Abstract
Waste generated during used cooking oil (UCO) collection poses significant environmental challenges due to its high oil content. This study investigates the efficacy of coagulation and flocculation in separating oil from cooking oil secondary waste (COSW), which typically contains 53% oil. Two additives, aluminum sulfate (Alum—Al2(SO4)3) and polyacrylamide (PAM), were employed to enhance the separation process. Experimental results demonstrate that the combined application of coagulation and flocculation using these additives achieved 82% oil removal efficiency. These findings suggest a promising approach for recovering valuable oil resources from COSW while addressing environmental concerns associated with its improper disposal.
1. Introduction
Urbanization, while enhancing our lives, brings with it a host of issues, including the emission of hazardous gases, the production of waste, and the subsequent environmental contamination. A widely accepted approach to tackle this is by converting waste into valuable products or energy [1]. With petroleum-based products becoming less sustainable and more expensive, the world is increasingly turning to alternative solutions. The need for food commodities like grains, vegetables, milk and milk products, and cooking oil has increased with population growth. As a result, the volume of kitchen waste has also grown.
Cooking oil, one of the most essential ingredients in meal preparation, is used in homes, restaurants, and the food industry, specifically for frying. It is dangerous for humans to consume this greasy excrement as it could cause serious health issues, such as cancer and digestive diseases [2]. As a result of chemical processes after burning cooking oil, particulate particles, total polar solids, and polymeric molecules form [3]. Waste containing oil and fat has the lowest decomposition rate among all kitchen wastes [4], making it difficult to handle. Cooking oil waste produces odor, a severe environmental problem; one liter of oil disposed into natural waters is estimated to contaminate up to one million liters of water [5]. It increases the organic burden on water bodies and creates a thin layer on top of the water, lowering the dissolved oxygen concentration needed for aquatic life [6].
Used cooking oil (UCO) (also known as waste cooking oil (WCO)) is vegetable or animal oil that has been used in various fields, including food production, but is no longer appropriate for cooking. UCO is a waste stream that can be challenging and needs to be carefully handled. A range of residential, commercial, and industrial sources produce it, which may cause issues if disposed of incorrectly [7]. The “fat, oil, and grease (FOG)” fraction is a common name for the recoverable organic fraction from cooking oil secondary waste (COSW). According to the literature, 30–50% of COSW comprises fats, oils, and greases, while 40–50% comprises water and the rest is biosolids [8,9]. However, these amounts can vary due to various feedstocks. This substance sometimes has high levels of free fatty acids (FFAs), sulfur, peroxide value, metals, and other pollutants. The grease or oil may be rancid if the peroxide value is high [10]. Although COSW is not hazardous, proper transportation equipment is still necessary [11]. In recent years, there has been a trend towards utilizing COSW due to stricter regulations on organic waste disposal. On the other hand, FOG made from COSW contains both saturated and unsaturated fatty acids, such as oleic acid and palmitic acid, which reduces its potential for reuse [12]. COSW can also be sold as a co-fuel for incineration or used to prepare esters (such as FOG methyl esters) [13]. However, according to an assessment of the most recent state-of-the-art study in COSW management, gaps in technological and scientific understanding limit the broad use of this waste on an industrial scale [8,14].
Moreover, the discharge of greasy wastewater into the environment is a major problem that has attracted the attention of academics. As a stream of warm, greasy wastewater cools down in a sewer system, waste edible fats and oils solidify and combine with other discarded materials to form blockages higher up in the drainpipe [8]. Furthermore, fats may encourage the development of mycelium, a component of fungi that consumes oxygen to degrade organic compounds. Grease-containing sewage water reduces oxygen availability, which increases expenses and energy needs for wastewater treatment plants (WWTPs) because oxygen is needed for biological wastewater treatment. As a result, it makes drying sewage sludge challenging [15]. Generally, this discharge not only causes environmental problems but also leads to economic losses [16].
It is challenging to separate an emulsion due to the viscous interface that forms between the oil and water phases and its high FFA concentration that stabilizes the emulsion. This transitional region of light particles, water droplets, and clinging organic molecules is well known to be highly tenacious and to obstruct efficient dewatering. The rag layer floats on the water’s surface because it has a lower density than the free water phase. Occasionally, the rag layer may split, and some may sink to the free water layer’s base. The floc looks denser than water because oil can drain from mineral particles. The total amount of oils and grease in COSW may differ significantly with different feedstock and collecting techniques [17,18].
There have been several methods for the management of COSW, namely land application, headwork injection, dewatering and landfill, soap production (soap chips), the extraction of oil for biofuel production [19], anaerobic digestion [17,20,21], Fenton oxidation [22], compost [23], and active carbon [24].
Biodiesel, as an alternative to fossil diesel, has been developed from oils derived from plants or animals. Due to the significant global environmental and climatic problems with conventional diesel, biodiesel offers a green, sustainable alternative fuel [25]. Biodiesel can largely replace fossil fuels. This product’s sources include wastewater grease, UCO, animal fats, and vegetable oils [15]. Vegetable oils and animal fats are mostly composed of triglycerides, which are fatty acid esters linked to glycerol. Any source of fatty acids can be used to produce biodiesel. Therefore, biodiesel production should be possible using any lipid from an animal or plant [7]. Due to their specifications, UCO and waste from edible oil refinery plants are highly recommended. These wastes have significant potential to be converted into biodiesel and other environmentally friendly fuels without harming the environment. However, their collection and preparation result in secondary residue with inferior original quality attributes [15,26]. When COSW is intended for biodiesel feed, the greasy matter should be separated from water and solids. The grease is a proper replacement for fuel in boilers or as a feedstock for biodiesel, and the resulting solids can be utilized as a feedstock for anaerobic digestion or composting [19].
There are various problems with using COSW to produce biodiesel, one of which is its high sulfur content in some cases. Sulfur reduction is quite difficult; just a few biodiesel plants have developed handling techniques. Distillation plus activated carbon can surpass the 15 ppm sulfur threshold required for on-road fuel sales in the US. However, the approach is expensive and probably not suitable for commercial use [15]. The remaining waste, which includes food leftovers and wastewater, becomes a problem once the FOG parts have been removed, and as a result of processing, extra waste streams of liquid and solid byproducts are produced. This is because only the FOG component of COSW can be refined for biodiesel production [20]. Moreover, significant physical filtration is required because of the biodiesel produced from dewatered COSW’s extremely high gel point. Generally, biodiesel production is challenging due to COSW’s high FFA content (15–100% FFA), solidification at room temperature, and water contamination. Using conventional technology, commonly known as alkali-catalyzed transesterification, high levels of FFAs require several expensive operations and real-time system adjustments [12]. Specifically, soap and water are produced by the interaction of FFAs and the alkali catalyst. When the FFA level is greater than 3%, the soap helps to create an emulsion during the water wash and inhibits the separation of the glycerol from the methyl esters [26].
Numerous techniques have been developed to manufacture biodiesel from low-quality FOG derived from plants and animals. Techniques for pretreating catalysts with strong acids have produced high-quality finished goods without soap forms and with good conversion yields [27]. Despite this, the acid’s corrosive nature, slow reaction rate, and higher temperature requirements prevent the approach from being used for esterification reactions. Another potential approach for manufacturing biodiesel from feedstock with a high FFA level is eliminating FFA content and other undesirable components utilizing feedstock purification procedures like refining, bleaching, and deodorization [27].
Enzymatic lipase transesterification is another practical method for making biodiesel. Enzymes do not make soap and can esterify FFA and triglycerides in a single step without additional washing. Enzymes have tolerated the level of FFA in the feedstock well, but they are expensive and unable to provide the level of reaction completeness required to meet the ASTM fuel standards [27].
There are presently few commercial technologies that are effective. According to a 2013 report from the US company BlackGold Biofuels, handling high concentrations and very variable volumes of FFAs in a single process without making any adjustments or producing soap is possible. They have developed techniques for fuel purification, like desulfurization, and are currently trying to sell their technique, but it costs much money and might not be justified for the relatively small volumes [15]. The Pacific Biodiesel Technologies approach prevents yield loss using feedstock with up to 50% FFA. Another example is RPM Sustainable Technologies, which uses base-catalyzed transesterification and a specialized acid-catalyzed esterification pre-processor to transform dewatered brown grease into ASTM/EN-grade biodiesel fuel.
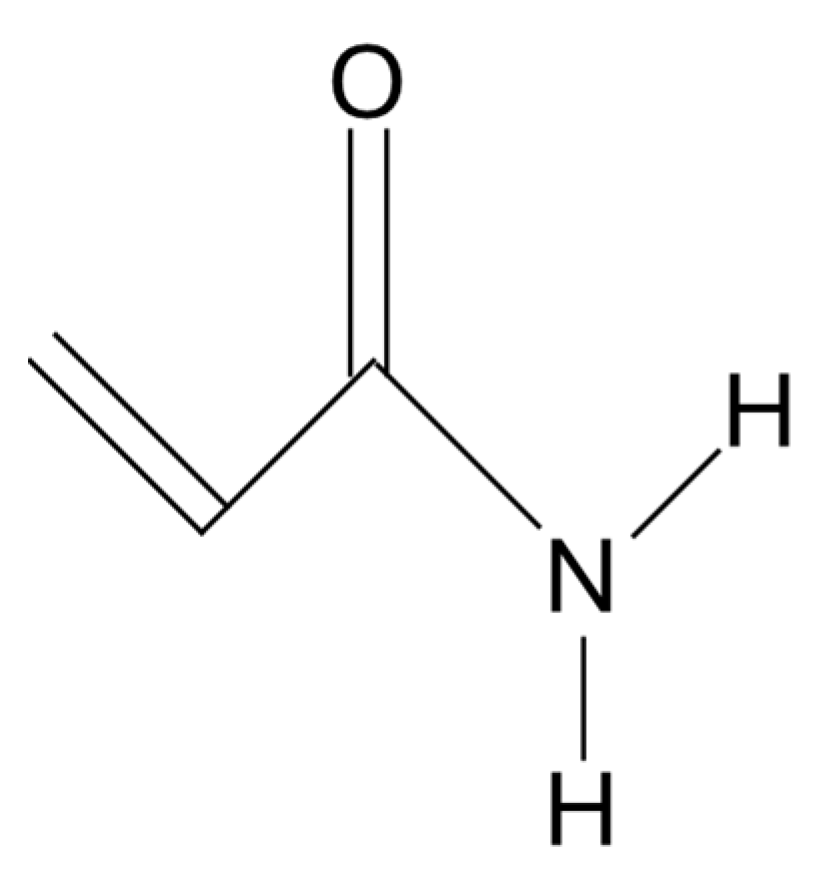
Any polymer with acrylamide as one of the monomers (Figure 1) is generally referred to as “polyacrylamide” [28].
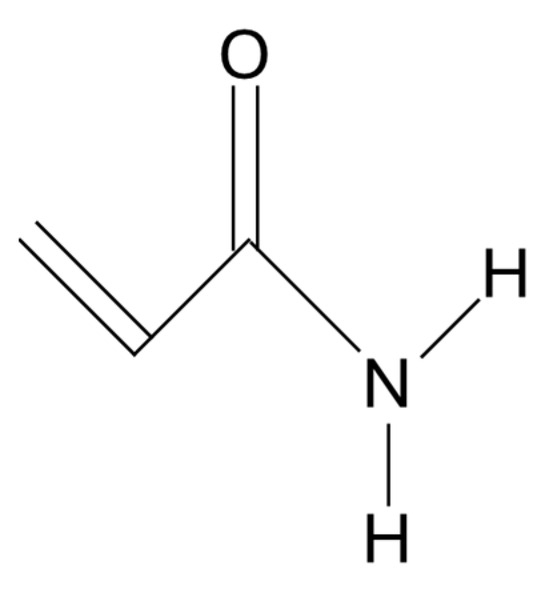
Figure 1.
Acrylamide monomer.
Poly(prop-2-enamide) (according to its IUPAC nomenclature) is a water-soluble polymer created by the polymerization of either acrylamide monomers or N,N′-methylenebis(acrylamide) [29]. PAM is used extensively in environmental systems, including as a viscosity enhancer in enhanced oil recovery (EOR), as a friction reducer in high-volume hydraulic fracturing (HVHF) [30], as a flocculant in water treatment and sludge dewatering [30], and as a soil conditioning agent in agricultural applications and other land management practices [31]. The most popular anionic PAM used in oil and gas development and soil conditioning is a hydrolyzed form of polyacrylamide (HPAM), a copolymer of acrylamide and acrylic acid [32]. In this water-in-oil emulsion, the polymer is dispersed in the aqueous phase and encapsulated by a continuous oil phase that is stabilized by surfactants [28]. While cationic polyacrylamides have improved performance at high salinity because of lower electrostatic repulsion from electrolyte shielding, anionic polyacrylamides perform better as drag reducers in freshwater environments [28,33].
PAM is frequently employed as a flocculant in the purification of drinking water [34]. PAM can build bridges between destabilized particles to produce micron-sized aggregates with good settling qualities [35]. PAM of various types—cationic, nonionic, and anionic—has been investigated for flocculation. Because there are more binding sites, there is an increase in the capacity for flocculation and adsorption as MW increases [30]. Although interactions with tiny particles are reduced, polymer molecules with an MW larger than approximately 106 Da are more likely to entangle [31]. While increasing temperature increases the adsorption of anionic PAM to clays due to the reduction in hydrogen bonds between PAM and water and the greater penetration of PAM into the interior clay structure, high salinity and low pH reduce electrostatic interactions [36].
It is well known that PAM can degrade through several methods, considerably increasing its mobility and perhaps releasing acrylamide monomer, a recognized toxin and probable carcinogen. This is true even if the PAM utilized in environmental systems has a very high MW [28].
Due to their outstanding flocculation function resulting from the synergistic effects of charge neutralization and bridging adsorption, cationic and multifunctional polyacrylamide are crucial industrial reagents, especially for wastewater treatment [37]. Coagulation, the first phase in wastewater treatment, works to neutralize by reducing electric loads and helping link colloidal particles with sizes ranging from 0.01 to 1 μm. Following coagulation, flocculation encourages contact between colloidal particles to create agglomerates, which call for flocculants such as poly(acrylamide)s [38].
Polyacrylamide can also be utilized as a nitrogen source [39,40]. Guoliang Sang et al. [41], Lanmei Zhao et al. [42], Boya Xiong et al. [28], Eldon A. Smith et al. [43], and Marcus J. Caulfield et al. [44,45] have all presented evidence that the usage of PAM does not leave hazardous residue in the ecosystem. PAM solids can be used as fertilizers because they include numerous organic chemicals as well as nitrogen and phosphorus molecules. However, the impacts of various PAMs on plants should be addressed in addition to flocculation efficiency [46].
The overall goal of this study is to design, validate, and develop routes to recover oil from COSW and reduce the amount of generated waste in order to align with the circular economy concept. Two sets of experiments were conducted for this study. The first stage involved pretests designed to identify the key parameters and their respective levels influencing the process. In the second stage, the findings were utilized from these preliminary studies to design the main experiments systematically. The aim is to develop processes adapted to the specific characteristics, conditions, and challenges related to oil recovery. It is expected that by using this procedure, the final recovered oil efficiency will be increased, which is important from an environmental and economical point of view. Furthermore, the recovered oil would be a suitable feed for biodiesel production.
2. Materials and Methods
Secondary waste collected from the COSW collection system in Iran was used as a sample. A sample of COSW was analyzed to determine the physical and chemical features of the waste. Laboratory-grade polyacrylamide (anionic and cationic PAM—(C3H5NO)n) and aluminum sulfate (Alum—Al2(SO4)3) were obtained from Sigma-Aldrich. The PAM and Alum were dissolved in 30 mL deionized water, separately, to reach their respective concentrations (As shown in Figure 2).

Figure 2.
Collected cooking oil secondary waste (COSW).
To determine the effective parameters for the separation and extraction of oil from COSW, as well as the parameter intervals, a series of experiments were designed and carried out as pretests. In these pretests, temperature and COSW content were designed to be fixed at 60 °C and 500 mL, respectively. To test the impacts of additives on the separation procedure, various additives (cationic PAM dissolved in water, cationic PAM not dissolved in water, anionic PAM, cationic PAM with Alum) and retention times (60 min, 180 min, and 4 days) were investigated.
In this project, PAM and Alum are used to separate oil from secondary waste. The effect of various parameters such as temperature, mixing time, and amounts of chemicals used was investigated in the main tests. There are several contributing factors in the experiment, and thus, performing all required tests would be time-consuming and a waste of energy. As a solution to this issue, it is possible to perform the analysis of the results with proper accuracy by designing experiments using the RSM method.
In this research, the steps are divided into two main parts:
- Preparation of COSW and additives;
- Additive utilization to separate oil from the COSW.
To determine effective factors, it should be noted that the factors should be dependent. The factors that are analyzed in this research are as follows:
- Mixing time;
- Temperature;
- PAM content (concentration);
- Aluminum sulfate content.
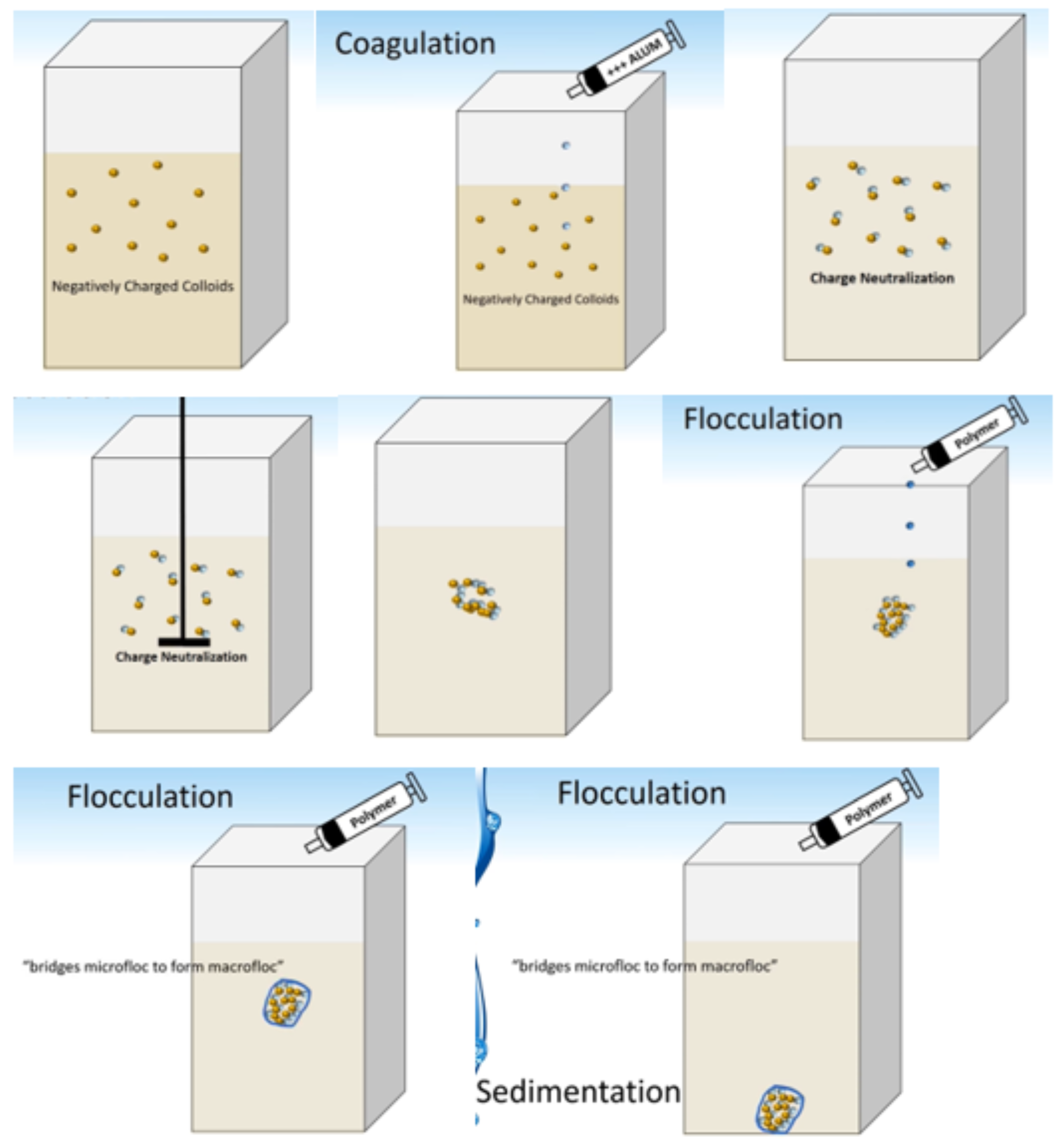
The main idea of separation in this research is based on coagulation and flocculation of the materials (Figure 3).
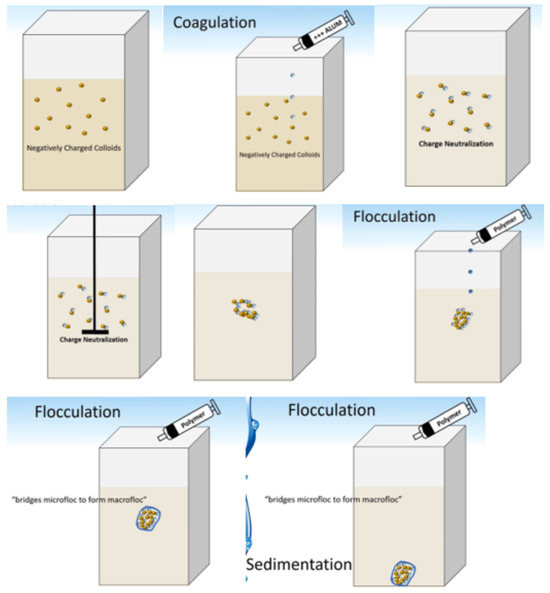
Figure 3.
Schematic of coagulation–flocculation process.
Based on the results derived from the conducted pretests at the previous stage, the values as well as the levels for temperature, PAM content, alum content, and time were selected. The effective factors and the number of selected levels are given in Table 1.

Table 1.
Design of the experiment for temperature, additive content, and time.
The selected levels, chosen based on the pretests, are shown in Table 2.

Table 2.
Variables and levels for temperature, additive content, and time.
The design of the experiments is given in Table 3.

Table 3.
Output of Design Expert V11 software for the experiment design.
3. Result and Discussion
A year-long monitoring of COSW amounts received at a primary collection center in Iran, which acts as the main hub for the country, revealed contributions from over 100 waste-generating centers. In 2022, these centers collectively generated 500 tons of COSW. The quality parameters of COSW obtained from the Iranian UCO management company are summarized in Table 4.

Table 4.
Qualities of collected COSW (input materials).
The retention (settling) time for COSW samples was analyzed to determine optimal separation conditions. During the initial phase, separation occurred more rapidly, with diminishing rates over time. After 3 h, the separation rate became negligible, though minor separation was observed over extended durations (up to 4 days). Based on these findings, a retention time of 3 h was deemed sufficient for the experiments. The results of the pretests are shown in Table 5 and Figure 4.

Table 5.
Pretest results to determine the effective parameters for the separation and extraction of oil from COSW.
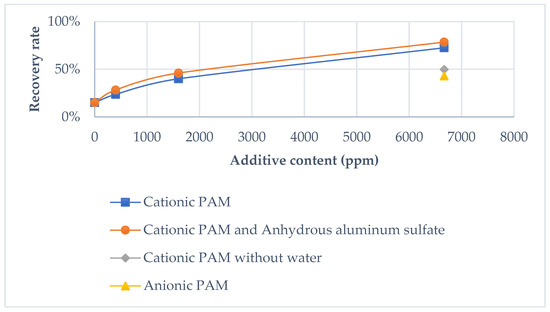
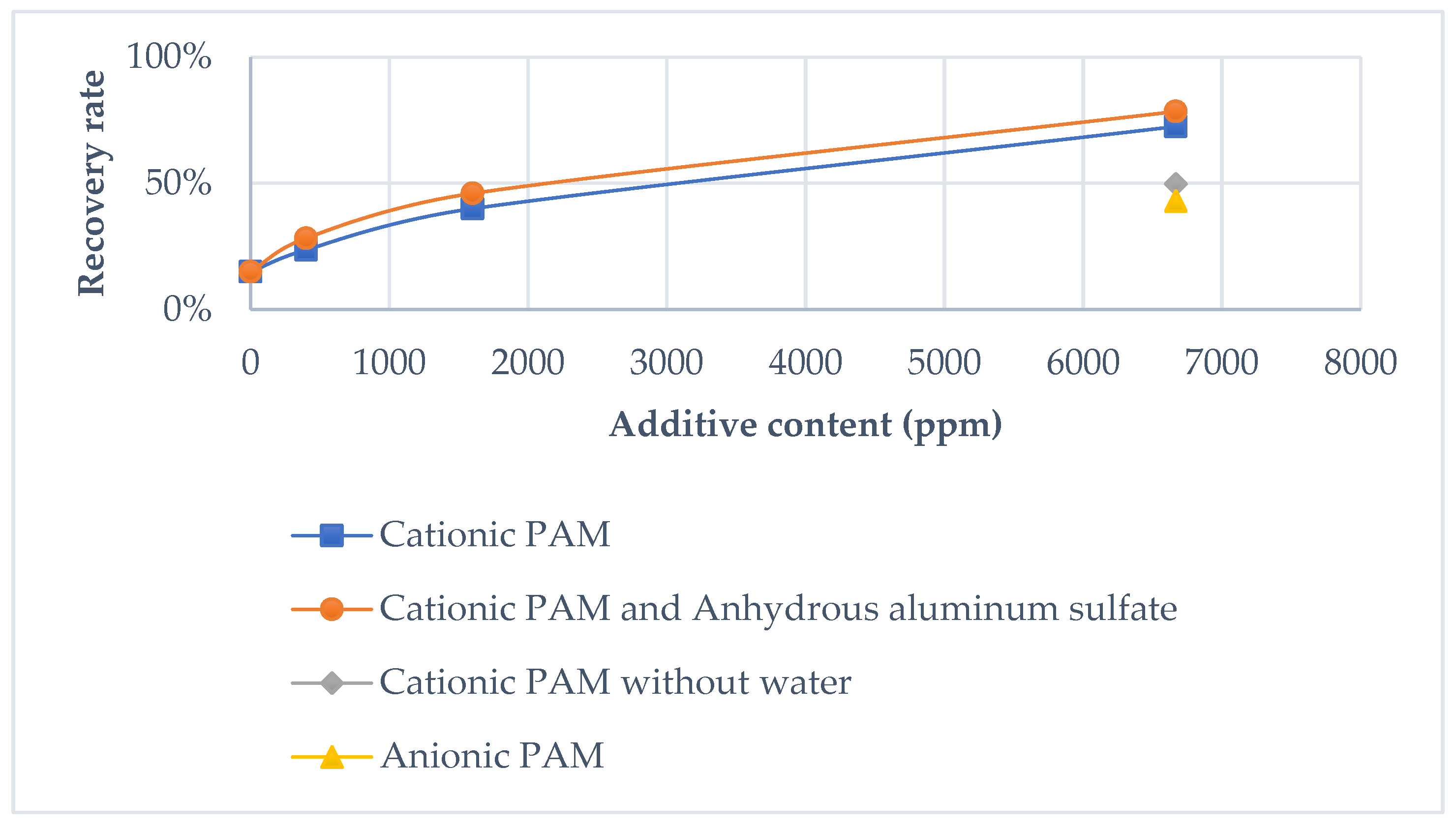
Figure 4.
Oil recovery rate using different additives.
Preliminary experiments were conducted to determine the influencing parameters and their levels, with the results presented in Table 5 and Figure 4. Each test began with 500 mL of COSW, containing 53% oil (approximately 265 mL). Oil content was quantified using a centrifuge.
In the initial test, the system recovered 15% of the oil without any additives. Subsequent tests demonstrated significant improvements when additives were introduced:
- Cationic PAM enhanced recovery to 88.6%.
- Anionic PAM achieved a recovery rate of 43%.
These results indicate the positive effect of PAM additives on recovery, with cationic PAM providing superior performance compared to anionic PAM.
Tests 3 and 4 highlighted a direct correlation between additive amounts and recovery efficiency. Increasing the cationic PAM quantity by 14-fold raised the recovery rate from 23.7% to 88.6%. However, when cationic PAM was added without prior blending with water (Test 5), the recovery yield decreased.
Further tests (samples 7, 8, and 9) showed that adding Al2(SO4)3·18H2O as a secondary additive slightly improved the recovery rate.
The retention time, determined to be 3 h, was incorporated into the experimental design. Table 6 presents the levels of four independent variables examined in this study alongside the respective results. Regression analysis and ANOVA were conducted to analyze the experimental data, with oil recovery percentage chosen as the primary response variable.

Table 6.
Oil recovery rate for designed experiments.
Regression Model Equation and ANOVA
Based on the obtained results and the statistical analyses performed on them, it is appropriate to use a second-order statistical model for the recovery of oil. In selecting the model, the regression coefficients were evaluated many times by checking the results and analysis of variance (ANOVA) and then the appropriate coefficients were selected.
The modeled equation is as follows:
Recovery rate =
−245.44604 + 6.17800 × (Temp.) + 0.007711 × (PAM) + 0.014296 × (Alum) + 2.29213
× (Time) − 0.041714 [(Temp.)]2 − 6.09844 × 10−7 × [(PAM)]2 − 1.22766 × 10−6 × [(Alum)]2
− 0.036019 × (Time)
−245.44604 + 6.17800 × (Temp.) + 0.007711 × (PAM) + 0.014296 × (Alum) + 2.29213
× (Time) − 0.041714 [(Temp.)]2 − 6.09844 × 10−7 × [(PAM)]2 − 1.22766 × 10−6 × [(Alum)]2
− 0.036019 × (Time)
The developed equation effectively elucidates the influence of each parameter on the response level. The model’s validity was assessed through variance analysis. A p-value below 0.05 signifies the significance of a model term, while a value exceeding 0.1 suggests its insignificance. As shown in Table 7, Table 8 and Table 9, the obtained p-values for lack of fit, the adjusted R-squared (predicted), and the high F-value (112.26) collectively indicate strong model adequacy. Furthermore, the high values of the correlation coefficient and adjusted correlation coefficient demonstrate the model’s ability to accurately predict the response level based on the considered factors.

Table 7.
Fit summary results from analysis of Design Expert software.

Table 8.
ANOVA analysis for reduced quadratic model resulting from analysis of Design Expert software.

Table 9.
Final modeled equation for oil recovery rate from COSW. (* weightage based on individual parameters).
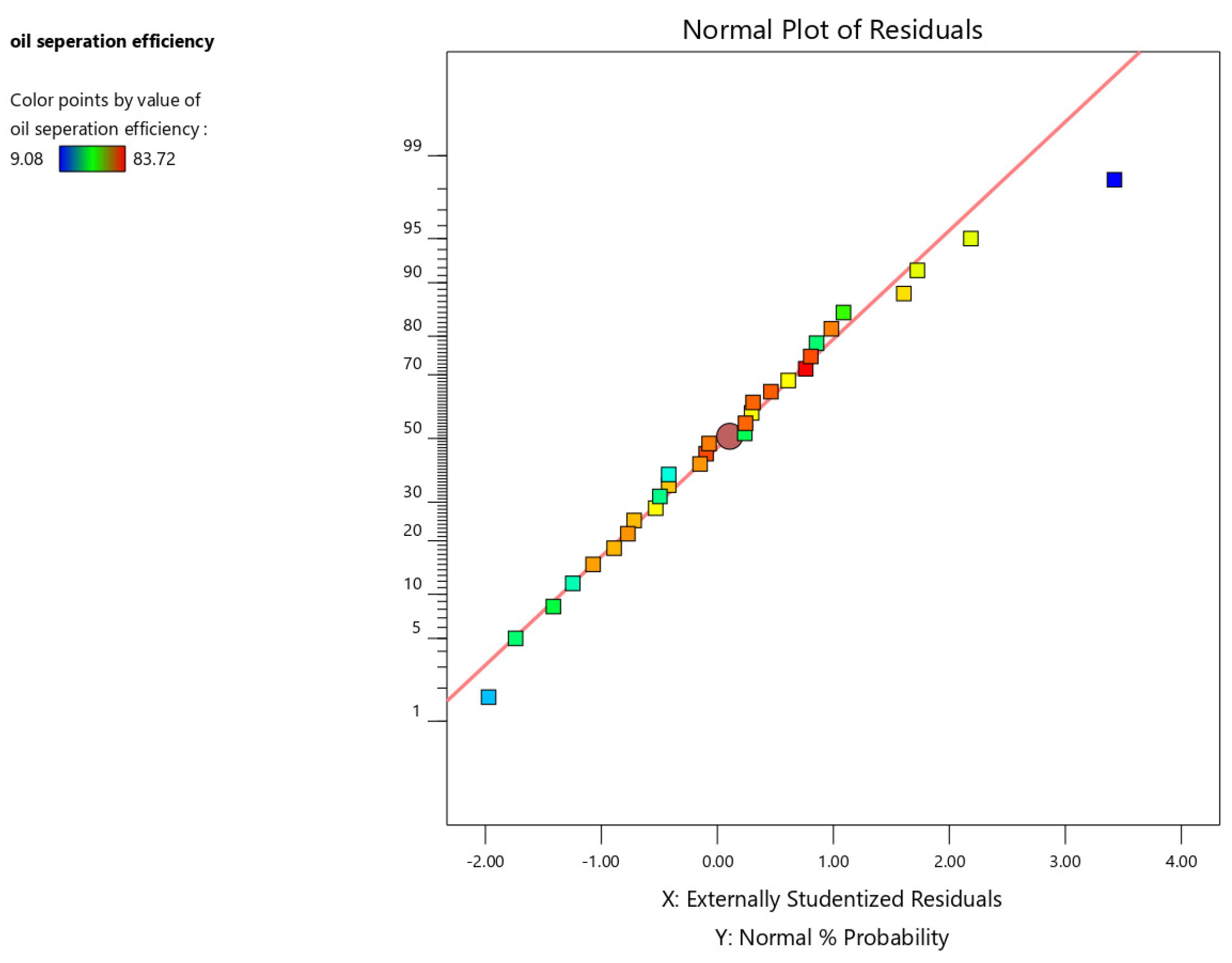
The normal probability plot provides a visual assessment of residual normality. Ideally, the points should exhibit a linear trend, indicating normally distributed residuals. Figure 5 demonstrates that the residuals for this dataset closely adhere to a normal distribution.
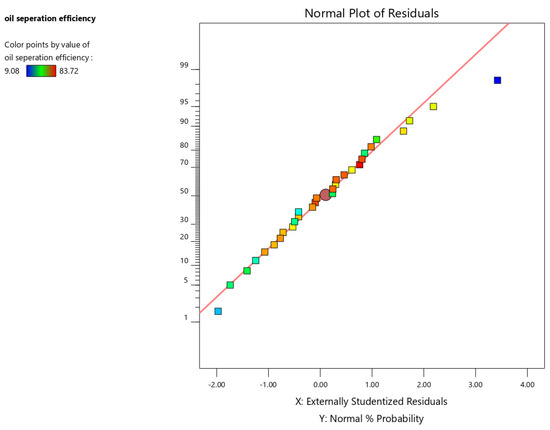
Figure 5.
Normal probability plot of the residuals for oil separation efficiency.
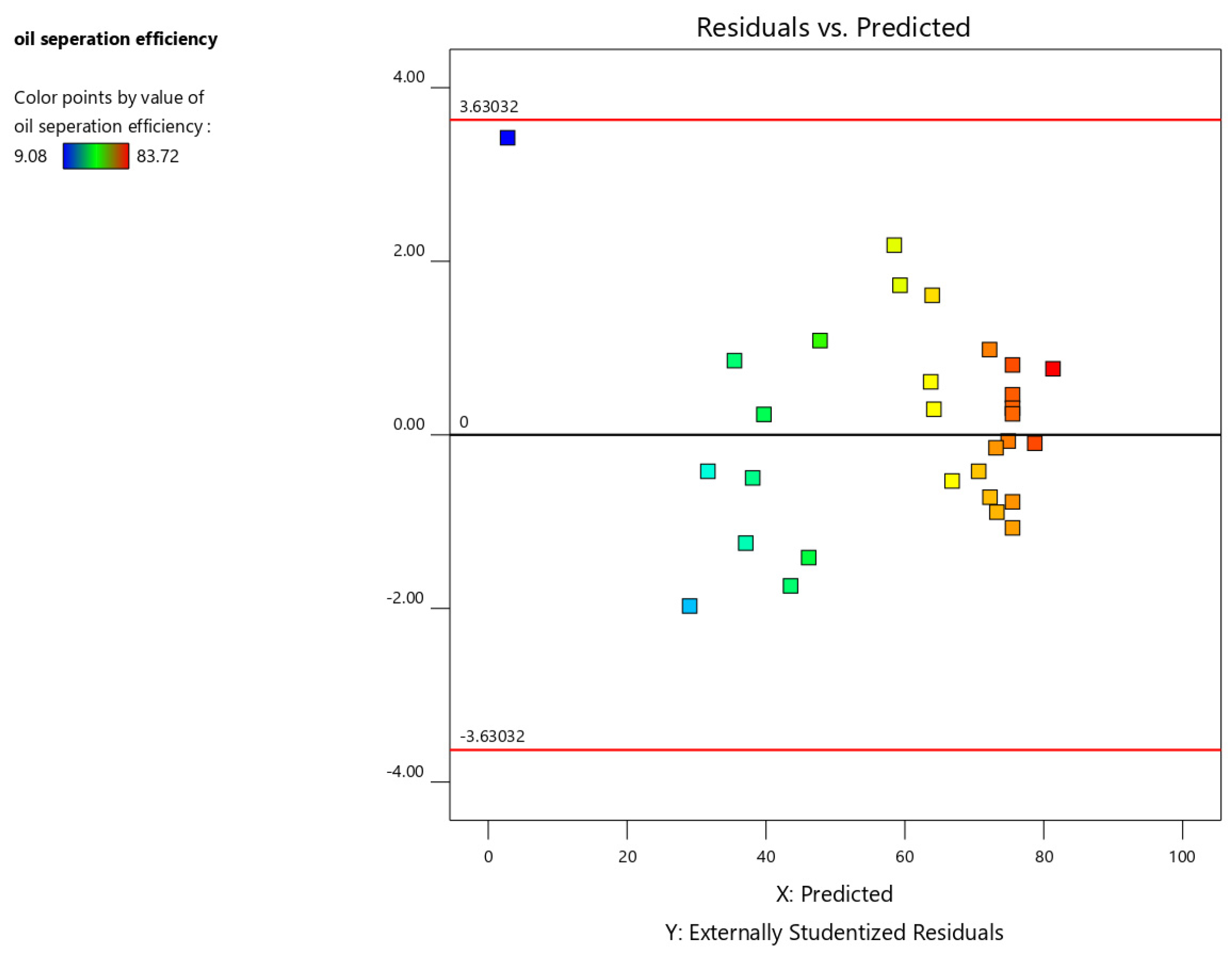
The residual vs. predicted plot assesses the model’s fit by plotting the residuals against the predicted values. Ideally, the points should be randomly scattered around the zero line. A discernible pattern in the plot may indicate either an inadequate model fit or non-normality of the response variable, potentially necessitating data transformation for further analysis. Points exceeding the critical value lines, determined based on the specified alpha level, are potential outliers and warrant further investigation.
Figure 6 shows a random scatter of residuals around zero, suggesting that the model adequately fits the data and that the assumptions of the model are not violated.
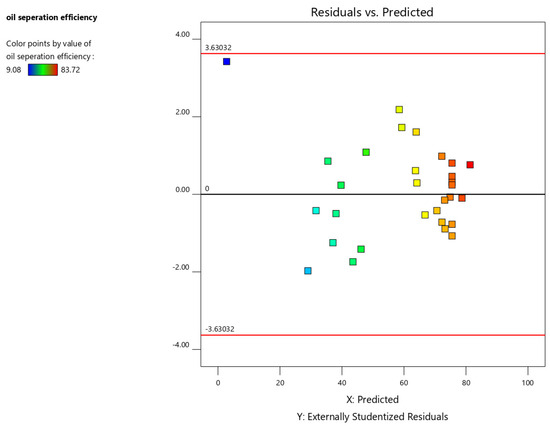
Figure 6.
Residual vs. predicted plot for oil separation efficiency.
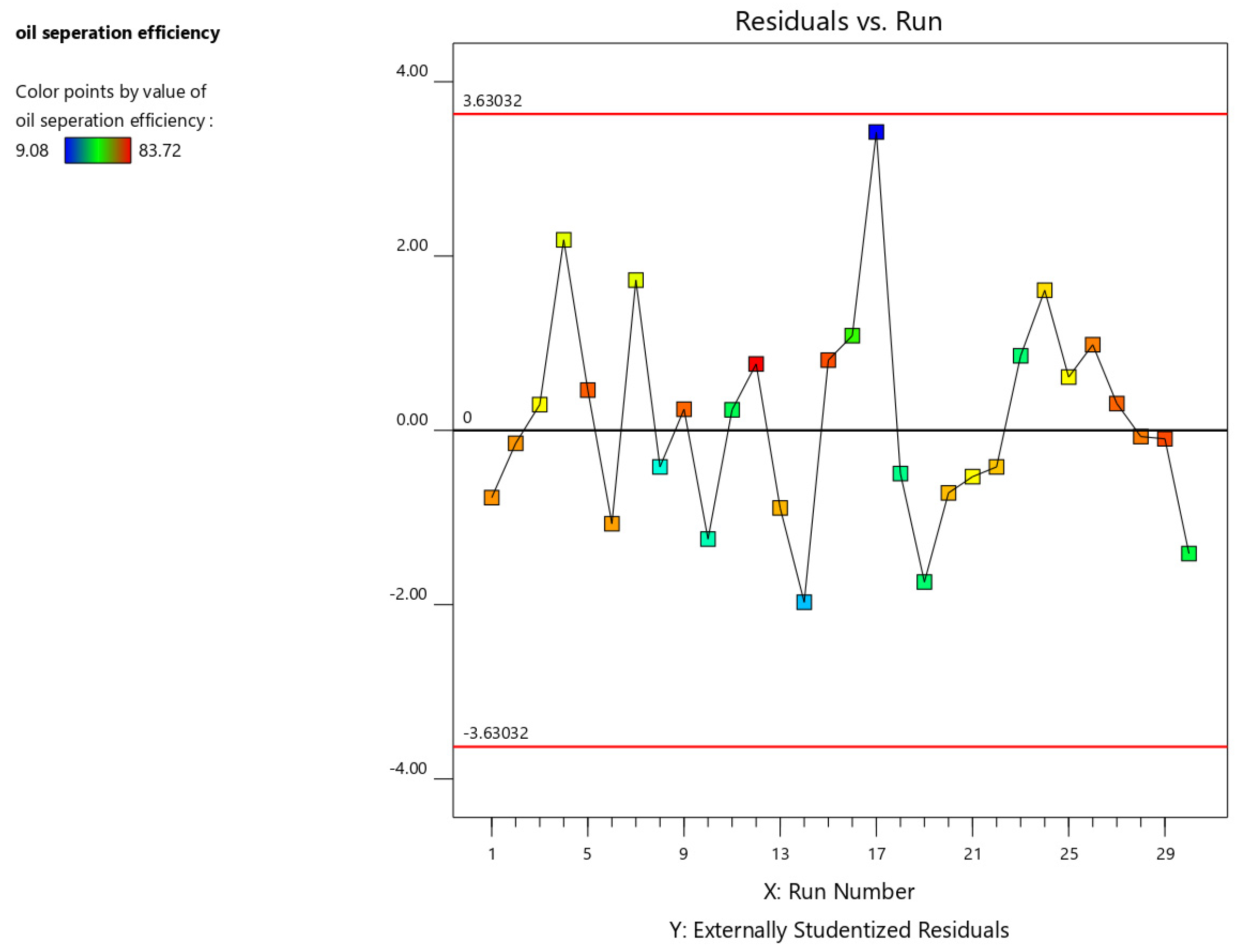
The residual vs. order plot examines (Figure 7) the influence of run order on the residuals. A random distribution of points indicates that the experimental run order does not significantly impact the results. Conversely, a discernible pattern or trend suggests the presence of time-related factors affecting the experiment. These factors can be mitigated through randomization and/or blocking techniques. Points exceeding the critical value lines, determined based on the specified alpha level, are potential outliers and require further investigation.
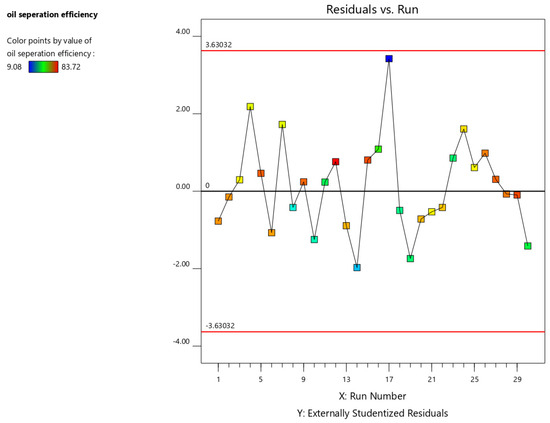
Figure 7.
Residual vs. order plot for oil separation efficiency.
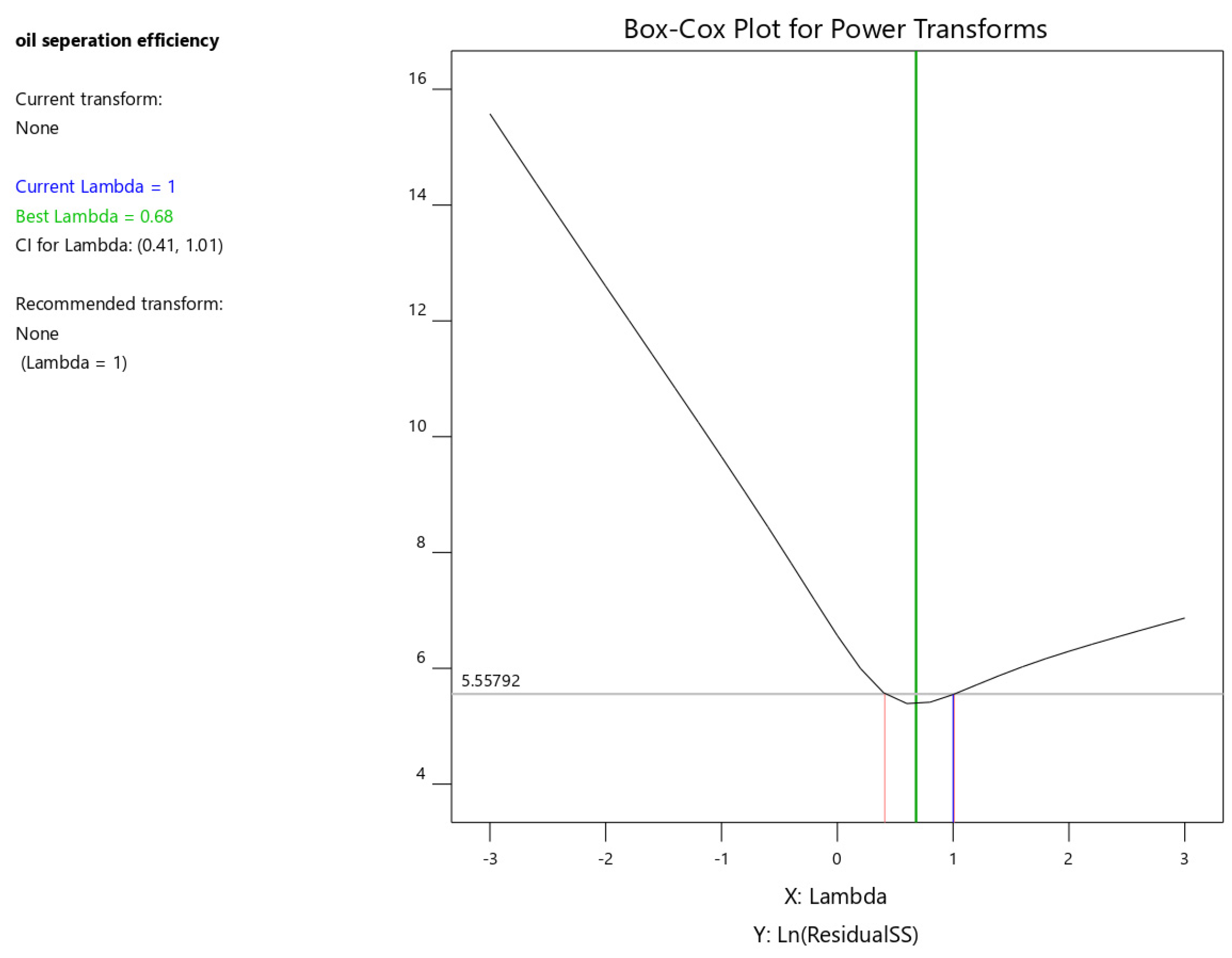
The Box–Cox transformation is a statistical method for transforming non-normally distributed data into a more normal distribution. This can improve the accuracy of linear regression models. The transformation is particularly useful for skewed data or data with outliers. By stabilizing variance, it enhances the reliability of subsequent statistical analyses.
The Box–Cox plot identifies the optimal power transformation for the response variable. It visualizes the best lambda value, its 95% confidence interval, and the current power transformation. The software typically recommends the standard transformation closest to the optimal lambda within the confidence interval.
As shown in Figure 8, the optimal lambda value is 0.68, while the software suggests a lambda of 1. This indicates that no power transformation is necessary for the current dataset.
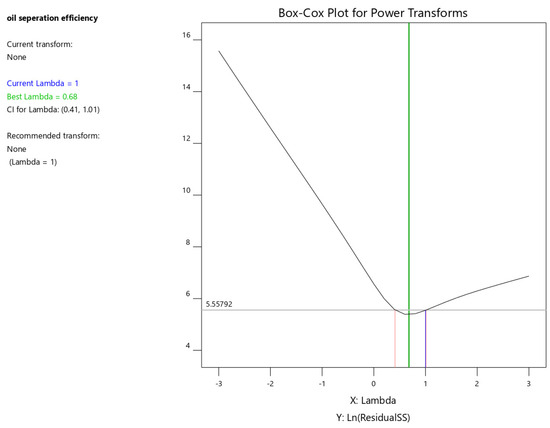
Figure 8.
Box–Cox plot for oil separation efficiency.
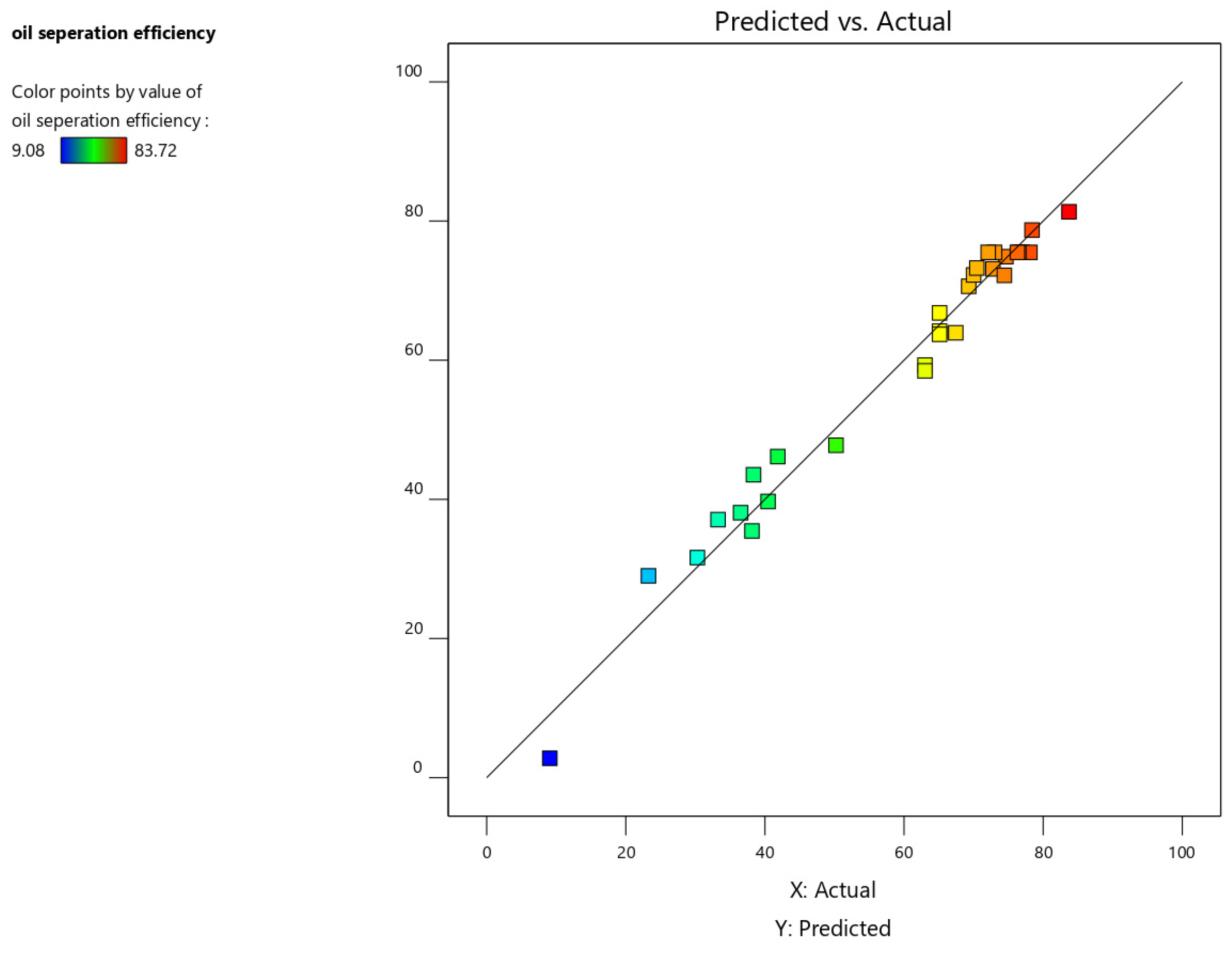
Figure 9 depicts the predicted response values plotted against the actual response values. This plot helps identify data points that the model struggles to predict accurately. Ideally, the data points should be evenly distributed around the 45-degree line. Deviations from this line may indicate a need for model improvement, such as through data transformation (refer to the Box–Cox plot) or the use of a higher-order model.
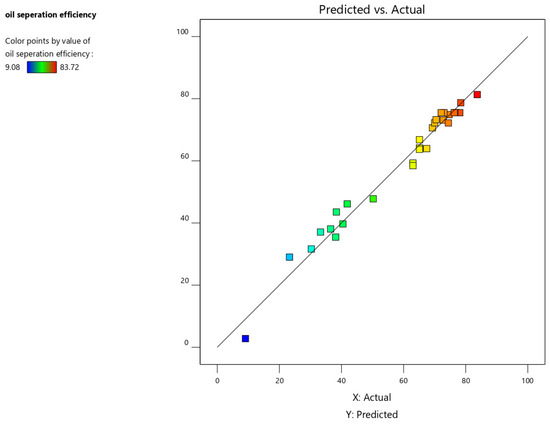
Figure 9.
Predicted vs. actual value plot for oil separation efficiency.
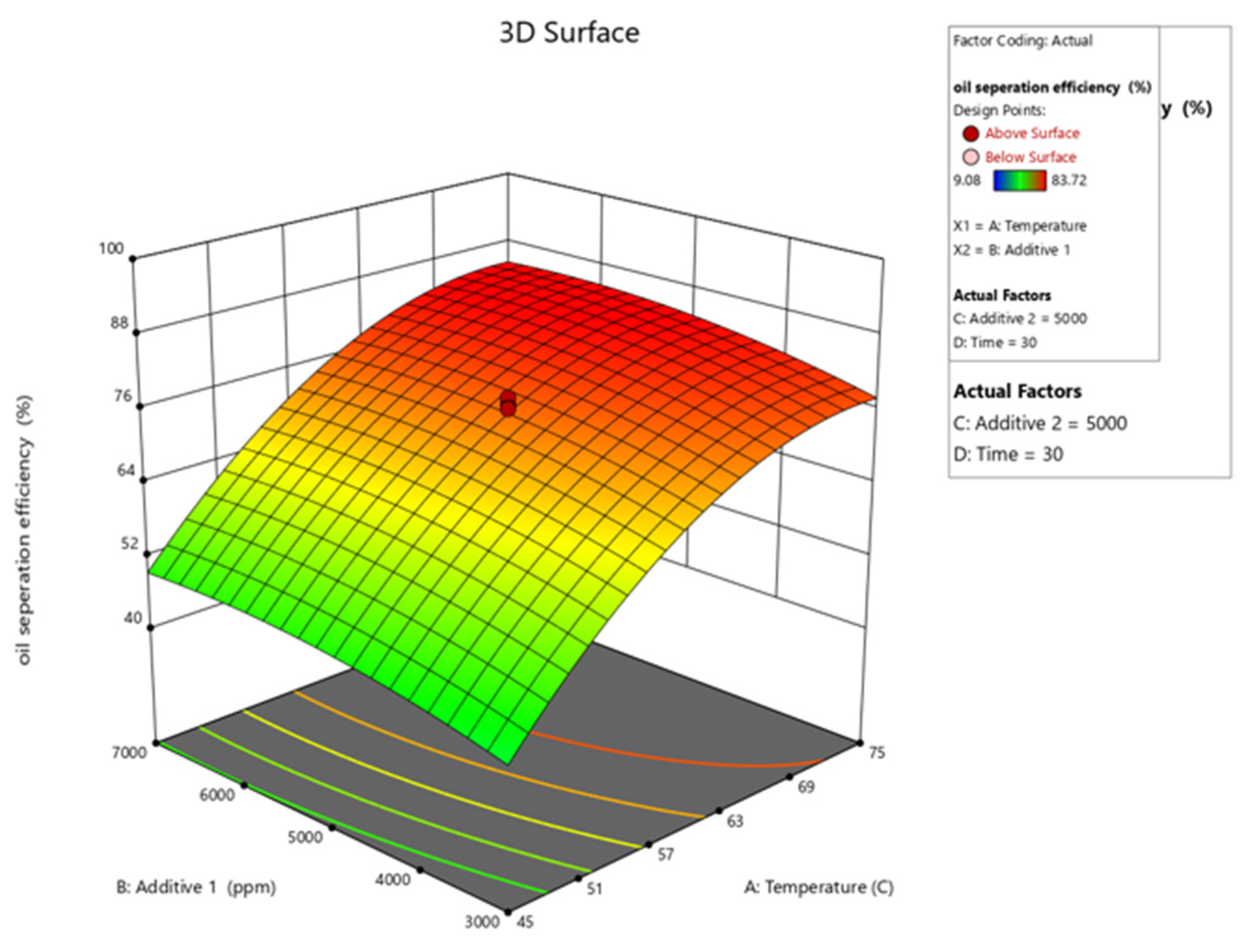
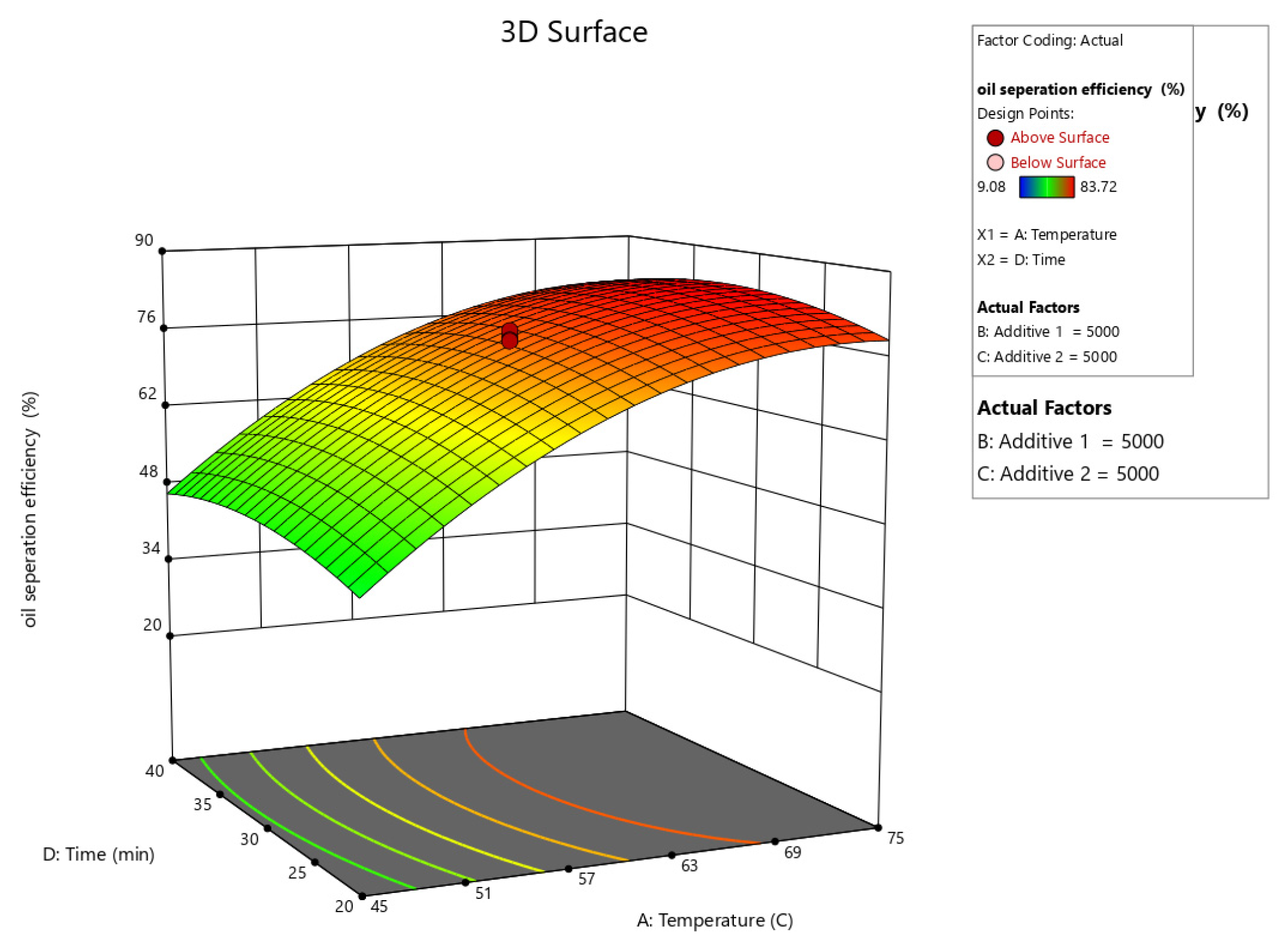
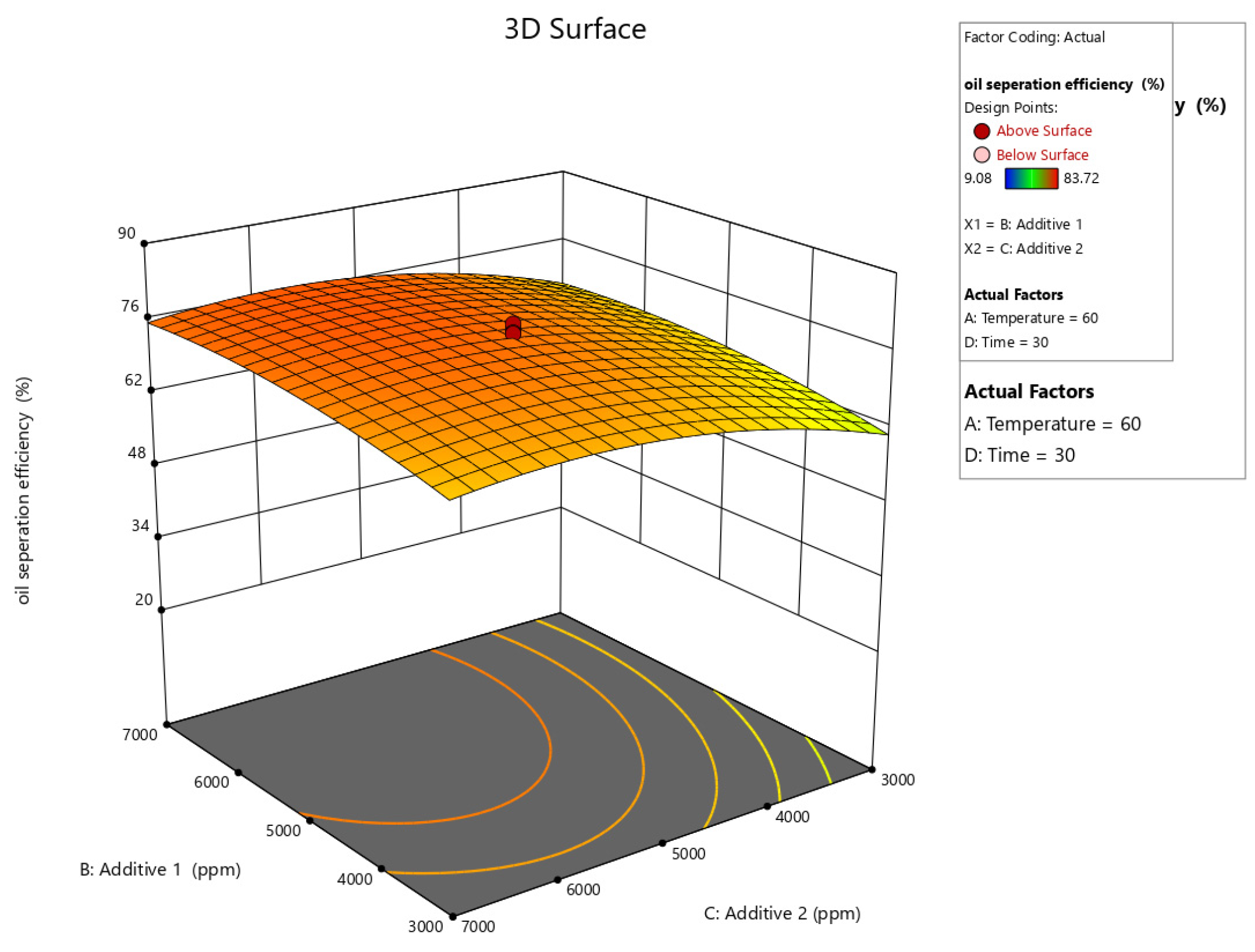
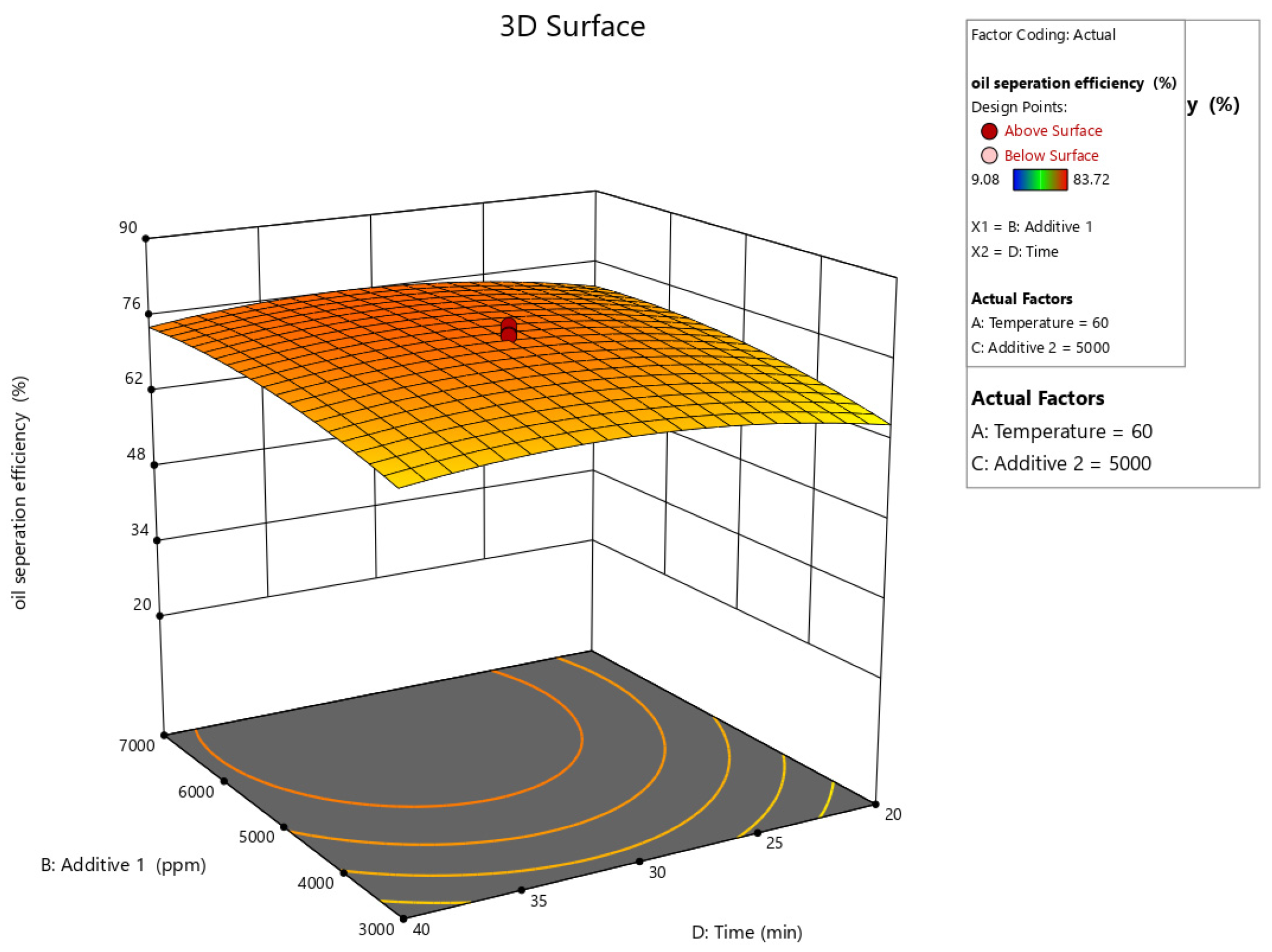
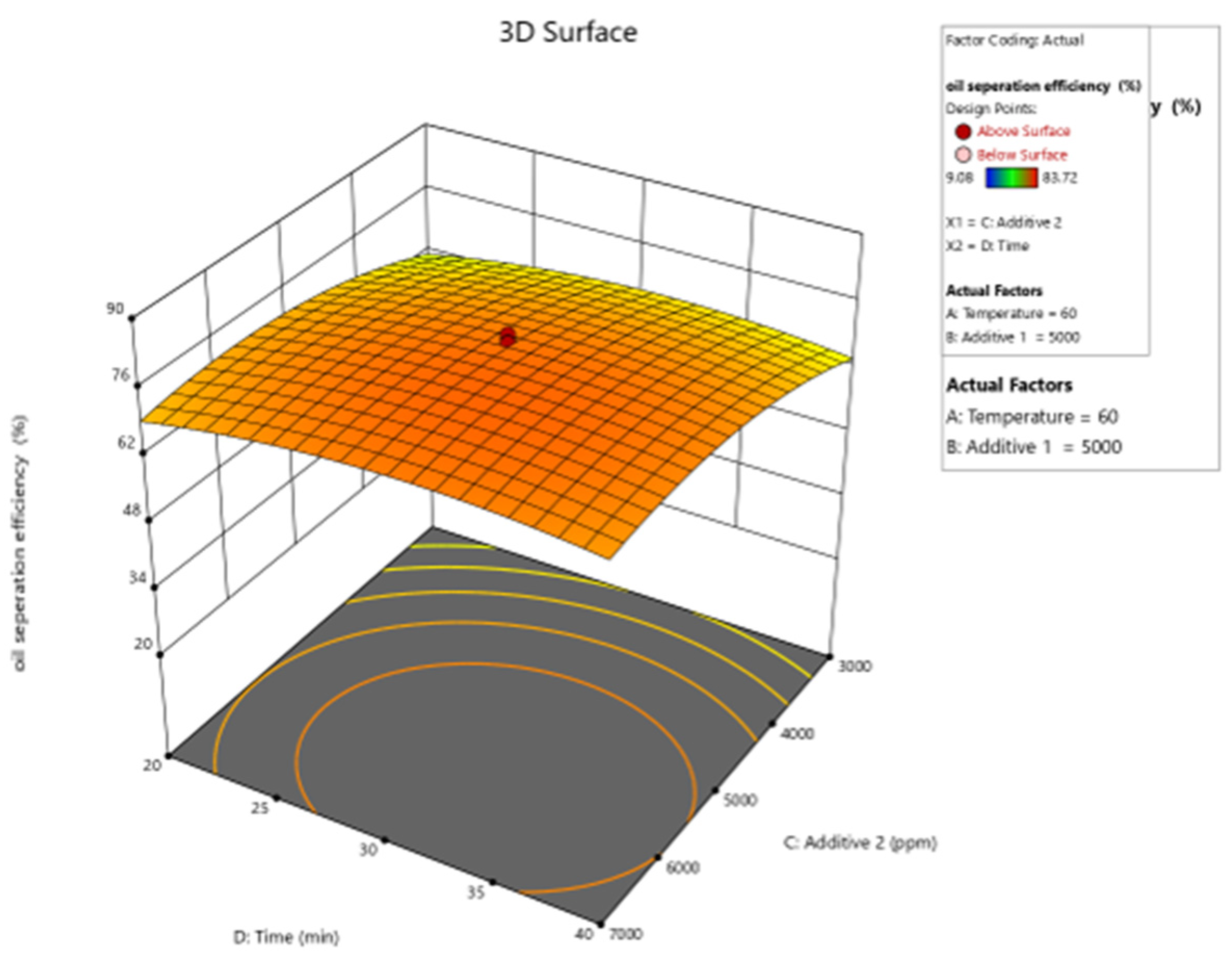
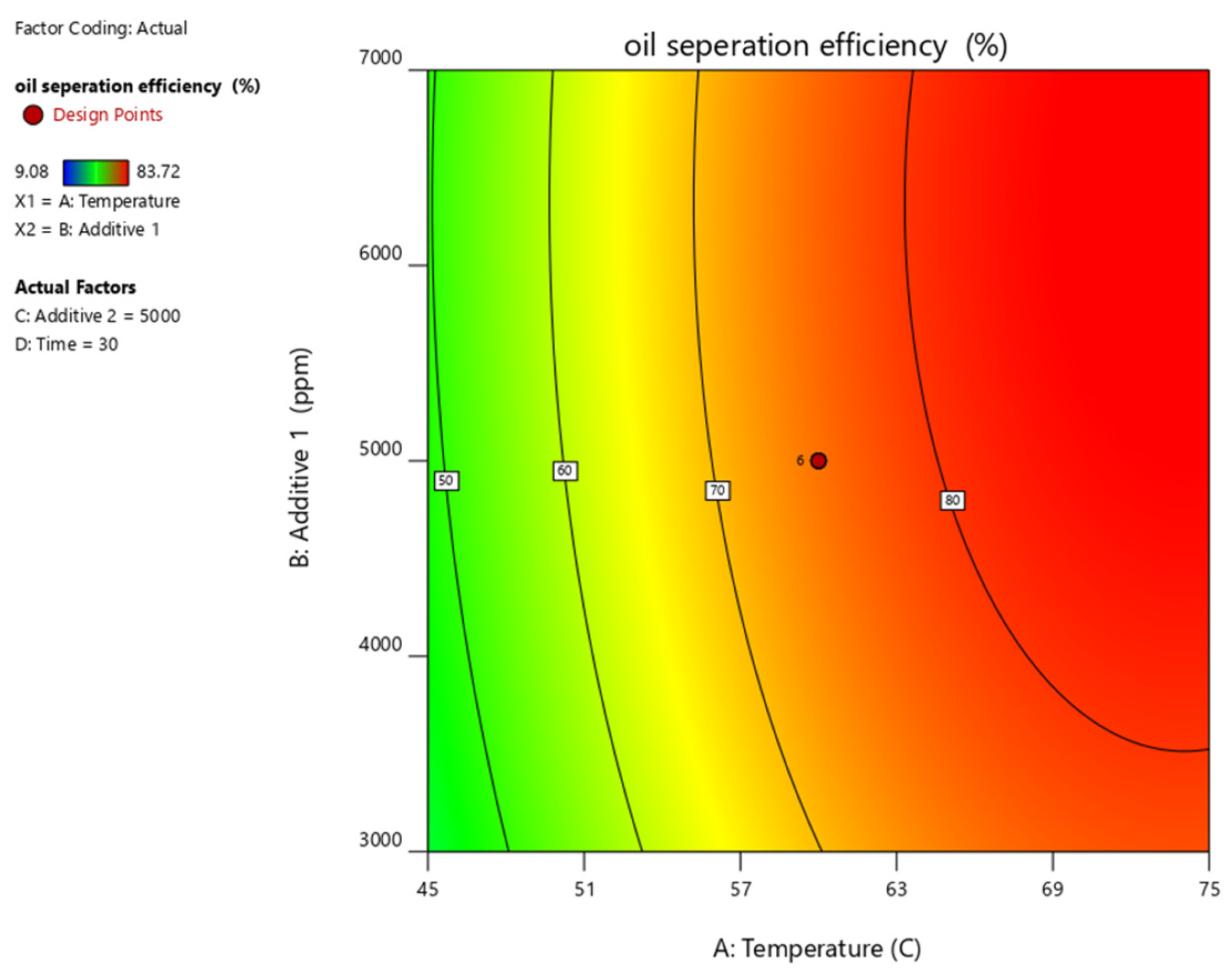
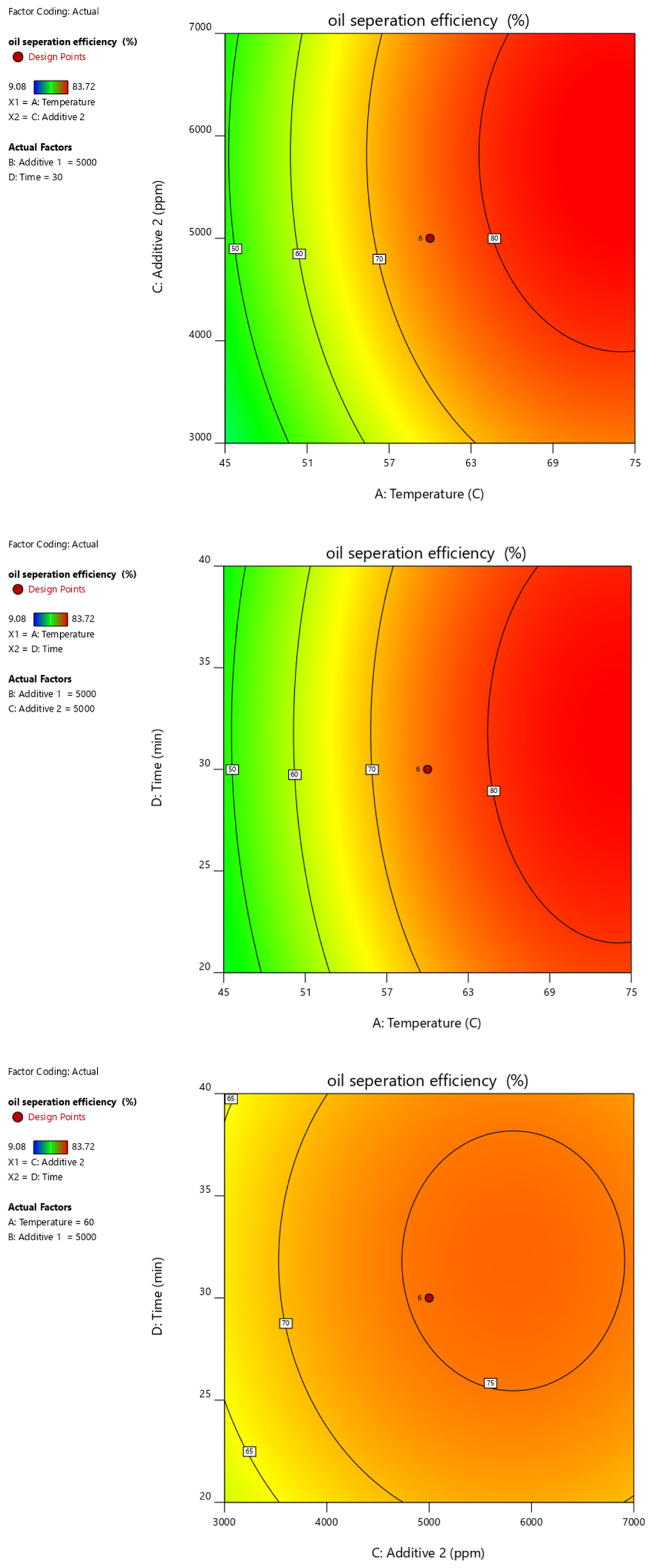
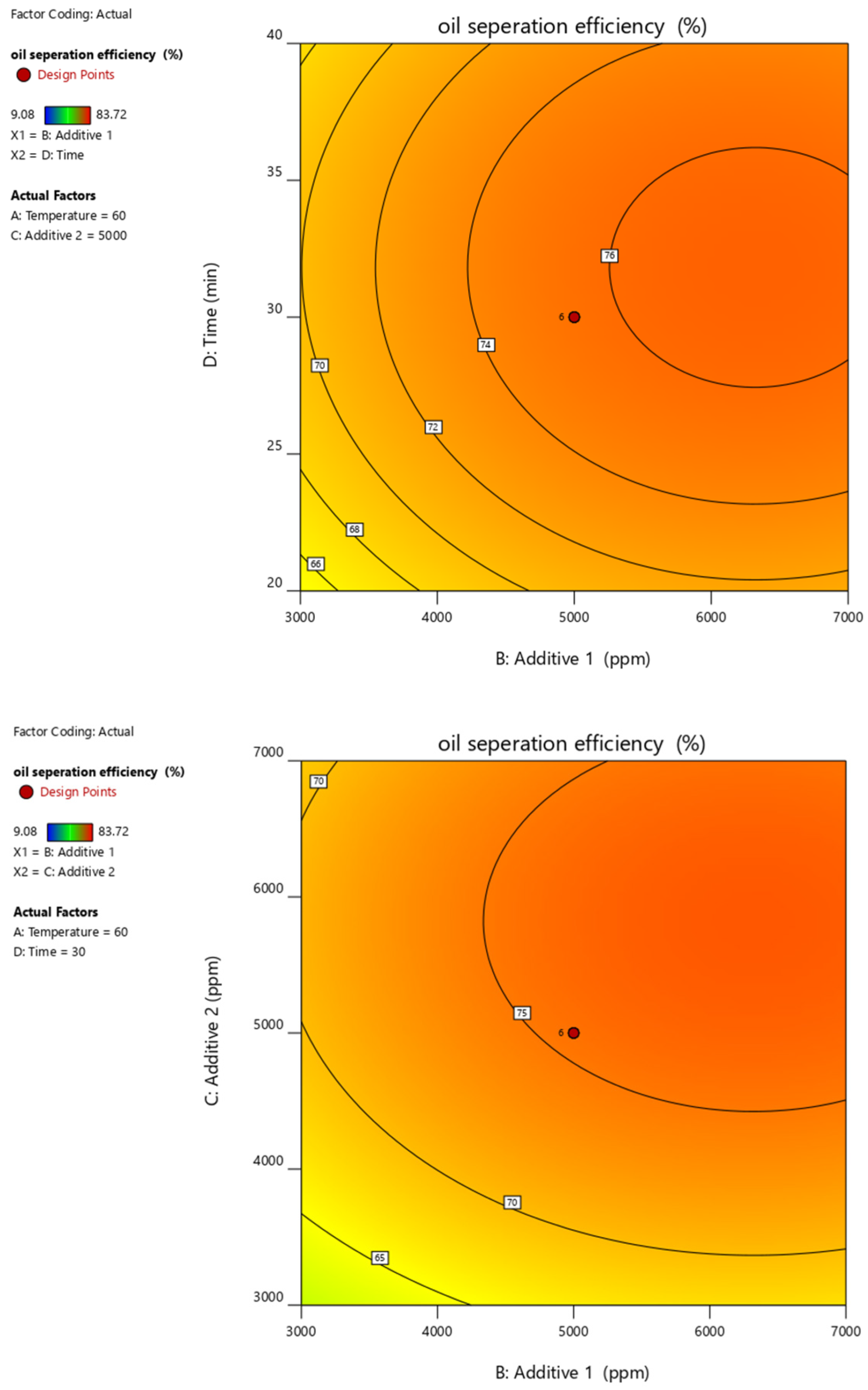
Figure 10, Figure 11, Figure 12, Figure 13 and Figure 14 present surface graphs and Figure 15 presents the oil separation efficiency contours illustrating the influence of various parameters and their combinations on oil separation efficiency. Increasing the temperature and the concentrations of additives 1 and 2 generally enhances oil separation efficiency. While mixing time has a minimal effect, temperature exerts a significant impact. At lower temperatures, oil separation is notably lower, especially in the absence of additives. Conversely, at a given temperature, increasing the concentration of additives 1 and 2 leads to improved oil separation. Although mixing time influences separation efficiency, its effect is less pronounced compared to temperature and additive concentrations.
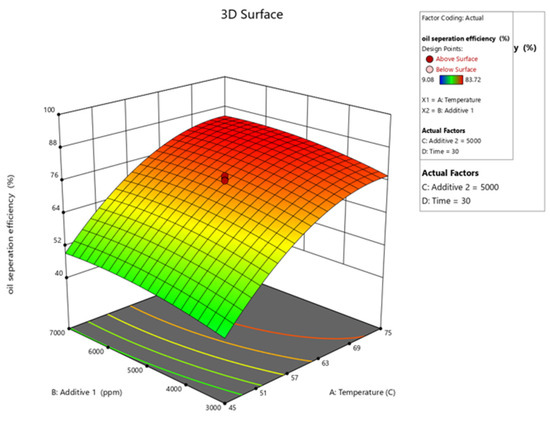
Figure 10.
Surface plot of oil separation efficiency (%) vs. temperature for additive 1.
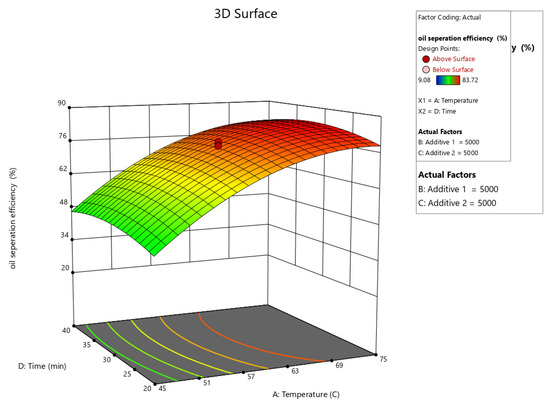
Figure 11.
Surface plot of oil separation efficiency (%) vs. temperature and time.
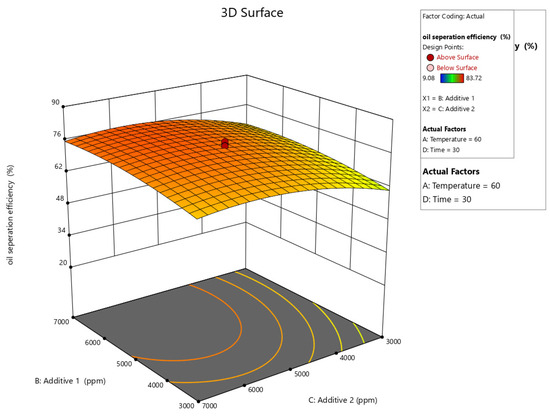
Figure 12.
Surface plot of oil separation efficiency (%) vs. additive 1 and additive 2.
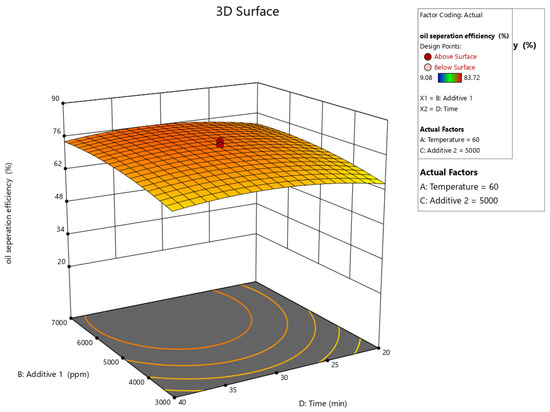
Figure 13.
Surface plot of oil separation efficiency (%) vs. additive 1 and time.
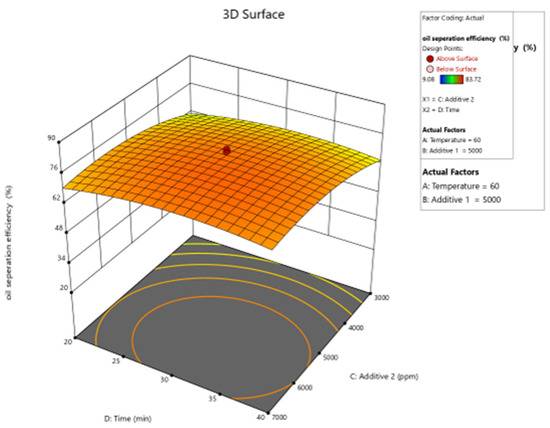
Figure 14.
Surface plot of oil separation efficiency (%) vs. time and additive 2.
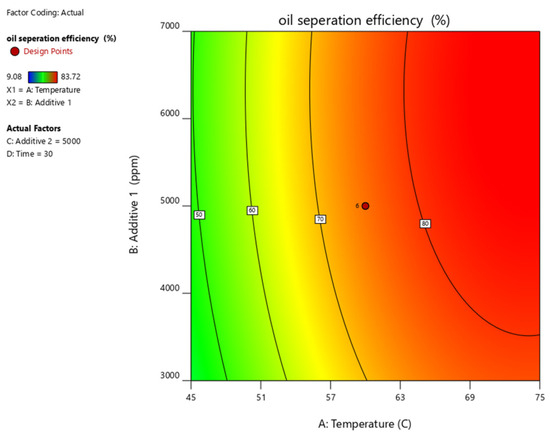
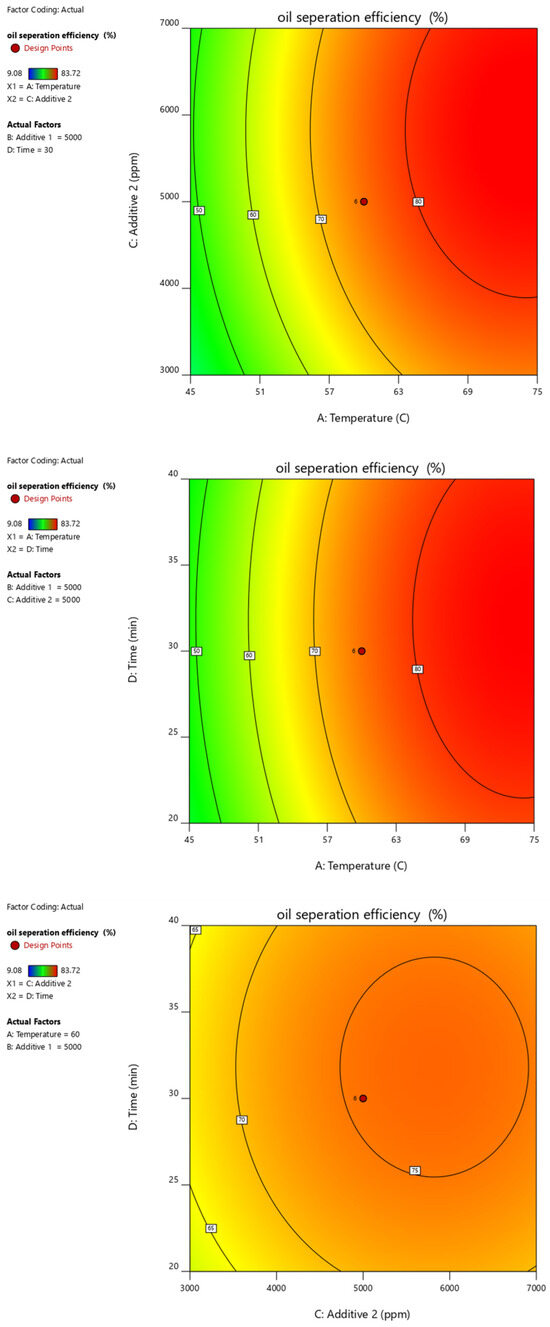
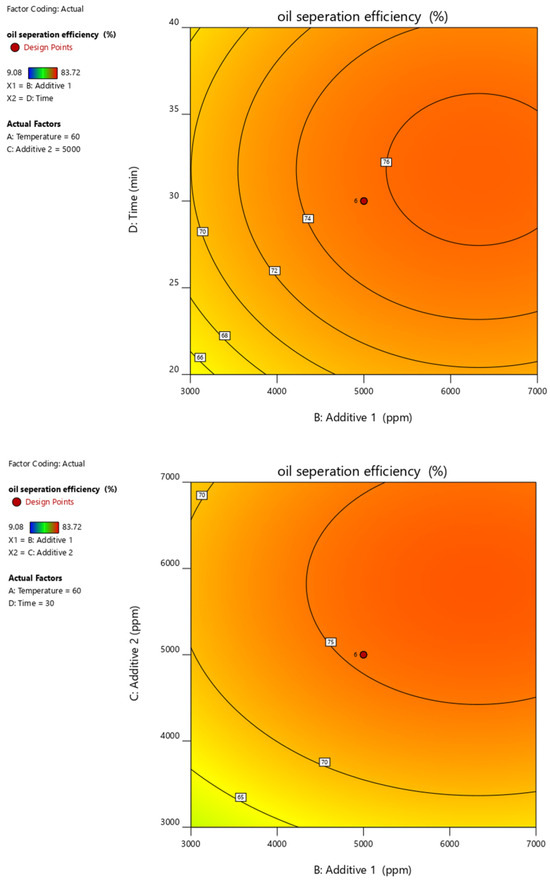
Figure 15.
Contour plots of oil separation efficiency (%) vs. temperature, time, additive 1 and additive 2 and their different combinations.
Based on experimental results, the software predicted the optimal sample conditions. The software generated seven solutions within specified ranges for temperature, additive 1, additive 2, and mixing time, with oil separation efficiency as the target (maximized between 9% and 100%). Option 1 was selected, achieving an oil separation efficiency of 85.759% (Table 10).

Table 10.
Best oil recovery rate according to the analysis provided by Design Expert V11 software.
The optimal sample was experimentally tested based on the software’s recommendations. The observed oil separation efficiency was 82.79%, exhibiting a 3.46% deviation from the software-predicted value of 85.76%.
The employed additives effectively separated a significant portion of oil from the COSW. This initial separation can be followed by further treatment steps, such as heating or three-phase decantation, to achieve higher oil recovery. While other coagulants and flocculants in combination with heat can be considered, thorough analysis of the resulting wastewater is crucial to assess and mitigate potential chemical hazards before discharge.
To minimize chemical usage, alternative separation methods can be explored, including coagulation followed by air flotation, decantation, filter press, or gravity separation. Heating can also enhance oil separation by reducing oil viscosity. Three-phase decantation can further improve this process by separating oil, water, and sludge into distinct streams.
A comprehensive evaluation of the environmental impact, cost-effectiveness, and potential hazards associated with each method is essential for selecting the most suitable approach. Future studies should focus on assessing these factors.
4. Conclusions
In this study, the separation of oil from cooking oil secondary waste collected from used cooking oil storage plants was studied. The waste is a complex mixture of oil, water, and solid particles. The goal of the study was to develop a method for separating the oil from the waste. The experiments successfully demonstrated the efficacy of coagulation and flocculation in separating cooking oil secondary waste (COSW). Through a series of preliminary experiments and the application of Design Expert software, optimized conditions for the process were established. The final model generated by the software accurately predicted the performance of the system, with experimental results closely aligning with the theoretical outcomes.
The optimized process, when applied to the optimum sample, achieved an impressive oil removal efficiency of 82.5%. This outcome underscores the potential of the proposed method for the efficient and sustainable treatment of UCO waste. Further research may focus on scaling up the process for industrial applications and exploring the economic viability of recovering valuable byproducts from the treated UCO.
Author Contributions
Conceptualization, M.S. and S.N.; methodology, M.S. and S.N.; software, M.S.; validation, S.N.; formal analysis, M.S.; investigation, M.S.; resources, M.S. and S.N.; data curation, M.S.; writing—original draft preparation, M.S. and S.N.; writing—review and editing, M.S. and S.N:; visualization, M.S.; supervision, S.N.; project administration, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Altın, R.; Cetinkaya, S.; Yücesu, H.S. The potential of using vegetable oil fuels as fuel for diesel engines. Energy Convers. Manag. 2001, 42, 529–538. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Dalai, A.K. Waste cooking oil an economical source for biodiesel: A review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Nakasaki, K.; Nagasaki, K.; Ariga, O. Degradation of fats during thermophilic composting of organic waste. Waste Manag. Res. 2004, 22, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.; Kucharska, K.; Kamiński, M. Ecological and health effects of lubricant oils emitted into the environment. Int. J. Environ. Res. Public Health 2019, 16, 3002. [Google Scholar] [CrossRef]
- Panadare, D.C. Applications of waste cooking oil other than biodiesel: A review. Iran. J. Chem. Eng. (IJChE) 2015, 12, 55–76. [Google Scholar]
- Abdullah, N.H.; Hasan, S.H.; Yusoff, N.R.M. Biodiesel production based on waste cooking oil (WCO). Int. J. Mater. Sci. Eng. 2013, 1, 94–99. [Google Scholar] [CrossRef]
- Mata, A.; Zhang, J.; Pridemore, J.; Johnson, K.; Holliday, N.; Helmstetter, A.; Lu, M. A Review of Grease Trap Waste Management in the US and the Upcycle as Feedstocks for Alternative Diesel Fuels. Environments 2024, 11, 159. [Google Scholar] [CrossRef]
- Forster, C. Oils, fats and greases in wastewater treatment. J. Chem. Technol. Biotechnol. 1992, 55, 402–404. [Google Scholar] [CrossRef]
- Xing, J.; Marques, M.; Li, L. Grease trap waste: A headache or a valuable energy source? In Proceedings of the Kalmar ECO-TECH’07, Kalmar, Sweden, 26–28 November 2007; pp. 389–400. [Google Scholar] [CrossRef]
- Hums, M.E.; Cairncross, R.A.; Spatari, S. Life-cycle assessment of biodiesel produced from grease trap waste. Environ. Sci. Technol. 2016, 50, 2718–2726. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Lopes, E.; Zepka, L.Q.; Deprá, M.C. Handbook of Waste Biorefinery; Springer Nature: Cham, Switzerland, 2022; ISBN 978-3-031-06561-3. [Google Scholar] [CrossRef]
- Van Der Veen, S. Dewatering and Recovery of Fats, Oils and Grease (FOG) of Grease Trap Waste: A Design-Research of a New-Built Process; Oulu University of Applied Sciences: Oulu, Finland, 2013. [Google Scholar]
- Klaucans, E.; Sams, K. Problems with fat, oil, and grease (FOG) in food industry wastewaters and recovered FOG recycling methods using anaerobic co-digestion: A short review. Key Eng. Mater. 2018, 762, 61–68. [Google Scholar] [CrossRef]
- Kolet, M.; Zerbib, D.; Nakonechny, F.; Nisnevitch, M. Production of biodiesel from brown grease. Catalysts 2020, 10, 1189. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kuramochi, H.; Xu, K.-Q. Variable oil properties and biomethane production of grease trap waste derived from different resources. Int. Biodeterior. Biodegradation 2017, 119, 273–281. [Google Scholar] [CrossRef]
- Zolezzi, L.; O’Hara, E. Brown Grease Recovery for Biodiesel; EPA: Honolulu, HI, USA, 2010. [Google Scholar]
- Wang, L. Anaerobic Co-Digestion of Thickened Waste Activated Sludge with Grease Interceptor Waste; North Carolina State University: Raleigh, NC, USA, 2012. [Google Scholar]
- Davidsson, Å.; Lövstedt, C.; la Cour Jansen, J.; Gruvberger, C.; Aspegren, H. Co-digestion of grease trap sludge and sewage sludge. Waste Manag. 2008, 6, 986–992. [Google Scholar] [CrossRef]
- Kongnoo, A.; Suksaroj, T.; Intharapat, P.; Promtong, T.; Suksaroj, C. Decolorization and organic removal from palm oil mill effluent by Fenton’s process. Environ. Eng. Sci. 2012, 29, 855–859. [Google Scholar] [CrossRef]
- Haug, R.T. The Practical Handbook of Compost Engineering; Routledge: Abingdon-on-Thames, UK, 2018. [Google Scholar]
- Omidvarborna, H.; Kumar, A.; Kim, D.-S. Recent studies on soot modeling for diesel combustion. Renew. Sustain. Energy Rev. 2015, 48, 635–647. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel Production, Properties, and Feedstocks; Springer: New York, NY, USA, 2011. [Google Scholar]
- Schumacher, L.G.; Peterson, C.L.; Van Gerpen, J. Engine oil analysis of biodiesel-fueled engines. Appl. Eng. Agric. 2005, 21, 153–158. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. npj Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Andersen, F.A. Amended final report on the safety assessment of polyacrylamide and acrylamide residues in cosmetics. Int. J. Toxicol. 2005, 24, 21–50. [Google Scholar]
- Guezennec, A.-G.; Michel, C.; Bru, K.; Touze, S.; Desroche, N.; Mnif, I.; Motelica-Heino, M. Transfer and degradation of polyacrylamide-based flocculants in hydrosystems: A review. Environ. Sci. Pollut. Res. 2015, 22, 6390–6406. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Schlautman, M.A. Effects of polymer molecular weight on adsorption and flocculation in aqueous kaolinite suspensions dosed with nonionic polyacrylamides. Water 2015, 7, 5896–5909. [Google Scholar] [CrossRef]
- Levy, G.J.; Warrington, N.W. Polyacrylamide Addition to Soils: Impacts on Soil Structure and Stability; Functional Polymers in Food Science; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Pei, Y.; Zhao, L.; Du, G.; Li, N.; Xu, K.; Yang, H. Investigation of the degradation and stability of acrylamide-based polymers in acid solution: Functional monomer modified polyacrylamide. Petroleum 2016, 2, 399–407. [Google Scholar] [CrossRef]
- Wong, S.S.; Teng, T.T.; Ahmad, A.L.; Zuhairi, A.; Najafpour, G. Treatment of pulp and paper mill wastewater by polyacrylamide (PAM) in polymer induced flocculation. J. Hazard. Mater. 2006, 135, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.I.; Sáez, J.; Lloréns, M.; Soler, A.; Ortuño, J.F.; Meseguer, V.; Fuentes, A. Improvement of coagulation–flocculation process using anionic polyacrylamide as coagulant aid. Chemosphere 2005, 58, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Tekin, N.; Demirbaş, Ö.; Alkan, M. Adsorption of cationic polyacrylamide onto kaolinite. Microporous Mesoporous Mater. 2005, 85, 340–350. [Google Scholar] [CrossRef]
- Mansri, A.; Bouras, B.; Tennouga, L.; Clisson, G.; Grassl, B. Hydrophobic and electrostatic interactions in the mixture hydrolyzed polyacrylamide–N-dodecylpyridinium chloride (AD37–DPC) in aqueous solution. Res. Chem. Intermed. 2014, 40, 269–279. [Google Scholar] [CrossRef]
- Mansri, A.; Bendraoua, A.; Benmoussa, A.; Benhabib, K. New polyacrylamide [PAM] material formulations for the coagulation/flocculation/decantation process. J. Polym. Environ. 2015, 23, 580–587. [Google Scholar] [CrossRef]
- Haveroen, M.E.; MacKinnon, M.D.; Fedorak, P.M. Polyacrylamide added as a nitrogen source stimulates methanogenesis in consortia from various wastewaters. Water Res. 2005, 39, 3333–3341. [Google Scholar] [CrossRef]
- Wen, Q.; Chen, Z.; Zhao, Y.; Zhang, H.; Feng, Y. Biodegradation of polyacrylamide by bacteria isolated from activated sludge and oil-contaminated soil. J. Hazard. Mater. 2010, 175, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Sang, G.; Pi, Y.; Bao, M.; Li, Y.; Lu, J. Biodegradation for hydrolyzed polyacrylamide in the anaerobic baffled reactor combined aeration tank. Ecol. Eng. 2015, 84, 121–127. [Google Scholar] [CrossRef]
- Zhao, J.; Xue, Y.; Qiu, R.; Guo, W.; Fan, L.; Wang, P. Superoleophilic Ulva prolifera for oil/water separation: A repayment from the green tide. Chem. Eng. J. 2016, 292, 147–155. [Google Scholar] [CrossRef]
- Smith, E.A.; Prues, S.L.; Oehme, F.W. Environmental degradation of polyacrylamides. Ecotoxicol. Environ. Saf. 1997, 37, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, M.J.; Hao, X.; Qiao, G.G.; Solomon, D.H. Degradation on polyacrylamides. Part II. Polyacrylamide gels. Polymer 2003, 44, 3817–3826. [Google Scholar] [CrossRef]
- Caulfield, M.J.; Hao, X.; Qiao, G.G.; Solomon, D.H. Degradation on polyacrylamides. Part I. Linear polyacrylamide. Polymer 2003, 44, 1331–1337. [Google Scholar] [CrossRef]
- Lee, W.-C.; Chang, C.-C. Effectively Recycling Swine Wastewater by Coagulation–Flocculation of Nonionic Polyacrylamide. Sustainability 2022, 14, 1742. [Google Scholar] [CrossRef]
- ISIRI 4778; Microbiological Test Methods and—Properties of Tissues. Iranian Standards and Industrial Research Institute—ISIRI: Tehran, Iran.
- ISIRI 10501; Determination of Sponification Value of Animal and Vegetable Fats and Oils—Test Method. Iranian Standards and Industrial Research Institute—ISIRI: Tehran, Iran.
- ISIRI 19052; Cereals and Legumes Measurement of Nitrogen Content and Calculation of the Amount of Crude Protein—Kjeldahl Method. Iranian Standards and Industrial Research Institute—ISIRI: Tehran, Iran.
- ISIRI 10741-1; Determination of Phosphorus Content in Animal and Vegetable Fats and Oils. Iranian Standards and Industrial Research Institute—ISIRI: Tehran, Iran.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).