Abstract
The aim of this research was to produce konjac glucomannan (KGM) antimicrobial film with added sweet basil oil (SB) (Ocimum basilicum) as an antimicrobial agent for inhibiting coliform bacteria which is the type most often found in fresh-cut vegetables. The concentrations of SB oil in the emulsion that inhibited the most antimicrobial growth were 4% and 6% (v/v). Film-forming conditions were evaluated by varying the volume of KGM solution per area (0.325, 0.455 and 0.585 mL·cm−2) and the concentration of SB oil (4% and 6%). After mixing the film emulsions, the emulsions were dried on a tray dryer at 50 °C for 10 h. After drying, the results showed that KGM film made at 0.325 mL·cm−2 with SB oil at 4% resulted in the smoothest surface. When the film was tested against Escherichia coli, KGM at 0.325 mL·cm−2 with 4% SB oil and at 455 mL·cm−2 with 6% SB oil produced the greatest inhibition. Film with SB oil at 4% was used to study film properties. Physical properties of the film such as tensile strength (68.08 MPa) and % elongation (33.56%) as well as water vapor transmission rate (4.44 × 10−3 g·cm−2·h−1) were determined. The KGM/SB film did not show an antimicrobial effect on packages of fresh-cut baby cos lettuce or spring onion under the experimental conditions. Further work will be carried out to study more closely the controlled release properties of KGM/SB film to enhance its antimicrobial effects. These improvements could help to develop a more successful application for its use as a natural biopreservative in minimally-processed products like fresh-cut vegetables.
1. Introduction
Nowadays, consumers are aware of their health and the quality of food. Fresh and less processed foods are the first option for a healthy meal. Freshly-made salads from fresh-cut vegetables are increasing in popularity. However, there is often news about disease outbreaks from consuming fresh-cut vegetables.
Essential oils are aroma compounds found in plants that contain volatile and non-volatile components. The qualitative and quantitative composition of essential oils determines their characteristic. Sweet basil (Ocimum basilicum) is a common herb in Thai cuisine. Its essential oil contains bioactive volatile compounds such as linalool and methyl chavicol. These bioactive volatile compounds in sweet basil have antimicrobial properties against gram-positive bacteria [1].
Konjac glucomannan is a component derived from tubers of Amorphophallus konjac plants which grow in subtropical and tropical regions of China, Japan, and Thailand. Konjac glucomannan is composed of (1→4) linked β-d-mannose and β-d-glucose. It has a high molecular weight, is water soluble, has properties of film-formation, is edible, biodegrades, has a tendency to form a fine dense network upon drying, and is used in pharmaceutical and food applications [2,3,4,5]. Therefore, Konjac glucomannan could be a good option for entrapping target molecules. The objectives of this study were to develop natural and edible antimicrobial film by combining Konjac glucomannan with sweet basil oil and determine its mechanical and antimicrobial properties, as well as the performance of the film on the fresh cut vegetables baby cos (Lactuca sativa var. longifolia) and spring onion (Allium wakegi) during storage.
2. Materials and Methods
2.1. Materials
Konjac glucomannan (food grade) was obtained from the Yunnan Genyun Konjac Resource Corp., Kunming (Yunnan, China). Sweet basil (SB) oil was purchased from Thai-China Flavours and Fragrances Industry Co., Ltd. (Nonthaburi, Thailand). All fresh vegetables including baby cos and spring onion were purchased from the Samyan wet market, Bangkok, Thailand, on the day of the experiment. All vegetables were washed under running water for 30 s and soaked in a 50 ppm available chlorine solution for 2 min then drained and put aside for drying on a perforated tray before use. The bacterial strain Escherichia coli ATCC 25922 that was used for testing antimicrobial activity was obtained from standardized culture at the Department of Food Technology, Faculty of Science, Chulalongkorn University, Bangkok, Thailand.
2.2. Methods
2.2.1. Emulsion Preparation and Antimicrobial Assessment
Konjac glucomannan (KGM) solution at 1% (w/v) was prepared by slowly adding KGM powder into water and stirring with a magnetic stirrer for 3 h at ambient temperature, followed by adding 0.5 M KOH to reach 0.14% (v/v) KOH and stirring for another 15 min. The solution was left for 1 h, then glycerol was added to reach 0.3% (w/v), and the solution stirred for 20 min. SB oil was then added to the KGM solution to achieve concentrations of 1%, 2%, 3%, 4%, 5%, 6%, 7%, and 8% (v/v), and they were stirred at 20,000 rpm for 10 min at ambient temperature.
Asuitable concentration of SB oil for further work was selected by assessing antimicrobial activity using the paper disc diffusion method against E. coli. The method described by Mekkerdchoo et al. [6] was followed. Bacterial cultures were grown and inoculated in fresh nutrient broth (NB) medium at 37 °C for 24 h. The microorganism was inoculated on the surface of a nutrient agar plate by sterile cotton swab (initial concentration of E. coli was 107 log colony forming units (CFU)/mL). Subsequently, sterile paper discs (Antibiotica-Testblattchen paper disc, Duran, West Chester, PA, USA) (6 mm in diameter) saturated with SB oil at different concentrations (4% or 6% (w/w)) were placed on the surface of each inoculated plate. A standard antibiotic (tetracycline (Becton, Dickson and Company, Franklin Lakes, NJ, USA)) was simultaneously used as a positive control. The inoculated plates were incubated at 37 °C for 24 h under aerobic conditions. After this period, it was possible to observe a clear zone, free of visible bacterial growth. The antibacterial activity was evaluated by measuring the clear zone diameter. The sensitivity of bacterial growth to the antimicrobial solutions was determined by the diameter of the inhibition zone as: not sensitive = diameters less than 0.8 cm; sensitive = diameters 0.9–1.4 cm; very sensitive = diameters 1.5–1.9 cm; and extremely sensitive = diameters larger than 2.0 cm [7]. All treatments were performed in triplicate.
2.2.2. Film Preparation and Antimicrobial Assessment
The KGM/SB emulsion selected above was cast on hard adonized steel trays (14 cm diameter) at 0.325, 0.445 and 0.585 mL·cm−2. The film solutions were dried at 50 °C by using a hot air dryer for 10 h. After drying, antimicrobial films were conditioned at 67% RH before further testing. Antimicrobial activity of the films against E. coli were assessed by measuring the clear zone (cm) [6]. The bacteria culture was grown and inoculated in NB medium at 37 °C. After 24 h of growth, the microorganism was inoculated on the surface of an agar plate (initial concentration of E. coli was 107 log CFU/mL). Film samples were cut into circles (6 mm diameter) from different positions of the film and placed on the culture plate. Standard antibiotic (tetracycline) was used as positive control. The culture plate was incubated at 37 °C for 24 h under aerobic conditions. After this period, it was possible to observe a clear zone. All measurements were done in triplicate.
2.2.3. Evaluation of Mechanical Property of Antimicrobial Film
The mechanical properties of antimicrobial films that had good antimicrobial activity from above were assessed. The properties included tensile strength (TS), percent of elongation (%E) (ASTM standard test method D882 [8], film thickness (digital micrometer), and water vapor transmission rate (WVTR) [1]. All measurements were performed in triplicate.
2.2.4. Antimicrobial Assessment of Fresh-Cut Vegetables during Storage
Baby cos lettuce (95 g) was packed into plastic boxes along with a KGM/SB film cut into a 3 cm × 3 cm piece. It was stored at 4 ± 1 °C, and was sampled every day for 7 days to assess total plate count (TPC) and bacterial count of E. coli and coliform. Spring onion (40 g) was packed as above and stored at ambient temperature for 24 h, and then TPC and bacterial count of E. coli and coliform were assessed. The TPC analyses were as follows: 25 g of each vegetable, from 3 different packages, was collected and placed in a sterile plastic bag (Sterilin, Staffordshire, UK) with 90 mL of buffered 0.1% peptone water (Merck, Darmstadt, Germany). After 1 min in a Stomacher blender (Model 400 circulator, West Sussex, UK), appropriate dilutions (10−1 to 10−7) were prepared for TPC determination on plates with Plate Count Agar and incubated at 35 °C for 24 h [9]. The number of E. coli and coliform were assessed using 3 M PetrifilmTM E. coli/Coliform Count Plates (EC) by taking 25 g of sample by aseptic technique, placing the sample in 225 mL sterile peptone water in a bag, and mixing using a Stomacher mixer. Then, a serial dilution from the mixed solution was prepared at 10−1, 10−2, 10−3, 10−4, 10−5, 10−6, and 10−7. A 1 mL solution of each dilution was pipetted onto 3M PetrifilmTM, pressed with a spreader, and left for 1 min for setting the gel. The sample was incubated at 37 °C for 48 h. The number of coliform and E. coli colonies were measured following the 3M PetrifilmTM manual, counting gas bubble-producing red coliform colonies and blue E. coli colonies. All measurements were performed in triplicate [9].
2.2.5. Statistical Analyses
All data were statistically analyzed (SPSS 17.0 software; IBM SPSS, Chicago, IL, USA). Data are shown as mean values with the standard deviation. Differences between mean values were established using Duncan’s multiple range test with a level of significance of p ≤ 0.05.
3. Results and Discussion
3.1. Antimicrobial Assessment of KGM/SB Emulsion and Film
Microbial assessment of the emulsion was evaluated by the diameter of the clear inhibition zone against E. coli (Table 1). Contact area was used to evaluate growth inhibition underneath film discs in direct contact with target microorganisms in agar [10]. Pure sweet basil oil gave a clear zone of 13.2 ± 0.8 mm, lower than tetracycline at 23.7 ± 1.6 mm but indicating inhibition of E. coli. According to the sensitivity levels, the response to SB oil was sensitive, a diameter of 9–14 mm [7]. When adding SB oil to KGM solution from 1%–8%, antimicrobial efficiency was reduced due to the lower oil concentration in the emulsion resulting in a decrease in active compound in the aqueous phase of the agar layer where microbial proliferation takes place [11]. Even though there were no statistical differences in the inhibition zone between 2%, 3%, 5%, 7% and 8% KGM/SB emulsion, 4% and 6% emulsion were selected for further study due to giving the largest clear zones (7.8 and 8.2 mm, respectively). According to Ponce et al. [7], at this range of the clear zone, it would be considered not sensitive for use as an antimicrobial solution.

Table 1.
Antimicrobial activity of a KGM/SB emulsion film assessed by the clear zone (mm) against E. coli.
Emulsions of KGM/SB with 4% or 6% SB oil were cast into films at different ratios of volume per area. The concentrations of 4% SB oil in a 0.325 mL·cm−2 film and 6% SB oil in a0.455 mL·cm−2 film gave the highest diameter clear zones (Table 2). A dilution effect was only seen in the 4% SB oil samples. The physical appearance of the different KGM/SB films is shown in Figure 1. The 4% SB oil was more transparent than the 6% film. During drying, when water evaporated, the SB oil was likely becoming more concentrated and phase separation may have occurred. This was more apparent in the 6% KGM/SB film when volume-to-area ratio increased, and the phase separation occurred creating the opaque appearance. Thus, SB oil used at a high concentration, which is composed mostly of nonpolar compounds, may not mix well with KGM solution which contains a range of polarities.

Table 2.
Antimicrobial activity of a KGM/SB emulsion film assessed by the clear zone (mm) against E. coli at different volumes per area.
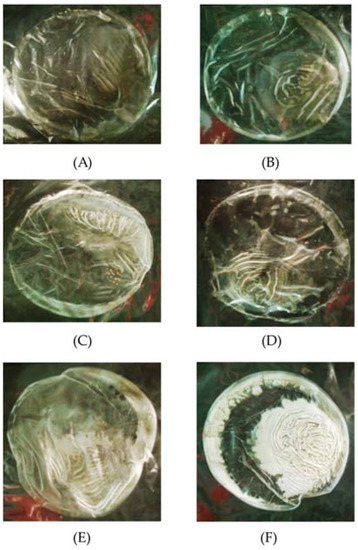
Figure 1.
Appearance of antimicrobial film after drying at different KGM-to-SB oil ratios. SB oil at 4% in 0.325 (A); 0.445 (B) and 0.585 (C) mL·cm−2 KGM solution; and at 6% in 0.325 (D); 0.445 (E) and 0.585 (F) mL·cm−2.
The KGM/SB film sample at 4% SB oil in 0.325 mL·cm−2 KGM was selected to determine mechanical properties (Table 3). Mechanical property determination is necessary for material that may be used as a food packaging [12]. The mechanical properties of antimicrobial film from a KGM/SB film sample at 4% SB oil in 0.325 mL·cm−2 KGM showed a lower tensile strength when compared to a previous study of mechanical properties of a 1% w/w KGM film (tensile strength = 88.1 ± 1.2 MPa) [10]. Normally, incorporation of additives into a film results in a lower tensile strength value [13]. On the other hand, %E and WVTR of this film were increased when compared to 1% KGM film (%E = 26.5 ± 1.8, WVTR = 5.4 × 10−12) [11]. These results indicated that additional film emulsion may decrease the homogeneity of the film matrix structure lowering the tensile strength and increasing %E, while increasing WVTR may have come from reducing the mass transfer resistance to water molecules [14,15]. Nevertheless, these changed physical properties improved film flexibility, and more moisture passing through the material could result in more rapid release of the antimicrobial agents in the blended film when the film is in contact with the surface of the food [10]. In addition, the film in this study showed a higher tensile strength and WVTR while %E was lower when compared to commercial plastic films which are more flexible and water resistant, such as low-density polyethylene (tensile strength = 14.9 ± 0.49 MPa, %E = 1073 ± 27.67, and WVTR = 4.98 × 10−13 g·cm−2·h−1) [16] and oriented polypropylene (tensile strength = 28 ± 1.05 MPa, %E = 780 ± 18.24, WVTR = 4.27 × 10−13) [17].

Table 3.
Mechanical property of antimicrobial film.
3.2. Microbial Assessment of Fresh Cut Vegetable during Storage
Due to the importance of microbial assessment in real food systems, two types of vegetables were selected for use in this study, baby cos lettuce (Figure 2 and Figure 3) and spring onion (Table 4).
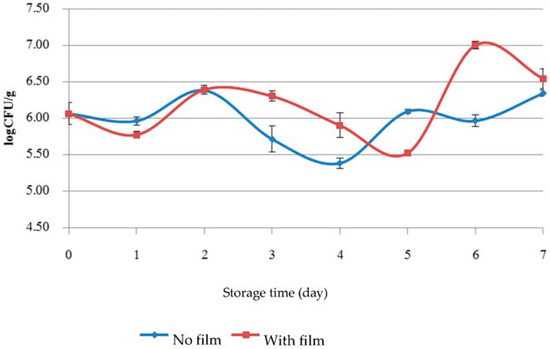
Figure 2.
Number of microorganisms by total plate count as colony forming units (CFU) recovered from baby cos lettuce during 7 days of cold storage.
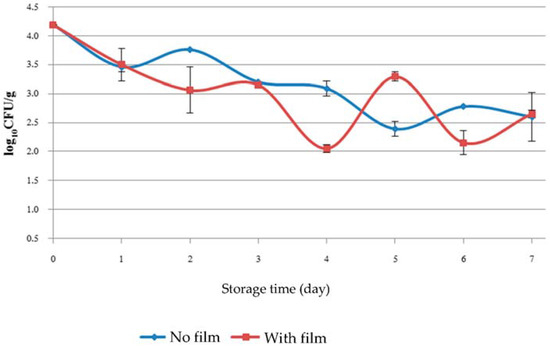
Figure 3.
Number of coliforms as colony forming units (CFU) recovered from baby cos lettuce during 7 days of cold storage.

Table 4.
Number of microorganisms as colony forming units (CFU) recovered from spring onion after 24 h.
KGM/SB film was not effective by total plate count analysis when used with low temperature storage at 4 ± 1 °C (Figure 2). This may be because low temperature suppressed the evaporation of the bioactive volatile compound SB oil to the surrounding air space. It is consistent with the results of Suppakul et al. [11] who studied efficacy of antimicrobial films containing constituents of SB oil used in cheese and found that lower temperatures suppressed the evaporation of bioactive volatile compound, while at a higher temperature the release rate of the bioactive compound from the film onto the food surface was increased. The mass transfer rate from the film surface to the air is dominant, because of convection and the larger concentration gradient than that in the film interior [14]. Any reduction in coliform CFU may be assumed due to the effect of cold storage more than the effect of antimicrobial film (Figure 3). E. coli was not found in the samples during storage for 7 days. Antimicrobials must directly contact the surface of food to exert the greatest antimicrobial effect [18]. Therefore, items with irregular shapes or rough surfaces like baby cos lettuce where KGM/SB film cannot directly contact the surfaces would result in bacterial cells that would be less exposed to antimicrobial compound.
For spring onion, after 24 h storage at ambient temperature, the KGM/SB film did not reduce microbial activity significantly when compared to no film (Table 4). Similar results were observed by Cano et al. [19] who studied incorporation of neem oil in starch-PVA films and found that the film did not improve the antimicrobial properties. Even more, the film seemed to promote the early growth of bacteria during the storage period. Ponce et al. [20] also found that the antibacterial activities of olive and rosemary oil emulsions were reduced when they were applied in the coating material. These results are probably due to the dispersion effect of the active compounds and the interactions among the oils and films. The low effectiveness of antimicrobial film with a real food system as in this study may occur for many reasons such as strong entrapment of the emulsion in the film structure, inhibiting diffusion to the film surface where microbial growth occurs [14], or essential oil emulsion strong interaction with hydroxyl groups of the polymers, thus limiting their diffusion to the film surface and their antimicrobial effects [19]. Moreover, the coliform bacteria that was used in this study were gram-negative which are generally more resistant to growth inhibition and the killing effects of various antibiotics and antimicrobial agents due to their strong surface hydrophilicity acting as a permeability barrier. The surface also possesses divalent cations that could stabilize the lipopolysaccharide association within the membrane, and may prevent active compounds from reaching the cytoplasmic membrane [11].
4. Conclusions
Sweet basil oil incorporated into konjac glucomannan film exhibited antimicrobial activity at a concentration of 4% (v/v) (volume-to-area ratio of 0.325 mL·cm−2) and a concentration of 6% (v/v), (volume-to-area ratio of 0.455 mL·cm−2). The 4% (v/v) (volume-to-area ratio of 0.325 mL·cm−2) film was clear with a smooth surface, a thickness of 0.074 mm, a tensile strength 6.81 MPa, elongation of 33.56%, and a water vapor transmission rate of 4.44 × 10−3 g·mm−2·h−1, respectively. In this preliminary study on application of the antimicrobial film to fresh-cut baby cos lettuce and spring onion in packages, KGM/SB film did not show an antimicrobial effect. However, there is a need for further study of the controlled release properties of KGM/SB film to enhance potential antimicrobial properties of the film against spoilage microorganisms and to extend shelf life of fresh-cut vegetables.
Acknowledgments
The authors would like to thank Chulalongkorn University for financial supporting of the study.
Author Contributions
“Chaleeda Borompichaicharkul, Panya Saeheng and Panuwat Eamsakulrat conceived and designed the experiments; Panya Saeheng and Panuwat Eamsakulrat performed the experiments; Chaleeda Borompichaichartkul, Orachorn Mekkerdchoo, Panya Saeheng and Panuwat Eamsakulrat analyzed the data; Chaleeda Borompichaichartkul and Orachorn Mekkerdchoo wrote the paper”. Authorship must be limited to those who have contributed substantially to the work reported.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Standard for Testing and Materials. Standard Test Method for Water Vapor Transmission of Material; ASTM E95-96; Annual Book of ASTM Standard; American Society for Testing and Materials Publishing: Philadelphia, PA, USA, 1995. [Google Scholar]
- American Standard for Testing and Materials. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM D882; Annual Book of ASTM Standard; American Society for Testing and Materials Publishing: Philadelphia, PA, USA, 2001. [Google Scholar]
- Davé, V.; McCarthy, S.P. Review of konjac glucomannan. J. Environ. Polym. Degrad. 1997, 5, 237–241. [Google Scholar]
- Dave, V.; Sheth, M.; McCarthy, S.P.; Ratto, J.A.; Kaplan, D.L. Liquid crystalline, rheological and thermal properties of konjac glucomannan. Polymer 1998, 39, 1139–1148. [Google Scholar] [CrossRef]
- Nakano, M.; Takikawa, K.; Arita, T. Release characteristics of dibucaine dispersed in konjac gels. J. Biomed. Mater. Res. 1979, 13, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Mekkerdchoo, O.; Patipasena, P.; Borompichaichartkul, C. Liposome encapsulation of antimicrobial extracts in pectin film for inhibition of food spoilage microorganisms. Asian J. Food Agro-Ind. 2009, 2, 817–838. [Google Scholar]
- Ponce, A.; Fritz, R.; del Valle, C.; Roura, S. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. Lebensm. Wiss. Technol. 2003, 36, 679–684. [Google Scholar] [CrossRef]
- Arunwatcharin, P. Pathogens and spoilage microorganism’s inhibition ability of Thai herb essential oils. In Proceeding of the 45th Conference on Agriculture and Home Economics, Agro-Industry, Kasetsart University, Bangkok, Thailand, 30 January–2 February 2007; pp. 508–515. (In Thai)
- Chacon, P.A.; Buffo, R.A.; Holley, R.A. Inhibitory effects of microencapsulated allyl isothiocyanate (AIT) against Escherichia coli O157:H7 in refrigerated, nitrogen packed, finely chopped beef. Int. J. Food Microbiol. 2006, 107, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Kennedy, J.F.; Peng, J.L.; Yie, X.; Xie, B.J. Preparation and performance evaluation of glucomannan-chitosan-nisin ternary antimicrobial blend film. Carbohydr. Polym. 2006, 65, 488–494. [Google Scholar] [CrossRef]
- Suppakul, P.; Sonneveld, K.; Bigger, S.W.; Miltz, J. Efficacy of polyethylene-based antimicrobial films containing principal constituents of basil. LWT-Food Sci. Technol. 2008, 41, 779–788. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Gustaw, W.; Świeca, M.; Baraniak, B. A study on the mechanical properties of pea protein isolate films. J. Food Process. Preserv. 2014, 38, 1726–1736. [Google Scholar] [CrossRef]
- Pranoto, Y.; Rakshit, S.K.; Salokhe, V.M. Physical and antimicrobial properties of alginate-based edible film incorporated with garlic oil. Food Res. Int. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Critzer, F.; Davidson, P.M.; Zivanovic, S.; Zhong, Q. Physical, mechanical, and antimicrobial properties of chitosan films with microemulsions of cinnamon bark oil and soybean oil. Food Hydrocoll. 2016, 52, 533–542. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Manikantan, M.R.; Varadharaju, N. Preparation and properties of linear low density polyethylene based nanocomposite films for food packaging. Indian J. Eng. Mater. Sci. 2012, 19, 54–66. [Google Scholar]
- Hyun, K.; Chong, W.; Koo, M.; Chung, I.J. Physical properties of polyethylene/silicate nanocomposite blown films. J. Appl. Polym. Sci. 2003, 89, 2131–2136. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.Z.; Wang, L.; Scullen, O.J.; Sommers, C.H. Antimicrobial films and coatings for inactivation of Listeria innocua on ready-to-eat deli turkey meat. Food Control 2014, 40, 64–70. [Google Scholar] [CrossRef]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and antimicrobial properties of starch-PVA blend films as affected by the incorporation of natural antimicrobial agents. Foods 2015. [Google Scholar] [CrossRef]
- Ponce, A.G.; Roura, S.I.; del Valle, C.E.; Moreira, M.R. Antimicrobial and antioxidant activities of edible coatings enriched with natural plant extracts: In vitro and in vivo studies. Postharvest Biol. Technol. 2008, 49, 294–300. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).