Abstract
Municipal solid waste (MSW) recycling is constrained by contamination, heterogeneity, and infrastructure built around material-specific pathways. We introduce effectiveness-normalized greenhouse gas (GHG) emissions as a system-level metric that adjusts reported process burdens by feedstock eligibility (Effectiveness Fraction, EF) and carbon recovery efficiency (CRE) to reflect real-world MSW conditions. Using published LCA data and engineering estimates, we benchmark six pathways, mechanical recycling, PET depolymerization, enzymatic depolymerization, pyrolysis, supercritical water gasification (SCWG), and Regenerative Robust Gasification (RRG), at the scale of mixed MSW. Normalizing for EF and CRE reveals large differences between process-level and system-level performance. Mechanical recycling and PET depolymerization show low process intensities yet high normalized impacts because they can treat only a small share of plastics in MSW. SCWG performs well at broader eligibility. RRG, a plasma-assisted molten-bath approach integrated with methanol synthesis, maintains the lowest normalized impact (~1.6 t CO2e per ton of recycled polymer) while accepting virtually all organics in MSW and vitrifying inorganics. Modeled methanol yields are ~200–300 gal·t−1 without external hydrogen and up to ~800 gal·t−1 with renewable methane reforming. The metric clarifies trade-offs for policy and investment by rewarding technologies that maximize diversion and carbon retention. We discuss how effectiveness-normalized results can be incorporated into LCA practice and Extended Producer Responsibility (EPR) frameworks and outline research needs in techno-economics, regional scalability, hydrogen sourcing, and uncertainty analysis. Findings support aligning infrastructure and procurement with robust, scalable routes that deliver circular manufacturing from heterogeneous MSW.
1. Introduction
The packaging industry plays a critical role in modern consumer markets, yet its environmental footprint—particularly from packaging waste—has become a major concern [1]. Traditional recycling systems rely heavily on mechanical recycling, which involves collecting, sorting, cleaning, and reprocessing polymers such as PET, HDPE, and PP. While technically all packaging materials are recyclable, practical recyclability is constrained by contamination, multilayer formats, and market demand. These limitations result in persistently low recycling rates and high contamination levels, undermining circular economy goals [2,3].
To address these challenges, interest is growing in robust recycling technologies that can process mixed and contaminated waste streams without extensive sorting. This review introduces Regenerative Robust Gasification (RRG)—a plasma-assisted, molten-bath gasification process capable of converting virtually all organics in municipal solid waste (MSW) into synthesis gas (syngas), which can then be upgraded to methanol for circular manufacturing [4]. Unlike material-specific recycling methods, RRG offers system-level scalability and resilience, enabling the production of high-quality recycled content from heterogeneous waste.
Recent advances in life cycle assessment (LCA) highlight the need for metrics that reflect real-world conditions rather than idealized feedstocks. We propose effectiveness-normalized greenhouse gas (GHG) emissions, which adjust reported process emissions by feedstock eligibility and carbon recovery efficiency [5]. Using this metric, we benchmark six recycling pathways—mechanical recycling, PET depolymerization, enzymatic depolymerization, pyrolysis, supercritical water gasification, and RRG—under realistic MSW composition scenarios [6,7].
Throughout this review, we use several related but distinct terms to describe advanced recycling technologies. Regenerative Robust Gasification (RRG) refers to a specific implementation of robust gasification that integrates plasma-assisted conversion in a molten-bath gasifier, with hydrogen enhancement to produce methanol from mixed municipal solid waste, specifically for circular manufacturing. Robust gasification is a broader category encompassing gasification systems capable of handling contaminated and heterogeneous waste streams with minimal sorting. Robust recycling is a conceptual term used to describe recycling approaches that maintain performance despite contamination and complexity, including but not limited to robust gasification. Plasma-assisted gasification refers specifically to gasification systems that use plasma arcs or torches to achieve high temperatures and is a key component of RRG. These terms are used deliberately to reflect their technical and functional distinctions.
This review aims to
- Examine the limitations of conventional recycling methods.
- Introduce and define the effectiveness-normalized GHG metric.
- Compare six recycling technologies using normalized performance indicators.
- Discuss the technical, economic, and policy implications of adopting RRG for packaging circularity and supply chain resilience.
1.1. Methodology and Scope
This review synthesizes published life cycle assessment (LCA) data and technical literature to compare recycling technologies under realistic municipal solid waste (MSW) conditions. LCAs follow accepted ISO-based structures; however, many assume high-purity feedstocks, which can misrepresent system-level performance for heterogeneous MSW. To address this limitation, we applied an effectiveness-normalization framework that adjusts reported greenhouse gas (GHG) emissions by feedstock eligibility and carbon recovery efficiency. This approach does not replace LCA methodology but complements it by exposing a structural weakness in comparative assessments and providing a fairer basis for evaluating technologies at scale. Details of normalization assumptions and calculations are provided in Appendix A.
1.2. Overview of Municipal Solid Waste and Conventional Recycling Methods
1.2.1. Municipal Solid Waste
Municipal Solid Waste (MSW) refers to everyday items discarded by households, businesses, and institutions. It includes packaging, food scraps, paper, plastics, textiles, and other materials collected through curbside programs or drop-off centers. In the United States, MSW generation increased from 88.1 million tons in 1960 to 292.4 million tons in 2018, averaging 4.9 pounds per person per day [8]. Of this, 32.1% was recycled, 11.8% was combusted with energy recovery, and about 6% underwent biochemical processing through anaerobic digestion. Global projections indicate MSW will reach 3.4 billion tons by 2050, with North America contributing nearly 400 million tons [9].
1.2.2. Landfilling
Landfilling remains the most common disposal method but poses significant environmental challenges. Anaerobic degradation of organic waste generates landfill gas—primarily methane and carbon dioxide—both potent greenhouse gases, with methane having a far higher global warming potential [10]. Although modern sanitary landfills incorporate gas capture systems, methane emissions persist, and landfill fires release toxic compounds such as dioxins [11]. These issues underscore the need to reduce reliance on landfilling.
1.2.3. Incineration (Waste-to-Energy)
Incineration, often implemented as waste-to-energy (WTE), reduces waste volume by up to 90% while generating electricity and steam [12]. However, it produces bottom and fly ash requiring specialized disposal and emits pollutants including dioxins, furans, and heavy metals, requiring advanced controls [13]. Compliance costs under frameworks such as the EU Waste Incineration Directive and U.S. Clean Air Act range from $70–$200 per ton, plus $20–$50 per ton for upgrades [14,15,16]. These economic and environmental constraints limit scalability despite energy recovery benefits.
1.2.4. Mechanical Recycling
Mechanical recycling reprocesses sorted plastics into pellets but is highly sensitive to contamination and composite packaging. While effective for clean PET, HDPE, and PP streams, contamination rates remain high—averaging 25% in Florida and reaching 90% in Cleveland despite decades of consumer education [2,3]. Toronto’s Blue Bin program reports that 30% of recyclables are landfilled due to contamination [17]. Multilayer flexible packaging and black plastics further challenge optical sorting systems, making many materials effectively non-recyclable [18].
These systemic limitations, combined with material degradation during repeated cycles and fluctuating market demand, undermine the economic viability and scalability of mechanical recycling for mixed MSW streams [19,20]. Quality degrades with each cycle, and food-contact applications require extra decontamination. Logistics include transport to MRFs and disposal of residues (20–30%). Despite decades of consumer education, contamination rates remain high—up to 90% in some regions—making mechanical recycling economically and technically limited for mixed MSW streams.
1.2.5. Comparison of Recycling Technologies
In addition to mechanical recycling, several other recycling technologies have been developed, each with distinct feedstock requirements and environmental profiles. This section describes these methods and compares their system-level performance with Regenerative Robust Gasification (RRG), the proposed approach in this review.
To contextualize the advantages of Regenerative Robust Gasification (RRG), we compare it with five other recycling technologies: mechanical recycling, PET depolymerization, enzymatic depolymerization, pyrolysis, and supercritical water gasification (SCWG). Each process differs in feedstock compatibility, energy intensity, and scalability.
A key challenge in comparing these technologies is that life cycle assessments (LCAs) often assume idealized, high-purity feedstocks, which misrepresent system-level performance. To address this, we introduce effectiveness-normalized GHG emissions, which adjust reported process emissions by two factors:
- −
- Effectiveness Fraction (EF): The share of plastics in MSW that a process can realistically handle.
- −
- Carbon Recovery Efficiency (CRE): The fraction of carbon retained in useful outputs rather than lost as CO2 or residues.
This normalization highlights the true system-level impact of each technology. For example, if a process only accepts 10% of plastics in MSW, its GHG emissions per ton of recycled polymer are multiplied by 10 to reflect its limited contribution to overall diversion.
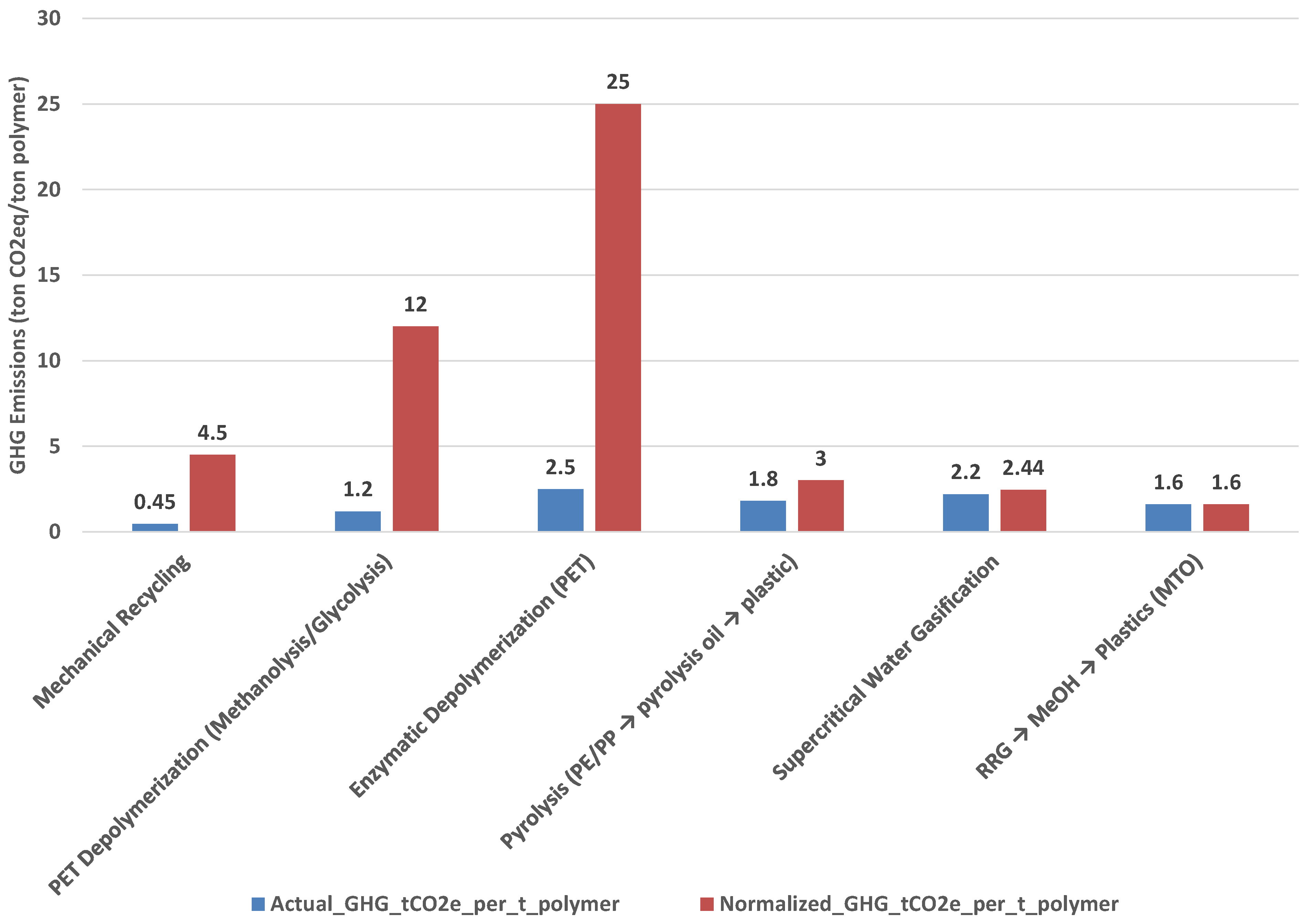
Table 1 summarizes feed limitations, primary outputs, actual GHG emissions, eligible fraction, and normalized GHG values for each process. Mechanical recycling and PET depolymerization exhibit low actual GHG values (0.45–1.2 t CO2e/t polymer), but their normalized impacts rise sharply (4.5–12.0 t CO2e) because they can only process about 10% of plastics in MSW. Enzymatic depolymerization fares worst, with normalized emissions exceeding 25.0 t CO2e. Pyrolysis performs better at system scale (3.0 t CO2e) due to broader compatibility with polyolefins, while SCWG achieves 2.44 t CO2e by accepting most plastics. In contrast, RRG maintains the lowest normalized impact—1.6 t CO2e per ton of recycled polymer—because it accepts virtually all organics in MSW and retains most carbon in circular products.

Table 1.
System-level comparison of six recycling technologies. Actual GHG values represent cradle-to-gate emissions per ton of recycled polymer (or polymer-equivalent). Normalized GHG values adjust for feedstock eligibility (Effectiveness Fraction, EF) and carbon recovery efficiency (CRE), providing a realistic basis for evaluating circularity potential.
This comparison underscores why normalization is critical for policy and infrastructure planning. Technologies that appear efficient in isolation may deliver limited system-level benefits when eligibility constraints are considered. By integrating EF and CRE into performance metrics, decision-makers can prioritize solutions that maximize diversion, carbon retention, and circularity.
1.3. Life Cycle Assessment Framework
Life cycle assessment (LCA) is an ISO-standardized methodology for evaluating environmental impacts across a product’s life cycle. Many published LCA studies assume high-purity feedstocks and idealized conditions, which can misrepresent system-level performance for heterogeneous municipal solid waste (MSW). In this review, we synthesized published LCA data that follow these accepted boundaries and functional units. To address the limitations of these assumptions, we applied an effectiveness-normalization framework that adjusts reported greenhouse gas (GHG) emissions by feedstock eligibility and carbon recovery efficiency. This approach does not replace standard LCA methodology but complements it by providing a fairer basis for comparing recycling technologies under real-world conditions. Details of normalization assumptions and calculations are provided in Appendix A.
Details of normalization assumptions and calculations are provided in Appendix A. (RRG) This normalization highlights the true system-level impact of each technology. For example, if a process only accepts 10% of plastics in MSW, its GHG emissions per ton of recycled polymer are multiplied by 10 to reflect its limited contribution to overall diversion.
Table 1 summarizes feed limitations, primary outputs, actual GHG emissions, eligible fraction, and normalized GHG values for each process. Mechanical recycling and PET depolymerization exhibit low actual GHG values (0.45–1.2 t CO2e/t polymer), but their normalized impacts rise sharply (4.5–12.0 t CO2e) because they can only process about 10% of plastics in MSW. Enzymatic depolymerization fares worse, with normalized emissions exceeding 25.0 t CO2e. Pyrolysis performs better at the system scale (3.0 t CO2e) due to broader compatibility with polyolefins, while SCWG achieves 2.44 t CO2e by accepting most plastics. In contrast, RRG maintains the lowest normalized impact—1.6 t CO2e per ton of recycled polymer—because it accepts virtually all organics in MSW and retains most carbon in circular products [5,6,7].
1.3.1. Mechanical Recycling
Mechanical recycling reprocesses sorted plastics into pellets but is highly sensitive to contamination, multilayer packaging, and black plastics that optical sorters cannot detect. Quality degrades with each cycle, and food-contact applications require extra decontamination. Logistics include transport to MRFs and disposal of residues (20–30%). Despite decades of consumer education, contamination rates remain high—up to 90% in some regions—making mechanical recycling economically and technically limited for mixed MSW streams [2,3].
1.3.2. PET Depolymerization (Methanolysis/Glycolysis)
Chemical depolymerization targets PET, converting it into monomers such as DMT and EG for repolymerization into virgin-quality PET [21,22]. Processes include methanolysis, glycolysis, and hydrolysis, each requiring PET-rich feedstock and extensive sorting to remove contaminants and multilayers. While effective for PET, these methods do not address mixed plastic waste, reinforcing the limitations of material-specific recycling.
1.3.3. Pyrolysis of Polyolefins
Pyrolysis thermally decomposes polyolefins (PE, PP) into oils, waxes, and gases without oxygen. It tolerates contamination better than mechanical recycling but excludes oxygenated polymers (PET, PVC) and halogenated plastics, which form toxic byproducts. Pyrolysis oil requires refining and mass balance accounting, limiting recycled content to <5% of refinery inputs [23]. These constraints make pyrolysis unsuitable for unsorted MSW [24].
1.3.4. Enzymatic Depolymerization
Enzymatic depolymerization hydrolyzes PET under mild conditions using specialized enzymes, offering high selectivity but facing major cost and stability challenges. Enzyme production is expensive, and activity is inhibited by dyes, multilayers, and additives, requiring extensive pre-treatment. Current economics restrict this method to clean PET streams, making it impractical for large-scale mixed waste processing [25].
1.3.5. Supercritical Water Gasification (Expanded)
SCWG operates at >374 °C and >22 MPa, converting wet organic waste into syngas. It accepts diverse feedstocks and reduces tar formation but demands high energy and corrosion-resistant materials, increasing costs. While promising for wet MSW fractions, SCWG remains capital-intensive and less proven at scale [6].
1.3.6. Regenerative Robust Gasification (RRG)
RRG uniquely processes virtually all organics in MSW without sorting, using plasma-assisted molten-bath gasification to produce syngas for methanol synthesis. High temperatures destroy persistent contaminants (e.g., PFAS) while vitrifying inorganics. Electrification enables carbon-free power integration, and methanol-to-olefins pathways deliver virgin-quality plastics, supporting circularity and carbon sequestration [4,5].
While these methods can achieve low emissions under ideal conditions, their limited feedstock compatibility significantly reduces system-level effectiveness compared to RRG, which accepts virtually all organics in MSW. Table 1 summarizes these recycling processes along with normalized estimated emissions (CO2eq). To enable fair comparison of greenhouse gas (GHG) emissions, we normalize reported GHG values based on the fraction of plastics in MSW eligible for each process. For example, if a process only accepts 5% of plastics in MSW, its GHG emissions per ton of recycled polymer are multiplied by 20 to reflect system-level impact. This normalization highlights the true effectiveness of each technology in diverting waste from landfills and producing virgin-quality recycled content.
Carbon Recovery Efficiency (CRE) is a key parameter in evaluating recycling technologies at a system level. It represents the fraction of carbon in the input waste that is retained in useful outputs—such as syngas, methanol, or polymer-equivalent products—rather than lost as CO2 emissions, char, or other non-recyclable residues. High CRE values indicate that a process effectively converts waste carbon into circular products, reducing reliance on virgin feedstock and improving overall carbon utilization.
Mathematically, CRE can be expressed as:
For this review, CRE values were derived from published LCAs and process data, reflecting conversion yields and downstream integration. CRE, together with the Effectiveness Fraction (EF), informs the effectiveness-normalized GHG metric used in Table 1 to compare technologies under real-world conditions.
GHG intensities in Table 1 represent cradle-to-gate emissions for each recycling process up to pellet or monomer stage, expressed per metric ton of recycled polymer (or polymer-equivalent for chemical pathways). Values were derived from recent LCAs and engineering estimates, including GREET-based models and published process data [26,27], [5,25]. To enable system-level comparison, we normalized actual GHG values by the inverse of the eligible fraction (EF) of plastics in MSW that each process can handle:
This approach accounts for feedstock eligibility and carbon recovery efficiency, ensuring that technologies are evaluated under real-world conditions rather than idealized assumptions. Detailed assumptions and sources are provided in Appendix A.
Figure 1 illustrates the contrast between reported process emissions (“actual GHG”) and system-level performance when normalized for feedstock eligibility and carbon recovery efficiency (“effectiveness-normalized GHG”). While mechanical recycling and PET depolymerization exhibit low actual GHG values, their normalized impacts increase sharply because these processes can only handle a small fraction of plastics in MSW (≈10%). Enzymatic depolymerization is particularly unfavorable, with normalized emissions exceeding 25 t CO2e per ton of recycled polymer. In contrast, robust technologies such as Regenerative Robust Gasification (RRG) and supercritical water gasification maintain low normalized impacts because they accept nearly all plastics and retain most carbon in circular products.
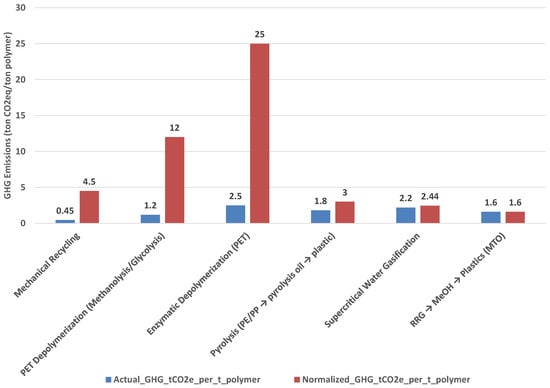
Figure 1.
Actual versus effectiveness-normalized GHG emissions (t CO2e per ton of recycled polymer) for six recycling technologies. Normalization accounts for feedstock eligibility and carbon recovery efficiency, revealing system-level performance differences. Material-specific processes (mechanical recycling, PET depolymerization) appear favorable in isolation but perform poorly when normalized, while robust technologies (RRG, SCWG) maintain low impacts.
This comparison underscores why LCAs based on idealized, high-purity feedstocks can mislead policy and investment decisions. Technologies that appear efficient in isolation may deliver limited system-level benefits when eligibility constraints are taken into account. Normalization provides a more realistic basis for evaluating recycling strategies, guiding infrastructure planning and Extended Producer Responsibility (EPR) frameworks toward solutions that maximize diversion and carbon retention.
1.3.7. Types of Gasifiers and Their Applications
There are many types of gasifiers, each suited to different feedstocks and applications. For this review, we emphasize that most traditional gasifiers are not suitable for “robust” mixed waste gasification. Currently, only two types of gasifiers plasma-assisted gasifiers, have been found to be suitable for commercial-scale gasification of mixed waste. Of these, only the molten-bath, plasma-assisted designs are proving to be sufficiently robust to process commercial quantities of mixed waste with limited sorting (Table 2).

Table 2.
Summary of gasifier types, robustness, and typical feedstocks. Plasma-assisted molten-bath designs provide the highest robustness, allowing for the processing of mixed MSW and hazardous waste streams with minimal sorting.
Gasification is a partial oxidation process that converts carbonaceous materials into synthesis gas (syngas), primarily carbon monoxide (CO) and hydrogen (H2). Unlike incineration, which fully oxidizes feedstock to CO2 and water, gasification retains chemical energy in syngas for downstream applications such as fuels, chemicals, and plastics. Typical reactions that characterize the gasification process are summarized in Table 3.

Table 3.
Primary chemical reactions in gasification, including oxidation, water-gas, Boudouard, methanation, and reforming steps. Reaction enthalpies (kJ/mol) indicate endothermic and exothermic contributions to process energy balance.
Modern systems vary by design:
- Fixed-bed and entrained-flow gasifiers are suited for uniform feedstocks like coal or biomass.
- Fluidized-bed systems can process sorted MSW fractions but require extensive pre-treatment.
- Plasma-assisted molten-bath gasifiers achieve extreme temperatures (>5000 °C), enabling robust conversion of mixed and contaminated waste streams with minimal sorting.
Regenerative Robust Gasification (RRG) integrates plasma-assisted molten-bath technology with hydrogen enhancement to optimize syngas composition for methanol synthesis. This approach supports circular manufacturing by producing virgin-quality plastics from unsorted municipal solid waste, while vitrifying inorganics into stable slag and destroying persistent contaminants such as PFAS.
1.3.8. Recent Innovations in Regenerative Robust Gasification
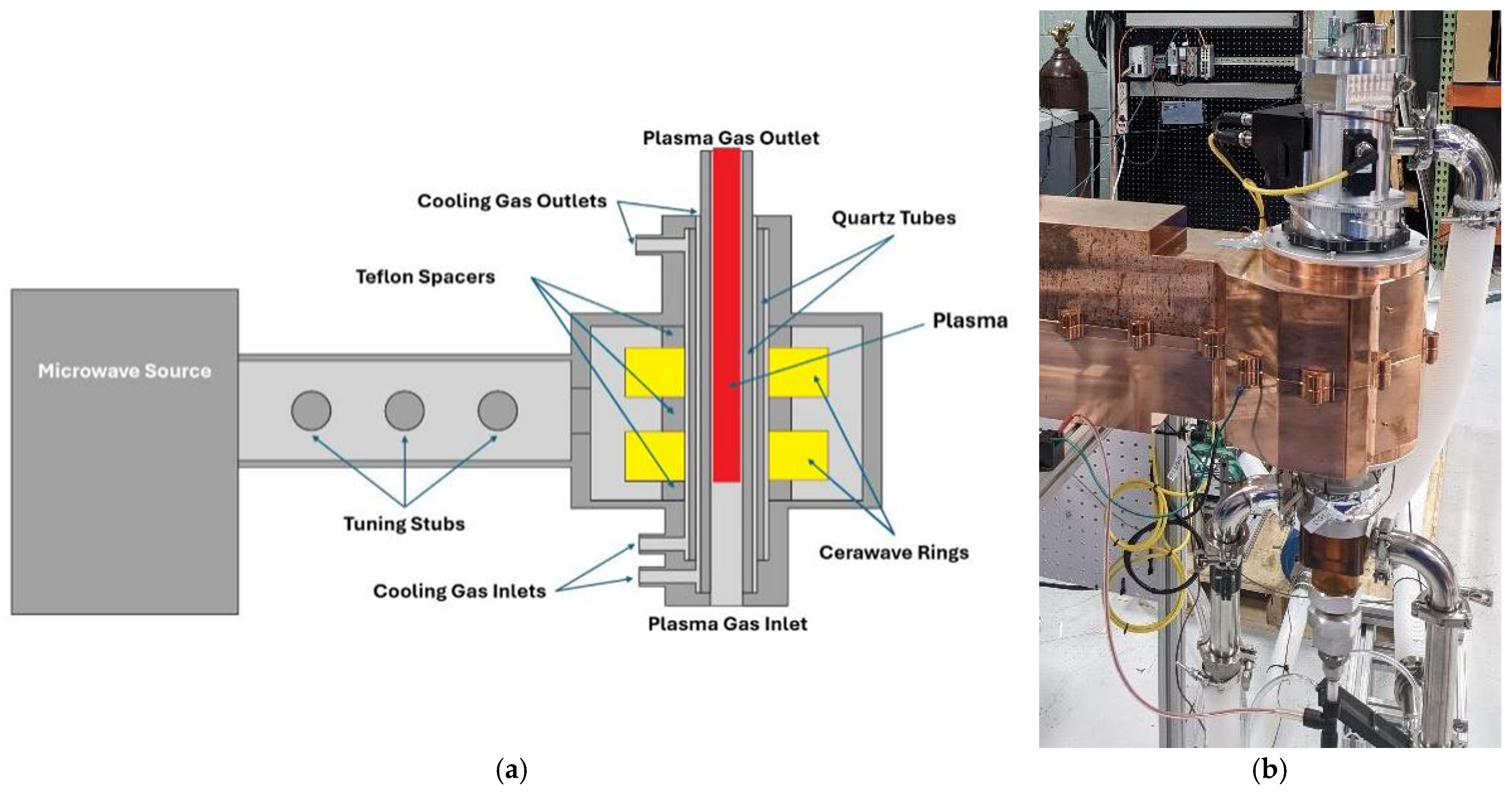
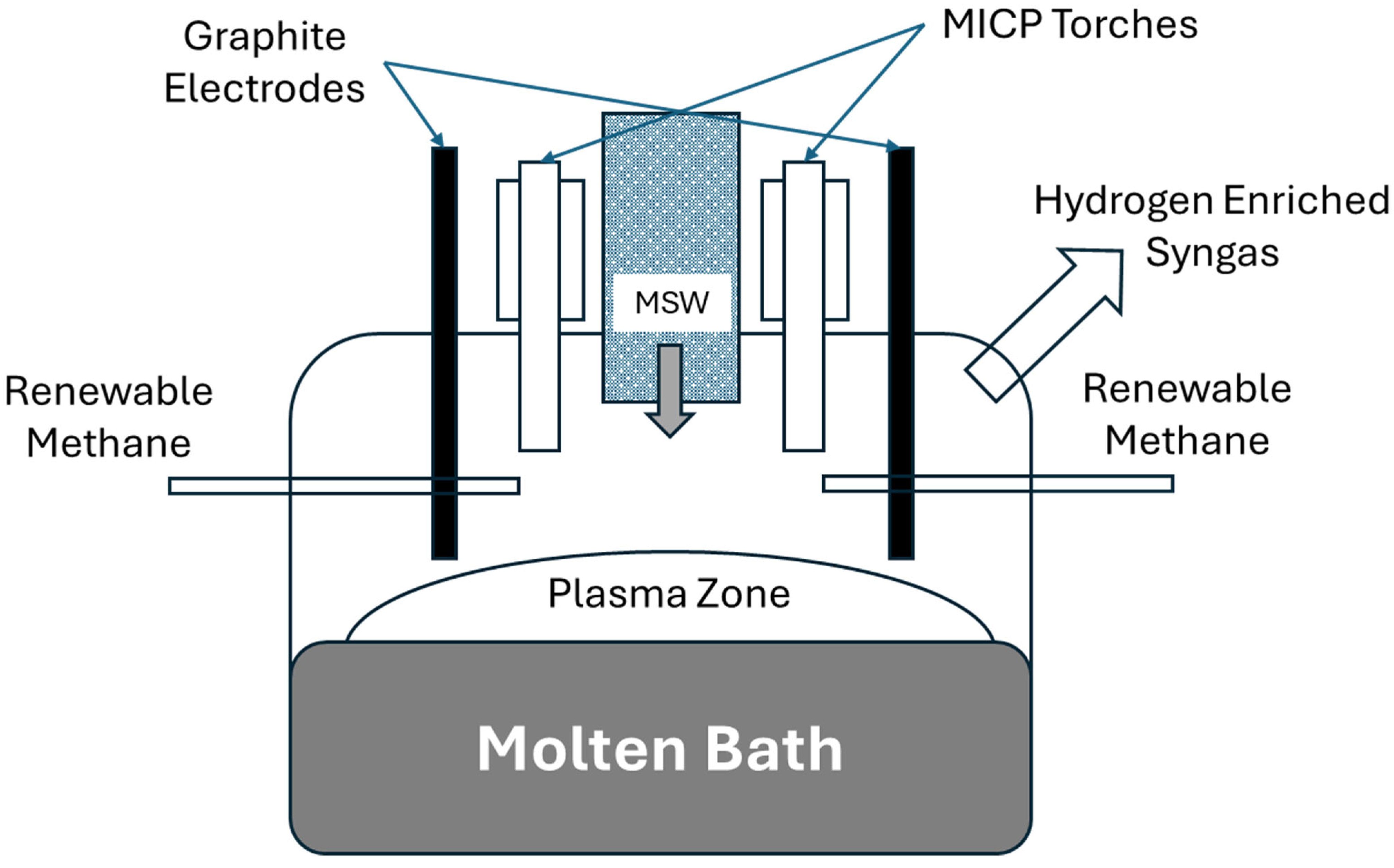
The Packaging Engineering Laboratory at the University of Florida recently introduced a molten metal bath gasifier combined with two 10 kW microwave induction coupled plasma torches (ReaSYN Technologies, MICP-10, Miami, FL, USA) (Figure 2). This led to the development of a larger system incorporating a molten-glass bath gasifier with graphite plasma arc electrodes and one 100 kW microwave induction coupled plasma torch developed in conjunction with ReaSYN Technologies, Inc. (ReaSYN Technologies, Inc., ReaSYN Able X1 robust gasifier with ReaSYN MICP-100 torch, Miami, FL, USA) (Figure 3). The robust gasification technology has been shown to process a wide variety of materials that are otherwise not recyclable [4]. Addition of the MICP torch provides an opportunity to fine-tune the syngas hydrogen-to-carbon monoxide ratio in real-time by exploiting steam and carbon dioxide thermolysis and/or renewable methane reformation, significantly boosting methanol yield from each ton of MSW processed. Table 4 shows methanol yield (gallons/ton MSW) predictions of robust gasification of MSW without and with the ReaSYN MICP torch due to steam thermolysis and renewable methane reformation.
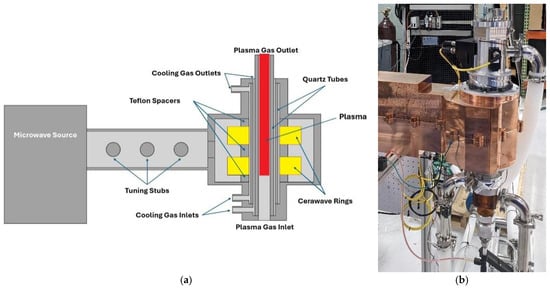
Figure 2.
Components of the ReaSYN MICP-100 plasma torch: (a) schematic diagram showing induction coil and plasma generation zone; (b) photograph of the assembled torch (ReaSYN Technologies, Miami, FL, USA).
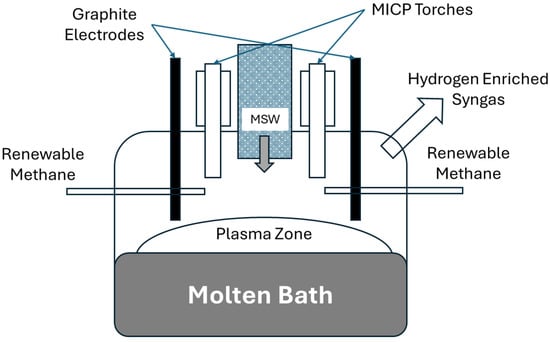
Figure 3.
Schematic of the ReaSYN Able X1 robust gasifier integrating molten-bath plasma arc electrodes with a microwave induction coupled plasma torch to optimize syngas composition and methanol yield.

Table 4.
Predicted methanol yields (gallons per ton of MSW) for RRG systems with and without microwave induction coupled plasma (MICP) torch enhancement. Hydrogen addition via steam thermolysis and methane reforming significantly increases methanol output.
2. (Regenerative) Circular Pathway and Methanol Conversion
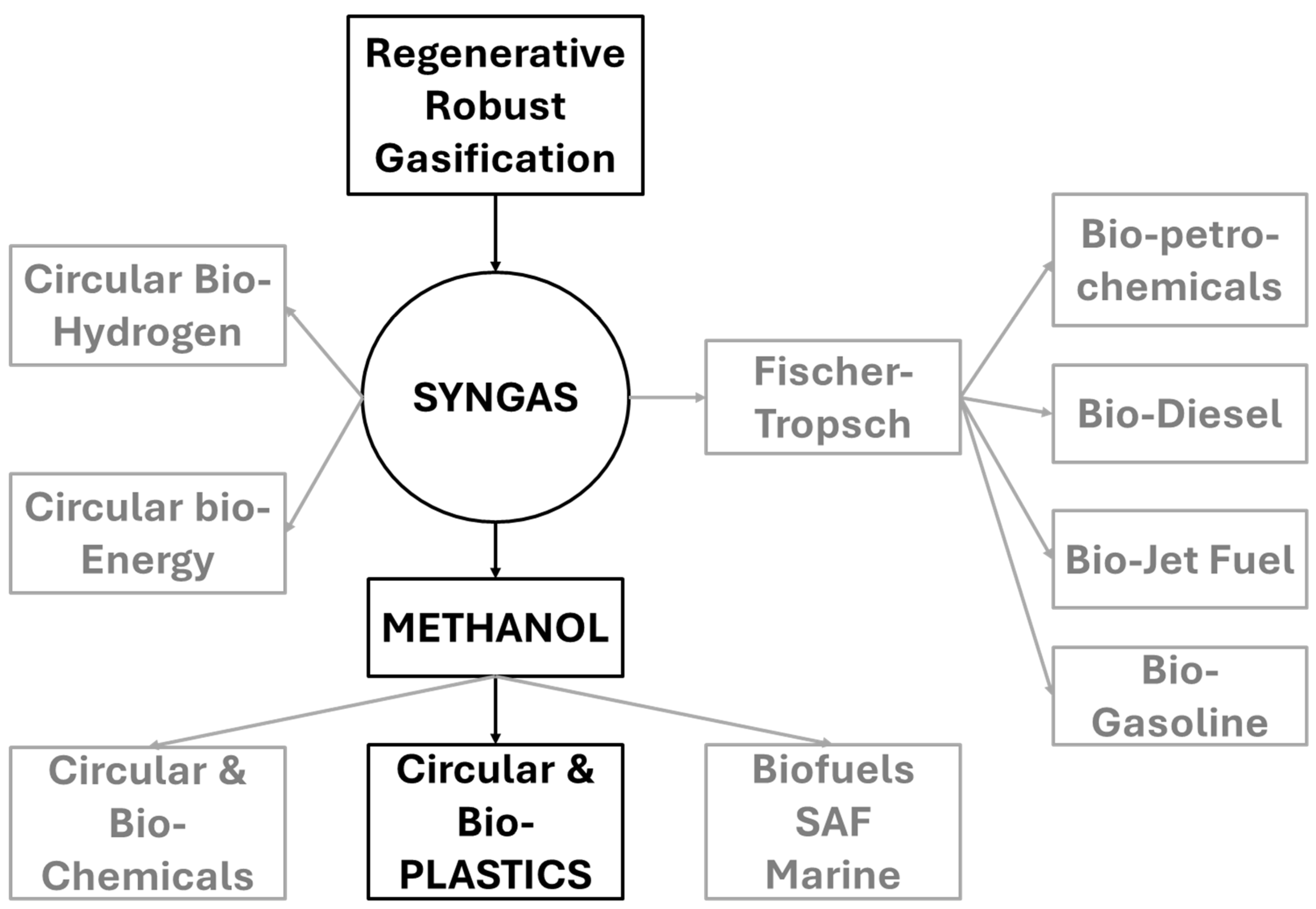
Synthesis gas is highly versatile. As shown in Figure 4, it can be used as a primary feedstock for circular manufacturing through the methanol pathway, as a feedstock for fuels and chemicals through the Fischer-Tropsch pathway, as a clean-burning fuel for power generation, as a rich source of hydrogen to support the hydrogen economy, as an alternative to natural gas, or as a reducing agent for chemical processing.
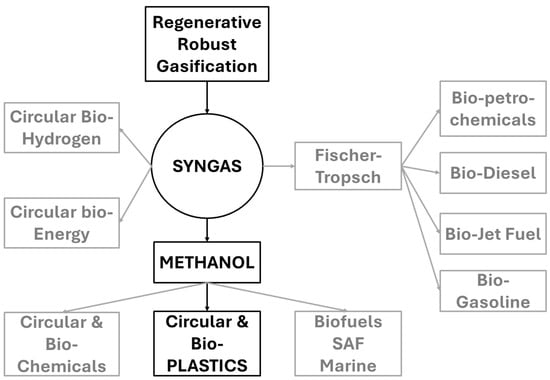
Figure 4.
Circular pathways enabled by syngas from RRG. Preferred route for plastics circularity—syngas → methanol → olefins—is highlighted. Alternative pathways include Fischer–Tropsch fuels, hydrogen production, and clean energy applications.
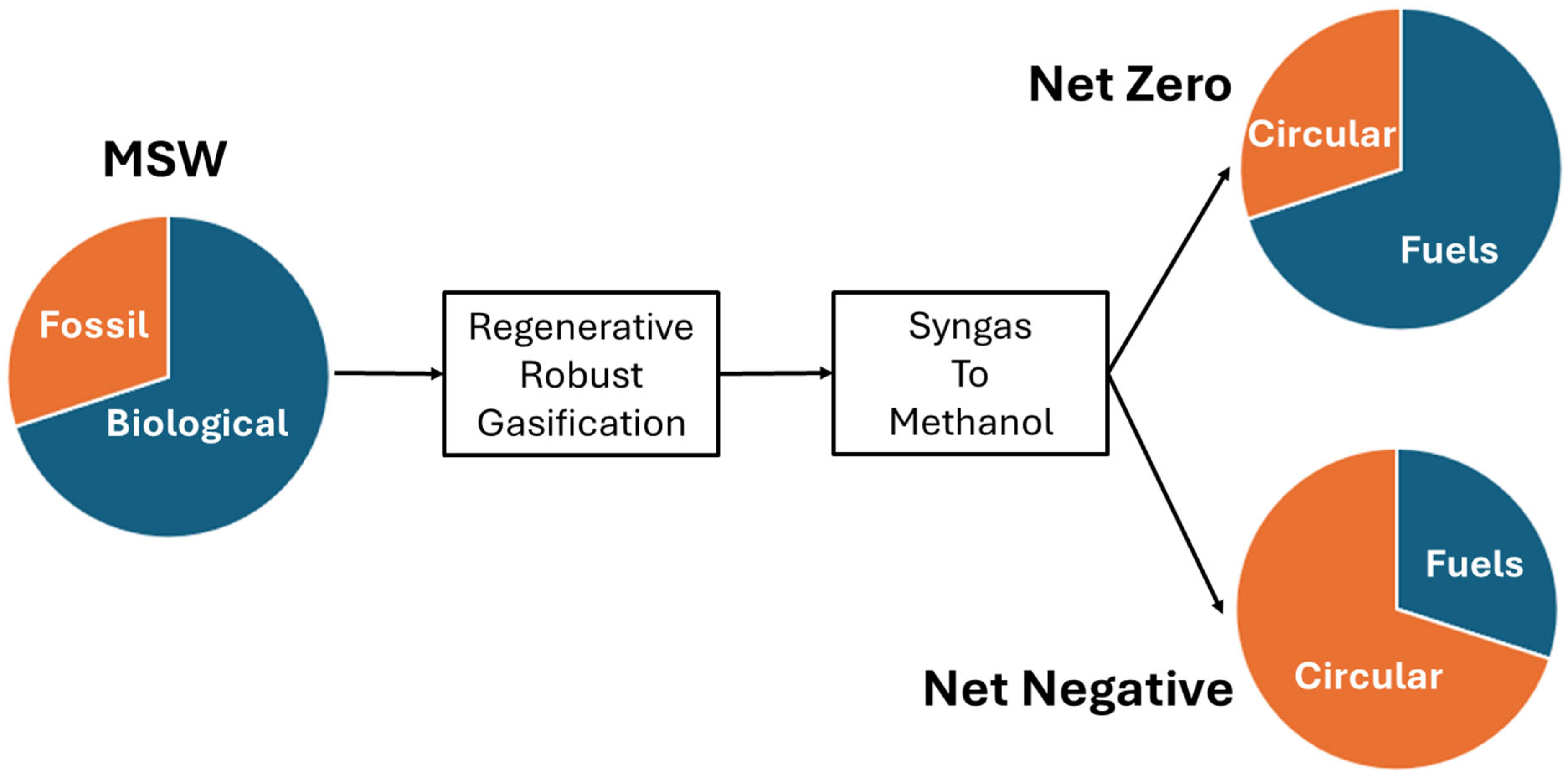
The methanol pathway provides the best opportunity to maximize carbon capture and circularity. Since the organic fraction of MSW (metal and glass excluded), is about 80% of biological origin, and about 20% is of fossil origin, and since all organic waste, regardless of origin, can be converted into methanol, RRG provides a unique opportunity to not only recycle all plastics, but to serve as a carbon sequestration process (Figure 5). RRG is an electrified process that is amenable to carbon-free power. Therefore, if all methanol is sent to circular manufacturing, RRG serves to sequester carbon from both fossil and biological sources, resulting in net negative carbon emissions. However, RRG can also serve to supply demand for clean fuels such as sustainable aviation fuel (SAF), sustainable marine fuel, and other transportation fuels at net zero or net negative so long as the fraction of methanol sent to circular manufacturing is equal to or greater than the fraction of fossil-based waste in MSW.
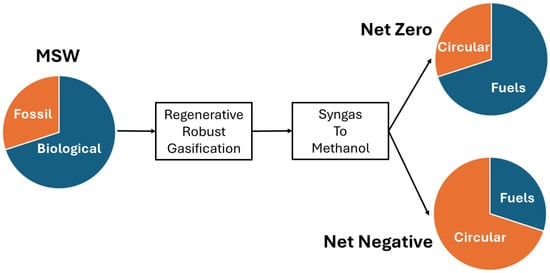
Figure 5.
Carbon impact of RRG under different methanol allocation scenarios. When methanol from both fossil and biogenic fractions of MSW is directed to circular manufacturing, RRG achieves net-negative emissions. Partial allocation supports net-zero targets while facilitating the production of clean fuels.
Process of Converting Syngas to Methanol
The conversion of syngas to methanol is a well-established industrial process that involves catalytic reactions. Syngas, a mixture of carbon monoxide (CO) and hydrogen (H2) is subjected to high pressures and temperatures in the presence of a catalyst, typically copper-based, to produce methanol (CH3OH). The overall reaction can be represented as:
CO + 2H2 → CH3OH
For optimal methanol production, the syngas should have a hydrogen to carbon monoxide ratio of at least 2:1. This ratio can be adjusted using the water-gas shift (WGS) reaction (Equation (9)), where steam reacts with carbon monoxide to produce additional hydrogen and carbon dioxide (CO2):
Methanol is a versatile chemical feedstock with several advantages for producing new plastics:
- High Purity: Methanol produced from syngas can be of high purity, making it suitable for various chemical processes.
- Renewable Source: When derived from waste syngas, methanol serves as a renewable feedstock, reducing reliance on fossil fuels.
- Circular Economy: Methanol can be converted into olefins, which are the building blocks for many plastics, enabling the production of virgin-like plastics from waste materials.
- Environmental Benefits: Using waste-derived methanol reduces greenhouse gas emissions and diverts waste from landfills, contributing to a more sustainable waste management system.
One of the most promising applications of waste-derived methanol is in the production of virgin-quality plastics. The process involves converting methanol into olefins, such as ethylene and propylene, through the methanol-to-olefins (MTO) process. These olefins can then be polymerized to produce high-quality plastics like polyethylene (PE) and polypropylene (PP), which are widely used in packaging.
The MTO process, developed by Mobil in the 1970s and actively marketed today by Honeywell UOP, is a well-established technology that has been commercialized at a large scale in China since about 2000. This commercially available pathway can produce new plastics with the same properties and quality as virgin materials made from virgin fossil fuels, but with a significantly lower environmental impact.
By leveraging waste-derived methanol, the packaging industry can achieve greater sustainability and circularity, reducing its environmental footprint and contributing to a more resilient and eco-friendly economy.
3. Limitations of Regenerative Robust Gasification (RRG)
While RRG offers unique advantages in processing mixed and contaminated waste streams, several limitations constrain its near-term scalability. First, plasma-assisted gasification systems are capital-intensive and require substantial electrical energy input, which can challenge economic viability unless low-cost renewable power is available. However, it is important to note that waste-to-energy (WTE) facilities are also capital-intensive at similar scales, yet RRG provides greater flexibility in feedstock acceptance and enables production of higher-value outputs with true circularity. Additionally, RRG facilities may partially offset their energy demand by utilizing a portion of the biological fraction of syngas or methanol as an internal source of carbon-neutral power, though this trade-off reduces the amount available for circular products. Second, syngas cleanup and conditioning for methanol synthesis involve complex operations and additional cost. Third, commercial deployment remains limited to pilot and demonstration projects, with few full-scale facilities in operation. Integration with existing waste management infrastructure and methanol conversion units also poses logistical challenges. Finally, regulatory frameworks for advanced recycling vary widely, creating uncertainty for investment and permitting. Addressing these limitations will require continued innovation, supportive policy, and strategic partnerships to realize RRG’s full potential.
4. Implications for the Packaging Industry
The adoption of regenerative robust gasification (RRG) technology in the packaging industry can significantly reduce landfill usage. Traditional landfilling practices producing landfill gas composed of methane and carbon dioxide. Methane is a potent greenhouse gas, with a global warming potential many times greater than that of carbon dioxide. By diverting waste from landfills to RRG systems, the production of landfill gas can be minimized, thereby reducing methane emissions and helping to mitigate climate change.
Furthermore, RRG technology can process mixed and contaminated waste streams without the need for extensive sorting, making it more efficient and adaptable to the diverse nature of packaging waste. This capability ensures that a larger proportion of waste is converted into valuable syngas rather than being sent to landfills, contributing to a significant reduction in landfill usage [4].
The effectiveness-normalized GHG data presented earlier in this review highlight the significant environmental advantage of Regenerative Robust Gasification (RRG) over conventional recycling methods. For example, while enzymatic PET depolymerization exhibits a normalized impact of 25.0 t CO2e per ton of recycled polymer, RRG achieves just 1.6 t CO2e—despite accepting 100% of the organic fraction of MSW. This performance aligns directly with packaging industry goals such as Coca-Cola’s target to use 35–40% recycled material in primary packaging by 2035 and Unilever’s goal to reach 25% recycled plastic content by 2025 [28]. Additionally, Unilever aims to make all rigid plastic packaging reusable, recyclable, or compostable by 2030, and flexible packaging by 2035 [29]. RRG’s ability to produce methanol from mixed waste offers a scalable and versatile feedstock for virgin-quality plastics, helping brands meet food-grade requirements without the limitations of mechanical recycling. By enabling high-volume, high-quality recycled content from unsorted waste, RRG supports both environmental and economic objectives, offering a practical pathway to meet ambitious circularity commitments and reduce reliance on virgin plastic.
4.1. Potential for Reducing Plastic Litter in Oceans and Terrestrial Environments
Plastic litter in oceans and terrestrial environments poses a severe threat to wildlife and ecosystems. Traditional recycling methods often fail to capture all types of plastics, especially those that are contaminated or composed of complex materials. RRG technology, however, can process virtually all types of plastics, converting them into syngas and subsequently into methanol and other valuable chemicals [30].
By enabling the recycling of a broader range of plastics, RRG technology can help reduce the amount of plastic litter that ends up in natural environments. This reduction in plastic waste not only protects wildlife but also contributes to cleaner oceans and landscapes, promoting environmental sustainability [2].
4.2. Cost Savings and Economics from Improved Recycling Rates
One of the significant economic advantages of RRG technology is the reduction in costs associated with sorting waste materials. Traditional mechanical recycling requires extensive sorting to separate different types of plastics and other materials, which is both labor-intensive and costly. RRG technology eliminates the need for such detailed sorting, as it can process mixed waste streams efficiently [30].
Additionally, the high efficiency of RRG systems in converting waste into syngas and subsequently into valuable chemicals like methanol improves overall recycling rates. Higher recycling rates mean that more waste is converted into useful products, reducing the costs associated with waste disposal and landfill usage [31].
Unlike traditional material-specific recycling methods, where relatively small amounts of different materials of marginal quality are aggregated for sale, the economics of RRG revolve around the demand for one highly versatile recycled-content feedstock such as methanol. RRG promises relatively large quantities of recycled-content methanol that can be exploited by many markets, making RRG more economically sustainable than traditional recycling.
4.3. Ambitious Recycling Commitments Facing Supply Reality
The Plastics Pact represents a network of initiatives that unite businesses, governments, and organizations behind a common vision to create a circular economy for plastics. Unfortunately, The Plastics Pact appears to be stuck in a paradigm that views materials based on how they perform in our traditional material-specific recycling system. The US Plastic Pact Roadmap 2.0 emphasizes reuse before recycling while establishing a “Problematic and Unnecessary Materials List”, an anathema to the notion of liberty and free-market economy [32]. RRG accepts all plastics without sorting, rendering such Problematic and Unnecessary Materials Lists unnecessary.
At its core, the Pact includes several key pledges: to make 100% of plastic packaging reusable, recyclable, or compostable by 2025; to effectively recycle or compost 50% of plastic packaging; to achieve 30% average recycled content across all plastic packaging; and to eliminate what some at the Ellen MacArthur Foundation deem to be problematic or unnecessary single-use plastic packaging items [33]. Major corporations including Coca-Cola, PepsiCo, Nestlé, and Unilever have committed to these targets, with some setting even more ambitious goals, such as Coca-Cola’s pledge to use at least 50% recycled material in packaging by 2030 [28].
The gap between these commitments and current reality is substantial. To meet the 30% recycled content target across the packaging industry, approximately 11–13 million metric tons of high-quality recycled plastic would be required annually [34]. For food contact applications specifically, which represent roughly 40% of plastic packaging, about 4–5 million metric tons of food-grade recycled plastic would be needed [1]. However, current production of food-grade recycled PET (rPET) and recycled HDPE (rHDPE) combined is estimated at only 1.2–1.5 million metric tons globally [35]. This significant shortfall is even more pronounced for polypropylene (PP), which has an extremely limited food-grade recycling infrastructure despite being widely used in food packaging applications [36].
The scarcity of high-quality recycled plastics has created substantial price premiums in the market. For traditionally processed recycled materials, even the highest quality grades are of lesser quality than virgin material. Food-grade recycled PET currently commands a premium of 30–45% over virgin PET, with prices fluctuating between $1600–$2100 per metric ton compared to $1200–$1400 for virgin material [37]. For food-grade recycled HDPE, premiums can reach 50–70% above virgin material prices [38], while food-grade recycled PP, when available, may cost twice as much as virgin PP [39]. These premiums reflect not only limited supply but also the additional costs associated with the specialized collection, sorting, and FDA-approved decontamination processes required for food-contact applications [40]. As companies compete for this limited supply to fulfill their public commitments, these price premiums are likely to persist or even increase in the near term, creating significant economic pressure on brands trying to meet their sustainability pledges [41].
RRG promises to close the gap between demand and supply for high-quality recycled plastics. Additionally, plastics produced from recycled-content methanol from the RRG process are equal in quality to virgin materials. Therefore, the economics of RRG are simpler than those of mechanical recycling. To the extent that industry values achieving and sustaining a circular economy for plastics, it need only demand materials manufactured from recycled-content methanol [42].
4.4. Policy and Economic Implications
Extended Producer Responsibility (EPR) is a policy approach that holds producers accountable for the end-of-life management of their products, typically through mandatory fees and participation in Producer Responsibility Organizations (PROs). While intended to promote circularity, current frameworks often entrench outdated mechanical recycling systems and fail to address technical limitations [43]. Critics argue these programs focus on cost-shifting rather than innovation, creating regulatory complexity and transparency issues [44]. Regional implementation challenges and legal disputes—such as lawsuits over constitutional concerns in Oregon—underscore the controversy surrounding EPR [45]. Furthermore, compliance mechanisms can disincentivize investment in transformative technologies like Regenerative Robust Gasification [46].
To overcome these barriers, policies should integrate effectiveness-normalized metrics [47], support infrastructure for waste-to-methanol conversion [42], align recycled content targets with scalable technologies [34], encourage procurement of methanol-derived plastics [28,29], and promote transparency in life cycle assessment reporting [48]. These measures would help move beyond legacy systems toward robust, circular solutions.
Supply Chain Resilience and Strategic Benefits: The COVID-19 pandemic has underscored a critical economic lesson: efficient supply chains are not necessarily resilient supply chains. The global disruption caused by the pandemic revealed vulnerabilities in highly optimized, just-in-time supply chains, prompting a reevaluation of how essential goods and resources are produced and distributed [49,50]. In this context, the distributed production of waste-derived methanol at virtually all landfills emerges as a strategic approach to bolster national interests by enhancing supply chain resilience.
Methanol, a versatile chemical feedstock, can be synthesized from various waste materials, transforming landfills into valuable production sites [51]. This process not only addresses waste management challenges but also contributes to a circular economy by converting waste into a resource [52]. Methanol’s utility extends beyond its role as a fuel; it serves as a precursor for a wide range of products, including plastics, pharmaceuticals, and synthetic fuels. By adopting processes that convert waste into methanol, nations can support manufacturing and clean energy demands, thereby reducing reliance on fossil fuels and enhancing energy security [53].
The distributed nature of waste-derived methanol production offers several advantages. First, it decentralizes production, reducing the risk of supply chain disruptions caused by localized events [54]. Second, it leverages existing waste streams, minimizing the need for new raw material extraction and reducing environmental impacts [33]. Third, it creates economic opportunities in diverse regions, fostering local industries and job creation.
Moreover, the conversion of waste to methanol aligns with broader environmental and sustainability goals. It promotes recycling and circularity, reducing the volume of waste sent to landfills and mitigating greenhouse gas emissions associated with waste decomposition [55]. This approach also supports the transition to a low-carbon economy by providing a renewable alternative to fossil-based feedstock [15].
In summary, the distributed production of waste-derived methanol at landfills serves national interests by improving supply chain resilience, supporting manufacturing and clean energy demands, and advancing environmental sustainability. The lessons learned from the COVID-19 pandemic highlight the importance of resilient supply chains, and the conversion of waste to methanol offers a practical and strategic solution to achieve this goal.
5. Conclusions
This review reframes the comparison of recycling technologies by introducing effectiveness-normalized GHG as a practical, system-level complement to conventional LCA when evaluating heterogeneous MSW. By explicitly accounting for what fraction of the stream a technology can actually process (EF) and how much carbon is retained in circular outputs (CRE), the metric exposes mismatches between favorable process averages and limited system impact. Three conclusions follow:
- System-level performance matters: Material-specific pathways (e.g., mechanical recycling, PET depolymerization) can exhibit low process intensities yet deliver high normalized impacts when limited eligibility forces the system to manage the remaining stream via higher-emitting or lower-value routes. In contrast, RRG maintains the lowest normalized GHG (~1.6 t CO2e per t polymer) because it accepts virtually all organics in MSW, converts them to syngas, and enables methanol-to-olefins for virgin-quality plastics while vitrifying inorganics.
- Infrastructure strategy should align with eligibility and carbon retention: For mixed residuals, robust platforms (RRG; promisingly, SCWG for wet fractions) better match real composition and contamination, improving diversion, carbon utilization, and supply resilience. Modeled methanol yields of ~200–300 gal·t−1 without hydrogen and up to ~800 gal·t−1 with renewable methane reforming illustrate how hydrogen management and integration choices translate to circular output.
- Policy and procurement can operationalize the metric: The effectiveness-normalized lens can be incorporated into future LCA practice (as a reporting companion to process intensities) and into EPR instruments to align fees, credits, and targets with system-level diversion and carbon recovery—rather than feedstock purity alone. Similarly, procurement can recognize polymer-equivalent recycled content from waste-derived methanol to unlock high-volume, food-grade circular supply independent of bale purity.
5.1. Implications for Policymakers
- Use effectiveness-normalized indicators alongside conventional LCA results when setting recycled-content targets, qualifying technologies, or awarding incentives.
- Support waste-to-methanol infrastructure where grid mix, renewable power, or renewable methane can lower marginal GHG and raise carbon retention.
- Allow polymer-equivalent accounting for methanol-derived plastics to reduce dependence on constrained, high-purity bales while safeguarding traceability and transparency.
- Encourage disclosure of EF and CRE in LCAs and compliance filings to ensure fair comparison across pathways and sites.
5.2. Limitations
Uncertainty remains for pathways with scarce, scale-consistent data (e.g., SCWG); results are sensitive to site energy mix, hydrogen source, and treatment of landfill methane avoidance. We intentionally report process burdens and apply normalization to reveal system-scale effects; avoided-burden crediting is not the driver of our rank order.
Author Contributions
Conceptualization, B.W.; C.L. and Z.B.; Methodology, C.S.; J.G. and B.W.; Software, C.S.; J.G. and Z.B.; Validation, C.L. and Z.B.; Formal Analysis, B.W.; Investigation, C.S.; J.G. and B.W.; Resources, B.W.; Data Curation, B.W.; Writing—Original Draft Preparation, C.S.; J.G. and B.W.; Writing—Review and Editing, B.W.; C.L. and Z.B.; Visualization, C.S.; J.G. and B.W.; Supervision, B.W. and Z.B.; Project Administration, B.W.; Funding Acquisition, B.W. and Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Consortium for Waste Circularity (https://wastecircularity.org/, Key Largo, FL, USA) and the Florida Agricultural Experiment Station, Gainesville, FL, USA.
Data Availability Statement
The original contributions presented in this study are included in the article and appendix. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors gratefully acknowledge the Consortium for Waste Circularity and all of its members for funding this research through UF/IFAS.
Conflicts of Interest
The authors declare the following potential conflict of interest: Since the submission of this manuscript, author Bruce Welt has begun serving as a consultant to ReaSYN Technologies, Inc., a company mentioned in this review. During preparation of this manuscript, author Swearingen began employment at InEnTec, LLC, a company involved in commercial robust gasification. These roles did not influence the preparation or content of the manuscript.
Abbreviations
| ACC | American Chemistry Council |
| BHET | Bis(hydroxyethyl) Terephthalate |
| CAA | Clean Air Act |
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| CPG | Consumer Packaged Goods |
| DMT | Dimethyl Terephthalate |
| EF | Eligible Fraction |
| EG | Ethylene Glycol |
| EPA | Environmental Protection Agency |
| EPR | Extended Producer Responsibility |
| GHG | Greenhouse Gas |
| HDPE | High-Density Polyethylene |
| IECR | Industrial Engineering Chemistry Research |
| IED | Industrial Emissions Directive |
| ISWA | International Solid Waste Association |
| MSW | Municipal Solid Waste |
| MTO | Methanol-to-Olefins |
| NESHAP | National Emission Standards for Hazardous Air Pollutants |
| NIR | Near-Infrared |
| NSPS | New Source Performance Standards |
| PC | Polycarbonate |
| PCR | Post-Consumer Recycled |
| PE | Polyethylene |
| PET | Polyethylene Terephthalate |
| PFAS | Perfluoroalkyl Substances |
| PP | Polypropylene |
| PS | Polystyrene |
| PVA | Polyvinyl Alcohol (PVOH) |
| PVC | Polyvinyl Chloride |
| RRG | Regenerative Robust Gasification |
| SAF | Sustainable Aviation Fuel |
| SCWG | Supercritical Water Gasification |
| TPA | Terephthalic Acid |
| WGS | Water-Gas-Shift |
| WID | Waste Incineration Directive |
| WTE | Waste-to-Energy |
Appendix A
- Purpose and Scope.
- Functional Units and Normalization.
Example: Mechanical recycling (EF = 0.10). If Actual GHG = 0.45 t CO2e/t polymer, then Normalized GHG = 0.45/0.10 = 4.5 t CO2e/t polymer.
Carbon Recovery Efficiency (CRE).
CRE represents the fraction of carbon in the input feedstock that is retained in useful outputs (e.g., syngas, methanol, or polymer-equivalent products) rather than lost as CO2 emissions, char, or other non-recyclable residues. It is calculated as:
CRE = Carbon in recycled product/Carbon in feedstock × 100%
CRE values were derived from published LCAs and process data for each technology, considering conversion yields and downstream integration. High CRE indicates better carbon utilization and supports circularity goals.
While Table 1 reports normalized GHG values, CRE is an underlying parameter influencing system-level performance and is used qualitatively in comparing technologies.
- Cross-cutting Assumptions
- Allocation: When sources report avoided burdens or credits (e.g., displacement of virgin resin), we prefer cut-off allocation for recycled content factors consistent with Franklin Associates/APR practice, but several chemical pathways rely on system expansion or mass balance; this is noted per pathway.
- (1)
- Mechanical Recycling (PET/HDPE/PP)
Basis and literature range: Recent LCIs for postconsumer recycled (PCR) PET, HDPE, and PP report cradle-to-gate GHG intensities typically on the order of 0.4–0.7 kg CO2e per kg PCR resin (≈0.4–0.7 t CO2e/t). EPA’s WARM documentation [56] and Franklin Associates/APR datasets [57] provide detailed factors for PET and HDPE; [58] show 20–30% GHG reductions vs. virgin for PET systems.
Normalization example: Eligible fraction EF ≈ 10% (clear PET, HDPE, and portions of PP suitable for closed-loop). Normalized GHG = 0.45/0.10 = 4.5 t CO2e/t polymer.
Caveats: Values vary with bale quality, decontamination for food-contact, and electricity mix.
- (2)
- PET Depolymerization (Methanolysis/Glycolysis)
Basis: Process-modeling studies indicate methanolysis energy demand typically ~6–10 MJ/kg PET depending on purification, with higher burdens than glycolysis and lower than hydrolysis under many configurations. Public LCI datasets (e.g., ecoinvent) include methanolysis modules; plant-specific LCA results vary with solvent recovery and distillation integration.
Normalization example: EF ≈ 10% (PET share in MSW practically recoverable for depolymerization). Normalized GHG = 1.2/0.10 = 12.0 t CO2e/t polymer.
Caveats: Site power mix, degree of pre-sorting, and monomer purification strongly affect results; treat 1.2 as a midpoint within literature ranges.
- (3)
- Enzymatic Depolymerization (PET)
Basis: Recent NREL LCA [59] finds current enzymatic PET recycling performing worse than virgin PET across most categories (1.2–17×), with hotspots in enzyme production, NaOH, and electricity. The 2.5 t CO2e/t figure reflects present-day, non-optimized systems.
Normalization example: EF ≈ 10% (PET). Normalized GHG = 2.5/0.10 = 25.0 t CO2e/t polymer.
Caveats: Strongly sensitive to enzyme loading, recycling of media, and heat integration; future reductions are plausible.
- (4)
- Pyrolysis of Polyolefins (PE/PP → pyrolysis oil → new plastics)
Basis: GREET-based LCAs [60] using multi-plant data show that integrating pyrolysis oil into steam crackers can yield 18–23% lower GHG than virgin plastics on a blended basis; absolute intensities depend on allocation (mass balance), oil yields, and hydrogen/utility integration. A central tendency around 1–2 t CO2e/t plastic is consistent with reported ranges for pyrolysis-to-plastic pathways.
Normalization example: EF ≈ 60% (polyolefin share suitable for pyrolysis). Normalized GHG = 1.8/0.60 = 3.0 t CO2e/t polymer.
Caveats: Results depend on cracker configuration, fraction of pyrolysis oil feed, and whether avoided incineration credits are taken.
- (5)
- Supercritical Water Gasification (SCWG)
Basis: Peer-reviewed reviews [61,62] document SCWG advantages for wet/waxy streams and reduced tar, but public cradle-to-gate LCAs with consistent functional units are scarce. Given high operating temperatures/pressures (≥374 °C, ≥22 MPa) and material/corrosion requirements, we use a conservative placeholder pending plant data.
Normalization example: EF ≈ 90%. Normalized GHG = 2.2/0.90 ≈ 2.44 t CO2e/t polymer.
Caveats: High uncertainty; recommend site-specific LCA when scale data are available.
- (6)
- Regenerative Robust Gasification (RRG) → Methanol → Plastics
Basis: Recent LCA for MSW → methanol with hydrogen enhancement reports net 183–709 kg CO2e per ton of methanol (100-year GWP), depending on H2 source and landfill methane credits. For polymer equivalence via methanol-to-olefins (MTO), assume ~1.7–2.0 t MeOH per t light olefins (carbon yield ≳90%); polymerization adds a modest increment. A central estimate maps to ~1–2 t CO2e/t polymer, consistent with the 1.6 t value used.
Normalization: EF = 100% (accepts all organics, including mixed plastics). Normalized GHG = 1.6/1.00 = 1.6 t CO2e/t polymer.
Caveats: Site energy mix, hydrogen source (only electrolytic vs. pyrolytic considered here), and treatment of landfill methane avoidance dominate results; we report process burdens exclusive of circular crediting.
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2021, 7, eabf0631. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hagelin-Weaver, H.; Welt, B. A Concise Review of Catalytic Synthesis of Methanol from Synthesis Gas. Waste 2023, 1, 228–248. [Google Scholar] [CrossRef]
- Robinson Plc. Annual Report. Guiding Our Sustainability Journey. 2023. Available online: https://robinsonpackaging.com/wp-content/uploads/2024/03/Annual-report-2023.pdf (accessed on 10 November 2025).
- Welt, B. Robust gasification trial results for a variety of difficult-to-recycle packaging-related materials. J. Appl. Packag. Res. 2023, 15, 6. Available online: https://repository.rit.edu/japr/vol15/iss1/6 (accessed on 10 November 2025).
- McNeeley, J.; Liu, Y. Life Cycle Assessment of Methanol Production from Municipal Solid Waste with Hydrogen Enhancement. Ind. Eng. Chem. Res. 2024, 63, 1234–1245. [Google Scholar]
- Cerqueira, C.Q.; Lora, E.E.S.; de Souza, L.L.P.; Leme, M.M.V.; Barros, R.M.; Venturini, O.J. Life cycle assessment of methanol production from municipal solid waste: Environmental comparison with landfilling and incineration. Resources 2025, 14, 12. [Google Scholar] [CrossRef]
- Nathrath, P.; Kroll, F.; Karmann, D.; Geißelbrecht, M.; Schühle, P. Methanol production in a sustainable, mild and competitive process: Concept launch and analysis. Green Chem. 2025, 27, 9268–9279. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. National Overview: Facts and Figures on Materials, Wastes and Recycling. 2018. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials (accessed on 10 November 2025).
- World Bank. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Recycling Basics. 2021. Available online: https://www.epa.gov/recycle/recycling-basics (accessed on 10 November 2025).
- Dabrowska, D.; Rykala, W.; Nourani, V. Causes, Types and Consequences of Municipal Waste Landfill Fires—Literature Review. Sustainability 2023, 15, 5713. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Advancing Sustainable Materials Management: 2018 Fact Sheet. 2018. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/advancing-sustainable-materials-management (accessed on 10 November 2025).
- U.S. Environmental Protection Agency. Air Emissions from MSW Combustion Facilities. 2021. Available online: https://www.epa.gov/stationary-sources-air-pollution/municipal-solid-waste-landfills-new-source-performance-standards (accessed on 10 November 2025).
- International Solid Waste Association (ISWA). Waste-to-Energy State-of-the-Art Report. 2015. Available online: https://www.iswa.org/wp-content/uploads/knowledge-base/ISWA6_7-000-2_WtE_State_of_the_Art_Report_2012_Revised_November_2013.pdf (accessed on 10 November 2025).
- European Commission. A Study on the Economic Valuation of Environmental Externalities from Landfill Disposal and Incineration of Waste: A Final Report. 2000. Available online: https://ec.europa.eu/environment/pdf/waste/studies/econ_eva_landfill_report.pdf (accessed on 10 November 2025).
- Astrup, T.; Møller, J.; Fruergaard, T. Incineration and Co-combustion of Waste: Accounting of Greenhouse Gases and Global Warming Contributions. Waste Manag. 2015, 37, 147–153. [Google Scholar] [CrossRef]
- The Sustainable Switch. Recycling in Toronto: How Does It Work Why Is It So Complicated? 2021. Available online: https://thesustainableswitchca.wordpress.com/2021/01/12/recycling-in-toronto-how-does-it-work-why-is-it-so-complicated/ (accessed on 10 November 2025).
- Toronto Environmental Alliance. Ontario’s Recycling System Needs to Be Overhauled-but Let’s Do It Right. 2022. Available online: https://www.torontoenvironment.org/tea_welcomes_a_stronger_producer_responsibility_system_for_ontario (accessed on 10 November 2025).
- California Management Review. America’s Broken Recycling System. 2023. Available online: https://cmr.berkeley.edu/2023/05/america-s-broken-recycling-system/ (accessed on 10 November 2025).
- Ragaert, K.; Delva, L.; Van Geen, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, T.; Hwang, H.; Sung, Y.; Kim, B.H. Development of Glycolysis Catalysts for PET Wastes Including Polyester Textiles. Fibers Polym. 2025, 26, 1–17. [Google Scholar] [CrossRef]
- Laldinpuii, Z.T.; Khiangte, V.; Lalhmangaihzuala, S.; Lalmuanpuia, C.; Pachuau, Z.; Lalhriatpuia, C.; Vanlaldinpuia, K. Methanolysis of PET Waste Using Heterogeneous Catalyst of Bio-waste Origin. J. Polym. Environ. 2022, 30, 1600–1614. [Google Scholar] [CrossRef]
- ISCC. Enabling a Circular Economy for Chemicals with the Mass Balance Approach. 2019. Available online: https://www.iscc-system.org/news/enabling-a-circular-economy-for-chemicals-with-the-mass-balance-approach/ (accessed on 10 November 2025).
- Creadore, M.; Castaldi, M. Quantitative Comparison of Life Cycle Assessments of Advanced Recycling Technologies for End-of-Life Plastics. ASME. J. Energy Resour. Technol. 2023, 145, 042201. [Google Scholar] [CrossRef]
- NREL. Life Cycle Assessment of Enzymatic PET Depolymerization. Green Chem. 2022, 24, 456–470. [Google Scholar] [CrossRef]
- Argonne National Laboratory. GREET Model Update: Plastic Pyrolysis Pathways. 2022. Available online: https://greet.es.anl.gov/publication-plastic-pyrolysis-2022 (accessed on 10 November 2025).
- Argonne National Laboratory. GREET Model Update: Circular Plastics and Pyrolysis. 2023. Available online: https://greet.es.anl.gov/publication-circular-plastics-2023 (accessed on 10 November 2025).
- The Coca-Cola Company. Sustainability. 2024. Available online: https://www.coca-colacompany.com/about-us/sustainability (accessed on 10 November 2025).
- Unilever. How We’re Aiming for Greater Impact with Updated Plastic Goals. 2024. Available online: https://www.unilever.com/news/news-search/2024/how-were-aiming-for-greater-impact-with-updated-plastic-goals/ (accessed on 10 November 2025).
- Barstow, T. Professor Says Full Plastic Recycling Could Be Within Reach. FlexPack Voice. 2024. Available online: https://flexpackvoice.com/issues/professor-says-full-plastic-recycling-could-be-within-reach/ (accessed on 10 November 2025).
- Royal Society of Chemistry (RSC); Institution of Chemical Engineers (IChemE); Institute of Materials, Minerals and Mining (IOM3). Jobs and Skills for a Circular Economy: A Cross-Sector Perspective from the Chemical and Materials Science and Engineering Communities. Royal Society of Chemistry. 2025. Available online: https://www.rsc.org/getContentAsset/43424aa3-2ef9-4a9d-b375-89b0c26e8492/f4c91d86-ac3e-4675-bdf3-f8325ded9710/Royal-Society-of-Chemistry-Skills-for-a-circular-economy-report.pdf. (accessed on 10 November 2025).
- U.S. Plastics Pact. U.S. Plastics Pact: Roadmap 2.0. 2025. Available online: https://usplasticspact.org/wp-content/uploads/2025/02/USPact_Roadmap2.0_Publication.pdf (accessed on 10 November 2025).
- Ellen MacArthur Foundation. Towards the Circular Economy: Economic and Business Rationale for an Accelerated Transition. 2013. Available online: https://content.ellenmacarthurfoundation.org/m/27265af68f11ef30/original/Towards-the-circular-economy-Vol-1.pdf (accessed on 10 November 2025).
- Closed Loop Partners. Accelerating Circular Supply Chains for Plastics. 2022. Available online: https://www.closedlooppartners.com/wp-content/uploads/2021/01/CLP_Circular_Supply_Chains_for_Plastics_Updated.pdf (accessed on 10 November 2025).
- McKinsey Company. Plastics Recycling: Using an Economic-Feasibility Lens to Select the Next Moves. 2023. Available online: https://www.mckinsey.com/industries/chemicals/our-insights/plastics-recycling-using-an-economic-feasibility-lens-to-select-the-next-moves (accessed on 10 November 2025).
- Circular Economy for Flexible Packaging. Design for Recycling Guidelines for Polypropylene Packaging. 2022. Available online: https://ceflex.eu/guidelines/ (accessed on 10 November 2025).
- S&P Global. Recycled Plastics Market Outlook. 2023. Available online: https://www.spglobal.com/commodityinsights/en/ci/products/recycled-plastics-prices.html (accessed on 10 November 2025).
- Independent Commodity Intelligence Services (ICIS). Pricing intelligence: Recycled plastics market data and analysis. 2023. Available online: https://www.icis.com/explore/services/pricing/ (accessed on 10 November 2025).
- Association of Plastic Recyclers. The APR Design Guide for Plastics Recyclability. 2022. Available online: https://plasticsrecycling.org/apr-design-guide (accessed on 10 November 2025).
- U.S. Environmental Protection Agency. Recycling Economic Information Report. 2021. Available online: https://www.epa.gov/smm/recycling-economic-information-rei-report (accessed on 10 November 2025).
- Rabobank. Despite Challenges, Plastic Recycling Poised to Grow. RaboResearch Report. 2023. Available online: https://www.recyclingmonster.com/article/rabobank-despite-challenges-plastic-recycling-poised-to-grow/3573 (accessed on 10 November 2025).
- Ferrari, M.; Loppe, J.; Vande Loo, J. ‘Green Methanol’ Is the Packaging Industry’s Future. Plastics Today. 2021. Available online: https://www.plasticstoday.com/packaging/-green-methanol-is-the-packaging-industry-s-future (accessed on 10 November 2025).
- Waste Watch Ottawa. Extended Producer Responsibility (EPR). 2022. Available online: https://wastewatchottawa.com/epr/ (accessed on 10 November 2025).
- Circular Materials. For Producers, By Producers. 4 November 2024. Available online: https://www.circularmaterials.ca/ (accessed on 10 November 2025).
- U.S. District Court for the District of Oregon. National Association of Wholesaler-Distributors v. Oregon Department of Environmental Quality; U.S. District Court for the District of Oregon: Portland, OR, USA, 2025. [Google Scholar]
- Leonard, M.M. Mechanical vs. Chemical Recycling: Friends or Foes? Envetec. 2025. Available online: https://envetec.com/news/2024/09/compare-recycling-types/ (accessed on 10 November 2025).
- Tabish, M.; Khan, S.A.R.; Yu, Z.; Tanveer, M. A thorough overview of the literature on waste recycling in the circular economy: Current practices and future perspectives. Environ. Sci. Pollut. Res. 2024, 31, 61377–61396. [Google Scholar] [CrossRef] [PubMed]
- Popowicz, M.; Katzer, N.J.; Kettele, M.; Schöggl, J.-P.; Baumgartner, R.J. Digital technologies for life cycle assessment: A review and integrated combination framework. Int. J. Life Cycle Assess. 2025, 30, 405–428. [Google Scholar] [CrossRef]
- Ivanov, D.; Dolgui, A. Viability of intertwined supply networks: Extending the supply chain resilience angles towards survivability. Int. J. Prod. Res. 2020, 58, 2904–2915. [Google Scholar] [CrossRef]
- Gereffi, G. What does the COVID-19 pandemic teach us about global value chains? J. Int. Bus. Policy 2020, 3, 287–301. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Beyond Oil and Gas: The Methanol Economy; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G. Waste-to-energy: A review of the status and benefits in China. Renew. Sustain. Energy Rev. 2016, 55, 115–128. [Google Scholar]
- Chopra, S.; Sodhi, M.S. Managing risk to avoid supply-chain breakdown. MIT Sloan Manag. Rev. 2004, 46, 53–61. [Google Scholar]
- United Nations, Department of Economic and Social Affairs. Global Sustainable Development Report, 2015th ed.; United Nations: New York, NY, USA, 2025; Available online: https://www.un.org/en/development/desa/publications/global-sustainable-development-report-2015-edition.html (accessed on 10 November 2025).
- U.S. Environmental Protection Agency. Documentation for Greenhouse Gas Emission and Energy Factors Used in the Waste Reduction Model (WARM). Office of Resource Conservation and Recovery. 2025. Available online: https://www.epa.gov/waste-reduction-model/documentation-waste-reduction-model (accessed on 10 November 2025).
- Franklin Associates/APR datasets. Life Cycle Impacts for Postconsumer Recycled Resins: PET, HDPE, and PP. 2018. Available online: https://plasticsrecycling.org/wp-content/uploads/2024/08/2018-APR-LCI-report.pdf (accessed on 10 November 2025).
- Shen, L.; Nieuwlaar, E.; Worrell, E.; Patel, M.K. Life cycle energy and GHG emissions of PET recycling: Change-oriented effects. Int. J. Life Cycle Assess. 2025, 16, 522–536. [Google Scholar] [CrossRef]
- Uekert, T.; DesVeaux, J.S.; Singh, A.; Nicholson, S.R.; Lamers, P.; Ghosh, T.; McGeehan, J.E.; Carpenter, A.C.; Beckham, G.T. Life cycle assessment of enzymatic poly(ethylene terephthalate) recycling. Green Chem. 2022, 24, 6531–6543. [Google Scholar] [CrossRef]
- Benavides, P.T.; Gracida-Alvarez, U.R.; Lee, U.; Wang, M.Q. Life-cycle Analysis of Conversion of Post-Use Plastic via Pyrolysis with the GREET Model; Argonne National Laboratory: Argonne, IL, USA, 2022; p. ANL-22/37. [Google Scholar] [CrossRef]
- Kladisios, P.; Sagia, A.S. A review on supercritical water gasification of biomass. Bioenergy Bioresour. Open Access 2022, 3, 7–10. [Google Scholar]
- Neves, F.; Soares, A.A.; Rouboa, A. Systematic review of biomass supercritical water gasification for energy production. Energies 2025, 18, 5374. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).