Abstract
Intercropping systems are a widely used agricultural practice by smallholder farmers to enhance food security and to use natural resources more efficiently. The objective of this study was to evaluate two lettuce cvs under an intercropping system. Enzymatic growth, yield and economic benefit were evaluated. The experiment was carried out from August to February in Jaboticabal (Brazil) using tomato plants as a main crop and Lucy Brown and Vanda cvs of lettuce as secondary crops. The catalase, superoxide dismutase and peroxidase activity of lettuce plants was analyzed. Mineral nutrient content, growth, yield parameters and commercial economic benefit were measured. Significant differences in stress and activity enzymatic indicators were found versus cultivars of lettuce. The loss of abiotic factors such as radiation or its effect on enzymatic stress indicators was greater than its effect on yield. Yield loss in the intercropping systems was different for each cultivar system, with cv Lucy Brown showing greater yield loss than cv Vanda. Economic benefit was only found for the Vanda lettuce cv. Although the benefits of intercropping have been demonstrated as very appropriate cultural management, the choice of cultivars involved might be the determining factor for the agronomic success of the system.
1. Introduction
Intercropping systems are a widely used agricultural practice, mainly by smallholder farmers, in many developed and developing countries, such as Asian, African and Latin American countries, to enhance food security and to use natural resources more efficiently [1]. In intercropping systems, two or more species are grown simultaneously in the same area for part or all of the growing season [2,3].
The advantages of using intercropping systems are the reduction in cultivated area with the same production, higher efficiency and effectiveness of the agricultural area; larger and faster growth of plant cover, leading to less soil erosion [4]; and a low incidence of diseases, pests and damage caused by weeds [5,6]. This system also increases the economic benefits compared with those of the sole cropping of soybean [7,8]. The success of this approach is attributed to the efficient utilization of water and light, thereby increasing crop yield and improving biodiversity and ecological services [9,10].
The production of horticultural crops in small farms, normally characterized by the intensive use of renewable and non-renewable resources, is the sector that can most take advantage and benefit from this agronomic practice due to the possibility of the cultivation of two or more species in the same area [11]. Numerous studies have published the beneficial results of intercropping in fields [4,7,8,11,12] and greenhouses [13,14]; on the other hand, there is little information on soilless culture [15]. The efficiency of the intercropping system will depend on the choice of the crops and the management of the cultivation system for minimizing the competition between species for the environmental resources and for maximizing the complementarity between them [12].
Studies have indicated that the intercropping of tomato (Solanum lycopersicon L.) and lettuce (Lactuca sativa L.) is feasible in both conventional soil [13,14] and soilless culture [15]. In greenhouse conditions, both tomato and lettuce meet the most important criterion and perhaps the first requirement for success in the association: the plants must be complementary in agrobotanical characteristics in order to take advantage of temporal and/or spatial compatibility [13], since they are planted at the same time, reducing competition mainly for light.
Among the factors that most influence crops, such as water, nutrients and space, light is the most important factor related to excessive yield due to crop mixtures that present temporal complementarity and high efficiency [16]. In the tomato–lettuce intercropping system, shading by the taller (tomato) crop modifies the light environment experienced by the lower (lettuce) crop in terms of both light quantity (PAR-photosynthetically active radiation) and quality (R:FR ratio), causing stress [17]. These stresses threaten plant growth and development, and may cause the plant to produce reactive oxygen species (ROS), which act as early signals of a plant’s defense response to external stress and serve as secondary messengers for subsequent defense reactions [18,19,20,21,22].
Other physiological aspects of the roots of both crops may be affected, since it is well known that the roots of a secondary crop in intercropping may affect the competence of the main crop in water and nutrient uptake, thus potentially affecting nutrient uptake [23] and stress indicators for both crops.
Malondialdehyde (MDA) is an active aldehyde, and its production can be used as a marker to measure the level of oxidative stress in an organism [24,25]. Various external stresses, such as low light, often induce the activity of free-radical detoxification enzymes in plants, such as SOD, catalase and peroxidase (POX) [26,27]. The SOD enzyme is considered the first line of enzymatic defense against oxidative stress [28], and catalyzes the dismutation of O2 into H2O2 and molecular oxygen, having a fundamental role in ROS detoxification [29,30]. The CAT enzyme can be considered a good stress indicator, containing high potential to directly demutate H2O2 into H2O and O2, which are indispensable for ROS detoxification under stress conditions [31]. POD also plays an essential role in protecting plant cells against oxidative damage [32,33]. When plants are subjected to different stresses such as, for example, low light, its activity increases, with the intention of decreasing stress for plants.
Multiple stress indicators have been described for various biotic or abiotic stresses for several crops; however, little work has been carried out to record the potential stress on the main and secondary crops in intercropping systems through MDA or other enzymatic indicators.
On the other hand, numerous studies have discussed the ability of intercropping systems to increase productivity, but there is little information on the feasibility of such a system in soilless crops [13,14,15] and furthermore on the combined effects of tomato and lettuce intercropping on antioxidant enzyme activities, mineral nutrition and productive characteristics of the secondary crop (lettuce). The objective of this study was to evaluate yield, production parameters, MDA, antioxidant enzyme activity, mineral nutrient content and profitability of tomato under soilless culture intercropping systems, using two different lettuce cvs as secondary crops.
2. Materials and Methods
The experiment was conducted at the Julio de Mesquita Filho campus of the São Paulo State University (UNESP), Jaboticabal, São Paulo, Brazil (21°15′22″ S and 48°15′58″ W and 575 m), from 28 August 2013 to 15 February 2014. The climate of the region is classified as subtropical, rainy during the summer and relatively dry in the winter. The annual means of precipitation and temperature are 1424.6 mm and 22.2 °C, respectively.
2.1. Treatments and Experimental Design
The experiment was an intercropping (I) system of tomato and lettuce vs. monoculture (M) of the two species, grown in a soilless growing system. Treatments were the monoculture of lettuce cv ‘Lucy Brown’ (LB, Crisphead group), lettuce cv ‘Vanda’ (V, loose-leaf group), tomato cv ‘Debora Victory’ (T) and the intercropping of lettuce and tomato (T + LB and T + V) (Figure 1). Each intercrop replicate consisted of twelve tomato plants intercropped with twelve lettuce plants of either cultivar. For monoculture, each replicate had twelve tomato plants or twelve lettuce plants (LB and V), and in intercropping, each replicate had twelve tomato plants and twelve lettuce plants (LB and V). The experimental design was a randomized block design, with five treatments, six blocks and twelve plants per species and replicate, with a total of 72 plants in each monoculture treatment and 144 in intercropping.
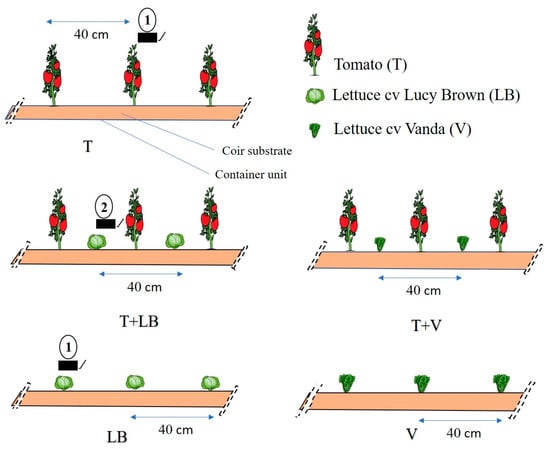
Figure 1.
Tomato–lettuce intercropping and monoculture systems. Number 1 indicates radiation measurement in monocropping and number 2 indicates radiation measurement in intercropping.
2.2. Plant Growing Conditions
The experiment was conducted in a greenhouse with a low-density polyethylene cover 150 μm thick and with side and front closures with black polypropylene mesh, providing 50% shade. The greenhouse is 48 m long, 12.8 m wide and 3.3 m high and oriented east–west. A thermo-reflective mesh with 50% shading was installed to control the temperature inside the greenhouse. Tomato and lettuce plants were transplanted at the same time in cultivation channels spaced 1.10 m × 0.40 m apart (between rows × between plants). Tomato cv Mayoral grafted onto cv Emperor seedlings and lettuce cv Romaine seedlings were transplanted into coir fiber substrate bags with six and four leaves, respectively. The tomato plants were transplanted on 28 August 2013, when the lettuce plants were also transplanted. The channels were made of zinc sheet (2.00 mm thick) coated on the inside with a black plastic film with a thickness of 100 µm, trapezoidal in shape, 0.18 m wide at the base, 0.38 m wide at the top, 0.20 m high and 5.00 m long, with a capacity of 280 L of coconut husk fiber. Tomato plants were grown 0.40 m apart, with a single stem, and were individually trellised and wrapped in plastic strips. Lettuce plants were transplanted at half the distance between the tomato plants (0.40 m apart). The culture channels contained commercial coir fiber substrate, with defined physical and physicochemical characteristics that have been described by Pozo et al. [34]. Irrigation was carried out in each cultivation channel by drip irrigation, with self-compensating drippers spaced at 0.40 m and sensors to measure substrate moisture. The automatic irrigation system was activated when the average moisture value recorded by the sensors in the canals reached −4.0 kPa. The nutrient solution used for all plants was that recommended by Sonneveld and Straver [35], with pH and electrical conductivity (EC) adjusted to 5.8 and 2.0, respectively. The macro and micronutrients in the nutrient solution are shown in Table 1.

Table 1.
Nutrient solution used for the crop.
2.3. Greenhouse Climate Measurements
Recordings of maximum and minimum temperature (°C), mean relative humidity (%) and photosynthetically active solar radiation (µmol m−2 s−1) were made every 15 min and then averaged for a day, using a WatchDog 2475 microprocessor recorder (Spectrum Technologies, Aurora, IL, USA) located inside the greenhouse. The average temperature during the growing cycle was 22.9 °C (maximum mean = 31.0 °C; minimum mean = 14.9 °C), and relative humidity ranged from 37 to 97%. Photosynthetically active radiation for both tomato and lettuce in monoculture was measured two centimeters above the plant canopy by quantum light sensors model 2475 (WatchDog model 2000 series; Spectrum Technologies, Aurora, IL, USA). In the case of intercropped lettuce, the measurement was made two centimeters above the plant canopy between the tomato plants (Figure 1).
2.4. Biochemical Evaluations of Lettuce
All biochemical evaluations were performed on the lettuce species, on the first fully expanded leaf of four lettuce plants of each treatment by six replicates, two days before harvest. The collected samples were placed in liquid nitrogen and stored in a freezer at −80 °C until extraction for lipid peroxidation and antioxidant enzyme activity.
2.4.1. Lipid Peroxidation
The lipid peroxidation content was determined according to the methodologies proposed by Shimizu et al. [36] and Gratão et al. [37]. Plant tissues were homogenized in a pestle and mortar with 20% (w/v) insoluble polyvynilpyrrolidone (PVPP) and 0.1% trichloroacetic acid (TCA). The homogenate was centrifuged at 10,000× g for 5 min. Some 250 μL of the supernatant was added to 1 mL 0.5% TBA in 20% TCA. This solution was incubated in a water bath at 95 °C for 20 min, and the reaction was stopped by quickly cooling in an ice-water bath. Absorbance of the formed TBARS was determined spectrophometrically at 535 nm. Measurements were corrected for unspecific turbidity by subtracting the absorbance at 600 nm. The concentration of MDA equivalents was calculated using the absorbance coefficient 1.55 × 10−5 mol−1 cm−1.
2.4.2. Antioxidant Enzyme Activities
For enzyme extraction, 200 mg of frozen leaf samples were macerated in liquid nitrogen and homogenized with 50 mM potassium phosphate buffer (pH 7.5) containing 2 mM EDTA, 5 mM 2–mercaptoethanol and 20% PVP in a final volume of 3 mL. The homogenate was centrifuged at 10,000× g for 30 min (Mod. Beckman Avanti J–25 centrifuge; Brand, Indianapolis, IN, USA), and the supernatant was stored in a freezer at −80 °C for antioxidant enzyme analysis. Three replicates in triplicate were used for enzyme determinations.
2.4.3. Catalase
Catalase (CAT, EC 1.11.1.6) determination was carried out in a spectrophotometer, where the reaction medium was composed of a mixture containing plant extract, 100 mM potassium phosphate buffer (pH 7.5) and 30% hydrogen peroxide. The reaction took place for one minute during which the degradation of hydrogen peroxide was monitored at an absorbance of 240 nm (BioTek Instruments, Winooski, VT, USA) [38]. Results were expressed in µmol min−1 mg−1 protein.
2.4.4. Superoxide Dismutase
Superoxide dismutase (SOD, EC 1.15.1.1) activity was determined through a reaction medium containing an aliquot of plant extract, 50 mM potassium phosphate buffer (pH 7.8), 50 mM methionine, 10 mM ethylene diaminetetraacetic acid (EDTA), 1 mM nitrotetrazolium chloride (NBT) and 0.1 mM riboflavin. The reaction medium was placed in test tubes that were kept in a chamber with fluorescent light of 15 W and temperature of 25 °C for 5 min, so that a reaction could occur through the degradation of the blue formazan compound. Subsequently, samples were read on a spectrophotometer at an absorbance of 560 nm (BioTek Instruments, Winooski, VT, USA), and the values were expressed in U SOD mg−1 protein [39].
2.4.5. Peroxidase
Peroxidase (POX EC 1.11.1.7) activity was verified according to the methodology of Teisseire and Guy [40]. Briefly, the reaction system was composed of 30 μL of enzyme extract, 50 mmol L−1 potassium phosphate buffer pH 6.5, 20 mmol L−1 pyrogal pH 7.8 and 5 mmol L−1 H2O2, totaling a volume of 1.0 mL. The reaction was carried out at room temperature for 5 min. Purpurogallin formation was measured in a UV-visible spectrophotometer at 430 nm (BioTek Instruments, Winooski, VT, USA), and the molar extinction coefficient (2.5 mmol L−1 cm−1) was used to calculate the specific activity of the enzyme expressed as μmol H2O2 min−1mg−1 of protein.
2.5. Efficiency of Nutrient Absorption in Lettuce
Foliar content of nitrogen (N), phosphorus (P), potassium (K) and magnesium (Mg) in g kg−1, and iron (Fe) in mg kg−1, was evaluated in lettuce plants. For this purpose, the main vein of the outer leaf, at the head formation stage, was collected from three plants of each treatment, chosen at random, according to the methodology of Malavolta et al. [41]. Leaf samples were gently washed, oven-dried at 65 °C and ground to <1 mm in a Wiley mill. Ground leaves were digested in sulfuric acid to quantify Kjeldahl–N and in a mixture of nitric and perchloric acids to determine P, K, Mg and Fe content by plasma emission spectroscopy (inductively coupled plasma atomic emission spectroscopy (ICP–OES), Santa Clara, CA, USA) [41].
2.6. Plant Growth Biometric Parameters
Lettuce growth was verified by obtaining a fresh mass harvested 35 days after transplanting, collecting four plants per treatment. For the tomato plants, leaf area (m2) and dry mass (g plant−1) were evaluated at the end of the last fruit harvest, using three plants from each treatment. Tomatoes were harvested at the time of ripening between February and April and commercially classified according to their equatorial diameter [42] as G (60–70 mm), M (50–60 mm) and MMM (<50 mm). Yields (kg m−2) were obtained for each class, in addition to total yield. A subsample of three tomatoes per plant per treatment was collected at each harvest and a homogeneous mixture was formed to measure soluble solids (°Brix).
2.7. Economic Factors
Economic revenues of the tomato and lettuce crops were calculated for the monocultures and intercrops to evaluate the economic feasibility of intercropping as a function of the number of plants per square meter. Thus, the net revenue (NR) that corresponded to the estimated monetary value of the tomato, lettuce or intercropped production was first calculated, considering the price of the tomatoes in each commercial class (G = 0.68 EUR kg−1, M = 0.53 EUR kg−1 and MMM = 0.41 EUR kg−1). A mean price of 0.59 EUR kg−1 was used for lettuce cv Lucy Brown and 0.85 EUR kg−1 was used for cv Vanda. The prices were obtained from CEAGESP [43]. To calculate the operating cost, the number of plants per square meter was considered the only differential between the planting systems. The company’s profit (EUR m−2) was calculated as the difference between the NR and the cost of the plants.
2.8. Statistical Analysis
Parameters were compared by analysis of variance (ANOVA). When necessary, means were separated by Tukey’s test at 5% (p < 0.05) for each treatment and sample of Biochemical Evaluations of each lettuce cv, using AgroEstat software, version 1.1, São Paulo State University (UNESP), Jaboticabal, Brazil [44].
3. Results and Discussion
3.1. Incident Radiation
Figure 2A shows the photosynthetically active radiation (PAR) intercepted during the lettuce intercropping cycle. During the first two weeks there was no significant difference between crop systems; however, with the intercropping system, as the tomato plants grew, radiation reaching the lettuce decreased. The total loss of incident radiation on the lettuces in the intercropping system compared to those grown by monoculture was 48.7% (Figure 2B), while Tringovska et al. [14], with the same crops (tomato and lettuce), reported a lower plant growth loss of 27%. In the same intercropping situation, also with tomato and lettuce at a latitude of 32° (Almeria, Spain), Cunha–Chiamolera et al. [45] published a mean PAR value of 8 E m−2 day−1, meaning that 30% of radiation was lost, while in Jaboticabal (Brazil) at a latitude of 21° and a mean radiation of 12 E m−2 day−1, a 50% reduction in PAR was also obtained.
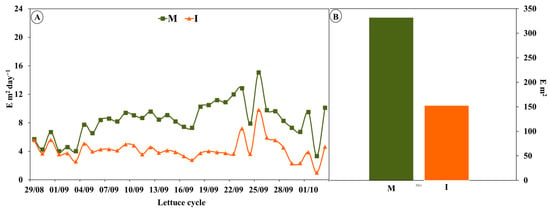
Figure 2.
Reduction of photosynthetically active radiation in tomato and lettuce plants in intercropping (I) compared to monoculture (M). (A) Time of crop. (B) Total cycle.
After the first two weeks, there was a significant decrease in incident PAR in intercropping lettuce. This progressive reduction is due to the shading that produces tomato plants, which show a greater vertical plant growth, favored in part by the form of tutoring. It is well known that during intercropping, light is frequently the most important factor related to crop yields [2]. It is well known that light has significant effects on plants: either the presence of neighboring plants or self-shading within the canopy could reduce the availability of PAR and alter light quality for each plant [46].
3.2. Biochemical Indicators in Plants
3.2.1. Lipid Peroxidation Measurement
Numerous authors have reported lipid peroxidation, expressed as MDA content, as a good indicator of stress [32,47,48]. Figure 3 shows MDA levels in the two lettuce cvs and cropping systems. The levels for cv Lucy Brown were significantly higher than those for Vanda in monoculture. In addition, in both intercropping systems, MDA levels were higher than for monoculture. An average reduction in radiation of 50% in the intercropping system led to a very significant increase in MDA of 60.9% and 58.2% in cvs Lucy Brown and Vanda, respectively. MDA content is closely related to the antioxidant defense system through the action of enzymes that can eliminate ROS [49,50,51]. The high levels in both lettuces in intercropping show that the plants suffered under low light conditions. Therefore, MDA was a good indicator of potential light stress, as already published by Wang et al. [52] for garlic plants with eggplant intercropping. Our MDA values were similar to those found by Alves et al. [51] working on salt stress in lettuce seedlings of the same cultivars we used.
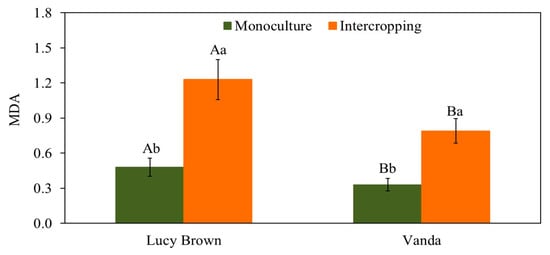
Figure 3.
Lipid peroxidation (MDA) (μmol mg−1 fresh biomass) in lettuce plants. Capital and lowercase letters indicate significant differences according to Tukey’s test at 5% (n = 20) probability of cultivars in the same cropping system and in the same cultivar between cropping systems, respectively.
3.2.2. Antioxidant Enzyme Activities
The effect of light reduction on the defense system of lettuce was measured through antioxidative enzymes such as CAT, SOD and POX content. Lettuce plants in intercropping systems significantly increased their content of enzymes with antioxidant capacity (Figure 4). Figure 4A shows significant differences in CAT enzyme activity within cropping systems and between cultivars. In monoculture, no significant differences were observed between cultivars, but in intercropping, the enzymatic activity was higher for Lucy Brown. However, the mean increase in enzyme activity was similar for Lucy Brown and Vanda, with 64.3% and 66.9%, respectively. Contrary to what was found for CAT, the highest SOD enzyme activity was found in cv Vanda (Figure 4B). In monoculture, the highest activity was found in cv Lucy Brown, with an increase of 62%. However, when comparing cultivation systems for the same cultivar, the difference in enzyme activity was 37.8% and 71.1% for Lucy Brown and Vanda, respectively. This shows that, for SOD enzyme activity, cv Vanda was more affected. POX enzyme activity was very similar to that of SOD in monoculture and intercropping, with higher activity for cv Lucy Brown (Figure 4C). However, when enzyme activity was evaluated within the same cultivar, the difference between cultivars was similar, with values of 69.5% and 73.5% for Lucy Brown and Vanda, respectively.
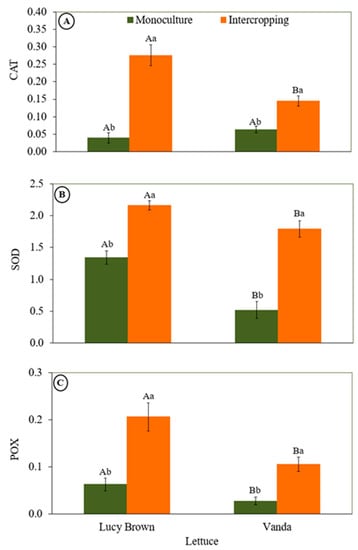
Figure 4.
Specific activity of antioxidant enzymes. (A) Catalase (CAT, µmol min−1 mg−1 protein), (B) superoxide dismutase (SOD, U SOD mg−1 protein) and (C) peroxidase (POX, μmol of H2O2 min−1 mg−1 protein) in lettuce plants. Capital and lowercase letters indicate significant differences according to Tukey’s test at 5% (n = 20) probability of cultivars in the same cropping system and in the same cultivar between cropping systems, respectively.
Under limiting conditions, such as low light, plants activate a defense mechanism by increasing antioxidant metabolites, so that they can compensate for stress and ROS homeostasis and maintain crop yield [53,54]. Plants have different antioxidant defense systems, and depending on the degree of stress, the various defense mechanisms may suffer irreversible damage, not being efficient against ROS sequestration and degradation [37,54]. When the plants were subjected to low light, there was an increase in CAT, SOD and POX activity. That caused the enzymes to increase their activities in an attempt to prevent the plants from damage caused by oxidative stress, according to what has been reported by Alves et al. [30]. Kolahi et al. [55] also observed an increase in the activity of the same enzymes (CAT, SOD and POX) when lettuce was subjected to stress.
However, all CAT, SOD and POX enzymes were good indicators of stress in plants. Xu et al. [53] and Alves et al. [54] had already reported the increase of CAT, SOD and POX as stress indicators. Although the mean increase in enzyme activity response was similar for the two lettuce varieties, each cultivar showed different proportional increases, while CAT was shown to be the best indicator for Lucy Brown, and SOD and POX for Vanda. Therefore, these results suggest that different indicators of enzyme activity under light stress can be found for each variety of crops such as lettuce.
3.3. Efficiency of Nutrient Absorption in Lettuce
When lettuce was growing in monoculture, N, P and K values were similar in both lettuce cultivars, while Mg and Fe content in Lucy Brown was higher than in Vanda (Table 2). On the other hand, when lettuce was growing in intercropping with tomato, all levels of nutrient content showed significant differences between the two cultivars. While Vanda showed higher levels of N, P and K content, the values of Mg and Fe content were higher in Lucy Brown. With the exception of P in the intercropping cv Vanda, N and P content was within the ranges published by Trani and Raij [56] for lettuce (30–50 g kg−1 and 4–7 g kg−1, respectively). The Mg content was below the recommended (4–7 g kg−1), and the K and Fe content was all above (50–80 g kg−1 and 50−150 mg kg−1, respectively) [56]. Similar results were found by Nascimento et al. [11] in lettuce plants for nitrogen content.

Table 2.
Foliar levels of nitrogen, phosphorus, potassium, magnesium and iron in two lettuce cultivars, Lucy Brown and Vanda, subjected to two cropping systems, monoculture and intercropping.
With the exception of K, the effect of competition on the uptake of lettuce roots growing with tomato plants was significantly affected (% ∆ I−M). P in the case of cv Lucy Brown was very significantly reduced by up to 31%, while this decrease uptake caused by intercropping competition was recorded for Fe in cv Vanda (Table 2).
Mineral metabolism can be altered by different plant stresses. Mineral composition and the maintenance of mineral balance are important for plant growth and development [57]. Physiological stresses can result in rapidly increasing amounts of ROS in plant cells, damaging cell structure and decreasing the nutritional quality of their fruits and vegetative parts [20,58,59,60,61].
It is possible to observe how the stress suffered by the plants negatively affected the absorption of some nutrients, causing K and Fe toxicity, in addition to Mg deficiency. The assessment of the nutrient balance of the plants can show relevant information about the nutritional status in various growth conditions [62]. Other authors also found the plants were affected in relation to nutrient uptake, when subjected to stress [11,63,64].
3.4. Lettuce Growth
The fresh weight values of Lucy Brown lettuces in monoculture were more than those for Vanda (Figure 5). The decrease in intercropping yield for Lucy Brown (77.2%) was higher than the 50% reduction in average incident radiation, while Vanda’s loss of fresh mass was lower (33.2%). Barros Junior et al. [63] and Barbosa et al. [65] also found a similar trend when working with Lucy Brown and Vanda cvs. This difference in the growth of the cultivars is related to the greater need of light in the Lucy Brown cv compared to the Vanda cv [66]. This loss of yield for lettuce was also recorded by Yan et al. [67], Cecílio Filho et al. [68], Su et al. [69] and Tringovska et al. [14], who used intercropping systems with lettuce and tomatoes.
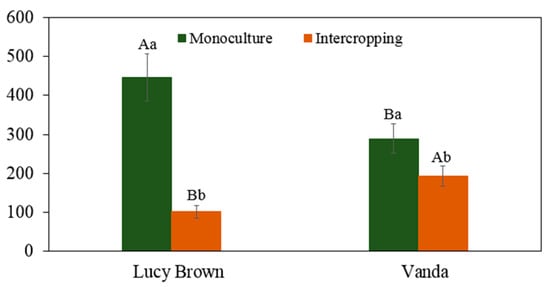
Figure 5.
Total fresh mass (g plant−1) of lettuce plants. Capital and lowercase letters indicate significant differences according to Tukey’s test at 5% (n = 20) probability of cultivars in the same cropping system and in the same cultivar between cropping systems, respectively.
3.5. Tomato Growth
Intercropping should provide the benefit of a secondary crop without a significant loss of the main crop. Tomato growth was not affected by the intercropping system with lettuce in the parameters evaluated (Table 3 and Table 4). Tomato development and productivity were within the expected limits for the culture, showing that it is viable for tomato intercropping, as long as it is with smaller cultures [14,70].

Table 3.
Leaf area, dry mass and total soluble solid content of monoculture tomato crop and in intercropping with two lettuce cultivars.

Table 4.
Tomato fruit size by class and yield (kg m2) in monoculture and in intercropping with lettuce cultivars.
There was significant difference in leaf area, dry mass and total soluble solids values. A similar trend was reported by Cecílio Filho et al. [68] and Tringovska et al. [14], who, when using tomato and lettuce in an intercropping system, also did not find significant losses in the main crop. Tomato growth has not suffered with the presence of lettuces, due to the high degree of complementarity in the cultures, since lettuces have a low bearing, not affecting the levels of solar radiation and nutrient absorption of tomatoes [71].
3.6. Economic Benefit
Table 5 shows values for the tomato crop considering the price of each size as a function of the market standard [43]. The net revenue of intercropping tomato in comparison to monoculture was −7.08% and 9.06% when grown with Lucy Brown and Vanda lettuce, respectively. This results in a total profit of −12.80% and 5.65% for Lucy Brown and Vanda, respectively. These data are similar to those of Cecílio Filho et al. [68], in tomato intercropping with lettuce, in which they also found losses in income when tomato was grown together with Lucy Brown. The intercropping of tomato and lettuce cv Vanda showed higher tomato profitability compared to monoculture. It was observed that the cost with lettuce was lower than the cost with tomato; however, the profit was lower. The cv Vanda presented a higher profit when compared to cv Lucy Brown. The benefit of intercropping between tomato and Vanda was higher (7.77 EUR m−2) than for tomato and Lucy Brown (6.03 EUR m−2). This higher value was also due to the higher productivity obtained by the higher yield of class-G tomatoes, which have a higher market value, and the higher economic contribution of lettuce, despite the loss of size and value of intercropped lettuce in relation to monoculture. These results agree with those observed in other studies that evaluated the agronomic and economic viability of tomato and lettuce intercropping [13,68].

Table 5.
Net revenue, cost of plant and profit (EUR m−2) of tomato and lettuce (Lucy Brown and Vanda) in monoculture and intercropping.
One of the questions that arise when working with vegetable intercropping is whether the productive performance of the intercropping systems evaluated is translated into economic efficiency [72,73]. The different economic performance of the two varieties in the intercropping system suggests that a detailed study of the cultivars within the same secondary crop is necessary. This may be linked to the minimum light requirements of each variety [66].
4. Conclusions
Tomato plants do not express an overall negative effect from the incorporation of lettuce in intercropping. Instead, lettuces grown in intercropping register competition stress from both abiotic factors and competition with tomato, shown by (1) stress indicators, (2) enzymatic indicators, (3) mineral composition and (4) their vegetative development.
Metabolic and stress indicators, as well as nutrient content, have been shown to be excellent indicators of potential loss due to the effect of competition (spatial and for resources in the rhizosphere).
In general terms, it can be stated that there is a proportional loss due to the use of intercropping systems, which leads to a loss of important resources necessary for the crops. If we evaluate their proportion, we find (1) a significant decrease in abiotic resources (especially radiation to secondary crops), (2) an exchange in metabolism through enzymatic and stress indicators, and a negative effect (3) both in the main crop in intercropping (in our case, tomato) and (4) in the secondary crop (in our case, lettuce). Points 1 to 4 are proportionally ordered from largest to smallest.
However, the final economic gains in one of the varieties indicate that there is a clear benefit of the lettuce and tomato intercropping system depending on an appropriate choice of secondary vegetable cultivar.
In summary, our work suggests that there is an opportunity for small and medium-sized farmers to benefit from the implementation of simple farm management.
Author Contributions
Conceptualization, T.P.L.C.-C., D.M.M.S. and A.B.C.F.; methodology, T.P.L.C.-C., D.M.M.S. and A.B.C.F.; formal analysis, T.P.L.C.-C., D.M.M.S., A.B.C.F., F.M.C. and M.U.; investigation, T.P.L.C.-C., A.B.C.F. and F.M.C.; data curation, T.P.L.C.-C., D.M.M.S., A.B.C.F., F.M.C. and M.U.; writing—original draft preparation, T.P.L.C.-C., D.M.M.S. and A.B.C.F.; writing—review and editing, T.P.L.C.-C., F.M.C., R.G.G.-G., S.N. and M.U.; supervision, D.M.M.S. and A.B.C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, Y.; Gong, W.; Yang, F.; Wang, X.; Yong, T.; Yang, W. Responses to shade and subsequent recovery of soya bean in maize-soya bean relay strip intercropping. Plant Prod. Sci. 2016, 19, 206–214. [Google Scholar] [CrossRef]
- Francis, C.A. Biological efficiencies in multiple-cropping systems. Adv. Agron. 1989, 42, 1–42. [Google Scholar] [CrossRef]
- Willey, R.W. Intercropping: Its importance and research needs. I. Competition and yield advantages. Field Crop Abst. 1979, 32, 1–10. [Google Scholar]
- Rao, M.R.; Willey, R.W. Evaluation of yield stability in intercropping: Studies on sorghum/pigeonpea. Exp. Agric. 1980, 16, 105–116. [Google Scholar] [CrossRef]
- Sarrantonio, M.; Gallandt, E. The role of cover crops in North American cropping systems. J. Crop Prod. 2008, 8, 53–74. [Google Scholar] [CrossRef]
- Huang, C.; Liu, Q.; Gou, F.; Li, X.; Zhang, C.; Werf, W.V.D.; Zhang, F. Plant growth patterns in a tripartite strip relay intercrop are shaped by asymmetric aboveground competition. Field Crops Res. 2017, 201, 41–51. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, W.; Wu, Q. Effects of shading in maize/soybean relay-cropping system on the photosynthetic characteristics and yield of soybean. Acta Agron. Sin. 2007, 9, 1502–1507. [Google Scholar]
- Yang, F.; Wang, X.; Liao, D.; Lu, F.; Gao, R.; Liu, W.; Yong, T.; Wu, X.; Du, J.; Yang, W. Yield response to different planting geometries in maize–soybean relay strip intercropping systems. Agron. J. 2015, 107, 296–304. [Google Scholar] [CrossRef]
- Chen, Y. Development of agricultural recycle economy in arid areas of Hexi Corridor. J. Anhui Agric. Sci. 2011, 6, 3726–3728. [Google Scholar] [CrossRef]
- Rahman, T.; Liu, X.; Hussain, S.; Ahmed, S.; Chen, G.; Yang, F.; Chen, L.; Du, J.; Liu, W.; Yang, W. Water use efficiency and evapotranspiration in maize-soybean relay strip intercrop systems as affected by planting geometries. PLoS ONE 2017, 12, e0178332. [Google Scholar] [CrossRef]
- Nascimento, C.S.; Cecílio Filho, A.B.; Mendoza-Cortez, J.W.; Nascimento, C.S.; Bezerra Neto, F.; Grangeiro, L.C. Effect of population density of lettuce intercropped with rocket on productivity and land-use efficiency. PLoS ONE 2018, 13, e0194756. [Google Scholar] [CrossRef]
- Bezerra Neto, F.; Andrade, F.V.; Negreiros, M.Z.; Santos Júnior, J.J. Desempenho agroeconômico do consórcio cenoura x alface lisa em dois sistemas de cultivo em faixa. Hortic. Bras. 2003, 21, 635–641. [Google Scholar] [CrossRef]
- Cecílio Filho, A.B.; Rezende, B.L.A.; Barbosa, J.C.; Feltrim, A.L.; Silva, G.S.; Grangeiro, L.C. Interaction between lettuce and tomato plants, in intercropping cultivation, established at different times, under protected cultivation. Hortic. Bras. 2008, 26, 158–164. [Google Scholar] [CrossRef]
- Tringovska, I.; Yankova, V.; Markova, D.; Mihov, M. Effect of companion plants on tomato greenhouse production. Sci. Hortic. 2015, 186, 31–37. [Google Scholar] [CrossRef]
- Cunha-Chiamolera, T.P.L.; Urrestarazu, M.; Cecílio-Filho, A.B.; Morales, I. Agronomic and economic feasibility of tomato and lettuce intercropping in a soilless system as a function of the electrical conductivity of the nutrient solution. HortScience 2017, 52, 1195–1200. [Google Scholar] [CrossRef]
- Malézieux, E.; Crozat, Y.; Dupraz, C.; Laurans, M.; Makowski, D.; Ozier-Lafontaine, H.; Rapidel, B.; Tourdonnet, S.; Valantin-Morison, M. Mixing plant species in cropping systems: Concepts, tools and models. A review. Agron. Sustain. Dev. 2009, 29, 43–62. [Google Scholar] [CrossRef]
- Liu, X.; Rahman, T.; Song, C.; Su, B.; Yang, F.; Yong, T.; Wu, Y.; Zhang, C.; Yang, W. Changes in light environment, morphology, growth and yield of soybean in maize-soybean intercropping systems. Field Crops Res. 2017, 200, 38–46. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Rizhsky, L.; Hegie, A.; Koussevitzky, S.; Mittler, R. Double mutants deficient in cytosolic and thylakoid ascorbate e peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol. 2007, 144, 1777–1785. [Google Scholar] [CrossRef]
- Möller, I.M.; Sweetlove, L.J. ROS signalling—Specificity is required. Trends Plant Sci. 2010, 15, 370–374. [Google Scholar] [CrossRef]
- Wrzaczek, M.; Brosché, M.; Kangasjärvi, J. ROS signaling loops—Production, perception, regulation. Curr. Opin. Plant Biol. 2013, 16, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.M.; Huang, L.F.; Zhang, X.; Wang, M.L.; Xu, G.Y.; Xia, X.J. OsCML4 improves drought tolerance through scavenging of reactive oxygen species in rice. J. Plant Biol. 2015, 58, 68–73. [Google Scholar] [CrossRef]
- Cordovilla, M.P.; Bueno, M.; Aparicio, C.; Urrestarazu, M. Effects of salinity and the interaction between Thymus vulgaris and Lavandula angustifolia on growth, ethylene production and essential oil contents. J. Plant Nutr. 2014, 37, 875–888. [Google Scholar] [CrossRef]
- Davey, M.; Stals, E.; Panis, B.; Keulemans, J.; Swennen, R. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar] [CrossRef]
- Rio, D.D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovas. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Tang, K.; Zhan, J.C.; Yang, H.R.; Huang, W.D. Changes o fresveratrol and antioxidant enzymes during UV-induced plant defense response in peanut seedlings. J. Plant Physiol. 2010, 167, 95–102. [Google Scholar] [CrossRef]
- Rai, A.C.; Singh, M.; Shah, K. Effect of water withdrawal on formation of free radical, proline accumulation and activities of antioxidant enzymes in ZAT12-transformed transgenic tomato plants. Plant Physiol. Biochem. 2012, 61, 108–114. [Google Scholar] [CrossRef]
- Gratão, P.L.; Monteiro, C.C.; Tezotto, T.; Carvalho, R.F.; Alves, L.R.; Peters, L.J.; Azevedo, R.A. Cadmium stress antioxidante responses and root-to-shoot communication in grafted tomato plants. Biometals 2015, 28, 803–816. [Google Scholar] [CrossRef]
- Abouzari, A.; Fakheri, B.A. Reactive oxygen species: Generation, Oxidative Damage, and Signal Transduction. Int. J. Life Sci. 2015, 9, 3–17. [Google Scholar] [CrossRef]
- Alves, R.C.; Oliveira, K.R.; Lúcio, J.C.B.; Silva, J.S.; Carrega, W.C.; Queiroz, S.F.; Gratão, P.L. Exogenous foliar ascorbic acid applications enhance salt-stress tolerance in peanut plants throughout an increase in the activity of major antioxidant enzymes. S. Afr. J. Bot. 2022, 150, 759–767. [Google Scholar] [CrossRef]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant Biosys. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in theentire antioxidant defence grid. O.M. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Pozo, J.; Alvaro, J.E.; Morales, I.; Requena, J.; La Malfa, T.; Mazuela, P.; Urrestarazu, M. A new local sustainable inorganic material for soilless culture in Spain: Granulated volcanic rock. HortScience 2014, 49, 1537–1541. [Google Scholar] [CrossRef]
- Sonneveld, C.; Straver, N.B. Nutrient solution for vegetables and flowers grown in water or substrates. Voedingspolossingen glastijnbouw 1994, 8, 1–33. [Google Scholar]
- Shimizu, N.; Hosogi-Park, P.J. Reactive oxygen species (ROS) generation ROS-induced lipid peroxidation are associated with plasma membrane modifications in host cells in response to AK-toxin I from Alternaria alternate Japonese pear pathotype. J. Gen. Plant Pathol. 2006, 72, 6–15. [Google Scholar] [CrossRef]
- Gratão, P.L.; Monteiro, C.C.; Carvalho, R.F.; Tezotto, T.; Piotto, F.A.; Peres, L.E.P.; Azevedo, R.A. Biochemical dissection of diageotropica and never ripe tomato mutants to Cd stressful conditions. Plant Physiol. Biochem. 2012, 56, 79–96. [Google Scholar] [CrossRef]
- Cia, M.C.; Guimarães, A.C.R.; Medici, L.O.; Chabregas, S.M.; Azevedo, R.A. Antioxidant response to water deficit by drought-tolerant and -sensitive sugarcane varieties. Ann. Appl. Biol. 2012, 161, 313–324. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Teisseire, H.; Guy, V. Copper-induced changes in antioxidant enzymes activities in fronds of duckweed (Lemna minor). Plant Sci. 2000, 153, 65–72. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do Estado Nutricional da Plantas: Princípios e Aplicações; Potafós Publ.: Piracicaba, Brazil, 1997; p. 319. [Google Scholar]
- Commission Implementing Regulation (EU) No 543/2011 of 7 June 2011 Laying Down Detailed Rules for the Application of Council Regulation (EC) No 1234/2007 in Respect of the Fruit and Vegetables and Processed Fruit and Vegetables Sectors. January 2023. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32011R0543 (accessed on 15 March 2023).
- CEAGESP. Cotações: Legumes. Available online: http://www.ceagesp.com.br/ (accessed on 17 February 2023).
- Barbosa, J.C.; Maldonado, J.W. AgroEstat—Sistema para Análises Estatísticas de Ensaios Agronômicos; FCAV/UNESP: Jaboticabal, Brazil, 2015. [Google Scholar]
- Cunha-Chiamolera, T.P.L.; Morales, I.; Cecílio-Filho, A.B.; Urrestarazu, M. Viabilidad de los cultivos asociados de hortalizas en los sistemas sin suelo. Agrícola Vergel 2015, 34, 148–152. [Google Scholar]
- Gong, W.Z.; Jiang, C.D.; Wu, Y.S.; Chen, H.H.; Liu, W.Y.; Yang, W.Y. Tolerance vs. avoidance: Two strategies of soybean (Glycine max) seedlings in response to shade in intercropping. Photosynthetica 2015, 53, 259–268. [Google Scholar] [CrossRef]
- Correia, B.; Hancock, R.; Amaral, J.; Gómez-Cadenas, A.; Valledor, L.; Pinto, G. Combined drought and heat activities protective responses in Eucalyptus globulus that are not activated when subjected to drought and heat stress alone. Front. Plant Sci. 2018, 9, 819. [Google Scholar] [CrossRef]
- Nascimento, C.S.; Nascimento, C.S.; Lopes, G.; Carrasco, G.; Gratão, P.L.; Cecílio Filho, A.B. Biofortified rocket (Eruca sativa) with selenium by using the nutrient film technique. Horticulturae 2022, 8, 1088. [Google Scholar] [CrossRef]
- Roy, P.R.; Ul-Arif, M.T.; Akter, T.; Ray, S.R.; Sayed, M.A. Exogenous ascorbic acid and hydrogen peroxide alleviates salt-induced oxidative stress in rice (Oryza sativa L.) by enhancing antioxidant enzyme activities and proline content. Adv. Reinar. Biol. 2016, 10, 148–154. [Google Scholar]
- Niu, L.; Liao, W. Hydrogen peroxide signaling in plant development and abiotic responses: Crosstalk with nitric oxide and calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef]
- Alves, R.C.; Nicolau, M.C.M.; Checchio, M.V.; Sousa-Junior, G.S.; Oliveira, F.A.; Prado, M.R.; Gratão, P.L. Salt stress alleviation by seed priming with silicon in lettuce seedlings: An approach based on enhancing antioxidant responses. Bragantia 2020, 79, 19–29. [Google Scholar] [CrossRef]
- Wang, M.; Wu, C.; Cheng, Z.; Meng, H. Growth and physiological changes in continuously cropped eggplant (Solanum melongena L.) upon relay intercropping with garlic (Allium sativum L.). Front. Plant Sci. 2015, 6, 262. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, Y.; Xu, Z.; Chen, Z.; Duan, L. Combining physiological and metabolomic analysis to unravel the regulations of coronatine alleviating water stress in tobacco (Nicotiana tabacum L.). Biomolecules 2020, 10, 835. [Google Scholar] [CrossRef]
- Alves, R.C.; Rossatto, D.R.; Silva, J.S.; Checchio, M.V.; Oliveira, K.R.; Oliveira, F.A.; Queiroz, S.F.; Cruz, M.C.P.; Gratão, P.L. Seed priming with ascorbic acid enhances salt tolerance in micro-tom tomato plants by modifying the antioxidant defense system components. Biocatal. Agric. Biotechnol. 2021, 31, 101927. [Google Scholar] [CrossRef]
- Kolahi, M.; Mohajel-Kazemi, E.; Yazdi, M.; Goldson-Barnaby, A. Oxidative stress induced by cadmium in lettuce (Lactuca sativa Linn.): Oxidative stress indicators and prediction of their genes. Plant Physiol Biochem. 2020, 146, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Trani, P.E.; van Raij, B. Hortaliças. In Recomendações de Adubação e Calagem para o Estado de São Paulo (Boletim Técnico, 100), 2nd ed.; van Raij, B., Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; IAC: Campinas, Brazil, 1997; pp. 155–164. [Google Scholar]
- Ferreira, R.L.C.; Prado, R.M.; Souza Junior, J.P.; Gratão, P.L.; Tezotto, T.; Cruz, F.J.R. Oxidative stress, nutritional disorders, and gas exchange in lettuce plants subjected to two selenium sources. J. Soil Sci. Plant Nutr. 2020, 20, 1215–1228. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Dean, R.T.; Davies, M.J. Radical chemistry of epigallocatechin gallate and its relevance to protein damage. Arch. Biochem. Biophys. 2003, 414, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; VanBreusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 2006, 28, 1091–1101. [Google Scholar] [CrossRef]
- Singh, O.P.; Usha, K.; Saboki, E.; Srivastav, M.; Dahuja, A.; Singh, B. Enzymatic reactive oxygen species (ROS) scavenging system in mango varieties resistant and susceptible to malformation. Sci. Hortic. 2012, 38, 81–89. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M. Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Ríos, J.J.; Blasco, B.; Leyva, R.; Sanchez-Rodriguez, E.; Rubio-Wilhelmi, M.M.; Romero, L.; Ruiz, J.M. Nutritional balance changes in lettuce plant grown under different doses and forms of Se. J. Plant Nutr. 2013, 36, 1344–1354. [Google Scholar] [CrossRef]
- Barros Júnior, A.P.; Cecílio Filho, A.B.; Rezende, B.L.; Pôrto, D.R.Q.; Prado, R.M. 2011. Nitrogen fertilization on intercropping of lettuce and rocket. Hortic. Bras. 2011, 29, 398–403. [Google Scholar] [CrossRef]
- Cecílio Filho, A.B.; Alves, A.U.; Galati, V.C.; Bezerra Neto, F.; Barbosa, J.C.; Machado, B.Q.V. Intercropping of eggplant and tomato as function of times of transplant and cropping season. Rev. Caatinga. 2022, 35, 276–287. [Google Scholar] [CrossRef]
- Barbosa, A.P.; Arieira, J.O.; Hora, R.C.; Silva, A.F.M.; Kondo, P.N.Y.; Silva, M.R.; Severino, J.J.; Silva, T.R.B. An agronomic and economic evaluation of lettuce cultivars intercropped with rocket over two cultivation seasons. Afr. J. Agric. Res. 2015, 1, 1083–1090. [Google Scholar] [CrossRef]
- Bezerra Neto, F.; Gomes, E.G.; Araújo, R.R.; Oliveira, E.Q.; Nunes, G.H.S.; Grangeiro, L.C.; Azevedo, C.M.S.B. Evaluation of yield advantage indexes in carrot-lettuce intercropping systems. Interciencia 2010, 35, 59–64. [Google Scholar]
- Yan, Y.; Gong, W.; Yang, W.; Wan, Y.; Chen, X.; Chen, Z.; Wang, L. Seed treatment with uniconazole powder improves soybean seedling growth under shading by corn in relay strip intercropping system. Plant Prod. Sci. 2010, 13, 367–374. [Google Scholar] [CrossRef]
- Cecílio Filho, A.B.; Rezende, B.L.A.; Barbosa, J.C.; Grangeiro, L.C. Agronomic efficiency of intercropping tomato and lettuce. An. Acad. Bras. Cienc. 2011, 83, 1109–1119. [Google Scholar] [CrossRef]
- Su, B.Y.; Song, Y.X.; Song, C.; Cui, L.; Yong, T.W.; Yang, W.Y. Growth and photosynthetic responses of soybean seedlings to maize shading in relay intercropping system in Southwest China. Photosynthetica 2014, 52, 332–340. [Google Scholar] [CrossRef]
- Carballo-Méndez, F.J.; Urrestarazu, M.; Rodríguez-Ortiz, J.C.; Morales, I. Electrical conductivity of the nutrient solution on the vegetative propagation of bell pepper and tomato. Cienc. Rural. 2023, 53, e20210730. [Google Scholar] [CrossRef]
- Sandhu, R.K.; Boyd, N.S.; Qiu, Q.; Guan, Z.; Licata, M. 2020. Optimization of planting dates of Jalapeno pepper (Capsicum annuum ‘Jalapeño’ L.) and cantaloupe (Cucumis melo var. cantalupensis Ser.) relay cropped with strawberry (Fragaria x ananassa Duchesne). PLoS ONE 2020, 15, e0236677. [Google Scholar] [CrossRef] [PubMed]
- Andrade Filho, F.C.; Oliveira, E.Q.; Lima, J.S.S.; Moreira, J.N.; Silva, I.N.; Lins, H.A.; Cecílio Filho, A.B.; Barras Júnior, A.P.; Bezerra Neto, F. Agro-economic viability from two croppings of broadleaf vegetables intercropped with beet fertilized with roostertree in different population densities. Revista Facultad Ciencias Agrarias UNCuyo 2019, 52, 210–222. [Google Scholar]
- Cecílio Filho, A.B.; Machado, B.Q.V.; Alves, A.U.; Pereira, B.J.; Guerra, N.M.; Bezerra Neto, F. 2022. Bio-agronomic efficiency indices of eggplant and tomato intercropping. Hortic. Bras. 2022, 40, 181–189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).