Abstract
Two new cobalt(II) complexes with an unsymmetrical bidentate ligand, 2-(1,4,5,6-tetrahydropyrimidin-2-yl)-6-methoxyphenol (H2mthp), were synthesized and crystallographically characterized. Tetra- and hexa-coordinate mononuclear complexes were selectively obtained by adjusting the stoichiometry of the base. The coordination geometry of hexa-coordinated complex was severely distorted from an ideal octahedron, due to the NO5 coordination environment from the mixed coordination of one Hmthp− and two H2mthp ligands. Both complexes formed one-dimensional chain networks by hydrogen-bond and N-H···π interactions. Single-molecule magnet behavior was observed for the tetrahedral complex under zero magnetic field. The relatively short Co···Co distances induced non-zero intermolecular magnetic coupling, which split the ground ±Ms levels to suppress quantum-tunneling of magnetization. In the octahedral complex, by contrast, the distance was not short enough to induce the coupling. As a consequence, single-molecule magnetic behavior was observed for the octahedral complex only in the presence of an external static field.
1. Introduction
Single-molecule magnets (SMMs) [1] are of considerable current interest due to their potential applications in high-density storage, spintronics and quantum computing [2,3,4,5]. To exhibit slow relaxation of magnetization, a bistable spin ground state with a large negative axial zero-field splitting (ZFS) is important because the spin-reversal barrier U is proportionally dependent on the axial ZFS parameter D, where U = |D|S2 for an integer spin system and |D|(S2−1/4) for a half-integer spin system). SMMs with a single paramagnetic center are called single-ion magnets (SIMs) [6]. Owing to the simplicity and facile design of the molecule, many lanthanide and first-row transition metal complexes have been studied to prepare SMMs with a strong magnetic anisotropy [7,8,9,10,11,12,13]. Cobalt(II) complexes are one of the most studied metal ions as SIMs because tetra- and hexa-coordinated cobalt(II) complexes often exhibit strong magnetic anisotropy [8,9,10,11,12,13,14,15,16,17]. In most of the examples of 3d metal-based SIMs, however, slow magnetic relaxation was not observed in the absence of an external magnetic field, due to fast relaxation via quantum-tunneling of magnetization (QTM). For a Kramers ion, QTM arises from the mixing of ±Ms level hyperfine or dipolar interactions [11]. Avoiding a close interaction between SMMs in the long distance is one of the possible solutions to avoid the QTM phenomena by dipolar interactions. On the other hand, we recently reported zero-field SIM behaviors in tetrahedral cobalt(II) complexes with one-dimensional hydrogen-bonded networks [12,13]. It is suggested that relatively short intermolecular distances (ca. 6 Å) and one-dimensional alignments of the complexes suppress the QTM by splitting the ground ±Ms levels by intermolecular magnetic coupling [18,19,20,21]. However, the correlation between intermolecular distances and the alignments of the SMMs within the chain structure remain unclear. In this paper, we synthesized an analogous tetrahedral cobalt(II) complex and a severely distorted octahedral cobalt(II) complex with one-dimensional networks (Scheme 1). The effects of intrachain distances and alignments of SIMs on QTM phenomena were investigated, and zero-field and field-induced SIM behaviors were observed in tetrahedral and octahedral complexes, respectively.
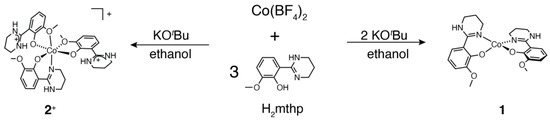
Scheme 1.
Chemical structures and preparation schemes of a tetrahedral and octahedral cobalt(II) complexes 1 and 2+.
2. Results and Discussion
2.1. Preparation of the Ligand and Cobalt(II) Complexes
The ligand precursor 2-(1,4,5,6-tetrahydropyrimidin-2-yl)-6-methoxyphenol (H2mthp) was prepared by a similar method to that reported for an analogous ligand in the literature [13]. The mononuclear bis-bidentate type complex [Co(Hmthp)2]·C2H5OH was synthesized by a reaction of Co(BF4)2·6H2O, H2mthp and KOtBu in 1:3:2 ratio in ethanol (Scheme 1). An excessive amount of the ligand precursor was required for the synthesis to prevent the formation of Co(OH)2 as impurity. It should be noted that the use of other bases, such as triethylamine, was not successful, presumably because triethylamine was not strong enough to deprotonate H2mthp. In the case of a smaller molar ratio of KOtBu (1:3:1 ratio), a mononuclear tris-bidentate cobalt(II) complex [Co(Hmthp)(H2mthp)2]BF4 (2BF4) was obtained. Both cobalt(II) complexes tended to lose crystallinity in air by efflorescence, and solvent molecules of crystallization were substituted by water.
2.2. Crystal Structures of H2mthp and Cobalt(II) Complexs
The ligand precursor H2mthp was crystallized in chiral orthorhombic space group P212121. In the crystal, the asymmetric unit consisted of two independent H2mthp molecules and existed in zwitterionic form, with a deprotonated phenol group and a protonated imino group precedented in analogous compounds (Figure S1) [22]. The X-ray crystallographic analysis revealed that 1·C2H5OH and 2BF4 were tetra-coordinated and hexa-coordinated cobalt(II) complexes, respectively (Figure 1 and Figure 2). In 1·C2H5OH, the crystallographic asymmetric unit consisted of 1 and an ethanol molecule of crystallization. The cobalt(II) ion was coordinated by phenolato-O and imino-N donors of Hmthp− ligand in a bidentate fashion to afford a pseudo-tetrahedral coordination geometry (Table 1). The structural parameter τ4 for 1 was calculated to be 0.77 (τ4 = [360° − (α + β)]/141°, where α and β were the largest two angles in the coordination sphere), which was smaller than those of analogous complexes, indicating that the coordination geometry was more distorted from an ideal tetrahedron [23]. The mean plane angle between two bidentate ligands (O1-Co1-N2 plane to O3-Co1-N4 plane angle = 76.7°) deviated from 90°. Hydrogen-bonding interactions were observed between the N-H group of the ligand and phenolato-O atom of the neighboring molecule to construct one-dimensional hydrogen-bonded networks of 1. As the crystallographic inversion centers were located between two neighboring molecules in a chain, the magnetic anisotropy axes of the molecules should be in the same axis. Two intrachain Co···Co distances were not equivalent (5.979(4) and 6.106(4) Å). The interchain closest Co···Co distances were much longer (≥9 Å) than the intrachain ones, implying that the interchain magnetic interaction were negligible.
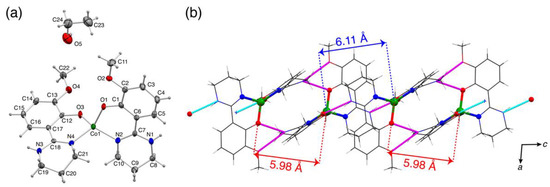
Figure 1.
(a) Molecular structure of 1·C2H5OH (50% probability levels). (b) Hydrogen-bonded networks of 1·C2H5OH along c axis.
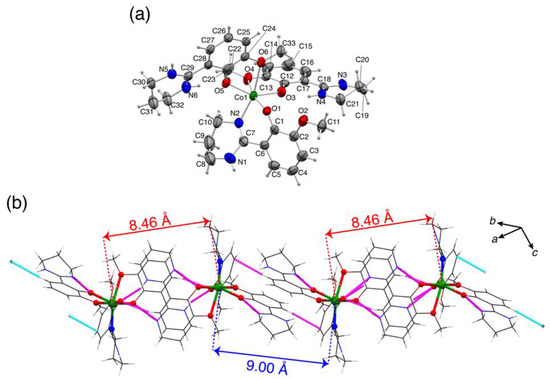
Figure 2.
(a) Molecular structure of 2+ in 2BF4·1.5C2H5OH (50% probability levels). (b) One-dimensional networks of 2+ by hydrogen-bond and N-H···π interactions.

Table 1.
Selected bond parameters for 1·C2H5OH.
The 2BF4 was crystallized in a triclinic system P as 2BF4·1.5C2H5OH (Figure 2). The hexa-coordinated geometry of the Co center was severely distorted by a NO5 coordination mode. In 2+ cation, the Co center was coordinated by one Hmthp− and two H2mthp ligands. Hmthp− acted as a bidentate ligand with phenolato-O atom and imino-N atoms as donor atoms, as was also observed in 1, whereas H2mthp ligands coordinated via phenolato-O and methoxy-O atoms in zwitterionic form, as observed in the crystal of H2mthp. The protonation state of the ligands was consistent with the stoichiometry of the reaction condition (H2mthp:KOtBu = 3:1). The coordination bonds with methoxy-O atoms were significantly longer than those with the other donor atoms (by 0.3 Å), implying the weak coordination of the methoxy-O atoms. The bond parameters of Co ion were within the range of a typical divalent high-spin cobalt(II) complex (Table 2). The considerable distortion of the coordination geometry can be explained by the severe steric requirement of the six-membered chelate mode of the ligand in an octahedral geometry. The mean plane angles between the phenyl and tetrahydropyrimidinyl 6-membered rings of a Hmthp− ligand was 38.6(3)°, indicating that the ligand underwent a strong steric hindrance on O-N chelate mode in an octahedral coordination geometry [24]. It was noted that such distortion of the ligand was not observed in 1 because a tetrahedral coordination geometry accepted much larger bite angles than an octahedral one (109.5° for tetrahedral and 90° for octahedral geometry) and, the mean plane angles observed in 1 (15.4(3)° and 5.5(2)°) were comparable to those of H2mthp. As a result, the bite angle of the six-membered chelate mode (phenolato- and imino-N atoms) in 2+ was restricted to 91.44(14)°, although the angle of the five-membered chelate rings (phenolato-O and methoxy) accepted much smaller bite angles (72.58(11)° or 71.15(12)°). Although one-dimensional chain networks were formed by hydrogen-bond and N-H···π interactions, the shortest Co···Co distance was not the intrachain one but the interchain one (8.239(1) Å). This long intermolecular Co···Co distance suggested that the intermolecular magnetic interaction was negligible.

Table 2.
Selected bond parameters for 2BF4·1.5C2H5OH.
2.3. Magnetic Properties
2.3.1. Static Magnetic Properties
The temperature dependence of magnetic susceptibility was measured for 1·C2H5OH and 2BF4·1.5C2H5OH to reveal their magnetic properties (Figure 3). The χMT product at 300 K for 1·C2H5OH (ca. 2.5 cm3 mol−1 K), which was higher than the spin-only value 1.876 for the S = 3/2 system, was a typical value for tetrahedral cobalt(II) complexes. Upon cooling, the χMT products were almost constant, down to 100 K, followed by a steep drop of value because of ZFS. To determine the g-factors and the axial ZFS parameter (D), the temperature dependence of the χMT data and the field dependence of the magnetization were simultaneously fitted to the following spin Hamiltonian:
where β and zJ are Bohr magneton and the intermolecular interaction, respectively (Table 3). The transversal ZFS parameter (E) was not considered as it was difficult to determine from the magnetic data. Intermolecular magnetic interactions were considered for 1·C2H5OH to analyze the effect of intermolecular coupling on the magnetic relaxation dynamics. Although Hmthp− was a derivative of the Hthp− ligand, with the same coordination mode, the obtained g-factors for 1·C2H5OH were rather closer to those of [Co(Hmimn)2]·CH3OH than [Co(Hthp)2] [12,13]. This suggested that the electronic structure of the phenolate donor had stronger influence on the g-factors. The large negative D value, owing to the second order spin-orbit coupling, was suggestive of SIM behavior with a spin-reversal barrier of ca. 100 cm−1 (2|D|).
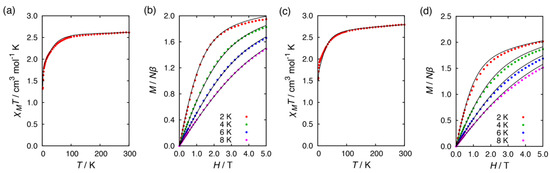
Figure 3.
(a) Temperature dependence of χMT product for 1·C2H5OH. (b) The field dependence of the magnetization at 2, 4, 6, and 8 K for 1·C2H5OH. (c) Temperature dependence of χMT product for 2BF4·1.5C2H5OH. (d) The field dependence of the magnetization at 2, 4, 6, and 8 K for 2BF4·1.5C2H5OH. Solid lines correspond to the fit using the MagSakiTetra program.

Table 3.
Experimental g-factors (gx, gy, gz), axial ZFS parameters (D), intermolecular magnetic interactions (zJ), and temperature-independent paramagnetism (TIP).
The χMT product for 2BF4·1.5C2H5OH (ca. 2.8 cm3 mol−1 K) was also higher than the spin-only value because of unquenched orbital contribution on the magnetic moment. The simultaneous fitting of the χMT vs. T and M vs. H plots was performed. Intermolecular interaction was not considered, because the intermolecular Co···Co distance was significantly long. The obtained zero-field splitting parameter D was negative and relatively small among octahedral CoII SIMs, presumably due to severely distorted octahedral geometry [9,16,17].
2.3.2. Dynamic magnetic properties
Alternating current (ac) susceptibility measurements for 1·C2H5OH exhibited significant frequency dependence on the out-of-phase signal (χM”) in the absence of an external magnetic field at 1.9 K (Figure 4). This was indicative of slow magnetic relaxation in the absence of an external field. It should be noted that zero-field SIM behavior in a 3d metal complex is still very rare owing to fast relaxation via QTM. Upon heating, however, the χM” vs. frequency plot showed no change except the weakening of the χM” signal. This temperature independence is a characteristic feature of QTM. By applying an external field, on the other hand, another relaxation process appeared in the lower frequency region in the χM” vs. frequency plot (Figure S3 and Figure 4b). The temperature dependence was measured in the presence of 3.5 kOe, where the QTM relaxation pathway was completely suppressed and relatively small contribution of the direct relaxation process was expected. To elucidate the relaxation dynamics, the relaxation time τ was extracted by the generalized Debye-fit model:
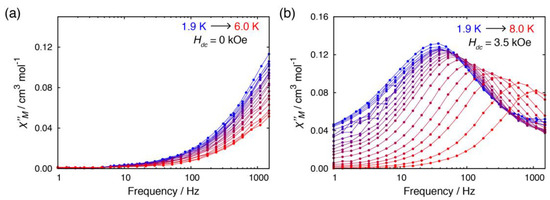
Figure 4.
Temperature dependence of out-of-phase (χM”) susceptibilities for 1·C2H5OH (a) in the absence of the dc field and (b) in the presence of dc field (Hdc = 3.5 kOe). Lines are guide for the eyes.
The relaxation dynamics could not be fitted with a single relaxation process, as it showed different trends below and above ca. 4 K (Figure 5). The dynamics was nicely fitted with a combination of two processes, direct and Raman relaxation processes:
where A, C, and n are coefficients, H is the magnetic field, T is the temperature (A = 0.667 K−1 kOe−4 s−1, C = 1.23×10−1 s−1 K−5.2, n = 5.2). In the low temperature region (<4 K), the direct process, which involves relaxation from −Ms to +Ms with emission of a single lattice phonon, was the predominant relaxation process. This predominance could be elucidated by the presence of a relatively strong external field (3.5 kOe) as the τ was inversely proportional to the powers of an external field. Above 4 K, the relaxation dynamics were taken over by the Raman process, in which relaxation between ±Ms states occurred via virtual state. The n value should be ≤9 for a Kramers ion when both an acoustic and an optical phonon are considered, and the obtained n value for 1·C2H5OH was consistent with the expected value [25,26].
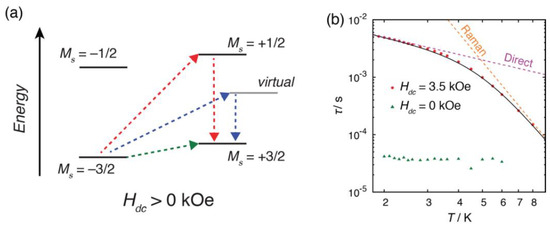
Figure 5.
(a) Possible spin-lattice relaxation processes of the Co complexes under an external magnetic field. The red, blue and green arrows indicate Orbach, Raman and direct relaxation processes, respectively. (b) Temperature dependence of relaxation time τ. The dashed lines indicate fitted lines for a single relaxation process of Raman and direct process. The solid black lines indicate the sum of the relaxation processes.
As observed in previously reported three analogous compounds [12,13], 1·C2H5OH exhibited one-dimensional hydrogen-bonded networks as well as zero-field slow magnetic relaxation. We reported that the relatively short intermolecular Co···Co distance induced a static exchange bias field to split the ground ±Ms levels. As this splitting of the ground ±Ms levels effectively suppressed the QTM phenomena, formation of hydrogen-bonded networks was a feasible approach for zero-field SIMs. In the case of one-dimensional chain, the efficiency of suppression of QTM was dependent on the molecular orientation and symmetry of the alignment of the chain. As the Co centers in the chain structures were antiferromagnetically coupled, alternating spin orientation should be the ground state. In this state, QTM would be suppressed, because the two neighboring molecules in a chain induced a dipolar field to split the ground ±Ms levels. When the dipolar field of the two neighboring molecules were stochastically oriented to opposite direction, on the other hand, the dipolar field would be canceled and QTM could not be suppressed, as observed in [Co(Hthp)2] (Himn− = 2-(1,4,5,6-tetrahydropyrimidin-2-yl)phenolate). This was not the case when the two intrachain Co···Co distances were significantly different because the dipolar field from the two neighboring molecules would not be canceled, as observed in [Co(Himn)2] (Himn− = 2-(2-midazlinyl)phenolate). In 1·C2H5OH, two intrachain distances were slightly different (ca. 0.1 Å) but the difference was only half the value of [Co(Himn)2]. Consequently, the dipolar field was almost canceled, and the QTM was only partially suppressed in the absence of an external field. These results indicated that the difference in the intrachain Co···Co distances was an important factor to suppress the QTM as well as the intermolecular magnetic coupling.
Ac susceptibility measurement was also performed on complex 2BF4·1.5C2H5OH. Unlike 1·C2H5OH, this complex did not exhibit slow magnetic relaxation in the absence of an external magnetic field, presumably because the Co···Co distance in 2BF4·1.5C2H5OH was not short enough to cause intermolecular magnetic coupling to suppress the QTM (Figure S6). In the presence of a magnetic field, on the other hand, QTM was well suppressed, meaning that 2+ cation was a field-induced SIM (Figure 6). To investigate the relaxation dynamics, temperature dependence of the ac susceptibility was measured under a static field of 1.0 kOe. The τ vs. T plot suggested that there were two relaxation processes present and they switched at ca. 4 K. Below 4 K, the double-logarithmic plot showed a large degree of linearity suggesting a power law (τ ∝ T−n). The relaxation dynamics was well-fitted by combining the Raman and Orbach relaxation processes:
where τ0 is the pre-exponential factor, ∆Orbach is the relaxation barrier, and kB is the Boltzmann constant (C = 45.5 s−1 K−2.6, n = 2.6, τo = 2.45×10−9 s, ∆Orbach = 36.7 cm−1). The n value was within the range of expected value for the Raman process. It was noted that the combination of the phonon-bottlenecked direct process (low temperature region) and the Raman process (high temperature region) did not give a reasonable fit. The relaxation barrier for the Orbach process ∆Orbach was slightly smaller than the expected value from the ZFS parameter 2|D|. Thus, the relaxation dynamics of high-temperature region of 2BF4·1.5C2H5OH were dominated by the Orbach process.
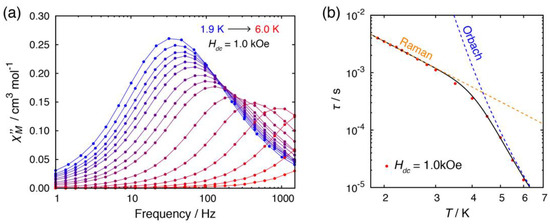
Figure 6.
(a) Temperature dependence of out-of-phase (χM”) susceptibilities for 2BF4·1.5C2H5OH in the presence of dc field (Hdc = 1.0 kOe). Lines are guide for the eyes. (b) Temperature dependence of relaxation time τ. The dashed lines indicate fitted lines for a single relaxation process of Raman and Orbach process. The solid black lines indicate the sum of the relaxation processes.
4. Materials and Methods
4.1. General Consideration
All the chemicals were used as received without further purification, except for Co(BF4)2·6H2O, which was recrystallized from a water/ethanol mixed solvent before use. Elemental analyses (C, H, and N) were performed at the Research Institute for Instrumental Analysis, Kanazawa University. 1H NMR measurements were carried out at 22 °C on a JEOL 400SS spectrometer. Chemical shifts were referenced to the solvent residual peak [27]. Infrared spectra were measured on a JASCO FT/IR-4200 spectrometer.
4.2. Preparations
2-(1,4,5,6-tetrahydropyrimidin-2-yl)-6-methoxyphenol (H2mthp). A mixture of methyl 3-methoxysalicylate (9.11g, 50 mmol) and 1,3-diaminopropane (12 mL) was refluxed overnight. The unreacted 1,3-diaminopropane was evaporated off under ambient pressure, followed by the addition of 5 mL ethanol. After cooling, the yellowish residue was collected by filtration and washed with ethanol. Yield: 7.02 g, 68%. 1H NMR (399 MHz, Methanol-d4) δ 7.02 (d, J = 8.8 Hz, 1H, aryl-H), 6.83 (d, J = 7.7 Hz, 1H, aryl-H), 6.45 – 6.38 (m, 1H, aryl-H), 3.78 (s, 3H, -CH3), 3.59 – 3.46 (m, 4H, -CH2-), 2.02 (quin, J = 5.8 Hz, 2H, -CH2-).
[Co(Hmthp)2]·C2H5OH (1·C2H5OH). An ethanol solution (10 mL) of Co(BF4)2·6H2O (34.1 mg, 0.10 mmol) was slowly added to a mixture of ethanol (10 mL), H2mthp (62.0 mg, 0.30 mmol) and KOtBu (23.0 mg, 0.20 mmol) in a Schlenk flask under Ar atmosphere. The reaction mixture was allowed to stand at room temperature for a few days, and red crystals were obtained. Yield: 11.3 mg, 22%. The crystallinity of this compound was easily lost in air, due to its efflorescent nature, and solvent molecules of crystallization were substituted by water. Anal Calcd for [Co(Hmthp)2]·0.7H2O = C22H27CoN4O4.7: C, 54.82; H, 5.73; N, 11.62%. Found: C,54.64; H, 5.58; 11.62%.
[Co(Hmthp)(H2mthp)2]BF4·1.5C2H5OH (2BF4·1.5C2H5OH). An ethanol solution (5 mL) of Co(BF4)2·6H2O (35.0 mg, 0.10 mmol) was slowly added to a mixture of ethanol (5 mL), H2mthp (62.2 mg, 0.30 mmol) and KOtBu (10.4 mg, 0.09 mmol) in a Schlenk flask under Ar atmosphere. The reaction mixture was allowed to stand at room temperature for a few weeks, and purple crystals were obtained. Yield: 46.9 mg, 61%. The crystallinity of this compound was easily lost in air, due to its efflorescent nature, and solvent molecules of crystallization were substituted by water. Anal Calcd for [Co(Hmthp)(H2mthp)2]BF4·2H2O = C33H45BCoF4N6O8: C, 49.58; H, 5.67; N, 10.51%. Found: C, 49.59; H, 5.76; 10.08%.
4.3. Crystallography
Crystallographic data are summarized in Table 4. Single-crystal X-ray diffraction data were obtained with a Rigaku XtaLAB AFC11 diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). A single crystal was mounted with a glass capillary and flash-cooled with a cold N2 gas stream. Data were processed using the CrysAlisPro software packages. The structures were solved by intrinsic phasing methods using the SHELXT [28] software packages, and refined on F2 (with all independent reflections) using the SHELXL [29] software packages. The non-hydrogen atoms were refined anisotropically. In 2BF4·1.5C2H5OH, a BF4− anion and ethanol molecules of crystallization were disordered at two possible positions. The rigid-bond restraint (RIGU) and SIMU command were employed for these disordered atoms except for one of two parts of BF4− anion (B1, F1, F2, F3, and F4). All B-F bonds were restrained to the same distances by the SADI command. The C-O bonds for ethanol molecules and B-F bonds for the minor part of BF4− (B2, F5, F6, F7, and F8) were constrained by the DFIX command. The DANG command was employed to restrain C-C-O angles of the ethanol molecules.

Table 4.
Crystallographic data and refinement parameters of 1, 2BF4·1.5C2H5OH and H2mthp.
4.4. Magnetic Measurements
Magnetic susceptibility measurements were performed with a MPMS-7 or MPMS-XL7 SQUID magnetometer. Susceptibility data were recorded in the temperature range from 1.9 to 300 K with a static field of 5.0 kOe. The polycrystalline samples were grounded into fine powders by an agate mortar in dried condition. The samples were loaded into a gelatin capsule and covered in liquid paraffin to prevent field-induced orientation of crystals. All data were corrected for diamagnetism of the sample by means of Pascal’s constants [30]. Temperature dependence of the magnetic susceptibility and field dependence of the magnetization data were fitted using the MagSakiTetra W0913 program [31]. The dynamic susceptibility was measured with alternating-current (ac) fields of 3 Oe magnitude and a constant direct current (dc) field of 0–4.0 kOe in the frequency range from 1 to 1500 Hz. The relaxation time τ was extracted from fitting to the generalized Debye model. The fit was performed using the CC-Fit program [32].
5. Conclusions
In this study, tetra-coordinated and a hexa-coordinated CoII complexes, containing the same ligand, were prepared and characterized by X-ray crystallography and magnetometry. In the crystal, the tetracoordinated complex 1·C2H5OH possessed pseudo-tetrahedral coordination geometry and formed one-dimensional chain networks by intermolecular hydrogen-bonding interactions. Two intrachain Co···Co distances were slightly different (ca. 0.1 Å) and were short enough to induce a weak intermolecular magnetic coupling. Zero-field SIM behavior was observed for 1·C2H5OH because QTM was partially suppressed by the non-zero intermolecular magnetic coupling. The partial suppression of QTM indicated that the difference in intrachain Co···Co distances is important to suppress QTM completely. On the other hand, the hexacoordinated complex 2BF4·1.5C2H5OH exhibited a severely distorted octahedral coordination geometry with one-dimensional networks by N-H···π and hydrogen-bonding interactions. The intermolecular Co···Co distances were above the range of intermolecular magnetic coupling, and SIM behavior was observed only in the presence of an external field. Thus, these results indicated that not only non-zero magnetic coupling, but also the difference of intrachain distances are important to achieve zero-field SIM.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/magnetochemistry9010017/s1. Figure S1: Molecular structure of H2mthp (50% probability levels); Figure S2: Temperature dependence of (a) the in-phase χM’ vs. frequency plots and (b) out-of-phase χM” vs. frequency plots for 1·C2H5OH in the absence of a dc field with ac frequency of 1–1488 Hz; Figure S3: Dc field dependence of (a) the in-phase χM” vs. frequency plots and (b) out-of-phase χM” vs. frequency plots for 1·C2H5OH at 1.9 K with ac frequency of 1–1488 Hz; Figure S4: Temperature dependence of (a) the in-phase χM’ vs. frequency plots and (b) out-of-phase χM” vs. frequency plots for 1·C2H5OH in the presence of 3.5 kOe with ac frequency of 1–1488 Hz; Figure S5: Cole–Cole plot for 1·C2H5OH (a) in the absence and (b) in the presence of 3.5 kOe dc field; Figure S6: Dc field dependence of (a) the in-phase χM’ vs. frequency plots and (b) out-of-phase χM”vs. frequency plots for 2·1.5C2H5OH at 1.9 K with ac frequency of 1–1488 Hz; Figure S7: Temperature dependence of (a) the in-phase χM’ vs. frequency plots and (b) out-of-phase χM” vs. frequency plots for 2·1.5C2H5OH in the presence of 1.0 kOe with ac frequency of 1–1488 Hz; Figure S8. Cole–Cole plot for 2·1.5C2H5OH in the presence of 1.0 kOe dc field; Table S1: Hydrogen-bond distances and angles; Table S2: Cole-Cole fit values for 1·C2H5OH in 3.5 kOe dc field from 1.9 to 8.0 K; Table S3: Cole-Cole fit values for 1·C2H5OH in 0 kOe dc field from 1.9 to 6.0 K; Table S4. Cole-Cole fit values for 2BF4·1.5C2H5OH in 1.0 kOe dc field from 1.9 to 6.0 K. Figure S9. 1H NMR spectrum of H2mthp in CD3OD. Figure S10. Infrared spectra of (a) pristine 1·C2H5OH, (b) hydrated 1·C2H5OH, (c) pristine 2BF4·1.5C2H5OH, (d) hydrated 2BF4·1.5C2H5OH, and H2mthp (nujol mull).
Author Contributions
Conceptualization, R.M.; methodology, R.M.; investigation, R.M. and H.S.; resources, R.M. and Y.H.; writing—original draft, R.M.; writing—review and editing, R.M., H.S. and Y.H.; visualization, R.M. project administration, R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly funded by a Grant-in-Aid for Scientific Research No. 19K15525 from MEXT, Japan. This work was partially supported by The Mitani Foundation for Research and Development.
Data Availability Statement
The crystallographic data are available from the Cambridge Crystallographic Data Centre (CCDC). Other data not presented in Supplementary Materials are available on request from the corresponding author.
Acknowledgments
We thank Yuya Imai (Kanazawa University) for 1H NMR measurement. A part of this work was conducted in the Institute for Molecular Science, supported by the Advanced Research Infrastructure for Materials and Nanotechnology (JPMXP1222MS1013 and JPMXP1222MS1013b of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Mannini, M.; Pineider, F.; Sainctavit, P.; Danieli, C.; Otero, E.; Sciancalepore, C.; Talarico, A.M.; Arrio, M.-A.; Cornia, A.; Gatteschi, D.; et al. Magnetic memory of a single-molecule quantum magnet wired to a gold surface. Nat. Mater. 2009, 8, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Ardavan, A.; Rival, O.; Morton, J.J.L.; Blundell, S.J.; Tyryshkin, A.M.; Timco, G.A.; Winpenny, R.E.P. Will Spin-Relaxation Times in Molecular Magnets Permit Quantum Information Processing? Phys. Rev. Lett. 2007, 98, 057201. [Google Scholar] [CrossRef] [PubMed]
- Stamp, P.C.E.; Gaita-Ariño, A. Spin-based quantum computers made by chemistry: Hows and whys. J. Mater. Chem. 2009, 19, 1718–1730. [Google Scholar] [CrossRef]
- Gaita-Ariño, A.; Luis, F.; Hill, S.; Coronado, E. Molecular spins for quantum computation. Nat. Chem. 2019, 11, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.Y.; Kaizu, Y. Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- Harman, W.H.; Harris, T.D.; Freedman, D.E.; Fong, H.; Chang, A.; Rinehart, J.D.; Ozarowski, A.; Sougrati, M.T.; Grandjean, F.; Long, G.J.; et al. Slow Magnetic Relaxation in a Family of Trigonal Pyramidal Iron(II) Pyrrolide Complexes. J. Am. Chem. Soc. 2010, 132, 18115–18126. [Google Scholar] [CrossRef]
- Rechkemmer, Y.; Breitgoff, F.D.; van der Meer, M.; Atanasov, M.; Hakl, M.; Orlita, M.; Neugebauer, P.; Neese, F.; Sarkar, B.; van Slageren, J. A four-coordinate cobalt(II) single-ion magnet with coercivity and a very high energy barrier. Nat. Commun. 2016, 7, 10467. [Google Scholar] [CrossRef]
- Mitsuhashi, R.; Pedersen, K.S.; Ueda, T.; Suzuki, T.; Bendix, J.; Mikuriya, M. Field-induced single-molecule magnet behavior in ideal trigonal antiprismatic cobalt(II) complexes: Precise geometrical control by a hydrogen-bonded rigid metalloligand. Chem. Commun. 2018, 54, 8869–8872. [Google Scholar] [CrossRef]
- Mitsuhashi, R.; Hosoya, S.; Sunatsuki, Y.; Suzuki, T.; Mikuriya, M. Field-induced single-ion magnet behaviors in 1-dimensionally assembled tetrahedral cobalt(II) complexes with halide donors. Inorg. Chim. Acta 2022, 529, 120667. [Google Scholar] [CrossRef]
- Zadrozny, J.M.; Long, J.R. Slow magnetic relaxation at zero field in the tetrahedral complex [Co(SPh)4]2−. J. Am. Chem. Soc. 2011, 133, 20732–20734. [Google Scholar] [CrossRef]
- Mitsuhashi, R.; Hosoya, S.; Suzuki, T.; Sunatsuki, Y.; Sakiyama, H.; Mikuriya, M. Hydrogen-bonding interactions and magnetic relaxation dynamics in tetracoordinated cobalt(II) single-ion magnets. Dalton Trans. 2019, 48, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, R.; Hosoya, S.; Suzuki, T.; Sunatsuki, Y.; Sakiyama, H.; Mikuriya, M. Zero-field slow relaxation of magnetization in cobalt(II) single-ion magnets: Suppression of quantum tunneling of magnetization by tailoring the intermolecular magnetic coupling. RSC Adv. 2020, 10, 43472–43479. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, T.T.; Barbosa, V.M.M.; Oliveira, W.X.C.; Pedroso, E.F.; García, D.M.A.; Nunes, W.C.; Pereira, C.L.M. Field-Induced Slow Magnetic Relaxation of a Six-Coordinate Mononuclear Manganese(II) and Cobalt(II) Oxamate Complexes. Inorg. Chem. 2020, 59, 12983–12987. [Google Scholar] [CrossRef]
- Yao, B.; Singh, M.K.; Deng, Y.-F.; Wang, Y.-N.; Dunbar, K.R.; Zhang, Y.-Z. Trigonal Prismatic Cobalt(II) Single-Ion Magnets: Manipulating the Magnetic Relaxation Through Symmetry Control. Inorg. Chem. 2020, 59, 8505–8513. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.R.; Singh, M.K.; Dunbar, K.R. Geometrical control of the magnetic anisotropy in six coordinate cobalt complexes. Chem. Commun. 2020, 56, 8492–8495. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.-F.; Singh, M.K.; Gan, D.; Xiao, T.; Wang, Y.; Liu, S.; Wang, Z.; Ouyang, Z.; Zhang, Y.-Z.; Dunbar, K.R. Probing the Axial Distortion Effect on the Magnetic Anisotropy of Octahedral Co(II) Complexes. Inorg. Chem. 2020, 59, 7622–7630. [Google Scholar] [CrossRef]
- Wernsdorfer, W.; Aliaga-Alcalde, N.; Hendrickson, D.N.; Christou, G. Exchange-biased quantum tunnelling in a supramolecular dimer of single-molecule magnets. Nature 2002, 416, 406–409. [Google Scholar] [CrossRef]
- Hill, S.; Edwards, R.S.; Aliaga-Alcalde, N.; Christou, G. Quantum Coherence in an Exchange-Coupled Dimer of Single-Molecule Magnets. Science 2003, 302, 1015–1018. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Wernsdorfer, W.; Abboud, K.A.; Christou, G. A supramolecular aggregate of four exchange-biased single-molecule magnets. J. Am. Chem. Soc. 2011, 133, 20688–20691. [Google Scholar] [CrossRef]
- Han, T.; Giansiracusa, M.J.; Li, Z.H.; Ding, Y.S.; Chilton, N.F.; Winpenny, R.E.P.; Zheng, Y.Z. Exchange-Biasing in a Dinuclear Dysprosium(III) Single-Molecule Magnet with a Large Energy Barrier for Magnetisation Reversal. Chem.—A Eur. J. 2020, 26, 6773–6777. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, R.; Suzuki, T.; Sunatsuki, Y. Four-Electron Oxidative Dehydrogenation Induced by Proton-Coupled Electron Transfer in Ruthenium(III) Complex with 2-(1,4,5,6-Tetrahydropyrimidin-2-yl)phenolate. Inorg. Chem. 2013, 52, 10183–10190. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 36, 955–964. [Google Scholar] [CrossRef]
- Mitsuhashi, R.; Suzuki, T.; Hosoya, S.; Mikuriya, M. Hydrogen-Bonded Supramolecular Structures of Cobalt(III) Complexes with Unsymmetrical Bidentate Ligands: mer/fac Interconversion Induced by Hydrogen-Bonding Interactions. Cryst. Growth Des. 2017, 17, 207–213. [Google Scholar] [CrossRef]
- Scott, P.L.; Jeffries, C.D. Spin-lattice relaxation in some rare-earth salts at helium temperatures; observation of the phonon bottleneck. Phys. Rev. 1962, 127, 32–51. [Google Scholar] [CrossRef]
- Shrivastava, K. Theory of Spin–Lattice Relaxation. Phys. Status Solidi B 1983, 117, 437. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Sakiyama, H. Development of MagSaki(Tetra) Software for the Magnetic Analysis of Tetranuclear High-spin Cobalt(II) Complexes. J. Comput. Chem. Jpn.-Int. Ed. 2016, 2, 2016-0001. [Google Scholar] [CrossRef]
- Chilton, N.F. CC-Fit; The University of Manchester: Manchester, UK, 2014; Available online: http://www.nfchilton.com/cc-fit.html (accessed on 4 November 2019).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).