Foliar Application of Selenium under Nano Silicon on Artemisia annua: Effects on Yield, Antioxidant Status, Essential Oil, Artemisinin Content and Mineral Composition
Abstract
1. Introduction
2. Material and Methods
2.1. Growing Conditions and Experimental Protocol
2.2. Colloidal Solution of Silicon Nanoparticles
2.3. Sample Preparation
2.4. Dry Matter
2.5. Nitrates
2.6. Elemental Composition
2.7. Ascorbic Acid
2.8. Photosynthetic Pigments
2.9. Total Polyphenols (TP)
2.10. Antioxidant Activity (AOA)
2.11. Artemisinin
2.12. Proline
2.13. Malonic Dialdehyde
2.14. Selenium
2.15. Essential Oil
2.16. Statistical Analysis
3. Results and Discussion
3.1. Morphological Characteristics
3.2. Biochemical Characteristics
3.3. Photosynthetic Pigments
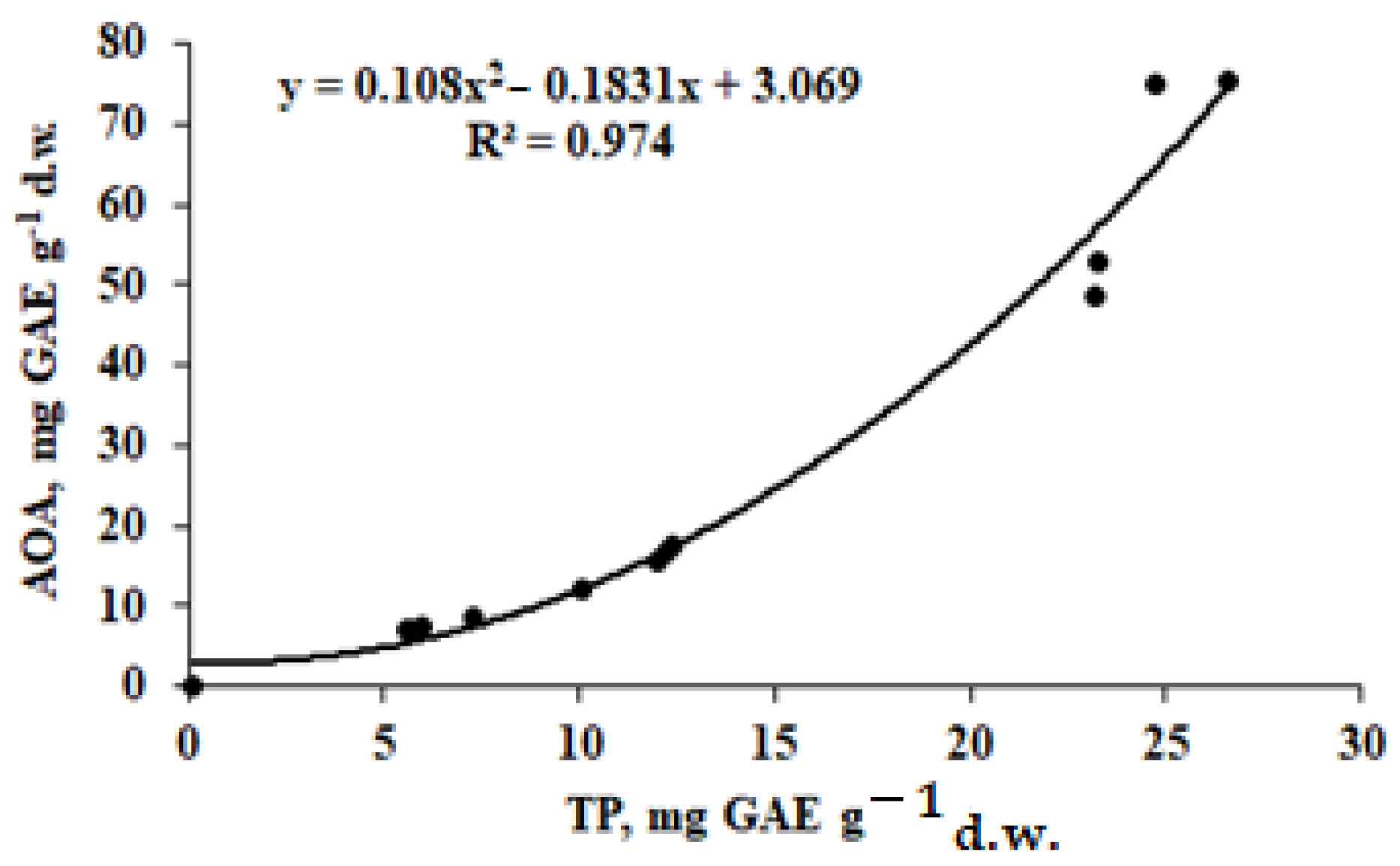
3.4. Antioxidant Status
3.5. Essential Oil Accumulation
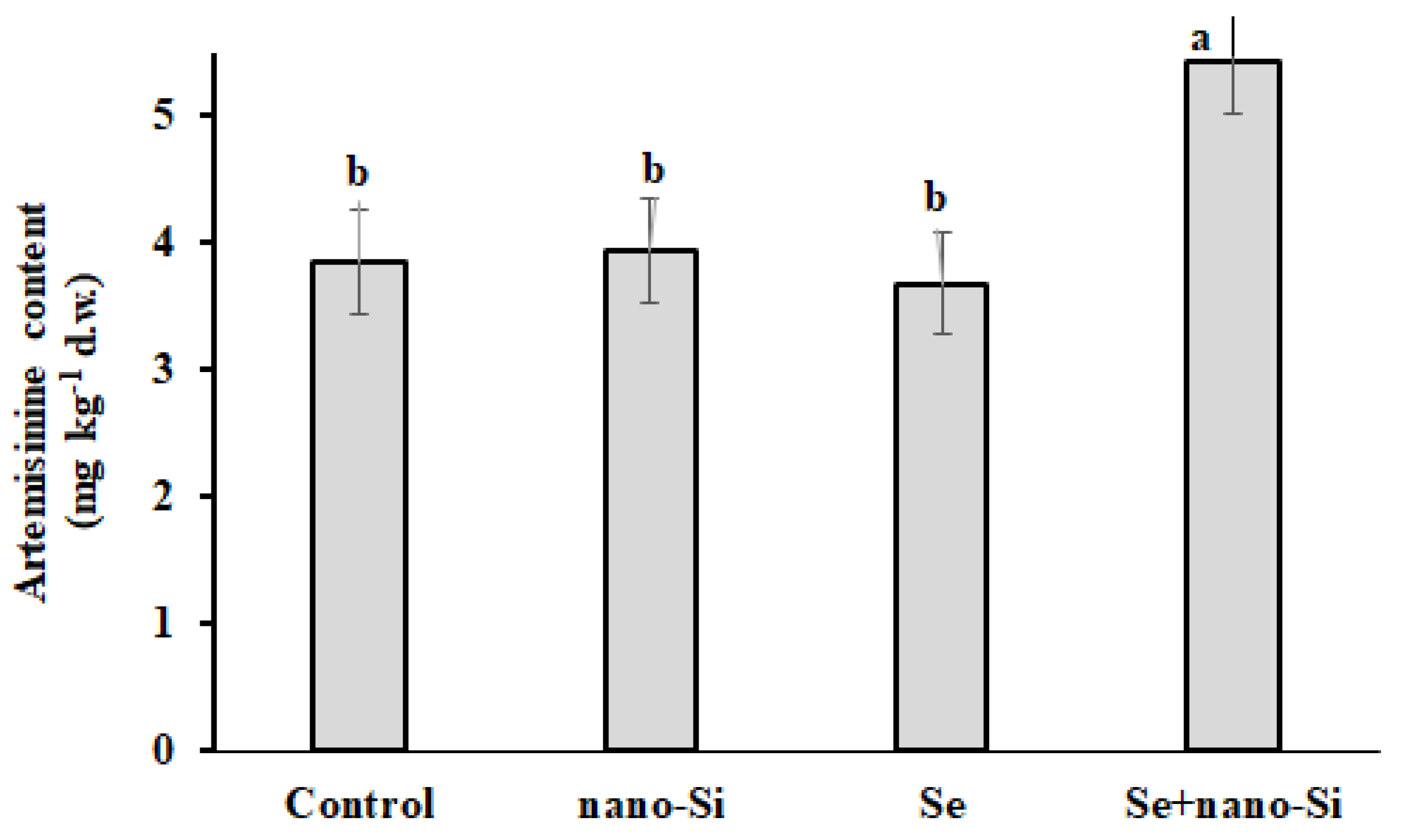
3.6. Artemisinin Accumulation
3.7. Mineral Composition
3.7.1. Se Accumulation
3.7.2. Other Elements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, W.E.; Peh, H.Y.; Chan, T.K.; Wong, W.S. Artemisinins: Pharmacological actions beyond anti-malarial. Pharmacol. Ther. 2014, 142, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.; Yoshida, M.; Davis, C.E.; Greenhill, N.S.; Davis, P.F. An extract of the medicinal plant Artemisia annua modulates production of inflammatory markers in activated neutrophils. J. Inflamm. Res. 2015, 8, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, A.; Hayat, M.Q.; Ashraf, M. Ethnopharmacology of Artemisia annua L.: A Review. In Artemisia Annua—Pharmacology and Biotechnology; Aftab, T., Ferreira, J., Khan, M., Naeem, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Oh, S.-I.; Kim, J.-S.; Kim, C.-K.; Yi, S.S.; Kim, S.-J.; Park, S.-K. Artemisia annua increases resistance to heat and oxidative stresses, but has no effect on lifespan in Caenorhabditis elegans. Food Sci. Technol. 2016, 36, 356–361. [Google Scholar] [CrossRef][Green Version]
- Fatima, K.; Abbas, S.R.; Zia, M.; Sabir, S.M.; Khan, R.T.; Khan, A.A.; Hassan, Z.; Zaman, R. Induction of secondary metabolites on nanoparticles stress in callus culture of Artemisia annua L. Braz. J. Biol. 2021, 81, 474–483. [Google Scholar] [CrossRef]
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Sweeney, E.M.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Salehi, M.; Karimzadeh, G.; Naghavi, M.R.; Badi, H.N.; Monfared, S.R. Expression of key genes affecting artemisinin content in five Artemisia species. Sci. Rep. 2018, 8, 12659. [Google Scholar] [CrossRef]
- Lu, F.; He, X.L.; Culleton, R.; Cao, J. A brief history of artemisinin: Modes of action and mechanisms of resistance. Chin. J. Nat. Med. 2019, 17, 331–336. [Google Scholar] [CrossRef]
- Wani, K.I.; Choudhary, S.; Zehra, A.; Naeem, M.; Weathers, P.; Aftab, T. Enhancing artemisinin content in and delivery from Artemisia annua: A review of alternative, classical, and transgenic approaches. Planta 2021, 254, 29. [Google Scholar] [CrossRef]
- Li, J.; Zhou, B. Biological Actions of Artemisinin: Insights from Medicinal Chemistry Studies. Molecules 2010, 15, 1378–1397. [Google Scholar] [CrossRef]
- Klayman, D.L. Artemisia annua: From weed to respectable antimalarial plant. In Human Medicinal Agents from Plants; Kinghom, A.D., Balandrin, M.F., Eds.; American Chemical Society Symposium Series; ACS Publications: Washington, DC, USA, 1993; pp. 242–255. [Google Scholar]
- Dite, Z.; Suvada, R.; Toth, T.; Jun, P.E.; Pis, V.; Dite, D. Current Condition of Pannonic Salt Steppes at Their Distribution Limit: What Do Indicator Species Reveal about Habitat Quality? Plants 2021, 10, 530. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Alamgir, K.; Pan, L.; Zhu, Y.; Yang, X. Global assessment of the distribution and conservation status of key medicinal plants (Artemisia annua L.): The roles of climate and anthropogenic activities. Sci. Total Environ. 2022, 821, 153378. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Rakoto, M.L.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a Traditional Plant Brought to Light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef] [PubMed]
- Namuli, A.; Bazira, J.; Casim, T.U.; Engeu, P.O. A review of various efforts to increase artemisinin and other antimalarial compounds in Artemisia annua L plant. Cogent Biol. 2018, 4, 1513312. [Google Scholar] [CrossRef]
- Todeschini, V.; Anastasia, F.; Massa, N.; Marsano, F.; Cesaro, P.; Bona, E.; Gamalero, E.; Oddi, L.; Lingua, G. Impact of Phosphatic Nutrition on Growth Parameters and Artemisinin Production in Artemisia annua Plants Inoculated or Not with Funneliformis mosseae. Life 2022, 12, 497. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, L.; Peng, M.; Wu, X.; Li, F.; Wang, Z. Effects of zinc manganese and boron on artemisinin and yields of Artemisia annua Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi. China J. Chin. Mater. Medica 2010, 5, 275–278. [Google Scholar]
- Aftab, T.; Khan, M.M.A.; Idrees, M.; Naeem, M.; Hashmi, N. Moinuddin Effect of Salt Stress on Growth, Membrane Damage, Antioxidant Metabolism and Artemisinin Accumulation in Artemisia annua L. Plant Stress 2010, 4, 3643. [Google Scholar]
- Rostkowska, C.; Mota, C.; Oliveira, T.C.; Santiago, F.M.; Oliveira, L.A.; Korndörfer, G.H.; Lana, R.M.Q.; Rossi, M.; Mogueira, N.L.; Simonnet, X.; et al. Si-Accumulation in Artemisia annua Glandular Trichomes Increases Artemisinin Concentration, but Does Not Interfere in the Impairment of Toxoplasma gondii Growth. Front. Plant Sci. 2016, 7, 1430. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Kharchenko, V.A.; Caruso, G. Selenium: Prospects of functional food production with high antioxidant activity. In Chapter 7 in Reference Series in Phyto-Chemistry. Plant Antioxidants and Health; Ekiert, H., Ramawat, K.G., Arora, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 149–176. [Google Scholar]
- Golubkina, N.A.; Kosheleva, O.V.; Krivenkov, L.V.; Dobrutskaya, H.G.; Nadezhkin, S.M.; Caruso, G. Intersexual differences in plant growth, yield, mineral composition and antioxidants of spinach (Spinacia oleracea L.) as affected by selenium form. Sci. Hortic. 2017, 225, 350–358. [Google Scholar] [CrossRef]
- Golubkina, N.; Zayachkovsky, V.; Sheshnitsan, S.; Skrypnik, L.; Smirnova, T.; Antoshkina, M.; Fedotov, M.; Caruso, G. Prospects of garlic extracts, selenium and silicon application for plants protection against herbivory. Review. Agriculture 2022, 12, 64. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef]
- Golubkina, N.; Moldovan, A.; Fedotov, M.; Kekina, H.; Kharchenko, V.; Folmanis, G.; Alpatov, A.; Caruso, G. Iodine and Selenium Biofortification of Chervil Plants Treated with Silicon Nanoparticles. Plants 2021, 10, 2528. [Google Scholar] [CrossRef] [PubMed]
- Association Official Analytical Chemists (AOAC). The Official Methods of Analysis of AOAC International; 22 Vitamin C.; AOAC: Rockville, MD, USA, 2012. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Golubkina, N.A.; Kekina, H.G.; Molchanova, A.V.; Antoshkina, M.S.; Nadezhkin, S.M.; Soldatenko, A.V. Plants Antioxidants and Methods of Their Determination; INFRA-M: Moscow, Russia, 2020. [Google Scholar] [CrossRef]
- Numonov, S.; Sharopov, F.; Salimov, A.; Sukhrobov, P.; Atolikshoeva, S.; Safarzoda, R.; Habasi, M.; Aisa, H.A. Assessment of Artemisinin Contents in Selected Artemisia Species from Tajikistan (Central Asia). Medicines 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Ouertani, R.N.; Abid, G.; Karmous, C.; Chikha, M.B.; Boudaya, O.; Mahmoudi, H.; Mejri, S.; Jansen, K.; Ghorbel, A. Evaluating the contribution of osmotic and oxidative stress components on barley growth under salt stress. AoB Plants 2021, 1, plab034. [Google Scholar] [CrossRef]
- Alfthan, G.V. A micromethod for the determination of selenium in tissues and biological fluids by single-test-tube fluorimetry. Anal. Chim. Acta 1984, 165, 187–194. [Google Scholar] [CrossRef]
- Heath, R.L.; Parker, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Golubkina, N.; Kekina, H.; Caruso, G. Yield, Quality and Antioxidant Properties of Indian Mustard (Brassica juncea L.) in Response to Foliar Biofortification with Selenium and Iodine. Plants 2018, 7, 80. [Google Scholar] [CrossRef]
- Sali, A.; Zeka, D.; Fetahu, S.; Rusinovci, I.; Kaul, H.-P. Selenium supply affects chlorophyll concentration and biomass production of maize (Zea mays L.). Die Bodenkult. J. Land Manag. Food Environ. 2008, 69, 249–255. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Feizi, M.; Kumari, A.; Khan, M.; Mandzhieva, S.; Sushkova, S.; El-Ramady, H.; Verma, K.K.; Singh, A.; et al. Effects of Silicon and Silicon-Based Nanoparticles on Rhizosphere Microbiome, Plant Stress and Growth. Biology 2021, 10, 791. [Google Scholar] [CrossRef]
- Siddiqui, H.; Ahmed, K.B.M.; Sami, F.; Hayat, S. Silicon nanoparticles and plants: Current knowledge and future perspectives. In Sustainable Agriculture Reviews 41: Nanotechnology for Plant Growth and Development; Hayat, S., Pichtel, J., Faizan, M., Fariduddin, Q., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 129–142. [Google Scholar]
- Tzenkova, R.; Kamenarska, Z.; Draganov, A.; Atanassov, A. Composition of A. annua essential oil obtained from species growing wild in Bulgaria. Biotechnol. Biotechnol. Equip. 2010, 24, 1833–1835. [Google Scholar] [CrossRef]
- Mukarram, M.; Khan, M.M.A.; Corpas, F.J. Silicon nanoparticles elicit an increase in lemongrass (Cymbopogon flexuosus (Steud.) Wats) agronomic parameters with a higher essential oil yield. J. Hazard. Mater. 2021, 412, 125254. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Santomauro, F.; Sacco, C.; Bergonzi, M.C.; Donato, R. Essential oil of Artemisia annua L.: An extraordinary component with numerous antimicrobial properties. Evid.-Based Complement. Altern. Med. 2014, 2014, 159819. [Google Scholar] [CrossRef]
- Verdian-rizi, M.R.; Sadat-Ebrahimi, E.; Hadjiakhoondi, A.; Fazeli, M.R.; Pirali Hamedani, M. Chemical Composition and Antimicrobial Activity of Artemisia annua L. Essential Oil from Iran. J. Med. Plants 2008, 7, 58–62. [Google Scholar]
- Juteau, F.; Masotti, V.; Bessière, J.M.; Dherbomez, M.; Viano, J. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia 2002, 73, 532–535. [Google Scholar] [CrossRef]
- Golubkina, N.; Logvinenko, L.; Molchanova, A.; Caruso, G. Genetic and Environmental Influence on Macro- and Microelement Accumulation in Plants of Artemisia Species. In Chapter 17 in Plants Micronutrients. Deficiency and Toxicity Management; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2020; pp. 389–416. [Google Scholar]
- Ahmad, B.; Khan, M.M.A.; Jaleel, H.; Shabbir, A.; Sadiq, Y.; Uddin, M. Silicon nanoparticles mediated increase in glandular trichomes and regulation of photosynthetic and quality attributes in Mentha piperita L. J. Plant Growth Regul. 2020, 39, 346–357. [Google Scholar] [CrossRef]
- Afshari, M.; Pazoki, A.; Sadeghipour, O. Foliar-applied Silicon and its Nanoparticles Stimulate Physio-chemical Changes to Improve Growth, Yield and Active Constituents of Coriander (Coriandrum Sativum L.) Essential Oil Under Different Irrigation Regimes. Silicon 2021, 13, 4177–4188. [Google Scholar] [CrossRef]
- Skrypnik, L.; Novikova, A.; Tokupova, E. Improvement of Phenolic Compounds, Essential Oil Content and Antioxidant Properties of Sweet Basil (Ocimum basilicum L.) Depending on Type and Concentration of Selenium Application. Plants 2019, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Mohammadreza, V. Variation in the essential oil composition of Artemisia annua L. of different growth stages cultivated in Iran. Afr. J. Plant Sci. 2008, 2, 16–18. [Google Scholar]
- Coşge, B.; Kiralan, M.; Yaman, C. The Effect of Different Harvest Stages on Chemical Composition and Antioxidant Capacity of Essential Oil from Artemisia annua L. J. Agric. Sci. 2015, 21, 71–77. [Google Scholar] [CrossRef][Green Version]
- Delabays, N.; Simonne, X.; Gaudin, M. The Genetics of Artemisinin Content in Artemisia annua L. and the Breeding of High Yielding Cultivars. Curr. Med. Chem. 2002, 8, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D. The biosynthesis of artemisinin (qinghaosu) and the phytochemistry of Artemisia annua L. (qinghao). Molecules 2010, 15, 7603–7698. [Google Scholar] [CrossRef] [PubMed]
- Domokos, E.; Jakab-Farkas, L.; Darko, B.; Biro-Janka, B.; Mara, G.; Albert, C.; Balog, A. Increase in Artemisia annua plant biomass artemisinin content and guaiacol peroxidase activity using the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front. Plant Sci. 2018, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xie, C.; Duan, B.; Chen, S. Mapping the potential distribution of high artemisinin-yielding Artemisia annua L. (Qinghao) in China with a geographic information system. Chin. Med. 2010, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Widiyastuti, Y.; Subositi, D. Photoperiod effect on the growth and artemisinin content of Artemisia Annua grown in tropical region. In Proceedings of the AIP Conference Proceedings, Yogyakarta, Indonesia, 19–20 October 2018; Volume 2099, p. 020027. [Google Scholar] [CrossRef]
- Verma, R.K.; Chauhan, A.; Verma, R.S.; Gupta, A. Influence of planting date on growth, artemisinin yield, seed and oil yield of Artemisia annua L. under temperate climatic conditions. Ind. Crops Prod. 2011, 34, 860–864. [Google Scholar] [CrossRef]
- Marchese, J.A.; Ferreira, J.F.S.; Rehder, V.L.G.; Rodrigues, O. Water deficit effect on the accumulation of biomass and artemisinin in annual wormwood (Artemisia annua L., Asteraceae). Braz. J. Plant Physiol. 2010, 22, 1–9. [Google Scholar] [CrossRef]
- Marchese, J.A.; Rehder, V.L.G. The influence of temperature in the production of artemisinin in Artemisia annua L. Braz. J. Med. Plant 2001, 4, 89–93. [Google Scholar]
- Marchese, J.A.; Rehder, V.L.G.; Casiraghi, V.; Tedesco, A.C.; Lira, R. Flowering in plants of Artemisia annua L. submitted to different conditions of photoperiod and temperature. Acta Hortic. 2002, 569, 275–280. [Google Scholar] [CrossRef]
- Ferreira, J.F.S. Nutrient deficiency in the production of artemisinin, dihydroartemisinic acid, and artemisinic acid in Artemisia annua L. J. Agric. Food Chem. 2007, 55, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.S.; Karhagomba, I.B.; Hirt, H.M.; Wemakor, E. The potential of Artemisia annua L. as a locally produced remedy for malaria in the tropics: Agricultural, chemical and clinical aspects. J. Ethnopharmacol. 2000, 73, 487–493. [Google Scholar] [CrossRef]
- Łukaszewicz, S.; Politycka, B.; Smoleń, S. Effects of selenium on the content of essential micronutrients and their translocation in garden pea. J. Elem. 2018, 23, 1307–1317. [Google Scholar] [CrossRef]
- Golubkina, N.; Amagova, Z.; Matsadze, V.; Zamana, S.; Tallarita, A.; Caruso, G. Effects of arbuscular mycorrhizal fungi on yield, biochemical characteristics and elemental composition of garlic and onion under selenium supply. Plants 2020, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B. Effect of selenium on selected macronutrients in maize plants. J. Elem. 2008, 13, 513–519. [Google Scholar]
- Golubkina, N.; Gomez, L.D.; Kekina, H.; Cozzolino, E.; Simister, R.; Tallarita, A.; Torino, V.; Koshevarov, A.; Cuciniello, A.; Maiello, R.; et al. Joint Selenium–Iodine Supply and Arbuscular Mycorrhizal Fungi Inoculation Affect Yield and Quality of Chickpea Seeds and Residual Biomass. Plants 2020, 9, 804. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; García-Caparrós, P.; Parvin, K.; Zulfiqar, F.; Ahmed, N.; Fujita, M. Selenium Supplementation and Crop Plant Tolerance to Metal/Metalloid Toxicity. Front. Plant Sci. 2022, 12, 792770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of Nanoparticles Alleviates Heavy Metals Stress and Promotes Plant Growth: An Overview. Nanomaterials 2021, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of silicon on heavy metal uptake at the soil-plant interphase: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium Biofortification and Interaction With Other Elements in Plants: A Review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef] [PubMed]


| Month | 2020 | 2021 | ||
|---|---|---|---|---|
| Mean Temperature °C | Humidity % | Mean Temperature °C | Humidity % | |
| May | 13.5 | 66 | 13.5 | 66 |
| June | 21.0 | 73 | 17.2 | 69 |
| July | 23.81 | 74.0 | 20.1 | 72 |
| August | 17.8 | 72 | 17.8 | 75 |
| September | 11.9 | 80 | 11.9 | 80 |
| Control | Si | Se | Si + Se | |
|---|---|---|---|---|
| Plant height (cm) | 123 ± 15 a | 131 ± 17 a | 127 ± 16 a | 127 ± 15 a |
| Leaf length (cm) | 24 ± 4 b | 25 ± 3 b | 27 ± 4 b | 45 ± 7 a |
| Number of leaves | 14 ± 1 b | 19 ± 2 a | 19 ± 2 a | 20 ± 3 a |
| Leaf weight (g) | 26.03 ± 2.05 c | 50.08 ± 5.10 b | 52.63 ± 5.05 b | 115.14 ± 9.71 a |
| Stem weight (g) | 32.3 ± 2.78 c | 41.94 ± 3.98 b | 39.05 ± 3.04 b | 76.79 ± 8.02 a |
| Leaf weight (% of plant weight) | 44.6 ± 4.0 b | 56.4 ± 4.2 a | 57.4 ± 4.1 a | 60.0 ± 5.0 a |
| Parameter | Plant Part | Control | Nano-Si | Se | Se + Nano-Si |
|---|---|---|---|---|---|
| Dry matter (%) | Leaves | 13.0 ± 1.0 a | 15.0 ± 1.2 a | 13.7 ± 1.1 a | 13.3 ± 1.0 a |
| Stems | 13.4 ± 1.1 a | 15.6 ± 1.3 a | 14.5 ± 1.3 a | 14.3 ± 1.2 a | |
| Roots | 16.6 ± 1.4 a | 17.3 ± 1.5 a | 17.5 ± 1.5 a | 16.3 ± 1.4 a | |
| Nitrates (mg g−1 d.w.) | Leaves | 3.00 ± 0.25 a | 3.04 ± 0.22 a | 2.90 ± 0.21 a | 2.76 ± 0.21 a |
| Stems | 5.38 ± 0.48 a | 4.34 ± 0.35 b | 3.93 ± 0.37 b | 4.69 ± 0.37 ab | |
| Stems/leaves | 1.79 ± 0.03 a | 1.43 ± 0.03 b | 1.36 ± 0.02 b | 1.70 ± 0.04 a | |
| TDS (mg g−1 d.w.) | Leaves | 67.7 ± 6.1 a | 67.2 ± 6.1 a | 73.1 ± 6.9 a | 67.1 ± 6.1 a |
| Stems | 127.7 ± 11.1 a | 97.0 ± 8.8 b | 89.4 ± 8.2 b | 105.5 ± 9.0 ab | |
| Stems/leaves | 1.89 ± 0.06 a | 1.44 ± 0.06 bc | 1.22 ± 0.05 c | 1.57 ± 0.04 b |
| Parameter | Control | Nano-Si | Se | Se + Nano-Si |
|---|---|---|---|---|
| Chl a (mg g−1 f.w.) | 1.32 ± 0.11 b | 1.01 ± 0.09 c | 1.66 ± 0.13 a | 1.45 ± 0.12 ab |
| Chl b (mg g−1 f.w.) | 3.48 ± 0.30 a | 2.73 ± 0.21 b | 3.89 ± 0.32 a | 3.61 ± 0.31 a |
| Total chl (mg g−1 f.w.) | 4.80 ± 0.42 a | 3.74 ± 0.33 b | 5.55 ± 0.50 a | 5.06 ± 0.45 a |
| Carotene (mg g−1 f.w.) | 0.56 ± 0.04 b | 0.49 ± 0.03 b | 0.68 ± 0.06 a | 0.70 ± 0.06 a |
| Chl b/Chl a | 2.64 ± 0.02 a | 2.70 ± 0.02 a | 2.34 ± 0.03 a | 2.49 ± 0.02 a |
| Carotene /total chl (%) | 11.9 ± 0.01 a | 13.1 ± 0.01 a | 12.3 ± 0.01 a | 13.8 ± 0.02 a |
| Tissue | Control | Si | Se | Se + Si | |
|---|---|---|---|---|---|
| AA (mg 100 g−1 f.w.) | Leaves | 74.2 ± 7.1 b | 106.0 ± 8.5 a | 90.6 ± 8.5 a | 106.3 ± 8.2 a |
| AOA (mg GAE g−1 d.w.) | Leaves | 48.7 ± 4.2 b | 75.1 ± 7.0 a | 75.2 ± 7.1 a | 52.7 ± 5.1 b |
| Stems | 12.2 ± 1.2 b | 17.4 ± 1.6 a | 16.6 ± 1.6 a | 15.7 ± 1.5 a | |
| Roots | 7.1 ± 0.7 a | 6.9 ± 0.6 a | 8.5 ± 0.7 a | 7.5 ± 0.7 a | |
| TP (mg GAE g−1 d.w.) | Leaves | 23.2 ± 2.1 a | 24.7 ± 2.2 a | 26.6 ± 2.3 a | 23.3 ± 2.1 a |
| Stems | 10.1 ± 1.0 a | 12.4 ± 1.2 a | 12.2 ± 1.1 a | 12.0 ± 1.1 a | |
| Roots | 5.7 ± 0.5 b | 5.6 ± 0.5 b | 7.3 ± 0.7 a | 6.0 ± 0.6 ab | |
| MDA (mM g−1 d.w.) | Leaves | 0.38 ± 0.04 a | 0.31 ± 0.03 a | 0.35 ± 0.03 a | 0.36 ± 0.03 a |
| Proline (mg g−1 d.w.) | Leaves | 8.14 ± 0.81 ab | 8.21 ± 0.81 ab | 7.67 ± 0.78 b | 9.41 ± 0.91 a |
| Artemisinin (mg kg−1 d.w.) | Leaves | 3.85 ± 0.17 b | 3.94 ± 0.11 b | 3.68 ± 0.14 b | 5.42 ± 0.25 a |
| Parameter | Control | Si | Se | Si + Se |
|---|---|---|---|---|
| Essential oil yield (%) | 0.20 ± 0.01 a | 0.15 ± 0.01 b | 0.15 ± 0.01 b | 0.10 ± 0.01 c |
| Number of components | 81 ± 2 a | 50 ± 1 c | 71 ± 1 b | 72 ± 2 b |
| The Main Oil Components, % | ||||
| Eucalyptol | 3.32 ± 0.30 b | 7.85 ± 0.75 a | 1.83 ± 0.16 c | 3.06 ± 0.29 b |
| Artemisia ketone | 6.28 ± 0.63 c | 23.73 ± 2.33 a | 4.39 ± 0.41 d | 8.36 ± 0.82 b |
| Camphor | 32.83 ± 2.90 a | 34.35 ± 3.04 a | 23.02 ± 2.04 b | 21.61 ± 2.10 b |
| Germacrene D | 9.25 ± 0.91 b | 5.03 ± 0.50 c | 11.77 ± 1.13 a | 10.17 ± 1.02 ab |
| Element | Control | Si | Se | Se + Si |
|---|---|---|---|---|
| Leaves | ||||
| Ca | 10,778 ± 1057 a | 8678 ± 812 b | 6789 ± 628 c | 8253 ± 800 bc |
| K | 75,681 ± 7405 a | 71,512 ± 6998 a | 45,027 ± 4220 b | 52,833 ± 5179 b |
| Mg | 3718 ± 357 a | 2810 ± 277 b | 2743 ± 268 b | 2679 ± 260 b |
| Na | 514 ± 50 b | 438 ± 44 b | 491 ± 48 bc | 677 ± 67 a |
| P | 6177 ± 602 a | 5172 ± 511 ab | 5400 ± 535 a | 5150 ± 506 ab |
| Stems | ||||
| Ca | 9328 ± 901 a | 7006 ± 688 b | 6821 ± 632 b | 8044 ± 795 a |
| K | 78,648 ± 7004 a | 72,945 ± 6987 a | 69,363 ± 6123 a | 75,420 ± 7224 a |
| Mg | 1512 ± 150 a | 1495 ± 145 a | 1129 ± 110 b | 1327 ± 133 a |
| Na | 312 ± 29 a | 314 ± 30 a | 381 ± 34 a | 315 ± 30 a |
| P | 4799 ± 456 a | 3391 ± 329 b | 4571 ± 446 a | 3817 ± 377 b |
| Element | Control | Si | Se | Se + Si |
|---|---|---|---|---|
| Leaves | ||||
| Al | 61.3 ± 6.1 b | 57.2 ± 5.7 b | 128.0 ± 12.8 a | 41.1 ± 4.0 c |
| As | 0.15 ± 0.01 a | 0.15 ± 0.01 a | 0.09 ± 0.01 b | 0.08 ± 0.01 b |
| Cd | 0.46 ± 0.04 a | 0.29 ± 0.03 b | 0.25 ± 0.02 b | 0.20 ± 0.02 c |
| Cr | 0.42 ± 0.04 b | 0.46 ± 0.05 b | 0.62 ± 0.06 a | 0.32 ± 0.03 c |
| Ni | 1.49 ± 0.15 a | 1.17 ± 0.11 b | 1.45 ± 0.14 a | 0.91 ± 0.09 b |
| Pb | 0.66 ± 0.06 a | 0.78 ± 0.07 a | 0.39 ± 0.04 c | 0.53 ± 0.05 b |
| Sr | 13.10 ± 1.30 a | 11.8 ± 1.10 ab | 9.68 ± 0.92 c | 10.82 ± 1.03 bc |
| V | 0.68 ± 0.06 a | 0.75 ± 0.07 a | 0.77 ± 0.07 a | 0.42 ± 0.04 b |
| Stems | ||||
| Al | 104.0 ± 10.4 a | 55.3 ± 5.5 b | 47.4 ± 4.7 b | 45.1 ± 4.5 b |
| As | 0.06 ± 0.01 a | 0.04 ± 0.01 b | 0.03 ± 0.001 c | 0.03 ± 0.001 c |
| Cd | 0.82 ± 0.08 a | 0.87 ± 0.08 a | 0.87 ± 0.09 a | 0.73 ± 0.07 a |
| Cr | 1.45 ± 0.14 b | 2.35 ± 0.22 a | 1.44 ± 0.14 b | 2.99 ± 0.30 a |
| Ni | 1.62 ± 0.16 a | 1.74 ± 0.17 a | 1.23 ± 0.12 b | 1.59 ± 0.15 ab |
| Pb | 3.36 ± 0.33 a | 1.21 ± 0.12 b | 0.83 ± 0.08 c | 0.98 ± 0.10 bc |
| Sr | 28.17 ± 2.80 a | 23.53 ± 2.35 a | 25.94 ± 2.45 a | 25.91 ± 2.51 a |
| V | 0.32 ± 0.03 a | 0.23 ± 0.02 b | 0.14 ± 0.01 c | 0.15 ± 0.01 c |
| Element | Control | Si | Se | Se + Si |
|---|---|---|---|---|
| Leaves | ||||
| B | 54.81 ± 5.46 a | 37.77 ± 3.80 b | 31.95 ± 3.20 bc | 29.69 ± 3.00 c |
| Co | 0.12 ± 0.01 de | 0.14 ± 0.01 cd | 0.10 ± 0.01 e | 0.06 ± 0.01 f |
| Cu | 20.17 ± 2.02 a | 19.76 ± 2.00 a | 16.41 ± 1.62 b | 16.18 ± 1.60 b |
| Fe | 277.0 ± 27.5 a | 240.0 ± 24.1 ab | 216.0 ± 21.3 b | 148.0 ± 14.7 c |
| Li | 0.06 ± 0.01 d | 0.16 ± 0.02 a | 0.14 ± 0.01 b | 0.08 ± 0.01 c |
| Mn | 40.60 ± 4.03 a | 30.01 ± 3.00 b | 25.72 ± 2.55 c | 22.19 ± 2.19 c |
| Mo | 1.22 ± 0.12 b | 2.18 ± 0.22 a | 1.18 ± 0.11 b | 1.26 ± 0.12 b |
| Si | 10.39 ± 1.04 a | 10.11 ± 1.00 a | 10.41 ± 1.02 a | 9.32 ± 0.92 a |
| Zn | 111.0 ± 11.2 a | 114.0 ± 11.3 a | 83.1 ± 8.3 b | 68.1 ± 6.8 c |
| Stems | ||||
| B | <0.021 a | <0.021 a | <0.021 a | <0.021 a |
| Co | 0.23 ± 0.02 a | 0.15 ± 0.01 bc | 0.14 ± 0.01 c | 0.17 ± 0.02 b |
| Cu | 11.34 ± 1.13 a | 9.07 ± 0.90 a | 11.03 ± 0.11 | 9.38 ± 0.09 a |
| Fe | 170.0 ± 17.0 a | 103.0 ± 10.1 b | 77.7 ± 7.7 c | 87.8± 8.7 bc |
| Li | 0.15 ± 0.01 a | 0.15 ± 0.02 a | 0.09 ± 0.01 b | 0.10 ± 0.01 b |
| Mn | 24.09 ± 2.40 a | 21.84 ± 2.12 a | 17.45 ± 1.73 b | 16.73 ± 1.64 b |
| Mo | 0.86 ± 0.08 b | 1.21 ± 0.12 a | 0.99 ± 0.10 ab | 1.04 ± 0.10 ab |
| Si | 9.14 ± 0.91 a | 8.04 ± 0.80 a | 6.64 ± 0.65 b | 7.85 ± 0.78 ab |
| Zn | 96.6 ± 9.6 b | 107.0 ± 10.0 b | 106.0 ± 10.0 b | 159.0 ± 15.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.; Logvinenko, L.; Konovalov, D.; Garsiya, E.; Fedotov, M.; Alpatov, A.; Shevchuk, O.; Skrypnik, L.; Sekara, A.; Caruso, G. Foliar Application of Selenium under Nano Silicon on Artemisia annua: Effects on Yield, Antioxidant Status, Essential Oil, Artemisinin Content and Mineral Composition. Horticulturae 2022, 8, 597. https://doi.org/10.3390/horticulturae8070597
Golubkina N, Logvinenko L, Konovalov D, Garsiya E, Fedotov M, Alpatov A, Shevchuk O, Skrypnik L, Sekara A, Caruso G. Foliar Application of Selenium under Nano Silicon on Artemisia annua: Effects on Yield, Antioxidant Status, Essential Oil, Artemisinin Content and Mineral Composition. Horticulturae. 2022; 8(7):597. https://doi.org/10.3390/horticulturae8070597
Chicago/Turabian StyleGolubkina, Nadezhda, Lidia Logvinenko, Dmitry Konovalov, Ekaterina Garsiya, Mikhail Fedotov, Andrey Alpatov, Oksana Shevchuk, Liubov Skrypnik, Agnieszka Sekara, and Gianluca Caruso. 2022. "Foliar Application of Selenium under Nano Silicon on Artemisia annua: Effects on Yield, Antioxidant Status, Essential Oil, Artemisinin Content and Mineral Composition" Horticulturae 8, no. 7: 597. https://doi.org/10.3390/horticulturae8070597
APA StyleGolubkina, N., Logvinenko, L., Konovalov, D., Garsiya, E., Fedotov, M., Alpatov, A., Shevchuk, O., Skrypnik, L., Sekara, A., & Caruso, G. (2022). Foliar Application of Selenium under Nano Silicon on Artemisia annua: Effects on Yield, Antioxidant Status, Essential Oil, Artemisinin Content and Mineral Composition. Horticulturae, 8(7), 597. https://doi.org/10.3390/horticulturae8070597










