Abstract
Low temperature is an abiotic stress factor limiting the distribution of fruit tree cultivation areas. As temperate deciduous fruit trees, apple (Malus domestica) trees go dormant in the winter to adapt to or avoid damage caused by low temperatures. The capacity for cold resistance is closely linked to the physiological, biochemical, and structural characteristics of one-year-old branches. In this study, we investigated such changes in the branches of cold-resistant ‘Hanfu’ (HF) and cold-sensitive ‘Naganofuji 2’ (CF) apple varieties. The relative electrical conductivity, malondialdehyde content, and reactive oxygen species content of HF branches were lower than those of CF branches, while the antioxidant enzyme activity was higher in HF. The proline, soluble protein, and soluble sugar contents in both varieties showed an initial increase, followed by a subsequent decrease. Sucrose and sorbitol were the main sugar components, but sucrose and fructose were higher in HF than in CF. The periderm, phloem, and xylem of HF branches were also found to be thicker than those of CF branches, while the vessel diameter was smaller and the density greater. The results of this study provide a theoretical reference for further research on the low temperature adaptability of apple tree branches during dormancy.
1. Introduction
Apples (Malus domestica) are an economically important fruit in China, with major apple-producing regions including the traditional Bohai Bay and northwest Loess Plateau regions, as well as distinctive apple-producing regions such as the southwest cool highlands, Xinjiang, and the northeast. The climatic characteristics of apple-producing regions vary substantially, with the northeast region being a high-latitude area with a cold climate [1]. In this region, low temperatures are an important environmental factor affecting apple cultivation and the safe overwintering of trees. As a deciduous fruit tree in temperate regions, apple trees adapt or avoid low temperature damage in winter through dormancy, and they enhance cold resistance by regulating their own physiological and metabolic changes [2,3]. Previous studies have shown that continuous low temperatures can lead to membrane damage, reduced selective permeability, extravasation of internal solutes, and increased relative electrical conductivity. Therefore, relative electrical conductivity is often used to evaluate the cold resistance of plants [4]. In addition, low temperature stress can induce the production of reactive oxygen species (ROS), which can be used as a signal molecule to make plants respond to low temperature. However, with the deepening of stress, the increased production of ROS can cause plant membrane lipid peroxidation and induce the production of malonaldehyde (MDA). Liu et al. found that low temperatures induced considerable accumulation of MDA, superoxide anion (O2−), and hydrogen peroxide (H2O2) in banana leaves [5]. Therefore, maintaining the dynamic balance of ROS is essential for the survival of plants under stress conditions. Superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) are important protective enzymes against ROS induced damage [6]. Similarly, Wei et al. found that low temperatures substantially increased the activities of SOD, CAT, and POD, which enhanced the cold tolerance of tobacco seedlings after treatment at 4 °C [7]. In addition, the ascorbate-glutathione (AsA-GSH) cycle is an important pathway for plants to maintain intracellular redox balance, including ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), monodehydroascorbate reductase (MDHAR), and glutathione reductase (GR) [8,9]. Rani et al. found that cold acclimation increased the APX and GR activities of cold-tolerant genotype chickpeas, and reduced the damage induced by low temperatures to the leaves of cold-tolerant genotype chickpeas [10]. It has also been reported that osmotic adjustment substances such as soluble sugar, proline, soluble protein, and various low molecular weight solutes can protect plants from damage under low temperature stress [3]. Yu et al. found that the total soluble sugar content of peach (Prunus persica) tree bark tissue increased considerably during cold acclimation [11]. Sucrose, glucose, fructose, and sorbitol were the main sugars in peach tree bark, and the content of sucrose and sorbitol in the bark tissue of ‘Janghowon Hwangdo’ trees, which exhibit strong cold resistance, was substantially higher than that in ‘Hikawa Bakuho’ trees, which display weak cold resistance. Kwon et al. also found that the soluble sugar content in peach tree seedlings of the cold-tolerant variety ‘Someee’ was markedly higher than that in seedlings of the cold-sensitive variety ‘Odoroki’ during cold acclimation; sucrose was the main soluble sugar, and its content was most closely related to cold resistance [12]. Under low temperature stress, not only do the internal physiological and biochemical activities of plants change, but also the tissue structure changes. The branch structure is the main index to evaluate cold tolerance [13]. The periderm, formed during secondary growth in branches, is an important plant tissue that protects internal tissues such as phloem and xylem from biotic and abiotic stresses [14]. Previous studies have found that Hibiscus cultivars with a greater number of cell layers in the periderm cork layer exhibit stronger cold resistance [15]. Hajihashemi et al. observed the stem structure of nine Stevia rebaudiana varieties and found that cold stress substantially increased the phloem thickness in all varieties and promoted the production of phloem fibers [16]. Pandey et al. showed that xylem was more sensitive to environmental factors [17]. With a decrease in temperature, the diameter of xylem vessels decreased, and the density increased, which effectively reduced the occurrence of an air embolism.
‘Hanfu’ (HF) is a cold-resistant, high-quality, large-sized apple cultivar mainly planted in the characteristic apple-producing areas of Northeast China. It can survive the winter under conditions between −12 °C and −10 °C, which is the average temperature range in these areas in January [18]. ‘Naganofuji 2’ (CF) is the main cultivar in China’s Bohai Bay production region, and its cold resistance is weaker than that of HF [1], which makes it unsuitable for cultivation in the northeast production region. Past research on the effect of low temperature stress on apple trees primarily focused on damage under treatment at 4 °C, or different degrees of freezing damage to branches, and mainly evaluated the strength of cold resistance. However, few studies have analyzed the cold resistance of apple trees under natural low temperature conditions. Considering that apple seedlings are propagated asexually through grafting to maintain the favorable traits of the scion, we used cold-resistant Pingyi Tiancha (Malus hupehensis Rehd. var. pingyiensis Jiang) as the rootstock, and grafted apple varieties (HF and CF) with different degrees of cold resistance. The aim of this study was to evaluate the physiological and biochemical changes, as well as the differences in branch structure, in these two apple varieties under natural low temperatures during dormancy. The results of this study contribute to improving our understanding of the response of apple trees to low temperature stress during dormancy.
2. Materials and Methods
2.1. Plant Materials and Experimental Design
The experiment was carried out in the fruit tree base of Shenyang Agricultural University, Shenyang, Liaoning Province, China (41°49′ N, 123°34′ E). The orchard soil in the study area was classified as brown soil, and the following physical characteristics were recorded: pH 6.3, organic matter content 7.20 g/kg, alkali-hydrolyzable nitrogen 49.5 mg/kg, available phosphorus 35.3 mg/kg, and available potassium 76.1 mg/kg. In mid-April 2020, scions of ‘Hanfu’ (HF) and ‘Naganofuji 2’ (CF) were top-grafted (>1 m from the ground) onto four-year-old Pingyi Tiancha (M. hupehensis Rehd. var. pingyiensis Jiang) rootstocks in the field (diameter of approximately 3–4 cm) using the single-bud scion grafting method. This rootstock has strong cold tolerance and is an apple rootstock variety widely used in cold regions of China. The same rootstock was used for both varieties to control for any differences in the effects of the rootstock on the cold tolerance of the scion. Orchard management was carried out uniformly according to local industry standards throughout the growing season. During the dormant period, samples were collected on 15 November 2020, 15 December 2020, 1 January 2021, 15 January 2021, 31 January 2021, and 15 March 2021. In the Shenyang area, the lowest average temperatures typically occur in January (daily average high temperature: −4.8 °C, average low temperature: −15.1 °C); thus, intensive sampling was conducted during this period. The collected one-year-old branches were cut into lengths of approximately 0.5–1 cm, taking care to avoid buds. One segment was used to determine the relative electrical conductivity, and the remainder were frozen in liquid nitrogen and stored in an ultralow-temperature refrigerator at −80 °C for further analysis of physiological and biochemical indicators. Based on the meteorological data provided by the National Meteorological Science Data Center (http://data.cma.cn/site/index.html) (accessed on 5 November 2021), we calculated the temperature data of the Shenyang area from 2018 to 2021.
2.2. Determination of Relative Electrical Conductivity
The relative electrical conductivity of branches was determined according to the method of Xu et al. [19]. The one-year-old branches of apple trees at different dormancy stages were cut into 1–2 mm segments. The initial conductivity (EL1) was measured using a conductivity meter (DDS-307, Rex, Shanghai, China). Each sample was placed in a boiling water bath for 30 min and then oscillated at room temperature (150 rpm) for 10 h. The final conductivity (EL2) was measured, and the relative electrical conductivity value was calculated as EL1/EL2 (%).
2.3. Determination of MDA, O2−, and H2O2 Contents
The content of MDA was determined according to the method of He et al. [20]. Briefly, a 0.1 g sample was weighed and extracted with 3.0 mL of 100 g/L trichloroacetic acid solution. Next, 2.0 mL of 0.67% thiobarbiturate was added for color development. The absorbance values at 450 nm, 532 nm, and 600 nm wavelengths were measured.
The content of O2− was determined according to the method of Zhou et al. [21]. Briefly, a 0.1 g sample was weighed and extracted with 2 mL of 50 mmol phosphate buffer (pH 7.8). Next, 0.1 mL of hydroxylamine hydrochloride, 1 mL of p-aminobenzenesulfonic acid, and 1 mL of α-naphthylamine were added for color development, and a colorimetric assay was performed at 530 nm.
The content of H2O2 was determined according to the method of Zhou et al. [21]. Briefly, a 0.06 g sample was weighed and extracted with 2 mL of 5% trichloroacetic acid. Next, 100 μL of 20% titanium tetrachloride, 200 μL of concentrated ammonia, and 3 mL of 1 mol concentrated sulfuric acid were added for color development, and a colorimetric assay was performed at 410 nm.
2.4. Determination of Antioxidative Enzyme Activities
The activities of SOD, POD, CAT, APX, MDHAR, DHAR, and GR were determined according to the method of Li et al. [22]. Briefly, for the determination of SOD, a 0.1 g sample was weighed and extracted with 1 mL of 50 mmol phosphate buffer (pH 7.8). Next, 1.75 mL of 50 mmol phosphate buffer (pH 7.8) was added, and then 0.3 mL each of methionine (130 mmol), nitrogen blue tetrazole (750 μmol), edathamil disodium (100 μmol), and riboflavin (20 μmol) were added to determine the absorbance at 560 nm.
The 0.1 g sample was weighed, and the test solution of POD, CAT, and APX was extracted with 0.1 g of polyvinylpyrrolidone and 2 mL of 50 mmol phosphate buffer (pH 7.8). Briefly, for the determination of POD, a mixture of 50 mL of 50 mmol phosphate buffer (pH 7.8), 28 μL of guaiacol, 19 μL of 30% H2O2, and 200 μL of enzyme solution was reacted for 30 min, and a colorimetric assay was performed at 470 nm. Briefly, for the determination of CAT, a mixture of 1 mL of 50 mmol Tris-HCl, 1.75 mL of water, and 0.05 mL of enzyme solution was reacted for 3 min, and then 0.2 mL of H2O2 was added to determine the absorbance value at 240 nm. Briefly, for the determination of APX, a mixture of 0.65 mL of water, 0.1 mL of 50 mmol phosphate buffer (pH 7.8), 0.1 mL of 2 mmol ascorbic acid, 0.05 mL of enzyme solution, and 0.1 mL of 2 mmol H2O2 was used to determine the absorbance value at 290 nm. Briefly, to determine MDHAR, a mixture of 2.7 mL of 2 mmol AsA, 0.12 mL AsA oxidase, 0.09 mL of 2 mmol nicotinamide adenine dinucleotide phosphate, and 0.09 mL of enzyme solution was used to determine the absorbance value at 340 nm. Subsequently, to determine DHAR, a mixture of 0.57 mL of 0.1 mol phosphate buffer (pH 7.0), 0.1 mL of 1 mmol ethylenediaminetetraacetic acid (EDTA), 0.1 mL of 10 mmol oxidized ascorbate, 0.1 mL of 2 mmol GSH, 0.1 mL water, and 0.03 mL of enzyme solution was used to determine the absorbance value at 265 nm. Finally, to determine GR, a mixture of 0.1 mL of 1 mol 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 0.1 mL of 5 mmol EDTA, 0.1 mL of 1 mmol nicotinamide adenine dinucleotide phosphate, 0.1 mL of 5 mmol oxidized GSH, 0.55 mL water, and 0.05 mL of enzyme solution was used to determine the absorbance value at 340 nm.
2.5. Determination of Proline, Soluble Protein, and Soluble Sugar Contents
The content of proline was determined according to the method of He et al. [23]. Briefly, a 0.1 g sample was weighed and extracted with 1.5 mL of 3% sulfosalicylic acid. Next, 1 mL of supernatant, 1 mL of glacial acetic acid, and 1 mL of acidic ninhydrin were added for color development, and a colorimetric assay was performed at 520 nm.
The content of soluble protein was determined according to the method of Xu et al. [1]. Briefly, a 0.1 g sample was weighed and extracted with 0.1 g of polyvinylpyrrolidone and 2 mL of 50 mmol phosphate buffer (pH 7.8). Next, 0.03 mL of the enzyme solution was mixed with 0.97 mL of distilled water and 5.0 mL of Coomassie Brilliant Blue solution. Finally, the mixture was allowed to rest for 2 min, and then a colorimetric assay was performed at 595 nm.
The content of soluble sugar was determined according to the method of Yemm et al. [24]. Briefly, a 0.1 g sample was weighed and extracted with 3 mL of 80% ethanol. Next, 4 mL of anthrone was added for color development, and a colorimetric assay was performed at 620 nm.
2.6. Determination of Sucrose, Glucose, Fructose, and Sorbitol Contents
The content of sucrose, glucose, fructose, and sorbitol was determined according to the method of Huang et al. [25]. A 0.2 g sample was weighed and extracted with 3 mL of 80% ethanol. The sugar content was determined using an Agilent 1260 high-performance liquid chromatograph (Agilent, Santa Clara, CA, USA) equipped with a quaternary pump, an automatic sampler, and a differential refractive index detector. The chromatographic column was Caromix Ca-NP (7.8 × 300 mm, 10 μm), and the column temperature was 80 °C.
2.7. Anatomical Structure Analysis of One-Year-Old Branches
On 15 November 2020, 15 January 2021, and 15 March 2021, upright and uniform one-year-old branches free of pests and diseases were selected for sampling. Segments with 1 cm diameters were taken from the middle of the branches, while avoiding bud eyes, and a fixative (FAA: formaldehyde, alcohol, acetic acid) was applied. The branches were immersed in ethylene glycol ether acetate for 6 h at 37 °C, stored overnight at 37 °C, and then exposed to room temperature for 20–30 min. Subsequently, they were soaked in anhydrous ethanol for 20 min, 95% ethanol for 10 min, 90% ethanol for 10 min, and 80% ethanol for 10 min, and then they were washed with deionized water. Next, the branches were placed in safranine dye solution for 3–5 s, and the excess dye was washed off with deionized water. The branches were sequentially placed in 50%, 70%, and 80% gradient alcohol for 3–8 s each. Then, the branches were placed in solid green dye solution for 4–6 s and dehydrated with anhydrous ethanol afterward. Finally, the branches were placed in xylene for 5 min and then sealed with neutral gum. The cross-section of each branch was observed using an upright optical microscope (Eclipse E100, Nikon, Tokyo, Japan), images were captured using a Nikon DS-U3 microscope camera controller (Nikon, Tokyo, Japan), and the anatomical structure of the branches was analyzed using CaseViewer software (version 2.4.0). Vessel density was calculated by using Adobe Photoshop software to draw a square box of 200 × 200 pixels on each image and the number of vessels in the box was counted. If the box boundary vessel area was >1/2, then it was counted; otherwise, it was not counted.
2.8. Statistical Analyses
Each treatment in this study was performed in triplicate, and the data are presented as the mean ± standard deviation. Statgraphics (STN, St Louis, MO, USA) was used for statistical analysis, prior to which all data were tested for normality using descriptive methods. Standard skewness and standard kurtosis between −2 and +2 were considered to satisfy normal distribution. A one-way ANOVA with Duncan’s test was used to identify significant differences (p < 0.05). Physiological and biochemical data were analyzed using Pearson’s correlation analysis.
3. Results
3.1. Changes in Temperature during Dormancy
To explore the physiological and biochemical responses of one-year-old apple tree branches to natural low temperatures during winter dormancy in the cold region of Northeast China, we analyzed the changes in the stress-resistance indices of two apple varieties with different degrees of cold resistance. First, we calculated the temperature changes in Shenyang during the dormancy period from 2018 to 2021. Figure 1 shows that the temperature change trend during the dormancy period from 2018 to 2021 was similar in each year. The temperature was low during January, with the highest temperature in this month being below zero. During March, the highest temperature reached above zero, while the lowest temperature was below zero. The phenomenon of alternating high and low temperatures can lead to a freeze–thaw pattern, which can damage trees.
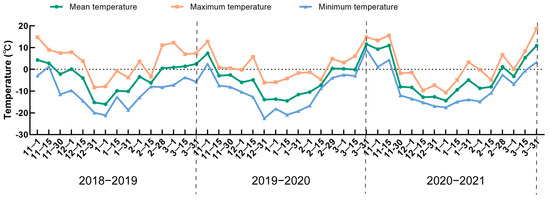
Figure 1.
Temperature statistics during dormancy in Shenyang from 2018 to 2021.
3.2. Effects of Natural Low Temperatures on Physiology and Biochemistry of Apple Tree Branches during Dormancy
Relative electrical conductivity is an important index used to evaluate the degree of plant damage. In this study, the relative electrical conductivity of HF and CF showed a trend of first increasing and then decreasing (Figure 2A). In samples collected on 15 December 2020, the relative electrical conductivity of HF and CF was measured, and the difference was not significant. However, the relative electrical conductivity of CF was significantly higher than that of HF in other periods. The change trend of MDA content, which is used as an indicator of damage to plant cells due to stress, was similar to that of the relative electrical conductivity, decreasing in March, and the MDA content of CF was significantly higher than that of HF. Figure 2B shows that the O2− content of HF and CF increased rapidly from December to January, increasing by 16.34% and 10.13%, respectively. In March, the content of O2− in HF decreased, while the content of O2− in CF increased, and the difference was significant. The change trend of H2O2 content in HF and CF branches during dormancy first increased and then decreased. The H2O2 content of HF reached a peak (47.32 mmol/g) in samples collected on 15 January 2021, while that of CF reached a peak (55.15 mmol/g) in samples collected on 31 January 2021. The H2O2 content of HF and CF in the two periods was significantly different. During different stages of dormancy, the SOD activity of HF was significantly higher than that of CF, while from 15 December 2020 to 31 January 2021, the POD activity of HF and CF changed slightly; the activities of SOD, POD, and CAT decreased on 15 March 2021, but the antioxidant enzyme activity of HF was significantly higher than that of CF. Whether HF or CF, APX activity peaked on 15 January 2021, and HF was significantly higher than CF. The activity of MDHAR and GR peaked on 31 January 2021. The DHAR activity of HF and CF increased first, then decreased, while HF and CF peaked on 15 and 31 January 2021, respectively. The DHAR activity of the two varieties decreased on 15 March 2021, and HF was considerably higher than CF (Figure 2B).
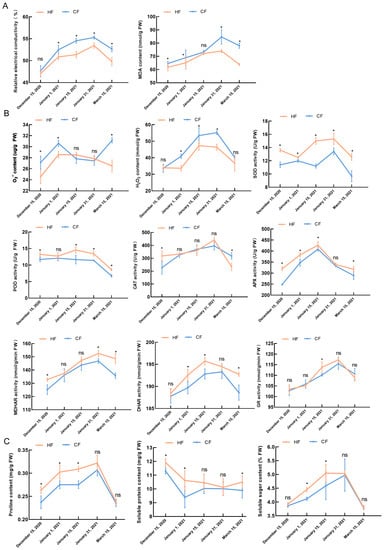
Figure 2.
Physiological and biochemical changes in apple tree branches during dormancy: (A) degree of cell membrane damage during dormancy; (B) generation and removal of reactive oxygen species (ROS) during dormancy; (C) content of osmotic adjustment substances during dormancy. Bars and error bars indicate mean ± standard deviation from three biological replicates. ‘Hanfu’ (HF); ‘Naganofuji 2’ (CF). * indicate significant differences (p < 0.05). ns indicates no significance.
Figure 2C shows that, during dormancy, the content of proline in HF (0.32 mg/g) and CF (0.30 mg/g) was the highest in samples collected on 31 January 2021, but the difference was not significant. The soluble protein content of HF (11.91 mg/g) and CF (11.30 mg/g) was the highest in samples collected on 15 December 2020, and the difference was significant. We found that the soluble sugar content of HF and CF increased rapidly during January, and the soluble sugar content of HF was significantly higher than that of CF in samples collected on 1 January 2021 and 15 January 2021, but there was no significant difference in the soluble sugar content between the two varieties in samples collected on 31 January 2021.
Sucrose, fructose, glucose, and sorbitol are important components of soluble sugar. The change trend of sucrose content in HF and CF was relatively stable in samples collected between 15 December 2020 and 15 January 2021, and the sucrose content in HF was significantly higher than that in CF. In samples collected between 15 January 2021 and 31 January 2021, the glucose content of CF decreased slightly, while the glucose content of HF changed more gradually. The fructose content of CF (2.69 mg/g) was the highest in samples collected on 15 January 2021 and was not significantly different from that of HF. The fructose content of HF reached a maximum value of 3.19 mg/g in samples collected on 31 January 2021, which was significantly higher than that of CF. During dormancy, there was no significant difference in sorbitol content between the two varieties, except in samples collected on 1 January 2021, which displayed a higher sorbitol content in HF than CF. In addition, we found that the contents of sucrose and sorbitol in branches were relatively high (Figure 3). In summary, the differences between the two varieties during dormancy confirmed that, in HF, the cell membrane was relatively stable, the scavenging ability of ROS was strong, a high amount of osmotic adjustment substances could be accumulated, and the cold tolerance was strong.
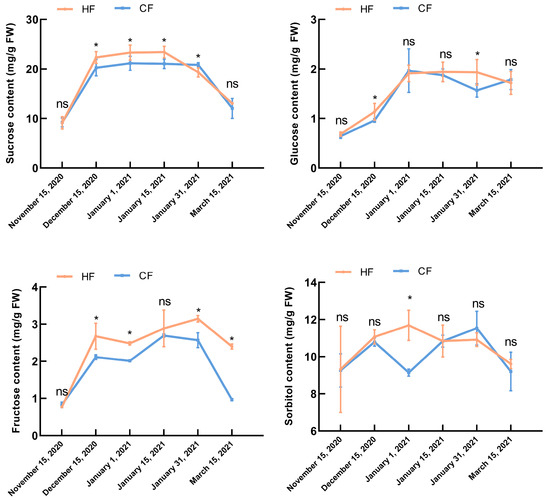
Figure 3.
Sugar content changes in apple tree branches during dormancy. Bars and error bars indicate mean ± standard deviation from three biological replicates. ‘Hanfu’ (HF); ‘Naganofuji 2’ (CF). * significant differences (p < 0.05). ns indicates no significance.
3.3. Effects of Natural Low Temperatures on the Anatomical Structure of One-Year-Old Apple tree Branches
The periderm is composed of the cork layer, the cork cambium, and the inner layer of the cork, which plays a protective role. In samples collected on 15 November 2020, the thickness and proportion of the periderm of HF were significantly greater than those of CF, which is less resistant to cold (Table 1), and although the cork layer on the outer periderm of the two varieties is composed of three layers of cells, the cork layer cells of HF were full in shape and arranged closely and uniformly (Figure 4A). The phloem is composed of the primary and secondary phloem, contains a large number of sieve tubes and companion cells, and is located between the cortex and the cambium. In this study, the phloem thickness of HF during dormancy was significantly greater than that of CF, and the proportion of phloem was also significantly higher in HF than in CF (Table 1, Figure 4). The cambium is composed of multiple layers of narrow and closely arranged cells, which are meristems that produce secondary xylem and phloem. We determined that the cambium thickness of CF was larger than that of HF in samples collected on 15 November 2020, and the difference was significant. However, in samples collected on 15 January 2021 and 15 March 2021, there was no significant difference in the thickness and proportion of cambium between the two varieties (Table 1, Figure 4). Xylem is an important cold-resistant tissue in branches. We found that the xylem thickness of HF was significantly greater than that of CF during dormancy, and the proportion of xylem in HF branches was also significantly higher than that in CF. The vessels in the xylem transport water and inorganic salts upward. We found that during dormancy, the number of vessels in HF was significantly higher than that in CF, within a certain range. In samples collected on 15 November 2020 and 15 January 2021, the vessel area of HF was significantly smaller than that of CF (Table 1, Figure 4). The pith is located in the central part of the branch, which is composed of parenchyma cells and can store nutrients. We observed that the parenchyma cells in the pith of the two varieties were closely arranged. In samples collected on 15 March 2021, the pith radius of HF was significantly larger than that of CF, and HF branches had a higher proportion of pith than CF (Table 1, Figure 4C).

Table 1.
Anatomical structure indices of the cross-sections of one-year-old apple tree branches.
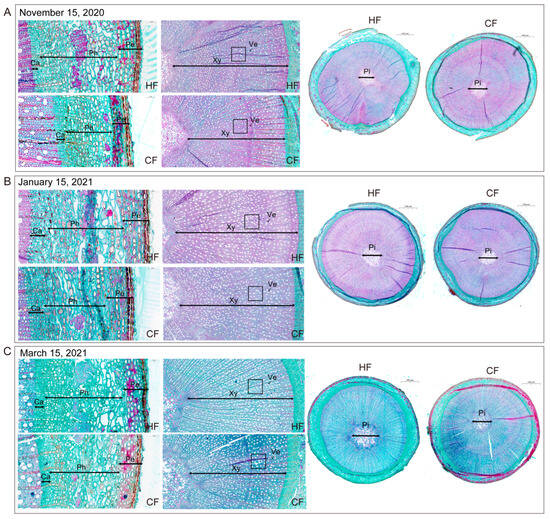
Figure 4.
Anatomical structure of cross-sections of one-year-old apple tree branches in dormant period: (A) 15 November 2020; (B) 15 January 2021; and (C) 15 March 2021. periderm (Pe); phloem (Ph); cambium (Ca); xylem (Xy); vessel (Ve); pith (Pi); ‘Hanfu’ (HF); ‘Naganofuji 2’ (CF).
3.4. Correlation Analysis
We analyzed the correlation between the physiological and biochemical data of HF and CF. As shown in Figure 5A, there were significant positive correlations between MDA and glucose and relative electrical conductivity (p < 0.05), a highly significant positive correlation between O2− and glucose (p < 0.01), a highly significant positive correlation between SOD and fructose (p < 0.01), and significant negative correlations between glucose and relative electrical conductivity and soluble protein (p < 0.05). Figure 5B shows that there were significant positive correlations between H2O2 and CAT and soluble sugar (p < 0.05), a highly significant positive correlation between CAT and relative electrical conductivity (p < 0.001), and a significant positive correlation between sucrose and POD (p < 0.001).
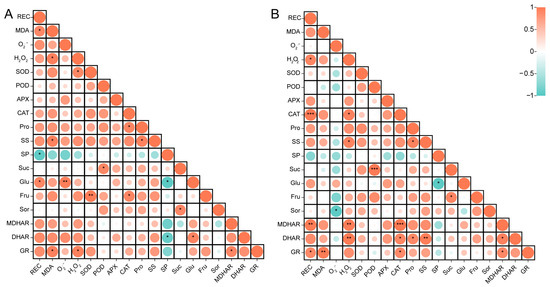
Figure 5.
Pearson correlation analysis between physiological and biochemical indices of HF (A) and CF (B). Relative electrical conductivity (REC); malondialdehyde (MDA); superoxide dismutase (SOD); peroxidase (POD); ascorbate peroxidase (APX); catalase (CAT); proline (Pro); soluble sugar (SS); soluble protein (SP); sucrose (Suc); glucose (Glu); fructose (Fru); sorbitol (Sor); monodehydroascorbate reductase (MDHAR); dehydroascorbate reductase (DHAR); glutathione reductase (GR). The levels of significance are indicated as follows: * p < 0.05; ** p < 0.01; *** p < 0.001.
4. Discussion
Dormancy is an important strategy for the survival of apple trees and other temperate perennial woody plants under low temperature conditions [26,27]. The shortening of the photoperiod and the decrease in temperature are important conditions for inducing dormancy [28]. Although, fruit trees in the Rosaceae family, such as apple, are insensitive to changes in the photoperiod and initiate dormancy mainly in response to low temperatures [29,30]. However, global warming may lead to a lack of sufficient low temperature accumulation during winter dormancy, thereby impacting the normal phenological stages of deciduous fruit trees, such as affecting germination uniformity, fruit ripening, or leaf abscission [31,32]. In addition, an increase in temperature may also promote the extension of the growing season, resulting in a delay in apple dormancy, which will lead to changes in the content and structure of metabolites [33] and reduce the cold resistance of apples. Therefore, this study analyzed the physiological and biochemical changes during dormancy in apple varieties with different degrees of cold resistance under natural low temperature conditions in winter from 2018 to 2021. Figure 1 shows that the lowest temperature of each year appeared around January, while the lowest temperature during the dormancy period during 2020–2021 increased significantly. Therefore, an increase in temperature during dormancy may affect the cold accumulation of plants. In addition, an increase in temperature during ecodormancy may induce the early germination of buds and increase the risk of low temperature damage [34].
The temperature, whether continuously low during winter dormancy or repeatedly alternating between high and low in late winter and early spring, is an important environmental factor affecting the optimal cultivation area of apple trees. The difference in the cold resistance of apple varieties can be reflected in the changes in physiological and biochemical indices under low temperature stress. Xu et al. found that under natural low temperature conditions in mid-November, the membrane structure of cold-resistant HF was more stable, and the antioxidant enzyme activity and osmotic adjustment substance content were higher [16]. The plasma membrane in plants plays a central role in regulating various cellular processes in response to low temperature injury [35]. Previous studies have suggested that low temperatures can increase membrane permeability, leading to the leakage of internal solutes, decreased osmotic potential, and cell death due to water loss in severe cases [36]. Therefore, the relative electrical conductivity value is considered to be an important index to evaluate the degree of plant membrane damage [37]. In this study, we found that the relative electrical conductivity and MDA content of HF and CF gradually increased with the deepening of dormancy and reached a maximum at the end of January (Figure 2A), which was similar to the results of previous studies on the cold resistance of Quercus during dormancy [38]. In addition, the relative electrical conductivity and MDA content of CF were significantly higher than those of HF. The results showed that CF was severely damaged by low temperatures during winter dormancy in the cold region of Northeast China, and its cold resistance was weaker than that of HF. Wang et al. evaluated the cold resistance of different apple rootstocks and found that the relative electrical conductivity and MDA content of GM256, with strong cold resistance, were lower under different low temperature treatments, while the relative electrical conductivity and MDA content of M9, with weak cold resistance, were higher [39].
Low temperature stress can induce an increase in ROS content in plants, and with the deepening of stress, the accumulation of ROS exceeds the range that plants can tolerate, leading to adverse effects [40]. In this study, in both HF and CF during 15 December 2020 to 31 January 2021, the O2− content increased at first and then decreased in mid and late January, while the H2O2 content increased slightly. This result may be attributed to the increase in SOD activity during the internal dormancy period. SOD is first activated as the first line of defense of the antioxidant system, which promotes the conversion of O2− to H2O2. Sedaghat et al. also found that the SOD activity in the terminal buds of figs increased during dormancy, which may be beneficial to germination [41]. Previous studies have suggested that ROS produced in the early stage of stress may act as a signal that promotes an active response to various abiotic stresses and the establishment of plant defense systems; ROS also play a role in the process of endodormancy release [32]. As an important part of the antioxidant system, SOD, POD, CAT antioxidant enzymes and APX, DHAR, MDHAR, and GR in the AsA-GSH cycle pathway are important ways for plants to maintain the dynamic balance of ROS under stress conditions. In this study, the activities of SOD, POD, and CAT in HF and CF changed significantly at different dormancy stages. The activities of SOD and POD in HF with strong cold resistance during dormancy were significantly higher than those in CF, which may lead to their different adaptability to environmental stress during dormancy. (Figure 2B). Meng et al. conducted low temperature treatment on maize with different cold tolerance and found that the SOD activity of the cold-tolerant RM line was higher at low temperatures, while the POD activity decreased. [42]. Jing et al. analyzed the changes in antioxidant enzyme activities of apple dwarfing interstocks with different degrees of cold resistance during overwintering. It was found that GM256, with strong cold resistance, had higher SOD and POD activities, and the production rate of superoxide anions was lower. An increase in antioxidant enzyme activity may reduce the damage to the membrane system caused by low temperatures and enhance the cold resistance of plants [43]. The AsA-GSH cycle is important for cells to resist ROS damage, and the activity of these enzymes is positively correlated with plant stress tolerance. In this study, the content of H2O2 in cold-resistant HF was low, which may be associated with the AsA-GSH cycle-related enzymes. As an essential enzyme in the ASA-GSH cycle, APX can use AsA to provide electrons and catalyze the reduction of H2O2 to H2O [44]. The higher APX activity in HF alleviated the damage of H2O2 to cells. The activity of APX first increased, effectively alleviating the cell damage caused by the high ROS levels, and then decreased after mid-January. Hernandez et al. found that the APX activity in peach buds in cold regions increased by 12 times during endodormancy and then decreased at the end of endodormancy, which was similar to the results of this study [45]. In addition, MDHAR and DHAR can promote the regeneration of AsA, which is involved in the removal of H2O2 by APX. GR promotes GSH production, and AsA and GSH produced in the ASA-GSH cycle can be used as non-enzymatic antioxidants to scavenge ROS [46]. In this study, the activities of MDHAR and DHAR were higher in cold-resistant HF, while the difference in GR activity was small, and the activities of MDHAR, DHAR, and GR decreased on 15 March 2021 in both HF and CF. This is similar to the findings of Wang et al. on two Wucai with different cold resistance under low temperature stress. WS-1 with strong cold resistance has higher activities of GR, DHAR, and MDHAR, which improves the antioxidant level under low temperature stress [47]. In addition, 0 °C environment increased the APX and GR activities of winter jujube and maintained the postharvest quality of winter jujube by reducing oxidative damage and enhancing antioxidant capacity [48]. In summary, with the deepening of dormancy and decrease in natural temperature, the activity of antioxidant enzymes increases, and promotes the AsA-GSH cycle to respond to low temperature stress, thereby enhancing the scavenging ability of ROS, reducing the degree of cell membrane damage and membrane lipid peroxidation, and maintaining the balance of reactive oxygen metabolism recovery.
Under low temperature stress, the accumulation of osmotic adjustment substances is an important way for plants to improve their tolerance for low temperatures. Proline can not only participate in osmotic balance and maintain membrane structure and protein stability but can also scavenge ROS [49]. Previous studies have found that spraying 20 mmol proline on citrus leaves can significantly improve their antioxidant capacity under −3 °C low temperature conditions, regulate the redox state of cells, and stabilize cell activity as an ROS scavenger under oxidative stress conditions [50]. In this study, the proline content in HF and CF fluctuated between December and January, and HF accumulated more proline content overall (Figure 2C). Zhao et al. found that a large amount of proline accumulated in wheat after cold acclimation [51]. Similarly, Qi et al. found that rapeseed seedlings with strong cold resistance could accumulate more proline under low temperature conditions and displayed higher antioxidant enzyme activity and reduced ROS production [52]. In summary, we concluded that proline accumulation during dormancy may improve the adaptability of HF to low temperatures by reducing ROS content and increasing cell osmotic potential. Additionally, the soluble protein content in HF and CF reached a maximum in December and then decreased to varying degrees (Figure 2C). Similarly, Karimi et al. found that the soluble protein content in grape buds decreased in March during cold acclimation [53]. The increase in soluble protein content during natural dormancy may be an important and effective means for apple trees to enhance membrane stability and frost resistance as the accumulation of soluble protein can improve the water holding capacity of cells.
It has been reported that soluble sugar serves important functions such as energy storage, signal transduction, ROS scavenging, and osmotic adjustment [32,36,54]. Furthermore, soluble sugar plays an important role in enhancing plant cold tolerance and is a physiological marker that reflects the depth of dormancy [55]. In this study, the soluble sugar content continued to increase during the dormant period, reaching a maximum at the end of January and then decreasing (Figure 2C). Zhang et al. found that the soluble sugar content in Prunus mume buds was low at the early stage of dormancy and then increased with the deepening of dormancy, which may be related to the promotion of starch hydrolysis by low temperatures [56]. The decrease in natural temperatures during dormancy increases the risk of plant injury due to freezing, and the accumulation of soluble sugar is a strategy for plants to cope with low temperature stress. Previous studies have found that peach trees in cold regions accumulate more soluble sugar than those in temperate regions during dormancy, and the contents of sorbitol and sucrose were the highest among the sugars [45]. Rady et al. found that the content of soluble sugar in dormant apple tree buds was also higher [57], which was consistent with the results of this study (Figure 2). The contents of sucrose and sorbitol in HF and CF were higher than those of glucose and fructose, which indicated that sucrose and sorbitol may participate in plant protection during winter dormancy by enhancing the tolerance of plants to low temperature stress. In addition, the contents of glucose and fructose in HF and CF also increased rapidly during dormancy (Figure 3), and the accumulated glucose and fructose may meet the carbon demand of apple trees under low temperature conditions [58,59]. The decrease in total soluble sugar content and different sugar components during March may be in preparation for growth recovery activities, such as germination and flowering [38].
The cold resistance of plants is related to both the external environment and internal physiological and biochemical activities, and it also varies depending on tissue structure. The living bark cells of many woody plants have been shown to be more resistant to cold than xylem cells are, and the pith in the center of the branch is more sensitive to freezing injury than the outer layers [60,61]. However, few studies have focused on the relationship between different tissue structures and cold resistance in apple tree branches. Therefore, this experiment evaluated the cold resistance of apple trees by analyzing the anatomical structure indices of pith, xylem, phloem, periderm, and vessels of one-year-old apple tree branches.
The periderm is a secondary protective tissue, which is the first line of defense for plants in resisting stress. Zhai et al. found that the periderm of cold-resistant Hibiscus was thicker and the number of cork layer cells was greater compared to those of non-cold-resistant varieties [12]. In this study, the cold-resistant HF had a thicker periderm, and the cork layer cells were arranged closely to reduce the evaporation of water (Figure 4A). Sieve tubes and companion cells in the phloem are the main components involved in transporting photosynthetic assimilation products. They can also store starch and other substances, increase the osmotic potential of cells, and prevent cell membranes from being damaged by dehydration or low temperatures. In addition, the phloem can support the change in carbon distribution and promote the accumulation of starch in flower buds and the development of leaf primordia in winter [27,62,63]. In this study, we found that the phloem of HF, with strong cold resistance, was thicker and accumulated a larger amount of soluble sugar (primarily sucrose and sorbitol) than that of CF (Figure 2C, Figure 3 and Figure 4). Similarly, in comparing the cold resistance of different varieties of raspberries, Chang et al. found that raspberries with stronger cold resistance often have thicker phloem [64].
We found that in mid-November, the cambium of CF, with weaker cold resistance, was thicker than that of HF (Figure 4A). This result may have been due to the stronger growth potential and vigorous vegetative growth of CF, which increased the differentiation of cambium tissue into xylem and phloem, thereby potentially increasing the risk of low temperature injury. In addition, the cambium tissue enters dormancy later than the cortex and xylem, making it susceptible to freeze damage if low temperatures occur in the early stage of endodormancy. However, when the plant completely enters the ecodormancy period (mid to late January in northern regions of China), even under conditions of severe cold temperatures, the cambium is more resistant to cold than the cortex or xylem are, and it is less prone to freeze injury. This may be due to the active embryonic cells of the cambium tissue that display strong meristem ability and can rapidly metabolize, self-repair, and maintain cell stability when freeze injury occurs. Moreover, it has been theorized that the cambium will gradually regain its vitality and begin partial lignification after the ecodormancy period when the plants experience a certain degree of low temperatures. When low temperatures occur in the late winter and early spring, microtubule depolymerization may occur; thus, the early activation of the cambium increases the risk of freezing injury [65]. Furthermore, the reactivation of cambium can also promote the conversion of starch into soluble sugar, increase the osmotic potential of cells, and provide the pressure required for cell expansion [66]. However, the results of this study showed that there was no significant difference in cambium thickness between the two varieties with different degrees of cold resistance in mid-January and mid-March (Figure 4B,C). We propose that the thicker cambium of CF during endodormancy is vulnerable to low temperature damage, which may be one of the reasons for its weak cold resistance.
The proportion of xylem is positively correlated with the cold resistance of plant varieties [67]. Ito et al. found that carbohydrates in Japanese pears were transported to stems during endodormancy, and this process may be affected by low temperatures, resulting in an increase in sorbitol content in the xylem and promoting the formation of cold tolerance [68]. In this study, we found that the thickness and proportion of xylem in HF were significantly greater than those of xylem in CF at different stages of dormancy (Figure 4), and the sorbitol content of HF was higher than that of CF during the endodormancy period (Figure 3). We speculate that the increase in xylem thickness and the accumulation of sorbitol may be adaptations of HF to low temperature conditions and that the secondary xylem tissue is the most cold resistant [69]. The vessels in the xylem play an important role in transporting water, and their density and area are also related to cold resistance. Guo et al. observed that the cold-resistant ‘Hunchun’ peach tree has thick vessels, which can ensure higher water transport efficiency and reduce freezing injury [70]. However, the relationship between vessel diameter and cold resistance in apple trees is inconsistent with that in peach trees. Pramsohler et al. explored the freezing pattern of apple trees during natural frost [61]. It was found that the temperature in branches with a small vessel diameter was lower than that in branches with a large vessel diameter, indicating that branches with a small vessel diameter had stronger cold resistance. Schweingruber et al. observed the anatomical characteristics of Campanulaceae plants from different regions and found that those living in cold regions have smaller vessel diameters in their stems than those living in warm regions [71]. It is likely that this feature may enable plants to cope with embolism and cavitation caused by freezing, thereby improving their tolerance to low temperatures. In addition, García-Cervigón et al. reached a similar conclusion that a decrease in vessel diameter and an increase in vessel density are adaptations of Nothofagus pumilio to its high-elevation and low temperature environment [72]. The results of the current study revealed that, during different periods of dormancy, the vessel density in the xylem of HF was significantly higher than that in the xylem of CF, and the vessel area of HF was significantly smaller than that of CF (Figure 4, Table 1). Therefore, the narrowing of vessels and the increase in vessel density of trees in cold environments are adaptive mechanisms to survive in low temperature stress environments [73,74]. Based on the study data, HF exhibits a reduced risk of embolism caused by severe winter temperatures or alternating high and low temperatures in early spring, and thus, it displays greater resistance to low temperatures.
5. Conclusions
In this study, we clarified the differences in physiological, biochemical, and structural characteristics of one-year-old apple tree branches of cold-resistant HF and cold-sensitive CF during natural dormancy. Compared with CF, HF displayed more stable membrane lipid structure and higher antioxidant enzyme activity. Additionally, HF accumulated a greater amount of proline and soluble sugar (primarily sucrose and sorbitol) than CF did with the natural decrease in temperature. A comparison of the structural characteristics of HF and CF one-year-old branches revealed that thicker periderm, phloem, and xylem tissues; smaller vessel diameters; and a higher vessel density likely enhance the cold resistance of apple tree branches. The results of this study contribute to an improved understanding of the physiological, biochemical, and anatomical changes in apple tree branches in response to low temperatures during dormancy. Furthermore, this study provides a reference for evaluating the cold resistance of apple trees.
Author Contributions
Conceptualization, D.Z. and S.Q.; methodology, G.X. and M.H.; software, G.X. and M.H.; validation, G.X. and M.H.; formal analysis, G.X. and M.H.; investigation, G.X.; resources, D.Z. and S.Q.; data curation, G.X. and M.H.; writing—original draft preparation, G.X.; writing—review and editing, D.Z., D.L. and S.Q.; visualization, G.X. and M.H.; supervision, D.Z. and S.Q.; project administration, S.Q.; funding acquisition, S.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the earmarked fund for CARS [grant number CARS-27], the Applied Basic Research Project of Science and Technology Department of Liaoning Province [grant number 2023JH2/101300131], the Scientific Research Funding Project of Liaoning Province [grant number LJKMZ20221026], and the Shenyang Science and Technology Plan Seed Industry Innovation Special [grant number 22-318-2-03].
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, G.; Zhou, J.; Lyu, D.; Qin, S. Cold resistance evaluation of four apple varieties. J. Fruit Sci. 2023, 40, 669–679. [Google Scholar]
- Theocharis, A.; Clément, C.; Barka, E.A. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar]
- Manasa, S.L.; Panigrahy, M.; Panigrahi, K.C.; Rout, G.R. Overview of cold stress regulation in plants. Bot. Rev. 2022, 88, 359–387. [Google Scholar]
- Wang, H.; Gong, M.; Xin, H.; Tang, L.; Dai, D.; Gao, Y.; Liu, C. Effects of chilling stress on the accumulation of soluble sugars and their key enzymes in Jatropha curcas seedlings. Physiol. Mol. Biol. Plants 2018, 24, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, H.; Wang, B.; Zhang, Y.; Wang, J.; Cheng, C.; Huang, Y. Exogenous melatonin enhances cold resistance by improving antioxidant defense and cold-responsive genes’ expression in banana. Horticulturae 2022, 8, 260. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [PubMed]
- Wei, Y.; Chen, H.; Wang, L.; Zhao, Q.; Wang, D.; Zhang, T. Cold acclimation alleviates cold stress-induced PSII inhibition and oxidative damage in tobacco leaves. Plant Signal. Behav. 2022, 17, 2013638. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, P.; Krasylenko, Y.; Zeiner, A.; Šamaj, J.; Takáč, T. Signaling Toward Reactive Oxygen Species-Scavenging Enzymes in Plants. Front. Plant Sci. 2021, 11, 618835. [Google Scholar] [PubMed]
- Fujita, M.; Hasanuzzaman, M. Approaches to Enhancing Antioxidant Defense in Plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
- Rani, A.; Kiran, A.; Sharma, K.D.; Prasad, P.V.V.; Jha, U.C.; Siddique, K.H.M.; Nayyar, H. Cold Tolerance during the Reproductive Phase in Chickpea (Cicer arietinum L.) Is Associated with Superior Cold Acclimation Ability Involving Antioxidants and Cryoprotective Solutes in Anthers and Ovules. Antioxidants 2021, 10, 1693. [Google Scholar] [CrossRef]
- Yu, D.J.; Hwang, J.Y.; Chung, S.W.; Oh, H.D.; Yun, S.K.; Lee, H.J. Changes in cold hardiness and carbohydrate content in peach (Prunus persica) trunk bark and wood tissues during cold acclimation and deacclimation. Sci. Hortic. 2017, 219, 45–52. [Google Scholar] [CrossRef]
- Kwon, J.H.; Nam, E.Y.; Yun, S.K.; Kim, S.J.; Yu, D.J.; Lee, H.J. Comparative carbohydrate metabolism in the shoots of a cold-hardy and a cold-sensitive peach (Prunus persica) cultivar during cold acclimation and deacclimation. Hortic. Environ. Biotechnol. 2022, 63, 39–53. [Google Scholar] [CrossRef]
- Buchner, O.; Neuner, G. Winter frost resistance of Pinus cembra measured in situ at the alpine timberline as affected by temperature conditions. Tree Physiol. 2011, 31, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Serra, O.; Mähönen, A.P.; Hetherington, A.J.; Ragni, L. The making of plant armor: The periderm. Annu. Rev. Plant Biol. 2022, 73, 405–432. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.T. A Structural and Physiological Study of Winter Hardiness of Different Variance-Typed Hibiscus syriacus. Master’s Thesis, Hebei Normal University of Science & Technology, Qinhuangdao, China, 2015. [Google Scholar]
- Hajihashemi, S.; Noedoost, F.; Geuns, J.M.C.; Djalovic, I.; Siddique, K.H.M. Effect of Cold Stress on Photosynthetic Traits, Carbohydrates, Morphology, and Anatomy in Nine Cultivars of Stevia rebaudiana. Front. Plant Sci. 2018, 9, 1430. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. Climatic influence on tree wood anatomy: A review. J. Wood Sci. 2021, 67, 24. [Google Scholar] [CrossRef]
- Qin, S.; Xu, G.; He, J.; Li, L.; Ma, H.; Lyu, D. A chromosome-scale genome assembly of Malus domestica, a multi-stress resistant apple variety. Genomics 2023, 115, 110627. [Google Scholar] [CrossRef]
- Xu, G.; Li, L.; Zhou, J.; Lyu, D.; Zhao, D.; Qin, S. Comparison of transcriptome and metabolome analysis revealed differences in cold resistant metabolic pathways in different apple cultivars under low temperature stress. Hortic. Plant J. 2023, 9, 183–198. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Ma, C.; Zhang, Y.; Polle, A.; Rennenberg, H.; Cheng, X.; Luo, Z.B. Overexpression of bacterial g-glutamylcysteine synthetase mediates changes in cadmium influx, allocation and detoxification in poplar. New Phytol. 2015, 205, 240–245. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, H.; He, J.; Lyu, D.; Li, H. Integration of cadmium accumulation, subcellular distribution, and physiological responses to understand cadmium tolerance in apple rootstocks. Front. Plant Sci. 2017, 8, 966. [Google Scholar] [CrossRef]
- Li, L.; Yang, B.; Zhao, X.; Wang, P.; Lyu, D.; Qin, S. Auxin Participates in the Regulation of the Antioxidant System in Malus baccata Borkh. Roots under Sub-Low Temperature by Exogenous Sucrose Application. Horticulturae 2023, 9, 297. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Luo, J.; Ma, C.; Li, S.; Qu, L.; Gai, Y.; Jiang, X.; Janz, D.; Polle, A.; et al. A transcriptomic network underlies microstructural and physiological responses to cadmium in Populus × canescens. Plant Physiol. 2013, 162, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Huang, Y.; Qin, S.; He, J.; Lyu, D. Integration of cell wall fraction, organic matter content, and membrane to understand crispness changes in apples. Sci. Hortic. 2023, 321, 112309. [Google Scholar] [CrossRef]
- Faust, M.; Erez, A.; Rowland, L.J.; Wang, S.Y.; Norman, H.A. Bud dormancy in perennial fruit trees; physiological basis for dormancy induction maintenance and release. HortScience 1997, 32, 623–629. [Google Scholar] [CrossRef]
- Fadón, E.; Herrero, M.; Rodrigo, J. Dormant flower buds actively accumulate starch over winter in sweet cherry. Front. Plant Sci. 2018, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Considine, M.J.; Considine, J.A. On the language and physiology of dormancy and quiescence in plants. J. Exp. Bot. 2016, 67, 3189–3203. [Google Scholar] [CrossRef]
- Heide, O.M.; Prestrud, A.K. Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiol. 2005, 25, 109–114. [Google Scholar] [CrossRef]
- Cooke, J.E.K.; Eriksson, M.E.; Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef]
- Beauvieux, R.; Wenden, B.; Dirlewanger, E. Bud dormancy in perennial fruit tree species: A pivotal role for oxidative cues. Front. Plant Sci. 2018, 9, 657. [Google Scholar] [CrossRef]
- Fadón, E.; Fernandez, E.; Behn, H.; Luedeling, E. A conceptual framework for winter dormancy in deciduous trees. Agronomy 2020, 10, 241. [Google Scholar] [CrossRef]
- Liu, H.; Lu, C.; Wang, S.; Ren, F.; Wang, H. Climate warming extends growing season but not reproductive phase of terrestrial plants. Global Ecol. Biogeogr. 2021, 30, 950–960. [Google Scholar] [CrossRef]
- Pagter, M.; Hausman, J.F.; Arora, R. Deacclimation kinetics and carbohydrate changes in stem tissues of Hydrangea in response to an experimental warm spell. Plant Sci. 2011, 180, 140–148. [Google Scholar] [CrossRef]
- Takahashi, D.; Li, B.; Nakayama, T.; Kawamura, Y.; Uemura, M. Plant plasma membrane proteomics for improving cold tolerance. Front. Plant Sci. 2013, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Yuanyuan, K.; Bruno, A.K.; Lele, Z.; Jincai, L. Cold Stress in Wheat: Plant Acclimation Responses and Management Strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef]
- Chen, L.J.; Xiang, H.Z.; Miao, Y.; Zhang, L.; Guo, Z.F.; Zhao, X.H.; Lin, J.W.; Li, T.L. An overview of cold resistance in plants. J. Agron. Crop Sci. 2014, 200, 237–245. [Google Scholar] [CrossRef]
- Morin, X.; Améglio, T.; Ahas, R.; Kurz-Besson, C.; Lanta, V.; Lebourgeois, F.; Miglietta, F.; Chuine, I. Variation in cold hardiness and carbohydrate concentration from dormancy induction to bud burst among provenances of three European oak species. Tree Physiol. 2007, 27, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Ya, H.U.; Chen, B.H.; Zhu, Y.F.; Dawuda, M.M.; Svetla, S. Physiological mechanisms of resistance to cold stress associated with 10 elite apple rootstocks. J. Integr. Agric. 2018, 17, 857–866. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Role of Reactive Oxygen Species and Hormones in Plant Responses to Temperature Changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Sedaghat, S.; Gaaliche, B.; Rahemi, M.; Zare, H.; Jafari, M. Enzymatic activity and physico-chemical changes of terminal bud in rain-fed fig (Ficus carica L.‘Sabz’) during dormant season. Hortic. Plant J. 2022, 8, 195–204. [Google Scholar] [CrossRef]
- Meng, A.; Wen, D.; Zhang, C. Maize Seed Germination Under Low-Temperature Stress Impacts Seedling Growth Under Normal Temperature by Modulating Photosynthesis and Antioxidant Metabolism. Front. Plant Sci. 2022, 13, 843033. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Liu, M.; Yin, B.; Liang, B.; Li, Z.; Zhang, X.; Xu, J.; Zhou, S. Effects of 10 Dwarfing Interstocks on Cold Resistance of ‘Tianhong 2’ Apple. Horticulturae 2023, 9, 827. [Google Scholar] [CrossRef]
- Li, S. Novel insight into functions of ascorbate peroxidase in higher plants: More than a simple antioxidant enzyme. Redox Biol. 2023, 64, 102789. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Díaz-Vivancos, P.; Martínez-Sánchez, G.; Alburquerque, N.; Martínez, D.; Barba-Espín, G.; Acosta-Motos, J.R.; Carrera, E.; García-Bruntón, J. Physiological and biochemical characterization of bud dormancy: Evolution of carbohydrate and antioxidant metabolisms and hormonal profile in a low chill peach variety. Sci. Hortic. 2021, 281, 109957. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhang, J. Advances in the research on the AsA-GSH cycle in horticultural crops. Front. Agric. China 2010, 4, 84–90. [Google Scholar] [CrossRef]
- Wang, J.; Fang, R.; Yuan, L.; Yuan, G.; Zhao, M.; Zhu, S.; Hou, J.; Chen, G.; Wang, C. Response of photosynthetic capacity and antioxidative system of chloroplast in two wucai (Brassica campestris L.) genotypes against chilling stress. Physiol. Mol. Biol. Plants 2020, 26, 219–232. [Google Scholar] [CrossRef]
- Sang, Y.; Yang, W.; Liu, Y.; Zhang, W.; Guo, T.; Shen, P.; Tang, Y.; Guo, M.; Chen, G. Influences of low temperature on the postharvest quality and antioxidant capacity of winter jujube (Zizyphus jujuba Mill. cv. Dongzao). LWT 2022, 154, 112876. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Abbas, S.; Hassan, M.U.; Saeed, F.; Haider, S.; Sharif, R.; Anand, A.; Corpas, F.J.; Jin, W.; et al. Assessment of proline function in higher plants under extreme temperatures. Plant Biol. 2023, 25, 379–395. [Google Scholar] [CrossRef]
- Mohammadrezakhani, S.; Rezanejad, F.; Hajilou, J. Effect of putrescine and proline on proflies of GABA, antioxidant activities in leaves of three Citrus species in response to low temperature stress. J. Plant Biochem. Biot. 2021, 30, 545–553. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, M.; Xu, K.; Li, J.; Li, S.; Zhang, S.; Yang, X. Integrated transcriptomics and metabolomics analyses provide insights into cold stress response in wheat. Crop J. 2019, 7, 857–866. [Google Scholar] [CrossRef]
- Qi, W.; Wang, F.; Ma, L.; Qi, Z.; Liu, S.; Chen, C.; Wu, J.; Wang, P.; Yang, C.; Wu, Y.; et al. Physiological and Biochemical Mechanisms and Cytology of Cold Tolerance in Brassica napus. Front. Plant Sci. 2020, 11, 1241. [Google Scholar] [CrossRef]
- Karimi, R.; Ershadi, A.; Esna-Ashari, M.; Boojar, M.M.A. Seasonal changes in soluble proteins, total phenol and malondialdehyde content and their relationship with cold hardiness of some grapevine cultivars. J. Crop. Improv. 2014, 16, 999–1013. [Google Scholar]
- Nägele, T.; Heyer, A.G. Approximating subcellular organisation of carbohydrate metabolism during cold acclimation in different natural accessions of Arabidopsis thaliana. New Phytol. 2013, 198, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Cuneo, I.F.; Luedeling, E.; Alvarado, L.; Farias, D.; Saa, S. Starch and hexoses concentrations as physiological markers in dormancy progression of sweet cherry twigs. Trees 2019, 33, 1187–1201. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, K.; Li, Q. Research on Chilling Requirements and Physiological Mechanisms of Prunus mume. Horticulturae 2023, 9, 603. [Google Scholar] [CrossRef]
- Rady, M.M.; Seif El-Yazal, M.A. Response of “Anna” apple dormant buds and carbohydrate metabolism during floral bud break to onion extract. Sci. Hortic. 2013, 155, 78–84. [Google Scholar] [CrossRef]
- Gholizadeh, J.; Sadeghipour, H.; Abdolzadeh, A.; Hemmati, K.; Hassani, D.; Vahdati, K. Redox rather than carbohydrate metabolism differentiates endodormant lateral buds in walnut cultivars with contrasting chilling requirements. Sci. Hortic. 2017, 225, 29–37. [Google Scholar] [CrossRef]
- Citadin, I.; Pertille, R.H.; Loss, E.M.S.; Oldoni, T.L.C.; Danner, M.A.; Júnior, A.W.; Lauri, P.É. Do low chill peach cultivars in mild winter regions undergo endodormancy? Trees 2022, 36, 1273–1284. [Google Scholar] [CrossRef]
- Quamme, H.; Weiser, C.J.; Stushnoff, C. The mechanism of freezing injury in xylem of winter apple twigs. Plant Physiol. 1973, 51, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Pramsohler, M.; Hacker, J.; Neuner, G. Freezing pattern and frost killing temperature of apple (Malus domestica) wood under controlled conditions and in nature. Tree Physiol. 2012, 32, 819–828. [Google Scholar] [CrossRef]
- Gordon, D.; Damiano, C.; DeJong, T.M. Preformation in vegetative buds of Prunus persica: Factors influencing number of leaf primordia in overwintering buds. Tree Physiol. 2006, 26, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.M.; Savage, J.A. Seasonal changes in temperate woody plant phloem anatomy and physiology: Implications for long-distance transport. AoB Plants 2021, 13, plab028. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Zhang, L.; Dong, Q.; Luan, H.; Jia, P.; Qi, G.; Guo, S.; Zhang, X. The anatomical structure character of raspberry stems is a key factor affecting its cold resistance. Flora 2023, 298, 152196. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol. Plant. 2013, 147, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Kudo, K.; Rahman, M.H.; Nakaba, S.; Yamagishi, Y.; Nabeshima, E.; Nugroho, W.D.; Oribe, Y.; Kitin, P.; Jin, H.; et al. Climate change and the regulation of wood formation in trees by temperature. Trees 2018, 32, 3–15. [Google Scholar] [CrossRef]
- Amrina, S.; Vivek, D.; Tejpal, G.; Singh, A.P.; Yelam, S.; Pandey, G.K. Simultaneous over-expression of PaSOD and RaAPX in transgenic Arabidopsis thaliana confers cold stress tolerance through increase in vascular lignifications. PLoS ONE 2014, 9, e110302. [Google Scholar]
- Ito, A.; Sugiura, T.; Sakamoto, D.; Moriguchi, T. Effects of dormancy progression and low-temperature response on changes in the sorbitol concentration in xylem sap of Japanese pear during winter season. Tree Physiol. 2013, 33, 398–408. [Google Scholar] [CrossRef]
- Ketchie, D.O.; Kammereck, R. Seasonal variation of cold resistance in Malus woody tissue as determined by differential thermal analysis and viability tests. Can. J. Bot. 1987, 65, 2640–2645. [Google Scholar] [CrossRef]
- Guo, X.; Xiao, X.; Xu, X.; Dong, F.; Zhang, L. Observation on the vessel elements of secondary xylem in late-ripening peach trees. J. Fruit Sci. 2008, 25, 22–26. [Google Scholar]
- Schweingruber, F.H.; Ríha, P.; Doležal, J. Variation in stem anatomical characteristics of Campanuloideae species in relation to evolutionary history and ecological preferences. PLoS ONE 2014, 9, e88199. [Google Scholar] [CrossRef]
- García-Cervigón, A.I.; Fajardo, A.; Caetano-Sánchez, C.; Camarero, J.J.; Olano, J.M. Xylem anatomy needs to change, so that conductivity can stay the same: Xylem adjustments across elevation and latitude in Nothofagus pumilio. Ann. Bot. 2020, 125, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Vicente, E.; Didion-Gency, M.; Morcillo, L.; Morin, X.; Vilagrosa, A.; Grossiord, C. Aridity and cold temperatures drive divergent adjustments of European beech xylem anatomy, hydraulics and leaf physiological traits. Tree Physiol. 2022, 42, 1720–1735. [Google Scholar] [CrossRef] [PubMed]
- Arnič, D.; Gričar, J.; Jevšenak, J.; Božič, G.; von Arx, G.; Prislan, P. Different Wood Anatomical and Growth Responses in European Beech (Fagus sylvatica L.) at Three Forest Sites in Slovenia. Front. Plant Sci. 2021, 12, 669229. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).