Indocyanine Green-Containing Magnetic Liposomes for Constant Magnetic Field-Guided Targeted Delivery and Theranostics
Abstract
1. Introduction
2. Materials and methods
2.1. Synthesis of Iron Oxide Magnetic Nanoparticles
2.2. Preparation of ICG-Containing Magnetic Liposomes
2.3. Characterization of MLPSICG
2.4. In Vitro MRI Relaxivity Measurements
2.5. In Vitro Fluorescent Measurements
2.6. In Vivo and Ex Vivo Fluorescent Imaging
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of MLPSICG
3.2. Magnetic Resonance Contrast Properties of MLPSICG
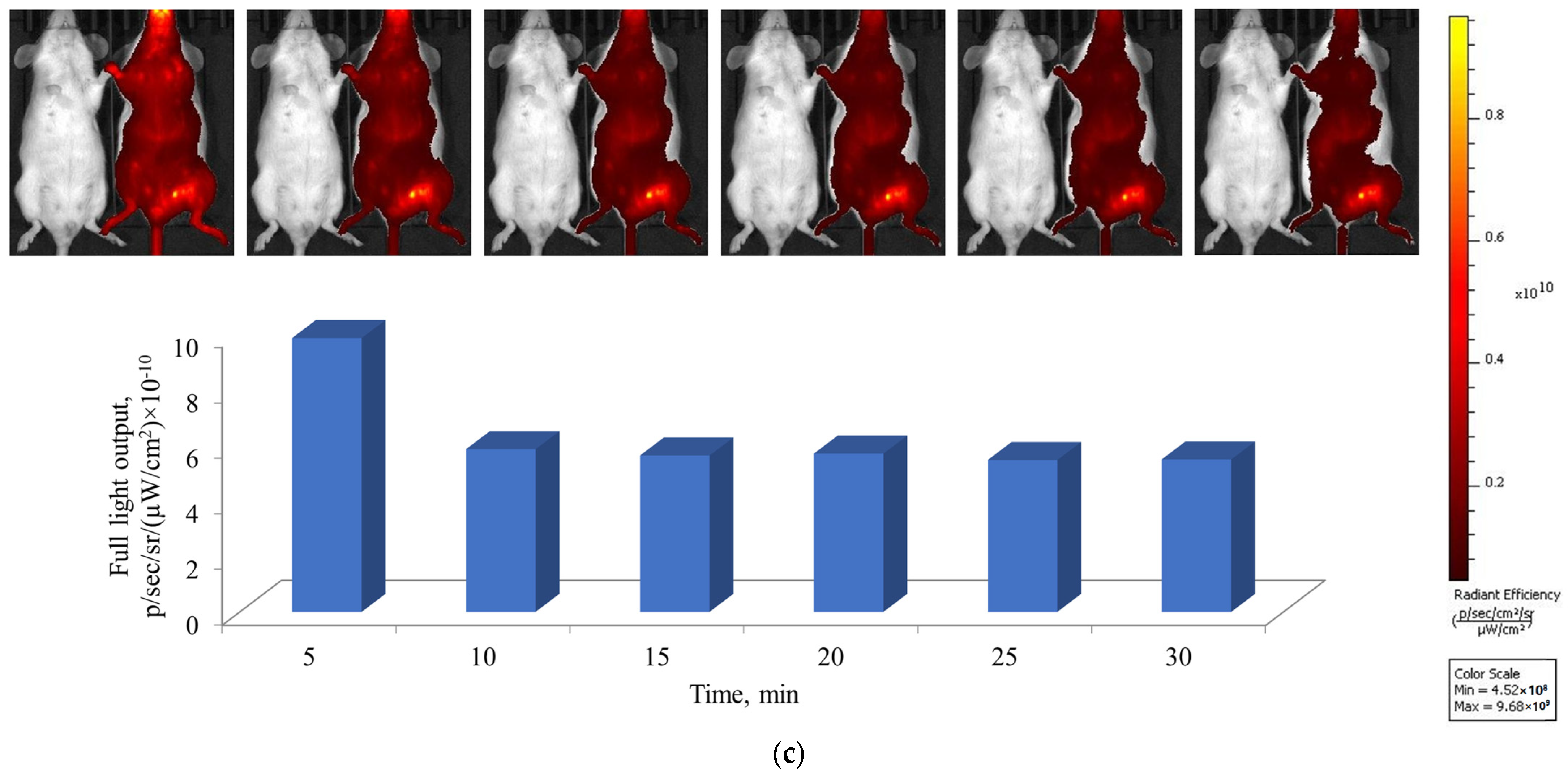
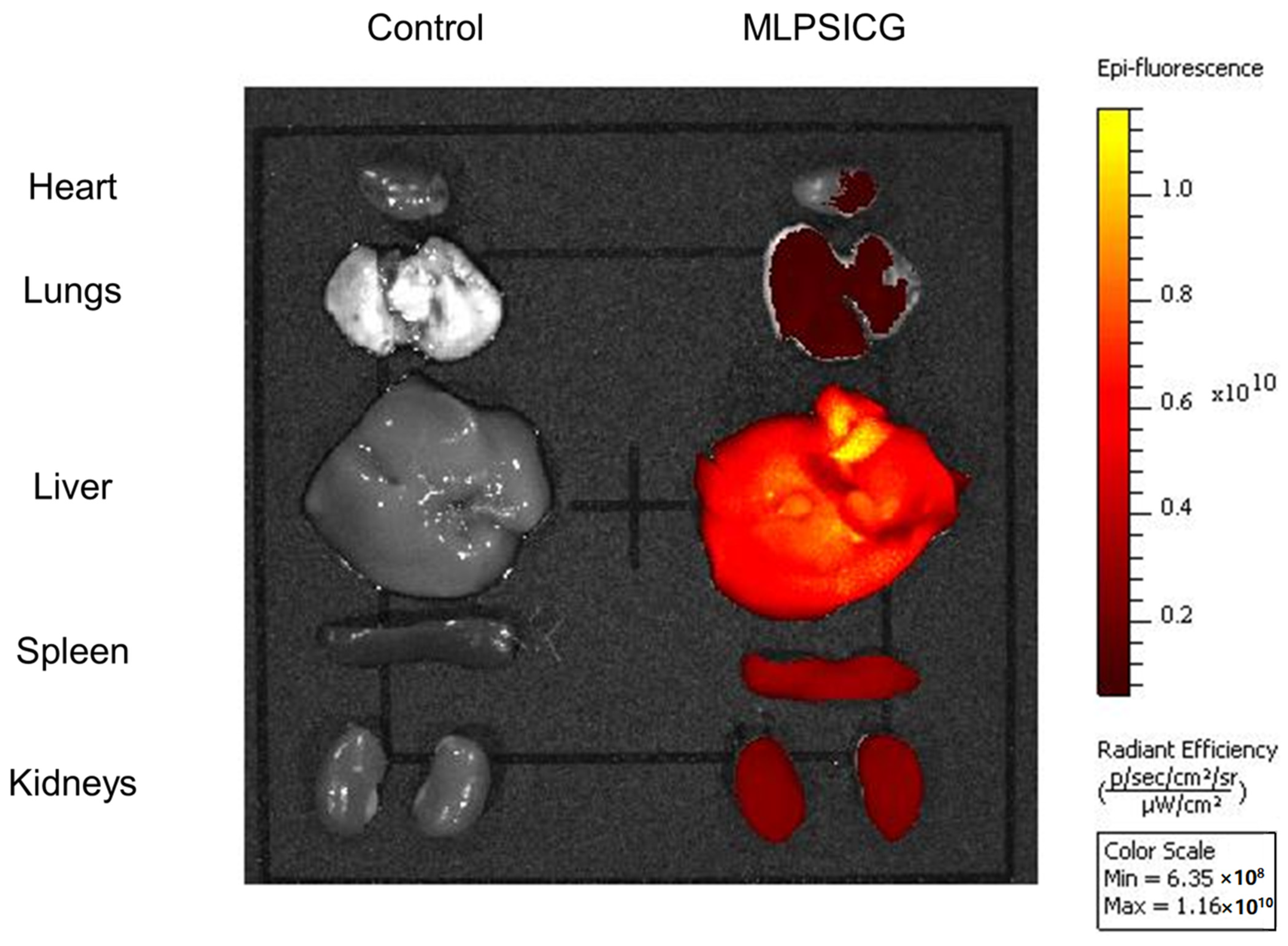
3.3. In Vitro, Ex Vivo, and In Vivo Fluorescent Properties of MLPSICG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freeman, A.I.; Mayhew, E. Targeted drug delivery. Cancer 1986, 58, 573–583. [Google Scholar] [CrossRef]
- Douglas, S.J.; Davis, S.S.; Illum, L. Nanoparticles in drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 1987, 3, 233–261. [Google Scholar] [PubMed]
- Kwon, I.K.; Lee, S.C.; Han, B.; Park, K. Analysis on the current status of targeted drug delivery to tumors. J. Control. Release 2012, 164, 108–114. [Google Scholar] [CrossRef]
- Bhatia, R.; Sharma, A.; Narang, R.K.; Rawal, R.K. Recent Nanocarrier Approaches for Targeted Drug Delivery in Cancer Therapy. Curr. Mol. Pharmacol. 2021, 14, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lin, W.; Zhu, L. Targeted Drug Delivery for the Treatment of Blood Cancers. Molecules 2022, 27, 1310. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Ahmad, A.; Khan, F.; Mishra, R.K.; Khan, R. Precision Cancer Nanotherapy: Evolving Role of Multifunctional Nanoparticles for Cancer Active Targeting. J. Med. Chem. 2019, 62, 10475–10496. [Google Scholar] [CrossRef] [PubMed]
- Galagudza, M.; Korolev, D.; Postnov, V.; Naumisheva, E.; Grigorova, Y.; Uskov, I.; Shlyakhto, E. Passive targeting of ischemic-reperfused myocardium with adenosine-loaded silica nanoparticles. Int. J. Nanomed. 2012, 7, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- de Steenwinkel, J.E.M.; van Vianen, W.; ten Kate, M.T.; Verbrugh, H.A.; van Agtmael, M.A.; Schiffelers, R.M.; Bakker-Woudenberg, I.A.J.M. Targeted drug delivery to enhance efficacy and shorten treatment duration in disseminated Mycobacterium avium infection in mice. J. Antimicrob. Chemother. 2007, 60, 1064–1073. [Google Scholar] [CrossRef]
- Nasr, S.H.; Rashidijahanabad, Z.; Ramadan, S.; Kauffman, N.; Parameswaran, N.; Zinn, K.R.; Qian, C.; Arora, R.; Agnew, D.; Huang, X. Effective atherosclerotic plaque inflammation inhibition with targeted drug delivery by hyaluronan conjugated atorvastatin nanoparticles. Nanoscale 2020, 12, 9541–9556. [Google Scholar] [CrossRef]
- Lammers, T.; Aime, S.; Hennink, W.E.; Storm, G.; Kiessling, F. Theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1029–1038. [Google Scholar] [CrossRef]
- Dasgupta, A.; Biancacci, I.; Kiessling, F.; Lammers, T. Imaging-assisted anticancer nanotherapy. Theranostics 2020, 10, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hao, X.; Liang, X.; Zhang, Q.; Zhang, C.; Zhou, G.; Shen, S.; Jia, G.; Zhang, J. Inorganic Nanomaterials as Carriers for Drug Delivery. J. Biomed. Nanotechnol. 2016, 12, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Bawab, A.A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, C.B., II; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef]
- Anilkumar, T.S.; Shalumon, K.T.; Chen, J.P. Applications of Magnetic Liposomes in Cancer Therapies. Curr. Pharm. Des. 2019, 25, 1490–1504. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Andrade, R.G.D.; Castanheira, E.M.S. Magnetoliposomes: Recent advances in the field of controlled drug delivery. Expert Opin. Drug Deliv. 2021, 18, 1323–1334. [Google Scholar] [CrossRef]
- Ferreira, R.V.; da Mata Martins, T.M.; Goes, A.M.; Fabris, J.D.; Cavalcante, L.C.D.; Outon, L.E.F.; Domingues, R.Z. Thermosensitive gemcitabine-magnetoliposomes for combined hyperthermia and chemotherapy. Nanotechnology 2016, 27, 085105. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.F.L.; Ferreira, R.V.; Pedersoli, D.C.; Paiva, P.R.P.; da Silva Cunha, P.; Goes, A.M.; Domingues, R.Z. Cytotoxic effect of thermosensitive magnetoliposomes loaded with gemcitabine and paclitaxel on human primary breast cancer cells (MGSO-3 line). J. Nanopart. Res. 2020, 22, 172. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, R.; Wu, H.; Li, M.; Zhou, G.; Ji, M. Thermoresponsive magnetoliposome encapsulating doxorubicin and high performance Ferumoxytol for effective tumor synergistic therapy in vitro. J. Drug Deliv. Sci. Technol. 2020, 57, 101677. [Google Scholar] [CrossRef]
- Kostevšek, N. A Review on the Optimal Design of Magnetic Nanoparticle-Based T2 MRI Contrast Agents. Magnetochemistry 2020, 6, 11. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Ma, J.; Li, Q.; Li, Y.; Zhou, X.; Zhao, D.; Song, H.; Chen, Q.; Zhu, X. Light/magnetic hyperthermia triggered drug released from multi-functional thermo-sensitive magnetoliposomes for precise cancer synergetic theranostics. J. Control. Release 2018, 272, 145–158. [Google Scholar] [CrossRef]
- Toro-Cordova, A.; Flores-Cruz, M.; Santoyo-Salazar, J.; Carrillo-Nava, E.; Jurado, R.; Figueroa-Rodriguez, P.A.; Lopez-Sanchez, P.; Medina, L.A.; Garcia-Lopez, P. Liposomes Loaded with Cisplatin and Magnetic Nanoparticles: Physicochemical Characterization, Pharmacokinetics, and In-Vitro Efficacy. Molecules 2018, 23, 2272. [Google Scholar] [CrossRef] [PubMed]
- Bolfarini, G.C.; Siqueira-Moura, M.P.; Demets, G.J.F.; Morais, P.C.; Tedesco, A.C. In vitro evaluation of combined hyperthermia and photodynamic effects using magnetoliposomes loaded with cucurbituril zinc phthalocyanine complex on melanoma. J. Photochem. Photobiol. B 2012, 115, 1–4. [Google Scholar] [CrossRef]
- Di Corato, R.; Béalle, G.; Kolosnjaj-Tabi, J.; Espinosa, A.; Clément, O.; Silva, A.K.A.; Ménager, C.; Wilhelm, C. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano 2015, 9, 2904–2916. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, X.; Xu, X.; Xia, X.; Wang, H.; Li, L.; Dong, W.; Ma, P.; Yang, Y.; Liu, Y.; et al. Thermal and magnetic dual-responsive liposomes with a cell-penetrating peptide-siRNA conjugate for enhanced and targeted cancer therapy. Colloids Surf. B Biointerfaces 2016, 146, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, R.; Reddy, N.G.; Prabhu, V.; Rishi, P.; Pereira, A.; Bhatt, A.; Yadav, N.K.; Chhablani, J. Indocyanine green angiography imaging findings in artery occlusions. Eur. J. Ophthalmol. 2022, 32, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Che, X. Effect of indocyanine green fluorescence angiography on preventing anastomotic leakage after colorectal surgery: A meta-analysis. Surg. Today 2021, 51, 1415–1428. [Google Scholar] [CrossRef]
- Kuo, W.S.; Chang, Y.T.; Cho, K.C.; Chiu, K.C.; Lien, C.H.; Yeh, C.S.; Chen, S.J. Gold nanomaterials conjugated with indocyanine green for dual-modality photodynamic and photothermal therapy. Biomaterials 2012, 33, 3270–3278. [Google Scholar] [CrossRef] [PubMed]
- Toropova, Y.G.; Golovkin, A.S.; Malashicheva, A.B.; Korolev, D.V.; Gorshkov, A.N.; Gareev, K.G.; Afonin, M.V.; Galagudza, M.M. In vitro toxicity of FemOn, FemOn-SiO2 composite, and SiO2-FemOn core-shell magnetic nanoparticles. Int. J. Nanomedicine 2017, 12, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, M.R.; Jaafari, M.R.; Shams, S.F.; Kashefi, M. Design and fabrication of multifunctional temperature-sensitive magnetoliposomal nanostructures. Mater. Today Commun. 2017, 13, 102–111. [Google Scholar] [CrossRef]
- Dmitrieva, M.V.; Lugen, B.; Polozkova, A.P.; Orlova, O.L.; Krasnyuk, I.I.; Krasnyuk, I.I., Jr. Selection of a cryoprotector for production a lyophilized liposomal dosage form of the indolocarbazole derivative LHS-1269. Russ. J. Biother. 2021, 20, 74–79. [Google Scholar] [CrossRef]
- Zheltova, V.; Vlasova, A.; Bobrysheva, N.; Abdullin, I.; Semenov, V.; Osmolowsky, M.; Voznesenskiy, M.; Osmolovskaya, O. Fe3O4@HAp core–shell nanoparticles as MRI contrast agent: Synthesis, characterization and theoretical and experimental study of shell impact on magnetic properties. Appl. Surf. Sci. 2020, 531, 147352. [Google Scholar] [CrossRef]
- Charles, S.W. Magnetic fluids (ferrofluids). In Magnetic Properties of Fine Particles, 1st ed.; Dormann, J.L., Fiorani, D., Eds.; North-Holland: Amsterdam, The Netherlands, 1992; pp. 267–276. ISBN 9780444597410. [Google Scholar]
- Bertotti, G. Hysteresis in Magnetism: For Physicists, Materials Scientists, and Engineers, 1st ed.; Academic Press: Amsterdam, The Netherlands, 1998; 558p, ISBN 9780120932702. [Google Scholar]
- Ryu, J.K.; Oh, J.H.; Kim, H.G.; Rhee, S.J.; Seo, M.; Jahng, G.H. Estimation of T2* Relaxation Times for the Glandular Tissue and Fat of Breast at 3T MRI System. J. Korean Soc. Magn. Reson. Med. 2014, 18, 1–6. [Google Scholar] [CrossRef][Green Version]
- Béalle, G.; Di Corato, R.; Kolosnjaj-Tabi, J.; Dupuis, V.; Clément, O.; Gazeau, F.; Wilhelm, C.; Ménager, C. Ultra magnetic liposomes for MR imaging, targeting, and hyperthermia. Langmuir 2012, 28, 11834–11842. [Google Scholar] [CrossRef]
- Marie, H.; Lemaire, L.; Franconi, F.; Lajnef, S.; Frapart, Y.M.; Nicolas, V.; Frébourg, G.; Trichet, M.; Ménager, C.; Lesieur, S. Superparamagnetic Liposomes for MRI Monitoring and External Magnetic Field-Induced Selective Targeting of Malignant Brain Tumors. Adv. Funct. Mater. 2015, 25, 1258–1269. [Google Scholar] [CrossRef]
- Garnier, B.; Tan, S.; Miraux, S.; Bled, E.; Brisson, A.R. Optimized synthesis of 100 nm diameter magnetoliposomes with high content of maghemite particles and high MRI effect. Contrast Media Mol. Imaging 2012, 7, 231–239. [Google Scholar] [CrossRef]
- Carvalho, A.; Gonçalves, M.C.; Martins, M.B.F.; Meixedo, D.; Feio, G. Relaxivities of magnetoliposomes: The effect of cholesterol. Magn. Reson. Imaging 2013, 31, 610–612. [Google Scholar] [CrossRef]
- Martínez-González, R.; Estelrich, J.; Busquets, M.A. Liposomes Loaded with Hydrophobic Iron Oxide Nanoparticles: Suitable T2 Contrast Agents for MRI. Int. J. Mol. Sci. 2016, 17, 1209. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Gonçalves, M.C.; Corvo, M.L.; Martins, M.B.F. Development of New Contrast Agents for Imaging Function and Metabolism by Magnetic Resonance Imaging. Magn. Reson. Insights 2017, 10, 1178623X17722134. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zheng, R.; Fang, X.; Wang, X.; Zhang, X.; Yang, W.; Sha, X. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials 2016, 92, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Philip, R.; Penzkofer, A.; Bäumler, W.; Szeimies, R.M.; Abels, C. Absorption and fluorescence spectroscopic investigation of indocyanine green. J. Photochem. Photobiol. A 1996, 96, 137–148. [Google Scholar] [CrossRef]
- Beziere, N.; Lozano, N.; Nunes, A.; Salichs, J.; Queiros, D.; Kostarelos, K.; Ntziachristos, V. Dynamic imaging of PEGylated indocyanine green (ICG) liposomes within the tumor microenvironment using multi-spectral optoacoustic tomography (MSOT). Biomaterials 2015, 37, 415–424. [Google Scholar] [CrossRef]
- Mérian, J.; Boisgard, R.; Bayle, P.A.; Bardet, M.; Tavitian, B.; Texier, I. Comparative biodistribution in mice of cyanine dyes loaded in lipid nanoparticles. Eur. J. Pharm. Biopharm. 2015, 93, 1–10. [Google Scholar] [CrossRef]
- Sadauskas, E.; Wallin, H.; Stoltenberg, M.; Vogel, U.; Doering, P.; Larsen, A.; Danscher, G. Kupffer cells are central in the removal of nanoparticles from the organism. Part. Fibre Toxicol. 2007, 4, 10. [Google Scholar] [CrossRef]
- Suganami, A.; Iwadate, Y.; Shibata, S.; Yamashita, M.; Tanaka, T.; Shinozaki, N.; Aoki, I.; Saeki, N.; Shirasawa, H.; Okamoto, Y.; et al. Liposomally formulated phospholipid-conjugated indocyanine green for intra-operative brain tumor detection and resection. Int. J. Pharm. 2015, 496, 401–406. [Google Scholar] [CrossRef]
- Zhao, P.; Zheng, M.; Yue, C.; Luo, Z.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Cai, L. Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipid-polymer nanoparticles. Biomaterials 2014, 35, 6037–6046. [Google Scholar] [CrossRef]
- Lee, C.H.; Cheng, S.H.; Wang, Y.J.; Chen, Y.C.; Chen, N.T.; Souris, J.; Chen, C.T.; Mou, C.Y.; Yang, C.S.; Lo, L.W. Near-Infrared Mesoporous Silica Nanoparticles for Optical Imaging: Characterization and In Vivo Biodistribution. Adv. Funct. Mater. 2009, 19, 215–222. [Google Scholar] [CrossRef]
- Huang, J.; Shu, Q.; Wang, L.; Wu, H.; Wang, A.Y.; Mao, H. Layer-by-layer assembled milk protein coated magnetic nanoparticle enabled oral drug delivery with high stability in stomach and enzyme-responsive release in small intestine. Biomaterials 2015, 39, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ashokan, A.; Gowd, G.S.; Somasundaram, V.H.; Bhupathi, A.; Peethambaran, R.; Unni, A.K.K.; Palaniswamy, S.; Nair, S.V.; Koyakutty, M. Multifunctional calcium phosphate nano-contrast agent for combined nuclear, magnetic and near-infrared in vivo imaging. Biomaterials 2013, 34, 7143–7157. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation kinetics of indocyanine green in aqueous solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Tse, B.W.C.; Yang, H.; Thorling, C.A.; Liu, Y.; Touraud, M.; Chouane, J.B.; Liu, X.; Roberts, M.S.; et al. Indocyanine green-incorporating nanoparticles for cancer theranostics. Theranostics 2018, 8, 1227–1242. [Google Scholar] [CrossRef]
- Sheng, Z.; Hu, D.; Zheng, M.; Zhao, P.; Liu, H.; Gao, D.; Gong, P.; Gao, G.; Zhang, P.; Ma, Y.; et al. Smart human serum albumin-indocyanine green nanoparticles generated by programmed assembly for dual-modal imaging-guided cancer synergistic phototherapy. ACS Nano 2014, 8, 12310–12322. [Google Scholar] [CrossRef]
- Zheng, M.; Zhao, P.; Luo, Z.; Gong, P.; Zheng, C.; Zhang, P.; Yue, C.; Gao, D.; Ma, Y.; Cai, L. Robust ICG theranostic nanoparticles for folate targeted cancer imaging and highly effective photothermal therapy. ACS Appl. Mater. Interfaces 2014, 6, 6709–6716. [Google Scholar] [CrossRef]
- Yan, F.; Wu, H.; Liu, H.; Deng, Z.; Liu, H.; Duan, W.; Liu, X.; Zheng, H. Molecular imaging-guided photothermal/photodynamic therapy against tumor by iRGD-modified indocyanine green nanoparticles. J. Control. Release 2016, 224, 217–228. [Google Scholar] [CrossRef]
- Zhao, P.; Zheng, M.; Luo, Z.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Ma, Y.; Cai, L. NIR-driven Smart Theranostic Nanomedicine for On-demand Drug Release and Synergistic Antitumour Therapy. Sci. Rep. 2015, 5, 14258. [Google Scholar] [CrossRef]
- Galagudza, M.M.; Korolev, D.V.; Sonin, D.L.; Alexandrov, I.V.; Minasian, S.M.; Postnov, V.N.; Kirpicheva, E.B.; Papayan, G.V.; Uskov, I.S. Passive and active target delivery of drugs to ischemic myocardium. Bull. Exp. Biol. Med. 2011, 152, 105–107. [Google Scholar] [CrossRef]
- Lipinski, M.J.; Albelda, M.T.; Frias, J.C.; Anderson, S.A.; Luger, D.; Westman, P.C.; Escarcega, R.O.; Hellinga, D.G.; Waksman, R.; Arai, A.E.; et al. Multimodality imaging demonstrates trafficking of liposomes preferentially to ischemic myocardium. Cardiovasc. Revasc. Med. 2016, 17, 106–112. [Google Scholar] [CrossRef]
- Paulis, L.E.; Geelen, T.; Kuhlmann, M.T.; Coolen, B.F.; Schäfers, M.; Nicolay, K.; Strijkers, G.J. Distribution of lipid-based nanoparticles to infarcted myocardium with potential application for MRI-monitored drug delivery. J. Control. Release 2012, 162, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Scherlag, B.S.; Dormer, K.; Rutel, I.; Huang, B.; Zhou, X.; Kuriakose, A.E.; Nguyen, K.K.; Po, S. Targeted Ganglionated Plexi Denervation Using Magnetic Nanoparticles Carrying Calcium Chloride Payload. JACC Clin. Electrophysiol. 2018, 4, 1347–1358. [Google Scholar] [CrossRef] [PubMed]

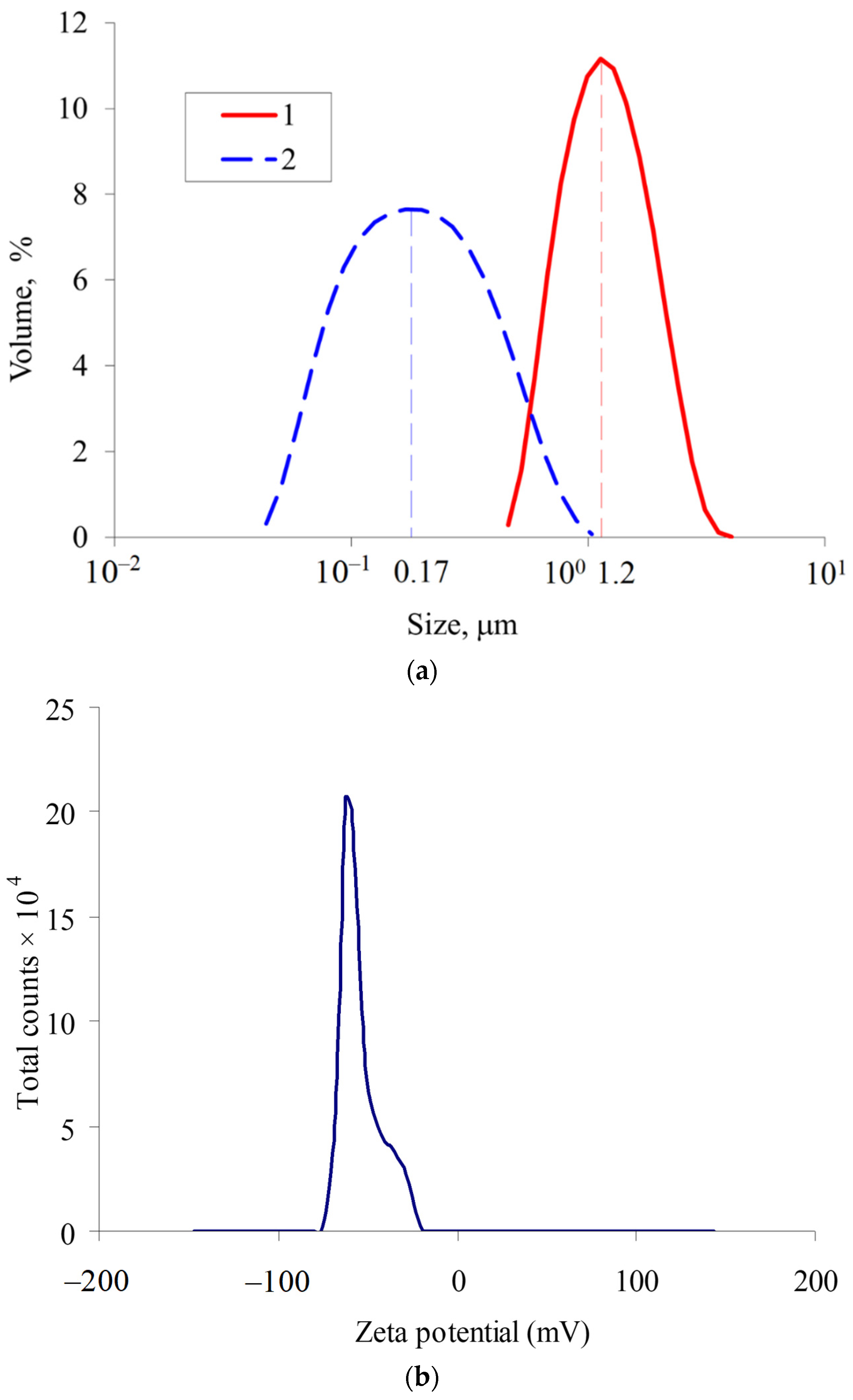
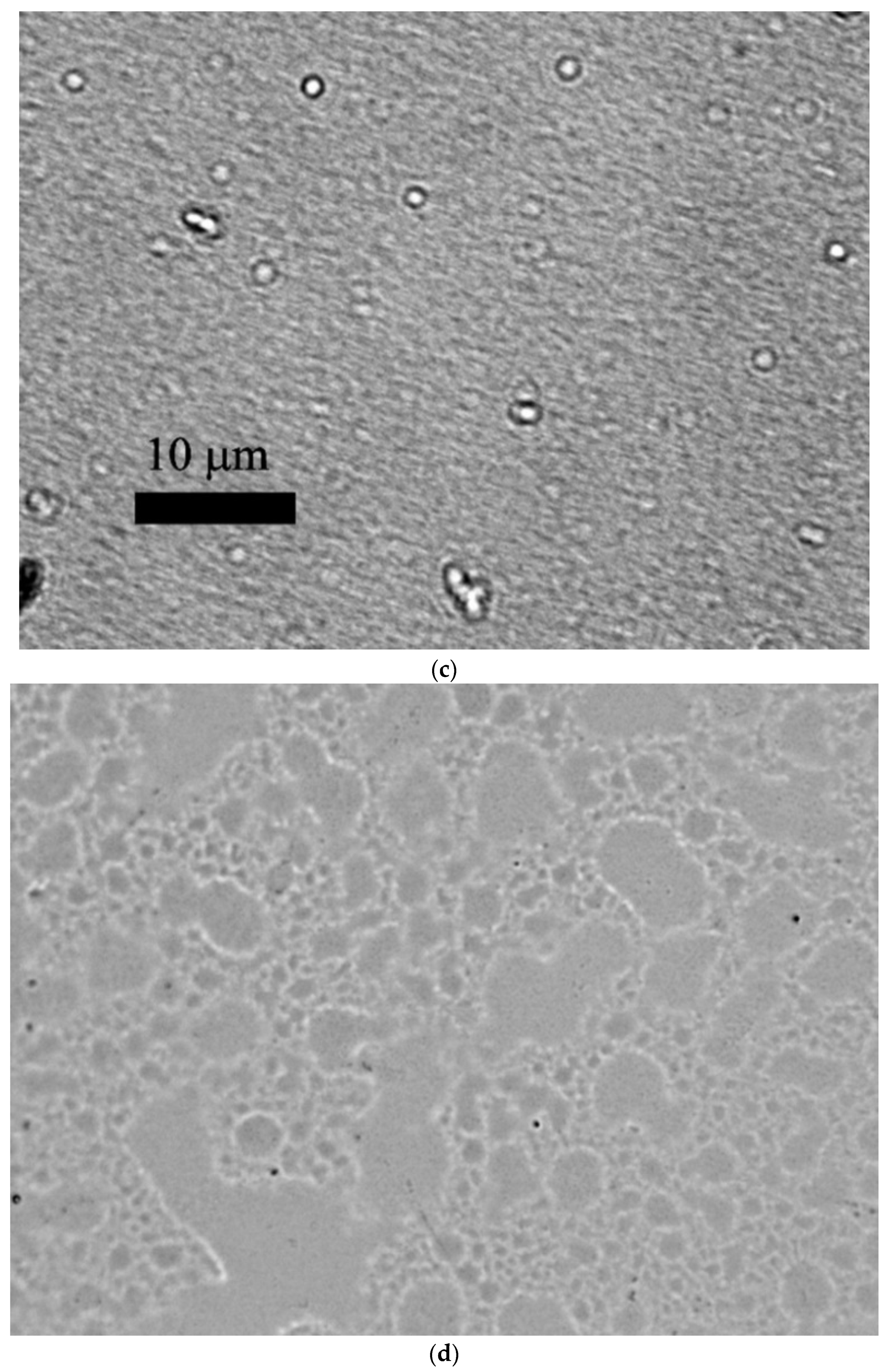



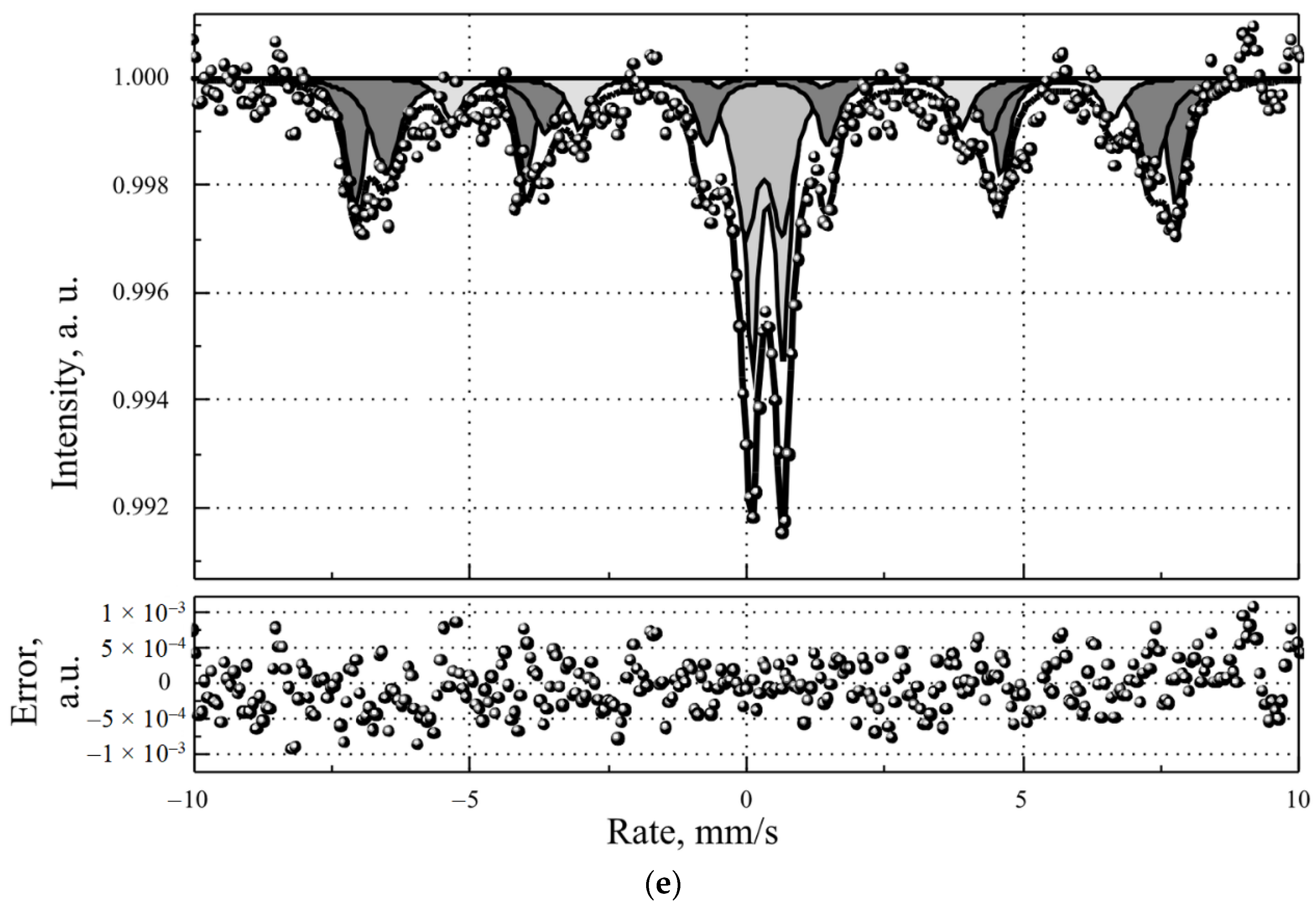

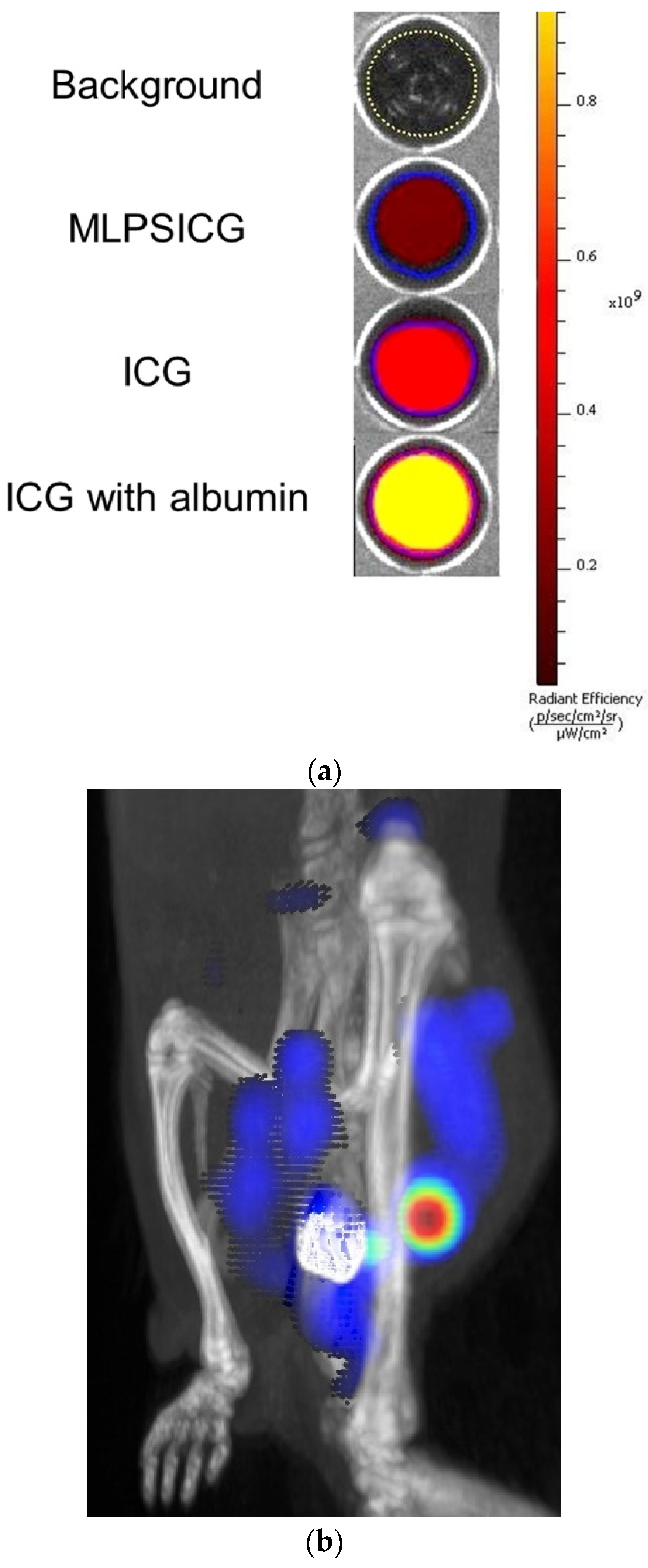
| N | Substance | Chem. Shift mm/s | Quadrupole Splitting mm/s | Ultrafine Magnetic Field, Tl | Proportion of Iron Atoms % |
|---|---|---|---|---|---|
| 1 | Fe+3 1st state | 0.37 ± 0.02 | 0.56 ± 0.05 | — | 20.3 |
| 2 | Fe+3 2nd state | 0.31 ± 0.07 | 0.67 ± 0.12 | — | 17.3 |
| 3 | Fe3O4 (A) | 0.32 ± 0.02 | 0.04 ± 0.04 | 46.108 ± 0.166 | 23.4 |
| 4 | Fe3O4 (B) | 0.38 ± 0.03 | 0.03 ± 0.06 | 43.068 ± 0.307 | 28.0 |
| 5 | α–FeOOH | 0.53 ± 0.05 | 0.21 ± 0.11 | 37.099 ± 0.434 | 11.0 |
| Organs | Full Light Output, p/sec/sr/(µW/cm2) × 10−10 | ||
|---|---|---|---|
| Control | MLPSICG | Difference | |
| Heart | 0.02 | 0.23 | 0.21 |
| Lungs | 0.10 | 1.53 | 1.43 |
| Liver | 0.39 | 28.90 | 28.51 |
| Spleen | 0.04 | 2.10 | 2.06 |
| Kidneys | 0.03 | 1.44 | 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korolev, D.V.; Shulmeyster, G.A.; Istomina, M.S.; Nikiforov, A.I.; Aleksandrov, I.V.; Semenov, V.G.; Galagudza, M.M. Indocyanine Green-Containing Magnetic Liposomes for Constant Magnetic Field-Guided Targeted Delivery and Theranostics. Magnetochemistry 2022, 8, 127. https://doi.org/10.3390/magnetochemistry8100127
Korolev DV, Shulmeyster GA, Istomina MS, Nikiforov AI, Aleksandrov IV, Semenov VG, Galagudza MM. Indocyanine Green-Containing Magnetic Liposomes for Constant Magnetic Field-Guided Targeted Delivery and Theranostics. Magnetochemistry. 2022; 8(10):127. https://doi.org/10.3390/magnetochemistry8100127
Chicago/Turabian StyleKorolev, Dmitry V., Galina A. Shulmeyster, Maria S. Istomina, Alexey I. Nikiforov, Ilia V. Aleksandrov, Valentin G. Semenov, and Michael M. Galagudza. 2022. "Indocyanine Green-Containing Magnetic Liposomes for Constant Magnetic Field-Guided Targeted Delivery and Theranostics" Magnetochemistry 8, no. 10: 127. https://doi.org/10.3390/magnetochemistry8100127
APA StyleKorolev, D. V., Shulmeyster, G. A., Istomina, M. S., Nikiforov, A. I., Aleksandrov, I. V., Semenov, V. G., & Galagudza, M. M. (2022). Indocyanine Green-Containing Magnetic Liposomes for Constant Magnetic Field-Guided Targeted Delivery and Theranostics. Magnetochemistry, 8(10), 127. https://doi.org/10.3390/magnetochemistry8100127






