Abstract
The first neutral 0D and 1D heterometallic assemblies based on orbitally degenerate heptacyanidorhenate(IV) were prepared and structurally characterized. An analysis of the magnetic data of polycrystalline samples showed that both compounds display slow magnetization relaxation at temperatures below 5 K. The very low temperature measurements of the magnetization on the single crystals demonstrate that for the 1D compound {[Mn(SB2+)Re(CN)7]·7H2O}n (1) and the 0D complex [Mn(SB2+)(H2O)Re(CN)7]·2H2O (2), the hysteresis loops open just below 2.2 and 1.8 K, respectively. Thus, heterometallic polymer 1 is the first single-chain magnet involving a pentagonal bipyramidal [ReIV(CN)7]3− synthon, and the binuclear complex 2 represents a single-molecule magnet.
1. Introduction
Orbitally degenerate 4d/5d cyanidometallates with unquenched orbital angular momentum are efficient sources of strong magnetic anisotropy in the design of molecular nanomagnets, which were first predicted theoretically [1,2] and then confirmed experimentally by synthesis of a number of single-molecule magnets (SMMs) based on these homoleptic complexes as highly anisotropic building blocks [3,4,5,6,7,8,9,10]. An important feature of these complexes is the absence of single-ion magnetic anisotropy due to their low-spin ground state (S = 1/2). In this case, magnetic anisotropy is produced cooperatively, in concert with attached high-spin 3d ions, through anisotropic exchange interactions, underlying an alternative strategy toward high-performance SMMs [1,2]. In contrast to rather numerous cyanide-bridged 1D magnetic systems involving 3d metal ions [11,12,13,14,15,16], the single-chain magnets (SCMs) incorporating homoleptic cyanide complexes of heavier d metal ions are considerably less common [17,18,19,20,21,22]. Among these, the unidimensional magnetic polymers based on low-spin (S = 1/2) orbitally degenerate cyanidometallates are particularly rare, with only a few SCMs based on hexacyanides of iron(III) [23,24,25,26] and osmium(III) [17], and four 1D coordination polymers involving heptacyanidomolibdate(III) [27,28], only three of which are SCMs.
The low-spin octahedral nd5 complexes [MIII(CN)6]3−, MIII = Fe, Ru, Os [6,24,29], as well as the pentagonal bipyramidal complexes [MoIII(CN)7]4− (4d3) [30] and [ReIV(CN)7]3− (5d3) [3,31], display anisotropic exchange interactions with linked high-spin 3d ions. An energy barrier U, which has to be surmounted to reverse the magnetization, determines the slow relaxation of magnetization in the low-dimensional (LD) assemblies comprising these tectons. The U value for an SMM depends on the uniaxial anisotropy energy of a molecule. For a 1D polymer, the appearance of slow magnetic relaxation was predicted by Glauber [32]. Unlike SMMs, the energy barrier in SCMs depends not only on the magnetic anisotropy strength but also on the magnitude of intrachain magnetic coupling [16]. Hence, it should be easier to increase the U value for SCMs than for SMMs. As previously stated, a neutral 1D polymer based on the low-spin hexacyanoferrate(III) and MnIII Schiff base (SB) complex can display SCM behavior [23]. This alternating [-MnIII-NC-FeIII-CN-] system comprises two sources of magnetic anisotropy: zero-field splitting (ZFS) of the [MnIIISB]3+ unit with an easy magnetization axis along the Jahn–Teller distortion direction, and angular orbital momentum L of the [Fe(CN)6]3− unit. Due to the relatively small spin–orbit coupling (SOC) of the latter (ζFe = 464 cm−1 [33]) L may be quite quenched depending on the degree of distortion from the perfect octahedral (Oh) geometry. This was confirmed by our comparative study [24] of two anionic SCMs with a general formula (X)2[MnIII(acacen)FeIII(CN)6] (X = Et4N+ or Ph4P+; acacen = N,N′-ethylenebis(acetylacetonylideneaminato) and the same magnetic core fragment (–Fe–CN–Mn–NC). These chains display (since these are inherent properties of substances, therefore it always exists, and not only when we studied theme) noticeably different blocking temperatures (Tb) of ~1.1 and 2.5 K, respectively. To prevent the L quenching and provide stronger exchange interactions (since the heavier metal ions possess more diffuse 4(5)d-orbitals), we used the complex [Os(CN)6]3− as a metalloligand with 5d5 electronic configuration and higher SOC [34,35]. As a result, the only SCM (this is not only the first SCM, but so far the only one) composed of hexacyanidoosmate(III) was prepared and studied [17].
Inspired by the above achievements, we aimed to obtain a similar anionic chain comprising another orbitally degenerate heptacyanidorhenate(IV) magnetic unit (Scheme 1a) not only possessing sufficiently strong spin–orbit splitting (ζRe = 2400 cm−1) [35], but also possessing a coordination polyhedron with a pronounced uniaxial symmetry. However, our attempts to prepare an SCM starting from [Mn(acacen)]+ and [Re(CN)7]3− resulted in highly anisotropic 3D [31] and 2D [19] networks. In addition, the efforts to prepare the anionic chains based on the triply charged octacyanidometallates of MoV and WV, were unsuccessful because the cationic complex [Mn(acacen)]+ (Scheme 1b) formed layered systems with the [MV(CN)8]3− in the presence of Ph4P+ [36] or PPN+ (bis(triphenylphosphine)iminium) [37].

Scheme 1.
Building units: (a) pentagonal bipyramidal [Re(CN)7]3− (D5h symmetry); (b) [Mn(acacen)]+.
The present study is a continuation of our previous research directed toward the design of LD bimetallic nanomagnets involving orbitally degenerate cyanidometallates and MnIII Schiff base complexes. Here, we present the synthesis, crystal structure description, and preliminary magnetic studies of two neutral assemblies incorporating a salen-type complex [MnIII(SB2+)]3+ (Scheme 2): the first SCM based on pentagonal bipyramidal ReIV heptacyanide (Scheme 1a), {[Mn(SB2+)Re(CN)7]·7H2O}n (1), and a binuclear compound, [Mn(SB2+)(H2O)Re(CN)7]·2H2O (2). The crystals of the latter were obtained as a result of a prolonged recrystallization process of the chain polymer.
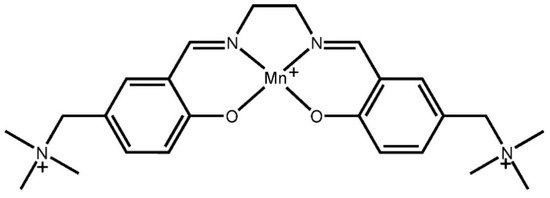
Scheme 2.
Molecular structure of triply charged salen-type [Mn(SB2+)]3+.
2. Results and Discussion
2.1. Synthetic Approach
Electroneutrality is a fundamental force in the self-assembly of heterometallic coordination compounds in solution. The MnIII complexes with Schiff bases of both the salen and acacen type ordinarily have a charge of +1; therefore, when they interact with triply charged anions of cyanidometallates, three times as many of them (3:1 ratio) are required to ensure the electroneutrality of the system. This combination does not guarantee the formation of LD heterometallic assemblies with the axial symmetry of a heterometallic system. However, at a ratio of 1:2, for the diamagnetic dianion [Fe(CN)5NO]2− and [MnIII(acacen)]+, the neutral 0D and 1D polynuclear compounds were obtained along with a layered material {[(MnIII(acacen))2Fe(CN)5NO]}n [38] depending on the solvent used.
Previously, we successfully obtained the first neutral heterobimetallic cyanide-bridged compounds involving one anisotropic MnIII complex and one octacyanidotungstate(V) per molecular unit. One of them—[Mn(SB2+)(H2O)WV(CN)8]·5H2O, obtained via slow diffusion of component solutions—is a discrete molecule, while the other—{[Mn(SB2+)(H2O)WV(CN)8]·8H2O}n, precipitated during a rapid mixing of the reagents—is a 1D polymer exhibiting SCM properties [18]. Therefore, in order to obtain the neutral low-dimensional species incorporating [ReIV(CN)7]3−, we used the same manganese(III) complex [Mn(SB2+)(H2O)2](ClO4)3·H2O. However, in the case of heptacyanidometallate(IV), the process of self-assembly of bimetallic compounds occurs somewhat differently. When layering an acetonitrile solution of (Bu4N)3[Re(CN)7] on an aqueous solution of a manganese(III) complex, the large dark crystals of a chain polymer (1) are formed on the walls of the test tube, and then crumble in air due to the partial loss of solvate water molecules. Compound 1 can also be obtained in the form of a finely crystalline powder by dropping a solution of an MnIII complex into a solution of cyanidometallate: [Mn(SB2+)(H2O)2](ClO4)3 + (Bu4N)3[Re(CN)7] + H2O → {[Mn(SB2+)Re(CN)7]·7H2O}n↓ + 3Bu4NClO4 (for more details, see the Section 4). The slow recrystallization of powdered compound 1 in aqueous media resulted in formation of crystals of 2—the binuclear compound [Mn(SB2+)(H2O)Re(CN)7]·2H2O—in a small amount: 1powder + H2O → 2crystals. According to X-ray powder diffraction data, the initial powder of the chain polymer was only partially transformed into a dimer species (see below).
2.2. Crystal Structure Description
The crystallographic data and structural refinement summary for 1 and 2 are included in Table S1 (see Supplementary Materials). Single crystal X-ray structural analysis revealed that compound 2 has a 0D molecular structure, while 1 is a 1D chain polymer. The molecular views of the repeating unit in the chain {[Mn(SB2+)Re(CN)7]}n (1) and an asymmetric unit of [Mn(SB2+)(H2O)Re(CN)7]·2H2O (2) are shown in Figure 1. Both compounds are neutral bimetallic assemblies consisting of one [Re(CN)7]3− anion and one cation ([Mn(SB2+)]3+ for 1 or [Mn(SB2+)(H2O)]3+ for 2).

Figure 1.
Molecular structure of (a) the repeating unit in the chain {[Mn(SB2+)Re(CN)7]}n (1) (solvent water molecules are omitted) and (b) the molecular unit [Mn(SB2+)(H2O)Re(CN)7](H2O)2 in 2. ORTEP diagrams are made with 50% probability thermal ellipsoids.
In both compounds, a slightly distorted pentagonal bipyramidal coordination environment of the Re center comprises seven cyanide ligands. In fact, [Re(CN)7]3− is much less distorted than its isoelectronic counterpart, [Mo(CN)7]4− [8,20,28]. The Re−C distances are in the range 2.061(5)–2.144(5) Å, with an average of 2.106(19) Å, similar to that observed for (Bu4N)3[Re(CN)7] (2.064(10)–2.123(11) Å) [39]. The coordination environment of the Mn ion is an elongated tetragonal bipyramid because of the Jahn–Teller distortion. The 2O and 2N donor atoms of the SB2+ ligand in the basal plane of the pyramid form shorter bonds of 1.877–1.989 Å, while the axial bonds are much longer, i.e., 2.242–2.299 Å.
The Mn–(N≡C)axial bond angle departs considerably from 180° and is equal to 144.2° and 145.1° for 1 and 2, respectively, being close to a value of 144.4° for {[Mn(SB2+)Fe(CN)6]}n [23]. It should be noted that such a flexion is typical of the cyanide-bridged MnIII–M(CN)n complexes [9,34,38,40,41,42]. However, for the complexes of SB2+, which is a sterically demanding ligand, this angle is especially small. For example, for a much less bulky SB complex [Mn(acacen)]+, the value of this angle for the ReIV-MnIII system varies in the range 152.9–163.7° [31,36,37]. At the same time, the Mn–(N≡C) angle is 162.6 and 160.1° in the discrete species [Mn(MeSB2+)(H2O)Fe(CN)6] [9] and [Mn(SB2+)(H2O)W(CN)8] (3) [18], respectively.
In contrast to the heterobimetallic {MnIII(SB)M(CN)m} complexes—where SB is a salen-type ligand, for which a dimerization of the MnIII(SB) fragments is quite widespread (see, for example, [25,43])—the trications [Mn(SB2+)H2O]3+ in the 0D neutral moieties based on hexa- and heptacyanidometallates are not dimerized in a crystal due to the trans location of the [Me3N+CH2] substituents relative to the SB2+ plane (Figure 1 and Figure S1). Meanwhile, the binuclear molecules of 3 [18] are dimerized due to hydrogen bounding, π–π stacking of the ligand aromatic rings, and non-valent CN…H interactions of the [Me3N+CH2] groups. As a result, the latter are in cis positions relative to one another.
As in the case of the neutral 1D polymer involving hexacyanidometallates [23], in its congener 1, the Jahn–Teller axes (JTA) of the [MnIII(SB2+)]3+ moieties are ideally aligned along the chain direction without a bending angle (Figure 2 and Figure S2), while the apical axes of the rhenium cyanide (AAR) are close to perpendicular at 104.1°, the latter being slightly flatter than the 97.9° observed in {[Mn(SB2+)Fe(CN)7]·4H2O}n [23]. A similar situation is observed for the packing of the 0D binuclear compound 2 (Figure S3), with JTA and AAR angles of 172.7° and of 67.7°, respectively.

Figure 2.
View of the chain motifs projected along the a-axis. Hydrogen atoms and interstitial water molecules have been omitted for clarity.
Visually, the small crystals of the binuclear compound 2 are more resistant to the loss of solvate water molecules compared with the large crystals of the chain compound 1, whose crystals crumble within a few days, depending on the temperature and humidity of the surrounding air. The elemental analysis data additionally testify in favor of the latter. This difference is due to the features of the packing and strength of hydrogen bonds in the crystals of 1 and 2. In the latter, a system of hydrogen bonds links the discrete binuclear molecules in the chains (Figure 3) bounded in the layers (Figure S4), which are interconnected by the sufficiently short contacts C≡N…H-C and C-O…HC of ~2.5 Å. The chain packing in a crystal of compound 1 leads to the formation of channels enclosing the majority of the solvate H2O molecules (Figure S5).

Figure 3.
The 1D motif formation in 2. A view along the b-axis.
2.3. Characterization of the Polycrystalline Samples
Since 1 and 2 have sufficiently similar chemical composition, which varies depending on partial loss of solvate water, the main methods for their identification are IR spectroscopy and X-ray phase analysis. Significant differences can be observed in the IR spectra of the compounds not only in the region of C≡N stretching vibrations, but also in the area associated with ligand vibrations (Figure 4). More spectroscopic information is presented in Figures S6–S8 in the SI.

Figure 4.
IR spectra for 1 and 2, registered for the single-crystal batches.
According to the IR and PXRD data (Figure S9), bimetallic complex 1 prepared by a precipitation technique (see the Section 4) is identical to the crystals obtained by a slow diffusion technique. As mentioned above, a small amount (singular) of crystals of 2 was obtained via prolonged recrystallization of the powdered compound 1 in water. According to the PXRD study (Figure S10), the original powder present in the reaction vessel was not completely converted from a chain into a dimer even after six months.
2.4. Investigation of Magnetic Properties
2.4.1. Static Magnetic Behavior
The temperature dependence of the dc molar susceptibility for the powder sample of 1, measured in an applied field of 1000 Oe, is shown in Figure 5 as a χT versus T plot. At 300 K, the observed χT value of 3.68 is close to the 3.60 emu K mol−1 expected for one MnIII (S = 2, g = 2.03) [31] and one ReIV (S = 1/2, g = 2.33) [39] as magnetically uncoupled spin carriers. Starting from room temperature, χT first decreases slightly and then reaches a shallow minimum of 3.28 emu K mol−1 at ~75 K. This behavior of χT is characteristic of antiferromagnetic interactions between the MnIII and ReIV centers within the chains [31,44]. Below 50 K, χT increases and reaches a sharp maximum of 9.30 emu·K·mol−1 at ~5 K, before dropping to 6.48 emu K mol−1 at 2 K.
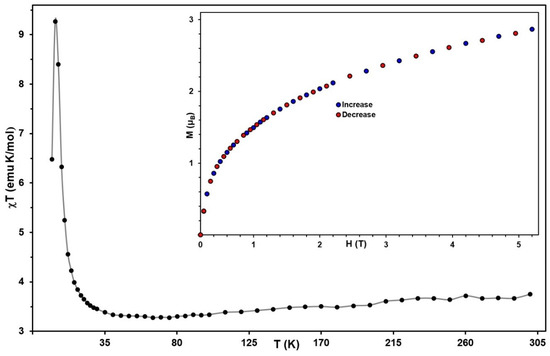
Figure 5.
Magnetic dc plots for 1. Temperature dependence of χT at H = 1000 Oe (solid line guides the eyes). Inset: magnetization versus the field at T = 2 K.
The non-compensation of the spins induces a ferrimagnetic arrangement along the chain, giving rise to a repeating unit with S = 3/2. As the temperature drops below 5 K, χT decreases sharply, owing to the field saturation of the magnetization and the magnetic anisotropy of the ReIV centers.
The magnetization vs. field curve of 1 measured at 2 K reveals complete reversibility of the magnetization (inset of the Figure 5). The M value reached at 50 kOe is equal to 2.86 μB per MnIII–ReIV unit, which is far from 5 μB (the theoretical value corresponding to the five unpaired electrons). The estimated saturation magnetic field (HA) for 1 using the experimental data M(H) is about 151 kOe, which is significantly greater than the values of 100, 108, and 120 kOe found for SCMs based on neutral [(SB2+)(Cr/Fe)(CN)6] units [23] and anionic [MnIII(acacen)FeIII(CN)6]2− [17] fragments, respectively.
The magnetic behavior of 2 was studied on a sample consisting of the crystals manually sorted from the possible powder impurities of the 1D precursor. At first glance, the magnetic behavior of 2 (Figure 6) was very similar to that of 1; the temperature dependence of χT also had a sharp maximum, and the shape of the plots for M(H) was also similar. However, the devil is in the details.
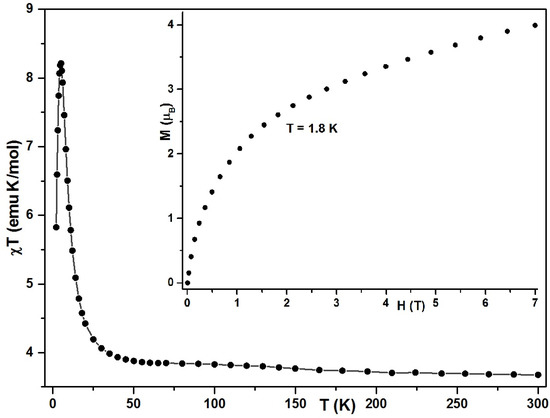
Figure 6.
Magnetic dc plots for 2. Temperature dependence of χT at H = 1000 Oe (solid line). Inset: magnetization versus the field at T = 2 K.
The χT value of 3.68 emu·K·mol−1 at 300 K precisely coincides with that registered for 2. Furthermore, unlike 1, χT increases slowly, pointing to a ferromagnetic interaction between the spins, with about 3.85 emu·K·mol−1 at 70 K, after which the χT values begin to rise, reaching a peak of 8.21 emu·K·mol−1 at 5 K before dropping sharply at lower temperatures. The presence of the pronounced peak on the χT plot at a temperature of about 5 K is quite unexpected for a 0D compound, since no such a peak is found in the temperature dependence of the related dimers [Et4N]2[Mn(saldmen)(H2O)Fe(CN)6]·MeOH·4H2O (3) [45] and [MnIII(MeSB2+)(H2O)Fe(CN)6]·7H2O·MeCN (4) (MeSB2+ = (R)-N,N′-(1-methylethylene) bis(5-trimethylammoniomethylsalicylideneiminate. For the latter, the χT value remains practically constant up to sufficiently low temperatures, and then drops sharply at T < 15 K. On lowering the temperature, the χT of 3 gradually increases from 3.45 emu·K·mol−1, reaching a flat maximum of 3.91 emu·K·mol−1 at 26 K, and then decreases to ~3.25 (3.88) emu·K·mol−1. The authors believe that this decrease is due to the ZFS of the MnIII ion and/or an intermolecular antiferromagnetic interaction. However, when dimer 3 is desolvated, its magnetic behavior changes significantly. Lowering the temperature results in a gradual decrease in χT, which shows a round minimum at ca. 60 K. It then increases abruptly to reach a value of 18.6 emu·K·mol−1 at 1.9 K (at Hdc = 600 Oe), which is much higher than that of the largest possible spin state ST = 5/2, where χT = 4.38 emu·K·mol−1. This behavior of 3′ is reminiscent of that shown by 1, with the difference that, for the latter, at low temperatures, the saturation of χT occurs at H = 1000 Oe instead of 600 Oe for 3′, the molar susceptibility of 3′ is not saturated at 1.9 K, and HDC = 1 Oe.
The variable-field plot of the magnetization for 2 is presented in Figure 6. It should be noted that the value of M of 3.58 μB at 50 kOe is close to the 3.45 μB shown by 3 and exceeds the value for 1 by 0.72 μB. For 2, the M value at the maximum available field of 70 kOe is 3.99 μB—the estimated for ferromagnetically coupled MnIII (S = 2) and ReIV (S = ½) with a gav = 2.0 magnetic field HA being about 120 kOe. This is less than the estimated lower limit of the saturation magnetic field, HA, for 2.
Unlike the chain, the 0D compound displays magnetic hysteresis at T = 1.8 K (Figure S11), with a very small coercive field of 156 Oe.
2.4.2. AC Magnetic Measurements
Although the χT plots of compounds 1 and 2 are similar at temperatures lower than 30 K, the dependences of the two compounds differ. The temperature-variable behavior of the ac molar susceptibilities of 1 measured in the frequency range of 1–1200 Hz in an oscillating ac magnetic field of 2.7 Oe and a zero dc field are presented in Figure S12 (SI). These data clearly indicate that both the real and imaginary parts of the susceptibility have a pronounced frequency dependence below 4 K.
Unfortunately, the lower temperature limit available for commercial SQUID magnetometers (down to ~2 K) is not sufficient to reliably determine the full set of relaxation parameters for the 1D compound, since the magnetization-blocking temperature Tb for 1 appears to be below 2 K. This conclusion follows from the fact that not a single curve from the set of plots of the imaginary part of the magnetic susceptibility χ”(T), at various frequencies, reaches a maximum. Below, we present a few SCM parameters estimated for 1 from the experimental data.
The correlation length, ξ, of a 1D classical polymer is directly proportional to the χT product in a zero applied field. In the particular cases of the Ising-like or anisotropic Heisenberg models, ξ and, thus, χT increase exponentially with decreasing temperature as follows: χT ≈ Ceff × exp(Δξ/kBT), where Ceff is the effective Curie constant and Δξ is the energy required to create a domain wall along the chain [12,46]. Therefore, to determine the SCM behavior of a material, the ln(χ′T) versus 1/T plot (χ′ being the real ac susceptibility data collected in Hdc = 0 at the lowest available ac frequency) must be thoroughly examined. Its linear dependence, with a slope corresponding to Δξ, proves the 1D magnetic nature of the material and the presence of significant magnetic anisotropy. Figure S14 (SI) shows the respective ln(χ′T) versus 1/T plots for 1. A linear region is observed in the temperature range 10–6 K, giving Ceff = 1.005 emu K mol−1 and Δξ/kB = 6.66 K. At 4.5 K, ln(χ′T) reaches a maximum ((χ′T)max = 10.3 emu K mol−1) and then experiences a linear decrease with decreasing temperature until χ′ is blocked under an oscillation of 1 Hz. An intersection of the two linear regions, occurring at ca. 4.5 K, corresponds to the crossover temperature (T*), where the magnetic correlation becomes physically limited by crystalline defects, and temperatures below T* comprise the finite-sized regime [47].
The dynamic magnetic behavior of the heterobinuclear compound 2 was studied for a polycrystalline sample in the frequency range of 1–1500 Hz under an oscillating ac magnetic field of 3.5 Oe. The dependences of the ac molar susceptibility at the dc fields of 0 and 200 Oe are practically the same for 2, as shown by the variable-field test carried out at 2 K (Figure S13). Therefore, a study of the temperature dependences of the ac molar susceptibility for 2 at different frequencies was performed at Hdc = 100 Oe (see Figure S15). Both sets of plots indicate the presence of two relaxation processes. This may be due either to a manifestation of two different relaxation mechanisms, or to the presence of a second magnetic phase. In our opinion, the latter option is the most plausible explanation, since a stratum of a desolvated solid could be formed on the surface of the compound 2 crystals.
Despite the fact that the binuclear molecules in compound 2 are connected in layers by a network of hydrogen bonds, such intermolecular coupling can appear only at very low temperatures, because they are too low in energy compared to the covalent bonds of bridging cyanides. Therefore, down to temperatures of ~10 K, the deviations from the high-temperature value of χT can only be caused by the M(CN)–Mn(SB) exchange interaction, as is observed for 1. At very low temperatures, the positive or negative “bend” of the χT plot is usually determined by the intermolecular interaction, whether ferro- (as in the case of 3′) or antiferromagnetic in nature. Examples of the latter include the complexes [MIIL2(ROH)2] (MII = Ni, Co; R = H, Me, Et; L is a CF3-decorated enamine ketone derivative of stable 3-imidazoline nitroxide) [48,49,50,51], in which the bis-chelate metal complexes are woven into a 2D network through hydrogen bonds between the ROH and nitroxyl groups of adjacent molecules: M-ROH…·O–N–(M). A removal of coordinated ROH molecules also leads to an essential change in the magnetic behavior due to the formation of covalent metal–radical bonds, leading to the formation of a 1D compound [52].
As can be seen in Figure 3, the dimer fragments of 2 are connected in a chain due to a set of hydrogen bonds: (Mn–OH2)…(solvate H2O)…(N≡C–Re). The removal of at least one solvate water molecule necessarily leads to the reorganization of the entire packing of binuclear fragments and, as a result, the modification of the intermolecular contacts and geometric parameters inside the dimer. With the complete removal of water from 2, the formation of a 1D polymer is possible, in which [Mn(SB2+)]3+ complexes are connected to ReIV ions by an apical and equatorial cyanide bridge (see Figure S16). Such an organization of the chain should promote mutual alignment of both the apical axes of the heptacyanidorhenate anions and the Jahn–Teller axes of the [Mn(SB2+)]3+ cations in the crystal, which is important to increase the coercivity of low-dimensional magnets. It should be noted that the determined by the Mn–(N≡C) angle mutual slope of the (N≡C–Re–C≡N)apical and N–Mn–N axes is responsible for the nature and magnitude of the intrachain exchange interaction affecting the spin reversal barrier height and the value of Tb.
2.4.3. Magnetic Measurements at Very Low Temperatures
By means of an in-house-made μ-SQUID system, additional M versus H data down to 30 mK (Figure 7 and Figure S17) were collected from single crystals. As shown in Figure 7 for 1, the M/Ms (Ms is a saturation field) versus H hysteresis loop opens below 1.6 K, and the temperature lower than 2 K was used to study the polycrystalline sample of 2. The coercivity of 1.3 T is observed for a crystal of 1 at 0.4 K. This value is somewhat less than the 1.68 T obtained for the Fe congener {[Mn(SB2+)Fe(CN)7]·4H2O}n [23]. Considering the 1D nature of 1, as demonstrated by the analysis of correlation length discussed above (ln(χ′T) versus 1/T plot; Figure S14), the slow relaxation of magnetization evident from the M versus H hysteresis loops strongly supports the view that 1, with Tb ≈ 1.6 K, is a new example of a single-chain magnet.
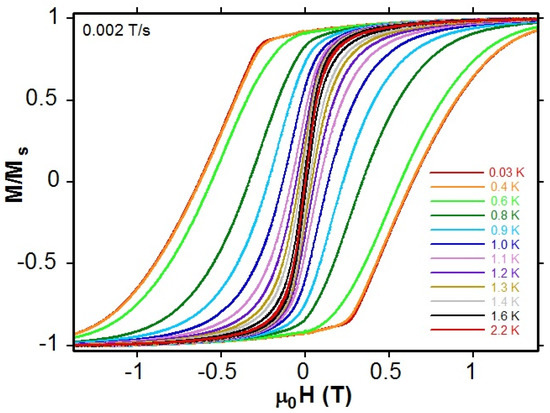
Figure 7.
Field dependences of the normalized magnetizations at 0.002 T s−1 measured at low temperatures from oriented single crystals of 1 with the Hdc applied along the easy magnetic axis.
Despite the presence of magnetic hysteresis, the pronounced frequency dependence of the molar magnetic susceptibility, and the 0D structure, the classification of the relaxation nature (SMM or SCM) of compound 2 is not clear, for several reasons. In contrast to 1, a magnetic hysteresis loop for 2 is already visible at 1.8 K on a polycrystalline sample (Figure S11). Moreover, as shown by low-temperature studies (Figure S18a), the shape of the hysteresis and the coercivity of 2 are preserved for a single crystal up to 0.8 K. At 0.5 K, the coercivity at H = 0 collapses and, starting from ~0.4 K, the shape of the hysteresis changes. This behavior may also be associated with the presence of the aforementioned dehydrated layer, which leads to the coexistence of two different magnetic phases with two different magnetization-blocking temperatures of ~1.8 and ~0.4 K—presumably for 1D and 0D species, respectively. This hypothesis is also supported by the presence of linear segments on the ln(X′T) vs. 1/T plot (Figure S14b).
3. Conclusions and Perspectives
The two neutral low-dimensional heterobimetallic compounds based on the binuclear magnetic unit [MnIII(SB2+)Re(CN)7] were obtained and structurally characterized. Preliminary studies of their magnetic behavior showed that compound 1, {[Mn(SB2+)Re(CN)7](H2O)7}n, is the first SCM involving orbitally degenerate pentagonal bipyramidal heptacyanidorhenate, and its blocking temperature is Tb ~1.6 K. The nature of the χT behavior in the high-temperature range for 1D and 0D differs significantly, confirming the ferrimagnetic and ferromagnetic character of magnetic exchange interactions (EIs) for the first and the second, respectively. However, at temperatures below 60 K, ferromagnetic EIs prevail in both compounds up to a temperature of 4.5 K, where a maximum is observed in the χT-T plot, and then χT rapidly decreases due to saturation in the field effect. For both compounds, even under a field of 70 kOe, the magnetization does not reach the maximum value of 5 μB expected for five unpaired electrons. This is due to the high anisotropy of these systems, as evidenced by the estimated values of the fields HA of 151 and 120 kOe for 1 and 2, respectively.
The results of static and dynamic magnetic measurements for 2 show that the studied sample likely contains two phases, since its small crystals were apparently dehydrated in the near-surface layer at the time of measurement. Analysis of the crystal packing of compound 2 shows that its structure is formed by hydrogen bonds’ 1D motifs, which, upon dehydration, could turn into covalently bonded chains that are structurally different from those of 1.
The unusual magnetic properties of both compounds originate from the interplay of Re–Mn anisotropic spin coupling and the ZFS effect of MnIII ions with a noncollinear orientation of the local magnetic axes in crystals of the compounds.
Unfortunately, the magnetization-blocking temperatures were low and were just outside the range of commercial SQUID magnetometers, so we were unable to obtain a dataset sufficient to obtain a complete set of quantitative parameters for magnetic relaxation of 1 and 2 by simulating the experimental data. We need to obtain low-temperature data for the decays with time of the normalized magnetizations measured from oriented single crystals to be able to estimate the barriers Δτ1 and Δτ2 for the two compounds [23]. In addition, we are planning studies aimed at obtaining compound 2 in a different way than the time-consuming recrystallization of 1, in order to ensure reproducible synthesis of the binuclear compound in quantities sufficient for detailed characterization (including TG, DSC, and PXRD methods) of the solvated phase of 2, as well as its dehydrated derivative. This will make it possible to conduct a complete study of the static and relaxation magnetic properties of each form separately. The obtained experimental data will be used for theoretical modeling in order to determine the magnetic characteristics for anisotropic exchange, as was done using microscopic theory for 3D [(MnIIIacacen)3ReIV(CN)7]n [31] and [{MnLN5(H2O)}2Mo(CN)7] [30]. In the longer term, we plan to produce doubly connected SCMs such as {[M(bida)(H2O)]2[Mo(CN)7]·6H2O}n [20].
4. Materials and Methods
All of the reagents and solvents (EKOS-1, Moscow, Russia) were used without further purification; [Mn(SB2+)(H2O)2](ClO4)3·H2O [41] and (Bu4N)3[Re(CN)7] [39] were prepared using published protocols.
The polycrystalline powder material of {[Mn(SB2+)Re(CN)7]·7H2O}n (1) was obtained via a precipitation method using a 1:3 mixture of water and acetonitrile as the solvent.
A dark-brown solution of [Mn(SB2+)(H2O)2](ClO4)3·2H2O (41 mg, 0.05 mmol) in 2.5 mL of solvent was added dropwise to a stirred light-yellow solution of (Bu4N)3[Re(CN)7 (55 mg, 0.05 mmol) in 2.5 mL of solvent. The reaction mixture was stirred while heating until boiling. The precipitated dark product was centrifuged and then washed twice with H2O (2 mL), twice with MeCN (2 mL), once with Et2O (2 mL), and then air-dried. The yield was 95%. C31H34MnN11O2Re·4.5(H2O) (914.89); CHN: calcd. C 40.70, H 4.74, N 16.84; found C 40.8, H 4.5, N 16.95. IR (ATR) ῡ(CN): 2111.8(sh), 2073.1, 2030.6, 1999.8(sh), 1969.0(sh) 1930.4 (sh) cm−1.
The crystals of 1 were obtained via slow diffusion of reagent solutions. First, 2 mL of an aqueous solution containing 41 mg of manganese complex was placed in a glass test tube (0.8 cm × 10 cm), on which a buffer layer was placed (1:1 mixture of H2O:MeCN, 1.5 mL). The top layer was a solution of rhenium cyanide (55 mg in 2 mL of MeCN). The tubes were closed with parafilm and stored in the dark at a temperature of +4 °C. Three weeks later, rather large dark-brown crystals and a polycrystalline powder were obtained. The crystals were manually separated from the powder (~9 mg) and used for X-ray diffraction studies and magnetic measurements.
The crystals of [Mn(SB2+)(H2O)Re(CN)7]·2H2O (2): The powder of 1 was placed in a test tube covered with several mL of distilled water. The test tube closed with parafilm was kept in a dark place at room temperature. A few months later, the water was partially evaporated, and small dark parallelepiped-like crystals formed on the walls of the test tube near the upper frontier of the liquid. IR (ATR) ῡ(CN): 2121.7(sh), 2081.2, 2019.5, 1932.7(sh), C31H34MnN11O2Re·3H2O (887.87); CHN: calcd. C 41.94, H 4.54, N 17.35; found C 42.1, H 4.4, N 17.4.
Elemental (C,H,N) analysis were carried out with a Euro-Vector 3000 analyzer (Eurovector, Redavalle, Italy). FTIR spectra were measured with a NICOLET spectrophotometer (Thermo Electron Scientific Instruments LLC, Madison, WI, USA) in the 4000–375 cm−1 range. Powder X-ray measurements were performed using Cu-Kα radiation (λ = 1.5418 Å) with an X’Pro powder diffractometer (PANalytical Inc., Almelo, The Netherlands) at room temperature. All magnetic measurements of properties were performed using a Quantum Design MPMS 5XL SQUID magnetometer (Quantum Design, Inc., San Diego, CA, USA) in the temperature range of 1.8–300 K and under a magnetic field of up to 50 kOe. The magnetic susceptibility χm is the molar magnetic susceptibility per mole of C31H34MnN11O2Re·7H2O and C31H34MnN11O2Re·3H2O units, and was corrected for the diamagnetic contribution calculated from Pascal’s constants [53]. Ultralow-temperature (>1.8 K) magnetization measurements on single crystals were performed using a μ-SQUID array [54].
Single-crystal XRD experimental details are presented in Table S1 (Supplementary Materials). Crystallographic data were deposited with the Cambridge Crystallographic Data Centre (deposit numbers CCDC 1569081-1569082). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/ (accessed on 10 August 2022) (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge, CB2 1EZ, UK; Fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/magnetochemistry8100126/s1, Figure S1: A view of chains’ packing in a crystal of 1 demonstrating a translocation of the [Me3N+CH2] substituents relative to the SB2+ plane. Hydrogen atoms are omitted for clarity; Table S1: SCXRD experimental details; Figure S2: A layer formation in a crystal of 1: H2O molecules bind the fragments [MnIII(SB2+)]3+ and [Re(CN)7]3− belonging to the different chains. The layers are interconnected by means of interstitial water molecules. The SB2+ ligands are reduced for clarity; Figure S3: A view of dimers’ packing in a crystal of 2. The SB2+ ligands are reduced, and hydrogen atoms are omitted for clarity; Figure S4: A layer formation in a crystal of 2: H2O molecules bind the fragments [MnIII(SB2+)]3+ and [Re(CN)7]3− belonging to the different chains. The layers are interconnected by means of interstitial water molecules. The hydrogen atoms are partially omitted for clarity; Figure S5: A view (in the c-axis direction) of channels in a crystal of 1: (a) filled by water molecules, (b) empty; Figure S6: IR spectrum for {[Mn(SB2+)Re(CN)7]·7H2O}n 1; Figure S7: IR spectrum for the precursor [Mn(SB2+)(H2O)2](ClO4)3·H2O; Figure S8: IR spectrum for the precursor (Bu4N)3[Re(CN)7]·H2O (KBr); Figure S9: PXRD patterns for the chain compound 1: polycrystalline sample (black), theoretically calculated (red); Figure S10: PXRD patterns for the dimer compound 2 (room temperature) and theoretical calculations (150 K) for 1 and 2; Figure S11: Magnetic hysteresis loop of 2 measured at 1.8 K on a polycrystalline sample (sw. rate—0.07 T·s−1); Figure S12: Variable temperature of the real, χ’ (top), and imaginary, χ″ (bottom), parts of the ac molar susceptibility data for 1 under Hdc = 0 Oe, Hac = 2.7 Oe. Solid lines are guides; Figure S13: Frequency dependence of the real (χ′) (top), and imaginary (χ″) (bottom), parts of the ac susceptibility for a polycrystalline sample of 2 in different dc-fields y and with applied a 3 Oe ac field. Solid lines are guides; Figure S14: Plots of ln(X′T) vs. 1/T (where X′ is the molar component of the ac susceptibility) for 1 (top) and 2 (bottom) collected in a zero applied dc field and at a frequency of 1 Hz. The dashed red lines correspond to a linear fit for the high-temperature region, giving Δξ/kB = 6.66 (Ceff = 1.005 emu·K/mol) and 4.21 K (Ceff = 1.526 emu·K/mol) for 1 and 2, respectively. The dashed green lines correspond to a linear fit for the low-temperature region. An intersection of the two linear regions corresponds to the crossover temperature T* ≈ 4.5 K, which is equal for both; Figure S15: Variable-temperature real, χ′ (top), and imaginary, χ″ (bottom), ac molar susceptibility data for 2 under Hdc = 100 Oe, Hac = 3.5 Oe. Solid lines are guides; Figure S16: Possible formation of a 1D polymer from 2 by desolvation. The [Mn(SB2+)]3+ complexes are connected to the ReIV ions by equatorial and apical cyanide bridges; Figure S17: Field dependences of the normalized magnetization at the field-sweeping rates of 0.002 T·s−1 (a), and 0.140 T·s−1 (b), measured at different temperatures on an oriented single crystal of 2 with the magnetic field applied along the easy magnetic axis; Figure S18: Field dependences of the normalized magnetization at 0.03 K (a), and 0.2 K (b) measured at different field sweeping rates on an oriented single crystal of 2 with the magnetic field applied along the easy magnetic axis. The maximum coercitivity reaches1043.5 Oe at 30 mK and SwRate of 0.280 T·s−1.
Author Contributions
Conceptualization, K.E.V.; funding acquisition, K.E.V.; investigation, K.E.V., W.W., and T.S.S.; supervision K.E.V.; visualization, K.E.V.; writing—original draft, K.E.V.; writing—review and editing, K.E.V. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation (No. 121031700313-8 and No. 121031700321-3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Marko Damjanovic for magnetic data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mironov, V.S.; Chibotaru, L.F.; Ceulemans, A. Mechanism of a Strongly Anisotropic MoIII−CN−MnII Spin−Spin Coupling in Molecular Magnets Based on the [Mo(CN)7]4− Heptacyanometalate: A New Strategy for Single-Molecule Magnets with High Blocking Temperatures. J. Am. Chem. Soc. 2003, 125, 9750–9760. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.S. New Approaches to the Problem of High-Temperature Single-Molecule Magnets. Dokl. Phys. Chem. 2006, 408, 130–136. [Google Scholar] [CrossRef]
- Freedman, D.E.; Jenkins, D.M.; Iavarone, A.T.; Long, J.R. A Redox-Switchable Single-Molecule Magnet Incorporating [Re(CN)7]3−. J. Am. Chem. Soc. 2008, 130, 2884–2885. [Google Scholar] [CrossRef] [PubMed]
- Zadrozny, J.M.; Freedman, D.E.; Jenkins, D.M.; Harris, T.D.; Iavarone, A.T.; Mathonière, C.; Clérac, R.; Long, J.R. Slow Magnetic Relaxation and Charge-Transfer in Cyano-Bridged Coordination Clusters Incorporating [Re(CN)7]3−/4−. Inorg. Chem. 2010, 49, 8886–8896. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Avendaño, C.; Dunbar, K.R. Molecular Magnetic Materials Based on 4d and 5d Transition Metals. Chem. Soc. Rev. 2011, 40, 3213. [Google Scholar] [CrossRef] [PubMed]
- Dreiser, J.; Pedersen, K.S.; Schnegg, A.; Holldack, K.; Nehrkorn, J.; Sigrist, M.; Tregenna-Piggott, P.; Mutka, H.; Weihe, H.; Mironov, V.S.; et al. Three-Axis Anisotropic Exchange Coupling in the Single-Molecule Magnets NEt4[MnIII2(5-Brsalen)2(MeOH)2MIII(CN)6] (M=Ru, Os). Chem. Eur. J. 2013, 19, 3693–3701. [Google Scholar] [CrossRef]
- Qian, K.; Huang, X.-C.; Zhou, C.; You, X.-Z.; Wang, X.-Y.; Dunbar, K.R. A Single-Molecule Magnet Based on Heptacyanomolybdate with the Highest Energy Barrier for a Cyanide Compound. J. Am. Chem. Soc. 2013, 135, 13302–13305. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-Q.; Shao, D.; Wei, X.-Q.; Shen, F.-X.; Shi, L.; Kempe, D.; Zhang, Y.-Z.; Dunbar, K.R.; Wang, X.-Y. Reversible On–Off Switching of a Single-Molecule Magnet via a Crystal-to-Crystal Chemical Transformation. J. Am. Chem. Soc. 2017, 139, 11714–11717. [Google Scholar] [CrossRef]
- Ishikawa, R.; Nakano, M.; Breedlove, B.K.; Yamashita, M. Syntheses, Structures, and Magnetic Properties of Discrete Cyano-Bridged Heterodinuclear Complexes Composed of MnIII(Salen)-Type Complex and MIII(CN)6 Anion (MIII = Fe, Mn, and Cr). Polyhedron 2013, 64, 346–351. [Google Scholar] [CrossRef]
- Shi, L.; Wei, X.; Wang, X.; Wu, D. Research Progress in Molecular Magnetic Materials Based on the [Mo(CN)7]4− Unit. Sci. Sin. Chim. 2020, 50, 1637–1653. [Google Scholar] [CrossRef]
- Miyasaka, H.; Saitoh, A.; Abe, S. Magnetic Assemblies Based on Mn(III) Salen Analogues. Coord. Chem. Rev. 2007, 251, 2622–2664. [Google Scholar] [CrossRef]
- Coulon, C.; Miyasaka, H.; Clérac, R. Single-Chain Magnets:Theoretical Approach and Experimental Systems BT. In Single-Molecule Magnets and Related Phenomena; Winpenny, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 163–206. ISBN 978-3-540-33240-4. [Google Scholar]
- Sun, H.-L.; Wang, Z.-M.; Gao, S. Strategies towards Single-Chain Magnets. Coord. Chem. Rev. 2010, 254, 1081–1100. [Google Scholar] [CrossRef]
- Dhers, S.; Feltham, H.L.C.; Brooker, S. A Toolbox of Building Blocks, Linkers and Crystallisation Methods Used to Generate Single-Chain Magnets. Coord. Chem. Rev. 2015, 296, 24–44. [Google Scholar] [CrossRef]
- Bar, A.K.; Pichon, C.; Sutter, J.-P. Magnetic Anisotropy in Two- to Eight-Coordinated Transition–Metal Complexes: Recent Developments in Molecular Magnetism. Coord. Chem. Rev. 2016, 308, 346–380. [Google Scholar] [CrossRef]
- Coulon, C.; Pianet, V.; Urdampilleta, M.; Clérac, R. Single-Chain Magnets and Related Systems BT. In Molecular Nanomagnets and Related Phenomena; Gao, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 143–184. ISBN 978-3-662-45723-8. [Google Scholar]
- Peresypkina, E.V.; Majcher, A.M.; Rams, M.; Vostrikova, K.E. A Single Chain Magnet Involving Hexacyanoosmate. Chem. Commun. 2014, 50, 7150–7153. [Google Scholar] [CrossRef]
- Majcher, A.M.; Pilet, G.; Mironov, V.S.; Vostrikova, K.E. Neutral Low-Dimensional Assemblies of a Mn(III) Schiff Base Complex and Octacyanotungstate(V): Synthesis, Characterization, and Magnetic Properties. Magnetochemistry 2017, 3, 16. [Google Scholar] [CrossRef]
- Vostrikova, K.E. Low-Dimensional Heterometallic Assemblies Involving Orbitally Degenerate Cyanometallate and Displaying Slow Magnetic Dynamics. J. Magn. Magn. Mater. 2018, 459, 71–77. [Google Scholar] [CrossRef]
- Shi, L.; Shao, D.; Wei, X.; Dunbar, K.R.; Wang, X. Enhanced Single-Chain Magnet Behavior via Anisotropic Exchange in a Cyano-Bridged MoIII–MnII Chain. Angew. Chem. Int. Ed. 2020, 59, 10379–10384. [Google Scholar] [CrossRef]
- Charytanowicz, T.; Jankowski, R.; Zychowicz, M.; Chorazy, S.; Sieklucka, B. The Rationalized Pathway from Field-Induced Slow Magnetic Relaxation in CoII–WIV Chains to Single-Chain Magnetism in Isotopological CoII–WV Analogues. Inorg. Chem. Front. 2022, 9, 1152–1170. [Google Scholar] [CrossRef]
- Tan, P.; Yang, Y.; Lv, W.; Jing, R.; Cui, H.; Zheng, S.-J.; Chen, L.; Yuan, A.; Chen, X.-T.; Zhao, Y. A Cyanometallate- and Carbonate-Bridged Dysprosium Chain Complex with a Pentadentate Macrocyclic Ligand: Synthesis, Structure, and Magnetism. New J. Chem. 2022, 46, 7892–7898. [Google Scholar] [CrossRef]
- Miyasaka, H.; Madanbashi, T.; Saitoh, A.; Motokawa, N.; Ishikawa, R.; Yamashita, M.; Bahr, S.; Wernsdorfer, W.; Clérac, R. Cyano-Bridged MnIII-MIII Single-Chain Magnets with MIII=CoIII, FeIII, MnIII, and CrIII. Chem. Eur. J. 2012, 18, 3942–3954. [Google Scholar] [CrossRef] [PubMed]
- Rams, M.; Peresypkina, E.V.; Mironov, V.S.; Wernsdorfer, W.; Vostrikova, K.E. Magnetic Relaxation of 1D Coordination Polymers X2[Mn(acacen)Fe(CN)6 ], X = Ph4P+, Et4N+. Inorg. Chem. 2014, 53, 10291–10300. [Google Scholar] [CrossRef] [PubMed]
- Ferbinteanu, M.; Miyasaka, H.; Wernsdorfer, W.; Nakata, K.; Sugiura, K.; Yamashita, M.; Coulon, C.; Clérac, R. Single-Chain Magnet (NEt4)[Mn2(5-MeOsalen)2Fe(CN)6] Made of MnIII−FeIII−MnIII Trinuclear Single-Molecule Magnet with an S = 9/2 Spin Ground State. J. Am. Chem. Soc. 2005, 127, 3090–3099. [Google Scholar] [CrossRef]
- Aguilà, D.; Jeannin, O.; Fourmigué, M.; Jeon, I.-R. MnIII–FeIII Heterometallic Compounds within Hydrogen-Bonded Supramolecular Networks Promoted by an [Fe(CN)5(CNH)]2− Building Block: Structural and Magnetic Properties. Inorg. Chem. 2018, 57, 7892–7903. [Google Scholar] [CrossRef]
- Wei, X.-Q.; Qian, K.; Wei, H.-Y.; Wang, X.-Y. A One-Dimensional Magnet Based on [MoIII(CN)7]4−. Inorg. Chem. 2016, 55, 5107–5109. [Google Scholar] [CrossRef]
- Wang, K.; Xia, B.; Wang, Q.-L.; Ma, Y.; Liao, D.-Z.; Tang, J. Slow Magnetic Relaxation Based on the Anisotropic Ising-Type Magnetic Coupling between the MoIII and MnII Centers. Dalton Trans. 2017, 46, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Pinkowicz, D.; Southerland, H.I.; Avendaño, C.; Prosvirin, A.; Sanders, C.; Wernsdorfer, W.; Pedersen, K.S.; Dreiser, J.; Clérac, R.; Nehrkorn, J.; et al. Cyanide Single-Molecule Magnets Exhibiting Solvent Dependent Reversible “On” and “Off” Exchange Bias Behavior. J. Am. Chem. Soc. 2015, 137, 14406–14422. [Google Scholar] [CrossRef]
- Mironov, V.S. Origin of Dissimilar Single-Molecule Magnet Behavior of Three Mn II 2 Mo III Complexes Based on [Mo III (CN) 7 ] 4– Heptacyanomolybdate: Interplay of Mo III –CN–Mn II Anisotropic Exchange Interactions. Inorg. Chem. 2015, 54, 11339–11355. [Google Scholar] [CrossRef] [PubMed]
- Samsonenko, D.G.; Paulsen, C.; Lhotel, E.; Mironov, V.S.; Vostrikova, K.E. [MnIII(SchiffBase)]3[ReIV(CN)7], Highly Anisotropic 3D Coordination Framework: Synthesis, Crystal Structure, Magnetic Investigations, and Theoretical Analysis. Inorg. Chem. 2014, 53, 10217–10231. [Google Scholar] [CrossRef]
- Glauber, R.J. Time-Dependent Statistics of the Ising Model. J. Math. Phys. 1963, 4, 294–307. [Google Scholar] [CrossRef]
- Graham, M.J.; Zadrozny, J.M.; Shiddiq, M.; Anderson, J.S.; Fataftah, M.S.; Hill, S.; Freedman, D.E. Influence of Electronic Spin and Spin–Orbit Coupling on Decoherence in Mononuclear Transition Metal Complexes. J. Am. Chem. Soc. 2014, 136, 7623–7626. [Google Scholar] [CrossRef]
- Vostrikova, K.E. Homoleptic Osmium Cyanide Complexes: Synthesis and Perspective Application in Molecular Magnetism. In Osmium: Synthesis Characterization and Applications; Wise, G., Ed.; Nova Science Publishers: New York, NY, USA, 2015; pp. 43–78. ISBN 978-1-63483-517-6. [Google Scholar]
- David, J.; Mendizábal, F.; Arratia-Pérez, R. Electronic Structure and Molecular Properties of the Heptacyanorhenate [Re(CN)7]3- and [Re(CN)7]4- Complexes. J. Phys. Chem. A 2006, 110, 1072–1077. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Vostrikova, K.E. 1567014: Experimental Crystal Structure Determination; Cambridge Crystallographic Data Centre: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Vostrikova, K.E. 1567013: Experimental Crystal Structure Determination; Cambridge Crystallographic Data Centre: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Peresypkina, E.V.; Vostrikova, K.E. 2[Mn(Acacen)]+ + [Fe(CN)5NO]2− Polynuclear Heterobimetallic Coordination Compounds of Different Dimensionality in the Solid State. Dalton Trans. 2012, 41, 4100. [Google Scholar] [CrossRef]
- Bennett, M.V.; Long, J.R.R. New Cyanometalate Building Units: Synthesis and Characterization of [Re(CN)7]3- and [Re(CN)8]3−. J. Am. Chem. Soc. 2003, 125, 2394–2395. [Google Scholar] [CrossRef]
- Lescouëzec, R.; Toma, L.M.; Vaissermann, J.; Verdaguer, M.; Delgado, F.S.; Ruiz-Pérez, C.; Lloret, F.; Julve, M. Design of Single Chain Magnets through Cyanide-Bearing Six-Coordinate Complexes. Coord. Chem. Rev. 2005, 249, 2691–2729. [Google Scholar] [CrossRef]
- Sakamoto, F.; Sumiya, T.; Fujita, M.; Tada, T.; Tan, X.S.; Suzuki, E.; Okura, I.; Fujii, Y. T-Site Selective Photocleavage of DNA by Cationic Schiff Base Complex of Manganese(III). Chem. Lett. 1998, 27, 1127–1128. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Podgajny, R.; Nowicka, B.; Chorazy, S.; Reczyński, M.; Sieklucka, B. Magnetic Clusters Based on Octacyanidometallates. Inorg. Chem. Front. 2015, 2, 10–27. [Google Scholar] [CrossRef]
- Sukhikh, T.; Vostrikova, K. Assembly of Mn(III) Schiff Base Complexes with Heptacyanorhenate(IV). Inorganics 2017, 5, 59. [Google Scholar] [CrossRef]
- Harris, T.D.; Bennett, M.V.; Clérac, R.; Long, J.R. [ReCl4(CN)2]2−: A High Magnetic Anisotropy Building Unit Giving Rise to the Single-Chain Magnets (DMF)4MReCl4(CN)2 (M = Mn, Fe, Co, Ni). J. Am. Chem. Soc. 2010, 132, 3980–3988. [Google Scholar] [CrossRef]
- Miyasaka, H.; Ieda, H.; Re, N.; Crescenzi, R.; Floriani, C. Assembling Bi-, Tri- and Pentanuclear Complexes into Extended Structures Using a Desolvation Reaction: Synthesis, Structure, and Magnetic Properties of Manganese(III)−Schiff-Base−Hexacyanoferrate Polymeric Compounds and Their Derived Extended Structures. Inorg. Chem. 1998, 37, 255–263. [Google Scholar] [CrossRef]
- Nakamura, K.; Sasada, T. Statistical Mechanics of Classical One-Dimensional Heisenberg Ferromagnets with Single-Site Anisotropy. J. Phys. C Solid State Phys. 1978, 11, 331–343. [Google Scholar] [CrossRef]
- Miyasaka, H.; Julve, M.; Yamashita, M.; Clérac, R. Slow Dynamics of the Magnetization in One-Dimensional Coordination Polymers: Single-Chain Magnets. Inorg. Chem. 2009, 48, 3420–3437. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, V.I.; Vostrikova, K.E.; Ikorskii, V.N.; Larionov, S.V.; Sagdeev, R.Z. The Low Temperature Ferromagnet dimethanol-bis-[2,2,5,5-tetramethyl-1-oxyl-3-imidazoline-4-(3′,3′,3′-trifluoromethyl-1′-propenyl-2′-oxyato)] Cobalt(II), CoL2(CH3OH)2. Dokl. Akad. Nauk SSSR 1989, 306, 660–662. [Google Scholar]
- Ovcharenko, V.I.; Vostrikova, K.E.; Romanenko, G.V.; Ikorski, V.N.; Podberezskaya, N.V.; Larionov, S.V. Synthesis, Crystal Structure and Magnetic Properties of Di(Methanol) and Di(ethanol)-bis-2,2,5,5-tetramethyl-1-oxyl-3-imidazoline-4-(3′,3′,3′-trifluoromethyl-1-propenyl-2′-oxyato Nickel(II)—A New Type of Low Temperature Ferromagnetics. Dokl. Akad. Nauk SSSR 1989, 306, 115–118. [Google Scholar]
- Vostrikova, K.E.; Ovcharenko, V.I.; Romanenko, G.V.; Ikorskii, V.N.; Podberezskaya, N.V.; Reznikov, V.A.; Volodarskii, L.B. Synthesis, Structure and Magnetic-Properties of Bis-chelate Complexes of Zinc(II), Copper(II), Cobalt(II) and Nickel(II) with Derivatives of 3-Imidazoline Nitroxyl Radical. Russ. J. Inorg. Chem. 1992, 37, 1755–1772. [Google Scholar]
- Ovcharenko, V.I.; Vostrikova, K.E.; Podoplelov, A.V.; Sagdeev, R.Z.; Romanenko, G.V.; Ikorskii, V.N. The Synthesis and Magnetic Properties of Two Different Water-Containing Layered Polymeric NiII Complexes with a 3-Imidazoline Nitroxide. The Low Efficiency of Exchange Interactions through the OH-bridges of Water Molecules. Polyhedron 1994, 13, 2781–2792. [Google Scholar] [CrossRef]
- Ovcharenko, V.I.; Romanenko, G.V.; Ikorskii, V.N.; Musin, R.N.; Sagdeev, R.Z. Polymorphous Modifications of a Ni2+ Complex with Stable Nitroxide Involving Ni2+–O–N Bonds. Quantum-Chemical Investigation of Exchange Interactions in Heterospin Systems. Inorg. Chem. 1994, 33, 3370–3381. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Wernsdorfer, W. Classical and Quantum Magnetization Reversal Studied in Nanometer-Sized Particles and Clusters. In Advances in Chemical Physics; John and Wiley and Sons: Hoboken, NJ, USA, 2001; pp. 99–190. ISBN 9780470141786. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).