Abstract
A series of heterometallic GdIII-VIV compounds were synthesized by the reaction of VOSO4·3H2O with cyclobutane-1,1-dicarboxylic acid salts M2(cbdc) (M = Na, Rb, Cs). The new compounds were formed by [Gd(VO)2(cbdc)4(H2O)8]− trinuclear anionic units that were similar in composition but differed in structure, depending on the nature of the alkali metal cation incorporated in the crystal structure of the compound. In the case of Na+, the {GdV2}− units were characterized by identical V···Gd distances and were linked into the 1D-polymeric chain [NaGd(VO)2(cbdc)4(H2O)10]n (1). In the systems with Rb+ and Cs+, the V···Gd distances were different, and the {GdV2}− units were linked into the 3D-framework {[RbGd(VO)2(cbdc)4(H2O)10]·2.5H2O}n (2) and the octanuclear molecule {[CsGd(VO)2(cbdc)4(H2O)11]·5H2O}2 (3), respectively. According to dc-magnetic measurements, the VIV and GdIII ions were ferromagnetically coupled in compound 1 (JVGd = 0.163 ± 0.008 cm−1), while in compounds 2 and 3, ferro- and weak antiferromagnetic exchange interactions were observed (JVGd = 0.989 ± 0.028 and −0.089 ± 0.008 cm−1 for 2, 0.656 ± 0.009 and −0.050 ± 0.004 cm−1 for 3). Analysis of the EPR spectra of 1 revealed the presence of weak magnetic anisotropy of GdIII ions (D ~ 0.08 cm−1 and E/D ~ 0.1–0.15). Ac-susceptibility measurements showed an occurrence the field-induced slow relaxation of magnetization in 1–3.
1. Introduction
The interest in the directed synthesis of heterometallic compounds where 3d and 4f metal ions are combined in a single molecule is caused by the wide range of physicochemical properties they exhibit, including luminescent properties [1,2], molecular magnetism [3,4], magnetocaloric effect [5,6,7,8,9], and catalytic activity [10,11,12]. In addition, studies on such heterometallic compounds are of fundamental concern in order to elucidate the exchange interactions between paramagnetic centers of different nature, 3d and 4f metal ions, and the effect of these interactions on the dynamic characteristics of a single-molecule magnet (SMM), for example, the magnitude of the energy barrier to magnetization reversal Δeff. From this point of view, the most convenient objects for studies by physicochemical methods include systems with a 3d center containing one unpaired electron (S = 1/2, quantum spin), namely a CuII (3d9) or VIV (3d1) ion. To date, a number of CuII–4f systems, from molecular complexes to coordination polymers, have already been well studied [8,13,14,15,16,17,18]. It has been shown that this class of compounds can be promising as magnetic coolers [4,19,20,21,22], and ferromagnetic interactions are often the prevailing type of spin-spin interactions in them [23,24,25,26]. Much less attention was paid to the synthesis of VIV–4f compounds and only a few studies on their magnetic behavior are known [27,28,29,30,31,32,33,34,35].
As it is known, the GdIII ion lacks intrinsic magnetic anisotropy, which is observed in DyIII, TbIII, and other heavy 4f-ions. Because of this, the GdIII ion by itself is ineffective in the design of SMM or single-ion magnets (SIM). Nevertheless, there are examples of GdIII complexes exhibiting slow magnetic relaxation, which is due to the phonon bottleneck effect [36,37,38,39,40,41,42,43,44] or by the presence of weak anisotropy caused by orbital nondegeneracy of the ground state 8S [44].
We previously synthesized and characterized a series of heterometallic LnIII-VIV compounds based on cyclobutane-1,1-dicarboxylate anions, in which three structural types were identified, depending on the nature (ionic radius) of LnIII [45]. A further study of this system showed that the nature of the alkali metal introduced into the reaction as a salt of a dicarboxylic acid also affected the structure of the compound formed. This work aimed at varying the alkali metal cation MI (MI = Na, Rb, Cs) in the MI-GdIII-VIV system and studying its effect on the composition, structure, and magnetic properties of the resulting compounds.
2. Results and Discussion
2.1. Synthesis
The reaction of VOSO4·3H2O, Na2(cbdc), and Gd(NO3)3·6H2O in a 1:2:1 ratio in water afforded crystals of the 1D-polymeric compound [NaGd(VO)2(cbdc)4(H2O)10]n (1). Replacement of sodium cyclobutane-1,1-dicarboxylate Na2(cbdc) with Rb2(cbdc) in a similar reaction gave the 3D-polymer {[RbGd(VO)2(cbdc)4(H2O)10]·2.5H2O}n (2), while the reaction with Cs2(cbdc) resulted in the crystallization of the octanuclear complex {[CsGd(VO)2(cbdc)4(H2O)11]·5H2O}2 (3) (Scheme 1).
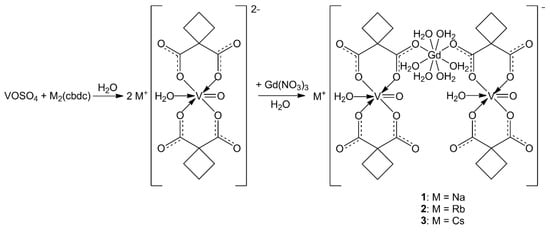
Scheme 1.
Scheme illustrating the formation of compounds 1–3.
2.2. Crystal Structure Description
Compounds 1 and 2 crystallize in monoclinic space group C2/c. Compound 2 contained 2.5 solvate water molecules. Compound 3 crystallizes in triclinic space group P with 5 solvate water molecules. The basic structural unit of all the three compounds is the [Gd(VO)2(cbdc)4(H2O)8]− ({GdV2}−) anionic complex with a trinuclear metal core. In this unit, gadolinium is the central atom, while the vanadium atoms are terminal ones and belong to bis-chelate moieties {(VO)(cbdc)2(H2O)} (see Scheme 1). In complex 1, the vanadium atoms were crystallographycally equivalent (Figure 1), while the terminal vanadium atoms, V1 and V2, in compounds 2 and 3 were nonequivalent (Figure 2 and Figure 3). The coordination polyhedron of vanadium (VO6) is a distorted octahedron formed by six O atoms. Four O atoms of two chelating cbdc2− anions lie in the equatorial plane of the polyhedron, while the axial positions are occupied by O atoms of the vanadyl moiety {V=O} and the water molecule. The central gadolinium atom in {GdV2}− was linked to two terminal vanadium atoms due to bridging coordination of two O atoms of cbdc2− anions in the {(VO)(cbdc)2(H2O)} moieties (Table 1 and Tables S1–S3).
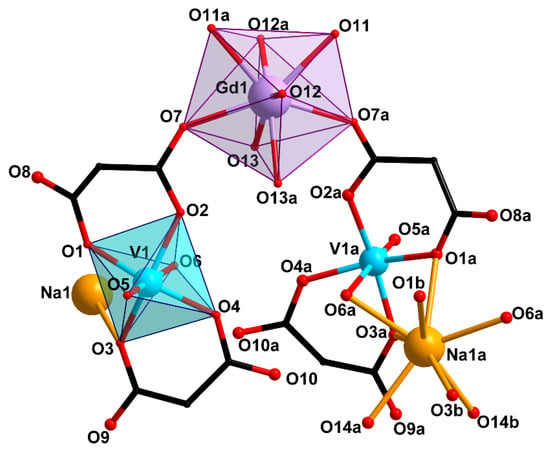
Figure 1.
Structure of trinuclear unit {GdV2}− and Na atom environment in compound 1 (H atoms and cyclic fragments of the carboxylate ligands are not shown). [Symmetry codes: (a) 1 − x, y, 0.5 − z; (b) 1 + x, y, z].
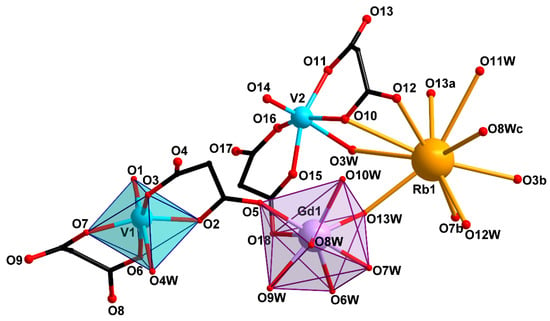
Figure 2.
Structure of trinuclear unit {GdV2}− and Rb atom environment in compound 2 (H atoms and cyclic fragments of the carboxylate ligands are not shown). [Symmetry codes: (a) 1 − x, y, 1.5 − z; (b) −0.5 + x, 0.5 + y, z; (c) 1 − x, 2 − y, 1 − z].
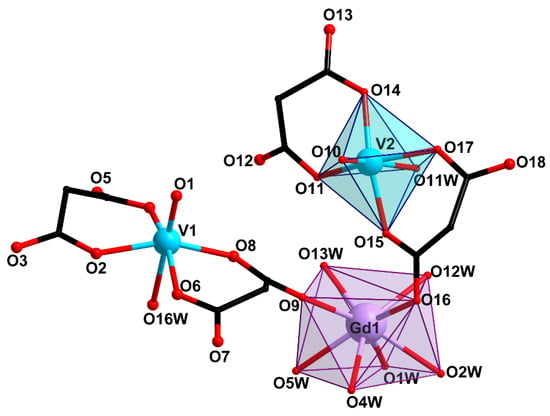
Figure 3.
Structure of trinuclear unit {GdV2}− in compound 3 (H atoms and cyclic fragments of the carboxylate ligands are not shown).

Table 1.
Selected bond lengths d in complexes 1–3.
The coordination polyhedra of gadolinium (GdO8) in 1–3 were analyzed using the SHAPE v2.1 program [46]. In 1, the GdO8 coordination polyhedron corresponded to a triangular dodecahedron [47]) formed by six O atoms of water molecules (O11, O12, O13) and two O atoms of carboxylate groups (O7) (Figure 1, Table S4). The GdO8 polyhedron in 2 can be described as a biaugmented (two-capped) trigonal prism [47]) with the basal planes formed by the O5, O8W, and O10W atoms on the one side, and the O18, O6W, and O13W atoms on the other side. The caps of the trigonal prism were formed by the O7W and O9W atoms located above the O6W–O8W–O10W–O13W and O5–O18–O6W–O8W faces, respectively (Figure 2, Table S4). The geometry of the GdO8 coordination polyhedron in 3 was closest to a tetragonal antiprism [47] with the basal planes formed by eight O atoms: four O atoms of water molecules (O1W, O2W, O4W, O5W) on the one side, and two O atoms of carboxylate groups (O9, O16) and two O atoms of water molecules (O12W, O13W) on the other side (Figure 3, Table S4).
In 1, the {GdV2}− units were linked in a 1D-polymeric chain by Na atoms coordinating the O atoms of carboxylate groups (O1 and O3) and water molecules (O6) belonging to the {(VO)(cbdc)2(H2O)} moieties of the neighboring {GdV2}− units (Figure 4, Table 1 and Table S1). The shortest V···V distance was observed between two metal atoms of the adjacent {GdV2}− units (6.234 Å). The water molecules coordinated to Gd (O11, O12, O13), V (O6), Na (O14) atoms, the O2, O3, O7, O8, O9, O10 carboxylate atoms, the O5 atoms of {V=O} moiety, and the CH2 groups of the cyclobutane moieties of the ligand were involved in the formation of intra- and intermolecular hydrogen bonds which additionally stabilized the crystal structure of 1 (Table S5).
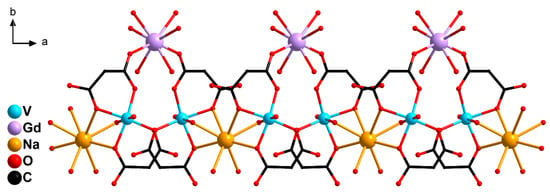
Figure 4.
A fragment of 1D-polymeric chain of 1: {GdV2}− units linked by Na+ cations (H atoms and cyclic fragments of the carboxylate ligands are not shown).
In 2, the {GdV2}− units were linked into a 3D-polymeric structure by Rb atoms bonding the O3, O7 carboxylate atoms of the {(V(1)O)(cbdc)2(H2O)} moieties and the O10, O12, O13 carboxylate atoms of the {(V(2)O)(cbdc)2(H2O)} moieties, as well as the water molecules coordinated to the V2 (O3W) and Gd (O8W, O13W) atoms (Figure 5, Table 1 and Table S2). Unlike the {[KGd(VO)2(cbdc)4(H2O)9]·3.5H2O}n structure obtained previously, in which the K atom additionally coordinated only one water molecule [45], the Rb atom in the structure of 2 bound two water molecules (O12W, O13W). The crystal structure of 2 was additionally stabilized by a network of hydrogen bonds formed by carboxylate O atoms (O10 and O17), water molecules coordinated to the V (O3W and O4W) and Gd (O9W, O10W, O13W) atoms, as well as solvate water molecules (O1W, O2W, O5W) (Table S6).
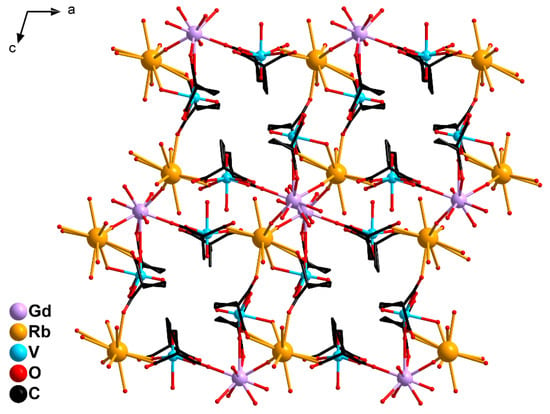
Figure 5.
A fragment of 3D-polymeric structure of 2: {GdV2}− units linked by Rb+ cations (H atoms are not shown).
In 3, two Cs atoms were bound with two {GdV2}− units via the O atoms of the carboxylate groups (O4, O8 and O12) and the water molecules coordinated to the V1 (O16W) and Gd (O13W) atoms. The Cs atoms connected the {GdV2} moieties into a dimer due to coordination of the carboxylate O13 atoms of the neighboring {(V(2)O)(cbdc)2(H2O)} moieties. Each Cs atom additionally coordinated three water molecules (O9W, O14W, O15W) (Figure 6, Table 1 and Table S3). The crystal structure of 3 was stabilized by an extensive network of hydrogen bonds formed with participation of all coordinated and solvate water molecules and the O atoms of carboxylate groups (O2, O3, O5, O6, O7, O11, O12, O13, O14, O18) (Table S7).
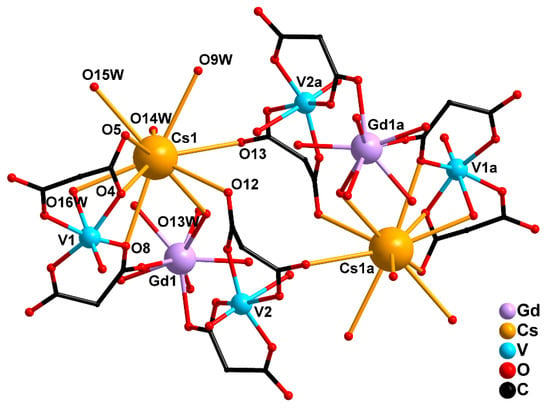
Figure 6.
Octanuclear structure of 3: two {GdV2}− units linked by two Cs+ cations (H atoms and cyclic fragments of the carboxylate ligands are not shown). [Symmetry code: (a) 2 − x, 2 − y, 1 − z].
Since the radius of Cs+ ion is larger than that of Rb+, the former participated in linking not only with neighboring {GdV2}− anions but also with nonequivalent bis-chelate moieties {(VO)(cbdc)2(H2O)} within one {GdV2}− unit. As a result, a decrease in the V···V distance (6.077 Å) was observed in the {GdV2}− unit of 3 in comparison with that in 1 (6.391 Å) and 2 (7.010 Å), along with a sharp decrease in the dimensionality from 3D-polymeric to molecular structure. In addition, despite the structural similarity of the heterometallic trinuclear anions {GdV2}− in all the three compounds, the presence in their structures of different alkali metal cations involved in linking the {GdV2} moieties to each other affected not only the structure dimensionality but also the V···Gd distances within the {GdV2} moieties (Figure 7).
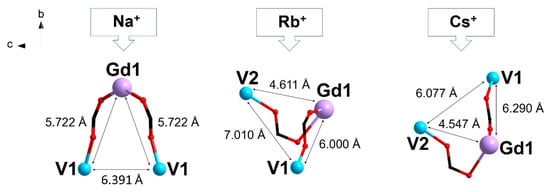
Figure 7.
Effect of the alkali metal cation M+ on the structure of the trinuclear anion {GdV2}− and the V···Gd, V···V distances in compounds 1–3.
2.3. EPR Spectroscopy
Figure 8 shows the X- and Q-band EPR spectra of solid samples 1–3 vs. temperature. The spectra of 2 and 3 showed negligible changes in the temperature range of 5–295 K. The spectra of 1 did exhibit some changes in the spectral shape between 295 and 80 K; however, these changes were insignificant and might be due to a variation in the linewidth of the multiple overlapped signals. Therefore, we assumed that the exchange couplings in the triad were rather small, as it is typical of GdIII complexes and as magnetic susceptibility measurements confirm. Therefore, all paramagnetic centers should contribute to the resulting EPR spectra. Judging by the total spectrum width and shape, we concluded that the EPR spectrum was dominated by S = 7/2 signals of GdIII with noticeable zero-field splitting. The signals of two VIV ions with S = 1/2 had narrower spectra but much smaller integral intensity; therefore, they were buried in the much stronger signal of GdIII (see Supplementary Materials for the supportive simulation).
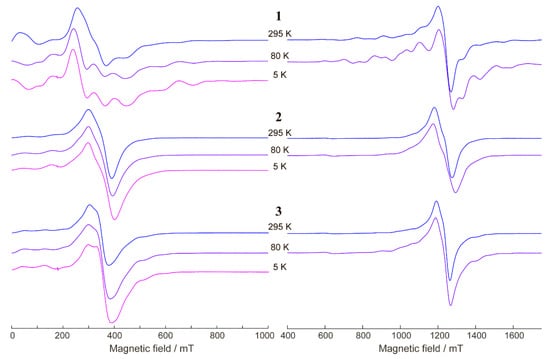
Figure 8.
X-band (left) and Q-band (right) EPR spectra of 1–3 (as indicated) at T = 295, 80, and 5 K. νmw ≈ 9.75 GHz at the X-band and νmw ≈ 34.20 GHz at the Q-band.
The zero-field splitting values D and E for 1 can be estimated simultaneously from the simulation of X/Q-band spectra (see Supplementary Materials). It was found that D was around ~0.08 cm−1 and E/D ~ 0.1–0.15. For the two other compounds, 2 and 3, the EPR spectra were less resolved (perhaps due to more efficient intermolecular exchange interactions), but it is obvious that the zero-field splitting had comparable or even smaller values.
2.4. Magnetic Properties
2.4.1. DC Susceptibility Measurements
The magnetic properties of compounds 1–3 were investigated by measuring the temperature dependences of molar magnetic susceptibility (χM) in the range of 2–300 K in a 5000 Oe dc-magnetic field. The experimental χMT values at 300 K for 1–3 (8.64, 8.74, and 8.89 cm3 K mol−1, respectively, see Figure 9, Figure 10 and Figure 11) were similar to the spin-only value χMTtheor = 8.74 cm3 K mol−1 expected for two non-interacting VIV ions (S = 1/2) and one GdIII ion (S = 7/2). The χMT(T) dependencies for 2 and 3 were similar in shape. The χMT values for 2 and 3 monotonically increased with decreasing temperature, reaching 8.99 and 9.03 cm3 K mol−1, respectively, at 45 K, then sharply grew to 9.75 and 9.71 cm3 K mol−1 at 4 K, and finally decreased to 8.63 and 9.03 cm3 K mol−1 at 2 K (Figure 9 and Figure 10). The χMT value for 1 monotonically increased with cooling, reached 8.94 cm3 K mol−1 at 8 K, and then sharply decreased to 8.05 cm3 K mol−1 at 2 K (Figure 11).
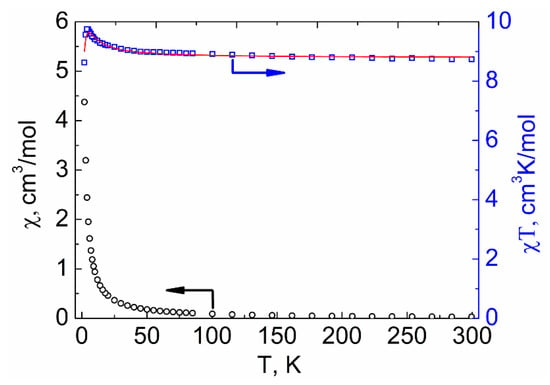
Figure 9.
Temperature dependencies of the χMT product (□) and χM (○) for compound 2. The red line shows the values calculated using PHI [48] program.
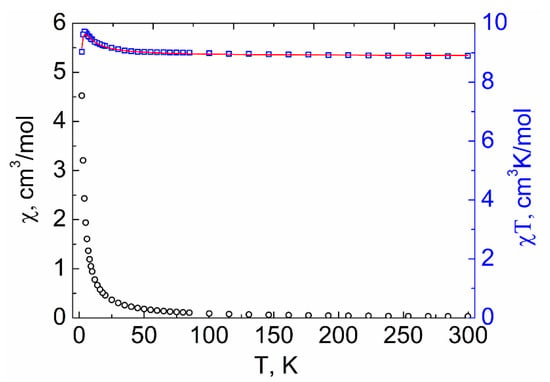
Figure 10.
Temperature dependencies of the χMT product (□) and χM (○) for compound 3. The red line shows the values calculated using PHI [48] program.
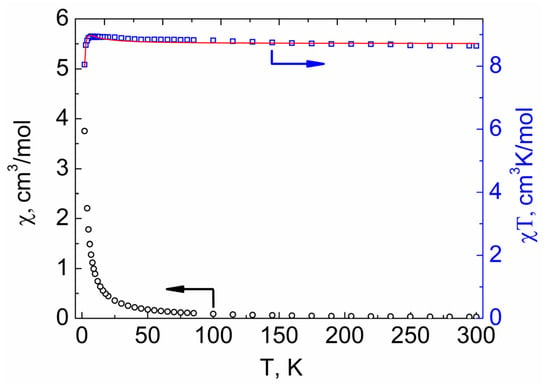
Figure 11.
Temperature dependencies of the χMT product (□) and χM (○) for compound 1. The red line shows the values calculated using PHI [48] program.
The interpretation of the experimental plots of χMT vs. T for complexes 2 and 3 was carried out using PHI program [48] (for details see Supplementary Materials). The parameter of isotropic exchange between VIV ions was neglected. The best fits of the experimental plots of χMT vs. T for 2 and 3 to the theoretically calculated curves were achieved with the following parameters: J1 (Gd···V1) = 0.989 ± 0.028 cm−1, J2 (Gd···V2) = −0.089 ± 0.008 cm−1, and g = 2.01 for 2 (Figure 9) and J1 (Gd···V1) = 0.656 ± 0.009 cm−1, J2 (Gd···V2) = −0.050 ± 0.004 cm−1, and g = 2.03 for 3 (Figure 10).
In order to clarify the correctness of the assignment of the J values to the V1-Gd and V2-Gd fragments in the compounds 2 and 3, using a DFT method, we computationally considered two separate GdIII{VO(cbdc)2(H2O)8} units of 2 and 3 and analyzed the forms of natural orbitals responsible for the character of the exchange interactions in VIV-GdIII pairs of different type. Following from the shapes of natural orbitals (Figure S24), there was an overlapping of the orbitals of the gadolinium(III) and oxygen atom of the vanadium fragment {V(2)O(cbdc)2(H2O), which provided an exchange channel leading to antiferromagnetic coupling in V(2)IV-GdIII pairs. In contrast, the orbitals of gadolinium and the closest oxygen atoms of {V(1)O(cbdc)2(H2O)} did not overlap, which resulted in ferromagnetic interactions between V(1) and Gd.
For the interpretation of the temperature dependence of χMT for complex 1, in addition to considering the magnetic exchange between GdIII and VIV ions, it is also necessary to take into account the exchange between VIV ions belonging to neighboring {GdV2}− units, which can interact through bridging Na+ ions (for details see Supplementary Materials). The best fit of the experimental plot of χMT vs. T for 1, obtained by the use of PHI [48] program, was achieved with the following parameters: J1 (Gd···V) = 0.163 ± 0.008 cm−1, J2 (V···V) = −1.095 ± 0.046 cm−1, g = 2.01.
There are several examples of GdIII-VIVO complexes in the literature for which the magnetic properties have been analyzed. The exchange parameters JGdV obtained for 1–3 were within the range of known values (Table 2) where interactions between paramagnetic centers can be ferro- or antiferromagnetic, as determined by the nature of the bridging moieties and the interatomic distance.

Table 2.
Magnetic properties of known GdIII-VIV complexes.
2.4.2. AC Susceptibility Measurements
It is known that slow relaxation of the magnetization effect can be observed both for GdIII- [36,37,38,39,40,41,42,43,44] and VIV-based complexes [49,50,51,52,53,54]. This fact inspired us to perform ac-magnetic measurements for heterometallic GdIII-VIV compounds 1–3. As a result, all the synthesized complexes were found to exhibit field-induced slow relaxation of magnetization. However, in the zero dc-magnetic field, no out-of-phase signal was detected for 1–3 (Figures S13–S16). To suppress the quantum tunneling of the magnetization process (QTM), which appeared to be the most likely reason for the absence of relaxation under the zero dc-field, the subsequent ac-measurements were carried out in dc-fields from 500 to 5000 Oe. In this way, we managed to quantify the optimal dc-field values for 1–3. The highest relaxation times were achieved by applying the optimal fields of 5000 Oe for 1 (Figures S13 and S14), and 2500 Oe for 2 (Figure S15) and 3 (Figure S16). The optimal field was estimated at 8 K for 1, in contrast to 2 and 3 (2 K), because the low-temperature magnetic behavior of 1 under ac-field was quite difficult to explain.
To further study the relaxation dynamics, the isotherms of the in-phase and out-of-phase components of ac-magnetic susceptibility were measured for 1–3 under optimal Hdc fields (Figures S19–S21). To produce the temperature dependencies of relaxation time (τ vs. 1/T) for 1–3, the χ″(ν) isotherms were approximated by the generalized Debye model. The plots of τ vs. 1/T obtained in this way (Figure 12) were approximated by the equations corresponding to different relaxation mechanisms and their combinations (see Tables S14–S16 in Supplementary Material). The best-fit parameters for the approximations of τ vs. 1/T plots for 1–3 are presented in Table 3. In the high-temperature range, all the τ vs. 1/T dependences were approximated using only the Orbach relaxation mechanism (τ = τ0∙exp{Δeff/kBT}) to estimate the value of the effective anisotropic barrier.
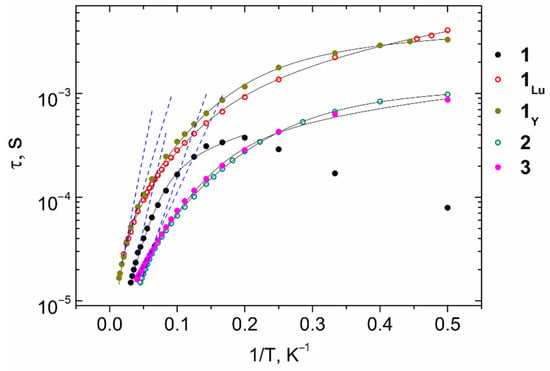
Figure 12.
τ vs. 1/T dependences for complexes 1–3, 1Y, and 1Lu under Hdc fields of 2500 (2, 3, 1Lu) and 5000 Oe (1, 1Y). Blue dashed lines represent fittings of the high-temperature range by the Orbach mechanism. Black solid lines represent fittings by the combinations of relaxation mechanisms presented in Table 3 and Table 4.

Table 3.
The best-fit parameters of magnetization relaxation for 1–3.
The best approximations of τ vs. 1/T dependences for complexes 1 and 3 were achieved using the Orbach and direct relaxation mechanisms (τ−1 = τ0−1∙exp(−Δeff/kBT) + AdirectTH4). The other combinations of the two relaxation mechanisms, as well as the single Orbach and Raman mechanisms, failed to fit the high-temperature range of the experimental data. The addition of a third relaxation mechanism led to overparameterization in all cases. In contrast, an ensemble of the Orbach, Raman, and QTM relaxation mechanisms (τ−1 = τ0−1exp(−Δeff/kBT) + CRamanTn_Raman + B) was used for the best-fit of the experimental data for 2. Adjunction of the third relaxation mechanism in this instance resulted in an improvement of the approximation in comparison with all options involving two relaxation mechanisms.
In our previous paper [55], we reported on two structural analogs of 1, [NaLn(VO)2(cbdc)4(H2O)10]n (1Y: Ln = Y, 1Lu: Ln = Lu). However, the SMM properties of those compounds were thoroughly studied in the present study (Figures S17, S18, S22 and S23). The corresponding fitting parameters of the τ vs. 1/T plots (Figure 12) are presented below (Table 4). The τ(1/T) best-fits in the entire temperature range for 1Y and 1Lu were achieved using the cooperative contribution of Orbach and Raman mechanisms (τ−1 = τ0−1∙exp(−Δeff/kBT) + CRamanTn_Raman) for 1Lu and the Orbach, Raman, and QTM mechanisms for 1Y. Other combinations of relaxation mechanisms either failed to fit the experimental data or led to over-parametrization.

Table 4.
The best-fit parameters of magnetization relaxation for 1Y and 1Lu.
At low temperatures, the relaxation of 1 was strikingly different from the relaxation of 2 and 3. A decrease in the relaxation time with a temperature decrease was observed. Because of this, the low-temperature section of the τ vs. 1/T plot cannot be approximated by a sum of any relaxation mechanisms. Such a change may originate from the absence of a network of hydrogen bonds in 1, in contrast to the structures of compounds 2 and 3.
The minimum V···V distance (6.077 Å) and one of the Gd···V distances (4.547 Å) in compound 3 led to the largest dipole-dipole interaction between ions in the GdIII···VIV and VIV···VIV pairs. These interactions possibly give rise to additional pathways of magnetization relaxation, and hence acceleration of relaxation [56]. Therefore, for compound 3, the effective magnetization reversal barrier, estimated from the high-temperature section by the Arrhenius equation, was the smallest.
Thus, as follows from a comparison of ac-magnetic data for 1, 1Y, and 1Lu, the slow relaxation of magnetization in GdIII-VIV compounds can be determined by the contributions of both GdIII and VIV ions. Moreover, compounds 1, 1Y, and 1Lu demonstrated the highest Δeff values (according to the approximation by a single Orbach mechanism) compared with those of 2 and 3, which can be explained by longer V···V (6.234 Å for 1 and 6.216 Å for 1Y) and Gd···V distances (5.722 Å for 1) weakening the dipole-dipole interaction between paramagnetic ions.
3. Materials and Methods
3.1. Synthesis
3.1.1. General Details
The synthesis of new compounds was carried out in air using distilled water. The following starting reagents were purchased from commercial sources and used without further purification: VOSO4·3H2O (>99%), Ba(NO3)2 (>98%), H2cbdc (99%, Acros Organics), Gd(NO3)3·6H2O (99.9%, Lanhit), NaOH (>99%), RbOH·xH2O (Sigma Aldrich), and CsOH·H2O (99.95%, Acros Organics). The complexes [NaLn(VO)2(cbdc)4(H2O)10]n (1Y: Ln = Y, 1Lu: Ln = Lu) were synthesized according to procedures described previously [56]. The IR spectra of complexes 1–3 were measured using a Spectrum 65 FT-IR spectrometer (Perkin-Elmer) by the ATR method in the range of 4000–400 cm−1. Elemental C,H,N-analysis was carried out using an EuroEA 3000 automatic analyzer (EuroVector).
3.1.2. Synthetic Procedure
The solution of VOSO4·3H2O (0.150 g, 0.69 mmol) in H2O (15 mL) was added with crystalline Ba(NO3)2 (0.181 g, 0.69 mmol), and the reaction mixture was stirred for 25 min at 23 °C. Weight amounts of H2cbdc (0.199 g, 1.38 mmol) and corresponding alkaline metal hydroxide (NaOH, 0.110 g, 2.76 mmol for 1; RbOH·xH2O, 0.283 g, 2.76 mmol for 2; CsOH·H2O, 0.414 g, 2.76 mmol for 3) were dissolved in H2O (10 mL) at 40 °C, and the resulting clear solution of M2(cbdc) was poured into the reaction mixture. The stirring was continued for 15 min, and crystalline Gd(NO3)3·6H2O (0.311 g, 0.69 mmol) was added. After 15 min, the reaction mixture was left for 1 h without disturbance. The BaSO4 precipitate was filtered off, and the resulting sky-blue solution (25 mL) was left to evaporate in air at 23 °C. Blue crystals precipitated in 3 weeks were suitable for X-ray diffraction. The crystals were filtered from the mother liquor, washed with H2O (t = 5 °C), and dried in air at 23 °C.
For [NaGd(VO)2(cbdc)4(H2O)10]n (1): the yield was 0.199 g (54.3 % based on VOSO4·3H2O). Calc. (%) for C24H44GdNaO28V2: C, 27.12; H, 4.17. Found (%) C, 27.04; H, 4.17. IR spectrum (ATR, ν/cm−1) (s = strong, m = medium, w = weak): 3643 v. w, 3347 m. br [ν(O–H)], 3227 w. br [ν(O–H)], 2999 w [ν(C–H)], 2956 w [ν(C–H)], 1634 w, 1579 s [νas(COO−)], 1553 s [νas(COO−)], 1443 m, 1431 m, 1390 s [νs(COO−)], 1348 s, 1253 w, 1244 w, 1229 m [ν(C–C)cycle], 1162 w, 1122 m [γ(C(–C)2)], 1063 w, 1011 w, 1000 w, 968 s [ν(V=O)], 952 m, 924 m, 876 w, 843 w, 808 w, 773 m, 725 s [δ(COO−)], 640 s, 561 s, 532 s, 467 s, 443 s, 420 s.
For {[RbGd(VO)2(cbdc)4(H2O)10]·2.5H2O}n (2): the yield was 0.113 g (28 % based on VOSO4·3H2O). Calc. (%) for C24H49GdRbO30.5V2: C, 24.63; H, 4.22. Found (%): C, 24.68; H, 4.50. IR spectrum (ATR, ν/cm−1) (s = strong, m = medium, w = weak): 3576 v. w, 3243 m. br [ν(O–H)], 3018 w [ν(C–H)], 2973 w [ν(C–H)], 2873 w, 1666 w, 1559 s [νas(COO−)], 1533 s [νas(COO−)], 1457 m, 1431 s, 1401 s [νs(COO−)], 1379 s [νs(COO−)], 1357 s, 1342 s, 1259 w, 1228 m [ν(C–C)cycle], 1169 w, 1124 m [γ(C(–C)2)], 1060 v. w, 1008 w, 994 s [ν(V=O)], 984 s [ν(V=O)], 961 w, 917 s, 879 w, 863 w, 776 s, 763 s [δ(COO−)], 734 s, 709 s [δ(COO−)], 654 s, 552 s, 537 s, 453 s, 433 s, 420 s.
For {[CsGd(VO)2(cbdc)4(H2O)11]·5H2O}2 (3): the yield was 0.097 g (32.8 % based on VOSO4·3H2O). Calc. (%) for C24H56CsGdO34V2: C, 22.50; H, 4.41. Found (%): C, 22.47; H, 4.49. IR spectrum (ATR, ν/cm−1) (s = strong, m = medium, w = weak): 3311 m. br, 3215 m. br [ν(O–H)], 3027 w, 3006 m [ν(C–H)], 2986 w [ν(C–H)], 2969 w [ν(C–H)], 1627 m, 1557 s [νas(COO−)], 1455 m, 1404 s [νs(COO−)], 1386 s [νs(COO−)], 1353 s, 1307 m, 1256 w, 1244 w, 1227 w [ν(C–C)cycle], 1182 w, 1163 w, 1126 w [γ(C(–C)2)], 1063 w, 1047 v. w, 1015 w, 998 w, 988 s [ν(V=O)], 976 s [ν(V=O)], 960 m, 934 w, 916 w, 882 m, 856 m, 781 s [δ(COO−)], 762 s [δ(COO−)], 696 s, 653 s, 615 s, 595 s, 546 s, 466 s, 449 s.
3.2. Single Crystal X-ray Diffraction
Single crystal X-ray diffraction study for the crystals of 1–3 was carried out on a Bruker SMART APEX II diffractometer equipped with a CCD detector (Mo-Kα, λ = 0.71073 Å, graphite monochromator) [57]. Semi-empirical absorption correction was applied by the SADABS program [58]. The structures were solved by direct methods and refined by the full-matrix least squares in the anisotropic approximation for non-hydrogen atoms. The calculations were carried out by the SHELX-2014 program package [59] using Olex2 1.2 [60]. The hydrogen atoms in structures 1–3 were positioned geometrically and refined using the riding model. The crystallographic parameters for 1–3 and the structure refinement details are given in Table 5.

Table 5.
Crystallographic parameters and structure refinement details for compounds 1–3.
CCDC 2019386–2019388 contain supplementary crystallographic data for 1–3. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
3.3. Powder X-ray Diffraction
The purity of the samples for magnetic measurements and EPR spectroscopy was achieved by powder X-ray diffraction. The powder patterns were obtained at room temperature on a Bruker D8 Advance diffractometer with a LynxEye detector in Bragg-Brentano geometry, with the sample dispersed thinly on a zero-background Si sample holder, λ(CuKα) = 1.54060 Å, θ/θ scan with variable slits (irradiated length 20 mm) from 4° to 65° 2θ, step size 0.020° (Figures S25–S27).
3.4. EPR Spectroscopy
Variable-temperature continuous wave X/Q-band EPR spectra were obtained using a Bruker Elexsys E580 spectrometer equipped with an Oxford Instruments temperature control system (T = 4–300 K). All spectral simulations were done using the EasySpin toolbox for Matlab [61].
3.5. Magnetic Measurements
Magnetic susceptibility measurements were performed with a Quantum Design PPMS-9 susceptometer. Dc-magnetic susceptibility measurements were performed in the 2–300 K temperature range in a 5000 Oe dc-magnetic field. For ac-susceptibility measurements, oscillating ac-magnetic fields of 5, 3, and 1 Oe within frequency ranges 10–100, 100–1000, and 1000–10,000 Hz, respectively, were applied. Measurements were performed on polycrystalline samples sealed in a polyethylene bag and covered with mineral oil in order to prevent field-induced orientation of the crystals. Magnetic data were corrected for the diamagnetic contribution evaluated from Pascal’s constants, the sample holder, and mineral oil.
3.6. DFT Calculations
The density functional theory calculations were performed by using the Gaussian 09 program package [62] with the UB3LYP functional [63]. The standard 6-31G (d,p) basis set was used for all atoms with the exception of Gd, for which the SDD basis set with effective core potential was employed. The relativistic effects were incorporated via the Douglas-Kroll-Hess (DKH) [64,65] method.
4. Conclusions
The radius of the alkali metal cation (Na, Rb, Cs) was shown to affect the structure of trinuclear heterometallic anions [Gd(VO)2(cbdc)4(H2O)8]− and their mutual arrangement in a crystal. With Na+, trinuclear {GdV2}− moieties were characterized by identical V···Gd distances and were connected into 1D-polymeric chains in the case of 1. With Rb+ and Cs+, the V···Gd distances were different, and [GdV2]− fragments were linked into a 3D-framework for 2 and a molecular complex for 3, respectively. Based on EPR spectroscopy data for 1, the presence of weak magnetic anisotropy of GdIII ions was identified (D ~ 0.08 cm−1 and E/D ~ 0.1–0.15). Fitting the χMT-temperature plots (excluding the magnetic anisotropy contributions of metal ions) showed the presence of ferromagnetic exchange interactions between GdIII and VIV ions in the trinuclear unit of 1, and both ferro- and antiferromagnetic exchange channels in 2 and 3. Ac-susceptibility measurements showed field-induced slow relaxation of magnetization in 1–3 that can be described using the sum of Orbach and direct mechanisms for 1 and 3, or the sum of Orbach, Raman, and QTM mechanisms for 2. Moreover, of all the compounds obtained, the highest values of the effective anisotropic energy barrier (according to the approximation by a single Orbach mechanism) were obtained for 1 and its structural analogs with diamagnetic YIII and LuIII ions.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/magnetochemistry7060082/s1: Table S1–S3: Selected bond lengths and angles for 1–3, Table S4: Continuous shape measures (CShM) for GdO8 coordination polyhedra in compounds 1–3, Figure S1: EPR spectra of 1 at T = 80 K, Figure S2: Simulation of the anticipated contributions of Gd(III) ion and two V(IV) ions into the overall EPR spectrum, Tables S5–S7: Hydrogen bonding parameters of structures 1–3, Figures S3-S5: Fitting the experimental 1/χM vs. T plots, Figures S6–S11 and Tables S8–S13: Fitting the experimental curves χMT vs. T for complexes 1–3, Figure S12: Fitting the experimental curve χMT vs. T for complex 1Lu, Figures S13–S23: AC magnetic data, Tables S14–S16: Fitting of the τ vs. 1/T dependences for 1–3; Figure S24: Natural orbitals of VIV-GdIII fragments of the compounds 2 and 3 calculated by the DFT method, Figures S25–S27: PXRD data.
Author Contributions
Conceptualization, methodology, E.S.B., M.A.K. and I.L.E.; formal analysis, J.K.V., A.A.K., K.A.B., E.A.U., N.N.E. and M.V.F.; investigation, validation, E.S.B., J.K.V., A.A.K., K.A.B., N.N.E. and M.V.F.; writing—original draft preparation, E.S.B., K.A.B., E.A.U. and M.V.F.; writing—review and editing, E.S.B., N.N.E., M.A.K. and I.L.E.; visualization, E.S.B., K.A.B., M.V.F. and M.A.K.; supervision, M.A.K. and I.L.E. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Russian Science Foundation, grant number 19-73-10181.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article and supplementary materials.
Acknowledgments
Single-crystal X-ray diffraction analysis for 1, powder X-ray diffraction analysis, IR spectroscopy, CHN analysis, and magnetic measurements for 1–3 were performed using the equipment of the JRC PMR IGIC RAS. X-ray diffraction data for 2 and 3 were collected using the equipment of Center for molecular composition studies of INEOS RAS. We are grateful to the Ministry of Science and Higher Education of the Russian Federation for the access to EPR equipment. We are grateful to Maxim A. Shmelev for the XPRD experiments and Alyona A. Starikova for the help with DFT calculations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, R.; Wang, L.; Xu, C.; Yang, H.; Chen, W.; Gao, G.; Liu, W. Anion-induced 3d-4f luminescent coordination clusters: Structural characteristics and chemical fixation of CO2 under mild conditions. Dalton Trans. 2018, 47, 7159–7165. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.-Y.; You, S.-Y.; Cui, H.-M.; Zeng, G.-P.; Cui, J.-Z. Tuning the luminescence of two 3d-4f metal-organic frameworks for the fast response and highly selective detection of aniline. Dalton Trans. 2017, 46, 16432–16438. [Google Scholar] [CrossRef]
- Dey, A.; Acharya, J.; Chandrasekhar, V. Heterometallic 3d-4f Complexes as Single—Molecule Magnets. Chem. Asian J. 2019, 14, 4433–4453. [Google Scholar] [CrossRef]
- Dey, A.; Bag, P.; Kalita, P.; Chandrasekhar, V. Heterometallic CuII-LnIII complexes: Single molecule magnets and magnetic refrigerants. Coord. Chem. Rev. 2021, 432, 213707. [Google Scholar] [CrossRef]
- Yin, J.-J.; Lu, T.-Q.; Chen, C.; Zhuang, G.-L.; Zheng, J.; Zheng, X.-Y.; Shao, F. Magnetocaloric Effect and Slow Magnetic Relaxation on Two Dimensional Layered 3d-4f Cluster-Based Metal-Organic Frameworks. Cryst. Growth Des. 2020, 20, 4005–4012. [Google Scholar] [CrossRef]
- Cremades, E.; Gómez-Coca, S.; Aravena, D.; Alvarez, S.; Ruiz, E. Theoretical Study of Exchange Coupling in 3d-Gd Complexes: Large Magnetocaloric Effect Systems. J. Am. Chem. Soc. 2012, 134, 10532–10542. [Google Scholar] [CrossRef]
- Lin, Q.; Li, J.; Dong, Y.; Zhou, G.; Song, Y.; Xu, Y. Lantern-shaped 3d–4f high-nuclearity clusters with magnetocaloric effect. Dalton Trans. 2017, 46, 9745–9749. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Zhang, D.-Q.; Hao, X.; Zhu, D.-B. Assembly of chiral 3d–4f wheel-like cluster complexes with achiral ligands: Single—molecule magnetic behavior and magnetocaloric effect. Inorg. Chem. Front. 2020, 7, 3340–3351. [Google Scholar] [CrossRef]
- Oyarzabal, I.; Echenique-Errandonea, E.; San Sebastián, E.; Rodríguez-Diéguez, A.; Seco, J.M.; Colacio, E. Synthesis, Structural Features and Physical Properties of a Family of Triply Bridged Dinuclear 3d-4f Complexes. Magnetochemistry 2021, 7, 22. [Google Scholar] [CrossRef]
- Griffiths, K.; Kostakis, G.E. Transformative 3d-4f coordination cluster carriers. Dalton Trans. 2018, 47, 12011–12034. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Griffiths, K.; Anson, C.E.; Powell, A.K.; Kostakis, G.E. A tetranuclear CuII2DyIII2 coordination cluster as a Suzuki (C–C) coupling reaction promoter. Dalton Trans. 2018, 47, 17202–17205. [Google Scholar] [CrossRef]
- Cancino, P.; Santibañez, L.; Stevens, C.; Fuentealba, P.; Audebrand, N.; Aravena, D.; Torres, J.; Martinez, S.; Kremere, C.; Spodine, E. Influence of the channel size of isostructural 3d-4f MOFs on the catalytic aerobic oxidation of cycloalkenes. New J. Chem. 2019, 43, 11057–11064. [Google Scholar] [CrossRef]
- Dermitzaki, D.; Psycharis, V.; Sanakis, Y.; Stamatatos, T.C.; Pissas, M.; Raptopoulou, C.P. Extending the family of heptanuclear heterometallic Cu5Ln2 (Ln = Gd, Tb, Dy) complexes: Synthesis, crystal structures, magnetic and magnetocaloric studies. Polyhedron 2019, 169, 135–143. [Google Scholar] [CrossRef]
- Zhang, H.-G.; Yang, H.; Sun, L.; Li, D.-C.; Dou, J.-M. A New Family of 2D Coordination Polymers Based on 3d–4f 15—Metallacrown-5 Units: Synthesis, Structure, and Single-Molecule Magnet Behavior. Eur. J. Inorg. Chem. 2019, 2019, 2839–2843. [Google Scholar] [CrossRef]
- Worrell, A.; Sun, D.; Mayans, J.; Lampropoulos, C.; Escuer, A.; Stamatatos, T.C. Oximato-Based Ligands in 3d/4f-Metal Cluster Chemistry: A Family of {Cu3Ln} Complexes with a “Propeller”-like Topology and Single-Molecule Magnetic Behavior. Inorg. Chem. 2018, 57, 13944–13952. [Google Scholar] [CrossRef]
- Zhao, X.; Li, G.; Ma, J.; Liu, W. Two Octanuclear {Cu4Ln4} (Ln = Dy or Tb) Complexes with a Butterfly-Shaped Unit Exhibiting Zero-Field Single-Molecule Magnet Behavior. Inorg. Chem. 2020, 59, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.Y.; Chen, P.Y.; Wu, M.Z.; Tian, L.; Liu, Z.Y. Synthesis of a series of hetero-multi-spin Ln2Cu3 complexes based on a methyl-pyrazole nitronyl nitroxide radical with slow magnetic relaxation behaviors. Dalton Trans. 2019, 48, 9187–9193. [Google Scholar] [CrossRef]
- Ghosh, T.K.; Maity, S.; Mayans, J.; Ghosh, A. Family of Isomeric CuII-LnIII (Ln = Gd, Tb, and Dy) Complexes Presenting Field-Induced Slow Relaxation of Magnetization Only for the Members Containing GdIII. Inorg. Chem. 2021, 60, 438–448. [Google Scholar] [CrossRef]
- Liu, J.-L.; Lin, W.-Q.; Chen, Y.-C.; Gómez-Coca, S.; Aravena, D.; Ruiz, E.; Leng, J.-D.; Tong, M.-L. CuII-GdIII Cryogenic Magnetic Refrigerants and Cu8Dy9 Single-Molecule Magnet Generated by In Situ Reactions of Picolinaldehyde and Acetylpyridine: Experimental and Theoretical Study. Chem. Eur. J. 2013, 19, 17567–17577. [Google Scholar] [CrossRef]
- Singh, M.K.; Rajeshkumar, T.; Kumar, R.; Singh, S.K.; Rajaraman, G. Role of (1,3) {Cu-Cu} Interaction on the Magneto-Caloric Effect of Trinuclear {CuII-GdIII-CuII} Complexes: Combined DFT and Experimental Studies. Inorg. Chem. 2018, 57, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Alexandropoulos, D.I.; Poole, K.M.; Cunha-Silva, L.; Sheikh, J.A.; Wernsdorfer, W.; Christou, G.; Stamatatos, T.C. A family of ‘windmill’-like {Cu6Ln12} complexes exhibiting single-molecule magnetism behavior and large magnetic entropy changes. Chem. Commun. 2017, 53, 4266–4269. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Das, S.; Palacios, M.A.; Colacio, E.; Chandrasekhar, V. Single-Molecule Magnet and Magnetothermal Properties of Two-Dimensional Polymers Containing Heterometallic [Cu5Ln2] (Ln = GdIII and DyIII) Motifs. Eur. J. Inorg. Chem. 2018, 2018, 1645–1654. [Google Scholar] [CrossRef]
- Alexandropoulos, D.I.; Cunha-Silva, L.; Tang, J.; Stamatatos, T.C. Heterometallic Cu/Ln cluster chemistry: Ferromagnetically-coupled {Cu4Ln2} complexes exhibiting single-molecule magnetism and magnetocaloric properties. Dalton Trans. 2018, 47, 11934–11941. [Google Scholar] [CrossRef] [PubMed]
- Kettles, F.J.; Milway, V.A.; Tuna, F.; Valiente, R.; Thomas, L.H.; Wernsdorfer, W.; Ochsenbein, S.T.; Murrie, M. Exchange Interactions at the Origin of Slow Relaxation of the Magnetization in {TbCu3} and {DyCu3} Single-Molecule Magnets. Inorg. Chem. 2014, 53, 8970–8978. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.-J.; Zhang, J.-C.; Jiang, Y.-X.; Tao, J.-X.; Wang, W.-Y.; Huang, X.-C. Two-dimensional heterometallic CuIILnIII (Ln = Tb and Dy) coordination polymers bridged by dicyanamides showing slow magnetic relaxation behavior. CrystEngComm 2019, 21, 5145–5151. [Google Scholar] [CrossRef]
- Chen, Y.; Long, Q.-Q.; Hu, Z.-B.; Wang, H.-S.; Huang, Z.-Y.; Chen, W.; Song, Y.; Zhang, Z.-C.; Yang, F.-J. Synthesis, crystal structures and magnetic properties of a series of pentanuclear heterometallic [CuII3LnIII2] (Ln = Ho, Dy, and Gd) complexes containing mixed organic ligands. New J. Chem. 2019, 43, 8101–8108. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dupuis, A.; Laurent, J.-P. An original heterodinuclear VO2+, Gd3+ complex with a nonet ground state. J. Chem. Soc. Dalton Trans. 1998, 735–736. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dahan, F.; Donnadieu, B.; Garcia-Tojal, J.; Laurent, J.-P. Versatility of the Nature of the Magnetic Gadolinium(III)–Vanadium(IV) Interaction—Structure and Magnetic Properties of Two Heterobinuclear [Gd, V(O)] Complexes. Eur. J. Inorg. Chem. 2001, 2001, 363–365. [Google Scholar] [CrossRef]
- Costes, J.-P.; Shova, S.; Clemente-Juan, J.M.; Suet, N. The first example of a hetero-tetranuclear [(VO)Gd]2 complex: Synthesis, crystal structure and magnetic properties of [VOLGd(hfa)2CH3OH]2·2CH3OH·2(CH3)2CO. Dalton Trans. 2005, 17, 2830–2832. [Google Scholar] [CrossRef]
- Shi, W.; Chen, X.-Y.; Zhao, Y.-N.; Zhao, B.; Cheng, P.; Yu, A.; Song, H.-B.; Wang, H.-G.; Liao, D.-Z.; Yan, S.-P.; et al. Synthesis, Crystal Structures, and Properties of Oxovanadium(IV)–Lanthanide(III) Heteronuclear Complexes. Chem. Eur. J. 2005, 11, 5031–5039. [Google Scholar] [CrossRef]
- Rocha, J.; Almeida Paz, F.A.; Shi, F.-N.; Ferreira, R.A.S.; Trindade, T.; Carlos, L.D. Photoluminescent Porous Modular Lanthanide–Vanadium–Organic Frameworks. Eur. J. Inorg. Chem. 2009, 2009, 4931–4945. [Google Scholar] [CrossRef]
- Ishida, T.; Watanabe, R.; Fujiwara, K.; Okazawa, A.; Kojima, N.; Tanaka, G.; Yoshii, S.; Nojiri, H. Exchange coupling in TbCu and DyCu single-molecule magnets and related lanthanide and vanadium analogs. Dalton Trans. 2012, 41, 13609–13619. [Google Scholar] [CrossRef] [PubMed]
- Hickson, J.R.; Horsewill, S.J.; Bamforth, C.; McGuire, J.; Wilson, C.; Sproules, S.; Farnaby, J.H. The modular synthesis of rare earth-transition metal heterobimetallic complexes utilizing a redox-active ligand. Dalton Trans. 2018, 47, 10692–10701. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Guha, A.K. Supramolecular assembly of binuclear [V2O3(NTA)2]4− unit with [Ce(H2O)9]3+: Synthesis, structure, luminescent, magnetic properties and TD-DFT calculation. Polyhedron 2017, 137, 1–9. [Google Scholar] [CrossRef]
- Shi, W.; Chen, X.-Y.; Zhao, B.; Yu, A.; Song, H.-B.; Cheng, P.; Wang, H.-G.; Liao, D.-Z.; Yan, S.-P. Construction of 3d-4f Mixed-Metal Complexes Based on a Binuclear Oxovanadium Unit: Synthesis, Crystal Structure, EPR, and Magnetic Properties. Inorg. Chem. 2006, 45, 3949–3957. [Google Scholar] [CrossRef]
- Orendáč, M.; Sedláková, L.; Čižmár, E.; Orendáčová, A.; Feher, A.; Zvyagin, S.A.; Wosnitza, J.; Zhu, W.H.; Wang, Z.M.; Gao, S. Spin relaxation and resonant phonon trapping in [Gd2(fum)3(H2O)4]3H2O. Phys. Rev. 2010, B81, 214410. [Google Scholar] [CrossRef]
- Martínez-Pérez, M.J.; Cardona-Serra, S.; Schlegel, C.; Moro, F.; Alonso, P.J.; Prima-García, H.; Clemente-Juan, J.M.; Evangelisti, M.; Gaita-Ariño, A.; Sesé, J.; et al. Gd-Based Single-Ion Magnets with Tunable Magnetic Anisotropy: Molecular Design of Spin Qubits. Phys. Rev. Lett. 2012, 108, 247213. [Google Scholar] [CrossRef]
- Rossin, A.; Giambastiani, G.; Peruzzini, M.; Sessoli, R. Amine-Templated Polymeric Lanthanide Formates: Synthesis, Characterization, and Applications in Luminescence and Magnetism. Inorg. Chem. 2012, 51, 6962–6968. [Google Scholar] [CrossRef] [PubMed]
- Arauzo, A.; Lazarescu, A.; Shova, S.; Bartolomé, E.; Cases, R.; Luzón, J.; Bartolomé, J.; Turta, C. Structural and magnetic properties of some lanthanide (Ln = Eu(III), Gd(III) and Nd(III)) cyanoacetate polymers: Field-induced slow magnetic relaxation in the Gd and Nd substitutions. Dalton Trans. 2014, 43, 12342–12356. [Google Scholar] [CrossRef]
- Khalfaoui, O.; Beghidja, A.; Long, J.; Boussadia, A.; Beghidja, C.; Guari, Y.; Larionova, J. Cinnamic acid derivative rare-earth dinuclear complexes and one-dimensional architectures: Synthesis, characterization and magnetic properties. Dalton Trans. 2017, 46, 3943–3952. [Google Scholar] [CrossRef] [PubMed]
- Girginova, P.I.; Pereira, L.C.J.; Coutinho, J.T.; Santos, I.C.; Almeida, M. Slow magnetic relaxation in lanthanide ladder type coordination polymers. Dalton Trans. 2014, 43, 1897–1905. [Google Scholar] [CrossRef]
- Holmberg, R.J.; Ho, L.T.A.; Ungur, L.; Korobkov, I.; Chibotaru, L.F.; Murugesu, M. Observation of unusual slow-relaxation of the magnetisation in a Gd-EDTA chelate. Dalton Trans. 2015, 44, 20321–20325. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Cosquer, G.; Izuogu, D.C.; Ohtsu, H.; Kawano, M.; Lan, Y.; Wernsdorfer, W.; Nojiri, H.; Breedlove, B.K.; Yamashita, M. Field-Induced Slow Magnetic Relaxation of GdIII Complex with a Pt−Gd Heterometallic Bond. Chem. Eur. J. 2017, 23, 4551–4556. [Google Scholar] [CrossRef]
- Handzlik, G.; Magott, M.; Arczyński, M.; Sheveleva, A.M.; Tuna, F.; Sarewicz, M.; Osyczka, A.; Rams, M.; Vieru, V.; Chibotaru, L.F.; et al. Magnetization Dynamics and Coherent Spin Manipulation of a Propeller Gd(III) Complex with the Smallest Helicene Ligand. J. Phys. Chem. Lett. 2020, 11, 1508–1515. [Google Scholar] [CrossRef]
- Bazhina, E.S.; Aleksandrov, G.G.; Kiskin, M.A.; Korlyukov, A.A.; Efimov, N.N.; Bogomyakov, A.S.; Starikova, A.A.; Mironov, V.S.; Ugolkova, E.A.; Minin, V.V.; et al. The First Series of Heterometallic LnIII-VIV Complexes Based on Substituted Malonic Acid Anions: Synthesis, Structure and Magnetic Properties. Eur. J. Inorg. Chem. 2018, 2018, 5075–5090. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE v.2.1, Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; Universitat de Barcelona: Barselona, Spain, 2013. [Google Scholar]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem. Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164. [Google Scholar] [CrossRef]
- Atzori, M.; Chiesa, A.; Morra, E.; Chiesa, M.; Sorace, L.; Carretta, S.; Sessoli, R. A two-qubit molecular architecture for electron-mediated nuclear quantum simulation. Chem. Sci. 2018, 9, 6183–6192. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Tesi, L.; Benci, S.; Lunghi, A.; Righini, R.; Taschin, A.; Torre, R.; Sorace, L.; Sessoli, R. Spin Dynamics and Low Energy Vibrations: Insights from Vanadyl-Based Potential Molecular Qubits. J. Am. Chem. Soc. 2017, 139, 4338–4341. [Google Scholar] [CrossRef]
- Atzori, M.; Morra, E.; Tesi, L.; Albino, A.; Chiesa, M.; Sorace, L.; Sessoli, R. Quantum Coherence Times Enhancement in Vanadium(IV)-based Potential Molecular Qubits: The Key Role of the Vanadyl Moiety. J. Am. Chem. Soc. 2016, 138, 11234–11244. [Google Scholar] [CrossRef]
- Atzori, M.; Tesi, L.; Morra, E.; Chiesa, M.; Sorace, L.; Sessoli, R. Room-Temperature Quantum Coherence and Rabi Oscillations in Vanadyl Phthalocyanine: Toward Multifunctional Molecular Spin Qubits. J. Am. Chem. Soc. 2016, 138, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Benci, S.; Morra, E.; Tesi, L.; Chiesa, M.; Torre, R.; Sorace, L.; Sessoli, R. Structural Effects on the Spin Dynamics of Potential Molecular Qubit. Inorg. Chem. 2018, 57, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Tesi, L.; Lunghi, A.; Atzori, M.; Lucaccini, E.; Sorace, L.; Tottia, F.; Sessoli, R. Giant spin–phonon bottleneck effects in evaporable vanadyl-based molecules with long spin coherence. Dalton Trans. 2016, 45, 16635–16643. [Google Scholar] [CrossRef]
- Kurganskii, I.V.; Bazhina, E.S.; Korlyukov, A.A.; Babeshkin, K.A.; Efimov, N.N.; Kiskin, M.A.; Veber, S.L.; Sidorov, A.A.; Eremenko, I.L.; Fedin, M.V. Mapping Magnetic Properties and Relaxation in Vanadium(IV) Complexes with Lanthanides by Electron Paramagnetic Resonance. Molecules 2019, 24, 4582. [Google Scholar] [CrossRef]
- Katoh, K.; Yamashita, S.; Yasuda, N.; Kitagawa, Y.; Breedlove, B.K.; Nakazawa, Y.; Yamashita, M. Control of the Spin Dynamics of Single-Molecule Magnets by using a Quasi One-Dimensional Arrangement. Angew. Chem. Int. Ed. 2018, 57, 9262–9267. [Google Scholar] [CrossRef] [PubMed]
- SMART (Control) and SAINT (Integration) Software, Version 5.0; Bruker AXS, Inc.: Madison, WI, USA, 1997.
- Sheldrick, G.M. SADABS, Program for Scaling and Correction of Area Detector Data; Göttingen University: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision E.01); Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Douglas, M.; Kroll, N.M. Quantum electrodynamical corrections to the fine structure of helium. Ann. Phys. 1974, 82, 89–155. [Google Scholar] [CrossRef]
- Hess, B.A. Applicability of the no-pair equation with free-particle projection operators to atomic and molecular structure calculations. Phys. Rev. A 1985, 32, 756–763. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).