Abstract
To understand the effect of ultraviolet (UV)-B irradiation on the antioxidant capacity and growth of lettuce (Lactuca sativa), we subjected lettuce plants to UV-B irradiation (15.55 kJ m−2 d−1) for 7 days and measured yield, photosynthetic performance, hydrogen peroxide (H2O2), reduced glutathione (GSH), and ascorbic acid (AsA) contents, and the enzyme activity and expression of genes involving AsA recycling. UV-B exposure did not significantly decrease the fresh/dry weight of the lettuce shoots. The net photosynthesis rate, internal CO2 concentration, transpiration rate, and stomatal conductance decreased during the first 4 days of irradiation and light but recovered at day 7. In UV-B-treated plants, the levels of AsA, GSH, and H2O2 increased significantly and simultaneously, with a positive correlation found between H2O2 and AsA or GSH levels. UV-B exposure upregulated the expression level of most genes encoding the enzymes involving AsA recycling but downregulated the associated enzymatic activities. The increase of AsA content in UV-B-exposed lettuce might contribute to the AsA–GSH cycle, leading to downregulation of ascorbate oxidase (AO) enzymatic activity and gene expression. UV-B irradiation had a greater impact on metabolite levels than time of UV-B treatment. These results suggest that AsA homeostasis in UV-B-treated lettuce is regulated through a feedback loop between the expression and activity of enzymes associated with AsA recycling. Short-term UV-B supplementation (24 h) could be a promising approach to enhance AsA content in lettuce.
1. Introduction
The ultraviolet (UV) radiation spectrum (100–400 nm) is divided into three wavebands: UV-A, UV-B, and UV-C. UV-B represents medium-wave UV radiation (280–315 nm) that is mostly absorbed by the stratospheric ozone (O3) layer. However, ozone depletion has led to the enhancement of UV-B radiation reaching the Earth, which has resulted in various environmental effects attracting the attention of researchers. UV-B radiation is generally considered as an environmental stress, causing changes in the morphology, physiology, and molecular processes of plants [1,2,3].
It is well known that UV-B radiation affects the chloroplast structure, especially photosystem II; thus, UV-B-induced damage leads to decreases in the chlorophyll a/b ratio and chlorophyll content, deregulates stomatal opening and closing, and reduces CO2 assimilation; therefore, reducing the overall photosynthesis efficiency and yield [4,5].
The increase in UV-B radiation usually inhibits the plant growth [6,7], decreasing leaf length and area, plant height, and shoot and stem dry mass, while increasing leaf thickness and axillary branching [8,9]. Moreover, UV-B influences the flowering process by reducing the flowering rate and expanding the size (diameter) of the flower [10,11].
UV-B radiation-induced stress leads to the overproduction of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), hydroxyl radical (HO–), or superoxide radical (O2•−) in the plant cell [12,13]. The ROS have high oxidative activity, which can damage cellular macromolecules, eventually inhibiting a variety of biochemical and physiological processes, thus affecting plant metabolism and growth [14,15]. To reduce ROS toxicity, plants have formed an effective and complex antioxidant resistance system involving both enzymatic and non-enzymatic mechanisms [16].
Ascorbic acid (AsA) as non-enzymatic antioxidant is the most abundant and important ROS-scavenging substance in plants [17]. UV-B exposure induces the production of AsA, which reacts with ROS and undergoes enzymatic oxidation to monodehydroascorbate (MDHA) and dehydroascorbate (DHA) through the activity of ascorbate peroxidase (APX) and ascorbate oxidase (AO), thus eliminating free radicals and protecting plants from oxidative stress [18]. It has been reported that UV-B induces AsA production in soybean sprouts [19], and that UV-B stress results in significant increases in the AsA content in Cassia auriculata seedlings [20]. The ascorbate-deficient mutant (vtc1) of Arabidopsis thaliana showed a highly sensitivity to UV-B irradiation [21]. Since the AsA contained in vegetables and fruit has beneficial effects on human health, UV-B irradiation can offer a effective approach to improve the quality of crops.
AsA accumulation in plants depends on its biosynthesis pathways and recycling processes. The four AsA biosynthetic mechanisms are the l-galactose [22], galacturonate [23], l-gulose [24], and myo-inositol [25] pathways, among which the pathway of l-galactose is predominant in most plants [26]. During recycling, MDHA and DHA are reconverted to AsA via the AsA–glutathione (GSH) cycle, which involve DHA reductase (DHAR), MDHA reductase (MDHAR), and GSH reductase (GR) (Figure S1) [17]. Accumulating evidence indicates that the AsA recycling pathway plays a critical role in the maintenance of the AsA pool and in the plant’s response to environmental stresses [27]. For Arabidopsis, when the plants were exposed to higher light and temperature conditions, DHAR expression increased and AsA levels were higher compared of those of control pants, leading to reduce levels of membrane damage [28]. In cucumber seedlings, the transcript levels and enzyme activities of AO, APX, GR, and DHAR presented significantly upregulation with UV-B exposure, suggesting that UV-B increases AsA levels by promoting the recycling pathway [29].
Lettuce is a vegetable crop grown worldwide with high economic value owing to its excellent nutrition and taste qualities. Due to its short growth cycle and suitability for soilless cultivation, lettuce can be cultivated in greenhouses and plant factories to ensure a good-quality and year-round supply. However, low light conditions and excessive fertilizer application result in a low AsA content in lettuce grown in protected facilities [30]. Light is a key regulator for AsA levels in plants [31]. Some studies have shown that high light intensity and continuous light-emitting diode (LED) light increase AsA levels in lettuce [32,33]; however, increasing the light intensity and irradiation time requires more energy, and thus costs, in production. Another study reported that lettuce grown under UV-transparent films exhibited a higher AsA content compared with that in lettuce under UV-blocking films in a greenhouse [34]. Regulation of the light quality (i.e., short-term UV-B irradiation supplementation) can provide a potential energy-efficient strategy to improve the AsA content of lettuce grown in plant factory using artificial light [35]. Our previous transcriptome comparison analysis showed that UV-B irradiation induces AsA accumulation in lettuce and changes the expression levels of related genes in the AsA recycling pathway (unpublished data). However, gaining a detailed understanding of the roles of UV-B irradiation in AsA levels and recycling in lettuce requires further exploration.
Application of UV-B irradiation is an effective technology to enhance the AsA level of plants and to regulate the plant growth, photosynthesis rate, and yield. Based on our previous results, we hypothesized that UV-B mediated regulation of growth and AsA content of lettuce involves the AsA recycling pathway at the level of enzymatic activities and gene expression. Therefore, the purpose of this study was to explore the impact of UV-B supplementation on lettuce by analyzing yield, photosynthetic capacity, and AsA accumulation, as well as gene expression levels and enzymes activities involving the AsA recycling pathway. We found that UV-B induced ROS production in parallel with AsA accumulation, which could be related to the upregulation of gene expression.
2. Materials and Methods
2.1. Plant Material and Treatments
Seeds of lettuce (Lactuca sativa L. cv. Green rose) were obtained from Shanghai Huihe Co., Ltd. and cultivated in Hoagland nutrient solution [36] after growing two true leaves in a plant factory at the Jiangxi Academy of Sciences under the following conditions: 20–22 °C, 60–80% humidity, and ambient CO2 concentration. Illumination was supplied for 16 h per day using LED tubes (Jinghe Lighting Co., Ltd., Nanchang, China), which provided red light (660 ± 10 nm) and blue (450 ± 10 nm) light at a 2:1 ratio; the photosynthetic photon flux density (PPFD) was 200 μmol m−2 s−1. After 10 d of growth, the plants had grown five or six leaves and were randomly separated into two parts (n = 15 each): one treated with supplementary UV-B irradiation and the other left untreated (control). Plant-weighted UV-B was applied with a radiation dose of 15.55 kJ m−2 d−1 using UV-B lamps (Phillips TL 100W/01, Eindhoven, The Netherlands), which received a total radiation dose of 15.55 kJ m−2 d−1 settled by controlling the height of the tube, measured using an LS125 UV light meter host (Linshang Technology Co., Ltd., Shenzhen, China) throughout the 7-day experimental period.
2.2. Sampling and Yield Measurements
Three samples were randomly selected from each group at 0 h, 1 h, 4 h, 24 h, 4 day, and 7 day of treatment. Fresh leaves without petioles were cut and immediately putted into liquid nitrogen for 10 min and preserved at −80 °C until further processing. The yield was measured at 7 d in the end of the experiment. Lettuce fresh shoots and roots were removed and weighed. Subsequently, the shoots and roots were dried at 105 °C in the oven for 5 min and again at 70 °C until the dry weight had stabilized.
2.3. Analysis of Photosynthesis Parameters
The net photosynthetic rate (An), stomatal conductance (gs), intercellular CO2 concentration (Ci), and transpiration rate (E) were monitored in the top third leaf of three randomly selected lettuce plants. Measurements were taken from 9 to 11 AM using an LI-6400XT photosynthesis instrument (Li-Cor Inc., Lincoln, NE, USA). Conditions of the leaf chamber were maintained at a flow rate of 500 μmol s−1, with a saturating photosynthetic active radiation (PAR) at 1000 μmol m−2 s−1, CO2 concentration at 400 μmol mol−1, relative humidity of 50–60% and leaf temperature at 25 °C. The measurements were repeated three times.
2.4. Biochemical Measurements
AsA and total-AsA (T-AsA) concentrations were determined by spectrophotometry as previously described by Gillespie and Ainsworth [37]. Approximately 0.1 g tissue were homogenized in 1 mL of trichloroacetic acid, centrifuged at 13,000× g over 10 min at 4 °C, and 0.15 mL of supernatant was blended with phosphate buffer (75 mM, 0.15 mL) and dithiothreitol (10 mM, 0.3 mL) and incubated for 15 min at 42 °C. Subsequently, 0.15 mL of N-ethylmaleimide was mixed and reacted at 37 °C for 1 min. Finally, trichloroacetic acid (10%, 0.75 mL), H3PO4 (43%, 0.6 mL), ethanol (70%, 0.6 mL), and FeCl3 (30%, 0.3 mL) were mixed and reacted at 42 °C for 40 min; and the T-AsA content was determined based on the absorbance measured at 525 nm. The AsA content was measured in similar steps except that 0.3 mL deionized H2O was used instead of dithiothreitol and N-ethylmaleimide.
The GSH content was determined as previously described by Rahman [38]. Approximately 0.1 g Leaves were homogenized in 1 mL of sulfosalicylic acid and transferred to centrifuge at 13,000× g over 15 min at 4 °C. The supernatant was blended with deionized H2O and phosphate-buffered saline and reacted at 25 °C for 10 min. GR was added to the incubation reaction for 3 min and the absorbance of the mixture was measured at 412 nm.
The H2O2 level was determined using the methods previously described by Brennan and Frenkel [39]. Approximately 0.1 g leaves were homogenized in 1 mL of acetone and transferred to centrifuge at 10,000× g over 20 min at 4 °C. Then the supernatant was collected, mixed with titanium sulfate and ammonia, and centrifuged at 4000× g for 15 min. The precipitate was dissolved in H2SO4 and the absorbance of the mixture was measured at 412 nm.
AO activity was determined as previously described by Esaka et al. [40]. Approximately 0.1 g leaves were extracted in 1 mL of pre-cooled pH 6.0 phosphate buffer. The reaction mixture contained the supernatant, 0.1 M sodium phosphate, 0.5 mM ethylenediaminetetraacetic acid, and 100 μM AsA, and the decrease in absorbance of the reaction solution was measured at 265 nm. For the APX activity assay, the method of Naknao and Asada was used by monitoring the decrease of the absorbance at 290 nm [41]. MDHAR activity assay was carried out in 1 mL reaction buffer containing pH 7.0, 0.2 mM NADH, 50 mM potassium phosphate, 2.5 mM AsA and 0.2 U AO based on the decrease in absorbance at 340 nm as described by Zhang and Kirkham [42]. The activity of DHAR was determined in 1 mL reaction buffer containing pH 6.3, 1 mM oxidized ascorbate, 50 mM potassium phosphate, and 2 mM GSH) by observing the GSH-dependent production of AsA based on the reduction of the absorbance at 265 nm [43]. The GR activity assay was carried out in 1 mL reaction buffer containing pH 7.8, 0.5 mM glutathione disulfide, 50 mM potassium phosphate, 0.15 mM NADPH, and 5 mM MgCl2 by monitoring the reduction of the absorbance of the mixture at 340 nm due to the GSH-dependent oxidation of NADPH [44].
In all assays, the absorbance of the reaction was determined with a UV-VIS spectrophotometer (V-5600, Metash, Shanghai, China).
2.5. Gene Expression Analysis
The total RNA was isolated from lettuce leaves by the CTAB method according to Chen et al. [45] and treated with DNase I (Aidlab, Beijing, China) to eliminate genomic DNA. RNA quality was checked on 1.5% agarose gels to avoid RNA degradation and contamination. RNA purity and concentration were determined by monitoring absorbance at 230, 260 and 280 nm using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Complementary DNA (cDNA) synthesis from total RNA was carried out using PC18-TRUEscript 1st Strand cDNA Synthesis Kit (Aidlab, Beijing, China) according to the manufacturer’s protocol. Quantitative polymerase chain reaction (qPCR) was performed in a final volume of 20 µL containing 0.5 µL cDNA, 10 µL SYBR Green PCR Master Mix (Takara, Dalian, China), 10 µM primers, and 8.5 µL ddH2O. Primers specific for five selected genes (AO, APX, MDHAR, DHAR, GR) (Table S1) were designed using Primer Premier 5.0 software. The qPCRs were performed on a CFX Opus 96 real-time PCR instrument (Bio-Rad, Hercules, CA, USA) and three biological and three technical replicates were conducted in the experiment. The lettuce GAPDH gene was used for data normalization, and the relative expression levels of genes were calculated and quantified using the 2−∆∆ Ct method [46].
2.6. Statistical Analysis
SPSS 20.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for the data analysis. The results for plant growth, biochemical parameters and gene expression levels were evaluated using one-way analysis of variance (ANOVA). The biochemical parameters and gene expression levels were subjected to two-way ANOVA, where UV-B treatment and time of UV-B treatment were considered as two factors. The method of Duncan’s new complex range test was used for multiple comparisons for analysis of the differences between treatments at each time point. All significance analyses were calculated using two-tailed tests. When the p-value was <0.05, the results were considered statistically significant. The Pearson correlation coefficient was used to detect any correlations between biochemical parameters; and gene expression levels.
3. Results
3.1. Effects of UV-B on Lettuce Biomass
After 7 d of UV-B irradiation, the fresh/dry weight of the lettuce shoots and roots in the UV-B group were lower than those in the control, although the difference between UV-B and control was not statistically significant, except for the root dry weight (Table 1).

Table 1.
Effects of ultraviolet (UV)-B exposure on lettuce mass.
3.2. Changes of Leaf Gas Exchanges Parameters during UV-B Radiation
Analysis of leaf gas exchanges parameters (Figure 1) revealed that in UV-B-irradiated lettuce plants, the An decreased after 1 h of treatment and showed a significant difference with that of the control group at 4 h (Figure 1A). The changes in Ci showed the opposite dynamics to those of An; however, no significant differences in Ci were found between UV-B-treated and control plants (Figure 1C). The gs and E had similar dynamics and were consistently lower in UV-B-treated plants up to 4 d, when the differences from those of the control were significant, but increased at 7 d (Figure 1B,D).
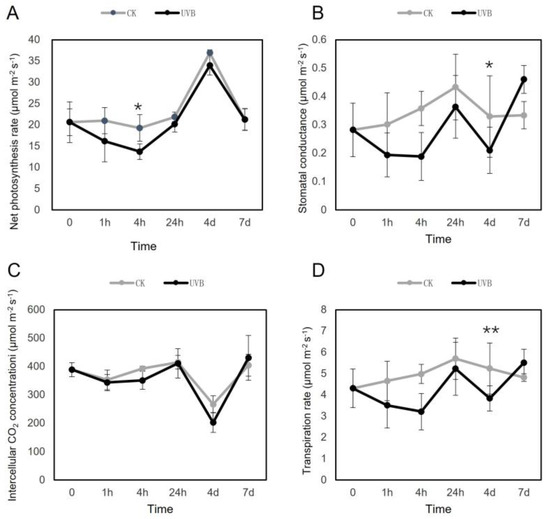
Figure 1.
Changes of leaf gas exchange parameters in lettuce treated with or without (control) ultraviolet (UV)-B. (A) net photosynthetic rate, (B) stomatal conductance, (C) intercellular CO2 concentration, (D) transpiration rate. The mean values and standard deviations are shown (n = 3); * and ** indicate significant difference between CK (control) and UV-B groups at each time point with p < 0.05 and p < 0.01, respectively.
3.3. Changes in AsA, GSH, and H2O2 Contents
Compared to that of the control, lettuce plants exposed to UV-B showed steady and significant increases in H2O2 production (Figure 2). Similar changes were observed in AsA and T-AsA contents, and UV-B-treated plants presented significantly higher contents than untreated plants, especially at 4 d and 7 d, when control plants showed a decrease in AsA and T-AsA contents compared to those measured at earlier time points. The GSH content was also increased after UV-B irradiation, and the difference from that of the control was significant at 4 h and 7 d. ANOVA indicated that the impacts of UV-B treatment, UV-B exposure time, and the UV-B × time interaction on H2O2 and GSH contents were significant (Table 2). The impacts of UV-B treatment and the UV-B × time interaction on AsA and T-AsA contents were also significant.
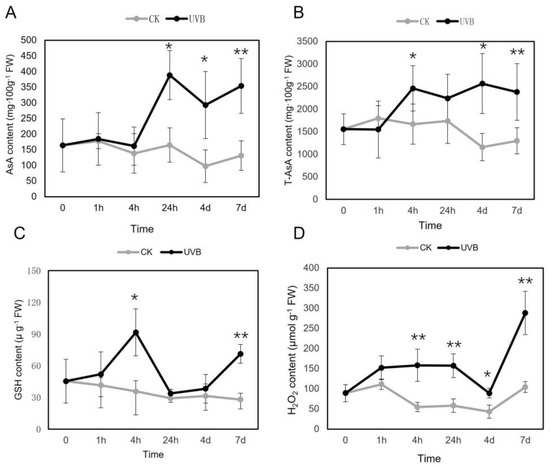
Figure 2.
Time-dependent changes of metabolite accumulation in lettuce treated with or without (control) ultraviolet (UV)-B. (A) AsA, (B) T-AsA, (C) GSH, and (D) H2O2 contents. The mean values and standard deviations are shown (n = 3); * and ** indicate significant difference between CK (control) and UV-B groups at each time point with p < 0.05 and p < 0.01, respectively. Abbreviations: AsA, ascorbic acid; T-AsA, total ascorbic acid; GSH, glutathione.

Table 2.
Analysis of variance results (p-values) of the effects of ultraviolet (UV)-B treatment and time of treatment on metabolite contents in lettuce.
3.4. UV-B Effects on the Activity of Enzymes Related to AsA Recycling and Their Gene Expression
AO activity was decreased in lettuce plants treated with UV-B, especially at 7 d (p < 0.01), which was consistent with the downregulation of AO gene expression (p < 0.05 at 4 h, 24 h, 4 d, and 7 d; Figure 3A,B). APX activity in the UV-B-treated group tended to be lower than that in the control (except for 7 d) (Figure 3C); however, APX gene expression was upregulated by UV-B, especially at 24 h (p < 0.01) (Figure 3D). MDHAR activity and gene expression showed similar changes after UV-B treatment, which were not significant compared with control levels (Figure 3E,F). GR activity was lower in UV-B-treated plants than in control plants at 4 h, 4 d, and 7 d (p < 0.05), whereas its mRNA expression was higher, especially at 7 d (p < 0.05) (Figure 3G,H). The results showed that no significant differences were found in DHAR activity and gene expression between the control and treatment groups except at 4 h (Figure 3I,J). ANOVA indicated that the effects of UV-B treatment, UV-B exposure time, and UV-B × time interaction on MDHAR and GR activities reached significant levels (Table 3). The analysis results showed that the effects of UV-B treatment and UV-B exposure time on AO activity was significant; however, the effects of UV-B treatment on APX and DHAR were not significant (Table 3). The effects of UV-B treatment, exposure time, and the UV-B × time interaction on AO gene expression were also significant; however, the effect of UV-B treatment on DHAR and MDHAR expression was not significant (Table 4).
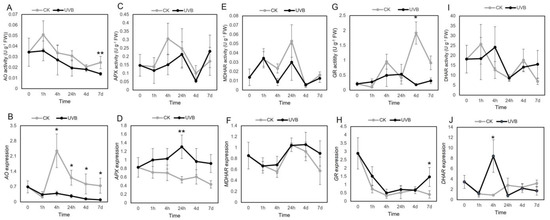
Figure 3.
Dynamic changes of enzyme activity and gene expression levels in lettuce treated with or without (control) ultraviolet (UV)-B. AO activity(A) and gene expression (B), APX activity (C) and gene expression (D), MDHAR activity (E) and gene expression (F), GR activity (G) and gene expression (H), DHAR activity (I) and gene expression (J). Upper and lower panels show catalytic activity and mRNA expression, respectively. The mean values and standard deviations are shown (n = 3); * and ** indicate significant difference between CK (control) and UV-B groups at each time point with p < 0.05 and p < 0.01, respectively. Abbreviations: AO, ascorbate oxidase; APX, ascorbate peroxidase; MDHAR, monodehydroascorbate reductase; GR, glutathione reductase; DHAR, dehydroascorbate reductase.

Table 3.
Analysis of variance results (p-values) of the effects of ultraviolet (UV)-B treatment and time of treatment on enzyme activities in lettuce.

Table 4.
Analysis of variance results (p- values) of the effects of ultraviolet (UV)-B treatment and time of treatment on gene expression in lettuce.
3.5. Correlation Analysis
Correlations between AsA, T-AsA, H2O2, and GSH contents; enzyme activities; and gene expression levels were determined (Table 5). AsA content was a significant positive correlated with the T-AsA and H2O2 contents and APX gene expression, and a significant negatively correlated with AO activity. H2O2 content was significant positively correlated with AsA, GSH contents and GR expression. GSH content was significantly positively correlated with H2O2 content and DHAR expression. APX activity and expression were significantly positively correlated with AO expression levels.

Table 5.
Correlation analysis (Pearson correlation coefficients) of the effects of ultraviolet-B exposure on metabolites contents, enzyme activities, and gene expression levels in lettuce.
4. Discussion
4.1. UV-B Radiation Dose Impacts the AsA Content and Biomass of Lettuce
In vegetable horticulture, the crop yield and product quality are matters of major concern for both producers and consumers. UV-B is generally regarded as an abiotic stress factor that impairs the photosynthetic process in plants [47], and the inhibitory effects of UV-B radiation on the biomass, leaf area, and stem height of various plant species have been documented [48]. Enhanced UV-B decreases the leaf E and gs, thereby reducing Ci, resulting in a reduction of the productivity and photosynthesis in crops [49]. However, recent studies have indicated that enhanced solar UV-B irradiation does not always cause environmental stress for plants [50]. Some studies have showed that photosynthesis is not highly sensitive to UV-B dose increase [51], and that excessive UV-B exposure has a significantly increase in the leaf area, fresh and dry biomass [52]. Moreover, application of supplemental UV-B irradiation (12.5 μmol·m−2 s−1) to lettuce during early development with 500 μmol m−2 s−1 delivered by metal halide induced photoprotective mechanisms, improved long-term characteristics such as plant robustness and the final yield [53,54]. These results suggested that UV-B may have a beneficial effect in promoting the quantity and nutritional quality of horticultural crops [55]. In this study, the supplementation of LED light (200 μmol m−2 s−1) with UV-B irradiation (15.55 kJ m−2 d−1) was slightly harmful to lettuce plants, as evidenced by decreased photosynthetic performance at the early stages; however, the photosynthesis rate improved during the later stages and was higher than that of the control group after 7 d, suggesting that the photosynthesis response to UV-B in lettuce is an acclimation process. Although a decrease in fresh/dry mass of lettuce shoots was observed after UV-B exposure, the change was no longer statistically significant after 7 d of UV-B radiation. The influences of UV-B on photosynthetic performance and crop yield may depend not only on plant adaptability and/or genetic traits but also on the UV-B dose and PAR intensity.
In this study, treatment with enhanced UV-B irradiation resulted in increased the AsA content of lettuce, which confirms the results of previous studies [56]. However, although UV-B stimulates ROS production, it does not always upregulate AsA biosynthesis [31]. For example, UV-B radiation has been reported to decrease the AsA content in the fruit of tomato (Lycopersicon esculentum) plants of the DRW and Esperanza genotypes but it increased the AsA content of the HP1 genotype [57]. UV-B irradiation significantly improved the AsA content in rice (Oryza sativa) after 14 d, followed by a gradual decrease in AsA, with no difference between the UV-B and control plants detected after 28 d [58]. These results suggested that the response of AsA content to UV-B may be species-specific and related to the dose applied. In our study, the lettuce AsA content increased significantly at 24 h of UV-B treatment and remained stable at 4 d and 7 d, suggesting that only a 24 h period of UV-B irradiation can markedly improve the AsA content of lettuce. Similar results were previously observed in broccoli, which exhibited a higher AsA content in UV-B exposed plants compared to that of control plants without UV-B treatment, although there were no significant differences between the 2.2, 8.8, and 16.4 KJ m−2 d−1 UV-B doses [59]. In combination, the results of the growth experiments suggest that 24 h UV-B irradiation could increase the AsA content of pre-harvest lettuce without significant negative effects on plant growth in the artifical lighting plant factory. Short-term UV-B exposure can increase the AsA content in crops, suggesting that UV-B supplementation may be a promising approach to enhance plant nutritional quality in commercial horticulture production [35].
In addition, UV-B irradiation plays avital role in the morphogenetic architecture of plants, and affects the production of metabolites and disease resistance in crops [49,60]. The influences of UV-B on plant secondary metabolism, synthesis of antioxidants, and resistance to pests and diseases have the potential to be beneficial for the development of sustainable technologies to enhance crop quality [55]. In this study, we focused on the UV-B-induced AsA accumulation in lettuce. However, further investigation into the production of secondary metabolites in response to UV-B would be beneficial, which could improve the commercial value of lettuce without compromising biomass production.
4.2. AsA Recycling Response to UV-B Radiation
In this study, we observed that UV-B exposure increased ROS (H2O2) accumulation, while simultaneously inducing the production of the non-enzymatic antioxidant AsA, which showed a significant positive correlation with H2O2 content of tobacco (Nicotiana tabacum). This suggest that AsA plays a major role in neutralizing H2O2, thereby enhancing the tolerance of lettuce to UV-B stress. A similar role of AsA has previously been reported in antioxidant signaling in response to stress [61]. The positive correlation between H2O2 and AsA levels is often related to enhanced tolerance to stress, as observed in plants such as brown mustard (Brassica juncea) [62], tomato [63], and wheat (Triticum aestivum) [64]. As the most powerful ROS scavenger, AsA directly donated electrons to free radicals, thereby protecting plants from oxidative damage [65]. AsA recycling plays a crucial role in maintaining the reduced forms of AsA and in the detoxification of ROS during UV-B radiation exposure in plants [66].
GSH is another non-enzymatic antioxidant that functions to donor electron for DHAR to catalyze the reduction of DHA to AsA, thus contributing to ROS scavenging and plant stress resistance [67]. In the present experiment, UV-B irradiation increased GSH levels in lettuce at 4 h (Figure 2C), which corresponded to a significant upregulation of T-AsA levels and DHAR gene expression. There was a positive correlation between GSH content and DHAR expression (Figure 2 and Figure 3), suggesting that GSH-dependent DHAR plays a role in AsA accumulation in response to UV-B exposure. As part of the AsA recycling pathway, the AsA–GSH cycle includes APX, DHAR, MDHAR, and GR, and maintains the reduced forms of AsA and GSH in the cell to protect plants against oxidative stress [68]. DHAR is a key enzyme involved in AsA recycling, which catalyzes the GSH-dependent reduction of dehydroascorbic acid to AsA by oxidized ascorbate. Overexpression of DHAR has been reported to enhance the plants tolerance to abiotic stresses [69]. For example, DHAR overexpression leads to enhanced AsA accumulation and improved tolerance to salt stress in the cherry tomato plant [70]. In the AsA–GSH cycle, AsA donates the electron to APX for H2O2 detoxification [71]. Our study found that AsA content was positively correlated with APX expression, indicating that APX has a key role in AsA accumulation in lettuce under UV-B. Similarly, the exposure of Arabidopsis thaliana leaves to high-intensity illumination led to a rapid increase of APX transcription [72]. Overexpression of Arabidopsis peroxisomal APX increases the resistance to H2O2 in tobacco [73]. Moreover, GR expression and GSH activity were significantly upregulated under UV-B irradiation for 7 d. Overexpression of chloroplasts GR promoted the accumulation of AsA and GSH in tobacco, and the transgenic plants had a stronger tolerant to high illumination and chilling stress [74]. Collectively, these results suggest that the AsA–GSH cycle plays an important role in AsA regeneration and, consequently, in stimulating protective responses to UV-B radiation in lettuce.
AO converts the AsA redox state into an oxidized state, and regulation of AO gene expression, which is important for AsA metabolism and responses to stress [40]. Our results revealed that both AO activity and AO expression level were significantly downregulated in lettuce after UV-B exposure, showing negative correlations with the AsA and T-AsA contents, suggesting that the decrease in AO enzymatic activity and gene expression contributed to AsA accumulation in lettuce. Consistent with our findings, prior studies demonstrated that the AO gene suppression in tomato fruit results in a significant increase in the AsA level and that the two parameters are correlated [75]. It has also been reported that overexpression of cucumber AO reduced AsA levels and increased the sensitivity to ozone injury in tobacco [76]. In the present study, the observed significant downregulation of AO expression at 4 h occurred earlier than the decrease in AO enzymatic activity at 24 h after UV-B irradiation, which was consistent with the changes of AsA levels under UV-B (Figure 2 and Figure 3). Therefore, we can assume that the decrease in AO enzymatic activity could be due to the downregulation of AO expression as a defensive response to maintain a high level of AsA to counteract increased H2O2 production in lettuce.
The ANOVA results (Table 2 and Table 3) showed that UV-B had a greater impact on the AsA, T-AsA, GSH, and H2O2 metabolite contents than the exposure time; however, the impact of the exposure time on the catalytic activity was significant for all analyzed enzymes. The higher gene expression level and lower enzyme activities in UV-B treatments than the control (Figure 3) indicated that in UV-B-irradiated lettuce, changes in gene expression had a faster and stronger effect on AsA metabolism than on enzymatic activity. In turn, the enzyme activities depended on the exposure time, and the directions of their changes in response to UV-B were opposite to those found in gene expression (except for AO), which might indicate post-transcriptional regulation during AsA accumulation. The same relationship between the catalytic activity and mRNA expression of AsA metabolism-related enzymes has been observed in lettuce treated under continuous light [77]. Moreover, the catalytic activities of APX, GR, DHAR, and MDHAR showed a tendency to decrease after UV-B exposure, which did not correspond to their transcriptional upregulation or to the increase in AsA content, suggesting a feedback mechanism to control AsA homeostasis in lettuce under UV-B irradiation. Such feedback inhibition regulation of the AsA level has been reported in pea [78] and tomato [79] plants, where the activities of the key enzymes related to AsA recycling and biosynthesis were inhibited by the increase in AsA content.
5. Conclusions
UV-B treatment significantly increased AsA content of lettuce via the AsA recycling pathway. The treatment did not significantly decrease fresh shoot weight. Supplementation of basic illumination conditions (red:blue light = 2:1) with UV-B irradiation (15.55 kJ m−2 d−1) augmented the AsA and T-AsA contents in lettuce. Although the photosynthetic rate of lettuce initially decreased in response to UV-B, it subsequently recovered during the adaptation process and the reduction of leaf weight became insignificant. UV-B exposure increased H2O2 accumulation and the production of the non-enzymatic antioxidants GSH and AsA, which showed significant positive correlations with the H2O2 content. The UV-B-induced AsA accumulation was mostly due to changes in the AsA–GSH cycle, and had a closer association with gene expression than with enzyme activity; however, the enzyme activity level might have a feedback regulatory effect on the AsA content. The decrease in AO enzymatic activity and transcript level may contributed to AsA accumulation in lettuce under UV-B exposure. Short-term UV-B supplementation (24 h) can be exploited as a promising method to enhance the AsA content and nutritional value of lettuce and other crops grown in greenhouse environments. Our study presents a practical approach for the use of UV-B supplementation to increase AsA levels in horticultural crops. The results also enhance our comprehending of the physiological and molecular mechanisms of adaptation to UV-B stress. Further investigation of the specific secondary metabolites involved in the plant response to UV-B irradiation would benefit horticulture development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9020200/s1, Table S1: Primers for real time RT-PCR analysis. Figure S1: The recycling pathway of l-ascorbic acid in plants [27]. L-ASA, l-ascorbic acid; H2O2, hydrogen peroxide; GSH, glutathione; MDHAR, monodehydroascorbate reductase; APX, ascorbate peroxidase; AO, ascorbate oxidase; DHA, dehydroascorbate; MDHA, monodehydroascorbate; DHAR, dehydroascorbate reductase; GSSG, glutathione disulfide; GR, glutathione reductase.
Author Contributions
Conceptualization, H.Z. and A.Z.; Methodology, S.L., L.Y. and H.Z. investigation, A.Y., L.L. and X.H.; Writing, H.Z., S.L. and A.Y.; Supervision, A.Z., S.L. and H.Z.; Funding acquisition, H.Z. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31960623). No additional external funding was received for this work. The funder played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
We thank Yanfang Yang for kindly providing advice for our manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dotto, M.; Casati, P. Developmental reprogramming by UV-B radiation in plants. Plant Sci. 2017, 264, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrihnan, P.; Masapapalli, S.K.; Datta, S. Light signaling and UV-B-mediated plant growth regulation. JIPB 2020, 62, 1270–1292. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Liu, H. How plants protect themselves from ultraviolet-B radiation stress. Plant Physiol. 2021, 187, 1096–1103. [Google Scholar] [CrossRef]
- Machado, F.; Dias, M.C.; de Pinho, P.G.; Araújo, A.M.; Pinto, D.; Silva, A.; Correia, C.; Moutinho-Pereira, J.; Santos, C. Photosynthetic performance and volatile organic compounds profile in Eucalyptus globulus after UVB radiation. Environ. Exp. Bot. 2017, 140, 141–149. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Zhu, J.; Yue, K.; Zhou, K. Characteristics of mango leaf photosynthetic inhibition by enhanced UV-B radiation. Horticulturae 2021, 7, 557. [Google Scholar] [CrossRef]
- Zhang, L.; Allen, L.H.; Vaughan, M.M.; Hauser, B.A.; Boote, K.J. Solar ultraviolet radiation exclusion increases soybean internode lengths and plant height. Agric. Forest Meteorol. 2014, 184, 170–178. [Google Scholar] [CrossRef]
- Jenkins, G.I. Photomorphogenic responses to ultraviolet-B light. Plant Cell Environ. 2017, 40, 2544–2557. [Google Scholar] [CrossRef]
- Innes, S.N.; Solhaug, K.A.; Arve, L.E.; Torre, S. UV radiation as a tool to control growth, morphology and transpiration of poinsettia (Euphorbia pulcherrima) in variable aerial environments. Sci. Hortic. 2018, 235, 160–168. [Google Scholar] [CrossRef]
- Torre, S.; Roro, A.G.; Bengtsson, S.; Mortensen, L.; Solhaug, K.A.; Gislerod, H.R.; Olsen, J.E. Control of plant morphology by UV-B and UV-B temperature interactions. Acta Hortic. 2012, 956, 207–214. [Google Scholar] [CrossRef]
- Llorens, L.; Badenes-Pérez, F.R.; Julkunen-Tiitto, R.J.; Zidorn, C.; Fereres, A.; Jansen, M.A. The role of UV-B radiation in plant sexual reproduction. Perspect. Plant Ecol. 2015, 17, 243–254. [Google Scholar] [CrossRef]
- Petropoulou, Y.; Georgiou, O.; Psaras, G.K.; Manetas, Y. Improved flower advertisement, pollinator rewards and seed yield by enhanced UV-B radiation in the Mediterranean annual Malcolmia maritima. New Phytol. 2001, 152, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radical Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Coaker, G.L. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 2019, 18, 100173. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Plant Sci. 2000, 19, 267–290. [Google Scholar]
- Conklin, P.L. Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ. 2001, 24, 383–394. [Google Scholar] [CrossRef]
- Xu, M.J.; Dong, J.F.; Zhu, M.Y. Effects of germination conditions on ascorbic acid level and yield of soybean sprouts. J. Sci. Food Agric. 2005, 85, 943–947. [Google Scholar] [CrossRef]
- Agarwal, S. Increased antioxidant activity in Cassia seedlings under UV-B radiation. Biol. Plant. 2007, 51, 157–160. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, L. Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Agius, F.; González-Lamothe, R.; Caballero, J.L.; Muñoz-Blanco, J.; Botella, M.A.; Valpuesta, V. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol. 2003, 21, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Wolucka, B.A.; Van, M.M. GDP-mannose 3′,5′-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J. Biol. Chem. 2003, 278, 47483–47490. [Google Scholar] [CrossRef] [PubMed]
- Lorence, A.; Chevone, B.I.; Mendes, P.; Nessler, C.L. myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004, 134, 1200–1205. [Google Scholar] [CrossRef]
- Ntagkas, N.; Woltering, E.; Nicole, C.; Labrie, C.; Marcelis, L.F.M. Light regulation of vitamin C in tomato fruit is mediated through photosynthesis. Environ. Exp. Bot. 2019, 158, 180–188. [Google Scholar] [CrossRef]
- Gallie, D.R. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. Environ. Exp. Bot. 2013, 64, 433–443. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Y.; Chen, W.; Tang, K.; Zhang, L. Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J. Integr. Plant Biol. 2010, 52, 400–409. [Google Scholar] [CrossRef]
- Liu, P.; Li, Q.; Gao, Y.; Wang, H.; Chai, L.; Yu, H.; Jiang, W. A new perspective on the effect of UV-B on L-Ascorbic acid metabolism in cucumber seedlings. J. Argic. Food Chem. 2019, 67, 4444–4452. [Google Scholar] [CrossRef]
- Massot, C.; Génard, M.; Stevens, R.; Gautier, H. Fluctuations in sugar content are not determinant in explaining variations in vitamin C in tomato fruit. Plant Physiol. Biochem. 2010, 48, 751–757. [Google Scholar] [CrossRef]
- Ntagkas, N.; Woltering, E.J.; Marcelis, L.F.M. Light regulates ascorbate in plants: An integrated view on physiology and biochemistry. Environ. Exp. Bot. 2018, 147, 271–280. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Yang, Q.; Zhang, Y.; Zhou, C.; Shao, M. Regulation of ascorbate accumulation and metabolism in lettuce by the red: Blue ratio of continuous light using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar]
- Zushi, K.; Suehara, C.; Shirai, M. Effect of light intensity and wavelengths on ascorbic acid content and the antioxidant system in tomato fruit grown in vitro. Sci. Hortic. 2020, 274, 109673. [Google Scholar] [CrossRef]
- Quinteroarias, D.G.; Acuña-Caita, J.F.; Asensio, C.; Valenzuela, J.L. Ultraviolet transparency of plastic films determines the quality of lettuce (Lactuca sativa L.) grown in a greenhouse. Agronomy 2021, 11, 358. [Google Scholar] [CrossRef]
- Loconsole, D.; Santamaria, P. UV lighting in horticulture: A sustainable tool for improving production quality and food safety. Horticulturae 2021, 7, 9. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agr. Expt. Sta. Circ. 1950, 347, 1–32. [Google Scholar]
- Gillespie, K.M.; Ainsworth, E.A. Measurement of reduced, oxidized and total ascorbate content in plants. Nat. Protoc. 2007, 2, 871–874. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Portoc. 2007, 1, 3159–3165. [Google Scholar] [CrossRef]
- Brennan, T.; Frenkel, C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef]
- Pignocchi, C.; Fletcher, J.M.; Wilkinson, J.E.; Barnes, J.D.; Foyer, C.H. The function of ascorbate oxidase in tobacco. Plant Physiol. 2003, 132, 1631–1641. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts- its inactivation in ascorbate-depleted medium and reactivation by monodeydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar]
- Zhang, J.; Kirkham, M.B. Enzymatic response of the ascorbate-glutathione cycle to drought in sorghum and sunflower plants. Plant Sci. 1996, 113, 139–147. [Google Scholar] [CrossRef]
- Arrigoni, O.; De Gara, L.; Tommasi, F.; Liso, R. Changes in ascorbate system during seed development of Vicia faba. Plant Physiol. 1992, 99, 235–238. [Google Scholar] [CrossRef]
- Dalton, D.A.; Langeberg, L.; Treneman, N.C. Correlations between the ascorbate-glutathione pathway and effectiveness in legume root nodules. Plant Physiol. 1993, 87, 365–370. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, H.W.; Wang, X.R.; Xie, X.L.; Yue, X.Y.; Tang, H.R. An alternative cetyltrimethylammounium bromidebased protocol for RNA isolation from blackberry (Rubus L.). Genet. Mol. Res. 2012, 11, 1773–1782. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-11 Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Goto, E.; Matsumoto, H.; Ishigami, Y.; Hikosaka, S.; Fujiwara, K.; Yano, A. Measurements of the photosynthetic rates in vegetables under various qualities of light from light-emitting diodes. Acta Hortic. 2020, 1037, 261–268. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Gourrierec, J.L.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Meyer, P.; Poel, B.; Coninck, B.D. UV-B light and its application potential to reduce disease and pest incidence in crops. Hortic. Res. 2021, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Schreiner, M. UVB and UVA as eustressors in horticultural and agricultural crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Caldwell, M.M.; Flint, S.D.; Robinson, S.A.; Bornman, J.F. Effects of solar ultraviolet radiation on terrestrial ecosystems. Patterns, mechanisms, and interactions with climate change. Photochem. Photobiol. Sci. 2011, 10, 226–241. [Google Scholar] [CrossRef]
- Sakalauskait, E.J.; Viskelis, P.; Dambrauskien, E.; Sakalauskien, S.; Samuolien, G.; Brazaityt, A.; Duchovskis, P.; Urbonavicien, D. The effects of different UV-B radiation intensities on morphological and biochemical characteristics in Ocimum basilicum L. J. Sci. Food Agric. 2013, 93, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Wargent, J.J.; Nelson, B.C.W.; Mcghie, T.K.; Barnes, P.W. Acclimation to UVB radiation and visible light in Lactuca sativa involves up-regulation of photosynthetic performance and orchestration of metabolome-wide responses. Plant Cell Environ. 2015, 38, 929–940. [Google Scholar] [CrossRef]
- Wargent, J.J.; Elfadly, E.M.; Moore, J.P.; Paul, N.D. Increased exposure to UV-B radiation during early development leads to enhanced photoprotection and improved long-term performance in Lactuca sativa. Plant Cell Environ. 2011, 34, 1401–1413. [Google Scholar] [CrossRef]
- Wargent, J.J.; Jordan, B.R. From ozone depletion to agriculture: Understanding the role of UV radiation in sustainable crop production. New Phytol. 2013, 197, 1058–1076. [Google Scholar] [CrossRef] [PubMed]
- Castagna, A.; Chiavaro, E.; Dallasta, C.; Rinaldi, M.; Galaverna, G.; Ranieri, A. Effect of postharvest UV-B irradiation on nutraceutical quality and physical properties of tomato fruits. Food Chem. 2013, 137, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Giuntini, D.; Graziani, G.; Lercari, B.; Fogliano, V.; Soldatini, G.F.; Ranieri, A. Changes in carotenoid and ascorbic acid contents in fruits of different tomato genotypes related to the depletion of UV-B radiation. J. Agric. Food Chem. 2005, 53, 3174–3181. [Google Scholar] [CrossRef]
- Dai, Q.; Yan, B.; Huang, S.; Liu, X.; Peng, S.; Miranda, M.L.L.; Chavez, A.Q.; Vergara, B.S.; Olszyk, D.M. Response of oxidative stress defense systems in rice (Oryza sativa) leaves with supplemental UV-B radiation. Physiol Plant. 1997, 101, 301–308. [Google Scholar] [CrossRef]
- Topcu, Y.; Dogan, A.; Sahin-Nadeem, H.; Polat, E.; Kasimoglu, Z.; Erkan, M. Morphological and biochemical responses of broccoli florets to supplemental ultraviolet-B illumination. Agric. Ecosyst. Environ. 2018, 259, 1–10. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Moreira-Rodríguez, M.; Benavides, J. UVA and UVB radiation as innovative tools to biofortify horticultural crops with nutraceuticals. Horticulturae 2022, 8, 387. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signaling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A.; Masood, A.; Per, T.S.; Asgher, M. Hydrogen peroxide alleviates nickel-inhibited photosynthetic responses through increase in use-efficiency of nitrogen and sulfur, and glutathione production in mustard. Front. Plant Sci. 2016, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Shi, K.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q. Hydrogen peroxide is involved in the cold acclimation-induced chilling tolerance of tomato plants. Plant Physiol. Bioch. 2012, 60, 141–149. [Google Scholar] [CrossRef]
- Lu, J.; Li, X.; Yang, Y.; Jia, L.Y.; You, J.; Wang, W.R. Effect of hydrogen peroxide on seedling growth and antioxidants in two wheat cultivars. Biol. Plant. 2013, 57, 487–494. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Young, T.E.; Ling, J.; Chang, S.S.; Gallie, D.R. Increasing vitamin C content of plants through enhanced ascorbate recycling. PANS 2003, 100, 3525–3530. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trived, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione reductase and glutathione: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Borhannuddin, A.T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Do, H.; Kim, I.S.; Jeon, B.W.; Lee, C.W.; Park, A.K.; Wi, A.R.; Shin, S.C.; Park, H.; Kim, Y.; Yoon, H.; et al. Structural understanding of the recycling of oxidized ascorbate by dehydroascorbate reductase (OsDHAR) from Oryza sativa L. japonica. Sci. Rep. 2016, 6, 19498. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Li, C.; Yu, X. Enhanced ascorbic acid accumulation through overexpression of dehydroascorbate reductase confers tolerance to methyl viologen and salt stresses in tomato. Czech J. Genet. Plant Breed. 2012, 48, 74–86. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Wang, Y.; Teng, R.M.; Zhuang, J. Cytosolic ascorbate peroxidase 1 modulates ascorbic acid metabolism through cooperating with nitrogen regulatory protein P-II in tea plant under nitrogen deficiency stress. Genomics 2020, 112, 3497–3503. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, S.; Reynolds, H.; Karpinska, B.; Wingsle, G.; Criessen, G.; Mullineaux, P. Systemic signalling and acclimation in response to excess excitation energy in Arabidopsis. Science 1999, 284, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Allen, R.D. Overexpression of an Arabidopsis peroxisomal ascorbate peroxidase gene in tobacco increases protection against oxidative stress. Plant Cell Physiol. 1999, 40, 725–11732. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Souriau, N.; Perret, S.; Lelandais, M.; Kunert, K.J.; Pruvost, C.; Jouanin, L. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol. 1995, 109, 1047–1057. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Shu, W.; Zhang, C.; Zhang, W.; Ye, Z. Suppressed expression of ascorbate oxidase gene promotes ascorbic acid accumulation in tomato fruit. Plant Mol. Biol. Rep. 2011, 29, 638–645. [Google Scholar] [CrossRef]
- Sanmartin, M.; Drogoudi, P.A.; Lyons, T.; Pateraki, I.; Barnes, J.; Kanellis, A.K. Over-expression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta 2003, 216, 918–928. [Google Scholar] [CrossRef]
- Zha, L.; Zhang, Y.; Liu, W. Dynamic responses of Ascorbate pool and metabolism in lettuce to long-term continuous light provided by red and blue LEDs. Environ. Exp. Bot. 2019, 163, 15–23. [Google Scholar] [CrossRef]
- Pallanca, J.; Smirnoff, N. The control of ascorbic acid synthesis and turnover in pea seedlings. J. Exp. Bot. 2000, 51, 669–674. [Google Scholar] [CrossRef]
- Mellidou, I.; Keulemans, J.; Kanellis, A.K. Davey, M.W. Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin C tomato cultivars. BMC Plant Biol. 2012, 12, 239. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).