Hydrogel Polymer Electrolytes for Aqueous Zinc-Ion Batteries: Recent Progress and Remaining Challenges
Abstract
1. Introduction
2. Overview of ZIBs
2.1. Energy Storage Mechanisms of Aqueous ZIBs
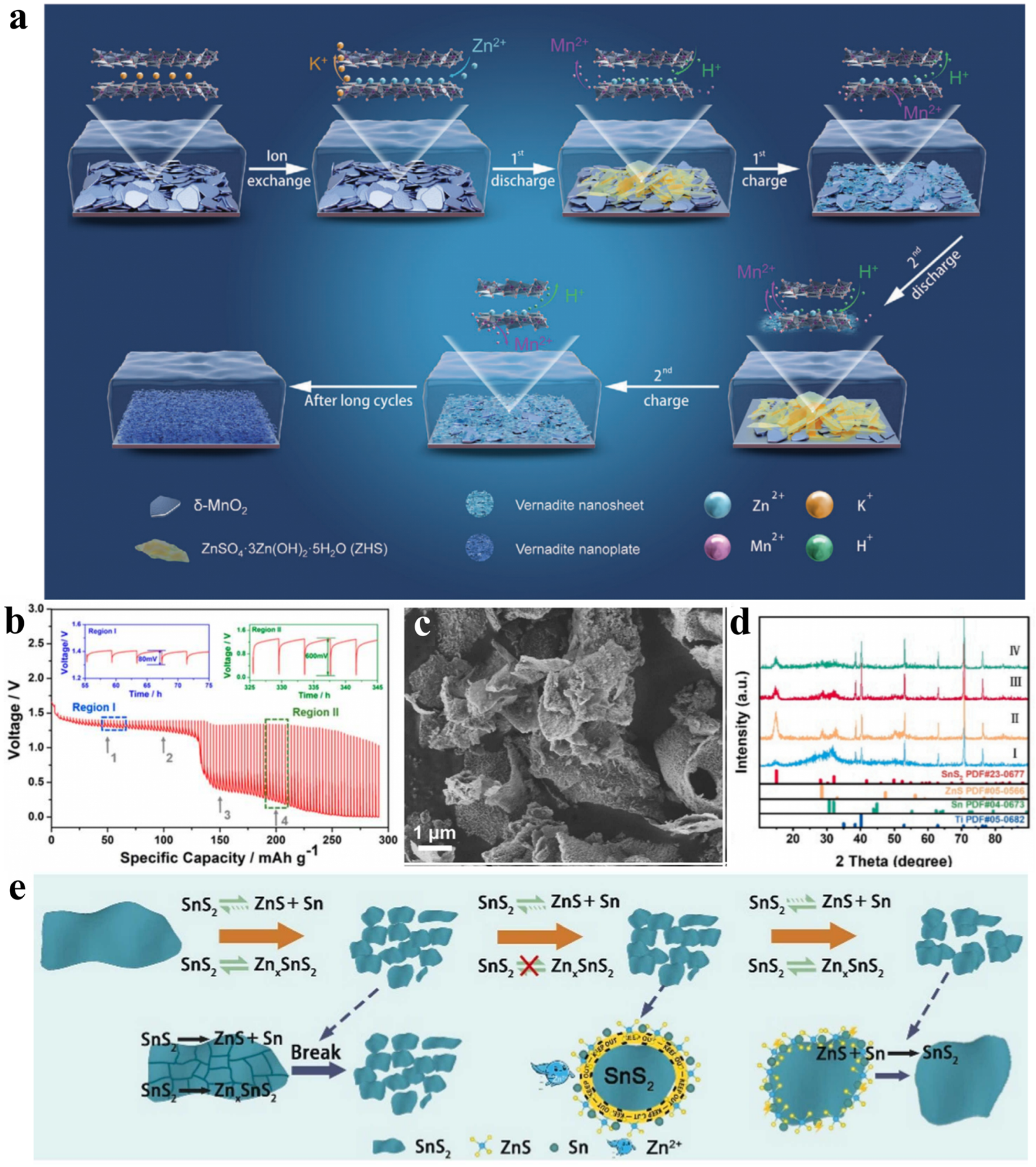
2.2. Anode Materials for Aqueous ZIBs
2.3. Cathode Materials for Aqueous ZIBs
2.4. Electrolytes for Aqueous ZIBs
3. Research Progress on HPEs in Aqueous ZIBs
3.1. PAM-Based Hydrogel Electrolytes
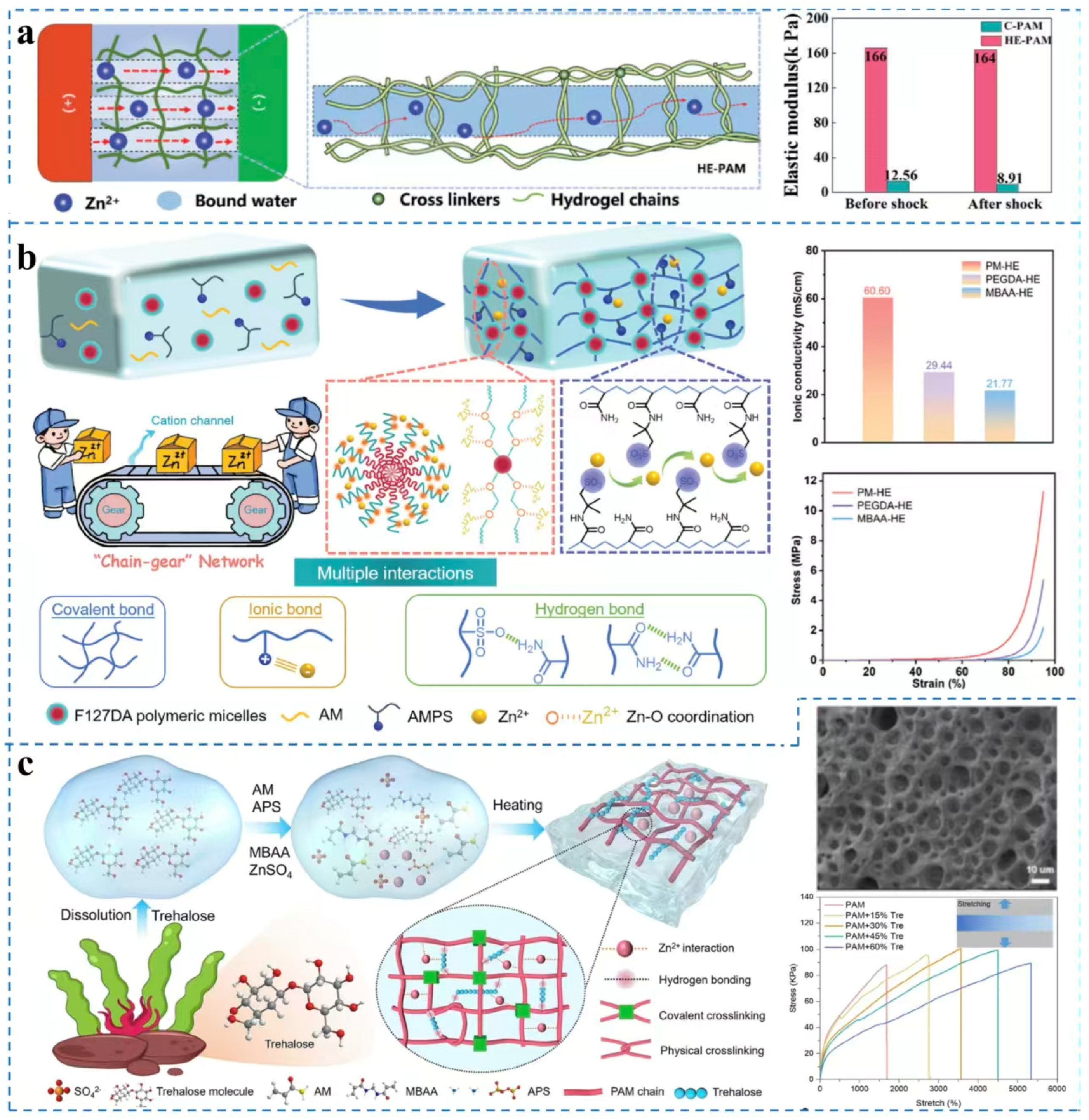
3.2. Polyvinyl Alcohol (PVA)

3.3. Polyacrylic Acid (PAA)
3.4. Low-Temperature Performance of HPEs
4. Outlook
4.1. Rational Design of High-Performance Multifunctional HPEs
4.2. In-Depth Understanding of Ion Transport and Interfacial Mechanisms
4.3. Interface Engineering and Stability Enhancement
4.4. Scalable Fabrication and Sustainability
4.5. Advanced Battery Configurations and Expanded Application
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jha, A.; Misra, A.K. Consequences of shifting to renewable energy on atmospheric carbon dioxide: A mathematical model. J. Appl. Math. Comput. 2024, 70, 4851–4876. [Google Scholar] [CrossRef]
- Kosowski, P. From Fossil Fuels to Renewables: Clustering European Primary Energy Production from 1990 to 2022. Energies 2024, 17, 5596. [Google Scholar] [CrossRef]
- Sun, H.; Xiang, X.; Wang, X.; Tsai, H.S.; Feng, W. Advanced photo-rechargeable lithium- and zinc-ion batteries: Progress and prospect. J. Power Sources 2024, 598, 234204. [Google Scholar] [CrossRef]
- Arsad, A.Z.; Hannan, M.A.; Al-Shetwi, A.Q.; Begum, R.A.; Hossain, M.J.; Ker, P.J.; Mahlia, T.M.I. Hydrogen electrolyser technologies and their modelling for sustainable energy production: A comprehensive review and suggestions. Int. J. Hydrogen Energy 2023, 48, 27841–27871. [Google Scholar] [CrossRef]
- Ruan, S.; Luo, D.; Li, M.; Wang, J.; Ling, L.; Yu, A.; Chen, Z. Synthesis and functionalization of 2D nanomaterials for application in lithium-based energy storage systems. Energy Storage Mater. 2021, 38, 200–230. [Google Scholar] [CrossRef]
- Mo, J.Y.; Jeon, W. The Impact of Electric Vehicle Demand and Battery Recycling on Price Dynamics of Lithium-Ion Battery Cathode Materials: A Vector Error Correction Model (VECM) Analysis. Sustainability 2018, 10, 2870. [Google Scholar] [CrossRef]
- Li, S.; Zhou, S.; Zhao, S.; Jin, T.; Zhong, M.; Cen, Z.; Gao, P.; Yan, W.; Ling, M. Room Temperature Resistive Hydrogen Sensor for Early Safety Warning of Li-Ion Batteries. Chemosensors 2023, 11, 344. [Google Scholar] [CrossRef]
- Forsyth, M.; Porcarelli, L.; Wang, X.; Goujon, N.; Mecerreyes, D. Innovative Electrolytes Based on Ionic Liquids and Polymers for Next-Generation Solid -State Batteries. Acc. Chem. Res. 2019, 52, 686–694. [Google Scholar] [CrossRef]
- Meng, R.; Li, H.; Lu, Z.; Zhang, C.; Wang, Z.; Liu, Y.; Wang, W.; Ling, G.; Kang, F.; Yang, Q.-H. Tuning Zn-Ion Solvation Chemistry with Chelating Ligands toward Stable Aqueous Zn Anodes. Adv. Mater. 2022, 34, 2200677. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, J.; Zhao, J.W.; Liu, K.; Yang, P.; Fan, H.J. Stable Zinc Anodes Enabled by a Zincophilic Polyanionic Hydrogel Layer. Adv. Mater. 2022, 34, 2202382. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, F.; Yang, F.; Liu, X.; Yu, Y.; Zheng, D.; Lu, X. Unshared Pair Electrons of Zincophilic Lewis Base Enable Long-life Zn Anodes under “Three High” Conditions. Angew. Chem.-Int. Ed. 2022, 61, e202208051. [Google Scholar] [CrossRef] [PubMed]
- Perez-Antolin, D.; Saez-Bernal, I.; Colina, A.; Ventosa, E. Float-charging protocol in rechargeable Zn-MnO2 batteries: Unraveling the key role of Mn2+ additives in preventing spontaneous pH changes. Electrochem. Commun. 2022, 138, 107271. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Yang, S.; Du, H.; Li, Y.; Wu, X.; Xiao, B.; He, Z.; Zhang, Q.; Wu, X. Advances in the structure design of substrate materials for zinc anode of aqueous zinc ion batteries. Green Energy Environ. 2023, 8, 1531–1552. [Google Scholar] [CrossRef]
- Naveed, A.; Yang, H.; Shao, Y.; Yang, J.; Yanna, N.; Liu, J.; Shi, S.; Zhang, L.; Ye, A.; He, B.; et al. A Highly Reversible Zn Anode with Intrinsically Safe Organic Electrolyte for Long-Cycle-Life Batteries. Adv. Mater. 2019, 31, 1900668. [Google Scholar] [CrossRef]
- Naveed, A.; Yang, H.; Yang, J.; Nuli, Y.; Wang, J. Highly Reversible and Rechargeable Safe Zn Batteries Based on a Triethyl Phosphate Electrolyte. Angew. Chem. Int. Ed. 2019, 58, 2760–2764. [Google Scholar] [CrossRef]
- Zhu, K.; Niu, X.; Xie, W.; Yang, H.; Jiang, W.; Ma, M.; Yang, W. An integrated Janus hydrogel with different hydrophilicities and gradient pore structures for high-performance zinc-ion batteries. Energy Environ. Sci. 2024, 17, 4126–4136. [Google Scholar] [CrossRef]
- Wu, K.; Cui, J.; Yi, J.; Liu, X.; Ning, F.; Liu, Y.; Zhang, J. Biodegradable Gel Electrolyte Suppressing Water-Induced Issues for Long-Life Zinc Metal Anodes. ACS Appl. Mater. Interfaces 2022, 14, 34612–34619. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Ran, F. Key approaches and challenges in fabricating advanced flexible zinc-ion batteries with functional hydrogel electrolytes. Energy Storage Mater. 2023, 56, 351–393. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Xin, W.; Peng, H.; Yan, Z.; Zhu, Z. Additive engineering for a hydrophilic/zincophilic polymeric layer towards dendrite-free zinc anode. Mater. Today Energy 2022, 29, 101130. [Google Scholar] [CrossRef]
- Qi, R.; Tang, W.; Shi, Y.; Teng, K.; Deng, Y.; Zhang, L.; Zhang, J.; Liu, R. Gel Polymer Electrolyte toward Large-Scale Application of Aqueous Zinc Batteries. Adv. Funct. Mater. 2023, 33, 2306052. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Z.; Jiang, J.; Wang, X.; Yu, C. Gelation mechanisms of gel polymer electrolytes for zinc-based batteries. Chin. Chem. Lett. 2024, 35, 109393. [Google Scholar] [CrossRef]
- Vandeginste, V.; Wang, J. A Review of the Synthesis of Biopolymer Hydrogel Electrolytes for Improved Electrode-Electrolyte Interfaces in Zinc-Ion Batteries. Energies 2024, 17, 310. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Zhang, P.T.; Fan, S.; Cai, M.H.; Hu, J.T.; Luo, Z.Y.; Mi, H.W.; Jiang, X.T.; Zhang, Q.L.; Ren, X.Z. Polypyrrole pre-intercalation engineering-induced NH4+ removal in tunnel ammonium vanadate toward high-performance zinc ion batteries. J. Colloid Interface Sci. 2024, 664, 168–177. [Google Scholar] [CrossRef]
- Ji, J.; Cheng, F.; Cai, W.; Ji, Y.; Liu, J.; Wang, Y.; Cai, C.; Fu, Y. Constructing ion “Tongs” and double-network for cellulose-based semi-solid electrolytes towards dendrite-free dual-ion batteries. Chem. Eng. J. 2024, 482, 149172. [Google Scholar] [CrossRef]
- Lee, C.J.; Wu, H.; Hu, Y.; Young, M.; Wang, H.; Lynch, D.; Xu, F.; Cong, H.; Cheng, G. Ionic Conductivity of Polyelectrolyte Hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 5845–5852. [Google Scholar] [CrossRef]
- Chen, J.; Zhai, Y.J.; Li, Y.J.; Zhang, X.Y.; Zhang, X.Q.; Chen, Y.X.; Zeng, Y.X.; Wu, X.Q.; Zheng, Q.J.; Lam, K.H.; et al. Optimizing Interplanar Spacing, Oxygen Vacancies and Micromorphology via Lithium-Ion Pre-Insertion into Ammonium Vanadate Nanosheets for Advanced Cathodes in Aqueous Zinc-Ion Batteries. Small 2024, 20, 2309412. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.S.; Ko, E.S.; Park, Y.Y.; Hong, D.; Lee, S.H.; Jeon, Y.P.; La, Y.; Kim, S.; Lee, I.-S.; Park, G.S.; et al. Hygroscopic and Malleable Dough-Type Zn-Air Battery in a Dry Condition Utilizing Deliquescence. Adv. Energy Mater. 2023, 13, 2300285. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, J.; Bi, S.; Wang, R.; Niu, Z. A Binary Hydrate-Melt Electrolyte with Acetate-Oriented Cross-Linking Solvation Shells for Stable Zinc Anodes. Adv. Mater. 2022, 34, 2201744. [Google Scholar] [CrossRef]
- Fu, C.; Wang, Y.; Lu, C.; Zhou, S.; He, Q.; Hu, Y.; Feng, M.; Wan, Y.; Lin, J.; Zhang, Y.; et al. Modulation of hydrogel electrolyte enabling stable zinc metal anode. Energy Storage Mater. 2022, 51, 588–598. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Mishra, K.; Yadav, N.; Hashmi, S.A. Recent progress in electrode and electrolyte materials for flexible sodium-ion batteries. J. Mater. Chem. A 2020, 8, 22507–22543. [Google Scholar] [CrossRef]
- Bai, L.; Ghiassinejad, S.; Brassinne, J.; Fu, Y.; Wang, J.; Yang, H.; Vlad, A.; Minoia, A.; Lazzaroni, R.; Gohy, J.-F. High Salt-Content Plasticized Flame-Retardant Polymer Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 44844–44859. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Chen, Z.; Jin, Y.; Chen, J. High voltage and self-healing zwitterionic double-network hydrogels as electrolyte for zinc-ion hybrid supercapacitor/battery. Int. J. Hydrogen Energy 2022, 47, 23909–23918. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, L.; Chen, J. Low-Temperature and High-Voltage-Tolerant Zinc-Ion Hybrid Supercapacitor Based on a Hydrogel Electrolyte. Chemelectrochem 2022, 9, e202200070. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Qin, B.; Passerini, S. Electrochemical intercalation of anions in graphite for high-voltage aqueous zinc battery. J. Power Sources 2020, 449, 227594. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, S.; Pan, Y.; Lu, B.; Liang, S.; Zhou, J. Dual mechanism of ion (de)intercalation and iodine redox towards advanced zinc batteries. Energy Environ. Sci. 2023, 16, 2358–2367. [Google Scholar] [CrossRef]
- Fan, H.; Wang, K.; Xu, Z. Tungsten Doping of Two-Dimensional VO2. Nanoribbons for Rapid Zinc Ion Storage Channels. Chemistryselect 2024, 9, e202401229. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, X.; Zhou, M.; Ju, Z.; Sun, X.; Sun, Y.; Huang, Z.; Li, H.; Ma, T. Proton Insertion Promoted a Polyfurfural/MnO2 Nanocomposite Cathode for a Rechargeable Aqueous Zn-MnO2 Battery. ACS Appl. Mater. Interfaces 2020, 12, 36072–36081. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, X.; Dong, M.; Xiong, Y.; Zhang, Z.; Ao, H.; Liu, M.; Zhu, Y.; Qian, Y. A large format aqueous rechargeable LiMn2O4/Zn battery with high energy density and long cycle life. Sci. China Mater. 2021, 64, 783–788. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, D.; Gan, Y. Traditional Electrochemical Zn2+ Intercalation/Extraction Mechanism Revisited: Unveiling Ion-Exchange Mediated Irreversible Zn2+ Intercalation for the δ-MnO2 Cathode in Aqueous Zn Ion Batteries. Adv. Energy Mater. 2024, 14, 2302655. [Google Scholar] [CrossRef]
- Li, D.; Ye, Z.; Ding, H.; Li, J.; Huang, H.; Yang, Z.; Su, J.; Zhu, J.; Zhang, W. Boosting proton intercalation via sulfur anion doping in V2O3 cathode materials towards high capacity and rate performance of aqueous zinc ion batteries. Energy Storage Mater. 2024, 71, 103635. [Google Scholar] [CrossRef]
- Li, F.; Ma, H.; Sheng, H.; Wang, Z.; Qi, Y.; Wan, D.; Shao, M.; Yuan, J.; Li, W.; Wang, K.; et al. Interlayer and Phase Engineering Modifications of K-MoS2@C Nanoflowers for High-Performance Degradable Zn-Ion Batteries. Small 2024, 20, 2306276. [Google Scholar] [CrossRef] [PubMed]
- Sada, K.; Darga, J.; Manthiram, A. Challenges and Prospects of Sodium-Ion and Potassium-Ion Batteries for Mass Production. Adv. Energy Mater. 2023, 13, 2302321. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, R.; Zhang, Q.; Ali, U.; Li, Y.; Hao, Y.; Jia, H.; Li, Y.; Zeng, G.; Sun, M.; et al. Ultrafine manganese hexacyanoferrate with low defects regulated by potassium polyacrylate for high-performance aqueous Zn-ion batteries. J. Energy Storage 2023, 72, 108535. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry With H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Deng, X.Y.; Ma, Z.Z.; Wang, X.G. Competition-cooperation mechanism between adsorption and conversion reaction for a novel SnS2@C composite cathode in zinc-ion batteries. J. Alloys Compd. 2025, 1014, 178855. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, N.; Qi, Y.; Zhu, Z.; Zhang, T.; Han, X.; Li, S.; Wu, J.; Qiu, J. Hollow iron carbides via nanoscale Kirkendall cavitation process for zinc-air batteries. Appl. Surf. Sci. 2022, 585, 152569. [Google Scholar] [CrossRef]
- Li, L.; Cheng, S.; Deng, L.; Liu, T.; Dong, W.; Liu, Y.; Huang, L.; Yao, H.; Ji, X. Effective Solution toward the Issues of Zn-Based Anodes for Advanced Alkaline Ni-Zn Batteries. ACS Appl. Mater. Interfaces 2023, 15, 3953–3960. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Zhang, R.; Shen, T.; Sun, J.; Hu, Z.; Li, L.; Yang, L.; Yu, H.Y. Advanced cellulose-based materials toward stabilizing zinc anodes. Sci. China Chem. 2024, 67, 1465–1484. [Google Scholar] [CrossRef]
- Li, M.; Lai, C.; He, X.; Zhang, Z.; Hu, J.; Shan, B.; Jiang, K.; Wang, K. Texturing Crystal Plane of Zinc Metal via Cleavage Fracture for a Dendrite-Free Zinc Anode. ACS Appl. Mater. Interfaces 2022, 14, 49719–49729. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Zhu, X.; Wan, Y.; Zhong, C. Interface Engineering of Zinc Electrode for Rechargeable Alkaline Zinc-Based Batteries. Small Methods 2023, 7, 2201277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Zhang, X.; Lin, C.; Zhang, H.; Miao, X.; Lin, J.; Chen, S.; Zhang, Y. A high-voltage aqueous rechargeable zinc-polyaniline hybrid battery achieved by decoupling alkali-acid electrolyte. Chem. Eng. J. 2022, 444, 136478. [Google Scholar] [CrossRef]
- Qu, W.; Cai, Y.; Chen, B.; Zhang, M. Heterointerface Engineering-Induced Oxygen Defects for the Manganese Dissolution Inhibition in Aqueous Zinc Ion Batteries. Energy Environ. Mater. 2024, 7, 12645. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, B.H.; Huang, J.; Xiao, K.; Liu, Z.Q. Microstructure Strain of ZnMn2O4 Spinel by Regulation of Tetrahedral Sites for High-Performance Aqueous Zinc-Ion Battery. Adv. Funct. Mater. 2024, 34, 2405680. [Google Scholar] [CrossRef]
- Juran, T.R.; Young, J.; Smeu, M. Density Functional Theory Modeling of MnO2 Polymorphs as Cathodes for Multivalent Ion Batteries. J. Phys. Chem. C 2018, 122, 8788–8795. [Google Scholar] [CrossRef]
- Wu, X.; Xiang, Y.; Peng, Q.; Wu, X.; Li, Y.; Tang, F.; Song, R.; Liu, Z.; He, Z.; Wu, X. Green-low-cost rechargeable aqueous zinc-ion batteries using hollow porous spinel ZnMn2O4 as the cathode material. J. Mater. Chem. A 2017, 5, 17990–17997. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Li, C.; He, Z.; Xiang, Y.; Xiong, L.; Chen, D.; Yu, Y.; Sun, K.; He, Z.; et al. The electrochemical performance improvement of LiMn2O4/Zn based on zinc foil as the current collector and thiourea as an electrolyte additive. J. Power Sources 2015, 300, 453–459. [Google Scholar] [CrossRef]
- Hoang, T.K.A.; Doan, T.N.L.; Cho, J.H.; Su, J.Y.J.; Lee, C.; Lu, C.; Chen, P. Sustainable Gel Electrolyte Containing Pyrazole as Corrosion Inhibitor and Dendrite Suppressor for Aqueous Zn/LiMn2O4 Battery. ChemSusChem 2017, 10, 2816–2822. [Google Scholar] [CrossRef]
- Cui, J.; Wu, X.; Yang, S.; Li, C.; Tang, F.; Chen, J.; Chen, Y.; Xiang, Y.; Wu, X.; He, Z. Cryptomelane-Type KMn8O16 as Potential Cathode Material—For Aqueous Zinc Ion Battery. Front. Chem. 2018, 6, 600352. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Zhang, X. Challenges and perspectives for manganese-based oxides for advanced aqueous zinc-ion batteries. InfoMat 2020, 2, 237–260. [Google Scholar] [CrossRef]
- Wei, T.; Li, Q.; Yang, G.; Wang, C. An electrochemically induced bilayered structure facilitates long-life zinc storage of vanadium dioxide. J. Mater. Chem. A 2018, 6, 8006–8012. [Google Scholar] [CrossRef]
- Tang, B.; Zhou, J.; Fang, G.; Guo, S.; Guo, X.; Shan, L.; Tang, Y.; Liang, S. Structural Modification of V2O5 as High-Performance Aqueous Zinc-Ion Battery Cathode. J. Electrochem. Soc. 2019, 166, A480–A486. [Google Scholar] [CrossRef]
- Xu, X.; Xiong, F.; Meng, J.; Wang, X.; Niu, C.; An, Q.; Mai, L. Vanadium-Based Nanomaterials: A Promising Family for Emerging Metal-Ion Batteries. Adv. Funct. Mater. 2020, 30, 1904398. [Google Scholar] [CrossRef]
- Dai, X.; Wan, F.; Zhang, L.; Cao, H.; Niu, Z. Freestanding graphene/VO2 composite films for highly stable aqueous Zn-ion batteries with superior rate performance. Energy Storage Mater. 2019, 17, 143–150. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Towards High-Voltage Aqueous Metal-Ion Batteries Beyond 1.5 V: The Zinc/Zinc Hexacyanoferrate System. Adv. Energy Mater. 2015, 5, 1501125. [Google Scholar] [CrossRef]
- Trocoli, R.; La Mantia, F. An Aqueous Zinc-Ion Battery Based on Copper Hexacyanoferrate. Chemsuschem 2015, 8, 481–485. [Google Scholar] [CrossRef]
- Li, Q.; Ma, K.; Hong, C.; Yang, Z.; Qi, C.; Yang, G.; Wang, C. High-voltage K/Zn dual-ion battery with 100,000-cycles life using zero-strain ZnHCF cathode. Energy Storage Mater. 2021, 42, 715–722. [Google Scholar] [CrossRef]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous Zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Kim, S.; Oguchi, H.; Toyama, N.; Sato, T.; Takagi, S.; Otomo, T.; Arunkumar, D.; Kuwata, N.; Kawamura, J.; Orimo, S.-i. A complex hydride lithium superionic conductor for high-energy-density all-solid-state lithium metal batteries. Nat. Commun. 2019, 10, 1081–1092. [Google Scholar] [CrossRef]
- Lu, Y.; Hou, X.; Miao, L.; Li, L.; Shi, R.; Liu, L.; Chen, J. Cyclohexanehexone with Ultrahigh Capacity as Cathode Materials for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 7020–7024. [Google Scholar] [CrossRef]
- Sheng, X.; Zhang, Z.; Ding, T.; Liao, J.; Zhou, X. Recent Advances in Amorphous FePO4 for Sodium-Ion Battery Cathodes. Chem. J. Chin. Univ. Chin. 2023, 44, e200336. [Google Scholar]
- Wu, Y.; Shi, M.; Luo, D.; Zhang, Z.; Li, Z.; Cheng, Z.; Kang, X. Synergetic effect of TiO2 coating and oxygen vacancy boosting LiMn2O4 cathode for stable aqueous zinc-ion batteries. J. Electroanal. Chem. 2023, 943, 117597. [Google Scholar] [CrossRef]
- Yesibolati, N.; Umirov, N.; Koishybay, A.; Omarova, M.; Kurmanbayeva, I.; Zhang, Y.; Zhao, Y.; Bakenov, Z. High Performance Zn/LiFePO4 Aqueous Rechargeable Battery for Large Scale Applications. Electrochim. Acta 2015, 152, 505–511. [Google Scholar] [CrossRef]
- Mainar, A.R.; Leonet, O.; Bengoechea, M.; Boyano, I.; de Meatza, I.; Kvasha, A.; Guerfi, A.; Alberto Blazquez, J. Alkaline aqueous electrolytes for secondary zinc-air batteries: An overview. Int. J. Energy Res. 2016, 40, 1032–1049. [Google Scholar] [CrossRef]
- Santos, F.; Tafur, J.P.; Abad, J.; Fernandez Romero, A.J. Structural modifications and ionic transport of PVA-KOH hydrogels applied in Zn/Air batteries. J. Electroanal. Chem. 2019, 850, 113380. [Google Scholar] [CrossRef]
- Zhou, M.; Fu, C.Y.; Qin, L.P.; Ran, Q.; Guo, S.; Fang, G.Z.; Lang, X.Y.; Jiang, Q.; Liang, S.Q. Intrinsic structural optimization of zinc anode with uniform second phase for stable zinc metal batteries. Energy Storage Mater. 2022, 52, 161–168. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, Y.; Li, Q.; Liang, G.; Yang, S.; Huang, Z.; Yang, Q.; Hu, H.; Li, X.; Chen, Z.; et al. An immiscible phase-separation electrolyte and interface ion transfer electrochemistry enable zinc/lithium hybrid batteries with a 3.5 V-class operating voltage. Energy Environ. Sci. 2023, 16, 4054–4064. [Google Scholar] [CrossRef]
- Deng, R.; He, Z.; Chu, F.; Lei, J.; Cheng, Y.; Zhou, Y.; Wu, F. An aqueous electrolyte densified by perovskite SrTiO3 enabling high-voltage zinc-ion batteries. Nat. Commun. 2023, 14, 4981–4987. [Google Scholar] [CrossRef]
- Alawi, M.J.; Gamal, H.; Rashad, M.; Alziyadi, M.O.; Shalaby, M.S. Zinc-ion batteries: Drawbacks, opportunities, and optimization performance for sustainable energy storage. J. Alloys Compd. 2025, 1012, 178455. [Google Scholar] [CrossRef]
- Cui, J.; Chen, Y.; Dong, Y.; Zhang, H.; Ivey, D.G. Sodium succinate as functional electrolyte additive to achieve highly reversible zinc-ion batteries. J. Mater. Chem. A 2024, 12, 28475–28485. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Zhang, L.; Wang, R.; Ma, Q.; Xiong, P.; Zhang, C. Minireview and Perspectives on Functional Electrolyte Additives for Aqueous Zinc-Ion Batteries. Energy Fuels 2024, 38, 15998–16009. [Google Scholar] [CrossRef]
- Wu, L.; Li, Z.; Xiang, Y.; Dong, W.; Qi, X.; Ling, Z.; Xu, Y.; Wu, H.; Levi, M.D.; Shpigel, N.; et al. Revisiting the Charging Mechanism of α-MnO2 in Mildly Acidic Aqueous Zinc Electrolytes. Small 2024, 20, 2404583. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 39–51. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, J.; Zheng, X.; Emwas, A.-H.; Lei, Y.; Mohammed, O.F.; Cui, Y.; Alshareef, H.N. Concentrated dual-cation electrolyte strategy for aqueous zinc-ion batteries. Energy Environ. Sci. 2021, 14, 4463–4473. [Google Scholar] [CrossRef]
- Parker, J.F.; Chervin, C.N.; Nelson, E.S.; Rolison, D.R.; Long, J.W. Wiring zinc in three dimensions re-writes battery performance-dendrite-free cycling. Energy Environ. Sci. 2014, 7, 1117–1124. [Google Scholar] [CrossRef]
- Burton, T.F.; Jommongkol, R.; Zhu, Y.; Deebansok, S.; Chitbankluai, K.; Deng, J.; Fontaine, O. Water-in-salt electrolytes towards sustainable and cost-effective alternatives: Example for zinc-ion batteries. Curr. Opin. Electrochem. 2022, 35, 101070. [Google Scholar] [CrossRef]
- Hou, W.; Araujo-Correa, A.E.; Qiu, S.; Velez, C.O.; Acosta-Tejada, Y.D.; Feliz-Hernández, L.N.; González-Nieves, K.; Morell, G.; Piñero Cruz, D.M.; Wu, X. Electrochemical Studies of Metal Phthalocyanines as Alternative Cathodes for Aqueous Zinc Batteries in “Water-in-Salt” Electrolytes. Batteries 2025, 11, 88. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, B.; Liu, X.; Liu, J.; Ding, J.; Zhong, C.; Hu, W. Water-in-salt electrolyte for safe and high-energy aqueous battery. Energy Storage Mater. 2021, 34, 461–474. [Google Scholar] [CrossRef]
- Liu, C.; Xu, W.; Zhang, L.; Zhang, D.; Xu, W.; Liao, X.; Chen, W.; Cao, Y.; Li, M.C.; Mei, C.; et al. Electrochemical Hydrophobic Tri-layer Interface Rendered Mechanically Graded Solid Electrolyte Interface for Stable Zinc Metal Anode. Angew. Chem. Int. Ed. 2024, 63, e202318063. [Google Scholar] [CrossRef]
- Xu, M.; Liu, F.; Chen, L.; Lei, Y.; Liu, Z.; Abdiryim, T.; Xu, F.; You, J.; Tan, Y.; Tan, Z.; et al. Zwitterionic poly(ionic liquid) hydrogel electrolytes with high-speed ion conduction channels for dendrite-free, long-enduring zinc-ion batteries and flexible electronics. Energy Storage Mater. 2025, 80, 104373. [Google Scholar] [CrossRef]
- Peng, H.; Wang, D.; Zhang, F.; Yang, L.; Jiang, X.; Zhang, K.; Qian, Z.; Yang, J. Improvements and Challenges of Hydrogel Polymer Electrolytes for Advanced Zinc Anodes in Aqueous Zinc-Ion Batteries. ACS Nano 2024, 18, 21779–21803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q.; Hong, H.; Yang, S.; Zhang, R.; Wang, X.; Jin, X.; Xiong, B.; Bai, S.; Zhi, C. Lean-water hydrogel electrolyte for zinc ion batteries. Nat. Commun. 2023, 14, 3890. [Google Scholar] [CrossRef] [PubMed]
- Radjendirane, A.C.; Sha, F.M.; Ramasamy, S.; Rajaram, R.; Angaiah, S. A Review on Recent Advances and Perspectives in Hydrogel Polymer Electrolytes for Aqueous Zinc-Ion Batteries. Energy Technol. 2024, 12, 2401105. [Google Scholar] [CrossRef]
- Cao, L.; Yang, M.; Wu, D.; Lyu, F.; Sun, Z.; Zhong, X.; Pan, H.; Liu, H.; Lu, Z. Biopolymer-chitosan based supramolecular hydrogels as solid state electrolytes for electrochemical energy storage. Chem. Commun. 2017, 53, 1615–1618. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, W.; Wei, M.; Zhang, P.; Zhao, S.; Pei, P.; Qiu, L.; Wang, H.; Meng, Z.; Wang, K. A photonic hydrogel for health self-monitoring of solid-state electrolytes in zinc-air batteries. Energy Storage Mater. 2022, 53, 136–147. [Google Scholar] [CrossRef]
- Gao, J.; Guo, F.; Ji, C.; He, X.; Mi, H.; Qiu, J. A flexible and stable zinc-ion hybrid capacitor with polysaccharide-reinforced cross-linked hydrogel electrolyte and binder-free carbon cathode. J. Mater. Chem. A 2022, 10, 24639–24648. [Google Scholar] [CrossRef]
- He, X.; Zhang, H.; Zhao, X.; Zhang, P.; Chen, M.; Zheng, Z.; Han, Z.; Zhu, T.; Tong, Y.; Lu, X. Stabilized Molybdenum Trioxide Nanowires as Novel Ultrahigh-Capacity Cathode for Rechargeable Zinc Ion Battery. Adv. Sci. 2019, 6, 1900151. [Google Scholar] [CrossRef]
- Gao, C.; Duan, Y.; Liu, Y.; Gu, J.; Guo, Z.; Huo, P. A high energy density supercapacitor fabricated with aqueous polymer electrolyte based on soybean protein isolate grafted by polyacrylic acid. J. Power Sources 2022, 541, 231658. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, M.; Shi, F.; Liu, X.; Tang, Z.; Wang, Y.; Huang, Y.; Hou, H.; Xie, X.; Zhi, C. An Intrinsically Stretchable and Compressible Supercapacitor Containing a Polyacrylamide Hydrogel Electrolyte. Angew. Chem. Int. Ed. 2017, 56, 9141–9145. [Google Scholar] [CrossRef]
- Yang, S.; Wu, Q.; Li, Y.; Luo, F.; Zhang, J.; Chen, K.; You, Y.; Huang, J.; Xie, H.; Chen, Y. A Bio-Inspired Multifunctional Hydrogel Network with Toughly Interfacial Chemistry for Dendrite-Free Flexible Zinc Ion Battery. Angew. Chem. Int. Ed. 2024, 63, e202409160. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Li, Y.; Cheng, X.; Yang, C.; Wang, K.-P.; Zhang, Q.; Wang, L. Lean-water hydrogel electrolyte with improved ion conductivity for dendrite-free zinc-Ion batteries. Chem. Eng. J. 2024, 490, 151524. [Google Scholar]
- Yan, H.; Zhang, X.; Yang, Z.; Xia, M.; Xu, C.; Liu, Y.; Yu, H.; Zhang, L.; Shu, J. Insight into the electrolyte strategies for aqueous zinc ion batteries. Coord. Chem. Rev. 2022, 452, 214297. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, Y.; Li, Z.; Tang, Z.; Pu, J.; Luo, L.; Ji, Y.; Xie, J.; Shu, Z.; Yao, Y.; et al. Highly-Entangled Hydrogel Electrolyte for Fast Charging/Discharging Properties in Aqueous Zinc Ion Batteries. Adv. Funct. Mater. 2024, 35, 2406620. [Google Scholar]
- Sun, M.; Ji, G.; Li, M.; Zheng, J. A Robust Hydrogel Electrolyte with Ultrahigh Ion Transference Number Derived from Zincophilic “Chain-Gear” Network Structure for Dendrite-Free Aqueous Zinc Ion Battery. Adv. Funct. Mater. 2024, 34, 2402004. [Google Scholar]
- Zeng, Z.; Liao, S.; Ma, G.; Qu, J. High-Conductivity and Ultrastretchable Self-Healing Hydrogels for Flexible Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2024, 16, 58961–58972. [Google Scholar]
- Long, J.; Han, T.; Lin, X.; Zhu, Y.; Liu, J.; Niu, J. A quasi-solid-state self-healing flexible zinc-ion battery using a dual-crosslinked hybrid hydrogel as the electrolyte and Prussian blue analogue as the cathode material. Chem. Sci. 2024, 15, 10200–10206. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Y.; Zhang, Y.; Yu, M.; Zhao, Q.; Briscoe, J.; Lu, Y.; Yao, J. Tough and recyclable hydrogel electrolytes with continuous ion migration pathways for dendrite-free zinc-ion batteries under harsh conditions. J. Mater. Chem. A 2025, 13, 13276–13285. [Google Scholar]
- Chen, M.; Zhou, W.; Wang, A.; Huang, A.; Chen, J.; Xu, J.; Wong, C.P. Anti-freezing flexible aqueous Zn–MnO2 batteries working at −35 °C enabled by a borax-crosslinked polyvinyl alcohol/glycerol gel electrolyte. J. Mater. Chem. A 2020, 8, 6828–6841. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Qi, L.; Li, M.; Wang, S.; Chen, J.; Sui, Z.; Bi, T.; Tang, Q.; Yu, L.; et al. A Bioinspired Gradient Hydrogel Electrolyte Network with Optimized Interfacial Chemistry toward Robust Aqueous Zinc-Ion Batteries. ACS Nano 2025, 19, 26770–26781. [Google Scholar] [CrossRef]
- Wu, R.; Yang, S.; Wang, R.; Guo, Y.; Du, P. Pullulan enhanced dual-network hydrogel electrolyte for high-performance flexible zinc-ion batteries. Chem. Eng. J. 2025, 509, 161223. [Google Scholar] [CrossRef]
- Du, L.L.; Wang, S.W.; Song, W.J.; Liu, H.; Wang, P.F.; Zhu, M.; Gong, Z.; Zhang, Y.H.; Wu, Y.H.; Shi, F.N. Liquid-permeable and solid-blocking hydrogel barrier layer enables dendrite-free growth of zinc anodes in aqueous zinc-ion batteries. J. Alloys Compd. 2025, 1031, 180826. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Wang, L.X.; Tang, X.N.; Li, L.; Shi, Y.; Shao, J.J. Application of poly(vinyl alcohol) conductive hydrogel electrolytes in zinc ion batteries. Chin. J. Inorg. Chem. 2025, 41, 893–902. [Google Scholar]
- Yu, J.; Li, M.; Kong, X.; Wang, T.; Zhang, H.; Zhu, X.; Zhao, J.; Ma, Z.; Yang, H. Polyanion hydrogel electrolyte with a high Zn2+ transference number for dendrite-free aqueous zinc-ion batteries. J. Mater. Chem. A 2025, 13, 16182–16192. [Google Scholar] [CrossRef]
- Chen, Y.; He, S.; Rong, Q. Stretchable, anti-drying, and self-healing hydrogel electrolytes for thermal adaptive zinc–air batteries with robust electrolyte/electrode interfaces. Mater. Today Chem. 2023, 33, e201726. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, S.; Huang, J.; Chen, L.; Bi, T.; Qi, L.; Cai, Z.; Zeng, X.; Hu, P.; Chen, W.; et al. Coupling of Mechanical, Self-Healing, Adhesion, and High-Ion Conducting Properties in Anti-Freezing Hydrogel Electrolytes of Zinc Ion Batteries via Fe3+-Carboxylate Coordination. Adv. Funct. Mater. 2025, 35, 2504726. [Google Scholar] [CrossRef]
- Song, Z.; Liu, X.; Ding, J.; Liu, J.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Poly(acrylic acid)-Based Composite Gel Polymer Electrolytes with High Mechanical Strength and Ionic Conductivity toward Flexible Zinc-Air Batteries with Long Cycling Lifetime. ACS Appl. Mater. Interfaces 2022, 14, 49801–49810. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Gui, Q.; Sun, S.; Zhao, P.; Mao, L.; Luo, T. High-Performance Fatigue-Resistant Dual-Polyrotaxane Hydrogel Electrolytes for Flexible Aqueous Zinc-Ion Batteries. Small 2025, 21, 2500124. [Google Scholar] [CrossRef]
- Wei, T.; Ren, Y.; Li, Z.; Zhang, X.; Ji, D.; Hu, L. Bonding interaction regulation in hydrogel electrolyte enable dendrite-free aqueous zinc-ion batteries from −20 to 60 °C. Chem. Eng. J. 2022, 434, 134646. [Google Scholar] [CrossRef]
- Ji, S.; Luo, H.; Qin, S.; Zhang, X.; Hu, Y.; Zhang, W.; Sun, J.; Xu, J.; Xie, H.; Yan, Z.; et al. Component Fluctuation Modulated Gelation Effect Enable Temperature Adaptability in Zinc-Ion Batteries. Adv. Energy Mater. 2024, 14, 2400063. [Google Scholar] [CrossRef]
- Gao, R.; Wang, J.; Song, Y.; Li, K.; Chen, Z.; Shen, Q.; Wang, Y. Polymer-Salt Effects with Enhanced Eutectic Behavior in Hydrogel Electrolytes for Aqueous Zinc Batteries at −70 °C. Adv. Funct. Mater. 2025, e14585. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Li, M.; Li, H.; Zhang, J.; Zhao, Y.; Xu, G.; Hu, J.; Lin, T.; Zhang, N. Lean-Water Gel Electrolyte Enables Zinc Ion Battery at −70 °C. Angew. Chem. Int. Ed. 2025, e202511520. [Google Scholar] [CrossRef]
- Ma, B.; Gao, Y.; Miao, L.; Xuan, H.; Tao, X.; Cheng, X.; Shi, P.; Huang, Y.; Zhao, Y.; Shao, Y.; et al. Unraveling the Ultrafast Deposition Kinetics Within Zincphilic and Hydrophobic Organic Interphases for Dendrite-Free and Long Lifespan Zinc Anodes. Adv. Funct. Mater. 2025, e13183. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, D.; Chang, L.; Zhang, Y.; Wang, W.; Zhang, W.; Zhu, Q. Interface engineering of electron-ion dual transmission channels for ultra-long lifespan quasi-solid zinc-ion batteries. Energy Storage Mater. 2025, 74, 103903. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, T.; Yang, J.L.; Liu, Y.; Xian, J.; Liu, K.; Zhao, Y.; Fan, H.J.; Yang, P. In-Situ Spontaneous Electropolymerization Enables Robust Hydrogel Electrolyte Interfaces in Aqueous Batteries. Angew. Chem. Int. Ed. 2024, 63, e202400230. [Google Scholar] [CrossRef]
- Liu, B.; Xu, Z.; Wei, C.; Zhu, Z.; Fang, Y.; Lei, X.; Zhou, Y.; Tang, C.; Ni, S.; Pan, H.; et al. Re-understanding and mitigating hydrogen release chemistry toward reversible aqueous zinc metal batteries. eScience 2025, 5, 100330. [Google Scholar] [CrossRef]
- Tan, H.; Meng, C.; Zhang, Y.P.; Zhao, J.Y.; Chen, H.; Chen, L.; Yin, Z.H.; Wu, X.L.; Liu, H.; Wang, J.J. Decoupling of Ion-Solvent Interactions via Compartmentalized Molecular Design for Ultra-Stable Aqueous Zinc Batteries. Adv. Funct. Mater. 2025, e16270. [Google Scholar] [CrossRef]
- Liu, T.; Du, X.; Wu, H.; Ren, Y.; Wang, J.; Wang, H.; Chen, Z.; Zhao, J.; Cui, G. A Bio-Inspired Methylation Approach to Salt-Concentrated Hydrogel Electrolytes for Long-Life Rechargeable Batteries. Angew. Chem. Int. Ed. 2023, 62, e202311589. [Google Scholar] [CrossRef]
- Wei, S.; Wang, Y.; Chen, S.; Song, L. Structure regulation and synchrotron radiation investigation of cathode materials for aqueous Zn-ion batteries. Chem. Sci. 2024, 15, 7848–7869. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Z.; Zhang, S.; Sun, L.; Li, M.; Yuwono, J.A.; Mao, J.; Hao, J.; Vongsvivut, J.P.; Xing, L.; et al. A biocompatible electrolyte enables highly reversible Zn anode for zinc ion battery. Nat. Commun. 2023, 14, 6526. [Google Scholar] [CrossRef]
- Han, J.; Jung, S.; Heo, S.E.; Choi, B.; Ryu, S.; Park, S.; Hong, J.; Yoo, J. Anisotropic Ion-Guiding Hydrogel Electrolyte with High-Water Affinity for Zn Ion Battery. Small 2025, 21, e2500799. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Gao, X.; Dong, H.; Zhao, X.; He, G.; Yang, H. In-Situ Self-Respiratory Solid-to-Hydrogel Electrolyte Interface Evoked Well-Distributed Deposition on Zinc Anode for Highly Reversible Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2025, 64, e202415251. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhao, H.; Li, J.; Xu, H.; Sun, P.; Xu, G.; Li, J.; Pan, L. Unlocking the mysteries of interfacial processes in zinc-ion batteries through multiscale advanced characterization techniques. Nano Res. 2025, e202511520. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Yu, L.; Lizundia, E.; Zeng, X.; Chen, L.; Lu, Z.; Qi, L.; Lin, Y.; Deng, H.; et al. Practical, sustainable, wide-temperature-adaptable zinc-metal batteries enabled by electrogelated recyclable biomacromolecular hydrogel electrolytes. Natl. Sci. Rev. 2025, 12, nwaf308. [Google Scholar] [CrossRef]
- Lu, H.; Hu, J.; Wei, X.; Zhang, K.; Xiao, X.; Zhao, J.; Hu, Q.; Yu, J.; Zhou, G.; Xu, B. A recyclable biomass electrolyte towards green zinc-ion batteries. Nat. Commun. 2023, 14, 4435. [Google Scholar] [CrossRef]

| Electrolyte | Tensile Strength (MPa) | Fracture Strain (%) | Ionic Conductivity (mS cm−1) | Zn2+ Transference Number | Zn||Zn Symmetric Cell (Cycle Time, Current Density/Capacity) | Full Cell Performance (Cathode||Anode, Cycles, Test Condition, Capacity Retention) | Refs. |
|---|---|---|---|---|---|---|---|
| PM-HE | 0.23 | 790 | 60.6 | 0.88 | >1500 h, 1 mA cm−2/1 mAh cm−2 | Zn||MnO2, 1000, 5 C, 91.6% | [105] |
| PAM/trehalose | 0.1 | 5338 | - | - | >2400 h, 1 mA cm−2/1 mAh cm−2 | Zn||MnO2, 3000, 10 A g−1, 62.7% | [101] |
| PAM−PAAS−QCS | 0.077 | 5100 | 33.61 | 0.72 | 1400 h, 0.5 mA cm−2/0.5 mAh cm−2 | Zn||PANI, 1500, 1 A g−1, 82.4% | [106] |
| PAM/PVA | 0.08 | 1490 | 6.7 | - | 500 h, 1 mA cm−2/1 mAh cm−2 | Zn||Co3[Fe(CN)6]2, 300, 1 A g−1, 79.5% | [107] |
| P3B2Z2 | 1.1 | 1455.6 | 19.4 | 0.53 | >1500 h, 2 mA cm−2/2 mAh cm−2 | Zn||KVOH, 1500, 5 A g−1, 77.5% | [108] |
| PVA/borax/glycerol | 0.1 | 490 | 29.6 | - | >1400 h, 2 mA cm−2/2 mAh cm−2 | Zn||rGO/MnO2, 2000, 1 A g−1, ~90% | [109] |
| PCG20-PC5 | 0.43 | 320 | 16.18 | 0.45 | >2200 h, 1 mA cm−2/1 mAh cm−2 | Zn|| MnO2, 800, 1 A g−1, 89.6% | [110] |
| PPZ | - | - | 30.1 | 0.84 | 1800 h, 0.5 mA cm−2/0.5 mAh cm−2 | Zn||PANI@TOC, 100, 2 mA cm−2, — | [112] |
| PMZA | - | - | 71.17 | 0.912 | 1800 h, 0.5 mA cm−2/0.5 mAh cm−2 | Zn||NVO, 7000, 5 A g−1, 100% | [114] |
| ATAC/EG/ PAA/Zn(OTF)2 | 0.08 | 570 | 7.5 | - | - | Zn||CC/Pt/C/RuO2, 127 h, 0.1 mA cm−2, — | [115] |
| PAA/CNF | 0.04 | ~220 | 32 | - | 4600 h, 0.5 mA cm−2/0.25 mAh cm−2, at −20 °C | Zn||FeHCF, 3000 h, 4 A g−1, 84.1% | [116] |
| PAA/Al2O3 | 0.1 | ~800 | 186 | - | - | Zn||Co3O4/C/Ni, 384 h, 2 mA cm−2, — | [117] |
| DPR/PAA | 0.38 | 1450 | 23.24 | - | >1700 h, 5 mA cm−2/5 mAh cm−2 | Zn||MnO2, 1000 h, 3 A g−1, 81.3% | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Xiong, S.; Li, J.; Wang, L.; Tang, X.; Li, L.; Sun, Q.; Shi, Y.; Shao, J. Hydrogel Polymer Electrolytes for Aqueous Zinc-Ion Batteries: Recent Progress and Remaining Challenges. Batteries 2025, 11, 380. https://doi.org/10.3390/batteries11100380
Zhu Z, Xiong S, Li J, Wang L, Tang X, Li L, Sun Q, Shi Y, Shao J. Hydrogel Polymer Electrolytes for Aqueous Zinc-Ion Batteries: Recent Progress and Remaining Challenges. Batteries. 2025; 11(10):380. https://doi.org/10.3390/batteries11100380
Chicago/Turabian StyleZhu, Zhaoxuan, Sihan Xiong, Jing Li, Lixin Wang, Xiaoning Tang, Long Li, Qi Sun, Yan Shi, and Jiaojing Shao. 2025. "Hydrogel Polymer Electrolytes for Aqueous Zinc-Ion Batteries: Recent Progress and Remaining Challenges" Batteries 11, no. 10: 380. https://doi.org/10.3390/batteries11100380
APA StyleZhu, Z., Xiong, S., Li, J., Wang, L., Tang, X., Li, L., Sun, Q., Shi, Y., & Shao, J. (2025). Hydrogel Polymer Electrolytes for Aqueous Zinc-Ion Batteries: Recent Progress and Remaining Challenges. Batteries, 11(10), 380. https://doi.org/10.3390/batteries11100380








