Abstract
Citrus species develop fruits through both sexual reproduction and parthenocarpy, following a growth pattern with an initial exponential phase dominated by cell division in the ovary wall, followed by a linear phase driven by cell expansion in juice vesicles. Sustained carbohydrate supply is essential to support the metabolic energy required for these processes, which are tightly regulated by hormonal signaling pathways involving gibberellins (GAs), auxins (IAA), cytokinins, and abscisic acid (ABA). Recent studies across cultivars have identified genes associated with hormone biosynthesis, carbohydrate metabolism, cell cycle regulation, and abscission in ovule and pericarp tissues. Manipulation of these hormones through targeted treatments and cultural practices has shown potential to enhance fruit set and growth. Notably, exogenous GA3 application promotes fruit set in parthenocarpic cultivars by upregulating GA20ox2/GA3ox and CYCA1.1, whereas synthetic auxins enhance fruit enlargement by improving assimilate partitioning and water uptake. Optimizing such treatments, however, requires a comprehensive understanding of physiological, environmental, and agronomic factors influencing fruit development. This review summarizes recent advances in hormonal and molecular regulation of citrus fruit set and developments, assesses applied strategies to improve productivity, and identifies current knowledge gaps needed to refine biotechnological and management aimed at enhancing both yield and fruit quality.
1. Introduction
Citrus fruit is classified as a modified berry known as hesperidium. It is composed of 9 to 14 carpels fused around the floral axis, forming locules where juice vesicles, or juice sacs, and seeds develop [1,2] (Figure 1). The pericarp is structurally differentiated into three distinct layers [2]. The exocarp, or flavedo, constitutes the outermost region of the fruit and comprises an epidermal layer and several layers of hypodermal cells forming a compact parenchyma. It is enveloped by a cuticle composed of crystalline-structured epicuticular waxes. The mesocarp, or albedo, consists of parenchymatous tissue characterized by multiple cell layers, large intercellular spaces, and a spongy texture. The endocarp, the innermost layer of the pericarp, forms part of the locular membrane. It includes an epidermis (the layer directly adjacent to the locule) and several layers of compact parenchymatous cells. As the fruit ripens, the rind—comprising both the mesocarp and exocarp—may progressively detach from the endocarp. The flavedo is responsible for the fruit’s characteristic coloration, which varies among different citrus species and varieties.

Figure 1.
Parts of a mature citrus fruit.
The growth and developmental pattern of citrus fruit undergoes the ovary growth and differentiation, ultimately maturing into a fully developed fruit. This process initiates after anthesis in spring and extends over a period of 6 to 9 months, depending on species and cultivars. In temperate regions with a Mediterranean climate, early-maturing varieties ripen in early autumn, whereas late-maturing cultivars may continue their development into winter. In subtropical regions, the fruiting period is typically 1 to 2 months shorter, and in tropical climates, it is further reduced. Measured in terms of size (diameter, length, or volume) or weight, fruit development follows a sigmoidal curve over time and can be divided into three distinct stages [1].
Stage I represents a phase of moderate growth, extending from anthesis to the completion of physiological fruitlet abscission, lasting slightly over two months. During this period, cell division is the predominant process, increasing cell number in all developing tissues. Fruit enlargement primarily results from the growth of ovary wall tissues (pericarp), which later differentiate into the fruit peel, and differentiation of the juice vesicles from the endocarp, which form the pulp. By the end of this stage, the pericarp attains its maximum thickness (Figure 2). This phase is commonly referred to as the fruit-setting stage.
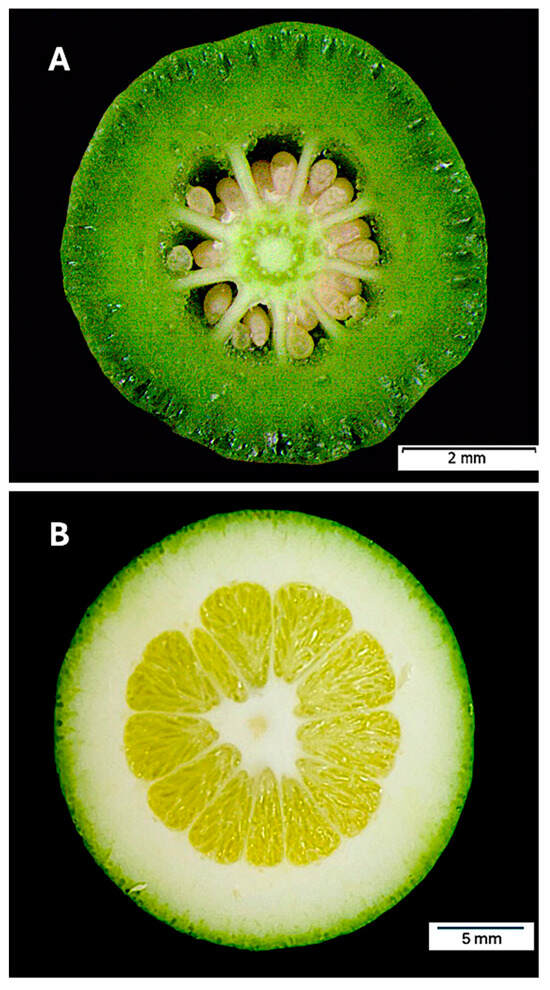
Figure 2.
Ovary at anthesis (A) and fruit at the end of physiological fruitlet abscission (B).
The mesocarp constitutes the pericarp region that undergoes the most significant expansion during Stage I of fruit development (Figure 2), driven by ovary growth. The highest rate of cell division occurs in the outer mesocarp, where a narrow layer of cells retains meristematic characteristics. These cells are small, possess thin walls, and are densely packed. As development progresses, these cells are displaced into deeper tissue layers, where they enlarge, their walls thicken, and intercellular spaces expand. The transition from the outer to the inner mesocarp is particularly evident at the organelle level [2]. Notably, plastids undergo substantial transformations: in the outer mesocarp, chloroplasts remain photosynthetically active but gradually degrade as the cells migrate inward, ultimately differentiating into amyloplasts. Throughout fruit development, both chloroplasts and amyloplasts progressively lose their starch reserves [3,4].
The innermost pericarp layer, the endocarp, exhibits minimal growth during Stage I, significantly less than that of the mesocarp (Figure 2). Consequently, the locule volume increases only moderately, primarily through cell division in the septa and tangential walls of the locules. In the septa, divisions occur periclinally, whereas in the tangential walls, they follow an anticlinal pattern. The septa correspond to the walls of the carpels. Within the endocarp, and extending laterally from it, each carpel contains two locular membranes composed of endocarp-like tissue, which converge at the floral axis. Between the membranes of adjacent locules, a parenchymatous mesocarp-like tissue forms the septum (Figure 3A). This tissue degrades readily, facilitating the natural separation of locules or segments in ripe fruits.
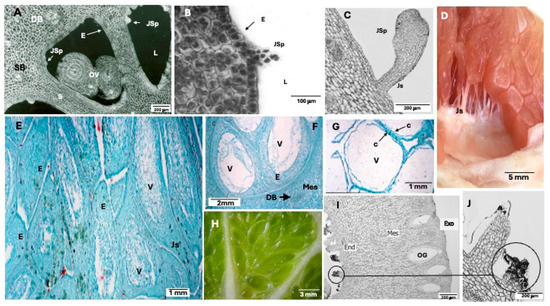
Figure 3.
Cross-section of an ovary showing well-formed ovules (A). Juice sac primordia (B), and in the early stage of development (C). Juice sac stalks maintaining contact with the endocarp (D). Cross-sections of the juice sacs (E–G) and a newly developed juice sac within the locule initiating juice accumulation (H). Carpelary emergence (I,J). DB: Dorsal bundle. SB: Septal bundel. S: septum. L: Locule. OV: Ovule. JSp: Juice sac primordia. Js: Juice stalk. E: Epidermis. V: vacuole. Mes: mesocarp. En: Endocarp. Exo: exocarp. OG: Oil gland. From [5] with permission.
Juice vesicles are delicate, filamentous structures that originate at anthesis from cells in the innermost layers of the endocarp. These cells differentiate into the epidermis and subepidermis of the locule, completing the process after style abscission. The epidermal cells of the endocarp primarily undergo anticlinal divisions; however, for reasons not yet fully understood, they occasionally shift to oblique and periclinal planes. This shift gives rise to a protrusion, or vesicular primordium (Figure 3A), which extends inward toward the locule while its daughter cells retain meristematic activity (Figure 3B,C). The terminal cells within the primordium undergo thickening to form the vesicle, whereas the basal cells differentiate into the stalk, which establishes a permanent structural connection between the vesicle and the endocarp (Figure 3D) [2].
The vesicle is enclosed by a robust epidermis covered with a waxy cuticle and contains large, highly vacuolated cells that store juice (Figure 3E–G), primarily composed of sugars, organic acids, and water. The stalk consists of an epidermis with a thin cuticle and parenchymal cells interconnected by plasmodesmata and plasmalemma invaginations, which facilitate the transport of substances. Notably, the stalk lacks vascular bundles. As juice vesicles grow and expand, they progressively fill the locular cavity (Figure 3H) [2].
Shortly before anthesis, carpel emergences begin to develop alongside the vesicles from initial cells distinct from the surrounding tissue lining the interior of the locular cavity (Figure 3I,J). These emergences comprise clusters of globular cells that actively proliferate and grow in a disordered manner toward the locule interior. Typically, the terminal cells enlarge, and their middle lamellae undergo softening, ultimately leading to their detachment. The initial development of carpel emergences progresses more rapidly than that of juice vesicles; however, their growth rate decreases considerably shortly thereafter and ceases entirely before the completion of Stage I. The cytoplasm of these globular cells is characterized by the presence of numerous Golgi vesicles and lipid bodies. Carpel emergences are hypothesized to function as secretory structures [2].
During Stage II, the fruit grows linearly as a result of the enlargement of its cells, which no longer divide (see Section 3). By the end of this stage, the fruit attains its maximum size [1].
In Stage III, fruit growth ceases, and instead, significant metabolic changes lead to ripening, senescence, and eventual abscission [1]. At this point, the fruit must be harvested for consumption. The study of this stage falls beyond the scope of this review.
This work addresses fruit setting and fruit development separately. The first section emphasizes current knowledge on the endogenous regulation, particularly carbohydrate availability and hormonal control, of the transition from ovary to developing fruit, with a special focus on the mechanisms underlying parthenocarpy. The second section reviews the role of auxins in cell enlargement as a prerequisite for water and solute uptake. Agronomic practices derived from these insights are also updated in both sections.
2. Fruit Setting
Orange and mandarin trees bloom profusely, with the number of flowers far exceeding the final fruit yield. Most of flowers abscise at various developmental stages, with an increasing trend as flowering intensity increases [6,7]. After anthesis, some ovaries show arrested development, turning completely yellow, while others grow more rapidly and maintain a dark green, glossy appearance. The former are shed, whereas the latter generally continue growth to maturity. As a result, from petals fall until late June or early July in the Northern Hemisphere (NH), a massive abscission of ovaries and fruitlets occurs, a phenomenon known as physiological fruitlet abscission.
This entire process is referred to as fruit set in citrus. For an individual flower, fruit set can be divided into two phases: the transition from the ovary’s slow developmental stage to the rapid growth phase of the young fruit, and the subsequent abscission process. Although these phases are governed by distinct regulatory mechanisms, they are primarily influenced by competition among developing fruitlets [8,9] and between fruitlets and concurrently growing vegetative shoots [10]. This competitive interaction determines fruit set [6], the main factor limiting productivity in cultivated citrus varieties.
2.1. Fertilization vs. Parthenocarpy
In general, citrus varieties require successful pollination, which involves the fertilization of ovules after anthesis to ensure proper ovary development and ultimately seed formation. This process requires the pollen grains to reach the stigma, their subsequent germination, and the elongation of the pollen tube through the style to reach the ovary and fertilize the ovules.
In citrus, the stigma is relatively large compared to the style diameter and is covered with papillae, whose cell number and size vary. These papillae play a crucial role in pollen hydration and germination. Upon germination, the pollen tube grows through the stylar canal, eventually reaching the ovule to facilitate fertilization.
The ovary and ovule emit signaling cues that guide and regulate the pollen tube as it navigates through the locule, ensuring its proper trajectory [11]. Three lines of evidence support this regulatory mechanism.
First, physiological changes in female tissues influence pollen tube growth. In fruit trees, the obturator, located at the base of the style, has been shown to modulate the trajectory of the pollen tube as it approaches the ovule. In citrus species, this role is fulfilled by papillary hairs [12]. Furthermore, pollen tube entry into the ovule is governed by a chemotropic response; in this process, the initial cells of the micropyle secrete specific compounds that facilitate pollen tube penetration [13].
Second, the embryo sac exerts control over pollen tube guidance. Synergid cells play a crucial role in attracting the pollen tube by synthesizing peptide signals [14].
Third, certain biochemical factors in the pistil are implicated in pollen tube development (see review by [15]). Among these: (1) Ca2+ plays a key role in regulating pollen tube growth, with concentrations in ovules and placenta found to be four times higher than in the rest of the style; moreover, Ca2+ is actively consumed in pollinated ovaries but remains unutilized in unpollinated ones; (2) specific molecules in the stigma and style, including kinases, enzymes associated with floral incompatibility systems, have been implicated in pollen tube regulation; and (3) adhesion-related molecules, as well as glycoproteins involved in pollen tube nutrition, have been identified.
However, the pollen tube must reach the ovule and accomplish fertilization within a restricted timeframe, as ovule receptivity persists for only a few days. This window is referred to as the effective pollination period (EPP), which varies across species and cultivars. EPP is defined as the difference between ovule longevity and the time required for the pollen tube to reach the embryo sac, also influenced by stigma receptivity.
In ‘Clemenules’ mandarin and ‘Valencia’ sweet orange, pollen tubes remain capable of fertilizing ovules up to 8 days after anthesis (DAA) [16]. This time to reach the ovary is significantly reduced by 2–3 days at high temperatures (20–30 °C) and increased at low temperatures (10 °C) [17,18]. However, in flowers pollinated at 10 DAA, pollen tubes fail to reach the ovary, as evidenced by the absence of seed development. This is because a physical barrier forms at the base of the stigma papillae at 10 DAA, preventing pollen tube progression into the stylar canals in these cultivars. This indicates how the stigma receptivity constrains the EPP. Conversely, in Satsuma mandarin, this limitation occurs earlier and although fertilization fails at 4 DAA, no such barrier is observed at the stigma, suggesting that ovule longevity is the primary limiting factor in this species [16]. Accordingly, in citrus flowers, receptivity varies by species.
In addition, since callose is a carbohydrate that impairs sugar transport to the ovule, its deposition at the chalazal end has been used as an early indicator of impending ovule abortion. In emasculated flowers of ‘Clemenules’ mandarin and ‘Valencia’ sweet orange, callose accumulation in unfertilized ovules occurs at 12 DAA. In contrast, in ‘Owari’ Satsuma mandarin, 100% of ovules exhibit signs of degeneration 4 days earlier [16].
Typically, successful pollination and fertilization reactivate cellular division, which slows during anthesis. This process is crucial for ovary development, ultimately leading to fruit and seed formation [19]. However, many Citrus varieties do not require ovule fertilization for ovary development. This phenomenon, known as parthenocarpy, is defined as the ability of a flower to set fruit without ovule fertilization, thereby preventing seed formation.
In citrus, parthenocarpy arises from homogenetic sterility, in which pollen cannot fertilize flowers of the same or a different variety—referred to as self-incompatibility and cross-incompatibility, respectively—or from gametic sterility. The latter includes androsterility or gynosterility, characterized by absent or defective development of pollen or ovules, respectively [20]. The Clementine mandarin and its mutations, along with certain hybrids, provide a representative example of self-incompatibility. In contrast, gametic sterility, marked by extensive degeneration of embryo sacs, is observed in sweet orange cultivars of the navel group, Satsuma mandarins, and ‘Marsh seedless’ grapefruit. However, a few sacs may fully develop and produce seeds, albeit in very limited numbers [21]. Interestingly, the presence of pollen tube growth inhibitors in the stigma or style can prevent fertilization, which can also be induced by environmental factors that cause asynchronous development of the pollen tube and the ovule. The complete absence of seeds occurs almost exclusively in triploid varieties.
Homogenetic sterility also induces facultative parthenocarpy in Citrus [22]. In this context, the incompatibility between pollen grains and the receptive stigma is governed by a self-sterility gene with multiple alleles. Only pollen grains carrying self-sterility alleles that do not match those of the stigma or style tissues are able to penetrate the ovule. Conversely, when alleles match, various immune responses, such as errors in pollen-stigma protein recognition, synthesis of pollen tube growth inhibitors in the stigma and style, and other biochemical barriers, restrict or entirely prevent pollen tube development. This mechanism ultimately leads to self-incompatibility, inhibiting autogamy.
In Citrus, the polymorphic locus controlling the gametophytic self-incompatibility system, known as the S gene, has been estimated to have several alleles [23]. To date, eighteen S-RNases have been reported in pummelo (S1–S9, S16), mandarin (S10–S11), sweet orange, clementine mandarin (S7 and S11) and in other citrus species (S12–S15, S17) [24,25]. The gametophytic S-RNase system associated with the self-incompatibility locus has been mapped to the proximal region of chromosome 7 in the clementine reference genome [22].
Facultative parthenocarpy occurs in varieties incapable of self-pollination when cross-pollination is absent. This phenomenon is observed in hybrid mandarins such as ‘Ellendale,’ ‘Nova,’ ‘Fortune,’ and ‘Nadorcott’ [26,27,28], as well as in highly parthenocarpic clementine cultivars such as ‘Marisol’ [29]. In contrast, obligatory parthenocarpy refers to varieties that always produce seedless fruits.
The mere deposition of pollen grains on the stigma, their germination, or the development of the pollen tube, in the absence of fertilization, can provide sufficient stimuli to initiate ovary development in the absence of seeds. These cases, which require some form of external stimulus, are classified as stimulated parthenocarpy. In contrast, ovary development occurring without any external stimulus is termed autonomous or vegetative parthenocarpy.
In both scenarios, tissue growth is governed by a genetic factor that sustains relatively high levels of hormones in the ovary during anthesis and immediately thereafter, regardless of pollination and fertilization of the ovules. This has been demonstrated in studies of citrus varieties with different parthenocarpic ability [19,29]. Certain self-incompatible varieties with low parthenocarpic ability exhibit reduced productivity unless pollinated, in which case they produce a high proportion of seeded fruits [21]. This phenomenon has been documented in some clementine cultivars arising from bud mutations. The hormonal basis of the stimulus that triggers fruit set is further supported by the ability to artificially induce parthenocarpic development through the application of specific phytohormones.
Parthenocarpy is of considerable agronomic importance in cultivated citrus varieties for fresh consumption, particularly mandarins, as seedlessness is widely regarded as a key quality attribute. However, cross-compatibility in Citrus species is generally high, and most cultivars produce functional pollen capable of fertilizing flowers of other varieties, often resulting in seeded fruit (Table 1).

Table 1.
Pollination among different species, varieties, and hybrids of the genus Citrus. Minimum, maximum, and (average) number of seeds produced per fruit. X: No seeds. ND: No data available. Adapted from [5], with permission.
To solve the problem of cross-pollination, the application of gibberellic acid (GA3) (10 mg L−1) prior to anthesis significantly increases the rate of ovule abortion, with earlier application resulting in greater efficacy. Specifically, seed reduction of 26% and 52% is achieved when applied 2 and 7 days before anthesis, respectively. When applied at anthesis, GA3 completely arrests pollen tube growth, preventing fertilization and seed formation [30]. Similarly, CuSO4·5H2O also induces seedless fruit. When applied locally to the flower before pollination, it reduces pollen germination and/or disrupts pollen tube development, thereby preventing the pollen tubes from reaching the embryo sac. However, due to the prolonged flowering period, it is recommended to apply CuSO4·5H2O to the entire tree when 60% of the flowers are at anthesis. This treatment reduces the number of seeds per fruit by 55–80% and increases the number of seedless fruits by 150–400%, depending on the year [31].
2.2. Transition from Ovary to Developing Fruit
During the week following anthesis, petal abscission takes place, and approximately two weeks later, the style and stigma also abscise, marking the transition from the ovary to the developing fruit.
The senescence and subsequent abscission of petals are closely associated with their endogenous ethylene production, which serves as the primary regulator of these processes [32]. Ethylene biosynthesis is strongly induced by indole-3-acetic acid (IAA) through the activation of ACC synthase, leading to the accumulation of 1-aminocyclopropane-1-carboxylic acid (ACC), the precursor of ethylene. The application of amino-oxyacetic acid (AOA) or silver thiosulfate (STS) to the petals, respectively, inhibiting ethylene biosynthesis and signaling, significantly delayed petal wilting, confirming ethylene’s role in this process. It is plausible that endogenous IAA in petals, which is hypothesized to contribute to their curvature at anthesis, triggers ethylene production, thereby promoting petal senescence and abscission [33].
The earliest external symptom of stigma-style senescence is the emergence of a bright ring above the junction between the style and the ovary. This ring gradually darkens to brown, while the style transitions from light green to yellowish before ultimately detaching from the ovary. Prior to abscission, the stigma papillae become highly vacuolated, with their cytoplasm reduced to a narrow band adjacent to the cell wall. The plastids lose their starch reserves, although dictyosomes remain apparently active. As senescence progresses, lignin deposition occurs progressively in the cell walls of the papillae, as well as in the epidermal cells and the outermost cortical layers [34]. Despite this, in some cultivars, a substantial proportion of fruit remain the style attached until ripening.
2.3. Abscission of Developing Fruitlets
Due to the plant’s limited capacity to sustain an excessive number of fruits, a substantial abscission of ovaries and developing fruits typically occurs after petal fall. Prior to this, a variable proportion of flower buds and open flowers may also undergo abscission [7,8]. Consequently, fruitlet abscission exhibits two waves that generally overlap to varying degrees, although their peaks are well-defined. In most cases, the proportion of flowers that successfully develop into mature fruits does not exceed 10%, with typical fruit set rates ranging between 5% and 1%. Under excessive flowering, fruit set can decline to as low as 0.1% or even lower [35,36].
Both waves correspond to characteristic developmental stages. The first wave primarily occurs within 3–4 weeks after petal fall, predominantly affecting ovaries and early-stage fruitlets (approximately 5–10 mm in diameter), although some entire flowers may also abscise. The second wave, commonly referred to as ‘June drop’ in the NH, and more precisely known as physiological fruitlet abscission, begins approximately two weeks after the first abscission period and extends from late May to late June (NH). Generally, a higher flower density results in more pronounced abscission peaks [7], although climatic conditions can modify the abscission wave by altering peak intensity, shifting their timing, or causing partial overlap. During this period, small developing fruits are shed until they reach a critical size threshold (1–2 cm in oranges or 0.75–1 cm in mandarins), beyond which the likelihood of abscission is significantly reduced. In general, most fruits that survive this second abscission phase remain on the tree until harvest.
The pattern of fruit set varies with cultivar, climatic conditions, and flowering intensity. Seeded varieties generally exhibit a higher fruit-setting capacity than seedless varieties [37,38,39,40]. Within seedless varieties, there is substantial variation in parthenocarpic ability [7].
The underlying cause of reproductive organ abscission is the formation of an abscission layer, where tissue weakening occurs [41]. During the first wave of fruit drop, shortly after anthesis, the abscission layer forms at the junction between the peduncle and the stem (abscission zone A, AZ-A). In a subsequent phase, as fruits reach a more advanced developmental stage, the second wave of abscission takes place, leading to separation at the junction between the ovary and the floral disk (abscission zone C, AZ-C).
The anatomical, ultrastructural, and biochemical modifications associated with the abscission of young and mature fruits at the AZ, as well as the regulatory effects of IAA and ethylene [41,42], closely resemble those occurring in the abscission zone of leaves [43,44]. Within the AZ-A, multiple layers of small cortical cells exhibit substantial starch deposits. In contrast, the cortical cells adjacent to the abscission zone are largely devoid of starch accumulation. At the onset of abscission, the cells within the AZ undergo division, starch granules are depleted, and the cytoplasm becomes increasingly electron-dense, accompanied by the emergence of numerous lysosomes [45]. As the process advances, vesicle production from the endoplasmic reticulum and the Golgi apparatus increases, leading to a release of hydrolytic enzymes, including cellulase and polygalacturonase. These vesicles fuse with the plasma membrane, facilitating the secretion of enzymatic contents into the cell wall. The degradation cascade initiates at the middle lamella, progressively dismantling the primary cell wall until structural disintegration is complete. Ultimately, as the separation zone tissue deteriorates, the ovaries abscise from the tree [46]. Ethylene enhances the enzymatic activity within the AZ-A by modulating auxin levels [47]. Moreover, exogenous auxin application at the distal end of the AZ delays abscission, whereas its application at the proximal end accelerates the process [48]. These findings suggest that spatiotemporal alterations in auxin gradients may serve as key regulatory signals triggering senescence and abscission [49,50].
Recent studies have begun to elucidate the gene networks regulating abscission in citrus, particularly within the AZ-C. Ethylene plays a central role in modulating gene expression in AZ-C cells, especially those involved in zone activation, cell wall modification, and lignin biosynthesis. In response to 1-aminocyclopropane-1-carboxylic acid (ACC) treatments, several genes encoding cell wall–degrading enzymes are upregulated, including polygalacturonases (CitPG16, CitPG20, CitPG41, CitPG42, CitPG43), pectate lyases (CitPL5), and cellulases (CitCEL3, CitCEL6, CitCEL10, CitCEL22) [51]. Following cell wall loosening, genes associated with lignin biosynthesis, such as CitPAL5 (phenylalanine ammonia-lyase), CitC3H1 (p-coumarate-3-hydroxylase), CitCAD3 (cinnamoyl alcohol dehydrogenase), and CitCASPs (Casparian strip membrane proteins), contribute to monolignol production and lignin polymerization [51].
In addition to transcriptional regulators, components of the RNA silencing machinery have been implicated in ethylene-mediated abscission responses. Small RNAs and their associated gene families—DICER-LIKE (CsDCL1), ARGONAUTE (CsAGO4a), and RNA-DEPENDENT RNA POLYMERASE (CsRDR1a)—show significant downregulation within 12–24 h of ethylene treatment. These genes are core components of the RNA-directed DNA methylation (RdDM) pathway, an epigenetic mechanism involved in gene silencing in plants [52].
Notably, abscission regulation in citrus is not exclusively governed by ethylene. The INFLORESCENCE DEFICIENT IN ABSCISSION–HAESA/HAESA-LIKE2 (IDA–HAE/HSL2) signaling pathway, well characterized in model species, acts independently of ethylene. The Citrus clementina IDA ortholog CitIDA3 can complement the ida mutant phenotype in Arabidopsis [53]. Furthermore, CitIDA3 expression is upregulated in Citrus cultivars prone to abscission during ripening and suppressed by 2,4-D treatment without affecting ethylene biosynthesis [54], indicating the presence of both ethylene-dependent and -independent regulatory modules in citrus abscission.
As developing fruits reach the second wave of abscission, the cells of the AZ-A lose their capacity to respond to hydrolytic enzymes, preventing fruit detachment in this zone. During ovary maturation, the pedicel diameter increases, and the primary vascular bundles, arranged in a circular pattern, transition into a vascular cylinder with secondary growth [2]. In this structure, the phloem forms an external ring, separated from the xylem by the cambium, which develops concentrically inward. The central region is occupied by medullary parenchyma cells, whose walls thicken and undergo lignification. Meanwhile, the AZ-A, located at the base of the peduncle, progressively disappears as secondary phloem and xylem fibers develop within the growing fruit, traversing the former abscission zone. Consequently, during the second abscission wave, fruit detachment occurs through the AZ-C, as also observed in mature fruits.
At the onset of the initial cellular activation of this abscission layer, the AZ-C splits into two groups, representing early and late stages of cell separation. In the early stage, an accumulation of amorphous material, resulting from the partial dissolution of the middle lamella and cell wall of AZ-C cells, makes the AZ-C clearly distinguishable. In the late stage, cell separation becomes evident in the central core of the AZ-C. Once the degradation of the cell wall and middle lamella is complete, large quantities of amorphous material accumulate in the central region [51].
Cells located on the distal side of the AZ-C accumulate starch grains, forming the starch-rich cell area (SA) [41,45,55,56]. Additionally, another distinct cellular region is present on the proximal side of the AZ-C (facing the pith), which consists of actively dividing cells (DA). The AZ-C is composed of 10–15 cell layers, encompassing different cellular regions characterized by two distinct cell morphologies and organellar compositions (e.g., SA cells contain amyloplasts). Cell wall degradation and cell degeneration primarily occur within the SA layers at the fracture plane, adjacent to the albedo, whereas cell expansion is predominant in the DA. Lignin deposition occurs in the central core of the AZ-C, between the axial vascular bundles, delineating the separation line between the calyx button and the fruit rind. Its timing is positively correlated with abscission kinetics [51]. As for the AZ-A, ethylene enhances the enzymatic activity involved in the activation of the AZ-C.
The pericarp cells of ovaries exhibiting signs of abscission are characterized by the presence of plasma membrane and tonoplast invaginations [57]. Mitochondria display a reduced number of cristae, while plastids undergo degradation of their endomembrane system. A key visual distinction between ovaries and developing fruitlets destined for abscission, compared to those that will persist on the tree, is the loss of chlorophyll.
2.4. Endogenous Regulation of Fruit Set
The regulation of fruit set is a critical physiological process, as it determines the final number of fruits that reach maturity and, consequently, has a significant impact on the yield.
This regulation involves both endogenous hormonal levels within the ovary and metabolic control mechanisms, particularly the capacity to allocate photoassimilates to developing fruitlets [58].
2.4.1. The Influence of Hormone Levels
A comprehensive review of the available literature on endogenous plant growth regulators and other endogenous compounds with analogous roles in fruit growth regulation was published by El-Otmani, Lovatt, Coggins, and Agustí in 1995 [59]. Since then, substantial advancements have been made, with a growing body of research published in recent years. This review synthesizes the latest findings on their role in fruit set regulation.
Pollination and subsequent fertilization of the ovules initiate the hormonal signaling cascade necessary for the transition from ovary to developing fruit [60]. Studies on fruit set regulation have established that key phytohormones, including gibberellins, auxins, cytokinins, abscisic acid, and ethylene, are involved in this process [61,62,63,64,65]. Their endogenous levels are tightly regulated by genes encoding enzymes responsible for biosynthesis and catabolism [19]. Typically, these hormones exhibit peak activity shortly after anthesis, underscoring their crucial role in the hormonal signaling network that governs fruit set.
In ovaries and developing fruit, gibberellins (GA) synthesized via two distinct biosynthetic pathways have been identified. In Citrus, the predominant pathway is the 13-hydroxylation route, whereas the non-hydroxylation pathway is less prevalent. This suggests that the former plays a key role in fruit development [64,66]. Within this pathway, gibberellin GA53 undergoes a series of metabolic steps to yield GA19, which is subsequently converted to GA20 by GA20-oxidase and further metabolized into GA1 by GA3-oxidase [64,67,68]. In Citrus, two paralogous genes encoding GA20-oxidases have been identified [69]. Gibberellin GA1 and its precursor GA20 are catabolized by GA2-oxidases, leading to the formation of GA8 and GA29, respectively, both of which are considered inactive forms.
GA1 is recognized as the biologically active GA and plays a pivotal role in fruit set and early citrus fruit development [62]. In both seedless and seed-bearing parthenocarpic varieties, a peak in GA1 concentration is observed in the ovary during anthesis. In seeded cultivars, such as the sweet orange ‘Pineapple’ and the mandarin ‘Murcott’, pollination induces GA1 biosynthesis in the ovaries. Conversely, when pollination is prevented through flower emasculation, GA1 levels decline (Figure 4A), leading to increased ovary abscission [39,60].
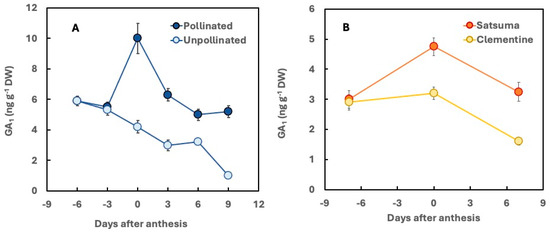
Figure 4.
Time-course of GA1 concentration in pollinated and unpollinated ovaries of sweet orange ‘Pineapple’ (A) and in developing ovaries from Satsuma and Clementine mandarins (B). Adapted from [60] (A) and [64] (B).
In ‘Pineapple’ sweet orange, exogenous application of gibberellic acid (GA3) enhances fruit set in unpollinated ovaries, whereas treatment of pollinated ovaries with paclobutrazol, a GA biosynthesis inhibitor, reduces GA content and promotes abscission [60]. In this cultivar, GA biosynthesis is most pronounced in ovules following pollination, coinciding with peak expression of GA20ox2, GA3ox1, and GA2ox1 genes [40].
In the parthenocarpic and therefore seedless cultivar ‘Washington’ navel, GA levels in both ovules and ovary walls are lower than in ‘Pineapple’ [40], and their synthesis appears to be constitutively regulated. The higher GA1 concentration observed in the seeded cultivar correlates with a reduced ovary and fruitlet abscission rate during the post-flowering period. Subsequently, during physiological fruitlet abscission, GA1 levels in the pericarp of developing fruits remain relatively high in both cultivars, reaching comparable levels despite an overall decline relative to those in the ovary wall after anthesis [40]. In seedless cultivars, the ability to sustain GA1 concentration in the fruit pericarp during physiological drop is associated with reduced abscission, supporting their parthenocarpic ability.
Similarly, the sterility induced in the ‘Moncalina’ mandarin by γ-ray irradiation of the ‘Moncada’ mandarin disrupts GA biosynthesis via the 13-hydroxylation pathway, as evidenced by lower levels of GA1 and its precursor GA19 in the ovaries compared to ‘Moncada’. This effect has been linked to the reduced expression of GA20ox2 and GA3ox1 genes in ‘Moncalina’, resulting in a significant decrease in GA production [39].
A comparative analysis of two seedless mandarin cultivars with differing parthenocarpic ability indicates that Satsuma mandarin, which exhibits high parthenocarpic ability, contains higher concentrations of GA from the 13-hydroxylation pathway (excluding the inactive GA8) compared to the clementine ‘Oroval,’ which has low parthenocarpic ability. At anthesis, GA1 levels in Satsuma ovaries were found to be considerably higher than those in clementine ovaries (Figure 4B) [63,64]. Similarly, in the clementine cultivars ‘Marisol’ and ‘Clemenules,’ which lack seed development in the absence of cross-pollination and exhibit high and low parthenocarpic ability, respectively, GA1 levels in the ovaries are significantly higher in the former than in the latter [29].
These findings suggest that GA1 plays a role in promoting ovary growth rather than repressing abscission [70]. In line with this, GA biosynthesis genes GA20ox2 and GA3ox2 are upregulated in Satsuma ovaries during anthesis, whereas their expression remains low in Clemenules mandarin. The activity of GA3ox2 correlates positively with GA1 concentration and CYCA1.1 gene expression in the ovary, which is linked to pericarp cell division [19]. Notably, in Satsuma, the upregulation of CYCA1.1 following anthesis occurs after the peak expression of GA20ox2 at anthesis and precedes its second activation 10 days post-anthesis, which coincides with a renewed overexpression of GA3ox2. This temporal pattern suggests that the cell division process itself may reactivate GA biosynthesis to sustain an adequate rate of cell proliferation [19].
The link between cell division activation and GA is further supported by studies investigating the effects of GA3 treatments on GA metabolism. In Satsuma mandarin, increased expression of the CYCA1.1 gene closely parallels an increase in endogenous GA1 concentration following exogenous GA3 application, leading to an increased rate of cell division. This effect is transient due to its intrinsic capacity for GA1 biosynthesis [19]. However, in Clementine ovaries, which lack the ability to synthesize GA1 at anthesis, GA3 application not only stimulates GA1 biosynthesis but also upregulates CYCA1.1 expression, promotes cell division, and significantly enhances fruit set (up to 95%). These findings provide evidence of the relationship between GAs, cell division, and fruit set [19].
This relationship is further reinforced by the spatiotemporal correlation between the accumulation of GA20ox2 transcripts and the temporal progression of cell division in the mesocarp and endocarp. In the ovary of the Satsuma mandarin, in situ hybridization analysis of GA20ox2 transcripts revealed a strong signal throughout the pericarp at anthesis, with the most intense cell division occurring in the mesocarp. In contrast, the Clementine ovary exhibited weak GA20ox2 expression, mainly in the exocarp, correlating with its lower mitotic activity. Thirty days later, in the Satsuma ovary, the GA20ox2 hybridization signal shifts to the endocarp and emerging vesicles, where cell division remains active. At this stage, mesocarp cells have ceased dividing and are enlarging, with no detectable hybridization signal (Figure 5; ref. [19]). Consequently, GA1 is identified as the active GA regulating fruit set in citrus.
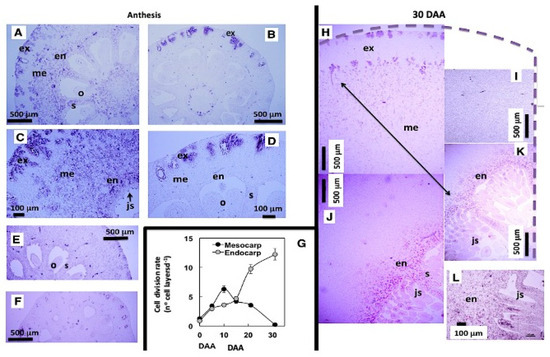
Figure 5.
In situ hybridization of GA20ox2 transcripts in Satsuma and Clementine ovaries. (A–D): Satsuma (A,C) and Clementine (B,D) ovaries at anthesis hybridized with the antisense probe showing GA20ox2 expression (purple staining); (E–F) Satsuma (E) and Clementine (F) ovaries hybridized with the sense probe as a negative control; (G)Time-course of cell division rate in Satsuma mesocarp and endocarp. Cross-section of the Satsuma ovary 30 days after anthesis (H–K); the 4 pictures are from the same fruit and rebuild the GA20ox2 signal observed along the pericarp (purple staining), which was high in the endocarp (J,K) and absent in the mesocarp (H–J); (L) detail of the signal observed in the endocarp and juice vesicles. en: endocarp; ex: exocarp; js: juice sacs; me: mesocarp; o: ovules; s: septa. DAA: days after anthesis. From [19] with permission.
Following petal abscission, the IAA content in the ovary increases sharply, reaching its peak shortly thereafter [63]. This pattern is observed in both seeded and seedless varieties, although the latter exhibit slightly higher concentrations, as demonstrated in the seeded ‘Duncan’ and seedless ‘Marsh’ grapefruit [71]. However, within seedless mandarin cultivars, no significant differences are detected regardless of their parthenocarpic ability [63]. This suggests that in these varieties, the IAA content of the ovary is not a limiting factor for fruit set and early fruit development. Conversely, in the seeded ‘Murcott’ mandarin, when pollination is experimentally prevented, IAA concentration remains at a minimal level, indicating that the substantial post-anthesis increase in IAA biosynthesis is pollination- and fertilization-dependent [39].
Similarly, the absence of pollination in the seeded ‘Pineapple’ orange leads to a reduction in IAA levels in both the ovule and pericarp but is also accompanied by a decline in GA precursors GA1 and GA20. However, exogenous IAA application to unpollinated ovaries upregulates GA20ox2 and GA3ox1 expression, restoring GA1 and GA20 levels, albeit exclusively in the ovules. This response is not detected in the pericarp, suggesting that the latter exhibits reduced sensitivity to IAA, at least in this variety [61]. These findings imply that IAA likely induces GA biosynthesis within the ovules, from where GAs are subsequently transported to the pericarp [72]. Supporting this hypothesis, treatment of pollinated ovaries with tri-iodobenzoic acid (TIBA), an inhibitor of IAA transport, results in decreased GA concentrations. This indicates that when IAA translocation from egg cells—where its biosynthesis occurs—is restricted, GA synthesis in the ovary is disrupted. Additionally, GA2ox, a gene encoding an enzyme involved in GA20 and GA1 catabolism, is upregulated in unpollinated ovaries and downregulated by exogenous IAA application [61]. Consequently, fruit set in non-parthenocarpic cultivars depends on successful pollination and fertilization, which in turn enhances GA biosynthesis via upregulation of GA20ox2 and GA3ox1 and repressing GA2ox1-mediated GA degradation. These regulatory effects are driven by IAA, whose post-pollination accumulation in the ovary promotes GA1 biosynthesis, thereby initiating the fruit developmental program [61]. Following petal abscission, IAA levels decline rapidly as the ovary develops, reaching minimal levels at the onset of physiological fruitlet abscission. This reduction coincides with increased conjugated IAA and enhanced auxin oxidase activity, which facilitates IAA catabolism [63]).
In both seeded- and seedless-bearing cultivars exhibiting high parthenocarpic ability, GA1 levels increase in the ovary immediately after anthesis, while abscisic acid (ABA) concentration remains low. However, in self-incompatible cultivars with limited natural parthenocarpy, the absence of pollination results in negligible GA1 accumulation. Instead, ABA levels transiently rise after a few days, leading to pronounced reproductive organ abscission. The failure of these cultivars to set fruit via parthenocarpy is attributed not only to the insufficient GA1 concentration but also to their limited capacity to inactivate ABA via conjugation [63]. Exogenous GA3 suppresses the post-anthesis ABA surge in ovaries of low-parthenocarpy varieties and mitigates their abscission. Conversely, inhibiting GA biosynthesis with paclobutrazol in parthenocarpic varieties increases both ABA accumulation and abscission rates. Furthermore, treatment with fluridone—an inhibitor of carotenoid biosynthesis and thus ABA synthesis—delays ovary and fruitlet abscission [65].
In unpollinated ovaries of seed-bearing cultivars, ABA levels rise post-anthesis due to an insufficient level of GA1, ultimately resulting in complete reproductive organ abscission. Elevated ABA concentrations promote the synthesis of 1-aminocyclopropane-1-carboxylic acid (ACC), a precursor to ethylene, which serves as a key effector of abscission [41,73]. In contrast, pollination and fertilization inhibit ABA accumulation in the ovary, thereby preventing its abscission [39].
In citrus, trans-zeatin (tZ) and 2-isopentenyl adenine (2-IP) have been identified as the predominant cytokinins in flower buds and ovaries, with zeatin riboside and isopentenyl adenosine present in lower concentrations [74]. The activity of these cytokinins increases markedly in the ovaries at anthesis, peaking around petal abscission, followed by a sharp decline over the subsequent two weeks of development. This temporal pattern is consistent across both seeded and seedless cultivars, albeit with quantitative variations among cultivars [75]. In seed-bearing cultivars, tZ and 2-IP levels are similar in pollinated and unpollinated ovaries, suggesting that the biosynthesis of these hormones is constitutive and independent of pollination or ovule fertilization [39].
2.4.2. Competition for Photoassimilates
Following petal abscission, a substantial number of ovaries remaining on the tree cannot fully develop into mature fruit due to limitations in carbon (C) availability, as their cumulative C demand would surpass the tree’s metabolic capacity [9]. Consequently, the net CO2 assimilation capacity of the leaves, particularly during critical periods of elevated C demand, is considered a key determinant of fruit set. In this context, the plant’s photosynthetic efficiency, and thus the availability of photoassimilates, is a primary determinant of the proportion of flowers that successfully set. The hypothesis that trees selectively retain only those fruits they can sustain, discarding those in poorer condition to receive the available nutrients, is highly suggestive and has been widely accepted. This suggests that citrus trees have an endogenous self-regulatory mechanism that modulates fruit production in accordance with their metabolic synthesis capacity [6,9].
In citrus, once mineral and water requirements are met, competition for carbohydrate becomes the primary factor driving fruitlet abscission [76,77,78]. During the fruit set period, ovaries or fruitlets that fail to sustain a high growth rate due to insufficient nutrient allocation are eventually shed. Consequently, peak competition for carbohydrates coincides with the highest rates of reproductive organ abscission. However, this competition is not limited to fruits alone; vegetative development also competes for C resources [76]. Studies on alternate bearing have shown that fruits accumulate substantial amounts of C at the expense of shoot development, with an inverse correlation between monthly C accumulation in fruits and shoot dry weight gain. The presence of a strong C sink, such as developing fruit, restricts C availability to other competing sinks, including growing shoots. Consequently, as fruit C demand increases, vegetative growth declines [10]. Experiments using 13C labeling indicate that 13CO2 uptake is influenced by fruit presence. Young shoots on fruit-bearing branches assimilate less isotope compared to those on non-fruiting branches. Moreover, within the same branch, growing fruit exhibits a higher 13C uptake than shoots [10].
This competition is partially mitigated by the ability of the ovaries to accumulate starch during floral ontogeny through a dual mechanism: (1) an autotrophic pathway, wherein source organs activate the expression of Rubisco small subunit (RbcS) genes [4,79], and (2) a heterotrophic pathway, involving the mobilization of stored reserves via sink organs that hydrolyze sucrose in the cytosol [80,81,82]. Throughout floral development, both the energy depletion signaling system, mediated by the expression of the SnRK1 (SNF1-related kinase 1) gene, a sensor of sugar deficiency, and carbon fixation via Rubisco are activated [4]. SnRK1 is induced under various energy-depleting stresses and plays a central role in maintaining energy homeostasis by modulating the activity of key metabolic enzymes [83], as well as regulating starch and sucrose synthesis and hydrolysis in relation to ovary abscission. Consequently, starch accumulation during ovary ontogeny is critical for fruit set, facilitating the ovary-to-fruit transition independently of leaf presence and maintaining sufficient glucose levels [3]. This temporary glucose supply alleviates competition between the ovary and young leaves until the latter become photosynthetically active and function as source organs [4].
However, the small ovary surface limits CO2 uptake. Furthermore, at the onset of the first wave of reproductive organ abscission, many ovaries undergo a phase of intense cell division, accompanied by maximum respiratory activity per unit of tissue weight. Consequently, during this initial stage, ovary development depends on both starch accumulation and photoassimilates, mainly from mature leaves of the previous year, not from newly emerged spring shoots [3,76]. The latter, which are still developing, function as C-consuming organs with a limited capacity for photoassimilate export and compete with the ovaries for C resources derived from the previous year’s leaves, which have a reduced capacity for CO2 assimilation and must also fulfill carbohydrate demands from other sink organs, particularly the roots [76].
Accordingly, at anthesis, the ovary marks a peak in soluble sugar concentration (glucose, fructose, and sucrose), which subsequently declines to low levels over the following two weeks. This transient increase in sugar concentration is attributed to elevated hormonal activity in the ovaries, which enhances their sink strength and promotes sucrose transport (see Section 2.4.1). Enzymes involved in sucrose metabolism, such as invertases, play a pivotal role in cleaving imported sucrose, thereby regulating C import rates into the developing fruit [81,84]. Comparative analysis of the expression of the sucrose synthase gene SUS1 and the cell wall invertase gene CWIN suggests that sugar unloading in the fruit occurs predominantly via a symplastic pathway [82].
These results suggest that during the early stages of development, reproductive organs rely mainly on their own carbohydrate reserves. However, as development progresses, they enter into intense competition for leaf-derived photosynthates. Consequently, a deficiency in these assimilates triggers the abscission of a significant proportion of ovaries and smaller fruitlets within the first month after petal fall [7] (see Section 2.3).
After that, during the second wave of reproductive organ abscission, termed physiological fruitlet abscission, there is a marked decline in the number of fruitlets retained on the tree relative to the initial ovary count. The remaining fruits enter a phase of accelerated growth, resulting in an increased carbohydrate demand per fruit compared to that of the initial ovaries. This process occurs coinciding with peak competition for carbohydrate resources and thus, when flowering is abundant, starch concentrations in mature leaves decline during the fruit set period, reaching a minimum towards the end of the fruit drop period. The mobilization of carbohydrate reserves stored in the leaves to support the developing fruitlets suggests that the carbohydrate demand during physiological fruitlet abscission exceeds the supply capacity of photosynthesis. Concurrently, young leaves emerging during the spring flush transition from acting as carbohydrate sinks to becoming sources of photoassimilates. This shift is evidenced by the efflux of 14C-labeled compounds and associated metabolic changes in the leaves [77]. Therefore, by the onset of fruit drop, newly developed leaves are sufficiently mature to supply photoassimilates to other organs. This function is particularly relevant in mixed inflorescences, where young leaves directly support the fruitlets they bear. Consequently, fruitlets on mixed shoots are at an advantage for fruit set compared to those on leafless inflorescences, which rely solely on assimilates from older leaves [8,76,85]. The second wave of abscission thus represents a fine-tuning mechanism by which the tree adjusts fruit load to match its CO2 assimilation potential. Hence, the availability of photoassimilates emerges as the primary limiting factor for fruit set during this period.
Multiple lines of evidence indirectly indicate that carbohydrates play a crucial role in fruit set: (1) Defoliation leads to ovary and small fruitlet abscission, whereas plants with a high leaf-to-flower ratio achieve significantly higher fruit set percentages, provided other factors remain constant [70,86]. Total defoliation, whether at anthesis or during the onset of physiological fruitlet abscission, results in the abscission of 100% of developing fruitlets. In contrast, removing 50% of the leaves leads to an intermediate level of abscission between fully defoliated and intact trees [3]. Furthermore, in fruits from defoliated branches, the expression of SnRK1 and RbcS genes is upregulated compared to fruits from non-defoliated branches and is activated earlier, suggesting that these genes detect the absence of carbohydrate sources and respond by mobilizing stored carbohydrates and enhancing their own biosynthesis [4]. (2) The presence of young leaves in close proximity to the ovary increases the likelihood of fruit set. Ovaries on inflorescences with young leaves (mixed shoots) exhibit a higher fruit set rate than those on leafless inflorescences (flower clusters) [8]; moreover, the greatest fruit set occurs in ovaries at the tip of a newly developed shoot, emphasizing the ovary’s dependence on nearby young leaves for successful development [76]. (3) The application of photosynthesis inhibitors during the fruit set period induces substantial fruitlet drop [82,87]. (4) Reducing photosynthetic activity through tree shading significantly decreases fruit set rates and overall yield [88]. (5) Continuous injection of sucrose solution into the trunk starting 30 days before anthesis enhances ovary retention [89]. (6) Girdling branches or scoring trunks or branches at petal fall markedly improves fruit set [90]. Scoring disrupts the phloem, reducing sucrose translocation to the roots, which act as strong sinks for photoassimilates [91]. Additionally, girdling has been shown to increase photosynthetic activity in leaves adjacent to the developing fruit [92] (see Section 2.6.2).
Lastly, deficiencies in certain mineral elements, particularly nitrogen [85,93], as well as phosphorus, potassium, magnesium, and iron [94,95], significantly reduce the plant’s photosynthetic capacity and negatively impact fruit set.
2.4.3. Hormone–Photoassimilate Interactions
Endogenous hormones and available carbohydrates in the ovaries and developing fruitlets interact during fruit set. It has been proposed that the gradient of photoassimilate concentration between the source (leaf) and the sink (fruit) may be the primary regulator of transport and partitioning patterns, while hormones act as modulators of multiple rate-limiting steps in this process [96]. In this context, studies using 14C-labeled carbohydrates have demonstrated that hormone application enhances carbohydrate mobilization towards the ovaries [97,98]. Moreover, the GA3 application has been shown to increase the photosynthetic rate by stimulating Rubisco activity [99] and facilitating carbon allocation to the ovary [100], reinforcing the necessity of a source–sink carbohydrate concentration gradient. This mechanism may partially explain why fruit set requires relatively high levels of GA1 in the ovary, as observed in seeded varieties that have undergone successful pollination and fertilization, or in cultivars with high parthenocarpic ability.
On the other hand, restricting carbohydrate supply to the ovaries and developing fruitlets during the fruit set period enhances ethylene release, which ultimately drives the abscission of reproductive organs. This process occurs in both the ZA-A and ZA-C abscission zones without distinction [70] (see Section 2.3). Evidence supporting this effect includes the observation that defoliation reduces sucrose and starch concentrations in developing fruitlets, leading to increased abscission rates correlated with elevated levels of ABA and ACC (Table 2). The sequence of events is as follows: a reduction in sucrose availability from the leaves increases ABA concentrations, which in turn induce ACC synthesis and subsequent ethylene release, which is the ultimate effector of abscission. Experimental studies using exogenous applications of these compounds to developing fruitlets successfully replicate and confirm this process. Under normal conditions, competition for available photoassimilates results in many ovaries and fruitlets receiving insufficient carbohydrate supply, triggering a hormonal cascade that leads to widespread abscission [7]. In this self-regulatory mechanism, sucrose, ABA, ACC, and ethylene act as key components, orchestrating fruit retention in accordance with the plant’s resource availability [70].

Table 2.
Concentrations of sucrose, starch, ABA, and ACC in developing fruitlets of defoliated (100%) at anthesis and non-defoliated Satsuma ‘Clausellina’ trees, and percentage of abscised fruit after 42 days. Control values correspond to non-defoliated trees. DW: dry weight. Adapted from [70].
In summary, the evidence indicates that following petal fall, a high endogenous concentration of GA1 in the ovary, along with an adequate supply of photoassimilates, primarily sucrose, is essential to initiate and sustain fruit growth. A deficiency in either of these compounds leads to fruitlet abscission during the fruit set phase.
2.5. Factors Affecting Fruit Set
Adverse environmental conditions, including elevated temperatures, water stress, and shading, disrupt CO2 assimilation and/or respiration, leading to energy deprivation [101] and triggering organ abscission. Other factors such as rootstock, mineral elements and flowering intensity also affect fruit set.
2.5.1. Temperature
Elevated temperatures (>38 °C) during the fruit set period, particularly when accompanied by dry winds and low soil moisture, significantly enhance fruitlet abscission rates [102,103]. A sudden rise in temperature, especially abrupt fluctuations, coupled with low atmospheric humidity, intensifies leaf transpiration, leading to water loss, and consequently, a transient decline in leaf water potential occurs, inducing temporary stomatal closure. The resulting decrease in stomatal conductance restricts gas exchange, ultimately reducing net CO2 assimilation [104], the process being species dependent [105]. This limitation exacerbates fruitlet abscission rates during periods of high photoassimilate demand by developing fruit, as previously noted. Moderate tree shading significantly enhances fruit set by lowering leaf temperature and increasing stomatal conductance [106].
2.5.2. Water Deficit
A soil water deficit during the fruit set significantly increases fruitlet abscission. Water scarcity induces ABA biosynthesis through the activation of genes such as 9-cis-epoxycarotenoid dioxygenase (CcNCED3), resulting in ABA accumulation [107,108]. This, in turn, stimulates ACC synthase activity in roots [109] and promotes ACC biosynthesis and accumulation. The latter is further facilitated by xylem flow blockage due to water stress [108]. Upon tree rehydration, xylem flow is restored, allowing ACC to be transported via the xylem stream from the roots to the leaves and fruitlets, where it is metabolized into ethylene, ultimately triggering leaf and fruitlet abscission. ABA accumulated in water-stressed roots can also be actively transported through the xylem to the leaves and fruits upon rehydration [108,109]. Once in the leaves, ABA induces stomatal closure, thereby reducing transpirational water loss. This response also limits net CO2 assimilation, consequently decreasing the supply of photoassimilates from the leaves to the developing fruitlets, which further promotes abscission.
Therefore, maintaining soil moisture above a critical threshold through appropriate irrigation frequency and water volume favors fruit set. In this context, high-frequency drip irrigation provides significant advantages, as it enables the maintenance of a consistently elevated moisture level in localized soil areas where the root system is concentrated.
2.5.3. Rootstock
Numerous studies have demonstrated substantial rootstock effects on fruit yield. These effects are typically the result of complex interactions among multiple factors, most notably the degree of rootstock adaptation to specific environmental and edaphic conditions. A strong positive correlation between tree size and yield per tree has also been consistently reported, underscoring the need to account for tree vigor when evaluating yield potential on a per-area basis. Poncirus trifoliata, Carrizo citrange (C. sinensis × P. trifoliata), Rough lemon (C. jambhiri), FA-5 (C. reshni × P. trifoliata), and C. macrophylla bear a good crop of fruit [110].
Tree spacing also influences fruit yield. In general, for oranges and mandarins, yield increases with higher tree density, although yield per tree generally declines or remains unaffected [111]. Dwarfing rootstocks enable high-density orchard systems.
2.5.4. Nitrogen
In addition to carbohydrates, the ovary and developing fruitlet require nitrogen (N) for protein synthesis from anthesis onwards. Under N-deficient conditions, when the availability of N to the fruitlet is inadequate, a pronounced fruitlet abscission occurs, leading to a significant reduction in yield. Consequently, enhancing N fertilization up to the optimal foliar N concentration results in an increased fruit set rate [112]. Specifically, the late winter application of low-biuret urea has been shown to enhance fruit setting potential, an effect attributed to an increase in the synthesis of polyamines, such as putrescine, spermidine, and spermine [113].
During the ovary and fruitlet drop period, N concentrations in mature leaves reach their lowest levels, a condition that is subsequently reversed by the end of spring [10]. This seasonal N deficiency is primarily attributed to the export of N reserves from the old leaves to support the high N demand associated with spring bud break, flowering, and fruitlet development [93,114]. The significant role of stored N during the early stages of fruit set results from the limited N uptake by the roots in late winter and early spring, which is due to reduced root growth and low soil temperatures [115]. However, by late spring, root N uptake increases substantially, compensating for the earlier seasonal N deficit [114,116]. Young leaves play a crucial role in N redistribution [117], as they initially assimilate most of the absorbed N, which is then retranslocated to support fruitlet development [114]. These processes suggest that an N deficiency during the post-flowering period, when ovary development is accelerated, can trigger fruit drop [118]. Moreover, N deficiency impairs N uptake and decreases N concentrations in leaves, stems, and roots, disrupting nutrient balance. This disruption negatively affects thylakoid structure, reducing levels of photosynthetic pigments and impairing photosynthetic electron transport, thereby decreasing CO2 assimilation, photosynthesis, and fruit set [119].
During the first half of spring, N uptake is primarily directed towards the young leaves, with a secondary allocation to the ovaries [120]. However, no competitive interaction has been observed between the N demands of the ovaries and shoot growth. In the fruit, N uptake increases until June, reaching a peak, and subsequently declines to very low levels, remaining relatively stable until ripening. Despite the low N utilization by the fruit during this period, shoot growth in summer and autumn is almost entirely suppressed, suggesting that N competition is not a critical factor limiting vegetative development [10]. In alternate bearing studies, trees supplemented with 15N demonstrated no significant differences in N enrichment between young shoots and fruit in ON and OFF trees, indicating that the presence of fruit does not affect the N availability for young shoots [10]. Moreover, the temporal evolution of N content (total N and protein N) in mature leaves and shoot bark did not support the hypothesis that fruit reduces the availability of reserve N for growing shoots. In fact, from late winter to early spring, N concentrations in mature leaves and shoot bark gradually decreased, which primarily reflected N consumption associated with spring sprouting, flowering, and freshly fruit set [93]. On the other hand, leaf N levels were consistently lower in OFF trees compared to ON trees, suggesting that vegetative growth represents a more substantial sink for N than reproductive organs, as evidenced by the higher N concentrations in young shoots compared to fruitlets [10]. Finally, in the shoot bark of OFF trees, the minimum N concentration was observed in April (NH), coinciding with peak N demand by the shoots, whereas in ON trees, this minimum occurred in June (NH), coinciding with the highest N demand by fruitlets. This temporal mismatch further supports the notion that new vegetative and reproductive organs do not compete for N reserves [10].
2.5.5. Mineral Deficiencies
Mineral nutrient deficiencies reduce fruit set by disrupting the photosynthetic process [119]. Comparisons of persisting and abscising fruitlets indicate a link between nutrient availability and abscission rate, especially under high competition [9,121]. In terms of crop load, treatments to correct these deficiencies are highly effective when applied during the petal-fall period [118].
In ‘Navelate’ sweet orange, a cultivar characterized by low parthenocarpic ability [122], the retranslocation rate of mineral elements is particularly low [94]. This is especially evident for potassium, which shows minimal accumulation in fruitlets undergoing physiological abscission [95]. Although the application of potassium nitrate during the spring flush promotes initial fruit set, this effect is transient and does not significantly impact final yield, primarily due to the inherently high rate of natural fruitlet abscission characteristic of this variety. This finding underscores the sensitivity of the fruit set process to deficiencies in specific mineral nutrients [123].
2.5.6. Flowering Intensity
The percentage of flowers that set fruit decreases as flowering intensity increases [6,7]. Across a broad range of flowering densities, the final yield remains independent of the total number of flowers produced. Consequently, when the same number of fruits is harvested, a higher flower count results in a lower fruit set percentage. When flowering is so limited that it constrains the potential yield, the fruit set percentage is typically high, even if the absolute number of harvested fruits is lower than average. This ratio tends to decline as normal flowering levels are reached [9]. However, excessive flowering can lead to a reduction in yield due to the overconsumption of nutrients by developing reproductive structures, intensifying competition and resulting in extensive abscission [8]. Additionally, in cases of extreme flowering, most inflorescences lack leaves, further contributing to a markedly low fruit set percentage [124] (Figure 5).
In these cases, the abscission of reproductive structures—including buds, flowers, and ovaries—is predominantly advanced to the initial fruit set phase. Conversely, under low flowering conditions, reproductive organs are mainly shed as developing fruitlets during the second wave of abscission [7] (see Section 2.3).
Gibberellic acid (GA3), at 25 to 100 mg L−1, reduces flowering by 45–75% when applied during floral bud induction [125] or early bud differentiation [124,126]. This effect has promoted its use to reduce excessive flowering and improve fruit set [127,128]. GA3 is effective in sweet oranges, Satsuma and Clementine mandarins, and hybrid cultivars.
The molecular mechanism underlying the GA3-induced reduction in flowering has been linked to the repression of the genes CiFT3 in the leaf and CsAP1, CsAP2, CcPI, and CcSEP3 in the bud [129,130,131], thereby explaining its role in disrupting flower induction, differentiation, and organogenesis.
Notably, this reduction in flowering can also enhance the effectiveness of specific techniques aimed at increasing fruit set [5,122] (see Section 2.3).
2.5.7. Type of Inflorescence
The likelihood of ovary set is largely influenced by the type of shoot on which it develops and the number of young leaves supporting its growth [76,128], as these factors determine the accessibility of nutrients. Consequently, ovaries from single-flowered leafy shoots have the highest setting rate, followed by those from axillary flowers in mixed inflorescences. In contrast, nearly all ovaries from leafless inflorescences, whether bearing one or multiple flowers, undergo abscission [118].
In addition, ovaries from inflorescences formed on vigorous, erect shoots set at lower rates than those on more horizontally oriented shoots. This phenomenon has been attributed to the slower phloem transport rate in horizontal shoots, which favors setting, reducing vegetative growth [118].
2.6. Fruit Setting Treatments
2.6.1. Application of Gibberellic Acid
In several citrus species and cultivars, the application of GA3 enhances fruit set [132]. Thus, when applied to small branches, GA3 significantly increases fruit set in the associated ovaries, although with variable efficacy. For instance, Soost and Burnett (1961) [133] reported only a 10% increase in fruit set in Clementine mandarins following the application of GA3 (potassium gibberellate) at concentrations ranging from 100 to 500 mg L−1. In contrast, García-Martínez and García-Papí (1979) [134] observed a 90% increase with applications at 50 mg L−1, at which the response plateaued. This discrepancy has been attributed to a potential phytotoxic effect induced by the higher concentrations used in the former study.
Additionally, studies show that localized application of GA3 to ovaries and leaves promotes fruit set in mandarins and hybrids [37,135,136], and oranges [132,135,137,138]. GA3 efficacy varies with concentration and timing.
However, applying GA3 to the entire tree immediately after anthesis does not enhance fruit set at the same rate, resulting in significantly lower fruit set. This suggests that selective treatment of localized canopy areas increases the competitiveness of treated ovaries compared to untreated ones, thereby promoting a higher fruit set rate. Nevertheless, post-flowering application of GA3 to the whole tree has been demonstrated as an effective strategy for improving fruit yield in mandarins [139,140,141,142] and hybrids [143]. In contrast, results in oranges have been largely ineffective [132,135,144], although some exceptions have been reported [122,127].
In the Mediterranean Basin, GA3 at concentrations ranging from 5 to 25 mg L−1 is employed to enhance fruit set in self-incompatible cultivars with low parthenocarpic ability, such as certain Clementine varieties. The optimal application timing is immediately after petal fall, as indicated by Moss (1972) [132]. However, due to the asynchronous nature of citrus flowering, it is recommended when approximately 80% of flowers have shed their petals [5].
The often-underestimated technical aspects of an application play a crucial role in influencing outcomes. Specifically, factors such as pH, surfactant type, relative humidity, temperature, and wind significantly affect GA3 uptake, with variations ranging from 2.2% to 28% of the applied quantity [145,146].
When applied to the entire tree, GA3 delays the abscission of reproductive organs by increasing the proportion that progress to the fruit development stage. However, this effect becomes significant only in trees with moderate flowering intensity, while trees with abundant flowering do not exhibit a comparable response [122]. This suggests that the aforementioned interactions between carbohydrates and hormones influence not only endogenous hormone activity but also the response to exogenously applied hormones.
GA3 application to flowers at anthesis upregulates GA20ox2 and GA3ox1 expression in the ovary, increasing GA20 and GA1 levels. These changes CcCYCA1.1 expression, promoting cell division and ovary growth, which leads to fruit set [19]. A summary of this process is provided in Figure 6.
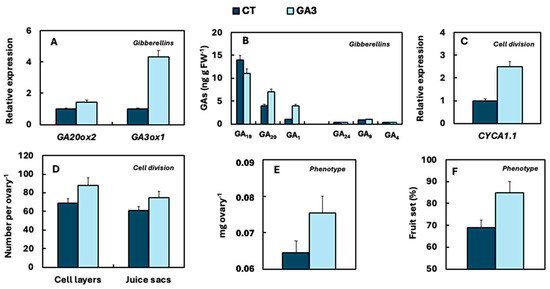
Figure 6.
In the clementine ovary, exogenous application of GA3 (10 mg L-1) at anthesis alters the 13-hydroxylation pathway by up-regulating GA3ox activity (A), leading to increased levels of GA20 and mainly GA1 (B). This hormonal modulation promotes cell division (C,D), ovary enlargement (E) and fruit set (F), i.e., the transition from ovary to developing fruitlet. No significant changes occur in the non-hydroxylation pathway (B). Analyses performed at 7 days (A–C) and 15 days after treatment (D–F). CT: untreated control fruits. Adapted with permission from [19].
The conclusion derived from the combined analysis of endogenous GA content data and the results from exogenous GA3 applications is that the fruit set process, particularly during its initial stages, necessitates a high concentration of GA in the ovary. This can derive from endogenous biosynthesis or exogenous application. However, in certain cultivars, despite an increase in the expression of CcGA3ox2 and even CcCYCA1.1 during anthesis, the relative expression remains low. This results in a reduced sink capacity of the ovary and an insufficient growth rate, which impedes successful fruit set. Consequently, these cultivars require exogenous GA3 application to achieve optimal fruit set. The variability in responses observed with foliar GA3 applications across different cultivars suggests that the effectiveness of the treatment is influenced by the endogenous GA1 concentration in the ovaries of each cultivar. Other factors, such as the availability of photoassimilates, competition for these resources, and specific crop management practices (e.g., irrigation, fertilization, pruning, striping, etc.), may also modulate the response to GA3 treatments. In trials conducted under the climatic conditions of the Mediterranean Basin, the response to GA3 application is not consistent across all cultivars [5,147] (Table 3).

Table 3.
Response to gibberellic acid applications (5 to 25 mg L−1) at petal fall for yield enhancement in various Citrus cultivars.
In addition to gibberellic acid, several lesser-known plant hormones have recently begun to be tested. For instance, salicilic acid applied at 10 μM, has shown effectiveness in reducing fruitlet abscission and increasing fruit yield of ‘Kinnow’ mandarin (C. reticulata) [148]. Likewise, strigolactone GR24 at 200 μmol L−1 increased fruit setting of ‘Hamlin’ sweet orange (C. sinensis), by efficient partitioning of photoassimilates facilitating the development of young fruits [149], and also epibrasinolide, a form of brassinosteroid, at 0.04–0.06 mg L−1, increased fruit setting rate of pummelo (C. grandis) [150]. In contrast, in ‘Hamlin’ and ‘Valencia’ oranges, MeJA (>10 mM) induces mature fruit abscission, associated with ethylene production, indicating its potential for mechanical harvesting [151]. To the best of our knowledge, studies involving this class of hormones remain scarce in citrus.
2.6.2. Girdling or Ringing Branches
Fruit set demands large amounts of carbohydrates, which explains why parthenocarpic cultivars with limited ability to synthesize GA1—and, consequently, to demand them—tend to exhibit poor fruit set and low yields. Furthermore, most of these cultivars do not respond to GA3 treatment to enhance fruit set, as previously mentioned. In such cases, girdling or ringing of branches are successfully used to mitigate fruitlet abscission and improve yield. The primary distinction between girdling and ringing lies in the width of the wound. Girdling involves removing a 1–2 cm strip of bark from scaffold branches, whereas ringing consists of a thin cut, approximately 1 mm wide, around the circumference of secondary branches, that penetrates the bark fully, without removing it and without damaging the underlying wood [152]. In fruit trees, the physiological response appears independent of the wound width [153]. Its effectiveness is primarily determined by the cultivar and, more crucially, by the timing of application, with harvest increases ranging from 30% to 45%, sometimes in combination with GA3 treatments [122].
The enhancement of fruit set induced by ringing at anthesis was attributed to changes in carbohydrate levels [91], mineral element composition [154], and a transient increase in GA1 concentration [155] above the girdling site. This response highlights the critical role of leaves as a primary source of metabolites, ensuring fruit retention during the early stages of development. Ringing has been successfully employed to promote fruit set in sweet orange, mandarins, and their hybrids [90,92,156].
However, when performed at the full physiological fruitlet abscission (35–40 days post-anthesis), ringing does not alter GA3ox1 expression [90]. Instead, it enhances carbohydrate availability to the fruit, preventing abscission. This suggests that carbohydrates are essential for fruit growth even during the initial phases of cell elongation. This finding is supported by Rivas et al. (2007) [92], who demonstrated that ringing delays fruitlet abscission by increasing the quantum yield efficiency of photosystem II (ΦPSII) in leaves of leafy flowering shoots. In contrast, ΦPSII decreases in young leaves of vegetative shoots, while no significant changes are observed in mature leaves. This enhancement in photosynthetic efficiency elevates carbohydrate concentrations in leaves, thereby favoring fruit set exclusively in leafy shoots.
As observed in many fruit tree species, girdling performed at the time of physiological fruitlet abscission also reduces summer sprouting [90,152]. This practice leads to a greater accumulation of carbohydrates in the fruit at the expense of developing shoots [10], suggesting the above-mentioned competitive relationship between reproductive and vegetative growth for carbohydrate [157]. Furthermore, in certain species and cultivars, successful parthenocarpic fruit set can only be achieved when vegetative sinks are removed [158], suggesting that the hypothesis attributing increased fruit set from girdling solely to enhanced carbohydrate supply [159,160,161,162] does not explain, by itself, the observed reduction in fruitlet abscission [163]. It has long been postulated that the high carbohydrate content in fruits borne on ringed branches inhibits their abscission by sustaining growth. However, the underlying mechanism may be the opposite: ringing prevents fruitlet abscission, thereby allowing the fruit to maintain its sink capacity, accumulate more carbohydrates, and continue growing. This, in turn, explains the higher efficiency of ΦPSII in the leaves of flowering shoots on girdled branches, as demonstrated by Rivas et al. (2007) [92].
This ring-mediated inhibition of fruitlet abscission has been proposed to occur via the induction of IAA export from the fruit to the abscission zone, thereby preventing its sensitization and subsequent activation [90], similar to the “primigenic dominance” proposed for apple by Bangerth [163,164]. Additionally, fruitlets on ringed branches retain their capacity for cell division, which is mediated by carbohydrate availability. Since ringing does not induce GA1 biosynthesis at this fruitlet stage, hormones other than gibberellins are likely involved in the regulation of fruitlet abscission.
Takahashi et al. (1975) [165] reported a peak in abscission in Satsuma mandarin occurring after a minimum in IAA content. Similarly, Talón et al. (1990b) [63] suggested that during the later stages of cell division and the early stages of cell enlargement, i.e., the onset of fruitlet abscission, the low parthenocarpic ability of ‘Clemenules’ mandarin is associated with a high concentration of conjugated IAA and a low ability to conjugate ABA. This coincides with the increased expression of the PIN1 gene reported by Mesejo et al. (2022) [90], i.e., with the polar transport of auxin [166]. Ringing-induced auxin transport from the fruitlet to the abscission zone AZ-C protects the fruitlet from abscission [164,167,168] by preserving vascular bundles function [51,90,169,170] and continuous carbohydrate transport, which may explain the increased ΦPSII in girdled leafy flowering shoots [92]. This mechanism is supported by the positive correlation between CcCYCA1.1 and CcPIN1 expression with ovary growth and the negative correlation with fruitlet abscission rate observed 10 days after girdling (Figure 7).
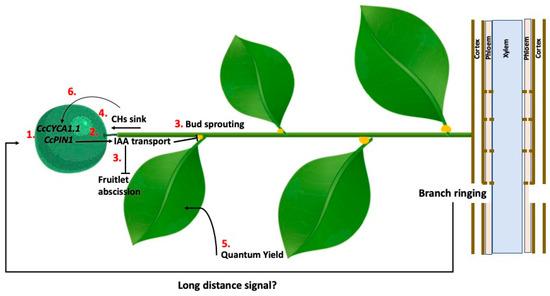
Figure 7.
The effect of ringing branches during the physiological fruitlet abscission to prevent fruitlet abscission is mediated by polar auxin transport from the fruit to the AZ-C, which maintains fruit sink activity and allows the fruit to continue growing. Numbers indicate the correlative events: (1) ringing increases CcPIN1 expression in the fruitlet, and (2) IAA polar transport across the abscission zone preventing abscission and (3) axillary bud sprouting; thus (4) fruitlet CHs sink activity is maintained, which (5) increases photosynthesis to (6) cover fruitlet cell division and growth. From [90] with permission.
Ringing branches also reduces carbohydrate concentrations in the roots [91], with this effect being more pronounced when ringing is performed during the vegetative growth stage.
A schematic overview of techniques to enhance fruit set in citrus trees, along with their timing, is presented in Figure 8.
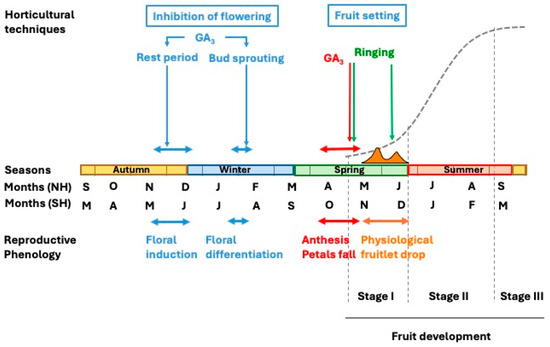
Figure 8.
Treatment periods (NH) for enhancing citrus fruit set. The vertical arrows indicate the specific timing of treatments, while the horizontal arrows indicate the corresponding application windows. The two branch ringing dates indicate that the treatment can be performed either at the end of petal fall or during the second wave of physiological fruitlet abscission. Adapted from [171].
3. Fruit Development
Stage II, also known as the linear growth phase, corresponds to a period of rapid fruit expansion that extends from the completion of physiological fruitlet abscission until just before the onset of color change. This stage lasts between 3 and 6 months, depending on whether the variety is early- or late-ripening, and at the end of this stage the fruit reaches its final size and begins to change color [1].
Once the cell division stage is complete, fruit growth proceeds in a linear manner, primarily driven by cell expansion and water accumulation [172]. Concurrently, epidermal cells of the vesicles elongate, while internal cells undergo significant enlargement. Within these cells, vacuoles undergo extensive development, confining the cytoplasm to a narrow layer adjacent to the cell wall (see Figure 3G).
Hydration is critical for cell wall extensibility by increasing the spacing between cellulose microfibrils, thereby enhancing their rearrangement. This process involves microfibril rotation [173] and is regulated by expansins [174], proteins essential for cell walls loosening during early citrus fruit expansion [175]. Water accumulation is mediated by aquaporins (AQPs), transmembrane protein channels localized in the plasma membrane (plasma membrane intrinsic proteins; PIPs), tonoplast (tonoplast intrinsic proteins; TIPs), and other intracellular membranes [176,177,178].
Expansins possess a binding domain that anchors them to the cellulose surface and a catalytic domain that interacts with hemicellulose, primarily xyloglucans, either on the microfibril surface or within the matrix between microfibrils. This facilitates the disruption of non-covalent bonds between the two polysaccharides, enabling their displacement in response to mechanical forces generated by cell turgor. This process is reversible, with new bonds between hemicellulose and cellulose forming as energy is released during the mechanical deformation of the cell wall components. The temporal delay between bonds breakage and new bonds allows for localized wall relaxation, which propagates as a wave-like movement of xyloglucans, facilitating the increase in cell volume required by the turgor pressure [174]. Several genes encoding expansins have been identified, with their expression levels increasing during the later stages of fruit development [179,180].
During this developmental stage, cell expansion is primarily driven by the biosynthesis of new cell wall components, with cellulose serving as the predominant structural constituent. Cellulose is synthesized by cellulose synthase, playing a central role in maintaining cell wall integrity. Conversely, the biosynthesis of other structural polymers, such as lignin and suberin, is downregulated, as indicated by the decreased activity of enzymes catalyzing the initial steps of their respective biosynthetic pathways [181]. Lignin enhances cell wall rigidity and mechanical strength, while suberin imparts hydrophobic properties, contributing to barrier function.
In addition, a significant subset of the differentially expressed genes is involved in cell wall modification, particularly those associated with the cell walls of juice sacs. In these structures, intercellular spaces of schizogenous and lysigenous origin progressively form as the surrounding cells expand [118]. Among these genes, pectinesterase plays a key role in the remodeling of pectin networks, affecting the textural properties of fruit pulp [182]. The expression of the gene encoding this enzyme declines as fruit development progresses [183].
AQPs mediate transcellular water transport [176,184,185] and facilitate the movement of small neutral solutes, including carbon, N, and micronutrients. These proteins are implicated in both solute transport and the developmental regulation of plant organs [186,187,188], exhibiting tissue- and organ-specific expression patterns [178]. In the context of water transport within plants, PIPs are likely more critical than TIPs in regulating water uptake, as the plasma membrane generally exhibits much lower water permeability than the tonoplast [177].
However, in citrus fruits, where vesicles contain approximately 85% water within their large vacuole, which occupies nearly the entire cell volume, the role of TIPs is of great importance. The transient expression pattern of γ-TIP aquaporins is likely associated with cell expansion, coinciding with the formation of the large central vacuole [189]. However, a specific study has yet to be conducted. Several genes encoding aquaporins have been identified in Citrus [190,191].
Consequently, the upregulation of AQPs genes may serve as a mechanism for the plant to regulate water flow across cells and contribute to the maintenance of cell turgor [192]. In this context, turgor pressure has been demonstrated to be critical for maintaining water potential and continuous water uptake during the development of fleshy and watery fruits [193]. More broadly, auxin regulates γ-TIP gene expression, cellular development and expansion, as well as pericarp growth in most fruits, both seeded and seedless fruits [194].
As a result, the juice vesicles expand until they reach their maximum size, fully occupying the internal space of the segments and developing the characteristic structure of mature tissue (Figure 1 and Figure 3G). In juice vesicles, the final stage of solute transport occurs either apoplastically or symplastically across non-vascular regions, involving distances ranging from a few cells to several millimeters. A cross-section of a juice sac stalk reveals a multicellular, non-vascular structure [195]. This characteristic is not exclusive to juice sacs, as most sucrose-importing cells are typically not in direct contact with the phloem [196,197,198]. Consequently, solute transfer to juice sacs occurs without the bidirectional water flow commonly associated with vascular sugar transport.
Exposure of trees to 14CO2 provides kinetic and autoradiographic data indicating that the post-phloem transport of photosynthates occurs between the segment epidermis and the parenchyma of the juice sac stalk. This provides evidence for directional movement towards the base of the juice sac stalk [195] driven by a sucrose gradient. However, during fruit expansion, this gradient declines not due to the dilution of sugars within the juice sac, but rather as a result of increased sucrose accumulation throughout the segment epidermis and vascular bundles. A similar gradient is observed when considering combined sucrose and hexose levels [195].
Although some synthesis of hexoses occurs in both vascular and non-vascular transport pathways, it is unlikely to be an essential step in the transfer of assimilates to the juice sacs during fruit development. Notably, the proportion of hexoses in these transport tissues is disproportionately low relative to the total photosynthates allocated to the juice sacs [195]. Furthermore, acid invertase activity in these tissues declines to barely detectable levels at this developmental stage [199]. Consequently, sucrose hydrolysis in this system appears to be more closely associated with storage rather than transport.
In any case, the osmotic effects generated by sucrose plus hexose gradients during juice sac development drive the influx of water and/or solutes [81], coinciding with the period of maximum dry weight increase. However, there are significant differences between the influx rates of water and carbon, particularly when respiratory losses are included in carbon demand calculations [195]. The progressive decline in this gradient marks the end of fruit growth.
An unresolved issue concerning non-vascular mass flow in this system may lie in the high turgor pressure of the juice sacs and their remarkable ability for water retention. A high turgor at the sink end of a mass flow pathway could generate a ‘back pressure’ that impedes transport [198,200]. However, elevated turgor pressure during juice sac expansion is essential, as the internal tissues of the citrus peel appear to expand only when pressure potentials rise high enough to overcome the physical constraints imposed by the skin [201,202].
The turgor pressure within the juice sacs is therefore sufficient to expand both the albedo and the flavedo. Thus, as the juice sacs fill, they exert considerable pressure on the fruit rind, which, upon stretching, undergoes a reduction in thickness. To accommodate growth, the cells of the epidermis, the flavedo, continue to divide anticlinally until the fruit ripens, whereas the cells of the albedo elongate parallel to the surface without undergoing division.
Consequently, cells of the hypodermis and the outer mesocarp change from a rounded or polygonal shape to an ellipsoidal morphology. By the end of stage II, the cell walls of the inner mesocarp develop protrusions that enlarge intercellular spaces. These protrusions continue to grow, forming cylindrical extensions that give the albedo cells an irregular shape. The distal ends of these extensions interconnect with those of adjacent cells, significantly expanding the intercellular spaces, ultimately leading to the characteristic spongy structure of the albedo [2].
The vascular bundles supplying the developing fruit can accommodate the mechanical stretching induced by growth due to the absence of sclerenchyma fibers and the annular or spiral lignified thickening of the walls of the tracheal element walls [2].
Studies using tritiated water have demonstrated that minimum fluid flows back out of the juice sacs. Consequently, cortex expansion tissue may play a crucial role in facilitating non-vascular transfer. This expansion is essential for turgor regulation, enabling mass flow and/or efficient sugar compartmentalization [198,203], as well as for the physical accommodation of solute influx.
A detailed overview of the mechanisms governing carbohydrate transport and unloading in citrus fruits can be found in the comprehensive review by Sadka et al. (2019) [81]. Furthermore, recent studies have begun to uncover the regulatory gene networks underlying these processes. In Satsuma mandarin, the transcription factor CitERF16 (CitETHYLENE RESPONSE FACTOR16) has been functionally characterized as an activator of CitSWEET11d, a clade-IV SWEET (SUGAR WILL EVENTUALLY BE EXPORTED TRANSPORTERS) involved in sucrose accumulation [204]. Moreover, the transcription factor CitZAT5 (ZINC FINGER OF ARABIDOPSIS THALIANA), which contributes to sucrose metabolism and fructose transport, was found positively regulate the expression of the sucrose synthase gene CitSUS5 and fructose transporter gene CitSWEET6, promoting sugar accumulation in citrus fruit [205].
CRISPR/Cas9-based genome editing has emerged as a powerful tool for functional genomics and targeted trait improvement in citrus. To date, its application has been relatively limited, with most studies focusing on enhancing disease resistance [206]. Recently, CRISPR/Cas9 has also been employed to modify fruit quality traits, such as pigmentation, by disrupting β-LCY2 in anthocyanin-rich sweet orange cultivars [207]. However, no published studies have yet applied CRISPR/Cas9 to investigate gene networks underlying citrus fruit set or developmental processes, highlighting a research gap.
3.1. Factors Affecting Fruit Development
Fruit growth is governed by the accumulation of dry matter and water and may be constrained by a limited sink capacity, i.e., the potential ability to accumulate carbohydrates, or by the plant’s ability to supply them, i.e., when source availability is restricted. If these limitations arise during the early stages of fruit development, the growth potential of the fruit may be irreversibly reduced. Indeed, in many mandarin cultivars, a strong correlation has been observed between them and fruit diameter at the end of physiological fruitlet abscission and at maturity [208].
During the initial growth stages, up to the completion of physiological fruitlet abscission, the vascular system of the fruit pedicel undergoes progressive development, with an increase in both the number of sieve tubes and xylem vessels. This vascular network fully develops at the onset of the rapid fruit growth phase [209]. A positive correlation between pedicel diameter and final fruit size has been documented in ‘Marsh’ grapefruit [210], ‘Valencia’ orange [211], and ‘Fortune’ and ‘Okitsu’ Satsuma mandarins [212,213].
Genetic factors regulate the size, shape, and color of fruit, defining the characteristic traits of each species and variety. Some species produce small fruits measuring only a few centimeters in diameter (2–3 cm), such as Persian lime (Citrus latifolia), nasnaran (Citrus amblycarpa) or calamondin (Citrus madurensis). In contrast, larger citrus fruits, like pummelos (Citrus grandis) and citrons (Citrus medica), can exceed 20 cm in diameter. However, within a given genotype, fruit size exhibits considerable variability, fluctuating within broad ranges among trees of the same variety, within fruits on the same tree, and even between years.
These differences are due to the ability of each species or variety to synthesize hormones in its ovaries. In plants where the hormonal content increases significantly at anthesis, the number of cells in the ovary walls also increases. This is due to the role of the hormones in promoting cell division, thereby influencing the potential for fruit development. This hormonal surge is typically more pronounced following self-pollination or cross-pollination, which explains why fruits from fertilized ovaries, i.e., those containing seeds, tend to be larger [214]. Analyses of endogenous plant hormones in pollinated and unpollinated ovaries of different species have led to the conclusion that fruit set is regulated by hormones synthesized in the developing seeds and/or in the ovary walls (see Section 2.4.1). In this context, hormones act as key signaling molecules that initiate and drive citrus fruit development.
Among these hormones, it is widely accepted that auxins play a key role in cell expansion [215]. In citrus, studies on the effects of IAA on fruit growth have shown that the fruit attains its maximum growth rate prior to reaching peak hormone concentration, although growth persists even when auxin levels are at their lowest [63]. This suggests that the role of IAA in fruit growth may be indirect, primarily localized to the seeds, where it promotes embryo development. This, in turn, generates a strong sink effect that influences the pericarp, which expands concomitantly. Supporting this hypothesis, a positive correlation has been observed between seed presence and fruit growth, with growth being more rapid and pronounced in fruits containing a greater number of developing seeds [171,214].
This effect suggests that the final size of the fruit is primarily determined during the early stages of its development, specifically when embryo growth initiates, although other factors may contribute at later stages. Consequently, hormone concentrations in the pericarp just before the onset of stage II can influence cell elongation [38,216] and thus overall fruit growth. In particular, the application of an auxin at this phenological stage induces this effect by enlarging the septa cells, which expand the locules, while the vesicles increase in volume due to the radial and tangential elongation of their epidermal cells, without a change in cell number [212]. This auxin-induced promotion of fruit growth has been linked to a reduction in cell turgor or wall pressure, which facilitates the action of expansins (see Section 3). Expansins lower the water potential of cells, thereby promoting the uptake of water and solutes, ultimately leading to an increase in cell volume.
3.1.1. The Characteristics of the Tree
The age of a tree significantly influences fruit size. Generally, young trees produce larger fruits with a lower juice content and a thicker, rougher rind. This phenomenon has been attributed to the high vigor typically exhibited by young trees, which reduces fruit set. As a result, fewer fruits that develop on the tree grow to sizes exceeding the standard for the variety, often displaying undesirable characteristics. However, these traits diminish as the tree matures. Upon reaching full adulthood and entering peak production, fruit size and other qualitative attributes tend to normalize. In contrast, older trees typically yield smaller fruits, partly due to their propensity for prolific flowering, which creates intense competition among reproductive organs, ultimately limiting ovary growth and negatively impacting final fruit size [118].
The flowering intensity, defined as the number of flowers produced per tree, plays a crucial role in determining fruit development and final size (see Section 3.2.3). Furthermore, a lower flower count increases the proportion of flowers borne on leafy inflorescences, and the presence of young leaves on the shoot stimulates fruit growth, with differences becoming evident as early as the final stages of fruit set and increasing progressively until ripening. However, during approximately one month around anthesis, young leaves act as competing sinks rather than sources, diverting assimilates away from developing fruits. This phenomenon is particularly relevant in citrus trees growing in tropical climates, where low photosynthetic rates exacerbate carbon limitation, ultimately reducing fruit size [217]. It is only during the transition to mature leaves that they assume their role as a carbohydrate source [76]. These findings highlight the critical role of young leaves in fruit development. Given that leaves serve as carbohydrate sources, fruit weight is influenced by the leaf-to-fruit ratio, i.e., larger leaf area per fruit generally results in greater fruit size, and vice versa [218]. This underscores the importance of an adequate carbohydrate flux from leaves to fruit, as fruit development is limited when carbohydrate supply is low and promoted when it is restored. Experimental evidence supports this: defoliation of Satsuma mandarin trees at the onset of stage II of fruit growth reduces fruit size by approximately 20% [3]; conversely, increasing carbohydrate availability by girdling enhances fruit size [218,219,220]. In addition, the position of the fruit in the canopy affects size, with fruit located inside the tree being smaller than those located at the periphery. This has been attributed to the greater photosynthetic activity of the outer vegetative layers, which provide a greater supply of assimilates to the fruit located outside the canopy [221,222].
In each tree, as with flowering intensity, the average final fruit size is inversely related to the number of fruits that reach maturity [6,118,223]. The correlation between these variables follows a potential function, so that only when the number of fruits falls below a certain threshold, varying by variety, does the curve exhibit a high slope and small variations in the number of fruits result in significant differences in fruit size. Conversely, above this threshold, i.e., at high fruit loads, fruit size remains relatively stable, even with further increases in fruit number [118]. According to Guardiola and García-Luis (2000) [224], this phenomenon is attributed to carbohydrate competition among reproductive organs, driven either by a limited assimilate supply (source limitation) from flowering to fruit maturity or by a reduced intrinsic capacity of the fruit to accumulate assimilates even under conditions of unlimited supply (sink limitation). Consequently, competition for photoassimilates and mineral nutrients increases as the fruit load per tree increases.
The hypothesis proposed by Brenner and Cheikh (1995) [96] for fruit set, that the photoassimilate concentration gradient between source (leaf) and sink (fruit) regulates transport, is not shared by Guardiola and García-Luis (2000) [224] in explaining fruit growth. Instead, they argue that the strength of the sink rather than assimilate supply is the main determinant of fruit growth rate during this period. In fact, increasing the assimilate supply by hand thinning or girdling has little effect on fruit growth in small-fruited Satsuma and Clementine mandarins, unless the trees are heavily thinned [225].
3.1.2. Environmental Factors
Fruit growth rate is primarily determined by temperature and relative humidity (RH) at each developmental stage. For a more detailed discussion, refer to the revision by Agustí (1999) [226] and the references therein.
Studies on Satsuma mandarin indicate that lower day/night temperature regimes result in smaller fruit size and suggest that night temperature, rather than daytime temperature, plays a key role in regulating fruit size [227,228,229]. Maximum fruit size is typically achieved within a temperature range of 20–25 °C, and when the temperature exceeds 30 °C, fruit growth is depressed [230,231]. This growth depression has been attributed to the depletion of carbohydrate reserves due to increased metabolic activity at high temperatures [232], resulting in a reduced carbohydrate supply to the developing fruit [233]. Similarly, fruit size in ‘Ruby Red’ grapefruit is positively correlated with spring temperature and negatively correlated with summer temperature [234].
In general, water deficit reduces fruit size [235]. In semi-arid regions, where RH is low and strong dry winds prevail during the fruit development period, fruit size is typically smaller. Persistently low RH values during the night further limit growth rates, with 37% RH considered a critical threshold [236].
High summer temperatures in conjunction with low RH lead to high evapotranspiration rates, leading to stomatal closure. This, in turn, reduces net CO2 assimilation and consequently limits carbohydrate supply to the fruit, ultimately restricting its growth rate [226]. In early autumn, decreasing temperatures produce a similar effect, further constraining fruit growth. In particular, temperatures below 5 °C during this period exert a pronounced inhibitory effect on fruit development [226].
This explains why in Mediterranean climates the fruit’s growth rate is slow and dependent on seasonal temperature changes, whereas in tropical regions it grows almost continuously during development. This results in a steady increase in fruit volume and a shorter time to reach ripeness [237].
Similarly, a comprehensive six-year study comparing two dry climates, hot and arid (BSh) and Mediterranean (Csa), found that fruit juice content was significantly lower in the former (43% w/w) than in the latter (53% w/w). However, fruit size remained unchanged due to increased peel growth under arid conditions [238].
Temperature and RH also influence fruit morphology. Elevated temperatures during the fruit-setting stage can lead to deformities, often resulting in a pear-shaped stem end [226]. In contrast, cold and humid climates promote the development of flattened Satsuma fruit [227,231]. Similarly, grapefruit grown in humid subtropical or tropical regions tends to exhibit a flattened shape, whereas those cultivated in arid environments typically develop a more spherical form [239].
Water availability plays a pivotal role in determining the final fruit size. Trees experiencing water deficits during the summer due to insufficient rainfall suffer a reduced rate of fruit development [240]. This phenomenon is mainly due to a decrease in stomatal conductance, leading to diminished transpiration and net CO2 assimilation. As a result, the water and carbohydrate supply to the fruit is restricted, slowing its growth. Upon drought relief, fruit growth resumes; however, the developmental delay may be irreversible, potentially resulting in smaller fruit [241]. Conversely, numerous studies on citrus fruits have shown that summer rainfall, and particularly autumn rainfall, which maintains high soil moisture levels, enhances final fruit size.
Additionally, a strong correlation has been established between the prevalence of small fruits per tree and the number of cloudy days throughout the year [242]. In certain tropical regions where fog is frequent, fruit size tends to be smaller due to reduced light intensity and lower temperatures.
3.1.3. Growing Conditions
In general, when water availability is not a limiting factor, clayey soils present challenges for root development and typically yield smaller fruits compared to sandy soils, where trees establish a more extensive and vigorous root system. Consequently, fruits grown in sandy soils tend to be larger, often exhibit higher juice density, and contain lower acid concentrations. Additionally, they ripen slightly earlier and develop a thinner, more delicate peel.
However, as previously mentioned, soil moisture is a critical determinant of final fruit size. Reduced water availability, either due to drought conditions or inadequate irrigation frequency and volume, negatively impacts fruit development. Even in regions with optimal annual rainfall, improper irrigation scheduling and water delivery can significantly limit fruit size [237].
Experiments conducted on ‘Washington’ navel and ‘Valencia’ sweet orange have shown that delaying irrigation until fruit growth ceases results in an inadequate recovery of growth, preventing the fruit from reaching the final size typical of the variety [243]. Moreover, when the total water supply remains insufficient throughout the fruit development period, trees adapt by reducing water consumption and limiting fruit size. And this adaptation is better or worse depending on the RH and ambient temperature. Conversely, in the absence of water deficits, i.e., when irrigation is not delayed and water supply is adequate, fruit size is modulated by variations in RH and ambient temperature [244].
In many citrus-growing regions, water scarcity has necessitated a shift in irrigation methods and/or the implementation of strategies to optimize water use. Concerning the former, traditional flood (gravity) irrigation has largely been replaced by microirrigation systems, such as drip and microsprinkler irrigation, as well as by furrow irrigation systems [245]. These systems enable water application at very frequent intervals, including multiple times per day, although the specific benefits of such high-frequency irrigation remain unclear [246]. Nevertheless, there is no consensus on the minimum water requirements at specific phenological stages to optimize fruit development under controlled deficit irrigation. Early studies [247] demonstrated that substantial water savings could be achieved by extending irrigation intervals; however, this approach resulted in a reduction in fruit size. Consequently, research has focused on developing alternative irrigation strategies aimed at enhancing water use efficiency (WUE) without compromising fruit size, quality, or yield [248].
The implementation of a moderate Regulated Deficit Irrigation (RDI) strategy, based on stem water potential values ranging from −1.3 to −1.5 MPa, can achieve water savings of 7% to 14% without significantly reducing fruit size when applied at the onset of the fruit growth stage in ‘Clemenules’ mandarin trees. However, more severe deficits, with stem water potential values between −1.5 and −1.7 MPa, lead to yield reductions due to a decrease in fruit size [249]. The extent of these effects depends on both the intensity and duration of plant water stress [250]. In addition, strategies such as Partial Root-Zone Drying have been associated with smaller fruit size and fewer fruits, likely due to decreased plant water potential and diminished photosynthetic activity [251].
The influence of fertilization on fruit quality is highly variable and depends significantly on the specific mineral element, as well as the timing and extent of any nutritional imbalances. In addition, there is a marked varietal sensitivity and rootstock-dependent responses to mineral nutrition in relation to fruit size. Chapman (1968) [252] and Embleton et al. (1973) [112] conducted extensive research on leaf mineral content as a diagnostic tool for fertilization and its correlation with fruit characteristics, and Wutscher (1979) [253] studied the effects of rootstocks on fruit quality. Many studies have been carried out on these topics, and the main findings in relation to fruit size are summarized below.
The effect of rootstock on fruit size can vary in importance depending on the scion cultivar and cultural practices. Citranges (C. sinensis × P. trifoliata), FA-5 (C. reshni × P. trifoliata), Rough lemon (C. jambhiri), Swingle citrumelo (C. paradisi × P. trifoliata), and C. macrophylla typically induce larger fruit, whereas Cleopatra mandarin (C. reshni) and P. trifoliata are associated with smaller fruit sizes. With regard to the effect of rootstock on fruit coloration and internal fruit quality traits, such as juice content, total soluble solids (TSS, oBrix), and titratable acidity, rootstocks such as P. trifoliata, various Citranges, including Benton, Carrizo, Troyer, and C-35, Cleopatra mandarin (C. reshni), FA-5 (C. reshni × P. trifoliata), and Swingle citrumelo improve fruit quality. In contrast, rootstocks such as Rough lemon and C. macrophylla have less favorable effects, causing the fruit to dry out prematurely [110].
Tree spacing also affects fruit size, which generally increases under low-density planting, as well as TSS in both oranges and mandarins [111].
Nitrogen deficiency generally results in smaller fruit, whereas excessive N fertilization has been reported to reduce orange fruit size due to an increase in fruit set [254]. In grapefruit, however, the results are inconsistent [255], and in Satsuma mandarin, increased N fertilization increases fruit size but negatively affects intrinsic quality, such as rough peel texture, lower sugar content, and higher acidity [256]. These apparent contradictions arise from the fact that citrus leaves are not highly sensitive indicators of N excess, making accurate diagnosis challenging. For example, even under conditions of excessive fertilization, Clementine trees efficiently accumulate N in their leaves, and in many cases an increased fruit growth rate can be observed without a corresponding change in leaf N concentration [257].
Phosphorus (P) does not influence fruit characteristics. In oranges and lemons, neither yield nor fruit size responds to increasing P, whereas in grapefruit, P has been reported to increase fruit size [258].
Field evidence suggests that potassium supplementation enhances fruit size in sweet oranges [259], grapefruits [260], limes [261], and lemons [262]. Accordingly, foliar potassium concentrations slightly above the established optimum range can contribute to moderate increases in fruit size without adversely affecting quality.
Deficiencies in calcium, magnesium, iron, zinc, and manganese negatively impact final fruit size, and their correction is essential for normal fruit development [252]. In general, correcting a mineral deficiency stimulates fruit growth. However, once the optimum foliar concentration is reached, further applications confer no additional benefit [112] and may even be detrimental.
Limited information is available on the effect of organic matter on fruit size. However, its incorporation into the soil improves soil structure, enhancing potassium penetration and phosphorus mobility. Additionally, organic manure appears to alleviate the severity of zinc deficiency [252].
Techniques to improve fruit set (see the Section 2.6) generally result in a reduction in fruit size by increasing the number of developing fruits.
The regulation of fruit size has long been recognized as one of the primary benefits of pruning. Pruning stimulates the emergence of new shoots, thereby facilitating normal fruit development in cases where leaf surface area may be insufficient. It also opens up a significant part of the canopy, enhancing light penetration and consequently increasing photosynthetic efficiency. In grapefruit, for instance, fruits on shaded branches are less than 60% the size of those exposed to direct light [218]. These combined effects reduce competition among flowers and/or developing fruits. Furthermore, pruning also removes fruits on weak branches unlikely to reach optimal size [263].
The timing of pruning is a critical factor to consider in orchard management. Since pruning is typically performed after harvest, early-maturing cultivars are generally pruned before those with later maturation. When early-maturing varieties undergo pruning prior to or during the flowering period, the subsequent reduction in flowering can slightly enhance fruit size; however, this may also stimulate increased vegetative regrowth, which can be either beneficial or undesirable depending on management objectives. Late-maturing cultivars present a distinct challenge due to overlapping harvest periods. In such cases, postponing pruning until early summer reduces the number of developing fruits and vegetative regrowth is less vigorous, contributing to improved fruit size. However, pruning during the summer may create an inverse relationship between the percentage of leaf area removed and fruit weight, as the daily carbohydrate supply must meet the fruit’s metabolic demands. Therefore, pruning should be light when fruit load is high and heavier when fruit load is low.
Therefore, no single pruning system or set of guidelines can be universally applied to the diverse field conditions encountered in citrus orchards. Furthermore, the impact of pruning on fruit size is generally limited and highly variable, as the response to pruning depends on multiple factors, including tree age and vigor, cultivar, fruiting habits, growing conditions, and cultural practices. Consequently, various strategies combining manual and mechanical pruning have been evaluated over the years. Studies have demonstrated that topping and light pruning can enhance fruit size in certain cultivars, such as ‘Clausellina’ mandarin, ‘Washington’ navel and ‘Hamlin’ sweet oranges, ‘Orlando’ Tangelo, and ‘Star Ruby’ grapefruit [264,265,266,267]. Conversely, neither manual pruning nor mechanical pruning (topping and hedging) significantly increased fruit diameter in other cultivars, including ‘Clemenules’ mandarin, ‘Orlando’ Tangelo, ‘Fino 95’ lemon, and ‘Salustiana’, ‘Valencia’ and other sweet oranges [268,269,270,271]. Notably, it has also been reported that fruit size in ‘Eureka’ lemon decreases as a result of topping and hedging [272].
3.2. Treatments to Increase Fruit Size
3.2.1. Girdling or Ringing
The most effective timing for girdling or ringing to increase fruit size is immediately after physiological fruitlet abscission. When performed at this stage, an increase in fruit diameter of approximately 2 to 4 mm is typically observed in mandarin, sweet orange, and grapefruit varieties [218,219,220,273], resulting in an improved distribution of commercial sizes. Delayed girdling or ringing reduces its effectiveness, although a measurable effect remains until late August (NH) [274]. The beneficial effects of girdling have been attributed to carbohydrate accumulation in the branches above the girdle, thereby increasing carbohydrate availability for fruit development [160].
Importantly, advancing the treatment has opposite effects. When girdling is performed during physiological fruitlet abscission, fruit set increases (see Section 2.6.2), ultimately leading to a reduction in fruit size [220,274].
3.2.2. Fruit Thinning
As there is an inverse relationship between the number of fruits per tree and their final size (see Section 3.1), removing some of the developing fruits allows the available photoassimilates to be redirected to the remaining fruits on the tree, allowing them to increase in size. This removal can be performed manually or chemically.
Manual thinning is performed in two ways: randomly or selectively. The random approach is applied once physiological fruitlet abscission has concluded. Earlier thinning does not make sense because (i) fruitlets naturally abscise, (ii) its effectiveness is minimal, and (iii) the sheer number of developing fruitlets makes the process unfeasible. Conversely, delaying thinning progressively reduces effectiveness [266].
In randomly conducted manual thinning, a minimum of 50% of the fruit must be removed. Studies on Satsuma mandarins ‘Clausellina’ [266] and ‘Silverhill’ [275] have shown that optimum results are achieved when two out of three developing fruits (66%) are removed. However, this practice results in a significant reduction in yield, which may only, in some cases, offset the economic benefits of increased fruit size. In sweet orange, thinning intensity appears to be less critical, with the removal of one-third to one-half of the developing fruit significantly increasing the size of ‘Valencia’ oranges [225].
When thinning intensity is evaluated based on leaf-to-fruit ratio, experiments conducted in New Zealand on Satsuma ‘Miyagaya’ [275] showed that unthinned trees and trees thinned to a 10:1 ratio produced an excessive number of undersized fruits. Trees thinned to a 15:1 ratio exhibited insufficient return flowering, whereas thinning at a 35:1 ratio or higher led to an excessive reduction in yield. A crop load of 20–25:1 was found to be sustainable.
Selective manual thinning involves the removal of underdeveloped or visibly defective fruit. This practice serves two main purposes: (1) to remove the smallest fruit, thereby increasing the average size of the remaining fruit, and (2) to improve the quality of the fruit by discarding those that show biotic or abiotic disorders. This thinning is usually delayed until the second half of summer, when the fruit can be clearly distinguishable [118].
Selective manual thinning has several advantages over random thinning. In particular, it is not as intense as random thinning, minimizing yield loss and making it more economically viable. In addition, the direct removal of defective fruit in the field improves crop quality while reducing post-harvest costs associated with packing and processing. Nevertheless, due to the limited number of fruits removed, has minimal impact on competition among developing fruits and does not significantly stimulate fruit growth.
The main drawback of hand thinning, whether random or selective, is its high labor cost. Consequently, it is typically used on specific mandarin varieties with high market value, improving grower profitability.
Chemical thinning reduces the number of developing fruitlets by inducing their abscission. The main advantage of this technique is that it greatly reduces the labor costs associated with manual thinning, although it has the disadvantage of being less precise and selective.
Similar to hand thinning, chemical thinners must effectively remove more than 50% of the developing fruits to significantly increase the size of the remaining fruits, although this inevitably leads to a reduction in overall crop yield. These compounds predominantly target smaller fruitlets, showing selectivity for less developed fruits. It is widely recognized that the optimal timing for chemical thinning is during the physiological fruitlet abscission period [127]. While chemical thinners do not directly enhance the growth of the remaining fruits, they indirectly contribute to an increase in their average size by eliminating smaller fruitlets (Figure 9A).
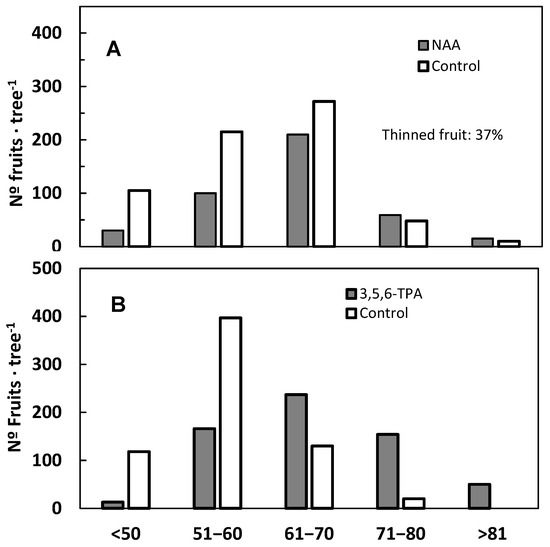
Figure 9.
Distribution of fruit diameters from trees treated with naphthaleneacetic acid (NAA; 50 mg L−1) during the physiological fruitlet abscission stage (A) and 3,5,6-trichloro-2-pyridyloxyacetic acid (3,5,6-TPA; 10 mg L−1) at the end of the physiological fruitlet abscission stage (B) compared to untreated control trees. Application at the first stage selectively reduces the number of small-sized fruits, whereas application at the second stage additionally increases the number of larger fruits. The total number of fruits harvested was comparable across treatments. Adapted from [5], with permission.
The most widely tested plant growth regulators for thinning include 2-chloroethyl phosphonic acid (ethephon), an ethylene-releasing agent [276]; naphthaleneacetic acid (NAA) [276,277]; ethyl 5-chloro-1H-3-indazolylacetate (ethychlozate; Figaron) [278,279]; 3-chlorophenoxyacetic acid (CPA) [278]; and 3,5,6-trichloro-2-pyridyloxyacetic acid (3,5,6-TPA) [280,281]. Other synthetic auxins, such as 2,4-dichlorophenoxyacetic acid (2,4-D) [276], 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) [276,278], S-ethyl 4-chloro-o-tolyloxythioacetic acid (Phenotiol) [281], and 2,4-dichlorophenoxypropionic acid (2A-DP) [212,282], have also been evaluated, but their thinning efficacy is variable, or they fail to induce thinning. Among these compounds, NAA, ethephon, ethychlozate, and 3,5,6-TPA have shown the most consistent thinning effects (see [127] for further details) and have been extensively studied [283].
The propensity of fruitlets to abscise has been correlated with sugar availability [3,89]. In citrus, it is suggested that C shortage during fruit set acts as a signal that triggers a hormonal sequence involving ethylene biosynthesis, which controls fruitlet abscission and adjusts the number of developing fruitlets to sugar availability [7,70,73]. So, the application of the synthetic auxins impairs temporal photosynthetic activity, and this phytotoxic effect reduces the production and supply of photosynthates to the fruitlet, which reduces the growth rate, triggers ethylene production and leads to abscission [87].
This phenomenon has been studied using NAA, ethychlozate [278] and 3,5,6-TPA [284], all of which cause photosynthetic impairment through leaf chlorosis. Specifically, 3,5,6-TPA reduces the effective quantum yield of photosystem II (ΦPSII) and photochemical quenching (qP), i.e., the energy used in photosynthesis, while increasing non-photochemical quenching (qN), i.e., the thermal dissipation of excess energy, and reducing CO2 assimilation [87]. However, this phytotoxic stress does not cause permanent damage on the photosynthetic apparatus, as its effects are fully reversible once the stress is alleviated [87,284].
The phytotoxicity induced by 3,5,6-TPA reduces CO2 fixation through repression of the RbcS gene [82], which impairs the catalytic efficiency of the Rubisco complex. Consequently, some key genes involved in its synthesis (SUS1 and SUSA) and transport to sink tissues (SUT3 and SUT4) are downregulated [82]. As a result, the sucrose content in the fruit of treated trees declines, along with its hydrolysis, as evidenced by the repression of the SUS1 invertase gene in the fruit. However, the expression of the cell wall invertase gene CWIN is only reduced several days later [82]. This temporal pattern suggests that sugar unloading in the fruit primarily occurs via the symplastic pathway (see Section 2.4.2).
3,5,6-TPA treatment also downregulates the expression of the PIN1 gene, i.e., IAA efflux towards the peduncle, as well as the expression of TRN2, which regulates auxin homeostasis in the fruit [82]. Both effects lead to an unprotected abscission layer and impaired development of vascular bundles [285], which ultimately lose their transport capacity [87]. Consequently, carbohydrate loading in the fruit is reduced, leading to the repression of CYCA1-1, i.e., the cell division rate, increases fruitlet ethylene production [87,284], and triggers abscission.
Multiple factors influence the efficacy of these treatments, including tree health, fruit load, cultivar, formulation, concentration, volume applied, the climatic conditions at the time of treatment, and the fruitlet size at the date of treatment. High temperatures (>30 °C) can lead to excessive thinning; to mitigate this risk, applications should be carried out early in the morning or late in the afternoon when temperatures are lower [278]. Consequently, treatment outcomes are often erratic at the same concentration, which is why thinner substances are rarely used in citrus.
It is important to note that this thinning effect ceases once physiological fruitlet abscission concludes and the fruit enters the linear growth phase (Stage II) [1]. However, when applied at this later stage, certain synthetic auxins act as fruit size enhancers (see Section 3.3).
3.2.3. Inhibition of Flowering
The intensity of flowering has a significant influence on fruit development and its final size. This hypothesis is supported by the observations that (1) at anthesis ovaries from trees with low flowering intensity are heavier than those from high flowering trees [9], (2) this is partially attributed to the higher proportion of leafy flowering shoots in trees with low flowering intensity [76], (3) the larger ovary size at the time of petal fall promotes faster early fruitlet growth, resulting in larger final fruit size [7], and (4) an inverse relationship between flower number and fruit size, independent of crop load, has been demonstrated in several orange and mandarin cultivars [5,223]. However, this effect is often obscured by its concomitant effect on fruit set (see Section 2.5.6).
Consequently, the application of gibberellic acid during the winter rest period, which significantly reduces flowering, can be used as an effective thinning strategy to increase fruit size. This approach has been shown to promote fruit enlargement in both Clementine mandarin [286] and ‘Owari’ Satsuma mandarin [287].
3.3. Increasing Fruit Size
Differences in cell number or cell size, or a combination of both, result in differences in fruit size. However, the relative contribution of each factor to the final fruit size remains unclear.
The potential to increase fruit size by promoting cell division is an underexplored approach. Treatment with plant growth regulators, such as gibberellins and cytokinins, applied at or shortly after anthesis enhances early fruit growth [134,288], but does not always increase fruit weight at harvest.
In contrast, the potential for increasing fruit size through cell expansion has been studied more extensively. Synthetic auxins applied at the onset of the rapid fruit growth stage (stage II), i.e., after physiological fruitlet abscission, promote cell expansion and increase fruit size.
Among synthetic auxins, 2,4-DP and 3,5,6-TPA are the most commonly used in oranges and mandarins. 2,4-DP, applied as butylglycol ester or 2-ethylhexyl ester at concentrations of 50 or 25 mg L−1, respectively, increases fruit diameter by 5% to 10% with minimal thinning effects [212,282]. Similarly, 3,5,6-TPA, as free acid, applied at concentrations of 10–20 mg L−1, induces a diameter increase of 5% to 15% [281].
These compounds increase vesicle size without affecting the number of vesicles. This effect occurs due to cell expansion rather than cell division [212,289,290].
The role of auxins in promoting cell expansion was established some years ago and shown to depend on the maintenance of cell turgor [291]. Studies on other fleshy fruits have shown that 3,5,6-TPA applied at the onset of the active fruit growth period (stage II) induces cell wall loosening, leading to a reduction in cell turgor pressure and water potential. This facilitates greater water uptake, enhancing carbohydrate accumulation, promoting fruit weight gain, and maintaining cell turgor [292].
This effect is also indirectly evidenced by the increase in peduncle size, as a consequence of the direct effect of synthetic auxin treatments on xylem and phloem enlargement [213]. This is consistent with the proposed mechanism of carbohydrate transport into the fruit, as previously described in vine canes [293].
The efficacy of these growth regulators is highest towards the end of the physiological fruitlet abscission, just before the period of maximum peel thickness and vesicle and locule expansion [281,294]. In practice, the optimum fruit diameter at the time of treatment is between 15 and 20 mm for mandarins and between 25 and 30 mm for oranges. Beyond this size, fruit sensitivity to treatment decreases, and higher concentrations may be required to achieve a significant increase in fruit size [212,282,288,289].
The frequency distribution of commercial fruit sizes after treatment with these synthetic auxins exhibits a significant shift towards larger calibers (Figure 9B), in contrast to thinning treatments applied during physiological fruitlet abscission, which do not shift towards larger sizes (Figure 9A) [274,280]. This response is general for all mandarin and sweet orange cultivars and results in a significant increase in high-quality commercial fruit and a reduction in discarded fruit. Typically, these treatments result in an average fruit diameter increase of 4 to 7 mm for mandarins and 3 to 5 mm for oranges.
Since these synthetic auxins also have a thinning effect, a minimal and selective reduction in smaller fruits can be achieved by optimizing the timing of application. The combined action of increasing fruit growth and selectively thinning smaller fruits makes this the most effective strategy to improve fruit size in citrus.
These compounds have largely replaced previously used substances, many of which are now deregistered. Among the most important were NAA, esters of 2,4,5-TP, 2,4-D, and 2,4,5-T, and phenotiol, typically applied at concentrations ranging from 5 to 20 mg L−1 [153,211,282,290,295,296].
A schematic overview of techniques to increase fruit size, along with their timing, is presented in Figure 10.
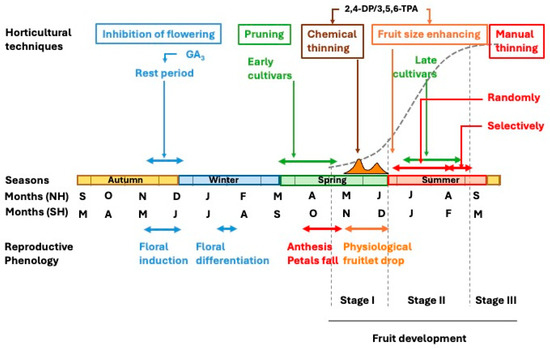
Figure 10.
Optimal periods (NH) for cultural practices and treatments to increase citrus fruit size throughout the phenological cycle. The vertical arrows indicate the specific treatments to be applied, while the horizontal arrows indicate the period during which each treatment should be applied. The colors relate to horticultural techniques and treatment periods. Adapted from [171].
4. Concluding Remarks
Citrus varieties generally require successful pollination for fertilization, a process involving the transfer of pollen grains to the stigma, germination and growth of a pollen tube toward the ovules. These events typically reactivate ovary cell division, thereby promoting fruit and seed development. Nevertheless, numerous Citrus cultivars are capable of fruit set through parthenocarpy, bypassing fertilization. In such cases, genetic factors maintain elevated hormone levels in the ovary during anthesis and the early stages of fruit set. Fruit set patterns depend on cultivar, climate, and flowering intensity, with seeded varieties generally displaying higher fruit set than seedless ones, although substantial variability exists within seedless cultivars.
Following petal fall, a considerable abscission of ovaries and young fruits occurs, driven by hormonal signals and carbon limitations. Two overlapping waves of fruitlet abscission are commonly observed. Hormonal cascades initiated by pollination, involving gibberellins, auxins, cytokinins, abscisic acid, and ethylene, coordinate the regulation of fruit set. Among these, gibberellin GA1, synthesized via the 13-hydroxylation pathway, plays a pivotal role, particularly in cultivars with high parthenocarpic ability. Upregulation of gibberellin biosynthesis genes, including GA20ox2 and GA3ox1, is associated with ovary growth, elevated GA1 concentrations, and increased CYCA1.1 expression, collectively indicating enhanced cell division during early fruit development.
Auxin levels, particularly IAA, rise sharply following anthesis. In seeded cultivars, IAA synthesis depends on successful pollination and fertilization. In unpollinated ovaries, low IAA levels reduce gibberellin biosynthesis, leading to ovary abscission; however, exogenous application of IAA can partially restore gibberellin biosynthesis. In seed-bearing cultivars, ABA levels increase in unpollinated ovaries, causing abscission due to insufficient GA1 content. Conversely, high-parthenocarpy ability cultivars maintain low abscisic acid concentrations, ensuring fruit retention. Cytokinins, including trans-zeatin (tZ) and 2-isopentenyl adenine (2-IP), are constitutively synthesized and largely independent of fertilization.
In addition to hormonal regulation, carbohydrate availability critically affects fruitlet survival. Soluble sugar concentrations peak at anthesis and then decline, leading to intense competition for assimilates among developing fruits. A shortage of carbohydrates induces fruitlet abscission, highlighting the role of sucrose supply during early fruit development.
Thus, high endogenous GA1 concentrations coupled with adequate carbohydrate availability are crucial for successful fruit set and growth. Application of exogenous gibberellic acid (5–25 mg L−1) at petal fall or girdling branches 25–30 days later has been shown to improve fruit set. The former upregulates GA20oxs expression in the ovary, leading to an increase in GA1 concentration, inducing CcCYCAs expression, stimulating cell division, promoting ovary growth, and ultimately facilitating fruit set. The latter induces auxin transport from the fruitlet to the abscission zone, protects the fruitlet from abscission, and preserves vascular bundles' function and carbohydrate transport to sustain growth.
Following the cell division phase, fruit growth progresses linearly, driven by cell expansion and water accumulation. Expansins mediate cell wall loosening, while aquaporins regulate water and nutrient influx, both processes essential for maintaining turgor pressure and supporting fruit enlargement. Hormonal regulation, particularly auxins, facilitates cell expansion, often through seed-mediated signaling pathways.
Auxin applications at the onset of the linear developmental stage enlarge septal cells and vesicles, increasing fruit size. Synthetic auxins such as 2,4-dichlorophenoxypropionic acid (2,4-DP) and 3,5,6-trichloro-2-pyridinyloxyacetic acid (3,5,6-TPA) significantly increase fruit diameter. Although manual and chemical thinning could optimize fruit size by reducing intra-plant competition, their use in citrus remains limited due to the extensive thinning required (>50% of the initial fruit set).
Author Contributions
Conceptualization, writing—original draft preparation, and supervision, M.A.; Writing—review and editing, resources, and supervision, C.R., A.M.-F. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bain, J.M. Morphological, anatomical, and physiological changes in the developing fruit of the Valencia orange, Citrus sinensis (L) Osbeck. Austr. J. Bot. 1958, 6, 1–24. [Google Scholar] [CrossRef]
- Schneider, H. The anatomy of citrus. In The Citrus Industry; Reuther, W., Batchelor, L.D., Webber, H.J., Eds.; University of California, Division of Agricultural Sciences: Berkeley, CA, USA, 1968; Volume II, pp. 2–85. [Google Scholar]
- Mehouachi, J.; Serna, D.; Zaragoza, S.; Agustí, M.; Talón, M.; Primo Millo, E. Defoliation increases fruit abscission and reduces carbohydrate levels in developing fruits and woody tissues of Citrus unshiu. Plant Sci. 1995, 107, 189–197. [Google Scholar] [CrossRef]
- Mesejo, C.; Martínez-Fuentes, A.; Reig, C.; Agustí, M. The flower to fruit transition in Citrus is partially sustained by autonomous carbohydrate synthesis in the ovary. Plant Sci. 2019, 285, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Agustí, M.; Mesejo, C.; Reig, C. Citricultura, 3rd ed.; Ed. Mundi-Prensa: Madrid, Spain, 2020; 488p. [Google Scholar]
- Goldschmidt, E.E.; Monselise, S.P. Physiological assumptions toward the development of a citrus fruiting model. Proc. Int. Soc. Citric. 1977, 2, 668–672. [Google Scholar]
- Agustí, M.; García Marí, F.; Guardiola, J.L. The influence of flowering intensity on the shedding of reproductive structures in sweet orange. Sci. Hortic. 1982, 17, 343–352. [Google Scholar] [CrossRef]
- Zucconi, F.; Monselise, S.P.; Goren, R. Growth abscission relationships in developing orange fruit. Sci. Hortic. 1978, 9, 137–146. [Google Scholar] [CrossRef]
- Guardiola, J.L.; García-Marí, F.; Agustí, M. Competition and fruit set in the Washington navel orange. Physiol. Plant. 1984, 62, 297–302. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Iglesias, D.J.; Reig, C.; Mesejo, C.; Agustí, M.; Primo-Millo, E. Carbon utilization by fruit limits shoot growth in alternate-bearing citrus trees. J. Plant Physiol. 2015, 176, 108–117. [Google Scholar] [CrossRef]
- Herrero, M. Ovary signals for directional pollen tube growth. Sex. Plant Reprod. 2001, 14, 3–7. [Google Scholar] [CrossRef]
- Distefano, G.; Gentile, A.; Herrero, M. Pollen–pistil interactions and early fruiting in parthenocarpic citrus. Ann. Bot. 2011, 108, 499–509. [Google Scholar] [CrossRef]
- Tilton, V.R.; Wilcox, L.W.; Palmer, R.G.; Albertsen, M.C. Stigma, style and obturator of soybean, Glycine max (L.) Merr. (Leguminoseae) and their function in the reproductive process. Am. J. Bot. 1984, 71, 676–686. [Google Scholar] [CrossRef]
- Okuda, S.; Tsutsui, H.; Shiina, K.; Sprunck, S.; Takeuchi, H.; Yui, R.; Kasahara, R.D.; Hamamura, Y.; Mizukami, A.; Susaki, D.; et al. Defensin-like polypeptide LUREs are pollen tuve attractants secreted from synergid cells. Nature 2009, 458, 357–361. [Google Scholar] [CrossRef]
- Shi, D.Q.; Yang, W.C. Pollen germination and tube growth. In Plant Developmental Biology—Biotechnological Perspectives; Pua, E.-C., Davey, M.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 1, pp. 245–282. [Google Scholar] [CrossRef]
- Mesejo, C.; Martínez-Fuentes, A.; Reig, C.; Agustí, M. The effective pollination period in ‘Clemenules’ mandarin, ‘Owari’ Satsuma mandarin and ‘Valencia’ sweet orange. Plant Sci. 2007, 173, 223–230. [Google Scholar] [CrossRef]
- Distefano, G.; Gentile, A.; Hedhly, A.; La Malfa, S. Temperatures during flower bud development affect pollen germination, self-incompatibility reaction and early fruit development of clementine (Citrus clementina Hort. ex Tan.). Plant Biol. 2018, 20, 191–198. [Google Scholar] [CrossRef]
- Montant, R.; Cuenca, J.; Vives, M.C.; Navarro, L.; Ollitrault, P.; Aleza, P. Influence of temperature on the progamic phase in Citrus. Environ. Exp. Bot. 2019, 166, 103806. [Google Scholar] [CrossRef]
- Mesejo, C.; Yuste, R.; Reig, C.; Martínez-Fuentes, A.; Iglesias, D.J.; Muñoz-Fambuena, N.; Bermejo, A.; Germaná, M.A.; Primo-Millo, E.; Agustí, M. Gibberellin reactivates and maintains ovary-wall cell division causing fruit set in parthenocarpic Citrus species. Plant Sci. 2016, 247, 13–24. [Google Scholar] [CrossRef]
- Vardi, A.; Levin, I.; Carmi, N. Induction of seedlessness in Citrus: From classical techniques to emerging biotechnological approaches. J. Am. Soc. Hortic. Sci. 2008, 133, 117–126. [Google Scholar] [CrossRef]
- Frost, H.B.; Soost, R.K. Seed reproduction: Development of gametes and embryos. In The Citrus Industry; Reuther, W., Batchelor, L.D., Webber, H.J., Eds.; University of California, Division of Agricultural Sciences: Berkeley, CA, USA, 1968; Volume II, pp. 290–324. [Google Scholar]
- Ollitrault, P.; Ahmed, D.; Costantino, G.; Evrard, J.C.; Cardi, C.; Mournet, P.; Perdereau, A.; Froelicher, Y. Segregation distortion for male parents in high density genetic maps from reciprocal crosses between two self-incompatible cultivars confirms a gametophytic system for self-incompatibility in Citrus. Agriculture 2021, 11, 379. [Google Scholar] [CrossRef]
- Ngo, B.X.; Kim, J.-H.; Wakana, A.; Isshiki, S.; Mori, T. Estimation of self-incompatibility genotypes of Citrus cultivars with Got-3 allozyme markers. J. Jpn. Soc. Hortic. Sci. 2011, 80, 284–294. [Google Scholar] [CrossRef]
- Honsho, C. Self-incompatibility related to seedless fruit production in citrus plants. Hortic. J. 2023, 92, 1–12. [Google Scholar] [CrossRef]
- Bennici, S.; Poles, L.; Di Guardo, M.; Percival-Alwyn, L.; Caccamo, M.; Licciardello, C.; Gentile, A.; Distefano, G.; La Malfa, S. The origin and the genetic regulation of the self-compatibility mechanism in clementine (Citrus clementina Hort. ex Tan.). Front. Plant Sci. 2024, 15, 1360087. [Google Scholar] [CrossRef]
- Vardi, A.; Neumann, H.; Frydman-Shani, A.; Yaniv, Y.; Spiegel-Roy, P. Tentative model on the inheritance of juvenility, self-incompatibility and parthenocarpy. Acta Hortic. 2000, 535, 199–206. [Google Scholar] [CrossRef]
- Distefano, G.; Casas, G.L.; Malfa, S.L.; Gentile, A.; Tribulato, E.; Herrero, M. Pollen tube behavior in different mandarin hybrids. J. Am. Soc. Hortic. Sci. 2009, 134, 583–588. [Google Scholar] [CrossRef]
- Gambetta, G.; Gravina, A.; Fasiolo, C.; Fornero, C.; Galiger, S.; Inzaurralde, C.; Rey, F. Self-incompatibility, parthenocarpy and reduction of seed presence in ‘Afourer’ mandarin. Sci. Hortic. 2013, 164, 183–188. [Google Scholar] [CrossRef]
- Mesejo, C.; Yuste, R.; Martinez-Fuentes, A.; Reig, C.; Iglesias, D.J.; Primo-Millo, E.; Agustí, M. Self-pollination and parthenocarpic ability in developing ovaries of self-incompatible Clementine mandarins (Citrus clementina). Physiol. Plant. 2013, 148, 87–96. [Google Scholar] [CrossRef]
- Mesejo, C.; Martínez-Fuentes, A.; Reig, C.; Agustí, M. Gibberellic acid impairs fertilization in Clementine mandarin under cross-pollination conditions. Plant Sci. 2008, 175, 267–271. [Google Scholar] [CrossRef]
- Mesejo, C.; Martínez-Fuentes, A.; Reig, C.; Rivas, F.; Agustí, M. The inhibitory effect of CuSO4 on Citrus pollen germination and pollen tube growth and its application for the production of seedless fruit. Plant Sci. 2006, 170, 37–43. [Google Scholar] [CrossRef]
- Ma, N.; Ma, C.; Liu, Y.; Shahid, M.O.; Wang, C.; Gao, J. Petal senescence: A hormone view. J. Exp. Bot. 2018, 69, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Zacarías, L.; Tudela, D.; Primo-Millo, E. Role of ethylene in the opening and senescence of citrus flowers. Sci. Hortic. 1991, 46, 55–60. [Google Scholar] [CrossRef]
- Cresti, M.; Ciampolini, F.; van Went, J.L.; Wilms, H.J. Ultrastructure and histochemistry of Citrus limon (L.) stigma. Planta 1982, 156, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Erickson, L.C.; Brannanman, B.L. Abscission of reproductive structures and leaves of orange trees. Proc. Am. Soc. Hortic. Sci. 1960, 75, 222–229. [Google Scholar]
- Monselise, S.P. Citrus fruit development: Endogenous systems and external regulation. Proc. Int. Soc. Citric. 1977, 2, 664–668. [Google Scholar]
- García-Papi, M.A.; García-Martínez, J.L. Fruit set and development in seeded and seedless Clementine mandarin. Sci. Hortic. 1984, 22, 113–119. [Google Scholar] [CrossRef]
- García-Papi, M.A.; García-Martínez, J.L. Endogenous plant growth substances content in young fruits of seeded and seedless Clementine mandarin as related to fruit set and development. Sci. Hortic. 1984, 22, 265–274. [Google Scholar] [CrossRef]
- Bermejo, A.; Primo-Millo, E.; Agustí, M.; Mesejo, C.; Reig, C.; Iglesias, D.J. Hormonal profile in ovaries of mandarin varieties with differing reproductive behaviour. J. Plant Growth Regul. 2015, 34, 584–594. [Google Scholar] [CrossRef]
- Bermejo, A.; Martínez-Alcántara, B.; Martínez-Cuenca, M.R.; Yuste, R.; Mesejo, C.; Reig, C.; Agustí, M.; Primo-Millo, E.; Iglesias, D.J. Biosynthesis and contents of gibberellins in seeded and seedless sweet orange (Citrus sinensis L. Osbeck) cultivars. J. Plant Growth Regul. 2016, 35, 1036–1048. [Google Scholar] [CrossRef]
- Goren, R. Anatomical, physiological, and hormonal aspects of abscission in citrus. Hortic. Rev. 1993, 15, 145–182. [Google Scholar] [CrossRef]
- Estornell, L.H.; Agustí, J.; Merelo, P.; Talón, M.; Tadeo, F.R. Elucidating mechanisms underlying organ abscission. Plant Sci. 2013, 199–200, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Agustí, J.; Merelo, P.; Cercós, M.; Tadeo, F.R.; Talón, M. Ethylene induced differential gene expression during abscission of citrus leaves. J. Exp. Bot. 2008, 59, 2717–2733. [Google Scholar] [CrossRef]
- Agustí, J.; Merelo, P.; Cercós, M.; Tadeo, F.R.; Talón, M. Comparative transcriptional survey between laser-microdissected cells from laminar abscission zone and petiolar cortical tissue during ethylene-promoted abscission in citrus leaves. BMC Plant Biol. 2009, 9, 127. [Google Scholar] [CrossRef]
- Shiraishi, M.; Yanagisawa, T. Anatomical and histochemical studies of starch distribution during abscission in filaments and petals of Wase Satsuma mandarin. J. Jpn. Soc. Hortic. Sci. 1988, 56, 365–374. [Google Scholar] [CrossRef]
- Huberman, M.; Goren, R. Exo- and endo-cellular cellulase and polygalacturonase in abscission zones of developing orange fruits. Physiol. Plant. 1979, 45, 189–196. [Google Scholar] [CrossRef]
- Taylor, J.E.; Whitelaw, C.A. Signals in abscission. New Phytol. 2001, 151, 323–340. [Google Scholar] [CrossRef]
- Addicott, F.T.; Lynch, R.S. Acceleration and retardation of abscission by indoleacetic acid. Science 1951, 114, 688–689. [Google Scholar] [CrossRef] [PubMed]
- Addicott, F.T.; Lynch, R.S.; Carns, H.R. Auxin gradient theory of abscission regulation. Science 1955, 121, 644–645. [Google Scholar] [CrossRef] [PubMed]
- Kućko, A.; Wilmowicz, E.; Ostrowski, M. Spatio-temporal IAA gradient is determined by interactions with ET and governs flower abscission. J. Plant Physiol. 2019, 236, 51–60. [Google Scholar] [CrossRef]
- Merelo, P.; Agustí, J.; Arbona, V.; Costa, M.L.; Estornell, L.H.; Gómez-Cadenas, A.; Coimbra, S.; Gómez, M.D.; Pérez-Amador, M.A.; Domingo, C.; et al. Cell wall remodeling in abscission zone cells during ethylene-promoted fruit abscission in Citrus. Front. Plant Sci. 2017, 8, 126. [Google Scholar] [CrossRef]
- Sabbione, A.; Daurelio, L.; Vegetti, A.; Talón, M.; Tadeo, F.; Dotto, M. Genome-wide analysis of AGO, DCL and RDR gene families reveals RNA-directed DNA methylation is involved in fruit abscission in Citrus sinensis. BMC Plant Biol. 2019, 19, 401. [Google Scholar] [CrossRef]
- Estornell, L.H.; Wildhagen, M.; Pérez-Amador, M.A.; Talón, M.; Tadeo, F.R.; Butenko, M.A. The IDA peptide controls abscission in Arabidopsis and Citrus. Front. Plant Sci. 2015, 6, 1003. [Google Scholar] [CrossRef]
- Mesejo, C.; Marzal, A.; Martínez-Fuentes, A.; Reig, C.; Agustí, M. On how auxin, ethylene and IDA-peptide relate during mature Citrus fruit abscission. Sci. Hortic. 2021, 278, 109855. [Google Scholar] [CrossRef]
- Wilson, W.C.; Hendershott, C. Anatomical and histochemical studies of abscission of oranges. Proc. Am. Soc. Hortic. Sci. 1968, 92, 203–210. [Google Scholar]
- Huberman, M.; Goren, R.; Zamski, E. Anatomical aspects of hormonal regulation of abscission in citrus. The shoot-peduncle abscission zone in the non-abscising stage. Physiol. Plant. 1983, 59, 445–454. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Stead, A.D. Abscission of flowers and floral parts. J. Exp. Bot. 1997, 48, 821–837. [Google Scholar] [CrossRef]
- Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits: A developmental perspective. Plant Cell 1993, 5, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- El-Otmani, M.; Lovatt, C.J.; Coggins, C.V.; Agustí, M. Plant growth regulators in Citriculture: Factors regulating endogenous levels in citrus tissues. Crit. Rev. Plant Sci. 1995, 14, 367–412. [Google Scholar] [CrossRef]
- Ben-Cheikh, W.; Perez-Botella, J.; Tadeo, F.R.; Talón, M.; Primo-Millo, E. Pollination increases gibberellin levels in developing ovaries of seeded varieties of citrus. Plant Physiol. 1997, 114, 557–564. [Google Scholar] [CrossRef]
- Bermejo, A.; Granero, B.; Mesejo, C.; Reig, C.; Tejedo, V.; Agustí, M.; Primo-Millo, E.; Iglesias, D.J. Auxin and gibberellin interact in citrus fruit set. J. Plant Growth Regul. 2018, 37, 491–501. [Google Scholar] [CrossRef]
- Talón, M.; Hedden, P.; Primo-Millo, E. Gibberellins in Citrus sinensis: A comparison between seeded and seedless varieties. J. Plant Growth Regul. 1990, 9, 201–206. [Google Scholar] [CrossRef]
- Talón, M.; Zacarías, L.; Primo-Millo, E. Hormonal changes associated with fruit set and development in mandarins differing in their parthenocarpic ability. Physiol. Plant. 1990, 79, 400–406. [Google Scholar] [CrossRef]
- Talón, M.; Zacarías, L.; Primo-Millo, E. Gibberellins and parthenocarpic ability in developing ovaries of seedless mandarins. Plant Physiol. 1992, 99, 1575–1581. [Google Scholar] [CrossRef]
- Zacarias, L.; Talon, M.; Ben-Cheikh, W.T.; Lafuente, M.; Primo-Millo, E. Abscisic acid increases in non-growing and paclobutrazol-treated fruits of seedless mandarins. Physiol. Plant. 1995, 95, 613–619. [Google Scholar] [CrossRef]
- Poling, S. Identification of endogenous gibberellins in immature navel orange fruit. J. Agric. Food Chem. 1991, 39, 677–680. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Ann. Rev. Plant Biol. 2008, 59, 225. [Google Scholar] [CrossRef] [PubMed]
- Talón, M.; Zacarías, L.; Primo-Millo, E. Role of gibberellins in parthenocarpic development of seedless mandarins. Proc. Int. Soc. Citric. 1994, 1, 485–488. [Google Scholar]
- Huerta, L.; García-Lor, A.; García-Martinez, J.L. Characterization of gibberellin20-oxidases in the Citrus hybrid Carrizo citrange. Tree Physiol. 2009, 29, 569–577. [Google Scholar] [CrossRef]
- Gómez-Cadenas, A.; Mehouachi, J.; Tadeo, F.R.; Primo-Millo, E.; Talón, M. Hormonal regulation of fruitlet abscission induced by carbohydrate shortage in citrus. Planta 2000, 210, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Ali-Dinar, H.M.; Krezdorn, A.H.; Wheaton, T.A. The sexual hormonal relation in citrus during fruit set. Acta Hortic. 1988, 218, 159–174. [Google Scholar] [CrossRef]
- Dorcey, E.; Urbez, C.; Blázquez, M.A.; Carbonell, J.; Pérez-Amador, M.A. Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J. 2009, 58, 318–332. [Google Scholar] [CrossRef]
- Iglesias, D.J.; Tadeo, F.R.; Primo-Millo, E.; Talon, M. Carbohydrate and ethylene levels related to fruitlet drop through abscission zone A in citrus. Trees 2006, 20, 348–355. [Google Scholar] [CrossRef]
- Hernández-Miñana, F.M.; Primo-Millo, J.; Primo-Millo, E. Endogenous cytokinins in developing fruits of seeded and seedless citrus cultivars. J. Exp. Bot. 1989, 40, 1127–1134. [Google Scholar] [CrossRef]
- Hernández-Miñana, F.M.; Primo-Millo, E. Studies on endogenous cytokinins in Citrus. J. Hortic. Sci. 1990, 65, 596–601. [Google Scholar] [CrossRef]
- Moss, G.I.; Steer, B.T.; Kriedemann, P.E. The regulatory role of inflorescence leaves in fruit-setting by sweet orange (Citrus sinensis). Physiol. Plant. 1972, 27, 432–438. [Google Scholar] [CrossRef]
- Powell, A.A.; Krezdorn, A.H. Influence of fruit setting treatment on translocation of 14C-metabolites in citrus during flowering and fruiting. J. Am. Soc. Hortic. Sci. 1977, 102, 709–714. [Google Scholar] [CrossRef]
- Goldschmidt, E.E.; Koch, K.E. Citrus. In Photoassimilate Distribution in Plants and Crops; Zamski, E., Schaffer, A., Eds.; Dekker: New York, NY, USA, 1996; pp. 797–823. [Google Scholar]
- Hiratsuka, S.; Suzuki, M.; Nishimura, H.; Nada, K. Fruit photosynthesis in Satsuma mandarin. Plant Sci. 2015, 241, 65–69. [Google Scholar] [CrossRef]
- Baroja-Fernández, E.; Muñoz, F.J.; Saikusa, T.; Rodríguez-López, M.; Akazawa, T.; Pozueta-Romero, J. Sucrose synthase catalyzes the de novo production of ADPglucose linked to starch biosynthesis in heterotrophic tissues of plants. Plant Cell Physiol. 2003, 44, 500–509. [Google Scholar] [CrossRef]
- Sadka, A.; Shlizerman, L.; Kamara, I.; Blumwald, E. Primary metabolism in citrus fruit as affected by its unique structure. Front. Plant Sci. 2019, 10, 1167. [Google Scholar] [CrossRef]
- Agustí, M.; Martínez-Fuentes, A.; Mesejo, C.; Marzal, A.; Reig, C. Expression of carbohydrate-related genes underlying 3,5,6-TPA-induced fruitlet abscission in citrus. Sci. Rep. 2024, 14, 26482. [Google Scholar] [CrossRef]
- Hulsmans, S.; Rodriguez, M.; De Coninck, B.; Rolland, F. The SnRK1 energy sensor in plant biotic interactions. Trends Plant Sci. 2016, 21, 648–661. [Google Scholar] [CrossRef]
- Feng, G.; Wu, J.; Xu, Y.; Lu, L.; Yi, H. High-spatiotemporal-resolution transcriptomes provide insights into fruit development and ripening in Citrus sinensis. Plant Biotechnol. J. 2021, 19, 1337–1353. [Google Scholar] [CrossRef]
- Lenz, F. Flower and fruit development in Valencia Late orange as affected by type of inflorescence and nutritional status. Hortic. Res. 1966, 6, 65–78. [Google Scholar]
- Ruan, Y.L. Fruit set, young fruit and feaf growth of Citrus unshiu in relation to assimilate supply. Sci. Hortic. 1993, 53, 99–107. [Google Scholar] [CrossRef]
- Mesejo, C.; Rosito, S.; Reig, C.; Martínez-Fuentes, A.; Agustí, M. Synthetic auxin 3,5,6-TPA provokes Citrus clementina (Hort. ex Tan) fruitlet abscission by reducing photosynthate availability. J. Plant Growth Regul. 2012, 31, 186–194. [Google Scholar] [CrossRef]
- Berüter, J.; Droz, P. Studies on locating the signal for fruit abscission in the apple tree. Sci. Hortic. 1991, 46, 201–214. [Google Scholar] [CrossRef]
- Iglesias, D.J.; Tadeo, F.R.; Primo-Millo, E.; Talón, M. Fruit set dependence on carbohydrate availability in citrus trees. Tree Physiol. 2003, 23, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Mesejo, C.; Martínez-Fuentes, A.; Reig, C.; Agustí, M. Ringing branches reduces fruitlet abscission by promoting PIN1 expression in ‘Orri’ mandarin. Sci. Hortic. 2022, 306, 111451. [Google Scholar] [CrossRef]
- Wallerstein, I.; Goren, R.; Monselise, S.P. The effect of girdling on starch accumulation in sour orange seedlings. Can. J. Bot. 1974, 52, 935–937. [Google Scholar] [CrossRef]
- Rivas, F.; Gravina, A.; Agustí, M. Girdling effects on fruit set and quantum yield efficiency of PSII in two Citrus cultivars. Tree Physiol. 2007, 27, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Legaz, F.; Serna, M.D.; Primo-Millo, E. Mobilization of the reserve N in citrus. Plant Soil 1995, 173, 205–210. [Google Scholar] [CrossRef]
- González-Ferrer, J.; Agustí, M.; Guardiola, J.L. Fruiting pattern and retranslocation of reserves in Navelate and Washington navel oranges. Proc. Int. Soc. Citric. 1984, 1, 194–200. [Google Scholar]
- García-Marí, F.; Agustí, M.; Barberá, J.; Guardiola, J.L. Potasio y desarrollo inicial del fruto en la variedad de naranjo Navelate. Rev. Agroquim. Tecnol. Aliment. 1980, 20, 257–272. [Google Scholar]
- Brenner, M.L.; Cheikh, N. The role of hormones in photosynthate partitioning and seed filling. In Plant Hormones: Physiology, Biochemistry, and Molecular Biology, 2nd ed.; Davies, P.J., Ed.; Klewer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 649–670. [Google Scholar]
- Kriedemann, P.E. The distribution of 14C-labelled assimilates in mature lemon trees. Aust. J. Agric. Res. 1970, 21, 623–632. [Google Scholar] [CrossRef]
- Mauk, C.S.; Bausher, M.G.; Yelenosky, G. Influence of growth regulator treatments on dry matter production, fruit abscission, and 14C-assimilate partitioning in Citrus. J. Plant Growth Regul. 1986, 5, 111–120. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, D.Q. Stimulation effect of gibberellic acid short-term treatment on leaf photosynthesis related to the increase in Rubisco content in broad bean and soybean. Photosynth. Res. 2001, 68, 39–47. [Google Scholar] [CrossRef]
- Jahnke, S.; Bier, D.; Estruch, J.J.; Beltrán, J.P. Distribution of photoassimilates in the pea plant: Chronology of events in nonfertilized ovaries and effects of gibberellic acid. Planta 1989, 180, 53–60. [Google Scholar] [CrossRef]
- Baena-González, E.; Sheen, J. Convergent energy and stress signaling. Trends Plant Sci. 2008, 13, 474–482. [Google Scholar] [CrossRef]
- Koo, R.C.J. Importance of moisture control in citrus groves. In Citrus World; University of Florida: Gainesville, FL, USA, 1967; pp. 13–16. [Google Scholar]
- Moss, G.I. The influence of temperature during flower development on the subsequent fruit-set of sweet orange (Citrus sinensis) ‘Washington Navel’. Hortic. Res. 1973, 13, 65–73. [Google Scholar]
- Syvertsen, J.P.; Goñi, C.; Otero, A. Fruit load and canopy shading affect leaf characteristics and net gas exchange of ‘Spring’ navel orange trees. Tree Physiol. 2003, 23, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.P.; Zhou, H.F.; Zhang, L.C. Photosynthetic characteristics and protective mechanisms against photooxidation during high temperature stress in two citrus species. Sci. Hortic. 2006, 108, 260–267. [Google Scholar] [CrossRef]
- Borges, A.; Da Cunha Barros, M.; Pardo, E.; García, M.; Franco, J.; Gravina, A. Cuajado de frutos en tangor ‘Ortanique’en respuesta a la polinización ya distintas situaciones de estrés ambiental. Agrociencia 2009, 13, 7–18. [Google Scholar] [CrossRef]
- Agustí, J.; Zapater, M.; Iglesias, D.J.; Cercós, M.; Tadeo, F.R.; Talón, M. Differential expression of putative 9-cis-epoxycarotenoid dioxygenases and abscisic acid accumulation in water stressed vegetative and reproductive tissues of citrus. Plant Sci. 2007, 172, 85–94. [Google Scholar] [CrossRef]
- Gómez-Cadenas, A.; Tadeo, F.R.; Talón, M.; Primo-Millo, E. Leaf abscission induced by ethylene in water stressed intact seedlings of Cleopatra mandarin requires previous abscisic acid accumulation in roots. Plant Physiol. 1996, 112, 401–408. [Google Scholar] [CrossRef]
- Tudela, D.; Primo-Millo, E. 1-Aminocyclopropane-1-carbox-ylic acid transported from roots to shoots promotes leaf abscission in Cleopatra Mandarin (Citrus reshni Hort. ex Tan.) seedlings rehydrated after water stress. Plant Physiol. 1992, 100, 131–137. [Google Scholar] [CrossRef]
- Bowman, K.D.; Joubert, J. Citrus rootstocks. In The Genus Citrus; Talón, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 105–127. [Google Scholar]
- Haque, M.A.; Sakimin, S.Z. Planting Arrangement and Effects of Planting Density on Tropical Fruit Crops. A Review. Horticulturae 2022, 8, 485. [Google Scholar] [CrossRef]
- Embleton, T.W.; Jones, W.W.; Labanauskas, C.K.; Reuther, W. Leaf analysis as a diagnostic tool and guide to fertilization. In The Citrus Industry; Reuther, W., Ed.; University of California, Division of Agricultural Sciences: Berkeley, CA, USA, 1973; Volume III, pp. 183–210. [Google Scholar]
- Lovatt, C.J.; Sagee, O.; Avi, A.G. Ammonia and/or its metabolites influence flowering, fruit set, and yield of the ‘Washington’ navel orange. Proc. Int. Soc. Citric. 1992, 1, 412–416. [Google Scholar]
- Martínez-Alcántara, B.; Quiñones, A.; Primo-Millo, E.; Legaz, F. Nitrogen remobilization response to current supply in young citrus trees. Plant Soil 2011, 342, 433–443. [Google Scholar] [CrossRef]
- Mooney, P.A.; Richardson, A.C. Seasonal trends in the uptake and distribution of nitrogen in satsuma mandarins. Proc. Int. Soc. Citric. 1992, 2, 593–597. [Google Scholar]
- Legaz, F.; Primo-Millo, E.; Primo-Yúfera, E.; Gil, C. Dinamics of 15N-labelled nitrogen nutrients in ‘Valencia’ orange trees. Proc. Int. Soc. Citric. 1981, 2, 575–582. [Google Scholar]
- Legaz, F.; Primo-Millo, E.; Primo-Yúfera, E.; Gil, C.; Rubio, J.L. Nitrogen fertilization in citrus—I. Absorption and distribution of nitrogen in calamondin trees (Citrus mitis Bl.), during flowering, fruit set and initial fruit development periods. Plant Soil 1982, 66, 339–351. [Google Scholar] [CrossRef]
- Agustí, M.; Primo-Millo, E. Flowering and fruit set. In The Genus Citrus; Talón, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 219–244. [Google Scholar]
- Huang, W.-T.; Xie, Y.-Z.; Chen, X.-F.; Zhang, J.; Chen, H.-H.; Ye, X.; Guo, J.; Yang, L.-T.; Chen, L.-S. Growth, mineral nutrients, photosynthesis and related physiological parameters of Citrus in response to nitrogen deficiency. Agonomy 2021, 11, 1859. [Google Scholar] [CrossRef]
- Akao, S.; Kubota, S.; Hayashida, M. Utilization of reserve nitrogen, especially autumn nitrogen, by Satsuma trees development of spring shoots. J. Jpn. Soc. Hortic. Sci. 1978, 47, 31–38. [Google Scholar] [CrossRef]
- Sanz, A.; Monerri, C.; González-Ferrer, J.; Guardiola, J.L. Changes in carbohydrates and mineral elements in Citrus leaves during flowering and fruit set. Physiol. Plant. 1987, 69, 93–98. [Google Scholar] [CrossRef]
- Agustí, M.; García-Marí, F.; Guardiola, J.L. Gibberellic acid and fruit set in sweet orange. Sci. Hortic. 1982, 17, 257–264. [Google Scholar] [CrossRef]
- Ogaki, C.; Fujita, K.; Ito, H. Investigations on the cause and control of alternate bearing of Unshu orange trees. VI. Seasonal change of nitrogen, phosphorus and potassium absorption in alternate and annual bearing trees. J. Jpn. Soc. Hortic. Sci. 1966, 35, 8–18. [Google Scholar] [CrossRef]
- García-Luis, A.; Almela, V.; Monerri, C.; Agustí, M.; Guardiola, J.L. Inhibition of flowering in vivo by existing fruits and applied growth regulators in Citrus unshiu. Physiol. Plant. 1986, 66, 515–520. [Google Scholar] [CrossRef]
- Monselise, S.P.; Halevy, A.H. Chemical inhibition and promotion of citrus flower bud induction. J. Proc. Am. Soc. Hortic. Sci. 1964, 84, 141–146. [Google Scholar]
- Guardiola, J.L.; Monerri, C.; Agustí, M. The inhibitory effect of gibberellic acid on flowering in Citrus. Physiol. Plant. 1982, 55, 136–142. [Google Scholar] [CrossRef]
- El-Otmani, M.; Coggins, C.V.; Agustí, M.; Lovatt, C.J. Plant growth regulators in Citriculture: World current uses. Crit. Rev. Plant Sci. 2000, 19, 395–477. [Google Scholar] [CrossRef]
- Agustí, M.; Reig, C.; Martínez-Fuentes, A.; Mesejo, C. Advances in Citrus flowering: A review. Front. Plant Sci. 2022, 13, 868831. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Fambuena, N.; Mesejo, C.; González-Mas, M.C.; Iglesias, D.J.; Primo-Millo, E.; Agustí, M. Gibberellic acid reduces flowering intensity in sweet orange [Citrus sinensis (L.) Osbeck] by repressing CiFT gene expression. J. Plant Growth Regul. 2012, 31, 529–536. [Google Scholar] [CrossRef]
- Goldberg-Moeller, R.; Shalom, L.; Shlizerman, L.; Samuels, S.; Zur, N.; Ophir, R.; Blumwald, E.; Sadka, A. Effects of gibberellin treatment during flowering induction period on global gene expression and the transcription of flowering control genes in citrus buds. Plant Sci. 2013, 198, 46–57. [Google Scholar] [CrossRef]
- Tang, L.; Lovatt, C.J. Effects of low temperature and gibberellic acid on floral gene expression and floral determinacy in ‘Washington’navel orange (Citrus sinensis L. Osbeck). Sci. Hortic. 2019, 243, 92–100. [Google Scholar] [CrossRef]
- Moss, G.I. Promoting fruit-set and yield in sweet orange using plant growth substances. Aust. J. Exp. Agric. Anim. Husb. 1972, 12, 96–102. [Google Scholar] [CrossRef]
- Soost, R.K.; Burnett, R.H. Effects of gibberellin on yield and fruit characteristicsof Clementine mandarin. Proc. Am. Soc. Hortic. Sci. 1961, 77, 194–-201. [Google Scholar]
- García-Martinez, J.L.; García-Papí, M.A. The influence of gibberellic acid, 2, 4-dichlorophenoxyacetic acid and 6-benzylaminopurine on fruit-set of Clementine mandarin. Sci. Hortic. 1979, 10, 285–293. [Google Scholar] [CrossRef]
- Krezdorn, A.H. The use of growth regulators to improve fruit set in citrus. Proc. First Int. Citrus Symp. 1969, 3, 1113–1119. [Google Scholar]
- Feinstein, B.; Monselise, S.P.; Goren, R. Studies on the reduction of seeds number in mandarins. HortScience 1975, 10, 385–386. [Google Scholar] [CrossRef]
- Hield, H.Z.; Coggins, C.W., Jr.; Garber, M.J. Gibberellin tested on Citrus: Fruit set on Bearss lime, Eureka lemon, and Washington navel orange increased by treatments in preliminary investigations. Calif. Agric. 1958, 12, 9–12. [Google Scholar]
- Krezdorn, A.H.; Cohen, M. The influence of chemical fruit set sprays on yield and quality of fruits. Proc. Fla. State Hortic. Soc. 1962, 75, 53–60. [Google Scholar]
- Coggins, C.W., Jr.; Hield, H.Z.; Burns, R.M.; Eaks, I.L.; Lewis, L.N. Gibberellin research with Citrus. Calif. Agric. 1966, 20, 12–13. [Google Scholar]
- del Rivero, J.M.; Veyrat, P.; Gomez de Barreda, D. Improving fruit set in Clementine mandarin with chemical treatments in Spain. Proc. 1st. Int. Citrus Cong. 1969, 3, 1121–1124. [Google Scholar]
- Damigella, P.; Tribulato, E.; Continella, G. Prove comparative con ácido gibberellico, incisione anulare e concimazione fogliare su Clementine (Citrus clementina. Hort.). Tecnol. Agric. 1970, 5, 508–525. [Google Scholar]
- Blondel, L. Activit‚s compar‚es des gibberellins et de l’incision annulaire sur la fructification du Clementinier in Corse. I Cong. Mund. Citric. 1977, 2, 375–383. [Google Scholar]
- Brosh, P.; Monselise, S.P. Increasing yields of ‘Topaz’ mandarin by gibberellin and girdling in the presence of ‘Minneola’ pollinizers. Sci. Hortic. 1977, 7, 369–372. [Google Scholar] [CrossRef]
- Hield, H.Z.; Coggins, C.W., Jr.; Garber, M.J. Effect of gibberellin sprays on fruit set of Washington navel trees. Hilgardia 1965, 36, 297–311. [Google Scholar] [CrossRef]
- Greenberg, J.; Goldschmidt, E.E. The effectiveness of GA3 application to citrus fruit. In Proceedings of the Citriculture, Sixth International Citrus Congress, Middle-East, Tel Aviv, Israel, 6–11 March 1988; Volume 1, pp. 339–342. [Google Scholar]
- Henning, G.L.; Coggins, C.W., Jr. Bioassay used to determine the impact of surfactants on the biological effectiveness of exogenous gibberellic acid. In Proceedings of the Citriculture, Sixth International Citrus Congress, Middle-East, Tel Aviv, Israel, 6–11 March 1988; Volume 1, pp. 325–331. [Google Scholar]
- Talón, M.; Juan, M.; Soler, J.; Agustí, M.; Primo-Millo, E. Criterios de racionalización de las aplicaciones de ácido giberélico para la mejora del cuajado de los frutos cítricos. Lev. Agric. 1999, 347, 128–133. [Google Scholar]
- Ashraf, M.Y.; Yaqub, M.; Akhtar, J.; Athat Khan, M.; Ali Khan, M.; Ebert, G. Control of excessive fruit drop and improvement in yield and juice quality of kinnow (Citrus deliciosa × Citrus nobilis) through nutrient management. Pak. J. Bot. 2012, 44, 259–265. [Google Scholar]
- Zheng, Y.; Kumar, N.; González, P.; Etxeberria, E. Strigolactones restore vegetative and reproductive developments in Huanglongbing (HLB) affected, greenhouse-grown citrus trees by modulating carbohydrate distribution. Sci. Hortic. 2018, 237, 89–95. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.; He, W.; Chen, Q.; Wang, X. Effect of spraying brassinolide on fruit quality of Citrus grandis cv. ‘Huangjinmiyou’ and ‘Hongroumiyou’. IOP Conf. Ser. Earth Environ. Sci. 2019, 358, 022029. [Google Scholar] [CrossRef]
- Hartmond, U.; Yuan, R.; Burns, J.K.; Grant, A.; Kender, W.J. Citrus fruit abscission induced by methyl-jasmonate. J. Am. Soc. Hortic. Sci. 2000, 125, 547–552. [Google Scholar] [CrossRef]
- Goren, R.; Huberman, M.; Goldschmidt, E.E. Girdling: Physiological and horticultural aspects. Hortic. Rev. 2004, 30, 1–36. [Google Scholar] [CrossRef]
- Agustí, M.; Andreu, I.; Juan, M.; Almela, V.; Zacarías, L. Effects of ringing branches on fruit size at maturity of peach and nectarine cultivars. J. Hortic. Sci. Biotechnol. 1998, 73, 537–540. [Google Scholar] [CrossRef]
- Kurtzman, R.H. Xylem sap flow as affected by metabolic inhibitors and girdling. Plant Physiol. 1966, 41, 641–646. [Google Scholar] [CrossRef]
- Mehouachi, J.; Iglesias, D.J.; Agustí, M.; Talón, M. Delay of early fruitlet abscission by branch girdling in citrus coincides with previous increases in carbohydrate and gibberellin concentrations. Plant Growth Regul. 2009, 58, 15–23. [Google Scholar] [CrossRef]
- Krezdorn, A.H. Fruit setting of Citrus. Proc. Int. Soc. Citric. 1981, 1, 249–253. [Google Scholar]
- Goldschmidt, E.E.; Golomb, A. Carbohydrate balance of alternate bearing citrus trees and the significance of reserves for flowering and fruiting. J. Am. Soc. Hortic. Sci. 1982, 107, 206–208. [Google Scholar] [CrossRef]
- Carbonell, J.; García-Martínez, J.L. Fruit-set of unpollinated ovaries of Pisum sativum L. Planta 1980, 147, 444–450. [Google Scholar] [CrossRef]
- García-Luis, A.; Fornes, F.; Sanz, A.; Guardiola, J.L. The regulation of flowering and fruit set in citrus: Relationship with carbohydrate levels. Isr. J. Bot. 1988, 37, 189–201. [Google Scholar]
- Mataa, M.; Tominaga, S.; Kozaki, I. The effect of time of girdling on carbohydrate contents and fruiting in Ponkan mandarin (Citrus reticulata Blanco). Sci. Hortic. 1998, 73, 203–211. [Google Scholar] [CrossRef]
- Goldschmidt, E.E. Carbohydrate supply as a critical factor for citrus fruit development and productivity. HortScience 1999, 34, 1020–1024. [Google Scholar] [CrossRef]
- Rivas, F.; Erner, Y.; Alós, E.; Juan, M.; Almela, V.; Agustí, M. Girdling increases carbohydrate availability and fruit-set in citrus cultivars irrespective of parthenocarpic ability. J. Hortic. Sci. Biotechnol. 2006, 81, 289–295. [Google Scholar] [CrossRef]
- Bangerth, F.K. Dominance among fruits/sinks and the search for a correlative signal. Physiol. Plant. 1989, 76, 608–614. [Google Scholar] [CrossRef]
- Bangerth, F.K. Abscission and thinning of young fruit and their regulation by plant hormones and bioregulators. Plant Growth Regul. 2000, 31, 43–59. [Google Scholar] [CrossRef]
- Takahashi, N.; Yamaguchi, I.; Kono, T.; Igoshi, M.; Hirose, K.; Suzuki, K. Characterization of plant growth substances in Citrus unshiu and their change in fruit development. Plant Cell Physiol. 1975, 16, 1101–1111. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin–cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009, 69, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.J.; Mullins, M.G. Auxin, ethylene and kinetin in a carrier-protein model system for the polar transport of auxins in petiole segments of Phaseolus vulgaris. New Phytol. 1969, 68, 977–991. [Google Scholar] [CrossRef]
- Yuan, R.; Kender, W.J.; Burns, J.K. Young fruit and auxin transport inhibitors affect the response of mature ’Valencia’ oranges to abscission materials via changing endogenous plant hormones. J. Am. Soc. Hortic. Sci. 2003, 128, 302–308. [Google Scholar] [CrossRef]
- Gälweiler, L.; Guan, C.; Muller, A.; Wisman, E.; Mendgen, K.; Yephremov, A.; Palme, K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 1998, 282, 2226–2230. [Google Scholar] [CrossRef]
- Aloni, R. The induction of vascular tissues by auxin. In Plant hormones. Biosynthesis, Signal Transduction, Action! Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 485–506. [Google Scholar]
- Agustí, M.; Primo-Millo, E. Fisiología de los Cítricos; Ed. Mundi-Prensa: Madrid, Spain, 2024; 392p. [Google Scholar]
- Wolf, S.; Hématy, K.; Höfte, H. Growth control and cell wall signaling in plant. Annu. Rev. Plant Biol. 2012, 63, 381–407. [Google Scholar] [CrossRef]
- Lloyd, C. Dynamic microtubules and the texture of plant cell walls. Inst. Rev. Cell Mol. Biol. 2011, 287, 287–329. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Expansive growth of plant cell walls. Plant Physiol. Biochem. 2000, 38, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Harpster, M.H.; Dunsmuir, P. Differential expression of expansin gene family members during growth and ripening of tomato fruit. Plant Mol. Biol. 1999, 39, 161–169. [Google Scholar] [CrossRef]
- Maurel, C. Aquaporins and water permeability of plant membranes. Annu. Rev. Plant Physiol. 1997, 48, 399–429. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.; Maurel, C. The role of aquaporins in root water uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef]
- Tyerman, S.D.; Niemietz, C.M.; Bramley, H. Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ. 2002, 125, 173–194. [Google Scholar] [CrossRef]
- Yu, K.; Xu, Q.; Da, X.; Guo, F.; Ding, Y.; Deng, X. Transcriptome changes during fruit development and ripening of sweet orange (Citrus sinensis). BMC Genom. 2012, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues de Souza-Neto, R.; Nogales da Costa, F.; Teper, D.; Barbosa, I.G.; Takita, M.A.; Benedetti, C.E.; Wang, N.; Alves de Souza, A. The expansin gene CsLIEXP1 is a direct target of CsLOB1 in Citrus. Phytopathology 2023, 113, 1266–1277. [Google Scholar] [CrossRef]
- Cercós, M.; Soler, G.; Iglesias, D.J.; Gadea, J.; Forment, J.; Talón, M. Global análisis of gene expression during development and ripening of citrus fruit fleah. A proposed mechanism for citric acid utilization. Plant Mol. Biol. 2006, 62, 513–527. [Google Scholar] [CrossRef]
- Waldron, K.W.; Parker, M.L.; Smith, A.C. Plant cell walls and food quality. Compr. Rev. Food Sci. Food Saf. 2003, 2, 128–146. [Google Scholar] [CrossRef]
- Licciardello, C.; Russo, M.P.; Vale’, G.; Recupero, R.G. Identification of differentially expressed genes in the flesh of blood and common oranges. Tree Genet. Genomes 2008, 4, 315–331. [Google Scholar] [CrossRef]
- Agre, P.; Sasaki, S.; Chrispeels, M.J. Aquaporins: A family of water channel proteins. Am. J. Physiol. 1993, 261, F461. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Eisenbarth, D.A.; Weig, A.R. Dynamics of aquaporins and water relations during hypocotyl elongation in Ricinus communis L. seedlings. J. Exp. Bot. 2005, 56, 1831–1842. [Google Scholar] [CrossRef]
- Lin, W.L.; Peng, Y.H.; Li, G.W.; Arora, R.; Tang, Z.C.; Su, W.A.; Cai, W.M. Isolation and functional characterization of PgTIP1, a hormoneautotrophic cells-specific tonoplast aquaporin in ginseng. J. Exp. Bot. 2007, 58, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ruan, X.; Zhang, J.; Wu, Y.; Wang, X.; Li, X. Cotton plasma membrane intrinsic protein 2s (PIP2s) selectively interact to regulate their water channel activities and are required for fibre development. New Phytol. 2013, 199, 695–707. [Google Scholar] [CrossRef]
- Ludevid, D.; Höfte, H.; Himelblau, E.; Chrispeels, M.J. The expression pattern of the tonoplast intrinsic protein c-TIP in Arabidopsis thaliana is correlated with cell enlargement. Plant Physiol. 1992, 100, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gamir, J.; Ancillo, G.; González-Mas, M.C.; Primo-Millo, E.; Iglesias, D.J.; Forner, M.A. Root signalling and modulation of stomatal clousure in flooded citrus seedlings. Plant Physiol. Biochem. 2011, 49, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, H.; Zhu, F.; Cheng, Y. Transcript profiles analysis of citrus aquaporins in response to fruit water loss during storga. Plant Biol. 2021, 23, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Alexandersson, E.; Fraysse, L.; Sjövall-Larsen, S.; Gustavsson, S.; Fellert, M.; Karlsson, M.; Johanson, U.; Kjellbom, P. Whole gene family expression and drought stress regulation of aquaporins. Plant Mol. Biol. 2005, 59, 469–484. [Google Scholar] [CrossRef]
- Gariglio, N.; Reig, C.; Agustí, M. Unraveling water relations in growing fruit: Insights from the epidermal growth regulation hypothesis. Front. Plant Sci. 2024, 15, 1495916. [Google Scholar] [CrossRef]
- Ozga, J.A.; Reinecke, D.M. Hormonal interactions in fruit development. J. Plant Growth Regul. 2003, 22, 73–81. [Google Scholar] [CrossRef]
- Koch, K.E.; Avigne, W.T. Postphloem, nonvascular transfer in citrus. Kinetics, metabolism, and sugar gradients. Plant Physiol. 1990, 93, 1405–1416. [Google Scholar] [CrossRef]
- Bennett, A.B.; Damon, S.; Osteryoung, K.; Hewitt, J. Mechanisms of retrieval and metabolism following phloem unloading. In Plant Biology. Phloem Transport; Cronshaw, J., Lucas, W.T., Giaquinta, R.T., Eds.; Alan Liss Inc.: New York, NY, USA, 1986; Volume 1, pp. 307–316. [Google Scholar]
- Offler, C.E.; Patrick, J.W. Cellular pathway and hormonal control of short distance transfer in sink regions. In Plant Biology. Phloem Transport; Cronshaw, J., Lucas, W.T., Giaquinta, R.T., Eds.; Alan Liss Inc.: New York, NY, USA, 1986; Volume 1, pp. 295–306. [Google Scholar]
- Wolswinkel, P. Phloem unloading and turgor-sensitive transport: Factors involved in sink control of assimilate partitioning. Physiol. Plant. 1985, 65, 331–339. [Google Scholar] [CrossRef]
- Lowell, C.A.; Tomlinson, P.T.; Koch, K.E. Sucrose-metabolizing enzymes in transport tissues and adjacent sink structures in developing citrus fruit. Plant Physiol. 1989, 90, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Kallarackal, J.; Milburn, J.A. Specific mass transfer and sink-controlled phloem translocation in castor bean. Aust. J. Plant Physiol. 1984, 11, 483–490. [Google Scholar] [CrossRef]
- Kaufmann, M.R. Water potential components in growing citrus fruits. Plant Physiol. 1970, 46, 145–149. [Google Scholar] [CrossRef]
- Mantell, A.; Goldschmidt, E.E.; Monselise, S.P. Turnover of tritiated water in calamondin plants. J. Am. Soc. Hortic. Sci. 1980, 105, 741–744. [Google Scholar] [CrossRef]
- Ho, L.C. Metabolsim and compartmentation of imported sugars in sink organs in relation to sink strength. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 355–378. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Lin, X.; Fang, H.; Shi, Y.; Grierson, D.; Chen, K. Transcription factor CitERF16 is involved in citrus fruit sucrose accumulation by activating CitSWEET11d. Front. Plant Sci. 2021, 12, 809619. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Shi, Y.; Liu, S.; Jin, R.; Sun, J.; Grierson, D.; Li, S.; Chen, K. The transcription factor CitZAT5 modifies sugar accumulation and hexose proportion in citrus fruit. Plant Physiol. 2023, 192, 1858–1876. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Wang, N. Highly efficient generation of canker-resistant sweet orange enabled by an improved CRISPR/Cas9 system. Front. Plant Sci. 2022, 12, 769907. [Google Scholar] [CrossRef]
- Salonia, F.; Ciacciulli, A.; Pappalardo, H.D.; Poles, L.; Pindo, M.; Larger, S.; Caruso, P.; Caruso, M.; Licciardello, C. A dual sgRNA-directed CRISPR/Cas9 construct for editing the fruit-specific β-cyclase 2 gene in pigmented citrus fruits. Front. Plant Sci. 2022, 13, 975917. [Google Scholar] [CrossRef]
- Koch, K.E. Carbohydrate-modulated gene expression in plants. Ann. Rev. Plant Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef]
- Erner, Y.; Shomer, I. Morphology and Anatomy of Stems and Pedicels of Spring Flush Shoots Associated with Citrus Fruit Set. Ann. Bot. 1996, 78, 537–545. [Google Scholar] [CrossRef]
- Bustan, A.; Erner, Y.; Goldschmidt, E.E. Interactions between developing Citrus fruits and their supportive vascular system. Ann. Bot. 1995, 76, 657–666. [Google Scholar] [CrossRef]
- Stewart, W.S.; Hield, H.Z.; Brannaman, B. Effects of 2,4-D and related substances on fruit-drop, yield, size, and quality of Valencia oranges. Hilgardia 1952, 21, 301–329. [Google Scholar] [CrossRef]
- El-Otmani, M.; Agustí, M.; Aznar, M.; Almela, V. Improving the size of ‘Fortune’ mandarin fruits by the auxin 2,4-DP. Sci. Hortic. 1993, 55, 283–290. [Google Scholar] [CrossRef]
- Mesejo, C.; Martínez-Fuentes, A.; Juan, M.; Almela, V.; Agustí, M. Vascular tissues development of citrus fruit peduncle is promoted by synthetic auxins. Plant Growth Regul. 2003, 39, 131–135. [Google Scholar] [CrossRef]
- Nitsch, J.P. Hormonal factors in growth and development. In The Biochemistry of Fruits and Their Products; Hulme, A.C., Ed.; Academic Press: London, UK, 1970; pp. 427–472. [Google Scholar]
- Rayle, D.L.; Cleland, R.E. The acid theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef]
- Nitsch, J.P. Perennation through seeds and other structures: Fruit development. In Plant Physiology, a Treatise; Steward, F.C., Ed.; Academic Press: London, UK, 1971; Volume 6A, pp. 413–501. [Google Scholar]
- Eissenstat, D.M.; Duncan, L.W. Root growth and carbohydrate responses in bearing citrus trees following partial canopy removal. Tree Physiol. 1992, 1, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Fishler, M.; Goldschmidt, E.E.; Monselise, S.P. Leaf area and fruit size on girdled grapefruit branches. J. Am. Soc. Hortic. Sci. 1983, 108, 218–221. [Google Scholar] [CrossRef]
- Cohen, A. Citrus fruit enlargement by means of summer girdling. J. Hortic. Sci. 1984, 59, 119–125. [Google Scholar] [CrossRef]
- Cohen, A. Effect of girdling date on fruit size of Marsh Seedless grapefruit. J. Hortic. Sci. 1984, 59, 567–573. [Google Scholar] [CrossRef]
- Mebelo, M.; Tominaga, S. Effects of time and degree of shading on fruit set, fruit development, leaf carbohydrates and photosynthesis in Ponkan (Citrus reticulata Blanco). Trop. Agric. 1998, 42, 103–110. [Google Scholar]
- Gimeno, V.; Simón, I.; Martínez, V.; Lidón, V.; Shahid, M.A.; Garcia-Sanchez, F. Effect of shade screen on production, fruit quality and growth parameters of ‘Fino 49’ lemon trees grafted on Citrus macrophylla and sour orange. Acta Hortic. 2015, 1065, 1845–1852. [Google Scholar] [CrossRef]
- Guardiola, J.L. Factors limiting productivity in Citrus. A physiological approach. Proc. Int. Citrus Congr. 1988, 1, 381–394. [Google Scholar]
- Guardiola, J.L.; García-Luis, A. Increasing fruit size in Citrus. Thinning and stimulation of fruit growth. Plant Growth Regul. 2000, 31, 121–132. [Google Scholar] [CrossRef]
- Moss, G.I.; Bevington, K.B.; Gallash, P.T.; El-Zeftawi, B.M. Alternate cropping of Valencia oranges. Sci. Bull. 1981, 88, 1–27. [Google Scholar]
- Agustí, M. Preharvest factors affecting postharvest quality of citrus fruit. In Advances in Postharvest Diseases and Disorders Control of Citrus Fruit; Schirra, M., Ed.; Research Signpost: Trivandrum, India, 1999; pp. 1–34. [Google Scholar]
- Kurihara, A. Fruit growth of satsuma orange under controlled conditions. I. Effects of preharvest temperature on fruit growth, color development, and fruit quality in satsuma orange. Bull. Hortic. Res. Sta. Jpn. 1969, Serie A 8, 15–30. [Google Scholar]
- Kurihara, A. Fruit growth of satsuma orange under controlled conditions. II. Effects of night temperature in the fall on fruit growth, color development, and fruit quality in satsuma orange. Bull. Hortic. Res. Sta. Jpn. 1971, Serie A 10, 29–37. [Google Scholar]
- Yamanishi, O.K. Effect of spring day/night temperatures on flower development, fruit set and fruit quality on strangulated pummelo trees. J. Jpn. Soc. Hortic. Sci. 1994, 63, 493–504. [Google Scholar] [CrossRef]
- Hilgeman, R.H.; Tucker, H.; Hales, T.A. The effect of temperature, precipitation, blossom date and yield upon the enlargement of Valencia oranges. Proc. Am. Soc. Hortic. Sci. 1959, 74, 266–279. [Google Scholar]
- Nii, N.; Harada, K.; Kadowaki, K. Effects of temperature on the fruit growth and quality of satsuma orange. J. Jpn. Soc. Hortic. Sci. 1970, 39, 309–317. [Google Scholar] [CrossRef]
- Kriedemann, P.E.; Barrs, H.D. Citrus orchards. In Water Deficits and Plant Growth; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA, 1981; pp. 325–417. [Google Scholar]
- Yamanishi, O.K. Trunk strangulation and winter heating effects on carbohydrate level and its relation with flowering, fruiting and yield of ‘Tosa Buntan’ pummelo growth in a plastic house. J. Hortic. Sci 1995, 70, 85–95. [Google Scholar] [CrossRef]
- Fucik, J.E.; Norwine, J. Climatologically parameters and grapefruit size relationships in Rio Grande Valley of Texas. J. Rio Grande Valley Hortic. Soc. 1979, 33, 83–90. [Google Scholar]
- Blanke, M.M.; Bower, J.P. Small fruit problem in citrus trees. Trees 1991, 5, 239–243. [Google Scholar] [CrossRef]
- Hales, T.A.; Mobayen, R.G.; Rodney, D.R. Effects of climatic factors on daily Valencia fruits volume increase. Proc. Am. Soc. Hortic. Sci. 1968, 92, 185–190. [Google Scholar]
- Reuther, W. Climate and citrus behavior. In The Citrus Industry; Reuther, W., Ed.; University of California, Division of Agricultural Sciences: Berkeley, CA, USA, 1973; Volume III, pp. 280–337. [Google Scholar]
- Mesejo, C.; Martínez-Fuentes, A.; Reig, C.; El-Otmani, M.; Agustí, M. Examining the impact of dry climates temperature on citrus fruit internal ripening. Sci. Hortic. 2024, 337, 113501. [Google Scholar] [CrossRef]
- Davies, S.F.; Albrigo, L.G. Citrus; CAB International Publisher: Wallingford, UK, 1994; 254p. [Google Scholar]
- Erickson, L.C.; Richards, S.J. Influence of 2, 4-D and soil moisture on size and quality of Valencia oranges. Proc. Am. Soc. Hortic. Sci. 1955, 65, 109–112. [Google Scholar]
- Cohen, A.; Goell, A. Fruit growth and dry matter accumulation in grapefruit during periods of water withholding and after reirrigation. Aust. J. Plant Physiol. 1988, 15, 633–639. [Google Scholar] [CrossRef]
- Waynick, D.D. Factors concerned in the growth of Valencia orange. Calif. Citrogr. 1928, 13, 200. [Google Scholar]
- Furr, J.R.; Taylor, C.A. Growth of lemon fruits in relation to moisture content of the soil. US Dept. Agric. Tech. Bull. 1939, 640, 71. [Google Scholar]
- Hilgeman, R.H.; Von Horn, C.W. Citrus growing in Arizona. Ariz. Agric. Expt. Sta. Bul 1954, 258, 35. [Google Scholar]
- Raza, A.; Zaka, M.A.; Khurshid, T.; Nawaz, M.A.; Ahmed, W.; Afzal, M.B. Different irrigation systems affect the yield and water use efficiency of Kinnow mandarin (Citrus reticulata Blanco). J. Anim. Plant Sci. 2020, 30, 1178–1186. [Google Scholar] [CrossRef]
- Carr, M.K.V. The water relations and irrigation requirements of citrus (Citrus spp.): A review. Expl. Agric. 2012, 48, 347–377. [Google Scholar] [CrossRef]
- Hutton, R.J.; Landsberg, J.J.; Sutton, B.G. Timing irrigation to suit citrus phenology: A means to reduce water use without compromising fruit yield and quality? Aust. J. Exp. Agric. 2007, 47, 71–80. [Google Scholar] [CrossRef]
- Raza, A.; Warraich, I.A.; Nawaz, M.A.; Asim, M.; Aziz, A.; Shireen, F.; Rehman, M.A. Does furrow irrigation system improve yield and weater use efficiency of Kinnow mandarin (Citrus reticulata Blanco)? Int. J. Agric. Ext. 2020, 8, 199–206. [Google Scholar] [CrossRef]
- Ballester, C.; Castel, J.; Intrigliolo, D.S.; Castel, J.R. Response of Clementina de Nules citrus trees to summer deficit irrigation. Yield components and fruit composition. Agric. Water Manag. 2011, 98, 1027–1032. [Google Scholar] [CrossRef]
- Ginestar, C.; Castel, J.R. Response of young ‘Clementine’ citrus trees to water stress during different phenological periods. J. Hortic. Sci. 1996, 71, 551–559. [Google Scholar] [CrossRef]
- Hutton, R.J.; Loveys, B.R. A partial root zone drying irrigation strategy for citrus—Effects on water use efficiency and fruit characteristics. Agric. Water Manag. 2011, 98, 1485–1496. [Google Scholar] [CrossRef]
- Chapman, H.D. The mineral nutrition of citrus. In The Citrus Industry; Reuther, W., Batchelor, L.D., Webber, H.J., Eds.; University of California, Division of Agricultural Sciences: Berkeley, CA, USA, 1968; Volume II, pp. 127–289. [Google Scholar]
- Wutscher, H.K. Rootstock effects on fruit quality. In Factors Affecting Fruit Quality; Ferguson, J.J., Wardowski, W.F., Eds.; University Florida Citrus Short Course Proceedings: Lake Alfred, FL, USA, 1988; pp. 24–34. [Google Scholar]
- Jones, W.W.; Embleton, T.W. Yield and fruit quality of ‘Washington’ navel orange trees as related to leaf nitrogen and nitrogen fertilization. Proc. Am. Soc. Hortic. Sci. 1967, 91, 138–142. [Google Scholar]
- Smith, P.F. Effects of nitrogen rates and timing of application on Marsh grapefruit in Florida. Proc. 1st Int. Citrus Symp. 1969, 3, 1559–1567. [Google Scholar]
- Yuda, E. Nutritional problems in citrus culture in Japan. Proc. Int. Soc. Citric. 1977, 1, 5–9. [Google Scholar]
- Guardiola, J.L.; Agustí, M. El diagnóstico foliar en los agrios. Un análisis crítico. Lev. Agríc. 1984, 249/250, 16–25. [Google Scholar]
- Bar-Akiva, A.; Hiller, V.; Pratt, J. Effect of phosphorus, and chicken manure on yield, fruit quality and leaf composition of grapefruit trees. Proc. Am. Soc. Hortic. Sci. 1968, 93, 145–152. [Google Scholar]
- Parker, E.R.; Jones, W.W. Oeange fruit sizes in relation to potassium fertilization in a long-term experiment in California. Proc. Am. Soc. Hortic. Sci. 1950, 55, 101–113. [Google Scholar]
- Smith, P.F.; Rasmussen, G.K. Relationship of fruit size, yield and quality of Marsh grapefruit to potash fertilization. Proc. Fla. State Hortic. Soc. 1960, 73, 42–49. [Google Scholar]
- Lynch, S.J.; Goldweber, S.; Rich, C. Some effects of nitrogen, phosphorus and potassium fertilization on the constituents of Persian lime fruits. Proc. Fla. State Hortic. Soc. 1953, 66, 224–227. [Google Scholar]
- Embleton, T.W.; Jones, W.W. Potassium and lemon fruit quality. Calif. Citrog. 1966, 51, 269. [Google Scholar]
- Gilfillan, I.M. Improvement of fruit size in SRA 89 clementines in the Eastern Cape. Citrus J. 1995, 5, 27–28. [Google Scholar]
- Phillips, R.L. Hedging angles for ‘Hamlin’ oranges. Proc. Fla. State Hortic. Soc. 1972, 85, 48–50. [Google Scholar]
- Zaragoza, S.; Alonso, E. Citrus pruning in Spain. Proc. Int. Soc. Citric. 1981, 1, 172–175. [Google Scholar]
- Zaragoza, S.; Trenor, I.; Alonso, E.; Primo-Millo, E.; Agustí, M. Treatments to increase the final fruit size on Satsuma ‘Clausellina’. Proc. Int. Soc. Citric. 1992, 2, 725–728. [Google Scholar]
- Yildirim, B.; Yeșiloğlu, T.; Incesu, M.; Kamiloğlu, M.; Özgüven, F.; Tuzcu, Ö.; Kaçar, Y.A. The effects of mechanical pruning on fruit yield and quality in ‘Star Ruby’ grapefruit. J. Food Agric. Environ. 2010, 8, 834–838. [Google Scholar]
- Wheaton, T.A.; Whitney, J.D.; Tucker, D.P.H.; Castle, W.S. Cross hedging, tree removal, and topping affect fruit yield and quality of citrus hedgerows. Proc. Int. Soc. Citric. 1984, 1, 109–114. [Google Scholar]
- Morales, P.; Davies, F.S.; Littell, R.C. Pruning and skirting affect canopy microclimate, yields, and fruit quality of ‘Orlando’ Tangelo. HortScience 2000, 35, 30–35. [Google Scholar] [CrossRef]
- Martín-Gorriz, B.; Martínez-Barba, C.; Torregrosa, A. Lemon trees response to different long-term mechanical and manual pruning practices. Sci. Hortic. 2021, 275, 109700. [Google Scholar] [CrossRef]
- Fonte, A.; Torregrosa, A.; Garcerá, C.; Mateu, G.; Chueca, P. Mechanical pruning of ‘Clemenules’ mandarins in Spain: Yield effects and economic analysis. Agronomy 2022, 12, 761. [Google Scholar] [CrossRef]
- Francis, H.L.; Miller, M.; Boswell, S.; Colladay, C. An economic analysis of three lemon pruning methods. Citrograph 1975, 61, 24–26. [Google Scholar]
- Agustí, M.; Almela, V.; Aznar, M. Rayado y tamaño final del fruto en los agrios. Actas Hortic. 1990, 6, 101–106. [Google Scholar]
- Agustí, M.; Martínez-Fuentes, A.; Mesejo, C. Citrus fruit quality. Physiological basis and techniques of improvement. Agrociencia 2002, Vi-2, 1–16. [Google Scholar]
- Harty, A.R.; Sutton, P.G. Crop regulation of Satsuma mandarin in New Zealand. Proc. Int. Soc. Citric. 1992, 2, 729–734. [Google Scholar]
- Wheaton, T.A. GFruit thinning of Florida mandarins using plant growth regulators. Proc. Int. Soc. Citric. 1981, 1, 263–268. [Google Scholar]
- Hirose, K.; Iwagaki, I.; Suzuki, K. IZAA (5-chloroindazol-8-acetic acid ethyl esther) as a new thinning agent of Satsuma mandarin (C. unshiu Marc.). Proc. Int. Soc. Citric. 1978, 270–273. [Google Scholar]
- Hirose, K. Development of chemical thinners for commercial use for Satsuma mandarin in Japan. Proc. Int. Soc. Citric. 1981, 1, 256–260. [Google Scholar]
- Noma, Y. Effect of ethyl 5-chloro-1H-3-indazolylacetate on fruit thinning of Satsuma mandarin (Citrus unshiu Marcovitch). Proc. Int. Soc. Citric. 1981, 1, 271–275. [Google Scholar]
- Agustí, M.; Almela, V.; Juan, M.; Primo-Millo, E.; Trénor, I.; Zaragoza, S. Effect of 3, 5, 6-trichloro-2-pyridyl-oxyacetic acid on fruit size and yield of ‘Clausellina’mandarin (Citrus unshiu Marc.). J. Hortic. Sci. 1994, 69, 219–223. [Google Scholar] [CrossRef]
- Agustí, M.; El-Otmani, M.; Aznar, M.; Juan, M.; Almela, V. Effect of 3,5,6-trichloro-2-pyridyloxyacetic acid on clementine early fruitlet development and fruit size at maturity. J. Hortic. Sci. 1995, 70, 955–962. [Google Scholar] [CrossRef]
- Agustí, M.; Almela, V.; Aznar, M.; El-Otmani, M.; Pons, J. Satsuma mandarin fruit size increased by 2, 4-DP. HortScience 1994, 29, 279–281. [Google Scholar] [CrossRef]
- Costa, G.; Botton, A.; Vizzotto, G. Fruit thinning: Advances and trends. Hortic. Rev. 2019, 46, 185–226. [Google Scholar] [CrossRef]
- Agustí, M.; Juan, M.; Almela, V. Response of ‘Clausellina’ Satsuma mandarin to 3,5,6-trichloro-2-pirydiloxyacetic acid and fruitlet abscission. Plant Growth Regul. 2007, 53, 129–135. [Google Scholar] [CrossRef]
- Aloni, R. Ecophysiological implications of vascular differentiation and plant evolution. Trees 2015, 29, 1–16. [Google Scholar] [CrossRef]
- Guardiola, J.L.; Agustí, M.; Barberá, J.; Sanz, A. Influencia del ácido giberélico en la maduración y senescencia del fruto de la mandarina Clementina (Citrus reticulata Blanco). Rev. Agroquim. Tecnol. Aliment. 1981, 21, 225–239. [Google Scholar]
- Agustí, M.; Almela, V.; Guardiola, J.L. The regulation of fruit cropping in mandarins through the use of growth regulators. Proc. Int. Soc. Citric. 1981, 1, 216–220. [Google Scholar]
- Guardiola, J.L.; Barrés, M.T.; Albert, C.; García-Luis, A. Effects of exogenous growth regulators on fruit development in Citrus unshiu. Ann. Bot. 1993, 71, 169–176. [Google Scholar] [CrossRef]
- Agustí, M.; Almela, V.; Aznar, M.; Pons, J.; El-Otmani, M. The use of 2,4-DP to improve fruit size in citrus. Proc. Int. Soc. Citric. 1992, 1, 423–427. [Google Scholar]
- Aznar, M.; Almela, V.; Juan, M.; El-Otmani, M.; Agustí, M. Effect of synthetic auxin phenotiol on fruit development of ‘Fortune’ mandarin. J. Hortic. Sci. 1995, 70, 617–621. [Google Scholar] [CrossRef]
- Cleland, R. A dual role of turgor pressure in auxin-induced cell elongation in Avena coleoptiles. Planta 1967, 77, 182–191. [Google Scholar] [CrossRef]
- Reig, C.; Mesejo, C.; Martínez-Fuentes, A.; Agustí, M. Synthetic auxin 3,5,6-TPA increases fruit size of loquat (Eriobotrya japonica Lindl.) by reducing cell turgor pressure. Sci. Hortic. 2016, 210, 213–219. [Google Scholar] [CrossRef]
- Skene, K.G.M. Hormonal effects on sugar release from vine canes. Ann. Bot. 1971, 35, 277–286. [Google Scholar] [CrossRef]
- Erner, Y.; Kaplan, Y.; Artzi, B.; Hamou, M. Increasing citrus fruit size using auxins and potassium. Acta Hortic. 1993, 329, 112–119. [Google Scholar] [CrossRef]
- Monselise, S.P. The use of growth regulators in citriculture; a review. Sci. Hortic. 1979, 11, 151–162. [Google Scholar] [CrossRef]
- Stewart, W.S.; Klotz, L.J.; Hield, H.Z. Effects of 2,4-D and related substances on fruit drop, yield, size and quality of Washington navel oranges. Hilgardia 1951, 21, 161–193. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.