Abstract
Persimmon fruits are often affected by large postharvest losses due to rapid ripening and the early onset of senescence. To reduce such losses in fresh fruits, the application of hydrocolloid-based edible coatings was conducted. Therefore, a plant hydrocolloid-based gum, tragacanth gum (TCG), was applied to persimmon fruits at 0.5%, 1%, and 1.5% TCG concentrations, and stored at 20 ± 2 °C and 80–85% relative humidity for 20 days (analysis at 0, 4th, 8th, 12th, 16th, and 20th day). As a result of TCG application on persimmon fruits, there were greatly suppressed respiration rates, ethylene production, weight loss, decay incidence, and H2O2 and malondialdehyde content. In addition, TCG-coated persimmon fruits had higher concentrations of bioactive compounds including phenols, flavonoids, carotenoids, ascorbic acid, and soluble tannin. Higher enzymatic antioxidant activities and lower softening enzyme activities were also recorded for TCG-coated persimmon fruits. Uncoated persimmon fruits quickly lost fruit quality attributes like color, firmness, taste, and aroma during storage compared to coated ones. Based on our findings, the use of TCG, especially at the concentration of 1% TCG, can be recommended to be applied as the edible coating to maintain the nutritional, biochemical, and commercial quality of persimmon fruits during ambient storage.
1. Introduction
Persimmon (Diospyros kaki Thunb.) is a climacteric fruit with distinct sensory quality and nutritional profile [1,2]. Fuyu persimmons, the leading non-astringent persimmon fruits, are the globally cultivated persimmons. Fuyu persimmon can be eaten from the crispy firm to ripe stages in many parts of Europe and Asia. Persimmon fruits contain a high concentration of biologically active compounds, such as ascorbic acid, carotenoids, and tannins, which are associated with a range of human health-promoting activities, such as antioxidative, anti-carcinogenic, and anti-mutagenic properties [1,3].
Like other climacteric fruits, persimmon fruit undergoes prompt ripening due to ethylene production and accelerated respiration [2]. Loss of firmness makes persimmon fruits vulnerable to mechanical damage and rot, ultimately restricting long-distance shipping [4,5]. Inhibiting ethylene biosynthesis or activity may be important in delaying ripening and extending the postharvest life of persimmon fruit [4].
Low temperatures, although effective in increasing the postharvest life of persimmon fruits, may cause chilling injury to persimmon fruit that reduces its economic and nutritional potential. The major chilling injury symptoms during low-temperature storage have been peel browning and pulp softening [6]. Storage conditions are always hospitable for fresh fruits and vegetables. Low or high temperature leads to the accumulation of reactive oxygen species (ROS) within fruit tissues that cause oxidative stress and ultimately senescence [7].
Edible coatings have been applied to extend the postharvest life of fruits and vegetables either as a whole or in fresh-cut form [8]. In addition to being cost-effective, edible coatings could be a good substitute for chemically manufactured preservatives due to their eco-friendly and biodegradable nature and natural sources [9,10]. Edible coating application on one hand reduces metabolic activities, such as respiration and ethylene production, and, on other hand, mitigates oxidative stress by decreasing the accumulation of malondialdehyde content and hydrogen peroxide and membrane leakage in coated fruits such as mango [11], persimmon [12], and guava [13]. Edible coatings suppress the accumulation of ROS by activating the enzymatic and non-enzymatic antioxidant defense mechanism, thus delaying senescence in coated produces [10,12,14]. Extension in the postharvest life of coated fruits may also be attributed to the deactivation of cell wall-degrading enzymes that have been reported in persimmon [12], strawberry [15], and apricot fruits [10].
Edible coatings based on natural gums have been widely applied for an extension in the postharvest of horticultural fresh produce [16]. Natural gums, a type of hydrocolloid, are polysaccharides comprised of sugars other than glucose and are chemically inert, inexpensive, biodegradable, odorless, non-toxic, and easily available. Gums are also known as hydrocolloids since they are water-soluble [9].
Tragacanth gum (TCG), also known as katira, is an anionic polysaccharide secreted by different species of Astragalus plants [17,18]. TCG exhibits exclusive biological and chemical features, including non-toxicity, biocompatibility, environment friendliness, and stability across a wide pH range [19]. Our studies have shown that the application of TCG to apricot and mango maintained a higher number of biochemical attributes and antioxidants and lowered oxidative stress by reducing ROS production and suppressing the activities of softening enzymes [10,20]. It has been reported that a combination of TCG with essential oil (EO) of Zataria multiflora slowed browning and inhibited microbiological infestation in button mushrooms [19]. Similarly, an edible coating based on TCG prevented browning, controlled microbial infestation, and preserved the quality of button mushrooms [21].
Therefore, the current research was performed with the hypothesis that TCG will preserve the fruit quality, delay senescence, and reduce fruit softening of stored persimmons by reducing transpirational losses, respiration rate, and ethylene production. Therefore, oxidative stress markers, enzymatic and non-enzymatic antioxidants, cell wall-degrading enzymes, and biochemical and sensory quality attributes of stored persimmon fruits were analyzed during storage conditions.
2. Materials and Methods
2.1. Fruit Material
Persimmon fruits (Diospyros kaki Thunb. cv. Fuyu) were harvested at the commercial maturity stage (yellowish orange peel color) from an orchard located in Swat, Pakistan (34°49′24.5208″ N 72°31′2.892″ E). The persimmon fruits (1000 fruits) were immediately transported (15 ± 2 °C, 75–80% relative humidity (RH)) to the laboratory in Bahauddin Zakariya University, Multan, Pakistan. The fruits were selected on the basis of uniform size, shape, and absence of any disease and defects. The fruits were then sanitized by immersing them in a 0.01% sodium hypochlorite solution for 2 min and then washed with distilled water to eliminate the sodium hypochlorite solution’s residues. Thereafter, the fruits were air-dried at 15 °C for 2 h.
2.2. Preparation and Application of the Edible Coating
Different tragacanth gum (TCG; Sigma-Aldrich, St. Louis, MO, USA) concentrations, such as 0%, 0.5%, 1%, and 1.5% w/v, were prepared by adding TCG powder to deionized water and homogenizing the solutions on a magnetic stirrer for 2 h at 70 °C. The solutions were then incubated for 24 h at 4 °C to hydrate the hydrocolloid gum [10]. After that, each coating solution was blended at 3000 rpm for 10 min. Finally, the solution was degassed by ultrasonication (37 kHz; Elmer, Germany) and placed at room conditions for 1 h. Glycerol (1%, v/v), as a plasticizer, and Tween 20 (0.1%, v/v), as a surfactant, were incorporated into the solutions to prepare the final formulation of edible coating.
On the day of the coating application, a sample of 45 fruits (15 fruits/replication) was taken randomly and immediately analyzed for quality attributes. The remaining fruits (900) were divided into four groups, each containing 225 fruits. The first group was dipped in deionized water (control) for 3 min, whereas the remaining three groups were immersed in respective coating solutions, 0.5%, 1%, and 1.5% TCG for 3 min. Afterward, treated fruits were air-dried for 2 h at 20 °C. The dried fruits were stored in clamshell clear PET boxes, having 5 mm diameter holes on the top and all sides of the boxes. Subsequently, the boxes were stored at 20 ± 2 °C and 80–85% relative humidity for 20 days.
The analyses of different parameters were carried out at the beginning (0 days), 4th day, 8th day, 12th day, 16th day, and 20th day of storage. On each sampling interval weight loss, sensory evaluation, total soluble solids, titratable acidity, and ascorbic acid measurements were conducted by taking fruits randomly from each replication. Some fruits from each replication were cursed in liquid nitrogen and stored at −80 °C for further analysis.
2.3. Fruit Weight Loss and Decay Incidence
The difference between the final and initial weights (±0.01 g) of each replication on respective sampling days was used to assess fruit weight loss (%). For decay incidence, several persimmon fruits that have visible fungal growth, rot, or lesion (≥1 mm diameter) were counted. Finally, a decay incidence was calculated by taking the ratio of the number of decayed fruits to the total number of fruits [16].
2.4. Respiration Rate and Ethylene Production
Two persimmon fruits from each replication were placed in plastic jars at room temperature (25 °C) for one hour to measure respiration rate and ethylene production. Respiration rate and ethylene production were measured by using the Three Gas Analyzer (F-950, Felix Instruments, Camas, WA, USA) instrument and presented as mmol CO2 kg−1 h−1 and µmol kg−1 h−1, respectively.
2.5. Fruit Senescence Characteristics
2.5.1. Membrane Leakage
Membrane leakage was determined according to the method of Yang et al. [22] with some modifications. A total of 10 discs (10 mm diameter and 3 mm thickness) were prepared from randomly selected persimmon fruits. Prepared discs were washed with deionized water and subsequently shaken in 50 mL of deionized water on an orbital shaker for 30 min. After shaking, electrical conductivity (EC1) was measured, then the samples were boiled for 30 min and cooled. EC2 was measured, and membrane integrity was calculated.
2.5.2. Malondialdehyde Content
A total of 1 g of fruit tissue was homogenized with 15 mL of trichloroacetic acid (TCA) (10%; v/v) and the homogenate was centrifuged for 20 min at 10,000× g. A total of 2 mL of supernatant was collected and mixed with 2 mL of 2-thiobarbituric acid (0.6%, w/v). The reaction mixture was heated for 20 min at 100 °C in a water bath. Subsequently, after cooling, the absorbance of the reaction mixture was measured at 450 nm, 532 nm, and 600 nm wavelengths [23] on the UV/VIS-spectrophotometer (UV-1602; BMS, Saint-Laurent, QC, Canada), and malondialdehyde content was determined as µmol kg−1 fresh weight (FW).
2.5.3. Hydrogen Peroxide (H2O2) Concentration
For H2O2 concentration, 1 g of persimmon fruit tissue was ground with 1 mL of TCA (0.1%) and centrifuged the resulting homogenate for 15 min at 12,000× g. Thereafter, the supernatant (0.5 mL) was collected and mixed with 0.5 mL of phosphate buffer (PhB) (10 mM, pH 7.0) and 1 mL of potassium iodide (1 M). Subsequently, the absorbance of the mixture was noted at 390 nm [24] on the UV/VIS-spectrophotometer (UV-1602; BMS, QC, Canada) and the concentration of H2O2 was denoted as µmol kg−1 FW.
2.6. Non-Enzymatic Antioxidant Measurements
Determination of total phenolic content (TPC) and total flavonoid content (TFC) was performed by the method of Ebrahimi and Rastegar [25] with slight modifications from fruit methanolic extracts. A total of 1 g of persimmon fruit tissue was homogenized with 10 mL of methanol (80%) and subsequently centrifuged for 10 min at 4000× g. The collected supernatant was used for the estimation of TPC and TFC.
2.6.1. Total Phenolic Content (TPC)
Total phenolic content was determined according to the modified method by Nasiri et al. [19]. A reaction mixture was prepared by adding 300 µL of methanolic extract to a solution prepared from 1.2 mL of sodium carbonate (7%) and 1.5 mL of Folin–Ciocalteu reagent (10%). The final mixture was incubated in dark conditions at room temperature (25 °C) for 1.5 h. Thereafter, the absorbance was recorded at 765 nm in the UV/VIS-spectrophotometer (UV-1602; BMS, QC, Canada). Gallic acid was used as standard, and the results were expressed as mg GAE 100 g−1 FW.
2.6.2. Total Flavonoid Content (TFC)
Total flavonoid content was estimated by using a colorimetric method [25]. A total of 500 µL of methanolic extract was added to 100 µL of aluminum chloride (10%) and 100 µL of acetate potassium (1 mM). The resulting mixture was incubated for 30 min and the absorbance was recorded at 415 nm by using the UV/VIS-spectrophotometer (UV-1602; BMS, QC, Canada). Quercetin was used as a standard, and the results were expressed as mg QE 100 g−1 FW.
2.6.3. Ascorbic Acid Concentration (AA)
A method by Ali et al. [26] with slight modifications was used for the estimation of ascorbic acid concentration. A total of 10 mL of extracted persimmon juice (the fruit was extracted with a blender and filtered through two layers of muslin cloth) was diluted with 90 mL of oxalic acid (0.4%) and then filtered. Thereafter, 10 mL of filtered aliquot was titrated against 2,6-dichloroindophenol until the light pink color was developed. The ascorbic acid concentration was expressed as mg 100 g−1 FW.
2.6.4. Free Radical Scavenging Activity (RSA)
Free radical scavenging activity (RSA) of persimmon fruits was determined by the spectrophotometry method using the free radical scavenging power of 2,2-diphenyl-1-picrylhydrazyl (DPPH) measurement [22]. A total of 1 g of persimmon fruit tissue was homogenized with 10 mL of ethanol (80%). After shaking (3 min) and filtration, the reaction mixture was prepared from 1 mL of fruit ethanolic extract and 2 mL of DPPH (0.1 mM), which were mixed and incubated for 30 min in dark conditions at room temperature (25 °C). Then the absorbance of the reaction mixture was recorded at 520 nm by the UV/VIS-spectrophotometer (UV-1602; BMS, QC, Canada). Trolox was used as a standard and free radical scavenging activity was expressed as mM Trolox 100 g−1 FW.
2.6.5. Soluble Tannin Content (ST)
Soluble tannin content was determined by using the method of Taira [27] with slight modifications. A total of 1 g of fruit sample was homogenized in 10 mL of methanol (80%) for 5 min, followed by centrifugation at 10,000× g at 4 °C for 10 min. Afterward, 1 mL of supernatant, 7 mL of distilled water, and 1 mL of Folin–Ciocalteu reagent, were added to a test tube and incubated for 5 min. Subsequently, 1 mL of sodium carbonate (10%) was added and incubated at room temperature (25 °C) for 1 h. After the incubation, absorbance was recorded at 725 nm in a UV/VIS-spectrophotometer (UV-1602; BMS, QC, Canada). Soluble tannin content was expressed as % (w/w).
2.7. Antioxidant Enzyme Activities
Enzymes activities measurement was conducted according to the method used by Saleem et al. [12]. A total of 1 g of nitrogen freeze persimmon fruit tissue was ground with 2 mL of PhB (100 mM, pH 7.2). Homogenate was centrifuged for 10 min at 12,000× g for collection of supernatants. The resulting supernatant, named as the enzyme extract, was used for further analysis.
2.7.1. Ascorbate Peroxidase (APX) Activity
APX activity was measured by following a method by Nakano and Asada [28] with some modifications. A reaction mixture was prepared by adding 100 µL of enzyme extract to 1700 µL of PhB (50 mM, pH 5.0), 100 µL of L-ascorbic acid (0.50 mM), and 100 µL of H2O2 (0.15 mM). The absorbance of the mixture was recorded at every 30-sec interval up to 2 min at 290 nm by the UV/VIS-spectrophotometer (UV-1602; BMS, QC, Canada), and mean values were used for the calculation of APX activity. APX activity was expressed as U mg−1 protein.
2.7.2. Catalase (CAT) Activity
CAT activity was calculated using a modified method by Liu et al. [29]. Within a test cuvette, 100 µL of enzyme extract, 2.4 mL of PBH (50 mM, pH 5.0), and 500 µL of H2O2 (20 mM) were added, and the change of absorbance at 240 nm was recorded at every 30-sec interval up to 2 min by the UV/VIS-spectrophotometer (UV-1602; BMS, QC, Canada) and mean values were used for the calculation of CAT activity (extinction coefficient, 39.4 mM−1 cm−1). CAT activity was expressed as U mg−1 protein.
2.7.3. Superoxide Dismutase (SOD) Activity
SOD enzyme activity was calculated by 50% inhibition of photochemical reduction in the nitro-blue-tetrazolium (NBT) as used by Ali et al. [26]. 100 µL of enzyme extract, 1 mL of distilled water, 500 µL of PhB (50 mM, pH 5.0), 100 µL of NBT (20 µM), 200 µL of methionine (22 µM), 200 µL of Triton-X (0.1 µM), and 100 µL of riboflavin (0.60 µM) were added in a test tube. The test tubes containing the reaction mixture were exposed to a UV-light lamp for 15 min. After completion of time, the absorbance was measured at 560 nm by the UV/VIS-spectrophotometer (UV-1602; BMS, QC, Canada) after 30-sec intervals up to 2 min. One unit of SOD activity was determined as the amount of enzyme causing 50% inhibition of NBT photoreduction and SOD activity was expressed as U mg−1 protein.
2.7.4. Peroxidase (POD) Activity
In the presence of H2O2, the formation of tetraguaiacol from guaiacol increases the absorbance of the reaction mixture at 470 nm which determines POD activity. Measurement of POD activity was conducted with a method of Liu et al. [29] with slight modifications. A mixture containing 200 µL of enzyme extract, 2 mL of PhB (50 mM, pH 5.0), and 100 µL of guaiacol (20 mM) was prepared. The reaction was initiated by adding 100 µL of H2O2 (40 mM) and the change in absorbance was recorded at every 30-sec interval up to 3 min at 470 nm by the UV/VIS-spectrophotometer (UV-1602; BMS, QC, Canada) and mean values were used for the calculation of POD activity. POD activity was expressed as U mg−1 protein.
2.7.5. Protein Content
The protein content was measured as suggested by Bradford [30]. For this purpose, bovine serum albumin was used as a protein standard and the results were expressed as mg mL−1.
2.8. Fruit Color and Total Carotenoid Content
The skin color of persimmon fruits was investigated by using a colorimeter (CR-400, Konica Minolta, Tokyo, Japan). Three different areas of fruit were used for the measurement of L*, a*, and b*, and these values were used for calculating hue angle and chroma. Before using the colorimeter, the colorimeter was warmed and calibrated.
Total carotenoid content was estimated according to the method used by Lichtenthaler [31]. A total of 1 g of persimmon tissue was homogenized with 5 mL of chilled acetone (80%) in dark and cool conditions. The homogenate was then centrifuged at 10,000× g for 5 min. The supernatant was collected, and reaming debris was re-extracted by adding 5 mL of acetone and repeating the above-mentioned process. Absorbance was noted at 470 nm, 646 nm, and 663 nm by the UV/VIS-spectrophotometer (UV-1602; BMS, Canada) after thoroughly mixing both supernatants. Total carotenoids were expressed as µg g−1 FW.
2.9. Determination of Fruit Tissue Softening
2.9.1. Fruit Firmness
Fruit firmness was measured by using a digital handheld fruit penetrometer (FR-5120, Lutron Electronics Enterprises, Taipei, Taiwan) with an 8 mm tip. Ten fruits were used per replication and average fruit firmness was expressed as newton (N).
2.9.2. Pectinmethylesterase (PME) Activity
Determination of PME activity was conducted according to the method used by Liu et al. [32]. A total of 4 g of frozen persimmon fruit tissue was ground with 10 mL of NaCl (8.8%, containing polyvinylpolypyrrolidone 2.5% w/v) solution. Homogenate was passed through a centrifugation process for 30 min at 12,000× g. The supernatant was collected, and its pH was adjusted to 7.50. A total of 100 μL of PME extract, 750 µL of deionized water, 2 mL of citrus pectin (0.5%), and 150 μL of bromothymol blue (0.01%, pH 7.50) were added to a test tube. Subsequently, the mixture was shaken well, and the absorbance was noted at 620 nm by the UV/VIS-spectrophotometer (UV-1602; BMS, QC, Canada). Enzyme activity was stated as U mg−1 protein.
2.9.3. Polygalacturonase (PG) and Cellulase (CX) Activity
For the extraction of the enzymes, a method by Awad and Young [33] with slight modifications was used. A total of 2 g of frozen persimmon fruit tissue was homogenized with 16 mL of cold sodium acetate buffer (NaAc-B) (40 mM, pH 5.5) along with 4 mL of NaCl (0.20 M) containing polyvinylpolypyrrolidone (2%, w/v). The homogenate was centrifuged at 4500× g for 30 min at 4 °C. The collected supernatant was used for the determination of PG and CX activity.
A mixture was prepared in a glass tube by adding 100 µL of enzyme extract, 500 µL of cold NaAc-B (0.20 M, pH 4.5), and 400 µL of citrus pectin (1%). The resulting mixture was incubated at 37 °C for 1 h. Thereafter, 1 mL of dinitrosalicylic acids (DNS) (1%) was added to the mixture and heated at 100 °C for 5 min. The absorbance was noted at 540 nm by the UV/VIS-spectrophotometer (UV-1602; BMS, QC, Canada) after cooling the reaction mixture. Enzyme (PG) activity was stated as U mg−1 protein [34].
A mixture containing 500 µL of cold NaAc-B (0.10 M, pH 5), 100 µL of enzyme extract, and 400 µL of carboxymethyl cellulose (1%) was prepared and subsequently incubated at 37 °C for 1 h. Thereafter, 1 mL of DNS (1%) was incorporated into the reaction mixture and heated at 100 °C for 5 min. The reaction mixture was allowed to cool at room temperature and the absorbance was measured at 520 nm by the UV/VIS-spectrophotometer (UV-1602; BMS, QC, Canada). Enzyme (CX) activity was stated as U mg−1 protein [35].
2.10. Determination of Fruit Quality
2.10.1. Total Soluble Solids and Titratable Acidity
Persimmon juice was extracted by a common juicer and filtered through a muslin cloth. Total soluble solids were measured by using a digital refractometer (Pal-1; Atago, Tokyo, Japan) and expressed as %. Titratable acidity (%) of persimmon fruit juice was determined by a method of Horwitz [36] with slight modifications. A total of 10 mL of extracted persimmon juice was diluted with 40 mL of distilled water and titrated against NaOH (0.1 N) until the solution attained pH 8.2.
2.10.2. Sensory Evaluation
Sensory attributes were evaluated with a panel test (10 trained sensory panelists) as used by Ali et al. [10]. The sensory attributes such as taste, aroma, and overall acceptability were assessed on a 9-point hedonic scale (9 = like extremely, 8 = like very much, 7 = like moderately, 6 = like, 5 = neither like nor dislike, 4 = dislike slightly, 2 = dislike very much slightly, 1 = dislike extremely). For the taste evaluation, the fruits were washed, sliced, and tested with peel.
2.11. Statistical Analysis
The obtained data were analyzed for normal distribution and homogeneity of variance. Further, the analysis of variance (ANOVA) technique was applied to evaluate the effects of coatings and storage days (two-factor factorial experiment) on the postharvest life of persimmon fruits under storage conditions. The means of the treatments were separated by using Tukey’s HSD test (p ≤ 0.05). Statistical analyses were carried out by using statistical software Statistix version 8.1 analytical software (Tallahassee, FL, USA).
3. Results and Discussion
3.1. Fruit Weight Loss
Throughout the 20-day storage period, all treatments presented a progressive weight loss tendency (Figure 1A). However, all TCG formulations had significant (p ≤ 0.05) effects in terms of reducing weight loss during the fruit storage period. In summary, on the 20th day, uncoated persimmon fruits exhibited higher (10%) weight loss whereas fruits coated with 1% TCG showed the least difference (8%) as compared with the beginning of storage.
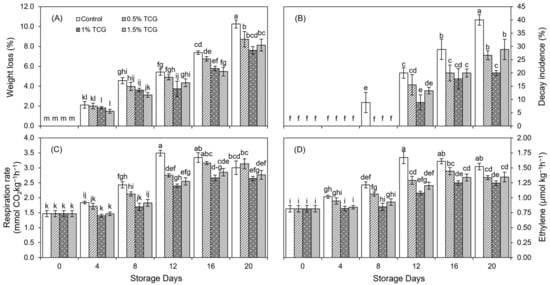
Figure 1.
Effects of the tragacanth gum application on persimmon fruits on: (A) weight loss; (B) decay incidence; (C) respiration; and (D) ethylene production. Persimmon fruits stored for 20 days (analysis at 0, 4th, 8th, 12th, 16th, and 20th day) at 20 ± 2 °C, 80–85% RH. Error bars show standard deviation of the means (three replications). Graph bars bearing similar letters are not significantly different according to Tukey’s HSD test at p ≤ 0.05. Assignment of more than three letters to a bar is denoted by “−” sign between the first and last letter.
Reduction in weight loss is akin to an economical loss. Weight loss from fruits and vegetables is mainly due to the respiration and transpiration of water and is also influenced by storage conditions [19]. The significant reduction in weight loss in TCG-coated fruits may be due to the semi-permeable barrier formed by the edible coating that restricts the movement of O2, CO2, water, and solutes between the coated fruit and its surrounding environment [10,21]. Our findings are in line with the studies on mango [37,38], guava [39], banana [40], and persimmon [12], where the application of natural gum-based edible coatings reduced weight loss.
3.2. Decay Incidence
Initial application of all TCG formulations significantly (p ≤ 0.05) inhibited the decay incidence in coated persimmon fruits up to the 8th day, compared with uncoated fruits (Figure 1B). On the 12th day, all treatments showed the presence of decayed persimmon fruits; however, the maximum decay incidence was observed in uncoated fruits (20% spoilage) followed by 16% and 13% spoilage for 0.5% and 1.5% TCG application, respectively. The minimum decay incidence (9% spoilage) was shown for 1% TCG. This fruit decaying trend persisted up to the end of storage, where uncoated fruits had twice (40% spoilage) as much decaying incidence as fruits coated with 1% TCG (20% spoilage).
Senescence and decay are the most important causes of postharvest losses in the horticultural industry. The edible coating inhibits the onset of the senescence and stabilizes cellular integrity, thereby significantly reducing microbial attacks on coated fruits. Moreover, the application of a coating based on natural gums may reduce pathogen proliferation by forming a barrier between a commodity and disease-causing pathogens, thus increasing the storage life of commodities [10,41,42]. Our findings are similar to those of Ali et al. [10] who found that TCG application to apricot fruits reduced microbial infestation. Similarly, studies using plant-based gums on tomatoes [16], sweet cherry [43], and apricot fruits [44] have shown the inhibition of the growth of microbes on coated fruits.
3.3. Respiration and Ethylene Production
Respiration in persimmon fruits coated with 1% and 1.5% TCG decreased initially up to the 4th day compared with other treatments (Figure 1C). From the 8th day, all treatments showed an enhancement in respiration rate. However, uncoated fruits attained a respiration peak earlier than TCG-coated fruits, on the 12th day, when their respiration rate was about 46% more than for the 1% TCG-coated fruits. TCG-coated fruits had a respiration peak later, and fruits with 1% TCG application had the highest respiration rate on the final day of storage.
Generally, there was an increase in ethylene production in all fruits (Figure 1D). Like respiration rate, ethylene production was also the highest (p ≤ 0.05) in uncoated fruits on the 12th day of the storage period. On this day, uncoated fruits had 55% more ethylene production than the 1% TCG-coated fruits. Although all TCG formulations attained an ethylene peak on the 16th day, the ethylene peak in the 1% TCG-treated fruits continued till the 20th day. On the 20th day, all other treatments showed a decrease in ethylene production.
The ripening of climacteric fruits is characterized by an increase in respiration rate and ethylene production. Ethylene regulates fruit ripening by regulating the expression of genes involved in carotenoid biosynthesis, chlorophyll degradation, and starch hydrolysis to simple sugars [37]. Generally, a barrier formed by a coating reduces gas exchange, including oxygen, between the fruits and the ambient environment [16]. The low respiration rate in TCG-coated persimmon fruits in this study might be attributed to the barrier properties of the coating [21]. The low respiration rate and ethylene generation in fruits coated with plant-based gums might be attributed to reduced gas exchange between the fruits and the surrounding environment, resulting in the limited availability of oxygen for respiration [37]. Furthermore, the use of coatings prepared from plant-based gums greatly inhibits respiration and ethylene production in coated fruits and vegetables, such as mango [37], sweet cherry [43], persimmon [12], and tomatoes [16].
3.4. Fruit Senescence Characteristics
3.4.1. Membrane Leakage
An increasing trend in membrane leakage was noticed over time for all treatments (Figure 2A). However, compared with uncoated fruits, TCG-coated persimmon fruits significantly (p ≤ 0.05) suppressed membrane leakage in coated persimmon fruits already on the 8th day. Among the different TCG formulations, the lowest membrane leakage was observed in fruit coated with 1% TCG during the experiment. On the 20th day, uncoated fruits showed about 39% higher membrane leakage (p ≤ 0.05) than the 1% TCG-coated fruits.
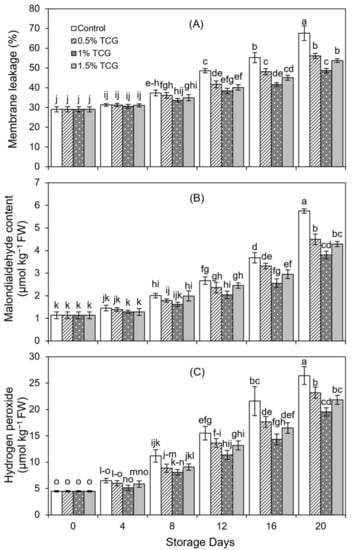
Figure 2.
Effects of the tragacanth gum application on persimmon fruits on: (A) membrane leakage; (B) malondialdehyde content; and (C) hydrogen peroxide. Persimmon fruits stored for 20 days (analysis at 0, 4th, 8th, 12th, 16th, and 20th day) at 20 ± 2 °C, 80–85% RH. Error bars show standard deviation of the means (three replications). Graph bars bearing similar letters are not significantly different according to Tukey’s HSD test at p ≤ 0.05. Assignment of more than three letters to a bar is denoted by “−” sign between the first and last letter.
The disintegration of the cell membrane occurs due to the disturbance in fatty acid composition and the release of solutes that boosted the membrane permeability. Also, the maintenance of cell membrane constituents is important while storing fruits or vegetables, especially at low temperatures [11]. As ripening continues, membrane leakage leads to senescence and dysfunction of the cell membrane which reduces postharvest life [10]. Our findings agree with the previous studies, where edible coatings based on natural gums have been shown to maintain membrane stability and delay senescence in bananas [40], mango [11], or persimmon fruits [12].
3.4.2. Malondialdehyde (MDA) Content
MDA content increased with advancing storage time in all treatments, although the edible coating had a significant effect in preventing the accumulation of MDA content in TCG-coated persimmon fruits (Figure 2B). On the 8th day, 1% TCG coating showed the significantly lowest MDA content, and this trend continued until the end of the experiment. On the 16th and 20th day, a sharp increase in MDA content was noted in all applied treatments, especially in uncoated persimmon fruits, with uncoated fruits showing 28%, 51%, and 34% higher MDA accumulation than persimmon fruits coated with 0.5%, 1%, and 1.5% TCG on D20 storage.
MDA is a stress indicator as it is the end product of the peroxidation of unsaturated fatty acids and generally is used for the assessment of membrane stability in plant tissues [6,11]. It should be noted that the concentration of MDA increased with time under storage conditions [13,43]. Higher production of ROS in fruits boosts the accumulation of MDA concentration [11,13]. On the other hand, plant-based gum as edible coatings markedly suppressed the overproduction of ROS by activating the defense mechanism which reduces the accumulation of MDA concentration in coated fruits [10,12]. Thus, the reduced accumulation of MDA in coated persimmon fruits may be due to the least oxidative stress caused by the application of plant-based gums as reported for other fruits [11,12,43].
3.4.3. Hydrogen Peroxide (H2O2)
With the passage of 20 days storage period, H2O2 content considerably increased (from 4.47 µM kg−1 to 26.43 µM kg−1) in uncoated fruits, compared to the content of 19.56 µM kg−1 in the 1% TCG-coated fruits (Figure 2C). Similar to MDA contents, a significant increase in H2O2 concentration was observed after the 12th day of storage. Statistical analysis showed that TCG coatings suppressed H2O2 formation by 23% on average in coated persimmon fruits compared to uncoating fruits on the 20th day.
H2O2 is a strong ROS and is associated with lower quality and reduced shelf life of harvested fruits [10]. Increased H2O2 generation has accelerated MDA accumulation and decreased membrane integrity in persimmon fruits [6,45]. In this study, the lowest concentration of H2O2 in TCG-coated fruits might be due to the activation of the antioxidant defense mechanism and the modification of the fruit surface for limiting the gaseous exchange that alleviated oxidative stress [10,11]. Our results are aligned with the previous studies that reported that plant-based gums as edible coating reduced the accumulation of H2O2 in persimmon [12], mango [11], and apricot fruits [44].
3.5. Non-Enzymatic Antioxidant Measurements
3.5.1. Total Phenolic Content (TPC)
With storage duration, TPC noticeably decreased across all treatments (Table 1). Nevertheless, TCG-based edible coating showed its significant effect as early as the 4th day which continued until the end of the experiment. Overall, the application of 1% TCG coating was shown to be most effective in preserving TPC in coated fruits as compared to other treatments. On the 20th day, fruits coated with 1% TCG showed the highest TPC (235.57 mg GAE 100 g−1 FW) along with fruits coated with 0.5% TCG (228.32 mg GAE 100 g−1 FW) compared to the lowest TPC (178.78 mg GAE 100 g−1 FW) shown for uncoated fruits.

Table 1.
Effects of tragacanth gum, as an edible coating, on total phenolic content (TPC), total flavonoid content (TFC), ascorbic acid (AA) content, radical scavenging activity (RSA), and soluble tannin (ST) content of persimmon fruits stored for 20 days.
TPC markedly scavenges the ROS in fruits, thus reducing oxidative damage to cells [11,46]. Prolonged storage causes oxidative damage, which results in the degradation of TPC in harvested fruits and vegetables [12]. Moreover, senescence causes the breakdown of cell wall constituents leading to the reduction in TPC in harvested fruits [13]. The application of edible coatings on fruit surfaces has a key role in the preservation of TPC, as it forms a modified atmosphere around the fruit surface and inhibits the respiration and oxidation rate of TPC by suppressing the activity of polyphenol oxidase [43,47]. A higher amount of TPC in TCG-coated persimmon fruits might be due to the barrier effect of plant-based gums which reduced the enzyme-based oxidation of TPC [10,21]. Higher TPC has been reported in various fruits coated with natural gums, such as mango [11,25], tomato [16], apricot [44], and guava [41].
3.5.2. Total Flavonoids Content (TFC)
Overall, a decreasing trend was observed for TFC with advancing days of storage (Table 1). However, from the 4th to the 20th day, TCG application inhibited TFC loss in coated persimmon fruits compared with uncoated fruits. At the end of the experiment, fruits coated with 1% TCG preserved higher TFC (32%), followed by 0.5% TCG (28%), and 1.5% TCG-coated (21%) compared with uncoated fruits.
Flavonoids show strong antioxidant activity and positively influence the post-harvest shelf life of harvested fruits [8,46]. Generally, TFC decreases with the advancement in the storage period due to the ripening and senescence processes [8,25]. Guava fruits coated with a combination of Arabic gum and garlic extract retained higher TFC as compared with uncoated fruits at the end of the 15-day storage period [41]. Similarly, Murmu and Mishra [48] reported higher TFC in Arabic gum-coated guava fruits. In this study, a higher amount of TFC in TCG-coated fruits may be due to reduced metabolic activities and delayed senescence [25,40].
3.5.3. Ascorbic Acid (AA) Content
The content of AA (51.30 mg 100 g−1 FW) decreased from the beginning (day 0) with time across all treatments (Table 1). Though, from the 4th day to the 20th day, the application of TCG coating significantly (p ≤ 0.05) prevented the oxidation of ascorbic acid in persimmon fruits. The persimmon fruits coated with 1% TCG showed 47% greater ascorbic acid content than for uncoated fruits, followed by 0.5% and 1.5% TCG-coated fruits showing 37% and 32% higher AA content than uncoated fruits on the 20th day.
Ascorbic acid is a water-soluble strong antioxidant that alleviates the oxidative stress caused by ROS. Ascorbic acid is reduced with storage time due to the ripening and senescence of harvested fruits and vegetables [19,37]. Oxygen availability to respiring fruit tissues negatively affects ascorbic acid concentration [25]. Retention of ascorbic acid in TCG-coated fruits is probably due to the fact that TCG coating limits oxygen supply, minimizing the respiration rate and subsequently maintaining higher ascorbic acid contents [21]. Additionally, Ali et al. [10] documented a higher amount of ascorbic acid in 1% TCG-coated apricot fruits than that in uncoated apricot. A high amount of ascorbic acid in coated fruit is probably due to the active antioxidative defensive mechanism which inhibits the loss of ascorbic acid [11,43].
3.5.4. Free Radical Scavenging Activity (RSA)
A slight increase in RSA was noted in the initial first four days of storage for 1% TCG-coated persimmon fruits compared to other treatments. From the 8th day to the 20th day the treatments exhibited a significant (p ≤ 0.05) difference in RSA (Table 1). TCG application considerably maintained a higher amount of RSA (156.18 mM TE 100 g−1 FW) in coated persimmon fruits over uncoated fruits (143.50 mM TE 100 g−1 FW) on the 20th day of storage. Additionally, on the 20th day the 1% TCG-coated persimmon fruits retained higher RSA (1.30-fold) than uncoated fruits which exhibited the lowest RSA (100.48. mM TE 100 g−1 FW).
Antioxidants have the ability to regulate oxidative reactions by scavenging ROS and controlling tissue damage and reducing the loss of functional properties [46]. The most important antioxidants belong ascorbic acid, carotenoids, phenolic, and flavonoid compounds [8,19]. In the current research, the higher amount of antioxidants in the coated persimmon fruits might be due to the persistent amount of AA and TPC [10]. The application of coatings, developed from plant-based gums, markedly preserves higher antioxidant activity in coated persimmon [12], guava [41], tomato [16], and jamun fruits [49], thereby delaying the senescence.
3.5.5. Soluble Tannin (ST) Content
Soluble tannin content showed a decreasing trend with the passage of time in all treatments (Table 1). However, up to the 4th day of storage, there was no significant difference among the treatments. Thereafter, significant differences (p ≤ 0.05) appeared among the treatments, where TCG-coated fruits had higher soluble tannin content as compared with the uncoated fruits. Furthermore, on the 20th day, 1% TCG-coated persimmon fruits exhibited higher soluble tannin content (0.080%), followed by fruits coated with 0.5% and 1.5% TCG (0.063% and 0.060%, respectively). Uncoated persimmon fruits showed the lowest soluble tannin content (0.036%).
Tannin content plays a significant role in fruit ripening and senescence and is generally reduced with the passage of time during storage [50]. The continuous reduction in soluble tannins may be due to the development of a complex between the tannin and the pectin released from the cell wall during tissue softening. During postharvest, the soluble tannin precipitates and turns insoluble after combining it with pectin [27]. In this study, the application of TCG maintained higher firmness by lowering the activity of cell wall-degrading enzymes, as seen in the results in Chapter 3.8. (Fruit Tissue Softening), and membrane leakage (Figure 2A), resulting in the inhibition-released soluble pectin from the cell wall. This retarded the decrease in tannin content in coated fruits. Higher soluble tannin content has been reported in edible coated persimmon fruits by Nasr et al. [50] and Xue et al. [51].
3.6. Antioxidant Enzymes Activities
As for the activity of APX enzyme, there was no significant difference among treatments up to the 4th day of the storage period (Figure 3A).
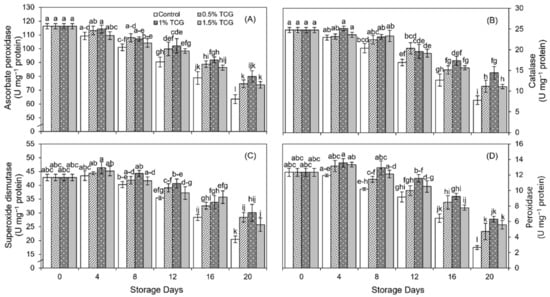
Figure 3.
Effect of tragacanth gum application, as an edible coating, on: (A) ascorbate peroxidase; (B) catalase; (C) superoxide dismutase; and (D) peroxidase enzymes of persimmon fruits stored for 20 days (analysis on 0th, 4th, 8th, 12th, 16th, and 20th day) at 20 ± 2 °C, 80–85% RH. Error bars show standard deviation of the means (three replications). Graph bars bearing similar letters are not significantly different according to Tukey’s HSD test at p ≤ 0.05. Assignment of more than three letters to a bar is denoted by “−” sign between the first and last letter.
Then, there was a significant difference (p ≤ 0.05) in APX activity between treatments on the 8th day, where uncoated fruits had lower activity than TCG-coated fruits. The difference in APX activity between different TCG concentrations first appeared on the 8th day, where the maximum APX activity was shown by the 0.5% and 1% TCG-coated fruits. This trend continued till the end of the experiment. On the last day, the application of 1% TCG markedly preserved higher APX activity (21%) in coated persimmon fruits than in uncoated persimmon fruits.
CAT exhibited a significant (p ≤ 0.05) drop in its activity in all treatments during the whole storage period except for 1% TCG, where it initially increased and then reduced for the rest of the storage period (Figure 3B). As compared to CAT activity at the beginning, uncoated persimmons showed 69% lower CAT activity on the 20th day, whereas the 1% TCG-coated persimmons showed 42% lower CAT activity. At the end of the storage period, the fruits coated with 0.5%, 1%, and 1.5% TCG levels showed a higher CAT activity (43%, 85%, and 42% less, respectively) than uncoated fruits. Thus, the 1% TCG-coated fruits had twice as much CAT activity as uncoated fruits on the 20th day.
SOD activity presented a developing trend up to the 4th day of storage in all treatments, then a reduction in SOD activity was initiated that persisted up to the end of the experiment (Figure 3C). Between the 8th and 20th day, the treatments showed a significant (p ≤ 0.05) effect on SOD activity. At the end of the storage period, the 0.5%, 1%, and 1.5% TCG-coated persimmons showed higher (1.38-fold, 1.47-fold, and 1.26-fold, respectively) SOD activity compared with uncoated fruits.
TCG coating application significantly (p ≤ 0.05) affected the POD activity. Contrary to uncoated fruits which had lower POD activity (11.94 U mg−1 protein) on the 4th day, TCG-coated persimmons improved POD activity from 12.34 U mg−1 protein at the beginning to 13.35 U mg−1 protein on the 4th day (Figure 3D). After that period, all treatments showed a continuous reduction in POD activity, where the most rapid reduction was noticed between the 16th and the 20th day. However, at the end of the storage period, the fruits coated with 1% and 1.5% TCG had noticeably higher POD activity (138% and 111%, respectively) than uncoated fruits.
Plants have antioxidant enzyme systems that play a key role in protecting the cells against oxidative stress, retaining fruit quality, and prolonging the postharvest life by scavenging ROS [7]. Antioxidative enzymes such as SOD converts superoxide into H2O2 and then APX, CAT, and POD suppress the detrimental effect of H2O2 [10]. Failure or reduction in the capacity of an antioxidant system might cause oxidative damage to the membranous cell structures, resulting in the early ripening and senescence of fresh fruits and vegetables [14]. It is noticeable that the application of edible coatings maintains higher antioxidant activity and enhances the postharvest life of strawberry [52], sweet cherry [14], and banana fruits [38]. Our findings are also in accordance with the previous studies where the application of coatings prepared from plant-based edible gums activated the ROS scavenging system and maintained higher activities of APX, CAT, SOD, and POD enzymes in coated fruits such as persimmon [12], apricot [10,44], and banana [50] during storage.
3.7. Fruit Color
3.7.1. Hue Angle and Chroma
Up to the 4th day of storage, there was no significant difference in the hue angle of persimmon fruits across all treatments (Table 2). From the 8th day, TCG-coated fruits showed a significant difference in hue angle. During 20 days of storage, TCG-coated persimmon fruits maintained a higher hue angle than uncoated fruits. Uncoated fruits had a significantly greater change in hue angle. On the 20th day, the 1% TCG-coated fruits had a 14% higher hue angle as compared with uncoated fruits. Similarly, chroma gradually increased in all treatments with the storage duration, but TCG application significantly reduced the change in chroma value in coated persimmon fruits (Table 2). On the 20th day, the 1% TCG-coated fruits showed a lower chroma value, which was 16% lower compared with that of uncoated fruits.

Table 2.
Effect of tragacanth gum as an edible coating on hue angle, chroma, and total carotenoids content of persimmon fruits stored for 20 days.
Hue angle is related to ripening phenomena and decreased with the advancement of ripening, as color changes from yellowish orange to red-orange [53]. Accumulation of carotenoid contents also caused the reduction in hue angle [54]. The higher hue angle in coated persimmon fruits might be due to the significant effect of plant-based edible coatings which inhibited respiration and ethylene production, thus delaying senescence by creating a modified atmosphere around the fruit surface as is reported in tomatoes [16]. Our results are also in accordance with the previous findings as edible coating application significantly maintained a higher hue angle in coated fruits such as fresh-cut persimmon [55], guava [56], and mango [57].
Chroma is an indicator of color saturation; a higher chroma showed higher saturation of colors [57]. It is noted that chroma increased with ripening due to color development in persimmon fruits [53]. The low chroma value of coated persimmon fruits is probably because of edible coating delaying ripening and senescence by reducing respiration and carotenoid accumulation [56,57]. The application of TCG-based coating has been shown to inhibit color change in mushrooms [19,21].
3.7.2. Total Carotenoids Content
Up to the 4th day of storage, uncoated fruits and those with 1.5% TCG application showed significantly (p ≤ 0.05) higher carotenoid content than that of other applied treatments (Table 2). From the 8th day, all TCG concentrations inhibited the development of carotenoids in coated persimmon fruits which continued until the end of the storage period. On the 20th day, uncoated persimmon fruits showed a higher accumulation of carotenoids (0.309 µg g−1 FW) than that of 1% TCG-coated fruits (0.248 µg g−1 FW), which was 1.24-fold higher than for 1% TCG-coated fruits on the 20th day.
Persimmon fruits are a great source of carotenoids and other bioactive phytochemicals [5]. In the early stage of development, dominant carotenoids are of chloroplast type which transforms into chromoplast gradually with the passage of time due to ripening activity [3]. In the current study, the greater amount of total carotenoids in uncoated persimmon fruits was probably due to the enhanced ripening activity. The application of plant-based coatings has been shown to suppress the accumulation of carotenoids and delay ripening and senescence [12,45].
3.8. Fruit Tissue Softening
3.8.1. Fruit Firmness
Fruit firmness progressively declined in both coated and uncoated persimmon fruits with the advancement of storage days (Figure 4A). Up to the 4th day, no significant effect of treatments was noticeable. However, on the 8th day, TCG application showed a significant effect on fruit firmness, as higher fruit firmness was recorded in TCG-coated persimmon fruits compared with uncoated fruits. This trend continued till the 20th day when uncoated fruits showed the lowest fruit firmness (11.77 N) which was 63% less than the firmness of the fruits coated with 1% TCG, which retained the greatest firmness on the 20th day.
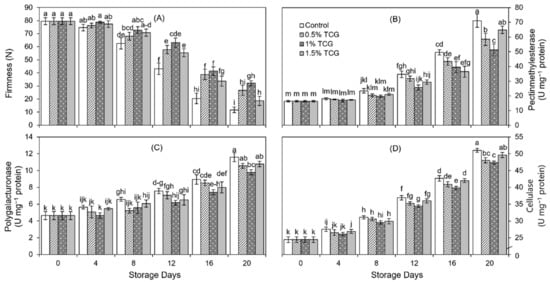
Figure 4.
Effects of tragacanth gum application as an edible coating on: (A) firmness; (B) activity of pectinmethylesterase; (C) activity of polygalacturonase; and (D) activity of cellulase of persimmon fruits stored for 20 days (analysis on 0th, 4th, 8th, 12th, 16th, and 20th day) at 20 ± 2 °C, 80–85% RH. Error bars show standard deviation of the means (three replications). Graph bars bearing similar letters are not significantly different according to Tukey’s HSD test at p ≤ 0.05. Assignment of more than three letters to a bar is denoted by “−” sign between the first and last letter.
Fruit firmness is an important fruit quality factor that determines customer acceptance; however, fruit firmness decreases with an increase in storage days and ripening. It is recommended that the fruits of cv. Fuyu or similar cultivars have a minimum firmness of more than 2.5 kg or 10 N. The fruits with a firmness of less than 1.8 kg force are not considered marketable [58]. Loss of firmness is associated with the change in the cell wall structure. The disintegration of the cell wall leads to the destruction of the physical structure within the cells, the rupturing of the cell membrane, and the leakage of its contents; all of that accelerate fruit tissue damage [51]. In coated persimmons, limited availability of O2 and high level of CO2 caused by the application of TCG coating may reduce the activity of cell wall-degrading enzymes and ensure higher fruit firmness [21]; this was also observed in the present study. Our results agree with the previous studies where edible coatings prepared from different plant-based gums maintained higher firmness in mushroom [19], banana [40], sweet cherry [43], and mango fruits [25].
3.8.2. Softening Enzyme Activities
As for the activity of the PME enzyme, there was no significant difference among treatments up to the 4th day of the storage period (Figure 4B). Thereafter, there was a significant difference (p ≤ 0.05) in the PME activity of treatments on the 8th day, where uncoated fruits had higher activity than TCG-coated fruits. The difference in PME activity between different TCG concentrations first appeared on the 12th day, where the minimum PME activity was shown for the 1% TCG-coated fruits. This trend continued till the end of the experiment. On the 20th day, the 1% TCG-coated persimmons showed the lowest PME activity which was 28% lower than that shown for uncoated fruits, which had the highest PME activity.
PG activity showed a steady progressing trend with the storage period, but a rapid increment in PG activity was found from the 16th to the 20th day (Figure 4C). As compared to PG activity at the beginning, uncoated persimmons showed 1.5-fold higher PG activity on the 20th day, whereas the 1% TCG-coated persimmons showed a 1.11-fold higher PG activity. At the end of the storage period, the fruits coated with 0.5%, 1%, and 1.5% TCG levels showed lower PG activity (9%, 15%, and 7% less, respectively) than uncoated fruits.
As with PME and PG, there was no significant (p ≤ 0.05) difference in CX activity across all treatments up to the 4th day (Figure 4D). However, TCG coating application drastically lowered (4%) the CX activity during the entire storage period. Moreover, on the 20th day, uncoated persimmon fruits showed considerably higher CX activity (51.00 U mg−1 protein) in contrast to TCG-coated fruits, where the lowest CX activity (47.80 U mg−1 protein) was observed for the 1% TCG-coated fruits.
Softening in persimmon is due to the transformation of cell wall structures, the disintegration of cellulose, and the dissolution of pectin from protopectin. This process is catalyzed by a series of enzymes including PME, PG, and CX [2,5]. Softening is highly linked with the ripening process that is regulated by the rate of respiration and ethylene production [54]. The activity of hydrolyzing enzymes is upregulated by the overproduction of carbon dioxide in climacteric fruits [59]. The suppressed activity of PME and PG in coated fruits is most likely owed to lesser carbon dioxide generation due to low respiration rate, which reduces softening and delays the senescence in coated fruits [15]. In this study, we also found a lower rate of respiration and ethylene production (Figure 1C,D) along with suppressed activities of cell wall-degrading enzymes. Our findings are in line with previously reported work, whereby the application of coatings developed from plant-based gums repressed the activities of cell wall-degrading enzymes and reduced the postharvest oxidative stress in coated fruits by regulating gaseous exchange in apricots [10,44], persimmon [12], carambola [15], and jamun fruits [49].
3.9. Fruit Quality
3.9.1. Total Soluble Solids (TSS)
TSS considerably increased in uncoated fruits (from 13% to 22%) throughout the storage period (Table 3). Nevertheless, TCG coating application significantly (p ≤ 0.05) inhibited the development of TSS in coated persimmon fruits as compared with uncoated fruits. On the 20th day, uncoated persimmon fruits showed markedly higher TSS (32%) as compared with the 1% TCG-coated fruits.

Table 3.
Effect of tragacanth gum as an edible coating on total soluble solids (TSS), titratable acidity (TA), taste, aroma, and overall acceptability (score) of persimmon fruits stored for 20 days.
TSS is an important factor that defines consumers’ acceptance of harvested fruits and vegetables [46]. The continuous increase in TSS with the advancement of storage time is due to the hydrolysis of polysaccharides within the cell wall [46,49]. Increased respiration rate and water loss cause the conversion of starch into sugars which results in higher TSS accumulation in harvested fruits [43]. In our study, the slow accumulation of TSS in coated fruits is probably due to the significant effect of TCG on fruit ripening as also reported by Ali et al. [10]. Edible coatings modify the fruit’s internal physiology by creating a semipermeable layer over the fruit surface that limits moisture loss, respiration rate, sugar transformation, and other metabolic activities [43,46]. Coating with natural gums markedly suppressed TSS accumulation in stored horticultural commodities, such as tomato [16], mango [37], persimmon [12], banana [40], and apricot [44].
3.9.2. Titratable Acidity (TA)
With the passage of time, TA showed a constantly decreasing trend which was significantly (p ≤ 0.05) affected by the treatments. In the beginning, persimmon fruits showed 0.502% TA (content that to minimum point on the 20th day) (Table 3). On the 4th day, all treatments were statistically similar except for 1% TCG, which showed higher TA (0.492%). Thereafter, all TCG concentrations preserved significantly higher TA. However, on the 20th day, the 1% TCG-applied persimmon fruits had a considerably greater (1.62-fold) TA than that of uncoated fruits.
TA is linked with the concentration of organic acids present in fruits that play an important role in fruit quality [25]. Organic acids are also consumed during the ripening of fruits where the oxidation of organic acids occurs during the tricarboxylic acid cycle [60]. Coating with plant-based gum markedly suppresses respiration and thus also the oxidation of organic acid in coated fruits [38,44]. Our results are in line with the previous finding which reported the stabilizing effect of natural gums-based edible coatings on TA in coated fruits such as banana [40], mango [38], and jamun [49].
3.9.3. Sensory Evaluation
Statistical analysis showed that sensory attributes (taste, aroma, and overall acceptability) first progressed with the passage of time and then reduced in uncoated fruits (Table 3). On the other hand, TCG coatings, especially 1% TCG, led to steady development in sensory scores throughout the storage period (Figure 5). Hence, the 1% TCG-coated fruits showed the highest sensory score for the studied sensory traits at the end of the storage period. On the 20th day, uncoated fruits showed the lowest taste score (26% lower) compared with the 1% TCG-coated fruits. Similarly, on the 20th day, the 1% TCG-coated fruits showed the highest aroma score (8.58) compared with the lowest score achieved by uncoated fruits (5.75). Contrary to uncoated fruits, TCG-coated fruits showed gradual development in overall acceptability score. From the 16th to the 20th day, TCG-coated fruits showed a significantly higher overall acceptability score compared with uncoated fruits. On the 20th day, the overall acceptability scores shown by the treatments in descending order are as follows: 1% TCG > 0.5% TCG > 1.5% TCG > control.

Figure 5.
Effect of tragacanth gum application on visual appearance of persimmon fruits stored for 20 days at 20 ± 2 °C and 80–85% RH.
A higher taste score for uncoated fruits might be due to the accumulation of soluble sugars (Table 3) and the consumption of organic acid (Table 3) which ultimately improved the taste score. The retention of aroma for a longer duration by the fruits coated with plant-based gum might be due to preserved biochemical attributes [10]. Increased weight loss of fruits and vegetables increases the shriveling and deteriorates visual appearance. Ultimately, weight loss results in a significant reduction in consumer acceptance, purchase intentions, and market potential [10]. TCG application prevents weight loss, ensuring customer acceptability of coated fruits [10]. The application of coating based on natural gums has been shown to preserve the sensory attributes in coated tomato fruits [16]. The use of plant-based gum on fruit inhibited the loss in the sensory score and delayed the postharvest senescence by maintaining the fruit quality and customer acceptance [15]. Our results are also in line with Anjum et al. [41], whereby guava fruits coated with Arabic gum had higher sensory scores. In our study, the high ripening rate and weight loss of uncoated fruits caused a quick change in firmness, aroma, taste, and color, leading to a rapid loss of sensory attributes compared with TCG-coated fruits. Therefore, TCG-coated fruits showed higher sensory scores which may be due to the delays in ripening and senescence caused by low respiration rate and ethylene production (Figure 1C,D).
4. Conclusions
The application of TCG preserved fruit quality and prolonged the postharvest storage life of harvested persimmon fruits. The use of TCG reduced the physiological weight loss, decay incidence, respiration rate, ethylene production, membrane leakage, MDA, and H2O2 content in coated persimmon fruits. TCG-coated fruits accumulated more antioxidants such as flavonoids, polyphenols, ascorbic acid, and soluble tannin, and also showed higher APX, CAT, SOD, and POD activities. Reduced fruit firmness and activity of PME, PG, and CX enzymes were also determined in TCG-coated fruits. Similarly, higher fruit quality and sensory scores were maintained in TCG-applied fruits than in uncoated persimmon fruits. In addition, the application of 1% TCG on persimmon fruits comparatively better-maintained fruit quality compared with the 0.5% or 1.5% TCG-coated fruits. The reduced efficiency of 1.5% TCG-based coating might be due to a too high concentration of TCG for smooth- and thin-skinned fruit like persimmons. Therefore, the application of 1% TCG can be recommended for the storage of persimmon fruits under ambient storage conditions.
Author Contributions
Conceptualization, S.E. (Shaghef Ejaz), M.A.A., G.I. and R.A.M.; formal analysis, M.S.S., S.H. and G.I.; investigation, M.S.S. and S.A.; methodology, S.E. (Shaghef Ejaz) and M.A.A.; supervision, S.E. (Shaghef Ejaz) and M.A.A.; visualization, G.I. and R.A.M.; writing—original draft, M.S.S., S.A., S.E. (Sezai Ercisli), S.S. and J.M.; writing—review and editing, S.E. (Sezai Ercisli), S.S. and J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the internal grant of Tomas Bata University in Zlin (No. IGA/FT/2022/004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (S.E.)
Conflicts of Interest
The authors declare no conflict of interest.
References
- Denoya, G.I.; Pataro, G.; Ferrari, G. Effects of postharvest pulsed light treatments on the quality and antioxidant properties of persimmons during storage. Postharvest Biol. Technol. 2020, 160, 111055. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Lin, H.; Lin, M.; Chen, Y.; Lin, Y. 1-Methylcyclopropene containing-papers suppress the disassembly of cell wall polysaccharides in anxi persimmon fruit during storage. Int. J. Biol. Macromol. 2020, 151, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Plaza, L.; Colina, C.; De Ancos, B.; Sánchez-Moreno, C.; Pilar Cano, M. Influence of ripening and astringency on carotenoid content of high-pressure treated persimmon fruit (Diospyros kaki L.). Food Chem. 2012, 130, 591–597. [Google Scholar] [CrossRef]
- Luo, Z. Effect of 1-methylcyclopropene on ripening of postharvest persimmon (Diospyros kaki L.) fruit. LWT Food Sci. Technol. 2007, 40, 285–291. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Luo, Z.; Sun, J.; Li, L.; Yin, X.; Li, J.; Xu, Y. Effects of inside-out heat-shock via microwave on the fruit softening and quality of persimmon during postharvest storage. Food Chem. 2021, 349, 129161. [Google Scholar] [CrossRef]
- Niazi, Z.; Razavi, F.; Khademi, O.; Aghdam, M.S. Exogenous Application of hydrogen sulfide and γ-aminobutyric acid alleviates chilling injury and preserves quality of persimmon fruit (Diospyros kaki, Cv. Karaj) during cold storage. Sci. Hortic. 2021, 285, 110198. [Google Scholar] [CrossRef]
- Imahori, Y.; Bai, J.; Baldwin, E. Antioxidative responses of ripe tomato fruit to postharvest chilling and heating treatments. Sci. Hortic. 2016, 198, 398–406. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Mohamed Amin Tawakkal, I.S.; Muda Mohamed, M.T. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Saha, A.; Tyagi, S.; Gupta, R.K.; Tyagi, Y.K. Natural gums of plant origin as edible coatings for food industry applications. Crit. Rev. Biotechnol. 2017, 37, 959–973. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Sardar, H.; Saddiq, B. Tragacanth gum coating modulates oxidative stress and maintains quality of harvested apricot fruits. Int. J. Biol. Macromol. 2020, 163, 2439–2447. [Google Scholar] [CrossRef]
- Khaliq, G.; Muda Mohamed, M.T.; Ghazali, H.M.; Ding, P.; Ali, A. Influence of gum arabic coating enriched with calcium chloride on physiological, biochemical and quality responses of mango (Mangifera indica L.) fruit stored under low temperature stress. Postharvest Biol. Technol. 2016, 111, 362–369. [Google Scholar] [CrossRef]
- Saleem, M.S.; Ejaz, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Hussain, S.; Ali, S.; Canan, İ. Postharvest application of gum arabic edible coating delays ripening and maintains quality of persimmon fruits during storage. J. Food Process. Preserv. 2020, 44, e14583. [Google Scholar] [CrossRef]
- Etemadipoor, R.; Mirzaalian Dastjerdi, A.; Ramezanian, A.; Ehteshami, S. Ameliorative effect of gum arabic, oleic acid and/or cinnamon essential oil on chilling injury and quality loss of guava fruit. Sci. Hortic. 2020, 266, 109255. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Gol, N.B.; Chaudhari, M.L.; Rao, T.V.R. Effect of edible coatings on quality and shelf life of carambola (Averrhoa carambola L.) fruit during storage. J. Food Sci. Technol. 2015, 52, 78–91. [Google Scholar] [CrossRef]
- Shakir, M.S.; Ejaz, S.; Hussain, S.; Ali, S.; Sardar, H.; Azam, M.; Ullah, S.; Khaliq, G.; Saleem, M.S.; Nawaz, A.; et al. Synergistic effect of gum arabic and carboxymethyl cellulose as biocomposite coating delays senescence in stored tomatoes by regulating antioxidants and cell wall degradation. Int. J. Biol. Macromol. 2022, 201, 641–652. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Kadkhodaee, R.; Emadzadeh, B.; Koocheki, A. Preparation and characterization of tragacanth-locust bean gum edible blend films. Carbohydr. Polym. 2016, 139, 20–27. [Google Scholar] [CrossRef]
- Nazarzadeh Zare, E.; Makvandi, P.; Tay, F.R. Recent progress in the industrial and biomedical applications of tragacanth gum: A review. Carbohydr. Polym. 2019, 212, 450–467. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Tragacanth gum containing zataria multiflora boiss. essential oil as a natural preservative for storage of button mushrooms (Agaricus bisporus). Food Hydrocoll. 2017, 72, 202–209. [Google Scholar] [CrossRef]
- Ali, S.; Zahid, N.; Nawaz, A.; Naz, S.; Ejaz, S.; Ullah, S. Tragacanth Gum Coating Suppresses the Disassembly of Cell Wall Polysaccharides and Delays Softening of Harvested Mango (Mangifera indica L.) Fruit. Int. J. Biol. Macromol. 2022, 222, 521–532. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Application of tragacanth gum impregnated with satureja khuzistanica essential oil as a natural coating for enhancement of postharvest quality and shelf life of button mushroom (Agaricus bisporus). Int. J. Biol. Macromol. 2018, 106, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, F.; Cheng, J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011, 127, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Tian, S. Effect of oxalic acid on control of postharvest browning of litchi Fruit. Food Chem. 2006, 96, 519–523. [Google Scholar] [CrossRef]
- Velikova, V.; Loreto, F. On the relationship between isoprene emission and thermotolerance in phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ. 2005, 28, 318–327. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Rastegar, S. Preservation of mango fruit with guar-based edible coatings enriched with spirulina platensis and aloe vera extract during storage at ambient temperature. Sci. Hortic. 2020, 265, 109258. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shahid, M. Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruits. Food Chem. 2016, 206, 18–29. [Google Scholar] [CrossRef]
- Taira, S. Astringency in Persimmon. In Modern Method of Plant Analysis, Fruit Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin, Germany, 1996; pp. 97–110. [Google Scholar]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar]
- Liu, D.; Zou, J.; Meng, Q.; Zou, J.; Jiang, W. Uptake and accumulation and oxidative stress in garlic (Allium sativum L.) under lead phytotoxicity. Ecotoxicology 2009, 18, 134–143. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Liu, K.; Liu, J.; Li, H.; Yuan, C.; Zhong, J.; Chen, Y. Influence of postharvest citric acid and chitosan coating treatment on ripening attributes and expression of cell wall related genes in cherimoya (Annona cherimola Mill.) fruit. Sci. Hortic. 2016, 198, 1–11. [Google Scholar] [CrossRef]
- Awad, M.; Young, R.E. Postharvest variation in cellulase, polygalacturonase, and pectinmethylesterase in avocado (Persea americana Mill, cv. fuerte) fruits in relation to respiration and ethylene production. Plant Physiol. 1979, 64, 306–308. [Google Scholar] [CrossRef][Green Version]
- Yoshida, O.; Nakagawa, H.; Ogura, N.; Sato, T. Effect of heat treatment on the development of polygalacturonase activity in tomato fruit during ripening. Plant Cell Physiol. 1984, 25, 505–509. [Google Scholar]
- Deng, Y.; Wu, Y.; Li, Y. Changes in firmness, cell wall composition and cell wall hydrolases of grapes stored in high oxygen atmospheres. Food Res. Int. 2005, 38, 769–776. [Google Scholar] [CrossRef]
- Horwitz, W. Official and Tentative Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1960; pp. 314–320. [Google Scholar]
- Khaliq, G.; Muda Mohamed, M.T.; Ali, A.; Ding, P.; Ghazali, H.M. Effect of gum arabic coating combined with calcium chloride on physico-chemical and qualitative properties of mango (Mangifera indica L.) fruit during low temperature storage. Sci. Hortic. 2015, 190, 187–194. [Google Scholar] [CrossRef]
- Daisy, L.L.; Nduko, J.M.; Joseph, W.M.; Richard, S.M. Effect of edible gum arabic coating on the shelf life and quality of mangoes (Mangifera indica) during storage. J. Food Sci. Technol. 2020, 57, 79–85. [Google Scholar] [CrossRef]
- Etemadipoor, R.; Ramezanian, A.; Mirzaalian Dastjerdi, A.; Shamili, M. The Potential of gum arabic enriched with cinnamon essential oil for improving the qualitative characteristics and storability of guava (Psidium guajava L.) Fruit. Sci. Hortic. 2019, 251, 101–107. [Google Scholar] [CrossRef]
- Alali, A.A.; Awad, M.A.; Al-Qurashi, A.D.; Mohamed, S.A. Postharvest gum arabic and salicylic acid dipping affect quality and biochemical changes of ‘Grand Nain’ bananas during shelf Life. Sci. Hortic. 2018, 237, 51–58. [Google Scholar] [CrossRef]
- Anjum, M.A.; Akram, H.; Zaidi, M.; Ali, S. Effect of gum arabic and aloe vera gel based edible coatings in combination with plant extracts on postharvest quality and storability of ‘Gola’ guava fruits. Sci. Hortic. 2020, 271, 109506. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Jiyong, S.; Mahunu, G.K.; Zhai, X.; Mariod, A.A. Quality and postharvest-shelf life of cold-stored strawberry fruit as affected by gum arabic (Acacia Senegal) edible coating. J. Food Biochem. 2018, 42, e12527. [Google Scholar] [CrossRef]
- Dong, F.; Wang, X. Guar gum and ginseng extract coatings maintain the quality of sweet cherry. LWT Food Sci. Technol. 2018, 89, 117–122. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Saleem, M.S.; Tul-Ain Haider, S.; Ul Hasan, M. Effect of gum arabic coating on antioxidative enzyme activities and quality of apricot (Prunus armeniaca L.) fruit during ambient storage. J. Food Biochem. 2021, 45, e13656. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.S.; Ejaz, S.; Anjum, M.A.; Ali, S.; Hussain, S.; Nawaz, A.; Naz, S.; Maqbool, M.; Abbas, A.M. Aloe vera gel coating delays softening and maintains quality of stored persimmon (Diospyros kaki Thunb.) Fruits. J. Food Sci. Technol. 2022, 59, 3296–3306. [Google Scholar] [CrossRef]
- Kumar, N.; Ojha, A.; Upadhyay, A.; Singh, R.; Kumar, S. Effect of active chitosan-pullulan composite edible coating enrich with pomegranate peel extract on the storage quality of green bell pepper. LWT 2021, 138, 110435. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A Review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. The effect of edible coating based on arabic gum, sodium caseinate and essential oil of cinnamon and lemon grass on guava. Food Chem. 2018, 245, 820–828. [Google Scholar] [CrossRef]
- Khaliq, G.; Saleh, A.; Bugti, G.A.; Hakeem, K.R. Guggul gum incorporated with basil essential oil improves quality and modulates cell wall-degrading enzymes of jamun fruit during storage. Sci. Hortic. 2020, 273, 109608. [Google Scholar] [CrossRef]
- Nasr, F.; Pateiro, M.; Rabiei, V.; Razzvi, F.; Formaneck, S.; Gohari, G.; Lorenzo, J.M. Chitosan-Phenylalanine Nanoparticles (Cs-Phe Nps) Extend the Postharvest Life of Persimmon (Diospyros Kaki) Fruits under Chilling Stress. Coatings 2021, 11, 819. [Google Scholar] [CrossRef]
- Xue, J.; Huang, L.; Zhang, S.; Sun, H.; Gao, T. Study on the evaluation of carboxymethyl-chitosan concentration and temperature treatment on the quality of “Niuxin” persimmon during cold storage. J. Food Process. Preserv. 2020, 44, e14560. [Google Scholar] [CrossRef]
- Saleem, M.S.; Anjum, M.A.; Naz, S.; Ali, S.; Hussain, S.; Azam, M.; Sardar, H.; Khaliq, G.; Canan, İ.; Ejaz, S. Incorporation of ascorbic acid in chitosan-based edible coating improves postharvest quality and storability of strawberry fruits. Int. J. Biol. Macromol. 2021, 189, 160–169. [Google Scholar] [CrossRef]
- Kou, J.; Zhao, Z.; Wang, W.; Wei, C.; Guan, J.; Ference, C. Comparative study of ripening related gene expression and postharvest physiological changes between astringent and nonastringent persimmon cultivars. J. Am. Soc. Hortic. Sci. 2020, 145, 203–212. [Google Scholar] [CrossRef]
- Kou, J.; Wei, C.; Zhao, Z.; Guan, J.; Wang, W. Effects of ethylene and 1-methylcyclopropene treatments on physiological changes and ripening-related gene expression of ‘Mopan’ persimmon fruit during storage. Postharvest Biol. Technol. 2020, 166, 111185. [Google Scholar] [CrossRef]
- Sanchís, E.; González, S.; Ghidelli, C.; Sheth, C.C.; Mateos, M.; Palou, L.; Pérez-Gago, M.B. Browning inhibition and microbial control in fresh-cut persimmon (Diospyros kaki Thunb. Cv. Rojo Brillante) by apple pectin-based edible coatings. Postharvest Biol. Technol. 2016, 112, 186–193. [Google Scholar] [CrossRef]
- Formiga, A.S.; Pinsetta, J.S.; Pereira, E.M.; Cordeiro, I.N.F.; Mattiuz, B.H. Use of edible coatings based on hydroxypropyl methylcellulose and beeswax in the conservation of red guava ‘Pedro Sato’. Food Chem. 2019, 290, 144–151. [Google Scholar] [CrossRef]
- Sousa, F.F.; Pinsetta Junior, J.S.; Oliveira, K.T.E.F.; Rodrigues, E.C.N.; Andrade, J.P.; Mattiuz, B.H. Conservation of ‘Palmer’ mango with an edible coating of hydroxypropyl methylcellulose and beeswax. Food Chem. 2021, 346, 128925. [Google Scholar] [CrossRef]
- Bignell, G.; Bruun, D.; Oag, D.; Nissen, B.; George, A. Persimmon Postharvest Manual, 2nd ed.; Department of Agriculture and Fisheries: Brisbane City, QLD, Australia, 2017.
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in organic acid profiles during fruit development and ripening: Correlation or causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).