Abstract
Mount Etna, located on the eastern coast of Sicily, is Europe’s most active volcano. Over the past five years, it has experienced numerous significant eruptive episodes, with the most recent occurring in August 2024. During this event, substantial amounts of volcanic ash were dispersed over densely populated areas, particularly in the province of Catania. Environmental factors, such as volcanic eruptions, are known to influence inflammatory skin conditions, including atopic dermatitis. We analyzed a cohort of patients with atopic dermatitis who were exposed to volcanic ash during the Mount Etna eruption in August 2024, aiming to evaluate the impact of the eruption on respiratory and cutaneous symptoms, treatment response, use of protective equipment, and changes in EASI scores over an eight-week period. A total of 67 Caucasian atopic dermatitis patients (mean age 41.2) were assessed after a volcanic eruption. Symptom worsening occurred in 58.9% (respiratory) and 26.9% (skin) of patients. EASI scores significantly increased (p < 0.05). No clinical difference was found between treatment types or mask use, which did not prevent symptom exacerbation. Volcanic ash exposure significantly worsened respiratory and skin symptoms in atopic dermatitis patients, underscoring the need for improved protective measures and further research on environmental triggers of chronic inflammatory conditions.
1. Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin condition characterized by a compromised skin and mucosal barrier, making it prone to xerosis, environmental irritants, and allergens, which can trigger both skin and respiratory symptoms [1,2]. Globally, an estimated 204.05 million individuals are affected by AD, with a point prevalence of 2.6%, including 101.27 million adults and 102.78 million children [3,4,5,6,7]. As a complex, multi-factorial disease, AD is driven by a combination of genetic predisposition and environmental exposures. In susceptible individuals, early-life contact with allergens, climatic factors, and indoor and outdoor air pollutants is believed to significantly contribute to disease onset and progression [8,9]. On the morning of 4 August 2024, Etna’s “Voragine” crater intensified its Strombolian activity, culminating in impressive lava fountains [1]. This event caused significant fallout of ash and lapilli toward the east-southeast, involving several areas at the foot of Etna.
1.1. Effects of Volcanic Ash in Atopic Dermatitis
Volcanic ash—a major form of airborne particulate matter (PM)—is well-documented to have adverse health effects, contributing to both acute and chronic disease burden [10,11]. Epidemiological data link exposure to fine PM (PM2.5 and PM10) with increased risks of mortality, ischemic heart disease, respiratory illness, and exacerbation of chronic conditions such as asthma and chronic obstructive pulmonary disease [12,13]. While the respiratory impact of volcanic ash is more widely studied, its effects on the skin, especially in patients with pre-existing dermatological conditions like AD, remain underexplored [12,13,14]. Ash particles contain reactive transition metals (e.g., Fe, Cu, Ni), which promote oxidative stress through the generation of reactive oxygen species (ROS), thereby triggering inflammation and cellular damage [3,15]. Additionally, volcanic ash is a known source of polycyclic aromatic hydrocarbons (PAHs)—a class of persistent organic pollutants produced through the incomplete combustion of organic matter. PAHs are highly lipophilic and tend to accumulate in lipid-rich tissues, including the hypodermic layer. Due to their low volatility and strong affinity for PMs, PAHs are deposited in soil, water, and vegetation, and enter the human body through inhalation, ingestion, or cutaneous absorption. Some PAHs, such as benzo(a)pyrene and anthracene, are recognized skin irritants and sensitizers, and enhance immune activation and trigger inflammatory responses. These immune effects can exacerbate conditions such as AD by promoting cytokine release, allergic reactions, and autoimmune responses [16,17,18]. Recent studies have linked PAHs and PM exposure to AD exacerbation through several biological pathways. These include oxidative stress, cutaneous microbiome dysbiosis, disruption of the epidermal barrier, and activation of the aryl hydrocarbon receptor (AhR) pathway—a key regulator of the cutaneous immune system. Persistent activation of AhR by PAHs can induce the production of ROS, alter keratinocyte differentiation and lead to sustained inflammatory responses in AD-prone skin [9,16,17,19]. Additionally, the AhR pathway interacts with other signaling cascades such as the epidermal growth factor receptor (EGFR) and JAK/STAT pathways, influencing keratinocyte behavior and inflammatory responses [20,21,22]. AhR activation in keratinocytes has been linked to the upregulation of neurotrophic factors like artemin, potentially contributing to AD-associated pruritus. Moreover, air pollutants such as PAHs and PMs contribute to cutaneous microbiome dysbiosis, facilitating colonization by Staphylococcus aureus—a well-established driver of AD flares and symptom severity [23,24,25,26]. This microbial imbalance impairs filaggrin (FLG) function, disrupts tight junction integrity, and promotes immune activation, collectively leading to further compromise of the epidermal barrier. These overlapping factors may induce a sustained, systemic inflammatory state, exacerbating both cutaneous and respiratory symptoms in individuals with AD, exposed to environmental pollutants, including volcanic ash [8,9,19,27,28].
1.2. Mount Etna Features
Mount Etna, located in Sicily, Italy (37°44′ N, 15°00′ E), is Europe’s largest and one of the world’s most active volcanoes [29,30]. The stratovolcano is marked by near-continuous eruptive activity from summit craters and frequent lava flows from flank fissures and vents [29,31]. With an estimated volume of over 350 km3, Etna rises to approximately 3330 m in elevation, though this varies slightly with eruptive activity. The volcano’s base spans roughly 60 × 40 km and consists of a shield volcano, while its upper portion forms a stratovolcano [29,31].
Etna’s structure reflects a long history of overlapping eruptions—both explosive and effusive—forming a complex mantle of volcanic deposits [32]. It is situated along the subduction zone between the African and Eurasian tectonic plates. Its basaltic lava, primarily composed of alkali basalt and trachybasalt, is characterized by low silica content and basic composition, contributing to fluid lava flows and intermittent explosive events [30,32].
The August 2024 eruption produced mainly basaltic lava (alkali basalt and trachybasalt), which, despite its effusive nature, led to widespread dispersal of fine ash and lapilli—thereby increasing the potential for environmental exposure in surrounding communities [30,33].
1.3. Aim of the Study
This study aims to evaluate the association between acute exposure to volcanic ash following the August 2024 eruption of Mount Etna and the worsening of cutaneous and respiratory symptoms in adults with atopic dermatitis. We assessed changes in disease severity using objective (EASI) and subjective (NRS) measures across two timepoints, and examined whether treatment modality (systemic vs. topical) and use of personal protective equipment (PPE) influenced these outcomes.
2. Materials and Methods
A total of sixty-seven adult patients with a confirmed diagnosis of atopic dermatitis (AD) were enrolled in this observational, retrospective study following the August 2024 eruption of Mount Etna. Patient inclusion was based on documented exposure to volcanic ash, as determined by official ash dispersion maps from regional environmental protection agencies and PM10 concentration data from air quality monitoring stations. Patients were considered exposed if they resided in areas where PM10 levels exceeded 50 µg/m3 on at least one day between 4–7 August 2024.
Self-reported exposure characteristics were collected via a structured questionnaire. This included time spent outdoors during ash fallout (categorized as <30 min, 30–120 min, or >2 h per day) and participation in outdoor activities (e.g., commuting, physical labor, and recreation).
Inclusion criteria were (1) a dermatologist-confirmed diagnosis of AD according to established clinical criteria; (2) direct or indirect exposure to volcanic ash during the eruption period; and (3) age ≥ 18 years. Exclusion criteria were (1) the presence of other chronic inflammatory or autoimmune skin diseases (e.g., psoriasis, contact dermatitis); (2) diagnosed immunodeficiency disorders; and (3) active systemic infections at the time of assessment.
Comprehensive data were collected for each participant, including demographic information (age, sex, and residence), pre-existing respiratory or allergic conditions, and baseline AD status. Detailed clinical data were gathered regarding respiratory and cutaneous symptoms, medication use (topical or systemic), and adherence to protective measures, such as the use of face masks or air filtration devices.
Mask usage was further stratified by type: surgical masks, FFP2/FFP3 masks, or cloth/fabric masks. Consistent PPE use was defined as wearing a mask during all outdoor exposures over the 3-day high-exposure window (4–6 August 2024), based on self-report via questionnaire.
Meteorological data (wind direction/speed, rainfall) were obtained from regional weather stations to confirm ash dispersion and assess environmental variability. Rainfall on 5 August, which reduced airborne ash in certain areas, was considered during exposure classification. The study period coincided with Sicily’s late summer season, typically associated with low airborne pollen levels. Nevertheless, allergy-prone individuals were asked to distinguish usual allergic symptoms from eruption-related worsening.
High-resolution clinical photographs were obtained (with informed consent) to document visible cutaneous changes. AD severity was assessed using the Eczema Area and Severity Index (EASI), which quantitatively evaluates the extent and severity of eczematous lesions across four anatomical regions. In addition, both respiratory and skin symptom worsening were self-reported using a Numerical Rating Scale (NRS) from 1 to 10, with higher scores indicating greater subjective severity. Worsening of symptoms was defined as an increase of ≥2 points on the NRS.
Assessments were conducted at two timepoints: (T0) baseline, 24–48 h after the eruption, and (T8) eight-week follow-up, to capture both acute and subacute symptom progression or resolution. Patients were stratified into two treatment groups: (1) those receiving systemic therapies, including biologics (e.g., dupilumab) or Janus kinase (JAK) inhibitors; and (2) those managed exclusively with topical therapy, including corticosteroids, calcineurin inhibitors, or emollients.
2.1. Statistical Analyses
Statistical analyses included descriptive statistics for demographic and clinical characteristics. Within-subject changes in EASI and NRS scores were evaluated using paired t-tests. Between-group comparisons (e.g., systemic vs. topical treatment; PPE use vs. non-use) were assessed using independent t-tests. Multivariate linear regression models were used to examine the effects of exposure duration, mask type, and treatment on changes in EASI scores, adjusting for age, sex, and baseline severity. A p-value < 0.05 was considered statistically significant.
2.2. Ethical Considerations
All data were anonymized prior to analysis, and no procedures beyond routine clinical practice were performed. Approval from the institutional ethics committee was not required due to the retrospective, non-interventional nature of the study and the anonymized status of all patient data.
3. Results
3.1. Patient Characteristics
A total of 67 adults were enrolled in our cohort: 43 (64.2%) were female and 24 (35.8%) were male, with a female-to-male ratio of approximately 1.8:1. The mean age at enrollment was 41.2 years (±12.01 years; range 12–76 years), with 15 patients (22.4%) younger than 30, 37 patients (55.2%) between 30 and 50, and the remaining 15 patients (22.4%) older than 50. All participants self-identified as Caucasian (Figure 1, Table 1).
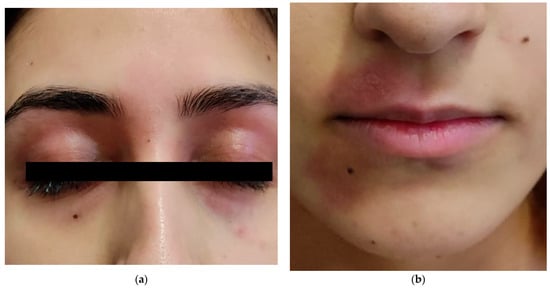
Figure 1.
Cutaneous symptoms exacerbation after volcanic ash exposure in a 20-year-old woman (a,b).

Table 1.
Demographic and clinical characteristics of atopic dermatitis patients following volcanic eruption.
The majority of subjects (n = 52; 78%) lived within a 30 km radius of Mount Etna, in municipalities known to receive the highest ash fall during eruptive events, whereas the other 15 patients (22%) resided in more distant towns where measured ash deposition was demonstrably lower. This allowed us to contrast the impact of heavy versus light volcanic particulate exposure on atopic dermatitis (AD) severity.
At baseline, 24 patients (35.8%) were receiving systemic therapy for moderate-to-severe AD. Of these, 18 (26.9% of the total cohort) were treated with the interleukin−4/13 receptor antagonist dupilumab, 5 patients (7.5%) with the JAK-1 inhibitor upadacitinib, and 1 (1.5%) with the anti-IL-13 monoclonal antibody tralokinumab. The remaining 43 individuals (64.2%) were managed with topical regimens only, consisting of mid- to high-potency corticosteroids and regular emollient application. All patients on systemic therapy had been receiving treatment for at least three months prior to the eruption, with 20 of 24 individuals (83.3%) having maintained the same regimen for over six months. No therapy changes were made between T0 and T8.
Lifestyle and comorbidity data revealed that 13 patients (19.4%) were smokers, potentially compounding cutaneous barrier dysfunction. Alcohol consumption patterns ranged from none (n = 42; 62.7%) to occasional (n = 20; 29.9%) and regular intake (n = 5; 7.5%). A total of 20 participants (17.9%) had a body mass index (BMI) in the overweight range (>25 kg/m2), linked to more recalcitrant AD. Only five individuals (7.5%) reported consistently wearing protective face masks when outdoors during periods of ash fall—a behavior that may mitigate, but clearly did not eliminate, exposure to airborne irritants.
3.2. Symptom Exacerbation After Volcanic Eruption
3.2.1. Symptom Assessment at T0—Overall Change
Of the 67 patients, 39 (58.9%) experienced worsening of respiratory symptoms—such as cough, wheezing, and/or dyspnea—within the first 48 h (T0) following the eruption. A total of 18 patients (26.9%) reported an exacerbation of cutaneous symptoms (pruritus, erythema, and oozing). Conversely, 22 individuals (32.1%) reported no appreciable change in their condition. Worsening was defined as an increase of two or more points on the NRS from baseline, which was considered a clinically meaningful change.
3.2.2. Symptom Assessment at T0—Respiratory Symptoms
Among the 39 patients with respiratory worsening, symptom severity was self-rated on a 0–10 Numerical Rating Scale (NRS). The mean respiratory NRS score was 3.7 ± 2.1 with a maximum score of 9, indicating that most patients experienced mild to moderate intensification of symptoms, with a small subset (n = 7) rating their dyspnea or cough in the moderate-to-severe range (NRS ≥ 5).
3.2.3. Symptom Assessment at T0—Cutaneous Symptoms
Eighteen patients reported skin flare-ups at T0, with a mean cutaneous NRS of 2.8 ± 1.9 and a maximum score of 7, reflecting generally mild flares, although four patients scored their itch or rash severity at 6 or higher.
3.2.4. Symptom Assessment at T8
At the 8-week follow-up visit (T8), cutaneous symptoms progressed substantially. The mean cutaneous NRS at eight weeks was 4.1 ± 2.5 (range: 0–10), with 24 patients (35.8%) now rating their itch or rash as moderate (NRS 4–6) and 6 patients (9.0%) as severe (NRS ≥ 7). Objective disease severity, measured via the Eczema Area and Severity Index (EASI), showed a marked increase. At T0, the mean EASI score was 8.12 ± 2.66 (range: 3–13), consistent with mild-to-moderate disease. At T8, the mean EASI had risen to 13.89 ± 4.19 (range: 5–22), indicating a shift toward moderate-to-severe disease. The increase in mean EASI (Δ = 5.77 points) was highly significant (paired t-test, p < 0.001), confirming that exposure to volcanic ash was associated with a clinically meaningful deterioration in atopic dermatitis severity over the two-month period.
3.3. Effect of Treatment Type
To explore the influence of baseline therapy on the impact of volcanic ash exposure, we performed a subgroup analysis comparing patients on systemic agents (n = 24; 35.8% of the cohort) with those managed exclusively with topical regimens (n = 43; 64.2%).
3.3.1. Objective Disease Severity (EASI)
In the systemic-therapy subgroup, the mean rise in EASI score from T0 to T8 was 5.1 ± 3.6 points. In the topical-therapy subgroup, the mean EASI increase was 6.4 ± 3.8 points. Although patients receiving systemic agents exhibited a numerically smaller EASI exacerbation (Δ = 1.3 points), this difference did not reach statistical significance (p = 0.11).
3.3.2. Patient-Reported Respiratory Symptoms (NRS)
Patients on systemic therapy rated their mean respiratory NRS at 3.1 ± 1.8, versus 4.0 ± 2.3 in the topical-only group. The 0.9-point lower mean in the systemic cohort suggested milder self-reported dyspnea and cough, but again, the between-group comparison was not statistically significant (p = 0.07).
3.3.3. Patients-Reported Cutaneous Symptoms (NRS)
Mean cutaneous NRS was 3.5 ± 2.0 for systemic therapy patients and 4.6 ± 2.7 for those on topical therapy alone. This difference trended in favor of systemic treatment, even if not statistically significant (p = 0.09).
3.4. Impact of Protective Measures and Geographic Exposure
Among the five patients who reported consistently wearing face masks during ash fall, two wore FFP2/FFP3 masks, two used surgical masks, and one used a cloth mask. Respiratory and cutaneous symptom worsening in this subgroup was comparable with the rest of the cohort. Their mean respiratory NRS score at T0 was 3.5 ± 1.5, only slightly lower than the 3.8 ± 2.2 reported by the 62 non-mask-wearers (p = 0.64). Similarly, mask-wearers’ mean cutaneous NRS of 3.9 ± 1.8 did not differ significantly from the 4.2 ± 2.6 seen in the non-mask group (p = 0.71). These findings suggest that facemask use alone—without additional measures such as protective eyewear or enhanced skin barrier strategies—was insufficient to prevent symptom exacerbation following Etna’s eruption.
When patients were stratified by distance from Mount Etna, those living within a 30 km radius (n = 52) experienced a significantly greater increase in EASI score over eight weeks than those beyond 30 km (n = 15). Specifically, the high-exposure group’s mean EASI rose by 6.2 ± 3.5 points, compared with a 4.1 ± 2.8-point increase in the lower-exposure group (p = 0.03). In parallel, residents closer to the volcano reported more severe respiratory symptoms, with a mean NRS of 4.2 ± 2.3 versus 3.1 ± 1.9 for those farther away (p = 0.04).
3.5. Multivariate Analysis of Predictors of AD Worsening
To explore independent predictors of disease exacerbation, we conducted a multivariate linear regression on the change in EASI score (ΔEASI), adjusting for age, baseline severity, treatment type, and mask usage. Among the included variables, only treatment type was significantly associated with disease worsening. Patients managed with topical therapy showed an increase in EASI scores compared with those receiving systemic therapy (β = 1.78, 95% CI: 0.20–3.36, p = 0.028). Other factors, such as mask type, baseline EASI, and age, did not reach statistical significance (Table 2).

Table 2.
Multivariate linear regression predicting change in EASI score (ΔEASI).
4. Discussion
Air pollution and particulate matter are well-established contributors to epidermal barrier dysfunction, immune dysregulation, oxidative stress, increased allergen sensitization, and inflammatory responses [34,35,36]. These mechanisms position them as key factors in the exacerbation of atopic dermatitis (AD) [9,24,25,37,38]. In our study, subjects with AD, residing in areas exposed to volcanic ash from Mount Etna, reported a notable worsening of both respiratory and cutaneous symptoms eight weeks after the eruption. While our findings suggest a significant exacerbation of AD symptoms following the volcanic eruption, it is important to consider background rates of seasonal or pollution-related flares. In Sicily, the summer season typically corresponds to lower airborne pollen levels, and no major urban pollution events were reported during the study window. Moreover, historical clinical records from our outpatient cohort indicate that August–September is not usually associated with a spike in AD exacerbations, suggesting that the observed symptom worsening was not attributable to baseline seasonal patterns.
Despite the theoretical benefits of systemic immunomodulation, unadjusted comparisons did not reveal statistically significant differences in clinical outcomes between treatment groups. However, multivariate regression—adjusting for age, baseline severity, and mask use—identified topical therapy as an independent predictor of greater EASI score increase, suggesting a modest but significant protective effect of systemic agents.
We defined symptom worsening as a ≥2-point increase on the Numerical Rating Scale (NRS), based on thresholds commonly used to indicate clinically meaningful change in chronic inflammatory conditions. Using this definition, over half of the participants reported respiratory symptom aggravation, and a substantial proportion experienced worsening skin symptoms, with a significant rise in both NRS and EASI scores by the eight-week follow-up.
Interestingly, consistent use of face masks—even high-filtration types like FFP2—did not significantly mitigate symptom exacerbation. This may be due to inadequate protection against percutaneous or ocular exposure or insufficient adherence to comprehensive protective measures.
4.1. Comparison with Previous Studies
Despite the growing understanding of environmental triggers in AD, data specifically addressing the impact of volcanic ash exposure remains limited. Few studies have examined this relationship in depth. One of the earliest investigations was conducted by Buist et al., who reported a four-year follow-up of 712 loggers exposed to varying levels of fresh volcanic ash following the 1980 eruptions of Mount St. Helens. Their findings indicated that ash exposure led to mucus hypersecretion and/or airway inflammation, which reversed upon reduction of exposure levels [39]. Hlodversdottir et al. investigated the Eyjafjallajökull eruption in Iceland, reporting respiratory symptoms and various physical complaints, including skin rashes and eczema [40,41]. Similarly, Carlsen et al. found that adults with high exposure to volcanic ash exhibited a higher incidence of respiratory symptoms such as cough, dyspnea, and sputum production 6–9 months post-eruption [41] Rojas-Ramos et al. conducted a prospective study on non-smoking farmers in Mexico exposed to ash from the Popocatépetl volcano, concluding that even short-term exposure may induce reversible airway inflammation [42].
4.2. Pathophysiological Insights
The exacerbation of AD and respiratory symptoms observed in the aftermath of the Mount Etna eruption may be mechanistically linked to the proinflammatory effects triggered by volcanic ash exposure. Several studies have demonstrated that inhalation of volcanic particulate matter—particularly ash containing crystalline silica such as cristobalite—can stimulate a robust inflammatory response through activation of the NLRP3 inflammasome [29,30]. Upon internalization by immune cells, such as macrophages, these particles cause lysosomal destabilization and mitochondrial stress, leading to the generation of reactive oxygen species (ROS). This process results in the assembly of the NLRP3 inflammasome complex, which activates caspase-1 and subsequently promotes the secretion of the mature form of interleukin-1β (IL-1β), a key cytokine in acute and chronic inflammation. In addition to IL-1β, studies have shown that volcanic ash exposure increases levels of interleukin-6 (IL-6), a multifunctional cytokine that plays a pivotal role in bridging innate and adaptive immunity and is strongly implicated in the pathogenesis of AD [31,32,33]. IL-6 contributes to the chronicity of AD by promoting Th2 polarization and enhancing the survival and proliferation of T cells involved in allergic inflammation [34]. Elevated IL-6 levels can also impair the skin barrier by disrupting keratinocyte differentiation and reducing the expression of key structural proteins such as filaggrin, which further predisposes the skin to irritation, microbial colonization, and allergen penetration. Another important cytokine upregulated in the context of ash exposure is interleukin-33 (IL-33), which has emerged as a central mediator of type 2 immune responses in AD [29]. While traditionally associated with myeloid cells, recent evidence indicates that NLRP3 is also expressed in epithelial cells and can function independently of the classical inflammasome pathway. In keratinocytes, NLRP3 acts as a transcriptional regulator, directly enhancing IL-33 expression via promoter binding and interaction with transcription factors such as IRF4. IL-33, once released by damaged or stressed epithelial cells, activates group 2 innate lymphoid cells (ILC2s), mast cells, and Th2 lymphocytes, leading to increased production of IL-5 and IL-13—cytokines that drive eosinophilic inflammation, pruritus, and epidermal thickening, hallmarks of AD [35]. Therefore, the upregulation of IL-1β, IL-6, and IL-33 in response to volcanic ash represents a plausible biological pathway linking environmental exposure to clinical deterioration in patients with pre-existing atopic conditions. These cytokines synergistically contribute to immune dysregulation, barrier dysfunction, and sustained skin inflammation. The combination of direct epithelial activation and systemic immune modulation may explain the rapid and severe worsening of AD symptoms following volcanic events, highlighting the need for preventive strategies and targeted monitoring in affected populations.
4.3. Study Limitations
This study has several limitations. The modest sample size (n = 67) and observational design limit causal inference. Exposure was based on geographic proximity and regional PM10 monitoring; however, individual-level exposure may have varied due to microclimatic differences and outdoor activity levels. Site-specific PM2.5 and cristobalite concentrations were not measured. A non-AD control group was not included, limiting the ability to determine whether observed effects were unique to AD. The cohort was also geographically diverse, and while this allowed analysis of dose–response trends, it introduced exposure variability. Additionally, female participants were overrepresented, reflecting typical AD demographics but potentially influencing outcomes. Symptom data were self-reported, which may introduce recall bias despite the use of validated tools (EASI, NRS). Lastly, only five participants consistently used face masks, limiting the power to evaluate PPE effectiveness, and the eight-week follow-up may not capture long-term effects.
5. Conclusions and Future Directions
Atopic dermatitis (AD) is a chronic, relapsing inflammatory skin disease associated with significant psychological burden and socio-economic impact. Although numerous therapeutic options have been approved in recent years, the prevention of disease flares is still one of the main critical aspects of AD management. Our findings highlight the need for targeted preventive measures, particularly in high-risk areas such as volcanic regions.
Enhanced protective measures and public health awareness campaigns are critical to minimizing the health risks in affected areas. Notably, the consistent worsening of symptoms observed in our study—even among individuals using protective masks—suggests that current interventions may be insufficient to fully counteract the effects of ash exposure.
A better understanding of the specific components of volcanic ash and their role in disease pathophysiology could be useful for guiding public health policies and individual protective measures in affected regions. Future research should explore the long-term effects of volcanic ash on chronic inflammatory disorders and evaluate the effectiveness of interventions such as barrier-enhancing topicals, air filtration, and broader protective guidelines.
Despite these limitations, our findings underscore the role of environmental triggers—particularly airborne particulate matter—in worsening AD symptoms. The fact that disease exacerbation occurred across treatment groups and was not prevented by mask usage highlights the need for broader preventive strategies, such as public health alerts, protective guidelines for at-risk populations, and perhaps the development of barrier-enhancing topical agents specifically for high-pollution environments.
Future studies should involve larger cohorts, objective exposure tracking, longer follow-up, and inclusion of molecular biomarkers to clarify the mechanisms linking volcanic ash exposure to skin inflammation.
Author Contributions
Conceptualization, F.T. and A.D.G.; methodology, F.T. and A.D.; validation, F.T., A.D.G. and A.R.; formal analysis, F.T. and E.Z.; investigation, F.T. and A.D.G.; resources, F.T. and A.D.G.; data curation, A.D.G.; writing—original draft preparation, F.T.; writing—review and editing, A.D.G., E.Z., A.R., A.D., S.P.N. and G.P.; visualization, S.P.N. and G.P.; supervision, A.D. and G.P.; project administration, S.P.N. and G.P.; funding acquisition, F.T., E.Z. and A.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to its retrospective design, use of anonymized data, and lack of any intervention or collection of identifiable personal information, in accordance with institutional and national ethical guidelines.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper and to publish photographic documentation.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EASI | Erythema Area Index |
References
- Keskinen-Rosenqvist, R.; Michélsen, H.; Schulman, A.; Wahlström, L. Physical symptoms 14 months after a natural disaster in individuals with or without injury are associated with different types of exposure. J. Psychosom. Res. 2011, 71, 180–187. [Google Scholar] [CrossRef]
- Afari, N.; Ahumada, S.M.; Wright, L.J.; Mostoufi, S.M. Psychological trauma and functional somatic syndromes: A systematic review and meta-analysis. Psychosom. Med. 2014, 76, 2–11. [Google Scholar] [CrossRef]
- GBD 2021 Asthma and Allergic Diseases Collaborators. Global, regional, and national burden of asthma and atopic dermatitis, 1990–2021, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Respir. Med. 2025, 13, 425–446. [Google Scholar] [CrossRef]
- Shin, Y.H.; Hwang, J.; Kwon, R.; Lee, S.W.; Kim, M.S.; GBD 2019 Allergic Disorders Collaborators; Shin, J.I.; Yon, D.K. Global, regional, and national burden of allergic disorders and their risk factors in 204 countries and territories, from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Allergy 2023, 78, 2232–2254. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, D.; Yang, Y.; Huang, Y.; Wang, L.; Yao, X.; Lu, Q. Global epidemiology of atopic dermatitis: A comprehensive systematic analysis and modelling study. Br. J. Dermatol. 2023, 190, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Puerta Durango, K.; Chiesa Fuxench, Z.C. Global Burden of Atopic Dermatitis: Examining Disease Prevalence Across Pedi-atric and Adult Populations World-Wide. Dermatol. Clin. 2024, 42, 519–525. [Google Scholar] [CrossRef] [PubMed]
- A study about how many people around the world have atopic dermatitis. Br. J. Dermatol. 2023, 190, e6. [CrossRef]
- Pan, Z.; Dai, Y.; Akar-Ghibril, N.; Simpson, J.; Ren, H.; Zhang, L.; Hou, Y.; Wen, X.; Chang, C.; Tang, R.; et al. Impact of Air Pollution on Atopic Dermatitis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2023, 65, 121–135. [Google Scholar] [CrossRef]
- Hendricks, A.J.; Eichenfield, L.F.; Shi, V.Y. The impact of airborne pollution on atopic dermatitis: A literature review. Br. J. Dermatol. 2020, 183, 16–23. [Google Scholar] [CrossRef]
- Gordian, M.E.; Özkaynak, H.; Xue, J.; Morris, S.S.; Spengler, J.D. Particulate air pollution and respiratory disease in An-chorage, Alaska. Environ. Health Perspect. 1996, 104, 290–297. [Google Scholar] [CrossRef]
- Thorsteinsson, T.; Jóhannsson, T.; Stohl, A.; Kristiansen, N.I. High levels of particulate matter in Iceland due to direct ash emissions by the Eyjafjallajkull eruption and resuspension of deposited ash. J. Geophys. Res. Solid Earth 2012, 117, B00C05. [Google Scholar] [CrossRef]
- Lombardo, D.; Ciancio, N.; Campisi, R.; Di Maria, A.; Bivona, L.; Poletti, V.; Mistretta, A.; Biggeri, A.; Di Maria, G. A ret-rospective study on acute health effects due to volcanic ash exposure during the eruption of Mount Etna (Sicily) in 2002. Multidiscip. Respir. Med. 2013, 8, 51. [Google Scholar] [CrossRef]
- Barone, G.; De Giudici, G.; Gimeno, D.; Lanzafame, G.; Podda, F.; Cannas, C.; Giuffrida, A.; Barchitta, M.; Agodi, A.; Mazzoleni, P. Surface reactivity of Etna volcanic ash and evaluation of health risks. Sci. Total Environ. 2021, 761, 143248. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.; De Giudici, G.; Gimeno, D.; Lanzafame, G.; Podda, F.; Cannas, C.; Giuffrida, A.; Barchitta, M.; Agodi, A.; Mazzoleni, P. Corrigendum to “Surface reactivity of Etna volcanic ash and evaluation of health risks” [Sci. Total Environ. 761 (2021), 143248]. Sci. Total Environ. 2022, 821, 153382. [Google Scholar] [CrossRef]
- Dattola, A.; Bennardo, L.; Silvestri, M.; Nisticò, S.P. What’s new in the treatment of atopic dermatitis? Dermatol. Ther. 2019, 32, e12787. [Google Scholar] [CrossRef]
- Mallah, M.A.; Changxing, L.; Mallah, M.A.; Noreen, S.; Liu, Y.; Saeed, M.; Xi, H.; Ahmed, B.; Feng, F.; Mirjat, A.A.; et al. Polycyclic aromatic hydrocarbon and its effects on human health: An overeview. Chemosphere 2022, 296, 133948. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Song, S.; Paek, D.; Park, C.; Lee, C.; Lee, J.H.; Yu, S.D. Exposure to ambient ultrafine particles and urinary 8-hydroxyl-2-deoxyguanosine in children with and without eczema. Sci. Total Environ. 2013, 458–460, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Han, Y.; Ahn, J.H.; Kim, S.W.; Lee, S.I.; Lee, K.H.; Ahn, K. Airborne formaldehyde causes skin barrier dysfunction in atopic dermatitis. Br. J. Dermatol. 2016, 175, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Andrysík, Z.; Vondráček, J.; Marvanová, S.; Ciganek, M.; Neča, J.; Pěnčíková, K.; Mahadevan, B.; Topinka, J.; Baird, W.M.; Kozubík, A.; et al. Activation of the aryl hydrocarbon receptor is the major toxic mode of action of an organic extract of a reference urban dust particulate matter mixture: The role of polycyclic aromatic hydrocarbons. Mutat. Res.—Fundam. Mol. Mech. Mutagen. 2011, 714, 53–62. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Wang, J. Source apportionment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Rizhao coastal area (China) using diagnostic ratios and factor analysis with nonnegative constraints. Sci. Total Environ. 2012, 414, 293–300. [Google Scholar] [CrossRef]
- Chakravarti, D.; Venugopal, D.; Mailander, P.C.; Meza, J.L.; Higginbotham, S.; Cavalieri, E.L.; Rogan, E.G. The role of polycyclic aro-matic hydrocarbon-DNA adducts in inducing mutations in mouse skin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008, 649, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Jinnestål, C.L.; Belfrage, E.; Bäck, O.; Schmidtchen, A.; Sonesson, A. Skin barrier impairment correlates with cutaneous Staphylococcus aureus colonization and sensitization to skin-associated microbial antigens in adult patients with atopic dermatitis. Int. J. Dermatol. 2014, 53, 27–33. [Google Scholar] [CrossRef]
- Tang, K.T.; Ku, K.C.; Chen, D.Y.; Lin, C.H.; Tsuang, B.J.; Chen, Y.H. Adult atopic dermatitis and exposure to air pollutants-a nationwide population-based study. Ann. Allergy Asthma Immunol. 2017, 118, 351–355. [Google Scholar] [CrossRef]
- Huang, C.C.; Wen, H.J.; Chen, P.C.; Chiang, T.L.; Lin, S.J.; Guo, Y.L. Prenatal air pollutant exposure and occurrence of atopic dermatitis. Br. J. Dermatol. 2015, 173, 981–988. [Google Scholar] [CrossRef]
- Brucker, N.; Moro, A.M.; Charão, M.F.; Durgante, J.; Freitas, F.; Baierle, M.; Nascimento, S.; Gauer, B.; Bulcão, R.P.; Bubols, G.B.; et al. Biomarkers of occupational exposure to air pollution, inflammation and oxidative damage in taxi drivers. Sci. Total Environ. 2013, 463–464, 884–893. [Google Scholar] [CrossRef]
- Vestergaard, C. Air Pollution and Atopic Dermatitis: Critical Windows of Risk in Early Life. Br. J. Dermatol. 2025, 192, 967. [Google Scholar] [CrossRef]
- Baek, J.O.; Cho, J.; Roh, J.Y. Associations between ambient air pollution and medical care visits for atopic dermatitis. Environ. Res. 2021, 195, 110153. [Google Scholar] [CrossRef] [PubMed]
- Mount Etna—UNESCO World Heritage Centre. Available online: https://whc.unesco.org/en/list/1427/ (accessed on 22 June 2025).
- Global Volcanism Program|Current Eruptions. Available online: https://volcano.si.edu/gvp_currenteruptions.cfm (accessed on 22 June 2025).
- MOUNT ETNA—World Heritage Datasheet. Available online: http://world-heritage-datasheets.unep-wcmc.org/datasheet/output/site/mount-etna/ (accessed on 22 June 2025).
- Global Volcanism Program|Etna. Available online: https://volcano.si.edu/volcano.cfm?vn=211060 (accessed on 22 June 2025).
- Global Volcanism Program|Report on Etna (Italy)—14 August–20 August 2024. Available online: https://volcano.si.edu/showreport.cfm?wvar=GVP.WVAR20240814-211060 (accessed on 22 June 2025).
- Dong, Y.-M.; Liao, L.-Y.; Li, L.; Yi, F.; Meng, H.; He, Y.-F.; Guo, M.-M. Skin inflammation induced by ambient particulate matter in China. Sci. Total Environ. 2019, 682, 364–373. [Google Scholar] [CrossRef]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.H.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Wullaert, A.; Bonnet, M.C.; Pasparakis, M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011, 21, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Lee, Y.L.; Su, H.J.; Sheu, H.M.; Yu, H.S.; Guo, Y.L. Traffic-related air pollution, climate, and prevalence of eczema in Tai-wanese school children. J. Investig. Dermatol. 2008, 128, 2412–2420. [Google Scholar] [CrossRef] [PubMed]
- Buist, A.S.; Vollmer, W.M.; Johnson, L.R.; Bernstein, R.S.; McCamant, L.E. A four-year prospective study of the respiratory effects of volcanic ash from Mt. St. Helens. Am. Rev. Respir. Dis. 1986, 133, 526–534. [Google Scholar] [CrossRef]
- Carlsen, H.K.; Hauksdottir, A.; Valdimarsdottir, U.A.; Gíslason, T.; Einarsdottir, G.; Runolfsson, H.; Briem, H.; Finnb-jornsdottir, R.G.; Gudmundsson, S.; Kolbeinsson, T.B.; et al. Health effects following the Eyjafjallajökull volcanic eruption: A cohort study. BMJ Open 2012, 2, e001851. [Google Scholar] [CrossRef]
- Hlodversdottir, H.; Petursdottir, G.; Carlsen, H.K.; Gislason, T.; Hauksdottir, A. Long-term health effects of the Eyjafjalla-jökull volcanic eruption: A prospective cohort study in 2010 and 2013. BMJ Open 2016, 6, e011444. [Google Scholar] [CrossRef]
- Rojas-Ramos, M.; Catalan-Vazquez, M.; Pozzo, A.L.M.D.; Garcia-Ojeda, E.; Villalba-Caloca, J.; Perez-Neria, J. A Seven Months Prospective Study of the Respiratory Effects of Exposure to Ash from Popocatepetl Volcano, Mexico. Environ. Geo-Chem. Health 2001, 23, 379–392. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).