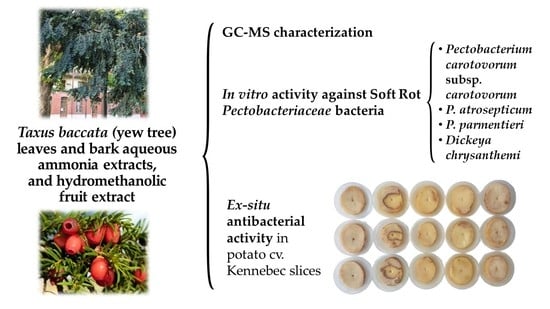
Phytochemical Screening and Antibacterial Activity of Taxus baccata L. against Pectobacterium spp. and Dickeya chrysanthemi
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Reagents
2.2. Bacterial Strains
2.3. Extracts Preparation
2.4. Extracts Characterization
2.5. In Vitro Antimicrobial Activity Assessment
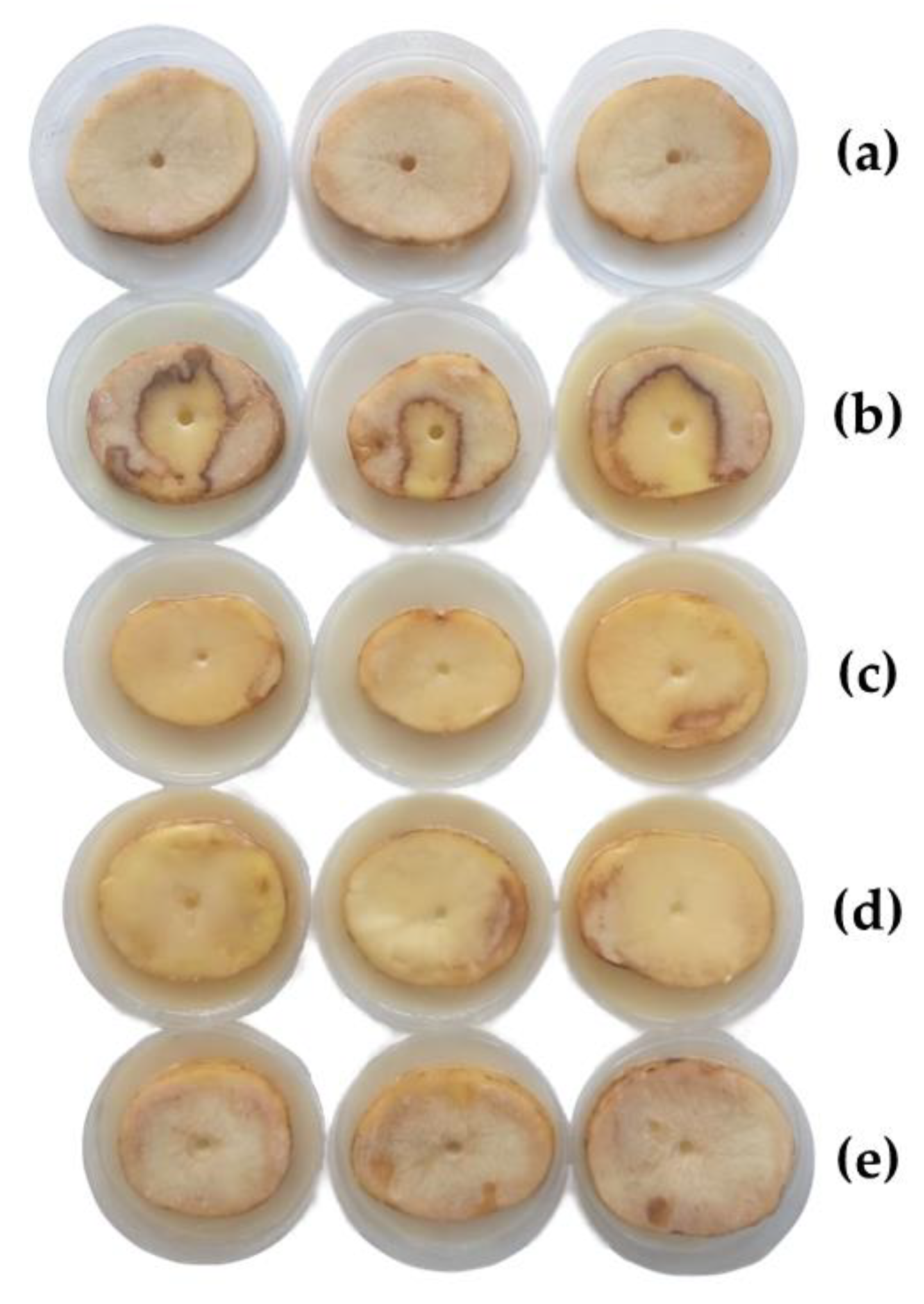
2.6. Protection Tests on Potato Slices
3. Results
3.1. Vibrational Characterization
3.2. GC–MS Chromatograms
3.3. Antibacterial Activity of the Extracts
3.3.1. In vitro Assays
3.3.2. Bioassays on Potato Slices
4. Discussion
4.1. Extract Components Identification
4.2. Phytochemical Profile
4.3. Comparison of Efficacy of T. baccata Extracts with Other Natural Compounds against SRP
4.4. Comparison with Other Extracts Tested for Potato Protection
4.5. Comparison with Conventional Antibiotics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC: Boca Raton, FL, USA, 2012; p. 3960. [Google Scholar]
- Grobosch, T.; Schwarze, B.; Stoecklein, D.; Binscheck, T. Fatal poisoning with Taxus baccata. Quantification of paclitaxel (taxol A), 10-deacetyltaxol, baccatin III, 10-deacetylbaccatin III, cephalomannine (taxol B), and 3,5-dimethoxyphenol in body fluids by liquid chromatography–tandem mass spectrometry. J. Anal. Toxicol. 2012, 36, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gou, X.; Bai, X.; Hou, X.; Li, D.; Zhong, G.; Jin, J.; Huang, M. Simultaneous determination of seven taxoids in rat plasma by UPLC–MS/MS and pharmacokinetic study after oral administration of Taxus yunnanensis extracts. J. Pharm. Biomed. Anal. 2015, 107, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Siegle, L.; Pietsch, J. Taxus ingredients in the red arils of Taxus baccata L. determined by HPLC-MS/MS. Phytochem. Anal 2018, 29, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.R.; Sauer, J.-M.; Hooser, S.B. Taxines: A review of the mechanism and toxicity of yew (Taxus spp.) alkaloids. Toxicon 2001, 39, 175–185. [Google Scholar] [CrossRef]
- Hiller, K.; Melzig, M.F. Lexikon der Arzneipflanzen und Drogen, 2nd ed.; Spektrum Akademischer Verlag Heidelberg: Heidelberg, Germany, 2010; p. 664. [Google Scholar]
- Wink, M.; Van Wyk, B.-E.; Wink, C. Handbuch der giftigen und psychoaktiven Pflanzen; Wissenschaftliche Verlagsgesellschaft Stuttgart: Stuttgart, Germany, 2008; p. 225. [Google Scholar]
- Teuscher, E.; Lindequist, U. Biogene Gifte: Biologie-Chemie-Pharmakologie-Toxikologie; Wissenschaftliche Verlagsgesellschaft Stuttgart: Stuttgart, Germany, 2010. [Google Scholar]
- Sharma, A.; Sharma, A.; Thakur, S.; Mutreja, V.; Bhardwaj, G. A brief review on phytochemistry and pharmacology of Taxus baccata L. Mater. Today Proc. 2022, 48, 1569–1574. [Google Scholar] [CrossRef]
- Furmanowa, M.; Kropczyńska, D.; Zobel, A.; Głowniak, K.; Sykłowska-Baranek, K.; Rapczewska, L. Detrimental effects of water extracts from surface and interior of Taxus baccata leaves on the two-spotted spider mite (Tetranychus urticae Koch). Die Pharmazie 2003, 58, 340–342. [Google Scholar]
- Dupuis, B.; Nkuriyingoma, P.; Van Gijsegem, F. Economic impact of Pectobacterium and Dickeya species on potato crops: A Review and case study. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Van Gijsegem, F., van der Wolf, J.M., Toth, I.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 263–282. [Google Scholar] [CrossRef]
- van der Wolf, J.M.; De Boer, S.H.; Czajkowski, R.; Cahill, G.; Van Gijsegem, F.; Davey, T.; Dupuis, B.; Ellicott, J.; Jafra, S.; Kooman, M.; et al. Management of diseases caused by Pectobacterium and Dickeya species. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Van Gijsegem, F., van der Wolf, J.M., Toth, I.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 175–214. [Google Scholar] [CrossRef]
- Perombelon, M.C.M.; Kelman, A. Ecology of the Soft Rot Erwinias. Annu. Rev. Phytopathol. 1980, 18, 361–387. [Google Scholar] [CrossRef]
- Czajkowski, R.; Pérombelon, M.C.M.; van Veen, J.A.; van der Wolf, J.M. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: A review. Plant Pathol. 2011, 60, 999–1013. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.A.X.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Abubakar, A.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J. Statistical optimization of aqueous ammonia pretreatment and enzymatic hydrolysis of corn cob powder for enhancing sugars production. Biochem. Eng. J. 2021, 174, 108106. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Balduque-Gil, J.; Barriuso-Vargas, J.J.; Casanova-Gascón, J.; González-García, V.; Cuchí-Oterino, J.A.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Holm oak (Quercus ilex subsp. ballota (Desf.) Samp.) bark aqueous ammonia extract for the control of invasive forest pathogens. Int. J. Mol. Sci. 2022, 23, 11882. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, E.; González-García, V.; Casanova-Gascón, J.; Barriuso-Vargas, J.J.; Balduque-Gil, J.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Valorization of Quercus suber L. bark as a source of phytochemicals with antimicrobial activity against apple tree diseases. Plants 2022, 11, 3415. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, E.; Martín-Ramos, P.; Martín-Gil, J.; Santiago-Aliste, A.; Hernández-Navarro, S.; Oliveira, R.; González-García, V. Bark extract of Uncaria tomentosa L. for the control of strawberry phytopathogens. Horticulturae 2022, 8, 672. [Google Scholar] [CrossRef]
- Petisco, C.; Vázquez de Aldana, B.R.; Zabalgogeazcoa, I.; Mediavilla, S.; García Criado, B. Determinación de lignina y celulosa en hojas de plantas leñosas mediante NIRS: Comparación de métodos estadísticos. In Proceedings of the Producciones Agroganaderas: Gestión Eficiente y Conservación del Medio Natural (Vol. I): XLV Reunión Científica de la SEEP, Oviedo, Spain, 30 May–3 June 2005; pp. 97–104. Available online: http://www.serida.org/seep2005/trabajos/libro.pdf (accessed on 1 February 2023).
- Charles, S.J.; Russell, G.K. Chemical Products from Bark Digested in Ammonia. U.S. Patent No. 2,823,223, 11 February 1958. [Google Scholar]
- CLSI. CLSI Standard M07-Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Abd-El-Khair, H.; Abdel-Gaied, T.G.; Mikhail, M.S.; Abdel-Alim, A.I.; El-Nasr, H.I.S. Biological control of Pectobacterium carotovorum subsp. carotovorum, the causal agent of bacterial soft rot in vegetables, in vitro and in vivo tests. Bull. Natl. Res. Cent. 2021, 45, 37. [Google Scholar] [CrossRef]
- Garge, S.S.; Nerurkar, A.S. Evaluation of quorum quenching Bacillus spp. for their biocontrol traits against Pectobacterium carotovorum subsp. carotovorum causing soft rot. Biocatal. Agric. Biotechnol. 2017, 9, 48–57. [Google Scholar] [CrossRef]
- Singh, A.A.; Singh, A.K.; Nerurkar, A. Disrupting the quorum sensing mediated virulence in soft rot causing Pectobacterium carotovorum by marine sponge associated Bacillus sp. OA10. World J. Microbiol. Biotechnol. 2021, 37, 5. [Google Scholar] [CrossRef]
- Kodithuwakku, U.; Alwis, C.D.; Prashantha, M.A.B.; Ratnaweera, D.R. One step synthesis of polythiophenes from the partially purified crude extract of the roots of Tagetes erecta. Int. J. Chem. 2016, 8, 1–14. [Google Scholar] [CrossRef]
- Tipson, R.S.; Isbell, H.S. Conformations of the pyranoid sugars. II. Infrared absorption spectra of some aldopyranosides. J. Res. Natl. Bur. Stand. A Phys. Chem. 1960, 64, 239. [Google Scholar] [CrossRef]
- Mohaček-Grošev, V.; Furić, K.; Ivanković, H. Observed bands in Raman and infrared spectra of 1,3-dioxolane and their assignments. Vib. Spectrosc 2013, 64, 101–107. [Google Scholar] [CrossRef]
- Fisher, D.; Palmer, L.I.; Cook, J.E.; Davis, J.E.; Read de Alaniz, J. Efficient synthesis of 4-hydroxycyclopentenones: Dysprosium(III) triflate catalyzed Piancatelli rearrangement. Tetrahedron 2014, 70, 4105–4110. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Ibrahim, S.R.M.; Omar, A.M.; Bagalagel, A.A.; Diri, R.M.; Noor, A.O.; Almasri, D.M.; Mohamed, S.G.A.; Mohamed, G.A. Thiophenes—Naturally occurring plant metabolites: Biological activities and in silico evaluation of their potential as cathepsin D inhibitors. Plants 2022, 11, 539. [Google Scholar] [CrossRef]
- Kawata, J.; Kameda, M.; Miyazawa, M. Constituents of essential oil from the dried fruits and stems of Akebia quinata (Thunb.) Decne. J. Oleo Sci. 2007, 56, 59–63. [Google Scholar] [CrossRef]
- Ketel, D.H. Distribution and accumulation of thiophenes in plants and calli of different Tagetes species. J. Exp. Bot. 1987, 38, 322–330. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Kaal, N.A.; Alterary, S.; Al-Showiman, S.S.; Farghaly, T.A.; Mubarak, M.S. Antimicrobial activity of thiophene derivatives derived from ethyl (E)-5-(3-(dimethylamino)acryloyl)-4-methyl-2-(phenylamino)thiophene-3-carboxylate. Chem. Cent. J. 2017, 11, 75. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.-J.; Chen, J.; Zhao, Q.-Q.; Li, Y.; Gao, K. Thiophene acetylenes and furanosesquiterpenes from Xanthopappus subacaulis and their antibacterial activities. Phytochemistry 2014, 106, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Meher, A.; Behera, B.; Nanda, B.K. GC-MS investigation of phytocomponents present in ethanolic extract of plant Ichnocarpus frutescens (L.) W.T.Aiton aerial part. Int. J. Pharm. Sci. Res. 2019, 10, 4711–4716. [Google Scholar] [CrossRef]
- Abdul Rahim, M.H.; Zakaria, Z.A.; Mohd Sani, M.H.; Omar, M.H.; Yakob, Y.; Cheema, M.S.; Ching, S.M.; Ahmad, Z.; Abdul Kadir, A. Methanolic extract of Clinacanthus nutans exerts antinociceptive activity via the opioid/nitric oxide-mediated, but cGMP-independent, pathways. Evid. Based Complement. Altern. Med. 2016, 2016, 1494981. [Google Scholar] [CrossRef]
- Ghosh, G.; Panda, P.; Rath, M.; Pal, A.; Sharma, T.; Das, D. GC-MS analysis of bioactive compounds in the methanol extract of Clerodendrum viscosum leaves. Pharmacogn. Res. 2015, 7, 110. [Google Scholar] [CrossRef]
- Kocaçalışkan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. Naturforsch. C J. Biosci. 2006, 61, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Langa-Lomba, N.; Sánchez-Hernández, E.; Buzón-Durán, L.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Activity of anthracenediones and flavoring phenols in hydromethanolic extracts of Rubia tinctorum against grapevine phytopathogenic fungi. Plants 2021, 10, 1527. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Physicochemical characterization and antimicrobial activity against Erwinia amylovora, Erwinia vitivora, and Diplodia seriata of a light purple Hibiscus syriacus L. cultivar. Plants 2021, 10, 1876. [Google Scholar] [CrossRef] [PubMed]
- Gharari, Z.; Shabani, H.; Bagheri, K.; Sharafi, A. Phytochemical composition profile of Scutellaria bornmuelleri methanolic extract using GC-MS analysis. Fut. Nat. Prod. 2022, 8, 7–14. [Google Scholar] [CrossRef]
- Kala, S.; Ammani, K. GC–MS analysis of biologically active compounds in Canthium parviflorum Lam. leaf and callus extracts. Int. J. Chemtech. Res. 2017, 10, 1039–1058. [Google Scholar] [CrossRef]
- Abubakar, M.; Majinda, R. GC-MS analysis and preliminary antimicrobial activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines 2016, 3, 3. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Subramaniam, P. Phytochemical profiling of leaf, stem, and tuber parts of Solena amplexicaulis (Lam.) Gandhi using GC-MS. Int. Sch. Res. Notices 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Ghaly, M.F.; Fahmi, S.M. Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J. Basic Microbiol. 2021, 61, 557–568. [Google Scholar] [CrossRef]
- Venditti, A. What is and what should never be: Artifacts, improbable phytochemicals, contaminants and natural products. Nat. Prod. Res. 2018, 34, 1014–1031. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Cuchí-Oterino, J.A.; Martín-Gil, J.; Lorenzo-Vidal, B.; Martín-Ramos, P. Dwarf pomegranate (Punica granatum L. var. nana): Source of 5-HMF and bioactive compounds with applications in the protection of woody crops. Plants 2022, 11, 550. [Google Scholar] [CrossRef]
- Nafea, E.; Moselhy, W.; Fawzy, A. Does the HMF value affect the antibacterial activity of the bee honey? Egypt. Acad. J. Biol. Sci. A Entomol. 2011, 4, 13–19. [Google Scholar] [CrossRef]
- Kaur, R.; Kaushal, S.; Sharma, P. Antimicrobial and antioxidant potential of pomegranate (Punica granatum L.) peel. Int. J. Chem. Stud. 2018, 6, 3441–3449. [Google Scholar]
- Khan, M.; Yusufzai, S.; Kaun, L.; Shah, M.; Idris, R. Chemical composition and antioxidant activity of essential oil of leaves and flowers of Alternanthera sessilis red from Sabah. J. Appl. Pharm. Sci. 2016, 6, 157–161. [Google Scholar] [CrossRef]
- Saravanakumari, P.; Mani, K. Structural characterization of a novel xylolipid biosurfactant from Lactococcus lactis and analysis of antibacterial activity against multi-drug resistant pathogens. Bioresour. Technol. 2010, 101, 8851–8854. [Google Scholar] [CrossRef] [PubMed]
- Küçük, H.B.; Yusufoğlu, A.; Mataracı, E.; Döşler, S. Synthesis and biological activity of new 1,3-dioxolanes as potential antibacterial and antifungal compounds. Molecules 2011, 16, 6806–6815. [Google Scholar] [CrossRef]
- Shobana, S.; Vidhya, V.; Ramya, M. Antibacterial activity of garlic varieties (ophioscordon and sativum) on enteric pathogens. Curr. Res. J. Biol. 2009, 1, 123–126. [Google Scholar]
- Teoh, Y.; Mashitah, M. Screening of antifungal activities from genera Trametes against growth of selected wood-degrading fungi from Malaysia. Aust. J. Bas. App. Sci. 2012, 6, 79–85. [Google Scholar]
- Akhtar, N.; Malik, A.; Ali, S.N.; Kazmit, S.U. Proceragenin, an antibacterial cardenolide from Calotropis procera. Phytochemistry 1992, 31, 2821–2824. [Google Scholar] [CrossRef]
- Meziani, S.; Oomah, B.D.; Zaidi, F.; Simon-Levert, A.; Bertrand, C.; Zaidi-Yahiaoui, R. Antibacterial activity of carob (Ceratonia siliqua L.) extracts against phytopathogenic bacteria Pectobacterium atrosepticum. Microb. Pathog. 2015, 78, 95–102. [Google Scholar] [CrossRef]
- Merad, N.; Andreu, V.; Chaib, S.; de Carvalho Augusto, R.; Duval, D.; Bertrand, C.; Boumghar, Y.; Pichette, A.; Djabou, N. Essential oils from two Apiaceae species as potential agents in organic crops protection. Antibiotics 2021, 10, 636. [Google Scholar] [CrossRef]
- Ashmawy, N.A.; Al Farraj, D.A.; Salem, M.Z.M.; Elshikh, M.S.; Al-Kufaidy, R.; Alshammari, M.K.; Salem, A.Z.M. Potential impacts of Pinus halepensis Miller trees as a source of phytochemical compounds: Antibacterial activity of the cones essential oil and n-butanol extract. Agrofor. Syst. 2018, 94, 1403–1413. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Elansary, H.O.; Ali, H.M.; El-Settawy, A.A.; Elshikh, M.S.; Abdel-Salam, E.M.; Skalicka-Woźniak, K. Bioactivity of essential oils extracted from Cupressus macrocarpa branchlets and Corymbia citriodora leaves grown in Egypt. BMC Complement Altern. Med. 2018, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.Z. Evaluation of the antibacterial and antioxidant activities of stem bark extracts of Delonix regia and Erythrina humeana grown in Egypt. J. Forest Prod. Ind 2013, 2, 48–52. [Google Scholar]
- Gormez, A.; Bozari, S.; Yanmis, D.; Gulluce, M.; Agar, G.; Sahin, F. The use of essential oils of Origanum rotundifolium as antimicrobial agent against plant pathogenic bacteria. J. Essent. Oil-Bear. Plants 2016, 19, 656–663. [Google Scholar] [CrossRef]
- Hajian-Maleki, H.; Baghaee-Ravari, S.; Moghaddam, M. Efficiency of essential oils against Pectobacterium carotovorum subsp. carotovorum causing potato soft rot and their possible application as coatings in storage. Postharvest Biol. Technol. 2019, 156, 110928. [Google Scholar] [CrossRef]
- Melkani, A.B.; Mohan, L.; Pant, C.C. Diterpene rich essential oil from Anisomeles indica (L.) O. Kuntz. and its antimicrobial activity. World J. Pharma. Res. 2016, 5, 932–943. [Google Scholar]
- Melkani, A.B.; Mohan, L.; Pant, C.C.; Negi, A.; Dev, V. Terpenoid composition and antibacterial activity of essential oil from Salvia hians Royle ex. Benth. J. Essent. Oil-Bear. Plants 2011, 14, 667–672. [Google Scholar] [CrossRef]
- Ouanas, S.; Hamelin, G.; Hervet, M.; Andrivon, D.; Val, F.; Yahiaoui-Zaidi, R. Protection against bacterial soft rot by olive extracts is related to general defence induction in potato tubers. Plant Pathol. 2017, 66, 404–411. [Google Scholar] [CrossRef]
- Vasilchenko, A.S.; Poshvina, D.V.; Sidorov, R.Y.; Iashnikov, A.V.; Rogozhin, E.A.; Vasilchenko, A.V. Oak bark (Quercus sp. cortex) protects plants through the inhibition of quorum sensing mediated virulence of Pectobacterium carotovorum. World J. Microbiol. Biotechnol. 2022, 38, 184. [Google Scholar] [CrossRef]
- Nyamari, J.K. Effect of Tagetes minuta and Capsicum frutescens extracts on Pectobacterium carotovorum, growth, yield and quality of potatoes (Solanum tuberosum). MSc Thesis, Egerton University, Njoro, Kenya, 2017. [Google Scholar]
- Miller, S.A.; Ferreira, J.P.; LeJeune, J.T. Antimicrobial use and resistance in plant agriculture: A One Health perspective. Agriculture 2022, 12, 289. [Google Scholar] [CrossRef]
- Gleason, M.; Paduch-Cichal, E.; Skutnik, E.; Kret, D.; Mirzwa-Mróz, E.; Gadomska-Gajadhur, A.; Schollenberger, M. The influence of plant essential oils on in vitro growth of Pectobacterium and Dickeya spp. bacteria. Acta Sci. Pol. Hortorum Cultus 2021, 20, 19–29. [Google Scholar] [CrossRef]
- Ashmawy, N.A.; Salem, M.Z.M.; El-Hefny, M.; Abd El-Kareem, M.S.M.; El-Shanhorey, N.A.; Mohamed, A.A.; Salem, A.Z.M. Antibacterial activity of the bioactive compounds identified in three woody plants against some pathogenic bacteria. Microb. Pathog. 2018, 121, 331–340. [Google Scholar] [CrossRef]
- Jafarpour, M.; Golparvar, A.R.; Lotfi, A. Antibacterial activity of essential oils from Thymus vulgaris, Trachyspermum ammi and Mentha aquatica against Erwinia carotovora in vitro. J. Med. Herb. 2013, 4, 115–118. [Google Scholar]
- Rakib, A.A.-A.; Mustafa, A.A.; Haidar, H.N. Antibacterial activity of clove, cinnamon, and datura extracts against Erwinia carotovora subsp. atroseptica causative agent of black stem and soft rot on potato. J. Med. Plant Res. 2012, 6, 1891–1895. [Google Scholar]
- Viswanath, H.; Bhat, K.; Bhat, N.; Wani, T.; Mughal, M.N. Antibacterial efficacy of aqueous plant extracts against storage soft rot of potato caused by Erwinia carotovora. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2630–2639. [Google Scholar] [CrossRef]
- Ashmawy, N.A.; Behiry, S.I.; Al-Huqail, A.A.; Ali, H.M.; Salem, M.Z.M. Bioactivity of selected phenolic acids and hexane extracts from Bougainvilla spectabilis and Citharexylum spinosum on the growth of Pectobacterium carotovorum and Dickeya solani bacteria: An opportunity to save the environment. Processes 2020, 8, 482. [Google Scholar] [CrossRef]
- Pretorius, J.C.; Magama, S.; Zietsman, P.C.; van Wyk, B.E. Growth inhibition of plant pathogenic bacteria and fungi by extracts from selected South African plant species. S. Afr. J. Bot. 2003, 69, 186–192. [Google Scholar] [CrossRef]
- Bhat, K.; Viswanath, H.; Bhat, N.; Wani, T. Bioactivity of various ethanolic plant extracts against Pectobacterium carotovorum subsp. carotovorum causing soft rot of potato tubers. Indian Phytopathol. 2017, 70, 463–470. [Google Scholar] [CrossRef]
- Mohan, L.; Negi, A.; Melkani, A.B.; Dev, V. Chemical composition and antibacterial activity of essential oil from Salvia mukerjeei. Nat. Prod. Commun. 2011, 6, 1949–1952. [Google Scholar] [CrossRef] [PubMed]
- Priydarshi, R.; Melkani, A.B.; Mohan, L.; Pant, C.C. Terpenoid composition and antibacterial activity of the essential oil from Inula cappa (Buch-Ham. ex. D. Don) DC. J. Essent. Oil Res. 2016, 28, 172–176. [Google Scholar] [CrossRef]
- Sqalli, H.; El Ouarti, A.; Farah, A.; Ennabili, A.; Haggoud, A.; Ibnsouda, S.; Houari, A.; Iraqui, M.H. Antibacterial activity of Thymus pallidus Batt. and determination of the chemical composition of its essential oil. Acta Bot. Gall. 2009, 156, 303–310. [Google Scholar] [CrossRef]
- Chudasama, K.S.; Thaker, V.S. Biological control of phytopathogenic bacteria Pantoea agglomerans and Erwinia chrysanthemi using 100 essential oils. Arch. Phytopathol. Pflanzenschutz 2014, 47, 2221–2232. [Google Scholar] [CrossRef]
- Mongkol, R.; Nilprapruck, P.; Yoshida, A. Antifungal and antibacterial activities of essential oil from Som Keaw (Citrus nobilis) in Thailand. Int. J. Agric. Technol. 2020, 16, 887–896. [Google Scholar]


| Leaf | Leaf Extract | Bark | Bark Extract | Red Aril | Red Aril Juice | Seed | Fruit Extract | Assignment |
|---|---|---|---|---|---|---|---|---|
| 3309 | 3343 | 3324 | 3177 | 3331 | 3281 | 3285 | 3285 | O−H str. |
| 3000 | 3006 | |||||||
| 2920 | 2915 | 2917 | 2928 | 2920 | 2931 | 2923 | 2917 | C−H str. |
| 2851 | 2848 | 2851 | 2853 | 2849 | C−H str. | |||
| 1727 | 1728 | 1726 | 1744 | C=O str. lactones | ||||
| 1698 | 1715 | OH cyclopentenones | ||||||
| 1652 | 1652 | C−S ring str. | ||||||
| 1636 | 1634 | furanes | ||||||
| 1615 | 1605 | 1605 | 1609 | 1605 | C=O lignin | |||
| 1540 | 1540 | 1549 | 1532 | COO− | ||||
| 1507 | 1516 | 1517 | N−H bending | |||||
| 1486 | dioxolanes | |||||||
| 1457 | 1463 | 1456 | thiophenes | |||||
| 1436 | 1423 | 1443 | −C=C− | |||||
| 1418 | 1398 | 1417 | 1409 | CH2/CH3 | ||||
| 1372 | 1374 | 1369 | 1373 | 1369 | 1378 | C−S ring str. and C−C ring aromatics | ||
| 1341 | 1347 | skeleton bending | ||||||
| 1313 | 1315 | 1314 | 1318 | 1312 | C–CH and C–OH | |||
| 1279 | 1271 | 1255 | C−O str. | |||||
| 1242 | 1245 | 1242 | 1235 | ring vib. thiophenes/xylopyranosides | ||||
| 1187 | 1193 | pyranose CO | ||||||
| 1159 | 1149 | 1143 | 1153 | 1146 | 1143 | 1143 | antisym. C−O−C | |
| 1098 | 1101 | 1104 | hydroxy-cyclopentenones | |||||
| 1053 | 1047 | 1053 | 1053 | C−O, flavonoids | ||||
| 1028 | 1028 | 1031 | 1015 | 1024 | 1031 | 1023 | furanes | |
| 925 | 918 | monosaccharides | ||||||
| 870 | 896 | 885 | 852 | 865 | α-glucopyranoses | |||
| 836 | 822 | 808 | 817 | pyranose ring br. | ||||
| 798 | 790 | 796 | C−H deform. (connect. thiophenes) | |||||
| 779 | 773 | 775 | C–H β out-of-p bending thiophenes | |||||
| 721 | 719 | C−S bend thiophene |
| Bacteria | Fruit Extract | Bark Extract | Leaf Extract |
|---|---|---|---|
| P. carotovorum subsp. carotovorum | 1500 | 500 | 187.5 |
| P. atrosepticum | 1500 | 500 | 187.5 |
| P. parmentieri | 1000 | 500 | 187.5 |
| D. chrysanthemi | 1500 | 750 | 187.5 |
| Bacteria | PG | AM | GM | CI | TC |
|---|---|---|---|---|---|
| P. carotovorum subsp. carotovorum | 0.38 | 0.19 | 0.75 | 0.003 | 1 |
| P. atrosepticum | 6 | 0.5 | 0.75 | 0.004 | 0.75 |
| P. parmentieri | 0.38 | 0.094 | 0.25 | 0.006 | 0.75 |
| D. chrysanthemi | 12 | 0.125 | 0.5 | <0.002 | 0.25 |
| Treatment | Relative Maceration (%) |
|---|---|
| Negative control (sterile water) | 0.0 ± 0.0 % d |
| Positive control (no treatment) | 97 ± 3.5 % a |
| Simultaneous inoculation and treatment application | 88 ± 1.4 % b |
| Pathogen inoculation 2 h before treatment application | 70 ± 4.3 % c |
| Treatment application 2 h before pathogen inoculation | 0.0 ± 0.0 % d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, E.; González-García, V.; Martín-Gil, J.; Lorenzo-Vidal, B.; Palacio-Bielsa, A.; Martín-Ramos, P. Phytochemical Screening and Antibacterial Activity of Taxus baccata L. against Pectobacterium spp. and Dickeya chrysanthemi. Horticulturae 2023, 9, 201. https://doi.org/10.3390/horticulturae9020201
Sánchez-Hernández E, González-García V, Martín-Gil J, Lorenzo-Vidal B, Palacio-Bielsa A, Martín-Ramos P. Phytochemical Screening and Antibacterial Activity of Taxus baccata L. against Pectobacterium spp. and Dickeya chrysanthemi. Horticulturae. 2023; 9(2):201. https://doi.org/10.3390/horticulturae9020201
Chicago/Turabian StyleSánchez-Hernández, Eva, Vicente González-García, Jesús Martín-Gil, Belén Lorenzo-Vidal, Ana Palacio-Bielsa, and Pablo Martín-Ramos. 2023. "Phytochemical Screening and Antibacterial Activity of Taxus baccata L. against Pectobacterium spp. and Dickeya chrysanthemi" Horticulturae 9, no. 2: 201. https://doi.org/10.3390/horticulturae9020201
APA StyleSánchez-Hernández, E., González-García, V., Martín-Gil, J., Lorenzo-Vidal, B., Palacio-Bielsa, A., & Martín-Ramos, P. (2023). Phytochemical Screening and Antibacterial Activity of Taxus baccata L. against Pectobacterium spp. and Dickeya chrysanthemi. Horticulturae, 9(2), 201. https://doi.org/10.3390/horticulturae9020201