Abstract
Aconitum violaceum Jacq. ex Stapf is a threatened medicinal plant with restricted global distribution. The highest frequency of seed germination was recorded on Murashige and Skoog’s (MS) basal medium, supplemented with 0.5 mg L−1 kinetin with a germination rate of 77.32% and mean germination time of 27 days. Among the various plant growth regulators examined, 0.1 mg L−1 kinetin (Kn) + 0.5 mg L−1 indole-3-acetic acid (IAA) proved to be effective for maximum embryogenic callus production (51.0%) within 31 days of inoculation. The conversion rate of somatic embryos into complete plantlets was highest in the MS medium augmented with 0.1 mg L−1 Kn + 0.5 mg L−1 IAA (68.00%), with an average root initiation time of 25 days. The rooted plantlets were subsequently hardened into jiffy pots with a combination of loamy soil, coco-peat, and vermicompost (1:1:1 v/v), and then transplanted into a greenhouse with a 60% survival rate. To our knowledge, this is the first study on direct in vitro propagation and embryogenic callus induction from seeds. The established regeneration protocol could be employed to propagate A. violaceum on a large scale in a short time. This would contribute significantly to its rapid propagation and germplasm conservation, and establish a framework for the domestication of this highly valued threatened medicinal plant.
1. Introduction
Aconitum violaceum Jacq. ex Stapf is a biennial herbaceous medicinal plant of the Ranunculaceae family and is endemic to the north-western Himalayan region of India, Pakistan, and Nepal [1,2,3]. In India, it is primarily found in the alpine and subalpine regions of the north-western Himalayas, at an elevation range of 3000–4000 m asl and shares its position with vulnerable plant species [4,5]. The toxic alkaloids of A. violaceum can be easily converted into less harmful alkaloids by heating or by using an alkaline treatment. They can then be employed in Ayurvedic and Unani medicines after they have been detoxified [6]. Traditionally, it is used to treat boils [7,8,9], asthma, high fever [7,8,9,10,11,12], gastric troubles [8,9,10,11,12], sciatic pains [8,11,13], intestinal worms [7,12,13], renal pain [7,11,12], and snake and scorpion bites [6]. This is due to its wide spectrum of biological activities, such as antioxidant [14], antimicrobial [14], anti-inflammatory [15,16,17], anti-malarial [15,16,17,18], anti-proliferative [16,17,18,19,20], analgesic, and antipyretic properties [14,15,17].
A. violaceum faces a great threat of extinction in its natural habitat due to various factors, such as physiological seed dormancy of >5 months [4,21,22], and long-term burying of seeds beneath snow (more than five months). Due to its specific ecological requirement, seed germination and seedling establishment in A. violaceum are quite challenging. A. violaceum grows along the margins of irrigation canals and at the edges of alpine streams; consequently, the majority of the seeds are susceptible to being carried away from their natural environment by running water, heavy rainfall, and floods, exposing them to adverse environmental conditions [4,21]. The plant maintains its spatial continuity through its rhizome. Therefore, uprooting whole plants due to collection, overgrazing, and pre-mature harvesting, alongside construction of high-altitude roads, dams, cemented water channels, and human settlements, also contribute to the decline of this species from its natural habitat. Beetles and aphids are another potential hazard to this species as they consume the flowers and other reproductive portions of the plant, thereby reducing the species’ sexual potential [22]. In addition, the species has a unique niche, which may limit its area of occupancy and dispersion [4].
When properly applied, mass propagation methods may help to reduce the extinction risk of vulnerable species by using procedures such as in vitro propagation [22]. In vitro regeneration techniques have been established for some Aconitum species such as A. chasmanthum Stapf ex Holmes [22], A. ferox [23], A. heterophyllum [24], A. nagarum [25], and A. vilmorinianum [26], but no such attempts have been made for A. violaceum. Giri et al. [24] regenerated complete plantlets from the somatic embryos of leaf and petiole explants in Aconitum heterophyllum. Hatano et al. [27] regenerated complete plantlets from the somatic embryo of anther explant in Aconitum carmichaeli. High concentrations of NAA (10 mg L−1) and darkness promote somatic embryo development in Ranunculus sceleratus [28]. After surveying extensive literature from various databases (DOAJ, Google Scholar, PubMed, Scihub, ScienceDirect, etc.) only a few studies have reported on the in vitro propagation of A. violaceum to date. Rawat et al. [29] attempted in vitro propagation from the nodal explant. Given the aforementioned issues, the current research on A. violaceum was carried out to develop: (1) direct in vitro seed germination protocols; (2) embryogenic callus production; and (3) complete plant regenerations from somatic embryos.
Due to its immense medicinal and economic value, establishing in vitro propagation protocols for A. violaceum would not only provide elite clones for pharmaceutical uses and facilitate rapid propagation and germplasm conservation, but it would also help to domesticate the plant and preserve wild populations.
2. Materials and Methods
2.1. Collection of Plant Material
The seeds of A. violaceum Jacq. ex Stapf were collected in the month of August (10–28) as soon as the follicle burst for seed dispersal, from the villages of Khawous and Numsuru (3220 m asl to 3340 m asl; 34°15.905 N to 34°12.705 N; 75°96.040 E to 75°96.720 E) in Ladakh, India. The plant specimen was identified and authenticated by the Center for Biodiversity and Taxonomy (CBT), Department of Botany, University of Kashmir, Hazratbal-Srinagar. A voucher specimen (accession number 3738-KASH) was deposited at the Kashmir University Herbarium (KASH). Seeds were collected in dry plastic and glass bottles and brought to the plant tissue culture laboratory, Department of Botany, University of Kashmir, where they were stored between 4 °C and 6 °C with 55 to 65% relative humidity for experimental purposes.
2.2. Chemicals
MS (Murashige and Skoog 1962) medium and plant growth regulators (PGRs), such as 2,4-dichlorophenoxyacetic acid (2,4-D), indole-3-acetic acid (IAA), kinetin (Kn), 6-benzylaminopurine (BAP), and 1-naphthaleneacetic acid (NAA), were purchased from Hi-Media, India. Mercuric chloride (HgCl2), sodium hypochlorite (NaClO), and sucrose were also purchased from Hi-Media, India, while agar (plant agar) was purchased from Sigma Aldrich, India.
2.3. Explant Selection, Culture Conditions, and Establishment of In Vitro Cultures
A. violaceum seeds were used as explants to start in vitro cultures. The seeds were immersed in tap water for 96 h (h) before inoculation on the MS medium. Prior to culture, seeds were rinsed for about 2 h under running tap water, surface sterilized with 1% NaClO (v/v) for 7 min with occasional agitation, washed 4–5 times with double-distilled autoclaved water, and then dipped in 70% ethanol for about 30–45 s. They were then washed with aseptic (autoclaved, double-distilled) water 3–4 times. The seeds were placed in the folds of sterile filter paper to absorb the leftover moisture. Seeds were then cultured in 30 mL borosilicate culture vials on the MS basal medium containing 0.8% (w/v) agar and sucrose (3% w/v), fortified with different PGRs at different concentrations (0.05–2.0 mg L−1), either individually or in combination. They were then incubated at 10 ± 2 °C with 50–55% humidity for a 12–12 h photoperiod (42–60 μmol m−2 s−1) in a plant growth chamber. Under the laminar air flow hood, all studies, from surface sterilization to inoculation, were carried out successfully. Data were represented as a mean of 50 replicates per repetition. Observations were recorded from each non-contaminated vial (experimental unit). Mean germination time (MGT) in days was calculated by following the methods of Darrudi et al. [30] using the following equation:
where
‘’ = number of newly germinated seeds after each incubation period
‘’ = number of days since the experiment began, and
‘’ = total number of seeds germinated at the end of the experiment.
Seed germination rate was calculated using the following equation:
where
% seed germination = (nx/Na) × 100
‘nx’ = total germinated seeds
‘Na’ = number of seeds used at the beginning of the experiment.
2.4. Embryogenic and Non-Embryogenic Callus Production
Mature and immature seeds of A. violaceum, taken from the wild plants, were cultured on the MS basal medium augmented with various concentrations of PGRs, such as Kn (0.05 mg L−1 to 3.0 mg L−1), IAA (0.1 mg L−1 to 1.5 mg L−1), and 2,4-D (0.05–2.5 mg L−1) with sucrose 3% (w/v) and 0.8 % agar for production of embryogenic and non-embryogenic calluses. The pH of the medium was adjusted to 5.8 ± 0.02 before autoclaving at 121 °C. Immature and mature seeds were surface sterilized with 1% sodium hypochlorite (NaClO) (v/v) for 7 min with occasional stirring, then rinsed 3–5 times with sterile water before being immersed in 70% ethanol for about 30 s and washed 3–4 times with sterile (autoclaved, double-distilled) water. The remaining moisture was removed by putting the seeds in the folds of sterile filter paper. Cultures were incubated at 10 ± 2 °C in a 16/8-h light/dark cycle or in completely dark conditions on racks fitted with cool fluorescent tube lights of 60.0 μmol m−2 s−1 illuminance and RH 55%. The frequency of embryogenic callus induction was studied after 6 to 8 weeks (wk) of incubation. Consequently, embryogenic and non-embryogenic calluses sub-cultured within 1 culture were used for whole plant regeneration. The experiment was conducted at least 3 times with a total of 10 replicates for each treatment (1 vial was considered as 1 replicate).
2.5. Effect of Light Requirement on Percentage of Somatic Embryo Formation
The effect of light conditions on the production of somatic embryos was investigated by keeping 50% of embryogenic calluses in the dark (the culture vials were wrapped in aluminum foil) and the other 50% were kept in a 16/8 h photoperiod for 10–30 days.
2.6. Whole Plant Regeneration from Somatic Embryos
To accelerate complete plant development from somatic embryos, the effects of Kn and IAA on further conversion of somatic embryos were studied. The somatic embryos were sub-cultured on the MS medium augmented with different concentrations of Kn (0.05–1.0 mg L−1) and IAA (0.1–1.0 mg L−1), either individually or in combination. After 8 weeks of culture, the average number of shoots and roots developed by somatic embryos were evaluated. All cultures were incubated at 10 ± 2 °C in a 16/8-h light/dark cycle with 60% RH. Each treatment consisted of 10 somatic embryos in 30 mL borosilicate culture vials and 150 mL conical flasks, and the experiment was repeated 3 times.
2.7. In Vitro Rooting
Multiple shoots developed directly from non-embryogenic and embryogenic calluses were sub-cultured on the MS basal medium and enriched with different combinations and concentrations of auxins and cytokinins. IAA concentrations were kept slightly higher compared to Kn. The effect of light and various concentrations of PGRs on the development of roots were also examined.
2.8. Hardening and Acclimatization of In Vitro Plantlets
Media traces from the roots of A. violaceum regenerated by different methods from seed explants were removed by thoroughly washing under tap water. The plantlets were then transplanted into jiffy pots composing a mixture of sterilized loamy soil, coco-peat, and vermicompost (1:1:1). The plantlets in the jiffy pots were enclosed in transparent polybags for 2 wks to ensure adequate humidity and were maintained in a growth chamber. After 3 wks, the hardened plantlets were transferred into a net-shade greenhouse for further acclimatization. Well-established plantlets were then transferred into the Kashmir University Botanical Garden (KUBG) and were kept in the shade with occasional watering. The phenotypic data, including the plant height, leaf number, floral bud numbers, and survival rate, was determined for up to 8 wks.
2.9. Experimental Design and Statistical Analysis
The data were evaluated using a factorial design that was completely randomized (CRD). Substantial variations between each treatment were assessed using Duncan’s multiple range test (DMRT) of one-way ANOVA using SPSS statistical software version 23 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, version 23.0. Armonk, NY, USA: IBM Corp.). Results were shown as mean value ± SEM (standard error mean) for each experiment. Graphs were prepared in origin pro (version 9, 2021; developed by originLab corporation, Northampton, MA, USA).
3. Results
3.1. Seed Germination
Seed germination rate was enhanced when the seeds were immersed in tap water for 96 h in winter and early spring, prior to culture. Among the various PGRs used, the highest rate of in vitro seed germination was recorded in winter and spring on the MS basal medium enriched with 0.5 mg L−1 Kn and 1.0 mg L−1 Kn with a MGT of 27.22 ± 0.70 and 26.88 ± 0.16 days, and percentage germination of 77.32 ± 0.38 and 75.33 ± 0.14. The number of shoot buds was also recorded as maximum (2.0 ± 0) in this treatment. The shoot length of each seed was recorded highest in the MS medium augmented with 1.0 mg L−1 Kn with an average length of 4.7 ± 0.11 cm (Table 1, Figure 1). Kn at low concentrations (<0.01 mg L−1) and high concentrations (>1.5 mg L−1) reduced seed germination percentage. Likewise, high concentrations of IAA (>1.5 mg L−1) also reduced the rate of seed germination. Thus, concentrations of Kn and IAA ranging between 0.1 and 1.5 mg L−1 were proven to be ideal for seed germination in A. violaceum. None of the seeds germinated in the control condition (MS basal) without PGRs (plant growth regulators). The data were recorded for up to eight weeks, which was represented by mean value ± SEM (standard error mean).

Table 1.
Effects of plant growth regulators (IAA, Kn, BAP, and NAA) on direct in vitro seed germination of wild-growing Aconitum violaceum Jacq. ex Stapf. in Ladakh, India.

Figure 1.
Direct in vitro seed germination of Aconitum violaceum Jacq. ex Stapf on the MS basal medium enriched with different concentrations of PGRs, either individually or in combination: (A) 0.1 mg L−1 Kn; (B) 0.35 mg L−1 Kn; (C) 0.5 mg L−1 Kn; (D) 1.0 mg L−1 Kn; (E) 1.5 mg L−1 Kn; (F) 0.1 mg L−1 IAA; (G) 0.5 mg L−1 IAA; (H) 1.0 mg L−1 IAA; (I) 0.1 mg L−1 BAP; (J,K) germinated seeds with well-formed roots before hardening; and (L) hardened plantlets in the jiffy pots composing a mixture of loamy soil, coco-peat, and vermicompost (1:1:1 v/v). Scale bar (A–I) represents 5 mm; (J–L) represents 1 cm.
3.2. Effects of Kn, IAA, and 2, 4-D on Embryogenic and Non-Embryogenic Callus Formation
The impacts of Kn and IAA on embryogenic callus production from immature and mature seed cultures were explored. Various concentrations of Kn, either individually or in combination with IAA, were found to be efficient in inducing a nodular mass of embryogenic callus. However, the highest proportion of embryogenic callus production was achieved on the MS basal medium supplemented with 0.1 mg L−1 Kn + 0.5 mg L−1 IAA, with a percent culture response of 51.0 ± 1.0% and a mean initiation time of 31.0 ± 0.57 days, followed by 0.1 mg L−1 Kn alone (35.53 ± 2.23). At concentrations ranging between 0.1 and 1.5 mg L−1 Kn and IAA, the proportion of somatic embryo formation is high. Raising the concentrations further dramatically reduces somatic embryo development and promotes callus induction. The MS media enriched with 2,4-D at concentrations 0.1 to 0.5 mg L−1 produced only non-embryogenic calluses (Figure 4A). Establishment of embryogenic callus induction was examined in this study (Table 2, Figure 2A–L). When the immature seeds were incubated on the MS medium augmented with various doses of Kn and IAA (0.1 mg L−1 to 0.5 mg L−1), either individually or in combination, two different types of calluses were generated simultaneously. Light green transparent calluses developed from immature and mature seeds after four to six weeks of culture, whereas creamy nodular calluses were mainly produced after six to eight weeks of incubation (Figure 2A,B). During callus proliferation, nodular calluses developed into many globular staged embryos (Figure 2C,D) after eight weeks of culture. Globular stage somatic embryos further transformed into subsequently staged embryos (Figure 2E,H).

Table 2.
Effects of Kn, IAA, and 2, 4-D on embryogenic and non-embryogenic callus formation from the immature and mature seeds of Aconitum violaceum.
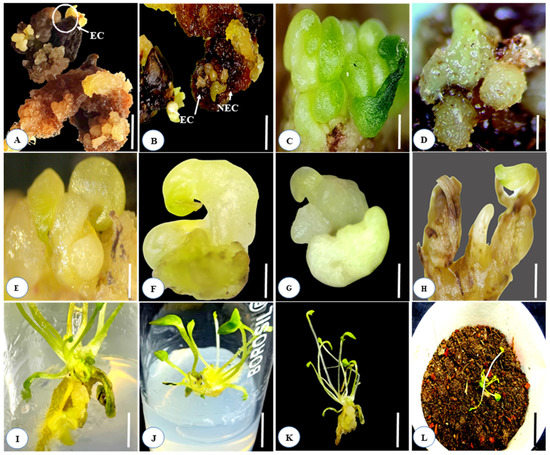
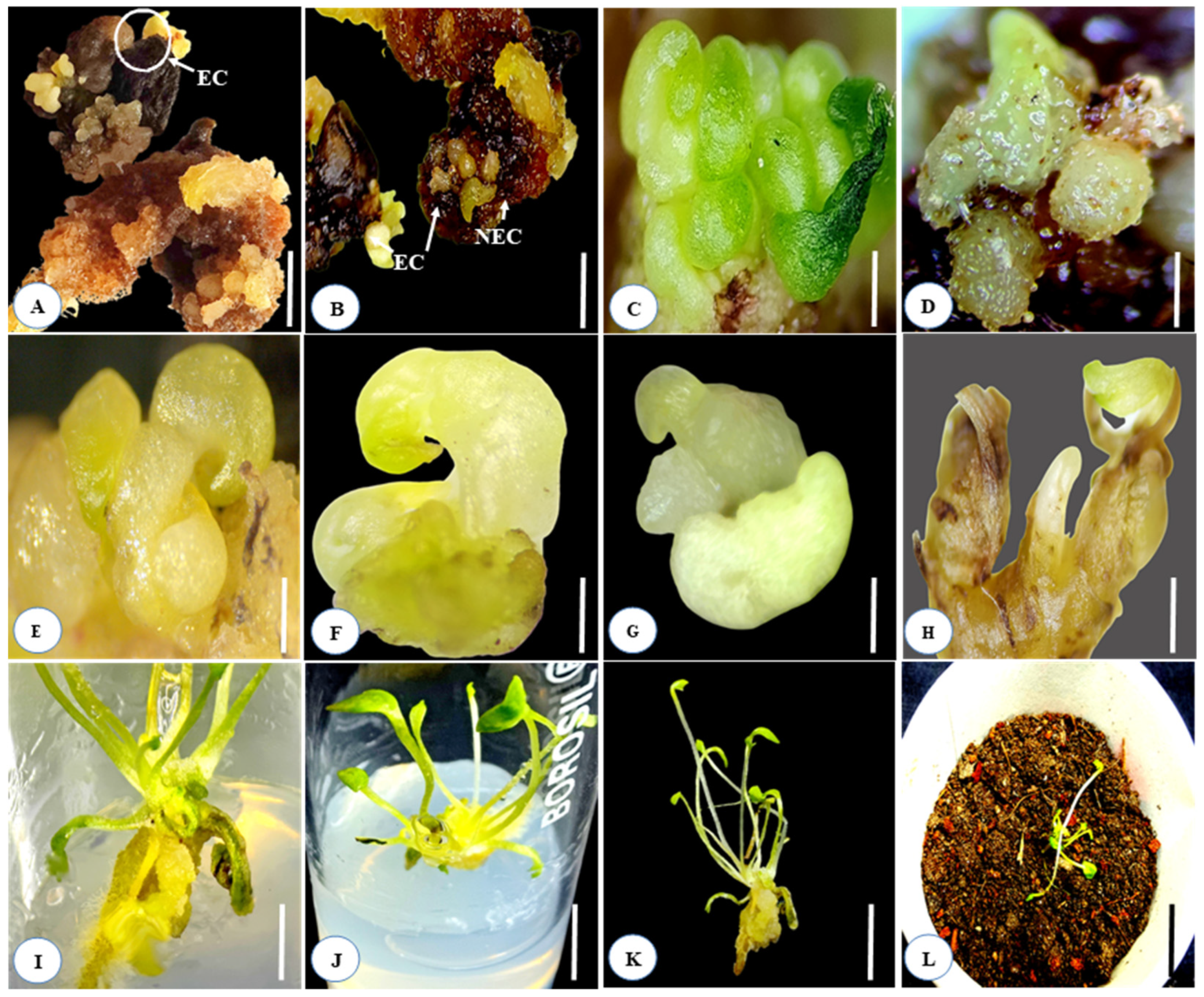
Figure 2.
Direct somatic embryogenesis and complete plant regeneration from immature and mature seed cultures of Aconitum violaceum in the MS medium fortified with various concentrations of PGRs, either separately or in combination: (A,B) embryogenic and non-embryogenic callus formation from the immature and mature seed cultures, enriched with 0.1 mg L−1 Kn + 0.5 mg L−1 IAA and 0.1 mg L−1 Kn; (C,D) globular-shaped somatic embryo development; (E–H) torpedo and subsequent stages of somatic embryo formation; (I) direct germination of somatic embryos on the MS medium, augmented with Kn 0.1 + IAA 0.5 mg L−1; (J) multiple shoot and root development from the cultured somatic embryos; (K) well-developed rooted plantlets before hardening; (L) hardening of plantlet in jiffy pots. Scale bar (A–I) represents 5 mm; (J–L) represents 1 cm.
3.3. Effect of Light on Percentage of Somatic Embryo Formation
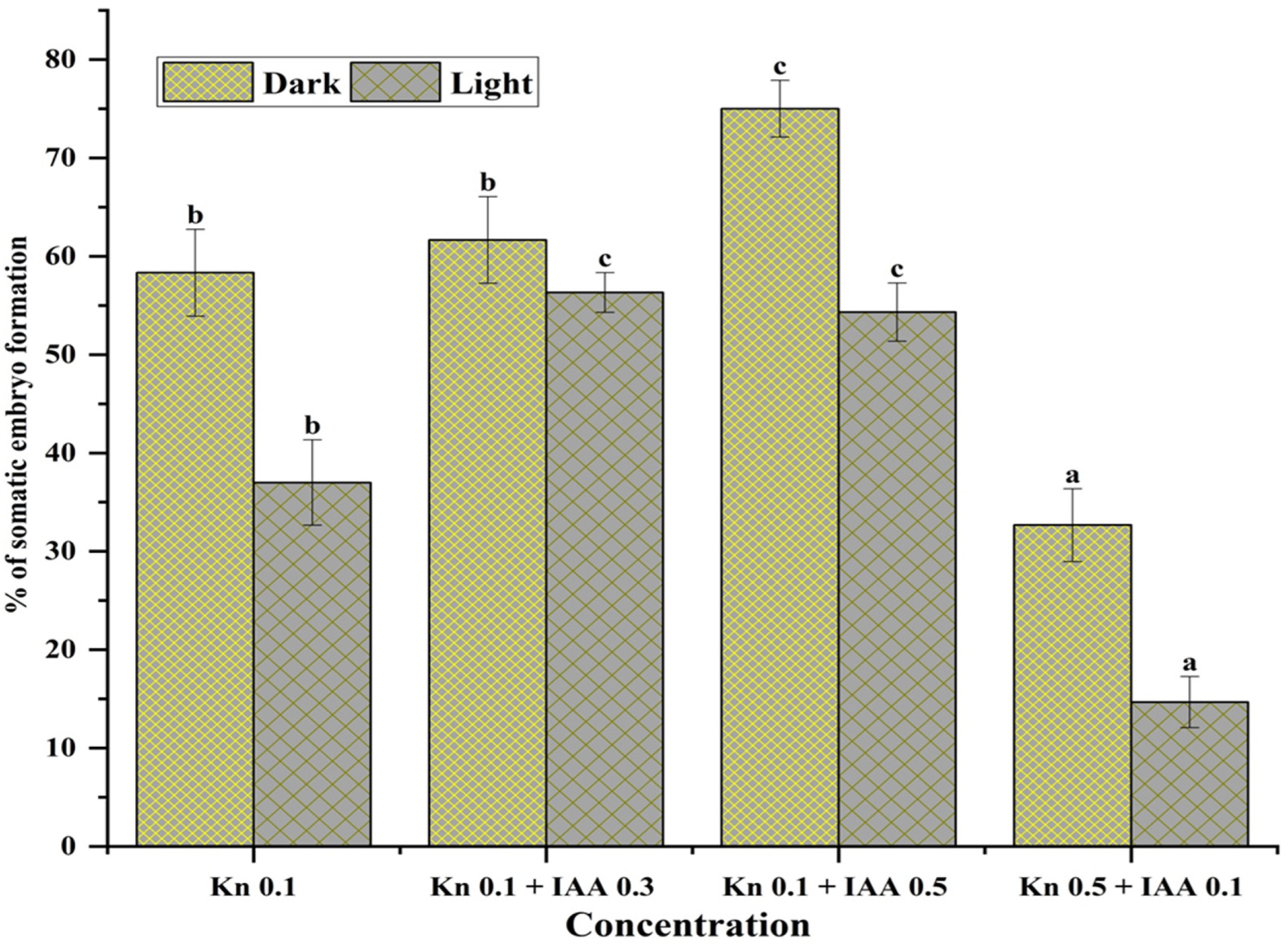
The MS medium cultured vials augmented with 0.1 mg L−1 Kn and 0.5 mg L−1 IAA, incubated in the dark, produced more somatic embryos (77.34 ± 0.05%) than the cultured vials fortified with the same PGRs (0.1 mg L−1 Kn and 0.5 mg L−1 IAA) incubated in the light (56.21 ± 0.07). Likewise, other concentrations showed similar patterns of somatic embryo formation. Thus, it was demonstrated that darkness had a significant impact on somatic embryo formation in Aconitum violaceum (Figure 3). In all trials, cultures that were incubated in the dark for a minimum of 10–25 days may facilitate the induction of somatic embryos.
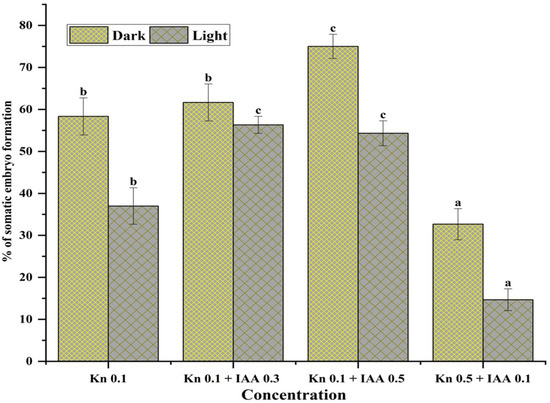
Figure 3.
Influence of light conditions and concentrations of Kn and IAA (mg L−1) on somatic embryo formation from the embryogenic callus of Aconitum violaceum. Mean value ± standard error mean followed by different letters in each column represents that the observations were substantially different according to DMRT (one-way ANOVA) at p ≤ 0.05. Abbreviations: Kn: kinetin. IAA: indole-3-acetic acid.
3.4. Complete Plant Development from Somatic Embryos
To regenerate complete plantlets from somatic embryos, the effects of Kn and IAA on further differentiation of somatic embryos were studied. Somatic embryos of different stages were sub-cultured on the MS basal medium augmented with various concentrations of Kn (0.05–1.0 mg L−1) and IAA (0.1–1.0 mg L−1), either individually or in combination (Figure 2I). However, somatic embryo conversion into plantlets was recorded only in 0.1 mg L−1 Kn, 0.1 mg L−1 Kn + 0.3 mg L−1 IAA, and 0.1 mg L−1 Kn + 0.5 mg L−1 IAA. The highest percentage of whole plant regeneration from somatic embryos was achieved on Kn 0.1 + IAA 0.5 mg L−1 with a percentage culture response of 68.00 ± 1.52%. These observations clearly illustrate that the 0.1 mg L−1 Kn + 0.5 mg L−1 IAA treatment had a more stimulatory effect than other treatments employed to convert somatic embryos into normal plantlets (Table 3, Figure 2J and Figure 4F,G). The healthy plantlets were transferred into earthen/plastic pots consisting of a mixture of garden soil, peat moss, and vermicompost in a ratio of (1:1:1) and kept in a plant growth chamber (Figure 2L and Figure 4H–J). These findings clearly demonstrate that complete plant regeneration via somatic embryogenesis could be possible.

Table 3.
Effect of Kn and IAA on whole plant regeneration from somatic embryos and in vitro rooting in Aconitum violaceum.

Figure 4.
Callus development and plant regeneration from the seeds of Aconitum violaceum in MS basal medium enriched with various concentrations of PGRs, either alone or in combination: (A) callus induction in MS medium + 0.5 mg L−1 2,4-D; (B) callus induction in MS medium + 0.1 mg L−1 Kn; (C) multi-shoot formation in MS medium + 0.1 mg L−1 Kn; (D) root induction when sub-culture in MS medium is augmented with 0.1 mg L−1 Kn + 0.5 mg L−1 IAA; (E) embryogenic callus production and root induction in MS medium fortified with 0.1 mg L−1 Kn; (F,G) multiple shoot and root development from embryogenic callus when sub-cultured in MS medium enriched with 0.1 mg L−1 Kn + 0.5 mg L−1 IAA; (H,I) hardened plants in the pots containing loamy soil, coco-peat, and vermicompost (1:1:1 v/v); (J) plant acclimatized in green house. Scale bar (A–G) represents 5 mm; (H–J) represents 1 cm.
3.5. In Vitro Rooting
The in vitro developed plantlets, through various methods (i.e., somatic embryogenesis and callusing), developed roots through sub-culturing in the MS basal medium enriched with the same concentrations (concentrations at which somatic embryos were developed) or different concentrations of Kn and IAA (Table 3, Figure 4D–G). The multi-shoot plantlets from sub-culturing in the MS medium, enriched with rooting hormone, developed roots within four weeks of inoculation. In most cases, more than three roots were formed per shoot in each treatment. The highest proportion of root formation occurred on the MS basal medium fortified with 0.1 mg L−1 Kn + 0.5 mg L−1 IAA and 30 g L−1 sucrose, with a percentage response of 68.00 ± 1.52% and average root length 4.66 ± 0.23, followed by 0.1 mg L−1 Kn + 0.3 mg L−1 IAA with a percentage response of 56.33 ± 4.91% and average root length of 5.66 ± 0.88. Among all the PGRs tested, the Kn and IAA combination was proven to be most effective for healthy root development and formation of maximum root–shoot ratio. The root induction ratio (number of roots formed per shoot) is further enhanced by dim light or complete darkness. Several cultured vials were wrapped in aluminum foil to reduce the intensity of light; these vials developed roots rapidly compared to vials that were kept in light (16/8-h photoperiod). Further root induction in A. violaceum was facilitated by a low concentration of PGRs. Increasing the concentration of PGRs promotes further callusing on the developed roots.
3.6. Hardening and Acclimatization of In Vitro Plantlets
The plantlets were then transferred into jiffy pots, filled with a mixture of sterilized garden soil/loamy soil, coco-peat/peat moss, and vermicompost (1:1:1). The plantlets in the jiffy pots were enclosed in transparent polybags for two weeks to ensure adequate humidity and were kept in the growth chamber. After the third week, the hardened plantlets were transferred into the greenhouse for further acclimatization. Healthy and well-established plantlets were then transplanted into the Kashmir University Botanical Garden (KUBG) and kept in the shade with occasional watering. After three to five weeks, well-established plantlets produced three to five leaves (Figure 5B). Transplanted plantlets attained a maximum height of 25.33 ± 1.76 after eight weeks (Table 4; Figure 5D). Overall, 55% of the transplanted plantlets survived and reached the budding stage (Figure 5D).

Figure 5.
Hardening and acclimatization of in vitro raised plantlets of Aconitum violaceum Jacq. ex Stapf: (A,B) three to four week old hardened plantlets; (C) five to six week old acclimatized plants; (D) eight week old acclimatized plants. Scale bar represents 1 cm.

Table 4.
Morphological data of tissue culture-raised plantlets of Aconitum violaceum after acclimatization in the field.
4. Discussion
Aconitum species are primarily harvested on a large scale for their rhizome, which has excellent pharmacological properties and is widely used in indigenous medicines, indirectly resulting in the decline of several Aconitum species from their native habitat [31]. Seed-based propagation is the most efficient, affordable, and practicable technique for most species to be cultivated on a large scale for commercial purposes [31]. Aconitum species are mostly wild-growing at high elevations, so their propagation through seeds at low altitudes is constrained by ecological factors such as soil fertility, soil textures, pH, humidity, temperature, phytosociology, nature of vegetation, and dormancy of seeds [4,32,33]. Thus, providing pre-sowing treatments (chilling, water soaking, hot water treatment, or with different chemicals etc.) can enhance its cultivation at lower elevations [32]. Soaking seeds in cold water or hot water is often used in seed germination of Aconitum spp [33] and Iris spp [34]. Cold scarification is the most efficient strategy for breaking dormancy in Ranunculaceae [35,36,37]. The seeds stratified at 4 °C for 4–10 days enhanced the germination rate in A. chasmanthum and A. nagarum when cultured on the MS basal medium [22,25]. Similarly, A. violaceum seeds also require chilling treatment for successful germination. A. violaceum seeds were cultured in different seasons to determine the organogenic response. However, the seeds sown in winter and early spring showed a better germination rate of >77% and gave peculiar organogenic responses (i.e., non-embryogenic and embryogenic callus formation, somatic embryos development, multiple shooting and rooting, and direct seed germination) on the MS basal medium supplemented by different PGRS (auxins and cytokinins), either alone or in combination. Similar results were achieved by Deb and Lunghu with A. nagarum [25]. Among the different PGRs used, Kn and IAA were found to be best for direct seed germination, embryogenic callus induction, and multiple shoot regeneration in A. violaceum. Seeds stored at lower temperatures (1 °C to 6 °C) showed a better rate of germination during an in vitro culture. Similarly, seeds from A. heterophyllum that have chilled for 25–40 days showed a germination rate of 60% [38]. These studies suggest that cold storage and cold-frame sowing or culturing of A. violaceum seeds showed a high frequency of germination. Other studies have also suggested that a low temperature is the most significant factor in the germination and in vitro propagation of Aconitum spp, which was also verified in the current study. The proposed in vitro regeneration will be helpful in the domestication of understudied plant species at lower altitudes. In its natural habitat, A. violaceum regenerates with rhizomes and the new stocks grow healthy and vigorous. The seed also has the potential to germinate into new seedlings; however, the plantlets of germinated seeds are delicate and susceptible to environmental fluctuation and rarely reach maturity stage. Most of the seeds face juvenile mortality at early stages due to extreme diurnal climatic fluctuation in their habitat.
The key rationale is that plants reproduced from direct somatic embryogenesis are commonly more uniform than plants regenerated indirectly by callus tissues [39]. Secondary embryogenesis approaches allow for the rapid production of enormous populations in a short period of time [40]. Secondary somatic embryos could also be developed from the surfaces of somatic embryos [41]. In the current study, somatic embryos were generated from immature and mature seed cultures of A. violaceum. Prior to culture on the growth medium, all seeds were stored at a low temperature (1 °C to 6 °C). The seeds cultured in winter and early spring produced a nodular mass of embryogenic potential callus on the MS basal medium enriched with various concentrations of auxins and cytokinins. Similar findings were observed by Vandelook et al. [42] in Aconitum lycoctonum, where a low temperature (below 10 °C) was suitable for the growth and germination of embryos. Giri et al. [24] developed complete plantlets from somatic embryos of leaf and petiole explants of Aconitum heterophyllum on the MS basal media augmented with 1 mg L−1 2,4-D and 0.5 mg L−1 Kn, or 5 mg L−1 NAA and 1 mg L−1 BAP. Among the various growth regulators studied, Kn and IAA were found to be the most effective for the direct production of an embryogenic callus from immature and mature seed cultures. At lower concentrations, Kn alone was able to induce a nodular mass of embryogenic callus, but the same was insufficient for the conversion of somatic embryos into complete plantlets. However, Kn in combination with IAA converts embryogenic calluses into somatic embryos and facilitates the regeneration of complete plantlets. Similarly, Kn also enhanced embryogenic callus induction in Drimiopsis kirkii [43], and in Iris species such as Iris sanguinea [44,45]. The addition of IAA in the range of 0.1–1.0 mg L−1 accelerated the rate of somatic embryo germination, which eventually reached to 68.00 ± 1.52%. The dark conditions promote somatic embryo formation and development of roots in in A. violaceum. Similar results were reported by Xu Kd [28] in Ranunculus sceleratus, where darkness enhanced the frequency of somatic embryo formation. Cold storage can enhance somatic embryo conversion frequency, potentially caused by epigenetic changes triggered by temperature stress [46]. Moreover, in vitro culturing of seeds on the MS basal medium fortified with various concentrations of 2,4-D induced callusing. Increasing the concentration of 2,4-D to >1.0 mg L−1 resulted in a decrease in the percentage of callus induction, which is consistent with the findings of Li et al. [47,48], who found that increasing the concentration of 2,4-D from 11.3 to 18 µM decreased callus induction in Rosa hybrida. Lower concentrations of PGRs and a low temperature promotes optimal growth and development of in vitro culture of A. violaceum. From the current investigation, we observed that the optimal ambient temperature in the growth chamber should be maintained at 8 °C–10 °C, and relative humidity of 50–55% should be maintained for complete regeneration of plantlets through somatic embryogenesis. Moreover, pre-soaking in cold water for 96 h enhances the rate of seed germination, embryogenic callus production, and other organogenic responses. Optimal application of Kn along with IAA is sufficient for direct seed germination, multiple shoot induction, somatic embryo induction, and complete plant regeneration from somatic embryos in a short time. In the tissue culture-raised plantlets, the mortality rate was highest in the juvenile stage. The acclimatized plantlets required slightly acidic, porous, and loamy soil for successful establishment. The plantlets grew well in semi-shaded places with temperature fluctuations ranging from 10 °C to 20 °C. A further rise in temperature would not be feasible for the survival of A. violaceum.
5. Conclusions
The present study is the first to report on the development of an in vitro propagation protocol for seed germination and somatic embryo formation from seeds of the threatened endemic plant species, A. violaceum. Furthermore, the study revealed that seeds are suitable explants for efficient multiplication and restoration of A. violaceum within a short period of time (approximately three to five months), starting from the initiation of seed germination or somatic embryo development to final tissue culture-raised plantlets. The regeneration protocols established here could be useful for mass multiplication and conservation of this important economic plant species. In addition, this work may be useful in the discovery of physiologically active secondary metabolites from in vitro-derived plantlets under controlled circumstances and their commercial utilization.
Author Contributions
Conceptualization, A.H. and S.S.; methodology, A.H., S.S., S.R. and I.A.N.; software, A.H., S.S., I.A.N., S.R. and N.A.W.; validation, A.H. and S.S.; formal analysis, A.H., S.S. and S.R; investigation, A.H., S.S. and S.R.; resources, E.A.M., D.O.E.-A., H.S., R.C., K.Y. and H.O.E.; data curation, writing—original draft preparation, A.H., S.S., I.A.N. and N.A.W.; writing—review and editing, A.H., S.S., I.A.N. and S.R.; funding acquisition, A.H., S.S., I.A.N., N.A.W., E.A.M., D.O.E.-A., H.S., R.C., K.Y. and H.O.E. All authors have read and agreed to the published version of the manuscript.
Funding
King Saud University (RSP-2021/118).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The first author acknowledges the Council of Scientific and Industrial Research (CSIR), Pusa, New Delhi, for providing financial assistance as SRF during this study. The authors extend their deep appreciation to the Researchers Supporting Project (RSP-2021/118), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ved, D.; Saha, D.; Ravikumar, K.; Haridasan, K. Aconitum violaceum . In The IUCN Red List of Threatened Species; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2015; p. e.T50126562A79581679. [Google Scholar] [CrossRef]
- Kala, C.P. Status and conservation of rare and endangered medicinal plants in the Indian Trans-Himalaya. Biol. Conserv. 2000, 93, 371–379. [Google Scholar] [CrossRef]
- Chaudhary, L.B.; Rao, R.R. Notes on the genus Aconitum L. (Ranunculaceae) in North West Himalaya (India). Feddes Repert. 1998, 109, 527–537. [Google Scholar] [CrossRef]
- Hadi, A.; Singh, S.; Nawchoo, I.A.; Rafiq, S.; Ali, S. Impacts of Habitat Variability on the Phenotypic Traits of Aconitum Violaceum Jacq. Ex Stapf. At different altitudes and environmental conditions in the Ladakh Himalaya, India. Plant Sci. Today 2022, 9. [Google Scholar] [CrossRef]
- CAMP. Threat assessment and management priorities of selected medicinal plants of Western Himalayan states, India. In Proceedings of the Conservation Assessment of Medicinal Plants Workshop, Shimla, Banglore, India, 22–26 May 2003. [Google Scholar]
- Anonymous. The wealth of India. Dictionary of Indian raw material and industrial products. In Raw Materials; National Institute of Science Communication and Information resources (NISCAIR): New Delhi, India, 1998; Volume I CSIR, p. 253. [Google Scholar]
- Sabir, S.; Arshad, M.; Hussain, M.; Sadaf, H.M.; Sohail; Imran, M.; Yasmeen, F.; Saboon; Chaudhari, S.K. A probe into biochemical potential of Aconitum violaceum: A medicinal plant from Himalaya. Asian Pac. J. Trop. Dis. 2016, 6, 502–504. [Google Scholar] [CrossRef]
- Hadi, A.; Singh, S.; Ali, S.; Mehdi, M. Traditional uses of medicinal plants by indigenous tribes of Ladakh union territory. Res. J. Agril. Sci. 2022, 13, 68–77. [Google Scholar]
- Pandey, M.R. Use of medicinal plants in traditional Tibetan therapy system in upper Mustang, Nepal. Our Nat. 2006, 4, 69–82. [Google Scholar] [CrossRef]
- Ameri, A. The effects of Aconitum alkaloids on the central nervous system. Prog. Neurobiol. 1998, 56, 211–235. [Google Scholar] [CrossRef]
- Bhattarai, S.; Chaudhary, R.P.; Quave, C.L.; Taylor, R.S.L. The use of medicinal plants in the trans-Himalayan arid zone of Mustang district, Nepal. J. Ethnobiol. Ethnomed. 2010, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Lone, P.A.; Bhardwaj, A.K.; Shah, K.W.; Tabasum, S. Ethnobotanical survey of some threatened medicinal plants of Kashmir Himalaya, India. J. Med. Plant Res. 2014, 8, 1362–1373. [Google Scholar]
- Gairola, S.; Sharma, J.; Bedi, Y.S. A cross-cultural analysis of Jammu, Kashmir and Ladakh (India) medicinal plant use. J. Ethnopharmacol. 2014, 155, 925–986. [Google Scholar] [CrossRef]
- Khan, F.A.; Khan, S.; Khan, N.M.; Khan, H.; Khan, S. Antimicrobial and antioxidant role of the aerial parts of Aconitum violaceum. J. Mex. Chem. Soc. 2021, 65, 84–93. [Google Scholar] [CrossRef]
- Yadav, S.; Verma, D.L. Acylated flavonol glycosides from the flowers of Aconitum violaceum Staph. Nat. Sci. 2010, 8, 239–243. [Google Scholar]
- Miana, G.A.; Ikram, M.; Khan, M.I.; Sultana, F. Alkaloids of Aconitum violaceum. Phytochemistry 1971, 10, 3320–3322. [Google Scholar] [CrossRef]
- Kunwar, R.M.; Shrestha, K.P.; Bussmann, R.W. Traditional herbal medicine in Far-west Nepal: A pharmacological appraisal. J. Ethnobiol. Ethnomed. 2010, 6, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uprety, Y.; Asselin, H.; Boon, E.K.; Yadav, U.; Shrestha, K.K. Indigenous use and bio-efficacy of medicinal plants in the Rasuwa District, Central Nepal. J. Ethnobiol. Ethnomed. 2010, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Dall’Acqua, S.; Shrestha, B.B.; Gewali, M.B.; Jha, P.K.; Carrara, M.; Innocenti, G. Diterpenoid alkaloids and phenol glycosides from Aconitum naviculare (Bruhl) Stapf. Nat. Prod. Commun. 2008, 3, 1985–1989. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.M.; Yan, L.Y.; He, H.Y.; Wei, X.M. Norditerpenoid alkaloids from Aconitum spicatum Stapf. J. Integr. Plant Biol. 2006, 48, 364–369. [Google Scholar] [CrossRef]
- Mir, A.H.; Tyub, S.; Kamili, A.N. Ecology, distribution mapping and conservation implications of four critically endangered endemic plants of Kashmir Himalaya. Saudi J. Biol. Sci. 2020, 27, 2380–2389. [Google Scholar] [CrossRef]
- Rafiq, S.; Wagay, N.A.; Bhat, I.A.; Kaloo, Z.A.; Rashid, S.; Lin, F.; El-Abedin, T.K.Z.; Wani, S.H.; Mahmoud, E.A.; Almutairi, K.F.; et al. In vitro Propagation of Aconitum chasmanthum Stapf Ex Holmes: An Endemic and Critically Endangered Plant Species of the Western Himalaya. Horticulturae 2021, 7, 586. [Google Scholar] [CrossRef]
- Singh, M.; Chettri, A.; Pandey, A.; Sinha, S. In vitro propagation and phytochemical assessment of Aconitum ferox Wall: A threatened medicinal plant of Sikkim Himalaya. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 90, 313–321. [Google Scholar] [CrossRef]
- Giri, A.; Ahuja, P.S.; Ajay Kumar, P.V. Somatic embryogenesis and plant regeneration from callus cultures of Aconitum heterophyllum Wall. Plant Cell Tissue Organ Cult. 1993, 32, 213–218. [Google Scholar] [CrossRef]
- Deb, C.R.; Langhu, T. Development of in vitro propagation protocol of Aconitum nagarum Stapf. Plant Cell Biotechnol. Mol. Biol. 2017, 18, 324–332. [Google Scholar]
- Mou, Z.; Ye, F.; Shen, F.; Zhao, D. In vitro Germination and Micropropagation of Aconitum vilmorinianum: An Important Medicinal Plant in China. Python-Int. J. Exp. Bot. 2022, 1–18. [Google Scholar] [CrossRef]
- Hatano, K.; Shoyama, Y.; Nishioka, I. Somatic embryogenesis and plant regeneration from the anther of Aconitum carmichaeli Debx. Plant Cell Rep. 1987, 6, 446–448. [Google Scholar] [CrossRef]
- Xu, K.; Wang, W.; Yu, D.; Li, X.-L.; Chetn, J.M.; Feng, B.J.; Zhao, Y.W.; Cheng, M.J.; Liu, X.X.; Li, C.W. NAA at a high concentration promotes efficient plant regeneration via direct somatic embryogenesis and SE-mediated transformation system in Ranunculus sceleratus. Sci. Rep. 2020, 9, 18321. [Google Scholar] [CrossRef] [Green Version]
- Rawat, J.M.; Agnihotri, R.K.; Nautiyal, S.; Rawat, B.; Chandra, A. In vitro propagation, genetic and secondary metabolite analysis of Aconitum violaceum Jacq.: A threatened medicinal herb. Acta Physiol. Plant. 2013, 35, 2589–2599. [Google Scholar] [CrossRef]
- Darrudi, R.; Hassandokht, M.R.; Nazeri, V. Effects of KNO3 and CaCl2 on seed germination of Rheum khorasanicum B. Baradaran & A. Jafari. J. Appl. Sci. Res. 2014, 10, 171–175. [Google Scholar]
- Nidhi, S.; Vikas, S.; Barkha, K.; Dobriyal, A.K.; Jadon, V.S. Advancement in research on Aconitum sp. (Ranunculaceae) under different area: A review. Biotechnology 2010, 9, 411–427. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.K.; Sharma, S.; Sharma, S.S. Seed germination behaviour of some medicinal plants of Lahaul and Spiti cold desert (Himachal Pradesh): Implications for conservation and cultivation. Cur. Sci. 2006, 90, 1113–1118. [Google Scholar]
- Srivastava, N.; Sharma, V.; Kamal, B.; Jadon, V.S. Aconitum: Need for sustainable exploitation (with special reference to Uttarakhand). Int. J. Green Pharm. 2010, 4, 220–228. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Dong, K.H.; Yang, J.F. Efects of diferent treatment methods on seed germination of Iris lactea Pall. Anim. Husb. Feed. Sci. 2013, 34, 23. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Deep complex morphophysiological dormancy in seeds of the mesic woodland herb Delphinium tricorne (Ranunculaceae). Int. J. Plant Sci. 1994, 155, 738–743. [Google Scholar] [CrossRef]
- Walck, J.L.; Baskin, C.C.; Baskin, J.M. Seeds of Thalictrum mirabile (Ranunculaceae) require cold stratification for loss of non- deep simple morphophysiological dormancy. Can. J. Bot. 1999, 77, 1769–1776. [Google Scholar] [CrossRef]
- Forbis, T.A.; Floyd, S.K.; DeQueiroz, A. The evolution of embryo size in angiosperms and other seed plants: Implications for the evolution of seed dormancy. Evolution 2002, 56, 2112–2125. [Google Scholar] [CrossRef]
- Paramanick, D.; Panday, R.; Shukla, S.S.; Sharma, V. Primary pharmacological and other important findings on the medicinal plant “Aconitum heterophyllum” (aruna). J. Pharmacopunct. 2017, 20, 89. [Google Scholar] [CrossRef] [Green Version]
- Maheswaran, G.; Williams, E.G. Direct somatic embryoid formation in immature embryos of Trifolium repens, T. pretense and Medicago sativa, and rapid clonal propagation of T. repens. Ann. Bot. 1984, 54, 201–211. [Google Scholar] [CrossRef]
- Joseph, R.; Yeoh, H.H.; Loh, C.S. Induced mutations in cassava using somatic embryos and the identification of mutant plants with altered starch yield and composition. Plant Cell Rep. 2004, 23, 91–98. [Google Scholar] [CrossRef]
- Chen, J.L.; Beversdorf, W.D.A. Combined use of microprojectile bombardment and DNA imbibition enhances transformation frequency of canola (Brassica napus L.). Theoret. Appl. Genet. 1994, 88, 187–192. [Google Scholar] [CrossRef]
- Vandelook, F.; Lenaerts, J.; Jozef, A.V.A. The role of temperature in post dispersal embryo growth and dormancy break in seed of Aconitum lycoctomum L. Flora-Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 536–542. [Google Scholar] [CrossRef]
- Lan, T.H.; Hong, P.I.; Huang, C.C.; Chang, W.C.; Lin, C.S. High-frequency direct somatic embryogenesis from leaf tissues of Drimiopsis kirkii Baker (giant squill). Vitr. Cell. Dev. Biol. Plant 2009, 45, 44–47. [Google Scholar] [CrossRef]
- Jevremovic, S.; Jeknic, Z.; Subotic, A. Micropropagation of Iris sp. Methods Mol. Biol. 2013, 11013, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Boltenkov, E.V.; Zarembo, E.V. In vitro regeneration and callogenesis in tissue culture of foral organs of the genus Iris (Iridaceae). Biol. Bull. 2005, 32, 138. [Google Scholar] [CrossRef]
- Rhie, Y.H.; Lee, S.Y. Seed dormancy and germination of Epimedium koreanum Nakai. Sci. Hortic. 2020, 272, 109600. [Google Scholar] [CrossRef]
- Montalban, I.; Garcia-Mendiguren, O.; Goicoa, T.; Ugarte, M.; Moncalean, P. Cold storage of initial plant material afects positively somatic embryogenesis in Pinus radiata. New For. 2015, 46, 309–317. [Google Scholar] [CrossRef]
- Li, X.; Krasnyanski, S.; Korban, S.S. Somatic embryogenesis, secondary somatic embryogenesis, and shoot organogenesis in Rosa. J. Plant Physiol. 2002, 159, 313–319. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).