Abstract
Watermelon is a crucial horticultural crop worldwide but its genetic base has become extremely narrow owing to long-term cultivation. Induced mutagenesis can create a range of variations with distinctive agricultural characteristics. To broaden the genetic diversity of watermelon, we established a mutagenesis library containing over 4000 M1 seeds from an inbred line ‘M08’, which was irradiated by 350 Gy of 60Co γ-rays for 3 h. The rates of germination, emergence, and survival of the M1 seeds were reduced by 5.88%, 18.66%, and 41.96%, respectively. After phenotypic screening, 20 and 10 types of morphological changes were observed in the M1 and M2 generations, with approximately 10.57% and 14.17% mutation frequencies, respectively. Six mutants with desirable horticultural alterations were selected for additional presentation, including the leaf color mutant C1-NO.1, the yellow peel mutant C1-NO.2, the pericarp thickening mutant C1-NO.3, the pericarp thinning mutant C1-NO.4, the seedless mutant C1-NO.5, and the C2-No.1 mutant with normal female flowers and malformed male flowers. Moreover, the three mutants M1-3, M2-1, and M1-5 were identified from our EMS-induced M2 library, exhibiting the fusiform fruit, the dark green peel, and the yellow leaves, respectively. Compared to the wild type (WT), the photosynthetic pigments and parameters were negatively impacted in the yellow-leaf mutant M1-5. For example, the total chlorophyll was 1.22 and 2.22 mg/g in the young and mature leaves of M1-5, respectively, which were significantly lower than those in the WT (2.58 and 2.90 mg/g, respectively). Notably, some mutagenesis phenotypes could be stably inherited, including traits such as yellow leaf color, fusiform fruit shape, and thickening and thinning pericarp. Taken together, these results indicate that these two mutant libraries serve as essential resources to discover new phenotypic germplasms, thereby facilitating the genetic breeding and functional gene exploration in watermelon.
1. Introduction
Watermelon (Citrullus lanatus L., 2n = 2x = 22) is a globally important cucurbit crop that is highly valuable and nutritious. For example, the abundant nutrients in watermelon, including vitamins, mineral salts, and amino acids (citrulline), provide numerous benefits for human health. Since it is one of the top five most consumed fresh fruits, watermelon had a global yield of 103.93 million tons in 2018, with 13.62 kg consumed per person [1]. China is the largest producer and consumer of watermelon in the world and it produced 63.0 million tons of watermelon cultivated on 1.51 million hm2 in 2018 [1,2,3].
Watermelon has been reported to have originated in Africa and to have been cultivated for at least 4000 years [2,4]. Because of the long-term cultivation and selection for desirable fruit quality, modern watermelon cultivars currently have a narrow genetic base [3,5]. The genus Citrullus contains seven species [3,6]. To broaden the genetic diversity, three of these species (C. colocynthis, C. amarus, and C. mucosospermus) have been used to cross with cultivated watermelons in breeding programs [3,5,6]. However, the reproductive barriers and linkage drags limit the speed of genetic improvement in watermelon [3]. Therefore, the development of mutant libraries is considered to be a direct and effective approach to broaden the genetic base of watermelon and improve the breeding efficiency of economically important traits.
To date, several methods have been successfully utilized to construct plant mutant libraries, including physical (60Co γ-rays and X-rays), chemical (ethyl methanesulfonate [EMS] and N-nitro-N-methylurea), and biological (T-DNA and transposons) approaches [7]. EMS has been widely adopted as a practical mutagen and is highly preferred owing to its high mutagenicity and ease of handling. For example, it has been used to create mutants in Arabidopsis [8], tomato (Solanum lycopersicum L.) [9,10], eggplant (Solanum melongena L.) [11], and strawberry (Fragaria × ananassa) [12]. Moreover, there are also numerous studies on EMS-induced mutant libraries in Cucurbitaceae crops. For example, EMS-induced mutant libraries in cucumber (Cucumis sativus L.) had been successfully constructed and some valuable germplasm resources were subsequently identified, including those related to flower size, leaf color, and fruit shape and peel [7,13]. Importantly, several regulatory genes for agronomic traits were cloned using EMS-induced mutant lines, including the gene for yellow leaf CscpFtsY [14], unusual flower and tendril CsUFO [15], and curly leaf CsPHB [16]. In melon (Cucumis sp.), the CRTISO gene responsible for the accumulation of carotenoids was also cloned from an EMS-induced mutant ‘yofI’ with yellow-orange flesh [17]. In watermelon (Citrullus lanatus L.), a photosensitive flesh line ‘psf’ was identified from the EMS-induced mutant library; a G-A transversion leads to a premature stop codon in the causal gene ClZISO, which results in morphological changes [18]. A pollen-EMS mutagenesis method was used to construct a library that contained 200,000 M1 seeds and two genes responsible for fruit shape and male sterility were identified, respectively [3]. Similarly, 60Co γ-rays have also been used to construct mutant libraries in plants. For instance, 10 accessions of tetraploid wheat were irradiated with 60Co γ-rays, which resulted in several novel mutation resources for wheat breeding [19]. The efficiency of different doses of 60Co γ-rays was extensively investigated in tomato and potato (Solanum tuberosum L.) [10,20]. Similarly, 60Co γ-rays were also utilized to induce mutations in cucurbit crops, such as bitter gourd (Momordica charantia L.) [21], squash (Cucurbita spp.), and pumpkin (Cucurbita moschata Duchesne) [22]. Although various doses of 60Co γ-rays have been used to determine the optimal dose to construct the watermelon library [23,24], a large-scale mutant library has not yet been created.
In this study, a watermelon mutant library that contained 4000 M1 seeds was successfully constructed using 60Co γ-ray irradiation. After undergoing morphological characterization, a serious of phenotypic mutants were identified, with 20 and 10 types of morphological changes observed in the M1 and M2 generations, respectively. Taken together with the mutant library established by our group through EMS treatment [25,26], nine representative mutants were characterized in detail. It is notable that some mutagenesis phenotypes could be stably inherited, including traits such as yellow leaves, fusiform fruit shape, and thickening and thinning pericarps. In summary, these two watermelon mutant libraries including the nine well-represented mutants can provide promising germplasm resources to breed new varieties of watermelon and explore novel gene functions.
2. Materials and Methods
2.1. Plant Materials
The watermelon germplasm ‘M08’ used in this study was provided by the Watermelon and Melon Research Group at Northwest A&F University (Yangling, China) [27]. To construct a mutagenesis library, dry seeds were irradiated with 350 Gy of 60Co γ-rays for 3 h at the Hefei Institute of Physical Sciences, Chinese Academy of Sciences, Hefei, China.
2.2. Construction of the Mutant Library
To obtain a large-scale M1 library, approximately 2000 irradiated seeds were independently germinated in the spring of 2021 and 2022, factoring in the constraints of available experimental field space and human resources. After the germination rate had been calculated (number of germinated seeds/total number of seeds × 100%), the seeds were sown in plastic trays with 50 holes filled with commercial peat-based compost. In addition, the emergence rate and seedling rate were recorded according to the relevant index calculation formulae previously described [28]. The seedlings were then transferred to farms at Northwest A&F University for morphological screening and self-pollination to produce the M2 generation. Among the M2 families, we randomly selected 17 independently self-crossed lines and grew 15 plants for each line. Morphological observations of the M2 plants were also performed in the greenhouses of Northwest A&F University.
As described in our previous studies [25,26], we established an EMS mutant library using the watermelon line ‘M08’ as the material. To validate the morphological variants of three valuable mutants (M1-3, M2-1, and M1-5), their M2 offspring were subsequently planted at the farms of Northwest A&F University (Yangling, China).
2.3. Phenotypic Characterization of the Mutants
The morphological changes were identified visually during the whole duration of the developmental stages. For the cotyledon phenotype, the color, size, and number were primarily inspected when the plantlets were grown in trays. The phenotypic alterations of other agricultural traits, such as the leaf morphologies, plant architecture, floral organs, and fruit changes, were recorded after the seedlings had been transferred to the field. A unique ID was assigned to each mutant to correlate with their phenotypic characteristics. Normal plants of germplasm ‘M08’ were used as the control to identify mutants.
2.4. Measurement of the Physiological Indices of Mutant M1-5
At the stem elongation stage, we selected the young and mature leaves from the mutant M1-5 and wild type (WT) to analyze the contents of pigment and photosynthetic characters. Fresh leaves were cut into small pieces and 0.1 g samples were placed into 10 mL 95% ethanol at room temperature for 24 h. After centrifugation at 3500× g for 10 min, the contents of chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Chl a + b), and carotenoids (Caro) were measured colorimetrically at 665 nm, 649 nm, and 470 nm using a spectrophotometer, as previously described [29,30]. Three technical replicates were performed for each sample. A volume of 95% ethanol was used as the blank control. Moreover, photosynthesis-related indices, including the net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular carbon dioxide concentration (Ci), and transpiration rate (Tr), were measured using an LI-6800 portable photosynthetic system (LICOR, Lincoln, NE, USA). The conditions were a temperature of 25 ± 2 °C, a CO2 concentration of 380 µmol mol−1, and a photosynthetic photon flux density (PPFD) of 500 µmol m−2 s−1, as previously described [1]. The amounts of pigments were calculated using the following equations:
Ca = 13.95 × A665 − 6.88 × A649
Cb = 13.95 × A649 − 6.88 × A655
Chl a = (Ca × V)/(W × 1000)
Chl b = (Cb × V)/(W × 1000)
Caro = (1000 × A470 − 2.05 × Ca − 114.8 × Cb)/245
A665, A649, and A470 were the absorbance values at wavelengths 665 nm, 649 nm, and 470 nm, respectively. V and W represented the volume of extracted liquid and sample weight, respectively.
2.5. Statistical Analysis
All of the data were presented as the mean ± SD for at least three independent replicates. The significance was analyzed using a one-way analysis of variance (ANOVA) with a Duncan’s test (p < 0.05) via SPSS 25.0 (IBM, Inc., Armonk, NY, USA).
3. Results
3.1. Negative Effect of 60Co-γ Radiation on Seed Viability
After the seeds had been irradiated with 60Co γ-rays, their viability was evaluated to assess the effect of a dosage of 350 Gy radiation. Compared to the normal seeds, the germination rate, the emergence rate, and the seedling rate were obviously reduced by 5.88%, 18.66%, and 41.96%, respectively (Table 1). These effects suggested that the 60Co radiation had a negative impact on seed vigor. In addition, during the germination process, we observed that the normal seeds began germinating after approximately 24 h in the incubator; whereas, the seeds exposed to 60Co-γ radiation generally started germinating after 36 h, indicating that the 60Co-γ radiation delayed and inhibited seed germination.

Table 1.
Effects of 60Co-γ radiation on the germination rate, emergence rate, and seedling rate of watermelon.
3.2. Observation and Analysis of the M1 and M2 Generations
To construct a large library of M1 mutants, approximately 2000 seeds were sown independently in 2021 and 2022 and 952 and 903 individuals were obtained respectively. Among the M1 population (Table 2), 196 mutant plants with phenotypic variations were identified and they exhibited 20 different types of mutations based on the characteristics of plant architecture, leaves, and flowers. The overall mutation frequency was approximately 10.57% while those for the leaves, stems, flowers, and fruits were 4.74%, 0.11%, 4.37%, and 1.13%, respectively. Among the mutants of leaf organs, several plants with crumpled leaf edges and yellowed leaves were discovered (Figure 1). However, in the mutants of flower organs, variations were observed in the number of petals and the presence of bisexual flowers.

Table 2.
Summary of mutant types in the 60Co γ-ray-induced library in the M1 generation.

Figure 1.
Representative mutant phenotypes in the M1 generation. (A1): Leaf (WT); (A2): Crumpled leaf edges; (A3): Chimera color leaf; (A4): Yellow leaf; (A5): Yellow leaf. (B1): Male flower (WT); (B2): Increased number of pale-yellow petals; (B3): Narrow petal shape; (B4): Deformed petals and stamens; (B5): Decreased number of pale-yellow petals. (C1): Female flower (WT); (C2): Deformed petals and stigmas; (C3): Clustered flowers; (C4): Deformed stigmas; (C5): Bisexual flower.
We selected 17 self-crossed lines of the M2 population. Each consisted of 15 plants. A total of 247 plants ultimately survived. Among them, 10 types of mutations involving 35 individuals were identified, with a mutation frequency of 14.17% (Table 3). Compared to the normal plants, these mutations involved variant cotyledons, leaf margins, and pericarps (Figure 2). For example, we observed that one line had completely yellow cotyledon individuals during the seedling stage (Figure 2B) and the ratio of yellow plants to normal plants was approximately 1:4 (4 yellow cotyledon and 16 normal seedlings). However, the yellow plants grew significantly weaker and they gradually wilted and eventually died, possibly owing to a decrease in their photosynthetic capacity.

Table 3.
Summary of mutant types in the 60Co γ-ray-induced library in the M2 generation.

Figure 2.
Representative mutant phenotypes in the M2 generation. (A): Cotyledon (WT); (B): Yellow cotyledon; (C): Yellow leaf; (D): Deformed cotyledon; (E): Leaf (WT); (F): Crumpled leaf edges; (G): Young fruit (WT); (H): Light stripes; WT: wild type.
3.3. Morphological Screening of the Mutations Identified by 60Co γ-ray Irradiation and EMS Treatments
As described above, hundreds of mutants were primarily identified from the M1 and M2 generations. They exhibited various changes in phenotype. Considering the fruits as an important trait of watermelon, we primarily narrowed the search to mutants related to this character. Among all of the morphological mutations observed following 60Co-γ irradiation, we selected five from the M1 population and one from the M2 population for details. They were designated C1-No.1, C1-No.2, C1-No.3, C1-No.4, C1-No.5, and C2-No1. The C1-No.1 plant was a mutant with yellow leaves during the growth period (Figure 3A,B). It harbored abnormal male flowers that made it difficult for self-pollination to produce the fruits. Compared to the WT, the pericarp of C1-No.2 was light green at the early stage of fruit development and it gradually became a milky white at the mature period (Figure 3C–E). The normal rind thickness of germplasm M08 was approximately 1.1 cm. In contrast, the rind thickness of the mutants C1-No.3 and C1-No.4 were 2.4 cm and 0.4 cm, respectively, which represented an increase of 118.18% and a decrease of 63.64%, respectively (Figure 3F–H). As a seedless mutant, C1-No.5 contained only one seed and had a lighter pink flesh compared to the WT (Figure 3F,I). More importantly, the mutant traits of C1-No.3 and C1-No.4 had been proven to be stably inherited through their self-crossed offspring (Figure S1A,B). In the M2 generation, a new male-sterile mutant, designated C2-No.1, was discovered with normal female flowers and malformed male flowers (Figure S1C).

Figure 3.
Five representative mutants from a 60Co γ-ray-induced library. (A): Normal seedling (WT); (B): C1-NO.1; (C): Mature fruit (WT); (D,E): C1-NO.2, young and mature stages, respectively; (F): Mature fruit (WT); (G): C1-NO.3; (H): C1-NO.4; (I): C1-NO.5; WT: wild type.
We used watermelon germplasm ‘M08’ to construct an EMS mutant library in our previous studies [23,24] and identified some valuable mutants in the M1 generation. In this study, we presented three of them, which were designated M1-3, M2-1, and M1-5. The mutant M1-3, which was characterized by fusiform fruit morphology in the M1 generation, had been proven to be stably inherited in the M2 offspring (Figure 4A,B). Compared to the WT, the M2-1 plant fruits were dark green with clear stripes (Figure 4A,C). In addition, as a leaf color mutant, the new young leaves of M1-5 were yellow while its mature leaves gradually turned green at the stem elongation stage. However, the mature leaves were still lighter than those of the WT (Figure 4D,E).
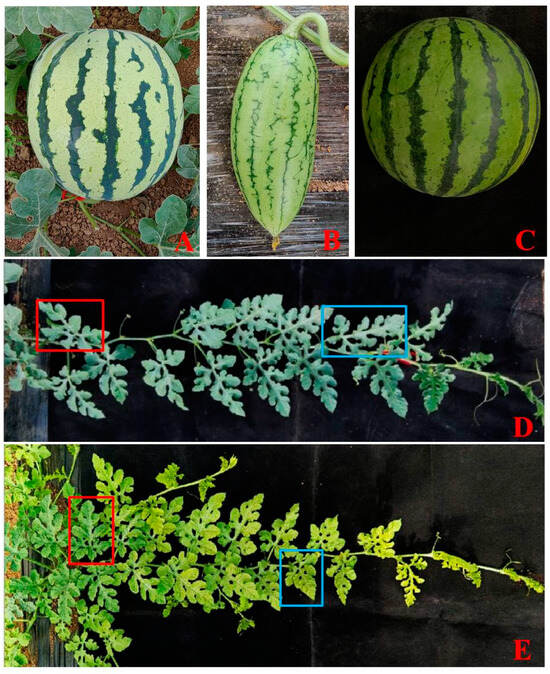
Figure 4.
Three representative mutants from the EMS-induced library. (A): Mature fruit (WT); (B): M1-3; (C): M2-1; (D): Seedlings (WT); (E): M1-5. Blue boxes: young leaves; Red boxes: mature leaves. EMS: ethyl methanesulfonate; WT: wild type.
3.4. Statistical Analyses of the Contents of Photosynthetic Pigments and Photosynthetic Parameters in Mutant M1-5
Leaf color may not only affect watermelon yield but can also serve as a morphological marker to assess the authenticity of hybrids. Hence, we further investigated the photosynthetic pigment content and photosynthetic parameters of M1-5 at the stem elongation stage. As expected, the content of carotenoids and total chlorophyll, including chlorophyll a and b, were consistently lower in the young leaves compared to the mature leaves in both the M1-5 and WT plants (Table 4). In addition, the ratios of Chl a/b and Caro/Chl in the new leaves of M1-5 were significantly higher than those in the mature leaves. However, the ratios did not change much in the WT, which was consistent with the changes in leaf color (Table 4 and Figure 4D,E). Furthermore, we investigated the differences in Pn, Gs, Tr, and Ci between the WT and M1-5. Compared with that of the WT, the Pn of young and mature M1-5 leaves decreased by 32.58% and 24.52%, respectively, which was similar to the trend of Gs (Figure 5A,B). The Ci indicated that the concentration of carbon dioxide (CO2) in M1-5 was significantly higher than that in the WT, suggesting that the mutant utilized a lower rate of CO2 (Figure 5C). In addition, the Tr of mature leaves in the WT was obviously stronger than that in the other leaves (Figure 5D). Taken together, these results indicated that the photosynthetic pigments and parameters were negatively impacted in the M1-5 mutant.

Table 4.
Contents of photosynthetic pigments in the young and mature leaves of the mutant M1-5 and the WT.
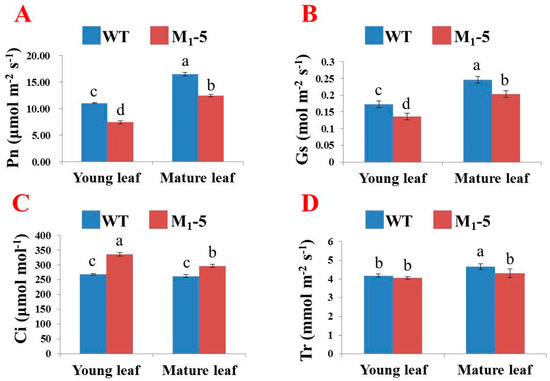
Figure 5.
Photosynthetic parameters of the WT and yellow-leaf mutant M1-5, including the Pn (A), Gs (B), Ci (C), and Tr (D). The data are represented as the mean ± SD. The different letters indicate significance according to the Duncan’s test (p < 0.05). Pn: net photosynthetic rate, Gs: stomatal conductance, Ci: intercellular CO2 concentration, Tr: transpiration rate; WT: wild type.
4. Discussion
Watermelon has been cultivated for at least 4000 years, which has led to a very low degree of genetic diversity [2,4]. Thus, the construction of a mutant library will provide potential favorable germplasm to breed new varieties and resources to explore gene functions. Although EMS has been widely used as a stable and effective mutagen, the amount of the mutagen that is lethal to one-half of the experimental population (LD50) and the efficiency of mutagenesis vary among plant species. For example, the treatment of 1% EMS for 24 h was much closer to the LD50 for the cucumber cultivar ‘Shannong No.5’, which resulted in an 18.3% frequency of mutation [13]. However, the 1.5% EMS treatment for 12 h was optimal for the cucumber inbred line ‘406’ based on the LD50 [7]. For the watermelon line ‘302’, the mutants were induced by 1.5% EMS for 10 h [18] while 1.2% EMS for 12 h was the best treatment to construct a mutant library of the watermelon germplasm ‘Shihong’ [31]. In our previous research [25,26], we established a watermelon mutant library using the inbred line ‘M08’ treated with 1.5% EMS for 12 h and obtained a series of mutagenesis lines in the M1 progenies with a total mutation frequency of 20.56%, which is much higher than that of the pollen-EMS mutation library of 15.38% [3].
Similarly, 60Co γ-rays have also been widely utilized in plant breeding to induce the desired phenotypic mutagenesis and various species respond differentially to a suitable dose of radiation. For instance, a dose of radiation that ranged from 200 to 250 Gy displayed much higher mutagenic efficiency in bitter gourd [21] compared to the safely recommended doses between 200 and 250 Gy in the winter squash and pumpkin lines [22]. In watermelon, dry seeds of the variety ‘Bojura’ were treated with 60Co γ-rays at 80, 100, 200, 250, 350, and 450 Gy, respectively, revealing that the survival rate (66.20% and 62.07%) of the higher doses (350 and 450 Gy) was close to the LD50 [23]. Similarly, the germination rate of the watermelon genotype ‘Yalıncak’ was 51.11% after exposure to 350 Gy of 60Co γ-rays [24]. Nevertheless, to our knowledge, a large-scale mutant library has not been generated using the 60Co γ-rays irrigation method to date. In this previous study, 4000 seeds of the watermelon inbred line ‘M08’ were used to construct an irradiation mutant library. The rate of seed germination was approximately 89.84% (Table 1), which was much higher than those of the genotypes ‘Yalıncak’ and ‘Bojura’ [23,24], despite the fact that a similar dosage of 60Co γ-rays (350 Gy) was applied. Since research to determine the optimal mutation dose in watermelon is currently limited, studies in other species of vegetables suggest that higher dosages of irradiation have an adverse effect on plant growth [24]. In this study, the rate of seeds from the irradiated line ‘M08’ was only 51.18% (Table 1), which was slightly lower than that of the genotype ‘Bojura’ at dosages of 350 Gy (66.20%) and 450 Gy (62.07%) [23]. Taken together, these data indicate that the suitable dosage varies among genotypes and higher levels of 60Co γ-ray irradiation have a negative effect on both seed germination and plant growth.
In general, most of the mutants induced by EMS or 60Co γ-rays may be harmful to plants or unfavorable from a breeding perspective. For example, we found a yellow-cotyledon mutagenic line in the M2 families (Figure 2B) and four yellowing seedlings were observed among a total of 20 plants, suggesting that this phenotypic change in morphology is controlled by a recessive gene. However, the mutagenic seedlings gradually withered and eventually died, possibly owing to their decrease in photosynthetic capacity. Undoubtedly, some mutants indeed exhibit desirable horticultural traits that can be used for the further breeding of new varieties or the exploration of gene functions. For example, fruit shape is one of the major objectives in watermelon breeding, which can influence consumer preference and fruit transportation. In the pollen-EMS mutant library, a mutant with elongated fruit was identified to be incompletely dominant compared to the spherical fruit [3]. In this study, we also discovered a fruit mutant, M1-3, which exhibits a stably inherited fusiform fruit shape (Figure 4A,B). This phenotype is different from the previously published elongated fruit shape [3], indicating that mutant M1-3 is a new type of germplasm. However, further genetic analysis and functional gene mining of this morphological trait are needed to investigate this hypothesis. Male sterility has been recognized as a useful trait to utilize hybrid vigor and produce hybrid seeds in watermelon breeding. To date, only one dominant and two recessive male sterile genes have been cloned from watermelon mutants [3,32,33]. In this study, we also reported a male-sterile mutant, C2-No.1, that produces malformed male flowers (Figure S1C), which has potential applications in the utilization of watermelon hybrid vigor. The colorful flesh of watermelon can attract consumers and benefit human health. Using the EMS-induced mutant ‘psf’, the ClZISO gene was predicted to be responsible for the photosensitive flesh, thus providing the theoretical basis for color breeding in watermelon [18]. In this study, a different mutant, C1-No.5, was characterized with a lighter pink flesh and seedless phenotype (Figure 3F,I), implying its significant value in the exploration of gene function and breeding of new varieties. Additionally, other mutants with stably inherited phenotypes, such as the rind thickness mutants C1-No.3 and C1-No.4 (Figure 3F–H), are also new valuable germplasms to develop new varieties of watermelon with favorable characteristics.
The morphological changes in leaves are a typical observation in mutant libraries. In both the M1 and M2 generations, the leaf-type variant mutants were the majority in our study (Table 2 and Table 3), which is consistent with the findings in other species, such as cucumber [7], strawberry [12], and eggplant [11]. Among these leaf-type mutants, changes in color were the most common mutants. For example, leaf mutants accounted for the highest proportion in the cucumber EMS-induced library and the changes in color varied from light green to pale yellow and dark green [7]. Considering that leaves serve as crucial organs to produce photosynthates for plant growth, leaf-color-type mutants are recognized as vital types of germplasm to study chloroplast and photomorphogenesis. In watermelon, a chlorophyll-deficient mutant ‘Yl2’ with yellow leaves was identified from an EMS-induced library; an observation of its anatomy revealed that the yellow leaves had fewer chloroplasts and thylakoids [34]. In this study, a mutant designated M1-5 was obtained; its young leaves were yellow but the mature leaves gradually turned green at the stem elongation stage (Figure 4D,E). These changes are similar to the phenotype of a natural virescent-leaf-color mutant ‘63’ [35]. According to the data of photosynthetic pigments and parameters between the M1-5 and WT (Table 4 and Figure 5), we deduced that the development of the chloroplast and efficiency of the photosynthetic performance in the former were possibly inhibited, which is consistent with the findings in mutants Yl2 and ‘63’ [34,35].
5. Conclusions
We established a large-scale watermelon mutant library using a treatment of irrigation with 60Co γ-rays. Hundreds of mutants that exhibited various phenotypic changes were identified from the M1 and M2 generations. In detail, six mutants related to fruit (C1-NO.2, C1-NO.3, C1-NO.4, C1-NO.5, M1-3, and M2-1), along with one flower (C2-No.1) and two leaf color (C1-NO.1 and M1-5) mutants, were additional mutants. The analysis of photosynthetic pigment and parameters between the WT and yellow-leaf mutant, M1-5, at the stem elongation stage indicated that the development of chloroplasts and the efficiency of photosynthetic performance were possibly inhibited in this mutant. Notably, the yellow leaf, as well as the fusiform fruit shape and thickening and thinning pericarp, can be stably inherited, which can be used in the genetics of watermelon breeding and the exploration of functional genes. Moreover, the large-scale watermelon mutant library could provide more valuable germplasm resources for further research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9101133/s1, Figure S1, Phenotypes of C1-NO.3 (A), C1-NO.4 (B), and C2-No.1 (C) in the M2 generation.
Author Contributions
Conceptualization, L.Y.; methodology, R.Y. and C.W. (Chunhua Wei); software, L.Y. and Y.H.; validation, X.C., X.H., M.F., C.W. (Chunxia Wang), Z.W. and Z.Y.; formal analysis, L.Y. and C.W. (Chunxia Wang); investigation, L.Y., Y.H., M.F., X.C. and Z.W.; resources, Y.Z., J.M. and J.Y.; writing—original draft preparation, L.Y. and C.W. (Chunhua Wang); writing—review and editing, X.Z., R.Y. and C.W. (Chunhua Wei); visualization, X.Z.; supervision, H.L., R.Y. and C.W. (Chunhua Wei); project administration, R.Y. and C.W. (Chunhua Wei); funding acquisition, R.Y. and C.W. (Chunhua Wei). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the High-quality Development and Ecological Protection Science and Technology Innovation Project of Ningxia Academy of Agriculture and Forestry Sciences (NGSB-2021-7), the Seed Innovation Project of Northwest A&F University (2452022116), the National Natural Science Foundation of Shaanxi Province, China [No. 2023-JC-YB-199], and the Key Research and Development Project of Yangling Seed Industry Innovation Center (Ylzy-sc-01).
Data Availability Statement
All relevant data can be found within this manuscript and the supporting information files.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, K.; Lan, Z.; Liu, Q.; Xue, Y.; Yan, J.; Su, Z.; Cheng, M.; Luan, F.; Zhang, X.; Li, H. Screening of rootstocks with resistance to chilling and continuous cropping but without compromising fruit quality for protected watermelon production. Veg. Res. 2022, 2, 10. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Du, H.; Zhao, H.; Mao, A.; Zhang, X.; Jiang, L.; Zhang, H.; Wen, C.; Xu, Y. Genetic relationship and pedigree of Chinese watermelon varieties based on diversity of perfect SNPs. Hortic. Plant J. 2022, 8, 489–498. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, S.; Zhang, Y.; Tan, J.; Li, X.; Chu, X.; Xu, B.; Tian, Y.; Sun, Y.; Li, B.; et al. A telomere-to-telomere gap-free reference genome of watermelon and its mutation library provide important resources for gene discovery and breeding. Mol. Plant 2022, 15, 1268–1284. [Google Scholar] [CrossRef] [PubMed]
- Paris, H.S. Origin and emergence of the sweet dessert watermelon, Citrullus lanatus. Ann. Bot. 2015, 116, 133–148. [Google Scholar] [CrossRef]
- Wu, S.; Wang, X.; Reddy, U.; Sun, H.; Bao, K.; Gao, L.; Mao, L.; Patel, T.; Ortiz, C.; Abburi, V.L.; et al. Genome of ‘Charleston Gray’, the principal American watermelon cultivar, and genetic characterization of 1,365 accessions in the U.S. National Plant Germplasm System watermelon collection. Plant Biotechnol. J. 2019, 17, 2246–2258. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, S.; Sun, H.; Wang, X.; Wu, S.; Lin, T.; Ren, Y.; Gao, L.; Deng, Y.; Zhang, J.; et al. Resequencing of 414 cultivated and wild watermelon accessions identifies selection for fruit quality traits. Nat. Genet. 2019, 51, 1616–1623. [Google Scholar] [CrossRef]
- Chen, C.; Cui, Q.-Z.; Huang, S.-W.; Wang, S.-H.; Liu, X.-H.; Lu, X.-Y.; Chen, H.-M.; Tian, Y. An EMS mutant library for cucumber. J. Integr. Agr. 2018, 17, 1612–1619. [Google Scholar] [CrossRef]
- Greene, E.A.; Codomo, C.A.; Taylor, N.E.; Henikoff, J.G.; Till, B.J.; Reynolds, S.H.; Enns, L.C.; Burtner, C.; Johnson, J.E.; Odden, A.R.; et al. Spectrum of chemically induced mutations from a large-scale reverse-genetic screen in Arabidopsis. Genetics 2003, 164, 731–740. [Google Scholar] [CrossRef]
- Menda, N.; Semel, Y.; Peled, D.; Eshed, Y.; Zamir, D. In silico screening of a saturated mutation library of tomato. Plant J. 2004, 38, 861–872. [Google Scholar] [CrossRef]
- Sikder, S.; Biswas, P.; Hazra, P.; Akhtar, S.; Chattopadhyay, A.; Badigannavar, A.M.; D’Souza, S.F. Induction of mutation in tomato (Solanum lycopersicum L.) by gamma irradiation and EMS. Indian J. Genet. Plant Breed. 2013, 73, 392. [Google Scholar] [CrossRef]
- Subramaniam, R.; Kumar, V.S. Ethyl methanesulphonate (EMS)-mediated mutagenesis induces genetic and morphological variations in eggplant (Solanum melongena L.). Int. J. Plant Biol. 2023, 14, 53. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; Zhao, C.; Cui, Y.; Cai, Z.; Zhao, J.; Zheng, Y.; Xue, L.; Lei, J. Establishment of a mutant library of Fragaria nilgerrensis schlechtendal ex J. Gay via EMS mutagenesis. Horticulturae 2022, 8, 61. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Li, J.; Yang, X.; Ren, Z. Ethyl methanesulfonate (EMS)-mediated mutagenesis of cucumber (Cucumis sativus L.). Agr. Sci. 2014, 05, 716–721. [Google Scholar] [CrossRef][Green Version]
- Zha, G.; Yin, J.; Cheng, F.; Song, M.; Zhang, M.; Obel, H.O.; Wang, Y.; Chen, J.; Lou, Q. Fine mapping of CscpFtsY, a gene conferring the yellow leaf phenotype in cucumber (Cucumis sativus L.). BMC Plant Biol. 2022, 22, 570. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, H.; Pan, J.; Du, H.; Zhang, K.; Zhang, L.; Yu, Y.; He, H.; Cai, R.; Pan, J. CsUFO is involved in the formation of flowers and tendrils in cucumber. Theor. Appl. Genet. 2021, 134, 2141–2150. [Google Scholar] [PubMed]
- Rong, F.; Chen, F.; Huang, L.; Zhang, J.; Zhang, C.; Hou, D.; Cheng, Z.; Weng, Y.; Chen, P.; Li, Y. A mutation in class III homeodomain-leucine zipper (HD-ZIP III) transcription factor results in curly leaf (cul) in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2019, 132, 113–123. [Google Scholar] [CrossRef]
- Galpaz, N.; Burger, Y.; Lavee, T.; Tzuri, G.; Sherman, A.; Melamed, T.; Eshed, R.; Meir, A.; Portnoy, V.; Bar, E.; et al. Genetic and chemical characterization of an EMS induced mutation in Cucumis melo CRTISO gene. Arch. Biochem. Biophys. 2013, 539, 117–125. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Guo, S.; Ren, Y.; Li, M.; Wang, J.; Yu, Y.; Zhang, H.; Gong, G.; He, H.; et al. ClZISO mutation leads to photosensitive flesh in watermelon. Theor. Appl. Genet. 2022, 135, 1565–1578. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, J.; Jiang, Y.; Wang, X.; Gu, M.; Wang, Y.; Kang, H.; Fan, X.; Sha, L.; Zhang, H.; et al. 100 Gy 60Coγ-Ray induced novel mutations in tetraploid wheat. Sci. World J. 2014, 2014, 725813. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Z.; Huang, T.; Tang, Z.; Li, L.; Ding, X.; Lin, P.; Chen, M. SRAP Marker analysis of genomic mutation induced by 60Co γ-Ray irradiation in potato (Solanum tuberosum). Agr. Sci. Technol. 2013, 14, 1092. [Google Scholar]
- Dutta, S.; Hazra, P.; Saha, S.; Acharya, B.; Bhattacharjee, T.; Maurya, P.K.; Banerjee, S.; Chakraborty, I.; Chattopadhyay, A. Applied mutagenesis could improve economically important traits in bitter gourd (Momordica charantia L.). J. Genet. 2021, 100, 43. [Google Scholar] [CrossRef]
- Kurtar, E.; Balkaya, A.; Kandemir, D. Determination of semi-lethal (LD50) doses for mutation breeding of Turkish winter squash (Cucurbita maxima Duch.) and pumpkin (Cucurbita moschata Duch.). Fresenius Environ. Bull. 2017, 26, 3209–3216. [Google Scholar]
- Velkov, N.; Tomlekova, N.; Sarsu, F. Sensitivity of watermelon variety Bojura to mutant agents 60Co and EMS. J. Biosci. Biotechnol. 2016, 1, 105–110. [Google Scholar]
- Kantoğlu, K.Y. Effects of gamma irradiation on seed germination in watermelon (Citrullus lanatus (Thunb.) matsum & nakai) and determination of effective mutation dose. Alatarim 2022, 21, 10–17. [Google Scholar]
- Wang, C.; He, Y.; Li, C.; Zeng, Z.; Yang, J.; Ma, H.; Wei, C.; Zhang, X. Optimation of EMS mutation in watermelon germplasm M08. China Cucurbits Veg. 2021, 34, 26–31. [Google Scholar] [CrossRef]
- Yin, L.; Wang, C.; Li, C.; Ding, X.; Pan, X.; Yu, R.; Ma, H.; Chen, X.; Yang, J.; Zhang, X.; et al. Screening and phenotypic analysis of EMS induced mutants in watermelon. Acta Hortic. Sin. 2023; accepted. [Google Scholar]
- Wang, Z.; Yang, Y.; Yadav, V.; Zhao, W.; He, Y.; Zhang, X.; Wei, C. Drought-induced proline is mainly synthesized in leaves and transported to roots in watermelon under water deficit. Hortic. Plant J. 2022, 8, 615–626. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, N.; Gao, Q.; Liu, L.; Zhang, Y.; Feishi, L. Analysis of EMS mutation conditions of watermelon seed. J. Northeast Agric. Univ. 2015, 46, 35–39. [Google Scholar]
- Xu, X.; Lu, X.; Tang, Z.; Zhang, X.; Lei, F.; Hou, L.; Li, M. Combined analysis of carotenoid metabolites and the transcriptome to reveal the molecular mechanism underlying fruit colouration in zucchini (Cucurbita pepo L.). Food Chem. Mol. Sci. 2021, 2, 100021. [Google Scholar] [CrossRef]
- Zong, H.; Liu, S.; Xing, R.; Chen, X.; Li, P. Protective effect of chitosan on photosynthesis and antioxidative defense system in edible rape (Brassica rapa L.) in the presence of cadmium. Ecotox. Environ. Saf. 2017, 138, 271–278. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, X.; Li, L.; Hou, S.; Huo, Z.; Zhang, X. Optimization of conditions for mutagenesis of watermelon seeds with ethyl methanesulfonate. China Cucurbits Veg. 2022, 35, 81–85. [Google Scholar]
- Zhang, R.; Chang, J.; Li, J.; Lan, G.; Xuan, C.; Li, H.; Ma, J.; Zhang, Y.; Yang, J.; Tian, S.; et al. Disruption of the bHLH transcription factor Abnormal Tapetum 1 causes male sterility in watermelon. Hortic. Res. 2021, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Sim, T.-Y.; Ryu, J.; Rhee, S.-J.; Kim, Y.; Lee, G.P. Identification of a candidate locus and development of a molecular marker for male sterility in watermelon. Hortic. Sci. Technol. 2021, 39, 673–683. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, C.; Gu, Y.; Cheng, R.; Huang, D.; Liu, X.; Sun, Y. Physiological and transcriptomic analysis of a yellow leaf mutant in watermelon. Sci. Rep. 2023, 13, 9647. [Google Scholar] [CrossRef]
- Xu, M.; Gao, M.; Guo, Y.; Bao, X.; Liu, X.; Gao, Y. Photosynthetic characteristics of virescent mutant in watermelon. J. Northwest Univ. Nat. Sci. Ed. 2022, 50, 91–96+106. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).