Genetic Assessment, Propagation and Chemical Analysis of Flowers of Rosa damascena Mill. Genotypes Cultivated in Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Genetic Assessment
2.2.1. DNA Extraction
2.2.2. Molecular Markers and PCR Amplification
2.2.3. Data Analysis
2.3. Propagation
2.3.1. Rooting of Shoot Cuttings
2.3.2. Data Analysis
2.4. Chemical Substances Analysis
2.4.1. Volatile Substances
2.4.2. Non-Volatile Substances
2.4.3. Data Analysis
3. Results and Discussion
3.1. Genetic Assessment
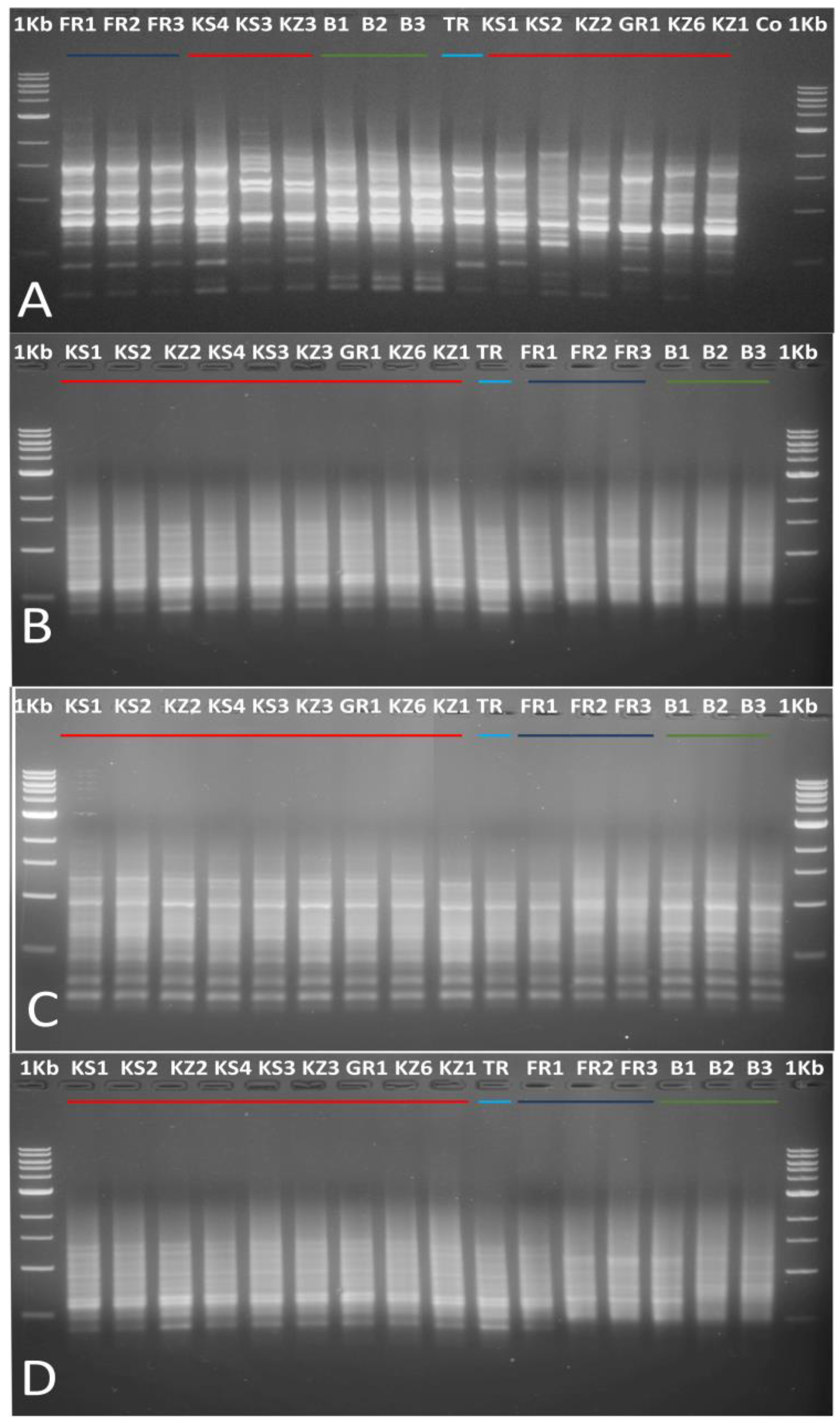
3.1.1. Use of ISSR and SCoT Markers
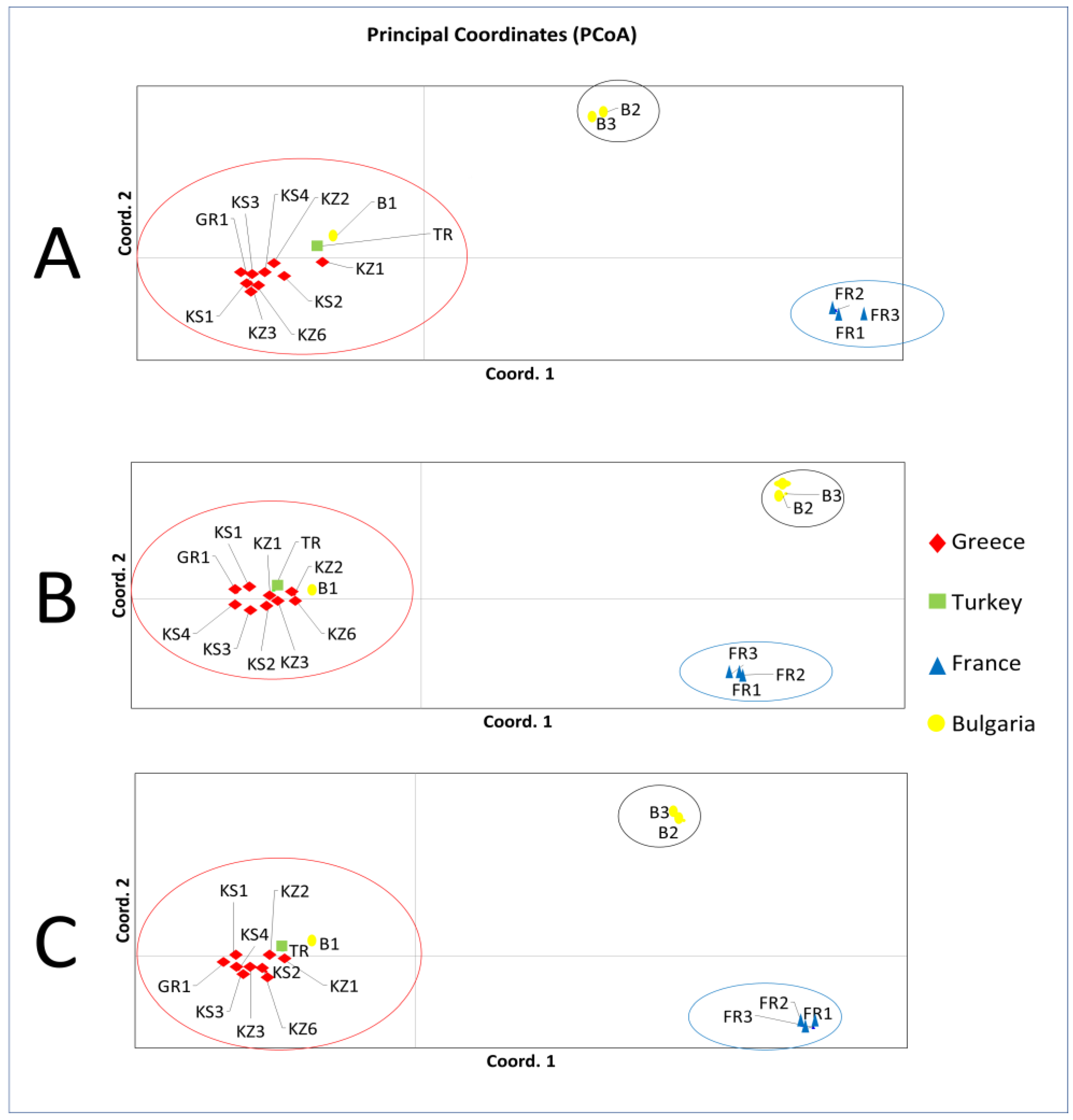
3.1.2. Principal Coordinates Analysis (PCoA)
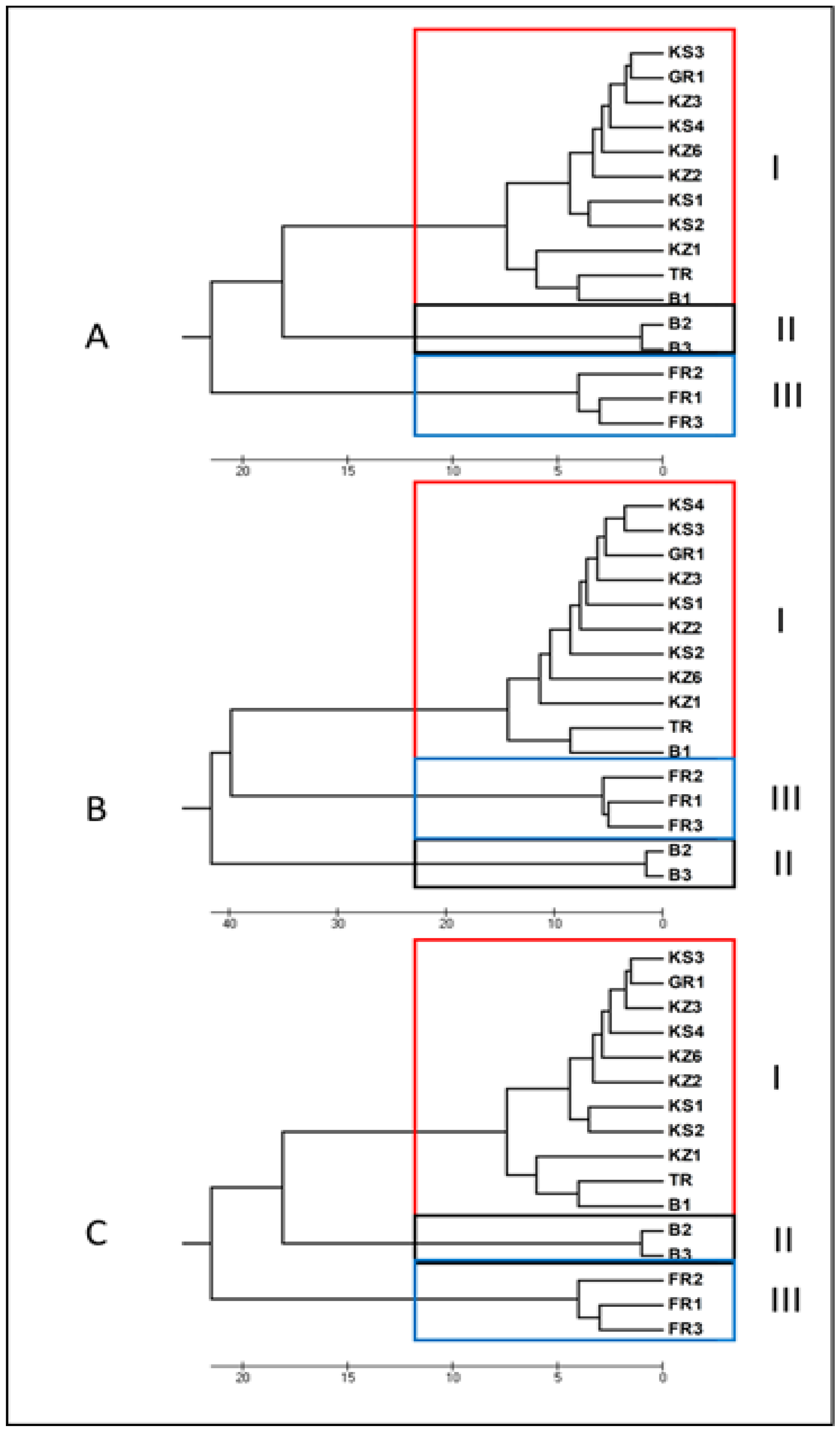
3.1.3. Unweighted Pair Group Method Analysis (UPGMA)
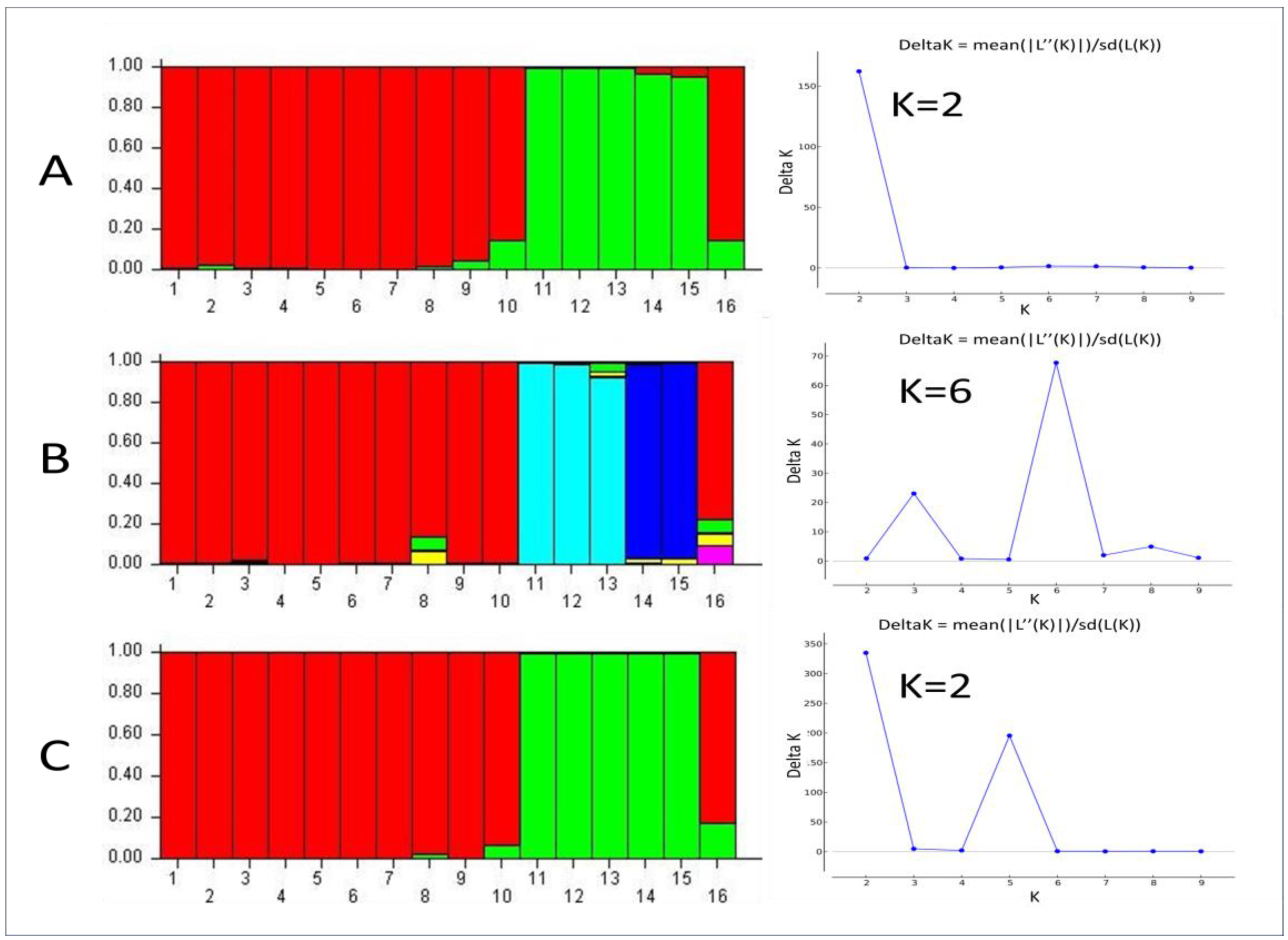
3.1.4. STRUCTURE Analysis
3.2. Propagation
Rooting of Shoot Cuttings
3.3. Chemical Substances Analysis
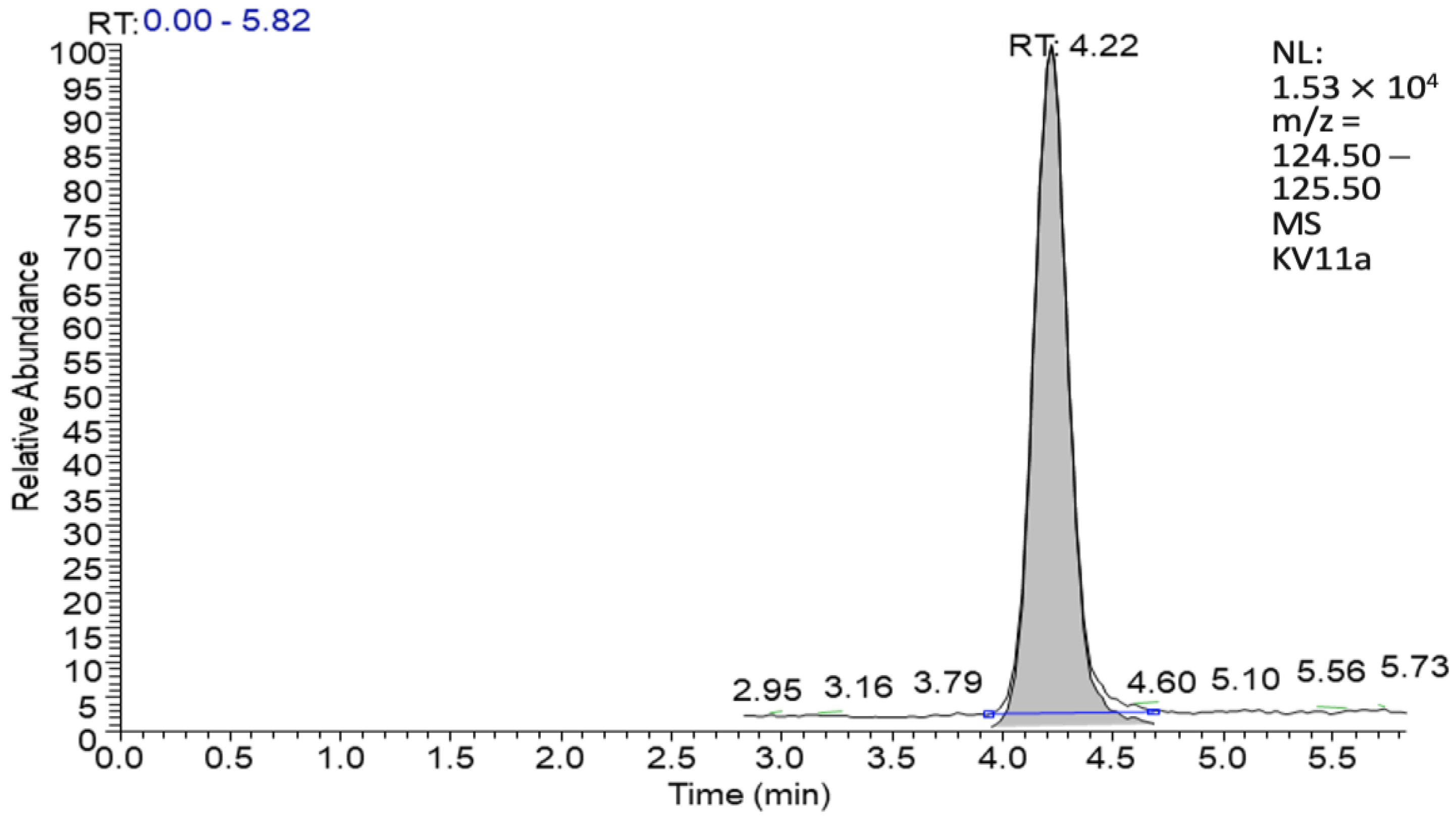
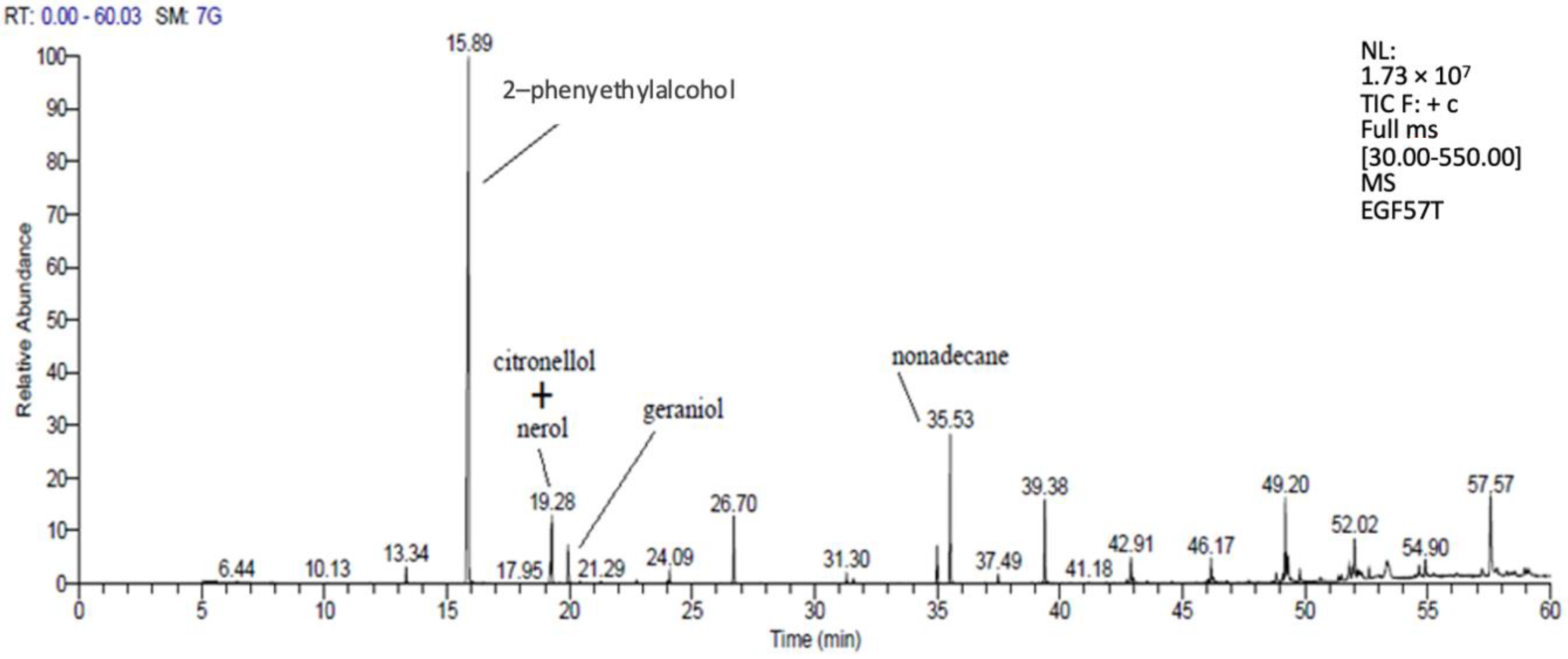
3.3.1. Volatile Substances Analysis with GC-MS
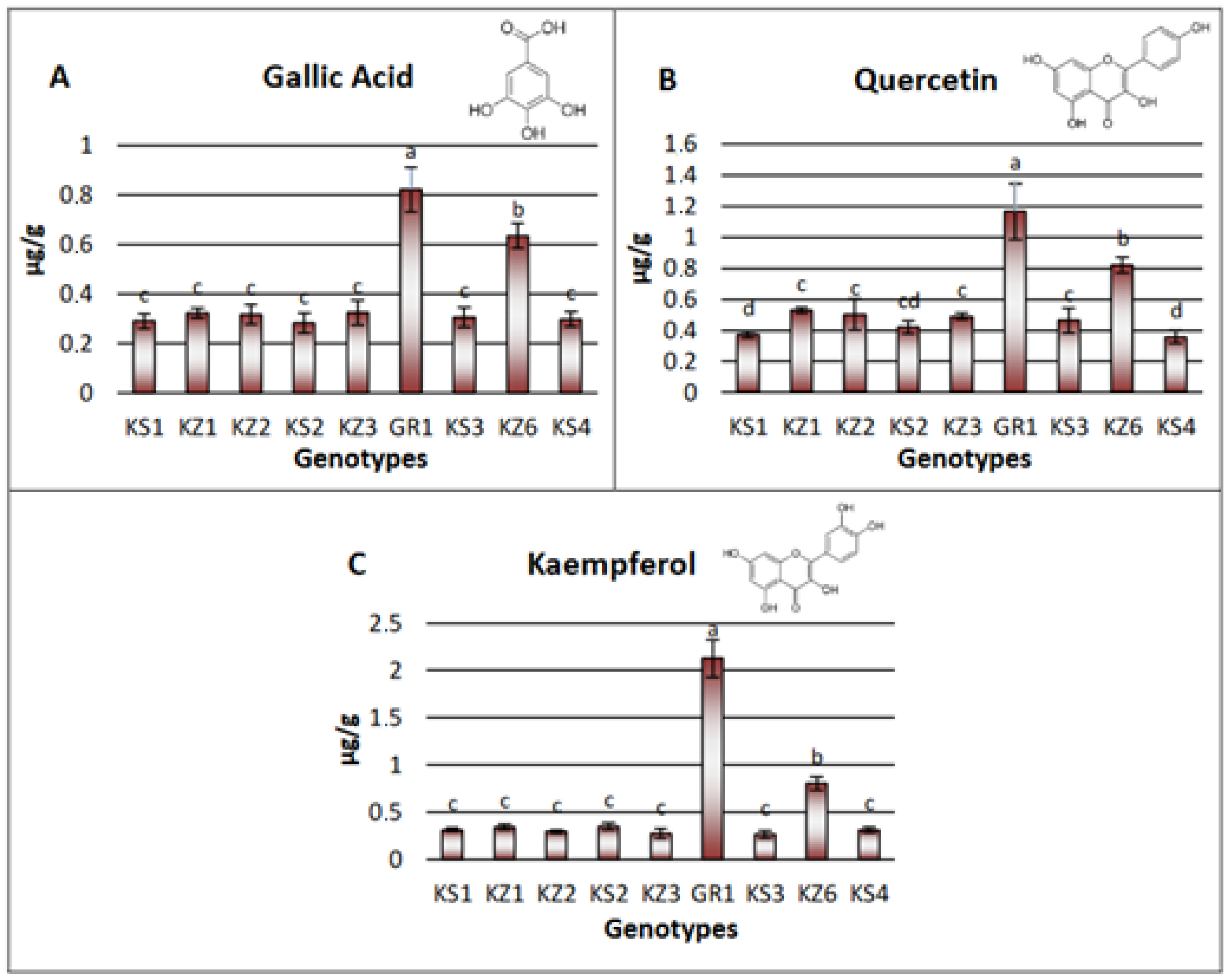
3.3.2. Non-Volatile Substances Analysis with LC-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsanaktsidis, C.G.; Tamoutsidis, E.; Kasapidis, G.; Itziou, A.; Ntina, E. Preliminary results on attributes of distillation products of the rose Rosa damascene as a dynamic and friendly to the environment rural crop. APCBEE Procedia 2012, 1, 66–73. [Google Scholar] [CrossRef][Green Version]
- Nasri, F.; Fadakar, A.; Saba, M.K.; Yousefi, B. Study of indole butyric acid (IBA) effects on cutting rooting improving some of wild genotypes of Damask roses (Rosa damascena Mill.). J. Agric. Sci. 2015, 60, 263–275. [Google Scholar] [CrossRef]
- Nazamuddin, M.; Wadud, A.; Najeed, J.; Tanwir, A.M.; Asim, M.K.; Aisha, P. Gul-e-surkh (Rosa damascena Mill.) and its therapeutic uses described in unani literature. Res. Pharm. Health Sci. 2018, 4, 411–414. [Google Scholar]
- Basim, E.; Basim, H. Antibacterial activity of Rosa damascena essential oil. Fitoterapia 2003, 74, 394–396. [Google Scholar] [CrossRef]
- Mahboubi, M. Rosa damascena as holy ancient herb with novel applications. J. Tradit. Compl. Med. 2016, 6, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, E.; Younis, I.Y.; Farag, M.A. Metabolites profiling of Egyptian Rosa damascena Mill. flowers as analyzed via ultra-high-performance liquid chromatography-mass spectrometry and solid-phase microextraction gas chromatography-mass spectrometry in relation to its anti-collagenase skin effect. Ind. Crops Prod. 2020, 155, 112818. [Google Scholar] [CrossRef]
- Ahmadi, S.J.; Mortazaeinezhad, F.; Zeinali, H.; Askari-Khorasgani, O.; Pessarakli, M. Evaluation of various Rosa damascena Mill. genotypes grown under rainfed semi-arid condition. Commun. Soil Sci. Plant Anal. 2019, 50, 2534–2543. [Google Scholar] [CrossRef]
- Niazi, M.; Hashempur, M.H.; Taghizadeh, M.; Heydari, M.; Shariat, A. Efficacy of topical rose (Rosa damascena Mill.) oil for migraine headache: A randomized double-blinded placebo-controlled cross-over trial. Compl. Ther. Med. 2017, 34, 35–41. [Google Scholar] [CrossRef]
- Baydar, N.G.; Baydar, H.; Debener, T. Analysis of genetic relationships among Rosa damascena plants grown in Turkey by using AFLP and microsatellite markers. J. Biotechnol. 2004, 111, 263–267. [Google Scholar] [CrossRef]
- Agaoglu, Y.S.; Ergül, A.; Baydar, N. Molecular analysis of genetic diversity oil rose (Rosa damascena Mill.) grown isparta (Turkey) region. Biotechnol. Biotechnol. Equip. 2000, 14, 16–18. [Google Scholar] [CrossRef]
- Farooq, A.; Kiani, M.; Khan, M.A.; Riaz, A.; Khan, A.A.; Anderson, N.; Byrne, D.H. Microsatellite analysis of Rosa damascena from Pakistan and Iran. Hort. Environ. Biotechnol. 2013, 54, 141–147. [Google Scholar] [CrossRef]
- Baydar, H.; Erbaş, S.; Kazaz, S. Variations in floral characteristics and scent composition and the breeding potential in seed-derived oil-bearing roses (Rosa damascena Mill.). Turk. J. Agric. For. 2016, 40, 560–569. [Google Scholar] [CrossRef]
- Nedeltcheva-Antonova, D.; Stoicheva, P.; Antonov, L. Chemical profiling of Bulgarian rose absolute (Rosa damascena Mill.) using gas chromatography–mass spectrometry and trimethylsilyl derivatives. Ind. Crops Prod. 2017, 108, 36–43. [Google Scholar] [CrossRef]
- Rusanov, K.; Kovacheva, N.; Rusanova, M.; Atanassov, I. Low variability of flower volatiles of Rosa damascena Mill. plants from rose plantations along the Rose Valley, Bulgaria. Ind. Crops Prod. 2012, 37, 6–10. [Google Scholar] [CrossRef]
- Rusanov, K.; Kovatcheva, N.; Stefanova, K.; Atanassov, A.; Atanassov, I. Rosa damascena—genetic resources and capacity bulding for molecular breeding. Biotechnol. Biotechnol. Equip. 2009, 23, 1436–1439. [Google Scholar] [CrossRef][Green Version]
- Iwata, H.; Kato, T.; Ohno, S. Triparental origin of Damask roses. Gene 2000, 259, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Yokoya, K.; Roberts, A.; Mottley, J.; Lewis, R.; Brandham, P. Nuclear DNA amounts in roses. Ann. Bot. 2000, 85, 557–561. [Google Scholar] [CrossRef]
- Saffari, V.R.; Sharifi-Sirchi, G.R.; Torabi-Sirchi, M.H. Enhancing rooting consistency in Rosa damascena scions. Afr. J. Biotechnol. 2011, 10, 16495–16500. [Google Scholar] [CrossRef]
- Ginova, A.; Tsvetkov, I.; Kondakova, V. Rosa damascena Mill.—An overview for evaluation of propagation methods. Bulg. J. Agric. Sci. 2012, 18, 545–556. [Google Scholar]
- Pati, P.K.; Prakash, O.; Sharma, M.; Sood, A.; Ahuja, P.S. Growth performance of cuttings raised from in vitro and in vivo propagated stock plants of Rosa damascena Mill. Biol. Plant. 2004, 48, 609–611. [Google Scholar] [CrossRef]
- Abbas, H.; Jaskani, M.J.; Hussain, Z.; Asif, M. Response of rose cuttings against root promoting hormones during spring and autumn. Int. J. Biol. Biotechnol. 2006, 3, 201–204. [Google Scholar]
- Aithida, A.; Tahiri, A.; Oubassine, Y.; Cherif, K.; Mokhtari, M.; Hassani, L.A.I. Agromorphological characterization of the perfume rose (Rosa damascena Mill.) of KelâaM’Gouna-Dades, Morocco. Atlas J. Plant Biol. 2019, 8, 86–95. [Google Scholar] [CrossRef]
- Panwar, S.; Singh, K.P.; Sonah, H.; Deshmukh, R.; Banyal, N.; Prasad, K.V.; Sharma, T.R. Molecular fingerprinting and assessment of genetic diversity in rose (Rosa × hybrida). Indian J. Biotechnol. 2015, 14, 518–524. [Google Scholar]
- Aldhebiani, A.Y.; Al Saud, N.S.; Yaslam, W.A.; Hassan, S.M. Molecular characterization of Rosa damascena Mill growing in Taif and Almadinah using ISSR and SSR markers. Res. J. Biotechnol. 2018, 13, 11–19. [Google Scholar]
- El-Assal, S.E.-D.; El-Awady, M.A.; El-Tarras, A.; Shehab, G. Assessing the genetic relationship of Taif rose with some rose genotypes (Rosa sp.) based on random amplified polymorphic DNA, inter simple sequence repeat and simple sequence repeat markers. Amer. J. Biochem. Biotechnol. 2014, 10, 88–98. [Google Scholar] [CrossRef][Green Version]
- Jabbarzadeh, Z.; Knosh-khui, M.; Salehi, H.; Saberivand, A. Inter simple sequence repeat (ISSR) markers as reproducible and specific tools for genetic diversity analysis of rose species. Afr. J. Biotechnol. 2010, 9, 6091–6095. [Google Scholar] [CrossRef]
- Amer, S.; Basaid, S.A.; Ali, E. Molecular identification of Rosa × damascena growing in Taif region (Saudi Arabia). Int. J. Plant Biol. 2016, 7, 6307–6317. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, V.; Ul Haq, S.; Jatav, P.K.; Kothari, S.L.; Kachhwaha, S. Assessment of genetic diversity in 29 rose germplasms using SCoT marker. J. King Saud Univ. Sci. 2019, 31, 780–788. [Google Scholar] [CrossRef]
- Korkmaz, M.; Yildirim Dogan, N. Analysis of genetic relationships between wild roses (Rosa L. Spp.) growing in Turkey. Erwerbs-Obstbau. 2018, 60, 305–310. [Google Scholar] [CrossRef]
- Mostafavi, A.S.; Omidi, M.; Azizinezhad, R.; Etminan, A.; Badi, H.N. Genetic diversity analysis in a mini core collection of Damask rose (Rosa damascena Mill.) germplasm from Iran using URP and SCoT markers. J. Genet. Eng. Biotechnol. 2021, 19, 144–157. [Google Scholar] [CrossRef]
- Lawrence, B.M. The isolation of aromatic materials from natural plant products. In A Manual on the Essential Oil Industry; De Silva, K.T., Ed.; United Nations lndustrial Development Organization: Vienna, Austria, 1995; pp. 57–154. [Google Scholar]
- Yousefi, B.; Tabaei-Aghdaei, S.R.; Darvish, F.; Assareh, M.H. Flower yield performance and stability of various Rosa damascena Mill. landraces under different ecological conditions. Sci. Hortic. 2009, 121, 333–339. [Google Scholar] [CrossRef]
- Baydar, H.; Baydar, N.G. The effect of harvest date, fermentation duration and Tween 20 treatment on essential oil content and composition of idustrial oil rose (Rosa damascena Mill.). Ind. Crops Prod. 2005, 21, 251–255. [Google Scholar] [CrossRef]
- Kazaz, S.; Erbaş, S.; Baydar, H. The effects of storage temperature and duration on essential oil content and composition oil rose (Rosa damascena Mill.). Turk. J. Field Crops 2009, 14, 89–96. [Google Scholar]
- Kazaz, S.; Erbaş, S.; Baydar, H.; Dilmacunal, T.; Koyuncu, M.A. Cold storage of oil rose (Rosa damascena Mill.) flowers. Sci. Hortic. 2010, 126, 284–290. [Google Scholar] [CrossRef]
- Dobreva, A.; Kovacheva, N. Daily dynamics of essential oils of Rosa damascena Mill. and Rosa alba L. Agric. Sci. Technol. 2010, 2, 71–74. [Google Scholar]
- Yang, L.; Jianwu, R.; Wang, Y. Chemical investigation of volatiles emitted from flowers of three varieties of Damask rose cultivated in Beijing. Hortc. Environ. Biotechnol. 2014, 55, 524–530. [Google Scholar] [CrossRef]
- Khare, S.; Gupta, M.; Cheema, H.S.; Maurya, A.K.; Rout, P.; Darokar, M.P.; Pal, A. Rosa damascena restrains Plasmodium falciparum progression in vitro and impedes malaria pathogenesis in murine model. Biomed. Pharmacother. 2018, 97, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Ouerghemmi, S.; Sebei, H.; Siracusa, L.; Ruberto, G.; Saija, A.; Khebour Allouche, F.; Dhaouadi, K.; Cimino, F.; Cristani, M. LC-DAD-ESI-MS and HPLC-DAD phytochemical investigation and in vitro antioxidant assessment of Rosa sp. stem pruning products from different northern areas in Tunisia. Phytochem. Anal. 2020, 31, 98–111. [Google Scholar] [CrossRef]
- Ncube, N.S.; Afolayan, A.J.; Okoh, A.I. Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. Afr. J. Biotechnol. 2008, 7, 1797–1806. [Google Scholar] [CrossRef]
- Charles, D.J.; Simon, J.E. Comparison of extraction methods for the rapid determination of essential oil content and composition of basil. J. Am. Soc. Hortic. Sci. 1990, 115, 458–462. [Google Scholar] [CrossRef]
- Green, R.J. Antioxidant Activity of Peanut Plant Tissues. Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 2004. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Tsaktsira, M.; Chavale, E.; Kostas, S.; Pipinis, E.; Tsoulpha, P.; Hatzilazarou, S.; Ziogou, F.-T.; Nianiou-Obeidat, I.; Iliev, I.; Economou, A.; et al. Vegetative propagation and ISSR-based genetic identification of genotypes of Ilex aquifolium ‘Agrifoglio Commune’. Sustainability 2021, 13, 10345. [Google Scholar] [CrossRef]
- Prevost, A.; Wilkinson, M.J. A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor. Appl. Genet. 1999, 98, 107–112. [Google Scholar] [CrossRef]
- De Riek, J.; Calsyn, E.; Everaert, I.; Van Bockstaele, E.; De Loose, M. AFLP based alternatives for the assessment of distinctness, uniformity and stability of sugar beet varieties. Theor. Appl. Genet. 2001, 103, 1254–1265. [Google Scholar] [CrossRef]
- Varshney, R.K.; Chabane, K.; Hendre, P.S.; Aggarwal, R.K.; Graner, A. Comparative assessment of EST-SSR, EST-SNP and AFLP markers for evaluation of genetic diversity and conservation of genetic resources using wild, cultivated and elite barleys. Plant Sci. 2007, 173, 638–649. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA 4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of idividuals using the software STRUCTURE: A simulation stydy. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Earl, D.A.; von Holdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implimating the Evanno method. Conserv. Gen. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Rusanov, K.E.; Kovacheva, N.M.; Atanassov, I.I. Comparative GC/MS analysis of rose flower and distilled oil volatiles of the oil bearing rose Rosa damascena. Biotechnol. Biotechnol. Equip. 2011, 25, 2210–2216. [Google Scholar] [CrossRef]
- Baydar, N.G.; Baydar, H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crops Prod. 2013, 41, 375–380. [Google Scholar] [CrossRef]
- Roldán-Ruiz, I.; Dendauw, J.; Van Bockstaele, E.; Depicker, A.; De Loose, M. AFLP markers reveal high polymorphic rates in ryegrasses (Lolium spp.). Mol. Breed. 2000, 6, 125–134. [Google Scholar] [CrossRef]
- Powell, W.W.; Koput, K.W.; Smith-Doerr, L. Interorganizational collaboration and the locus of innovation: Networks of learning in biotechnology. Adm. Sci. Quart. 1996, 41, 116–145. [Google Scholar] [CrossRef]
- Babaei, A.; Tabaei-Aghdaei, S.R.; Khosh-Khui, M.; Omidbaigi, R.; Naghavi, M.R.; Esselink, G.D.; Smulders, M.J.M. Microsatellite analysis of Damask rose (Rosa damascena Mill.) accessions from various regions in Iran reveals multiple genotypes. BMC Plant Biol. 2007, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Kiani, M.; Zamani, Z.; Khalighi, A.; Fatahi, R.; Byrne, D.H. Wide genetic diversity of Rosa damascena Mill. germplasm in Iran as revealed by RAPD analysis. Sci. Hortic. 2008, 115, 386–392. [Google Scholar] [CrossRef]
- Rusanov, K.; Kovacheva, N.; Vosman, B.; Zhang, L.; Rajapakse, S.; Atanassov, A.; Atanassov, I. Microsatellite analysis of Rosa damascena Mill. accessions reveals genetic similarity between genotypes used for rose oil production and old Damask rose varieties. Theor. Appl. Genet. 2005, 111, 804–809. [Google Scholar] [CrossRef]
- Kiani, M.; Zamani, Z.; Khalighi, A.; Fatahi, R.; Byrne, D.H. Microsatellite analysis of Iranian Damask rose (Rosa damascena Mill.) germplasm. Plant Breed. 2009, 129, 551–557. [Google Scholar] [CrossRef]
- Lau, J.W.; Green, P.J. Bayesian model-based clustering procedures. J. Comput. Graph. Stat. 2007, 16, 526–558. [Google Scholar] [CrossRef]
- Porras-Hurtado, L.; Ruiz, Y.; Santos, C.; Phillips, C.; Carracedo, A.; Lareu, M.V. An overview of STRUCTURE: Applications, parameter settings, and supporting software. Front. Genet. 2013, 4, 1–13. [Google Scholar] [CrossRef]
- Khatik, V.K.; Mishra, A. Influence of growing media and indole butyric acid on Damask rose cuttings (Rosa damascena Mill.) var. Ranisahiba. Vegetos—Int. J. Plant Res. 2017, 30, 262–266. [Google Scholar] [CrossRef]
- Akram, M.; Riaz, M.; Munir, N.; Akhter, N.; Zafar, S.; Jabeen, F.; Shariati, M.A.; Akhtar, N.; Riaz, Z.; Altaf, S.H.; et al. Chemical constituents, experimental and clinical pharmacology of Rosa damascena: A literature review. J. Pharm. Pharmacol. 2020, 72, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, Y.; Hashimoto, S.; Nii, H.; Furukawa, K. The volatile constituents of Shi Mei (Rosa davurica Pall.) flower concrete from China. Flavour Fragr. J. 1990, 5, 115–120. [Google Scholar] [CrossRef]
- Villa, C.; Robustelli Della Cuna, F.S.; Russo, E.; Ibrahim, M.F.; Grignani, E.; Preda, S. Microwave-assisted and conventional extractions of volatile compounds from Rosa x damascena Mill. fresh petals for cosmetic applications. Molecules 2022, 27, 3963. [Google Scholar] [CrossRef] [PubMed]
- Tambe, E.; Gotmare, S.R. Study of variation and identification of chemical composition in Rosa species oil collected from different countries. IOSR J. Appl. Chem. 2016, 9, 11–18. [Google Scholar] [CrossRef]
- Zhao, C.-Y.; Xue, J.; Cai, X.-D.; Guo, J.; Li, B.; Wu, S. Assessment of the key aroma compounds in rose-based products. J. Food Drug Anal. 2016, 24, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, M.; Mostafavi, A.; Shamspur, T. Effect of storage on essential oil content and composition of Rosa damascena Mill. petals under different conditions. J. Essent. Oil Bear. Plants 2011, 14, 430–441. [Google Scholar] [CrossRef]
- Erbaş, S.; Baydar, H. Variation in scent compounds of oil-bearing rose (Rose damascena Mill.) produced by headspace solid phase microextraction, hydrodistillation and solvent extraction. Rec. Nat. Prod. 2016, 10, 555–565. [Google Scholar]
- Mirzaei, M.; Sefidkon, F.; Ahmadi, N.; Shojaeiyan, A.; Hosseini, H. Damask rose (Rosa damascena Mill.) essential oil is affected by short- and long-term handling. Ind. Crops Prod. 2016, 79, 219–224. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, S.; Sood, S.; Kaundal, M.; Agnihotri, V.K. Effect of manure and inorganic fertilizers on growth, yield, and essential oil of Damask rose (Rosa damascena Mill.) and chemical properties of soil in western Himalayas. J. Plant Nutr. 2017, 40, 1604–1615. [Google Scholar] [CrossRef]
- Ben Mrid, R.; Ramdan, R.; El Karbane, M.; Nhiri, M. Promising effects of Rosa damascena petal extracts as antioxidant and antibacterial agents. Pak. J. Pharm. Sci. 2021, 34, 1–8. [Google Scholar] [CrossRef]

| No. | Genotype Code | Origin | Latitude N | Longitude E |
|---|---|---|---|---|
| 1 | KS1 | Kastoria, Greece | 40.508266° | 21.337528° |
| 2 | KS2 | Pentavrisos, Greece | 40.459530° | 21.132097° |
| 3 | KS3 | Dispilio, Greece | 40.477315° | 21.292740° |
| 4 | KS4 | Argos Orestiko, Greece | 40.435072° | 21.263327° |
| 5 | KZ1 | Platania, Greece | 40.380573° | 21.349774° |
| 6 | KZ2 | Simantro, Greece | 40.385791° | 21.320717° |
| 7 | KZ3 | Neapoli, Greece | 40.327133° | 21.375617° |
| 8 | KZ6 | Sideras, Greece | 40.381999° | 21.700040° |
| 9 | GR1 | Mega Sirinio, Greece | 40.114581° | 21.403893° |
| 10 | TR | Insparta, Turkey | 37.770464° | 30.496718° |
| 11 | FR1 | Doue La Fontaine, France | 47.185548° | −0.312815° |
| 12 | FR2 | Doue La Fontaine, France | 47.185410° | −0.313332° |
| 13 | FR3 | Doue La Fontaine, France | 47.185530° | −0.313543° |
| 14 | B1 | Tarnicheni, Bulgaria | 42.632029° | 25.139176° |
| 15 | B2 | Skobelevo, Bulgaria | 42.668283° | 25.196214° |
| 16 | B3 | Skobelevo, Bulgaria | 42.670284° | 25.196456° |
| Marker | Primer | Sequence (5′→3′) | Ta 1 (°C) |
|---|---|---|---|
| UBC-810 | GAG AGA GAG AGA GAG AT | 54 | |
| UBC-815 | CTC TCT CTC TCT CTC TG | 54 | |
| ISSR | UBC-834 | AGA GAG AGA GAG AGA GYT | 54 |
| UBC-841 | GAG AGA GAG AGA GAG AYC | 54 | |
| UBC-845 | CTC TCT CTC TCT CTC TRG | 54 | |
| 301 | CAA CAA TGG CTA CCA CCA | 50 | |
| 303 | CAA CAA TGG CTA CCA CCG | 50 | |
| SCoT | 313 | ACG ACA TGG CGA CCA TCG | 50 |
| 315 | ACG ACA TGG CGA CCG CGA | 50 | |
| 330 | CCA TGG CTA CCA CCG GCG | 50 |
| Time (min) | % B 1 |
|---|---|
| 0 | 40 |
| 3 | 100 |
| 3.5 | 100 |
| 3.51 | 40 |
| 15 | 40 |
| Substance | Parent Ion | Product Ion 1 | Collision Energy (V) | Product Ion 2 | Collision Energy (V) | Retention Time (min) |
|---|---|---|---|---|---|---|
| Gallic acid | 169 | 79.2 | 34 | 125 | 18 | 4.33 |
| Epicatechin | 289 | 109 | 30 | 245 | 18 | 2.57 |
| Syringic acid | 197.1 | 153.1 | 14 | 182.1 | 17 | 3.05 |
| Quercetin | 301.1 | 151.1 | 26 | 179.1 | 21 | 9.14 |
| Kaempferol | 285 | 185.1 | 29 | 239.1 | 30 | 10.98 |
| Marker | Primer Name | TAB 1 | NPB 2 | PPB 3 (%) | PIC 4 | RP 5 | MI 6 |
|---|---|---|---|---|---|---|---|
| UBC-810 | 23 | 18 | 78.26 | 0.28 | 9.38 | 2.95 | |
| UBC-815 | 13 | 8 | 61.54 | 0.20 | 3.50 | 1.05 | |
| ISSR | UBC-834 | 20 | 17 | 85.00 | 0.27 | 7.38 | 2.71 |
| UBC-841 | 15 | 13 | 86.67 | 0.33 | 7.00 | 2.72 | |
| UBC-845 | 17 | 14 | 82.35 | 0.27 | 6.25 | 2.25 | |
| 301 | 10 | 8 | 80.00 | 0.33 | 5.25 | 1.75 | |
| 303 | 23 | 19 | 82.61 | 0.26 | 6.63 | 2.78 | |
| SCoT | 313 | 20 | 17 | 85.00 | 0.3 | 8.38 | 2.88 |
| 315 | 16 | 10 | 62.50 | 0.22 | 5.38 | 1.49 | |
| 330 | 23 | 17 | 73.91 | 0.27 | 9.25 | 3.04 |
| Genotype | Rooting (%) | Number of Roots | Length of Roots (cm) |
|---|---|---|---|
| KS1 | 55.0 ± 12.9 1 a | 7.1 ± 1.2 1 a | 8.0 ± 2.4 1 a |
| KS2 | 62.5 ± 9.6 a | 6.2 ± 1.8 a | 9.6 ± 3.8 a |
| KS3 | 60.0 ± 8.2 a | 5.9 ± 1.4 a | 8.3 ± 2.5 a |
| KS4 | 57.5 ± 12.5 a | 7.2 ± 2.1 a | 6.9 ± 2.7 a |
| KZ1 | 67.5 ± 12.6 a | 5.6 ± 1.6 a | 9.5 ± 3.7 a |
| KZ2 | 55.0 ± 12.9 a | 6.4 ± 1.2 a | 6.7 ± 2.3 a |
| KZ3 | 70.0 ± 14.1 a | 6.9 ± 1.6 a | 8.2 ± 2.7 a |
| KZ6 | 65.0 ± 12.9 a | 6.2 ± 1.8 a | 7.6 ± 2.1 a |
| GR1 | 60.0 ± 14.1 a | 6.7 ± 2.0 a | 7.1 ± 3.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziogou, F.-T.; Kotoula, A.-A.; Hatzilazarou, S.; Papadakis, E.-N.; Avramis, P.-G.; Economou, A.; Kostas, S. Genetic Assessment, Propagation and Chemical Analysis of Flowers of Rosa damascena Mill. Genotypes Cultivated in Greece. Horticulturae 2023, 9, 946. https://doi.org/10.3390/horticulturae9080946
Ziogou F-T, Kotoula A-A, Hatzilazarou S, Papadakis E-N, Avramis P-G, Economou A, Kostas S. Genetic Assessment, Propagation and Chemical Analysis of Flowers of Rosa damascena Mill. Genotypes Cultivated in Greece. Horticulturae. 2023; 9(8):946. https://doi.org/10.3390/horticulturae9080946
Chicago/Turabian StyleZiogou, Fotios-Theoharis, Aikaterini-Angeliki Kotoula, Stefanos Hatzilazarou, Emmanouil-Nikolaos Papadakis, Panos-George Avramis, Athanasios Economou, and Stefanos Kostas. 2023. "Genetic Assessment, Propagation and Chemical Analysis of Flowers of Rosa damascena Mill. Genotypes Cultivated in Greece" Horticulturae 9, no. 8: 946. https://doi.org/10.3390/horticulturae9080946
APA StyleZiogou, F.-T., Kotoula, A.-A., Hatzilazarou, S., Papadakis, E.-N., Avramis, P.-G., Economou, A., & Kostas, S. (2023). Genetic Assessment, Propagation and Chemical Analysis of Flowers of Rosa damascena Mill. Genotypes Cultivated in Greece. Horticulturae, 9(8), 946. https://doi.org/10.3390/horticulturae9080946





