Developing a Chemical Process for Optimizing Oil Extraction from Cooking Oil Secondary Waste
Abstract
1. Introduction
2. Materials and Methods
- Preparation of COSW and additives;
- Additive utilization to separate oil from the COSW.
- Mixing time;
- Temperature;
- PAM content (concentration);
- Aluminum sulfate content.
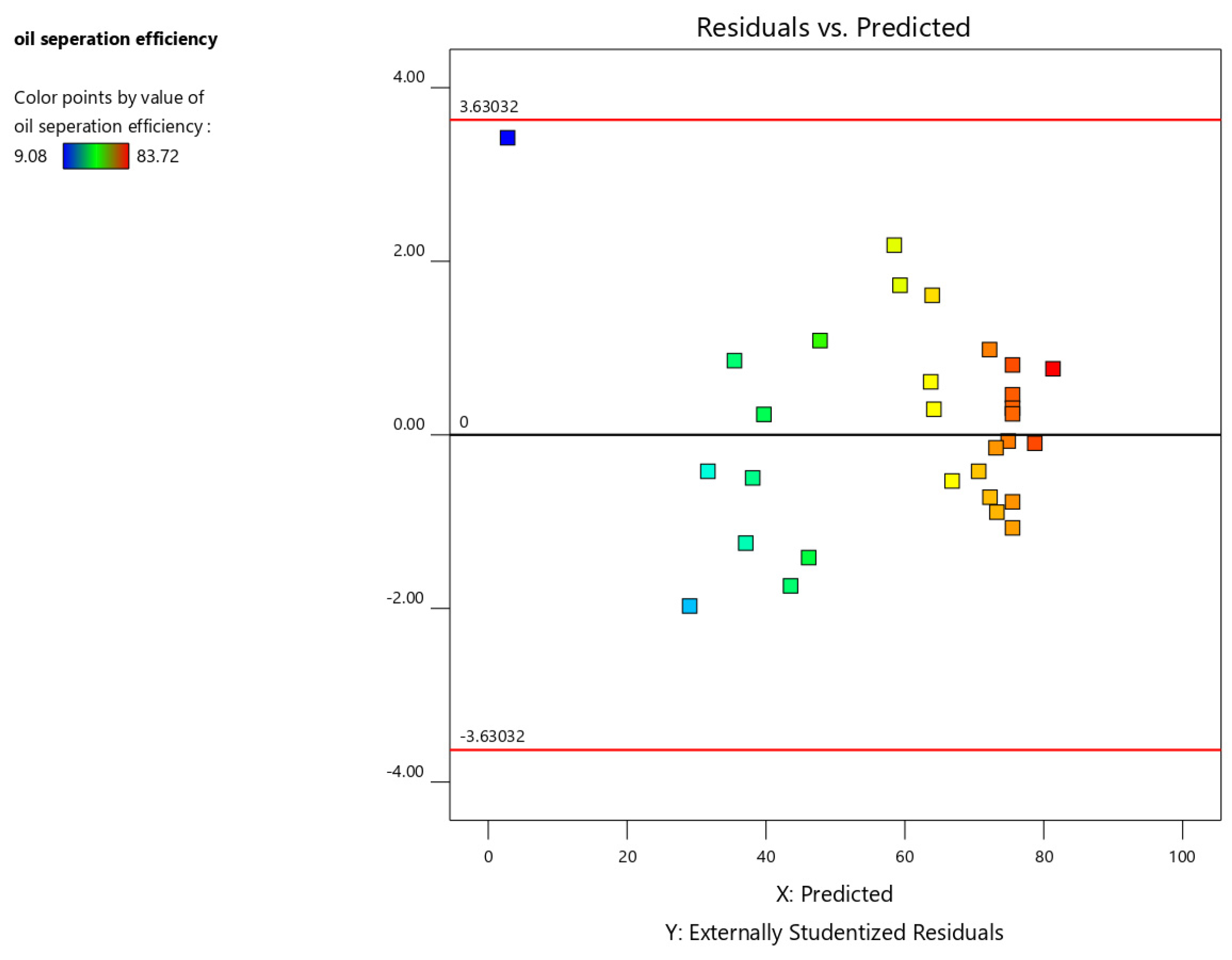
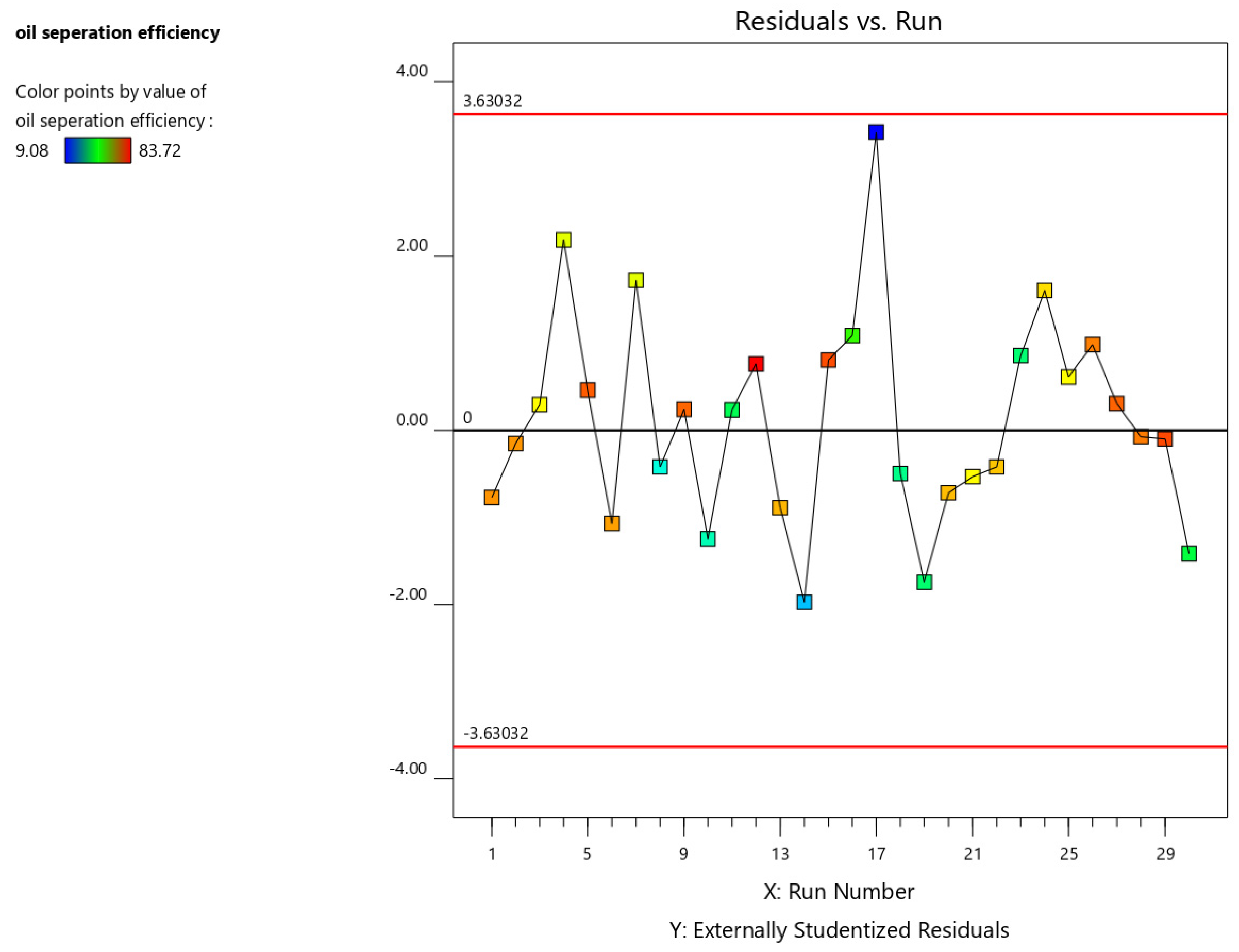
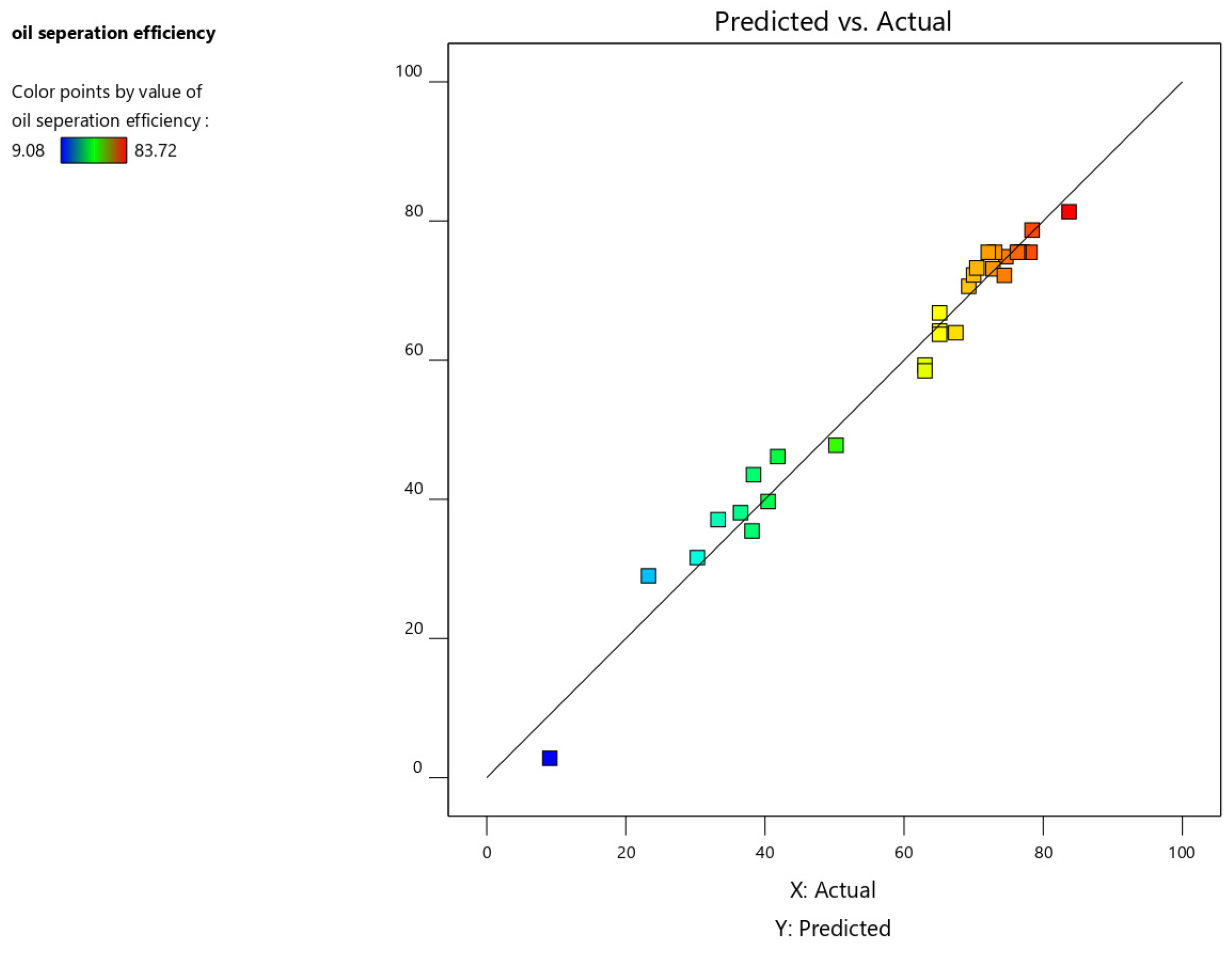
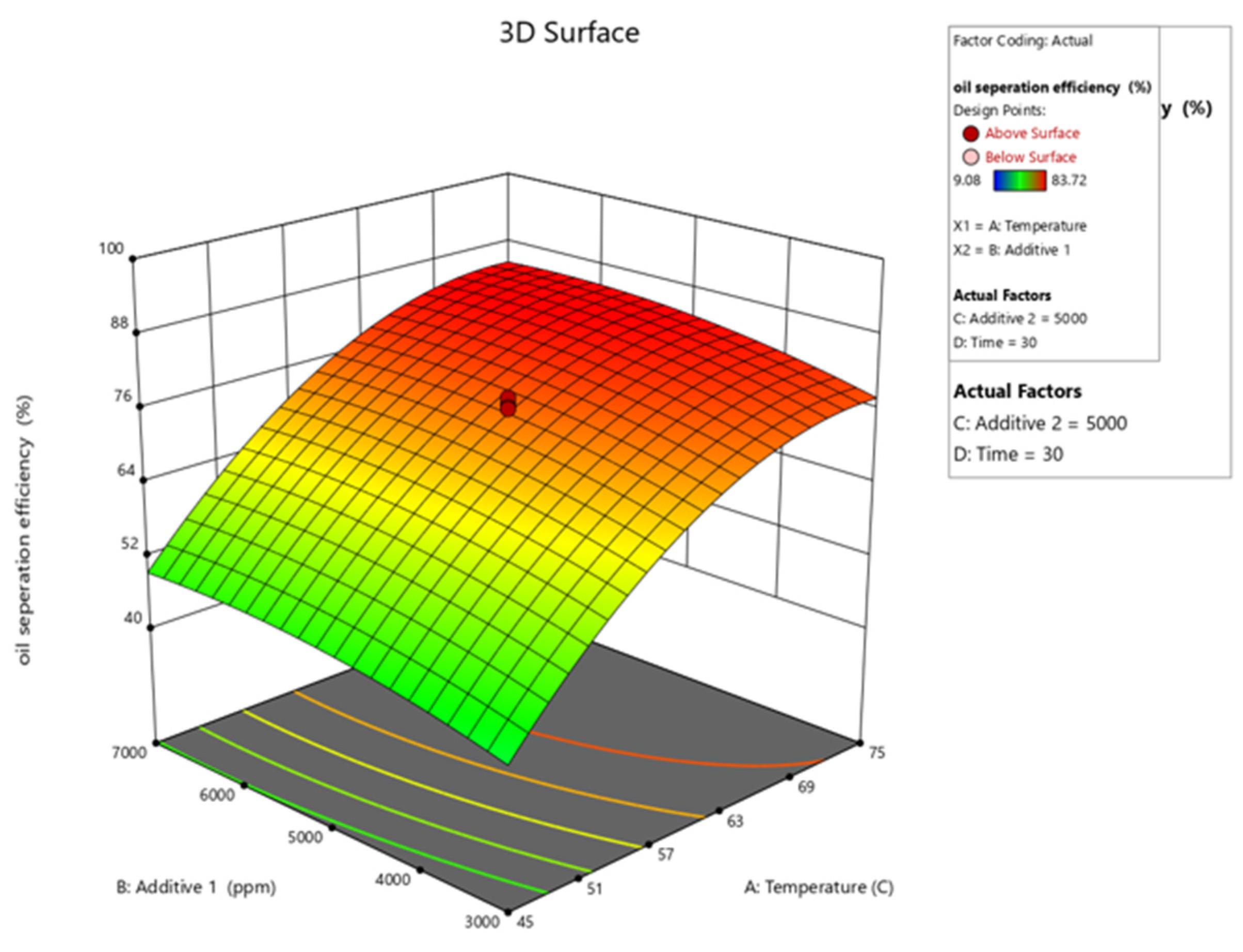
3. Result and Discussion
- Cationic PAM enhanced recovery to 88.6%.
- Anionic PAM achieved a recovery rate of 43%.
Regression Model Equation and ANOVA
−245.44604 + 6.17800 × (Temp.) + 0.007711 × (PAM) + 0.014296 × (Alum) + 2.29213
× (Time) − 0.041714 [(Temp.)]2 − 6.09844 × 10−7 × [(PAM)]2 − 1.22766 × 10−6 × [(Alum)]2
− 0.036019 × (Time)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Altın, R.; Cetinkaya, S.; Yücesu, H.S. The potential of using vegetable oil fuels as fuel for diesel engines. Energy Convers. Manag. 2001, 42, 529–538. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Dalai, A.K. Waste cooking oil an economical source for biodiesel: A review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Nakasaki, K.; Nagasaki, K.; Ariga, O. Degradation of fats during thermophilic composting of organic waste. Waste Manag. Res. 2004, 22, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.; Kucharska, K.; Kamiński, M. Ecological and health effects of lubricant oils emitted into the environment. Int. J. Environ. Res. Public Health 2019, 16, 3002. [Google Scholar] [CrossRef]
- Panadare, D.C. Applications of waste cooking oil other than biodiesel: A review. Iran. J. Chem. Eng. (IJChE) 2015, 12, 55–76. [Google Scholar]
- Abdullah, N.H.; Hasan, S.H.; Yusoff, N.R.M. Biodiesel production based on waste cooking oil (WCO). Int. J. Mater. Sci. Eng. 2013, 1, 94–99. [Google Scholar] [CrossRef]
- Mata, A.; Zhang, J.; Pridemore, J.; Johnson, K.; Holliday, N.; Helmstetter, A.; Lu, M. A Review of Grease Trap Waste Management in the US and the Upcycle as Feedstocks for Alternative Diesel Fuels. Environments 2024, 11, 159. [Google Scholar] [CrossRef]
- Forster, C. Oils, fats and greases in wastewater treatment. J. Chem. Technol. Biotechnol. 1992, 55, 402–404. [Google Scholar] [CrossRef]
- Xing, J.; Marques, M.; Li, L. Grease trap waste: A headache or a valuable energy source? In Proceedings of the Kalmar ECO-TECH’07, Kalmar, Sweden, 26–28 November 2007; pp. 389–400. [Google Scholar] [CrossRef]
- Hums, M.E.; Cairncross, R.A.; Spatari, S. Life-cycle assessment of biodiesel produced from grease trap waste. Environ. Sci. Technol. 2016, 50, 2718–2726. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Lopes, E.; Zepka, L.Q.; Deprá, M.C. Handbook of Waste Biorefinery; Springer Nature: Cham, Switzerland, 2022; ISBN 978-3-031-06561-3. [Google Scholar] [CrossRef]
- Van Der Veen, S. Dewatering and Recovery of Fats, Oils and Grease (FOG) of Grease Trap Waste: A Design-Research of a New-Built Process; Oulu University of Applied Sciences: Oulu, Finland, 2013. [Google Scholar]
- Klaucans, E.; Sams, K. Problems with fat, oil, and grease (FOG) in food industry wastewaters and recovered FOG recycling methods using anaerobic co-digestion: A short review. Key Eng. Mater. 2018, 762, 61–68. [Google Scholar] [CrossRef]
- Kolet, M.; Zerbib, D.; Nakonechny, F.; Nisnevitch, M. Production of biodiesel from brown grease. Catalysts 2020, 10, 1189. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kuramochi, H.; Xu, K.-Q. Variable oil properties and biomethane production of grease trap waste derived from different resources. Int. Biodeterior. Biodegradation 2017, 119, 273–281. [Google Scholar] [CrossRef]
- Zolezzi, L.; O’Hara, E. Brown Grease Recovery for Biodiesel; EPA: Honolulu, HI, USA, 2010. [Google Scholar]
- Wang, L. Anaerobic Co-Digestion of Thickened Waste Activated Sludge with Grease Interceptor Waste; North Carolina State University: Raleigh, NC, USA, 2012. [Google Scholar]
- Davidsson, Å.; Lövstedt, C.; la Cour Jansen, J.; Gruvberger, C.; Aspegren, H. Co-digestion of grease trap sludge and sewage sludge. Waste Manag. 2008, 6, 986–992. [Google Scholar] [CrossRef]
- Kongnoo, A.; Suksaroj, T.; Intharapat, P.; Promtong, T.; Suksaroj, C. Decolorization and organic removal from palm oil mill effluent by Fenton’s process. Environ. Eng. Sci. 2012, 29, 855–859. [Google Scholar] [CrossRef]
- Haug, R.T. The Practical Handbook of Compost Engineering; Routledge: Abingdon-on-Thames, UK, 2018. [Google Scholar]
- Omidvarborna, H.; Kumar, A.; Kim, D.-S. Recent studies on soot modeling for diesel combustion. Renew. Sustain. Energy Rev. 2015, 48, 635–647. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel Production, Properties, and Feedstocks; Springer: New York, NY, USA, 2011. [Google Scholar]
- Schumacher, L.G.; Peterson, C.L.; Van Gerpen, J. Engine oil analysis of biodiesel-fueled engines. Appl. Eng. Agric. 2005, 21, 153–158. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. npj Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Andersen, F.A. Amended final report on the safety assessment of polyacrylamide and acrylamide residues in cosmetics. Int. J. Toxicol. 2005, 24, 21–50. [Google Scholar]
- Guezennec, A.-G.; Michel, C.; Bru, K.; Touze, S.; Desroche, N.; Mnif, I.; Motelica-Heino, M. Transfer and degradation of polyacrylamide-based flocculants in hydrosystems: A review. Environ. Sci. Pollut. Res. 2015, 22, 6390–6406. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Schlautman, M.A. Effects of polymer molecular weight on adsorption and flocculation in aqueous kaolinite suspensions dosed with nonionic polyacrylamides. Water 2015, 7, 5896–5909. [Google Scholar] [CrossRef]
- Levy, G.J.; Warrington, N.W. Polyacrylamide Addition to Soils: Impacts on Soil Structure and Stability; Functional Polymers in Food Science; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Pei, Y.; Zhao, L.; Du, G.; Li, N.; Xu, K.; Yang, H. Investigation of the degradation and stability of acrylamide-based polymers in acid solution: Functional monomer modified polyacrylamide. Petroleum 2016, 2, 399–407. [Google Scholar] [CrossRef]
- Wong, S.S.; Teng, T.T.; Ahmad, A.L.; Zuhairi, A.; Najafpour, G. Treatment of pulp and paper mill wastewater by polyacrylamide (PAM) in polymer induced flocculation. J. Hazard. Mater. 2006, 135, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.I.; Sáez, J.; Lloréns, M.; Soler, A.; Ortuño, J.F.; Meseguer, V.; Fuentes, A. Improvement of coagulation–flocculation process using anionic polyacrylamide as coagulant aid. Chemosphere 2005, 58, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Tekin, N.; Demirbaş, Ö.; Alkan, M. Adsorption of cationic polyacrylamide onto kaolinite. Microporous Mesoporous Mater. 2005, 85, 340–350. [Google Scholar] [CrossRef]
- Mansri, A.; Bouras, B.; Tennouga, L.; Clisson, G.; Grassl, B. Hydrophobic and electrostatic interactions in the mixture hydrolyzed polyacrylamide–N-dodecylpyridinium chloride (AD37–DPC) in aqueous solution. Res. Chem. Intermed. 2014, 40, 269–279. [Google Scholar] [CrossRef]
- Mansri, A.; Bendraoua, A.; Benmoussa, A.; Benhabib, K. New polyacrylamide [PAM] material formulations for the coagulation/flocculation/decantation process. J. Polym. Environ. 2015, 23, 580–587. [Google Scholar] [CrossRef]
- Haveroen, M.E.; MacKinnon, M.D.; Fedorak, P.M. Polyacrylamide added as a nitrogen source stimulates methanogenesis in consortia from various wastewaters. Water Res. 2005, 39, 3333–3341. [Google Scholar] [CrossRef]
- Wen, Q.; Chen, Z.; Zhao, Y.; Zhang, H.; Feng, Y. Biodegradation of polyacrylamide by bacteria isolated from activated sludge and oil-contaminated soil. J. Hazard. Mater. 2010, 175, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Sang, G.; Pi, Y.; Bao, M.; Li, Y.; Lu, J. Biodegradation for hydrolyzed polyacrylamide in the anaerobic baffled reactor combined aeration tank. Ecol. Eng. 2015, 84, 121–127. [Google Scholar] [CrossRef]
- Zhao, J.; Xue, Y.; Qiu, R.; Guo, W.; Fan, L.; Wang, P. Superoleophilic Ulva prolifera for oil/water separation: A repayment from the green tide. Chem. Eng. J. 2016, 292, 147–155. [Google Scholar] [CrossRef]
- Smith, E.A.; Prues, S.L.; Oehme, F.W. Environmental degradation of polyacrylamides. Ecotoxicol. Environ. Saf. 1997, 37, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, M.J.; Hao, X.; Qiao, G.G.; Solomon, D.H. Degradation on polyacrylamides. Part II. Polyacrylamide gels. Polymer 2003, 44, 3817–3826. [Google Scholar] [CrossRef]
- Caulfield, M.J.; Hao, X.; Qiao, G.G.; Solomon, D.H. Degradation on polyacrylamides. Part I. Linear polyacrylamide. Polymer 2003, 44, 1331–1337. [Google Scholar] [CrossRef]
- Lee, W.-C.; Chang, C.-C. Effectively Recycling Swine Wastewater by Coagulation–Flocculation of Nonionic Polyacrylamide. Sustainability 2022, 14, 1742. [Google Scholar] [CrossRef]
- ISIRI 4778; Microbiological Test Methods and—Properties of Tissues. Iranian Standards and Industrial Research Institute—ISIRI: Tehran, Iran.
- ISIRI 10501; Determination of Sponification Value of Animal and Vegetable Fats and Oils—Test Method. Iranian Standards and Industrial Research Institute—ISIRI: Tehran, Iran.
- ISIRI 19052; Cereals and Legumes Measurement of Nitrogen Content and Calculation of the Amount of Crude Protein—Kjeldahl Method. Iranian Standards and Industrial Research Institute—ISIRI: Tehran, Iran.
- ISIRI 10741-1; Determination of Phosphorus Content in Animal and Vegetable Fats and Oils. Iranian Standards and Industrial Research Institute—ISIRI: Tehran, Iran.








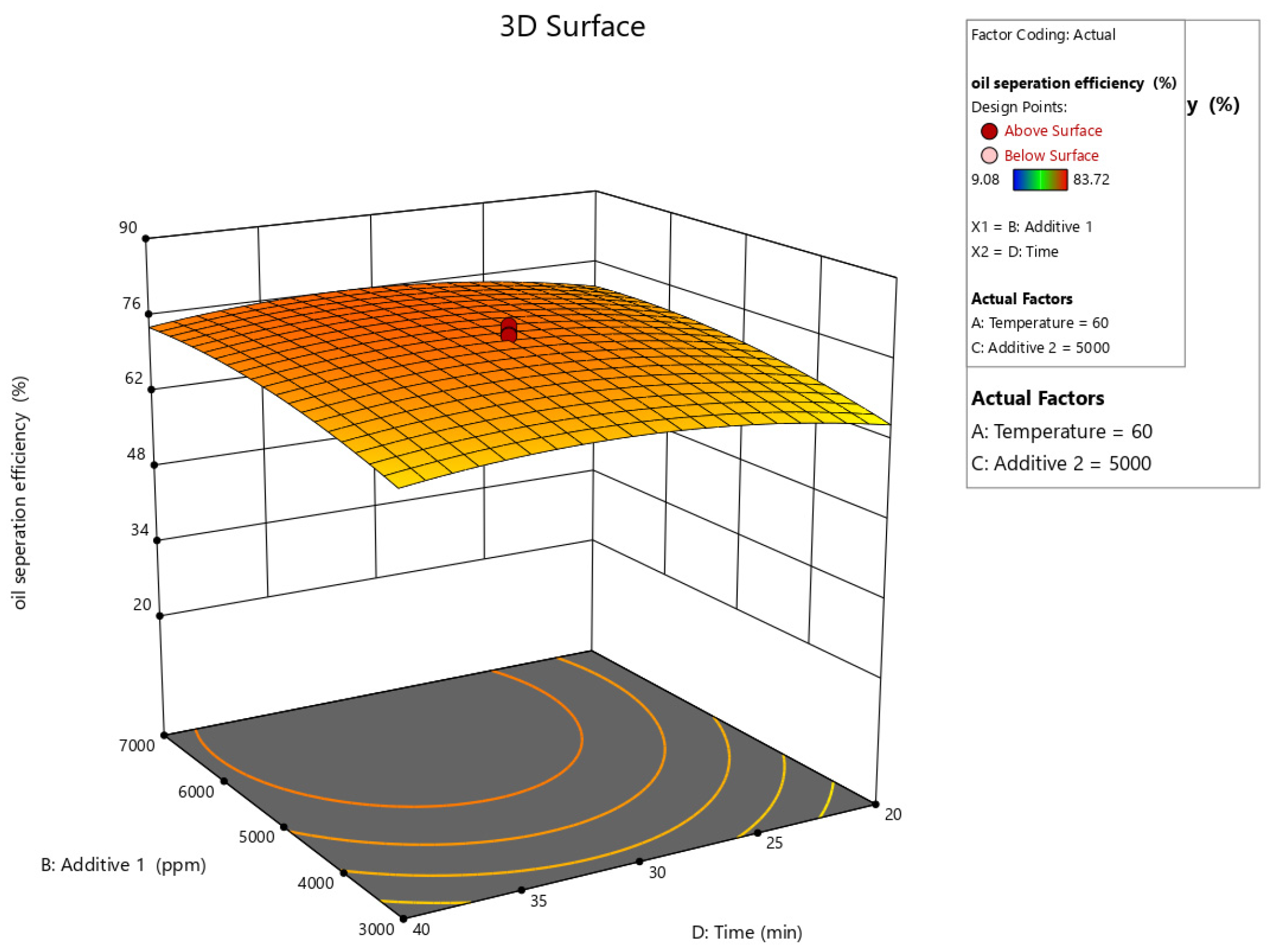
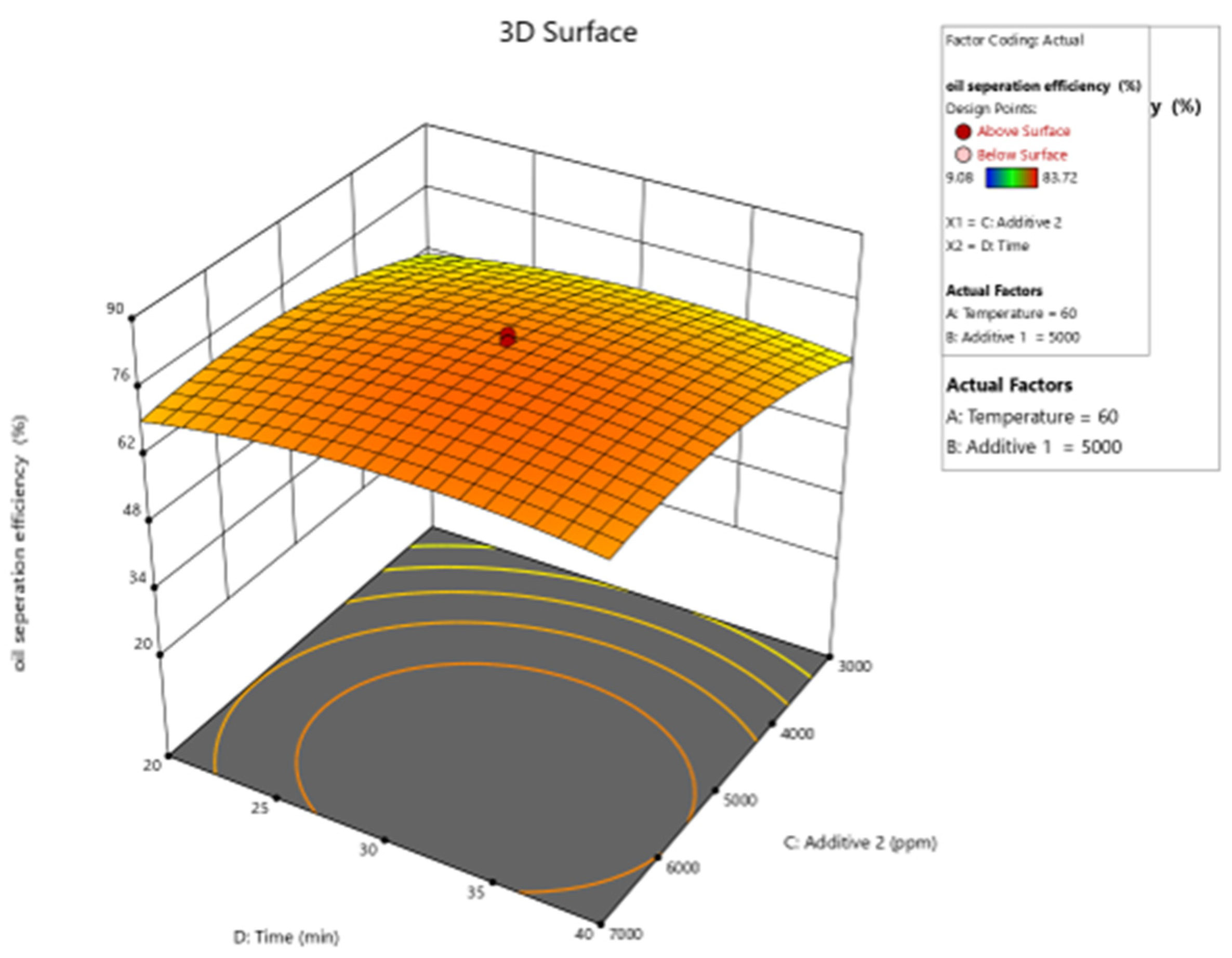
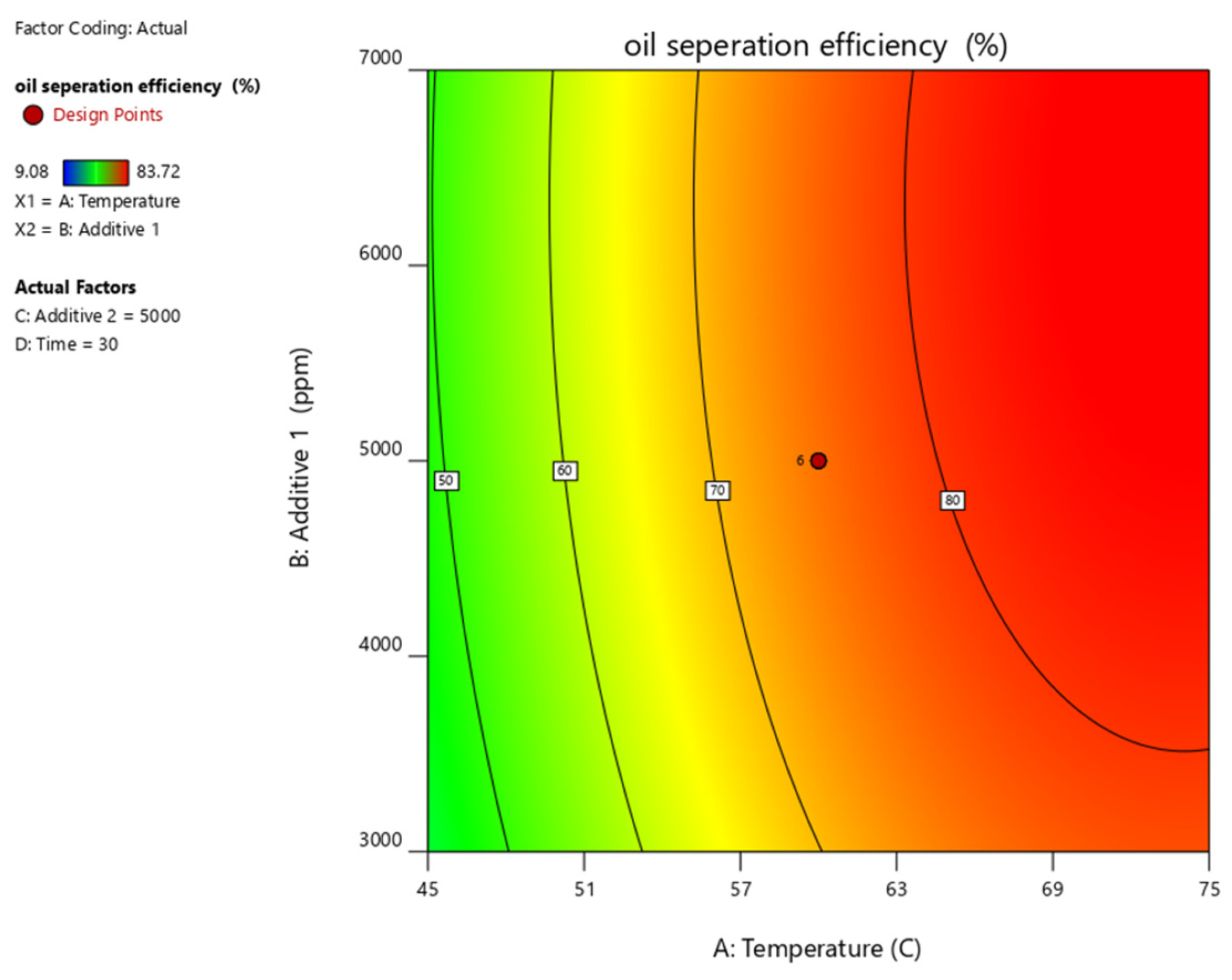
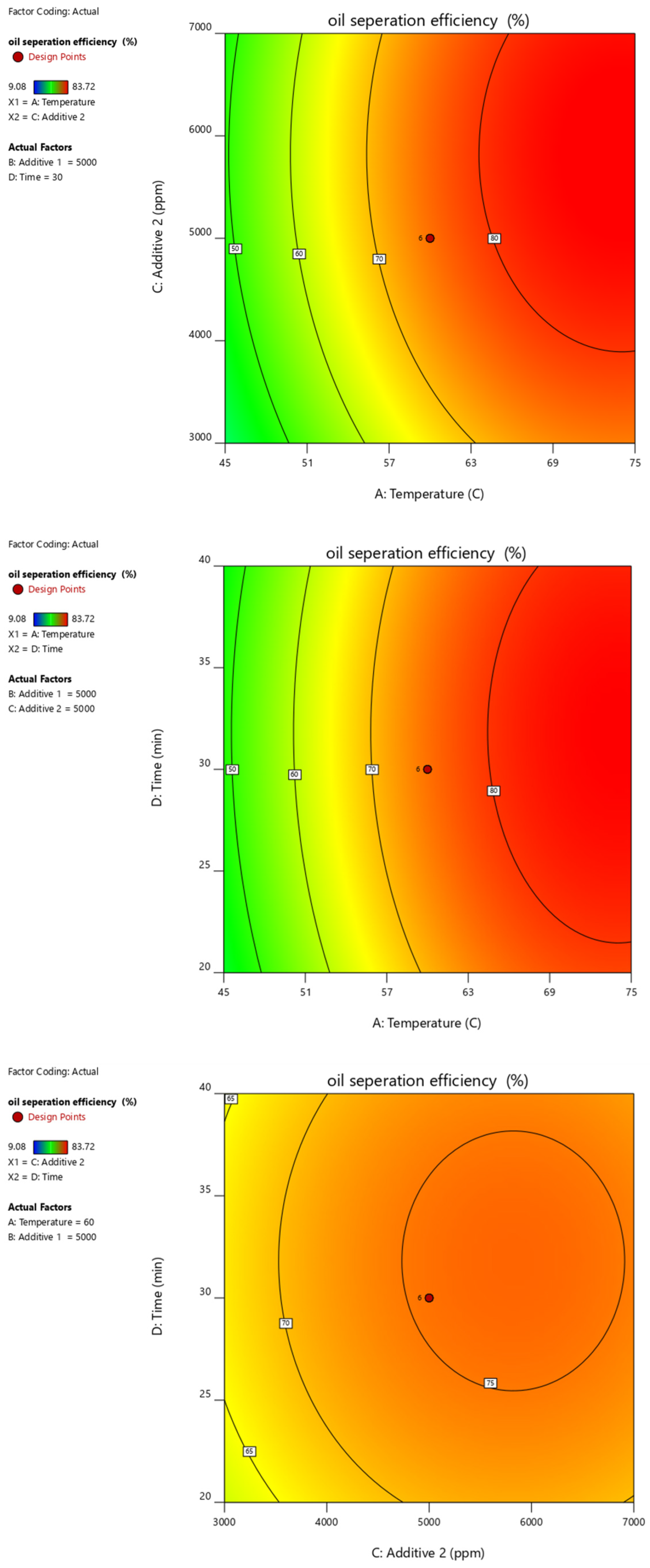
| Parameter | Units | Min. | Max. | Coded Low | Coded High | Mean |
|---|---|---|---|---|---|---|
| Temperature | °C | 30 | 90 | −1↔45 | +1↔75 | 60 |
| PAM content | ppm | 1000 | 9000 | −1↔3000 | +1↔7000 | 5000 |
| Alum content | ppm | 1000 | 9000 | −1↔3000 | +1↔7000 | 5000 |
| Time | Min. | 10 | 50 | −1↔20 | +1↔40 | 30 |
| Factor | Symbol | Unit | Selected Levels | ||||
|---|---|---|---|---|---|---|---|
| Mixing time | MT | min | 10 | 20 | 30 | 40 | 50 |
| Temperature | T | °C | 30 | 45 | 60 | 75 | 90 |
| PAM content | PC | ppm | 1000 | 3000 | 5000 | 7000 | 9000 |
| Aluminum sulfate content | AS | ppm | 1000 | 3000 | 5000 | 7000 | 9000 |
| Run | Mixing Time (min) | Temperature (°C) | PAM Content (ppm) | Aluminum Sulfate Content (ppm) |
|---|---|---|---|---|
| 1 | 30 | 60 | 5000 | 5000 |
| 2 | 30 | 90 | 5000 | 5000 |
| 3 | 20 | 75 | 3000 | 3000 |
| 4 | 10 | 60 | 5000 | 5000 |
| 5 | 30 | 60 | 5000 | 5000 |
| 6 | 30 | 60 | 5000 | 5000 |
| 7 | 30 | 60 | 1000 | 5000 |
| 8 | 40 | 45 | 3000 | 3000 |
| 9 | 30 | 60 | 5000 | 5000 |
| 10 | 20 | 45 | 3000 | 7000 |
| 11 | 40 | 45 | 3000 | 7000 |
| 12 | 40 | 75 | 7000 | 7000 |
| 13 | 40 | 75 | 7000 | 3000 |
| 14 | 20 | 45 | 3000 | 3000 |
| 15 | 30 | 60 | 5000 | 5000 |
| 16 | 30 | 60 | 5000 | 1000 |
| 17 | 30 | 30 | 5000 | 5000 |
| 18 | 40 | 45 | 7000 | 3000 |
| 19 | 20 | 45 | 7000 | 7000 |
| 20 | 20 | 75 | 3000 | 7000 |
| 21 | 40 | 75 | 3000 | 3000 |
| 22 | 20 | 75 | 7000 | 3000 |
| 23 | 20 | 45 | 7000 | 3000 |
| 24 | 30 | 60 | 5000 | 9000 |
| 25 | 50 | 60 | 5000 | 5000 |
| 26 | 30 | 60 | 9000 | 5000 |
| 27 | 30 | 60 | 5000 | 5000 |
| 28 | 40 | 75 | 3000 | 7000 |
| 29 | 20 | 75 | 7000 | 7000 |
| 30 | 40 | 45 | 7000 | 7000 |
| Test | Standard | Unit | Result |
|---|---|---|---|
| Total Fatty Matter | In House | % | 53 |
| Free fatty acid (as Lauric acid) | ISIRI 4778 [46] | g/100 g | 1.1 |
| Saponification value | ISIRI 10501 [47] | - | 160.5 |
| Color | - | - | Brown |
| Nitrogen | ISIRI 19052 [48] | % | 0.35 |
| Potassium | In House | mg/kg | 278.5 |
| Phosphorus | ISIRI 10741-1 [49] | % | 0.04 |
| Density | In House | g/ml | 0.99 |
| Viscosity | In House | cps | 441.7 |
| No. | Additive No. 1 Content | Stirring Time (min) | Retention Time (min) | Additive No. 2 Content | Stirring Speed (rpm) | Recovery Yield (%) |
|---|---|---|---|---|---|---|
| 1 | None | 30 | 180 | - | 700 | 15.00% |
| 2 | 400 ppm Cationic PAM | 30 | 180 | - | 700 | 23.71% |
| 3 | 1600 ppm Cationic PAM | 30 | 180 | - | 700 | 39.63% |
| 3 (repeated) | 1600 ppm Cationic PAM | 30 | 180 | - | 700 | 40.79% |
| 4 | 6667 ppm Cationic PAM | 30 | 180 | - | 700 | 73.11% |
| 4 (repeated) | 6667 ppm Cationic PAM | 30 | 180 | - | 700 | 72.30% |
| 5 | 200 mg without water Cationic PAM | 30 | 180 | - | 700 | 49.84% |
| 6 | 6667 ppm Anionic PAM | 30 | 180 | - | 700 | 43.00% |
| 7 | 400 ppm Cationic PAM | 30 | 180 | 4800 ppm | 200 | 28.30% |
| 8 | 1600 ppm Cationic PAM | 30 | 180 | 4800 ppm | 500 | 47.21% |
| 8 (repeated) | 1600 ppm Cationic PAM | 30 | 180 | 4800 ppm | 500 | 44.88% |
| 9 | 6667 ppm Cationic PAM | 30 | 180 | 4800 ppm | 700 | 78.50% |
| 10 | 6667 ppm Cationic PAM | 30 | 180 | - | 500 | 61.50% |
| 11 | 6667 ppm Cationic PAM | 40 | 180 | - | 700 | 66.11% |
| 12 | 6667 ppm Cationic PAM | 20 | 180 | - | 700 | 70.20% |
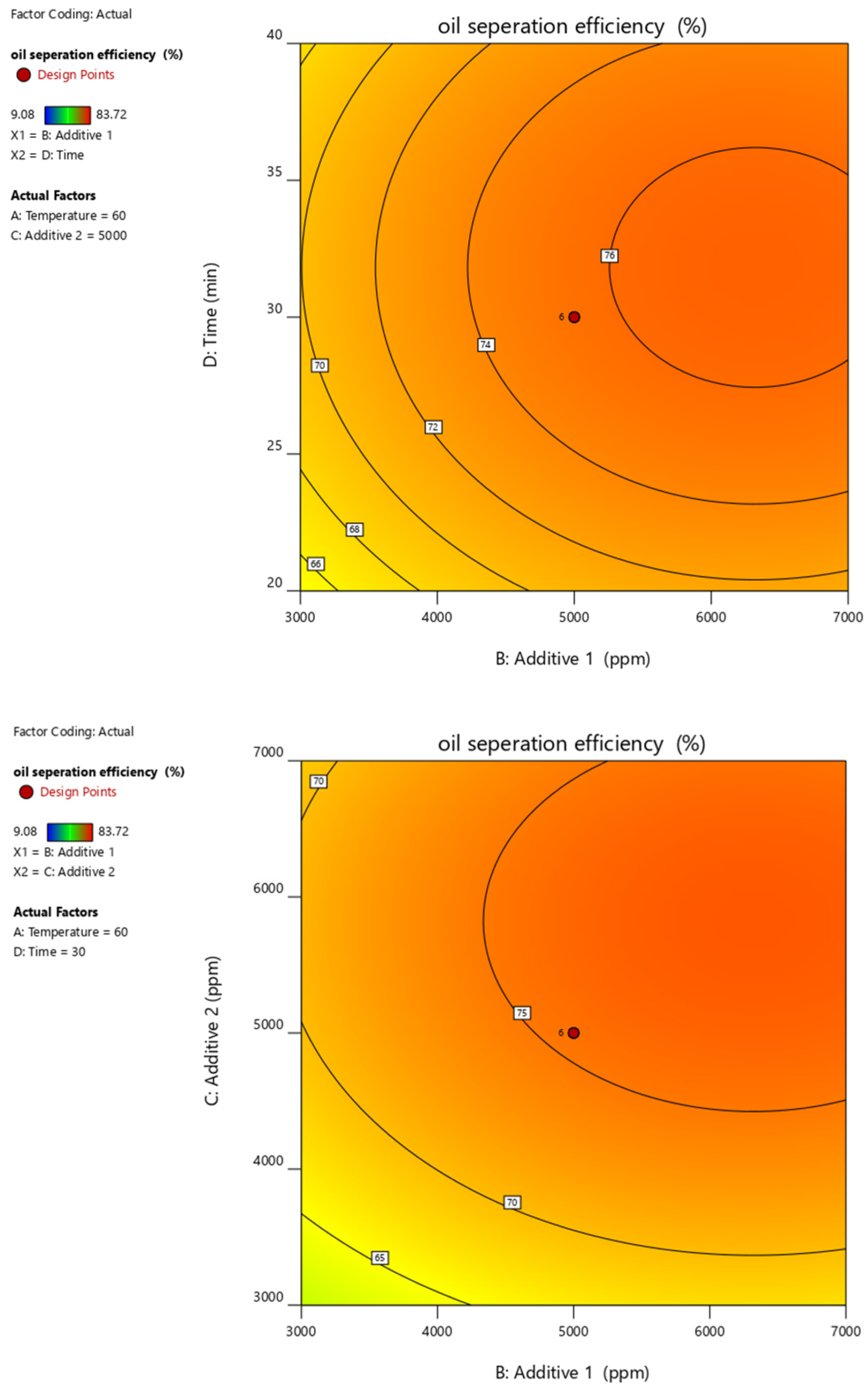
| Run | MT (min) (Xa) | T (°C) (Xb) | PC (ppm) (Xc) | AS (ppm) (Xd) | Recovery Rate (%) (Y) |
|---|---|---|---|---|---|
| 1 | 30 | 60 | 5000 | 5000 | 73.02 |
| 2 | 30 | 90 | 5000 | 5000 | 72.79 |
| 3 | 20 | 75 | 3000 | 3000 | 65.12 |
| 4 | 10 | 60 | 5000 | 5000 | 63.02 |
| 5 | 30 | 60 | 5000 | 5000 | 77.01 |
| 6 | 30 | 60 | 5000 | 5000 | 72.10 |
| 7 | 30 | 60 | 1000 | 5000 | 63.02 |
| 8 | 40 | 45 | 3000 | 3000 | 30.30 |
| 9 | 30 | 60 | 5000 | 5000 | 76.30 |
| 10 | 20 | 45 | 3000 | 7000 | 33.26 |
| 11 | 40 | 45 | 3000 | 7000 | 40.46 |
| 12 | 40 | 75 | 7000 | 7000 | 83.72 |
| 13 | 40 | 75 | 7000 | 3000 | 70.47 |
| 14 | 20 | 45 | 3000 | 3000 | 23.26 |
| 15 | 30 | 60 | 5000 | 5000 | 78.10 |
| 16 | 30 | 60 | 5000 | 1000 | 50.23 |
| 17 | 30 | 30 | 5000 | 5000 | 9.08 |
| 18 | 40 | 45 | 7000 | 3000 | 36.51 |
| 19 | 20 | 45 | 7000 | 7000 | 38.37 |
| 20 | 20 | 75 | 3000 | 7000 | 70.00 |
| 21 | 40 | 75 | 3000 | 3000 | 65.12 |
| 22 | 20 | 75 | 7000 | 3000 | 69.30 |
| 23 | 20 | 45 | 7000 | 3000 | 38.14 |
| 24 | 30 | 60 | 5000 | 9000 | 67.44 |
| 25 | 50 | 60 | 5000 | 5000 | 65.12 |
| 26 | 30 | 60 | 9000 | 5000 | 74.42 |
| 27 | 30 | 60 | 5000 | 5000 | 76.51 |
| 28 | 40 | 75 | 3000 | 7000 | 74.65 |
| 29 | 20 | 75 | 7000 | 7000 | 78.40 |
| 30 | 40 | 45 | 7000 | 7000 | 41.86 |
| Source | Sequential p-Value | Lack of Fit p-Value | Adjusted R2 | Predicted R2 | |
|---|---|---|---|---|---|
| Linear | <0.0001 | 0.0008 | 0.6769 | 0.6356 | |
| 2FI | 0.9998 | 0.0004 | 0.5791 | 0.4433 | |
| Quadratic | <0.0001 | 0.0912 | 0.9612 | 0.8953 | Suggested |
| Cubic | 0.7738 | 0.0199 | 0.9498 | −0.3852 | Aliased |
| Source | Sum of Squares | DF | Mean Square | F Value | p-Value (Prob > F) | |
|---|---|---|---|---|---|---|
| Model | 10,976.31 | 8 | 1372.04 | 112.26 | <0.0001 | Significant |
| A—Temperature | 7421.57 | 1 | 7421.57 | 607.23 | <0.0001 | |
| B—PAM | 249.61 | 1 | 249.61 | 20.42 | 0.0002 | |
| C—Alum | 391.40 | 1 | 391.40 | 32.02 | <0.0001 | |
| D—Time | 41.19 | 1 | 41.19 | 3.37 | 0.0806 | |
| A2 | 2416.18 | 1 | 2416.18 | 197.69 | <0.0001 | |
| B2 | 163.22 | 1 | 163.22 | 13.35 | 0.0015 | |
| C2 | 661.42 | 1 | 661.42 | 54.12 | <0.0001 | |
| D2 | 355.84 | 1 | 355.84 | 29.11 | <0.0001 | |
| Residual | 256.66 | 21 | 12.22 | |||
| Lack of fit | 228.25 | 16 | 14.27 | 2.51 | 0.1572 | Not significant |
| Pure error | 28.41 | 5 | 5.68 | |||
| Cor total | 11,232.97 |
| Oil Separation Efficiency | = |
|---|---|
| −245.44604 | |
| +6.17800 | * Temperature |
| +0.007711 | * PAM |
| +0.014296 | * Alum |
| +2.29213 | * Time |
| −0.041714 | * Temperature2 |
| −6.09844 × 10−7 | * PAM2 |
| −1.22766 × 10−6 | * Alum2 |
| −0.036019 | * Time2 |
| No. | Temperature (°C) | PAM Content (ppm) | Aluminum Sulfate Content (ppm) | Time (min) | Oil Separation Efficiency (%) | Desirability | |
|---|---|---|---|---|---|---|---|
| 1 | 74.068 | 6322.347 | 5820.937 | 31.810 | 85.759 | 0.843 | Selected |
| 2 | 74.064 | 6333.563 | 5827.408 | 31.791 | 85.759 | 0.843 | |
| 3 | 74.492 | 6324.325 | 5827.131 | 31.823 | 85.571 | 0.843 | |
| 4 | 74.223 | 6298.826 | 5756.872 | 39.355 | 93.706 | 0.821 | |
| 5 | 74.999 | 5962.815 | 5106.786 | 23.784 | 82.689 | 0.810 | |
| 6 | 75.000 | 4515.853 | 4723.054 | 25.809 | 80.948 | 0.790 | |
| 7 | 74.997 | 3984.408 | 5542.943 | 20.00 | 77.262 | 0.750 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narra, S.; Shahpasand, M. Developing a Chemical Process for Optimizing Oil Extraction from Cooking Oil Secondary Waste. Recycling 2025, 10, 35. https://doi.org/10.3390/recycling10020035
Narra S, Shahpasand M. Developing a Chemical Process for Optimizing Oil Extraction from Cooking Oil Secondary Waste. Recycling. 2025; 10(2):35. https://doi.org/10.3390/recycling10020035
Chicago/Turabian StyleNarra, Satyanarayana, and Masoud Shahpasand. 2025. "Developing a Chemical Process for Optimizing Oil Extraction from Cooking Oil Secondary Waste" Recycling 10, no. 2: 35. https://doi.org/10.3390/recycling10020035
APA StyleNarra, S., & Shahpasand, M. (2025). Developing a Chemical Process for Optimizing Oil Extraction from Cooking Oil Secondary Waste. Recycling, 10(2), 35. https://doi.org/10.3390/recycling10020035







