Abstract
Metal-oxygen batteries (especially Li-O2 battery) with ultrahigh theoretical energy density are of great promise for long-range vehicle electrification. However, the limited enduring stability and low-rate property further restricted the large-scale commercial application of metal-oxygen batteries. We firstly report the fabrication of a TiOX@Ti3C2TX with multilayer structure and its utilization as cathode for Li-O2 batteries. The TiOX protective layer was fabricated in situ to directly optimize surface properties of Ti3C2TX, as well as to strengthen surface active functional groups. The initial discharge capacity of as-prepared TiOX@Ti3C2TX cathode reaches 7100 mAh g−1 at 2500 mA g−1, as well as delivers impressive cycling stability (>100 cycles) at 2500 mA g−1. Experimental analysis reveals that the in situ TiOX protective layer enhanced active functional-groups and the improved complete decomposition of discharge products Li2O2 are three critical factors for promoting the electrochemical performance of LOBs. This work exhibits a new insight into the design of MXene electrocatalysts for metal-oxygen batteries.
1. Introduction
Under the threat of serious environmental pollution and energy crisis, energy storage and conversion technologies have attracted extensive worldwide attention [1,2,3]. Due to the ultra-high theoretical energy density, lithium-oxygen batteries (LOBs) have been considered as a competitive candidate for next generation electrochemical energy storage devices [4,5]. Major obstacles to the practical application of LOBs are the limited enduring stability, large polarization, and low-rate capability, which could be ascribed to the sluggish dynamics of oxygen reduction reaction and oxygen evolution reactions (ORR and OER) [6,7]. To this end, great efforts have been devoted to solve the above-mentioned challenges in recent years, mainly including the synthesis of high-efficiency dual-functional catalysts [8,9], the exploration of stable electrolytes [10,11], Li-anode modification engineering [12,13], and the fabrication of selective membrane [14,15]. It is noted that efficient bifunctional catalyst with high stability is of paramount significance to promote the sluggish kinetics of ORR and OER both occurring on the cathode [16,17].
Recently, carbonaceous materials, noble metals, metal oxides, metal sulfides, and compound matrices have already been intensively investigated as promising catalysts for LOBs [18,19]. Nevertheless, noble metals deliver high capacity and low overpotential, but are hindered by high cost and low reserves [20,21]. Carbonaceous materials are characterized by high conductivity, large surface area, and easy chemical functionalization, which are widely applied practically in electrochemical energy storage [22,23]. Carbon tends to be easily oxidized at high potentials and oxygen-rich organic environment, resulting in poor cycling lifespan and high voltage polarization [24]. Besides, the indistinctive electrochemical property and poor adjustability of metal oxides needs to be addressed urgently [25,26].
Impressively, featured by superior physical/chemical stability, tunable structure/properties, and excellent conductivity (~9880 S cm−1), Ti3C2Tx MXene (where Tx represents various surface terminations) are already widely applied in nitrogen reduction, hydrogen evolution, carbon dioxide reduction, and batteries [27,28,29]. With unique flexible layered structure and high tensile strength, Ti3C2Tx can act as a conductive matrix, which could significantly reduce ion diffusion barrier and tolerate a large volume change derived from the deposition of Li2O2 in LOBs [30,31]. The abundant active functional groups (-O, -F, -OH, etc.) on the surface of Ti3C2Tx can be served as fine catalytic active sites to promote the adsorption and desorption ability of discharge products, thus optimizing the kinetics [32]. Song et al. introduced lithium ions into the layered structure of Ti3C2Tx by electrostatic interaction and successfully optimized the O termination groups [33]. Furthermore, the synergistic effect between uniform O-terminal surface and abundant intercalated Li+ could provide remarkable stable catalytic sites (=O, Li+) and enhanced structural stability for Ti3C2Tx, leading to high electrochemical performance. Il-Kwon Oh and co-worker also synthesized Ti3C2Tx MXene with carboxylic acid graft, which showed superior electrocatalytic properties for ORR and OER due to the formation of metalloporphyrin structure and unpaired electrons [34]. Interestingly, Long et al. prepared in situ vacancy-rich TiO2 nanoparticles on Ti3C2Tx nanosheets by applied thermodynamic metastable Ti atoms as nucleation sites, achieving good rate capability and cycle stability in LOBs [35]. However, Ti3C2Tx MXene is extremely unstable in oxygen-rich environments (especially O2−, O− in LOBs) and gradually transforms into an undesired passivation layer (TiO2) on the surface. As a result, Ti3C2Tx MXene always suffer due to weakened electrocatalytic activity [36,37]. Therefore, how to design active Ti3C2Tx with high-durability remains a great challenge.
Herein, we reported a facile in situ surface engineering to form a TiOX protective layer on Ti3C2TX by one-step hydrothermal reaction for the first time. The TiOX protective layer was adopted to directly optimize surface properties of Ti3C2TX, as well as to strengthen surface active functional groups, thus improving the electrochemical activity and stability of Ti3C2TX. The LOBs based on TiOX-Ti3C2TX cathode exhibit superior specific capability (7169 mAh g−1) and endurable stability at ultra-high rate (2500 mA g−1). Further analysis indicates that TiOX@Ti3C2TX could promote fast charge transfer kinetics and the decomposition of insoluble discharge products. This work provides a new and easy way to improve the stability and electrochemical activity of MXene catalysts for LOBs.
2. Materials and Methods
2.1. Synthesis of TiOX-Ti3C2TX
One gram of lithium fluoride was dissolved in 20 mL hydrogen chloride (9 M) solution with magnetic stirring for 30 min. Then, 1 g Ti3AlC2 powder was slowly dropped into the above solution under vigorous stirring for 30 min. The mixture was sealed in a 50 mL PTFE-lined stainless-steel autoclave and heated at 90 °C for 24 h. The resultant was centrifuged with deionized water and ethanol at 10,000 rpm for 5–6 times until the pH value was adjusted to ~6. Finally, the collected precipitate was dried at 60 °C and was calcined at 300 °C for 2.5 h.
2.2. Electrochemical Measurement
The as-prepared sample was evenly sprayed onto the carbon paper (ϕ = 13 mm) with mass loading of 0.6 ± 0.1 mg cm−2 to mark it as the cathode. Furthermore, 1 M lithium bis (trifluoromethane sulfonamide, LiTFSI) was dissolved in dimethyl sulfoxide, the resulting solution acted as an electrolyte. The lithium metal foil with diameter of 16 mm was chosen as the anode. The LOBs were prepared and assembled in a glove box filled with Ar. The assembled LOBs were measured by LAND CT 3001A to test the electrochemical performance. The electrochemical impedance spectroscopy was carried out by CHI 1600E.
3. Results
The preparation of TiOX-Ti3C2TX is illustrated in Figure 1a. Briefly, TiOX-Ti3C2TX could be successfully obtained by one-step hydrothermal treatment acting as a simple acid etching. SEM analysis was performed to characterize the morphologic changes of the sample in Figure 1b and Figure S1. After acid etching activation, MAX was successfully stripped into a multilayer sheet structure to provide greater contact area and expose more active sites. The XRD patterns of as-prepared samples are shown in Figure 1c. The peaks of the MAX are identical to the Ti3AlC2, which is consistent with a previous report. After etching, the peak located at 39° attributed to the typical Ti3AlC2 (104) plane disappears, indicating that the Al layer has been selectively removed. The FTIR spectra of MXene (TiOX-Ti3C2TX) and MAX (Ti3AlC2) are shown in Figure 1d. The sharp peaks at 669, 1630, and 3480 cm−1 are consistent with the functional groups’ stretching vibrations (C-O, -COOH, -OH). Impressively, the intensity of these functional groups is significantly enhanced, and a new peak representing the Ti-O bond (559 cm−1) appears after the hydrothermal modification, indicating that a new layer of titanium oxide and a variety of active functional groups are fabricated on the surface of Ti3C2TX.
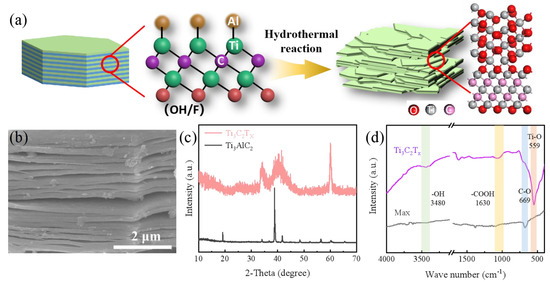
Figure 1.
(a) Schematic description of the preparation of TiOX-Ti3C2TX; (b) SEM image of TiOX-Ti3C2TX; XRD patterns (c) and FTIR curve (d) of as-prepared samples.
The TEM image (Figure 2a,b) shows TiOX-Ti3C2TX with a uniform multilayer sheet structure. The corresponding SAED pattern (Figure 2b inset) obviously exhibits the co-existence of (002), (110) planes of Ti3C2TX. The HRTEM image (Figure 2c,d) clearly demonstrates interplanar spacing of 0.253 nm and 0.234nm, related to (110) plane of Ti3C2TX and (004) plane of TiO2, respectively [38,39]. Interestingly, there are also considerable amorphous structures near the TiO2 crystalline phase. This phenomenon could be ascribed to the fact that the TiO2 layer is of hybrid amorphous/crystalline structure. The corresponding EDS mappings (Figure 2e) show that Ti, O, and C elements are evenly distributed across the surface of TiOX-Ti3C2TX. Besides, as shown in Figure S2 and Table S1, there is obviously no Al element present on the surface of TiOX-Ti3C2TX, indicating that the Al has been selectively removed.
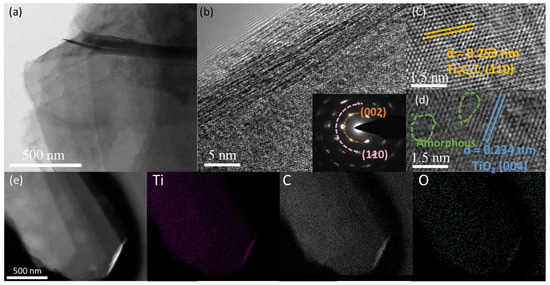
Figure 2.
(a) TEM image, (b) SAED pattern, (c,d) HR-TEM image, and (e) EDS mapping images of TiOX-Ti3C2TX.
The full XPS spectra of TiOX-Ti3C2TX are shown in Figure 3a, there are obvious Ti, O, F, and C peaks in the TiOX-Ti3C2TX curve, which indicates that after hydrothermal treatment, the unwanted Al-based impurities on the surface of MXene are completely removed and oxide passivation layers are successfully formed in situ. The Ti 2p spectra of TiOX-Ti3C2TX (Figure 3b) could be well fitted into seven peaks. The peaks’ centra at 455.3 and 462.6 eV are indexed to Ti-C and C-Ti-Fx bonding. The typical peak at 459.3 eV could be indexed to Ti-O, indicating the presence of a TiO2 protective layer. Moreover, The C 1s spectra of TiOX-Ti3C2TX (Figure 3c) show three peaks at 286.10, 284.80, and 282.70 eV, which correspond to C-O, C-C, and C-Ti, respectively. Besides, the characteristic peaks at 289.49 and 282.32 eV can be ascribed to COO/C-F and C-Ti-Tx bond in TiOX-Ti3C2TX [40]. The O 1s spectra in Figure 3d can be well deconvoluted into two characteristic peaks. The obvious peaks located at 530.10 and 530.80 eV are related to the existence of lattice oxygen and O vacancy. Notably, the peak located at 532.28 eV could be ascribed to O-H. Besides, the intensity of the O-H peak is significantly larger than those of lattice oxygen peak and O vacancy peak, suggesting an enhanced functional-group coupling on the surface of Ti3C2, which is consistent with the previous FTIR data analysis. In general, after hydrothermal treatment, the surface of Ti3C2 formed a TiOX protective layer with rich O vacancy and functional-groups with high activity, leading to higher electrochemical performance and stability.
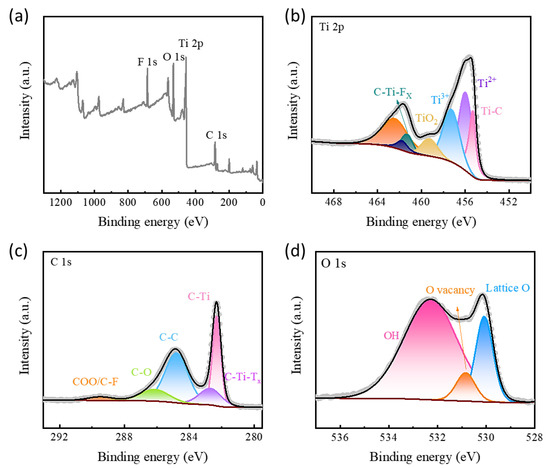
Figure 3.
XPS surveys (a) and high-resolution Ti 2p (b), C 1s (c), O 1s (d) spectra for TiOX-Ti3C2TX.
The electrocatalytic activity of TiOX-Ti3C2TX was examined as cathode in 2032-coin type LOBs. As shown in Figure 4a, the operating principle of Li-O2 battery could be summarized as follows: O2 (coming from the positive pole) can react with Li+ (coming from the negative pole) to generate discharge product Li2O2 on the surface of the cathode in discharge, which could be fully decomposed after charging. As shown in Figure S3, the cyclic voltammetry (CV) curves of TiOX-Ti3C2TX-based cathode delivers a relatively high ORR onset potential (2.83 V) and a low OER onset potential (3.41 V), indicating a promising bifunctional oxygen catalytic performance. The initial full discharge/charge profiles of the LOBs assembled with TiOX-Ti3C2TX at 2500 mA g−1 can be observed in Figure 4b. TiOX-Ti3C2TX delivers a significantly remarkable discharge capacity of 7169 mAh g−1. Especially, TiOX-Ti3C2TX shows a low charge voltage platform, which is less than 4V during the limited specific capacity of 1000 mAh g−1, which is favorable for improving the cycle efficiency, as well as extenuating the decomposition of the organic electrolyte. To explore the potential of TiOX-Ti3C2TX-based LOBs towards practical application, the full charge and discharge specific capacity of catalyst-based LOBs, normalized by the weight of actual electrodes (carbon paper + active materials), are presented in Figure S4. The LOBs based on TiOX-Ti3C2TX in this work demonstrate a competitive specific capacity (639 mAh g−1). Figure 4c shows the rate performance of TiOX-Ti3C2TX-based LOBs under different current densities. TiOX-Ti3C2TX demonstrates a negligible difference in the discharge/charge curves when the applied current densities from 100 up to 2500 mA g−1 and then back to 100 mA g−1. Notably, the LOB with TiOX-Ti3C2TX shows relatively lower discharge–charge voltage gap even after high-rate charging and discharging. In detail, TiOX-Ti3C2TX maintained a stable discharge–charge voltage gap from ~1.16 V at 100 mA g−1 to ~1.64 V at 2500 mA g−1. Furthermore, TiOX-Ti3C2TX shows little difference in the voltage curve after 50 cycling at 2500 mA g−1, and delivering a stable voltage gap of ~1.72 V. Impressively, TiOX-Ti3C2TX even sustained for almost 400 h and delivers low over-potential (~1.72 V) upon returning to low current density (100 mA g−1). Circulation durability of the as-prepared LOBs was measured at 2500 mA g−1 with a cut-off limited capacity of 1000 mAh g−1. In Figure 4d, the TiOX-Ti3C2TX-based cathode can continuously sustain for 100 h (over 100 cycles) without distinct attenuation at ultra-high rate of 2500 mA g−1, which is better than that of the Ti3C2TX-based cathode. These indicate that the loading of TiOX protective layer and active functional-groups could significantly improve the electrochemical activity and stability of Ti3C2 MXene. The comparisons between recent reports and our work are listed in Table S2, regarding some key parameters such as first discharge capacity, cycling current density, and cycle stability. By comparison, the performance of the TiOX-Ti3C2TX-based cathode in this work is pretty good in LOBs.
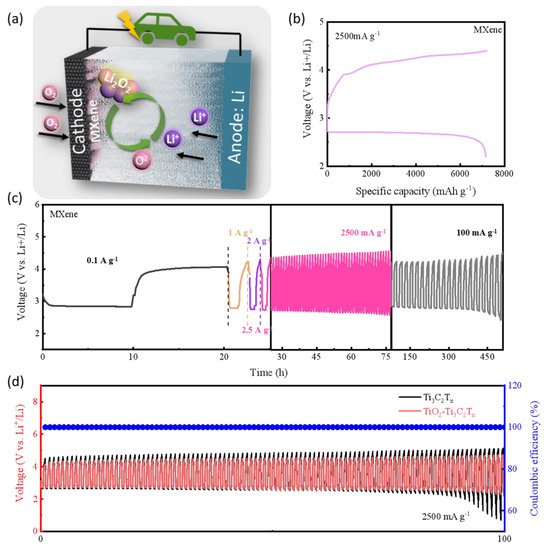
Figure 4.
(a) Working mechanism of LOBs. (b) Initial full discharge/charge curves of TiOX-Ti3C2TX-based LOBs at 2500 mA g−1; (c) rate performances of TiOX-Ti3C2TX-based LOBs; (d) cycling stability of as-prepared catalyst-based LOBs at 2500 mA g−1.
To further analyze the catalytic mechanism of TiOX-Ti3C2TX, battery impedance after assembly, first discharge, and charge process have been tested by electrochemical impedance spectra (EIS) as shown in Figure 5. The as-prepared TiOX-Ti3C2TX cathode delivers relevant ohmic resistance (Ro, 5.74 Ω) either after assembly or after the first cycle, but distinctly different charge transfer resistance (Rct). Typically, TiOX-Ti3C2TX-based LOBs showed higher Rct of 285.10 Ω after assembly than that of other stages. In detail, the TiOX-Ti3C2TX cathode delivered Rct of 186.59 Ω after the first discharge and Rct of 91.40 Ω after the first discharge. The equivalent circuit shows that a new interface is formed on the surface of the cathode after discharge, which can be attributed to the formation of insoluble discharge products. By comparison, TiOX-Ti3C2TX exhibited lower charge Rct values than that of discharge, indicating that the TiOX-Ti3C2TX could efficiently promote fast charge transfer kinetics and the decomposition of insoluble discharge products. Besides, the highest Rct values after assembly elucidated that the first electrochemical cycle reaction can effectively remove the impurities on the surface of TiOX-Ti3C2TX, which leads to reduced interface impedance and promotes the transmission of electrons.
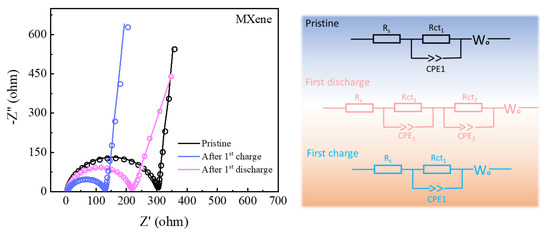
Figure 5.
EIS of TiOX-Ti3C2TX-based LOBs after the 1st discharge-charge and their corresponding analog circuit diagram.
Although TiOX-Ti3C2TX exhibits excellent electrochemical performance in LOBs, its stability still needs further optimization. Tailoring the surface functional groups could effectively improve the catalytic activity of Ti3C2TX, which could also optimize the electronic properties. However, Ti3C2TX MXene is composed of mixed surface functional groups, which causes great disparity with the theoretical study. Therefore, theoretical analysis on mixed-functional groups of Ti3C2TX will be the next main research to clarify their effects in altering the electrochemical properties of Ti3C2TX.
4. Conclusions
In conclusion, we creatively fabricated an in situ TiOX protective layer on the surface of Ti3C2TX by a facile hydrothermal reaction. A TiOX protective layer was adopted to directly optimize surface properties of Ti3C2TX. The LOBs based on TiOX-Ti3C2TX cathode exhibit superior specific capability (7169 mAh g−1) and endurable stability at ultra-high rate (2500 mA g−1). Such enhanced rate performances could be attributed to the three key aspects: (1) TiOX behaves as in situ protective layer to optimize the structural stability of Ti3C2TX, accompanied with enhancing the intensity of active functional-groups; (2) the uniformly-loaded active functional-groups (-F, -OH) could effectively optimize the catalytic performance of TiOX@Ti3C2TX; and (3) TiOX@Ti3C2TX could promote fast charge transfer kinetics and the decomposition of insoluble discharge products. This work provides an efficient way to improve the stability and electrochemical activity of MXene catalysts for LOBs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/batteries9040205/s1, Figure S1: SEM image of TiOX-Ti3C2TX; Figure S2: EDS mapping images of Al element for TiOX-Ti3C2TX; Figure S3: Cyclic voltammetry (CV) curves of TiOX-Ti3C2TX; Figure S4: Initial full discharge/charge curves of TiOX-Ti3C2TX based LOBs at 2500 mA g−1 (The specific capacities are normalized by the weight of actual whole electrodes); Table S1: EDS elemental analysis of TiOX-Ti3C2TX; Table S2: Comparison of battery performance of TiOX-Ti3C2TX based electrode with other reported electrodes [39,41,42,43,44].
Author Contributions
Z.S., investigation, conceptualization, writing—original draft preparation; S.Z., data curation, methodology, supervision; J.Z., writing—review and editing, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities, grant number 2022QN1003; the China Postdoctoral Science Foundation, grant number 2021M703495; the Natural Science Foundation of Jiangsu Province, grant number BK20221133.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical reasons.
Conflicts of Interest
We declare that the publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out., there is no professional or other personal interest of any nature or kind in any product, service and company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “High-conductive multilayer TiOX-Ti3C2TX Electrocatalyst for Longevous Metal-Oxygen Battery under a High Rate”.
References
- Zhang, Z.; Ding, T.; Zhou, Q.; Sun, Y.; Qu, M.; Zeng, Z.; Ju, Y.; Li, L.; Wang, K.; Chi, F. A review of technologies and applications on versatile energy storage systems. Renew. Sust. Energy Rev. 2021, 148, 111263. [Google Scholar] [CrossRef]
- Hussain, I.; Lamiel, C.; Sahoo, S.; Ahmad, M.; Chen, X.; Javed, M.S.; Qin, N.; Gu, S.; Li, Y.; Nawaz, T.; et al. Factors affecting the growth formation of nanostructures and their impact on electrode materials: A systematic review. Mater. Today Phys. 2022, 27, 100844. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Y.; Ke, X.; Zhang, Z.; Wu, W.; Lin, G.; Zhou, Z.; Shi, Z. Coupling of triporosity and strong Au–Li interaction to enable dendrite-free lithium plating/stripping for long-life lithium metal anodes. J. Mater. Chem. A 2020, 8, 18094–18105. [Google Scholar] [CrossRef]
- Balaish, M.; Jung, J.-W.; Kim, I.-D.; Ein-Eli, Y. A Critical Review on Functionalization of Air-Cathodes for Nonaqueous Li–O2 Batteries. Adv. Funct. Mater. 2020, 30, 1808303. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, Q.; Lu, Y.; Hao, Z.; Ni, Y.; Chen, J. An Ionic Liquid Electrolyte with Enhanced Li+ Transport Ability Enables Stable Li Deposition for High-Performance Li-O2 Batteries. Angew. Chem. Int. Edit. 2021, 60, 25973–25980. [Google Scholar] [CrossRef]
- Cao, D.; Bai, Y.; Zhang, J.; Tan, G.; Wu, C. Irreplaceable carbon boosts Li-O2 batteries: From mechanism research to practical application. Nano Energy 2021, 89, 106464. [Google Scholar] [CrossRef]
- Cao, D.; Zheng, L.; Li, Q.; Zhang, J.; Dong, Y.; Yue, J.; Wang, X.; Bai, Y.; Tan, G.; Wu, C. Crystal Phase-Controlled Modulation of Binary Transition Metal Oxides for Highly Reversible Li–O2 Batteries. Nano Lett. 2021, 21, 5225–5232. [Google Scholar] [CrossRef]
- Liu, G.; Wang, N.; Qi, F.; Lu, X.; Liang, Y.; Sun, Z. Novel Ni–Ge–P anodes for lithium-ion batteries with enhanced reversibility and reduced redox potential. Inorg. Chem. Front. 2023, 10, 699–711. [Google Scholar] [CrossRef]
- Hong, Y.-S.; Zhao, C.-Z.; Xiao, Y.; Xu, R.; Xu, J.-J.; Huang, J.-Q.; Zhang, Q.; Yu, X.; Li, H. Safe Lithium-Metal Anodes for Li-O2 Batteries: From Fundamental Chemistry to Advanced Characterization and Effective Protection. Batter. Supercaps 2019, 2, 638–658. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Liu, J.; Hou, C.; Xiu, Z.; Fan, Y.; Zhao, L.; Zhai, Y.; Li, H.; Zeng, J.; et al. Interfacial Super-Assembled Porous CeO2/C Frameworks Featuring Efficient and Sensitive Decomposing Li2O2 for Smart Li–O2 Batteries. Adv. Energy Mater. 2019, 9, 1901751. [Google Scholar] [CrossRef]
- Hu, Z.; Xie, Y.; Yu, D.; Liu, Q.; Zhou, L.; Zhang, K.; Li, P.; Hu, F.; Li, L.; Chou, S.; et al. Hierarchical Ti3C2Tx MXene/Carbon Nanotubes for Low Overpotential and Long-Life Li-CO2 Batteries. ACS Nano 2021, 15, 8407–8417. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tian, M.; Wang, H.; Wei, C.; Sun, Z.; Rummeli, M.H.; Strasser, P.; Sun, J.; Yang, R. Mildly Oxidized MXene (Ti3C2, Nb2C, and V2C) Electrocatalyst via a Generic Strategy Enables Longevous Li–O2 Battery under a High Rate. ACS Nano 2021, 15, 19640–19650. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Liu, H.; Xing, Y.; Zhao, L.; Shang, Y.; Huang, Y.; Chen, N.; Li, L.; Wu, F.; Chen, R. Local Strong Solvation Electrolyte Trade-Off between Capacity and Cycle Life of Li-O2 Batteries. Adv. Funct. Mater. 2021, 31, 2101831. [Google Scholar] [CrossRef]
- Li, J.; Ding, S.; Zhang, S.; Yan, W.; Ma, Z.-F.; Yuan, X.; Mai, L.; Zhang, J. Catalytic redox mediators for non-aqueous Li-O2 battery. Energy Storage Mater. 2021, 43, 97–119. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Li, F.; Zheng, L.; Xu, J.; Yu, J. A Bifunctional Photo-Assisted Li–O2 Battery Based on a Hierarchical Heterostructured Cathode. Adv. Mater. 2020, 32, 1907098. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Peng, S.; Yuan, Y.; Wang, J.; Du, Y.; Zhang, W.; Han, K.; Ji, Y.; Dang, F. Ti3C2Tx MXene cathode catalyst with efficient decomposition Li2O2 and high-rate cycle stability for Li-O2 batteries. Electrochim. Acta 2021, 388, 138622. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, L.; Xu, H.; Huang, Q.; Wang, Y.; Hou, C.; Hou, Y.; Wang, J.; Dang, F.; Zhang, J. Tunable Cationic Vacancies of Cobalt Oxides for Efficient Electrocatalysis in Li–O2 Batteries. Adv. Energy Mater. 2020, 10, 2001415. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Scheffler, R.; Speidel, A.; Girishkumar, G.; Luntz, A.C. On the Mechanism of Nonaqueous Li–O2 Electrochemistry on C and Its Kinetic Overpotentials: Some Implications for Li–Air Batteries. J. Phys. Chem. C 2012, 116, 23897–23905. [Google Scholar] [CrossRef]
- Nam, S.; Mahato, M.; Matthews, K.; Lord, R.W.; Lee, Y.; Thangasamy, P.; Ahn, C.W.; Gogotsi, Y.; Oh, I.-K. Bimetal Organic Framework–Ti3C2Tx MXene with Metalloporphyrin Electrocatalyst for Lithium–Oxygen Batteries. Adv. Funct. Mater. 2022, 33, 2210702. [Google Scholar] [CrossRef]
- Peng, C.; Wei, P.; Li, X.; Liu, Y.; Cao, Y.; Wang, H.; Yu, H.; Peng, F.; Zhang, L.; Zhang, B.; et al. High efficiency photocatalytic hydrogen production over ternary Cu/TiO2@Ti3C2Tx enabled by low-work-function 2D titanium carbide. Nano Energy 2018, 53, 97–107. [Google Scholar] [CrossRef]
- Plunkett, S.T.; Kondori, A.; Chung, D.Y.; Wen, J.; Wolfman, M.; Lapidus, S.H.; Ren, Y.; Amine, R.; Amine, K.; Mane, A.U.; et al. A New Cathode Material for a Li–O2 Battery Based on Lithium Superoxide. ACS Energy Lett. 2022, 7, 2619–2626. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, Q.; Mu, X.; Deng, H.; He, P.; Yu, J.; Zhou, H. Advanced Hybrid Electrolyte Li-O2 Battery Realized by Dual Superlyophobic Membrane. Joule 2019, 3, 2986–3001. [Google Scholar] [CrossRef]
- Song, S.; Yin, F.; Fu, Y.; Ren, J.; Ma, J.; Liu, Y.; Ma, R.; Ye, W. Simultaneous regulation of Li-ion intercalation and oxygen termination decoration on Ti3C2Tx MXene toward enhanced oxygen electrocatalysis for Li-O2 batteries. Chem. Eng. J. 2023, 451, 138818. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, K.; Zhang, C.; Yu, H.; Wang, X.; Yang, D.; Wang, J.; Huang, G.; Zhang, S. A Novel Material for High-Performance Li–O2 Battery Separator: Polyetherketone Nanofiber Membrane. Small 2022, 18, 2201470. [Google Scholar] [CrossRef]
- Sung, M.-C.; Lee, G.-H.; Kim, D.-W. Kinetic insight into perovskite La0.8Sr0.2VO3 nanofibers as an efficient electrocatalytic cathode for high-rate Li-O2 batteries. InfoMat 2021, 3, 1295–1310. [Google Scholar] [CrossRef]
- Guo, S.; Wang, J.; Sun, Y.; Peng, L.; Li, C. Interface engineering of Co3O4/CeO2 heterostructure in-situ embedded in Co/N-doped carbon nanofibers integrating oxygen vacancies as effective oxygen cathode catalyst for Li-O2 battery. Chem. Eng. J. 2023, 452, 139317. [Google Scholar] [CrossRef]
- Wang, D.; Mu, X.; He, P.; Zhou, H. Materials for advanced Li-O2 batteries: Explorations, challenges and prospects. Mater. Today 2019, 26, 87–99. [Google Scholar] [CrossRef]
- Abdul, M.; Mohd, Z.; Qasim, A.; Ahmed, M.; Ahmad, H.; Elsayed t El Fatimah, M.A.; Norah, S.A.; Shafaqat, A.; Muhammad, S.J. In Situ Nitrogen Functionalization of 2D-Ti3C2TX-MXenes for High-Performance Zn-Ion Supercapacitor. Molecules 2022, 27, 7446. [Google Scholar] [CrossRef]
- Yan, Y.; Shu, C.; Zheng, R.; Li, M.; Ran, Z.; He, M.; Ren, L.; Du, D.; Zeng, Y. Long-cycling lithium-oxygen batteries enabled by tailoring Li nucleation and deposition via lithiophilic oxygen vacancy in Vo-TiO2/Ti3C2TX composite anodes. J. Energy Chem. 2022, 65, 654–665. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Li, M.; Zheng, L.; Guan, D.; Huang, X.; Xu, J.; Yu, J. Porous Materials Applied in Nonaqueous Li–O2 Batteries: Status and Perspectives. Adv. Mater. 2020, 32, 2002559. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, N.; Bi, Z.; Gao, S.; Dai, Q.; Yang, T.; Wang, J.; Jia, Z.; Peng, Z.; Huang, J.; et al. Clear Representation of Surface Pathway Reactions at Ag Nanowire Cathodes in All-Solid Li–O2 Batteries. ACS Appl. Mater. Interfaces 2021, 13, 39157–39164. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, R.; Shu, C.; Long, J. Promoting the Electrocatalytic Activity of Ti3C2Tx MXene by Modulating CO2 Adsorption through Oxygen Vacancies for High-Performance Lithium-Carbon Dioxide Batteries. ChemElectroChem 2020, 7, 4922–4930. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Lu, S. Recent Advances in Layered Ti3C2Tx MXene for Electrochemical Energy Storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Huang, G.; Yu, Y.; Li, C.-L.; Li, J.-C.; Yan, J.-M.; Zhang, X.-B. Soluble and Perfluorinated Polyelectrolyte for Safe and High-Performance Li-O2 Batteries. Angew. Chem. Int. Edit. 2022, 61, e202116635. [Google Scholar] [CrossRef]
- Yoo, E.; Zhou, H. LiF Protective Layer on a Li Anode: Toward Improving the Performance of Li–O2 Batteries with a Redox Mediator. ACS Appl. Mater. Interfaces 2020, 12, 18490–18495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kong, N.; Uzun, S.; Levitt, A.; Seyedin, S.; Lynch, P.A.; Qin, S.; Han, M.; Yang, W.; Liu, J.; et al. Scalable Manufacturing of Free-Standing, Strong Ti3C2Tx MXene Films with Outstanding Conductivity. Adv. Mater. 2020, 32, 2001093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Ma, J.; Huang, A.; Yuan, M.; Li, Y.; Sun, G.; Chen, C.; Nan, C. Oxygen Vacancy-Rich RuO2–Co3O4 Nanohybrids as Improved Electrocatalysts for Li–O2 Batteries. ACS Appl. Mater. Interfaces 2021, 13, 39239–39247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhang, F.; Zhan, F.; Yi, D.; Yang, Y.; Cui, W.; Wang, X. Fe3+-stabilized Ti3C2Tx MXene enables ultrastable Li-ion storage at low temperature. J. Mater. Sci. Technol. 2021, 67, 156–164. [Google Scholar] [CrossRef]
- Zheng, R.; Shu, C.; Hou, Z.; Hu, A.; Hei, P.; Yang, T.; Li, J.; Liang, R.; Long, J. In Situ Fabricating Oxygen Vacancy-Rich TiO2 Nanoparticles via Utilizing Thermodynamically Metastable Ti Atoms on Ti3C2Tx MXene Nanosheet Surface To Boost Electrocatalytic Activity for High-Performance Li–O2 Batteries. ACS Appl. Mater. Interfaces 2019, 11, 46696–46704. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, K.; Gu, Q.; Tao, L.; Li, Y.; Tan, H.; Zhou, J.; Zhang, W.; Li, H.; Guo, S. Lewis-Acidic PtIr Multipods Enable High-Performance Li–O2 Batteries. Angew. Chem. Int. Edit. 2021, 60, 26592–26598. [Google Scholar] [CrossRef]
- Zheng, X.; Yuan, M.; Guo, D.; Wen, C.; Li, X.; Huang, X.; Li, H.; Sun, G. Theoretical Design and Structural Modulation of a Surface-Functionalized Ti3C2Tx MXene-Based Heterojunction Electrocatalyst for a Li–Oxygen Battery. ACS Nano 2022, 16, 4487–4499. [Google Scholar] [CrossRef]
- Lu, Y.; Ang, H.; Yan, Q.; Fong, E. Bioinspired Synthesis of Hierarchically Porous MoO2/Mo2C Nanocrystal Decorated N-Doped Carbon Foam for Lithium–Oxygen Batteries. Chem. Mater. 2016, 28, 5743–5752. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Wang, Y.; Zhang, S.; Kang, Z.; Xie, H.; Sun, L. MoO2 nanoparticles/carbon textiles cathode for high performance flexible Li-O2 battery. J. Energy Chem. 2020, 47, 66–71. [Google Scholar] [CrossRef]
- Cao, X.; Sun, Z.; Zheng, X.; Jin, C.; Tian, J.; Li, X.; Yang, R. MnCo2O4/MoO2 Nanosheets Grown on Ni foam as Carbon- and Binder-Free Cathode for Lithium–Oxygen Batteries. ChemSusChem 2018, 11, 574–579. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).