Diversity of Iron Oxides: Mechanisms of Formation, Physical Properties and Applications
Abstract
1. Introduction
2. Physical Properties of Various Iron Oxide Compounds
3. Mechanisms of Iron Oxide Formation
3.1. The Structures Containing Pure Phases of FeO, Fe4O5, Fe3O4, and α-, β-, γ-, δ-, ε- and ζ-Polymorphs of Fe2O3
| Composition | Main Mechanisms of Iron Oxide Formation | Declared Applications | Phase Verification Techniques | Refs. |
|---|---|---|---|---|
| FeO NPs | Thermal decomposition of the iron(II) precursor, mechanochemical reduction of magnetite, flame synthesis, laser target interaction in liquid carrier media | Biomedicine, electronics, spintronics, magnetic force microscopy, metastability studies | XRD 1, UV–Vis 2, MALDI-TOF MS 3, EELS 4, F-AAS 5 HAADF-STEM 6, | [41,74,75,76,77,78,79,80] |
| Ultra-thin FeO film | Oxidation of iron monocrystal surface | Iron oxidation kinetics study | RMDS 7 | [141] |
| Electron-beam deposition on Au(111) surface | Iron catalysis, electronics, biomedicine | STM 8 | [142] | |
| Millimeter-sized iron oxide particles | Magnetite reduction with iron as reducing agent | Catalysts for ammonia synthesis | TG-DSC 9 | [40] |
| FeO layer on the metal alloy surface | Invar oxidation in a static carbon dioxide atmosphere | Iron oxidation kinetics study | XRD, TG-DSC, TEM 10 | [143] |
| Wüstite inclusions in titanomagnetite particles | Titanomagnetite ironsand-fluidized bed reduction by hydrogen | Commercial iron making | XRD | [144] |
| FeO powder | Reduction of hematite in a gas-controlled electric furnace | Earth’s mantle sound velocity studies | XRD, IXS 11 | [145] |
| FeO inclusions in the mold flux | Iron oxide formation in molten mold flux | Study of the oxidation mechanism of mold flux-covered molten iron | XRF 12 | [146] |
| FeO inclusions within the dense iron shell | Porous hematite gas reduction under isothermal conditions | Industrial exploitation of low-grade iron ores | TG-DSC, XRD | [147] |
| FeO clusters within the stable iron oxide matrix | The reduction of magnetite/hematite at temperatures of 400~500 °C | Iron catalysis | Quantitative theoretical analysis | [148] |
| Composition | Main Mechanisms of Iron Oxide Formation | Declared Applications | Phase Verification Techniques | Refs. |
|---|---|---|---|---|
| Fe3O4 NPs | Co-precipitation from iron salt solution, co-precipitation from iron oxyhydroxide solution, solvothermal synthesis, electrochemical formation from a pure iron, thermal decomposition of the iron oleate complex, biomimetic process with use of a leaf extract, nucleation mediated by iron-binding protein Mms6, biogeneration with a use of amyloid peptide Aβ42 | Biomedicine, magnetic separation, antimicrobial and antioxidant applications, contaminant removal, black pigment production, ferrofluids | TEM, XRD, SAXS 1, RS 2, FTIR 3, XPS 4, HAADF-STEM, EELS, TG-DSC, UV–Vis, PL 5, MSP 6, SAED 7 | [81,82,83,84,85,86,87,88,89,90,91,149,150,151,152,153,154,155,156,157,158,159,160,161,162] |
| Bacterial magnetosomes | Bacterial biomineralization, transient phosphate-rich ferric hydroxide reduction to magnetite, formation by dissimilatory iron-reducing bacteria | Biomedicine, paleomagnetism, microbial iron cycle studies, bioremediation of toxic compounds | HAADF-STEM, TEM, XAS 8, SAED, XMCD 9 | [93,94,95,96,97,98,163] |
| Inclusions of Fe3O4 within ore samples | Abiotic hydrothermal mineralization, iron oxide formation derived from continental weathering, extrusive magmatic formation from iron oxide-melt liquid | Geochemistry, environmental magnetism studies, early Earth iron cycle studies, origin and evolution of iron oxides studies | XRD, RS, XPS, EDS 10, ICP-AES 11 | [99,100,101,102] |
| External magnetite layer on a metal surface | Oxidation of a steel surface, slow oxidation of green rusts at room temperature, high-temperature corrosion | Corrosion studies | XRD, EDS, XRF, RS, XPS, AES 12 | [105,106,107,108,109,110] |
| Fe3O4 microparticles | Microbial-induced precipitation with the use of Sporosarcina pasteurii | Green synthesis of magnetite | EDS | [28] |
| Aging of ferrous hydroxide gels at elevated temperatures | Colloidal crud formation studies | XRD, TEM | [162] | |
| Self-assembled Fe3O4 mesocrystalline films | Heat-up method with the use of iron(III) chloride and sodium oleate | Biomedicine and industrial applications | TEM, SAED, XAS, SAXS | [163] |
| Fe0/Fe3O4 composite | Controlled reduction of the starting Fe3O4 with H2 | Treatment of wastewater | MSP, XRD | [164] |
| Magnetite nanowires | Supercritical fluid inclusion within a mesoporous silica matrix | Soft magnetic materials | TEM, SAED, XRD, FTIR | [165] |
| Inclusions of Fe3O4 NPs | Bacterial reduction of amorphous hydrous ferric oxide | Biogeochemistry | TEM, SAED, XRD, EDS | [166] |
| Fe3O4 layer on the zerovalent iron surface | Surface oxidation of iron by oxygen in an aqueous medium | Organic pollutant removal | EDS, XRD | [167] |
| Epoxy/magnetite nanocomposites | Reduction of anhydrous ferric chloride by ammonium hydroxide | Marine coatings of steel | FTIR, XRD, TEM | [168] |
| Iron oxide nanocomposite hydrogel | Co-precipitation process by ammonium hydroxide | Biomedicine | XRD, TEM, TG-DSC, EDS | [169] |
| Surface film containing Fe3O4 NPs | Bacterial mineralization in the air–water interface in Arctic tundra waters | Anaerobic microbial carbon cycle | TEM, EDS, STEM, EELS, FTIR, RS | [170] |
| Nanocomposite hydrogel with Fe3O4 NPs | Reduction with ammonia from a remixed solution of FeCl3 and FeCl2 | Biomedicine | TEM, XRF, EDS, TG-DSC, FTIR | [171] |
| Biochar composite with Fe3O4 NPs | One-pot solvothermal method using phoenix tree leaf-derived biochar | Treatment of wastewater | TEM, XRD, FTIR, XPS, ICP-AES | [172] |
| Fe3O4 NP inclusions in the surface layer | Formation of NPs along with cracks and pores during pre-oxidation | Plasma nitriding of steel | XRD | [173] |
| Chitosan/graphene oxide composite with Fe3O4 | Co-precipitation of Fe3O4 and chitosan/graphene oxide | Organic pollutant removal | XRD, XPS, RS, FTIR | [174] |
| Fe3O4 layer on carbon fibers of a carbon paper | Deposition on the carbon paper gas diffusion layer at the cathode | Corrosion studies | XRD, EDS | [175] |
| Mesocrystals assembled from Fe3O4 nanocubes | Heat-up method with the use of iron(III) chloride and sodium oleate | Mesocrystal applications | TEM | [176] |
| Fe3O4 nanorods | Formation in electron-beam-induced deposition from iron pentacarbonyl | Electronics | TEM, EELS | [177] |
| Lipase immobilized on coated Fe3O4 NPs | Solvothermal method with the use of FeCl3·6H2O and ethylene glycol | Biodiesel production | TEM, XRD, FTIR | [178] |
| Spherical mesoporous magnetite aggregates | Precipitation from iron(III) ethoxide with ethanol in the surfactant solution | Catalysis, sustainability | FTIR, XPS, EDS, TEM, MSP | [179] |
| Perfluorocarbon-loaded hydrogel microcapsules | Coaxial interface shearing double emulsion method | Biomedicine | – | [180] |
| Mesoporous magnetite | Ball milling of Fe3O4 and SiO2 followed by partial reduction | Recyclable absorbent for toxic Cr(VI) ions | TEM, XRD, XPS, ICP-AES | [181] |
| Magnetite crystal model | Local spin-density approximation density-functional calculation | Magnetite electron structure studies | Density-functional calculations | [43] |
| Spherulite nanostructure with inclusions of Fe3O4 | Electron-beam irradiation of the precursor solution with iron nitrate | Crystal growth dynamics studies | TEM, STEM, EDS | [182] |
| Composition | Main Mechanisms of Iron Oxide Formation | Declared Applications | Phase Verification Techniques | Refs. |
|---|---|---|---|---|
| α-Fe2O3 NPs | Hydrothermal synthesis, precipitation from a ferric salt solution using a natural leaf extract, precipitation and aging of ferrihydrite in an oxidized system, direct transformation of α-FeOOH via high-energy ball milling | Biomedicine, bioremediation of toxic compounds, photocatalysis, geochemistry, electronics, antibacterial activity studies, geochemistry | XRD, FTIR, UV–Vis, EDS, TEM, RS, XPS, XAS, ICP-AES, EPR 1, HAADF-STEM, WAXS 2 | [105,106,107,108,109,110,111,180,181,182,183,184,185,186,187,188,189,190,191,192] |
| Inclusions of α-Fe2O3 in ore samples | Precipitation from oxygenated iron-rich water or biomineralization, dissolution of Fe(III) hydroxides by Fe(III)-reducing bacteria, terrestrial subglacial oxidation of glacial iron fluvial deposition | Terrestrial iron oxide concretion studies, Precambrian iron formation studies, Antarctic glacier studies, biogeochemistry | EDS, RS, TEM, HAADF-STEM, XRD, SAED, FTIR, UV–Vis | [112,113,114,115,116,117,118,119] |
| α-Fe2O3 layer on a metal surface | Anodic potentiostatic oxidation of stainless steel sheet | Anodic passivation of stainless steel | AES | [193] |
| Oxidation of steel in an O2-N2 atmosphere at high temperature | Improvement of steel coating quality | TEM, EDS, GD-OES 3 | [194] | |
| Corrosion of chromia-forming alloys in simplified combustion atmosphere | Fireside corrosion studies | EDS, XRD | [195] | |
| Porous α-Fe2O3 nanostructures | Hydrothermal synthesis from FeCl3·6H2O in a microwave reactor | Lithium-ion batteries | XRD, TEM, SAED, XPS, TG-DTG | [196] |
| Sol–gel transformations of precursors in self-organized nanocellulose | Energy conversion and storage | XRD, TEM, SAED, XPS, TG-DSC | [197] | |
| Martian hematite deposits | Precipitation from oxygenated iron-rich water or biomineralization | Search for evidence of life on Mars | EDS, TEM | [198] |
| Hematite layers on sandstone grains | Precipitation from oxidizing iron-saturated fluid | Geochemistry | XRD, ICP-MS 4 | [199] |
| Double-walled hematite nanotubes | Growth of Fe nanowires inside porous templates and oxidation | Photocatalysis, biomedicine | XRD, EELS, HAADF-STEM, RS | [200] |
| Coral-like and nanowire α-Fe2O3 | Thermal oxidation of iron foils in air- and water vapor-assisted conditions | Removal of Cr ions from aqueous systems | XRD, RS, TEM, XPS | [201] |
| α-Fe2O3 NPs on mineral surfaces | Weathering of Fe-bearing silicate minerals or partial oxidation of Fe3O4 | Paleoclimate studies | XRD, TEM, SAED | [202] |
| α-Fe2O3 nanorods | Controlled aqueous growth from FeCl3·6H2O and NaNO3 | Photoelectrochemical water splitting | XRD | [203] |
| Inclusions of α-Fe2O3 in regolith simulant | Ball milling of commercial α-Fe2O3 samples in isopropyl alcohol | Combustion studies | XRD, TG-DSC | [204] |
| Inclusions of α-Fe2O3 in stone matrix | Bacterial mineralization | Heritage sciences | XRD, EDS, RS | [205] |
| Inclusions of α-Fe2O3 in auriferous quartz | Terrigenous abiotic mineralization | Geochemistry | EDS | [206] |
| Hematite layers on sandstone grains | Terrigenous co-precipitation with sandstone and uranium | Geochemistry of radionuclides | Gamma-ray spectrometry, ICP-MS | [207] |
| Hematite inclusions encapsulated in chert | Dehydration of the interstitial goethite to hematite microplates | Geochemistry | TEM, XRD, EDS | [208] |
| Hollow α-Fe2O3 nanofibers | Electrospinning with a use of iron chloride and poly(vinylpyrrolidone) | Photoelectrochemical water splitting | EDS, TEM, SAED, TG-DSC, UV–Vis | [209] |
| Fossilized bacteria with α-Fe2O3 | Biomineralization by anoxygenic photoferrotrophy | Biogeochemistry | RS | [210] |
| Porous α-Fe2O3 xerogel and aerogel | Sol–gel synthesis from Fe(III) salts with addition of propylene oxide | Catalysis, sensors, biology | TEM | [211] |
| Iron oxide nanostructures | Microbial Fe(II) oxidation of carbonate green rust by Fe(II)-oxidizing bacteria | Precambrian iron formation studies | MSP | [212] |
| Iron oxide biogenic precipitates | Bacterial mineralization | Biogenic iron oxide formation studies | XAS | [213] |
| Steel-wearing ejected debris with α-Fe2O3 | Steel fretting wear controlled by oxygen ingress to the contact | Steel fretting wear studies | XRD | [214] |
| α-Fe2O3 NPs on a steel surface | Oxidation of iron-bonded diamond precision-polishing wheel | Grinding of hard and brittle materials | XRD, XPS, TEM | [215] |
| Nanostructured α-Fe2O3 films | Electrochemical anodization of steel in an alkaline solution | Photocatalysis, anti-bioadhesion | RS, UV–Vis | [216] |
| Monodispersed micaceous α-Fe2O3 | Hydrothermal synthesis from iron chromium hydroxide precursors | Iron chromium grinding waste recycling | ICP-AES, XRD, XPS | [217] |
| Nanoporous α-Fe2O3 layer on an iron foil | Anodization of iron is an ethylene glycol and NH4F aqueous solution | Photocatalysis | TEM, RS, XRD, UV–Vis, EDS, EELS | [218] |
| Natural α-Fe2O3 from the iron deposits | Terrigenous abiotic mineralization | Photocatalytic recycling of toxic wastewater | RS, EDS, UV–Vis | [219] |
| Nanocomposite containing α-Fe2O3 | Wet impregnation of Co3O4 powder with an Fe(NO3)⋅9H2O solution | Catalysis | XPS, XRD, TG-DSC, EDS, TEM | [220] |
| Stepped α-Fe2O3 (0001) surfaces | First principles spin-polarized density-functional theory simulation | Chloride-induced iron depassivation studies | Density-functional theory calculations | [221] |
| α-Fe2O3 powder | In situ generation of iron oxide via decomposition of Fe(NO3)3·9H2O | Catalysis | XRD | [222] |
| α-Fe2O3 nanorods | Hydrothermal precipitation and air calcination of goethite nanorods | Catalysis, lithium-ion batteries, sensors | XRD, MSP, UV–Vis, EDS, TG-DSC | [223] |
| α-Fe2O3 nano- and microparticles | Chemically synthesized commercial α-Fe2O3 samples | Mechanisms of oxide toxicity toward bacteria | FTIR, XAS | [224] |
| α-Fe2O3 nanowires | Heating of iron wires suspended between two electric contacts | Vacuum electronic devices | TEM, EDS, XPS, RS | [225] |
| α-Fe2O3 layer on zerovalent iron NPs | Iron oxide film formation under aerobic conditions | Remediation of water pollutants | TEM, FTIR, XPS, XRD | [226] |
| Inclusions of α-Fe2O3 in rock varnish | Terrigenous abiotic mineralization or biotic processes | Geomicrobiology | XRD, RS, EDS | [227] |
| Nanolayers of α-Fe2O3 in polymer composite | Iron pentacarbonyl transformation with diamond anvil cells in Ar gas | High-energy density solid studies | RS, TEM, XRD | [228] |
| Jian ware blue-colored glaze with α-Fe2O3 | Calcination of a milled mix at a high temperature in oxidizing atmosphere | Ancient ceramics studies | XRD, UV–Vis, TEM, XPS | [229] |
| Inclusions of α-Fe2O3 in sediment samples | Microbial reduction of surface Fe(III) by iron-reducing bacteria | Microbial iron reduction studies | XRD | [230] |
| Core-shell iron/iron oxide NPs | Zerovalent Fe core-controlled oxidation during deposition | Oxide formation under e-beam radiation studies | TEM, EELS | [231] |
| α-Fe2O3 film on a dielectric substrate | Liquid-phase atomic layer deposition of crystalline hematite | Catalysis, sensors, lithium-ion batteries | XRD, UV–Vis | [232] |
| Cube-shaped α-Fe2O3 microstructures | Facile hydrothermal method using hydrated ferric nitrate and NaOH | Ethanol gas sensing | XRD, FTIR, EDS, RS | [233] |
| Iron oxide/Ti composites | Plasma electrolytic oxidation, impregnation and annealing | Phenol photodegradation | XRD, EDS, FTIR, XPS | [234] |
| Microporous α-Fe2O3 NPs | Precipitation from iron(II) sulfate using a natural leaf extract | Sustainability | XRD, UV–Vis, XPS, FTIR | [235] |
| Inclusions of α-Fe2O3 in artificial clay | Fe(OH)3 colloid mixing into chemically pure kaolin | Laterite engineering | XRD | [236] |
| Iron oxide nanotubes | Potentiostatic anodization of iron foil in electrolytes containing NH4F | Catalysis, sensors, supercapacitors | XRD, TEM, SAED | [237] |
| α-Fe2O3 thin film | Spray pyrolysis from FeCl3 and methanol solution | Electrochemical supercapacitors | XRD, UV–Vis | [44] |
| Corroded steel tube samples with α-Fe2O3 | Steel corrosion in an aqueous medium with oxygen and chlorine | Pipeline corrosion assessment | XRD, EDS, TEM, SAED | [238] |
| Inclusions of α-Fe2O3 in stone samples | Formation by washing and leaching of a stone object by rainwater | Limestone artifact studies | RS, FTIR, EDS, XRF | [239] |
| Iron oxide-loaded slag | Precipitation from FeCl3 solution with NaOH into melted slag | Arsenic removal from water | ICP-AES, XRD | [240] |
| 3D-ordered macroporous α-Fe2O3 | Impregnation of polymer matrices and high-temperature calcination | Catalysis | XRD, TG-DSC, FTIR, SAED, UV–Vis, XPS | [241] |
| α-Fe2O3/mesoporous silica core-shell NPs | Solvothermal synthesis from ferric nitrate with sol–gel silica coating | Catalysis, biomedicine | XRD, TEM, FTIR, UV–Vis | [242] |
| Spindle-shaped α-Fe2O3 mesocrystal | Interface-driven nucleation by ferrihydrate oxidation and attachment | Thermoelectronics, photonics, catalysis, photovoltaics | TEM, SAED, FTIR, EDS | [243] |
| Hematite nanopillars | Electron-beam evaporation using anodized aluminum oxide templates with well-defined pore diameters | Photoelectrochemical water splitting | XRD, XPS, UV–Vis | [244] |
| Composition | Main Mechanisms of Iron Oxide Formation | Declared Applications | Phase Verification Techniques | Refs. |
|---|---|---|---|---|
| γ-Fe2O3 NPs | Solvothermal synthesis from iron salts, bacterial mineralization, lepidocrocite calcination in an air atmosphere, hydrothermal and solvothermal synthesis from salt solutions | Catalysis, biomedicine, nucleation and formation of biogenic iron oxide studies, electronics, maghemite to hematite transition studies, sensors | TEM, EDS, SAED, XRD, XPS, EPR, FTIR, UV–Vis, ICP-AES, HAADF-STEM, MSP, in situ total scattering, XAS, SAXS, RS | [93,125,126,127,128,129] |
| γ-Fe2O3 NPs in silica matrix | Gas-phase synthesis in a furnace aerosol reactor from iron pentacarbonyl | Biomedicine | XRD, TEM, EDS, FTIR, UV–Vis | [245] |
| Dehydration of iron(III) hydroxide to magnetite followed by oxidation | Catalysis | XRD, FTIR | [246] | |
| γ-Fe2O3 powder | Chemically synthesized commercial γ-Fe2O3 samples | Catalytic oxidation of S(IV) | ICP-MS, FTIR | [247] |
| 26-faceted maghemite polyhedrons | Direct burning of ferrocene in different solvents in an alcohol lamp | Lithium-ion batteries | XRD, TEM | [248] |
| Magnetic polymeric NPs with γ-Fe2O3 | Co-precipitation of FeCl3/FeCl2·4H2O with NH4OH solution | Biomedicine | TEM, TG-DSC, FTIR | [249] |
| γ-Fe2O3 NP superlattice thin films | Chemically synthesized commercial γ-Fe2O3 samples | Electronics, optical coatings | Grazing incidence small angle X-ray scattering | [250] |
| Maghemite-decorated graphene nanoscrolls | Hydrolysis of FeCl3·6H2O and W(CO)6, promoted with hydrazine | Energy storage | TEM, XPS, TG-DSC, RS | [251] |
| Hollow iron oxide NPs | Gas-phase vaporization synthesis of Fe NPs and oxidation to γ-Fe2O3 | Optics, nanoelectronics | TEM, HAADF-STEM, EDS | [252] |
| Mesoporous iron oxide | Inverse micelle synthesis from Fe(NO3)3·9H2O butanol solution | Arsenic removal from water | XRD, FTIR, RS, XPS | [253] |
| Composition | Main Mechanisms of Iron Oxide Formation | Declared Applications | Phase Verification Techniques | Refs. |
|---|---|---|---|---|
| Pristine and co-substituted ε-Fe2O3 | Simulated crystal structure with a use of density-functional calculations | Magnetoelectric material development | Density-functional theory calculations | [254] |
| ε-Fe2O3 embedded in biomimetic graphene | Precipitation from ferric and ferrous chloride with a biocompatible polymer | Biomedicine | XRD, TEM, SAED, RS, XPS, TG-DSC, FTIR | [130] |
| Epitaxially stabilized thin-film ε-Fe2O3 | Epitaxy on (100)-oriented yttrium-stabilized zirconia substrates | Electronics, permanent magnets, biomedicine | XRD, HAADF-STEM | [136] |
| ε-Fe2O3 in ancient black glazed wares | Surface iron enrichment and a firing of wares under reducing conditions | Electronics, spintronics | XRF, XAS, XRD, RS, TEM, EDS | [132] |
| ε-Fe2O3 NPs | Hydrolysis of tetraethoxysilane in a solution of ferric nitrate and annealing | Wireless technologies, electronics | XRD, TEM, THz-TDS 1 | [255] |
| ε-Fe2O3 inclusions in fired clay samples | Stabilization of ε-Fe2O3 NPs in a matrix of silicates during firing of clays | Paleomagnetism | XRD, EDS | [134] |
| Y3Fe5O12 matrix including ε-Fe2O3 | Formation of ε-Fe2O3 in the Y3Fe5O12 matrix using the sol–gel method | Magnetoelectric material development | XRD, XPS, TG-DSC, FTIR | [256] |
| δ-Fe2O3 in layered double hydroxyl | Dry impregnation of layered double hydroxyl structure with ferric nitrate | Photocatalysis | XRD, FTIR, XRF, TG-DSC, UV–Vis | [22] |
| ε-Fe2O3-SiO2 | Reverse micelle method with the use of ferric nitrate | Oxidative dehydrogenation of n-butene | XRD | [136] |
| β-Fe2O3 | Milling of Fe2(SO4)3 and NaCl and calcination at 550 °C in air | |||
| Ga-substituted ε-Fe2O3 NPs | Calcination of a mesoporous silica impregnated with metal nitrates | Biomedicine | XRD, XRF, TEM, ICP-MS | [131] |
| ε-Fe2O3 in archeological brick and baked clay | High-temperature firing of bricks and clays in air | Archaeomagnetism, paleomagnetism | RS | [138] |
| ε-Fe2O3 in archeological samples, ε-Fe2O3 NPs | Sol–gel synthesis from ferric and barium nitrate with tetraethyl orthosilicate | XRD, RS | [135] | |
| ε-Fe2O3 coatings on Si(100) substrates | One-pot sol–gel recipe assisted by glycerol in an acid medium | Paleomagnetism, biomedicine, electronics | RS, XAS, EELS, HAADF-STEM | [257] |
| ε-Fe2O3/SiO2 composite powder | Sol–gel synthesis from ferric and barium nitrate with tetraethyl orthosilicate | Electronics | XRD, TEM | [133] |
| ε-Fe2O3 nanorods | Chemical vapor deposition from the Fe organic liquid source | Photocatalysis, electronics | XPS | [258] |
| ε-Fe2O3/SiO2 composite | Sol–gel synthesis from nitrate with tetraethyl orthosilicate and nitric acid | Electronics, spintronics, magnetizable printing | TG-DSC, XRD, TEM | [259] |
| ε-Fe2O3 NPs | Immersion of mesoporous silica with an FeSO4 or Fe(C10H9CHO) solution and high-temperature calcination | High-coercivity material development | TEM, XRD, MSP, TEM, SAED | [20,41] |
| β-Fe2O3 NPs | Sensors, lithium-ion batteries | |||
| Epitaxial ε-Fe2O3 films on GaN substrate | Pulsed laser deposition on the Ga-terminated surface of the GaN (0001) | Electronics | XRD, RHEED 2, TEM, XAS, XMCD | [260] |
| Silica-coated ε-Fe2O3 NPs | Sol–gel treatment of β-FeOOH nanorods with tetraethoxysilane and calcination | Electronics | XRD, TEM, EDS, MSP | [261] |
| ε-Fe2O3 in a Hare’s Fur Jian ware | High-temperature firing of local iron-rich area on the ceramic glaze | Magnetoresistance materials | XRF, XAS, EDS, XRD, RS | [140] |
| Metal-substituted ε-Fe2O3 | Impregnation of mesoporous silica NPs with rhodium-substituted ε-Fe2O3 | Electronics, magnetic force microscopy, biomedicine | XRD | [262] |
| β-Fe2O3 NPs | Thermally-induced solid-state reaction of NaCl with Fe2(SO4)3 in air | Sensors, lithium-ion batteries | XRD, MSP, TEM, SAED | [20] |
| ζ-Fe2O3 | Pressure treatment of β-Fe2O3 NPs at pressures above 30 GPa | n/a | ||
| ε-Fe2O3 in a thin MgO(111) layer | Pulsed laser deposition from MgO and Fe2O3 targets ablated using a KrF laser | Electronics | RHEED, XRD, neutron reflectometry | [263] |
| Single crystal of Fe4O5 | Synthesis in the diamond anvil cell at high pressure after laser heating | Solid Earth studies | Density-functional theory calculations | [23] |
| Nanometer-scale lamellae of Fe4O5 | High-pressure and high-temperature multi-anvil synthesis | Deep Earth studies | XRD, TEM, SAED, EDS, STEM | [264] |
| Powder of Fe4O5 | High-pressure and high-temperature direct synthesis from a mixture of Fe3O4 and Fe | Electronics | XRD, neutron diffraction | [265] |
| β-Fe2O3 NPs | Thermally-induced solid-state reaction of NaCl with Fe2(SO4)3 in air | Optoelectronics, sensors, lithium-ion batteries | XRD, MSP, TEM | [64] |
| Hydrolysis of 2M FeCl3 in boiling water and cooling down slowly at room temperature | Biomedicine | UV–Vis, TEM, XRD, FTIR, EDS, SAED | [63] |
3.2. The Structures Containing Iron Oxide Atomic Clusters and an Amorphous Iron Oxide Phase
3.3. The Structures Containing Two Co-Existing Iron Oxide Crystal Phases
| Composition | Main Mechanisms of Iron Oxide Formation | Declared Applications | Phase Verification Techniques | Refs. |
|---|---|---|---|---|
| Iron oxide atomic clusters | Combustion synthesis from Fe(CO)5 mixed with hydrogen and oxygen, high irradiance laser ionization from pressed Fe2O3 and Fe3O4 tablets, biomineralization inside the ferritin shell, reaction of laser ablated iron foil with 5% O2 seeded in a helium carrier gas | Catalysis, biomedicine, electronics, sensors, prediction of the magnetic properties of FeOx NPs, natural iron storage process studies, photovoltaics | MBMS 1, PMS 2, RMDS 3, TEM, LI-TOFMS 4, density-functional theory calculations, European Synchrotron Radiation Facility | [271,272,273,274,275,276,277,281] |
| Surface iron oxide layer on metal | Multicycling of an iron foil electrode between the switching potentials, formation of iron oxide species after reaction with Cr(VI) and Cu(II) | Chemical water treatment, production of molecular hydrogen, removal of contaminants | RMDS, XRD, XPS, FTIR, EDS | [278,279,282] |
| Amorphous ferric oxides | Adding Fe(II) or Fe(III) to seawater | Bioavailable iron studies | XAS, XRD | [266] |
| Addition of Fe(III) to synthetic buffered solution or soluble microbial systems | Chemical water treatment | UV–Vis | [267] | |
| Amorphous Fe2O3 in a silica matrix | Impregnation of mesoporous silica with ferric nitrate and calcination | Antibiotic adsorption | TEM, XRD, FTIR, UV–Vis | [268] |
| Poorly crystalline iron oxides | Iron oxide biomineralization by iron-reducing bacteria | Geochemistry | ICP-MS | [270] |
| Amorphous iron oxide nanostructures | Photothermal reaction inside a droplet of iron(III) acetylacetonate solution | Electronics, sensors | TEM, SAED, EDS, RS | [269] |
| Two-dimensional iron oxide on Au(111) | Evaporating iron atoms, annealing and cooling down to 300 K in O2 | Catalysis | STM, density-functional theory calculations | [142] |
| Iron oxide layer on zerovalent iron NPs | Zerovalent iron corrosion in an electrolyte solution | Treatment of contaminated aquifers | UV–Vis, XAS | [283] |
| Ferric oxide NPs | Protein-promoted conversion of Fe(II) into insoluble ferric iron oxides | Mitochondrial iron mishandling studies | UV–Vis | [284] |
| Ultra-thin iron oxide nanowhiskers | Iron oleate complex followed by selective decomposition at 150 °C | Biomedicine | TG-DSC, TEM, SAED, RS, XPS, FTIR | [285] |
| High valent iron oxo complexes | Fluorine-substituted Fe−tetra-amidomacrocyclic ligand oxidation | Photocatalysis | UV–Vis, EPR, high-resolution mass spectrometry | [286] |
| FeO(111)-like film on Fe(110) surface | Initial oxidation of Fe(110) in oxygen via Frank–Van der Merwe mechanism | Catalysis, pigments, electronics | XPS, XAS, STM, AES, LEED 5, STS 6 | [280] |
| Colloidal Fe-FexOy composite NPs | Oxidation of metal NPs via a nanoscale Kirkendall process | Clean fuels, catalysis, electrochemical energy | TEM, SAXS, WAXS, RMDS | [287] |
| Biogenic microtubular iron oxides | Biotic formation of organic sheaths and subsequent abiotic deposition of Fe | Catalysis, pigments | EDS, RS, TEM, XRD, STEM | [288] |
| Iron oxide model thin-film electrodes | Thermal oxidation of pure metal iron substrates at 300 ± 5 °C in air | Lithium-ion batteries | RS, XPS, SIMS 7 | [289] |
| Iron(III) oxide/ hydroxide nanonetworks | Synthesis of iron(III) oxide/hydroxide xerogels from a hydrated ferric nitrate | Electronics, catalysis, sensors | XPS, FTIR, XRD, TEM | [290] |
| Fe0-iron oxide core-shell NPs | Precipitation from ferrous sulfate with leaf extracts | Removal of nitrate in aqueous solution | EDS, XRD, FTIR | [291] |
| Soil samples with amorphous iron oxides | Abiotic mineralization in soil pore structures | Soil weathering studies | XRD, ICP-AES | [292] |
| Reticular pipeline cracks filled with iron oxide | Decarburization and diffusive oxidation of steel matrix | Corrosion resistance studies | EDS | [293] |
| Composition | Main Mechanisms of Iron Oxide Formation | Declared Applications | Phase Verification Techniques | Refs. |
|---|---|---|---|---|
| Saprolitic soil samples | Aerobic weathering of Fe-bearing minerals | Pedogenic process studies | XRF, UV–Vis, XRD | [298] |
| Loess and paleosol samples with iron oxides | Aerobic weathering of Fe-bearing silicate minerals | XRD, UV–Vis | [297] | |
| Oxidized iron items | Soil iron corrosion limited by the diffusion of dissolved oxygen | Heritage science | EDS, XRD, RS | [295] |
| Surface iron oxide layer on metal | Anodic film formation on steel immersed in sour acid media | Corrosion resistance studies | XRD, EDS | [302] |
| Graphene-iron oxide nanotube composite | An adept template-free hydrothermal route from ferrous sulfate | Removal of the toxic heavy metal Cr(VI) | EDS, XRD, FTIR, UV–Vis, TEM | [299] |
| Polyacrylonitrile/iron oxide composite | Hydrothermal method for in situ growth of iron oxide; iron alkoxide hydrolysis | Removal of Congo red dye from water | FTIR, XRD, EDS, ICP-AES | [300] |
| Carbon/FexOy magnetic composites | Mechanical mixing and thermal treatment under N2 atmosphere | Wastewater treatment | XRD, TG-DSC, EDS, FTIR | [301] |
| Isoelement synthetic heterostructures | Hydrothermal method combined with controlled partial annealing process | Visible-light photocatalysis | XRD, TEM, XPS, UV–Vis, EPR | [296] |
| Composition | Main Mechanisms of Iron Oxide Formation | Declared Applications | Phase Verification Techniques | Refs. |
|---|---|---|---|---|
| Inclusions of iron oxides in ore samples | Precipitation during protracted hydrothermal fluid/rock interaction, biological oxidation of Fe(II) by photoautotrophs, microbial sedimentary ferric iron flux, infiltration by hypogene and supergene fluids during or after deformation | Banded iron formation studies, geochemistry, late Archean and early Paleoproterozoic studies, iron oxide copper gold system studies | ICP-MS, EDS, XRF, SAED, TEM, XRD, ICP-AES, TG-DSC | [303,304,305,306,307,308,309,310,311] |
| Surface iron oxide layer on metal | Tribo-oxidation wear of the cast iron disc | Brake system wear studies | EDS, XRD, TEM, SAED | [315] |
| Iron oxide NPs | Anodization of Fe sheet in ethylene glycol electrolyte and calcination | Biomedicine, catalysis, photovoltaics, electronics | XRD, EDS, XPS, RS, FTIR | [312] |
| Iron oxide inclusions in concrete samples | Corrosion of a steel-reinforcing bar in air-entrained concrete with chlorides | Corrosion resistance studies | EDS | [318] |
| Iron oxide nanosheets and nanowires | Thermal oxidation of iron foils in the presence of water vapor | Cr(VI) removal | XRD, TEM, RS, UV–Vis | [319] |
| Iron oxide hollow spheres | Microwave–hydrothermal ionic liquid method, calcination and autocatalysis | Photocatalysis | XRD, TEM, UV–Vis | [314] |
| Inclusions of iron oxides in mineralized rocks | Abiotic formation of a mineral deposit | Geochemistry | XRF | [320] |
| Theoretically calculated iron oxide phases | Radiation-chemical oxidation of Fe depending on pH and oxygen content | Precambrian studies | Kinetics of iron oxidation calculations | [321] |
| Iron oxide NPs supported on biogenic silica | Iron oxide NP impregnation under hydrothermal conditions and calcination | Rhodamine B photocatalytic degradation | EDS, XRD, UV–Vis, TEM | [316] |
| Sediment samples with inclusions of iron oxides | Mineralization by variable diagenetic processes | Rock magnetism studies | XRD, EDS | [322] |
| Iron oxide nanorods | Sols of ferric hydroxide radiolysis in water under gamma irradiation | Electronics, biomedicine | XRD, TEM | [314] |
| Spinel-bearing peridotite | Oxidation of ferrous iron in olivine and pyroxene into ferric iron | Serpentinization studies | FTIR, EDS | [323] |
| Iron oxide inclusions in kaolin clay samples | Abiotic chemical precipitation | Clay chemistry and morphology studies | ICP-AES, XRD, XRF, TG-DSC | [324] |
| Precipitates containing iron oxide inclusions | Biomineralization by photosynthetic Fe(II)-oxidizing bacteria | Banded iron formation studies | XRD, EDS | [325] |
| Iron-mineralized biofilms | Dissolution and re-precipitation of iron oxide minerals | Bioremediation of iron ore mines | – | [326] |
| Iron oxide nanotubes | Template-based electrodeposition and calcination under oxidizing atmospheres | Biomedicine, electronics, gas sensors, catalysis | TEM, XRD, SAED | [317] |
| Iron oxide powder | Hydrothermal process with a use of pyrite cinder lixivium | Pyrite cinder reutilization | FTIR, XRD, TEM, SAED | [327] |
| Growth model for submarine deposits | Transformation of primary (hydr)oxides via reduction by organic matter | Banded iron formation studies | – | [328] |
| Composition | Main Mechanisms of Iron Oxide Formation | Declared Applications | Phase Verification Techniques | Refs. |
|---|---|---|---|---|
| Fe-rich carbonates with inclusions of iron oxides | Laser heating of natural goethite in a diamond anvil cell in CO2 | Earth’s mantle studies | XRD, XAS, TEM, EELS, HAADF-STEM, SAED | [329] |
| Samples with partially reduced FeO and Fe3O4 | Porous iron growth from wüstite in CO/CO2 and H2/H2O systems | Porous iron growth mechanism studies | – | [330] |
| Fe/oxide core-shell NPs | Formation of Fe3O4 during the oxidation of Fe NPs; high-temperature reduction of Fe3O4 to FeO by an electron-beam | Environmental remediation, electronics, catalysis, biomedicine, energy storage | TEM, SAED, EELS, HAADF-STEM, EDS | [331] |
| Composition | Main Mechanisms of Iron Oxide Formation | Declared Applications | Phase Verification Techniques | Refs. |
|---|---|---|---|---|
| Iron oxide NPs | Thermal decomposition of iron oleate, continuous flow synthesis, co-precipitation of Fe3+/Fe2+ ions, aerosol spray pyrolysis with the use of ferric nitrate and ferric chloride, precipitation from iron salts with natural leaf extract | Biomedicine, soil remediation, metal removal, wastewater treatment, electronics, catalysis, energy storage, groundwater remediation | TEM, XRD, FTIR, SAED, TG-DSC, UV–Vis, SAXS, neutron diffraction, EDS, MSP, EELS, EPR, ICP-MS, XAS, RS | [45,332,333,334,335,336,337,338,339,340,352,353,354,355,358,359,360,361,362] |
| Surface iron oxide layer on metal | Oxidation of a pure iron surface in oxygen, electrochemical reduction of lepidocrocite and ferrihydrite, in situ formation on an iron surface depending on the applied potential | Iron oxidation studies, atmospheric steel corrosion studies, groundwater remediation, corrosion protection studies | XPS, XRD, XAS, RS, AES, ellipsometry | [345,346,347,348,349] |
| Oxidation layer on archaeological steel | Combined iron oxidation/iron(III) oxyhydroxide reduction without O2 | Corrosion studies on ancient metallic objects | EDS, RS | [343] |
| Iron oxide-TiO2 nanorod heterostructures | Precipitation by injection of Fe(CO)5 into stirred TiO2 containing mixture | Optoelectronics, biomedicine, catalysis | XRD, XAS, ICP-AES, TEM, UV–Vis | [363] |
| Iron oxide in nanoscrolls and nanoribbons | Precipitation from ferric and ferrous chloride with ammonia solution | Lithium-ion storage, photocatalysis, biosensors | TEM, FTIR | [364] |
| Iron oxide hollow core/Shell NPs | Solvothermal synthesis from FeCl3 and urea in ethylene glycol and calcination | Biomedicine | XRD, TEM, TG-DSC, UV–Vis | [365] |
| Thin-film nanocomposite membrane with iron oxide | In situ synthesis from aqueous solutions containing ferric chloride | Biofouling protection | EDS, TEM, XPS, UV–Vis, XRD, TG-DSC | [366] |
| Magnetoferritin iron oxide NPs | Controlled mineralization from recombinant human H-chain ferritin | Biomedicine | TEM | [367] |
| Iron oxide-based hollow magnetic nanoparticles | Synthesis from iron pentacarbonyl in 1-octadecene and oleylamine | Exchange bias studies | XRD, TEM, FTIR, MSP, F-AAS | [368] |
| Albumin protein-based magnetic NPs | Co-precipitation of FeCl2 and FeCl3 by ammonia in the presence of protein | Biomedicine | TEM, TG-DSC | [369] |
| Composite of organic matrix and iron oxide NPs | Thermal decomposition of iron(III) oleate complex | Biomedicine | TEM | [370] |
| Iron oxide powder | Photochemical oxidation of siderite (FeCO3) by ultraviolet radiation | Banded iron formation studies | XRD | [371] |
| Interfacial iron oxide layer on iron artifacts | Iron corrosion in an anoxic environment after a pH increase at the interface | Anoxic corrosion of archaeological steel studies | HAADF-STEM, RS, EDS, SAED, SIMS | [344] |
| Iron oxide hydroxyapatite core/shell nanocomposites | Precipitation from ferric and ferrous chloride with ammonia under N2 | Biomedicine | TEM, FTIR, XRD, AAS, EDS | [372] |
| Chitosan-based beads with iron oxide NPs | Co-precipitation from ferric and ferrous chloride with NaOH solution | Remediation of water sources | XRD, FTIR, TG-DSC, EDS | [356] |
| Silica–iron oxide nanocomposite | Co-precipitation from ferric and ferrous chloride with ammonia solution | Toxic species removal | XRD, TEM, FTIR, UV–Vis, SAED | [357] |
| Vertical tube-shaped iron-oxide accumulations | Deep water corrosion of carbon steel | Marine corrosion studies | EDS | [351] |
| Hydrogels with embedded iron oxide NPs | In situ mineralization of iron ions in a hydrogel matrix | Dye removal | XRD, FTIR, TG-DSC, TEM | [373] |
| Corroded reinforced concrete | Iron corrosion in a laboratory corrosion chamber | Steel rebar corrosion studies | XRD, EDS | [350] |
| Porous hollow iron oxide NPs on carbon nanotubes | Etching of Fe-FexOy intermediate with nitric acid aqueous solution and drying | Biomedicine, catalysis, separation | TEM, XRD | [374] |
| Iron oxide embedding of bacterial cells | Biomineralization by thermophilic iron-reducing bacteria | Biogenic iron mineral formation studies | XRD | [375] |
| Activated carbon aerogel with iron oxide inclusions | Hydrothermal synthesis from ferrous sulfate with ammonia | Catalytic oxidation of pesticides | XRD, FTIR, XPS, TEM | [376] |
| Polyglycerol-grafted iron oxide NPs | Thermal decomposition of iron(III) acetylacetonate in triethylene glycol | Biomedicine | TEM, TG-DSC, FTIR, ICP-AES | [377] |
3.4. The Structures Containing Three or More Co-Existing Iron Oxide Phases
3.5. The Main Characterization Techniques Used to Verify Phase Composition
3.6. The Analysis of the Distribution of Iron Oxide Compounds by their Frequency of Mention
3.7. The Main Mechanisms of Iron Oxide Formation
4. The Main Applications of the Structures Containing Iron Oxides
5. Summary and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jolivet, J.P.; Chanéac, C.; Tronc, E. Iron oxide chemistry. From molecular clusters to extended solid networks. Chem. Commun. 2004, 5, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Barnard, A.S. Naturally occurring iron oxide nanoparticles: Morphology, surface chemistry and environmental stability. J. Mater. Chem. A 2013, 1, 27–42. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron oxide surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef]
- Simonson, B.M. Origin and evolution of large Precambrian iron formations. Spec. Pap. Geol. Soc. Am. 2003, 370, 231–244. [Google Scholar] [CrossRef]
- Skirrow, R.G. Iron oxide copper-gold (IOCG) deposits—A review (part 1): Settings, mineralogy, ore geochemistry and classification. Ore Geol. Rev. 2022, 140, 104569. [Google Scholar] [CrossRef]
- Kharitonskii, P.; Bobrov, N.; Gareev, K.; Kosterov, A.; Nikitin, A.; Ralin, A.; Sergienko, E.; Testov, O.; Ustinov, A.; Zolotov, N. Magnetic granulometry, frequency-dependent susceptibility and magnetic states of particles of magnetite ore from the Kovdor deposit. J. Magn. Magn. Mater. 2022, 553, 169279. [Google Scholar] [CrossRef]
- Navrotsky, A.; Mazeina, L.; Majzlan, J. Size-driven structural and thermodynamic complexity in iron oxides. Science 2008, 319, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Strbak, O.; Dobrota, D. Archean Iron-Based Metabolism Analysis and the Photoferrotrophy-Driven Hypothesis of Microbial Magnetotaxis Origin. Geomicrobiol. J. 2019, 36, 278–290. [Google Scholar] [CrossRef]
- Narayanan, S.; Shahbazian-Yassar, R.; Shokuhfar, T. Transmission electron microscopy of the iron oxide core in ferritin proteins: Current status and future directions. J. Phys. D. Appl. Phys. 2019, 52, 453001. [Google Scholar] [CrossRef]
- Svobodova, H.; Kosnáč, D.; Tanila, H.; Wagner, A.; Trnka, M.; Vitovič, P.; Hlinkova, J.; Vavrinsky, E.; Ehrlich, H.; Polák, Š.; et al. Iron-oxide minerals in the human tissues. Biometals 2020, 33, 1–13. [Google Scholar] [CrossRef]
- Grüttner, C.; Müller, K.; Teller, J.; Westphal, F. Synthesis and functionalisation of magnetic nanoparticles for hyperthermia applications. Int. J. Hyperth. 2013, 29, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Kment, Š.; Sivula, K.; Naldoni, A.; Sarmah, S.P.; Kmentová, H.; Kulkarni, M.; Rambabu, Y.; Schmuki, P.; Zbořil, R. FeO-based nanostructures and nanohybrids for photoelectrochemical water splitting. Prog. Mater. Sci. 2020, 110, 100632. [Google Scholar] [CrossRef]
- Kralj, S.; Marchesan, S. Bioinspired Magnetic Nanochains for Medicine. Pharmaceutics 2021, 13, 1262. [Google Scholar] [CrossRef]
- Tran, H.V.; Ngo, N.M.; Medhi, R.; Srinoi, P.; Liu, T.; Rittikulsittichai, S.; Lee, T.R. Multifunctional Iron Oxide Magnetic Nanoparticles for Biomedical Applications: A Review. Materials 2022, 15, 503. [Google Scholar] [CrossRef]
- Lam, A.; Hyler, F.; Stagg, O.; Morris, K.; Shaw, S.; Velázquez, J.M.; Navrotsky, A. Synthesis and thermodynamics of uranium-incorporated α-Fe2O3 nanoparticles. J. Nucl. Mater. 2021, 556, 153172. [Google Scholar] [CrossRef]
- Huber, S.E.; Mauracher, A.; Sukuba, I.; Urban, J.; Maihom, T.; Probst, M. Electron impact ionisation cross sections of iron oxides. Eur. Phys. J. D 2017, 71, 335. [Google Scholar] [CrossRef]
- Fabrichnaya, O.; Saxena, S.K.; Richet, P.; Westrum, E.F. Thermodynamic Data, Models, and Phase Diagrams in Multicomponent Oxide Systems: An Assessment for Materials and Planetary Scientists Based on Calorimetric, Volumetric and Phase Equilibrium Data; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004; ISBN 978-3-642-08445-4. [Google Scholar]
- Carter, C.B.; Norton, M.G. Equilibrium Phase Diagrams. In Ceramic Materials; Springer: New York, NY, USA, 2013; pp. 123–139. [Google Scholar] [CrossRef]
- Machala, L.; Tuček, J.; Zbořil, R. Polymorphous transformations of nanometric iron(III) oxide: A review. Chem. Mater. 2011, 23, 3255–3272. [Google Scholar] [CrossRef]
- Tuček, J.; Machala, L.; Ono, S.; Namai, A.; Yoshikiyo, M.; Imoto, K.; Tokoro, H.; Ohkoshi, S.I.; Zbořil, R. Zeta-Fe2O3—A new stable polymorph in iron(III) oxide family. Sci. Rep. 2015, 5, 15091. [Google Scholar] [CrossRef]
- Kim, H.J. Short review on Fe2O3 polymorphs and ε-Fe2O3 thin films. New Phys. Sae Mulli 2018, 68, 819–828. [Google Scholar] [CrossRef]
- Kerchich, S.; Boudjemaa, A.; Chebout, R.; Bachari, K.; Mameri, N. High performance of δ-Fe2O3 novel photo-catalyst supported on LDH structure. J. Photochem. Photobiol. A Chem. 2021, 406, 113001. [Google Scholar] [CrossRef]
- Lavina, B.; Dera, P.; Kim, E.; Meng, Y.; Downs, R.T.; Weck, P.F.; Sutton, S.R.; Zhao, Y. Discovery of the recoverable high-pressure iron oxide Fe4O5. Proc. Natl. Acad. Sci. USA 2011, 108, 17281–17285. [Google Scholar] [CrossRef]
- Huang, S.; Hu, Q. Medium-range structure motifs of complex iron oxides. J. Appl. Phys. 2022, 131, 070902. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliveira, L.C.A.; Murad, E. Iron oxide catalysts: Fenton and Fentonlike reactions—A review. Clay Miner. 2012, 47, 285–302. [Google Scholar] [CrossRef]
- Pouran, S.R.R.; Raman, A.A.A.; Daud, W.M.A.W. Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J. Clean. Prod. 2014, 64, 24–35. [Google Scholar] [CrossRef]
- Mu, Y.; Jia, F.; Ai, Z.; Zhang, L. Iron oxide shell mediated environmental remediation properties of nano zero-valent iron. Environ. Sci. Nano 2017, 4, 27–45. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, G.; Qi, H. Precipitation of magnetic iron oxide induced by sporosarcina pasteurii cells. Microorganisms 2021, 9, 331. [Google Scholar] [CrossRef]
- Antone, A.J.; Sun, Z.; Bao, Y. Preparation and application of iron oxide nanoclusters. Magnetochemistry 2019, 5, 45. [Google Scholar] [CrossRef]
- De Yoreo, J.J. In-situ liquid phase TEM observations of nucleation and growth processes. Prog. Cryst. Growth Charact. Mater. 2016, 62, 69–88. [Google Scholar] [CrossRef]
- Faivre, D.; Godec, T.U. From bacteria to mollusks: The principles underlying the biomineralization of iron oxide materials. Angew. Chem.-Int. Ed. 2015, 54, 4728–4747. [Google Scholar] [CrossRef] [PubMed]
- Fortin, D.; Langley, S. Formation and occurrence of biogenic iron-rich minerals. Earth-Sci. Rev. 2005, 72, 1–19. [Google Scholar] [CrossRef]
- Obara, N.; Murao, R.; ShinOda, K.; Suzuki, S. Structural Changes of Minerals in Iron Ores via Aqueous Solution. Tetsu-Hagane J. Iron Steel Inst. Jpn. 2021, 107, 534–541. [Google Scholar] [CrossRef]
- Fatimah, I.; Fadillah, G.; Yudha, S.P. Synthesis of iron-based magnetic nanocomposites: A review. Arab. J. Chem. 2021, 14, 103301. [Google Scholar] [CrossRef]
- Ohkoshi, S.I.; Sakurai, S.; Jin, J.; Hashimoto, K. The addition effects of alkaline earth ions in the chemical synthesis of ε-Fe2O3 nanocrystals that exhibit a huge coercive field. J. Appl. Phys. 2005, 97, 10K312. [Google Scholar] [CrossRef]
- Ohkoshi, S.I.; Tokoro, H. Hard magnetic ferrite: ε-Fe2O3. Bull. Chem. Soc. Jpn. 2013, 86, 897–907. [Google Scholar] [CrossRef]
- Testov, D.O.; Gareev, K.G.; Khmelnitskiy, I.K.; Kosterov, A.; Surovitskii, L.; Luchinin, V.V. Influence of the Preparation Technique on the Magnetic Characteristics of ε-Fe2O3-Based Composites. Magnetochemistry 2023, 9, 10. [Google Scholar] [CrossRef]
- Kharitonskii, P.; Kamzin, A.; Gareev, K.; Valiullin, A.; Vezo, O.; Sergienko, E.; Korolev, D.; Kosterov, A.; Lebedev, S.; Gurylev, A.; et al. Magnetic granulometry and Mössbauer spectroscopy of FemOn-SiO2 colloidal nanoparticles. J. Magn. Magn. Mater. 2018, 461, 30–36. [Google Scholar] [CrossRef]
- Machala, L.; Zboril, R.; Gedanken, A. Amorphous iron(III) oxide—A review. J. Phys. Chem. B 2007, 111, 4003–4018. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Han, W.; Huo, C.; Cen, Y. Development and application of wüstite-based ammonia synthesis catalysts. Catal. Today 2020, 355, 110–127. [Google Scholar] [CrossRef]
- Sakurai, S.; Namai, A.; Hashimoto, K.; Ohkoshi, S. First Observation of Phase Transformation of All Four Fe2O3 Phases (γ → ε → β → α-Phase). J. Am. Chem. Soc. 2009, 131, 18299–18303. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Chen, Z.; Deegan, B.; O’Brien, S. Wüstite nanocrystals: Synthesis, structure and superlattice formation. J. Mater. Res. 2007, 22, 1987–1995. [Google Scholar] [CrossRef]
- Zhang, Z.; Satpathy, S. Electron states, magnetism, and the Verwey transition in magnetite. Phys. Rev. B 1991, 44, 13319–13331. [Google Scholar] [CrossRef]
- Yadav, A.A.; Deshmukh, T.B.; Deshmukh, R.V.; Patil, D.D.; Chavan, U.J. Electrochemical supercapacitive performance of Hematite α-Fe2O3 thin films prepared by spray pyrolysis from non-aqueous medium. Thin Solid Films 2016, 616, 351–358. [Google Scholar] [CrossRef]
- Roca, A.G.; Marco, J.F.; Del Puerto Morales, M.; Serna, C.J. Effect of nature and particle size on properties of uniform magnetite and maghemite nanoparticles. J. Phys. Chem. C 2007, 111, 18577–18584. [Google Scholar] [CrossRef]
- Jin, J.; Ohkoshi, S.I.; Hashimoto, K. Giant Coercive Field of Nanometer-Sized Iron Oxide. Adv. Mater. 2004, 16, 48–51. [Google Scholar] [CrossRef]
- Schrettle, F.; Kant, C.; Lunkenheimer, P.; Mayr, F.; Deisenhofer, J.; Loidl, A. Wüstite: Electric, thermodynamic and optical properties of FeO. Eur. Phys. J. B 2012, 85, 164. [Google Scholar] [CrossRef]
- Faure, B.; Salazar-Alvarez, G.; Bergström, L. Hamaker constants of iron oxide nanoparticles. Langmuir 2011, 27, 8659–8664. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.M. Forward and inverse modelling of the electrical properties of magnetite intruded by magma, Egypt. Geophys. J. Int. 2013, 194, 1527–1540. [Google Scholar] [CrossRef]
- Lunt, R.A.; Jackson, A.J.; Walsh, A. Dielectric response of Fe2O3 crystals and thin films. Chem. Phys. Lett. 2013, 586, 67–69. [Google Scholar] [CrossRef]
- Ghigna, T.; Zannoni, M.; Jones, M.E.; Simonetto, A. Permittivity and permeability of epoxy-magnetite powder composites at microwave frequencies. J. Appl. Phys. 2020, 127, 045102. [Google Scholar] [CrossRef]
- Bhavani, P.; Reddy, N.R.; Reddy, I.V.S. Synthesis and physical characterization of γ-Fe2O3 and (α + γ)-Fe2O3 nanoparticles. J. Korean Phys. Soc. 2017, 70, 150–154. [Google Scholar] [CrossRef]
- Gich, M.; Frontera, C.; Roig, A.; Fontcuberta, J.; Molins, E.; Bellido, N.; Simon, C.; Fleta, C. Magnetoelectric coupling in ε-Fe2O3 nanoparticles. Nanotechnology 2006, 17, 687. [Google Scholar] [CrossRef]
- Dubrovskiy, A.A.; Balaev, D.A.; Krasikov, A.A.; Yakushhkin, S.S.; Kirillov, V.L.; Martyanov, O.N. Magnetodielectric effect in a metamaterial consisting of xerogel with embedded ε-Fe2O3 iron oxide nanoparticles. Solid State Commun. 2019, 289, 27–29. [Google Scholar] [CrossRef]
- Gheisari, M.; Mozaffari, M.; Acet, M.; Amighian, J. Preparation and investigation of magnetic properties of wüstite nanoparticles. J. Magn. Magn. Mater. 2008, 320, 2618–2621. [Google Scholar] [CrossRef]
- Chen, C.J.; Chiang, R.K.; Lai, H.Y.; Lin, C.R. Characterization of monodisperse wüstite nanoparticles following partial oxidation. J. Phys. Chem. C 2010, 114, 4258–4263. [Google Scholar] [CrossRef]
- Özdemir, Ö. Coercive force of single crystals of magnetite at low temperatures. Geophys. J. Int. 2000, 141, 351–356. [Google Scholar] [CrossRef]
- Tadic, M.; Panjan, M.; Tadic, B.V.; Lazovic, J.; Damnjanovic, V.; Kopani, M.; Kopanja, L. Magnetic properties of hematite (α-Fe2O3) nanoparticles synthesized by sol-gel synthesis method: The influence of particle size and particle size distribution. J. Electr. Eng. 2019, 70, 71–76. [Google Scholar] [CrossRef]
- Araujo, R.N.; Nascimento, E.P.; Raimundo, R.A.; Macedo, D.A.; Mastelaro, V.R.; Neves, G.A.; Morales, M.A.; Menezes, R.R. Hybrid hematite/calcium ferrite fibers by solution blow spinning: Microstructural, optical and magnetic characterization. Ceram. Int. 2021, 47, 33363–33372. [Google Scholar] [CrossRef]
- Mirza, I.M.; Ali, K.; Sarfraz, A.K.; Ali, A.; Haq, A.U. A study of dielectric, optical and magnetic characteristics of maghemite nanocrystallites. Mater. Chem. Phys. 2015, 164, 183–187. [Google Scholar] [CrossRef]
- Shokrollahi, H. A review of the magnetic properties, synthesis methods and applications of maghemite. J. Magn. Magn. Mater. 2017, 426, 74–81. [Google Scholar] [CrossRef]
- Danno, T.; Nakatsuka, D.; Kusano, Y.; Asaoka, H.; Nakanishi, M.; Fujii, T.; Ikeda, Y.; Takada, J. Crystal structure of β-Fe2O3 and topotactic phase transformation to α-Fe2O3. Cryst. Growth Des. 2013, 13, 770–774. [Google Scholar] [CrossRef]
- Kumar, A.; Singhal, A. Synthesis of colloidal β-Fe2O3 nanostructures—Influence of addition of Co2+ on their morphology and magnetic behavior. Nanotechnology 2007, 18, 475703. [Google Scholar] [CrossRef]
- Malina, O.; Tuček, J.; Jakubec, P.; Kašlík, J.; Medřík, I.; Tokoro, H.; Yoshikiyo, M.; Namai, A.; Ohkoshi, S.I.; Zbořil, R. Magnetic ground state of nanosized β-Fe2O3 and its remarkable electronic features. RSC Adv. 2015, 5, 49719–49727. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, X.; Feng, J.; Huang, H.; Guo, Y.; Li, Z.; Zou, Z. Paving the road toward the use of β-Fe2O3 in solar water splitting: Raman identification, phase transformation and strategies for phase stabilization. Natl. Sci. Rev. 2020, 7, 1059–1067. [Google Scholar] [CrossRef]
- Levy, D.; Giustetto, R.; Hoser, A. Structure of magnetite (Fe3O4) above the Curie temperature: A cation ordering study. Phys. Chem. Miner. 2012, 39, 169–176. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Banerjee, S.K. High temperature stability of maghemite (γ-Fe2O3). Geophys. Res. Lett. 1984, 11, 161–164. [Google Scholar] [CrossRef]
- Jordan, K.; Cazacu, A.; Manai, G.; Ceballos, S.F.; Murphy, S.; Shvets, I.V. Scanning tunneling spectroscopy study of the electronic structure of Fe3O4 surfaces. Phys. Rev. B—Condens. Matter Mater. Phys. 2006, 74, 085416. [Google Scholar] [CrossRef]
- Liu, H.; Di Valentin, C. Band Gap in Magnetite above Verwey Temperature Induced by Symmetry Breaking. J. Phys. Chem. C 2017, 121, 25736–25742. [Google Scholar] [CrossRef]
- Bowker, M.; Hutchings, G.; Davies, P.R.; Edwards, D.; Davies, R.; Shaikhutdinov, S.; Freund, H.J. Surface structure of γ-Fe2O3(111). Surf. Sci. 2012, 606, 1594–1599. [Google Scholar] [CrossRef]
- Silva, M.F.; De Oliveira, L.A.S.; Ciciliati, M.A.; Silva, L.T.; Pereira, B.S.; Hechenleitner, A.A.W.; Oliveira, D.M.F.; Pirota, K.R.; Ivashita, F.F.; Paesano, A.; et al. Nanometric particle size and phase controlled synthesis and characterization of γ-Fe2O3 or (α + γ)-Fe2O3 by a modified sol-gel method. J. Appl. Phys. 2013, 114, 104311. [Google Scholar] [CrossRef]
- Elouafi, A.; Moubah, R.; Tizliouine, A.; Derkaoui, S.; Omari, L.H.; Lassri, H. Effects of Ru doping and of oxygen vacancies on the optical properties in α-Fe2O3 powders. Appl. Phys. A Mater. Sci. Process. 2020, 126, 228. [Google Scholar] [CrossRef]
- Nasu, T.; Yoshikiyo, M.; Tokoro, H.; Namai, A.; Ohkoshi, S.I. First-Principles Calculations and Optical Absorption Spectrum of a Light-Colored Aluminum-Substituted ε-Iron Oxide Magnet. Eur. J. Inorg. Chem. 2017, 2017, 531–534. [Google Scholar] [CrossRef]
- Dadashi, S.; Poursalehi, R.; Delavari, H. Structural and Optical Properties of Pure Iron and Iron Oxide Nanoparticles Prepared via Pulsed Nd:YAG Laser Ablation in Liquid. Procedia Mater. Sci. 2015, 11, 722–726. [Google Scholar] [CrossRef]
- Feld, A.; Weimer, A.; Kornowski, A.; Winckelmans, N.; Merkl, J.P.; Kloust, H.; Zierold, R.; Schmidtke, C.; Schotten, T.; Riedner, M.; et al. Chemistry of Shape-Controlled Iron Oxide Nanocrystal Formation. ACS Nano 2019, 13, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Kampferbeck, M.; Klauke, L.R.; Weller, H.; Vossmeyer, T. Little Adjustments Significantly Simplify the Gram-Scale Synthesis of High-Quality Iron Oxide Nanocubes. Langmuir 2021, 37, 9851–9857. [Google Scholar] [CrossRef] [PubMed]
- Marinca, T.F.; Chicinaş, H.F.; Neamţu, B.V.; Isnard, O.; Pascuta, P.; Lupu, N.; Stoian, G.; Chicinaş, I. Mechanosynthesis, structural, thermal and magnetic characteristics of oleic acid coated Fe3O4 nanoparticles. Mater. Chem. Phys. 2016, 171, 336–345. [Google Scholar] [CrossRef]
- Leszczyński, B.; Hadjipanayis, G.C.; El-Gendy, A.A.; Załȩski, K.; Śniadecki, Z.; Musiał, A.; Jarek, M.; Jurga, S.; Skumiel, A. The influence of oxidation process on exchange bias in egg-shaped FeO/Fe3O4 core/shell nanoparticles. J. Magn. Magn. Mater. 2016, 416, 269–274. [Google Scholar] [CrossRef]
- Narnaware, P.K.; Ravikumar, C. Mechanistic Insights into the Formation and Growth of Anisotropic-Shaped Wüstite-Spinel Core-Shell Iron Oxide Nanoparticles in a Coordinating Solvent. J. Phys. Chem. C 2020, 124, 25010–25027. [Google Scholar] [CrossRef]
- Kluge, S.; Deng, L.; Feroughi, O.; Schneider, F.; Poliak, M.; Fomin, A.; Tsionsky, V.; Cheskis, S.; Wlokas, I.; Rahinov, I.; et al. Initial reaction steps during flame synthesis of iron-oxide nanoparticles. CrystEngComm 2015, 17, 6930–6939. [Google Scholar] [CrossRef]
- Ahn, T.; Kim, J.H.; Yang, H.M.; Lee, J.W.; Kim, J.D. Formation pathways of magnetite nanoparticles by coprecipitation method. J. Phys. Chem. C 2012, 116, 6069–6076. [Google Scholar] [CrossRef]
- Besenhard, M.O.; LaGrow, A.P.; Hodzic, A.; Kriechbaum, M.; Panariello, L.; Bais, G.; Loizou, K.; Damilos, S.; Cruz, M.M.; Thanh, N.T.K.; et al. Co-precipitation synthesis of stable iron oxide nanoparticles with NaOH: New insights and continuous production via flow chemistry. Chem. Eng. J. 2020, 399, 125740. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Wang, Z.; Zhou, Z. Solvothermal synthesis of size-controlled monodispersed superparamagnetic iron oxide nanoparticles. Appl. Sci. 2019, 9, 5157. [Google Scholar] [CrossRef]
- Grabs, I.M.; Bradtmöller, C.; Menzel, D.; Garnweitner, G. Formation mechanisms of iron oxide nanoparticles in different nonaqueous media. Cryst. Growth Des. 2012, 12, 1469–1475. [Google Scholar] [CrossRef]
- Kozakova, Z.; Kuritka, I.; Kazantseva, N.E.; Babayan, V.; Pastorek, M.; Machovsky, M.; Bazant, P.; Saha, P. The formation mechanism of iron oxide nanoparticles within the microwave-assisted solvothermal synthesis and its correlation with the structural and magnetic properties. Dalton Trans. 2015, 44, 21099–21108. [Google Scholar] [CrossRef]
- Lozano, I.; Casillas, N.; de León, C.P.; Walsh, F.C.; Herrasti, P. New Insights into the Electrochemical Formation of Magnetite Nanoparticles. J. Electrochem. Soc. 2017, 164, D184–D191. [Google Scholar] [CrossRef]
- Kertmen, A.; Torruella, P.; Coy, E.; Yate, L.; Nowaczyk, G.; Gapiński, J.; Vogt, C.; Toprak, M.; Estradé, S.; Peiró, F.; et al. Acetate-Induced Disassembly of Spherical Iron Oxide Nanoparticle Clusters into Monodispersed Core-Shell Structures upon Nanoemulsion Fusion. Langmuir 2017, 33, 10351–10365. [Google Scholar] [CrossRef]
- Nene, A.; Takahashi, M.; Somani, P.R. Fe3O4 and Fe Nanoparticles by Chemical Reduction of Fe(acac)3 by Ascorbic Acid: Role of Water. World J. Nano Sci. Eng. 2016, 6, 20–28. [Google Scholar] [CrossRef]
- Perecin, C.J.; Tirich, B.M.; Nagamine, L.C.C.M.; Porto, G.; Rocha, F.V.; Cerize, N.N.P.; Varanda, L.C. Aqueous synthesis of magnetite nanoparticles for magnetic hyperthermia: Formation mechanism approach, high water-dispersity and stability. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127169. [Google Scholar] [CrossRef]
- Tahir, A.; Saeed, A.; Ramzan, I.; Hayat, S.S.; Ahmad, W.; Naeem, S.; Afzal, M.; Mukhtar, A.; Mehmood, T.; Khan, B.S. Mechanism for the formation of magnetite iron oxide nanostructures by Ficus carica dried fruit extract using green synthesis method. Appl. Nanosci. 2021, 11, 1857–1865. [Google Scholar] [CrossRef]
- Gan, L.; Lu, Z.; Cao, D.; Chen, Z. Effects of cetyltrimethylammonium bromide on the morphology of green synthesized Fe3O4 nanoparticles used to remove phosphate. Mater. Sci. Eng. C 2018, 82, 41–45. [Google Scholar] [CrossRef]
- Kashyap, S.; Woehl, T.J.; Liu, X.; Mallapragada, S.K.; Prozorov, T. Nucleation of iron oxide nanoparticles mediated by mms6 protein in situ. ACS Nano 2014, 8, 9097–9106. [Google Scholar] [CrossRef]
- Baaziz, W.; Ghica, C.; Cypriano, J.; Abreu, F.; Anselme, K.; Ersen, O.; Farina, M.; Werckmann, J. New phenotype and mineralization of biogenic iron oxide in magnetotactic bacteria. Nanomaterials 2021, 11, 3189. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shimon, S.; Stein, D.; Zarivach, R. Current view of iron biomineralization in magnetotactic bacteria. J. Struct. Biol. X 2021, 5, 100052. [Google Scholar] [CrossRef]
- Lovley, D.R.; Stolz, J.F.; Nord, G.L.; Phillips, E.J.P. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature 1987, 330, 252–254. [Google Scholar] [CrossRef]
- Garber, A.I.; Cohen, A.B.; Nealson, K.H.; Ramírez, G.A.; Barco, R.A.; Enzingmüller-Bleyl, T.C.; Gehringer, M.M.; Merino, N. Metagenomic Insights into the Microbial Iron Cycle of Subseafloor Habitats. Front. Microbiol. 2021, 12, 667944. [Google Scholar] [CrossRef] [PubMed]
- Staniland, S.; Ward, B.; Harrison, A.; Van Der Laan, G.; Telling, N. Rapid magnetosome formation shown by real-time X-ray magnetic circular dichroism. Proc. Natl. Acad. Sci. USA 2007, 104, 19524–19528. [Google Scholar] [CrossRef] [PubMed]
- Rahn-Lee, L.; Komeili, A. The magnetosome model: Insights into the mechanisms of bacterial biomineralization. Front. Microbiol. 2013, 4, 352. [Google Scholar] [CrossRef]
- Hu, X.; Chen, H.; Beaudoin, G.; Zhang, Y. Textural and compositional evolution of iron oxides at Mina Justa (Peru): Implications for mushketovite and formation of IOCG deposits. Am. Mineral. 2020, 105, 397–408. [Google Scholar] [CrossRef]
- Hua, X.; Zheng, Y.; Xu, Q.; Lu, X.; Cheng, H.; Zou, X.; Song, Q.; Ning, Z. Interfacial reactions of chalcopyrite in ammonia–ammonium chloride solution. Trans. Nonferrous Met. Soc. China Engl. Ed. 2018, 28, 556–566. [Google Scholar] [CrossRef]
- Velasco, F.; Tornos, F.; Hanchar, J.M. Immiscible iron- and silica-rich melts and magnetite geochemistry at the El Laco volcano (northern Chile): Evidence for a magmatic origin for the magnetite deposits. Ore Geol. Rev. 2016, 79, 346–366. [Google Scholar] [CrossRef]
- Warrier, A.K.; Sebastian, J.G.; Amrutha, K.; Sali, A.S.Y.; Mahesh, B.S.; Mohan, R. Magnetic properties of surface sediments in Schirmacher Oasis, East Antarctica: Spatial distribution and controlling factors. J. Soils Sediments 2021, 21, 1206–1221. [Google Scholar] [CrossRef]
- Dodd, M.S.; Wang, H.; Li, C.; Towner, M.; Thomson, A.R.; Slack, J.F.; Wan, Y.S.; Pirajno, F.; Manikyamba, C.; Wang, Q.; et al. Abiotic anoxic iron oxidation, formation of Archean banded iron formations, and the oxidation of early Earth. Earth Planet. Sci. Lett. 2022, 584, 117469. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Q.; Boyko, V.; Avetisyan, K.; Findlay, A.J.; Huang, F.; Wang, Z.; Chen, Z. Isotopic reconstruction of iron oxidation-reduction process based on an Archean Ocean analogue. Sci. Total Environ. 2022, 817, 152609. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, X.; Tan, H.; Deng, S. Investigation on high temperature corrosion of water-cooled wall tubes at a 300 MW boiler. J. Energy Inst. 2020, 93, 377–386. [Google Scholar] [CrossRef]
- Martinelli, L.; Balbaud-Célérier, F.; Terlain, A.; Bosonnet, S.; Picard, G.; Santarini, G. Oxidation mechanism of an Fe-9Cr-1Mo steel by liquid Pb-Bi eutectic alloy at 470 °C (Part II). Corros. Sci. 2008, 50, 2537–2548. [Google Scholar] [CrossRef]
- Misawa, T.; Hashimoto, K.; Shimodaira, S. The mechanism of formation of iron oxide and oxyhydroxides in aqueous solutions at room temperature. Corros. Sci. 1974, 14, 131–149. [Google Scholar] [CrossRef]
- Odziemkowski, M.S.; Schuhmacher, T.T.; Gillham, R.W.; Reardon, E.J. Mechanism of oxide film formation on iron in simulating groundwater solutions: Raman spectroscopic studies. Corros. Sci. 1998, 40, 371–389. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.; Li, X.; Pan, Y.; Li, J. Effect of Cr on the passive film formation mechanism of steel rebar in saturated calcium hydroxide solution. Appl. Surf. Sci. 2016, 389, 1182–1191. [Google Scholar] [CrossRef]
- Castro-Ocampo, O.; Celaya, C.A.; González-Reyes, L.; Hernández-Pérez, I.; Garibay-Febles, V.; Jaramillo-Quintero, O.A.; Lara-García, H.A.; Muñiz, J.; Suárez-Parra, R. Understanding hydroxyl radicals addition to CO2 on α-Fe2O3(1 1 0) surface photocatalyst for organic compounds production. Fuel 2022, 310, 122465. [Google Scholar] [CrossRef]
- Kusior, A.; Michalec, K.; Jelen, P.; Radecka, M. Shaped Fe2O3 nanoparticles-synthesis and enhanced photocatalytic degradation towards RhB. Appl. Surf. Sci. 2019, 476, 342–352. [Google Scholar] [CrossRef]
- Sivaranjani, R.; Thayumanavan, A.; Sriram, S. Photocatalytic activity of Zn-doped Fe2O3 nanoparticles: A combined experimental and theoretical study. Bull. Mater. Sci. 2019, 42, 185. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Chemerisov, S.D.; Marin, T.W. Photocatalytic decomposition of carboxylated molecules on light-exposed martian regolith and its relation to methane production on mars. Astrobiology 2010, 10, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Bolanz, R.M.; Bläss, U.; Ackermann, S.; Ciobotǎ, V.; Rösch, P.; Tarcea, N.; Popp, J.; Majzlan, J. The effect of antimonate, arsenate, and phosphate on the transformation of ferrihydrite to goethite, hematite, feroxyhyte, and tripuhyite. Clays Clay Miner. 2013, 61, 11–25. [Google Scholar] [CrossRef]
- Börsig, N.; Scheinost, A.C.; Shaw, S.; Schild, D.; Neumann, T. Uptake mechanisms of selenium oxyanions during the ferrihydrite-hematite recrystallization. Geochim. Cosmochim. Acta 2017, 206, 236–253. [Google Scholar] [CrossRef]
- Brandt, F.; Schäfer, T.; Claret, F.; Bosbach, D. Heterogeneous formation of ferric oxide nanoparticles on chlorite surfaces studied by X-ray absorption spectromicroscopy (STXM). Chem. Geol. 2012, 329, 42–52. [Google Scholar] [CrossRef]
- Chan, M.A.; Johnson, C.M.; Beard, B.L.; Bowman, J.R.; Parry, W.T. Iron isotopes constrain the pathways and formation mechanisms of terrestrial oxide concretions: A tool for tracing iron cycling on Mars? Geosphere 2006, 2, 324–332. [Google Scholar] [CrossRef]
- Chan, M.A.; Ormö, J.; Park, A.J.; Stich, M.; Souza-Egipsy, V.; Komatsu, G. Models of iron oxide concretion formation: Field, numerical, and laboratory comparisons. Geofluids 2007, 7, 356–368. [Google Scholar] [CrossRef]
- Gao, B.F.; Wu, C.Z.; Yang, T.; Santosh, M.; Dong, L.H.; Zhao, T.Y.; Ye, H.; Lei, R.X.; Li, W. The Neoproterozoic “Blood Falls” in Tarim Craton and Their Possible Connection With Snowball Earth. J. Geophys. Res. Earth Surf. 2019, 124, 229–244. [Google Scholar] [CrossRef]
- Magnol, R.V.; Macedo, M.Q.; de Macêdo, M.C.S.; Scandian, C. Tribological characterization of jaspilite by linear scratch test. Wear 2019, 426–427, 142–150. [Google Scholar] [CrossRef]
- Rasmussen, B.; Muhling, J.R. Development of a greenalite-silica shuttle during incursions of hydrothermal vent plumes onto Neoarchean shelf, Hamersley region, Australia. Precambrian Res. 2021, 353, 106003. [Google Scholar] [CrossRef]
- Parker, C.W.; Wolf, J.A.; Auler, A.S.; Barton, H.A.; Senko, J.M. Microbial reducibility of Fe(III) phases associated with the genesis of iron ore caves in the Iron Quadrangle, Minas Gerais, Brazil. Minerals 2013, 3, 395–411. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, L.; Jia, N.; Wang, Y.; Sun, L. Microbial biomineralization of iron seepage water: Implication for the iron ores formation in intertidal zone of Zhoushan Archipelago, East China Sea. Geochem. J. 2009, 43, 167–177. [Google Scholar] [CrossRef]
- Singh, M.; Singhal, J.; Prasad, K.A.; Rajesh, V.J.; Ray, D.; Sahoo, P. Spectral characteristics of banded iron formations in Singhbhum craton, eastern India: Implications for hematite deposits on Mars. Geosci. Front. 2016, 7, 927–936. [Google Scholar] [CrossRef]
- Bandi, S.; Srivastav, A.K. Understanding the Growth Mechanism of Hematite Nanoparticles: The Role of Maghemite as an Intermediate Phase. Cryst. Growth Des. 2021, 21, 16–22. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Ovejero, J.G.; Pablo-Sainz-Ezquerra, A.M.; Cuya, J.; Jeyadevan, B.; Veintemillas-Verdaguer, S.; Tartaj, P.; Morales, M.D.P. Unravelling an amine-regulated crystallization crossover to prove single/multicore effects on the biomedical and environmental catalytic activity of magnetic iron oxide colloids. J. Colloid Interface Sci. 2022, 608, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Gavilán, H.; Sánchez, E.H.; Brollo, M.E.F.; Asín, L.; Moerner, K.K.; Frandsen, C.; Lázaro, F.J.; Serna, C.J.; Veintemillas-Verdaguer, S.; Morales, M.P.; et al. Formation Mechanism of Maghemite Nanoflowers Synthesized by a Polyol-Mediated Process. ACS Omega 2017, 2, 7172–7184. [Google Scholar] [CrossRef]
- Jensen, K.M.Ø.; Andersen, H.L.; Tyrsted, C.; Bøjesen, E.D.; Dippel, A.C.; Lock, N.; Billinge, S.J.L.; Iversen, B.B.; Christensen, M. Mechanisms for iron oxide formation under hydrothermal conditions: An in situ total scattering study. ACS Nano 2014, 8, 10704–10714. [Google Scholar] [CrossRef]
- Kabelitz, A.; Guilherme, A.; Joester, M.; Reinholz, U.; Radtke, M.; Bienert, R.; Schulz, K.; Schmack, R.; Kraehnert, R.; Emmerling, F. Time-resolved in situ studies on the formation mechanism of iron oxide nanoparticles using combined fast-XANES and SAXS. CrystEngComm 2015, 17, 8463–8470. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Roychowdhury, A.; Das, D.; Nayar, S. Multi-functional biomimetic graphene induced transformation of Fe3O4 to ε-Fe2O3 at room temperature. RSC Adv. 2015, 5, 89488–89497. [Google Scholar] [CrossRef]
- Královec, K.; Havelek, R.; Koutová, D.; Veverka, P.; Kubíčková, L.; Brázda, P.; Kohout, J.; Herynek, V.; Vosmanská, M.; Kaman, O. Magnetic nanoparticles of Ga-substituted ε-Fe2O3 for biomedical applications: Magnetic properties, transverse relaxivity, and effects of silica-coated particles on cytoskeletal networks. J. Biomed. Mater. Res.—Part A 2020, 108, 1563–1578. [Google Scholar] [CrossRef] [PubMed]
- Dejoie, C.; Sciau, P.; Li, W.; Noé, L.; Mehta, A.; Chen, K.; Luo, H.; Kunz, M.; Tamura, N.; Liu, Z. Learning from the past: Rare e-Fe2O3 in the ancient black-glazed Jian (Tenmoku) wares. Sci. Rep. 2014, 4, 4941. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Chen, K. Refining single-crystalline epsilon iron oxide nanorods via low-temperature aging. Adv. Powder Technol. 2019, 30, 3021–3027. [Google Scholar] [CrossRef]
- Gurylev, A.; Kharitonskii, P.; Kosterov, A.; Berestnev, I.; Sergienko, E. Magnetic properties of fired clay (bricks) possibly containing epsilon iron (III) oxide. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1347. [Google Scholar] [CrossRef]
- López-Sánchez, J.; Palencia-Ortas, A.; del Campo, A.; McIntosh, G.; Kovacheva, M.; Martín-Hernández, F.; Carmona, N.; de la Fuente, O.R.; Marín, P.; Molina-Cardín, A.; et al. Further progress in the study of epsilon iron oxide in archaeological baked clays. Phys. Earth Planet. Inter. 2020, 307, 106554. [Google Scholar] [CrossRef]
- Corbellini, L.; Lacroix, C.; Harnagea, C.; Korinek, A.; Botton, G.A.; Ménard, D.; Pignolet, A. Epitaxially stabilized thin films of ϵ-Fe2O3 (001) grown on YSZ (100). Sci. Rep. 2017, 7, 3712. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, T.; Ikenaga, N. Oxidative dehydrogenation of n-butene to buta-1,3-diene with novel iron oxide-based catalyst: Effect of iron oxide crystalline structure. Mol. Catal. 2021, 507, 111560. [Google Scholar] [CrossRef]
- López-Sánchez, J.; McIntosh, G.; Osete, M.L.; del Campo, A.; Villalaín, J.J.; Pérez, L.; Kovacheva, M.; de la Fuente, O.R. Epsilon iron oxide: Origin of the high coercivity stable low Curie temperature magnetic phase found in heated archeological materials. Geochem. Geophys. Geosystems 2017, 18, 2646–2656. [Google Scholar] [CrossRef]
- Kosterov, A.; Kovacheva, M.; Kostadinova-Avramova, M.; Minaev, P.; Salnaia, N.; Surovitskii, L.; Yanson, S.; Sergienko, E.; Kharitonskii, P. High-coercivity magnetic minerals in archaeological baked clay and bricks. Geophys. J. Int. 2021, 224, 1256–1271. [Google Scholar] [CrossRef]
- Tao, S.; Liu, S.; Yuan, Y.; Dong, J.; Li, Q. A Microstructural and Compositional Study of ε-Fe2O3 Crystals in the Hare’s Fur Jian Ware. Crystals 2022, 12, 367. [Google Scholar] [CrossRef]
- Jeon, B.; Van Overmeere, Q.; Van Duin, A.C.T.; Ramanathan, S. Nanoscale oxidation and complex oxide growth on single crystal iron surfaces and external electric field effects. Phys. Chem. Chem. Phys. 2013, 15, 1821–1830. [Google Scholar] [CrossRef]
- Jiang, Y.; Bu, S.; Zhou, D.; Shi, X.; Pan, F.; Ji, Q.; Niu, T. Two-Dimensional Iron Oxide on Au(111): Growth Mechanism and Interfacial Properties. J. Phys. Chem. C 2021, 125, 24755–24763. [Google Scholar] [CrossRef]
- Menecier, S.; Valette, S.; Denoirjean, P.; Lefort, P. Invar oxidation in CO2:Kinetics and mechanism of formation of a wustite layer. J. Therm. Anal. Calorim. 2012, 107, 607–616. [Google Scholar] [CrossRef]
- Prabowo, S.W.; Longbottom, R.J.; Monaghan, B.J.; del Puerto, D.; Ryan, M.J.; Bumby, C.W. Phase transformations during fluidized bed reduction of New Zealand titanomagnetite ironsand in hydrogen gas. Powder Technol. 2022, 398, 117032. [Google Scholar] [CrossRef]
- Tanaka, R.; Sakamaki, T.; Ohtani, E.; Fukui, H.; Kamada, S.; Suzuki, A.; Tsutsui, S.; Uchiyama, H.; Baron, A.Q.R. The sound velocity of wüstite at high pressures: Implications for low-velocity anomalies at the base of the lower mantle. Prog. Earth Planet. Sci. 2020, 7, 23. [Google Scholar] [CrossRef]
- Wang, M.; Kobayashi, Y.; Endo, R.; Susa, M. Formation kinetics of iron oxide in mould flux during continuous casting. ISIJ Int. 2013, 53, 56–61. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, J.; Qin, B.; Dong, Y.; Lu, Y.; Li, Y.; Hao, W.; Zhang, Y. Reduction kinetics of hematite ore fines with H2 in a rotary drum reactor. Powder Technol. 2018, 332, 18–26. [Google Scholar] [CrossRef]
- Chen, Z.; Chou, K.C.; Morita, K. Mechanism of metastable Wüstite formation in the reduction process of iron oxide below 570 °C. Mater. Trans. 2016, 57, 1660–1663. [Google Scholar] [CrossRef]
- Vasylkiv, O.; Bezdorozhev, O.; Sakka, Y. Synthesis of iron oxide nanoparticles with different morphologies by precipitation method with and without chitosan addition. J. Ceram. Soc. Jpn. 2016, 124, 489–494. [Google Scholar] [CrossRef]
- Manikandan, A.; Vijaya, J.J.; Mary, J.A.; Kennedy, L.J.; Dinesh, A. Structural, optical and magnetic properties of Fe3O4 nanoparticles prepared by a facile microwave combustion method. J. Ind. Eng. Chem. 2014, 20, 2077–2085. [Google Scholar] [CrossRef]
- Cabrera, L.; Gutierrez, S.; Menendez, N.; Morales, M.P.; Herrasti, P. Magnetite nanoparticles: Electrochemical synthesis and characterization. Electrochim. Acta 2008, 53, 3436–3441. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, T.H.; Choi, S.; Kim, K.S.; Park, D.W. Preparation of silica coated iron oxide nanoparticles using non-transferred arc plasma. Adv. Powder Technol. 2012, 23, 701–707. [Google Scholar] [CrossRef]
- Kamura, A.; Idota, N.; Sugahara, Y. Nonaqueous synthesis of magnetite nanoparticles via oxidation of tetrachloroferrate anions by pyridine-N-oxide. Solid State Sci. 2019, 92, 81–88. [Google Scholar] [CrossRef]
- Pedrosa, J.; Costa, B.F.O.; Portugal, A.; Durães, L. Controlled phase formation of nanocrystalline iron oxides/hydroxides in solution—An insight on the phase transformation mechanisms. Mater. Chem. Phys. 2015, 163, 88–98. [Google Scholar] [CrossRef]
- Samulewski, R.B.; Gonçalves, J.M.; Urbano, A.; Da Costa, A.C.S.; Ivashita, F.F.; Paesano, A.; Zaia, D.A.M. Magnetite synthesis in the presence of cyanide or thiocyanate under prebiotic chemistry conditions. Life 2020, 10, 34. [Google Scholar] [CrossRef]
- Shen, L.; Qiao, Y.; Guo, Y.; Tan, J. Preparation and formation mechanism of nano-iron oxide black pigment from blast furnace flue dust. Ceram. Int. 2013, 39, 737–744. [Google Scholar] [CrossRef]
- Sun, S.; Gebauer, D.; Cölfen, H. Alignment of Amorphous Iron Oxide Clusters: A Non-Classical Mechanism for Magnetite Formation. Angew. Chem.-Int. Ed. 2017, 56, 4042–4046. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, D.D.; Abd Hamid, S.B. One step facile synthesis of ferromagnetic magnetite nanoparticles. J. Magn. Magn. Mater. 2016, 414, 204–208. [Google Scholar] [CrossRef]
- Demirbas, A.; Kislakci, E.; Karaagac, Z.; Onal, I.; Ildiz, N.; Ocsoy, I. Preparation of biocompatible and stable iron oxide nanoparticles using anthocyanin integrated hydrothermal method and their antimicrobial and antioxidant properties. Mater. Res. Express 2019, 6, 125011. [Google Scholar] [CrossRef]
- Tahirbegi, I.B.; Pardo, W.A.; Alvira, M.; Mir, M.; Samitier, J. Amyloid Aβ42, a promoter of magnetite nanoparticle formation in Alzheimer’s disease. Nanotechnology 2016, 27, 465102. [Google Scholar] [CrossRef]
- Baumgartner, J.; Morin, G.; Menguy, N.; Gonzalez, T.P.; Widdrat, M.; Cosmidis, J.; Faivre, D. Magnetotactic bacteria form magnetite from a phosphate-rich ferric hydroxide via nanometric ferric (oxyhydr)oxide intermediates. Proc. Natl. Acad. Sci. USA 2013, 110, 14883–14888. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Matijević, E. Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels. J. Colloid Interface Sci. 1980, 74, 227–243. [Google Scholar] [CrossRef]
- Brunner, J.; Baburin, I.A.; Sturm, S.; Kvashnina, K.; Rossberg, A.; Pietsch, T.; Andreev, S.; Sturm, E.; Cölfen, H. Self-Assembled Magnetite Mesocrystalline Films: Toward Structural Evolution from 2D to 3D Superlattices. Adv. Mater. Interfaces 2017, 4, 1600431. [Google Scholar] [CrossRef]
- Costa, R.C.C.; Moura, F.C.C.; Ardisson, J.D.; Fabris, J.D.; Lago, R.M. Highly active heterogeneous Fenton-like systems based on Fe0/Fe3O4 composites prepared by controlled reduction of iron oxides. Appl. Catal. B Environ. 2008, 83, 131–139. [Google Scholar] [CrossRef]
- Crowley, T.A.; Ziegler, K.J.; Lyons, D.M.; Erts, D.; Olin, H.; Morris, M.A.; Holmes, J.D. Synthesis of metal and metal oxide nanowire and nanotube arrays within a mesoporous silica template. Chem. Mater. 2003, 15, 3518–3522. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Zachara, J.M.; Kennedy, D.W.; Dong, H.; Onstott, T.C.; Hinman, N.W.; Li, S.M. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim. Cosmochim. Acta 1998, 62, 3239–3257. [Google Scholar] [CrossRef]
- Fujioka, N.; Suzuki, M.; Kurosu, S.; Kawase, Y. Linkage of iron elution and dissolved oxygen consumption with removal of organic pollutants by nanoscale zero-valent iron: Effects of pH on iron dissolution and formation of iron oxide/hydroxide layer. Chemosphere 2016, 144, 1738–1746. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.M.; El-Saeed, A.M.; El-Mahdy, G.M.; Al-Lohedan, H.A. Application of magnetite nano-hybrid epoxy as protective marine coatings for steel. RSC Adv. 2015, 5, 101923–101931. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hooshyar, Z.; Asli, M.J.; Shahidi, F.E.; Dianatnejad, N. Synthesis of a novel supermagnetic iron oxide nanocomposite hydrogel based on graft copolymerization of poly((2-dimethylamino)ethyl methacrylate) onto salep for controlled release of drug. Mater. Sci. Eng. C 2014, 36, 277–286. [Google Scholar] [CrossRef]
- Jubb, A.M.; Eskelsen, J.R.; Yin, X.; Zheng, J.; Philben, M.J.; Pierce, E.M.; Graham, D.E.; Wullschleger, S.D.; Gu, B. Characterization of iron oxide nanoparticle films at the air–water interface in Arctic tundra waters. Sci. Total Environ. 2018, 633, 1460–1468. [Google Scholar] [CrossRef]
- Kurdtabar, M.; Koutenaee, R.N.; Bardajee, G.R. Synthesis and characterization of a novel pH-responsive nanocomposite hydrogel based on chitosan for targeted drug release. J. Polym. Res. 2018, 25, 119. [Google Scholar] [CrossRef]
- Liang, S.; Shi, S.; Zhang, H.; Qiu, J.; Yu, W.; Li, M.; Gan, Q.; Yu, W.; Xiao, K.; Liu, B.; et al. One-pot solvothermal synthesis of magnetic biochar from waste biomass: Formation mechanism and efficient adsorption of Cr(VI) in an aqueous solution. Sci. Total Environ. 2019, 695, 133886. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.C.; Sun, F.; Hu, J. Characterization and effect of pre-oxidation on D.C. plasma nitriding for AISI4140 steel. Vacuum 2014, 109, 170–174. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Q.; Zang, Z.; Han, R. Adsorptive removal of sulfosalicylic acid from aqueous medium by iron(III)-loaded magnetic chitosan/graphene oxide. J. Colloid Interface Sci. 2022, 606, 1249–1260. [Google Scholar] [CrossRef]
- Mo, J.; Steen, S.M.; Zhang, F.Y.; Toops, T.J.; Brady, M.P.; Green, J.B. Electrochemical investigation of stainless steel corrosion in a proton exchange membrane electrolyzer cell. Int. J. Hydrogen Energy 2015, 40, 12506–12511. [Google Scholar] [CrossRef]
- Brunner, J.J.S.N.; Maier, B.; Kirner, F.; Sturm, S.; Cölfen, H.; Sturm, E.V. Self-Assembled Faceted Mesocrystals: Advances in Optimization of Growth Conditions. Cryst. Growth Des. 2021, 21, 5490–5495. [Google Scholar] [CrossRef]
- Shimojo, M.; Takeguchi, M.; Mitsuishi, K.; Tanaka, M.; Furuya, K. Mechanisms of crystalline iron oxide formation in electron beam-induced deposition. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2007, 46, 6247–6249. [Google Scholar] [CrossRef]
- Touqeer, T.; Mumtaz, M.W.; Mukhtar, H.; Irfan, A.; Akram, S.; Shabbir, A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Fe3O4-PDA-lipase as surface functionalized nano biocatalyst for the production of biodiesel using waste cooking oil as feedstock: Characterization and process optimization. Energies 2020, 13, 177. [Google Scholar] [CrossRef]
- Yu, B.Y.; Kwak, S.Y. Assembly of magnetite nanocrystals into spherical mesoporous aggregates with a 3-D wormhole-like pore structure. J. Mater. Chem. 2010, 20, 8320–8328. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, M.; Zhu, Y.; Huang, F.; Si, T.; Xu, R.X. Perfluorocarbon-Loaded Hydrogel Microcapsules from Interface Shearing for Magnetic Guided Ultrasound and Laser Activation. Front. Phys. 2020, 8, 581519. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Bao, N.; Ding, J. Scalable route to mesoporous iron oxides and their Cr(VI) ions uptake capacity study. Mater. Chem. Phys. 2014, 144, 512–518. [Google Scholar] [CrossRef]
- Zheng, W.; Hauwiller, M.R.; Liang, W.I.; Ophus, C.; Ercius, P.; Chan, E.M.; Chu, Y.H.; Asta, M.; Du, X.; Alivisatos, A.P.; et al. Real time imaging of two-dimensional iron oxide spherulite nanostructure formation. Nano Res. 2019, 12, 2889–2893. [Google Scholar] [CrossRef]
- Aisida, S.O.; Ugwu, K.; Akpa, P.A.; Nwanya, A.C.; Nwankwo, U.; Bashir, A.K.H.; Madiba, I.G.; Ahmed, I.; Ezema, F.I. Synthesis and characterization of iron oxide nanoparticles capped with Moringa Oleifera: The mechanisms of formation effects on the optical, structural, magnetic and morphological properties. In Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; Volume 36, pp. 214–218. [Google Scholar]
- Baalousha, M.; Manciulea, A.; Cumberland, S.; Kendall, K.; Lead, J.R. Aggregation and surface properties of iron oxide nanoparticles: Influence of pH and natural organic matter. Environ. Toxicol. Chem. 2008, 27, 1875–1882. [Google Scholar] [CrossRef]
- Bhar, R.; Kaur, G.; Mehta, S.K. Exploring drying pattern of a sessile droplet of genomic DNA in the presence of hematite nanoparticles. Sci. Rep. 2018, 8, 6352. [Google Scholar] [CrossRef]
- Fomin, A.; Poliak, M.; Rahinov, I.; Tsionsky, V.; Cheskis, S. Combined particle mass spectrometer-quartz crystal microbalance apparatus for in situ nanoparticle monitoring during flame assisted synthesis. Combust. Flame 2013, 160, 2131–2140. [Google Scholar] [CrossRef]
- Lemine, O.M. Transformation of goethite to hematite nanocrystallines by high energy ball milling. Adv. Mater. Sci. Eng. 2014, 2014, 589146. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, R.; Ma, L.; Fu, H.; Lin, X.; Parker, S.C.; Molinari, M. Adsorption of phosphate and cadmium on iron (oxyhydr)oxides: A comparative study on ferrihydrite, goethite, and hematite. Geoderma 2021, 383, 114799. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, S.; Liu, F.; Liang, Y.; Shi, Z. Effects of humic acid and fulvic acid on the sequestration of copper and carbon during the iron oxide transformation. Chem. Eng. J. 2020, 383, 123194. [Google Scholar] [CrossRef]
- Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Gadi, S.; Cherukuri, C.S.L.; Barla, S.; Pammi, S.V.N. Antibacterial efficacy of green synthesized α-Fe2O3 nanoparticles using Sida cordifolia plant extract. Heliyon 2019, 5, e02765. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Jeevanandam, P. Synthesis of self-assembled prismatic iron oxide nanoparticles by a novel thermal decomposition route. RSC Adv. 2013, 3, 189–200. [Google Scholar] [CrossRef]
- Soltis, J.A.; Feinberg, J.M.; Gilbert, B.; Penn, R.L. Phase Transformation and Particle-Mediated Growth in the Formation of Hematite from 2-Line Ferrihydrite. Cryst. Growth Des. 2016, 16, 922–932. [Google Scholar] [CrossRef]
- Ramasubramanian, N.; Preocanin, N.; Davidson, R.D. Analysis of Passive Films on Stainless Steel by Cyclic Voltammetry and Auger Spectroscopy. J. Electrochem. Soc. 1985, 132, 793–798. [Google Scholar] [CrossRef]
- Jin, X.; Hu, G.; Wang, L.; Wang, H. Characterization of surface layers of oxidation-reduction treated Si and Mn added advanced high strength steel. Surf. Coatings Technol. 2020, 382, 125172. [Google Scholar] [CrossRef]
- Lutz, B.S.; Holcomb, G.R.; Meier, G.H. Determination of the Initiation and Propagation Mechanism of Fireside Corrosion. Oxid. Met. 2015, 84, 353–381. [Google Scholar] [CrossRef]
- Guo, W.; Sun, W.; Lv, L.P.; Kong, S.; Wang, Y. Microwave-Assisted Morphology Evolution of Fe-Based Metal-Organic Frameworks and Their Derived Fe2O3 Nanostructures for Li-Ion Storage. ACS Nano 2017, 11, 4198–4205. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Fominykh, K.; Fattakhova-Rohlfing, D.; Zeller, P.; Döblinger, M.; Bein, T. Nanocellulose-assisted formation of porous hematite nanostructures. Inorg. Chem. 2015, 54, 1129–1135. [Google Scholar] [CrossRef]
- Allen, C.C.; Probst, L.W.; Flood, B.E.; Longazo, T.G.; Schelble, R.T.; Westall, F. Meridiani Planum hematite deposit and the search for evidence of life on Mars—Iron mineralization of microorganisms in rock varnish. Icarus 2004, 171, 20–30. [Google Scholar] [CrossRef]
- Beitler, B.; Parry, W.T.; Chan, M.A. Fingerprints of fluid flow: Chemical diagenetic history of the Jurassic Navajo Sandstone, southern Utah, USA. J. Sediment. Res. 2005, 75, 547–561. [Google Scholar] [CrossRef]
- Azevedo, J.; Fernández-García, M.P.; Magén, C.; Mendes, A.; Araújo, J.P.; Sousa, C.T. Double-walled iron oxide nanotubes via selective chemical etching and Kirkendall process. Sci. Rep. 2019, 9, 11994. [Google Scholar] [CrossRef] [PubMed]
- Budiman, F.; Tan, W.K.; Kawamura, G.; Muto, H.; Matsuda, A.; Razak, K.A.; Lockman, Z. Formation of Dense and High-Aspect-Ratio Iron Oxide Nanowires by Water Vapor-Assisted Thermal Oxidation and Their Cr(VI) Adsorption Properties. ACS Omega 2021, 6, 28203–28214. [Google Scholar] [CrossRef]
- Chen, T.; Xie, Q.; Xu, H.; Chen, J.; Ji, J.; Lu, H.; Balsam, W. Characteristics and formation mechanism of pedogenic hematite in Quaternary Chinese loess and paleosols. Catena 2010, 81, 217–225. [Google Scholar] [CrossRef]
- De Carvalho, V.A.N.; Luz, R.A.D.S.; Lima, B.H.; Crespilho, F.N.; Leite, E.R.; Souza, F.L. Highly oriented hematite nanorods arrays for photoelectrochemical water splitting. J. Power Sources 2012, 205, 525–529. [Google Scholar] [CrossRef]
- Delgado, A.; Cordova, S.; Shafirovich, E. Thermite reactions with oxides of iron and silicon during combustion of magnesium with lunar and Martian regolith simulants. Combust. Flame 2015, 162, 3333–3340. [Google Scholar] [CrossRef]
- Dias, L.; Rosado, T.; Candeias, A.; Mirão, J.; Caldeira, A.T. A change in composition, a change in colour: The case of limestone sculptures from the Portuguese National Museum of Ancient Art. J. Cult. Herit. 2020, 42, 255–262. [Google Scholar] [CrossRef]
- Dunn, S.C.; von der Heyden, B.P. Gold remobilization in gossans of the Amani area, southwestern Tanzania. Ore Geol. Rev. 2021, 131, 104033. [Google Scholar] [CrossRef]
- Ebaid, Y.Y.; Nasr, M.M.; Santos, J.K.B.; Makhlouf, O. Behavior of uranium series in groundwater of the Wajid Formation, Wadi AdDawasir, Saudi Arabia. Environ. Monit. Assess. 2020, 192, 564. [Google Scholar] [CrossRef] [PubMed]
- Egglseder, M.S.; Cruden, A.R.; Tomkins, A.G.; Wilson, S.A.; Dalstra, H.J.; Rielli, A.; Li, C.; Baumgartner, J.; Faivre, D. Tiny particles building huge ore deposits—Particle-based crystallisation in banded iron formation-hosted iron ore deposits (Hamersley Province, Australia). Ore Geol. Rev. 2019, 104, 160–174. [Google Scholar] [CrossRef]
- Einert, M.; Ostermann, R.; Weller, T.; Zellmer, S.; Garnweitner, G.; Smarsly, B.M.; Marschall, R. Hollow α-Fe2O3 nanofibres for solar water oxidation: Improving the photoelectrochemical performance by formation of α-Fe2O3/ITO-composite photoanodes. J. Mater. Chem. A 2016, 4, 18444–18456. [Google Scholar] [CrossRef]
- Fru, E.C.; Ivarsson, M.; Kilias, S.P.; Bengtson, S.; Belivanova, V.; Marone, F.; Fortin, D.; Broman, C.; Stampanoni, M. Fossilized iron bacteria reveal a pathway to the biological origin of banded iron formation. Nat. Commun. 2013, 4, 2050. [Google Scholar] [CrossRef] [PubMed]
- Gash, A.E.; Tillotson, T.M.; Satcher, J.H.; Poco, J.F.; Hrubesh, L.W.; Simpson, R.L. Use of epoxides in the sol-gel synthesis of porous iron(III) oxide monoliths from Fe(III) salts. Chem. Mater. 2001, 13, 999–1007. [Google Scholar] [CrossRef]
- Han, X.; Tomaszewski, E.J.; Sorwat, J.; Pan, Y.; Kappler, A.; Byrne, J.M. Oxidation of green rust by anoxygenic phototrophic Fe(II)-oxidising bacteria. Geochem. Perspect. Lett. 2020, 12, 52–57. [Google Scholar] [CrossRef]
- Kikuchi, S.; Makita, H.; Takai, K.; Yamaguchi, N.; Takahashi, Y. Characterization of biogenic iron oxides collected by the newly designed liquid culture method using diffusion chambers. Geobiology 2014, 12, 133–145. [Google Scholar] [CrossRef]
- Kirk, A.M.; Sun, W.; Bennett, C.J.; Shipway, P.H. Interaction of displacement amplitude and frequency effects in fretting wear of a high strength steel: Impact on debris bed formation and subsurface damage. Wear 2021, 482–483, 203981. [Google Scholar] [CrossRef]
- Kuai, J.; Zhang, J.; Ardashev, D.V.; Zhang, H. Properties of α-Fe2O3 in Oxide Film of α-Fe Grinding Wheel without Abrasive Particles. Ferroelectrics 2019, 548, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.F. Photocatalytic anti-bioadhesion and bacterial deactivation on nanostructured iron oxide films. J. Mater. Chem. B 2018, 6, 1458–1469. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, S.G.; Chang, C.C.; Volinsky, A.A. Preparation and formation mechanism of monodisperse micaceous iron oxide from iron chromium grinding waste. Powder Technol. 2018, 329, 401–408. [Google Scholar] [CrossRef]
- Martín-González, M.; Martinez-Moro, R.; Aguirre, M.H.; Flores, E.; Caballero-Calero, O. Unravelling nanoporous anodic iron oxide formation. Electrochim. Acta 2020, 330, 135241. [Google Scholar] [CrossRef]
- Mechakra, H.; Sehili, T.; Kribeche, M.A.; Ayachi, A.A.; Rossignol, S.; George, C. Use of natural iron oxide as heterogeneous catalyst in photo-Fenton-like oxidation of chlorophenylurea herbicide in aqueous solution: Reaction monitoring and degradation pathways. J. Photochem. Photobiol. A Chem. 2016, 317, 140–150. [Google Scholar] [CrossRef]
- Natile, M.M.; Glisenti, A. New NiO/Co3O4 and Fe2O3/Co3O4 nanocomposite catalysts: Synthesis and characterization. Chem. Mater. 2003, 15, 2502–2510. [Google Scholar] [CrossRef]
- Pang, Q.; DorMohammadi, H.; Isgor, O.B.; Árnadóttir, L. The Effect of Surface Defects on Chloride-Induced Depassivation of Iron—A Density Functional Theory Study. Corrosion 2020, 76, 690–697. [Google Scholar] [CrossRef]
- Peng, L.; Peng, H.; Li, N.; Zhong, W.; Mao, L.; You, K.; Yin, D. Facile access to nitroalkanes: Nitration of alkanes by selective CH nitration using metal nitrate, catalyzed by in-situ generated metal oxide. Catal. Commun. 2020, 142, 106035. [Google Scholar] [CrossRef]
- Popov, N.; Bošković, M.; Perović, M.; Zadro, K.; Gilja, V.; Krehula, L.K.; Robić, M.; Marciuš, M.; Ristić, M.; Musić, S.; et al. Effect of Ru3+ ions on the formation, structural, magnetic and optical properties of hematite (α-Fe2O3) nanorods. J. Magn. Magn. Mater. 2021, 538, 168316. [Google Scholar] [CrossRef]
- Qu, C.; Qian, S.; Chen, L.; Guan, Y.; Zheng, L.; Liu, S.; Chen, W.; Cai, P.; Huang, Q. Size-dependent bacterial toxicity of hematite particles. Environ. Sci. Technol. 2019, 53, 8147–8156. [Google Scholar] [CrossRef]
- Rackauskas, S.; Nasibulin, A.G.; Jiang, H.; Tian, Y.; Kleshch, V.I.; Sainio, J.; Obraztsova, E.D.; Bokova, S.N.; Obraztsov, A.N.; Kauppinen, E.I. A novel method for metal oxide nanowire synthesis. Nanotechnology 2009, 20, 165603. [Google Scholar] [CrossRef] [PubMed]
- Ratanaphain, C.; Viboonratanasri, D.; Prompinit, P.; Krajangpan, S.; Khan, E.; Punyapalakul, P. Reactivity characterization of SiO2-coated nano zero-valent iron for iodoacetamide degradation: The effects of SiO2 thickness, and the roles of dehalogenation, hydrolysis and adsorption. Chemosphere 2022, 286, 131816. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Yan, Y.; Nie, Y.; Lu, A.; Wu, X.; Li, Y.; Wang, C.; Ding, H. Natural extracellular electron transfer between semiconducting minerals and electroactive bacterial communities occurred on the rock varnish. Front. Microbiol. 2019, 10, 293. [Google Scholar] [CrossRef]
- Ryu, Y.J.; Kim, M.; Yoo, C.S. Phase Diagram and Transformations of Iron Pentacarbonyl to nm Layered Hematite and Carbon-Oxygen Polymer under Pressure. Sci. Rep. 2015, 5, 15139. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Wang, F.; Zhu, J.; Zhang, B.; Zhao, T.; Wang, Y.; Wang, J. Effect of phase separation on the Jian ware blue colored glaze with iron oxide. Ceram. Int. 2018, 44, 16407–16413. [Google Scholar] [CrossRef]
- Sun, J.; Wei, L.; Yin, R.; Jiang, F.; Shang, C. Microbial iron reduction enhances in-situ control of biogenic hydrogen sulfide by FeOOH granules in sediments of polluted urban waters. Water Res. 2020, 171, 115453. [Google Scholar] [CrossRef]
- Sundararajan, J.A.; Kaur, M.; Qiang, Y. Mechanism of electron beam induced oxide layer thickening on iron-iron oxide core-shell nanoparticles. J. Phys. Chem. C 2015, 119, 8357–8363. [Google Scholar] [CrossRef]
- Taniguchi, A.; Taniguchi, T.; Wagata, H.; Katsumata, K.I.; Okada, K.; Matsushita, N. Liquid-phase atomic layer deposition of crystalline hematite without post-growth annealing. CrystEngComm 2019, 21, 4184–4191. [Google Scholar] [CrossRef]
- Umar, A.; Ibrahim, A.A.; Kumar, R.; Albargi, H.; Alsaiari, M.A.; Ahmed, F. Cubic shaped hematite (α-Fe2O3) micro-structures composed of stacked nanosheets for rapid ethanol sensor application. Sens. Actuators B Chem. 2021, 326, 128851. [Google Scholar] [CrossRef]
- Vasilyeva, M.S.; Rudnev, V.S.; Zvereva, A.A.; Ustinov, A.Y.; Arefieva, O.D.; Kuryavyi, V.G.; Zverev, G.A. FeOx, SiO2, TiO2/Ti composites prepared using plasma electrolytic oxidation as photo-Fenton-like catalysts for phenol degradation. J. Photochem. Photobiol. A Chem. 2018, 356, 38–45. [Google Scholar] [CrossRef]
- Vinayagam, R.; Pai, S.; Varadavenkatesan, T.; Narasimhan, M.K.; Narayanasamy, S.; Selvaraj, R. Structural characterization of green synthesized α-Fe2O3 nanoparticles using the leaf extract of Spondias dulcis. Surf. Interfaces 2020, 20, 100618. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Z.Z.; Niu, Y.; Lyu, H.B. Macromechanics, microstructure and particle contact model of artificially cemented laterite. Mater. Express 2021, 11, 732–739. [Google Scholar] [CrossRef]
- Xie, K.; Guo, M.; Huang, H.; Liu, Y. Fabrication of iron oxide nanotube arrays by electrochemical anodization. Corros. Sci. 2014, 88, 66–75. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, X.; Elsayed, Y.E.A.N.; Hong, C.; Yadav, A.; Rogowska, M.; Ambat, R. Characteristics of scales and their impacts on under-deposit corrosion in an oil production well. Mater. Corros. 2021, 72, 1051–1064. [Google Scholar] [CrossRef]
- Zha, J.; Wei, S.; Wang, C.; Li, Z.; Cai, Y.; Ma, Q. Weathering mechanism of red discolorations on Limestone object: A case study from Lingyan Temple, Jinan, Shandong Province, China. Herit. Sci. 2020, 8, 54. [Google Scholar] [CrossRef]
- Zhang, F.S.; Itoh, H. Iron oxide-loaded slag for arsenic removal from aqueous system. Chemosphere 2005, 60, 319–325. [Google Scholar] [CrossRef]
- Zhang, R.; Dai, H.; Du, Y.; Zhang, L.; Deng, J.; Xia, Y.; Zhao, Z.; Meng, X.; Liu, Y. P123-PMMA dual-templating generation and unique physicochemical properties of three-dimensionally ordered macroporous iron oxides with nanovoids in the crystalline walls. Inorg. Chem. 2011, 50, 2534–2544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fang, K.; Lin, M.; Hou, B.; Zhong, L.; Zhu, Y.; Wei, W.; Sun, Y. Controlled fabrication of iron oxide/mesoporous silica core-shell nanostructures. J. Phys. Chem. C 2013, 117, 21529–21538. [Google Scholar] [CrossRef]
- Zhu, G.; Sushko, M.L.; Loring, J.S.; Legg, B.A.; Song, M.; Soltis, J.A.; Huang, X.; Rosso, K.M.; De Yoreo, J.J. Self-similar mesocrystals form via interface-driven nucleation and assembly. Nature 2021, 590, 416–422. [Google Scholar] [CrossRef]
- Baldovi, H.G. Optimization of α-Fe2O3 nanopillars diameters for photoelectrochemical enhancement of α-Fe2O3-TiO2 heterojunction. Nanomaterials 2021, 11, 2019. [Google Scholar] [CrossRef]
- Basak, S.; Tiwari, V.; Fan, J.; Achilefu, S.; Sethi, V.; Biswas, P. Single step aerosol synthesis of nanocomposites by aerosol routes: γ-Fe2O3/SiO2 and their functionalization. J. Mater. Res. 2011, 26, 1225–1233. [Google Scholar] [CrossRef]
- Ikoma, S.; Ohki, K.; Yokoi, H. Preparation and characterization of silica-supported maghemite. J. Ceram. Soc. Jpn. 1992, 100, 864–866. [Google Scholar] [CrossRef]
- Coddens, E.M.; Huang, L.; Wong, C.; Grassian, V.H. Influence of Glyoxal on the Catalytic Oxidation of S(IV) in Acidic Aqueous Media. ACS Earth Space Chem. 2019, 3, 142–149. [Google Scholar] [CrossRef]
- Inamdar, S.; Choi, H.S.; Kim, M.S.; Chaudhari, K.; Yu, J.S. Flame synthesis of 26-faceted maghemite polyhedrons grown via 14-faceted polyhedrons and their carbon composites for Li-ion battery application. CrystEngComm 2012, 14, 7009–7014. [Google Scholar] [CrossRef]
- Kaewsaneha, C.; Elaissari, A.; Opaprakasit, P.; Sreearunothai, P.; Tangboriboonrat, P. Poly(styrene-b-acrylic Acid) Nanoparticles with High Magnetic Loading for Magnetic Hyperthermia Cancer Therapy. ACS Appl. Nano Mater. 2021, 4, 1841–1848. [Google Scholar] [CrossRef]
- Mishra, D.; Greving, D.; Confalonieri, G.A.B.; Perlich, J.; Toperverg, B.P.; Zabel, H.; Petracic, O. Growth modes of nanoparticle superlattice thin films. Nanotechnology 2014, 25, 205602. [Google Scholar] [CrossRef]
- Sharifi, T.; Gracia-Espino, E.; Barzegar, H.R.; Jia, X.; Nitze, F.; Hu, G.; Nordblad, P.; Tai, C.W.; Wågberg, T. Formation of nitrogen-doped graphene nanoscrolls by adsorption of magnetic γ-Fe2O3 nanoparticles. Nat. Commun. 2013, 4, 2319. [Google Scholar] [CrossRef]
- Wang, C.M.; Baer, D.R.; Thomas, L.E.; Amonette, J.E.; Antony, J.; Qiang, Y.; Duscher, G. Void formation during early stages of passivation: Initial oxidation of iron nanoparticles at room temperature. J. Appl. Phys. 2005, 98, 094308. [Google Scholar] [CrossRef]
- Wen, Z.; Lu, J.; Zhang, Y.; Cheng, G.; Huang, S.; Chen, J.; Xu, R.; Ming, Y.A.; Wang, Y.; Chen, R. Facile inverse micelle fabrication of magnetic ordered mesoporous iron cerium bimetal oxides with excellent performance for arsenic removal from water. J. Hazard. Mater. 2020, 383, 121172. [Google Scholar] [CrossRef]
- Ahamed, I.; Skomski, R.; Kashyap, A. Controlling the magnetocrystalline anisotropy of ε-Fe2O3. AIP Adv. 2019, 9, 035231. [Google Scholar] [CrossRef]
- Gorbachev, E.; Soshnikov, M.; Wu, M.; Alyabyeva, L.; Myakishev, D.; Kozlyakova, E.; Lebedev, V.; Anokhin, E.; Gorshunov, B.; Brylev, O.; et al. Tuning the particle size, natural ferromagnetic resonance frequency and magnetic properties of ϵ-Fe2O3 nanoparticles prepared by a rapid sol-gel method. J. Mater. Chem. C 2021, 9, 6173–6179. [Google Scholar] [CrossRef]
- Jang, M.S.; Ok, H.J.; Oh, I.; Kang, G.H.; Yoo, J.W.; Lee, K.S. Formation of Y3Fe5O12 matrix including ε-phase Fe2O3 with the giant coercive field via optimized sol–gel method. J. Magn. Magn. Mater. 2022, 552, 169218. [Google Scholar] [CrossRef]
- López-Sánchez, J.; Serrano, A.; del Campo, A.; Muñoz-Noval, Á.; Salas-Colera, E.; Cabero, M.; Varela, M.; Abuín, M.; Castro, G.R.; Rubio-Zuazo, J.; et al. A combined micro-Raman, X-ray absorption and magnetic study to follow the glycerol-assisted growth of epsilon-iron oxide sol-gel coatings. J. Alloys Compd. 2022, 892, 162061. [Google Scholar] [CrossRef]
- Maccato, C.; Carraro, G.; Peddis, D.; Varvaro, G.; Barreca, D. Magnetic properties of ε iron(III) oxide nanorod arrays functionalized with gold and copper(II) oxide. Appl. Surf. Sci. 2018, 427, 890–896. [Google Scholar] [CrossRef]
- Nikolić, V.N.; Tadić, M.; Panjan, M.; Kopanja, L.; Cvjetićanin, N.; Spasojević, V. Influence of annealing treatment on magnetic properties of Fe2O3/SiO2 and formation of ε-Fe2O3 phase. Ceram. Int. 2017, 43, 3147–3155. [Google Scholar] [CrossRef]
- Suturin, S.M.; Korovin, A.M.; Sitnikova, A.A.; Kirilenko, D.A.; Volkov, M.P.; Dvortsova, P.A.; Ukleev, V.A.; Tabuchi, M.; Sokolov, N.S. Correlation between crystal structure and magnetism in PLD grown epitaxial films of ε-Fe2O3 on GaN. Sci. Technol. Adv. Mater. 2021, 22, 85–99. [Google Scholar] [CrossRef]
- Tadic, M.; Milosevic, I.; Kralj, S.; Hanzel, D.; Barudzija, T.; Motte, L.; Makovec, D. Surface-induced reversal of a phase transformation for the synthesis of ε-Fe2O3 nanoparticles with high coercivity. Acta Mater. 2020, 188, 16–22. [Google Scholar] [CrossRef]
- Tokoro, H.; Namai, A.; Ohkoshi, S.I. Advances in magnetic films of epsilon-iron oxide toward next-generation high-density recording media. Dalton Trans. 2021, 50, 452–459. [Google Scholar] [CrossRef]
- Ukleev, V.; Volkov, M.; Korovin, A.; Saerbeck, T.; Sokolov, N.; Suturin, S. Stabilization of ϵ-Fe2O3 epitaxial layer on MgO(111)/GaN via an intermediate γ-phase. Phys. Rev. Mater. 2019, 3, 094401. [Google Scholar] [CrossRef]
- Myhill, R.; Ojwang, D.O.; Ziberna, L.; Frost, D.J.; Ballaran, T.B.; Miyajima, N. On the P–T–fO2 stability of Fe4O5, Fe5O6 and Fe4O5-rich solid solutions. Contrib. Mineral. Petrol. 2016, 171, 51. [Google Scholar] [CrossRef]
- Ovsyannikov, S.V.; Bykov, M.; Bykova, E.; Kozlenko, D.P.; Tsirlin, A.A.; Karkin, A.E.; Shchennikov, V.V.; Kichanov, S.E.; Gou, H.; Abakumov, A.M.; et al. Charge-ordering transition in iron oxide Fe4O5 involving competing dimer and trimer formation. Nat. Chem. 2016, 8, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Bligh, M.W.; Waite, T.D. Formation, reactivity, and aging of ferric oxide particles formed from Fe(II) and Fe(III) sources: Implications for iron bioavailability in the marine environment. Geochim. Cosmochim. Acta 2011, 75, 7741–7758. [Google Scholar] [CrossRef]
- Bligh, M.W.; Maheshwari, P.; Waite, T.D. Formation, reactivity and aging of amorphous ferric oxides in the presence of model and membrane bioreactor derived organics. Water Res. 2017, 124, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Brigante, M.; Parolo, M.E.; Schulz, P.C.; Avena, M. Synthesis, characterization of mesoporous silica powders and application to antibiotic remotion from aqueous solution. Effect of supported Fe-oxide on the SiO2 adsorption properties. Powder Technol. 2014, 253, 178–186. [Google Scholar] [CrossRef]
- Greenberg, E.; Armon, N.; Kapon, O.; Ben-Ishai, M.; Shpaisman, H. Nanostructure and Mechanism of Metal Deposition by a Laser-Induced Photothermal Reaction. Adv. Mater. Interfaces 2019, 6, 1900541. [Google Scholar] [CrossRef]
- Chow, S.S.; Taillefert, M. Effect of arsenic concentration on microbial iron reduction and arsenic speciation in an iron-rich freshwater sediment. Geochim. Cosmochim. Acta 2009, 73, 6008–6021. [Google Scholar] [CrossRef]
- Karakaya, Y.; Kluge, S.; Wiggers, H.; Schulz, C.; Kasper, T. Investigation of the combustion of iron pentacarbonyl and the formation of key intermediates in iron oxide synthesis flames. Chem. Eng. Sci. 2021, 230, 116169. [Google Scholar] [CrossRef]
- Paur, H.R.; Baumann, W.; Mätzing, H.; Seifert, H. Formation of nanoparticles in flames; Measurement by particle mass spectrometry and numerical simulation. Nanotechnology 2005, 16, S354–S361. [Google Scholar] [CrossRef]
- Poliak, M.; Fomin, A.; Tsionsky, V.; Cheskis, S.; Wlokas, I.; Rahinov, I. On the mechanism of nanoparticle formation in a flame doped by iron pentacarbonyl. Phys. Chem. Chem. Phys. 2015, 17, 680–685. [Google Scholar] [CrossRef]
- Xie, Y.; Dong, F.; Heinbuch, S.; Rocca, J.J.; Bernstein, E.R. Investigation of the reactions of small neutral iron oxide clusters with methanol. J. Chem. Phys. 2009, 130, 114306. [Google Scholar] [CrossRef]
- Yan, B.; Li, L.; Yu, Q.; Hang, W.; He, J.; Huang, B. High irradiance laser ionization mass spectrometry for direct speciation of iron oxides. J. Am. Soc. Mass Spectrom. 2010, 21, 1227–1234. [Google Scholar] [CrossRef]
- Zeth, K.; Offermann, S.; Essen, L.O.; Oesterhelt, D. Iron-oxo clusters biomineralizing on protein surfaces: Structural analysis of Halobacterium salinarum DpsA in its low- and high-iron states. Proc. Natl. Acad. Sci. USA 2004, 101, 13780–13785. [Google Scholar] [CrossRef]
- Zeth, K. Dps biomineralizing proteins: Multifunctional architects of nature. Biochem. J. 2012, 445, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.E.G.; Doyle, R.L.; Brandon, M.P. Redox switching and oxygen evolution at oxidized metal and metal oxide electrodes: Iron in base. Phys. Chem. Chem. Phys. 2011, 13, 21530–21551. [Google Scholar] [CrossRef]
- Weng, X.; Jin, X.; Lin, J.; Naidu, R.; Chen, Z. Removal of mixed contaminants Cr(VI) and Cu(II) by green synthesized iron based nanoparticles. Ecol. Eng. 2016, 97, 32–39. [Google Scholar] [CrossRef]
- Soldemo, M.; Lundgren, E.; Weissenrieder, J. Oxidation of Fe(110) in oxygen gas at 400 °C. Surf. Sci. 2016, 644, 172–179. [Google Scholar] [CrossRef]
- Barcaro, G.; Monti, S. Modeling generation and growth of iron oxide nanoparticles from representative precursors through ReaxFF molecular dynamics. Nanoscale 2020, 12, 3103–3111. [Google Scholar] [CrossRef]
- Ai, L.; Zhou, Y.; Chen, M. Role of Dissolved Oxygen in Iron Oxidation in Supercritical Water: Insights from Reactive Dynamics Simulations. J. Phys. Chem. C 2019, 123, 15009–15016. [Google Scholar] [CrossRef]
- Kim, C.; Ahn, J.Y.; Kim, T.Y.; Hwang, I. Mechanisms of electro-assisted persulfate/nano-Fe0 oxidation process: Roles of redox mediation by dissolved Fe. J. Hazard. Mater. 2020, 388, 121739. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.A.; Gakh, O.; Park, S.; Cui, J.; Mooney, S.M.; Sampson, M.; Ferreira, G.C.; Isaya, G. Assembly of human frataxin is a mechanism for detoxifying redox-active iron. Biochemistry 2005, 44, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Palchoudhury, S.; An, W.; Xu, Y.; Qin, Y.; Zhang, Z.; Chopra, N.; Holler, R.A.; Turner, C.H.; Bao, Y. Synthesis and growth mechanism of iron oxide nanowhiskers. Nano Lett. 2011, 11, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Panda, C.; Debgupta, J.; Díaz, D.D.; Singh, K.K.; Gupta, S.S.; Dhar, B.B. Homogeneous photochemical water oxidation by biuret-modified Fe-TAML: Evidence of FeV(O) intermediate. J. Am. Chem. Soc. 2014, 136, 12273–12282. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zuo, X.; Sankaranarayanan, S.K.R.S.; Peng, S.; Narayanan, B.; Kamath, G. Quantitative 3D evolution of colloidal nanoparticle oxidation in solution. Science 2017, 356, 303–307. [Google Scholar] [CrossRef]
- Tamura, K.; Kunoh, T.; Nakanishi, M.; Kusano, Y.; Takada, J. Preparation and characterization of additional metallic element-containing tubular iron oxides of bacterial origin. ACS Omega 2020, 5, 27287–27294. [Google Scholar] [CrossRef]
- Tian, B.; Światowska, J.; Maurice, V.; Zanna, S.; Seyeux, A.; Klein, L.H.; Marcus, P. Combined surface and electrochemical study of the lithiation/delithiation mechanism of the iron oxide thin-film anode for lithium-ion batteries. J. Phys. Chem. C 2013, 117, 21651–21661. [Google Scholar] [CrossRef]
- Walker, J.D.; Tannenbaum, R. Characterization of the sol-gel formation of iron(III) oxide/hydroxide nanonetworks from weak base molecules. Chem. Mater. 2006, 18, 4793–4801. [Google Scholar] [CrossRef]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Wu, X.; Wei, Y.; Wang, J.; Wang, D.; She, L.; Wang, J.; Cai, C. Effects of soil physicochemical properties on aggregate stability along a weathering gradient. Catena 2017, 156, 205–215. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Li, J.; Zhao, J.; Yu, Y. Effects of metallurgical factors on reticular crack formations in Nb-bearing pipeline steel. High Temp. Mater. Process. 2020, 39, 81–87. [Google Scholar] [CrossRef]
- Gareev, K.G.; Grouzdev, D.S.; Kharitonskii, P.V.; Kirilenko, D.A.; Kosterov, A.; Koziaeva, V.V.; Levitskii, V.S.; Multhoff, G.; Nepomnyashchaya, E.K.; Nikitin, A.V.; et al. Magnetic properties of bacterial magnetosomes produced by magnetospirillum caucaseum so-1. Microorganisms 2021, 9, 1854. [Google Scholar] [CrossRef]
- Grevey, A.L.; Vignal, V.; Krawiec, H.; Ozga, P.; Peche-Quilichini, K.; Rivalan, A.; Mazière, F. Microstructure and long-term corrosion of archaeological iron alloy artefacts. Herit. Sci. 2020, 8, 57. [Google Scholar] [CrossRef]
- Bi, J.; Tao, Q.; Ren, J.; Huang, X.; Wang, T.; Hao, H. P25-inspired α/γ-Fe2O3 isoelement heterostructures for visible-light photocatalysis: Optimization of crystalline ratios and mechanism investigation. J. Alloys Compd. 2023, 937, 168422. [Google Scholar] [CrossRef]
- Ghafarpour, A.; Khormali, F.; Balsam, W.; Forman, S.L.; Cheng, L.; Song, Y. The formation of iron oxides and magnetic enhancement mechanisms in northern Iranian loess-paleosol sequences: Evidence from diffuse reflectance spectrophotometry and temperature dependence of magnetic susceptibility. Quat. Int. 2021, 589, 68–82. [Google Scholar] [CrossRef]
- Long, X.; Ji, J.; Balsam, W. Rainfall-dependent transformations of iron oxides in a tropical saprolite transect of Hainan Island, South China: Spectral and magnetic measurements. J. Geophys. Res. Earth Surf. 2011, 116, F03015. [Google Scholar] [CrossRef]
- Anju, M.; Renuka, N.K. A novel template free synthetic strategy to graphene-iron oxide nanotube hybrid. RSC Adv. 2015, 5, 78648–78654. [Google Scholar] [CrossRef]
- Patel, S.; Hota, G. Iron oxide nanoparticle-immobilized PAN nanofibers: Synthesis and adsorption studies. RSC Adv. 2016, 6, 15402–15414. [Google Scholar] [CrossRef]
- Sousa, L.S.; Chagas, P.; Oliveira, L.C.A.D.; Castro, C.S.D. Carbon/FexOy magnetic composites obtained from PET and red mud residues: Paracetamol and dye oxidation. Environ. Technol. 2019, 40, 2840–2852. [Google Scholar] [CrossRef]
- Hernández-Espejel, A.; Palomar-Pardavé, M.; Cabrera-Sierra, R.; Romero-Romo, M.; Ramírez-Silva, M.T.; Arce-Estrada, E.M. Kinetics and mechanism of the electrochemical formation of iron oxidation products on steel immersed in sour acid media. J. Phys. Chem. B 2011, 115, 1833–1841. [Google Scholar] [CrossRef]
- Angerer, T.; Thorne, W.; Hagemann, S.G.; Tribus, M.; Evans, N.J.; Savard, D. Iron oxide chemistry supports a multistage hydrothermal genesis of BIF-hosted hematite ore in the Mt. Tom Price and Mt. Whaleback deposits. Ore Geol. Rev. 2022, 144, 104840. [Google Scholar] [CrossRef]
- Ebotehouna, C.G.; Xie, Y.; Adomako-Ansah, K.; Gourcerol, B.; Qu, Y. Depositional environment and genesis of the nabeba banded iron formation (Bif) in the ivindo basement complex, republic of the congo: Perspective from whole-rock and magnetite geochemistry. Minerals 2021, 11, 579. [Google Scholar] [CrossRef]
- Egglseder, M.S.; Cruden, A.R.; Dalstra, H.J.; Nicholas, L. The role of deformation in the formation of banded iron formation-hosted high-grade iron ore deposits, Hamersley Province (Australia). Precambrian Res. 2017, 296, 62–77. [Google Scholar] [CrossRef]
- Kreiner, D.C.; Barton, M.D. Sulfur-poor intense acid hydrothermal alteration: A distinctive hydrothermal environment. Ore Geol. Rev. 2017, 88, 174–187. [Google Scholar] [CrossRef]
- Mercer, C.N.; Watts, K.E.; Gross, J. Apatite trace element geochemistry and cathodoluminescent textures—A comparison between regional magmatism and the Pea Ridge IOAREE and Boss IOCG deposits, southeastern Missouri iron metallogenic province, USA. Ore Geol. Rev. 2020, 116, 103129. [Google Scholar] [CrossRef]
- Sheshukov, O.Y.; Mikheenkov, M.A.; Vyaznikova, E.A.; Bykov, A.S.; Vedmid’, L.B. Changes in phase composition of soderites of the bakal deposit at heating. Izv. Ferr. Metall. 2018, 61, 891–897. [Google Scholar] [CrossRef]
- Sun, S.; Konhauser, K.O.; Kappler, A.; Li, Y.L. Primary hematite in Neoarchean to Paleoproterozoic oceans. GSA Bull. 2015, 127, 850–861. [Google Scholar] [CrossRef]
- Fischer, W.W.; Knoll, A.H. An iron shuttle for deepwater silica in late Archean and early Paleoproterozoic iron formation. GSA Bull. 2009, 121, 222–235. [Google Scholar] [CrossRef]
- Rego, E.S.; Busigny, V.; Lalonde, S.V.; Philippot, P.; Bouyon, A.; Rossignol, C.; Babinski, M.; de Cássia Zapparoli, A. Anoxygenic photosynthesis linked to Neoarchean iron formations in Carajás (Brazil). Geobiology 2021, 19, 326–341. [Google Scholar] [CrossRef]
- Ali, G.; Park, Y.J.; Hussain, A.; Cho, S.O. A novel route to the formation of 3D nanoflower-like hierarchical iron oxide nanostructure. Nanotechnology 2019, 30, 095601. [Google Scholar] [CrossRef]
- Cao, S.W.; Zhu, Y.J. Iron oxide hollow spheres: Microwave-hydrothermal ionic liquid preparation, formation mechanism, crystal phase and morphology control and properties. Acta Mater. 2009, 57, 2154–2165. [Google Scholar] [CrossRef]
- Gracien, E.B.; Ruimin, Z.; LiHui, X.; Kanza, L.K.; Lopaka, I. Effects of pH on the morphology of iron oxides synthesized under gamma-irradiation. J. Radioanal. Nucl. Chem. 2006, 270, 473–478. [Google Scholar] [CrossRef]
- Alemani, M.; Gialanella, S.; Straffelini, G.; Ciudin, R.; Olofsson, U.; Perricone, G.; Metinoz, I. Dry sliding of a low steel friction material against cast iron at different loads: Characterization of the friction layer and wear debris. Wear 2017, 376–377, 1450–1459. [Google Scholar] [CrossRef]
- Fatimah, I.; Amaliah, S.N.; Andrian, M.F.; Handayani, T.P.; Nurillahi, R.; Prakoso, N.I.; Wicaksono, W.P.; Chuenchom, L. Iron oxide nanoparticles supported on biogenic silica derived from bamboo leaf ash for rhodamine B photodegradation. Sustain. Chem. Pharm. 2019, 13, 100149. [Google Scholar] [CrossRef]
- Lim, J.H.; Min, S.G.; Malkinski, L.; Wiley, J.B. Iron oxide nanotubes synthesized via template-based electrodeposition. Nanoscale 2014, 6, 5289–5295. [Google Scholar] [CrossRef]
- Aligizaki, K.K.; De Rooij, M.R.; Macdonald, D.D. Analysis of iron oxides accumulating at the interface between aggregates and cement paste. Cem. Concr. Res. 2000, 30, 1941–1945. [Google Scholar] [CrossRef]
- Budiman, F.; Kian, T.W.; Razak, K.A.; Matsuda, A.; Lockman, Z. The Assessment of Cr(VI) Removal by Iron Oxide Nanosheets and Nanowires Synthesized by Thermal Oxidation of Iron in Water Vapour. Procedia Chem. 2016, 19, 586–593. [Google Scholar] [CrossRef]
- Carranza, E.J.M.; de Souza Filho, C.R.; Haddad-Martim, P.M.; Katsuta, N.; Shimizu, I. Macro-scale ore-controlling faults revealed by micro-geochemical anomalies. Sci. Rep. 2019, 9, 4410. [Google Scholar] [CrossRef] [PubMed]
- Ershov, B.G. Radiation-induced oxidation of iron in the ocean of the early Earth. Precambrian Res. 2021, 364, 106360. [Google Scholar] [CrossRef]
- Gaikwad, V.; Badesab, F.; Dewangan, P.; Kotha, M. Diagenesis of Magnetic Minerals in Active/Relict Methane Seep: Constraints From Rock Magnetism and Mineralogical Records From Bay of Bengal. Front. Earth Sci. 2021, 9, 638594. [Google Scholar] [CrossRef]
- Huang, R.; Lin, C.T.; Sun, W.; Ding, X.; Zhan, W.; Zhu, J. The production of iron oxide during peridotite serpentinization: Influence of pyroxene. Geosci. Front. 2017, 8, 1311–1321. [Google Scholar] [CrossRef]
- Johnston, C.J.; Pepper, R.A.; Martens, W.N.; Couperthwaite, S. Improvement of aluminium extraction from low-grade kaolinite by iron oxide impurities: Role of clay chemistry and morphology. Miner. Eng. 2022, 176, 107346. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Amskold, L.; Lalonde, S.V.; Posth, N.R.; Kappler, A.; Anbar, A. Decoupling photochemical Fe(II) oxidation from shallow-water BIF deposition. Earth Planet. Sci. Lett. 2007, 258, 87–100. [Google Scholar] [CrossRef]
- Levett, A.; Gagen, E.; Paz, A.; Vasconcelos, P.; Southam, G. Strategising the bioremediation of Brazilian iron ore mines. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2749–2771. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Y. Effect of Fe(II) on the formation of iron oxide synthesized from pyrite cinders by hydrothermal process. Powder Technol. 2011, 209, 119–123. [Google Scholar] [CrossRef]
- Ohmoto, H. Nonredox transformations of magnetite-hematite in hydrothermal systems. Econ. Geol. 2003, 98, 157–161. [Google Scholar] [CrossRef]
- Boulard, E.; Menguy, N.; Auzende, A.L.; Benzerara, K.; Bureau, H.; Antonangeli, D.; Corgne, A.; Morard, G.; Siebert, J.; Perrillat, J.P.; et al. Experimental investigation of the stability of Fe-rich carbonates in the lower mantle. J. Geophys. Res. Solid Earth 2012, 117, B02208. [Google Scholar] [CrossRef]
- Matthew, S.P.; Cho, T.R.; Hayes, P.C. Mechanisms of porous iron growth on wustite and magnetite during gaseous reduction. Metall. Trans. B 1990, 21, 733–741. [Google Scholar] [CrossRef]
- Meng, S.; Wu, J.; Zhao, L.; Zheng, H.; Jia, S.; Hu, S.; Meng, W.; Pu, S.; Zhao, D.; Wang, J. Atomistic Insight into the Redox Reactions in Fe/Oxide Core-Shell Nanoparticles. Chem. Mater. 2018, 30, 7306–7312. [Google Scholar] [CrossRef]
- Cotin, G.; Kiefer, C.; Perton, F.; Ihiawakrim, D.; Blanco-Andujar, C.; Moldovan, S.; Lefevre, C.; Ersen, O.; Pichon, B.; Mertz, D.; et al. Unravelling the thermal decomposition parameters for the synthesis of anisotropic iron oxide nanoparticles. Nanomaterials 2018, 8, 881. [Google Scholar] [CrossRef]
- Glasgow, W.; Fellows, B.; Qi, B.; Darroudi, T.; Kitchens, C.; Ye, L.; Crawford, T.M.; Mefford, O.T. Continuous synthesis of iron oxide (Fe3O4) nanoparticles via thermal decomposition. Particuology 2016, 26, 47–53. [Google Scholar] [CrossRef]
- Miguel-Sancho, N.; Bomati-Miguel, O.; Roca, A.G.; Martinez, G.; Arruebo, M.; Santamaria, J. Synthesis of magnetic nanocrystals by thermal decomposition in glycol media: Effect of process variables and mechanistic study. Ind. Eng. Chem. Res. 2012, 51, 8348–8357. [Google Scholar] [CrossRef]
- Nandakumaran, N.; Barnsley, L.; Feoktystov, A.; Ivanov, S.A.; Huber, D.L.; Fruhner, L.S.; Leffler, V.; Ehlert, S.; Kentzinger, E.; Qdemat, A.; et al. Unravelling Magnetic Nanochain Formation in Dispersion for in Vivo Applications. Adv. Mater. 2021, 33, 2008683. [Google Scholar] [CrossRef] [PubMed]
- Pušnik, K.; Goršak, T.; Drofenik, M.; Makovec, D. Synthesis of aqueous suspensions of magnetic nanoparticles with the co-precipitation of iron ions in the presence of aspartic acid. J. Magn. Magn. Mater. 2016, 413, 65–75. [Google Scholar] [CrossRef]
- Vogt, C.; Toprak, M.S.; Muhammed, M.; Laurent, S.; Bridot, J.L.; Müller, R.N. High quality and tuneable silica shell-magnetic core nanoparticles. J. Nanoparticle Res. 2010, 12, 1137–1147. [Google Scholar] [CrossRef]
- Wen, J.Z.; Richter, H.; Green, W.H.; Howard, J.B.; Treska, M.; Jardim, P.M.; Sande, J.B.V. Experimental study of catalyst nanoparticle and single walled carbon nanotube formation in a controlled premixed combustion. J. Mater. Chem. 2008, 18, 1561–1569. [Google Scholar] [CrossRef]
- Chen, Z. Size and shape controllable synthesis of monodisperse iron oxide nanoparticles by thermal decomposition of iron oleate complex. Synth. React. Inorganic Met. Nano-Metal Chem. 2012, 42, 1040–1046. [Google Scholar] [CrossRef]
- Besenhard, M.O.; Lagrow, A.P.; Famiani, S.; Pucciarelli, M.; Lettieri, P.; Thanh, N.T.K.; Gavriilidis, A. Continuous production of iron oxide nanoparticles: Via fast and economical high temperature synthesis. React. Chem. Eng. 2020, 5, 1474–1483. [Google Scholar] [CrossRef]
- Lin, J.; He, F.; Su, B.; Sun, M.; Owens, G.; Chen, Z. The stabilizing mechanism of cadmium in contaminated soil using green synthesized iron oxide nanoparticles under long-term incubation. J. Hazard. Mater. 2019, 379, 120832. [Google Scholar] [CrossRef]
- Slimani, S.; Meneghini, C.; Abdolrahimi, M.; Talone, A.; Murillo, J.P.M.; Barucca, G.; Yaacoub, N.; Imperatori, P.; Illés, E.; Smari, M.; et al. Spinel iron oxide by the co-precipitation method: Effect of the reaction atmosphere. Appl. Sci. 2021, 11, 5433. [Google Scholar] [CrossRef]
- Bernard, M.C.; Joiret, S. Understanding corrosion of ancient metals for the conservation of cultural heritage. Electrochim. Acta 2009, 54, 5199–5205. [Google Scholar] [CrossRef]
- Leon, Y.; Saheb, M.; Drouet, E.; Neff, D.; Foy, E.; Leroy, E.; Dynes, J.J.; Dillmann, P. Interfacial layer on archaeological mild steel corroded in carbonated anoxic environments studied with coupled micro and nano probes. Corros. Sci. 2014, 88, 23–35. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; McIntyre, N.S. Examination of the oxidation of iron by oxygen using X-ray photoelectron spectroscopy and QUASESTM. Surf. Sci. 2004, 565, 151–162. [Google Scholar] [CrossRef]
- Monnier, J.; Réguer, S.; Foy, E.; Testemale, D.; Mirambet, F.; Saheb, M.; Dillmann, P.; Guillot, I. XAS and XRD in situ characterisation of reduction and reoxidation processes of iron corrosion products involved in atmospheric corrosion. Corros. Sci. 2014, 78, 293–303. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Fushimi, K. Optical characterization of passive oxides on metals. Electrochemistry 2016, 84, 826–832. [Google Scholar] [CrossRef]
- Velimirovic, M.; Auffan, M.; Carniato, L.; Batka, V.M.; Schmid, D.; Wagner, S.; Borschneck, D.; Proux, O.; von der Kammer, F.; Hofmann, T. Effect of field site hydrogeochemical conditions on the corrosion of milled zerovalent iron particles and their dechlorination efficiency. Sci. Total Environ. 2018, 618, 1619–1627. [Google Scholar] [CrossRef]
- Sánchez-Moreno, M.; Takenouti, H.; García-Jareño, J.J.; Vicente, F.; Alonso, C. A theoretical approach of impedance spectroscopy during the passivation of steel in alkaline media. Electrochim. Acta 2009, 54, 7222–7226. [Google Scholar] [CrossRef]
- Song, Y.; Wightman, E.; Tian, Y.; Jack, K.; Li, X.; Zhong, H.; Bond, P.L.; Yuan, Z.; Jiang, G. Corrosion of reinforcing steel in concrete sewers. Sci. Total Environ. 2019, 649, 739–748. [Google Scholar] [CrossRef]
- Silva-Bedoya, L.M.; Watkin, E.; Machuca, L.L. Deep-sea corrosion rusticles from iron-hulled shipwrecks. Mater. Corros. 2021, 72, 1138–1151. [Google Scholar] [CrossRef]
- Bautista-Guzman, J.; Gomez-Morales, R.; Asmat-Campos, D.; Checca, N.R. Influence of the alcoholic/ethanolic extract of mangifera indica residues on the green synthesis of feo nanoparticles and their application for the remediation of agricultural soils. Molecules 2021, 26, 7633. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Castro, J.J.; Winkler, E.L.; Lima, E.; Zysler, R.D.; Morales, M.D.P.; Ovejero, J.G.; Streitwieser, D.A. Improving degradation of real wastewaters with self-heating magnetic nanocatalysts. J. Clean. Prod. 2021, 308, 127385. [Google Scholar] [CrossRef]
- Kastrinaki, G.; Lorentzou, S.; Karagiannakis, G.; Rattenbury, M.; Woodhead, J.; Konstandopoulos, A.G. Parametric synthesis study of iron based nanoparticles via aerosol spray pyrolysis route. J. Aerosol. Sci. 2018, 115, 96–107. [Google Scholar] [CrossRef]
- Lagrow, A.P.; Besenhard, M.O.; Hodzic, A.; Sergides, A.; Bogart, L.K.; Gavriilidis, A.; Thanh, N.T.K. Unravelling the growth mechanism of the co-precipitation of iron oxide nanoparticles with the aid of synchrotron X-ray diffraction in solution. Nanoscale 2019, 11, 6620–6628. [Google Scholar] [CrossRef]
- Patiño-Ruiz, D.A.; De Ávila, G.; Alarcón-Suesca, C.; González-Delgado, Á.D.; Herrera, A. Ionic cross-linking fabrication of chitosan-based beads modified with FeO and TiO2 nanoparticles: Adsorption mechanism toward naphthalene removal in seawater from cartagena bay area. ACS Omega 2020, 5, 26463–26475. [Google Scholar] [CrossRef]
- Ramkumar, J.; Majeed, J.; Chandramouleeswaran, S. Insight to sorption mechanism employing nanocomposite: Case study of toxic species removal. Microporous Mesoporous Mater. 2021, 314, 110858. [Google Scholar] [CrossRef]
- Abutalib, N.H.; Lagrow, A.P.; Besenhard, M.O.; Bondarchuk, O.; Sergides, A.; Famiani, S.; Ferreira, L.P.; Cruz, M.M.; Gavriilidis, A.; Thanh, N.T.K. Shape controlled iron oxide nanoparticles: Inducing branching and controlling particle crystallinity. CrystEngComm 2021, 23, 550–561. [Google Scholar] [CrossRef]
- Gerber, O.; Pichon, B.P.; Ihiawakrim, D.; Florea, I.; Moldovan, S.; Ersen, O.; Begin, D.; Grenèche, J.M.; Lemonnier, S.; Barraud, E.; et al. Synthesis engineering of iron oxide raspberry-shaped nanostructures. Nanoscale 2017, 9, 305–313. [Google Scholar] [CrossRef]
- Hu, Z.; Nourafkan, E.; Gao, H.; Wen, D. Microemulsions stabilized by in-situ synthesized nanoparticles for enhanced oil recovery. Fuel 2017, 210, 272–281. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, A.; Esparza, R.; Rosas, G.; Pérez, R. Effect of the Surfactant on the Growth and Oxidation of Iron Nanoparticles. J. Nanomater. 2015, 2015, 240948. [Google Scholar] [CrossRef]
- Suppiah, D.D.; Johan, M.R. Influence of solution pH on the formation of iron oxide nanoparticles. Mater. Res. Express 2019, 6, 015008. [Google Scholar] [CrossRef]
- Buonsanti, R.; Grillo, V.; Carlino, E.; Giannini, C.; Gozzo, F.; Garcia-Hernandez, M.; Garcia, M.A.; Cingolani, R.; Cozzoli, P.D. Architectural control of seeded-grown magnetic-semicondutor iron oxide-tiO2 nanorod heterostructures: The role of seeds in topology selection. J. Am. Chem. Soc. 2010, 132, 2437–2464. [Google Scholar] [CrossRef]
- Briones, F.C.; Guerrero-Contreras, J.; Benito-Santiago, S.E.; Llamazares, J.L.S.; Espinosa-Faller, F.J. Formation of magnetic nanoscrolls and nanoribbons in iron oxide-decorated graphene oxide. Mater. Lett. 2022, 309, 131321. [Google Scholar] [CrossRef]
- Cao, S.W.; Zhu, Y.J. Surfactant-free preparation and drug release property of magnetic hollow core/shell hierarchical nanostructures. J. Phys. Chem. C 2008, 112, 12149–12156. [Google Scholar] [CrossRef]
- Abou-Elanwar, A.M.; Shirke, Y.M.; Yoo, C.H.; Kwon, S.J.; Choi, W.K.; Lee, J.S.; Hong, S.U.; Lee, H.K.; Jeon, J.D. Water vapor dehumidification using thin-film nanocomposite membranes by the in situ formation of ultrasmall size iron-chelated nanoparticles. Appl. Surf. Sci. 2021, 542, 148562. [Google Scholar] [CrossRef]
- Aslan, T.N. Relaxivity properties of magnetoferritin: The iron loading effect. J. Biosci. Bioeng. 2022, 133, 474–480. [Google Scholar] [CrossRef]
- Acevedo, P.G.; Gómez, M.A.G.; Prieto, Á.A.; Garitaonandia, J.S.; Piñeiro, Y.; Rivas, J. Significant Surface Spin Effects and Exchange Bias in Iron Oxide-Based Hollow Magnetic Nanoparticles. Nanomaterials 2022, 12, 456. [Google Scholar] [CrossRef]
- Badri, W.; Tarhini, M.; Lgourna, Z.; Lebaz, N.; Saadaoui, H.; Zine, N.; Errachid, A.; Elaissari, A. Preparation and Characterization of Glued Corn Flakes-Like Protein-Based Magnetic Particles. Chem. Afr. 2020, 3, 803–811. [Google Scholar] [CrossRef]
- Giardiello, M.; Hatton, F.L.; Slater, R.A.; Chambon, P.; North, J.; Peacock, A.K.; He, T.; McDonald, T.O.; Owen, A.; Rannard, S.P. Stable, polymer-directed and SPION-nucleated magnetic amphiphilic block copolymer nanoprecipitates with readily reversible assembly in magnetic fields. Nanoscale 2016, 8, 7224–7231. [Google Scholar] [CrossRef]
- Kim, J.D.; Yee, N.; Nanda, V.; Falkowski, P.G. Anoxic photochemical oxidation of siderite generates molecular hydrogen and iron oxides. Proc. Natl. Acad. Sci. USA 2013, 110, 10073–10077. [Google Scholar] [CrossRef]
- Li, M.; Fu, S.; Cai, Z.; Li, D.; Liu, L.; Deng, D.; Jin, R.; Ai, H. Dual regulation of osteoclastogenesis and osteogenesis for osteoporosis therapy by iron oxide hydroxyapatite core/shell nanocomposites. Regen. Biomater. 2021, 8, rbab027. [Google Scholar] [CrossRef]
- Singh, N.; Riyajuddin, S.; Ghosh, K.; Mehta, S.K.; Dan, A. Chitosan-Graphene Oxide Hydrogels with Embedded Magnetic Iron Oxide Nanoparticles for Dye Removal. ACS Appl. Nano Mater. 2019, 2, 7379–7392. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, H.; Peng, F.; Wang, H. Facile synthesis of porous hollow iron oxide nanoparticles supported on carbon nanotubes. Mater. Lett. 2012, 67, 245–247. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Phelps, T.J.; Cole, D.R.; Horita, J.; Fortier, S.M.; Elless, M.; Valley, J.W. Physiochemical, mineralogical, and isotopic characterization of magnetite-rich iron oxides formed by thermophilic iron-reducing bacteria. Geochim. Cosmochim. Acta 1997, 61, 4621–4632. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Wang, Y.; Cao, T.; Zhao, G. Electro-Fenton oxidation of pesticides with a novel Fe3O4@Fe2O3/activated carbon aerogel cathode: High activity, wide pH range and catalytic mechanism. Appl. Catal. B Environ. 2012, 125, 120–127. [Google Scholar] [CrossRef]
- Zou, Y.; Ito, S.; Fujiwara, M.; Komatsu, N. Probing the Role of Charged Functional Groups on Nanoparticles Grafted with Polyglycerol in Protein Adsorption and Cellular Uptake. Adv. Funct. Mater. 2022, 32, 2111077. [Google Scholar] [CrossRef]
- Wlokas, I.; Faccinetto, A.; Tribalet, B.; Schulz, C.; Kempf, A. Mechanism of iron oxide formation from iron pentacarbonyl-doped low-pressure hydrogen/oxygen flames. Int. J. Chem. Kinet. 2013, 45, 487–498. [Google Scholar] [CrossRef]
- Zhu, W.; Winterstein, J.; Maimon, I.; Yin, Q.; Yuan, L.; Kolmogorov, A.N.; Sharma, R.; Zhou, G. Atomic structural evolution during the reduction of α-Fe2O3 nanowires. J. Phys. Chem. C 2016, 120, 14854–14862. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Yoon, S.-S.; Takahashi, M. Study on Formation Mechanism of Iron Oxide Nanoparticles. J. Korean Magn. Soc. 2012, 22, 167–172. [Google Scholar] [CrossRef]
- Lin, J.; Sun, M.; Su, B.; Owens, G.; Chen, Z. Immobilization of cadmium in polluted soils by phytogenic iron oxide nanoparticles. Sci. Total Environ. 2019, 659, 491–498. [Google Scholar] [CrossRef]
- Ning, D.; Shoshin, Y.; van Stiphout, M.; van Oijen, J.; Finotello, G.; de Goey, P. Temperature and phase transitions of laser-ignited single iron particle. Combust. Flame 2022, 236, 111801. [Google Scholar] [CrossRef]
- Fang, G.; Ding, W.; Liu, Y.; Zhang, J.; Xing, F.; Dong, B. Identification of corrosion products and 3D distribution in reinforced concrete using X-ray micro computed tomography. Constr. Build. Mater. 2019, 207, 304–315. [Google Scholar] [CrossRef]
- Moloko, K.G.; Van Der Merwe, J.W. Investigation of the mechanism for fireside corrosion in coal-fired boilers in South Africa. J. S. Afr. Inst. Min. Metall. 2021, 121, 305–316. [Google Scholar] [CrossRef]
- Pilić, Z.; Martinović, I. Electrochemical behaviour of iron and AISI 304 stainless steel in simulated acid rain solution. Int. J. Mater. Res. 2016, 107, 925–934. [Google Scholar] [CrossRef]
- Ishizaka, K.; Lewis, S.R.; Hammond, D.; Lewis, R. Chemistry of black leaf films synthesised using rail steels and their influence on the low friction mechanism. RSC Adv. 2018, 8, 32506–32521. [Google Scholar] [CrossRef]
- Melfo, W.M.; Dippenaar, R.J. In situ observations of early oxide formation in steel under hot-rolling conditions. J. Microsc. 2007, 225, 147–155. [Google Scholar] [CrossRef]
- Kelly, H.; Spilde, M.N.; Jones, D.S.; Boston, P.J. Insights into the geomicrobiology of biovermiculations from rock billet incubation experiments. Life 2021, 11, 59. [Google Scholar] [CrossRef]
- Peng, X.; Zhou, H.; Li, J.; Li, J.; Chen, S.; Yao, H.; Wu, Z. Intracellular and extracellular mineralization of a microbial community in the Edmond deep-sea vent field environment. Sediment. Geol. 2010, 229, 193–206. [Google Scholar] [CrossRef]
- Levett, A.; Gagen, E.J.; Southam, G. Small but mighty: Microorganisms offer inspiration for mine remediation and waste stabilisation. Microbiol. Aust. 2019, 40, 190–194. [Google Scholar] [CrossRef]
- Walter, X.A.; Picazo, A.; Miracle, M.R.; Vicente, E.; Camacho, A.; Aragno, M.; Zopfi, J. Phototrophic Fe(II)-oxidation in the chemocline of a ferruginous meromictic lake. Front. Microbiol. 2014, 5, 713. [Google Scholar] [CrossRef]
- Zeng, Z.; Tice, M.M. Electron Transfer Strategies Regulate Carbonate Mineral and Micropore Formation. Astrobiology 2018, 18, 28–36. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Vinokurov, Z.S.; Saraev, A.A.; Tsapina, A.M.; Trigub, A.L.; Gerasimov, E.Y.; Gladky, A.Y.; Fedorov, A.V.; Yakovlev, V.A.; Kaichev, V.V. The Influence of Cu and Al Additives on Reduction of Iron(III) Oxide: In Situ XRD and XANES Study. Inorg. Chem. 2019, 58, 4842–4850. [Google Scholar] [CrossRef]
- Coombes, M.J.; Olivier, E.J.; Prestat, E.; Haigh, S.J.; du Plessis, E.; Neethling, J.H. Iron-silica interaction during reduction of precipitated silica-promoted iron oxides using in situ XRD and TEM. Appl. Catal. A Gen. 2021, 613, 118031. [Google Scholar] [CrossRef]
- Yu, G.; Sun, B.; Pei, Y.; Xie, S.; Yan, S.; Qiao, M.; Fan, K.; Zhang, X.; Zong, B. FexOy@C spheres as an excellent catalyst for Fischer-Tropsch synthesis. J. Am. Chem. Soc. 2010, 132, 935–937. [Google Scholar] [CrossRef]
- Bica, I.; Anitas, E.M.; Anitas, E.M.; Choi, H.J.; Sfirloaga, P. Microwave-assisted synthesis and characterization of iron oxide microfibers. J. Mater. Chem. C 2020, 8, 6159–6167. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, Z.; Sun, Y.; Cao, X.; Hou, D.; Alessi, D.S.; Ok, Y.S.; Tsang, D.C.W. Unraveling iron speciation on Fe-biochar with distinct arsenic removal mechanisms and depth distributions of As and Fe. Chem. Eng. J. 2021, 425, 131489. [Google Scholar] [CrossRef]
- Wang, T.; Tian, Z.; Tunlid, A.; Persson, P. Nitrogen acquisition from mineral-associated proteins by an ectomycorrhizal fungus. New Phytol. 2020, 228, 697–711. [Google Scholar] [CrossRef]
- Celi, L.; Prati, M.; Magnacca, G.; Santoro, V.; Martin, M. Role of crystalline iron oxides on stabilization of inositol phosphates in soil. Geoderma 2020, 374, 114442. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhawan, N. Investigation of carbothermic microwave reduction followed by acid leaching for recovery of iron and aluminum values from Indian red mud. Miner. Eng. 2020, 159, 106653. [Google Scholar] [CrossRef]
- Dong, Q.; Li, X.; Wang, Z.; Bi, Y.; Yang, R.; Zhang, J.; Luo, H.; Niu, M.; Qi, B.; Lu, C. Effect of iron(III) ion on moso bamboo pyrolysis under microwave irradiation. Bioresour. Technol. 2017, 243, 755–759. [Google Scholar] [CrossRef]
- Hamed, M.H.; Mueller, D.N.; Müller, M. Thermal phase design of ultrathin magnetic iron oxide films: From Fe3O4 to γ-Fe2O3 and FeO. J. Mater. Chem. C 2020, 8, 1335–1343. [Google Scholar] [CrossRef]
- He, W.; Lv, X.; Pan, F.; Li, X.; Yan, Z. Novel preparation process of iron powders with semisteel by rotary cup atomizer. Powder Technol. 2019, 356, 1087–1096. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, H.; Gong, H.; Xing, H.; Yuan, B.; Fu, B.; Li, A.; Yao, H. Mechanism study of selenium retention by iron minerals during coal combustion. In Proceedings of the Combustion Institute; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; Volume 38, pp. 4189–4197. [Google Scholar]
- Marinca, T.F.; Chicinaş, H.F.; Neamţu, B.V.; Popa, F.; Chicinaş, I. Reactive spark plasma sintering of mechanically activated α-Fe2O3/Fe. Ceram. Int. 2017, 43, 14281–14291. [Google Scholar] [CrossRef]
- Mu, Y.; Ai, Z.; Zhang, L.; Song, F. Insight into core-shell dependent anoxic Cr(VI) removal with Fe@Fe2O3 nanowires: Indispensable role of surface bound Fe(II). ACS Appl. Mater. Interfaces 2015, 7, 1997–2005. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.; Zhang, S.; Zhou, J.; Fan, L.; Ren, F.; Jiang, C. Large-scale and controlled synthesis of iron oxide magnetic short nanotubes: Shape evolution, growth mechanism, and magnetic properties. J. Phys. Chem. C 2010, 114, 16092–16103. [Google Scholar] [CrossRef]
- Shen, H.; Liu, B.; Zhao, S.; Zhang, J.; Yuan, J.; Zhang, Y.; Zhang, S. Iron oxide and jadeite nucleation in high alumina glass-ceramics prepared from secondary aluminum dross. Ceram. Int. 2021, 47, 21744–21750. [Google Scholar] [CrossRef]
- Prim, S.R.; Folgueras, M.V.; de Lima, M.A.; Hotza, D. Synthesis and characterization of hematite pigment obtained from a steel waste industry. J. Hazard. Mater. 2011, 192, 1307–1313. [Google Scholar] [CrossRef]
- Chen, H.; Lin, H.; Zhang, P.; Yu, L.; Chen, L.; Huang, X.; Jiao, B.; Li, D. Immobilisation of heavy metals in hazardous waste incineration residue using SiO2–Al2O3–Fe2O3–CaO glass-ceramic. Ceram. Int. 2021, 47, 8468–8477. [Google Scholar] [CrossRef]
- Karnik, B.S.; Baumann, M.J.; Corneal, L.M.; Masten, S.J.; Davies, S.H. TEM characterization of iron-oxide-coated ceramic membranes. J. Mater. Sci. 2009, 44, 4148–4154. [Google Scholar] [CrossRef]
- Ocaña, M.; Caballero, A.; González-Carreño, T.; Serna, C.J. Preparation by pyrolysis of aerosols and structural characterization of Fe-doped mullite powders. Mater. Res. Bull. 2000, 35, 775–788. [Google Scholar] [CrossRef]
- Shi, Y.; Han, X.X.; Li, B.W.; Chen, Y.X.; Zhang, M.X. Modification of glass network and crystallization of CaO–Al2O3–MgO–SiO2 based glass ceramics with addition of iron oxide. Ceram. Int. 2020, 46, 9207–9217. [Google Scholar] [CrossRef]
- Karamanov, A.; Paunović, P.; Ranguelov, B.; Ljatifi, E.; Kamusheva, A.; Načevski, G.; Karamanova, E.; Grozdanov, A. Vitrification of hazardous Fe-Ni wastes into glass-ceramic with fine crystalline structure and elevated exploitation characteristics. J. Environ. Chem. Eng. 2017, 5, 432–441. [Google Scholar] [CrossRef]
- Karamanov, A.; Kamusheva, A.; Karashanova, D.; Ranguelov, B.; Avdeev, G. Structure of glass-ceramic from Fe-Ni wastes. Mater. Lett. 2018, 223, 86–89. [Google Scholar] [CrossRef]
- Shankhwar, N.; Srinivasan, A. Evaluation of sol-gel based magnetic 45S5 bioglass and bioglass-ceramics containing iron oxide. Mater. Sci. Eng. C 2016, 62, 190–196. [Google Scholar] [CrossRef]
- Zhou, W.; Ge, L.; Chen, Z.G.; Liang, F.; Xu, H.Y.; Motuzas, J.; Julbe, A.; Zhu, Z. Amorphous iron oxide decorated 3D heterostructured electrode for highly efficient oxygen reduction. Chem. Mater. 2011, 23, 4193–4198. [Google Scholar] [CrossRef]
- Nitnavare, R.; Bhattacharya, J.; Thongmee, S.; Ghosh, S. Photosynthetic microbes in nanobiotechnology: Applications and perspectives. Sci. Total Environ. 2022, 841, 156457. [Google Scholar] [CrossRef]
- Rufus, A.; Sreeju, N.; Philip, D. Synthesis of biogenic hematite (α-Fe2O3) nanoparticles for antibacterial and nanofluid applications. RSC Adv. 2016, 6, 94206–94217. [Google Scholar] [CrossRef]
- Dave, S.; Dave, S.; Mathur, A.; Das, J. Biological synthesis of magnetic nanoparticles. In Nanobiotechnology: Microbes and Plant Assisted Synthesis of Nanoparticles, Mechanisms and Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 225–234. ISBN 9780128228784. [Google Scholar]
- Buchanan, D.M.; Newsome, L.; Lloyd, J.R.; Kazemian, M.; Kaulich, B.; Araki, T.; Bagshaw, H.; Waters, J.; van der Laan, G.; N’Diaye, A.; et al. Investigating Nanoscale Electron Transfer Processes at the Cell-Mineral Interface in Cobalt-Doped Ferrihydrite Using Geobacter sulfurreducens: A Multi-Technique Approach. Front. Earth Sci. 2022, 10, 799328. [Google Scholar] [CrossRef]
- Kishor, B.; Rawal, N. Studies on natural biogenic iron oxides for removal of copper (II) ion from aqueous solution. Desalin. Water Treat. 2017, 88, 145–153. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.; Chen, X.; Song, H. Amorphous Fe2O3/Graphene Composite Nanosheets with Enhanced Electrochemical Performance for Sodium-Ion Battery. ACS Appl. Mater. Interfaces 2016, 8, 30899–30907. [Google Scholar] [CrossRef]
- Enman, L.J.; Kast, M.G.; Cochran, E.A.; Pledger, E.; Stevens, M.B.; Boettcher, S.W. Transition-Metal-Incorporated Aluminum Oxide Thin Films: Toward Electronic Structure Design in Amorphous Mixed-Metal Oxides. J. Phys. Chem. C 2018, 122, 13691–13704. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Ding, B.; He, C.; Zhang, C.; Li, J.; Wang, H.; Li, Z.; Wang, G.; Wang, Y.; et al. Synergistic Apoptosis-Ferroptosis: Oxaliplatin loaded amorphous iron oxide nanoparticles for High-efficiency therapy of orthotopic pancreatic cancer and CA19-9 levels decrease. Chem. Eng. J. 2023, 464, 142690. [Google Scholar] [CrossRef]
- Ioffe, M.; Kundu, S.; Perez-Lapid, N.; Radian, A. Heterogeneous Fenton catalyst based on clay decorated with nano-sized amorphous iron oxides prevents oxidant scavenging through surface complexation. Chem. Eng. J. 2022, 433, 134609. [Google Scholar] [CrossRef]
- Chandrababu, P.; Cheriyan, S.; Raghavan, R. Aloe vera leaf extract-assisted facile green synthesis of amorphous Fe2O3 for catalytic thermal decomposition of ammonium perchlorate. J. Therm. Anal. Calorim. 2020, 139, 89–99. [Google Scholar] [CrossRef]
- Lin, Z.; Du, C.; Yan, B.; Yang, G. Amorphous Fe2O3 for photocatalytic hydrogen evolution. Catal. Sci. Technol. 2019, 9, 5582–5592. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.; Li, X.; Xia, Z.; Zhang, Q.; Zhou, M.; Tian, W.; Wang, M.; Hu, H.; Li, Z.; et al. Graphene oxide-induced synthesis of button-shaped amorphous Fe2O3/rGO/CNFs films as flexible anode for high-performance lithium-ion batteries. Chem. Eng. J. 2019, 369, 215–222. [Google Scholar] [CrossRef]
- Persdotter, A.; Boll, T.; Jonsson, T. Minor element effect on high temperature corrosion of a low-alloyed steel: Insight into alkali- and chlorine induced corrosion by means of atom probe tomography. Corros. Sci. 2021, 192, 109779. [Google Scholar] [CrossRef]
- Jiang, L.; Fu, J.; He, C. Reliability analysis of wood-plastic composites in simulated seawater conditions: Effect of iron oxide pigments. J. Build. Eng. 2020, 31, 101318. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Bala, N. Electrophoretic deposition of Fe3O4 nanoparticles incorporated hydroxyapatite-bioglass-chitosan nanocomposite coating on AZ91 Mg alloy. Mater. Today Commun. 2021, 26, 101870. [Google Scholar] [CrossRef]
- Nikravesh, B.; Ramezanzadeh, B.; Sarabi, A.A.; Kasiriha, S.M. Evaluation of the corrosion resistance of an epoxy-polyamide coating containing different ratios of micaceous iron oxide/Al pigments. Corros. Sci. 2011, 53, 1592–1603. [Google Scholar] [CrossRef]
- Fan, C.; Shi, J.; Sharafeev, A.; Lemmens, P.; Dilger, K. Optical spectroscopic and electrochemical characterization of oxide films on a ferritic stainless steel. Mater. Corros. 2020, 71, 440–450. [Google Scholar] [CrossRef]
- Gareev, K.G.; Grouzdev, D.S.; Kharitonskii, P.V.; Kosterov, A.; Koziaeva, V.V.; Sergienko, E.S.; Shevtsov, M.A. Magnetotactic bacteria and magnetosomes: Basic properties and applications. Magnetochemistry 2021, 7, 86. [Google Scholar] [CrossRef]
- Toropova, Y.G.; Golovkin, A.S.; Malashicheva, A.B.; Korolev, D.V.; Gorshkov, A.N.; Gareev, K.G.; Afonin, M.V.; Galagudza, M.M. In vitro toxicity of FemOn, FemOn-SiO2 composite, and SiO2-FemOn core-shell magnetic nanoparticles. Int. J. Nanomed. 2017, 12, 593–603. [Google Scholar] [CrossRef]
- Toropova, Y.G.; Zelinskaya, I.A.; Gorshkova, M.N.; Motorina, D.S.; Korolev, D.V.; Velikonivtsev, F.S.; Gareev, K.G. Albumin covering maintains endothelial function upon magnetic iron oxide nanoparticles intravenous injection in rats. J. Biomed. Mater. Res.—Part A 2021, 109, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Kharitonskii, P.; Zolotov, N.; Kirillova, S.; Gareev, K.; Kosterov, A.; Sergienko, E.; Yanson, S.; Ustinov, A.; Ralin, A. Magnetic granulometry, Mössbauer spectroscopy, and theoretical modeling of magnetic states of FemOn–Fem−xTixOn composites. Chin. J. Phys. 2022, 78, 271–296. [Google Scholar] [CrossRef]
- Gareev, K.G.; Grouzdev, D.S.; Koziaeva, V.V.; Sitkov, N.O.; Gao, H.; Zimina, T.M.; Shevtsov, M. Biomimetic Nanomaterials: Diversity, Technology, and Biomedical Applications. Nanomaterials 2022, 12, 2485. [Google Scholar] [CrossRef] [PubMed]
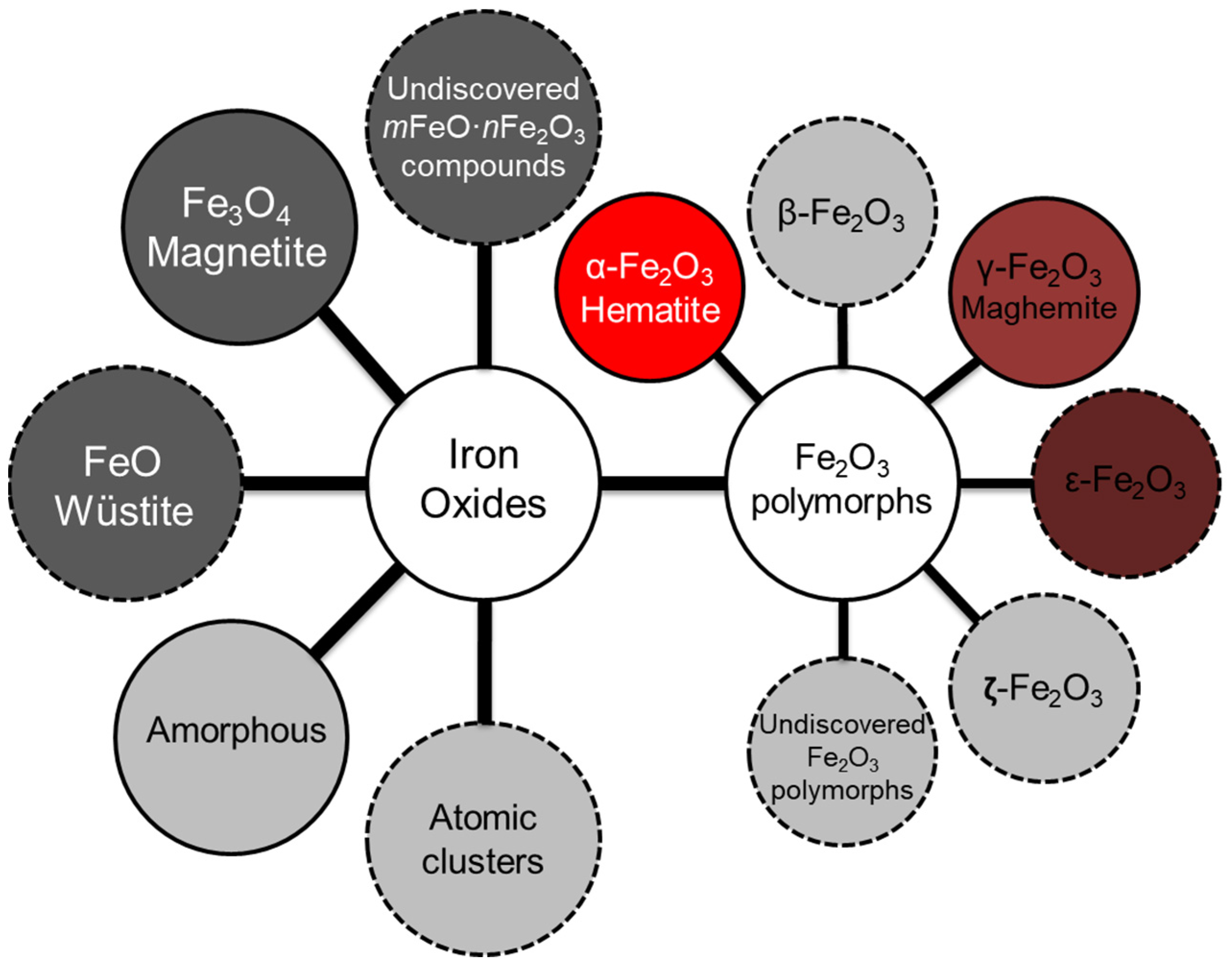


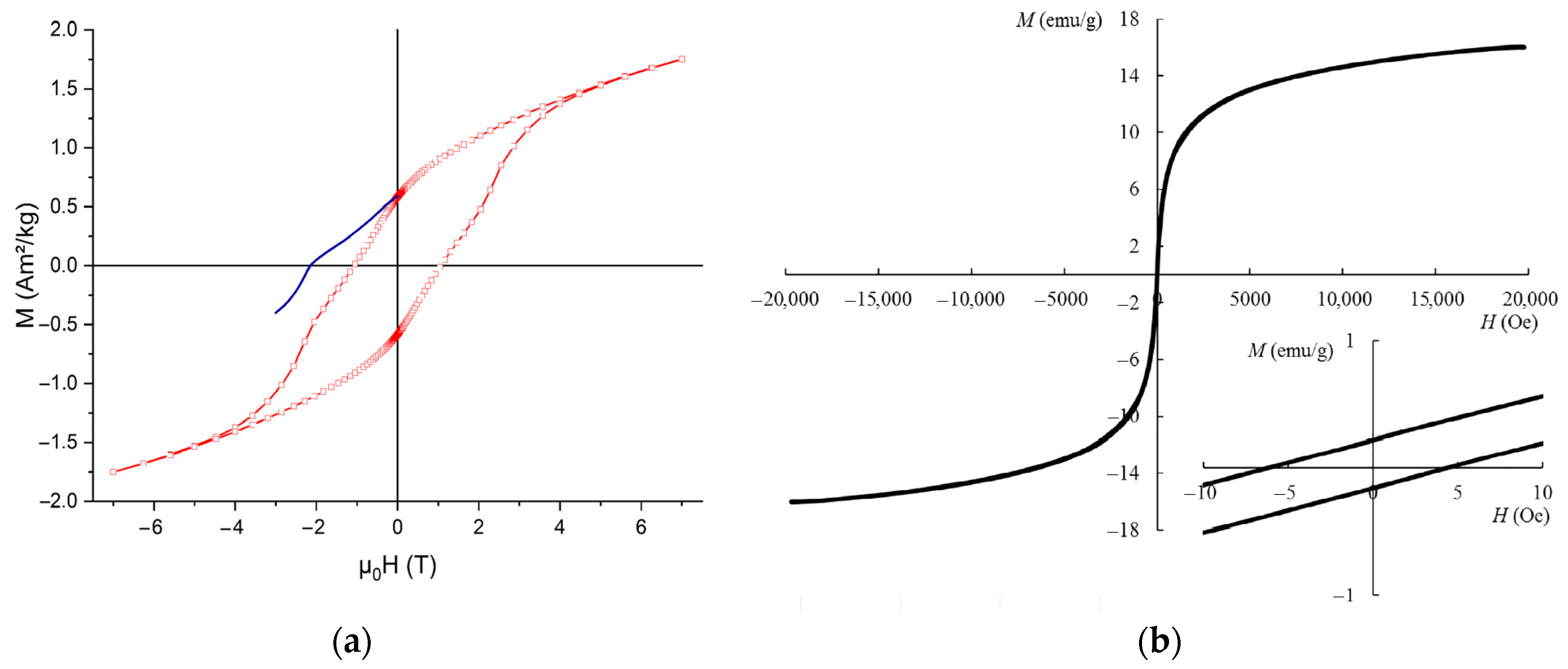
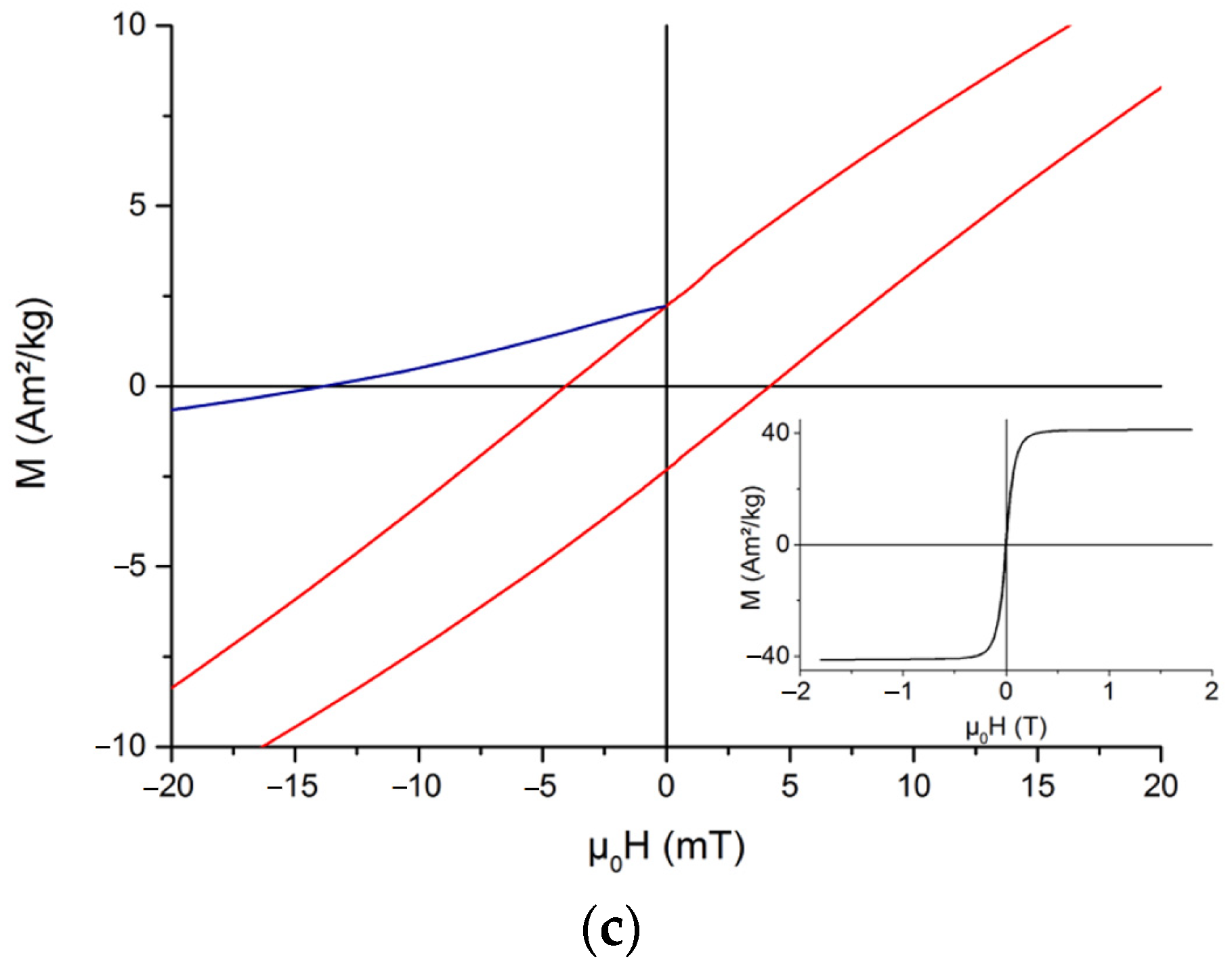
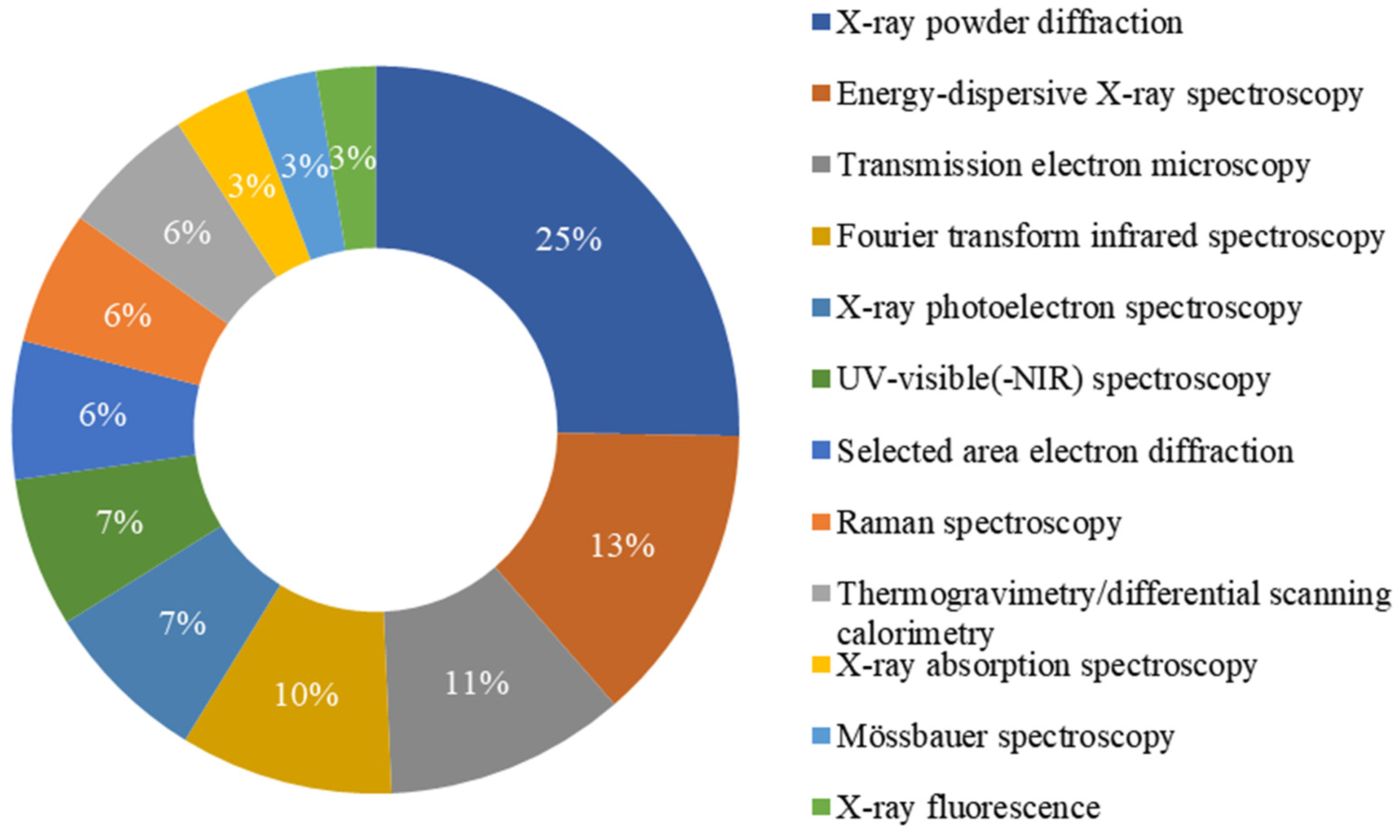

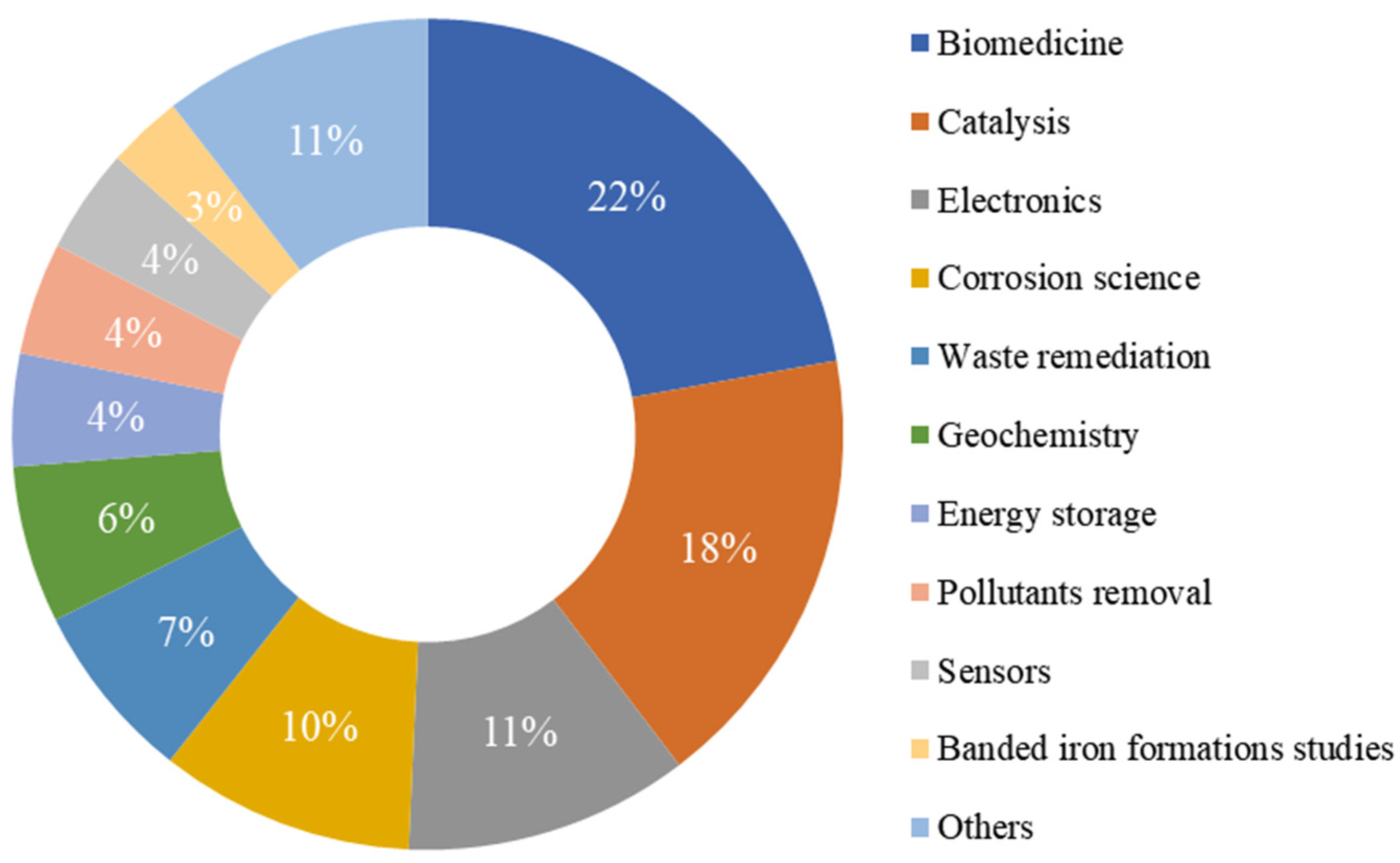
| Parameter | Iron Oxide Compound | |||||
|---|---|---|---|---|---|---|
| FeO | Fe3O4 | α-Fe2O3 | β-Fe2O3 | γ-Fe2O3 | ε-Fe2O3 | |
| Mineral name | Wüstite | Magnetite | Hematite | – | Maghemite | – |
| Crystal structure | Cubic [42] | Cubic spinel [43] | Rhombohedral [44] | Cubic [62] | Cubic spinel [45] | Orthorhombic [46] |
| Static dielectric permittivity | 22.6 [47] | 20–40 [48,49] | 12–26 [48,49,50] | n/a | 20 [48] | n/a |
| High-frequency dielectric permittivity | 10.8 [47] | 7–16 [51] | 7.6–7.9 [50] | n/a | 14.2 [52] | 4–10 [53,54] |
| Saturation mass magnetization 1 at 300 K, emu/g | 11–18 [55,56] | 92–94 [57] | 0.3–1.9 [58,59] | 0.02–0.05 [63,64] | 74–80 [60,61] | 15 [46] |
| Curie/Neel point, K | 196 [47,55] | 838–856 [66] | 948–963 [41,58] | 110–119 [62,64] | 618–928 [41,67] | 480–495 [41,46] |
| Optical band gap at 300 K, eV | 1.0 [47] | 0.2 [68,69] | 1.9–2.2 [59,70,71,72] | 1.7–1.9 [64,65] | 2.0 [60] | 2.0–2.4 [73] |
| Composition | Main Mechanisms of Iron Oxide Formation | Declared Applications | Phase Verification Techniques | Refs. |
|---|---|---|---|---|
| Iron oxide NPs | Thermal decomposition of an iron oleate in the presence of oleic acid, flame synthesis from Fe(CO)5, precipitation from ferric chloride in a natural leaf extract, thermal oxidation of polycrystalline Fe foils | Biomedicine, ferrofluids, electronics, immobilization of Cd in soils, catalysis, metal fuel | PMS, TEM, EDS, TG-DSC, SAED, XRD, XPS, FTIR, UV–Vis, EELS | [79,380,381,382,383,384] |
| Surface iron oxide layer on metal | Carbon steel corrosion at room temperature, iron carboxylate transformation to iron oxides, electrochemical anodization of metal in simulated acid rain solution | Corrosion resistance studies, railway industry, fireside corrosion studies | EDS, XRD, XRF, RS, XPS, FTIR, TG-DSC | [385,386,387,388,389] |
| Powder containing iron oxide microparticles | Carbothermal reduction of red mud by heating in a microwave furnace | Alumina production by-product recycling | XRD, EDS, XRF, TG-DSC | [400] |
| Iron oxide microfibers arranged in a complex hierarchical structure | Thermal decomposition of Fe(CO)5 and silicone oil and microwave vaporization | Environmental safety, biomedicine, sensors | EDS, XRD | [396] |
| Fe-based nanocomposite catalysts containing agglomerates of the two types | Melting of iron, aluminum and copper salts and reduction | Low-temperature catalytic oxidation of CO | XRD, TEM, XAS, EDS | [393] |
| Iron oxide powders containing hematite, magnetite and maghemite | Chemical precipitation from ferric nitrate and ferrous sulfate and heating | Inositol phosphate selective retention in soil | XRD | [399] |
| Silica–iron oxide nanocomposite with hematite, magnetite and wüstite | Silica promotion upon the reduction of amorphous iron oxide in hydrogen | Catalysis | XRD, TEM, EELS, STEM, SAED | [394] |
| Iron(II) and (III) oxides inclusions in char composites | Microwave pyrolysis of Moso bamboo samples with ferric chloride | Syngas production | XRD | [401] |
| Ultra-thin magnetic iron oxide films containing Fe3O4, γ-Fe2O3 and FeO | Thermally induced phase transformation of ultra-thin iron oxide films | All oxide heterostructures | XRD, XPS | [402] |
| Surface iron(II) and (III) oxide layer on iron granules | Atomization of the molten semi-steel with a rotary cup atomizer | Iron powder production | TG-DSC, XRD | [403] |
| Iron oxide (Fe3O4, γ-Fe2O3 and ɑ-Fe2O3) inclusions in fly ash samples | Coal combustion and flue gas cooling at various temperatures | Selenium adsorption by iron minerals | XRF, MSP, XPS | [404] |
| High-pressure metastable phases mFeO⋅nFe2O3 | Formation of complex iron oxide crystals under high-pressure conditions | Earth and planetary deep interior studies | – | [24] |
| Iron(III) oxide submicron inclusions in a biofilm on a basalt surface | Microbial direct or non-direct biomineralization | Biovermiculation studies | EDS | [388] |
| Inclusions of poorly crystalline iron(III) oxides in ore samples | Microbial biomineralization | Mine remediation, waste stabilization | SIMS | [390] |
| Iron oxide (Fe3O4, γ-Fe2O3 and ɑ-Fe2O3)/iron composite | Reactive spark plasma sintering of mechanically activated Fe powders | Magnetic material development | XRD, TG-DSC | [405] |
| Core−shell Fe@Fe2O3 nanowires containing Fe(II) and Fe (III) oxides | Ferric chloride reduction with sodium borohydride and surface oxidation | Cr(VI) removal | XRD, UV–Vis, TEM, XPS | [406] |
| Iron oxide submicron particles accumulated in the cytoplasm of cells | Intracellular or extracellular microbial biomineralization | Hydrothermal vent field studies | XRD, TEM, SAED, EDS | [389] |
| Deposit samples with inclusions of iron(II) and (III) oxides | Anoxygenic photosynthesis by a photoferrotrophic bacterium | Banded iron formation studies | – | [391] |
| Bovine serum albumin–iron oxide suspensions | Precipitation from ferric nitrate with NaOH in N2 atmosphere | Boreal forest studies | FTIR | [398] |
| Iron oxide (Fe3O4, γ-Fe2O3 and ɑ-Fe2O3) magnetic short nanotubes | Anion-assisted hydrothermal route by using phosphate and sulfate ions | Biomedicine, ferrofluids, electronics, spintronics | TEM, SAED, XRD | [407] |
| Fe-biochar composites containing iron(II) and (III) oxides | Pyrolysis of ferric chloride in a biochar matrix at various temperatures | Arsenic removal, environmental remediation | XRD, XPS, RS, FTIR | [397] |
| FexOy@C spheres embedded with highly dispersed iron oxide NPs | One-pot hydrothermal cohydrolysis-carbonization using iron | Catalysis | TEM, EDS, XAS, XRD, MSP | [395] |
| Powder with inclusions of submicron iron oxide particles | Reduction of solid ferric hydroxide by iron-reducing bacteria | Microbial iron reduction studies | EDS, RS | [392] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gareev, K.G. Diversity of Iron Oxides: Mechanisms of Formation, Physical Properties and Applications. Magnetochemistry 2023, 9, 119. https://doi.org/10.3390/magnetochemistry9050119
Gareev KG. Diversity of Iron Oxides: Mechanisms of Formation, Physical Properties and Applications. Magnetochemistry. 2023; 9(5):119. https://doi.org/10.3390/magnetochemistry9050119
Chicago/Turabian StyleGareev, Kamil G. 2023. "Diversity of Iron Oxides: Mechanisms of Formation, Physical Properties and Applications" Magnetochemistry 9, no. 5: 119. https://doi.org/10.3390/magnetochemistry9050119
APA StyleGareev, K. G. (2023). Diversity of Iron Oxides: Mechanisms of Formation, Physical Properties and Applications. Magnetochemistry, 9(5), 119. https://doi.org/10.3390/magnetochemistry9050119






