Abstract
Curcuma (turmeric) species are important culinary and medicinal plants, and the essential oils of Curcuma rhizomes have demonstrated promising pharmacological properties. The essential oils (EOs) of Curcuma species possess a wide variety of pharmacological properties, including anti-inflammatory, anticancerous, antiproliferative, hypocholesterolemic, antidiabetic, antirheumatic, hypotensive, antioxidant, antimicrobial, antiviral, antithrombotic, antityrosinase, and cyclooxygenase-1 (COX-1) inhibitory activities, among others. Curcuma oils are also known to enhance immune function, promote blood circulation, accelerate toxin elimination, and stimulate digestion. C. longa (turmeric) and C. zedoaria (zedoary) are the most extensively studied species of Curcuma due to their high commercial value. There is some interest in expanding the cultivation of Curcuma species to the southern regions in North America where the climate is favorable. The purpose of this work was to examine the rhizome essential oil composition of four species of Curcuma (C. aromatica, C. caesia, C. longa, C. zanthorrhiza) that were obtained from Vietnam and cultivated in North Alabama. The rhizome essential oils were obtained by hydrodistillation and analyzed by gas chromatographic techniques. The essential oils of C. aromatica were dominated by curzerenone (14.7–18.6%), germacrone (10.7–14.7%), 1,8-cineole (5.2–11.7%), and an unidentified component (8.7–11.0%). The major components in C. longa rhizome oil were ar-turmerone (8.3–36.1%), α-turmerone (12.7–15.2%), β-turmerone (5.0–15.4%), α-zingiberene (4.6–13.9%), and β-sesquiphellandrene (4.6–10.0%). The essential oils of C. caesia and C. zanthorrhiza were rich in curzerenone, curdione, and germacrone. These adapted turmeric varieties in North Alabama have potential use for medical purposes and medicinal plant oil market demands in the U.S.
1. Introduction
There are currently 93 recognized species of Curcuma L. (Zingiberaceae) [1]. These perennial rhizomatous herbs originated in subtropical and tropical areas of Asia, Australia, and South America [2], and a number of Curcuma species are cultivated in large scales in India, Nepal, Pakistan, Bangladesh, Indonesia, Malaysia, and Thailand [3]. Curcuma species are herbaceous perennial herbs with tuberous rhizomes (underground stems). Among them, some important species such as Curcuma amada Roxb. (mango ginger), Curcuma angustifolia Roxb. (wild arrowroot), C. aromatica Salisb. (wild turmeric), Curcuma caesia Roxb. (black turmeric), Curcuma decipiens Dalzell, Curcuma kwangsiensis S. G. Lee & C. F. Liang, Curcuma longa L., Curcuma montana Roxb., Curcuma ochrorhiza Valeton, Curcuma pierreana Gagnep., Curcuma roscoeana Wall., and Curcuma zedoaria (Christm.) Roscoe (zedoary) have economical value as they are used in medicine, cosmetics, and both the floricultural and culinary industries [4]. Turmeric is mainly used for culinary, medicinal, and aromatic purposes. Its rhizomes are the ancient colorful spice source and have a bitter and pungent taste and a pepper-like aroma. Turmeric is also known as the “Golden Spice of India” [5] or “Kitchen Queen” [6]. For example, it has been used in curries in India; in Japan and Korea it is popularly served as a herbal tea; and it is used as a preservative and a coloring agent in mustard sauce, cheese, butter, and chips in the western world [7]. Curcuminoids and the essential oil of turmeric are associated with a myriad of medicinal, culinary and industrial properties of curcuma species [8], which are derived from the underground plant part, rhizomes (actually the stem), which are tuberous, with a rough and segmented skin. The primary rhizome is known as “mother rhizome” or bulb, and is pear-shaped in the center (Figure 1). The branches of mother rhizomes are the secondary rhizomes, called lateral or “finger rhizomes” [9].

Figure 1.
Rhizomes of Vietnamese Curcuma species cultivated in North Alabama: Curcuma aromatica (green rhizome, CA22; white rhizome, CA46), Curcuma caesia (black rhizome, CC38), Curcuma zanthorrhiza (lime rhizome, CZ44), Curcuma longa (yellow rhizome, CL56; red rhizome, CL63). Photographs taken by Lam Duong.
Though turmeric has been known for its multiple uses for over 4000 years in India [10], its use as a medicinal and health supplement in the United States is of recent origin. The interest in turmeric in the U.S. has been increasing over the past two decades mainly due to a large number of scientific publications on its medicinal benefits [8]. To meet the growing demand for turmeric, the U.S. imports 90% of its market demand mainly from India. The U.S. import market was estimated at USD 87.28 million in 2018 [11]. The large market for turmeric in the United States suggests that there is opportunity for cultivation of turmeric in this country provided varieties with high curcumin yield and desirable essential oil composition are available.
Curcuma aromatica Salisb. (wild turmeric) is found naturally in South Asia, including southern China, Bhutan, Myanmar, India, Nepal, Sri Lanka [12], and Vietnam [13], and is widely cultivated in China, India, and Japan [14]. The plant is used in traditional medicines throughout its range for its wound-healing, anti-inflammatory, anti-tumor, immunomodulatory, antimicrobial effects and as an antidote for snake venom [15,16,17]. The rhizome essential oils are generally dominated by camphor, curzerenone, germacrone, curdione, and 1,8-cineole [14].
Curcuma caesia Roxb. (black turmeric) grows wild in northeastern and central India, Malaysia, Thailand, and Indonesia [14,18]. The rhizome of C. caesia has been used as a traditional medicine to treat leprosy, bronchitis, asthma, cancer, epilepsy, fever, wounds, impotence, fertility, vomiting, and pain [19]. Curcuma caesia is considered to be endangered in its native range in India [18], however, it has been underexplored in terms of cultivation and commercialization [20]. The major components in the rhizome essential oil of C. caesia from northeastern India were camphene, 1,8-cineole, camphor, borneol, (E)-β-caryophyllene, and ar-turmerone, which defined two chemotypes, a camphor/ar-turmerone chemotype and a 1,8-cineole/(E)-β-caryophyllene chemotype [20].
Curcuma longa L. (turmeric) is cultivated worldwide, especially in tropical countries in Asia, Australia, and the Neotropics [9]. It is a well-known medicinal agent and culinary ingredient. In addition to curcumin and other non-volatile curcuminoids, the essential oil of turmeric has been employed in the treatment of various maladies in humans and animals [21]. Turmeric essential oils are made up of hundreds of components and the major components, however, are α-turmerone, β-turmerone, ar-turmerone, β-sesquiphellandrene, α-zingiberene, germacrone, terpinolene, ar-curcumene, and α-phellandrene [3,22].
Curcuma zanthorrhiza Roxb. (Javanese turmeric) is often referred to in the literature as Curcuma xanthorrhiza Roxb., however, that name is not recognized by World Flora Online [23]. The plant is native to Indonesia, although is also cultivated in Malaysia, the Philippines, Thailand, Vietnam, and to a lesser extent in China, India, Japan, and South Korea [24]. Traditional medicinal uses of the plant include treatment for stomach illness, liver ailments, constipation, bloody diarrhea, dysentery, arthritis, rheumatism, fevers, hemorrhoids, vaginal discharge, and skin eruptions [24].
As part of our research program investigating potential cultivation of Curcuma in Alabama, Curcuma aromatica (both green- and white-colored rhizomes), C. caesia (black-colored rhizome), C. zanthorrhiza (lime-green rhizome), and C. longa (both yellow-, and red-colored rhizomes), obtained from Vietnam, were cultivated in North Alabama (Figure 1). The rhizome essential oils were obtained by hydrodistillation and analyzed by gas chromatographic methods. Both the “mother” or main rhizomes as well as the “daughter” or finger rhizomes were obtained and analyzed. The six Curcuma varieties used in this study were selected out of 64 genotypes according to three criteria: high yield but low curcuminoid content (variety, CL56), high yield but no curcumin content (CA22, CA46, CC38, and CZ44), and high yield and high curcumin content (CL63) based on unpublished data by the authors, Lam Duong and S.R. Mentreddy at Alabama A&M University.
2. Materials and Methods
2.1. Plant Material
The six Curcuma varieties used in this study were collected by Lam Duong from various locations in Vietnam: CA22 (Quang Nam province), CA46 (Gia Lai province), CC38 (Nghe An province), CL56 (Bac Giang province), CL63 (Quang Tri province), and CZ44 (Gia Lai province). The Curcuma rhizomes varieties were initially planted in a glass greenhouse at Alabama A&M University (Normal, AL, USA) and subsequently cultivated at the Alabama A&M Winfred Thomas Agricultural Research Station (Hazel Green, AL, USA, 34′89″ N, 86′56″ W) as previously described for C. longa cultivation [9]. Each of the fresh Curcuma rhizomes were collected on 18 March 2021, and stored at −20 °C until processed. The mother and daughter rhizomes were, separately, chopped and hydrodistilled using a Likens–Nickerson apparatus for 4 h (see Table 1).

Table 1.
Curcuma rhizome hydrodistillation yields.
2.2. Gas Chromatographic Analyses
The Curcuma rhizome essential oils were analyzed by gas chromatography—mass spectrometry (GC-MS) as previously reported [3]: Shimadzu GCMS-QP2010 Ultra (Shimadzu Scientific Instruments, Columbia, MD, USA), electron impact (EI) mode (70 eV), scan range 40–400 m/z, scan rate 3.0 scans/s; ZB-5ms GC column (60 m length × 0.25 mm diameter × 0.25 μm film thickness) (Phenomenex, Torrance, CA, USA), He carrier gas, 208.3 kPa head pressure, flow rate 2.0 mL/min, injector temperature 260 °C, ion source temperature 260 °C, oven temperature program 50 °C to 260 °C at 2 °C/min then held at 260 °C for 5 min; 0.3 μL of 5% w/v solutions in CH2Cl2 were injected, split ratio 1:24. Essential oil components were identified by comparison of MS fragmentation and retention index (RI) with those provided in the databases [25,26,27,28].
Gas chromatography with flame ionization detection (GC-FID) was carried out as previously reported [29]: Shimadzu GC 2010 (Shimadzu Scientific Instruments, Columbia, MD, USA) equipped with flame ionization detector, ZB-5 capillary column (60 m × 0.25 mm i.d.; film thickness 0.25 μm) (Phenomenex, Torrance, CA, USA); oven temperature programmed as above for GC-MS; injector and detector temperatures 260 °C; He carrier gas, flow rate 1.0 mL/min; 0.3 μL of 5% w/v solution in CH2Cl2 were injected, ratio 1:31. The percent compositions of the essential oils were calculated from peak areas with quantification using the external standard method; calibration curves of representative compounds from each class were used for quantification.
Chiral GC-MS was carried out as previously reported [29]: Shimadzu GCMS-QP2010S instrument (Shimadzu Scientific Instruments, Columbia, MD, USA), Restek B-Dex 325 column (Restek Corporation, Bellefonte, PA, USA); oven temperature program 50 °C to 120 °C at 1.5 °C/min, then to 200 °C at 2.0 °C/min; 0.1 μL of 5% w/v solutions in CH2Cl2 were injected, with a split ratio of 1:25. The enantiomers were determined by comparison of retention times with authentic samples obtained from Sigma-Aldrich (Milwaukee, WI, USA) and the relative enantiomer percentages were calculated from peak integration.
2.3. Statistical Analysis
Analysis of variance was conducted by one-way ANOVA followed by the Tukey test using Minitab® 18 (Minitab Inc., State College, PA, USA). Differences at p < 0.05 were considered to be statistically significant. For the agglomerative hierarchical cluster (AHC) analysis, the 12 essential oil compositions were treated as operational taxonomic units (OTUs), and the concentrations (percentages) of 18 of the most abundant essential oil components (curzerenone, curdione, germacrone, ar-turmerone, 1,8-cineole, α-turmerone, unidentified (RI = 1778), β-turmerone (=curlone), β-sesquiphellandrene, α-zingiberene, iso-curcumenol, curcumenone, trans-β-elemene, ar-curcumene, β-pinene, curcumenol, camphor, and curzerene) were used to determine the chemical associations between the Curcuma rhizome essential oil samples using XLSTAT Premium, version 2018.1.1.62926 (Addinsoft, Paris, France). Similarity was determined using the Pearson correlation, and clustering was defined using the unweighted pair-group method with arithmetic mean (UPGMA).
3. Results and Discussion
3.1. Chemical Composition of Curcuma Rhizome Essential Oils
The fresh rhizome samples were hydrodistilled to give colorless or pale-yellow essential oils in yields ranging from 0.41% to 1.13% (Table 1). The chemical composition of the Curcuma rhizome essential oils are compiled in Table 2. Gas chromatograms of each Curcuma variety are shown in Supplementary Figure S1.

Table 2.
Chemical compositions of the rhizome (mother and daughter) essential oils of Curcuma species from Vietnam, cultivated in North Alabama.
The essential oils from the green-colored mother and daughter rhizomes of C. aromatica (CA22) were dominated by curzerenone (18.6% and 14.7%, respectively), germacrone (14.7% and 10.7%, respectively), 1,8-cineole (11.7% and 6.6%, respectively), and an unidentified sesquiterpenoid (RI 1778, Supplementary Figure S2) (9.0 and 8.7%, respectively). Similarly, the white-colored mother and daughter rhizomes (C. aromatica, CA46) were dominated by the same components, curzerenone (14.9% and 14.8%), germacrone (14.5% and 12.5%), 1,8-cineole (10.2% and 5.2%), and the unidentified compound (RI 1778) (11.0% and 10.3%). The rhizome essential oil composition of C. aromatica show wide variation depending on geographical location [14]. For example, camphor was found to be a major component of C. aromatica rhizome essential oils from India (18.8–32.3%), whereas 8,9-dehydro-9-formylcycloisolongifolene (2.7–36.8%) was a dominant compound in the essential oils from China. Camphor was relatively minor in C. aromatica cultivated in North Alabama in this work (1.4–2.5%) and 8,9-dehydro-9-formylcycloisolongifolene was not observed. Curzerenone, germacrone, and 1,8-cineole, however, are relatively concentrated in Indian C. aromatica rhizome essential oils [14]. The rhizome essential oil of C. aromatica from Thailand showed camphor (26.9%), ar-curcumene (23.2%), and xanthorrhizol (18.7%) as the major components [30], whereas the rhizome essential oil from C. aromatica cultivated in Japan revealed β-turmerone (32.2 and 44.0%), 1,8-cineole (7.5 and 25.3%), and germacrone (4.6 and 9.6%) to be major compounds [31]. Notably, curzerenone, 8,9-dehydro-9-formylcycloisolongifolene, ar-curcumene, and xanthorrhizol were not detected in the essential oils from Japan. A recent examination of C. aromatica from different regions of eastern and southern India revealed relatively low concentrations of curzerenone (0.0–1.2%), but high concentrations of xanthorrhizol (8.8–24.4%), camphor (4.1–18.1%), germacrone (3.5–21.9%), neocurdione (5.8–14.6%), and 1,8-cineole (3.7–11.9%) [32].
The black-rhizome (C. caesia, CC38) essential oil, on the other hand, was rich in curzerenone (26.1% and 29.1%), curdione (28.7% and 35.6%), as well as iso-curcumenol (6.5% and 5.6%), for the mother and daughter rhizomes, respectively. In contrast, C. caesia rhizome essential oil from north India showed 8,9-dehydro-9-formylcycloisolongifolene, (11.7%), camphor (6.1%), 1,8-cineole (6.0%), and β-elemene (5.2%, reported as β-germacrene) as major components [33]. Neither curzerenone, curdione, nor iso-curcumenol were reported in the essential oil from north India, and 8,9-dehydro-9-formylcycloisolongifolene was not found in the essential oil from North Alabama. Note that curzerenone was determined to be artificially elevated in C. caesia essential oil due to the Cope rearrangement of furanodienone [34].
Likewise, the lime-colored rhizome essential oils of C. zanthorrhiza (CZ44,) were also rich in curzerenone (16.3% and 19.7%) and curdione (19.8% and 17.7%), in addition to germacrone (11.3% and 11.1%) for the mother and daughter rhizomes, respectively. This composition is in marked contrast to that reported for C. zanthorrhiza from Thailand with 1,8-cineole (37.6%) and curzerenone (13.7%) as the major components [30]. 1,8-Cineole was in lower concentration (7.2% and 2.5% for the mother and daughter rhizomes) in C. zanthorrhiza in this work. A C. zanthorrhiza rhizome essential oils from Bogor, Indonesia, on the other hand, was dominated by xanthorrhizol (26.8%), β-curcumene (17.0%), ar-curcumene (15.1%), camphor (9.1%), and germacrone (5.4%) [35]. Another sample of C. zanthorrhiza from West Java, Indonesia, was composed of β-curcumene (23.4%), ar-curcumene (22.1%), curzerene (6.0%), camphor (5.0%), and xanthorrhizol (4.7%) as the dominant constituents [36]. Neither β-curcumene nor xanthorrhizol were detected in the essential oil sample cultivated in North Alabama.
The major components of the rhizome essential oils from C. longa CL56 (yellow-colored rhizome) were α-turmerone (12.7% and 14.1%), α-zingiberene (11.4% and 13.9%), ar-turmerone (8.3% and 12.6%), and β-sesquiphellandrene (8.9% and 10.0%). The red-colored rhizome variety of C. longa (CL63) also yielded essential oils rich in ar-turmerone (36.1% and 31.3%), and α-turmerone (15.2% and 13.0%), as well as β-turmerone (=curlone) (15.4% and 13.0%). By comparison, the rhizome essential oils of C. longa cultivated in North Alabama, reported previously, showed α-turmerone (13.6–31.5%), ar-turmerone (6.8–32.5%), β-turmerone (4.8–18.4%), α-phellandrene (3.7–11.8%), 1,8-cineole (2.6–11.7%), α-zingiberene (0.9–12.5%), and β-sesquiphellandrene (0.7–8.0%) [9]. Two distinct chemical variations were found in the previous examination of C. longa cultivated in North Alabama. One group was dominated by turmerones (α-turmerone, ar-turmerone, and β-turmerone), while the second group had lower concentrations of turmerones but high concentrations of α-zingiberene and β-phellandrene. Thus, the red-colored rhizome (CL63) belongs to the turmerone-rich chemical group, while the yellow-colored rhizome (CL56) belongs to the second group (high in α-zingiberene).
In order to place the volatile phytochemistry of the Curcuma rhizome essential oils in this study into perspective, an agglomerative hierarchical cluster analysis (HCA) was carried out based on the relative concentrations of the major components (Figure 2). There are two clearly defined clusters with at least 50% similarity based on the HCA: Cluster 1 is a cluster made up of CA22 (green rhizome), CA46 (C. aromatica, white rhizome) CC38 (C. caesia, black rhizome), and CZ44 (C. zanthorrhiza, lime rhizome), essential oils and defined by relatively high concentrations of curzerenone (14.7–29.1%), curdione (1.3–35.6%), and germacrone (3.8–14.7%); and Cluster 2, a cluster of CL56 (C. longa, yellow rhizome) and CL63 (C. longa, red rhizome) rhizome essential oils that were dominated by ar-turmerone (8.3–36.1%), α-turmerone (12.7–15.2%), and β-turmerone (5.0–15.4%) (see Table 2).
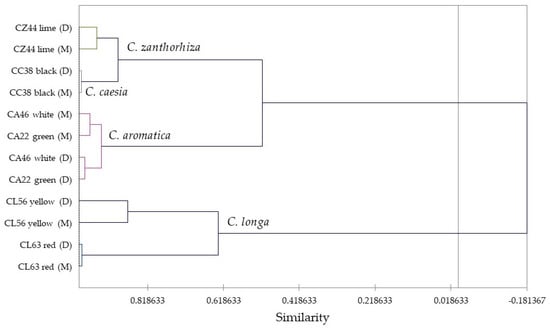
Figure 2.
Dendrogram obtained from the agglomerative hierarchical cluster analysis of the rhizome essential oil compositions of Vietnamese Curcuma species cultivated in North Alabama.
Interestingly, the volatile phytochemistry of C. caesia and C. zanthorrhiza rhizomes are very similar (about 90% similarity). Likewise, the green- and white-colored rhizome essential oils of C. aromatica are very similar (about 95% similarity). The yellow- and red-colored rhizome essential oils of C. longa showed somewhat lower similarity (about 60% similarity).
There are some significant differences in the concentrations of the major components in Cluster 1 (Figure 3). The concentration of curzerenone is significantly greater in C. caesia than in either C. aromatica or C. zanthorrhiza. The concentrations of curdione in C. aromatica are significantly lower than those in either C. caesia or C. zanthorrhiza. Germacrone was significantly lower in C. caesia than in either C. aromatica or C. zanthorrhiza.
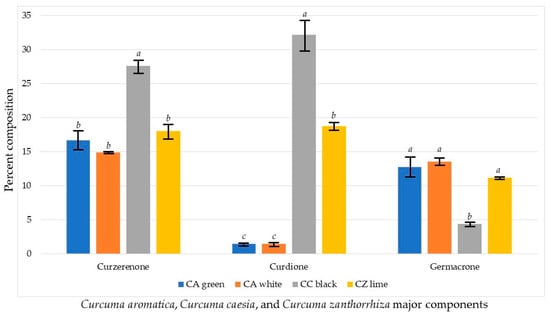
Figure 3.
Comparison of the main chemical components of Curcuma aromatica, Curcuma caesia, and Curcuma zanthorrhiza. For each chemical component, bars with the same letter are not significantly different at p ≤ 0.05.
The significant differences between the essential oils of yellow- and red-colored C. longa are the concentrations of ar-turmerone (much higher in the red rhizome variety) and β-turmerone (also higher in the red rhizome variety). The concentrations of α-turmerone in the red and yellow varieties are not significantly different (Figure 4). Nevertheless, although the compositions of yellow- and red-colored rhizomes of C. longa are notably different (60% similarity), they are comparable to the respective chemical profiles of C. longa from tropical Asian collections [3].
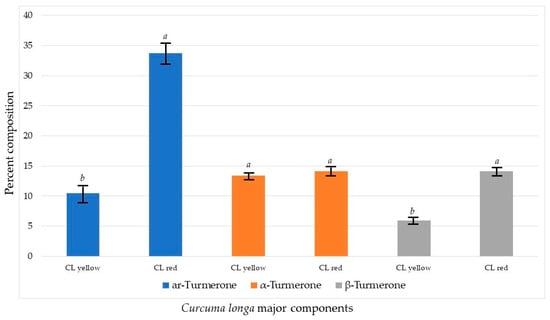
Figure 4.
Comparison of the main chemical components of Curcuma longa. For each chemical component, bars with the same letter are not significantly different at p ≤ 0.05.
3.2. Enantiomeric Distribution of Terpenoids in Curcuma Essential Oils
The enantiomeric distributions of terpenoid components in Curcuma rhizome essential oils have been determined by enantioselective GC-MS (Table 3). Although only found in trace quantities, when detected by chiral GC-MS (C. aromatica, C. caesia), the (−)-α-thujene predominated. (−)-α-Pinene was the dominant enantiomer in all samples. (−)-β-Pinene also predominated in all samples, but was especially dominant in the essential oils of C. zanthorrhiza (CZ44) and C. longa (CL56 and CL 63). (+)-Camphene was the dominant enantiomer in C. aromatica, C. caesia, and C. zanthorrhiza.

Table 3.
Enantiomeric distribution of terpenoid components in Vietnamese Curcuma rhizome essential oils cultivated in North Alabama.
α-Phellandrene and δ-3-carene, only detected in the essential oils of C. longa, were both exclusively the (+) enantiomers. (−)-Limonene was the major stereoisomer in the Curcuma essential oils, but was nearly racemic in C. zanthorrhiza. (−)-Sabinene predominated is all Curcuma rhizome essential oils where it was detected. (−)-Linalool was the major enantiomer in nearly all Curcuma samples, but was particularly abundant in C. caesia (CC38), C. zanthorrhiza (CZ44), and the yellow-rhizome C. longa (CL56). Interestingly, however, the red-rhizome C. longa (CL63) exhibited (+)-linalool as the major enantiomer. Likewise, (−)-α-terpineol was the dominant enantiomer in all Curcuma essential oils. Camphor was not found in C. longa, but (+)-camphor was the dominant enantiomer in C. aromatica, C. caesia, and C. zanthorrhiza. The major enantiomer of terpinen-4-ol in Curcuma essential oils was (−)-terpinen-4-ol, although the distribution was nearly racemic in C. zanthorrhiza.
δ-Elemene was nearly racemic in all of the Curcuma essential oils, whereas trans-β-elemene was exclusively the (−) enantiomer. Both (E)-β-caryophyllene and δ-cadinene were 100% (−) enantiomers, while germacrene D and β-bisabolene were exclusively the dextrorotatory stereoisomers.
4. Conclusions
The rhizome essential oils of C. aromatica, C. caesia, C. longa, and C. zanthorrhiza that were cultivated in North Alabama showed wide variation in composition compared to essential oils from other geographical locations. Nevertheless, the essential oil yields and composition provide evidence that Curcuma can be successfully cultivated in North Alabama and may provide additional sources of these species for both culinary and herbal medicinal uses. The knowledge of their relative oil yields and composition could help in value-addition for either fresh rhizomes or dry herbal markets. The four species showed specific essential oil components, which are known to have extensive pharmacological activity separately or in combination with curcuminoids. The species can be used to tailor herbal medicines to combat particular ailments. The cultivation of specific varieties to cater to niche markets could not only benefit the farmers, but also have an impact on the socio-economic sustainability of rural Alabama in particular and the southeastern U.S. in general. As far as we are aware, this is the first report of the essential oil compositions, including enantiomeric distributions for these Curcuma species cultivated in North America. Among the four species, the C. longa species that combine high yield with high curcumin have been found to be suitable for cultivation. However, the remaining species have economic potential, for example C. zanthorrhiza is well known for its antimicrobial activity against common human pathogens to cater to herbal companies interested in varieties that are high in a certain essential oil component.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8050360/s1, Figure S1: Gas chromatograms of rhizome essential oils of Curcuma varieties cultivated in North Alabama; Figure S2: Mass spectra of unidentified components of Curcuma rhizome essential oils.
Author Contributions
Conceptualization, S.R.M. and W.N.S.; methodology, L.D., S.R.M., P.S. and W.N.S.; formal analysis, S.R.M., R.S., P.S. and W.N.S.; investigation, L.D., S.R.M., R.S., P.S. and W.N.S.; resources, L.D. and S.R.M.; data curation, S.R.M. and W.N.S.; writing—original draft preparation, S.R.M. and W.N.S.; writing—review and editing, L.D., S.R.M., P.S. and W.N.S.; project administration, S.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the article.
Acknowledgments
The present work is a contribution of the Aromatic Plant Research Center (APRC, www.aromaticplant.org, accessed on 16 February 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Flora Online (WFO). Curcuma L. Available online: http://www.worldfloraonline.org/taxon/wfo-4000010089 (accessed on 26 February 2022).
- Ravindran, P.N.; Babu, K.N.; Shiva, K.N. Botany and crop improvement of Tumeric. In Turmeric: The Genus Curcuma; Ravindran, P.N., Babu, K.N., Sivaraman, K., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 15–70. [Google Scholar]
- Dosoky, N.S.; Satyal, P.; Setzer, W.N. Variations in the volatile compositions of Curcuma species. Foods 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.P. Turmeric (Curcuma longa L.) and Ginger (Zingiber officinale Rosc.)—World’s Invaluable Medicinal Spices; Springer Nature: Cham, Switzerland, 2019; ISBN 978-3-030-29188-4. [Google Scholar]
- Rathaur, P.; Raja, W.; Ramteke, P.W.; John, S.A. Turmeric: The golden spice of life. Int. J. Pharm. Sci. Res. 2012, 3, 1987–1994. [Google Scholar] [CrossRef]
- Lal, J. Turmeric, curcumin and our life: A review. Bull. Environ. Pharmacol. Life Sci. 2012, 1, 11–17. [Google Scholar]
- Gupta, S.C.; Sung, B.; Kim, J.H.; Prasad, S.; Li, S.; Aggarwal, B.B. Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol. Nutr. Food Res. 2013, 57, 1510–1528. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Setzer, W.N.; Duong, L.; Poudel, A.; Mentreddy, S.R. Variation in the chemical composition of five varieties of Curcuma longa rhizome essential oils cultivated in north Alabama. Foods 2021, 10, 212. [Google Scholar] [CrossRef]
- Prasad, S.; Aggarwal, B.B. Turmeric, the golden spice: From traditional medicine to modern medicine. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 263–288. ISBN 978-1439807132. [Google Scholar]
- Tridge Turmeric. Available online: https://www.tridge.com/intelligences/turmeric1/import (accessed on 14 April 2022).
- Wu, C.Y.; Raven, P.H.; Hong, D.Y. Zingiberaceae. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200028365 (accessed on 27 February 2022).
- Do, D.M.; Vo, T.H.; Nguyen, D.H.; Le, K.M.; Huynh, T.T.; Le, T.D.Q.; Huynh, T.H. Identification of Curcuma aromatica growing in Vietnam and its potential anticancer components. MedPharmRes 2019, 3, 12–18. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Sikha, A.; Harini, A.; Hegde Prakash, L. Pharmacological activities of wild turmeric (Curcuma aromatica Salisb): A review. J. Pharmacogn. Phytochem. 2015, 3, 1–4. [Google Scholar]
- Priyanka, R.; Vasundhara, M.; Manjunatha Reddy, A.H. Chemo-profiling of Curcuma aromatica Salisbury rhizomes and leaves from South India. J. Med. Plants 2019, 7, 260–262. [Google Scholar]
- Umar, N.M.; Parumasivam, T.; Aminu, N.; Toh, S.M. Phytochemical and pharmacological properties of Curcuma aromatica Salisb (wild turmeric). J. Appl. Pharm. Sci. 2020, 10, 180–194. [Google Scholar] [CrossRef]
- Borah, A.; Kumar, D.; Paw, M.; Begum, T.; Lal, M. A review on ethnobotany and promising pharmacological aspects of an endangered medicinal plant, Curcuma caesia Roxb. Turk. J. Bot. 2020, 44, 205–213. [Google Scholar] [CrossRef]
- Sharma, P.; Bajaj, S.; Fuloria, S.; Porwal, O.; Subramaniyan, V.; Ozdemir, M.; Meenakshi, D.U.; Kishore, N.; Fuloria, N.K. Ethnomedicinal and pharmacological uses of Curcuma caesia. Nat. Volatiles Essent. Oils 2021, 8, 14902–14910. [Google Scholar]
- Singh, S.; Sahoo, B.C.; Ray, A.; Jena, S.; Dash, M.; Nayak, S.; Kar, B.; Sahoo, S. Intraspecific chemical variability of essential oil of Curcuma caesia (black turmeric). Arab. J. Sci. Eng. 2021, 46, 191–198. [Google Scholar] [CrossRef]
- Rathore, P.; Dohare, P.; Varma, S.; Ray, A.; Sharma, U.; Jaganathanan, N.R.; Ray, M. Curcuma oil: Reduces early accumulation of oxidative product and is anti-apoptogenic in transient focal ischemia in rat brain. Neurochem. Res. 2008, 33, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.D.; Blázquez, M.A. Curcuma longa L. rhizome essential oil from extraction to its agri-food applications. A review. Plants 2021, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- WFO, World Flora Online. Curcuma zanthorrhiza Roxb. Available online: http://www.worldfloraonline.org/taxon/wfo-0000366175 (accessed on 26 February 2022).
- Rahmat, E.; Lee, J.; Kang, Y. Javanese turmeric (Curcuma xanthorrhiza Roxb.): Ethnobotany, phytochemistry, biotechnology, and pharmacological activities. Evid.-Based Complement. Altern. Med. 2021, 2021, 9960813. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Mondello, L. FFNSC 3; Shimadzu Scientific Instruments: Columbia, MD, USA, 2016. [Google Scholar]
- NIST. NIST17; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017.
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Dissertation, University of Alabama in Huntsville, Huntsville, AL, USA, 2015. [Google Scholar]
- Lawson, S.K.; Satyal, P.; Setzer, W.N. Phytochemical analysis of the essential oils from aerial parts of four Scutellaria “Skullcap” species cultivated in south Alabama: Scutellaria baicalensis Georgi, S. barbata D. Don, S. incana Biehler, and S. lateriflora L. Nat. Prod. Commun. 2021, 16, 1934578x211025930. [Google Scholar] [CrossRef]
- Jarikasem, S.; Thubthimthed, S.; Chawananoraseth, K.; Suntorntanasat, T.; Brophy, J.J. Essential oils from three Curcuma species collected in Thailand. Acta Hortic. 2005, 677, 37–41. [Google Scholar] [CrossRef]
- Kojima, H.; Yanai, T.; Toyota, A. Essential oil constituents from Japanese and Indian Curcuma aromatica rhizomes. Planta Med. 1998, 64, 380–381. [Google Scholar] [CrossRef]
- Behura, S.; Sahoo, A.; Singh, S.; Jena, S.; Kar, B.; Nayak, S. Variation in essential oil yield and volatile constituents of Curcuma aromatica rhizome from different regions of eastern and southern India. J. Essent. Oil-Bear. Plants 2021, 24, 1248–1255. [Google Scholar] [CrossRef]
- Kumar, A.; Navneet; Gautam, S.S. Volatile constituents of Curcuma caesia Roxb. rhizome from north India. Natl. Acad. Sci. Lett. 2020, 43, 607–610. [Google Scholar] [CrossRef]
- Mahanta, B.P.; Sut, D.; Lal, M.; Haldar, S. Hydrodistillation alters the compositional originality in black turmeric (Curcuma caesia Roxb.) essential oil. J. Essent. Oil Res. 2021, 33, 240–246. [Google Scholar] [CrossRef]
- Fitria, R.; Seno, D.S.H.; Priosoeryanto, B.P.; Hartanti; Nurcholis, W. Volatile compound profiles and cytotoxicity in essential oils from rhizome of Curcuma aeruginosa and Curcuma zanthorrhiza. Biodiversitas 2019, 20, 2943–2948. [Google Scholar] [CrossRef][Green Version]
- Septama, A.W.; Tasfiyati, A.N.; Kristiana, R.; Jaisi, A. Chemical profiles of essential oil from Javanese turmeric (Curcuma xanthorrhiza Roxb.), evaluation of its antibacterial and antibiofilm activities against selected clinical isolates. S. Afr. J. Bot. 2022, 146, 728–734. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).