Abstract
Enormous amount of scrap is generated on the shopfloor during manufacturing. Energy needed to melt increasing quantities of scrap will be ever increasing, and so will be the loss of metal during melting. Hence, conversion of scrap directly into marketable products by solid state processing methods is economical due to a lower energy requirement and a greater yield compared to the melting route. This makes the process more environmentally friendly. However, not all materials can be recycled in a solid state, with equal ease. One therefore needs to quantitatively assess the recyclability of a given kind of scrap. In the present work, a procedure to assess the recyclability of finely divided ferrous metallic scrap generated on the shopfloor is demonstrated. Recyclability includes material and the process used for recycling. For instance, a given material might be more recyclable using one process compared to the other. In the present study, the potential of powder technology (powder metallurgy (PM) and metal injection molding (MIM) based processes are compared for solid-state conversion of scrap directly into usable products. Grinding sludge collected in the shopfloor was pulverized and used as raw material. Properties of sintered parts were found to be significantly better due to in-situ reduction and densification during sintering. A quantitative measure of recyclability, namely, the Recyclability Index (RI) was defined to compare the manufacturability of different products. Recycled ferrous parts manufactured by PM route are found to have a greater RI (superior recyclability) than those manufactured by the MIM route. Complex reduction and sintering mechanisms in MIM parts, particularly, the kinetics of diffusion and volumetric shrinkage, limit suitability of MIM for recycling. In contrast, few industrial parts were developed and manufactured by conventional PM based approach to demonstrate the suitability of this novel recycling process especially for manufacture of porous parts.
1. Introduction
Conventional recycling relies on secondary sources (graded metallic scrap and industrial waste) to reduce the cost of manufacture using metal from the primary sources. Nearly 40 percent of steel is produced from secondary sources worldwide [1,2]. Secondary Steelmaking using an electric arc furnace uses metallic scrap as the primary raw material. This process is found to be feasible and economical when a large quantity of scrap is available [3]. Solid state approaches used for recycling non-ferrous metallic scrap, and on the other hand, process scrap is available in limited quantities but requires high capacity presses for deformation and furnaces for heating [4]. The powder technology based novel solid-state recycling process has the following advantages for the recycling of particulate ferrous metallic scrap:
- Alloying by adding different metallic powders thereby achieving unique properties and microstructure.
- Sintering stage can be used for the thermochemical treatment of materials.
- Low energy consumption compared to the melting route and shorter overall production time.
- Significant cost benefits due to a significantly cheaper raw material, i.e., the powder.
- Net-shape manufacturing. Little or no scrap is further generated during processing.
- High process yield (high metal recovery).
It is therefore important to quantify the properties and characteristics of products made by recycling. Usually, recycled manufacturing scrap constitutes metallic particles of greater purity than the ore from which virgin metal is extracted. However, unless the relationship between the product properties and manufacturing parameters is standardized and controlled, getting the desired quality is difficult. For instance, with particulate raw material (powders), large and complex shaped parts are difficult to make. Moreover, low ductility and low strength of parts, not to mention the atmospheric contamination due to powder and dust, and the emission from furnaces, are commonly faced issues [5,6] that need to be overcome to achieve the desired product quality.
Currently, applications of powder metallurgy (PM) may be classified into four major domains, namely, structural, tribological, cutting tools, and magnetic parts. However, parts produced by PM based recycling will not be suitable for all these domains and applications within these domains. Powder Metallurgy based recycling technique yields products of limited strength due to a high level of porosity, which is inevitable. Thus, this method of recycling suits manufacture of parts for applications where porosity is desirable and limited structural strength is expected [7,8].
Metal injection molding (MIM) is best suited for high-volume production of small metal parts. As with injection molding, these parts can be geometrically complex and have thin walls and fine details. As powdered metal is not melted in the MIM process, high-temperature alloys can be recycled to make tools without any adverse effect on tool life [9].
A brief overview of PM and MIM process steps in the context of recycling in process ferrous scrap is given below:
For the PM process, pulverization of identified metallic scrap (a mixture of oxides of iron in various proportions of oxygen depending on the source of scrap) is performed by crushing and ball-milling. Brittle iron oxide (e.g., forging scale) is crushed by a jaw crusher followed by ball milling. In ball-milling, harder ceramic (ZrO2) balls impinge on the brittle iron oxide refining the particle size. After sieving the powder, relatively coarser particles are used for conventional PM. In the MIM process, fine particles are needed to get adequate mouldability of the feedstock by injection and moulding, besides getting excellent mechanical properties and dimensional stability of sintered parts.
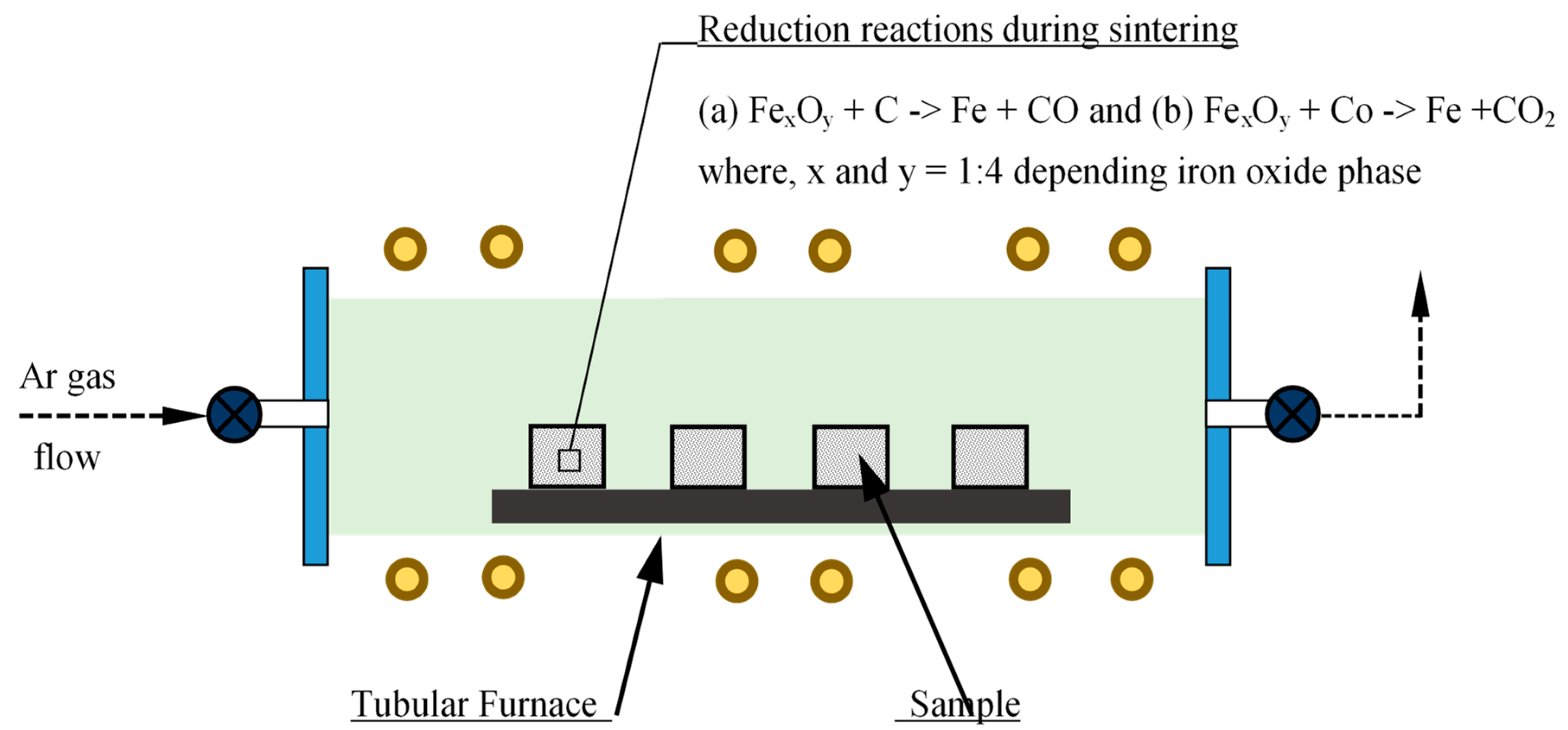
Phases of iron oxide in scrap were determined quantitatively to calculate the quantity of (carbonaceous) reducing agents required for carbothermic reduction during sintering. A calculated amount of reducing agent (here carbonaceous material) is mixed with iron oxide powder for in-situ carbothermic reduction during sintering. Mixing was performed using a mixer grinder mixing, turbula mixing, and low-energy ball milling, as well as a combination of these. The powder mixture was uniaxially compacted using either a hydraulic press or a mechanical press, which enabled compaction at different strain rates. Compaction of powder mixture to different sample shapes such as a solid cylinder and cylindrical bushes of various dimensions was also performed using dies designed and manufactured for the purpose. Compacted samples were then heated to have in-situ reduction and densification during the PM sintering cycle. A laboratory scale tubular furnace with inert gas flow arrangement as well as vacuum was utilized [10]. A series of reduction reactions took place during the initial phase of sintering, as represented schematically in Figure 1.
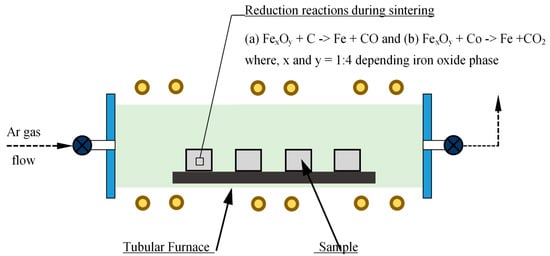
Figure 1.
Schematic representation of in-situ carbothermic reduction during sintering of PM compacts.
The first step of MIM process is to create a feedstock (a mixture) of metal powder, a reducing agent as before, and a polymer. The metal powder was mixed with a thermoplastic binder, cooled, and then granulated into a homogeneous feedstock in the form of pellets. Usually, sigma blade mixer was used to prepare the feedstock, which typically had 60% metal and 40% polymer by volume. The feedstock was shaped using the equipment and tooling similar to what is used in plastic injection molding. However, the mold cavities have to be designed approximately 20% larger to account for the shrinkage of the moulded product during sintering. During the molding cycle, the feedstock is melted and injected into the mold cavity, where it cools and solidifies into the shape of the part. The molded “green” part is ejected. In the subsequent step, polymer binders are removed from the solidified moulded shape. In some cases, solvent debinding is first performed in which the “green” part is placed in water or chemical bath (depending on the solubility of the binder) to dissolve the sacrificial binder component. The solvent debinded part is heated at a gentle heating rate in a low-temperature oven to remove the residual solvent left behind in the pores. After this step, thermal debinding or pre-sintering is performed in vacuum to remove the polymer binder by evaporation. As a result, the remaining “brown” metal part contains approximately 40% voids by volume. The final step of sintering is required to consolidate the loosely bound powder in the “brown” part, resulting in a high-density (95–99%) metal part. Sintering is usually carried out in an inert gas atmosphere or in the vacuum. Sintering temperatures are usually close to 85% of the metal’s melting point [11,12].
Literature available so far does not contribute to the knowledge of the effect of process variables on the process of solid state recycling, and the resulting product properties.
The primary objective of the present work is to establish a correlation between the properties of the product (at the end of in-situ reduction during sintering wherein metallic iron is obtained from iron oxide), and process variables (thermal debinding temperature, sintering temperature, sintering time, thermal debinding atmosphere, and sintering atmosphere). Finally, an attempt is made to analyze the recyclability of metallic scrap by the PM and MIM process.
2. Materials and Methods
Metallic waste generation in a forging plant at every intermediate process starting from hot forging, cutting to final finishing operation, was analyzed. Oxide scales, chips, and trimmed flash are usually recycled in a conventional steel making process by melting, whereas metallic constituents in grinding sludge are used for landfilling. Grinding sludge/swarf is a crude mixture of metallic (about 50 wt. % of iron oxides and pure metal particles) and nonmetallic constituents such as dust, cutting fluids, and lubricants. Many new techniques have been adopted for recycling such scrap by hot consolidation for manufacturing, pigments for paint industry, etc. These processes do not provide direct application of these essential metallic resources. Powder technology (particularly PM and MIM), as discussed in the previous section, is used to get usable products directly after recycling. Grinding sludge was dried to remove moisture, roasted under controlled conditions to obtain pyrolytic carbon from nonmetallic constituents, and ball milled for pulverization.
SEM-EDS study of powder samples was performed in order to find the semi quantitative elemental composition and the grade of steel. The powder was first coated with an ultra-thin film of gold in an ion sputter (Anatech, Model: Hummer VI-A) and was observed under a Scanning electron microscope (Hitachi, Model: S-3400N). The XRD analysis was carried out using Analytical Diffractometer (X’Pert) for determining the iron oxide phases of the sludge. A diffraction pattern was obtained over a range 5° to 90° at an average increment size of 0.015°. As each of the oxide phases exhibits characteristic reflection peaks corresponding to its d-values, these sets of d-values were matched with standards for iron oxide phases and various species of oxides were identified in the sludge. Table 1 gives the chemical composition of the powdered grinding sludge, which served as the raw material for this study.

Table 1.
Semi-quantitative abundance of major and minor elements in powdered grinding sludge sample.
Iron (Fe) and oxygen (O) are the major constituents in powder sample, whereas carbon (C) is a minor element. The presence of alloying elements such as Mn, Si, and Al in powder form in grinding sludge confirms that scrap is derived from finishing operations of micro-alloyed steel. The XRD analysis also confirmed the presence of Fe3O4 (92.8 wt. %), FeO (1.9 wt. %), Fe(CO)5 (1.5 wt. %) and negligible portion or absence of Fe2O3 in the Grinding Sludge (GS). Similar observations have been reported by F. Demirci et al. [13] using a microstructural examination of a section of the mill scale. The absence of the Fe2O3 phase in grinding sludge is due to a very short exposure of iron to high temperature and rapid cooling of the grinding chip. Neither the temperature attained nor the duration of exposure is adequate to form Fe2O3 during the grinding process [13]. Determined values of iron oxide phases were used to calculate the amount of carbon needed for carbothermic reduction.
The theoretical requirement of carbon for reduction of 1 g of Grinding Sludge powder (92.78% Fe3O4, 1.92% FeO, 1.44% Fe, and 3.84% C by wt.) may be calculated as:
* ( ) represents mass obtained from source, and [ ] represents mass obtained from reaction.
∴ Total theoretical C needed per gram of grinding sludge (by wt.) = 0.0247 + 0.0738 = 0.0985 g
As carbon is the limiting reactant (controls carbothermic reduction reaction) and stoichiometric proportion of carbon may not produce the desired degree of reduction [14]. Carbon is mixed with powdered scrap in 25% excess than the stoichiometric quantity [13,15]. Commercial pure iron powder and carbonyl iron powder (as detailed in Table 2) were used as benchmarks in PM and MIM, respectively, to facilitate normalization.

Table 2.
Physical properties of source material used in the present study.
CP, GS, and GSGR powder mixtures were compacted to cylindrical shaped samples having 10 mm height as well as diameter. Sintered cylindrical samples (CP iron, GS, and GSGR) obtained after conducting 33 full factorial experiments were used for characterization of properties. Important process parameters, namely, compaction pressure (levels: 900, 1050, and 1200 MPa), sintering temperature (levels: 1100, 1200, and 1300 °C), sintering atmosphere (levels: Vacuum, argon gas, nitrogen gas) were varied according to experimental settings (listed in Appendix A). Other parameters such as graphite addition (25% excess than a stoichiometric requirement), reduction temperature (100 °C below sintering temperature), reduction time 30 min, and sintering time 60 min were kept constant. Three samples per experimental condition were used for measurement of Degree of Reduction, i.e., DOR (for GS and GSGR), sintered density, hardness, and yield strength (in compression).
Previous studies have established that an organic binder system (Paraffin wax-HDPE-S.A) improves the ease and homogeneity of melt flow by reducing interparticle friction during powder injection molding by covering the entire surface area of the hard metallic powder particles. The rheology of the feedstock depends on the nature, size, and shape of the metal or ceramic particles [16,17]. Thus, in the present study, a binder system having 70 wt. % paraffin wax, 25 wt. % HDPE and 5 wt. % stearic acid (S.A) was mixed with metallic powder using a Sigma Blade Mixer running at 50 rpm [18] to prepare two separate MIM feedstocks, one designated as CI 90 (90% solid loading of Carbonyl Iron powder), and the other GSGR75 (75% solid loading of a mixture of 65.77 wt. % Grinding Sludge and 9.22 wt. % GRaphite).
Dogbone and cylindrical shaped samples were injection molded for tensile and compression testing, respectively. Solvent debinding was performed on parts of different shapes made from different feedstock materials. Parts were debinded in n-Heptane at 56 °C. To analyze the sintered properties of MIM test samples made at different sintering temperatures and sintering atmospheres, a full factorial (32) design was used. Nine experiments with three repetitions per experiment were performed to study the effect of sintering atmosphere and temperature, each at three levels. Nine experimental settings (sintering cycles) used to perform debinding and sintering of samples are given in Appendix B. Three samples per experimental condition were used for measurement of DOR (for GSGR75), sintered density, yield strength, UTS, elongation, and elastic modulus. The average values and standard deviation of properties were determined to represent the corresponding experimental characteristics.
3. Results and Discussion
Process quality characteristics like Degree of Reduction (DOR) and Degree of Densification (DOD) were determined by Equations (4) and (5).
During carbothermic reduction, the weight of the sample progressively decreased as oxygen from the oxide was lost. The initial and final weight of the sample was measured. Weight change is the sum total of the change in the weight of the constituents (oxide powder plus graphite). The ideal (theoretical) weight of the reduced sample was calculated.
For example, if the compacted sample having 86.9565 wt. % iron oxide powder and 13.0435 wt. % graphite (if 25% excess graphite added) weighed 3 g, then the theoretical reduced weight of iron can be determined from relation (6) as,
(** Amount of iron obtained after reduction of 1 g of scrap powder, considering iron oxide phases in scrap and stoichiometric calculations illustrated in relation (1) and (2) 0.692 + 0.012 of iron in scrap).
The density of the cylindrical compact was calculated by measuring its weight using a weighing balance of 0.01 g accuracy and volume using dimensions measured by the calipers to 0.01 mm precision.
“Degree of Densification” of the sintered iron samples was calculated using relation (5) by substituting the densities of the part into it before sintering (green density), after sintering (sintered density), and assuming = 7.86 g/cm3.
DOR, DOD, and mechanical properties of sintered parts are influenced by material and process parameters (such as the composition of the scrap, reducing agent and reducing gas, sintering time, compaction, injection, sintering and debinding temperatures, the particle size of iron oxide, compaction pressure, etc.) of the recycling process. Effect of these parameters on process characteristics and mechanical properties of recycled and sintered parts are discussed elsewhere [19,20]. However, the correlation between process characteristics, particularly Degree of Reduction (DOR) and properties of sintered parts is essential to understand the improvement in properties of GSGR and GSGR75 via in-situ carbothermic reduction by powder technology (PM and MIM) based recycling approach in the present work. The recycled material and part made from the recycled material should have comparable properties as that of virgin wrought material besides similar or lesser energy consumption. Following sections discuss the correlation between Degree of Reduction (DOR) and sintered properties along with a novel quantitative evaluation of recyclability using the Powder Technology based recycling approach. Optimized material and process parameter settings for Powder Metallurgy based recycling were selected based on process characteristics (DOR and DOD), and properties of compacted and sintered PM parts, as illustrated in References [10,15]. The latter part of this section demonstrates the suitability and prospective applications of the proposed recycling technique.
3.1. Correlation between DOR and Sintered Properties of PM and MIM Parts
In PM sintering, in the absence of separately added graphite powder, in-situ reduction in a grinding sludge (GS) sample occurs by carbothermic reduction of iron oxide through pyrolytically generated carbon. Carbon (~4.32% by wt.) in grinding sludge is generated by pyrolytic transformation of nonmetallic constituents during roasting of the GS. In the GSGR samples, carbothermic reduction of iron oxide occurred due to both pyrolytically generated carbon and 25% excess graphitic carbon added for carbothermic reduction.
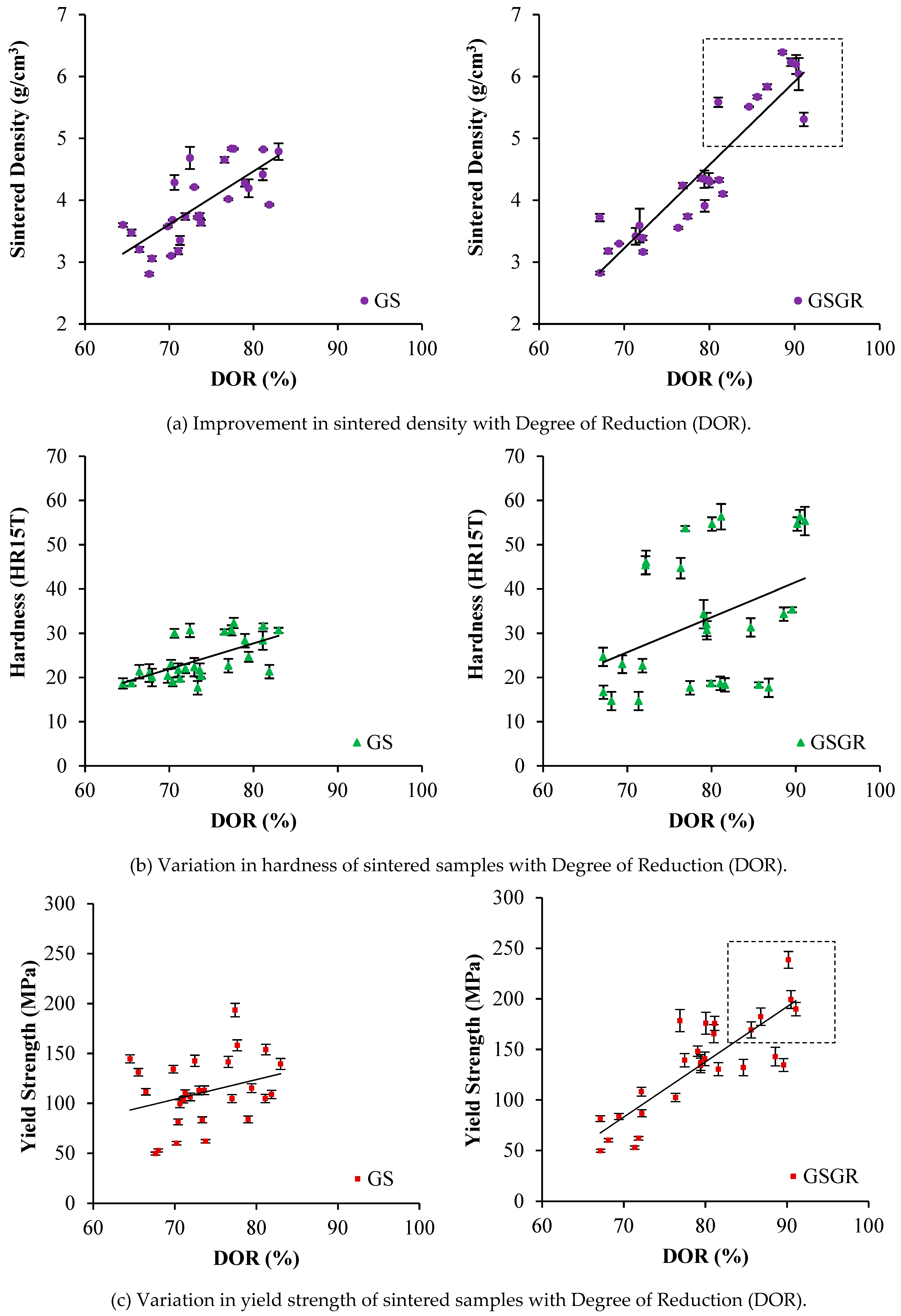
Sintered density, hardness, and yield strength show an increasing trend with DOR. The reason is the characteristics (properties and DOR) are simultaneously influenced by in-situ reduction and setting of control parameters. Sintered densities of GS and GSGR samples were similar for 65% to 80% DOR (Figure 2a). However, sintered density of GSGR samples further improved from 5.5 to 6.5 g/cm3 with an increase in DOR facilitated by 25% excess (compared to stoichiometry) carbon, compaction pressure (1050 MPa), sintering temperature (1200–1300 °C), and argon gas atmosphere in sintering. Hardness measured on the surface of cylindrical sintered samples does not correlate well with DOR (which is a bulk characteristic) [21]. The yield strength of sintered samples also correlated well with DOR because the increase in the metallic transformation resulting from reduction and densification enable a proportional increase in yield strength of the GSGR samples.
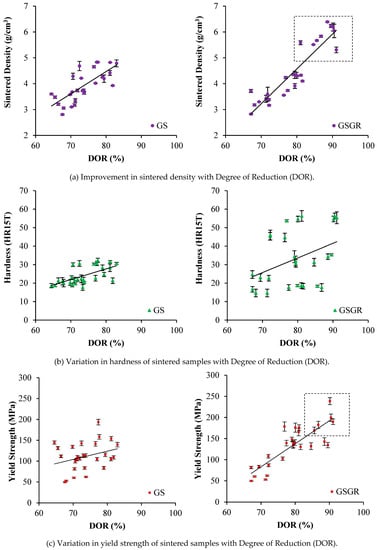
Figure 2.
Influence of Degree of Reduction on sintered properties of GS and GSGR parts manufactured via Powder Metallurgy route.
The effect of sintering parameters (sintering temperature and sintering atmosphere) on CI90 and GSGR75 feedstocks was studied for suitability of MIM for recycling. These parts were characterized to recognize the physical and mechanical property changes with sintering parameters and feedstock materials. Properties of sintered samples of CI90 were used as a reference to normalize the improvement in properties of sintered GSGR75 samples obtained from the MIM process.
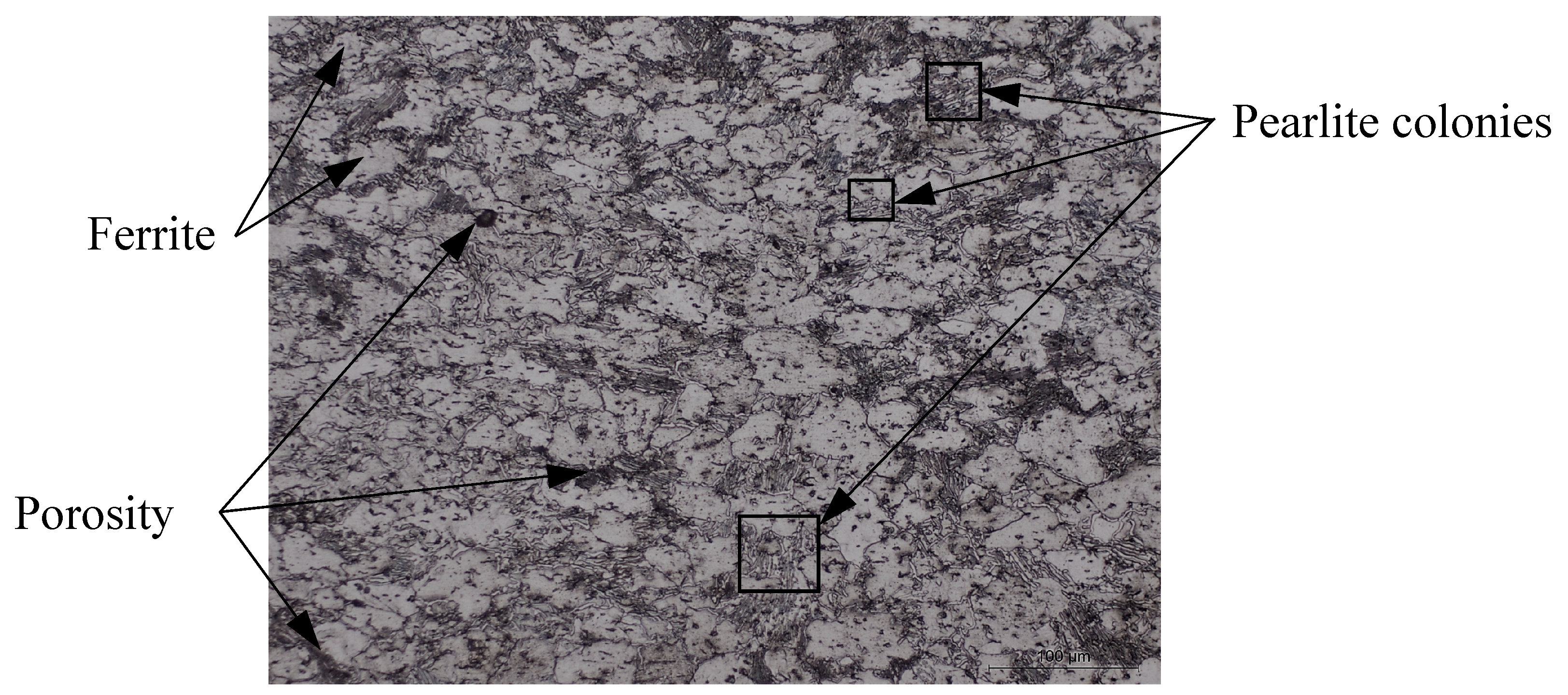
In-situ reduction of grinding sludge took place in GSGR75 samples by carbothermic reduction of iron oxide during sintering of MIM parts. The carbon is sourced from pyrolytically generated carbon and externally added graphite in 25% excess for carbothermic reduction. The trend of increasing sintered properties of GSGR75 parts with DOR is similar to that of GSGR, as shown in Figure 2. However, the correlation between sintered properties and DOR is weak in MIM-based GSGR75 parts, as compared to PM based GSGR. The weak correlation is because of the heterogeneous distribution of graphite in grinding sludge (arises from low solid loading in GGS75 i.e., 75 wt. %) limits the carbothermic reduction, densification, and properties of sintered parts. Figure 3 shows micrographs of sintered GSGR at 1200 °C and argon gas atmosphere. Grain boundaries of free ferrite, pearlite colonies (laminar structure of ferrite-cementite phases), and pores are clearly visible.
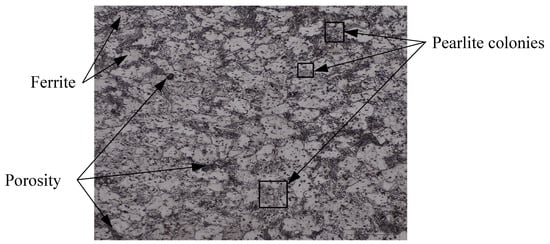
Figure 3.
Micrograph of cross section of cylindrical shaped GSGR (PM) sample sintered at 1200 °C with argon gas purge.
The quantification (DOR and DOD) during the recycling process via PM and MIM suggest a lower degree of completion of reduction and densification. During recycling, the optimal parametric setting provides ~92% DOR and ~70% DOD by PM technique; and ~69% DOR and ~74% DOD by the MIM technique. Additional improvement in these characteristics is not possible because:
- The residual carbon after carbothermic reduction during sintering hinders the physical contact between iron particles, which limits the extent of reduction and prolongs the process of necking for densification in PM. Particle contact developed during sintering of injection molded part of GSGR75 feedstock is even weaker due to substantial pore volume created during debinding, i.e., before the start of the sintering stage.
- The existence of pores during sintering is prolonged (densification gets delayed) because of the presence of irregularly sized and shaped pores, which hinder even volumetric diffusion during densification.
- The energy change during carbothermic reduction is considerably higher than the interfacial energy of particles. This does not support the pore removal mechanism (which requires high interfacial energy) and hence leads to decrease in densification. A more substantial volume difference between reactant (iron oxide and carbon) and the product (iron) opposes reduction and densification due to the high energy required to enhance the volumetric diffusion.
- In the case of MIM, finer powder particles accelerate densification because of higher activation energy for surface diffusion compared to reduction reaction rate.
- If densification started earlier complete reduction would be expected (especially in the case of very high sintering temperatures). However, bulk diffusion and grain coarsening would enhance the densification rate of reduced iron, which would entrap the unreduced portion and decrease the extent of reduction and sintered density.
- The growing preliminary iron grains surround the secondary (small) pores formed in a close-packed arrangement leading to low shrinkage. A considerable shrinkage is observed when large pores get eliminated.
3.2. Evaluation of Recyclability Index of GS, GSGR (PM) and GSGR75 (MIM)
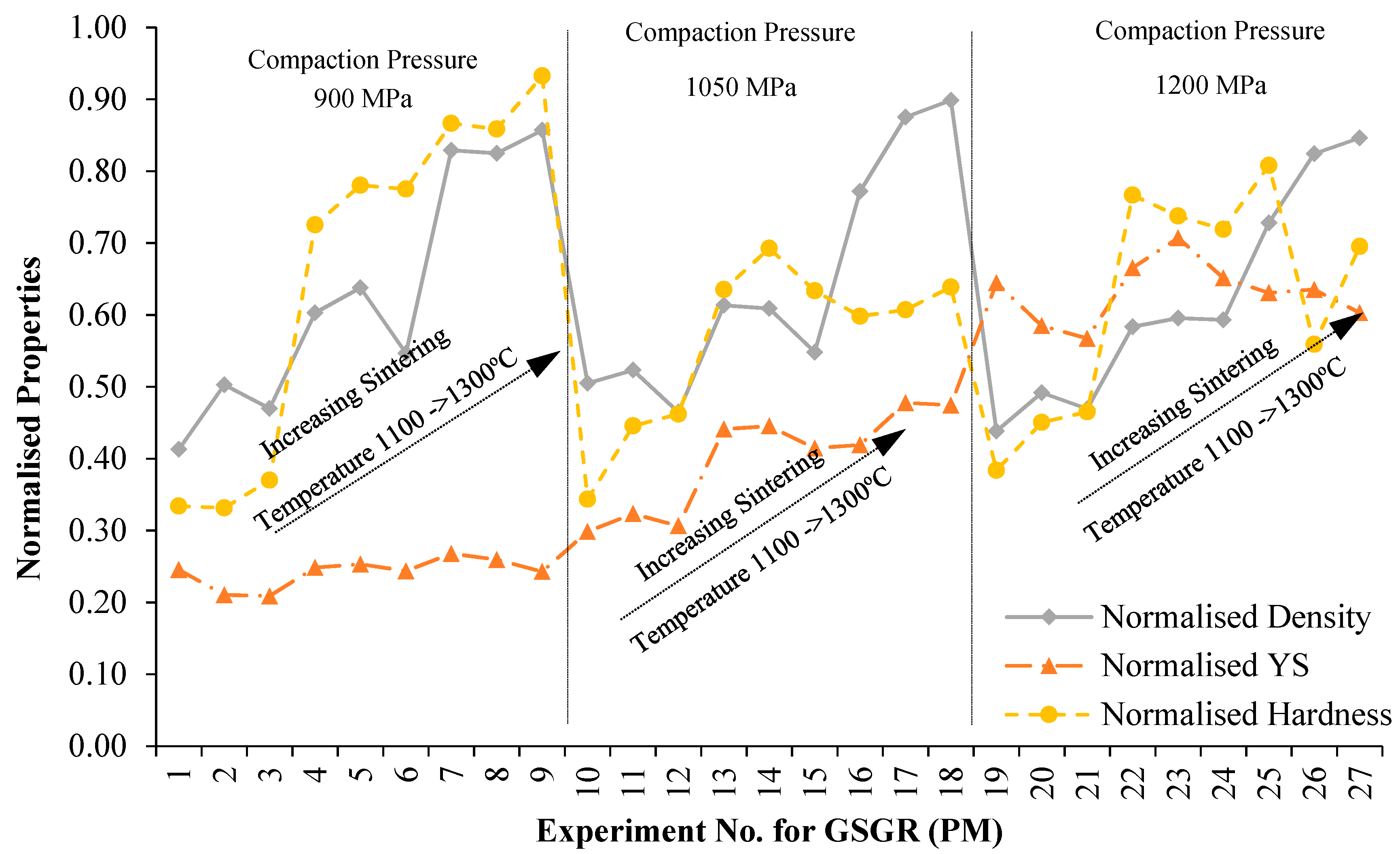
As a general observation from Figure 2, measured properties showed more improvement in GSGR than in GS with increasing DOR, though samples of the two feedstocks were processed under identical conditions. To evaluate relative improvement of properties by in-situ carbothermic reduction of grinding sludge being recycled, samples of CP iron processed under similar conditions were characterized and used as a reference (for normalizing). Sintered properties of GSGR samples were normalized with CP iron properties for the same experimental setting (Exp. No. 1–27) and plotted, as shown in Figure 4. Normalized properties of GSGR show an increasing trend with sintering temperature from 1100°C to 1300 °C for any of the selected compaction pressures. The variation in compaction pressure does not show significant improvements in the normalized properties of GSGR samples. Furthermore, these individual normalized properties were averaged and represented as a Recyclability Index (relation 7) corresponding to the experimental setting for GS and GSGR samples. For example, Recyclability Index for corresponding to Exp. No.14 for GSGR is calculated as:
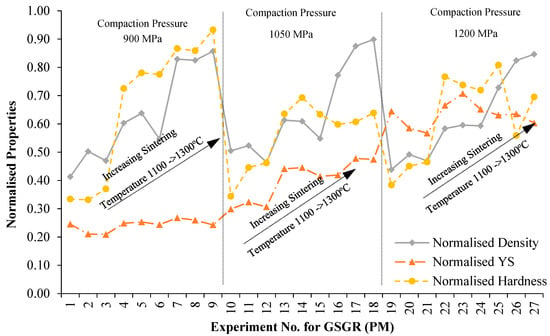
Figure 4.
Variation in normalized properties of GSGR (PM) sintered samples with experimental condition (parametric setting corresponding to the Exp. No. is given in Table A1).
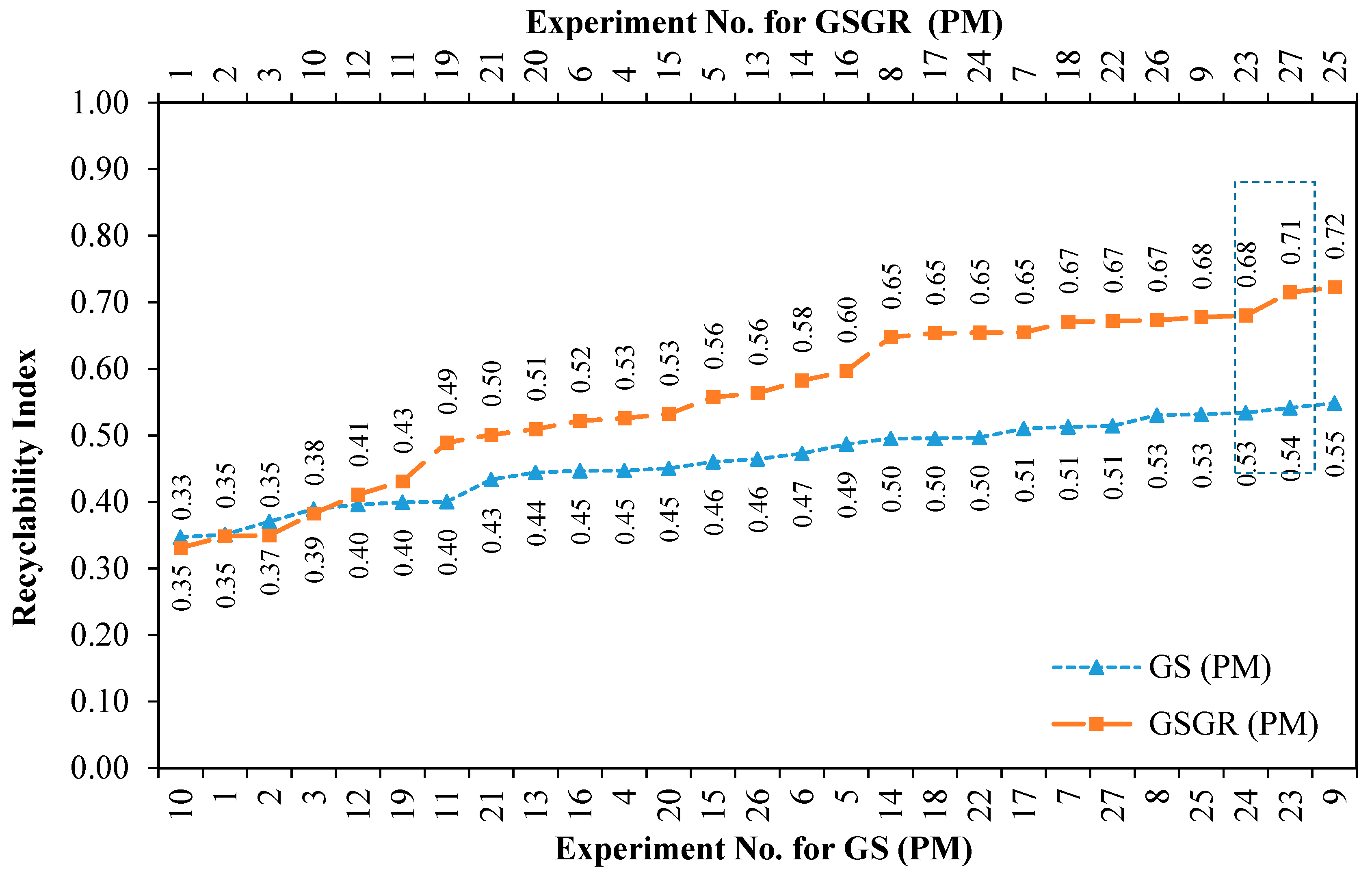
Recyclability Index (averaged sintered properties) quantifies the overall normalized property of sintered samples for a given experimental setting. This effectively quantifies improvement in sintered properties by carbothermic reduction. This improvement was greater in GSGR than GS as the energy input for processing CP iron, GS, and GSGR are identical. This Recyclability Index is only used to characterize the relative improvement in properties (for structural strength) of GS and GSGR with corresponding properties of CP iron under identical processing conditions. Recyclability Index is arranged in ascending order with corresponding experiment number for GS and GSGR samples, as given in Figure 5. Except for four experimental conditions (Exp. 1, 2, 3, and 10), Recyclability Index of GSGR is higher than that of GS. These experimental conditions have a sintering temperature of 1100 °C and lower compaction pressure, which resulted in a limited carbothermic reduction, residues of graphite, and partial metallic transformation of grinding sludge. The highest magnitude of Recyclability Index is observed in GSGR samples from Experiments 25 and 27, which is almost 17% more than the corresponding highest value for the GS samples.
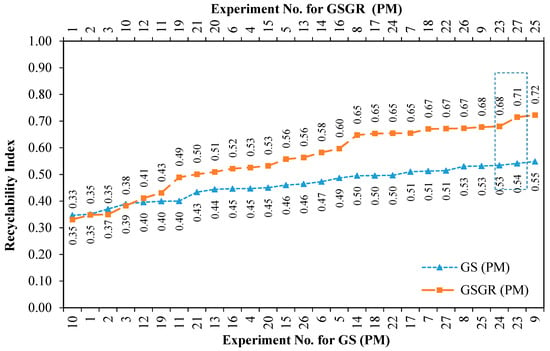
Figure 5.
Improvement in Recyclability Index of grinding sludge by carbothermic reduction for different experimental settings using Powder Metallurgy route.
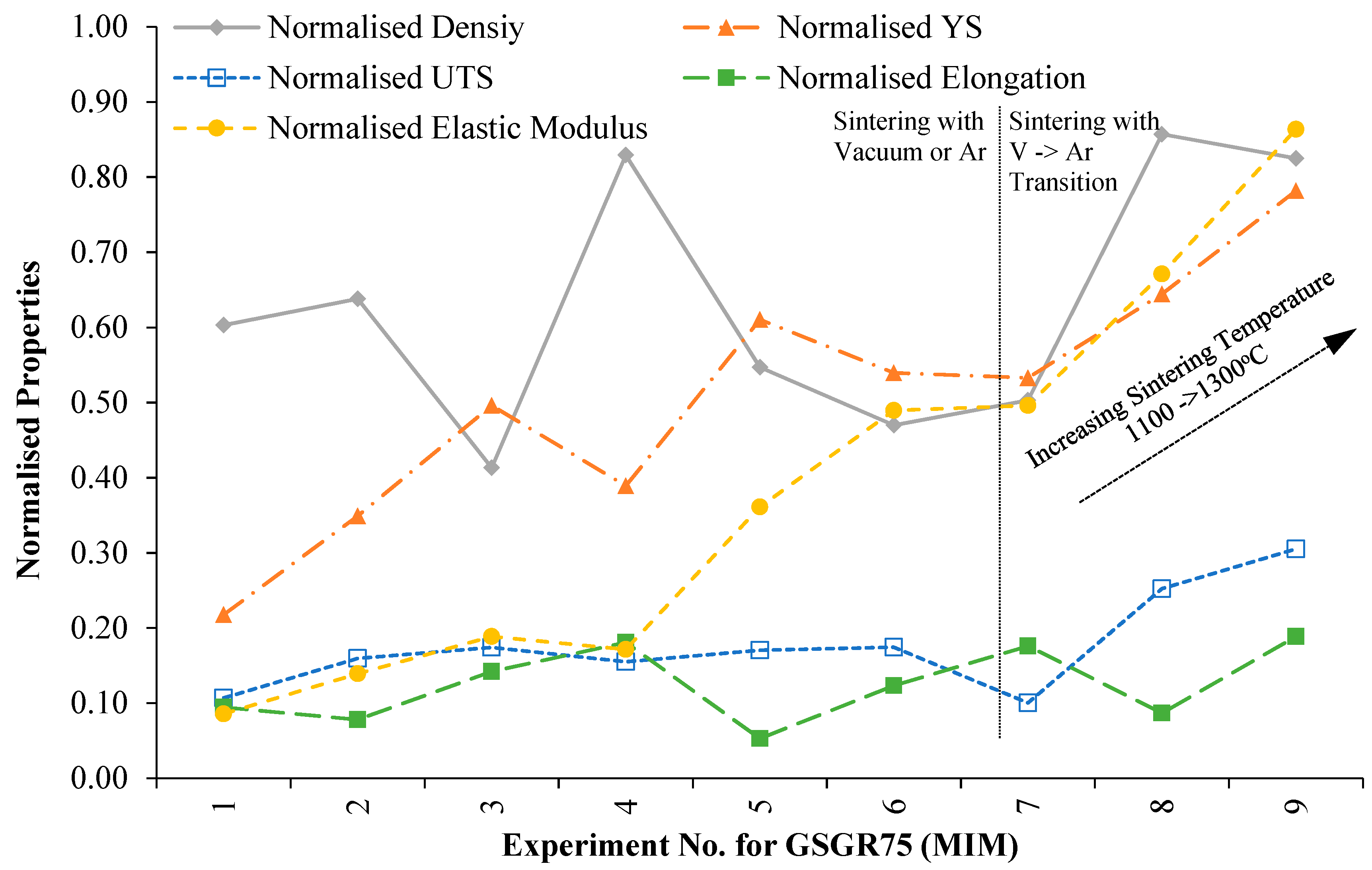
In order to evaluate the suitability of MIM-based recycling approach, sintered properties of GSGR75 samples were normalized with C90 properties for the corresponding experimental setting (Exp. No. 1–9 from Appendix B) and plotted in Figure 6. Normalized properties of GSGR75 show an increasing trend with sintering temperature from 1100°C to 1300 °C especially in experiments wherein samples were thermally debinded in vacuum followed by immediate sintering in argon.
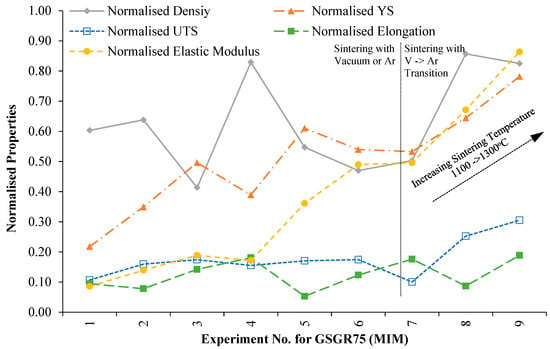
Figure 6.
Variation in normalized properties of GSGR75 (MIM) sintered samples with experimental condition.
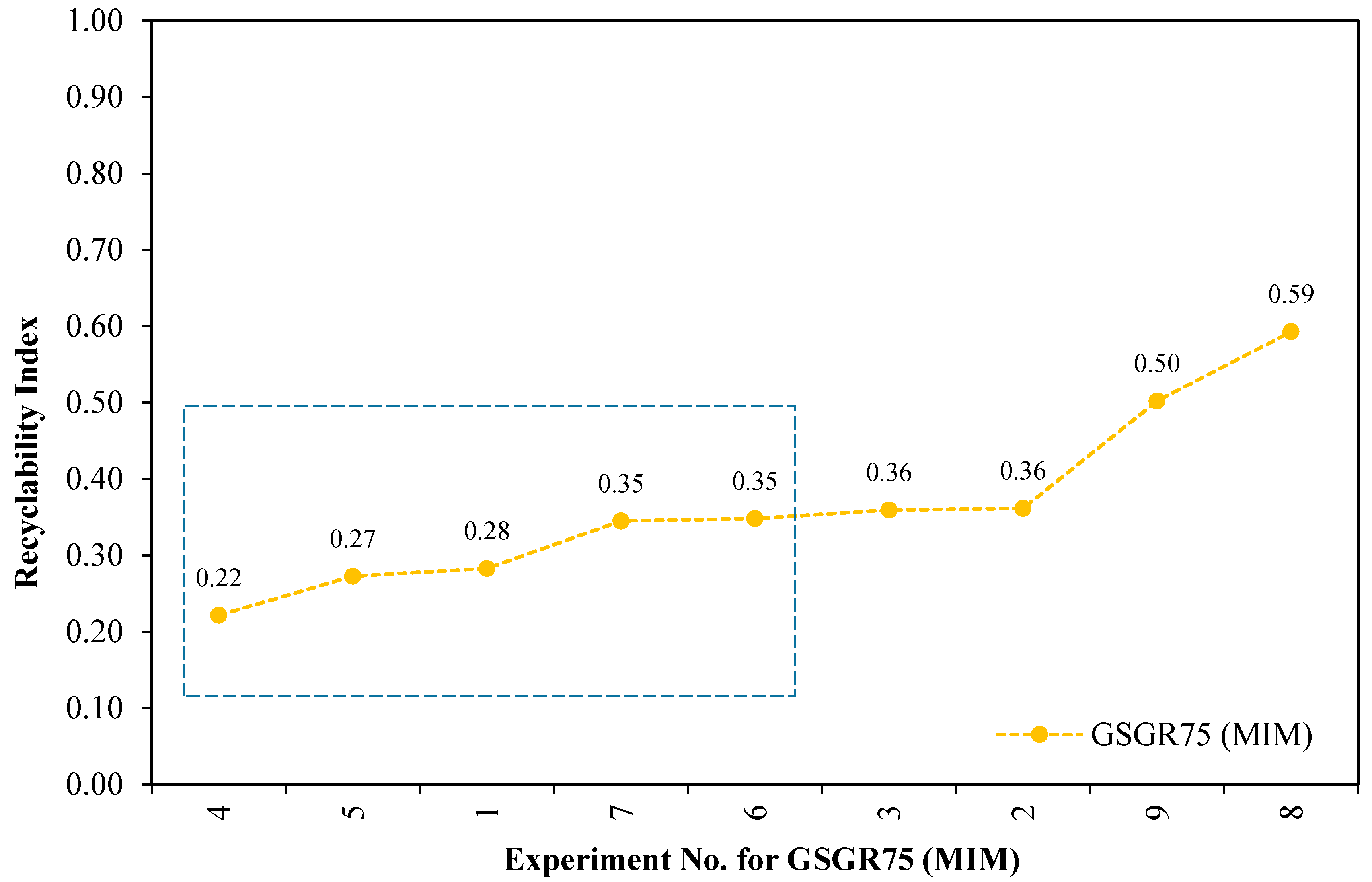
Furthermore, these individual normalized properties were averaged and represented as a Recyclability Index (see relation 6) corresponding to the experimental setting for GGSR75 samples. These averaged numbers (Recyclability Index) quantify the overall normalized property of sintered samples for a given experimental setting (Exp. No. 1–9 from Appendix B). This Recyclability Index is useful for comparison between the overall structural strength of GSGR75 and CI90 with equivalent energy input for MIM processing. Recyclability Index is arranged in ascending order with corresponding experiment number for GSGR75 and given in Figure 6.
Maximum Recyclability Index (0.59) of GSGR75 is observed from Figure 7 with Exp. No. 8 (sintering temperature: 1300 °C and sintering atmosphere: V-Ar wherein samples were thermally debinded in vacuum followed by immediate sintering in argon). Experiments (Exp. No. 1, 4, 7) with a sintering temperature of 1100 °C and vacuum sintering result in limited carbothermic reduction and formation of highly porous structure.
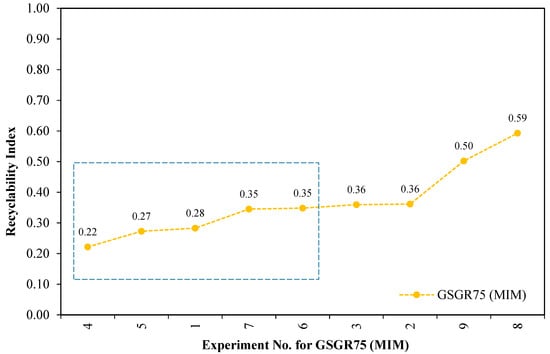
Figure 7.
Recyclability of grinding sludge by carbothermic reduction for different experimental settings using Metal Injection Molding (MIM) route.
Based on the experimental observations, the following mechanism of solid state sintering for recycling based on powder technology is proposed. Evolution of properties is also consistent with the degree of physical changes after sintering. The carbothermic reduction involves about 17 to 23 (depending upon the presence of iron oxide phase and corresponding reaction rate favoring conditions) volume percent change due to rearrangement iron lattice and atomic oxygen transport [22,23].
In the present study, densification proceeds through the following stages during sintering:
- Particle contact develops during compaction or injection, and the contacting particles start necking by surface and grain boundary diffusion. This leads to the formation of pores of various sizes.
- Pores tend to become rounded to lower the surface energy. The rate of reduction of porosity diminishes with decreasing pore size and hence resulting in lowered densification rate as sintering progresses.
- Combination of volumetric (bulk) diffusion and grain coarsening further enhances the densification rate and tends to eliminate the remaining porosity [24].
3.3. Applicability of Recycling and Manufacture of Porous PM Parts
Solid state recycling attempted in the present study involves in-situ carbothermic reduction of iron oxide from a source of scrap and densification during sintering of PM and MIM compacts. Full reduction and densification is not possible at the same time in such a complex carbothermic reduction involving multiple stages and modes of reduction. However, based on the experimental observations and characterization of sintered properties, some optimal parametric settings have been arrived at.
In recycled parts, a maximum of 70% of the properties of sintered Carbonyl iron can be achieved in PM, while about 59% properties of the sintered carbonyl iron can be achieved in MIM after recycling. The deviation in the properties of recycled parts from those of sintered carbonyl iron properties is caused by the difference in the chemical composition and level of porosity after sintering. The chemical composition of recycled iron (PM) parts (1.12 wt. % C, 0.42 wt. % Si, 0.76 wt. % Mn, and balance Fe) is similar to that of a high carbon steel, which contains approximately 0.7–2.5 wt. % carbon. Recycled iron parts obtained by the MIM process may have an even higher carbon content (1.37 wt. %).
The quality characteristics of the usable products made from downgraded metallic scrap (grinding sludge) using the powder technology based recycling process may be identified as the degree of reduction, the degree of densification, sintered density, and mechanical properties. Material and processing conditions of sintered samples of ~0.7 Recyclability Index are further utilized for simultaneous powder metallurgy based recycling in which in-situ reduction of grinding sludge shaped into a product shape is used to manufacture porous products. The proposed recycling technique is used in manufacturing simple geometric shapes like a solid cylinder, bush, circular ring, and plain bearing.
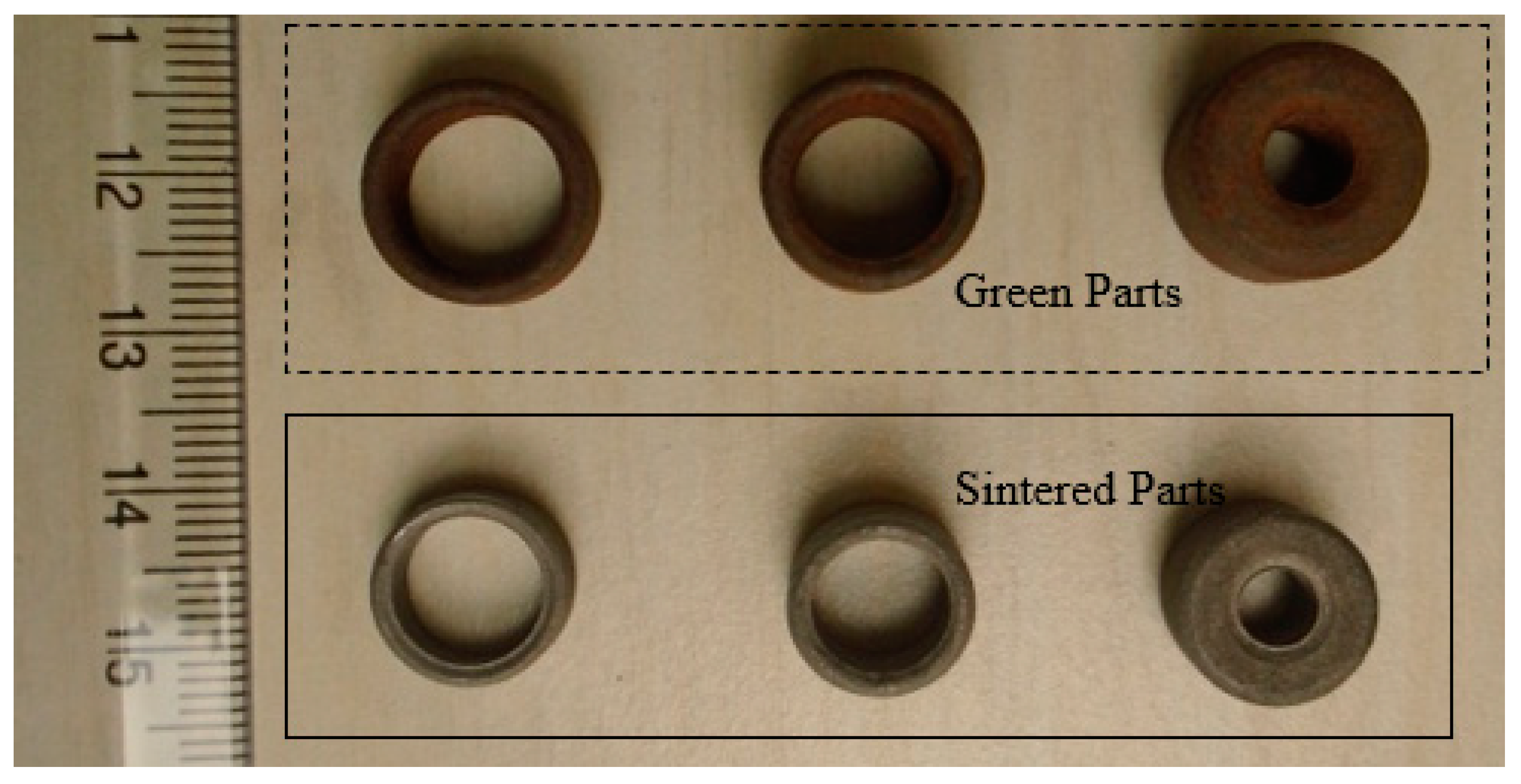
A mixture of grinding sludge and graphite (25% excess than the stoichiometric requirement for carbothermic reduction) GSGR was prepared. The mixed powder was then compacted at 1050 MPa compaction pressure. Compacted (green) samples were then sintered in a tubular furnace with a constant flow of argon gas at 150 mL/min. Parts sintered at 1200 °C are porous and shows considerable shrinkage, as shown in Figure 8.
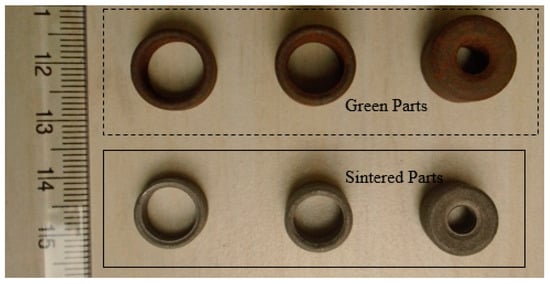
Figure 8.
Green and sintered PM parts of GSGR material.
Compaction die design considerations need to be developed for implementation of PM based recycling-for porous product manufacturing. This is because GSGR parts show significant volumetric shrinkage after sintering than CI parts because of simultaneous effect of carbothermic reduction and densification during sintering. These porous parts may work well for air film rolls/bearings, instrument filters, flow restrictors, flame arrestors, etc., in view of the performance requirements like filtration, flow control, porosity, and distribution. However, structural properties like sintered density and mechanical properties will have some limitations on account of porosity that is needed for functional needs of a porous part.
4. Conclusions
Extensive experimental studies were conducted to confirm possible implementation of powder technology (powder metallurgy and metal injection molding) for the development of a manufacturing process involving recycling of downgraded shop floor grinding sludge. Distinct experiments were performed to optimize PM and MIM based processing, on the sintered properties. It may be concluded that the interaction of sintering temperature (1200 °C) and sintering atmosphere (a constant flow of argon gas at 150 mL/min) significantly improves the characteristics of sintered parts after in-situ reduction and densification.
The mechanical properties of GSGR (PM) parts are better correlated with the degree of reduction (DOR) than GSGR75 (MIM) parts because of controlled carbothermic reduction and volumetric shrinkage in the sintering step of the PM process. A quantitative measure, namely, “Recyclability Index” was established to relate the effect of process parameters and process characteristics (DOR and DOD) with the sintered properties.
Recyclability Index of GSGR (PM) samples is significantly higher (~17%) than GS (PM) samples thanks to additional stoichiometric carbon (for completing in-situ carbothermic reduction). Recyclability Index of GSGR75 at the optimal parametric setting is 59% (meaning parts produced from this recycling technique have 59% physical and mechanical properties compared to those of the pure iron dense parts). Hence, the proposed MIM-based recycling approach would not be practically suitable for manufacturing of usable porous products. However, RI of GSGR parts is enough to implement powder metallurgy route for recycling to manufacture porous parts. Thus, PM based recycling of grinding sludge is possible for manufacturing of net-shaped porous products. Significantly high volumetric shrinkage during sintering also demands further optimization of compaction die design for dimensional control of sintered products.
Author Contributions
K.R. designed and performed the experiments. K.R. and P.D. analyzed the data with equal contribution. K.R. wrote the paper, and he is the corresponding author.
Funding
This research received no external funding.
Acknowledgments
The authors of this research paper acknowledge the support extended by the Department of Mechanical Engineering, IIT Bombay, that provided all necessary experimental and analytical resources.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| Acronyms | |
| PIM/MIM | : Powder/Metal Injection Moulding |
| PM | : Powder Metallurgy |
| RI | : Recyclability Index |
| ZrO2 | : Zirconia |
| GS | : Grinding Sludge |
| GSGR | : Grinding Sludge–Graphite mixture |
| CI | : Carbonyl Iron |
| CI90 | : 90% solid loading of Carbonyl Iron powder |
| GSGR75 | : 75% solid loading of GSGR powder |
| DOR | : Degree of Reduction |
| DOD | : Degree of Densification |
Appendix A

Table A1.
Experimental condition for Full Factorial 33 experiments conducted for PM study.
Table A1.
Experimental condition for Full Factorial 33 experiments conducted for PM study.
| Experiment No. | Experimental Settings for CP Fe, GS and GSGR | ||
|---|---|---|---|
| Compaction Pressure (MPa) | Sintering Temperature (°C) | Sintering Atmosphere | |
| 1 | 900 | 1100 | Vacuum |
| 2 | 900 | 1100 | Argon |
| 3 | 900 | 1100 | Nitrogen |
| 4 | 900 | 1200 | Vacuum |
| 5 | 900 | 1200 | Argon |
| 6 | 900 | 1200 | Nitrogen |
| 7 | 900 | 1300 | Vacuum |
| 8 | 900 | 1300 | Argon |
| 9 | 900 | 1300 | Nitrogen |
| 10 | 1050 | 1100 | Vacuum |
| 11 | 1050 | 1100 | Argon |
| 12 | 1050 | 1100 | Nitrogen |
| 13 | 1050 | 1200 | Vacuum |
| 14 | 1050 | 1200 | Argon |
| 15 | 1050 | 1200 | Nitrogen |
| 16 | 1050 | 1300 | Vacuum |
| 17 | 1050 | 1300 | Argon |
| 18 | 1050 | 1300 | Nitrogen |
| 19 | 1200 | 1100 | Vacuum |
| 20 | 1200 | 1100 | Argon |
| 21 | 1200 | 1100 | Nitrogen |
| 22 | 1200 | 1200 | Vacuum |
| 23 | 1200 | 1200 | Argon |
| 24 | 1200 | 1200 | Nitrogen |
| 25 | 1200 | 1300 | Vacuum |
| 26 | 1200 | 1300 | Argon |
| 27 | 1200 | 1300 | Nitrogen |
Appendix B

Table A2.
Experimental 32 design matrix and sintering condition for MIM sintering cycle.
Table A2.
Experimental 32 design matrix and sintering condition for MIM sintering cycle.
| Cycle | A: Sintering Temperature (°C) | B: Furnace Atmosphere during Thermal Debinding and Sintering | Coded Representation |
|---|---|---|---|
| 1 | 1100 | TD: Vacuum followed by S: Vacuum | 1100V |
| 2 | 1200 | TD: Vacuum followed by S: Vacuum | 1200V |
| 3 | 1300 | TD: Vacuum followed by S: Vacuum | 1300V |
| 4 | 1100 | TD: Vacuum, S: Ar gas separate | 1100Ar |
| 5 | 1200 | TD: Vacuum, S: Ar gas separate | 1200Ar |
| 6 | 1300 | TD: Vacuum, S: Ar gas separate | 1300Ar |
| 7 | 1100 | TD: Vacuum, S: Ar gas immediate transition | 1100V-Ar |
| 8 | 1200 | TD: Vacuum, S: Ar gas immediate transition | 1200V-Ar |
| 9 | 1300 | TD: Vacuum, S: Ar gas immediate transition | 1300V-Ar |
where TD: Thermal debinding and S: Sintering.
References
- Rick LeBlanc, an Overview of Metal Recycling, Its Importance and Recycling Processes. Available online: www.thebalance.com/an-introduction-to-metal-recycling-4057469 (accessed on 10 February 2018).
- Xylia, M.; Silveira, S.; Duerinck, J.; Meinke-Hubeny, F. Meinke-Hubeny, Weighing regional scrap availability in global pathways for steel production processes. Energy Effic. 2018, 11, 1135–1159. [Google Scholar] [CrossRef]
- Sibley, S.F.; Butterman, W.C. Metals recycling in the United States. Resour. Conserv. Recycl. 1995, 15, 259–267. [Google Scholar] [CrossRef]
- Güley, V.; Güzel, A.; Jäger, A.; Khalifa, N.B.; Tekkaya, A.E.; Misiolek, W.Z. Misiolek, Effect of die design on the welding quality during solid state recycling of AA6060 chips by hot extrusion. Mater. Sci. Eng. A 2013, 574, 163–175. [Google Scholar] [CrossRef]
- Yu Yao Kebang Powder Metallurgy Co. Ltd. 2015. Available online: www.kbpm.cn/en/th.asp (accessed on 10 February 2015).
- Asian Powder Metallurgy Association (APMA) Report 2009. Available online: www.apma.asia/#GlobalTransition (accessed on 10 February 2015).
- German, R.M. Powder Metallurgy of Iron and Steel; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Ramakrishnan, P. History of powder metallurgy. Indian J. Hist. Sci. 1983, 18, 109–114. [Google Scholar]
- Upadhyaya, G.S. Powder Metallurgy Technology; Cambridge International Science Publishing: Great Abingdon, UK, 1997. [Google Scholar]
- Rane, K.K.; Date, P.P. Sustainable recycling of ferrous metallic scrap using powder metallurgy process. J. Sustain. Metall. 2017, 3, 251–264. [Google Scholar] [CrossRef]
- Berginc, B.; Kampus, Z.; Sustarsic, B. The use of the Taguchi approach to determine the influence of injection-moulding parameters on the properties of green parts. J. Achiev. Mater. Manuf. Eng. 2006, 15, 63–70. [Google Scholar]
- Heaney, D.F. Qualification method for powder injection moulded components. P/M Sci. Technol. Briefs 2004, 6, 21–27. [Google Scholar]
- Demirci, F.; Turan, A.; Yucel, O. An investigation on the direct reduction of mill scale from continuous steel casting. In Proceedings of the International Iron & Steel Symposium, Karabuk University, Kılavuzlar, Turkey, 2–4 April 2012. [Google Scholar]
- Rane, K.K.; Date, P.P. Pulverisation of forging scales and grinding sludge: A new technique for metallic recycling for Forging Industries. Waste Manag. Resour. Util. 2014, 2014, 637–642. [Google Scholar]
- Rane, K.K.; Date, P.P. Reduction and densification characteristics of iron oxide metallic waste during solid state recycling. Adv. Powder Technol. 2015, 26, 126–138. [Google Scholar] [CrossRef]
- German, R.M.; Bose, A. Injection Moulding of Metal and Ceramic; Metal Powder Industries Federation: Princeton, NJ, USA, 1997. [Google Scholar]
- Karatas, C.; Kocer, A.; Unal, H.; Saritas, S. Rheological properties of feedstocks prepared with steatite powder and polyethylene-based thermoplastic binders. J. Mater. Process. Technol. 2004, 152, 77–83. [Google Scholar] [CrossRef]
- Rane, K.K.; Date, P.P. Rheological investigation of MIM feedstocks for reducing frictional effects during Injection Molding. Proc. Adv. Mater. Res. 2014, 966–967, 196–205. [Google Scholar] [CrossRef]
- Urval, R.; Lee, S.; Atre, S.V.; Park, S.J.; German, R.M. Optimization of process conditions in Powder Injection Moulding of Microsystem components using a Robust Design Method: Part I. Primary Design Parameters. Powder Metall. 2008, 51, 133–142. [Google Scholar] [CrossRef]
- Sidambe, A.T.; Figueroa, I.A.; Hamilton, H.; Todd, I. Improved Processing of Titanium Alloys by Metal Injection Moulding. IOP Conf. Ser. Mater. Sci. Eng. 2011, 26, 109–117. [Google Scholar] [CrossRef]
- Laska, M.; Kazior, J. Influence of various process parameters on the density of sintered aluminium alloys. Acta Polytech. 2012, 52, 93–95. [Google Scholar]
- Prakash, S. Reduction and sintering of fluxed iron ore pellets-a comprehensive review. J. S. Afr. Min. Metall. 1995, 96, 3–16. [Google Scholar]
- Srinivasan, N. Reduction of iron oxides by carbon in a circulating fluidized bed reactor. Powder Technol. 2002, 124, 28–39. [Google Scholar] [CrossRef]
- German, R.M. Sintering simplified: Surface area, density, and grain size relations. Mater. Sci. Forum 2016, 835, 50–75. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).