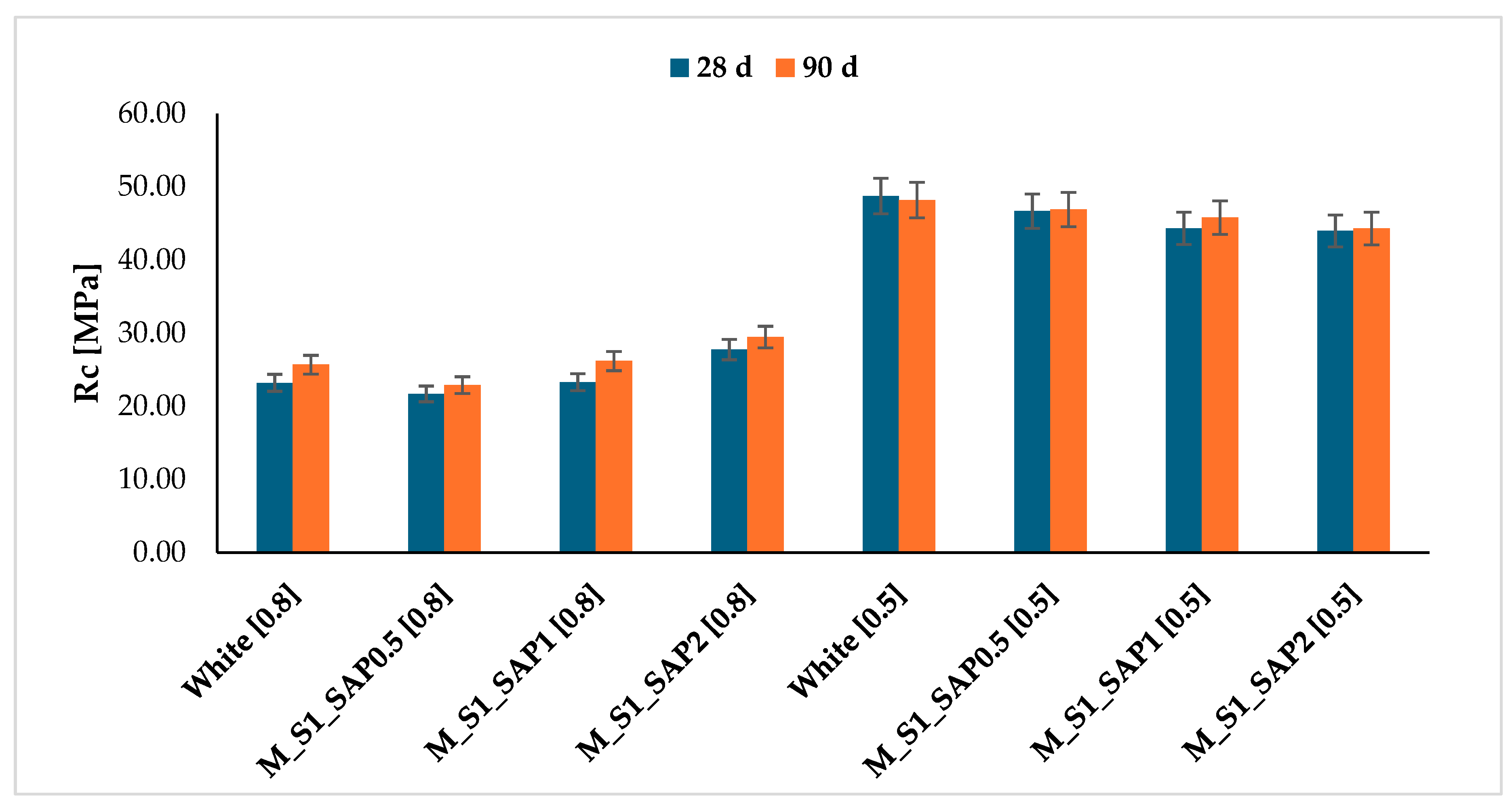Abstract
Port maintenance causes large quantities of dredged sediment throughout the world. The disposal of this material in authorised landfills is economically disadvantageous, as well as being at odds with a circular economy model with a reduced impact on the environment. The application of stabilization/solidification treatment to dredged marine sediments allows an improvement of their physical and mechanical properties, together with the production of cement-based materials that can be used for road construction, as well as for making blocks and bricks. In this study, an experimental laboratory investigation is carried out on two samples of sandy sediments collected from the Mola di Bari harbour (Southern Italy), to identify sustainable management options for recovering materials that will be dredged. To assess the influence on mortars made from sediments with variable organic matter content and seawater, these were characterised from a chemical–physical point of view before and after washing treatment and oxidative processes. The products of the Stabilization/Solidification (S/S) treatment were evaluated in terms of workability, flexural and compressive strengths, and, furthermore, a microstructural study was conducted using SEM-EDX and optical microscopy to analyse the internal structure of the materials. The mechanical performance evaluation clearly demonstrated organic matter’s negative impact on strength development, resulting in a 16% reduction. Pre-treatments, such as sediment washing, effectively improved the performance of treated sediments (e.g., 24% increase in compressive strength). This study aims to demonstrate the benefits of recycling marine sediments in cement-based materials, highlighting how this process can enhance circularity and sustainability while reducing the environmental impact of dredging activities.
1. Introduction
To guarantee the security of vessels in transit and concurrently enhance accessibility, it is imperative to undertake periodic dredging operations in harbours. As indicated in a report published by UNEP [1], 6 billion tons of sediment are extracted from the seabed on a worldwide basis annually as a component of dredging operations. The report also estimates that between 9.7 and 15.5 million tonnes were dredged within 200 nautical miles of the Italian coast in 2022. In most cases, dredged materials are classified as waste and disposed of with high environmental risks [2]. However, new management strategies are being developed to reduce landfilling and increase reuse, with sediment being regarded as a resource [3]. This implies the development of sustainable solutions for the recycling of dredging sediments.
Various project experiences and comparative assessments consistently highlight the economic and environmental benefits of sediment reuse [4,5]. For instance, a cost–benefit analysis conducted by Bortali et al. (2023) [6] in the port of Safi case study revealed a 74% reduction in overall costs, thus highlighting the significant advantages of reusing dredged marine materials in the construction sector. This finding is supported by the research conducted by Lakhiar et al. (2024) [7], which demonstrated that sandy sediments integrated into composite mixtures (BFRP) exhibited a diminished environmental impact and a 67% reduction in costs.
Dredged marine sediments typically have high moisture content and poor mechanical properties, so their reuse is challenging [8]. Proper management is fundamental for developing more circular systems with a lower environmental impact. In recent decades, research has focused on developing treatments that allow dredged sediments to be reused as secondary raw materials. One of the most interesting technologies is stabilisation/solidification (S/S) [9]. This treatment is a chemical/physical process that aims at waste inertization in a stable matrix with appropriate technical properties, such as permeability and mechanical strength [10]. S/S involves the addition of binders and additives [11,12]. Sediments treated in accordance with this process can be recovered for use in a variety of applications, including road construction [13,14], cement mortars [15], and filling material and blocks [16], as well as raw material in brick production [17,18].
The potential utilisation of marine sediments within cementitious material production cycles is contingent upon their mineral and chemical composition (e.g., siliceous, calcareous, clayey), in addition to their grain size characteristics. When these parameters are adequate, it is possible to reuse the sediment as a cement substitute [19,20] or as an acceptable fine aggregate substitute for producing of cementitious materials [21,22]. Research by Loudini et al. (2020) [23] confirmed the feasibility of using dredged marine sandy sediments as road-based layer material. Similarly, a study by Limeira et al. (2010) [24] found that concrete incorporating marine sediments with equivalent gradings satisfied the required mechanical criteria, establishing a minimum compressive strength of 30 MPa for port road pavement construction. Couvidat et al. (2016) [25] provide an example of this, examining the replacement of sand with a coarse fraction of sediment (d = 80 μm) for the production of non-structural mortars. As Hayek et al. (2023, 2024) [26,27] reported, replacing 30% of the aggregate with sandy marine sediments had no adverse effect on the durability of the concrete structure while reducing natural resource consumption and CO2 emissions.
Specifically, the particle size, mineralogical composition, and organic matter (OM) content of dredged sediment (DS) can significantly influence its reuse as a natural aggregate in the construction industry and thus within cement mixes. Sediments characterised by particles smaller than 65 μm may be challenging to reuse according to Messina et al. (2017) [28]. Conversely, sediment particles characterised by larger diameters and rich in Si, such as SiO2, have good potential for reuse as sand in concrete production [29]. Depending on the site’s location, DS may contain varying amounts of organic matter (OM), from low to high OM content [30]. When high percentages of up to 30% by mass are reached, applicability in the construction industry becomes difficult as a function of negative variations in the engineering properties of the material [31,32]. High OM content in sediments has resulted in subsequent complications during the hydration phase within cement mortars [33].
Hamouche & Zentar [34] demonstrated that when the percentage content of OM varies from 5 to 15% within the sediment, it loses its bearing capacity by approximately 60%. Ekwue’s [35] findings revealed a direct correlation between the increase in organic matter content and the rise in the optimum water content for compaction, concurrently accompanied by a decrease in bulk density.
Furthermore, DS is a supersaturated material with a high water content, due to the presence of free water, interstitial water bound by surface tension to the DS particles, capillary water, and chemically bound water. This amount of water increases the volume of the solid structure, making the dredged material difficult to manage [36,37]. Therefore, dewatering dredged sediments is essential to reduce their volume, thereby significantly reducing transportation and disposal costs and promoting their reusability [38]. Currently, the state of the art involves the application of different dewatering technologies based on mechanical, biological or chemical approaches [39,40,41], as well as innovative solutions which involve the use of superabsorbent polymers (SAP) in stabilization/solidification treatments [42,43,44,45].
The present study investigates the effect of pre-treatments on improving the mechanical behaviour of treated sediments from Mola di Bari harbour (Southern Italy). To adequately develop hydration reactions and the consequent mechanical strength, sediment washing (with tap water and hydrogen peroxide) and chemical dewatering (with superabsorbent polymers) have been tested to recycle dredged sediment as aggregates for cement-based materials. The cement-based materials obtained from S/S treatments were characterized both in their fresh state (i.e., workability tests) and after 28 and 90 days of curing (flexural and compression strength); additionally, optical and scanning electron microscopy (SEM) measurements were conducted to analyse the morphology and microstructure of the specimens, as well as their compositions. This experimental plan will enable the study of organic matter, salinity and moisture on cement-based material mechanical behaviour, for sustainable sediment management.
2. Results and Discussion
2.1. Characterization of the Sediments
The samples subjected to experimental testing were analysed in terms of particle size and chemical and physical characteristics of moisture, organic matter, and ash at all stages of the study, as detailed in Table 1. The physico–chemical analyses confirmed the different composition of the samples in terms of the percentage of volatile/organic fraction, which was found to be more prevalent in the sample designated as S1.

Table 1.
Chemical–physical characterisation of S1 and S2 samples.
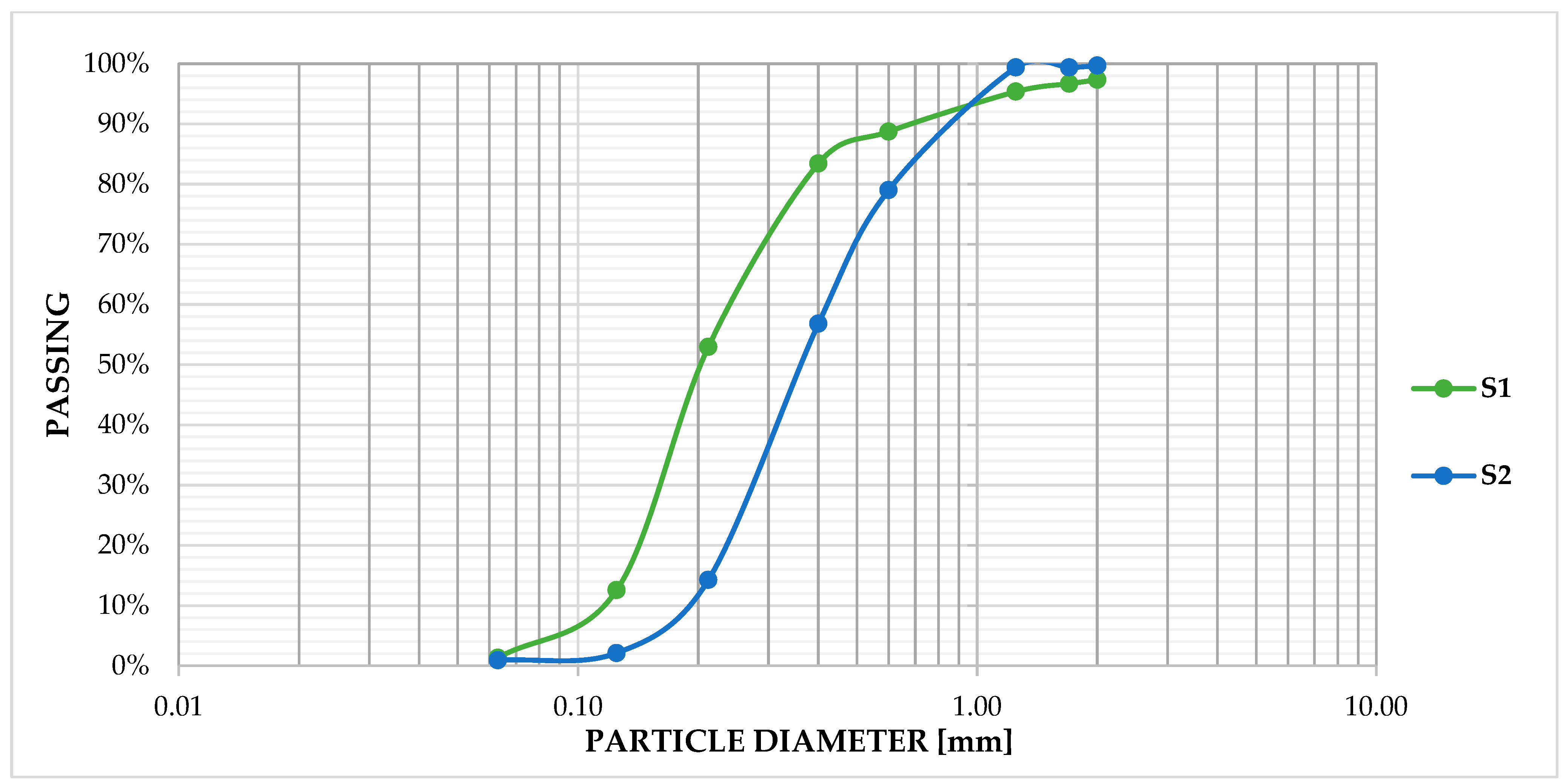
Sifting of S1 and S2 was carried out according to SNPA/18 2018 [46]. Moreover, washing treatment indicated that the sediments were solid particles purely in the sand range. Specifically, 90% of samples S1–S2 were in the fine–coarse sand range (0.063–0.6 mm) and the remaining 10% in the very coarse sand range (0.6–2 mm) according to the Udden–Wentworth scale [47,48], with a D50 of 0.35 and 0.2 mm for samples S2 and S1, respectively (Figure 1).
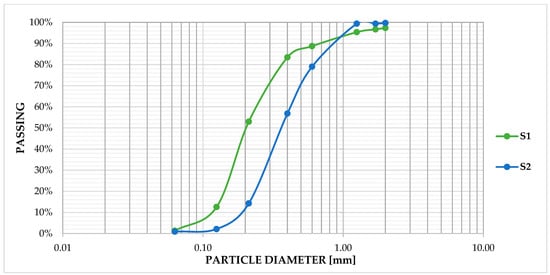
Figure 1.
Grain size curve sample S1 and S2.
The two sediment samples (S1 and S2) are illustrated in microscopic detail in Figure 2 and Figure 3.
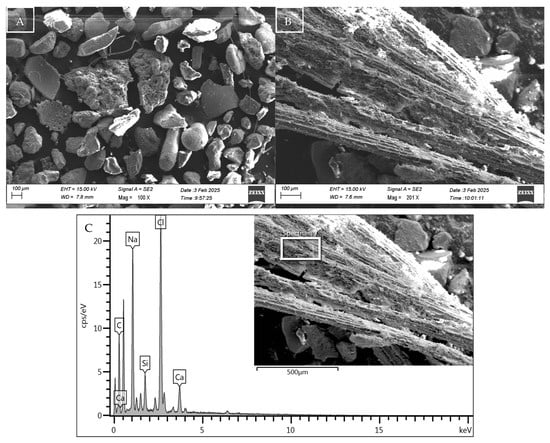
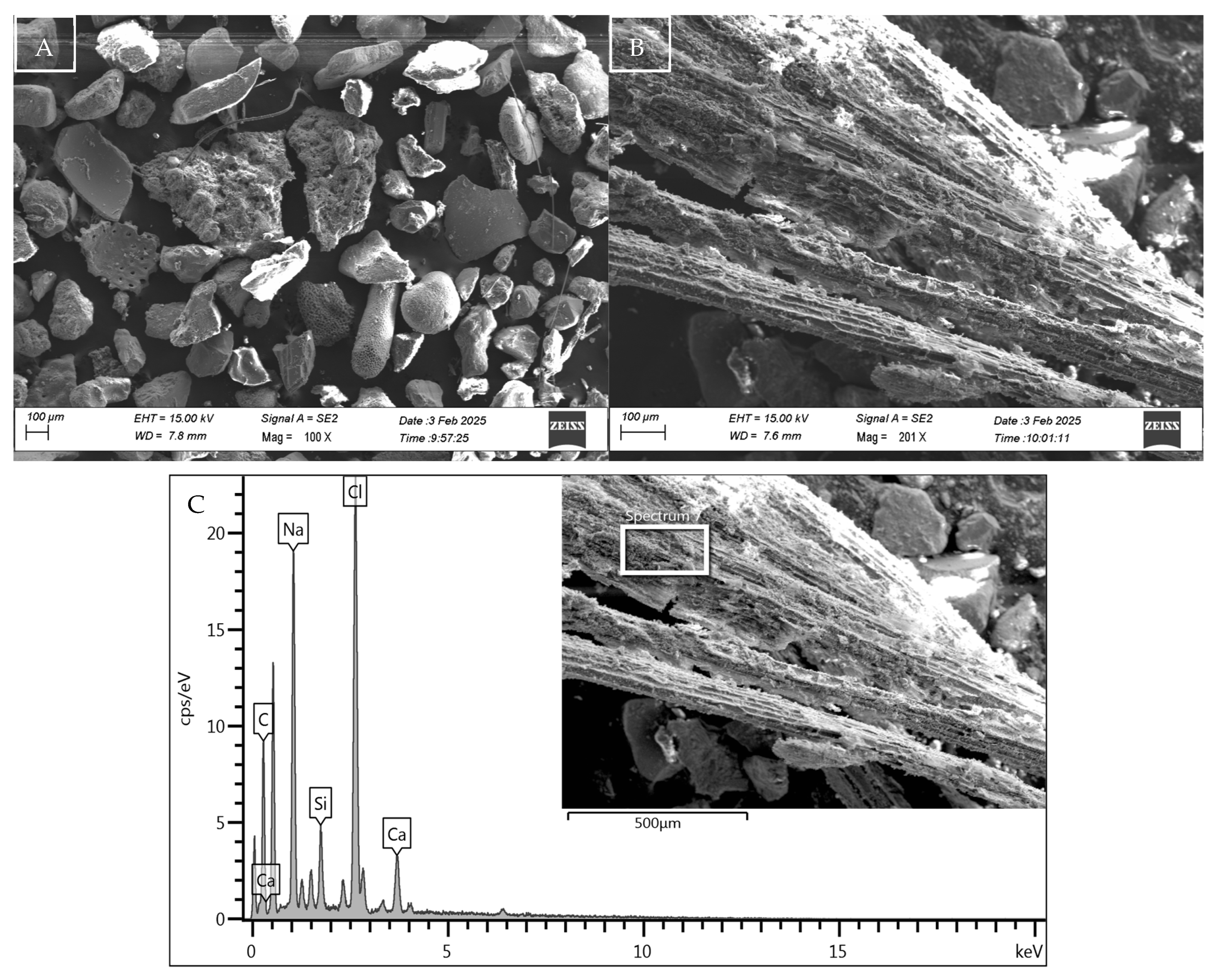
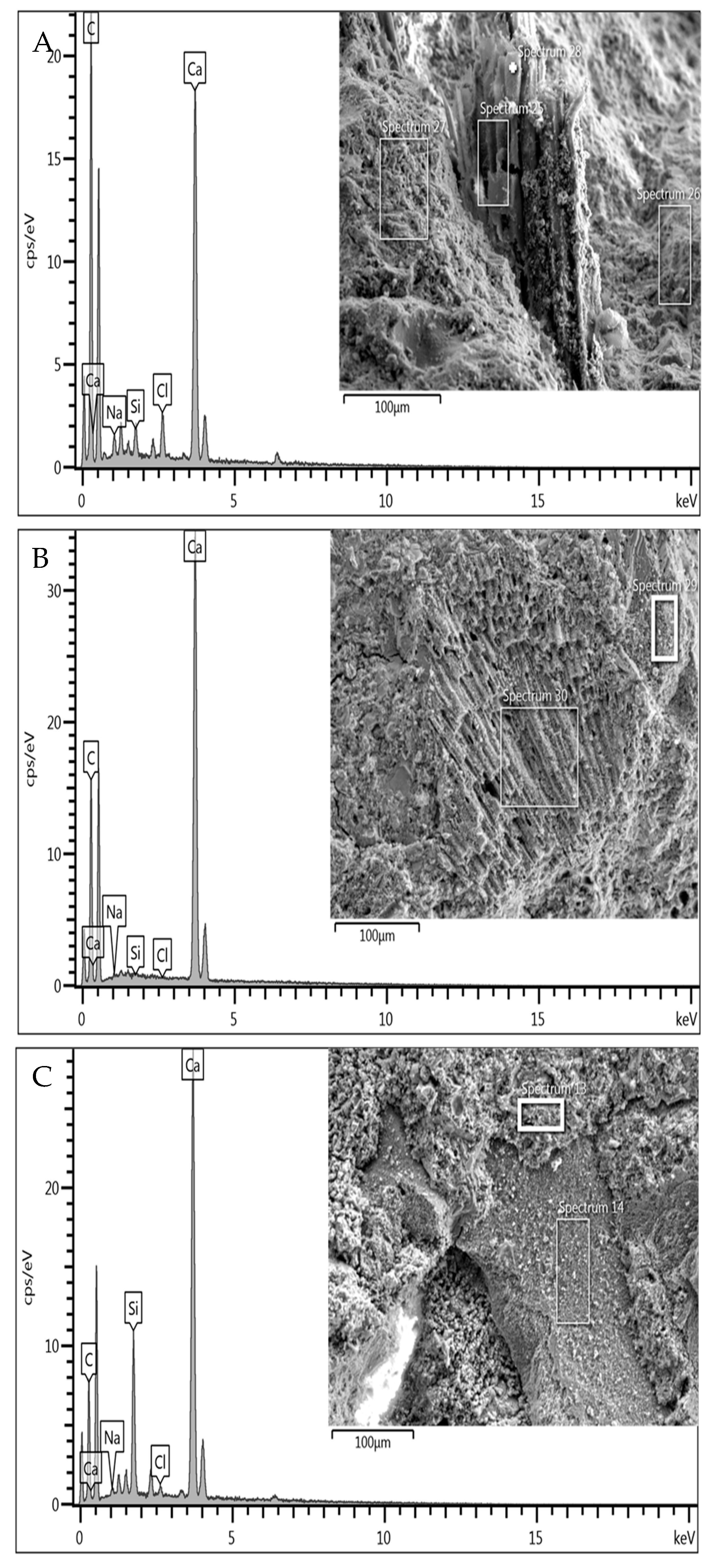
Figure 2.
Microstructure, morphology, and EDX spectra of sediment sample S1 (A), with details in (B) of the decomposed organic algae particle present and its composition in (C).
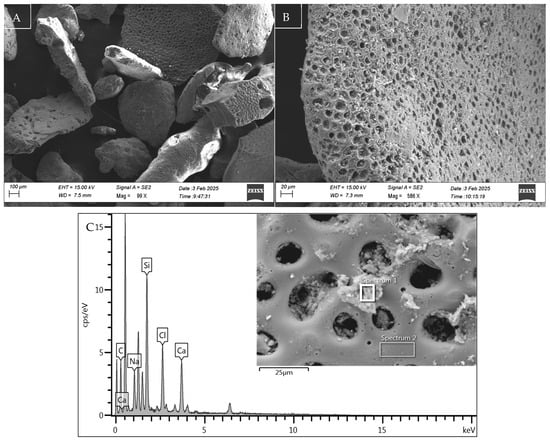
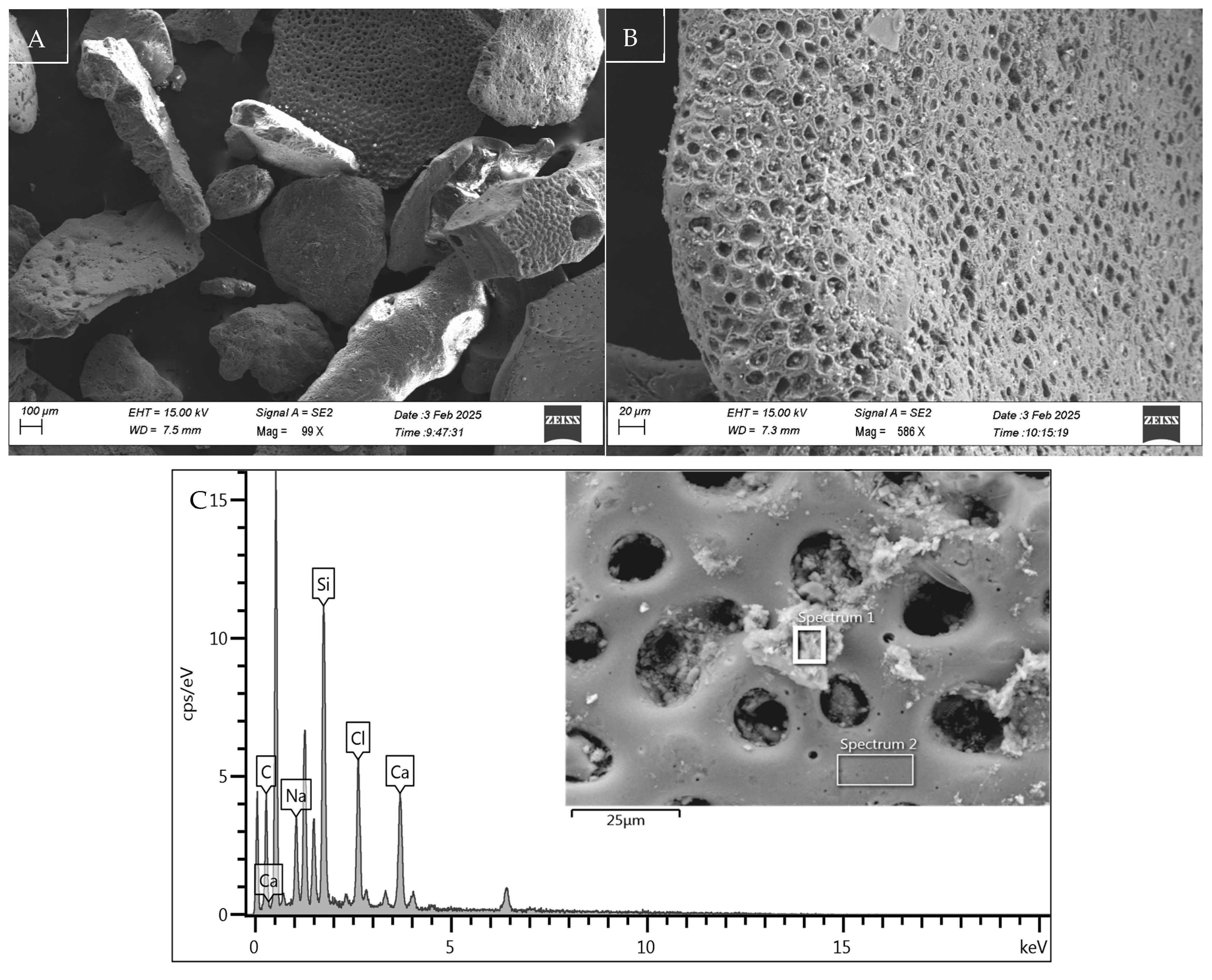
Figure 3.
Microstructure, morphology, and EDX spectra of sediment sample S2 (A), with details of the microporous structure of a sediment particle in (B) and its composition in (C).
The study at the microstructural level, developed through SEM-EDX analysis, highlighted the microporous nature of the sediment particles (S1, S2) used in the experimental tests. The essential differentiating component between the samples, the presence of organic fibres in sediment S1, is illustrated in Figure 2B, where an image of its microstructure in its natural state is shown. This is later confirmed by the product of EDX analysis (Figure 2C), in which the organic nature of the material is highlighted due to the significant peak of C together with the presence of elements such as Na and Cl, due to the marine origin of the material used. This information corroborates the chemical and physical characterisation data for sample S1, which demonstrates a higher presence of solid volatiles intrinsically associated with organic fibre.
As illustrated in Figure 3C, the spectrum of sediment particle S2 demonstrates a convergence of the representative peaks of the EDX analysis with the mineralogical composition of the sediments in the area under study, particularly the southern portion of the Adriatic Sea. As evidenced by Lucchini et al. 2003 [49] and by the data of the oceanographic campaign ‘PERTRE’ (one of the European ‘PERSEUS’ project missions) carried out by the Italian Institute for Marine Biological Resources and Biotechnology (IRBM-CNR) in 2016, there is confirmation of the reduced organic content shown in the characterisation data of Table 1.
2.2. Effect of Organic Matter on the Physical–Mechanical Performance of Cement Mortars
The chemical–physical characterisation of the non-treated, water-treated, and H2O2 pre-treated sediments is described in the following section.
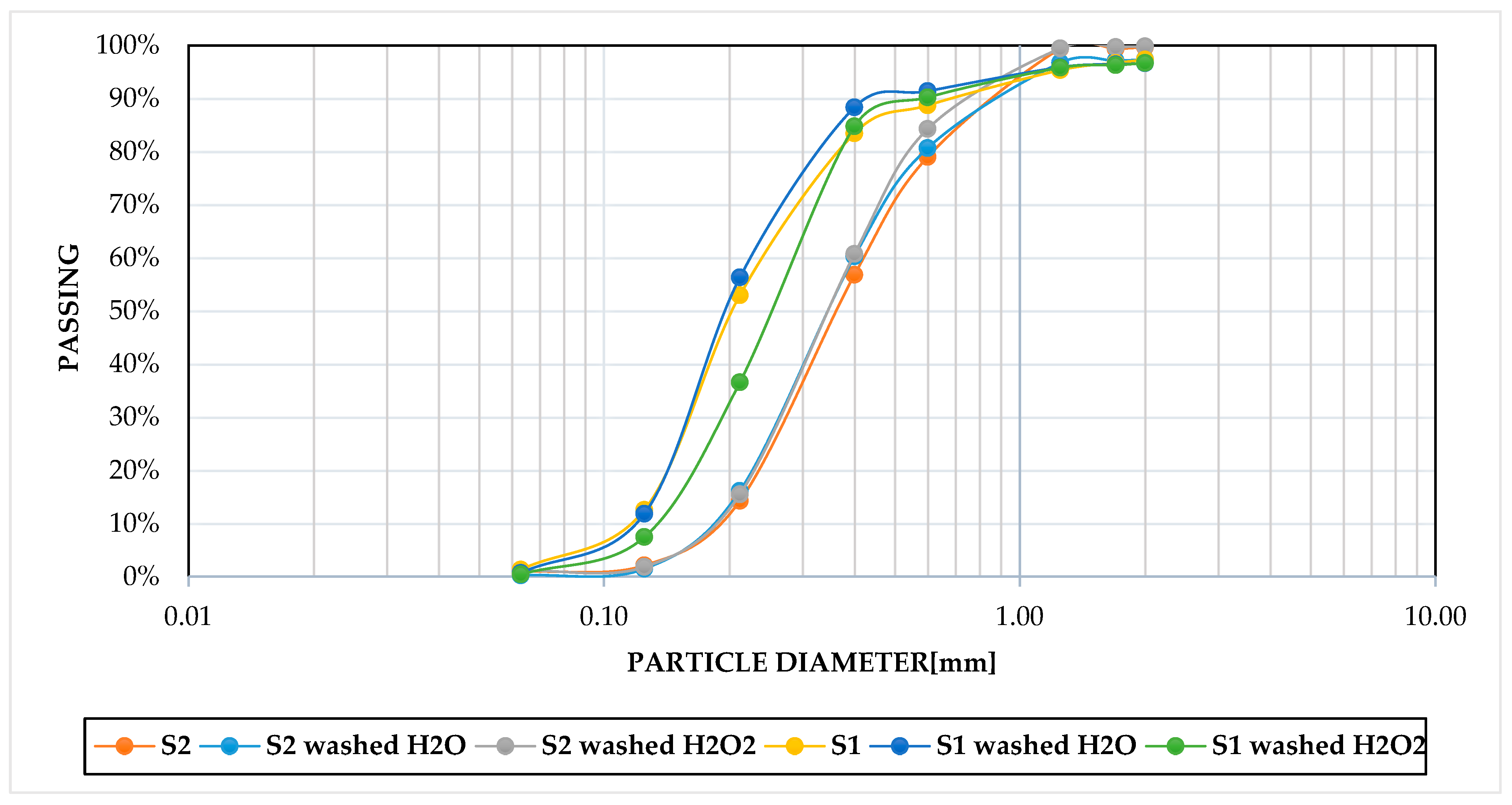
The chemical–physical analysis of the samples subjected to washing and oxidation treatments indicated a progressive reduction in volatile/organic fraction (Table 2). Indeed, samples S1 and S2, which underwent washing with H2O, exhibited 15% and 13% reductions of the indicated fraction, respectively. Moreover, after washing with an oxidiser, the maximum reduction was observed at 40% and 31.5%, respectively. After the treatments with H2O and H2O2, the grain size distribution was examined, and the data were compared to the initial condition. The most notable alteration was observed in sample S1 following the oxidation washing with H2O2, which indicated a slight reduction in the proportion of medium-sized sands (0.6–0.2 mm) and an increase in the finer fraction (0.2–0.063 mm) with a representative D50 of 0.28 mm. This particle size distribution is presumably correlated to the variation in organic content within the sample under study (Figure 3). In contrast, the variations of the particle size distribution following the treatments on sample S2 are not significant (Figure 4) because of the low organic content, so the D50 was constant following the treatments.

Table 2.
Chemical–physical characteristics of samples S1 and S2 subjected to H2O and H2O2 pre-treatment.
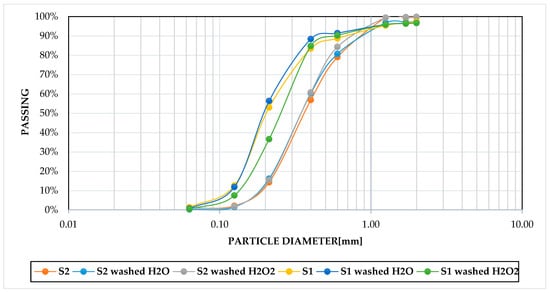
Figure 4.
Grain-size distributions of S1 and S2, treated and untreated.
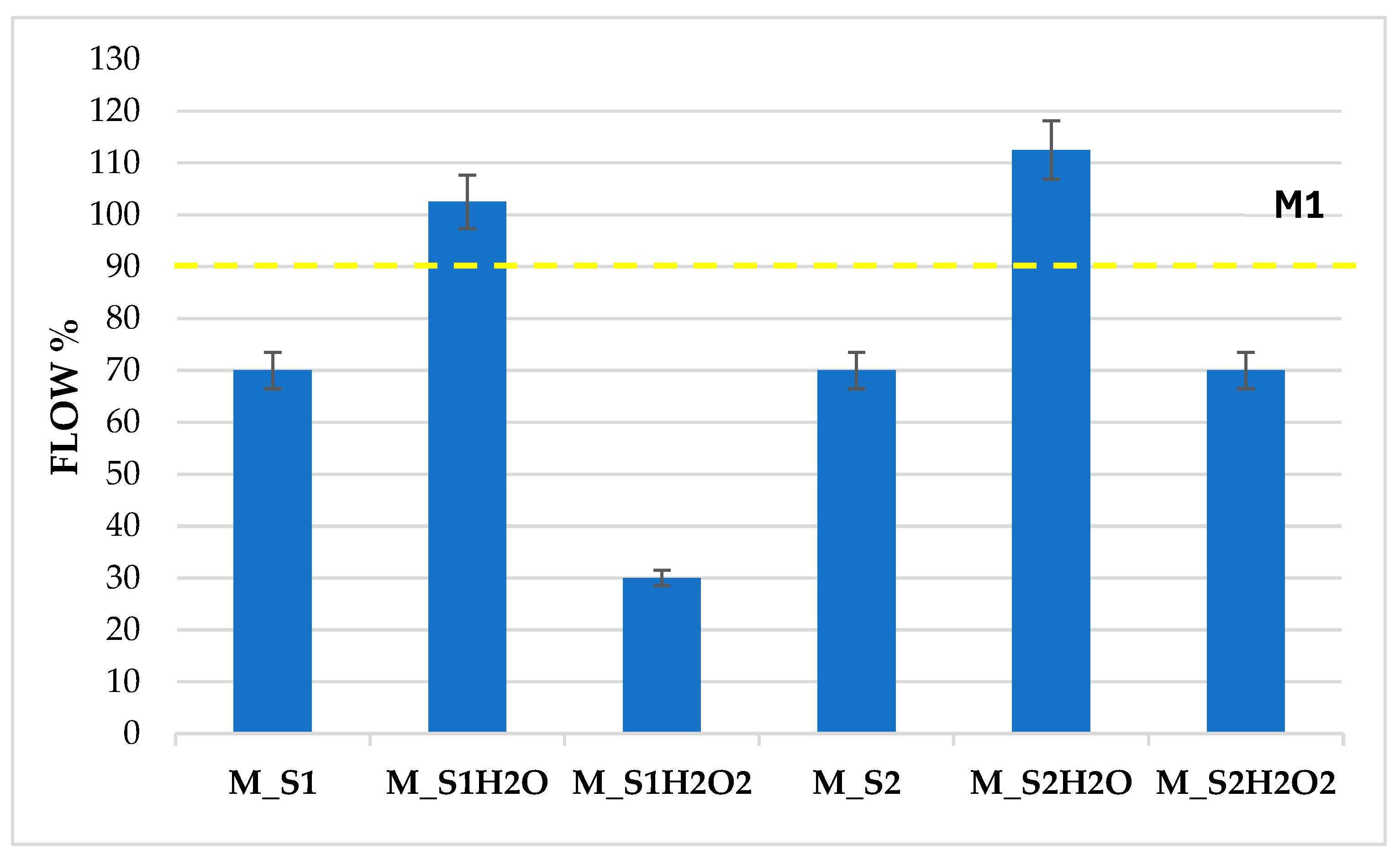
The mortars produced from sediment samples S1 and S2, respectively M_S1 and M_S2, showed 70% workability, slightly lower than the reference value of the normalised mortar M1 with a flow of 90%. This result is ascribed to the sediment, which is characterised by organic substances that absorb a higher quantity of water, thus reducing workability (Figure 5).
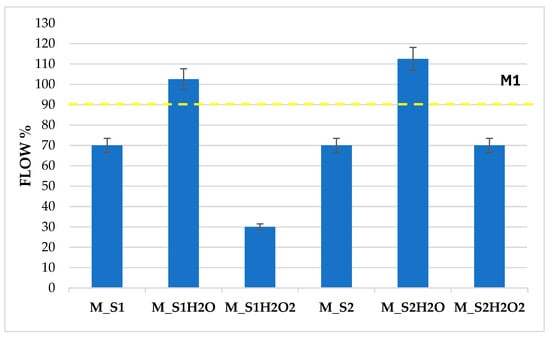
Figure 5.
Flow test on samples M_S1–M_S2 and the same treated with H2O and H2O2.
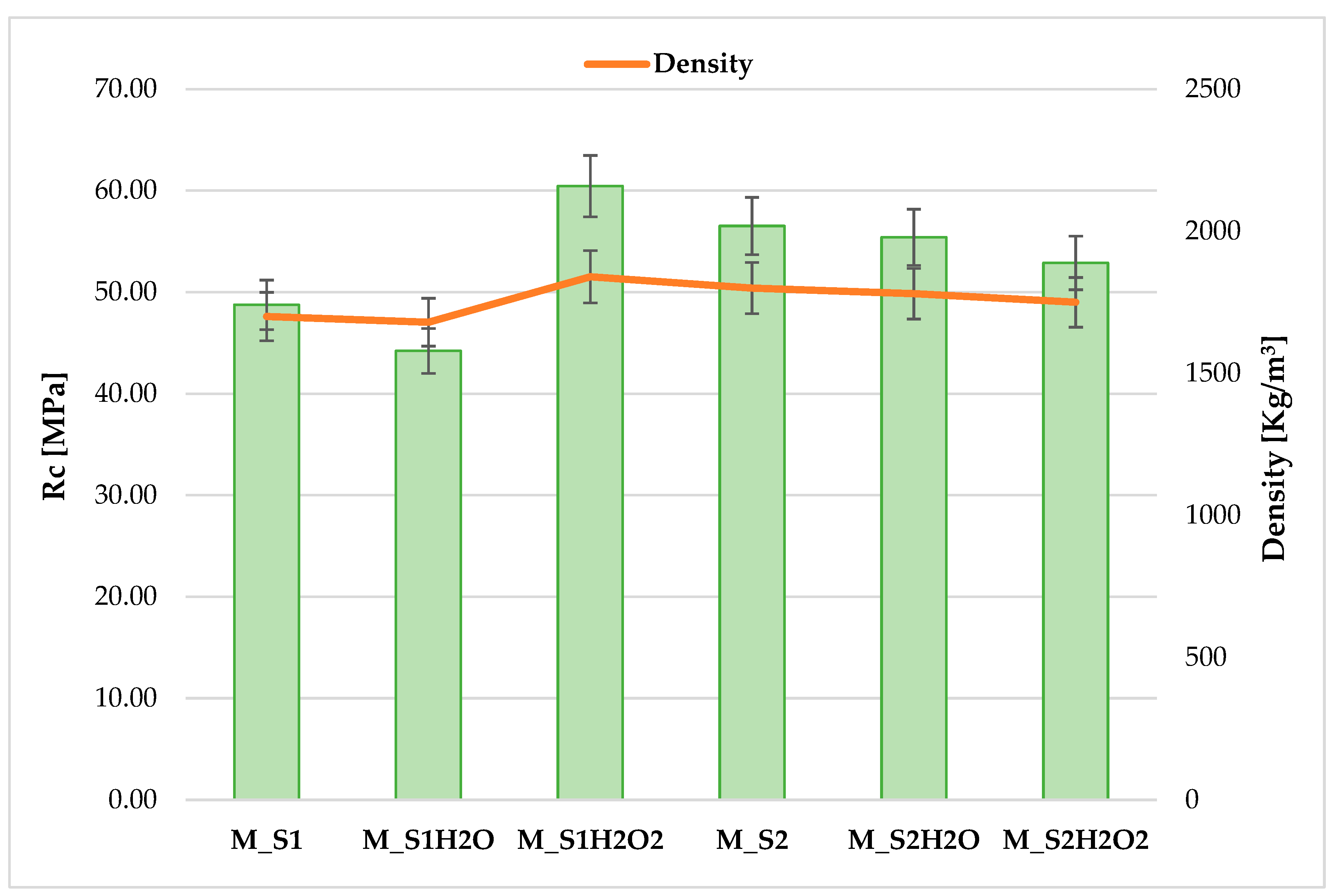
M_S1 and M_S2 samples showed interesting flexural and compression results, comparable with the reference (M1), as shown in Table 3. The M_S2 sample showed higher strength than the S1 sample (16%). This discrepancy can be attributed to the chemical composition of the sediments. In chemical–physical characterisation, sample S1 contained more organic particles, which were identified through optical analysis as seaweed residues (Posidonia). Experimental tests have confirmed that these particles inhibit hydration reactions and harm mechanical strength development, as shown in the study of Du et al. 2020 [50].

Table 3.
Overview of the physical–mechanical performance of cement mortars produced by mixing the S1–S2 sediment sample and after washing with H2O and H2O2.
It is hypothesised that the ionic substances present in seawater may affect the performance of cement-based materials, given the higher concentration of these substances in seawater compared to freshwater. Such effects may impede the development of the setting and rheological properties of the cement, consequently influencing the mechanical and durability properties of the concrete [51,52]. However, in contrast to these studies, other researchers have indicated that various chemicals, such as chloride ions present in seawater, can enhance the strength of concrete in the short term, in some cases with higher values than the conventional conglomerates [53,54]. As stated by Qu et al. (2021) [55], saline ions within the interstitial water in marine sediments have been observed to promote compressive strength development in the early stages of the cement hydration process. Furthermore, this trend has remained constant over time, exceeding the strengths achievable in the presence of fresh water.
The chloride ions from marine sediment, present in the system in the form of NaCl, CaCl2, MgCl2, and KCl, can react with tri-calcium aluminate (C3A) and/or gypsum (CaSO4), forming Friedel’s/Kuzel salts, which can expand to fill the pores. These reactions consume calcium hydroxide (CH), a cement hydration product, and further form a new compound such as Friedel’s/Kuzel’s salts, as shown below in Equations (1) and (2) (Yadav et al., 2024) [56].
This effect may explain the time stability of the mechanical strengths. The free ions retained in the system, leading to an increase of pH and also alkalinity of the pose solution, means the metastable hydroxy-AFm (alumino-ferrite monosubstituted) hydrates hardly coexist with aqueous Cl− [57,58], Equation (3).
Friedel’s salt is prone to instability and influenced by the alkalinity level; as alkalinity decreases due to carbonation, the salt becomes more soluble and releases chlorides into the pore solution [55].
The aforementioned indications were consequently evaluated by using H2O as a cleaning agent in a 1:1 ratio by weight to the quantity of sample to be cleaned until ionic equilibrium was attained, as determined by a pH meter/conductance meter from PC5000 EUTECH Instruments (the average pH alteration in a heterogeneous solution was from 8.33 to 7.4).
The sediment washing treatment, which involved flowing water, reduced the salt concentration within the sedimentary material. This was achieved by removing ions present in the solution, including sodium, chlorides, magnesium, sulphate, calcium, and carbonates. Therefore, this change is associated with the enhanced workability observed in the washed sediments, which exhibited flow peaks higher than 100% for samples M_S1H20 and M_S2H20. At 28 days of curing, the hardened cement mortars subjected to sediment washing exhibited reduced flexural and compressive strength compared to the corresponding mortars that were characterised by interstitial seawater. This decline is associated with decreased salt concentration and reduced density in the hardened mortars, which leads to a more porous structure following washing with H2O.
Organic materials generally display hydrophobic behaviour, which is detrimental to developing hydration reactions. However, hydrophilic behaviour can be obtained through oxidising treatment. The oxidation process results in the formation of defects and holes on the surface of the carbon material, which can facilitate enhanced physical interactions and, consequently, a stronger anchorage and greater strength in the hardened cement mortar. Furthermore, the oxidation process can alter the chemical composition of the organic material’s surface. These chemical modifications are associated with forming oxygen-containing functional groups on the surface, which enhance the interaction with cement, thereby facilitating the interaction between silicates and aluminates with the generated groups. Because of the oxidation process, the polarity of these groups is increased, as is the wettability of the material. This results in a reduction in the workability of cement mortars due to the reduction in the availability of water to lubricate fresh mixes. Additionally, physical alterations are observed because the oxidation treatments enhance the surface texture of the organic material, thereby facilitating the incorporation of fresh cement paste into the voids created on the treated algae surface [59].
Following oxidative washing with H2O2, the most evident results were obtained on the sample with the most significant organic component, S1. Indeed, following oxidation, S1 underwent a percentage reduction in organic compounds, which was accompanied by a correlated reduction in ∆ flow of 40%, in the corresponding mortar M_S1H2O2, due to the greater absorption of H2O by the degraded organic component. In contrast, following oxidation, S2 suffered a minimal percentage reduction in OM and a zero reduction in ∆ flow, in the corresponding mortar M_S2H2O2.
The data obtained in mortars from sediments treated with hydrogen peroxide (H2O2) yielded favourable results for sample S1, with a strength increase of 24% compared to the same in the as-is condition. By degrading organic particles, the reagent responsible for oxidation led to a decrease in the inhibition of mechanical strength development by organic particles. Furthermore, for sediment S2, which is less rich in organic component, oxidation with H2O2 did not bring any substantial changes in the mechanical behaviour of the sediment compared to the control sample (Figure 6).
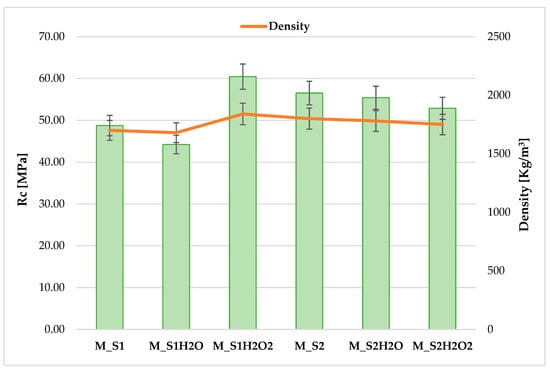
Figure 6.
Overall compressive strength test trend for cement mixes produced from sediment samples S1–S2 and washed with H2O and H2O2.
Following the SEM-EDX investigation of the mortar samples with S1 sediment and the same subjected to oxidising washing with H2O2 (M_S1, M_S1H2O2), a variation in terms of the chemical composition of the organic fibres was highlighted, with a reduction in the intensity of the C peak within the spectra obtained from the analysis (Figure 7A,B). Furthermore, an alteration in the fibre structure was observed, which, following the oxidising treatment, was found to be more degraded and amalgamated within the conglomerates.
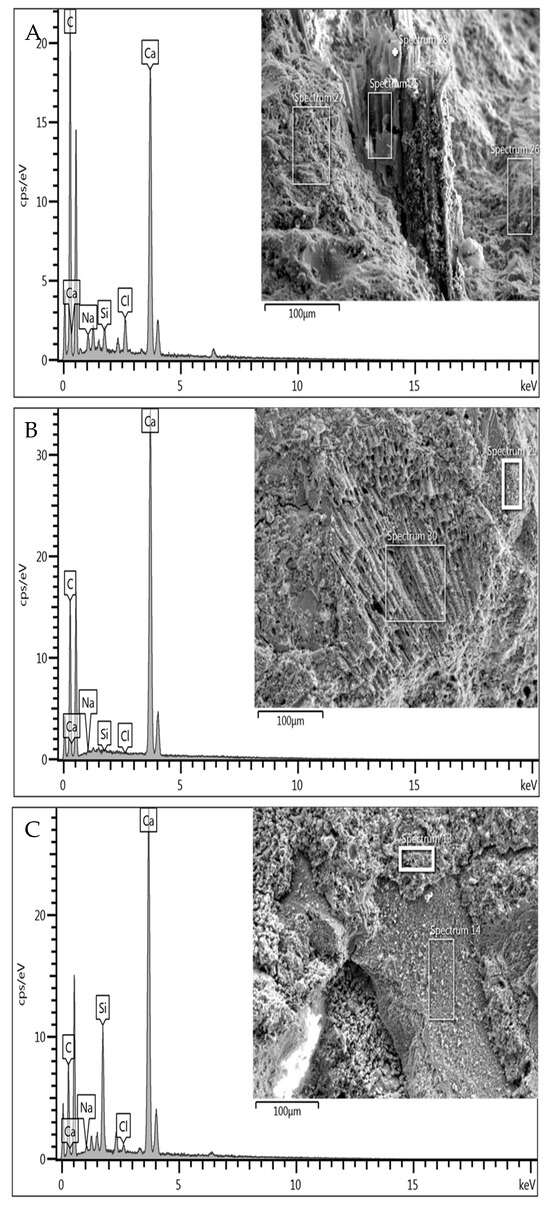
Figure 7.
SEM-EDX analysis of conglomerate samples M_S1 (A), M_S1H2O2 (B), M_S2 (C).
Conversely, the Si peak became more relevant within the spectrum in mortars obtained with sediment S2, which has fewer organic fibres. Mortar is a data point that distinguishes the marine sediments under study, as defined in the literature on their chemical–mineralogical composition. The microstructural analysis correlated the increased compressive strength of M_S1_H2O2 mortar samples compared to M_S1 and the reduction in the organic content of the S1 sediment sample pre- and post-oxidative treatment. This, in turn, is correlated with the degradation of the organic particles’ structure, as visually evidenced in Figure 7B. Indeed, as evidenced by Figure 7A,B and the accompanying EDX spectra, the degradation of organic particles coupled with a reduction in available organic content facilitated the development of a positive response and increased compressive strength in the conglomerate sample produced.
A similar observation can be made for the M_S2 mortar sample, produced from untreated S2 sediment. This sample, less rich in organic components as discernible from Figure 7C, exhibits fewer discontinuities and greater interaction between aggregate and hydration products, thereby achieving higher compressive strength values even without pre-treatments.
2.3. Influence of SAP Additive on the Rheological and Mechanical Behaviour of Mortars
The study aimed to examine the impact of a superabsorbent reagent on marine dredged sediment cement mortars. The investigation was focused on sample M_S1, which exhibited a higher organic residue and interstitial water content, to show how variations in water retention, resulting from differing mix compositions and reagent dosages, might influence the rheological and mechanical performance of cement mixes under identical operational conditions. From a technical and procedural point of view, the additive was added simultaneously during the mixing phase with cement Portland 42.5 R and wet marine sediments.
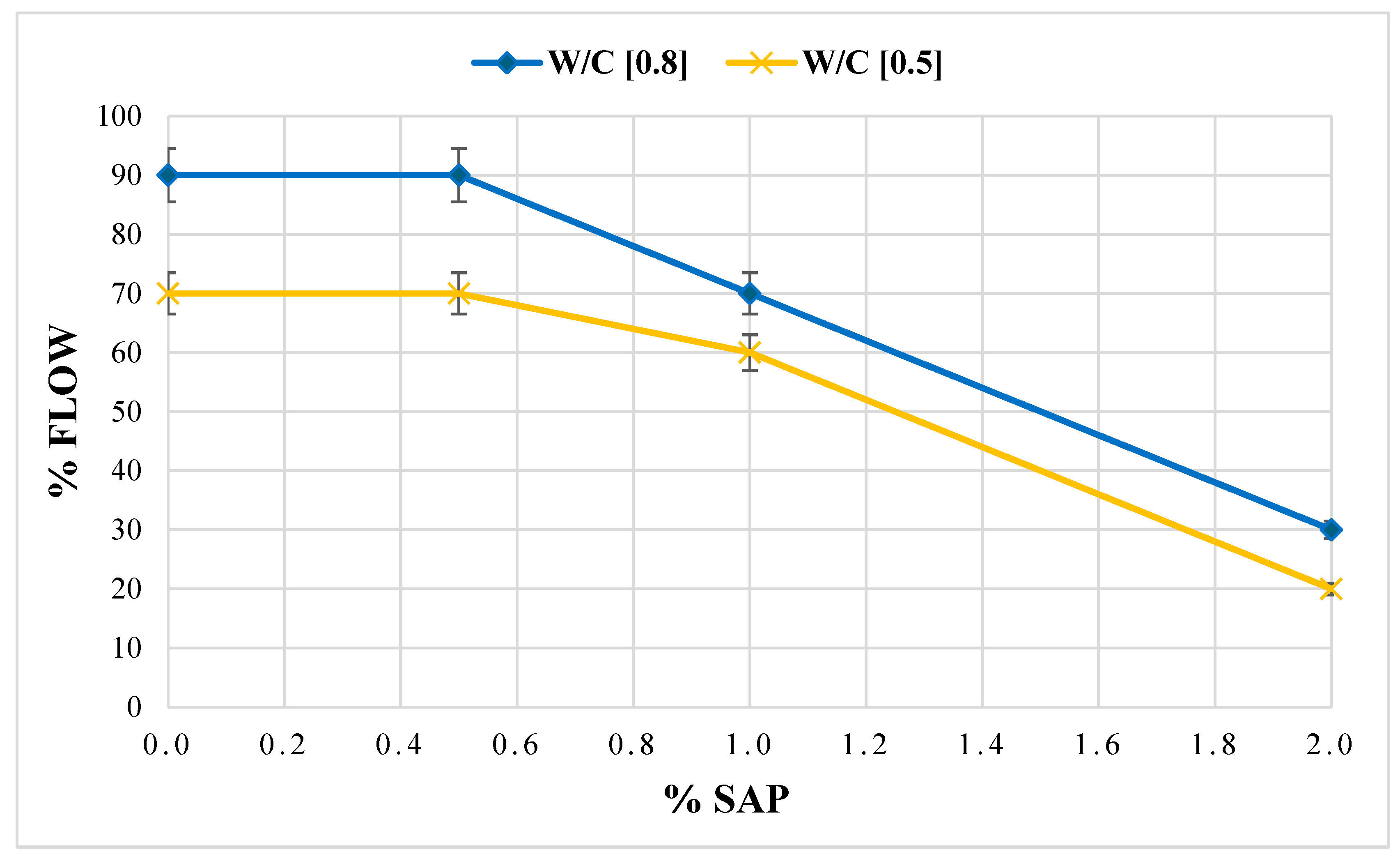
The data demonstrates a distinct response to the workability test of the mortars when the dosage of SAP is varied. The percentage of workability of the mixture consisting only of the S1 sediment sample (White) mixed with a w/c ratio of 0.8 is equivalent to the indicative value of the normalised mortar M1. Moreover, mortar samples showed a progressive reduction in spreading capacity with increasing additive concentration. Considering the aforementioned data, it can be stated that the mortar’s workability reduction is correlated with the instantaneous capacity of water absorption by the superabsorbent additive once the minimum dosage threshold is exceeded (0.5%) (Figure 8).
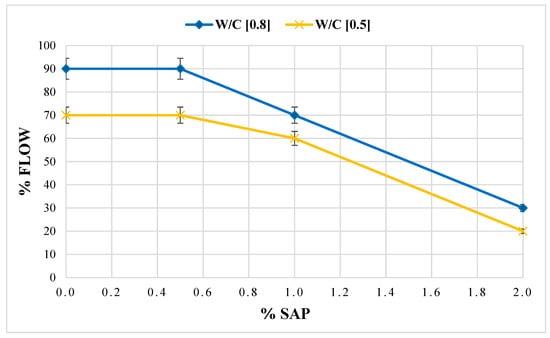
Figure 8.
Flow test performance of cement mortars with SAP at varying mix quantities and w/c ratio 0.8–0.5.
A comparative analysis of the data obtained from the flexural and compressive fracture tests conducted on mortars treated with SAP revealed, in the case of a w/c ratio of 0.8, a progressive increase in strength with the quantity of additive incorporated into the mixtures.
Samples with a water/cement ratio of 0.5 showed values in the same range as the reference, but with a declining trend at increasing SAP concentration. This phenomenon can be attributed to the capture of water available in the mortar, which consequently limits the hydration reactions and, in turn, the compressive strengths after 28 days of curing. This effect was observed for a water-to-cement ratio of 0.5, in contrast to that of 0.8, due to lower water content (Figure 9 and Table 4).
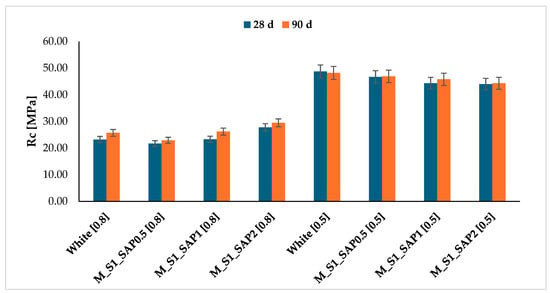
Figure 9.
Time development up to 90 days of cement mortars as the characteristic w/c mixing ratio and SAP dosage vary.

Table 4.
Overview of the physical and mechanical performance of cement mortars produced by mixing the S1 sediment sample with SAP additive.
The development of strength in mortar samples containing SAP was studied over 90 days, with samples characterised by both w/c ratios of 0.8 and 0.5. This is illustrated in Figure 9. The analysis confirms the increasing trend in the strength development for the samples mixed with a w/c ratio of 0.8. In contrast, the decreasing development of resistance is confirmed for the samples with a reduced water content of 0.5 after 90 days. Specifically, after 90 days of curing, the samples M_S1_SAP2 with a w/c ratio of 0.8 exhibited a 15% increase in strength compared to the white sample [0.8].
The results indicate that the additive SAP is a promising option for reducing excess water in dredged sediments. However, the dosage must be carefully calibrated to avoid compromising the post-curing strength.
2.4. Effect of Sodium Silicate Application on the Mechanical Performance of Concrete Mixes
The effects of sodium silicate in cementitious mortars were studied uniquely on sample S2, with a lower organic fraction. As observed, this tends to reduce the development of hydration reactions and, consequently, the resulting mechanical performance of conglomerates (Table 5).

Table 5.
Summary overview of the physical–mechanical performance of cement mortars produced by mixing the S2 sediment sample and sodium silicate additive.
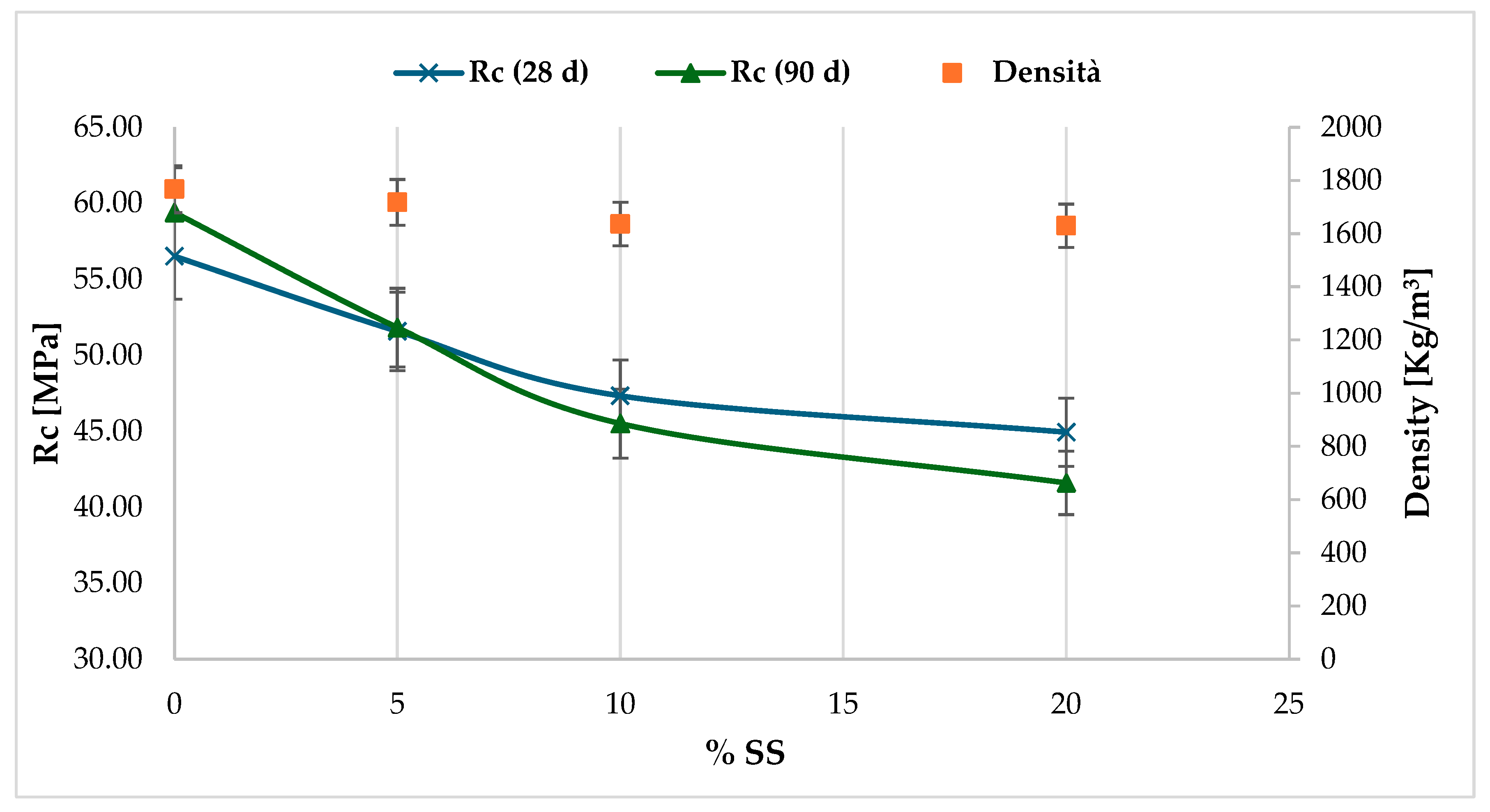
The S2 sediment sample White was considered a reference. It exhibited a lower workability compared to the normalised M1 standard. Starting from a weight percentage of 10% SS, a progressive reduction in the spreading capacity was verified. This confirms the capacity of sodium silicate to accelerate setting (Figure 10).
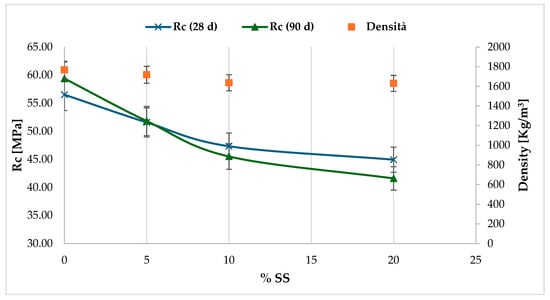
Figure 10.
Evaluation of the correlation between the compressive mechanical performance of conglomerates with the increase in the percentage of sodium silicate additive mixed and the curing period.
The fracture test results demonstrate a gradual decline in strength. The compressive strength values were below the threshold values, with a notable decrease observed in specimens with sodium silicate dosages ≥ 10%.
At first glance, this unusual strength behaviour can be correlated to the additive’s chemical composition and its interactions with the sediments in the mixes.
Sodium silicate is regarded as an accelerating admixture in the cement field. It can increase the degree of hydration of the cement during the initial few hours, thereby reducing the setting time and accelerating the hardening process over the first 24 h. Conversely, alkaline accelerators such as sodium silicate have been found to impair mechanical performance when subjected to long curing times [60].
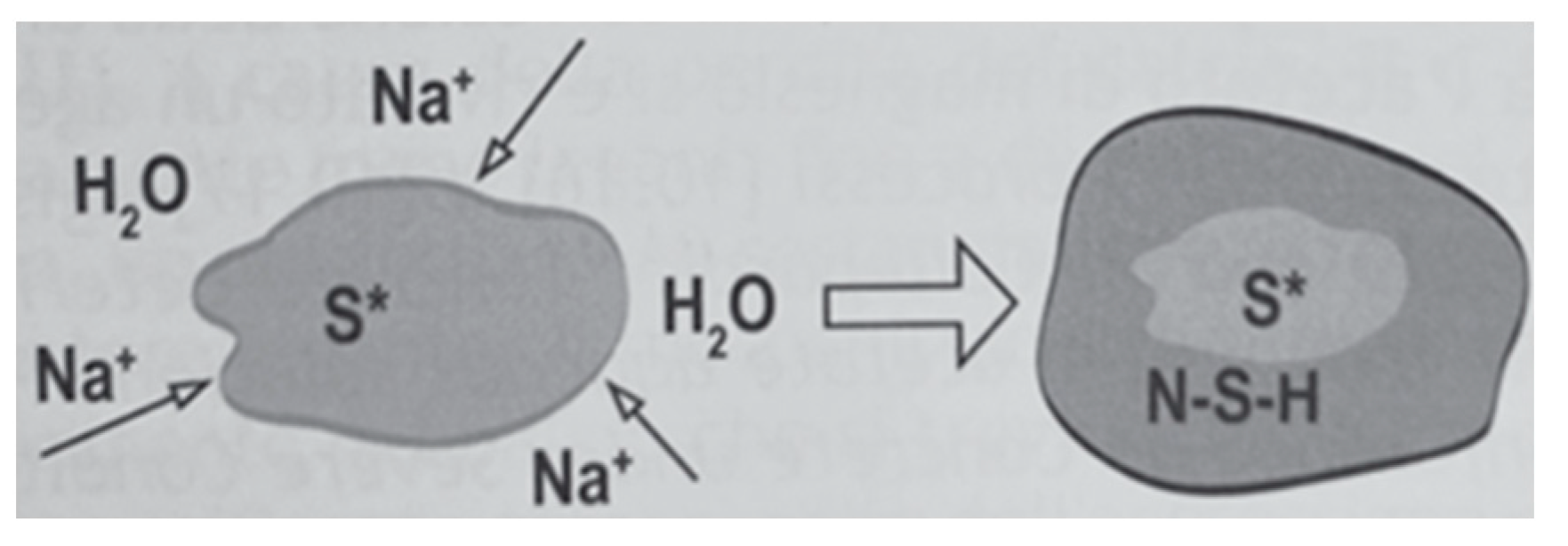
The reduction in strength observed in mortars containing sodium silicate can be attributed to the chemical reaction between alkali introduced by the additive and the silica present in the sediment (reaction between silica and alkali in the moisture (4)) [60]. The alkali-silica reaction (ASR) is a chemical phenomenon that causes the aggregate to expand due to the formation of a viscous gel, which increases its volume by water absorption , as shown in Figure 11. This increase in volume generates expansive pressure within the silica aggregate or at the interface between cement paste and aggregate, resulting in a loss of stiffness and strength.
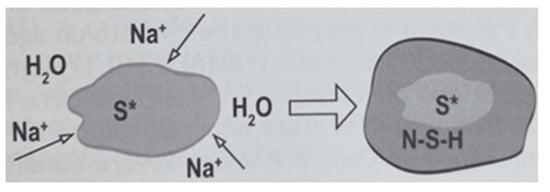
Figure 11.
Illustration of the ASR reaction [61].
Microstructural analyses of sample M_S2_SS10, particularly relevant due to the loss of mechanical properties, revealed the presence of micrometre-scale fractures within the cementitious matrix. This discontinuity at the matrix level, developed in the mortars where the sodium silicate-based additive was added, confirms the critical issues identified in the mechanical compressive strength tests. In contrast, for the mortar sample with 5% SS (M_S2_SS5), the negative influences in terms of mechanical strength are lower, as shown by the Rc data in Table 5, and this is confirmed at the microscopic level (Figure 12C) where no fractures are present within the matrix.

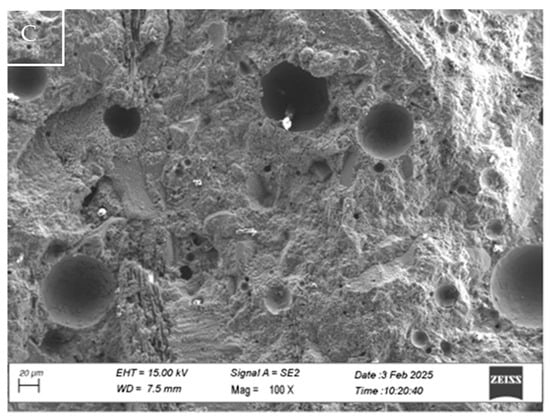
Figure 12.
SEM analysis of M_S2_SS10 (A,B) and M_S2_SS5 (C) conglomerate samples.
2.5. Specimen Stability and Macrostructure
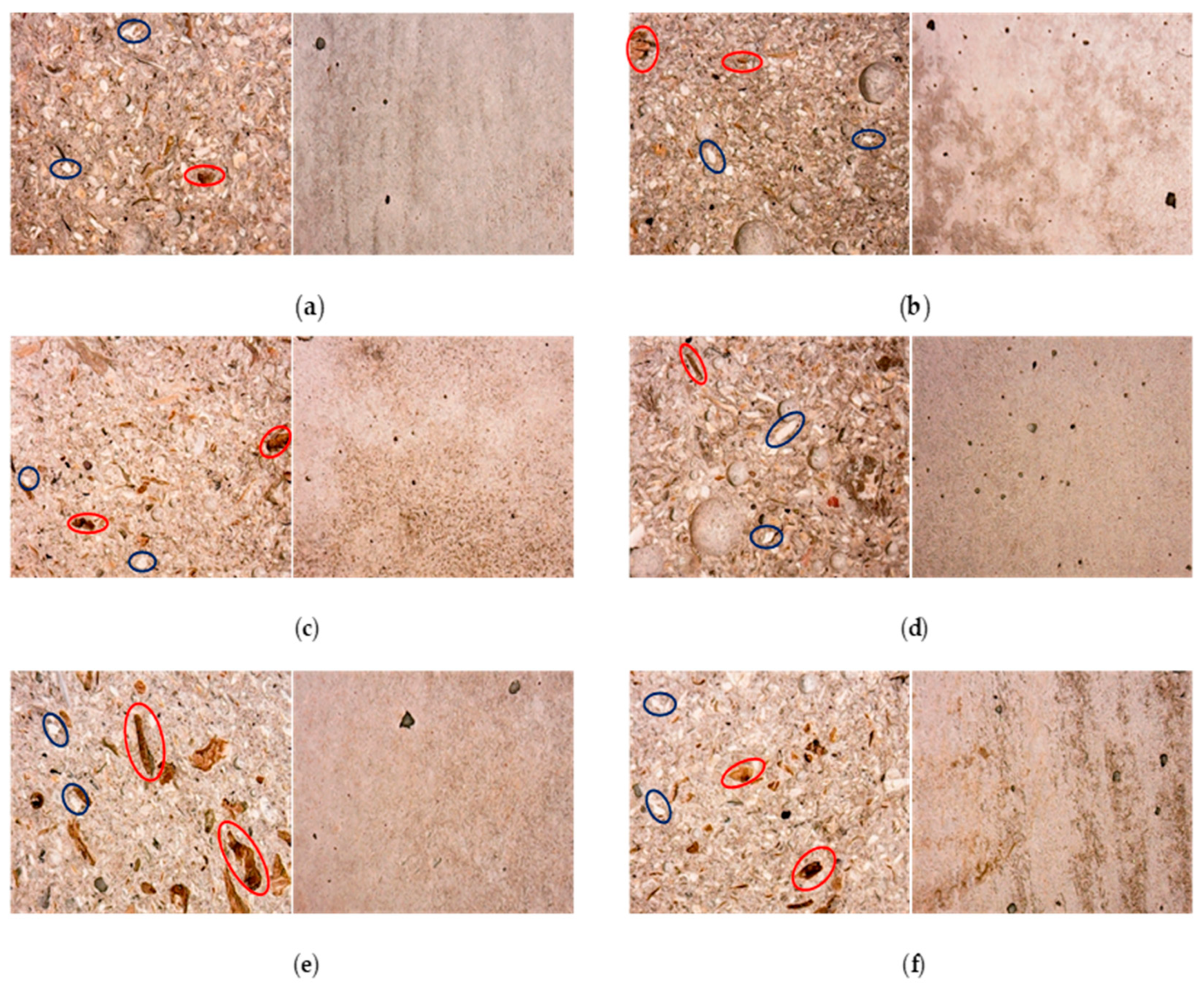
The potential development of surface efflorescence resulting from the presence of seawater in the mortars and shrinkage was analysed under an optical microscope. Generally speaking, the samples are homogeneous without segregation effects. Figure 13 reports the macrostructure of the composites, showing the best figures in terms of compressive strengths. As illustrated, natural components such as algae and sediment particles, visible in the image at approximately 1 mm, are indicated in red and blue, respectively.
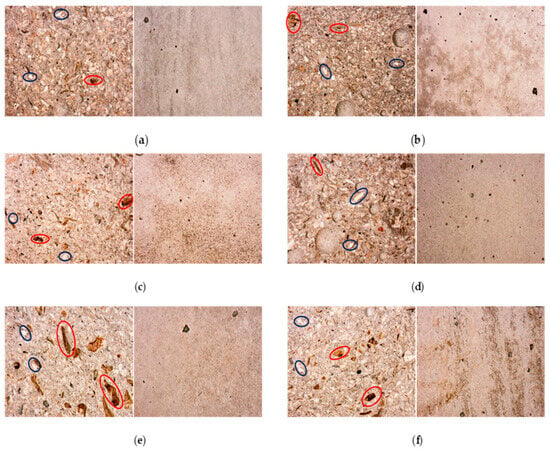
Figure 13.
Detail of structure of mechanically tested specimens, specifically, the cutting face (left) and outer surface (right) of the specimens are visible, respectively M_S2 (a), M_S1H2O2 (b), M_S1_SAP2.0 [0.8] (c), M_S2_SS10 (d), White [0.5] (e), M_S1_SAP0.5 [0.5] (f) at 28 days of ageing.
The evaluation of the dimensions of the mortar specimens indicated no variations during the observations at 1, 7, 28, and 90 days of curing, thereby denoting the absence of shrinkage in the concrete specimens with constant dimensions of 4 × 4 × 16 mm.
During the entire monitoring period, no efflorescence was observed on the surface of the samples.
3. Experimental Section
3.1. Materials
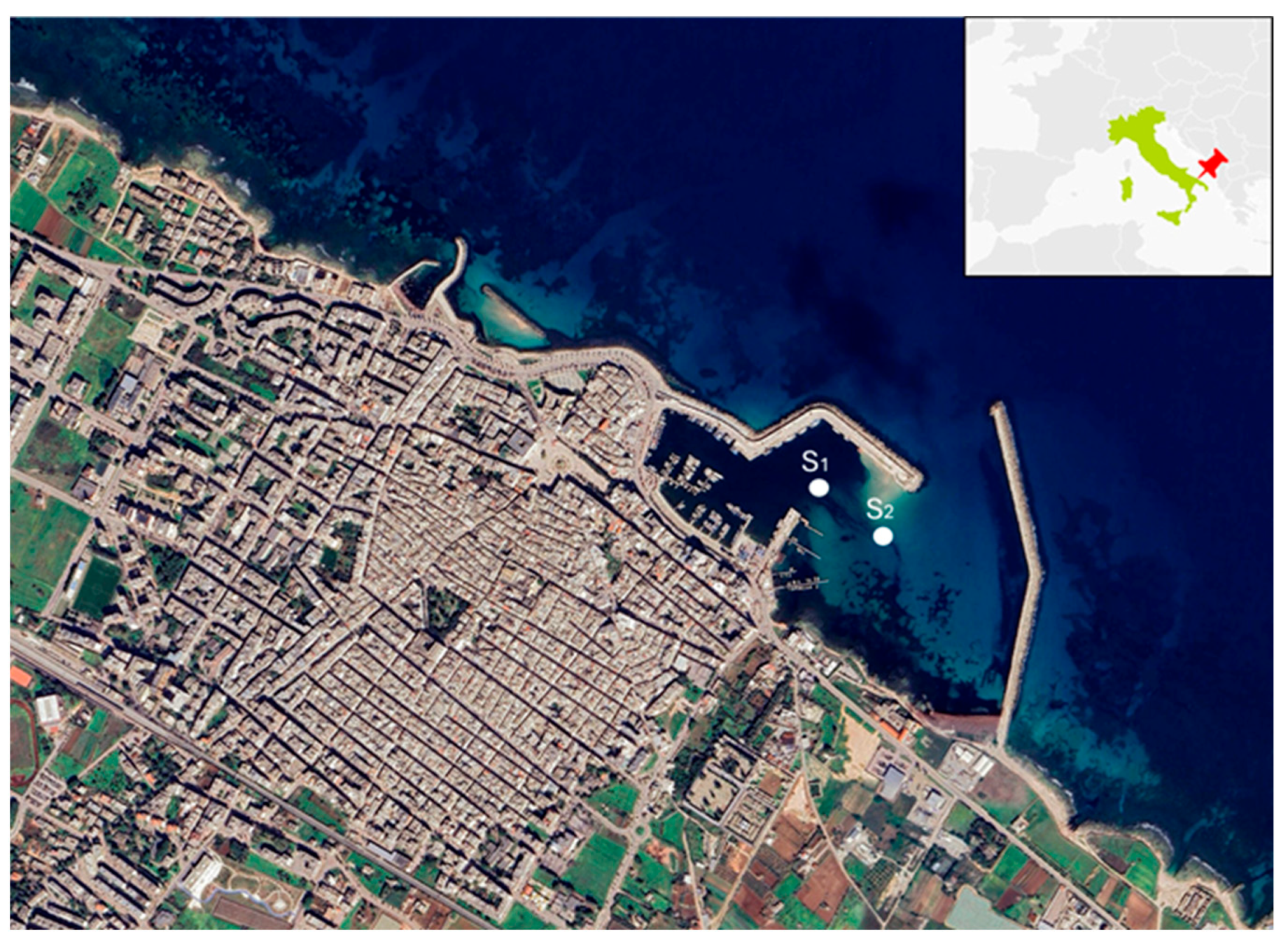
Marine sediments were collected from the Mola di Bari harbour (Figure 14). The harbour is subjected to silting phenomena that affect the basin’s navigability; therefore, periodic dredging of the sediment piles is necessary [62].
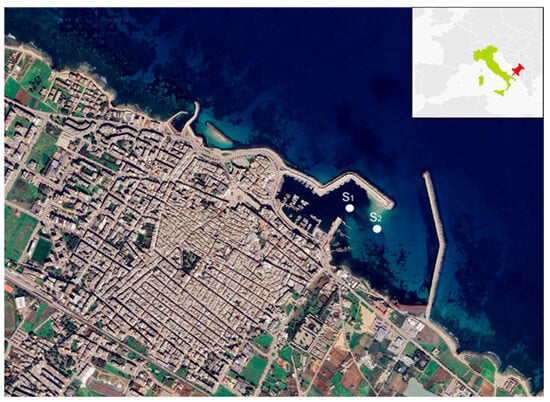
Figure 14.
Port of Mola di Bari (S1 and S2 are the location of sampling points).
Two samples, S1 and S2 (Figure 15), were collected from the surface layer of the seabed (0–100 cm) by expert divers, with a quantity of 50 kg per sample collected according to the methodology validated by Todaro et al. 2023 [63]. Each sample was subjected to chemical–physical characterisation and particle size analysis. The determination of particle size, moisture content, and organic matter of the sediments was conducted following the standard protocols of the ISPRA (Institute for Environmental Protection and Research) and the U.S. EPA (ICRAM-ARAT 2007 [64]).

Figure 15.
(a) S1 and (b) S2 sediment samples.
Portland cement II/A LL 42.5R from Buzzi Unicem SpA (Italy) was used as a binder in the S/S process.
Several treatments were employed to promote the circularity principle and recycle marine sediments in cement mortars. An initial sediment washing treatment with water was conducted. Repeated washes (4–5) of the sediment samples were carried out using the washing agent in a weight-to-weight ratio of 1 until a neutral pH was achieved in the resulting heterogeneous solution. Subsequently, the effect of an oxidising agent, such as hydrogen peroxide (H2O2, Sigma Aldrich, St. Louis, MO, USA), was examined in solution with a 30% w/w concentration. In the present study, the oxidising power of hydrogen peroxide has been exploited to decompose organic substances present in the sediment, including algae fibres (Posidonia) deposited in the layers of the material under study. The procedure performed is indicated in guideline SNPA/18 2018 [46].
In this study, to reduce the water content in DS, hence reducing the demand for the binder (Portland cement) required to develop adequate mechanical properties in hydration products, a granular cross-linked polyacrylate superabsorbent polymer (SAP) with specific properties shown in Table 1 was used. The additive is in a granular-powdery state under anhydrous conditions; however, upon contact with water, it converts to a gel state. The tea bag method determined the additive’s water retention ability [65], which confirmed the high adsorption capacity (g/g) listed in Table 6. The bulk density was determined following ISO 697 [66], while the information on particle size distribution was obtained using the UNI EN 933-1:2012 [67] procedure.

Table 6.
Properties of the superabsorbent polymer.
Sodium Silicate (SS) solution (Rm > 3.2, C < 40%) produced by INGESSIL S.r.l., Montorio, Italy, is a colourless and odourless liquid with a pH of 10.5–11.5 (20 °C), 10–310 mPa-s (20 °C) viscosity and a relative density of 1.18–1.32 g/mL. In the present research, this admixture was applied to evaluate alternative uses of cement mortars based on dredged marine sediment, focusing on verifying the effect of setting acceleration on post-curing mechanical strengths.
3.2. Methods
A variable quantity of marine sediment (bare and pre-treated) was employed and combined with cement to obtain mortars with a w/c (water–cement) ratio of 0.5 and an s/c (sediment–cement) ratio of 1. To ensure adequate mixing between the components per EN 196-1:2016 [68], an RMU-compliant automatic mixer was used. The mortars were subjected to a workability test [69] and then compacted in steel moulds, divided into three chambers of prismatic shape and measuring 4 × 4 × 16 cm [68]. Subsequently, the samples were demoulded and subjected to a curing phase of 28 and 90 days at a controlled temperature and humidity before flexural and compressive tests.
The initial step involved applying a solidification procedure to untreated sediment samples (S1 and S2). This procedure was then verified on the same samples previously pre-treated with H2O. The solidification procedure was successfully applied to the samples purified from the organic fraction through oxidation treatment with hydrogen peroxide.
The effect of additional substances on the sediment was assessed with the superabsorbent solid SAP, which was combined with wet sediment samples containing varying quantities of interstitial water to ascertain the effectiveness of the additive in different conditions. Subsequently, the sodium silicate setting accelerator was employed to examine its influence on cement mortars produced from marine sediments.
In addition, to ascertain the quality of the mixtures produced, samples of normalised mortar (M1) were made according to UNI EN 197-1 [70] (composed of 450.0 g of cement, 250.0 g of water, and 1350.0 g of normalised sand). These samples were used as a reference for evaluating the non-conventional mortars studied. Figure 16 shows specimens in the curing phase.

Figure 16.
Details of specimens in the curing phase.
Table 7 below provides an overview of the mixtures used to produce cement mortars derived from marine sediments S1 and S2. This analysis provides a detailed breakdown of the percentages of the constituents: sediments (SED), water (W) and cement (CEM), together with the specific w/c and s/c ratios. It is divided into successive experimental phases. The initial phase concerns mortars made with untreated sediments. The next phase concerns mortars containing sediments pre-treated with H2O and H2O2. The third phase concerns mortar mixtures obtained by mixing the S1 sediment sample with the SAP additive, the percentage of which was calculated based on the H2O content in the sediment sample. The final phase concerns mortar mixtures obtained from sediment S2 mixed with cement and sodium silicate (SS), the percentage of which was calculated based on the amount of dry sediment.

Table 7.
Mix design cement mortars produced from wet sediment samples S1 and S2.
The suitability of the fresh cement mortars was evaluated through a spreading test (flow test). This was conducted with the aid of the RMU’s manual shake table, following the technical procedure defined in UNI 7044:1972 [69]. Once the test is completed, the percentage of mortar diffusion can be calculated by measuring the diameter of the two orthogonal axes (D1, D2) the average of which is defined as Dm and is compared to the initial representative value (Di) of the mortar sample (Equation (5) [71]):
Furthermore, the consistency of cement mortars can be evaluated by comparing them with the control mortar using the ∆ flow data (Equation (6) [72]).
The mechanical strength of the mortars was determined through uniaxial compression and flexural tests, carried out on prismatic specimens following a conventional curing period of 28 and 90 days, respectively. The mechanical tests were conducted using a MaTest hydraulic press, equipped with an electronic pressure transducer integrated within the hydraulic circuit, capable of measuring the load with an accuracy of 0.01 kN. Under the standards specified in UNI EN 196-1:2016 [68], the press was equipped with a bending compressor to facilitate testing. Repetitions were carried out for each mix to obtain a representative average value of the flexural strengths. The characteristic flexural strength value Rf was thus obtained (Equation (7)):
F indicates the measured load at failure in bending (KN), L indicates the distance between the supports (100 mm), B indicates the width of the test specimen (40 mm), and H indicates the height of the test specimen (40 mm).
After flexural fracture, each mortar sample was subjected to compressive stress tests per UNI EN 196-1:2016 [68]. Thereafter, the characteristic compressive strength value, designated as Rc, was calculated using the methodology outlined in Equation (8):
The maximum breaking load (Fn) is expressed in kilonewtons (KN), while the load cross-sectional area (A) is expressed in square millimetres (mm2).
To study the effect of washing treatments on a microscopic level, the influence of organic matter on the development of strength in cement mortars, and the effect of adding sodium silicate, scanning electron microscopy with energy dispersive X-ray (SEM-EDX) measurements were used. The sediment samples (S1 and S2), as well as the conglomerate samples (M_S1, M_S1H2O2, M_S2, M_S2_SS5 and M_S2_SS10), were analysed. Initially, these samples were applied to a graphite adhesive bifacial disc, followed by metallization with gold.
These samples were analysed using the FESEM-EDX Carl Zeiss SIGMA 300VP microscope(Carl Zeiss Microscopy GmbH, Jena, Germany), employing an acceleration voltage of 15.00 kV and a working distance variable between 7.3 and 7.8 mm [73].
The microstructural analytical program included observing the elements C, Na, Si, Cl, and Ca in the samples under study through micrographs and element maps using Oxford Instruments AZtec software 6.1 version.
4. Conclusions
This study aimed to evaluate the influence of organic matter and seawater on the mechanical strength and microstructure of cementitious mortars and conglomerates produced by stabilization/solidification treatment of dredged marine sediments mixed with Portland cement 42.5 R. The effect of sediment washing with water and hydrogen peroxide was evaluated. Moreover, the effect of pore water variation by adding a superabsorbent reagent (SAP) and sodium silicate on the potential recovery of dredged marine sediment was analysed. The results demonstrate the following:
- Cementitious mortars produced from wet marine sediments and mixed with w/c ratios of 0.5 and s/c of 1 were characterised by physical–mechanical performances comparable to those of a standard mortar, provided that no organic element within the marine sediments inhibited the cement’s hydration reactions.
- Washing of sediment with water on the S1 and S2 samples before the stabilisation/solidification tests reduced the saline concentration, increasing the workability of the fresh mortars. However, this treatment also reduced the flexural and compressive strengths of the mortars, with a decrease in density.
- The mortars produced from sediments treated with H2O2 exhibited higher strength, with a 24% increase observed in the sample with higher organic content (S1) compared to the as-is condition. This improvement can be attributed to the reduction of organic matter, which inhibits cement reactions.
- Mortars produced from dredged marine sediments that are particularly rich in interstitial water must be managed in order to reduce the cement requirement in the mixes in accordance with sustainability principles. One potential solution is dosing an appropriate amount of SAP into cement mortars, which is technically feasible and represents a viable alternative. The development of this type of treatment would facilitate a more sustainable cement supply chain and appropriate management of the waste produced by dredging activities.
- The admixture of sodium silicate can accelerate setting for dosages exceeding 10% by weight of dry sediment, concurrently resulting in a reduction in strength as the curing period (28–90 days) extends. This phenomenon can be attributed to the progression of the alkali–silica reaction (ASR). Therefore, to avoid deterioration of the mechanical properties of cement mixes, additives should be limited to a maximum of 5% by mass of the additive.
The experimental tests developed in this study highlight the significant technical benefits of reusing dredged marine sediments from the port of Mola di Bari as substitute aggregates in the construction industry, complying with the relevant current national standards, thus promoting a concrete and advantageous solution that focuses on sustainability and circularity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/recycling10050169/s1.
Author Contributions
C.M.C.: Data curation, Writing—Original draft preparation. A.P.: Writing—Reviewing. F.T.: Visualization, Investigation. M.N.: Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- United Nations Enviromental Programme. 2024. Available online: https://unepgrid.ch/en/marinesandwatch (accessed on 20 November 2024).
- Todaro, F.; De Gisi, S.; Notarnicola, M. Contaminated marine sediments: Waste or resource? an overview of treatment technologies. Proc. Environ. Sci. Eng. Man. 2016, 3, 157–164. [Google Scholar]
- Apitz, S.E. Waste or resourse? Classifying and scoring dredged material management strategies in terms of waste hierarchy. J. Soils Sediments 2010, 10, 1657–1668. [Google Scholar] [CrossRef]
- Studds, P.; Miller, Z.M. Sustainable material reuse solutions for dredged sediments. Int. J. Sustain. Eng. 2010, 3, 33–39. [Google Scholar] [CrossRef]
- Smith, B.T.; Howard, I.L.; Vahedifard, F. Lightly cemented dredged sediment for sustainable reuse. Environ. Geotech. 2016, 5, 324–335. [Google Scholar] [CrossRef]
- Bortali, B.; Rabouli, M.; Yessari, M.; Hajjaji, A. Characterizinf Harbour Dredged Sediment for Sustainable Reuse as Costruction Material. Sustainability 2023, 15, 1834. [Google Scholar] [CrossRef]
- Lakhiar, M.; Zhao, X.; Guo, Y.; Chen, S. Investigations on the mechanical and life cycle properties of novel marine-based sustainable BFRP composites. Constr. Build. Mater. 2024, 453, 139105. [Google Scholar] [CrossRef]
- Akcil, A.; Erust, C.; Ozdemiroglu, S.; Fonti, V.; Beolchini, F. A review of approaches and techniques used in aquatic contaminated sediments: Metal removal and stabilization by chemical and biotechnological processes. J. Clean. Prod. 2015, 86, 24–36. [Google Scholar] [CrossRef]
- Samuel Suman Raj, D.; Aparna, C.; Rekha, P.; Hima Bindhu, V.; Anianeyulu, Y. Stabilisation and solidification technologies for the remediation of contamnated soils and sediments: An overview. Land Contam. Reclam. 2005, 13, 23–48. [Google Scholar] [CrossRef]
- Woo, H.E.; Tran, T.V.; Lee, I.C.; Kim, J.O.; Hibino, T.; Nohara, H.; Kim, K. Remediation of contaminated sediment originating from wastewater at T harbor areas using slaked lime-fly ash-cement mixture. Estuar. Coast Shelf Sci. 2019, 227, 106340. [Google Scholar] [CrossRef]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction-Part 2. Appl. Biomater. Funct. Mater. 2018, 16, 207–221. [Google Scholar] [CrossRef]
- Petrella, A.; Spasiano, D.; Rizzi, V.; Cosma, P.; Race, M.; De Vietro, N. Lead ion sorption by perlite and reuse of the ex-hausted material in the construction field. Appl. Sci. 2018, 8, 1882. [Google Scholar] [CrossRef]
- Siham, K.; Fabrice, B.; Edine, A.N.; Patrick, D. Marine dredged sediments as new materials resource for road construction. Waste Manag. 2008, 28, 919–928. [Google Scholar] [CrossRef]
- Kasmi, A.; Abriak, N.E.; Benzerzour, M.; Azrar, H. Environmental impact and mechanical behavior study of experimental road made with river sediments: Recycling of river sediments in road construction. J. Mater. Cycles Waste Manag. 2017, 19, 1405–1414. [Google Scholar] [CrossRef]
- Zhao, Z.; Berzerzour, M.; Abriack, N.-E.; Damidot, D.; Courard, L.; Wang, D. Use of uncontaminated marine sediments in mortar and concrete by partial substitution of cement. Cem. Concr. Compos. 2018, 93, 155–162. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Li, J.-S.; Baek, K.; Hou, D.; Ding, S.; Poon, C.-S. Recycling dredged sediment into fill materials, partition blocks, and paving blocks: Technical and economic assessment. J. Clean. Prod. 2018, 199, 69–76. [Google Scholar] [CrossRef]
- Cappuyns, V.; Deweirt, V.; Rousseau, S. Dredged sediment as a resource for brick production: Possibilities and barriers from a consumers’ perspective. Waste Manag. 2015, 38, 372–380. [Google Scholar] [CrossRef]
- H Slimanou, H.; Eliche-Quesada, D.; Kherbache, S.; Bouzidi, N.; Tahakourt, A.K. Harbor Dredged Sediment as raw material in fired clay brick production: Characterization and properties. J. Build. Eng. 2020, 28, 105208. [Google Scholar] [CrossRef]
- Dalton, J.L.; Gardner, K.H.; Seager, T.P.; Weimer, M.L.; Spear, J.C.M.; Magee, B.J. Properties of Portland cement made from contaminated sediments. Resour. Conserv. Recycl. 2004, 41, 227–241. [Google Scholar] [CrossRef]
- Aouad, G.; Laboudigue, A.; Gineys, N.; Abriak, N. Dredged sediments used as novel supply of raw material to produce portland cement clinker. Cem. Concr. Compos. 2012, 34, 788–793. [Google Scholar] [CrossRef]
- Agostini, F.; Skoczylas, Z.; Lafhai, Z. About a possible valorisation in cementitious materials of polluted sediments after treatment. Cem. Concr. Compos. 2007, 29, 270–278. [Google Scholar] [CrossRef]
- Agostini, F.; Davy, C.A.; Skoczylas, F.; Dubois, T. Effect of microstructure and curing conditions upon the performance of a mortar added with Treated Sediment Aggregates (TSA). Cem. Concr. Res. 2010, 40, 1609–1619. [Google Scholar] [CrossRef]
- Loudini, A.; Ibnoussina, M.; Witam, O.; Limam, A.; Turchanina, O. Valorisation of dredged marine sediments for use as road material. Case Stud. Costr. Mater. 2020, 13, e00455. [Google Scholar] [CrossRef]
- Limeira, J.; Agulló, L.; Etxeberri, M. Dredged marine sand as construction material. In Proceedings of the XIèmes Journées 990 Nationales Génie Côtier—Génie Civil, Les Sables d’Olonne, France, 22–25 June 2010. [Google Scholar] [CrossRef]
- Couvidant, J.; Benzaazoua, M.; Chatain, V.; Bouamrane, A.; Bouzahzah, H. Feasibility of the reuse of total and processed contaminated marine sediments as fine aggregates in cemented mortars. Constr. Build. Mater. 2016, 112, 892–902. [Google Scholar] [CrossRef]
- Hayek, M.; Soleimani, T.; Salgues, M.; Suoche, J.-C.; Garcia-Diaz, E. Valorization of uncontaminated dredged marine sediment through sand substitution in marine grade concrete. Eur. J. Environ. Cicil Eng. 2023, 27, 4008–4025. [Google Scholar] [CrossRef]
- Hayek, M.; Soleimani, T.; Salgues, M.; Souche, J.-C. Utilizing Fine Marine Sediment as a Partial Substitute for Sand in Self-Compacting Concrete Specially Designed for Application in Marine Environments. Sustainability 2024, 16, 2538. [Google Scholar] [CrossRef]
- Messina, F.; Ferone, C.; Molino, A.; Roviello, G.; Colangelo, F.; Molino, B.; Cioffi, R. Synergistic recycling of calcined clayey sediments and water potabilization sludge as geopolymer precursors: Upscaling from binders to precast paving cement-free bricks. Construct. Build. Mater. 2017, 133, 14–26. [Google Scholar] [CrossRef]
- Beddaa, H.; Ouazi, I.; Ben Fraj, A.; Lavergne, F.; Torrenti, J.M. Reuse potential of dredged river sediments in concrete: Effect of sediment variability. J. Clean. Prod. 2020, 265, 16. [Google Scholar] [CrossRef]
- Hussan, A.; Levacher, D.; Mezazigh, S.; Jardin, L. Co-valorization of sediments incorporating high and low organic matter with alkali-activated GGBS and hydraulic binder for use in road construction. J. Build. Eng. 2023, 66, 105848. [Google Scholar] [CrossRef]
- Fořt, F.; Afolayan, A.; Kočí, V.; Scheinherrovà, L.; Jan, J.; Borovec, J.; Cerný, R. Potential of water sediments in construction materials: Current approaches and critical consideration of future challenges. Heliyon 2025, 11, e41121. [Google Scholar] [CrossRef] [PubMed]
- Rakshith, S.; Singh, D. Utilization of dredged sediments: Contemporary issues. J. Waterw. Port Coast. Ocean Eng. 2017, 143, 04016025. [Google Scholar] [CrossRef]
- Kujala, K.; Mäkikyrö, M.; Lehto, O. Effect of humus on the binding reaction in stabilized soils. In Proceedings of the International Conference on Ground Improvement Geosystems, 1, 2, IS-Tokyo’96, Grouting and Mixing, Tokyo, Japan, 14–17 May 1996; pp. 415–420. [Google Scholar]
- Hamouche, F.; Zentar, F. Effects of organic matter on physical properties of dredged marine sediments. Waste Biomass Valorization 2020, 11, 389–401. [Google Scholar] [CrossRef]
- Ekwue, E.I. Organic-matter effects on soil strength properties. Soil Tillage Res. 1990, 16, 289–297. [Google Scholar] [CrossRef]
- Martellotta, A.; Levacher, D.; Gentile, F.; Piccinni, A. Estimation of silting evolution in the camastra reservoir and proposals for sediment recovery. J. Mar. Sci. Eng. 2024, 12, 250. [Google Scholar] [CrossRef]
- Hyrycz, M.; Ochowiak, M.; Krupinska, A.; Wlodarczak, S.; Matuszak, M. A review of flocculants as an efficient method for increasing the efficiency of municipal sludge dewatering: Mechanisms, performances, influencing factors and perspectives. Sci. Total Environ. 2022, 820, 153328. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.; Jain, B.; Hu, X.; Sancheti, G. A Review of Beneficial Use and Management of Dredged Material. Waste 2023, 1, 815–840. [Google Scholar] [CrossRef]
- Yang, H.; Feng, Q.; Zhu, J.; Liu, G.; Dai, Y.; Zhou, Q.; Xia, S.; Wu, Z.; Zhang, Y. Towards sustainable futures: A review of sediment remediation and resource valorization techniques. J. Clean. Prod. 2024, 435, 140529. [Google Scholar] [CrossRef]
- Beddaa, H.; Ben Fraj, A.; Ducl’eroir, S. Experimental study on river sediment incorporation in concrete as a full aggregate replacement: Technical feasibility and economic viability. Construct. Build. Mater. 2021, 313, 125425. [Google Scholar] [CrossRef]
- Bozic, M.; Zibret, L.; Kvock, D.; Pranjic, A.; Gregorc, B.; Ducman, V. Drava river sediment in clay brick production: Characterization, properties, and environmental performance. J. Build. Eng. 2023, 71, 106470. [Google Scholar] [CrossRef]
- Bian, X.; Wang, Z.F.; Ding, G.Q.; Cao, Y.P. Compressibility of cemented dredged clay at high water content with super-absorbent polymer. Eng. Geol. 2016, 208, 198–205. [Google Scholar] [CrossRef]
- Bian, X.; Cao, Y.P.; Wang, Z.F.; Ding, G.Q.; Lei, G.H. Effect of super-absorbent polymer on the undrained shear behavior of cemented dredged clay with high water content. ASCE J. Mater. Civ. Eng. 2017, 29, 04017023. [Google Scholar] [CrossRef]
- Bian, X.; Ding, G.Q.; Wang, Z.F.; Cao, Y.P.; Ding, J.W. Compression and strength behavior of cement-lime-polymer solidified dredged material at high water content. Mar. Georesources Geotechnol. 2017, 35, 840–846. [Google Scholar] [CrossRef]
- Bian, X.; Zeng, L.; Deng, Y.; Li, X. The Role of Superabsorbent Polymer on Strength and Microstructure Development in Cemented Dredged Clay with HighWater Content. Polymer 2018, 10, 1069. [Google Scholar] [CrossRef]
- Romano, E.; Ausili, A.; Bergamin, L.; Celia Magno, M.; Pierfranceschi, G.; Venti, F. Analisi granulometriche dei sedimenti marini. In Linee Guida; Italian National System for Environmental Protection: Roma, Italy, 2022; Volume 18, p. 111. ISBN 978-88-448-0925-6. [Google Scholar]
- Udden, J.A. Geology of Muscatine County. Iowa Geol. Surv. Annu. Rep. 1898, 9, 247–388. [Google Scholar] [CrossRef][Green Version]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Lucchini, F.; Dinelli, E.; Mordenti, A. Geochemical records of paleoenvironmental changes during late Quaternary in the Adriatic Sea sediments. Geo-Acta 2003, 2, 43–62. [Google Scholar]
- Du, C.; Zhang, J.; Yang, G.; Yang, Q. The influence of organic matter on the strength development of cement-stabilized marine soft clay. Georesources Geotechnol. 2020, 39, 983–993. [Google Scholar] [CrossRef]
- Qu, F.; Li, W.; Dong, W.; Tam, V.W.; Yu, T. Durability performance deterioration of concrete under marine environment from material to structure: A critical review. J. Build. Mater. 2020, 35, 102074. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, Y.; Zhang, N.; Hu, S.; Yang, F.; Lu, L.; Wang, J. Durability and mechanism of high-salt resistance concrete exposed to sewage-contaminated seawater. Constr. Build. Mater. 2020, 257, 119534. [Google Scholar] [CrossRef]
- Etxeberria, M.; Fernandez, J.M.; Limeria, J. Secondary aggregates and seawater employment for sustainable concrete dyke blocks production: Case study. Constr. Build. Mater. 2016, 113, 586–595. [Google Scholar] [CrossRef]
- Li, Q.; Geng, H.; Shui, Z.; Huang, Y. Effect of metakaolin addiction and seawater mixing on the properties and hydration of concrete. Appl. Clay Sci. 2015, 115, 51–60. [Google Scholar] [CrossRef]
- Qu, F.; Li, W.; Wang, K.; Tam, V.W.Y.; Zhang, S. Effects of seawater and undesalted sea sand on the hydration products, mechanical properties and microstructures of cement mortar. Constr. Build. Mat. 2021, 310, 125229. [Google Scholar] [CrossRef]
- Yadav, P.; Petrella, A.; Todaro, F.; De Gisi, S.; Vitone, C.; Petti, R.; Notarnicola, M. Ex Situ Stabiliza-tion/Solidification Approaches of Marine Sediments Using Green Cement Admixtures. Materials 2024, 17, 3597. [Google Scholar] [CrossRef]
- Balonis, M.; Lothenbach, B.; Le Saout, G.; Glasser, F.P. Impact of chloride on the mineralogy of hydrated Portland cement systems. Cem. Concr. Res. 2010, 40, 1009–1022. [Google Scholar] [CrossRef]
- Basheer, L.; Kropp, J.; Cleland, D.J. Assessment of the durability of concrete from its permeation properties: A review. Constr. Build. Mater. 2001, 15, 93–103. [Google Scholar] [CrossRef]
- Català, G.; Ramos-Fernández, E.V.; Zornosa, E.; Andion, L.G.; Garcés, P. Influence of the Oxidation Process of Carbon Material on the Mechanical Properties of Cement Mortars. J. Mater. Civ. Eng. 2011, 23, 321–329. [Google Scholar] [CrossRef]
- Lukas, W.; Kusterle, W. The influence of water glass on the technological parameters of shotcrete. In Shotcrete for Underground Support V, Proceedings of the Engineeting Foundation Conference, Uppsala, Sweden, 3–7 June 1990; American Society of Civil Engineers: New York, NY, USA, 1993; pp. 197–212. [Google Scholar]
- Collepardi, M.; Collepardi, S.; Troli, R. Il Nuovo Calcestruzzo, 7th ed.; Trevistampa: London, UK, 2022; ISBN : 9788890361982. [Google Scholar]
- Ferreira, A.M.G.S.; Santos, C.S.N. Sedimentation and Erosion in Harbor Estuaries. In Sedimentation Engineering; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Todaro, F.; Colangelo, F.; De Gisi, S.; Farina, I.; Ferone, C.; Labianca, C.; Petrella, A.; Cioffi, R.; Notarnicola, M. Recycling of Contaminated Marine Sediment and Industrial By-Products through Combined Stabilization/Solidification and Granulatio Treatment. Materials 2023, 16, 2399. [Google Scholar] [CrossRef] [PubMed]
- Manual for the Handling of Marine Sediments 2007, Italian Ministry of the Environment and Land. Available online: https://www.isprambiente.gov.it/it (accessed on 6 November 2024).
- Zhao, S.; Jensen, O.M.; Hasholt, M.T. Measuring absorption of superabsorbent polymers in cementitious environments. Mater. Struct. 2020, 53, 11. [Google Scholar] [CrossRef]
- International Standard (ISO). Surface Active Agents—Washing Powders—Determination of Apparent Density—Method by Measuring the Mass of a Given Volume. ISO 697:1981. Available online: https://www.iso.org/standard/4897.html (accessed on 13 February 2025).
- Italian Organization for Standardization (UNI). Tests to Determine the Geometric Characteristics of Aggregates—Part 1: Determination of Particle Size Distribution—Sieve Analysis. EN 933-1. Available online: https://store.uni.com/uni-en-933-1-2012 (accessed on 14 February 2025).
- Italian Organization for Standardization (UNI). Methods of Testing Cement-Part 1: Determination of Strength. EN 196-1. Available online: https://store.uni.com/uni-en-196-1-2016 (accessed on 20 November 2024).
- Italian Organization for Standardization (UNI). Determination of Consistency of Cement Mortars Using a Flow Table. UNI 7044:1972. Available online: https://store.uni.com/uni-7044-1972 (accessed on 21 November 2024).
- Italian Organization for Standardization (UNI). Cement Composition, Specifications and Conformity Criteria for Common Cements. EN 197-1. Available online: https://store.uni.com/uni-en-197-1-2011 (accessed on 14 November 2024).
- A Martellotta, A.M.N.; Petrella, A.; Gentile, F.; Levacher, D. Reuse of Lake Sediments in Sustainable Mortar. Environments 2023, 10, 149. [Google Scholar] [CrossRef]
- Todaro, F.; Petrella, A.; Santomasi, G.; De Gisi, S.; Notarnicola, M. Environmental Sustainable Cement Mortars Based on Polyethylene Terephtalate from Recycling Operations. Materials 2023, 16, 2111. [Google Scholar] [CrossRef]
- Dobiszewska, M.; Pichór, W.; Tracz, T.; Petrella, A.; Notarnicola, M. Effect of Glass Powder on the Cement Hydration, Microstructure and Mechanical Properties of Mortar. Mater. Proc. 2023, 13, 40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).