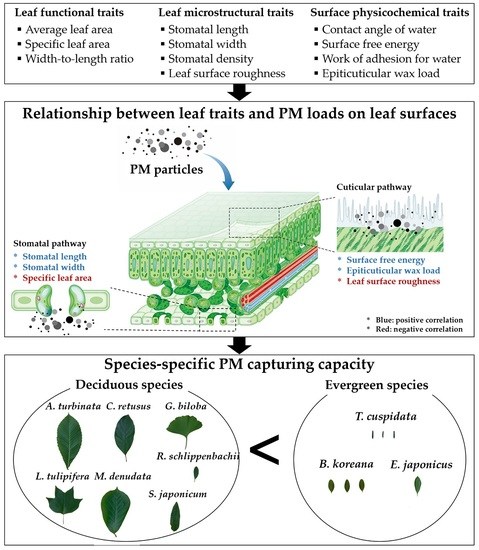
Relationship between Leaf Traits and PM-Capturing Capacity of Major Urban-Greening Species
Abstract
1. Introduction
2. Materials and Methods
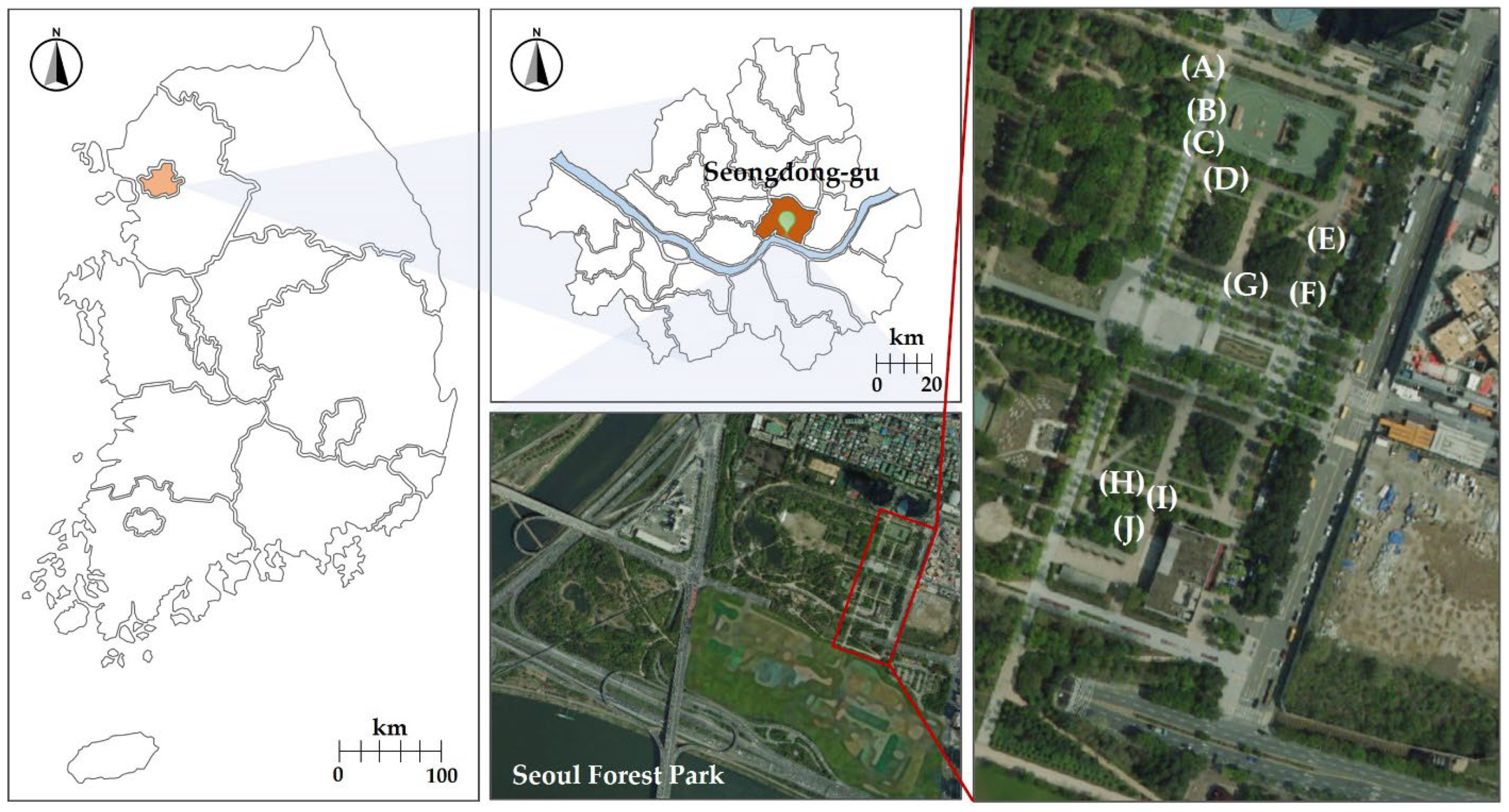
2.1. Study Site and Plant Material
2.2. Sampling
2.3. Leaf Functional Traits
2.4. Leaf Micromorphological Traits
2.5. Leaf Physicochemical Traits
2.6. Quantitative Assessment of PM
2.7. Leaf Area Index (LAI)
2.8. Statistical Analysis
3. Results
3.1. Leaf Functional Traits
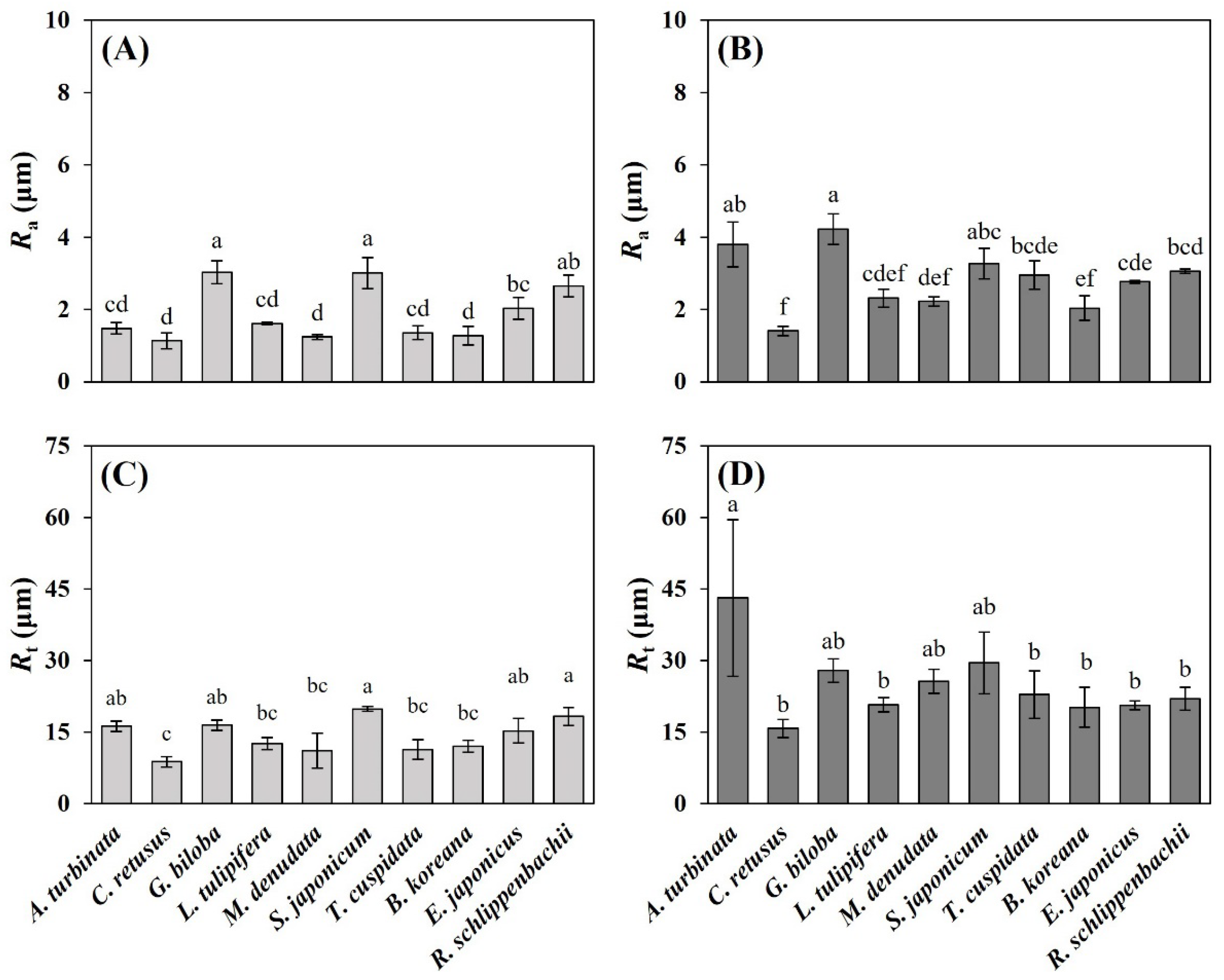
3.2. Leaf Micromorphological Traits
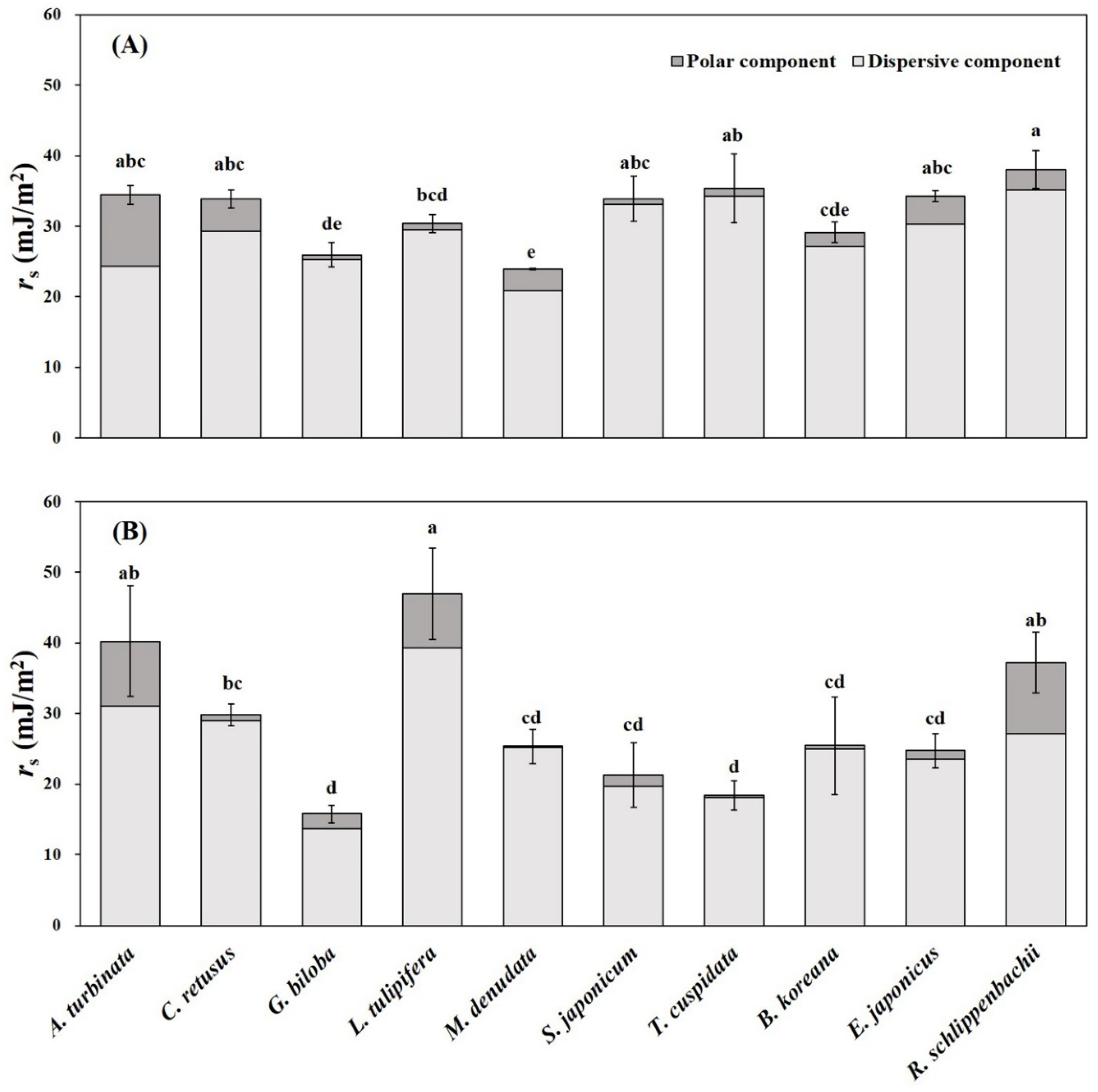
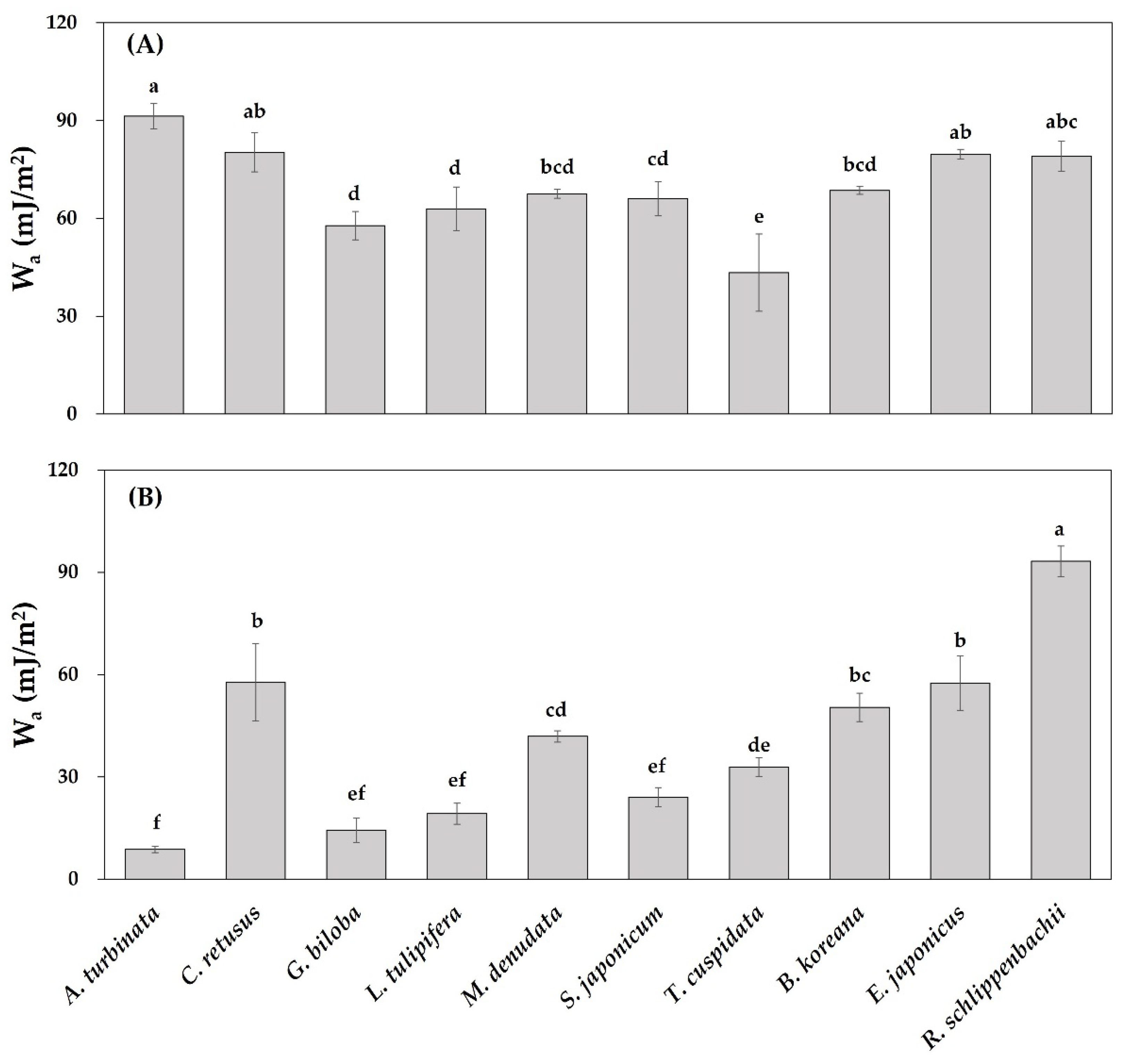
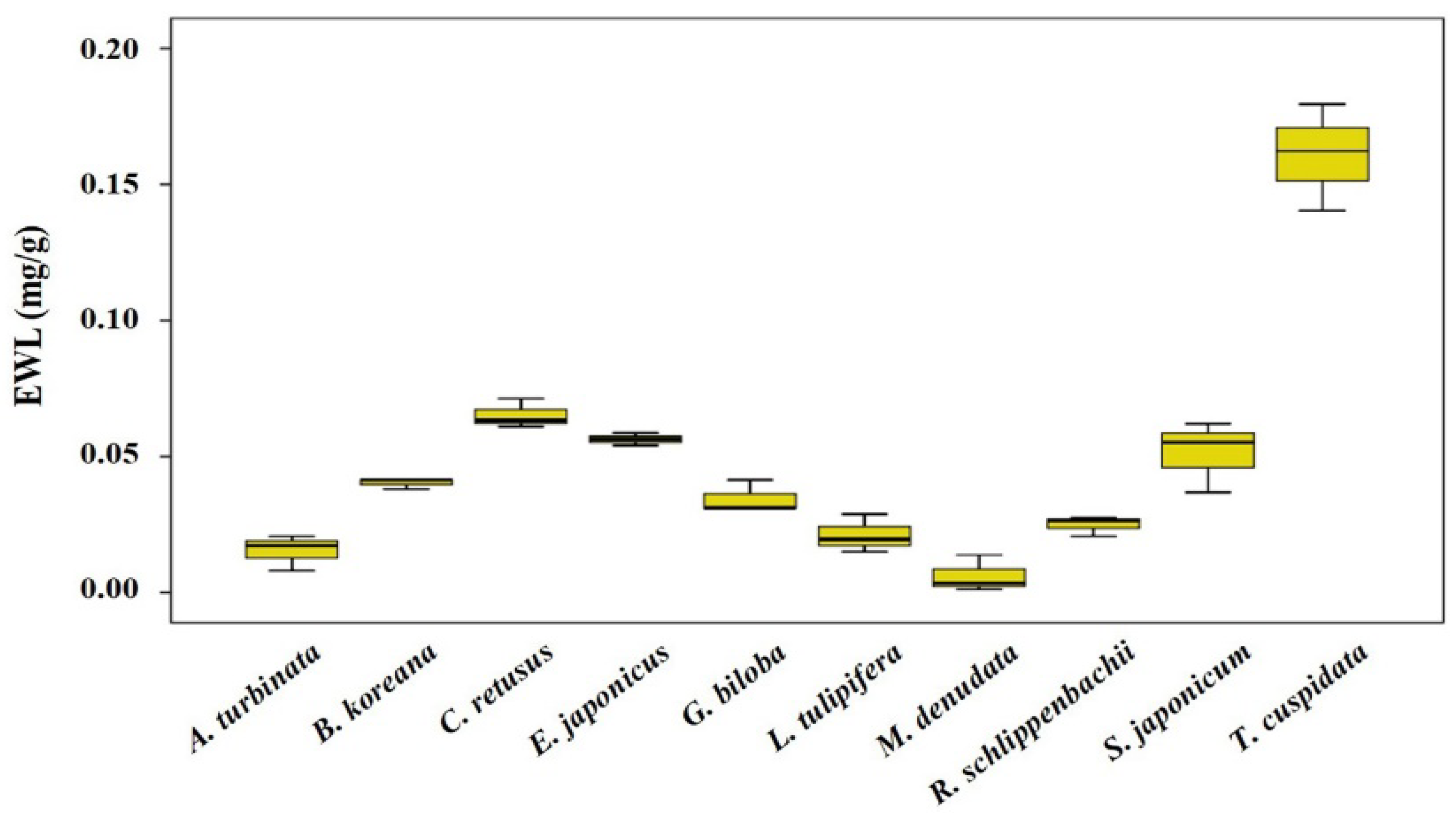
3.3. Leaf Physicochemical Traits
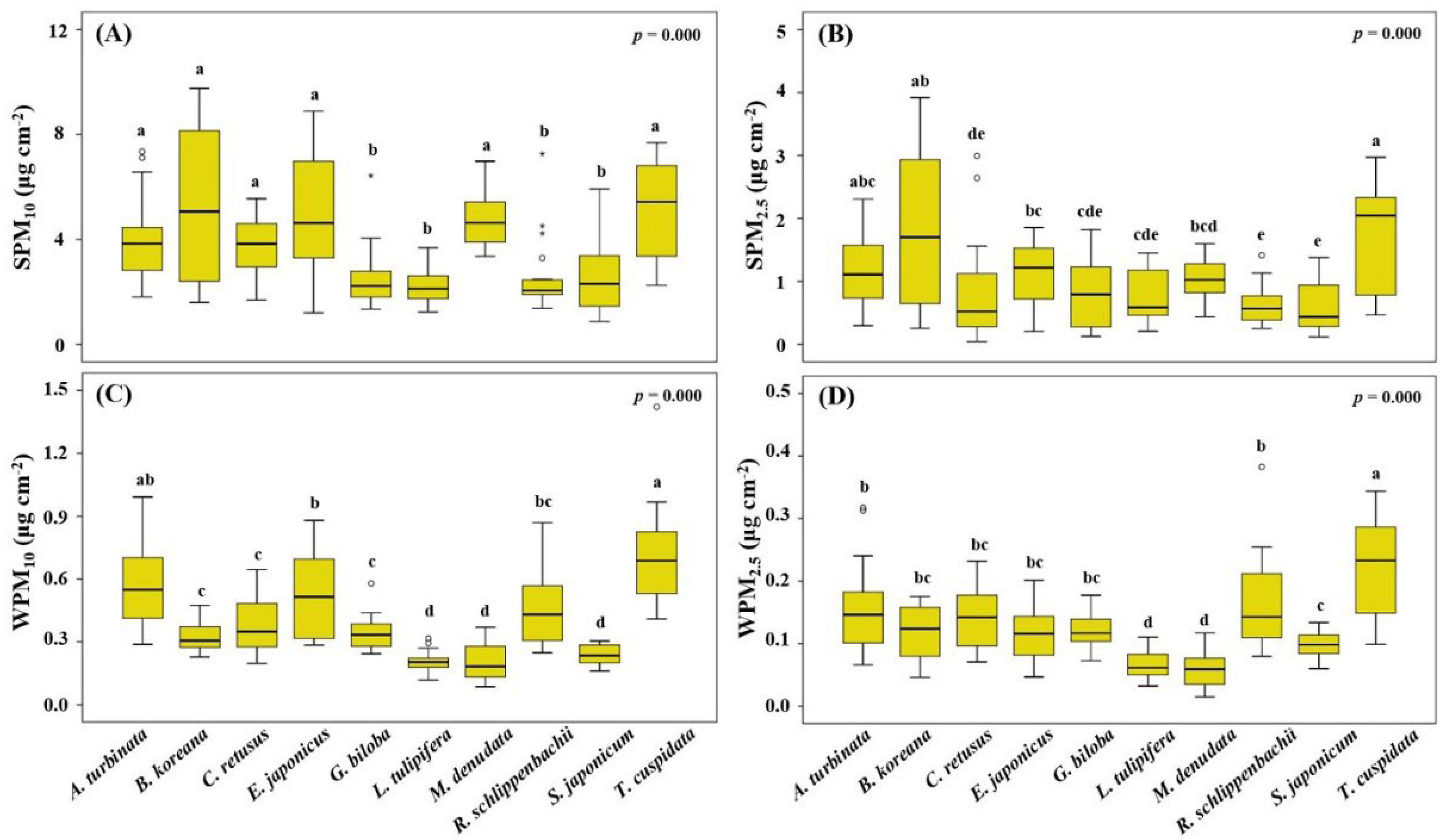
3.4. PM Load on Leaf Surfaces and in Wax Layers
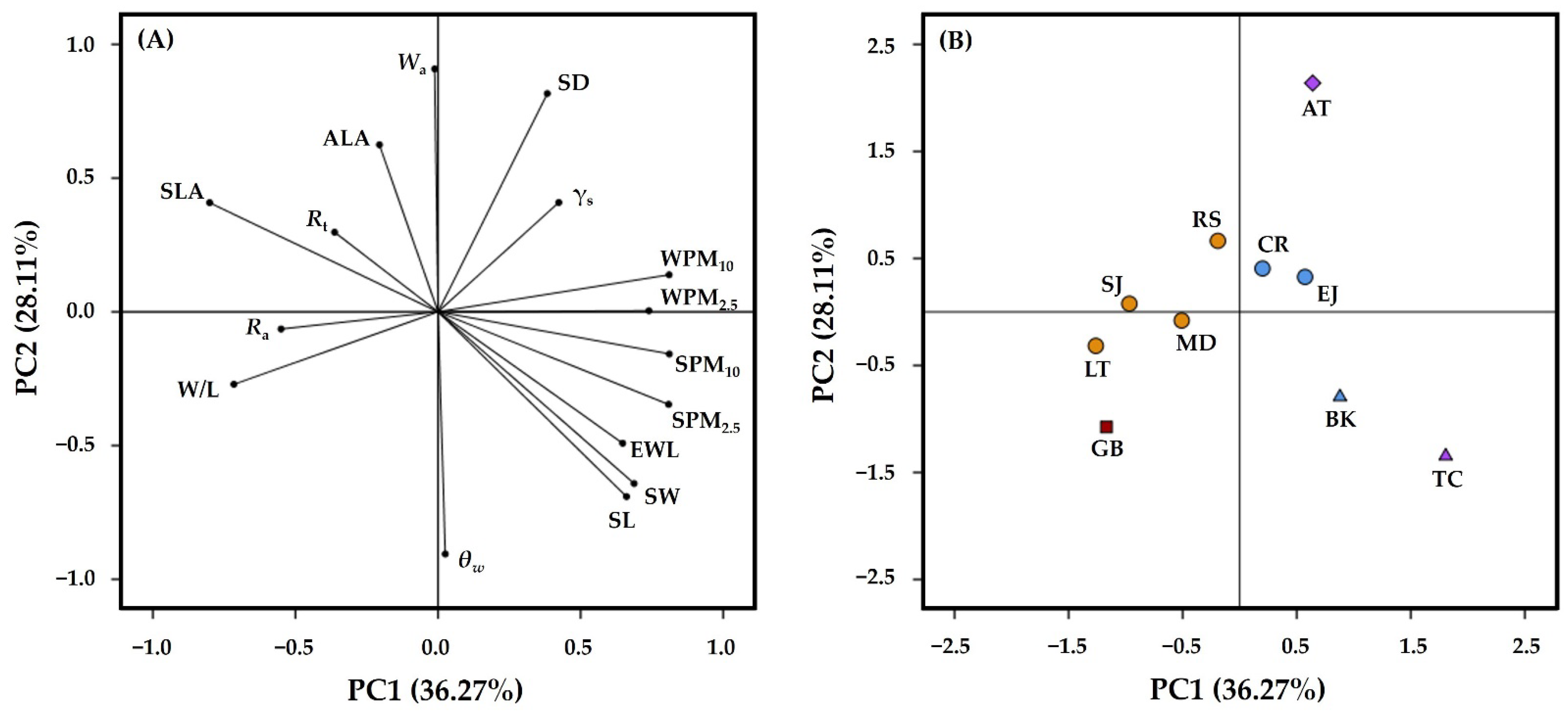
3.5. Relationship between Leaf Traits and PM Capturing Capability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, M.; Xin, Z.; Yu, X. Spatio-temporal variations in PM leaf deposition: A meta-analysis. Environ. Pollut. 2017, 231, 207–218. [Google Scholar] [CrossRef]
- Rai, P.K. Impacts of particulate matter pollution on plants: Implications for environmental biomonitoring. Ecotoxicol. Environ. Saf. 2016, 129, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Chiam, Z.; Song, X.P.; Lai, H.R.; Tan, H.T.W. Particulate matter mitigation via plants: Understanding complex relationships with leaf traits. Sci. Total Environ. 2019, 688, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, H.; Liao, W.; Wang, Z. Accumulation of particles on the surface of leaves during leaf expansion. Sci. Total Environ. 2015, 532, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, M.S.; de Oliveira Galvão, M.F.; de Medeiros, S.R.B. Cell death pathways of particulate matter toxicity. Chemosphere 2017, 188, 32–48. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; World Health Organization: Geneva, Switzerland, 2016; pp. 44–47. Available online: https://apps.who.int/iris/handle/10665/250141 (accessed on 23 December 2021).
- Beckett, K.P.; Freer-Smith, P.H.; Taylor, G. Urban woodlands: Their role in reducing the effects of particulate pollution. Environ. Pollut. 1998, 99, 347–360. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Chen, L.; McNulty, S. An investigation on the leaf accumulation-removal efficiency of atmospheric particulate matter for five urban plant species under different rainfall regimes. Atmos. Environ. 2019, 208, 123–132. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, R.; Kumari, K.M.; Srivastava, S.S. Atmospheric dry deposition to leaf surfaces at a rural site of India. Chemosphere 2004, 55, 1097–1107. [Google Scholar] [CrossRef]
- Yli-Pelkonen, V.; Setälä, H.; Viippola, V. Urban forests near roads do not reduce gaseous air pollutant concentrations but have an impact on particles levels. Landsc. Urban Plan. 2017, 158, 39–47. [Google Scholar] [CrossRef]
- Yang, J.; McBride, J.; Zhou, J.; Sun, Z. The urban forest in Beijing and its role in air pollution reduction. Urban For. Urban Green. 2005, 3, 65–78. [Google Scholar] [CrossRef]
- Nowak, D.J. Institutionalizing urban forestry as a “biotechnology” to improve environmental quality. Urban For. Urban Green. 2006, 5, 93–100. [Google Scholar] [CrossRef]
- Freer-Smith, P.H.; Beckett, K.P.; Taylor, G. Deposition velocities to Sorbus aria, Acer campestre, Populus deltoides × trichocarpa ‘Beaupré’, Pinus nigra and × Cupressocyparis leylandii for coarse, fine and ultra-fine particles in the urban environment. Environ. Pollut. 2005, 133, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Maher, B.A.; Li, F.; Wang, X.; Sun, X.; Zhang, H. Particulate matter deposited on leaf of five evergreen species in Beijing, China: Source identification and size distribution. Atmos. Environ. 2015, 105, 53–60. [Google Scholar] [CrossRef]
- Popek, R.; Gawrońska, H.; Wrochna, M.; Gawroński, S.W.; Sæbø, A. Particulate matter on foliage of 13 woody species: Deposition on surfaces and phytostabilisation in waxes–a 3-year study. Int. J. Phytoremediat. 2013, 15, 245–256. [Google Scholar] [CrossRef]
- Sgrigna, G.; Baldacchini, C.; Dreveck, S.; Cheng, Z.; Calfapietra, C. Relationships between air particulate matter capture efficiency and leaf traits in twelve tree species from an Italian urban-industrial environment. Sci. Total Environ. 2020, 718, 137310. [Google Scholar] [CrossRef]
- Sæbø, A.; Popek, R.; Nawrot, B.; Hanslin, H.M.; Gawronska, H.; Gawronski, S.W. Plant species differences in particulate matter accumulation on leaf surfaces. Sci. Total Environ. 2012, 427, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Weerakkody, U.; Dover, J.W.; Mitchell, P.; Reiling, K. Particulate matter pollution capture by leaves of seventeen living wall species with special reference to rail-traffic at a metropolitan station. Urban For. Urban Green. 2017, 27, 173–186. [Google Scholar] [CrossRef]
- Weerakkody, U.; Dover, J.W.; Mitchell, P.; Reiling, K. Evaluating the impact of individual leaf traits on atmospheric particulate matter accumulation using natural and synthetic leaves. Urban For. Urban Green. 2018, 30, 98–107. [Google Scholar] [CrossRef]
- Zhang, X.; Lyu, J.; Han, Y.; Sun, N.; Sun, W.; Li, J.; Liu, C.; Yin, S. Effects of the leaf functional traits of coniferous and broadleaved trees in subtropical monsoon regions on PM2.5 dry deposition velocities. Environ. Pollut. 2020, 265, 114845. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Zhang, L.; Zou, R.; Zhang, Z. Variation in tree species ability to capture and retain airborne fine particulate matter (PM2.5). Sci. Rep. 2017, 7, 3206. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, B.; Niu, X. Relationship between leaf surface characteristics and particle capturing capacities of different tree species in Beijing. Forests 2017, 8, 92. [Google Scholar] [CrossRef]
- He, C.; Qiu, K.; Alahmad, A.; Pott, R. Particulate matter capturing capacity of roadside evergreen vegetation during the winter season. Urban For. Urban Green. 2020, 48, 126510. [Google Scholar] [CrossRef]
- Tian, L.; Yin, S.; Ma, Y.; Kang, H.; Zhang, X.; Tan, H.; Meng, H.; Liu, C. Impact factor assessment of the uptake and accumulation of polycyclic aromatic hydrocarbons by plant leaves: Morphological characteristics have the greatest impact. Sci. Total Environ. 2019, 652, 1149–1155. [Google Scholar] [CrossRef]
- Wang, H.; Shi, H.; Li, Y.; Yu, Y.; Zhang, J. Seasonal variations in leaf capturing of particulate matter, surface wettability and micromorphology in urban tree species. Front. Environ. Sci. Eng. 2013, 7, 579–588. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Meng, H.; Zhang, T. How does leaf surface micromorphology of different trees impact their ability to capture particulate matter? Forests 2018, 9, 681. [Google Scholar] [CrossRef]
- Muhammad, S.; Wuyts, K.; Nuyts, G.; De Wael, K.; Samson, R. Characterization of epicuticular wax structures on leaves of urban plant species and its association with leaf wettability. Urban For. Urban Green. 2020, 47, 126557. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Seasonal changes of leaf surface contamination in beech, oak, and ginkgo in relation to leaf micromorphology and wettability. New Phytol. 1998, 138, 91–98. [Google Scholar] [CrossRef]
- Sgrigna, G.; Sæbø, A.; Gawronski, S.; Popek, R.; Calfapietra, C. Particulate Matter deposition on Quercus ilex leaves in an industrial city of central Italy. Environ. Pollut. 2015, 197, 187–194. [Google Scholar] [CrossRef]
- Kwak, M.J.; Lee, J.K.; Park, S.; Kim, H.; Lim, Y.J.; Lee, K.A.; Son, J.-A.; Oh, C.-Y.; Kim, I.; Woo, S.Y. Surface-based analysis of leaf microstructures for adsorbing and retaining capability of airborne particulate matter in ten woody species. Forests 2020, 11, 946. [Google Scholar] [CrossRef]
- Abbott, J.R.; Zhu, H. 3D optical surface profiler for quantifying leaf surface roughness. Surf. Topogr. Metrol. Prop. 2019, 7, 045016. [Google Scholar] [CrossRef]
- de Groot, P. Principles of interference microscopy for the measurement of surface topography. Adv. Opt. Photonics 2015, 7, 1–65. [Google Scholar] [CrossRef]
- Holder, C.D. Leaf water repellency of species in Guatemala and Colorado (USA) and its significance to forest hydrology studies. J. Hydrol. 2007, 336, 147–154. [Google Scholar] [CrossRef]
- Alghunaim, A.; Kirdponpattara, S.; Newby, B.M.Z. Techniques for determining contact angle and wettability of powders. Powder Technol. 2016, 287, 201–215. [Google Scholar] [CrossRef]
- Wang, H.; Shi, H.; Li, Y.; Wang, Y. The effects of leaf roughness, surface free energy and work of adhesion on leaf water drop adhesion. PLoS ONE 2014, 9, e107062. [Google Scholar] [CrossRef] [PubMed]
- Gindl, M.; Sinn, G.; Gindl, W.; Reiterer, A.; Tschegg, S. A comparison of different methods to calculate the surface free energy of wood using contact angle measurements. Colloids Surf. A Physicochem. Eng. Asp. 2001, 181, 279–287. [Google Scholar] [CrossRef]
- Young, T. III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Fowkes, F.M. Determination of interfacial tensions, contact angles, and dispersion forces in surfaces by assuming additivity of intermolecular interactions in surfaces. J. Phys. Chem. 1962, 66, 382. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Ebercon, A.; Blum, A.; Jordan, W.R. A rapid colorimetric method for epicuticular wax contest of sorghum leaves. Crop Sci. 1977, 17, 179–180. [Google Scholar] [CrossRef]
- Huggins, T.D.; Mohammed, S.; Sengodon, P.; Ibrahim, A.M.H.; Tilley, M.; Hays, D.B. Changes in leaf epicuticular wax load and its effect on leaf temperature and physiological traits in wheat cultivars (Triticum aestivum L.) exposed to high temperatures during anthesis. J. Agron. Crop Sci. 2018, 204, 49–61. [Google Scholar] [CrossRef]
- Dzierżanowski, K.; Popek, R.; Gawrońska, H.; Sæbø, A.; Gawroński, S.W. Deposition of particulate matter of different size fractions on leaf surfaces and in waxes of urban forest species. Int. J. Phytoremediat. 2011, 13, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Black, T.A.; Adams, R.S. Evaluation of hemispherical photography for determining plant area index and geometry of a forest stand. Agric. For. Meteorol. 1991, 56, 129–143. [Google Scholar] [CrossRef]
- Thimonier, A.; Sedivy, I.; Schleppi, P. Estimating leaf area index in different types of mature forest stands in Switzerland: A comparison of methods. Eur. J. For. Res. 2010, 129, 543–562. [Google Scholar] [CrossRef]
- Aryal, B.; Neuner, G. Leaf wettability decreases along an extreme altitudinal gradient. Oecologia 2010, 162, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.; Shi, Y.; Tang, J.; Liu, X.; Feng, C.; Zhang, Y. Spatial-temporal variability and dust-capture capability of 8 plants in urban China. Pol. J. Environ. Stud. 2018, 28, 453–462. [Google Scholar] [CrossRef]
- Liang, D.; Ma, C.; Wang, Y.Q.; Wang, Y.J.; Chen-xi, Z. Quantifying PM2.5 capture capability of greening trees based on leaf factors analyzing. Environ. Sci. Pollut. Res. 2016, 23, 21176–21186. [Google Scholar] [CrossRef]
- Simon, E.; Baranyai, E.; Braun, M.; Cserháti, C.; Fábián, I.; Tóthmérész, B. Elemental concentrations in deposited dust on leaves along an urbanization gradient. Sci. Total Environ. 2014, 490, 514–520. [Google Scholar] [CrossRef]
- Fernández, V.; Khayet, M.; Montero-Prado, P.; Heredia-Guerrero, J.A.; Liakopoulos, G.; Karabourniotis, G.; del Río, V.; Domínguez, E.; Tacchini, I.; Nerín, C.; et al. New insights into the properties of pubescent surfaces: Peach fruit as a model. Plant Physiol. 2011, 156, 2098–2108. [Google Scholar] [CrossRef]
- Adriaenssens, S.; Staelens, J.; Wuyts, K.; De Schrijver, A.; Van Wittenberghe, S.; Wuytack, T.; Kardel, F.; Verheyen, K.; Samson, R.; Boeckx, P. Foliar nitrogen uptake from wet deposition and the relation with leaf wettability and water storage capacity. Water Air Soil Pollut. 2011, 219, 43–57. [Google Scholar] [CrossRef]
- Zhang, T.; Bai, Y.; Hong, X.; Sun, L.; Liu, Y. Particulate matter and heavy metal deposition on the leaves of Euonymus japonicus during the East Asian monsoon in Beijing, China. PLoS ONE 2017, 12, e0179840. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Cha, S.J.; Lee, J.K.; Park, J.H. Evaluation of accumulated particulate matter on roadside tree leaves and its metal content. J. Appl. Biol. Chem. 2020, 63, 161–168. [Google Scholar] [CrossRef]
- Tomašević, M.; Vukmirović, Z.; Rajšić, S.; Tasić, M.; Stevanović, B. Characterization of trace metal particles deposited on some deciduous tree leaves in an urban area. Chemosphere 2005, 61, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Gong, J.; Yang, B.; Zhang, Z.; Wang, B.; Zhu, C.; Shi, J.; Yue, K. Comparison of the suitability of plant species for greenbelt construction based on particulate matter capture capacity, air pollution tolerance index, and antioxidant system. Environ. Pollut. 2020, 263, 114615. [Google Scholar] [CrossRef]
- Kwak, M.J.; Lee, J.; Kim, H.; Park, S.; Lim, Y.; Kim, J.E.; Baek, S.G.; Seo, S.M.; Kim, K.N.; Woo, S.Y. The removal efficiencies of several temperate tree species at adsorbing airborne particulate matter in urban forests and roadsides. Forests 2019, 10, 960. [Google Scholar] [CrossRef]
- Liu, J.; Cao, Z.; Zou, S.; Liu, H.; Hai, X.; Wang, S.; Duan, J.; Xi, B.; Yan, G.; Zhang, S.; et al. An investigation of the leaf retention capacity, efficiency and mechanism for atmospheric particulate matter of five greening tree species in Beijing, China. Sci. Total Environ. 2018, 616, 417–426. [Google Scholar] [CrossRef]
- Lhotská, M.; Zemanová, V.; Pavlík, M.; Pavlíková, D.; Hnilička, F.; Popov, M. Leaf fitness and stress response after the application of contaminated soil dust particulate matter. Sci. Rep. 2022, 12, 10046. [Google Scholar] [CrossRef]
| Species | ALA | SLA | W/L Ratio |
|---|---|---|---|
| Aesculus turbinata | 225.4 ± 34.4 a | 169.9 ± 26.1 b | 0.42 ± 0.01 ef |
| Chionanthus retusus | 82.3 ± 5.4 c | 132.7 ± 5.9 cd | 0.58 ± 0.08 cd |
| Ginkgo biloba | 45.1 ± 3.8 d | 143.2 ± 7.1 bcd | 1.46 ± 0.10 a |
| Liriodendron tulipifera | 161.5 ± 22.7 b | 228.5 ± 15.2 a | 1.29 ± 0.01 b |
| Magnolia denudata | 147.2 ± 16.1 b | 145.6 ± 11.3 bcd | 0.67 ± 0.06 c |
| Styphnolobium japonicum | 19.6 ± 1.9 de | 228.7 ± 18.4 a | 0.33 ± 0.01 f |
| Taxus cuspidata | 1.1 ± 0.1 e | 57.9 ± 6.9 e | 0.17 ± 0.02 g |
| Buxus koreana | 1.4 ± 0.1 e | 55.5 ± 1.7 e | 0.48 ± 0.04 de |
| Euonymus japonicus | 19.1 ± 1.7 de | 118.8 ± 10.8 d | 0.51 ± 0.02 de |
| Rhododendron schlippenbachii | 16.4 ± 2.5 de | 153.9 ± 6.3 bc | 0.45 ± 0.05 e |
| Species | Stomata | Trichome | |||||
|---|---|---|---|---|---|---|---|
| Density | Size (μm) | Types z | Size (μm) y | ||||
| (No. mm−2) | Length | Width | Adaxial | Abaxial | Adaxial | Abaxial | |
| A. turbinata | 439 ± 44 | 12.3 ± 3.1 | 4.9 ± 1.8 | NG | 176 ± 64 | ||
| C. retusus | 157 ± 16 | 19.4 ± 2.4 | 7.5 ± 1.1 | PG | NG, PG | 191 ± 48 | |
| G. biloba | 84 ± 21 | 22.3 ± 3.6 | 14.8 ± 2.8 | ||||
| L. tulipifera | 135 ± 8 | 19.9 ± 5.3 | 7.6 ± 2.1 | ||||
| M. denudata | 208 ± 2 | 19.4 ± 3.2 | 6.4 ± 1.3 | NG | NG | 215 ± 28 | 411 ± 79 |
| S. japonicum | 161 ± 20 | 12.4 ± 1.7 | 6.2 ± 1.6 | NG, SG | NG | 202 ± 31 | 436 ± 111 |
| T. cuspidata | 163 ± 34 | 42.3 ± 3.3 | 33.5 ± 3.6 | ||||
| B. koreana | 175 ± 32 | 42.1 ± 2.5 | 32.4 ± 2.4 | NG | 50 ± 6 | ||
| E. japonicus | 229 ± 16 | 26.7 ± 1.3 | 20.6 ± 0.9 | ||||
| R. schlippenbachii | 174 ± 23 | 17.0 ± 1.7 | 8.4 ± 0.7 | NG | NG | 1336 ± 482 | 1355 ± 252 |
| Species | θw (°) | θd (°) | ||
|---|---|---|---|---|
| Adaxial | Abaxial | Adaxial | Abaxial | |
| Aesculus turbinata | 75.2 ± 3.2 e | 151.6 ± 1.5 a | 57.3 ± 1.0 bc | 69.8 ± 8.0 bc |
| Chionanthus retusus | 84.2 ± 4.7 de | 102.1 ± 9.4 g | 52.5 ± 3.0 cd | 59.6 ± 2.9 cd |
| Ginkgo biloba | 101.9 ± 3.5 b | 143.6 ± 4.7 ab | 64.8 ± 3.2 ab | 94.0 ± 2.3 a |
| Liriodendron tulipifera | 97.8 ± 5.4 b | 137.5 ± 3.6 bc | 57.3 ± 2.7 bc | 56.4 ± 5.3 cd |
| Magnolia denudata | 94.2 ± 1.0 bcd | 115.2 ± 1.5 ef | 69.1 ± 0.3 a | 69.3 ± 3.0 bc |
| Styphnolobium japonicum | 95.3 ± 4.2 bc | 132.1 ± 2.9 cd | 51.0 ± 5.8 cd | 81.9 ± 5.4 ab |
| Taxus cuspidata | 114.1 ± 9.9 a | 123.3 ± 2.6 de | 56.0 ± 7.6 bc | 82.1 ± 3.0 ab |
| Buxus koreana | 93.3 ± 1.0 bcd | 108.0 ± 3.4 fg | 59.2 ± 2.6 bc | 67.6 ± 9.9 bc |
| Euonymus japonicus | 84.5 ± 1.2 cde | 102.2 ± 6.4 g | 51.1 ± 1.2 cd | 67.6 ± 4.1 bc |
| Rhododendron schlippenbachii | 85.0 ± 3.7 cde | 73.7 ± 3.7 h | 43.4 ± 5.4 d | 51.9 ± 9.8 d |
| Leaf Traits | SPM10 | SPM2.5 | WPM10 | WPM2.5 | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | P | r | p | r | p | |
| ALA | −0.15 | 0.672 | −0.19 | 0.595 | −0.13 | 0.727 | −0.28 | 0.431 |
| SLA | −0.81 ** | 0.005 | −0.83 ** | 0.003 | −0.54 | 0.104 | −0.54 | 0.107 |
| W/L | −0.55 | 0.101 | −0.40 | 0.257 | −0.51 | 0.130 | −0.53 | 0.116 |
| SL | 0.69 * | 0.028 | 0.87 ** | 0.001 | 0.37 | 0.298 | 0.38 | 0.280 |
| SW | 0.67 * | 0.035 | 0.86 ** | 0.002 | 0.47 | 0.176 | 0.46 | 0.186 |
| SD | 0.29 | 0.415 | 0.18 | 0.612 | 0.41 | 0.243 | 0.18 | 0.619 |
| Ra (ad) | −0.66 * | 0.039 | −0.59 | 0.075 | −0.16 | 0.659 | −0.07 | 0.841 |
| Rt (ad) | −0.53 | 0.116 | −0.46 | 0.183 | −0.01 | 0.985 | −0.01 | 0.987 |
| θw (ad) | 0.08 | 0.817 | 0.30 | 0.395 | 0.00 | 0.995 | 0.13 | 0.718 |
| rs (ad) | −0.09 | 0.798 | −0.08 | 0.836 | 0.62 | 0.058 | 0.66 * | 0.037 |
| Wa (ad) | −0.08 | 0.834 | −0.30 | 0.406 | 0.02 | 0.963 | −0.12 | 0.747 |
| Ra (ab) | −0.34 | 0.332 | −0.18 | 0.616 | 0.28 | 0.425 | 0.23 | 0.523 |
| Rt (ab) | −0.12 | 0.745 | −0.06 | 0.876 | 0.18 | 0.612 | 0.08 | 0.830 |
| θw (ab) | −0.17 | 0.642 | 0.03 | 0.934 | −0.08 | 0.829 | −0.16 | 0.649 |
| rs (ab) | −0.37 | 0.292 | −0.28 | 0.428 | −0.13 | 0.722 | −0.18 | 0.626 |
| Wa (ab) | 0.13 | 0.730 | −0.06 | 0.861 | 0.10 | 0.774 | 0.19 | 0.599 |
| EWL | 0.41 | 0.236 | 0.49 | 0.153 | 0.64 * | 0.047 | 0.71 * | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Lee, J.K.; Kwak, M.J.; Lim, Y.J.; Kim, H.; Jeong, S.G.; Son, J.-a.; Oh, C.-Y.; Je, S.M.; Chang, H.; et al. Relationship between Leaf Traits and PM-Capturing Capacity of Major Urban-Greening Species. Horticulturae 2022, 8, 1046. https://doi.org/10.3390/horticulturae8111046
Park S, Lee JK, Kwak MJ, Lim YJ, Kim H, Jeong SG, Son J-a, Oh C-Y, Je SM, Chang H, et al. Relationship between Leaf Traits and PM-Capturing Capacity of Major Urban-Greening Species. Horticulturae. 2022; 8(11):1046. https://doi.org/10.3390/horticulturae8111046
Chicago/Turabian StylePark, Sanghee, Jong Kyu Lee, Myeong Ja Kwak, Yea Ji Lim, Handong Kim, Su Gyeong Jeong, Joung-a Son, Chang-Young Oh, Sun Mi Je, Hanna Chang, and et al. 2022. "Relationship between Leaf Traits and PM-Capturing Capacity of Major Urban-Greening Species" Horticulturae 8, no. 11: 1046. https://doi.org/10.3390/horticulturae8111046
APA StylePark, S., Lee, J. K., Kwak, M. J., Lim, Y. J., Kim, H., Jeong, S. G., Son, J.-a., Oh, C.-Y., Je, S. M., Chang, H., Kim, K., & Woo, S. Y. (2022). Relationship between Leaf Traits and PM-Capturing Capacity of Major Urban-Greening Species. Horticulturae, 8(11), 1046. https://doi.org/10.3390/horticulturae8111046