, K.; Harada, H.; Kanda, H.; Yamamoto, K. Successful treatment with tocilizumab in a case of Cogan’s syndrome complicated with aortitis. Mod. Rheumatol. 2013, 23, 577–581. [Google Scholar] [CrossRef]
- Jančatová, D.; Zeleník, K.; Komínek, P.; Matoušek, P. Atypical Cogan’s syndrome: A case report and summary of current treatment options. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 428–431. [Google Scholar] [CrossRef]
- Almorza Hidalgo, T.; García González, A.J.; Castañeda, S.; Tomero, E.G.; Pablos Álvarez, J.L. Cogan syndrome: Descriptive analysis and clinical experience of 7 cases diagnosed and treated in two third level hospitals. Reumatol. Clin. 2021, 17, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, J.; Wu, D.; Wu, T.; Zhu, J. Tocilizumab Successfully Treating Refractory Hearing Impairment in a Patient with Cogan Syndrome: A Case Report and Review of the Literature. Ear Nose Throat J. 2024, 01455613241237079. [Google Scholar] [CrossRef] [PubMed]
- Okubo, N.; Oba, Y.; Ikuma, D.; Mizuno, H.; Yamanouchi, M.; Suwabe, T.; Ubara, Y.; Sawa, N. A case of polyarteritis nodosa with severe lower limb ulcer that was treated with prednisolone and tocilizumab. Mod. Rheumatol. Case Rep. 2025, 9, rxae085. [Google Scholar] [CrossRef]
- Ostrovršnik, J.; Hočevar, A.; Lestan, B.; Sodin Šemrl, S.; Lakota, K.; Tomšič, M. Long-term follow-up on tocilizumab treatment of AA amyloidosis secondary to polyarteritis nodosa. Amyloid 2016, 23, 260–261. [Google Scholar] [CrossRef]
- Hočevar, A.; Lestan, B.; Šemrl, S.S.; Lakota, K.; Kojc, N.; Potočnik, N.; Tomšič, M. AA amyloidosis in a polyarteritis nodosa patient treated with tocilizumab. Amyloid 2013, 20, 275–276. [Google Scholar] [CrossRef]
- Inoue, N.; Shimizu, M.; Mizuta, M.; Yachie, A. Successful treatment of tumor necrosis factor inhibitor-resistant cutaneous polyarteritis nodosa with tocilizumab. Pediatr. Int. 2020, 62, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Rajderkar, D.A.; Modica, R.F. A Case of Polyarteritis Nodosa Associated with Vertebral Artery Vasculitis Treated Successfully with Tocilizumab and Cyclophosphamide. Case Rep. Pediatr. 2016, 2016, 7987081. [Google Scholar] [CrossRef]
- Carrión-Barberà, I.; Pros, A.; Salman-Monte, T.C.; Vílchez-Oya, F.; Sánchez-Schmidt, J.M.; Pérez-García, C.; Monfort, J. Safe and successful treatment of refractory polyarteritis nodosa with tocilizumab in a patient with past hepatitis B virus infection: A case-based review. Clin. Rheumatol. 2021, 40, 2065–2070. [Google Scholar] [CrossRef]
- Boistault, M.; Lopez Corbeto, M.; Quartier, P.; Berbel Arcobé, L.; Carsi Durall, A.; Aeschlimann, F.A. A young girl with severe polyarteritis nodosa successfully treated with tocilizumab: A case report. Pediatr. Rheumatol. Online J. 2021, 19, 168. [Google Scholar] [CrossRef]
- Krusche, M.; Ruffer, N.; Kötter, I. Tocilizumab treatment in refractory polyarteritis nodosa: A case report and review of the literature. Rheumatol. Int. 2019, 39, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Bodoki, L.; Végh, E.; Szekanecz, Z.; Szűcs, G. Tocilizumab Treatment in Polyarteritis Nodosa. Isr. Med. Assoc. J. 2019, 21, 560–562. [Google Scholar]
- Krusche, M.; Ruffer, N.; Schneider, U.; Meyer, M.; Burmester, G.; Kötter, I. Tocilizumab treatment for polyarteritis nodosa. Rheumatology 2020, 59, e63–e65. [Google Scholar] [CrossRef] [PubMed]
- Peking Union Medical College Hospital. Tocilizumab and Tofacitinib in the Treatment of Vascular Behçet’s Syndrome. ClinicalTrials.gov Identifier: NCT05845723. Updated 19 August 2024. Available online: https://clinicaltrials.gov/show/NCT05845723 (accessed on 25 January 2025).
- Benson, J.M.; Peritt, D.; Scallon, B.J.; Heavner, G.A.; Shealy, D.J.; Giles-Komar, J.M.; Mascelli, M.A. Discovery and mechanism of ustekinumab: A human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. mAbs 2011, 3, 535–545. [Google Scholar] [CrossRef] [PubMed]
- London, J.; Régent, A.; Dion, J.; Jilet, L.; Jachiet, M.; Lidove, O.; Cohen-Aubart, F.; Aractingi, S.; Guégan, S.; Pennaforte, J.-L.; et al. Efficacy and safety of ustekinumab in Behçet disease: Results from the prospective phase 2 STELABEC trial. J. Am. Acad. Dermatol. 2022, 87, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, G.; Fabiani, C.; Venerito, V.; Lapadula, G.; Iannone, F.; Cantarini, L. Ustekinumab efficacy and safety in mucocutaneous multi-refractory Behçet’s disease. Clin. Exp. Rheumatol. 2017, 35 (Suppl. S108), 130–131. [Google Scholar]
- Baerveldt, E.M.; Kappen, J.H.; Thio, H.B.; van Laar, J.A.; van Hagen, P.M.; Prens, E.P. Successful long-term triple disease control by ustekinumab in a patient with Behcet’s disease, psoriasis and hidradenitis suppurativa. Ann. Rheum. Dis. 2013, 72, 626–627. [Google Scholar] [CrossRef]
- Dick, A.D.; Tugal-Tutkun, I.; Foster, S.; Zierhut, M.; Liew, S.H.M.; Bezlyak, V.; Androudi, S. Secukinumab in the Treatment of Noninfectious Uveitis: Results of Three Randomized, Controlled Clinical Trials. Ophthalmology 2013, 120, 777–787. [Google Scholar] [CrossRef]
- Patel, D.D.; Lee, D.M.; Kolbinger, F.; Antoni, C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann. Rheum. Dis. 2013, 72 (Suppl. S2), ii116–ii123. [Google Scholar] [CrossRef]
- Letko, E.; Yeh, S.; Foster, C.S.; Pleyer, U.; Brigell, M.; Grosskreutz, C.L. Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology 2015, 122, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Fagni, F.; Bettiol, A.; Talarico, R.; Lopalco, G.; Silvestri, E.; Urban, M.L.; Russo, P.A.J.; Di Scala, G.; Emmi, G.; Prisco, D. Long-term effectiveness and safety of secukinumab for treatment of refractory mucosal and articular Behçet’s phenotype: A multicentre study. Ann. Rheum. Dis. 2020, 79, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Insalaco, A.; Sfriso, P.; Lopalco, G.; Emmi, G.; Cattalini, M.; Manna, R.; Cimaz, R.; Priori, R.; Talarico, R.; et al. A snapshot on the on-label and off-label use of the interleukin-1 inhibitors in Italy among rheumatologists and pediatric rheumatologists: A nationwide multi-center retrospective observational study. Front. Pharmacol. 2016, 7, 380. [Google Scholar] [CrossRef]
- Cavalli, G.; Dinarello, C.A. Anakinra Therapy for Non-cancer Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1157, Erratum in Front. Pharmacol. 2019, 10, 148. [Google Scholar] [CrossRef]
- Yang, J.; Jain, S.; Capparelli, E.V.; Best, B.M.; Son, M.B.; Baker, A.; Newburger, J.W.; Franco, A.; Printz, B.F.; He, F.; et al. Anakinra Treatment in Patients with Acute Kawasaki Disease with Coronary Artery Aneurysms: A Phase I/IIa Trial. J. Pediatr. 2022, 243, 173–180. [Google Scholar] [CrossRef]
- Anakinra for Behcet’s Disease. ClinicalTrials.gov. Identifier: NCT01441076. Updated 23 October 2024. Available online: https://clinicaltrials.gov/study/NCT01441076 (accessed on 28 January 2025).
- Broderick, C.; Kobayashi, S.; Suto, M.; Ito, S.; Kobayashi, T. Intravenous immunoglobulin for the treatment of Kawasaki disease. Cochrane Database Syst. Rev. 2023, 1, CD014884. [Google Scholar] [CrossRef]
- Cantarini, L.; Vitale, A.; Scalini, P.; Dinarello, C.A.; Rigante, D.; Franceschini, R.; Simonini, G.; Borsari, G.; Caso, F.; Lucherini, O.M.; et al. Anakinra treatment in drug-resistant Behcet’s disease: A case series. Clin. Rheumatol. 2015, 34, 1293–1301. [Google Scholar] [CrossRef]
- Blonz, G.; Lacroix, S.; Benbrik, N.; Warin-Fresse, K.; Masseau, A.; Trewick, D.; Hamidou, M.; Stephan, J.; Néel, A. Severe Late-Onset Kawasaki Disease Successfully Treated with Anakinra. J. Clin. Rheumatol. 2020, 26, e42–e43. [Google Scholar] [CrossRef]
- Gambacorta, A.; Buonsenso, D.; De Rosa, G.; Lazzareschi, I.; Gatto, A.; Brancato, F.; Pata, D.; Valentini, P. Resolution of Giant Coronary Aneurisms in a Child with Refractory Kawasaki Disease Treated with Anakinra. Front. Pediatr. 2020, 8, 195. [Google Scholar] [CrossRef]
- Bossi, G.; Codazzi, A.C.; Vinci, F.; Clerici, E.; Regalbuto, C.; Crapanzano, C.; Veraldi, D.; Moiraghi, A.; Marseglia, G.L. Efficacy of Anakinra on Multiple Coronary Arteries Aneurysms in an Infant with Recurrent Kawasaki Disease, Complicated by Macrophage Activation Syndrome. Children 2022, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Manubens, J.; Gelman, A.; Franch, N.; Teodoro, S.; Palacios, J.R.; Rudi, N.; Rivera, J.; Antón, J. A child with resistant Kawasaki disease successfully treated with anakinra: A case report. BMC Pediatr. 2017, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Shafferman, A.; Birmingham, J.D.; Cron, R.Q. High dose Anakinra for treatment of severe neonatal Kawasaki disease: A case report. Pediatr. Rheumatol. 2014, 12, 26. [Google Scholar] [CrossRef]
- Maggio, M.C.; Cimaz, R.; Alaimo, A.; Comparato, C.; Di Lisi, D.; Corsello, G. Kawasaki disease triggered by parvovirus infection: An atypical case report of two siblings. J. Med. Case Rep. 2019, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Mastrolia, M.V.; Abbati, G.; Signorino, C.; Pagnini, I.; Simonini, G.; De Benedetti, F.; Bracaglia, C. Early anti IL-1 treatment replaces steroids in refractory Kawasaki disease: Clinical experience from two case reports. Ther. Adv. Musculoskelet Dis. 2021, 13, 1759720X211002593. [Google Scholar] [CrossRef]
- Barbara, Z.; Ellenwood, S.P.; Loe, E.D.; Choi, J.; Ryan, K.; Joseph, N. Kawasaki Disease Complicated by Salmonella oranienburg Coinfection. Case Rep. Pediatr. 2021, 2021, 5584514. [Google Scholar] [CrossRef]
- Ono, R.; Tominaga, T.; Nonaka, T.; Takamura, Y.; Oishi, K.; Shiraishi, T.; Hashimoto, S.; Noda, K.; Sawai, T.; Okano, S.; et al. Intestinal Behçet’s and suspected intestinal Behçet’s disease: A report of four surgical cases. Surg. Case Rep. 2024, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Tremoulet, A.H. Pilot Study of Atorvastatin and Anakinra in Children with Coronary artery Abnormalities Secondary to Kawasaki Disease. ClinicalTrials.gov Identifier NCT04747847. Updated 18 January 2024. Available online: https://clinicaltrials.gov/study/NCT04747847 (accessed on 25 January 2025).
- Assistance Publique—Hôpitaux de Paris. A Trial Comparing the Efficacy and Safety of Anakinra Versus Intravenous Immunoglobulin (IVIG) Retreatment, in Patients with Kawasaki Disease Who Failed to Respond to Initial Standard IVIG Treatment (ANACOMP). ClinicalTrials.gov identifier NCT04656184. Ongoing. Updated 28 February 2024. Available online: https://clinicaltrials.gov/study/NCT04656184 (accessed on 25 January 2025).
- Simmoni, G. Meyer Children’s Hospital IRCCS. Non Inferiority Kawasaki Trial with Anakinra (NIKITA). ClinicalTrials.gov Identifier: NCT06697431. Available online: https://clinicaltrials.gov/study/NCT06697431 (accessed on 25 January 2025).
- Dubois, E.A.; Rissmann, R.; Cohen, A.F. Rilonacept and canakinumab. Br. J. Clin. Pharmacol. 2011, 71, 639–641. [Google Scholar] [CrossRef]
- Burns, J.C.; Koné-Paut, I.; Kuijpers, T.; Shimizu, C.; Tremoulet, A.; Arditi, M. Found in Translation: International initiatives pursuing interleukin-1 blockade for treatment of acute Kawasaki Disease. Arthritis Rheumatol. 2017, 69, 268–276. [Google Scholar] [CrossRef]
- Kisla Ekinci, R.M.; Balci, S.; Bisgin, A.; Hershfield, M.; Atmis, B.; Dogruel, D.; Yilmaz, M. Renal Amyloidosis in Deficiency of Adenosine Deaminase 2: Successful Experience with Canakinumab. Pediatrics 2018, 142, e20180948. [Google Scholar] [CrossRef]
- Emmi, G.; Silvestri, E.; Ciucciarelli, L.; Squatrito, D.; Emmi, L. Reply: Anti-IL1 blocking agents in drug-resistant Behçet’s syndrome: Our little case series. Clin. Exp. Rheumatol. 2014, 32, S172. [Google Scholar]
- Hashemzadeh, M.; Furukawa, M.; Goldsberry, S.; Movahed, M.R. Chemical structures and mode of action of intravenous glycoprotein IIb/IIIa receptor blockers: A review. Exp. Clin. Cardiol. 2008, 13, 192–197. [Google Scholar]
- Chandwaney, R.H.; Stathopoulos, T.; Sunew, J.; McPherson, D.; Davidson, C.J. Adjunctive therapies in the cath lab. Successful thrombolysis using the combination of tissue plasminogen activator and abciximab in an adult with Kawasaki’s disease. J. Invasive Cardiol. 2001, 13, 651–653. [Google Scholar]
- Johnson, P.N.; Kuhn, R.J. Combination thrombolytic and anti-platelet therapies in an infant with incomplete kawasaki disease and coronary aneurysms. J. Pediatr. Pharmacol. Ther. 2008, 13, 242–250. [Google Scholar] [CrossRef]
- Etheridge, S.P.; Tani, L.Y.; Minich, L.L.; Revenaugh, J.R. Platelet glycoprotein IIb/IIIa receptor blockade therapy for large coronary aneurysms and thrombi in Kawasaki disease. Catheter. Cardiovasc. Diagn. 1998, 45, 264–268. [Google Scholar] [CrossRef]
- Jone, P.N.; Tapia, D.; Davidson, J.; Fagan, T.E.; Browne, L.; Ing, R.J.; Kay, J. Successful Treatment of Myocardial Infarction in an Infant with Kawasaki Disease. Semin. Cardiothorac. Vasc. Anesth. 2015, 19, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Durall, A.L.; Phillips, J.R.; Weisse, M.E.; Mullett, C.J. Infantile Kawasaki disease and peripheral gangrene. J. Pediatr. 2006, 149, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Mok, G.C.; Sung, R.Y.; Yam, M.C.; Arifi, A.A.; Lam, W.W.; Fok, T.F. A child with Kawasaki disease who survived after rupture of a coronary artery aneurysm. Eur. J. Pediatr. 2003, 162, 634–636. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Liu, X.; Luo, J.; Gao, C.; Li, X. Low-dose IL-2 effectively restored decreased regulatory T cells in patients with Behçet’s disease. Clin. Exp. Rheumatol. 2021, 39, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, Y.; Chen, X.; Zhuang, Z.; Li, X.; Shen, E. Low-dose IL-2 therapy in autoimmune diseases: An update review. Int. Rev. Immunol. 2024, 43, 113–137. [Google Scholar] [CrossRef]
- Low-dose IL-2 Treatment on Behcet’s Disease. ClinicalTrials.gov. Identifier: NCT04065672. Updated 28 January 2025. Available online: https://clinicaltrials.gov/study/NCT04065672 (accessed on 28 January 2025).
- Liu, T.; Zhou, W.; Zhu, Y.; Xia, W.; Chen, J.; Xiao, X.; Huang, B.; Feng, R.; Yao, H.; Chen, S.; et al. Efficacy and Safety of Low-Dose Interleukin-2 for Behcet’s Syndrome: A Phase 2 Randomized, Double-Blind, Placebo-Controlled Clinical Trial [Preprint]. Nature Portfolio. 2024. [Google Scholar] [CrossRef]
- Lorenzon, R.; Ribet, C.; Pitoiset, F.; Aractingi, S.; Banneville, B.; Beaugerie, L.; Berenbaum, F.; Cacoub, P.; Champey, J.; Chazouilleres, O.; et al. The universal effects of low-dose interleukin-2 across 13 autoimmune diseases in a basket clinical trial. J. Autoimmun. 2024, 144, 103172. [Google Scholar] [CrossRef]
- Bluestone, J.A.; St Clair, E.W.; Turka, L.A. CTLA4Ig: Bridging the basic immunology with clinical application. Immunity 2006, 24, 233–238. [Google Scholar] [CrossRef]
- Maciel, M.L.; Novello, M.; Neves, F.S. Short-term efficacy of abatacept in the treatment of refractory ocular and cutaneous Behçet’s disease. Rheumatol. Adv. Pract. 2017, 1, rkx004. [Google Scholar] [CrossRef]
- NCT01693640. A Pilot Study of the Safety and Efficacy of Abatacept Injections in the Treatment of Behcet’s Syndrome. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01693640 (accessed on 19 February 2025).
- Ghoreschi, K.; Jesson, M.I.; Li, X.; Lee, J.L.; Ghosh, S.; Alsup, J.W.; Warner, J.D.; Tanaka, M.; Steward-Tharp, S.M.; Gadina, M.; et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J. Immunol. 2011, 186, 4234–4243. [Google Scholar] [CrossRef]
- Roy, D.; Sathyanarayana, V.A.; Nagaraju, B.; Rao, V.K.R. Tofacitinib as monotherapy in cutaneous polyarteritis nodosa: A case series. Rheumatol. Adv. Pract. 2023, 7, rkad049. [Google Scholar] [CrossRef]
- Zou, J.; Cai, J.F.; Ye, J.F.; Guan, J.L. Tofacitinib as an alternative therapy for refractory intestinal Behçet’s syndrome. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221124014. [Google Scholar] [CrossRef]
- Liu, J.; Hou, Y.; Sun, L.; Li, C.; Li, L.; Zhao, Y.; Zeng, X.; Zhang, F.; Zheng, W. A pilot study of tofacitinib for refractory Behçet’s syndrome. Ann. Rheum. Dis. 2020, 79, 1517–1520. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, S.; Shao, C.; Liu, Y. Cogan’s syndrome is more than just keratitis: A case-based literature review. BMC Ophthalmol. 2023, 23, 212. [Google Scholar] [CrossRef]
- Rimar, D.; Alpert, A.; Starosvetsky, E.; Rosner, I.; Slobodin, G.; Rozenbaum, M.; Kaly, L.; Boulman, N.; Awisat, A.; Ginsberg, S.; et al. Tofacitinib for polyarteritis nodosa: A tailored therapy. Ann. Rheum. Dis. 2016, 75, 2214–2216. [Google Scholar] [CrossRef]
- Lin, M.; Tang, J.; Huang, Z.; Gao, X.; Chao, K. Gastrointestinal: Refractory parastomal ulcers of Behcet’s disease responsive to tofacitinib. J. Gastroenterol. Hepatol. 2023, 38, 485. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Tang, Y.; Wang, S.; Cui, L.; Sun, X.; Wang, Z.; Liu, Y. Case report: Refractory intestinal Behçet’s syndrome successfully treated with tofacitinib: A report of four cases. Front. Immunol. 2022, 13, 981502. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.R.; Wong, T.W.; Hsu, S.M. Extended-release tofacitinib for refractory Behçet disease: A case report. Medicine 2022, 101, e29189. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Nakayamada, S.; Sakata, K.; Kitanaga, Y.; Ma, X.; Lee, S.; Ishii, A.; Yamagata, K.; Nakano, K.; Tanaka, Y. Janus Kinase Inhibitor Baricitinib Modulates Human Innate and Adaptive Immune System. Front. Immunol. 2018, 9, 1510. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Z.; Yao, Z.; Guo, Y. Successful treatment of cutaneous polyarteritis nodosa with baricitinib. J. Dermatol. Treat. 2024, 35, 2417965. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Liu, W.; Wang, Y.; Liu, J.; Zhang, L.; Zhang, S.; Tian, X.; Zhao, Y.; Zheng, W.; et al. Baricitinib for the treatment of refractory vascular Behçet’s disease. Clin. Immunol. 2023, 250, 109298. [Google Scholar] [CrossRef]
- Liu, J.; Yu, X.; Wang, Z.; Liu, W.; Liu, X.; Wang, X.; Zhang, M.; Zhao, Y.; Zhang, F.; Yang, H.; et al. Baricitinib for the treatment of intestinal Behçet’s disease: A pilot study. Clin. Immunol. 2023, 247, 109241. [Google Scholar] [CrossRef]
- Ramanan, A.V.; Guly, C.M.; Keller, S.Y.; Schlichting, D.E.; de Bono, S.; Liao, R.; Quartier, P. Clinical effectiveness and safety of baricitinib for the treatment of juvenile idiopathic arthritis-associated uveitis or chronic anterior antinuclear antibody-positive uveitis: Study protocol for an open-label, adalimumab active-controlled phase 3 clinical trial (JUVE-BRIGHT). Trials 2021, 22, 689. [Google Scholar] [CrossRef]
- Ito, H.; Noda, K.; Saruta, M.; Kurosaka, D. Case report: Peristomal pyoderma gangrenosum complicated by rheumatoid arthritis and Behçet’s disease successfully treated with baricitinib. Int. J. Rheum. Dis. 2024, 27, e15275. [Google Scholar] [CrossRef]
- Namour, F.; Anderson, K.; Nelson, C.; Tasset, C. Filgotinib: A Clinical Pharmacology Review. Clin. Pharmacokinet. 2022, 61, 819–832. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Watkins, T.R.; Nguyen, Q.D.; Sharma, S.; Scales, D.K.; Dacey, M.S.; Shah, R.E.; Chu, D.S.; Grewal, D.S.; Faia, L.J.; et al. Filgotinib in Active Noninfectious Uveitis: The HUMBOLDT Randomized Clinical Trial. JAMA Ophthalmol. 2024, 142, 789–797. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Bhatnagar, S.; Parmentier, J.M.; Nakasato, P.; Wung, P. Upadacitinib: Mechanism of action, clinical, and translational science. Clin. Transl. Sci. 2024, 17, e13688. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, A.; Iguchi, E.; Kim, D.S.; Siripongvutikorn, Y.; Nishigaichi, A.; Yoshimura, M.; Takamatsu, H.; Ohshima, S. Efficacy of Upadacitinib in refractory Polyarteritis Nodosa: A case report. Oxf. Med. Case Rep. 2025, 2025, omae199. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Li, J.; Liu, T.; Li, Y. Upadacitinib for Refractory Behçet’s Syndrome: A Multi-Center, Prospective, Open-label, Pilot Study. Arthritis Rheumatol. 2024, 76 (Suppl. S9), 5121–5122. Available online: https://acrabstracts.org/abstract/upadacitinib-for-refractory-behcets-syndrome-a-multi-center-prospective-open-label-pilot-study/ (accessed on 25 January 2025).
- Vitale, A.; Palacios-Olid, J.; Caggiano, V.; Ragab, G.; Hernández-Rodríguez, J.; Pelegrín, L.; Mejía-Salgado, G.; Zarate-Pinzón, L.; Gentileschi, S.; Sota, J.; et al. Efficacy and safety of Janus kinase inhibitors in non-infectious inflammatory ocular diseases: A prospective cohort study from the international AIDA network registries. Front. Med. 2024, 11, 1439338. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; He, D.; Peng, X.; Huang, Z.; Su, W. Successful Remission with Upadacitinib in Two Patients with Anti-TNF-Refractory Macular Edema Associated with Behçet’s Uveitis. Ocul. Immunol. Inflamm. 2024, 32, 1897–1900. [Google Scholar] [CrossRef]
- Kraev, K.; Uchikov, P.; Hristov, B.; Kraeva, M.; Basheva-Kraeva, Y.; Popova-Belova, S.; Sandeva, M.; Chakarov, D.; Dragusheva, S.; Geneva-Popova, M. Coexistence of ankylosing spondylitis and Behçet’s disease: Successful treatment with upadacitinib. Immun. Inflamm. Dis. 2024, 12, e1242. [Google Scholar] [CrossRef]
- Cui, F.; Shen, L.; Li, L.; Wang, H.; Wang, F.; Bi, S.; Liu, J.; Zhang, G.; Wang, F.; Zheng, H.; et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerg. Infect. Dis. 2017, 23, 765–772. [Google Scholar] [CrossRef]
- Pagnoux, C.; Mahr, A.; Cohen, P.; Guillevin, L. Presentation and outcome of gastrointestinal involvement in systemic necrotizing vasculitides: Analysis of 62 patients with polyarteritis nodosa, microscopic polyangiitis, Wegener granulomatosis, Churg-Strauss syndrome, or rheumatoid arthritis-associated vasculitis. Medicine 2005, 84, 115–128. [Google Scholar]
- Criado, P.R.; Marques, G.F.; Morita, T.C.; de Carvalho, J.F. Epidemiological, clinical and laboratory profiles of cutaneous polyarteritis nodosa patients: Report of 22 cases and literature review. Autoimmun. Rev. 2016, 15, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.; Roberts, F.; MacDuff, E. (Eds.) Chapter 7—Cardiovascular Diseases. In Pathology Illustrated, 7th ed.; Churchill Livingstone: Amsterdam, The Netherlands, 2011; pp. 157–244. [Google Scholar] [CrossRef]
- Grau, G.E.; Roux-Lombard, P.; Gysler, C.; Lambert, C.; Lambert, P.H.; Dayer, J.M.; Guillevin, L. Serum cytokine changes in systemic vasculitis. Immunology 1989, 68, 196–198. [Google Scholar] [PubMed]
- Kawakami, T.; Takeuchi, S.; Soma, Y. Serum levels of interleukin-6 in patients with cutaneous polyarteritis nodosa. Acta Derm Venereol. 2012, 92, 322–323. [Google Scholar] [CrossRef]
- Guillevin, L.; Mahr, A.; Callard, P.; Godmer, P.; Pagnoux, C.; Leray, E.; Cohen, P. Hepatitis B virus-associated polyarteritis nodosa: Clinical characteristics, outcome, and impact of treatment in 115 patients. Medicine 2005, 84, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Bursi, R.; Cafaro, G.; Perricone, C.; Riccucci, I.; Calvacchi, S.; Gerli, R.; Bartoloni, E. Contribution of Janus-Kinase/Signal Transduction Activator of Transcription Pathway in the Pathogenesis of Vasculitis: A Possible Treatment Target in the Upcoming Future. Front. Pharmacol. 2021, 12, 635663. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 17, 78. [Google Scholar] [CrossRef]
- Reddy, V.; Cohen, S. JAK Inhibitors: What Is New? Curr. Rheumatol. Rep. 2020, 22, 50. [Google Scholar] [CrossRef]
- Kostik, M.M.; Raupov, R.K.; Suspitsin, E.N.; Isupova, E.A.; Gaidar, E.V.; Gabrusskaya, T.V.; Kaneva, M.A.; Snegireva, L.S.; Likhacheva, T.S.; Miulkidzhan, R.S.; et al. The Safety and Efficacy of Tofacitinib in 24 Cases of Pediatric Rheumatic Diseases: Single Centre Experience. Front. Pediatr. 2022, 10, 820586. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Arditi, M. Kawasaki disease: Pathophysiology and insights from mouse models. Nat. Rev. Rheumatol. 2020, 16, 391–405. [Google Scholar] [CrossRef]
- Sagiv, E.; Slee, A.; Buffone, A.; Choueiter, N.F.; Dahdah, N.S.; Portman, M.A. Etanercept with IVIg for acute Kawasaki disease: A long-term follow-up on the EATAK trial. Cardiol. Young 2023, 33, 613–618. [Google Scholar] [CrossRef]
- Nomura, O.; Fukuda, S.; Ota, E.; Ono, H.; Ishiguro, A.; Kobayashi, T. Monoclonal antibody and anti-cytokine biologics for Kawasaki disease: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2021, 51, 1045–1056. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Fukazawa, R.; Nagi-Miura, N.; Ohno, N.; Suzuki, N.; Katsube, Y.; Kamisago, M.; Akao, M.; Watanabe, M.; Hashimoto, K.; et al. Interleukin-1beta Inhibition Attenuates Vasculitis in a Mouse Model of Kawasaki Disease. J. Nippon. Med. Sch. 2019, 86, 108–116. [Google Scholar] [CrossRef]
- Touzot, M.; Cacoub, P.; Bodaghi, B.; Soumelis, V.; Saadoun, D. IFN-a induces IL-10 production and tilt the balance between Th1 and Th17 in Behçet disease. Autoimmun. Rev. 2015, 14, 370e375. [Google Scholar] [CrossRef]
- Deniz, R.; Tulunay-Virlan, A.; Ture Ozdemir, F.; Unal, A.U.; Ozen, G.; Alibaz-Oner, F.; Aydin-Tatli, I.; Mumcu, G.; Ergun, T.; Direskeneli, H. Th17-Inducing Conditions Lead to in vitro Activation of Both Th17 and Th1 Responses in Behcet’s Disease. Immunol. Investig. 2017, 46, 518–525. [Google Scholar] [CrossRef]
- Evereklioglu, C.; Er, H.; Türköz, Y.; Cekmen, M. Serum levels of TNF-alpha, sIL-2R, IL-6, and IL-8 are increased and associated with elevated lipid peroxidation in patients with Behçet’s disease. Mediat. Inflamm. 2002, 11, 87–93. [Google Scholar] [CrossRef]
- Turan, B.; Gallati, H.; Erdi, H.; Gurler, A.; Michel, B.A.; Villiger, P.M. Systemic levels of the T cell regulatory cytokines IL-10 and IL-12 in Behçet’s disease; soluble TNFR-75 as a biological marker of disease activity. J. Rheumatol. 1997, 24, 128–132. [Google Scholar] [PubMed]
- Hamzaoui, K.; Houman, H.; Ben Dhifallah, I.; Kamoun, M.; Hamzaoui, A. Serum BAFF levels and skin mRNA expression in patients with Behçet’s disease. Clin. Exp. Rheumatol. 2008, 26 (Suppl. S50), S64–S71. [Google Scholar] [PubMed]
- Le Joncour, A.; Régnier, P.; Maciejewski-Duval, A.; Charles, E.; Barete, S.; Fouret, P.; Rosenzwajg, M.; Klatzmann, D.; Cacoub, P.; Saadoun, D. Reduction of Neutrophil Activation by Phosphodiesterase 4 Blockade in Behçet’s Disease. Arthritis Rheumatol. 2023, 75, 1628–1637. [Google Scholar] [CrossRef]
- Hatemi, G.; Mahr, A.; Ishigatsubo, Y.; Song, Y.-W.; Takeno, M.; Kim, D.; Melikoğlu, M.; Cheng, S.; McCue, S.; Paris, M.; et al. Apremilast for oral ulcers associated with Behçet’s syndrome: A phase 3 randomized, placebo-controlled study. N. Engl. J. Med. 2019, 381, 1918–1928. [Google Scholar] [CrossRef]
- Lavalle, S.; Caruso, S.; Foti, R.; Gagliano, C.; Cocuzza, S.; La Via, L.; Parisi, F.M.; Calvo-Henriquez, C.; Maniaci, A. Behçet’s Disease, Pathogenesis, Clinical Features, and Treatment Approaches: A Comprehensive Review. Medicina 2024, 60, 562. [Google Scholar] [CrossRef] [PubMed]
- Hatemi, G.; Mahr, A.; Takeno, M.; Kim, D.Y.; Saadoun, D.; Direskeneli, H.; Melikoğlu, M.; Cheng, S.; McCue, S.; Paris, M.; et al. Apremilast for oral ulcers associated with active Behçet’s syndrome over 68 weeks: Long-term results from a phase 3 randomised clinical trial. Clin. Exp. Rheumatol. 2021, 39 (Suppl. S132), 80–87. [Google Scholar] [CrossRef] [PubMed]
- Hamuryudan, V.; Mat, C.; Saip, S.; Ozyazgan, Y.; Siva, A.; Yurdakul, S.; Zwingenberger, K.; Yazici, H. Thalidomide in the treatment of the mucocutaneous lesions of the Behçet syndrome. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 1998, 128, 443–450. [Google Scholar] [CrossRef]
- Alpsoy, E.; Durusoy, C.; Yilmaz, E.; Ozgurel, Y.; Ermis, O.; Yazar, S.; Basaran, E. Interferon alfa-2a in the treatment of Behçet disease: A randomized placebo-controlled and double-blind study. Arch. Dermatol. 2002, 138, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Hatemi, G.; Christensen, R.; Bang, D.; Bodaghi, B.; Celik, A.F.; Fortune, F.; Gaudric, J.; Gul, A.; Kötter, I.; Leccese, P.; et al. 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann. Rheum. Dis. 2018, 77, 808–818. [Google Scholar] [CrossRef]
- Emmi, G.; Bettiol, A.; Hatemi, G.; Prisco, D. Behçet’s syndrome. Lancet 2024, 403, 1093–1108. [Google Scholar] [CrossRef]
- Desbois, A.C.; Biard, L.; Addimanda, O.; Lambert, M.; Hachulla, E.; Launay, D.; Ackermann, F.; Pérard, L.; Hot, A.; Maurier, F.; et al. Efficacy of anti-TNF alpha in severe and refractory major vessel involvement of Behcet’s disease: A multicenter observational study of 18 patients. Clin. Immunol. 2018, 197, 54–59. [Google Scholar] [CrossRef]
- Grayson, P.C.; Yazici, Y.; Merideth, M.; Sen, H.N.; Davis, M.; Novakovich, E.; Joyal, E.; Goldbach-Mansky, R.; Sibley, C.H. Treatment of mucocutaneous manifestations in Behçet’s disease with anakinra: A pilot open-label study. Arthritis Res Ther. 2017, 19, 69. [Google Scholar] [CrossRef]
- Murphy, R.; Moots, R.J.; Brogan, P.; Çelik, A.F.; Clement-Jones, M.; Coulson, I.; Croft, A.P.; Crozier, S.; Forrest, L.; Harrold, J.; et al. British Association of Dermatologists and British Society for Rheumatology living guideline for managing people with Behçets 2024. Br. J. Dermatol. 2024, 191, e8–e25. [Google Scholar] [CrossRef]
- Hatemi, G.; Tukek, N.B.; Esatoglu, S.N.; Ozguler, Y.; Taflan, S.S.; Uygunoglu, U.; Melikoglu, M.; Ugurlu, S.; Fresko, I.; Siva, A.; et al. Infliximab for vascular involvement in Behçet’s syndrome. Clin. Immunol. 2023, 253, 109682. [Google Scholar] [CrossRef]
- Toledo-Samaniego, N.; Oblitas, C.M.; Peñaloza-Martínez, E.; Del-Toro-Cervera, J.; Alvarez-Sala-Walther, L.A.; Demelo-Rodríguez, P.; Galeano-Valle, F. Arterial and venous involvement in Behçet’s syndrome: A narrative review. J Thromb Thrombolysis. 2022, 54, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Alibaz-Oner, F.; Direskeneli, H. Advances in the Treatment of Behcet’s Disease. Curr. Rheumatol. Rep. 2021, 23, 47. [Google Scholar] [CrossRef]
- Yurtdas, M.; Kasifoglu, T. Cardiovascular involvement in Behçet’s disease. Patholog. Res. Int. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Sulu, B.; Hatemi, G. New and future perspectives in Behçet’s syndrome. Arch. Rheumatol. 2024, 39, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kang, N.; Yu, X.; Zhang, X.; Duan, Q.; Ma, X.; Zhao, Q.; Wang, Z.; Wang, X.; Liu, Y.; et al. TNF inhibitors target a mevalonate metabolite/TRPM2/calcium signaling axis in neutrophils to dampen vasculitis in Behçet’s disease. Nat. Commun. 2024, 15, 9261. [Google Scholar] [CrossRef] [PubMed]
- Emmi, G.; Vitale, A.; Silvestri, E.; Boddi, M.; Becatti, M.; Fiorillo, C.; Fabiani, C.; Frediani, B.; Emmi, L.; Di Scala, G.; et al. Adalimumab-Based Treatment Versus Disease-Modifying Antirheumatic Drugs for Venous Thrombosis in Behçet’s Syndrome: A Retrospective Study of Seventy Patients with Vascular Involvement. Arthritis Rheumatol. 2018, 70, 1500–1507. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, S.Y.; Kim, K.N.; Jung, J.K.; Chung, W.T. Adalimumab treatment for life threatening pulmonary artery aneurysm in Behçet disease: A case report. Clin. Rheumatol. 2010, 29, 91–93. [Google Scholar] [CrossRef]
- Caruso, P.; Moretti, R. Focus on neuro-Behçet’s disease: A review. Neurol. India 2018, 66, 1619–1628. [Google Scholar] [CrossRef]
- Zając, H.; Turno-Kręcicka, A. Ocular Manifestations of Behçet’s Disease: An Update on Diagnostic Challenges and Disease Management. J. Clin. Med. 2021, 10, 5174. [Google Scholar] [CrossRef]
- Bettiol, A.; Hatemi, G.; Vannozzi, L.; Barilaro, A.; Prisco, D.; Emmi, G. Treating the Different Phenotypes of Behçet’s Syndrome. Front. Immunol. 2019, 10, 2830. [Google Scholar] [CrossRef]
- Guan, X.; Zhao, Z.; Xin, M.; Xia, G.; Yang, Q.; Fu, M. Long-term efficacy, safety, and cumulative retention rate of antitumor necrosis factor-alpha treatment for patients with Behcet’s uveitis: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2024, 27, e15096. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Gao, C.; Zhang, C.; Di, X.; Liang, W.; Sun, W.; Wang, Q.; Zheng, Z. Behcet’s disease with peripheral nervous system involvement successfully treated with golimumab: A case report and review of the literature. Rheumatol. Int. 2021, 41, 197–203. [Google Scholar] [CrossRef]
- Zhao, C.; Li, C.; Duan, F.J.; Yan, Q.; Zhang, Z.; Du, Y.; Zhang, W. Case Report: Repeated Low-Dose Rituximab Treatment Is Effective in Relapsing Neuro Behçet’s Disease. Front. Neurol. 2021, 12, 595984. [Google Scholar] [CrossRef] [PubMed]
- Jade, J.; Chung, K.; Arendse, M.; Hussain, Z.; White, D. Neuro-Behçet’s disease presenting with tumour-like lesions and responding to rituximab. J. Clin. Neurosci. 2016, 32, 139–141. [Google Scholar] [CrossRef]
- Messina, M.J.; Rodegher, M.; Scotti, R.; Martinelli, V. Treatment of myelitis in Behçet’s disease with rituximab. BMJ Case Rep. 2014, 2014, bcr2014204366. [Google Scholar] [CrossRef]
- Graßhoff, H.; Comdühr, S.; Monne, L.R.; Müller, A.; Lamprecht, P.; Riemekasten, G.; Humrich, J.Y. Low-Dose IL-2 Therapy in Autoimmune and Rheumatic Diseases. Front. Immunol. 2021, 12, 648408. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Full Prescribing Information: PROLEUKIN (Aldesleukin) for Injection, for Intravenous Use. Published Online February 2024. Available online: https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/f3c516ad-d405-4fbe-af6a-962080dbfa7d/spl-doc?hl (accessed on 1 January 2025).
- Sharma, V.; Deo, P.; Sharma, A. Deficiency of adenosine deaminase 2 (DADA2): Review. Best Pract. Res. Clin. Rheumatol. 2023, 37, 101844. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, S.; Aksu, K.; Kabasakal, Y.; Ekinci, N.S.; Alpsoy, E.; Karakas, A.A.; Yilmaz, S.B.; Yegin, O. The role of IL-17 inhibition in neuro-Behçet’s disease: A case series and literature review. Front. Neurol. 2021, 12, 595984. [Google Scholar]
- Liu, J.; Tian, J.; Wang, Z.; Wang, L.; Huang, C.; Zhou, J.; Zeng, X.; Zhao, Y.; Zheng, W. Secukinumab in the treatment of parenchymal neuro-Behçet’s syndrome. Rheumatology 2022, 61, e277–e279. [Google Scholar] [CrossRef]
- Schena, F.; Penco, F.; Volpi, S.; Pastorino, C.; Caorsi, R.; Kalli, F.; Fenoglio, D.; Salis, A.; Bertoni, A.; Prigione, I.; et al. Dysregulation in B-cell responses and T follicular helper cell function in ADA2 deficiency patients. Eur. J. Immunol. 2021, 51, 206–219. [Google Scholar] [CrossRef]
- Eli Lilly and Company. A Study of Baricitinib (LY3009104) in Participants from 2 Years to Less Than 18 Years Old with Active JIA-Associated Uveitis or Chronic Anterior Antinuclear Antibody-Positive Uveitis. ClinicalTrials.gov. ID: NCT04088409. Last Update posted 2025-01-24. Available online: https://clinicaltrials.gov/ct2/show/NCT04088409 (accessed on 3 January 2025).
- Watanabe, N.; Gao, S.; Wu, Z.; Batchu, S.; Kajigaya, S.; Diamond, C.; Alemu, L.; Raffo, D.Q.; Hoffmann, P.; Stone, D.; et al. Analysis of deficiency of adenosine deaminase 2 pathogenesis based on single-cell RNA sequencing of monocytes. J. Leukoc. Biol. 2021, 110, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Maccora, I.; Maniscalco, V.; Campani, S.; Carrera, S.; Abbati, G.; Marrani, E.; Mastrolia, M.V.; Simonini, G. A wide spectrum of phenotype of deficiency of deaminase 2 (DADA2): A systematic literature review. Orphanet. J. Rare Dis. 2023, 18, 117. [Google Scholar] [CrossRef]
- Pinto, B.; Deo, P.; Sharma, S.; Syal, A.; Sharma, A. Expanding spectrum of DADA2: A review of phenotypes, genetics, pathogenesis and treatment. Clin. Rheumatol. 2021, 40, 3883–3896. [Google Scholar] [CrossRef]
- Signa, S.; Bertoni, A.; Penco, F.; Caorsi, R.; Cafaro, A.; Cangemi, G.; Volpi, S.; Gattorno, M.; Schena, F. Adenosine Deaminase 2 Deficiency (DADA2): A Crosstalk Between Innate and Adaptive Immunity. Front. Immunol. 2022, 13, 935957. [Google Scholar] [CrossRef] [PubMed]
- Zavialov, A.V.; Yu, X.; Spillmann, D.; Lauvau, G.; Zavialov, A.V. Structural basis for the growth factor activity of human adenosine deaminase ADA2. J. Biol. Chem. 2010, 285, 12367–12377. [Google Scholar] [CrossRef]
- Ombrello, A.K.; Qin, J.; Hoffmann, P.M.; Kumar, P.; Stone, D.; Jones, A.; Romeo, T.; Barham, B.; Pinto-Patarroyo, G.; Toro, C.; et al. Treatment Strategies for Deficiency of Adenosine Deaminase 2. N. Engl. J. Med. 2019, 380, 1582–1584. [Google Scholar] [CrossRef] [PubMed]
- Hashem, H.; Bucciol, G.; Ozen, S.; Unal, S.; Bozkaya, I.O.; Akarsu, N.; Taskinen, M.; Koskenvuo, M.; Saarela, J.; Dimitrova, D.; et al. Hematopoietic Cell Transplantation Cures Adenosine Deaminase 2 Deficiency: Report on 30 Patients. J. Clin. Immunol. 2021, 41, 1633–1647. [Google Scholar] [CrossRef]
- Nihira, H.; Izawa, K.; Ito, M.; Umebayashi, H.; Okano, T.; Kajikawa, S.; Nanishi, E.; Keino, D.; Murakami, K.; Isa-Nishitani, M.; et al. Detailed analysis of Japanese patients with adenosine deaminase 2 deficiency reveals characteristic elevation of type II interferon signature and STAT1 hyperactivation. J. Allergy Clin. Immunol. 2021, 148, 550–562. [Google Scholar] [CrossRef]
- Ayan, G.; Basaran, O.; Firlatan, B.; Kilic, L.; Bilginer, Y.; Alikasifoglu, M.; Karadag, O.; Ozen, S. Long-term follow-up of anti-TNF treatment in adult and pediatric DADA2 patients: Insights from real-world data. Int. J. Rheum. Dis. 2024, 27, e15377. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Fan, X.; Ma, J.; Liu, X.; Li, C. ADA2 deficiency (DADA2) misdiagnosed as systemic onset juvenile idiopathic arthritis in a child carrying a novel compound heterozygous ADA2 mutation: A case report. Transl. Pediatr. 2023, 12, 97–103. [Google Scholar] [CrossRef]
- Sharabati, I.; Ayesh, B.M.; Qafesha, R.M.; Rasras, H.; Abunejma, F.M.; Abdulrazzak, M.; Jobran, A.W. Central retinal artery occlusion in a child with ADA2 deficiency: A case report. Ann. Med. Surg. 2024, 86, 2343–2347. [Google Scholar] [CrossRef]
- Dell’Orso, G.; Grossi, A.; Penco, F.; Caorsi, R.; Palmisani, E.; Terranova, P.; Schena, F.; Lupia, M.; Ricci, E.; Montalto, S.; et al. Case Report: Deficiency of Adenosine Deaminase 2 Presenting with Overlapping Features of Autoimmune Lymphoproliferative Syndrome and Bone Marrow Failure. Front. Immunol. 2021, 12, 754029. [Google Scholar] [CrossRef]
- Garg, N.; Kasapcopur, O.; Foster, J.; Barut, K.; Tekin, A.; Kızılkılıç, O.; Tekin, M. Novel adenosine deaminase 2 mutations in a child with a fatal vasculopathy. Eur. J. Pediatr. 2014, 173, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Dantas, J.G.; Biegelmeyer, E.; Zarur, E.B.; Pinheiro, F.A.G. Rare primary vasculitis: Update on multiple complex diseases and the new kids on the block. Adv. Rheumatol. 2024, 64, 79. [Google Scholar] [CrossRef]
- Greco, A.; Gallo, A.; Fusconi, M.; Magliulo, G.; Turchetta, R.; Marinelli, C.; Macri, G.; De Virgilio, A.; de Vincentiis, M. Cogan’s syndrome: An autoimmune inner ear disease. Autoimmun. Rev. 2013, 12, 396–400. [Google Scholar] [CrossRef]
- Marrero-Gonzalez, A.R.; Ward, C.; Nguyen, S.A.; Jeong, S.S.; Rizk, H.G. Audiovestibular outcomes in adult patients with cogan syndrome: A systematic review. Eur. Arch. Otorhinolaryngol. 2025, 282, 23–35. [Google Scholar] [CrossRef]
- Kahuam-López, N.; Vera-Duarte, G.R.; Pérez-Vázquez, A.K.; Navas, A.; Ramirez-Miranda, A.; Graue-Hernandez, E.O. Cogan syndrome: A case report and review of the literature. Digit. J. Ophthalmol. 2023, 29, 88–93. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).