Abstract
Thymus × citriodorus (Lamiaceae) is medicinal, essential oil-bearing hybrid, synthesizing significant amounts of geraniol. This hybrid is suitable for cultivation in an open ground in the Baltic region; therefore, increasing the yield and amounts of bioactive compounds by organic matter fertilization during organic farming is realized. The aim of the present study was to evaluate the effect of fertilization with liquid cattle dung and humus on the quantitative and qualitative composition of essential oils as well as on biomass for some morphometrical and anatomical parameters of Thymus × citriodorus that were grown in open ground for two years. Each fertilization treatment was carried out on four replications, and four separate experimental plots were used as control plots. For anatomical investigations, the impress method and light microscopy were used. The essential oils were isolated by hydrodistillation and analyzed by GC-FID and GC-MS. The results showed somewhat different effects of liquid cattle dung and humus on the investigated parameters of hybrid in the first (warmer and drier) and second (rainier and cooler) experimental years. Liquid cattle dung had positive effects on biomass, height and the area covered by plants and on the number and length of inflorescences as well as on the density of stomata in the lower epidermis in the first year, but in the second experimental year, effects were observed on the length of inflorescences only. The effect of humus on the density of glandular trichomes in the upper epidermis of leaves was positive in the first year, but negative in the second experimental year. Moreover, in the second experimental year, humus affected negatively the height of plants and the percentage of the essential oil. The conclusion was that although it is fertilized with the same organic fertilizers, different climatic conditions in different years can influence chemical, anatomical and morphometrical parameters of plants growing in an open ground.
1. Introduction
Thymus × citriodorus (Lamiaceae) is a medicinal, aromatic, essential oil-bearing hybrid of Thymus vulgaris and Thymus pulegioides geraniol chemotypes, suitable for cultivation in the Baltic region because of its improved winterhardiness in comparison with Thymus species such as Thymus vulgaris, Thymus capitatus or Thymus zygis [1,2]. The raw material of T. × citriodorus is widely used for the enrichment of the aroma and the taste of teas as well as for fish dishes, desserts, ice creams and chewing gum [2,3]. The essential oil of this hybrid is rich in geraniol, as well as in geranial, nerol and neral [1,4]. Geraniol is a commercially important chemical compound, and it is used as aroma in flavour and fragrance industries, for the production of insecticides and repellents and it also has antimicrobial (against Escherichia coli, Listeria monocytogenes and Salmonella enterica), antioxidant, anti-inflammatory and anticancer properties [5].
In nutrient-deficient soil, non-optimal physical, chemical and biological properties can have a negative effect on the growth of plants, can reduce their yield and the yield’s quality of plant material and can influence the amount and chemical composition of secondary metabolites, including essential oils [6,7,8]. The supply of main nutrients can be managed by fertilization [6]. The main soil nutrients that have the greatest impact on the growth and development of medicinal plants are nitrogen, potassium and phosphorus, as well as calcium, magnesium, manganese, boron, iron and zinc [9]. Organic fertilizers are the sources of naturally available minerals. They contain moderate amounts of essential plant nutrients; help mitigate the risk of eutrophication, ground water contamination and overfertilization; improve soil (microbiological, physicochemical and biochemical) properties and thus influence soil quality; and can help minimize environmental damage without reducing crop yields and achieve sustainable levels of agriculture production [10]. Liquid cattle dung, as one natural organic fertilizer, contains significant amounts of major nutrients such as nitrogen, phosphorus and potassium as well as magnesium, calcium, copper, boron and manganese. Liquid cattle dung also can improve soil texture and structure and render soil more alkaline (increase soil pH) [11,12,13]. Humus as a processed organic fertilizer is a major component of soil organic matter that improves fertility and physical, chemical and biological features of soil. Humus also improves uptake process of the macro- and microelements as well as water regime and reduces abiotic stress and uptake of toxin ions for plants [14,15,16]. Other environmental factors such as temperature, light, rainfall and wind can also influence growth and the development and secondary metabolites accumulation of medicinal and aromatic plants [17,18].
Published data indicate that the effect of fertilization on morphological, anatomical and chemical parameters of aromatic plants can be different. For example, organic fertilization positively influences plant biomass, plant height and carvacrol accumulation for Origanum vulgare (Lamiaceae) [19]. Other study shows the positive influence of fertilization with cattle manure on the number of steams, steam diameter, leaf area and yield of raw material of O. vulgare [20]. For Thymus vulgaris, organic fertilization increases the number of branches, biomass as well as the yield of essential oil and the amount of thymol [21]. Cattle manure has positive effects on the essential oil yield and composition of Apium graveolens [22]. Fertilization with manure increases stomata densities for Pogostemon cablin (Lamiaceae) [23]. The density of trichomes on Artemisia annua increased after applying nitrogen [24]. The aim of the present study was to evaluate the effect of fertilization with liquid cattle dung and humus on the quantitative and qualitative composition of essential oils as well as on biomass for some morphometrical and anatomical parameters of Thymus × citriodorus that were grown in open ground for two years.
2. Materials and Methods
2.1. Plant Cultivation and Fertilization
T. × citriodorus propagated vegetatively by plant division from one motherplant and was grown in open-field conditions in the territory of the Nature Research Centre (Mažieji Gulbinai, near Vilnius, Lithuania). These clones were planted in twelve separate experimental square plots in 2019, and in each experimental square plot, nine sub-individuals were present. The area of one experimental square plot was 1.44 m2 (Figure 1). A single clone planted gives rise over time to a bush representing a single plant.

Figure 1.
Thymus × citriodorus in experimental plots at the field collection.
The fertilization experiment started in spring of 2020 and continued for two years (2020–2021). Every fertilization treatment was carried out in four separate experimental plots, and four separate experimental plots were also appointed as controls. Plants were fertilized with liquid cattle dung and humus. Three weeks lasted from the final fertilization process to harvesting processes.
Cattle were fed with barley hay and flour. Humus was bought from a shopping center and contained humic acids (85%), potassium (12%), iron (1%), nitrogen (1.3%) and other minerals (0.7%). Fertilization experiments started at the beginning of the vegetation period (from the end of April to early May) and finished two weeks before blooming. The fertilization treatment with liquid cattle dung was carried out three times—once per two weeks (one liter of liquid cattle dung and 4 L of water for one experimental plot). Fertilization with humus also was performed three times—once per two weeks (10 g humus was dissolved in 5 L water for one experimental plot). Fertilization was carried out only through soil on cloudy but not on rainy days.
Meteorological data were obtained from the meteorological bulletins of Vilnius meteorology station of the Lithuanian Hydrometeorological Service under the Ministry of Environment. The meteorological data (temperature, rainfall and humidity) of 2020 and 2021 in which T. × citriodorus vegetation started to flower (April–June) are presented in Table 1.

Table 1.
The meteorological data of Vilnius meteorology station in the period April–August in 2020 and in the period April–June in 2021 (– denotes unanalyzed meanings, because the experiment was completed in June in 2021).
2.2. Collection and Analysis of Soil Material
For chemical analysis, soil samples were taken before every fertilization and before harvesting plant materials. Three sub-samples of soil in each of the four experimental plots of the same fertilization treatment were taken from a depth of 10–15 cm and mixed and dried at room temperature. Soil analysis was performed at the Agrochemical Research Laboratory of the Lithuania Research Centre for Agriculture and Forestry. Soil pH was determined potentiometrically in 1 M KCl extracts. Organic carbon in soil was established by a dry combustion method, which removed carbonates (in molecular form) and total nitrogen by the modified Kjeldahl method (in ionic from); mobile potassium, phosphorus, calcium and magnesium by Egner-Rim-Doming method (in ionic forms); mobile sulphur by the turbidimetric method (in ionic form); mobile copper by the atomic absorption spectrometry (in ionic from) using a 1 M HCl solution. Mobile boron was established spectrophotometrically (in molecular form, using a H2SO4 ratio 1:10; it boiled for 5 min); mobile manganese by atomic absorption spectrometry (in ionic from, using 0,1 M H2SO4, ratio 1:10); and mobile zinc by atomic absorption spectrometry (in ionic from, using ammonium acetate, pH 4.8, ratio 1:10). After fertilization with liquid cattle dung and humus, only those soil parameters were analyzed, the quantities of which could change according to the literature data [13,14,15,25].
Soil at the field collection of the Nature Research Centre by IUSS Working Group WRB comprised cambisol.
2.3. Analysis of Plants’ Morphometrical Parameters
Samples for morphometrical, anatomical and chemical analysis were collected on June 23 in the first experimental year and on June 27 in the second experimental year.
The following morphometrical parameters were estimated at the middle of blooming (end of June): plant height, area covered by plant and the number of inflorescences per plant. Plant height and area covered by plant were evaluated for each plant per each experimental plot. The area covered by plant estimation was examined in the following manner: The diameter of every plant was measured at the widest and the narrowest locations; the mean value of plant diameter was estimated from these two parameters; every plant was considered in terms of a circular shape, and the area covered by the plant was calculated by the formula for calcutating the area of a circle (S = πR2, where S denotes the area covered by the plant, and R denotes the radius of plant). The number of inflorescences was estimated for three plants per each experimental plot: for the largest, medium and the smallest plant. After measuring morphometrical parameters, the above-grounded parts of plants were harvested and weighed per each experimental plot. Moreover, data about the weight of fresh plant raw material per each experimental plot were recorded (biomass was related to the area of 1 m2). The length of fifty inflorescences per every experimental plot was also measured. Later, raw plant material of each experimental plot was dried at room temperature separately.
2.4. Analysis of Anatomical Parameters
Densities of glandular trichomes and stomata in a mm2 area and size (diameter) of glandular trichomes were estimated on the lower and upper epidermis in each fertilization treatment. An imprint method [26] was used for anatomical investigations: a thin layer of colorless nail polish was spread on the fresh leaf of the middle flowering stem. The formed skin of the nail polish was ripped off the leaf and observed with a Leica light microscope. Two leaves from each of the 12 stems (3 stems from every experimental plot) were used for the evaluation of anatomical parameters depending on each fertilization treatment. Apex, base, margins and midrib regions of leaves were not used in this analysis and avoided the effect of different parts of the same leaf causing variations in the investigated anatomical parameters.
2.5. Essential Oil Analysis
After cutting plant materials, the materials were dried at room temperature separately from each experimental plot. The essential oils from each sample (from leaves and flowers and stems were not used) were isolated separately by hydrodistillation in a Clevenger apparatus for two hours. The distillation of essential oils was carried out in 2–6 replications per each sample. Essential oils from each sample were kept in 2 mL bottles separately. The amount of essential oils was estimated by percentage (quantitative analysis of essential oils). For further investigations (qualitative analysis), essential oil solutions of 1 percentage were prepared in a mixture of diethyl ether and n-pentane (1:1). The identification of main compounds was based on a GC Plus-2010 instrument equipped with a GC-QP 2010 Plus Shimadzu series mass selective detector in an electron impact ionisation mode at 70 eV. The identification was carried out for chemical compounds that presented at least 10 percent of one sample. A separation of compounds was performed on fused silica (100% dimethyl polysiloxane column (30 m × 0.25 mm ID × 0.25 μm film thickness) (Resteck, Bellefonte, PA, USA) with spitless injection: helium as a carrier gas at a flow rate of 1.6 mL/min and injector and detector temperatures at 250 °C. The GC oven’s temperature was programmed as follows: initial temperature of 50 °C (isothermal for 7 min) increased to 250 °C at a rate of 4 °C/min (isothermal for 5 min), and it further increased at a rate of 30 °C/min to 300 °C; the final temperature was kept for 2 min. The identification of investigated compounds was based on a comparison of retention indices (RIs) [27], computer mass spectra library (NBS75K) and the analytical standards. Retention indices had been determined relative to the retention times of a series of alkanes (C7–C30) with linear interpolation. The qualitative analysis of the main compounds was carried out using a TRACE GC ULTRA (Thermo Fisher Scientific, Waltman, MA, USA) gas chromatograph with a flame ionisation detector (FID) on the silica capillary column TR5-MS (30 m × 0.25mm ID × 0.25 μm film thickness) (Thermo Electron Corporation, Waltman, MA, USA) under the same chromatographic conditions. The percentage amounts of investigated compounds were recalculated according to the areas of FID chromatographic peaks, assuming that all constituents of essential oil comprised 100%.
2.6. Statistical Analysis
Calculations of means and standard deviations (SD) were carried out for soil elements and morphometrical, anatomical and chemical parameters of T. × citriodorus. For coefficients of variation (CV), the determination of the minimum and maximum value was carried out for anatomical and chemical parameters of T. × citriodorus. The Mann–Whitney test was used to estimate differences between the effects of fertilization treatments and control on soil elements, biomass and chemical parameters of T. × citriodorus. Student’s t-test was used to estimate differences between effects of fertilization treatments and the control on the morphometrical and anatomical parameters of T. × citriodorus. The Mann–Whitney test’s p/U values and Student’s t-test’s p/t values for T. × citriodorus parameters are presented in Table A2. Correlation analyses were performed using Spearman’s rank correlation coefficient. Statistical data processing was carried out with the STATISTICA®7 and MS Excel software.
3. Results
3.1. Influence of Fertilization on Soil pH and Chemical Composition
An analysis of soil parameters in the first experimental year showed a significant (p < 0.05) influence of fertilization with humus on the amount of soil organic carbon. The mean amount of organic carbon was 0.17 p.p higher in comparison with the control (Table 2). The fertilization with liquid cattle dung increased the amount of potassium and the amount of organic carbon by 100 mg/kg and 0.09 p.p, respectively, but these differences were not statistically significant. The fertilization with humus increased the amount of sulfur until 0.17 mg/kg in soil, but this increase was also not significant in comparison with the control (Table 2).

Table 2.
Changes in soil pH and chemical composition during fertilization with liquid cattle dung and humus in the first experimental year. 1 denotes soil collection before the first fertilization with liquid cattle dung and humus; 2 denotes soil collection before the second fertilization with liquid cattle dung and humus; 3 denotes soil collection before the third fertilization with liquid cattle dung and humus; 4 denotes soil collection before the collection of plant raw material; SD—standard deviation; line (—) denotes unanalyzed soil parameters.
Liquid cattle dung significantly (p < 0.05) affected the amount of potassium in soil: Its amount was about three times higher in comparison with the control after fertilization with liquid cattle dung (Table 3). Although the fertilization with humus increased the amount of pottasium and sulphur by 16.30 mg/kg and 0.3 mg/kg, respectively, in comparison with the control, these changes were not significant (Table 3).

Table 3.
Changes in soil pH and chemical composition during fertilization with liquid cattle dung and humus in the second experimental year. 1denotes soil collection before the first fertilization with liquid cattle dung and humus; 2 denotes soil collection before the second fertilization with liquid cattle dung and humus; 3 denotes soil collection before the third fertilization with liquid cattle dung and humus; 4 denotes soil collection before the collection of plant raw material; SD—standard deviation; line (—) denotes unanalyzed soil parameters.
3.2. Influence of Fertilization on Biomass and Some Morphometrical Parameters of Plants
The results of the first experimental year showed that fertilization with liquid cattle dung significantly (p < 0.05) affected the investigated morphometrical parameters of T. × citriodorus: Biomass increased by 2.6 times; plant height and the area covered by plant increased by 1.4 times; the number of inflorescences per plant increased by 1.6 times; the length of inflorescences increased by 1.4 times in comparison with the control. Humus had not significant influences any morphometrical parameters in the first experimental year. The number of inflorescences was the most variable, the plant height was the least variable morphometrical parameter between all fertilization treatments in the first experimental year (Figure 2).
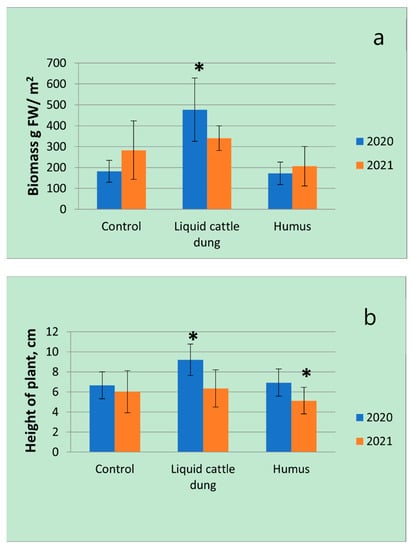
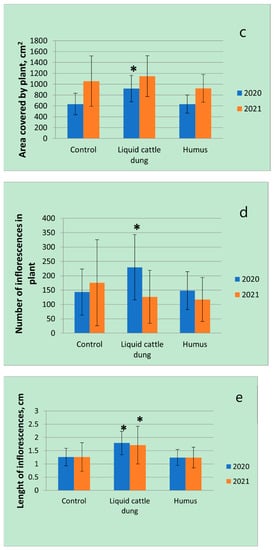
Figure 2.
Variations in morphometrical parameters (a—biomass; b—height; c—area covered by plant; d—number of inflorescences; e—length of inflorescences) of Thymus × citriodorus depending on the fertilization treatment in the first and second experimental years. * denotes statistically significant differences (p < 0.05); error bars denote standard deviation. FW—fresh weight.
In the second experimental year, the number of inflorescences ranged from around 115 (after humus application) to around 175 (control)—an increase of 52%—while biomass ranged from around 200 g (after humus application) to around 340 g (after liquid cattle dung application)—an increase of 70%. Fertilizations with liquid cattle dung significantly (p < 0.05) affected only the length of inflorescences: Inflorescences were 1.4 times larger in comparison with the control. Biomass, height and area covered by plants were a little larger after fertilization with liquid cattle dung in comparison with the control, but the results were not significant. It was observed that the height of T. × citriodorus plants significantly (p < 0.05) decreased in comparison with the control after fertilization with humus (Figure 2).
3.3. Influence of Fertilization on Anatomical Parameters of Thymus × citriodorus
The fertilization with liquid cattle dung in the first experimental year showed significant (p < 0.05) positive effects on stomata densities in the lower epidermis of leaves: Stomata density in mm2 was about 1.6 times higher in comparison with the control (Table 4). Humus also increased stomata densities in the lower epidermis of leaves, but these results were not significant (p > 0.05) in comparison to the control. The fertilization with liquid cattle dung and humus did not significantly affect the density of stomata in the upper epidermis and the density of glandular trichomes in the lower epidermis of leaves. The highest maximum values on the density of stomata and glandular trichomes in the upper epidermis of leaves were observed after humus fertilization. Humus significantly (p < 0.05) affected the density of glandular trichomes in the upper epidermis of leaves: There, the density of glandular trichomes was 1.6 times higher in comparison with the control. Humus increased the size of glandular trichomes in the lower epidermis of leaves but the result did not differ significantly from control.

Table 4.
Descriptive statistics of anatomical parameters in the upper and lower epidermis of Thymus × citriodorus depending on the fertilization treatment in the first and second experimental years. SD—standard deviation; CV—coefficient of variation.
Results showed that fertilization with humus in the second experimental year significantly and negatively influenced (p < 0.05) the density of glandular trichomes in the upper epidermis of leaves: The density of glandular trichomes decreased by 39.2% in comparison with the control. The density of glandular trichomes in the lower epidermis decreased after fertilization with liquid cattle dung and humus but significant differences between them and the control were not determined. The density of stomata in the lower epidermis of leaves was the lowest and in the upper epidermis—the highest after fertilization with liquid cattle dung in comparison with humus fertilization and the control. The size of glandular trichomes in the upper epidermis of leaves was the highest after fertilization with humus in comparison with fertilization with liquid cattle dung and the control. The least variable anatomical parameter was the density of stomata in the lower epidermis of leaves and the highest parameter was the density of glandular trichomes in the upper epidermis of leaves (except for the control). It is interesting that the maximum value of the size of glandular trichomes in the upper epidermis of leaves was observed even after fertilization procedures—127 μm (Table 4).
3.4. Inluence of Fertilization on Quantitative and Qualitative Composition of Essential Oil
Results of both experimental years showed that the highest percentages of essential oil in T. × citriodorus were attained after fertilization with liquid cattle dung, and the lowest percentages were attained after fertilization with humus (Figure 3). In comparison with the control fertilization with liquid cattle dung, there was an increased percentage of essential oils; with humus, the percentages decreased in both experimental years, and this became more pronounced in the second experimental year. In the second experimental year, the percentage of essential oil significantly (p < 0.05) decreased compared to the control by 37.04% after humus fertilization (Figure 3).
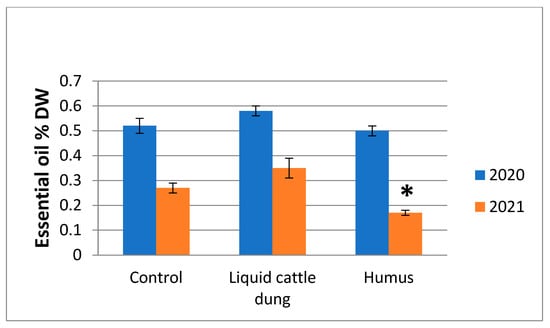
Figure 3.
Variations in percentage of Thymus × citriodorus essential oil depending on the fertilization treatment in the first and second experimental years. * denotes statistically significant differences (p < 0.05); error bars denote the standard error of mean. DW—dry weight.
Geraniol was the main chemical compound of essential oils in T. × citriodorus; it amounted to about a quarter of essential oils in both fertilization treatments and the control in the first experimental year. In the second experimental year, this chemical compound amounted to a quarter of essential oils after fertilization with liquid cattle dung and a fifth of essential oils after fertilization with humus. Fertilization with humus increased the percentage of geraniol and geranial in the first experimental year but decreased the percentage of geraniol, geranial and neral in the second experimental year. These changes in comparison with the control were not significant. The percentages of nerol and neralin essential oils were similar in all fertilization treatments in the first experimental year: nerol amounted about 14% and neral amounted to about 11% of essential oil (Figure 4, Table A1). In the second experimental year, the percentages of nerol and neral varied from the lowest to highest values (Table A1). Fertilization with liquid cattle dung decreased the percentage of germacrene D-4-ol in the first experimental year, but this result did not differ significantly from the control. In the second experimental year, both fertilization treatments increased the percentage of germacrene D-4-ol, but this increase also did not differ significantly from the control. Variations in the percentage of germacrene D-4-ol were the highest in comparison with the other four investigated chemical compounds in both experimental years (Figure 4, Table A1).
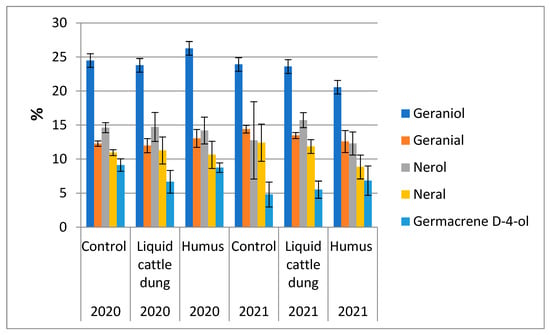
Figure 4.
Variations in the percentage of some chemical compounds in essential oils of Thymus × citriodorus depending on the fertilization treatment in the first and second experimental years.
A summary of findings of this research study is presented in Figure 5. A positive correlation (very strong, strong and weak) was observed between the year and area covered by plant (0.8), the upper leaf epidermis density of stomata (0.4), the upper leaf epidermis size of glandular trichomes (0.9), the lower leaf epidermis density of stomata (0.8), the lower leaf epidermis density of glandular trichomes (0.8), the lower leaf epidermis size of glandular trichomes (0.20) and the percentage of geranial (0.6), as well as between fertilization and the upper leaf epidermis density of stomata (0.70), the upper leaf epidermis density of glandular trichomes (0.9), the upper leaf epidermis size of glandular trichomes (0.2), the lower leaf epidermis size of glandular trichomes (0.2), the amount of essential oil (0.1) and the percentage of germacrene D-4-ol (0.9). For other parameters, negative correlations were observed.
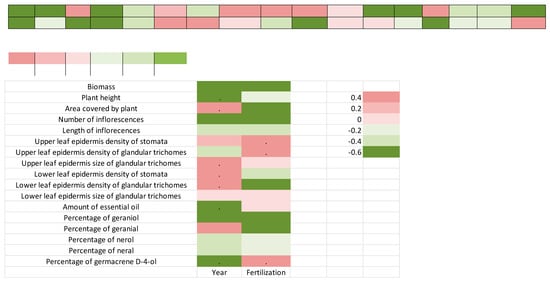
Figure 5.
Spearman’s correlation heatmap between the year and fertilization on the observed parameters. Dot in the figure denotes very strong correlations.
4. Discussion
The fertilization of plants from soil primarily affects the soil, its structure and its physical and chemical properties. The present study demonstrated that fertilization with humus significantly (p < 0.05) enriched soils with organic carbon in the first experimental year (Table 2). The literature data also suggest that humus-rich compost applications increased the amount of soil organic carbon [28], which has vital roles in the physical, chemical and biological behavior of the soil, including soil fertility, structure and water retention [29,30,31]. Results demonstrated that liquid cattle dung significantly (p < 0.05) increased the amount of potassium in the second experimental year (Table 2). Potassium is one essential macronutrient element of plants: It improves plant yield and the quality of produce, and it strengthens resistance against drought, salinity, high temperatures and abiotic and biotic stress. Potassium permits the activation of about 60 enzymes for participation in lipid and protein syntheses and in the circulation of hydrocarbons [32,33]. The main nutrient element in liquid cattle dung is potassium (this nutrient element does not evaporate from the soil after fertilization with liquid cattle dung and remains during the entire vegetation period), and nitrogen, phosphorus, calcium, sulphur, magnesium, manganese, copper, boron, zinc and auxins can become a part of liquid cattle dung [14,25].
Chemical and organic fertilizers can improve the metabolism of medicinal plants and, thus, can increase yields [34,35] and influence biomass and morphometrical parameters in medicinal plants of Ocimum, Thymus, Origanum and Mentha, all belonging to the Lamiaceae family [21,34,35]. Fertilization with liquid cattle dung significantly (p < 0.05) and positively affected morphometrical parameters of T. × citriodorus in the first experimental year and only the number of inflorescences in the second experimental year (Figure 2). Positive changes in morphometrical parameters may be related to soil enrichment with potassium and other elements after fertilization with liquid cattle dung. Fertilizations with the cow dung positively influenced biomass, plant height and the number of branches, as well as the number, length and thousand mass of leaves in Ocimum sanctum and Mentha arvensis (Lamiaceae) [36]. Fertilization with cattle manure positively affected the number of branches in two fertilization seasons and biomass only in the first fertilization season of T. vulgaris [21]. Fertilization with cattle manure positively affected biomass and leaf areas in Mentha × piperita [37]. The fertilization with humus negatively influenced (p < 0.05) the height of T. × citriodorus in the second experimental year (Figure 2b). Humus has positive impacts on a plant’s morphological parameters due to its improvements on soil fertility, water regimes and soil structure. The literature data suggest that low temperatures can reduce humus accumulation and a reduction in soil aeration [38]. According to the Lithuanian Hydrometeorological Service under the Ministry of Environment, temperatures in Lithuania at the end of April and at the beginning of May in 2021 (in the second experimental year) were lower than it customarily was in the past, and the month of May was very rainy, which could be a reason for the reduction in soil aeration. The literature data informed that most medicinal and aromatic plants have temperatures (for most medicinal plants, it is 15–25 °C) and water regimes for optimal growth and development [39,40,41]. Meanwhile, drought and heavy precipitation have negative effects for plant growth and development [41]. The application of humus-rich compost increases lettuce’s (Lactuca sativa L.) shoot growth [28], and liquid humus increases the total biomass, flower diameter, number, length and diameter of stems in Alpinia purpurata [42]. The fertilization treatment with humic acid did not increase the growth of lettuce [43] and the yield of foxtail millet [44].
Glandular trichomes can be found in approximately 30% of vascular plant species. Their functions are to secrete or store secondary metabolites, which contribute to increasing plant fitness relative to the environment [45]. Humus, unlike liquid cattle dung, in the first experimental year significantly (p < 0.05) and positively affected the density of glandular trichomes in the upper epidermis of leaves. However, in the second experimental year this effect was significantly (p < 0.05) negative (Table 4). According to the literature data, the density of glandular trichomes is higher in plants growing in dry environmental conditions [46]. Humus maintains a suitable water regime and structure of soil [16]. A suitable water regime and soil structure could affect the increase in the density of glandular trichomes in the upper epidermis in the first experimental year after humus fertilization. Meanwhile, a decrease in the density of glandular trichomes in the second experimental year could be related to the very rainy month of May in 2021 (147 mm and it is 2.6 times higher than it normally is): The plants have not been able to absorb humus because it could be more leached from the soil. Foliar fertilization with organic nitrogen, potassium and microelements negatively affected glandular trichomes densities in hop leaves (Humulus lupulus L. vs. Cascade) [47].
The fertilization with liquid cattle dung significantly (p < 0.05) and positively impacted the density of stomata in the lower epidermis of leaves of T. × citriodorus in the first experimental year (Table 4). As mentioned above, liquid cattle dung also promoted the growth of biomass and increased parameters of plant height, the area covered by plant, the number of inflorescences and length of inflorescences in comparison with the control in the first experimental year (Figure 2). A higher stomata density can improve plant biomass, but this does not occur in all plant species: It can depend on the species, environmental conditions and size of stomata (smaller stomata opened more rapidly); for example, a higher stomata density improves biomass in Arabidopsis thaliana [48,49]. Manure significantly and positively affected stomata densities at both sides (adaxial and abaxial); meanwhile, compost was affected positively in the adaxial side but negatively in the abaxial side of leaves in Pogostemon cablin (Lamiaceae) [23].
Investigations with Thymus migricus show positive effects of soil organic matter on essential oil contents in this species of genus Thymus [50]. Our results demonstrated that fertilization with humus significantly (p < 0.05) and negatively influenced the percentage of essential oil in T. × citriodorus in the second experimental year in comparison with the control (Figure 3). The percentage of essential oil in Artemisia sieberi plants after fertilization with humic acid was also lower in comparison with the control [51]. Plants use nutrient elements primarily for increasing biomass and only then synthesize secondary metabolites [52]. In the second experimental year, morphometrical parameters of T. × citriodorus after humus fertilization were also lower in comparison with the control (Figure 2). Worse climatic conditions (rainy and colder weather at the beginning of May in 2021) could disturb the assimilate of humus from soil and so can slacken the growth of biomass and the synthesis of secondary metabolites in the hybrid. The literature data also inform that medicinal plants accumulate more secondary metabolites in drier and warmer weather [17].
This study reported that fertilization with liquid cattle dung applications can help obtain higher yields of raw material of T. × citriodorus: Liquid cattle dung applications increased values of the morphometrical parameters of T. × citriodorus plants in both experimental years; in the first experimental year, all parameters significantly differed from the control. Humus had no effects or adversely affected morphometrical and anatomical parameters and the percentage of essential oils. Therefore, the cultivation of T. × citriodorus in soil with high amounts of humus can negatively affect both biomass and essential oil yields of this hybrid. Fertilizers and different climatic conditions in different years can influence chemical, anatomical and morphometrical parameters of plants that are grown in open grounds (Figure 5).
5. Conclusions
The fertilization treatment with liquid cattle dung (dosing: 0.7 L of liquid cattle dung and 2.8 L of water for 1m2) increased biomass and morphometrical parameters of Thymus × citriodorus plants. The fertilization treatment with liquid cattle dung is useful, particularly when the climate is not very rainy. Humus had unreliable or negative effects on the biomass and percentage of essential oils of Thymus × citriodorus; therefore, the fertilization treatment with humus or cultivation in the soil with large amounts of humus is not economically useful.
Author Contributions
Conceptualization, V.V. and K.L.; methodology, V.V. and K.L.; software, V.V. and K.L.; validation, V.V.; formal analysis, V.V., K.L. and I.S.; investigation, V.V. and K.L.; resources, V.V. and K.L.; data curation, V.V., K.L. and I.S.; writing—original draft preparation, K.L. and I.S.; writing—review and editing, V.V. and K.L.; visualization, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Social Fund under No. 09.3.3-LMT-K-712 “Development of Competences of Scientists, other Researchers and Students trough Practical Research Activities” measure.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated during this study are included in this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Descriptive statistics of some chemical compounds of Thymus × citriodorus essential oil depending on fertilization treatment in the first and the second experimental year. SD—standard deviation, CV—coefficient of variation.
Table A1.
Descriptive statistics of some chemical compounds of Thymus × citriodorus essential oil depending on fertilization treatment in the first and the second experimental year. SD—standard deviation, CV—coefficient of variation.
| Chemical Compound | Fertilization Treatment | |||
|---|---|---|---|---|
| Control | Liquid Cattle Dung | Humus | ||
| First experimental year | ||||
| Geraniol | Mean ± SD | 24.48 ± 1.13 | 23.78 ± 0.44 | 26.27 ± 2.73 |
| Min | 22.78 | 23.12 | 22.42 | |
| Max | 25.07 | 24.04 | 28.74 | |
| CV | 5 | 2 | 10 | |
| Geranial | Mean ± SD | 12.27 ± 0.39 | 11.97 ± 1.04 | 13.04 ± 1.31 |
| Min | 11.75 | 10.93 | 11.39 | |
| Max | 12.68 | 13.41 | 14.23 | |
| CV | 3 | 9 | 10 | |
| Nerol | Mean ± SD | 14.62 ± 0.73 | 14.73 ± 2.13 | 14.20 ± 1.97 |
| Min | 13.96 | 12.12 | 11.43 | |
| Max | 15.66 | 17.15 | 16.06 | |
| CV | 5 | 14 | 14 | |
| Neral | Mean ± SD | 10.95 ± 0.44 | 11.27 ± 1.99 | 10.63 ± 2.00 |
| Min | 10.33 | 9.26 | 7.84 | |
| Max | 11.33 | 13.27 | 12.52 | |
| CV | 4 | 18 | 19 | |
| Germacrene D-4-ol | Mean ± SD | 9.12 ± 0.91 | 6.66 ± 1.66 | 8.74 ± 0.70 |
| Min | 8.24 | 5.20 | 8.13 | |
| Max | 10.22 | 9.00 | 9.60 | |
| CV | 10 | 25 | 8 | |
| Second experimental year | ||||
| Geraniol | Mean ± SD | 23.91 ± 1.35 | 23.60 ± 2.58 | 20.55 ± 2.78 |
| Min | 22.42 | 20.06 | 17.62 | |
| Max | 25.72 | 26.11 | 23.15 | |
| CV | 6 | 11 | 14 | |
| Geranial | Mean ± SD | 14.40 ± 0.56 | 13.47 ± 0.45 | 12.59 ± 1.62 |
| Min | 13.90 | 13.08 | 10.78 | |
| Max | 15.18 | 14.11 | 13.92 | |
| CV | 4 | 4 | 13 | |
| Nerol | Mean ± SD | 12.75 ± 5.67 | 15.73 ± 1.10 | 12.29 ± 1.70 |
| Min | 5.14 | 14.38 | 10.65 | |
| Max | 17.32 | 17.06 | 14.04 | |
| CV | 45 | 7 | 14 | |
| Neral | Mean ± SD | 10.95 ± 0.44 | 11.27 ± 1.99 | 10.63 ± 2.00 |
| Min | 10.33 | 9.26 | 7.84 | |
| Max | 11.33 | 13.27 | 12.52 | |
| CV | 4 | 18 | 19 | |
| Germacrene D-4-ol | Mean ± SD | 9.12 ± 0.91 | 6.66 ± 1.66 | 8.74 ± 0.70 |
| Min | 8.24 | 5.20 | 8.13 | |
| Max | 10.22 | 9.00 | 9.60 | |
| CV | 10 | 25 | 8 | |

Table A2.
Mann–Whiney U-test (p/U) of biomass and chemical parameters of Thymus × citriodorus and Student t-test (p/t) of morphometrical and anatomical parameters of Thymus × citriodorus. Significant level was chosen as p < 0.05; the significant differences denote *, the statistical parameter that was not analysed denote –.
Table A2.
Mann–Whiney U-test (p/U) of biomass and chemical parameters of Thymus × citriodorus and Student t-test (p/t) of morphometrical and anatomical parameters of Thymus × citriodorus. Significant level was chosen as p < 0.05; the significant differences denote *, the statistical parameter that was not analysed denote –.
| Parameter of Thymus × citriodorus | p/U | p/t | ||
|---|---|---|---|---|
| Fertilization Treatment | ||||
| Liquid Cattle Dung | Humus | Liquid Cattle Dung | Humus | |
| First experimental year | ||||
| Biomass | 0.02/0.0 | 0.77/7.0 | – | – |
| Percentage of essential oil | 0.16/44.0 | 0.46/36.0 | – | – |
| Percentage of geraniol | 0.25/4.0 | 0.25/4.0 | – | – |
| Percentage of geranial | 0.39/5.0 | 0.39/5.0 | – | – |
| Percentage of neral | 1.00/8.0 | 0.77/7.0 | – | – |
| Percentage of nerol | 1.00/8.0 | 1.00/8.00 | – | – |
| Percentage of germacrene D-4-ol | 0.08/2.0 | 0.39/5.0 | – | – |
| Height of plant | – | – | 0.00/−7.38 * | 0.41/−0.83 |
| Area of plant | – | – | 0.00/−5.45 * | 0.97/0.04 |
| Number of inflorescences of plant | – | – | 0.04/−2.09 * | 0.93/−0.09 |
| Length of inflorescences | – | – | 0.00/−10.67 * | 0.73/0.34 |
| Density of stomata (upper epidermis) | – | – | 0.08/1.78 | 0.84/−0.19 |
| Density of glandular trichomes (upper epidermis) | – | – | 0.33/−0.98 | 0.00/−3.10 * |
| Size of glandular trichomes (upper epidermis) | – | – | 0.90/0.12 | 0.47/−0.72 |
| Density of stomata (lower epidermis) | – | – | 0.00/−4.00 * | 0.1/−1.70 |
| Density of glandular trichomes (lower epidermis) | – | – | 0.20/1.30 | 0.55/0.60 |
| Size of glandular trichomes (lower epidermis) | – | – | 0.10/1.65 | 0.13/−1.52 |
| Second experimental year | ||||
| Biomass | 0.39/5.0 | 0.25/4.0 | – | – |
| Percentage of essential oil | 0.18/158 | 0.04/31.5 | – | – |
| Percentage of geraniol | 1.00/8.0 | 0.08/1.0 | – | – |
| Percentage of geranial | 0.06/1.5 | 0.08/1.0 | – | – |
| Percentage of neral | 0.39/5.0 | 0.72/5.0 | – | – |
| Percentage of nerol | 0.77/7.0 | 0.72/5.0 | – | – |
| Percentage of germacrene D-4-ol | 0.39/5.0 | 0.29/3.0 | – | – |
| Height of plant | – | – | 0.48/−0.71 | 0.03/2.16 * |
References
- Ložienė, K.; Vaičiulytė, V.; Maždžierienė, R. Influence of metereological conditions on essential oil composition in geraniol bearing Thymus pulegioides and Thymus hybrid. Acta Physiol. Plant. 2021, 43, 27. [Google Scholar] [CrossRef]
- Toncer, O.; Karaman, S.; Diraz, E.; Sogut, T.; Kizil, S. Essential oil composition of Thymus × citriodorus (Pers.) Screb. at different harvest stages. Not. Bot. Horti. Agrobo. 2017, 42, 185–189. [Google Scholar] [CrossRef]
- Paslawska, M.; Sala, K.; Nawirska-Olzanska, A.; Stępien, B.; Pląskowska, E. Effect of different drying techniques on dehydratation kinetics, physical properties and chemical composition of lemon thyme. Nat. Prod. Commun. 2020, 15, 1–12. [Google Scholar] [CrossRef]
- Kizil, O.; Toncer, Ő. Essential oil and microelement composition of Thymus × citriodorus L. and Lippia citriodora H.B.K. Cercet. Agron Mold. 2016, 2, 97–105. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, M. Geraniol—A rewiev of commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Purbajanti, E.D.; Slamet, W.; Fushkak, E.; Rosyida, R. Effects of organic and inorganic fertilizers on growth, activity of nitrate reductase and chlorophyll contents of peanuts (Arachis hypogaea L.). Earth Environ. Sci. 2019, 250, 1–7. [Google Scholar] [CrossRef]
- Nurzynska-Wierdak, R. Does mineral fertilization modify essential oil content and chemical composition in medical plants. Acta Sci. Pol. Hortorumcultus 2013, 12, 3–16. [Google Scholar]
- Vaičiulytė, V.; Ložienė, K.; Taraškevičius, R.; Butkienė, R. Variation of essential oil composition of Thymus pulegioides relation in soil chemistry. Ind. Crops Prod. 2016, 95, 422–433. [Google Scholar] [CrossRef]
- Radanovic, D.; Mladenovic, S.A.; Markovic, T.L. Influence of soil characteristics and nutrient supply on medicinal and aromatic plants. In Proceedings of the III Conference of Medicinal and Aromatic Plants of Southeast European Countries, Nitra, Slovakia, 5–8 September 2004. [Google Scholar]
- Shaji, H.; Chandran, V.; Mathew, L. Organic fertilizers as a route to controlled release of nutrients. In Controlled Release Fertilizers for Sustainable Agriculture; Lewu, F.B., Volova, T., Sabu, T., Rakhimol, K.R., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2020; pp. 2231–2245. [Google Scholar] [CrossRef]
- Matsi, T.; Lithourgidis, A.S.; Barbayannis, N. Effect of liquid cattle manure on soil chemical properties and corn growth in northen Greece. Exp. Agric. 2014, 51, 435–450. [Google Scholar] [CrossRef]
- Evulo, B.S.; Hasan, K.O.; Ojeniyi, S.O. Comparative effect of cowdung manure on soil and leaf nutrient and yield of pepper. Int. J. Agric. Res. 2007, 2, 1043–1048. [Google Scholar] [CrossRef][Green Version]
- Prasad, A.; Kothari, N. Cow products: Boon to human health and food security. Trop. Anim. Health Prod. 2022, 54, 2–20. [Google Scholar] [CrossRef]
- Aşik, B.B.; Turan, M.A.; Celik, H.; Katkat, A.V. Effects of humic substances on plant growth and mineral nutrients uptake of wheat (Triticum durum cv. Salihli) under conditions of salinity. Asian J. Crop Sci. 2009, 1, 87–95. [Google Scholar] [CrossRef]
- Khaled, H.; Fawy, H.A. Effect of different levels of humic acids on the nutrient content, plant growth and soil properties under conditions of salinity. Soil WaterRes. 2009, 6, 21–29. [Google Scholar] [CrossRef]
- Celik, H.; Katkat, A.V.; Aşik, B.B.; Turan, M.A. Effect of humus on growth and nutrient uptake of maize under saline and calcareous soil conditions. Zemdirb. Agric. 2010, 97, 15–22. [Google Scholar]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: Aliterature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Roy, D.K. Use of medicinal plants and its vulnerability due to climatic change in Northern part of Bangladesh. Am. J. Plant. Sci. 2016, 7, 1782–1793. [Google Scholar] [CrossRef]
- Matlok, N.; Stępien, A.E.; Gorzelany, J.; Woinarowska-Novak, R.; Balawejder, M. Effects pf organic and mineral fertilizationon yield and selected quality parameters for dried herbs of two varieties of oregano (Origanum vulgare L.). Appl. Sci. 2020, 10, 5503. [Google Scholar] [CrossRef]
- Gerami, F.; Moghaddam, P.R.; Ghorban, R.; Hassani, A. Effects of irrigation levels and organic manure on morphological traits, essential oil content and yield of oregano (Origanum vulgare L.). An. Acad. Brass. Sci. 2016, 88, 2375–2385. [Google Scholar] [CrossRef]
- Hendawy, S.F.; Azza, A.; El-Din, E.; Aziz, E.E.; Omer, A.E. productivity and quality of Thymus vulgaris L. under organic fertilization conditions. Ozean J. Appl. Sci. 2010, 3, 203–216. [Google Scholar]
- Khalid, A.; Hussein, M.S. Effect of cattle and liquid manures on essential oil and antioxidant activities of celery (Apium graveolens L.) fruits. J. Essent-Oil Bear. Plants 2012, 15, 97–107. [Google Scholar] [CrossRef]
- Zahara, M.; Suwarniati, A.; Aini, Q.; Muslim, M. The effect of organic fertilizers on the leaf morphology and stomata density of Pogestemon cablin Benth. J. Nat. 2021, 21, 52–56. [Google Scholar] [CrossRef]
- Bilkova, I.; Kjaer, A.; van der Kooy, F.; Lommen, W.J.W. Effects of N fertilization on trichomes density, leaf size and artemisin production in Artemisia annua leaves. Acta Hortic. 2016, 1125, 369–376. [Google Scholar] [CrossRef]
- Onauskas, A.; Dambrauskas, K. Agrochemiko Žinynas; Mokslas: Vilnius, Lithuania, 1984; pp. 73–75. [Google Scholar]
- Dagys, A.; Bluzmanas, P.; Putrimas, A. Augalų Fiziologijos Laboratorinia Idarbai; Mintis: Vilnius, Lithuania, 1965; p. 309. [Google Scholar]
- Adams, R.P. Identificationof Essential Oil Components by Gas Chromatography/Mass Spectometry, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007; pp. 104–550. [Google Scholar]
- Solaiman, Z.M.; Yang, H.; Archdeacon, D.; Tippeti, O.; Tibi, M.; Whiteley, A.S. Humus–rich compost increases lettuce growth, nutrient uptake, mycorrhizal colonisation and soil fertility. Pedosphere 2019, 29, 170–179. [Google Scholar] [CrossRef]
- Mirchooli, F.; Kiani-Harchegani, M.; Darvishan, A.K.; Falahatkar, S.; Sadehi, S.H. Spatial distribution dependency of soil organic carbon content to important. Ecol. Indic. 2020, 116, 106473. [Google Scholar] [CrossRef]
- Pineiro, G.; Paruelo, J.M.; Oesterheld, M.; Jobbagy, J.G. Pathways of grazing effects on soil organic carbon and nitrogen. Regeland Ecol. Manag. 2010, 63, 109–119. [Google Scholar] [CrossRef]
- Stehouver, R. Soil chemistry and the quality of humus. Biocycle 2004, 45, 41. [Google Scholar]
- Dar, J.S.; Cheema, M.A.; Rehmani, M.I.A.; Khuhro, S.; Rajput, S.; Virk, A.L.; Hussain, S.; Bashir, M.A.; Alghanem, S.M.; Al-Zuaibr, F.M.; et al. Pottasium fertilization improves growth, yield and seed quality of sunflower (Hellianthus annus L.) under drought stress at different growth stages. PLoS ONE 2021, 16, e0256075. [Google Scholar] [CrossRef] [PubMed]
- Zahra, M.K.M.; Monib, S.I.; Abbdel-Al, S.; Heggo, A. Significance of soil inoculation with silicate bacteria. Zentrabl. Microbiol. 1984, 139, 349–357. [Google Scholar] [CrossRef]
- Roslon, W.; Osinska, E.; Bączek, K.; Węglarz, Z. The influence of organic–mineral fertilizers on yield and raw material quality of chosen plants of the Lamiaceae family from organic cultivation. Acta. Sci. Pol. Hortorum Cultus 2011, 10, 147–158. [Google Scholar]
- Samani, J.L.; Pirbalouti, A.G.; Malekpoor, F. Effect of organic and chemical fertilizers on growth parameters and essential oil of Iranian Basil (Ocimum basilicum L.). J. Crop Nutr. Sci. 2017, 3, 14–24. [Google Scholar]
- Rahman, K.M.; Sattar, M.A.; Rahman, G.M.M. Effect of fertilizer and manures on growth and yield of Tulsi and Pudina. J. Environ. Sci. Nat. Resour. 2014, 7, 13–16. [Google Scholar]
- Costa, A.G.; Bertolucci, S.K.V.; Chagas, J.H.; Ferraz, E.O.; Pinto, J.E.B.P. Biomass production, yield and chemical composition of peppermint essential oil using different organic fertilizer sources. Cienc. Agrotec. 2013, 37, 202–210. [Google Scholar] [CrossRef]
- Grigal, D.F.; Vance, E.D. Influence of soil organic matter on forest productivity. N. Z. J. For. Sci. 2000, 30, 169–205. [Google Scholar]
- Manukyan, A.; Schniztler, W.H. Influence of air temperature on productivity and quality of some medicinal plants under controlled environmet conditions. Eur. J. Hortic. Sci 2006, 71, 26–35. [Google Scholar]
- Nadjati, F.; Shabahang, J.; Dambhani, M.A.M. Effects on salinyti and temperature on germination and seedling growth of nine medicinal plant species. Seed Technol. 2010, 32, 93–107. [Google Scholar]
- Hui, D.; Yu, C.L.; Deng, Q.; Dzantor, E.K.; Zhou, S.; Dennis, S.; Sauve, R.; Jonhson, T.L.; Fay, P.A.; Shen, W.; et al. Effects of precipitation changes on swichgrass photosynthesis, growth and biomass: A mesocosm experiment. PLoS ONE 2018, 13, e0192555. [Google Scholar] [CrossRef]
- Saldana y Hernandez, M.I.; Gomez-Alvarez, R.; Rivera-Cruz, M.C.; Alvarez-Solis, J.D.; Pat-Fernandez, J.M.; Ortiz-Garcia, C.F. The influence of organic fertilizers on the properties of soil and production of Alpinia purpurata. Cien. Ing. Agr. 2014, 41, 215–224. [Google Scholar] [CrossRef]
- Hartz, T.K. Evaluation of Humic Substances Used in Commercial Fertilizer Formulation. Final Report, Frep Project 07-0174; 2007; pp. 1–23. Available online: https://www.cdfa.ca.gov/is/ffldrs/frep/pdfs/completedprojects/07-0174Hartz.PDF (accessed on 10 August 2022).
- Shen, J.; Gou, M.; Wang, Y.; Yuan, X.; Dong, S.; Song, X. An investigation into the beneficial effects and molecular mechanisms ob humic acid on foxtal millet after drought conditions. PLoS ONE 2020, 15, e0234029. [Google Scholar] [CrossRef]
- Huchhellman, A.; Boutry, M.; Hachez, C. Plant glandular trichomes: Natural cell factories of high biotechnological interest. Plant Psysiol. 2017, 175, 6–22. [Google Scholar] [CrossRef]
- Talebi, S.H.; Nohooji, M.G.; Yarmohammadi, M.; Khani, M.; Matsyura, A. Effect of altitude of essential oil composition in three Nepata species (N. sessilifolia, N. heliotropifolia, N. fissa). Mediterr. Bot. 2019, 40, 81–93. [Google Scholar] [CrossRef]
- Rodolfi, M.; Barbanti, M.; Giordano, C.; Rinaldi, M.; Fabbri, A.; Pretti, L.; Casolari, R.; Beghe, D.; Petruccelli, R.; Ganino, T. The effect of different organic fertilization on physiological and chemical characters in hop (Humulus lupulus L., cv Cascade) leaves and cones. Appl. Sci. 2021, 11, 6778. [Google Scholar] [CrossRef]
- Kardiman, R.; Ræbild, A. Relationship between stomata density, size and speed of opening Sumatran rainforest species. Tree Physiol. 2017, 38, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Sakoda, K.; Yamori, W.; Shimada, T.; Sugano, S.S.; Hara-Nishimura, I.; Tanaka, Y. Higher stomata density improves Photosynthesis induction and biomass production in Arabidopsis under fluctuating light. Front. Plant. Sci. 2020, 11, 589603. [Google Scholar] [CrossRef] [PubMed]
- Yavari, A.; Nazeri, V.; Sefidkon, F.; Hassani, M.E. Influence of some environmental factors on the essential oil variability of Thymus migricus. Nat. Prod. Commun. 2010, 5, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Sardashti, A.R.; Ganjali, A.; Kordi, A. Effect of humic substances on the quality of essential oils from medicinal plants. Bothalia 2014, 44, 280–300. [Google Scholar]
- Caceres, J.A.; Cuervo, J.L.A.; Rodriguez, L.C. Effect of organic fertilization on yield and quality of rosemary (Rosmarinus officinalis L.) essential oil. Agron. Colomb. 2017, 35, 232–237. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).