Abstract
Dissolved oxygen (DO) concentration in nutrient solution is critical for maximizing yield and optimizing quality traits of lettuce plants grown in floating systems. This study evaluated the effects of two aeration systems—a Venturi system (V) and a Venturi system combined with a nanobubble generator using electromagnetic waves (VN)—compared with a non-aerated control (C), on quali-quantitative traits of lettuce plants grown in a floating system over two consecutive harvests. Both aeration treatments significantly increased DO levels in the nutrient solution compared to C, with the VN treatment maintaining the highest value throughout the crop cycle. Although no significant differences in lettuce yield were observed, both aeration treatments enhanced the leaf concentration of P, Mn, Zn, and Cu in the second harvest, and Mg in both harvests. Moreover, the VN treatment lowered leaf nitrate concentration in both harvests compared to the other treatments. The increase in DO in the nutrient solution delayed leaf senescence, as evidenced by higher chlorophyll index and lower anthocyanin levels in the lettuce leaves harvested at the end of the trial for both aeration systems. These results suggest that aeration, particularly with nanobubbles, can be an effective and sustainable strategy to enhance the quality traits of lettuce grown in a floating system.
1. Introduction
Compared to traditional agriculture, hydroponic systems offer an effective approach for optimizing resource use efficiency and increasing productivity of vegetable crops. Moreover, the quality of products grown in hydroponics systems is often superior in terms of aesthetic characteristics, taste, and nutritional composition [1,2]. Different crops can be cultivated in hydroponics, although leafy vegetable species are among the most promising. In particular, it has been observed that one of the main advantages of growing lettuce in hydroponics is the shortening of the crop cycle in comparison with conventional cultivation methods [2,3]. Hydroponic systems are particularly suitable for the production of fresh-cut vegetables, as they allow for precise control of crop mineral nutrition and avoid contact of crops with soil, leading to high nutritional value, absence of contaminants, low microbial populations, and extended shelf-life of harvested leaves [4,5]. Management of nutrient solution, such as control of pH, electrical conductivity, temperature, and oxygen, is crucial for making hydroponic production successful [6,7].
Oxygen is essential for root activity, and its deficiency can lead to a reduction in root performance, resulting in a decrease in plant productivity and increased incidence of diseases [8,9]. Oxygen is relatively insoluble in water, and its concentration at saturation decreases as the temperature of the solution increases. Besides an oxygen depletion in nutrient solution under high-temperature conditions, the increase in temperature enhances the respiratory demand for oxygen by the roots [9,10]. Therefore, controlling dissolved oxygen (DO) levels in the nutrient solution is crucial, especially during the exponential growth of the crop and when the temperature exceeds the optimal range for crop growth [11].
Goto et al. [11] studied the effects of four DO concentrations (2.1, 4.8, 8.4, and 16.8 mg L−1) on lettuce grown in a hydroponic system without observing significant differences in fresh and dry weights of shoots and roots. Therefore, the authors suggested that the critical DO threshold for lettuce growth may lie below 2.1 mg L−1. The above findings were confirmed by Alvarado-Camarillo et al. [12], who observed a decrease in fresh weight, volume, and length of roots and fresh weight, dry weight, and length of shoots in lettuce grown in a non-aerated solution when the DO level in the solution dropped from 6.2 mg L−1 to 1.9 mg L−1 in aerated and non-aerated solutions, respectively. The reduction in lettuce biomass under low DO was associated with a decrease in P, K, and Mg concentration in the lettuce leaves. Similarly, Tesi et al. [13] reported a significant reduction in shoot biomass on a fresh and dry weight basis, and in root fresh weight and root length, in lettuce grown in a floating system under an un-aerated solution. They also observed that DO levels in the un-aerated solution reached a value close to zero after 3 weeks of cultivation, in contrast to the aerated treatment, where DO levels remained above 7 mg L−1.
Dissolved oxygen in the solution can also affect quality traits of lettuce, such as nitrate concentration. Tesi et al. [13] reported that nitrate concentration of lettuce leaves grown in a floating system increased from 1420 to 2704 mg/kg fresh weight when aeration was applied, providing a DO concentration above 7 mg L−1. On the contrary, Niñirola et al. [14] reported a decrease in nitrate concentration of lettuce leaves in cv Diveria under aerated conditions, while no significant effect of solution aeration on nitrate concentration of lettuce leaves was found in the cultivar Ganeria. Abu-Shahba et al. [15] recorded a significant increase in other important leaf quality traits like chlorophyll content, total phenols, flavonoids, ascorbic acid, α-tocopherol, and antioxidant activity in iceberg lettuce grown in a hydroponic system with aeration, especially when microbubble technology was used, in comparison with an un-aerated solution. Leaf concentrations of nitrogen, P, K, and Mg also increased under aeration, especially with microbubbles, while Ca concentration decreased. The negative impact of solution aeration on leaf Ca concentration may explain the higher incidence of tip burn recorded in lettuce grown under aerated solution, especially when combined with increasing concentrations of sodium chloride [13].
Furthermore, it has been reported that DO concentration in nutrient solutions can also impact the post-harvest quality of lettuce leaves. For instance, Niñirola et al. [14] reported a significant decrease in total phenols in lettuce leaves of two cultivars grown under aeration in comparison with un-aerated solution after 7 days of cold storage at 5 °C.
Oxygen can be supplied through various methods such as air stones, recirculation, mechanical mixing, oxygen gas injection, or nanobubbles. Air bubble generators have been reported as an effective way to enhance crop performance in hydroponics [16]. However, crop response can vary depending on the bubble size as reported by Park & Kurata [16] in lettuce grown in the deep flow technique, where the application of microbubbles enhanced fresh and dry weights of shoots, root dry weight, and leaf number and size in comparison with macrobubble treatment. Interestingly, the use of micro- or macrobubble generators induced only limited variation in DO concentration in the solutions (maximum 3%), suggesting that the observed differences in biomass accumulation and leaf size of lettuce were probably due to a better oxygen availability from the larger gas exchange surface area provided by microbubbles at the root interface. Similarly, Noh et al. [17] investigated the effects of microbubbles generated by a rotatory microbubble aerator with a pump on lettuce grown in the deep flow technique under saline conditions. They found that microbubble application in the solution increased leaf number, root length, and fresh and dry weights of shoots and roots compared to an un-aerated solution. Recently, several devices for generating nanobubbles have been proposed for solution oxygenation. Nanobubbles application could further enhance oxygen availability for roots due to the great stability, persistence, and large surface area for unit volume of nanobubbles [18,19]. Despite the positive agronomic results obtained in the irrigation of potted leafy vegetables with nanobubble-enriched water [20], limited information is available on the use of nanobubbles in hydroponically grown lettuce. Zhaolei et al. [21] reported that nanobubble application in lettuce grown under the nutrient film technique enhanced root growth, shoot fresh weight, total leaf area, and shelf-life of lettuce, suppressing algae growth in comparison with un-treated control. Also Yusuf et al. [22] reported an increase in the oxygen solution concentrations (from 4 to 6 mg L−1), number of leaves, and fresh weight of shoots in lettuce by applying nanobubbles for 15 min every 3 days in a hydroponic system compared to an un-treated control.
The above findings confirm the importance of nutrient solution aeration for maximizing the quali-quantitative traits of lettuce grown under hydroponics. However, the above studies reported contradictory results regarding the aeration-mediated impact on lettuce quality traits like nitrates and did not consider the long-term effect of solution aeration on lettuce performance in hydroponic systems under multiple harvests.
Starting from the above considerations, a greenhouse trial was conducted to evaluate the effects of two aeration systems—Venturi system (V) and Venturi system combined with a nanobubble generator using electromagnetic waves (VN)—on the yield and quality traits of lettuce growth in a floating system. The primary goal of the research was also to investigate nitrate-related performance of lettuce leaves in an aeration system and its interaction with multiple harvests.
2. Materials and Methods
2.1. Growth Conditions
The trial was conducted in winter 2022/2023 in a 300 m × 300 m polyethylene greenhouse situated at the experimental farm of Tuscia University, central Italy (latitude 42°25′ N, longitude 12°08′, altitude 310 m). During the growing cycle, the average daily mean, minimum, and maximum air temperatures were 21.0, 16.0, and 26.0 °C, respectively. The average daily mean, minimum, and maximum air humidities were 60.3, 20.7, and 100%, respectively. Growing tanks (0.36 m depth) were built using tuff bricks, resulting in a total area of 1.78 m2 each. Inside the tanks, plastic sheets 103-22B (Silostar, LIRSA S.p.A. Ottaviano, Italy) were placed, creating a closed system with polyethylene pipes (32 cm diameter) and three pumps PRPVC400C (Ribimex Italia S.r.l., Carrè, Italy). Lettuce seeds (Lactuca sativa L. var. acephala cv. Green Salad Bowl; SAIS S.p.A., Cesena, Italy) were sown on December 16 in polystyrene trays filled with vermiculite to ensure a final plant density of 618 plant m−2. Polystyrene trays were placed into tanks filled with nutrient solutions. The nutrient solution was prepared by dissolving the following fertilizers in pure water (mg/L): 722 Ca(NO3)2, 136 KH2PO4, 182 K2SO4, 203 KNO3, 384 Mg(NO3)2, and 81 NH4NO3. Micronutrients were added as 24 mg/L of a commercial fertilizer (Mikrom; Cifo S.p.A., Bologna, Italy) containing the following (g/kg): 5 B, 5 Cu, 40 Fe, 40 Mn, 2 Mo, 10 Zn, 18 Mg, and 24 S. During the growing cycle, the average nutrient solution electrical conductivity, pH, and temperature means were 1.70 ± 0.03, 6.0 ± 0.03, and 13.78 ± 0.19 °C, respectively.
2.2. Experimental Design and Treatments
Three treatments were tested as follows: a Venturi system (V), a Venturi system combined with a nanobubble generator using electromagnetic waves (VN), and an un-aerated solution as control (C). A Venturi system uses water flow to create suction of air into the system by constricting it in a cone-shaped tube. In the restriction, the water flow increases its velocity, reducing its pressure and producing a partial vacuum. As the fluid leaves the constriction, its pressure increases back to the ambient or pipe level. The Venturi system used in the current experiment was a Mazzei® Airjection® device MAI-A7 (Toro Ag Irrigation, Fiano Romano, Italy) having an average water flow of 10.8 L min−1. Previous studies reported that majority of the bubbles generated by Venturi injectors had a diameter of 100–300 µm [23].
The nanobubble generator prototype was obtained from Toro Company (Toro Ag Irrigation, Fiano Romano, Italy); its major components include two ceramic tubes for the formation and stabilization of nanobubbles, a generator of electromagnetic waves, and flow regulators, as described by Ahmed et al. [24]. Previous studies on similar devices demonstrated that these electromagnetic nanobubble generators can produce nanobubbles having a size below 100 nm [25]. Oxygenation treatments were applied 5 times a day, each lasting 15 min, at 8:00, 11:00, 14:00, 17:00, and 20:00.
A total of 15 experimental units (3 aeration treatments × 5 replicates) were allocated across the tanks.
The effectiveness of the aeration systems was evaluated during two consecutive crop harvests at the 39th and 60th days after sowing (DASs).
2.3. Nutrient Solutions Measurements
Dissolved oxygen levels in the nutrient solution were measured using the HI-9143 Oximeter (Hannah Instrument Italia S.r.l., Ronchi di Villafranca Padovana, Italy). The measuring probe was placed in the center of each plot, and oxygen readings were taken after stabilization. Measurements were performed on the 0th, 5th, 10th, 13th, 18th, 21st, 25th, 28th, 31st, 33rd, 35th, 41st, 45th, 49th, 52nd, and 54th DASs.
During the trial, the nutrient solution in the tanks was refilled twice: on the 18th and 43rd DAS.
2.4. Non-Destructive Physiological Measures
In both growing cycles, at 39 and 60 DASs, a non-destructive assessment was conducted to measure leaves’ chlorophylls (transmittance ratio T850/T720), flavonols (transmittance ratio F660/F325), and anthocyanin content (transmittance ratio F660/F525) using the Multi-Pigment-Meter (ADC BioScientific Ltd., Hoddesdon, UK). The same instrument automatically calculated the Nitrogen–Flavonol Index (NFI) as the ratio between the chlorophyll and flavonol content.
Additionally, the percent canopy cover of live green vegetation was monitored over time using the app Canopeo (https://canopeo.it.softonic.com/, accessed on 3 January 2024); Oklahoma State University). These measurements were performed on the 7th, 10th, 11th, 13th, 14th, 15th, 18th, 19th, 22nd, 24th, 26th, 28th, 31st, 33rd, 45th, 46th, 48th, 51st, 53rd, and 55th DASs.
2.5. Plant Biomass Determination, Nitrate Concentration, and Mineral Analysis
On the 39th and 60th DASs, lettuce plants were harvested to record the fresh weight of shoots. Shoot dry weight was determined after oven-drying plant tissues at 65 °C until the sample weight remained constant. Dry leaf samples were ground separately in a Wiley mill to pass through a 20-mesh screen.
Nitrate concentration in dry leaves was determined using the 5% salicylic acid method [26]. Specifically, 0.1 g of dry plant tissues was added to 10 mL of distilled water, homogenized, and centrifuged at room temperature. Then, 0.2 mL of the supernatant was incubated with 0.8 mL of salicylic acid for 30 min. Afterward, 19 mL of 1.7 N NaOH was added and incubated for 1 h. The nitrate concentration was determined spectrophotometrically at 410 nm using a Helios Beta Spectrophotometer (Thermo Electron Corporation, Altrincham, UK) and was expressed in mg kg−1 fresh weight (fw).
Leaf mineral assessment was performed on dry leaf samples. For nitrogen analysis, 0.25 g of the sample was used, while 0.5 g samples were allocated for P, K, Ca, Mg, Na, Fe, Mn, Zn, Cu, and B determination. Nitrogen concentration was determined using the Kjeldahl method [27], after mineralization with sulfuric acid, distillation, and titration with 0.1 N HCl. The other elements were analyzed with a two-step elemental analysis process. The samples were placed in tubes along with 65% HNO3 and MilliQ water, and then they were put in a digestor for 40 min (Multiwave GO Plus, Anton Paar GmbH, Rivoli, Torino, Italy). Following digestion, the samples were thinned with 1% HNO3 and examined using an atomic emission spectrometer (4210 MP-AES, Agilent Technologies, Santa Clara, CA, USA) connected with SPS 4 Autosampler (Agilent Technologies, Santa Clara, CA, USA). A standard sample was always prepared as a reference for the elemental analysis [28].
2.6. Chlorophyll and Total Carotenoids
On the 39th and 60th DASs, five plants were harvested and immediately frozen with liquid nitrogen and stored at −80 °C. Subsequently, the sample was ground with liquid nitrogen, and an aliquot of 0.5 g was used for analysis of chlorophyll and total carotenoids.
Chlorophyll a (Chla), chlorophyll b (Chlb), total chlorophyll (a + b), and carotenoid concentration in shoots were determined according to the Lichtenthaler & Wellburn method [29]. Extraction was conducted by adding 10 mL of 80% acetone into a glass tube, homogenization in ice, and centrifugation at 1400× g for 3 min at 4 °C. The absorbance of the supernatants was collected at 470, 663, and 647 nm with the Helios Beta Spectrophotometer (Thermo Electron Corporation, Altrincham, United Kingdom), and the final concentrations were calculated using the equations reported by Lichtenthaler & Wellburn [29].
2.7. Total Phenols, Flavonoids, and Antioxidant Activity
For total phenols and flavonoids, extraction was performed using 80% ethanol (pH 2), while for the ferric reducing antioxidant power assay (FRAP) and 2,2-diphenyl-l-picrylhydrazyl assay (DPPH), extraction was carried out with 80% methanol. Both extractions were conducted with 0.7 g of frozen samples and 15 mL of extracting solution, homogenization, and incubation for 1 h at room temperature. The extract was then centrifuged, and the supernatant was stored at −20 °C.
The total phenols content (TPC) was determined using the Folin–Ciocalteu method [30] with gallic acid (GAE) as a standard. Briefly, 0.2 mL of ethanolic extract was added with 1 mL of Folin–Ciocalteau’s reagent diluted (1:5, v:v) and 0.8 mL of 7.5% sodium carbonate (w/v). The absorbance of the solution was measured after 1 h at 765 nm, and the result was expressed as mM GAE for 100 g of FW.
The flavonoid contents were determined using the colorimetric method [31] with quercetin (QE) as a standard. Briefly, 0.7 mL of ethanolic extract was added with 0.15 mL of 5% (w/v) sodium nitrite, 0.15 mL of 10% (w/v) aluminum trichloride, and 1 mL of 1 M sodium hydroxide. The absorbance of the solution was measured at 510 nm, and the result was expressed as mM QE for 100 g of FW.
The FRAP assay [32] was conducted using 0.03 mL of methanolic extract, 0.09 mL of distilled water, and 0.9 mL of FRAP working solution, consisting of acetate buffer 300 mM (pH 3.6), 2,4,6-tris(2-pyridyl)-s-triazine 10 mM, and FeCl3 ∗ 6 H2O 20 Mm (10:1:1; v/v/v). After incubation at 37 °C for 30 min in the dark, the absorbance was read at 593 nm and, through curve calibration with Trolox, the results were expressed as mM Trolox for 100 g−1 of FW.
The DPPH assay was conducted using the Brand-Williams et al. method [33]. A 0.1 mL of sample was incubated with 0.1 mL of distilled water and 0.8 mL of DPPH 75 μM working solution. After 30 min in the dark, the absorbance was read at 517 nm and the results were expressed as mM Trolox for 100 g−1 of fw.
For all these determinations, the Helios Beta Spectrophotometer (Thermo Electron Corporation, Altrincham, UK) was used.
2.8. Statistical Analysis
All data were subjected to two-way analysis of variance (ANOVA) to understand if there was any interaction between the two factors (Aeration system and Harvest) on the dependent variables. Before analysis, the experimental data set was checked for normal distribution and homogeneity of variance using Levene’s test. Tukey’s test was carried out at p = 0.05 on each of the significant variables measured. The RStudio software version 4.4.2 (RStudio Team, Vienna, Austria) was used for statistical analysis.
3. Results
3.1. Dissolved Oxygen in Nutrient Solutions
In Figure 1, the dissolved oxygen (DO) levels in the nutrient solutions during the trial are reported. The treatments significantly affected DO from the 5th to the 54th day after sowing (DAS), with the highest values in the nanobubble generator treatment (VN), followed by the Venturi system (V) and un-aerated (C) treatment. DO gradually decreased from the 5th to the 18th DAS in all the treatments, and when the nutrient solutions were refilled (18th DAS) in all the tanks, the DO slightly increased till the 21st DAS. From the 21st to the 35th DAS, DO declined in all treatments, with a more pronounced decrease in the C treatment (from 8.38 to 2.35 mg L−1). Two days after the first harvest (41st DAS), DO levels were 2.32, 9.99, and 10.34 mg L−1 in the C, V, and VN treatments, respectively. From the 41st to the 45th DAS, an increase in DO was recorded in the C treatment (from 2.32 to 4.65 mg L−1) due to the refilling of nutrient solution, whereas the DO in the aerated treatment did not show significant variation (from 9.99 to 9.84 mg L−1 for V; from 10.34 to 10.06 mg L−1 for VN). From the 45th to the 49th DAS, DO markedly declined in all the treatments, and then remained stable until the end of measurements. At the end of the trial, DO levels were 2.98, 7.76, and 7.91 mg L−1 in C, V, and VN, respectively.
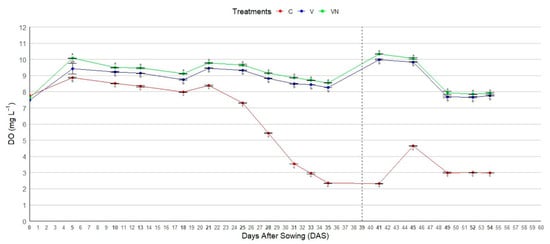
Figure 1.
Effect of aeration treatments [Venturi system (V), Venturi system combined with a nanobubble generator using electromagnetic waves (VN), and un-aerated solution as control (C)] on dissolved oxygen (DO) in the nutrient solutions during the growing cycle of lettuce. Dashed black line (39th DAS) corresponds to the first harvest. Bold numbers in the x-axis correspond to the measurement days. Means and standard errors of DO for each treatment are shown. Different letters within each day after sowing indicate significant differences according to Tukey’s test for p = 0.05. Vertical bars represent the standard errors of the means.
3.2. Canopy Cover and Spectral Indices of Leaves
During the growing cycle, canopy cover over time was similar among the treatments and reached 96.6% as average of all the treatments at the first harvest. At the second harvest, the maximum canopy cover was 89.7% as average of all the treatments.
Spectral leaf measurements obtained with the Multi-Pigment-Meter are shown in Table 1. The interaction between the harvest time and aeration system significantly affected the chlorophyll content, anthocyanins, and NFI. The leaf chlorophyll index and NFI were highest at the first harvest for all the treatments, while the lowest values were recorded in the un-aerated treatment at the second harvest; the II × V and II × VN treatments showed intermediate values.

Table 1.
Effect of aeration system and harvest time on multi-pigment readings (chlorophylls, flavonols, anthocyanins, and Nitrogen–Flavonol Index or NFI) of leaves in lettuce plants grown in floating system.
Similarly, the anthocyanin index was highest in the first harvest across all the treatments, while the lowest value was observed in both aeration systems; intermediate values were recorded in the un-aerated treatment at the second harvest. The flavonol index of the leaves was significantly affected only by harvest time, with the highest values in the second harvest.
3.3. Lettuce Yield
Harvest time had a significant effect on fresh and dry biomass of shoots, whereas no significant differences were observed for shoot dry matter (Table 2). Compared to the first harvest, the second harvest resulted in higher fresh and dry biomass of shoots (+42% and +36%, respectively).

Table 2.
Effect of aeration system and harvest time on fresh and dry biomass, and dry matter of shoots in lettuce plants grown in floating system.
No significant effects of aeration system and harvest time × aeration system interactions were recorded for fresh and dry biomass, and dry matter of shoots.
3.4. Photosynthetic Pigments of Leaves
Photosynthetic pigments of the lettuce leaves are reported in Table 3. Harvest factor had a significant impact on chlorophyll a, chlorophyll b, and total chlorophyll (a + b), with the highest values recorded at the second harvest. Neither the aeration system nor the interaction between the harvest time and aeration system affected the chlorophyll a, chlorophyll b, or total chlorophyll content.

Table 3.
Effect of aeration system and harvest time on chlorophyll a, chlorophyll b, total chlorophyll (a + b), and carotenoid concentration of leaves in lettuce plants grown in floating system.
No significant differences were recorded in carotenoid concentration in the lettuce leaves among treatments or their interactions.
3.5. Antioxidants and Nitrates in Leaves
Antioxidant activity, total phenols, flavonoids, and nitrates in the leaves are reported in Table 4.

Table 4.
Effect of aeration system and harvest time on ferric reducing antioxidant power assay (FRAP), 2,2-diphenyl--picrylhydrazyl assay (DPPH), total phenols, flavonoids, and nitrate concentration of leaves in lettuce plants grown in floating system.
The interaction between the harvest time and aeration system significantly affected the total phenols and flavonoids content. The highest total phenols were recorded in all the treatments at the first harvest and in the VN treatment at the second harvest, while the lowest value was observed in the V treatment at the second harvest; the un-aerated control at the second harvest provided intermediate values. Flavonoid concentration was highest in the II × C and II × VN treatments, whereas the lowest values were recorded in the I × C and I × VN treatments; intermediate values were observed in I × V and II × VN. The harvest time had a significant effect on FRAP, DPPH, and nitrate concentrations in the lettuce leaves, with the highest values recorded at the first harvest. No significant effects of the aeration system or interactions between the harvest time and aeration system were found for FRAP and DPPH.
The aeration system had a significant influence on nitrates accumulation in the lettuce leaves, with the lowest value recorded in VN, while similar values were observed in C and V. No significant interaction between the harvest time and aeration system was recorded for leaf nitrate concentrations.
3.6. Mineral Profile of Leaves
Macro- and microelement concentrations in the dry leaves are reported in Table 5 and Table 6, respectively.

Table 5.
Effect of the aeration system and harvest time on macronutrient concentration and sodium of leaves in lettuce plants grown in a floating system.

Table 6.
Effect of aeration system and harvest time on micronutrient concentration of leaves in lettuce plants grown in floating system.
The interaction between the harvest time and aeration system had a significant effect on the leaf N and P concentrations, while for the other macroelements, the interaction was not significant. For the leaf N concentration, the highest value was observed in I × V, followed by I × C and I × VN, and then by II × V and II × VN, while II × C provided the lowest value. Leaf P concentration was highest in II × V and II × VN, while the lowest value was recorded in II × C.
The harvest times had a significant effect on the leaf K, Ca, and Na concentrations: the highest values for K and Na were observed at the first harvest, whereas for Ca, the highest value was recorded at the second harvest. No significant difference was observed for the harvest time on the leaf Mg concentration.
The aeration systems significantly affected the leaf Ca, Mg, and Na concentrations, but not the K concentration. Leaf Ca concentration showed the lowest values in the VN treatment, while the highest values were recorded in the C and V treatments.
Both aeration systems (V and VN) increased the leaf Mg concentration, while for the leaf Na concentration, the highest values were recorded in C and VN.
The interaction between the harvest time and aeration system had significant effects on the Mn, Zn, and Cu concentrations in lettuce leaves. For the Mg and Zn concentrations, the highest values were obtained at the second harvest with the V treatment, while the lowest Mn concentrations were observed in the first harvest across all the treatments. The lowest Zn concentration was recorded in II × C. The highest Cu concentrations were found in I × C and II × VN, whereas the lowest value was obtained in I × V.
The interaction between the harvest time and aeration system did not affect the Fe and B concentrations in the lettuce leaves. However, significant differences were observed for the Fe and B concentrations, with the highest values recorded in the first harvest for the leaf Fe concentration, while the B concentration showed an opposite behavior, with the highest values in the second harvest.
The aeration system did not significantly affect the leaf Fe concentration, while significant differences were observed for the leaf B concentration, with the highest values in both aeration systems.
3.7. Principal Component Analysis (PCA)
To obtain an overview of yield and quality traits (photosynthetic pigments, antioxidant power, and mineral composition) of lettuce grown in floating system in response to aeration system (C, un-aerated treatment; V, Venturi system; VN, Venturi system combined with a nanobubble generator using electromagnetic waves) and harvest time (I and II), a principal component analysis (PCA) was performed for all the collected data. The first two principal components (PCs) explained a total variance of 80.2%, with PC1 and PC2 accounting for 65.3% and 14.9%, respectively (Figure 2).
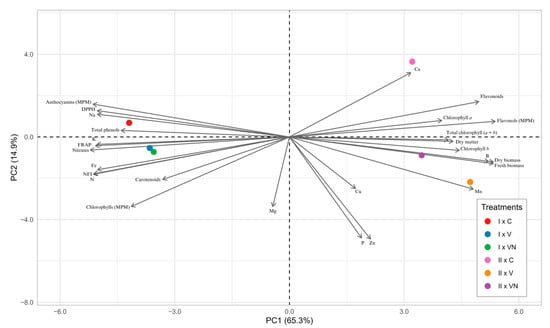
Figure 2.
Principal component loading plot and scores of principal component analysis (PCA) of growth parameters, mineral profile, spectral indices, antioxidants, nitrates, and photosynthetic pigments in lettuce leaves treated with different aeration systems [Venturi system (V), Venturi system combined with a nanobubble generator using electromagnetic waves (VN) and un-aerated solution as control (C)] and combined with two harvest times (I and II).
The interaction between the second harvest and Venturi system (II × V) contributed to the clear separation along PC1, while the distribution along PC2 was more influenced by the interaction between the second harvest and un-aerated solution (II × C).
During the first harvest, all the aeration treatments (I × C, I × V, I × VN) were positioned on the negative side of PC1, while in the second harvest, all the treatments (II × C, II × V, II × VN) were located on the positive side of PC1. For both harvest times, the un-aerated treatment (C) was on the positive side of PC2, while the V and VN treatments were on the negative side (Figure 2).
PC1 was positively correlated with growth parameters (fresh biomass, dry biomass, and dry matter), chlorophyll (a, b, and total), micro- (B, Mn, Cu, and Zn), and macronutrients (Ca and P; Figure 2). PC2 was positively correlated with antioxidant components (total phenols and flavonoids), spectral index (anthocyanins and flavonols), macronutrients (Ca and Na), and DPPH (Figure 2). Both aeration systems enhanced fresh biomass; dry biomass; dry matter; and mineral concentration of P, Mn, Zn, Cu, and B in lettuce leaves at the second harvest, while at the first harvest, the two aeration systems increased chlorophyll index, carotenoids, FRAP, NFI, nitrates, and mineral concentration of N, K, and Fe in lettuce leaves.
4. Discussion
Control of dissolved oxygen (DO) in nutrient solutions is essential for crop production in a hydroponic system, as the level of DO influences yield and quality traits of lettuce leaves [12,15]. In the current study, the aeration systems increased the DO in the nutrient solutions throughout the growing cycle. The dissolved oxygen decreased from the 22nd to the 35th DAS in all the treatments due to the intensive root respiration; however, the aeration systems mitigated the DO decline, maintaining average DO levels at 8.67 mg L−1 for the Venturi system (V) and slightly higher (8.98 mg L−1) for the Venturi system coupled with a nanobubble generator (VN). The highest DO in VN is due to the higher persistence into the water solution and surface-area-to-volume ratio of nanobubbles in comparison with the microbubbles generated by the Venturi system (V). In the un-aerated treatment, DO of the solution strongly declined, reaching 2.32 mg L−1 as minimum value at the 41st DAS. Despite the marked differences in DO between the aerated and un-aerated treatments, growth traits (canopy cover and fresh and dry shoot biomass) of the lettuce plants were similar among all the aeration treatments. These findings are in line with Alvarado-Camarillo et al. [12], who observed a significant reduction in lettuce yield only when DO dropped to 1.9 mg L−1 in the nutrient solution.
Lettuce is one of the most popular leafy vegetable in the world, and leaf quality traits are correlated with nutrients and antioxidant contents. Additionally, the presence of pigments such as chlorophylls, carotenoids, and anthocyanins plays an important role in leaf color and visual quality [34,35]. In this study, the effects of the aeration system were more evident at the second harvest (Table 1), where both aeration systems enhanced the leaf chlorophyll index and decreased the leaf anthocyanin index, indicating a delay in leaf senescence compared to the un-aerated treatment. Phenols are an important component of the plant cell wall, acting as protective agents against (a) biotic stresses; therefore, the V-mediated reduction in total phenols in the leaves harvested the second time in comparison to the VN treatment could negatively affect the shelf-life of the lettuce leaves during storage. Moreover, total phenols can benefit one’s health by reducing the risks of developing metabolic disorders [36]; therefore, lettuce leaves of the second harvest from the V treatment can be considered to have lower nutritional value than those from the VN treatment. Poor oxygen availability in the nutrient solution can reduce root uptake of nutrients and water in the following order of magnitude: K > N > P > H2O > Mg = Ca [37]. In the current trial, DO in the nutrient solutions also modulated the uptake of mineral elements, especially during the second harvest, where both aeration systems enhanced the concentrations of P, Mn, Zn, Cu, and B in the lettuce leaves compared to the un-aerated treatment, while total N concentration decreased. Similarly to the findings of Alvarado-Camarillo et al. [12], the leaf Mg concentration was enhanced by both aeration systems in both harvests. Abu-Shahba [15] also obtained an increase in P and Mg concentrations in lettuce leaves grown in hydroponics when aeration by microbubbles was imposed. Fruit and vegetables are important sources of minerals for the human diet, as reported by Levander [38]. Considering that fruits and vegetables can provide a significant total dietary intake of minerals (11% for P, 24% for Mg, 21% for Mn, 11% for Zn and 30% for Cu in United States) and the large consumption of lettuce in human diet, the aeration-mediated increase in P, Mg, Mn, Zn and Cu in lettuce leaves can contribute to enhance dietary mineral intake for humans. Despite the aeration system with nanobubbles reducing leaf Ca concentration in both harvests, tip burn was never observed in the lettuce leaves. Previous studies suggested that necrotic injury became apparent in the tip area of lettuce leaves when the Ca level dropped to 0.4 g kg−1 DW [39], which was not the case in the current experiment, where the Ca concentration in the leaves was always higher than 8 g kg−1 DW.
Leaf nitrate concentrations significantly affect the marketability of lettuce, with a threshold value of 5000 mg NO3 kg−1 fresh weight (EC Regulation 1258/2011; https://eur-lex.europa.eu/eli/reg/2011/1258/oj/eng, accessed on 3 January 2024) for lettuce harvested from October 1st to March 31st under greenhouse conditions. In all the treatments, the nitrate concentrations in the lettuce leaves were below the EC regulatory limit, with a decrease of 8.5% observed in the VN treatment compared to the un-aerated control (Table 4). The reduction in nitrates in the VN treatment was associated with higher dissolved oxygen levels in the nutrient solutions at both harvests (35th and 54th DASs with 8.55 and 7.91 mg L−1, respectively). The above findings are related to the high oxygen demand for the nitrate assimilation process as reported by [40], who observed a reduction in the activity of the key enzyme of the nitrate assimilation process (nitrate reductase) in soybean roots under hypoxic conditions. Ouyang et al. [41] also observed a reduction in nitrate concentration in lettuce leaves when DO of irrigation water reached a high level of 8.5 mg L−1. The highest value of DO in the VN treatment is attributed to the unique characteristics of nanobubbles: great stability, long persistence, and large surface area for unit of volume [18,19]. These features allow nanobubbles to remain attached longer to the plant’s root system, influencing root activity such as absorption and transport of nutrients in plants [42].
Yield and quality traits of lettuce were also influenced by harvest time. In this study, the second harvest showed an increase in fresh and dry shoot biomass compared to the first harvest. These findings are likely related to the varying number of sunlight hours in January and February (avg. 6.3 and 7.0 h, respectively). Similarly, Gonnella et al. [43] observed an increase in lettuce yield and a reduction in leaf nitrate content at the second harvest across both growing cycles (autumn–winter and winter–spring), with overall higher yield recorded in the winter–spring cycle compared to the autumn–winter cycle.
5. Conclusions
This study highlights the importance of oxygenation in nutrient solutions for hydroponic lettuce production. The use of aeration systems, particularly the Venturi system coupled with a nanobubble generator (VN), significantly increased the dissolved oxygen (DO) levels in the nutrient solution. Both aeration systems (VN and Venturi system, V) helped to mitigate the natural decline of DO due to intensive root respiration.
Despite similar growth performance among treatments in terms of yield parameters, the VN aeration system positively influenced several quality traits of lettuce leaves, like the uptake of essential minerals (P, Mg, Mn, Zn, and Cu) and reduced nitrate concentration in leaves. The increase in DO in the nutrient solution also delayed leaf senescence, as evidenced by a higher chlorophyll index and lower anthocyanin levels in the lettuce leaves harvested at the end of the trial.
Moreover, while a slight reduction in leaf Ca concentration was observed under the VN treatment, no symptoms of tip burn occurred, indicating that the Ca levels remained above the critical physiological threshold. Given the nutritional relevance of minerals in the human diet, the use of nanobubbles may offer an effective strategy for enhancing the nutritional quality of lettuce while maintaining production standards.
Future studies are necessary to explore the potential benefits of this innovative aeration system under different growing seasons, especially under hot climate conditions where oxygen solubility is reduced and root respiratory demand increases. Because the temperature effect on the nanobubble stability is negligible in a short time [44], the nanobubble system could provide positive results, especially under a high temperature regime where oxygen solubility is reduced and oxygen demand by roots is enhanced.
Author Contributions
Conceptualization, P.S. and G.C.; methodology, P.S. and G.C.; software, L.F.; validation, L.F., J.C.L.L., M.C., P.B. and G.C.; formal analysis, L.F., J.C.L.L. and G.C.; investigation, L.F., J.C.L.L., M.C., P.B. and G.C.; resources, G.C.; data curation, L.F., J.C.L.L. and G.C.; writing—original draft preparation, L.F. and G.C.; writing—review and editing, L.F., J.C.L.L., M.C., P.B., P.S. and G.C.; visualization, L.F. and J.C.L.L.; supervision, G.C.; project administration, G.C.; funding acquisition, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work is part of the Ph.D. thesis of Leonardo Fiore at Tuscia University and the Bachelor thesis of José Carlos Laban Lliuya, and it was partially funded by PNRR DM 352/2022, Parco Scientifico e Tecnologico del Lazio.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The graphical abstract has been created in BioRender (https://BioRender.com). The last access was performed on 4 September 2025.
Conflicts of Interest
Author Paolo Bonini was employed by the oloBion SL, and author Piero Santelli was employed by the TORO System Europe Srl. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Rajaseger, G. Hydroponics: Current Trends in Sustainable Crop Production. Bioinformation 2023, 19, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Fussy, A.; Papenbrock, J. Techniques—Chances, Challenges and the Neglected Question of Sustainability. Plants 2022, 11, 1153. [Google Scholar] [CrossRef]
- Touliatos, D.; Dodd, I.C.; Mcainsh, M. Vertical Farming Increases Lettuce Yield per Unit Area Compared to Conventional Horizontal Hydroponics. Food Energy Secur. 2016, 5, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Raffo, A.; Paoletti, F. Fresh-Cut Vegetables Processing: Environmental Sustainability and Food Safety Issues in a Comprehensive Perspective. Front. Sustain. Food Syst. 2022, 5, 681459. [Google Scholar] [CrossRef]
- De Corato, U. Improving the Shelf-Life and Quality of Fresh and Minimally-Processed Fruits and Vegetables for a Modern Food Industry: A Comprehensive Critical Review from the Traditional Technologies into the Most Promising Advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975. [Google Scholar] [CrossRef]
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A Review on Hydroponics and the Technologies Associated for Medium-and Small-Scale Operations. Agric 2022, 12, 646. [Google Scholar] [CrossRef]
- Ali Al Meselmani, M. Nutrient Solution for Hydroponics. In Recent Research and Advances in Soilless Culture; IntechOpen: London, UK, 2023; pp. 1–21. [Google Scholar] [CrossRef]
- Moreno Roblero, M.d.J.; Pineda Pineda, J.; Colinas León, M.T.; Sahagún Castellanos, J.; Moreno Roblero, M.d.J.; Pineda Pineda, J.; Colinas León, M.T.; Sahagún Castellanos, J. Oxygen in the Root Zone and Its Effect on Plants. Rev. Mex. Cienc. Agrícolas 2020, 11, 931–943. [Google Scholar]
- Al-Rawahy, M.S.; Al-Rawahy, S.A.; Al-Mulla, Y.A.; Nadaf, S.K. Influence of Nutrient Solution Temperature on Its Oxygen Level and Growth, Yield and Quality of Hydroponic Cucumber. J. Agric. Sci. 2019, 11, 75. [Google Scholar] [CrossRef]
- Graves, C.J. The Nutrient Film Technique. Hortic. Rev. (Am. Soc. Hortic. Sci.) 1983, 5, 1–44. [Google Scholar] [CrossRef]
- Goto, E.; Both, A.J.; Bright, L.D.; Langhans, R.W.; Leed, A.R. Effect of Dissolved Oxygen on Lettuce in Hydroponics. Int. Symp. Plant Prod. Closed Ecosyst. 1996, 440, 205–210. [Google Scholar]
- Alvarado-Camarillo, D.; Valdez-Aguilar, L.A.; González-Fuentes, J.A.; Rascón-Alvarado, E.; Peña-Ramos, F.M. Response of Hydroponic Lettuce to Aeration, Nitrate and Potassium in the Nutrient Solution. Acta Agric. Scand. Sect. B Soil Plant Sci. 2020, 70, 341–348. [Google Scholar] [CrossRef]
- Tesi, R.; Lenzi, A.; Lombardi, P. Effect of Salinity and Oxygen Level on Lettuce Grown in a Floating System. Acta Hortic. 2003, 609, 383–387. [Google Scholar] [CrossRef]
- Ninirola, D.; Egea-Gilabert, C.; Conesa, E.; Artés-Hernández, F.; Martínez, J.A.; Artés, F.; Fernández, J.A. Influence of Aeration of the Nutrient Solution on Quality Changes of Two Baby Leaf Lettuce Cultivars Grown in a Floating System at Harvest and during Shelf-Life as Fresh-Cut Product. Acta Hortic. 2018, 1209, 445–452. [Google Scholar] [CrossRef]
- Abu-Shahba, M.S.; Mansour, M.M.; Mohamed, H.I.; Sofy, M.R. Comparative Cultivation and Biochemical Analysis of Iceberg Lettuce Grown in Sand Soil and Hydroponics With or Without Microbubbles and Macrobubbles. J. Soil Sci. Plant Nutr. 2021, 21, 389–403. [Google Scholar] [CrossRef]
- Park, J.-S.; Kurata, K. Application of Microbubbles to Hydroponics Solution Promotes Lettuce Growth. Hort Technol. 2009, 19, 2011–2015. [Google Scholar]
- Noh, S.; Ahn, Y.; Fujiwara, K.; Park, J. Salinity Stress Relief of Lettuce through Microbubbles Generated in Hydroponic Cultivation. Hortic. Sci. Technol. 2022, 40, 21–29. [Google Scholar] [CrossRef]
- Parker, J.L.; Claesson, P.M.; Attard, P. Bubbles, Cavities, and the Long-Ranged Attraction between Hydrophobic Surfaces. J. Phys. Chem. 1994, 98, 8468–8480. [Google Scholar] [CrossRef]
- Azevedo, A.; Etchepare, R.; Calgaroto, S.; Rubio, J. Aqueous Dispersions of Nanobubbles: Generation, Properties and Features. Miner. Eng. 2016, 94, 29–37. [Google Scholar] [CrossRef]
- Jannesari, M.; Caslin, A.; English, N.J. Electric Field-Based Air Nanobubbles (EF-ANBs) Irrigation on Efficient Crop Cultivation with Reduced Fertilizer Dependency. J. Environ. Manag. 2024, 362, 121228. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, S.; Bhatia, S.; Sapkota, S.; Sofkova-Bobchevaa, S. Effect of Oxygen-Nanobubbles on the Growth of Lettuce in Hydroponics. Acta Hortic. 2023, 1377, 703–708. [Google Scholar] [CrossRef]
- Yusuf, A.; Asdak, C.; Muhaemin, M.; Fuadah, E.Z.; Dwiratna, S.; Nanda, M.A.; Sugiarto, A.T.; Alam, H.S. The Application of Nanobubble Technology in Hydroponic SWU-01 to Increase Dissolved Oxygen Concentration and Lettuce Plant Growth. J. Tek. Pertan. Lampung (J. Agric. Eng.) 2024, 13, 1395–1402. [Google Scholar] [CrossRef]
- Roshanti, F.; Sidhi, S.D.P.; Kamal, S.; Deendarlianto; Indarto. The Performance of Venturi Microbubble Generator Type with a 60° Twisted Baffles. Eng. Proc. 2023, 37, 116. [Google Scholar] [CrossRef]
- Ahmed, A.K.A.; Sun, C.; Hua, L.; Zhang, Z.; Zhang, Y.; Zhang, W.; Marhaba, T. Generation of Nanobubbles by Ceramic Membrane Filters: The Dependence of Bubble Size and Zeta Potential on Surface Coating, Pore Size and Injected Gas Pressure. Chemosphere 2018, 203, 327–335. [Google Scholar] [CrossRef] [PubMed]
- English, N.J. The Quest for Industrially and Environmentally Efficient Nanobubble Engineering: Electric-Field versus Mechanical Generation Approaches. Appl. Sci. 2024, 14, 7636. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Haroon, M.H.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1965, 9, 1149–1160. [Google Scholar]
- Tabatabai, M.A. Handbook of Reference Methods for Plant Analysis; CRC Press: Boca Raton, FL, USA, 1998; Volume 38, ISBN 9781574441246. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ”Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Franzoni, G.; Ferrante, A. Plant Extract Improves Quality Traits of Green and Red Lettuce Cultivars. Heliyon 2024, 10, e39224. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional Value, Bioactive Compounds and Health Benefits of Lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Rahman, M.; Rahaman, S.; Islam, R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, S.; et al. Role of Phenolic Compounds in Human Disease: Current. Molecules 2022, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Morard, P.; Silvestre, J. Plant Injury Due to Oxygen Deficiency in the Root Environment of Soilless Culture: A Review. Plant Soil 1996, 184, 243–254. [Google Scholar] [CrossRef]
- Levander, O.A. Fruit and Vegetable Contributions to Dietary Mineral Intake in Human Health and Disease. HortScience 1990, 25, 1486–1488. [Google Scholar] [CrossRef]
- Barta, D.J.; Tibbitts, T.W. Calcium Localization and Tipburn Development in Lettuce Leaves during Early Enlargement. J. Am. Soc. Hortic. Sci. 2000, 125, 294–298. [Google Scholar] [CrossRef]
- Brandão, A.D.; Sodek, L. Nitrate Uptake and Metabolism by Roots of Soybean Plants under Oxygen Deficiency. Braz. J. Plant Physiol. 2009, 21, 13–23. [Google Scholar] [CrossRef][Green Version]
- Ouyang, Z.; Tian, J.; Yan, X.; Shen, H. Effects of Different Concentrations of Dissolved Oxygen or Temperatures on the Growth, Photosynthesis, Yield and Quality of Lettuce. Agric. Water Manag. 2020, 228, 105896. [Google Scholar] [CrossRef]
- Shahzad, Z.; Canut, M.; Tournaire-Roux, C.; Martinière, A.; Boursiac, Y.; Loudet, O.; Maurel, C. A Potassium-Dependent Oxygen Sensing Pathway Regulates Plant Root Hydraulics. Cell 2016, 167, 87–98.e14. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M.; Serio, F. Yield and Quality of Greenhouse Multi-Leaf Lettuce Cultivars Grown in Soil and Soilless Culture under Mediterranean Conditions. Italus Hortus 2020, 27, 18–30. [Google Scholar] [CrossRef]
- Soyluoglu, M.; Kim, D.; Zaker, Y.; Karanfil, T. Stability of Oxygen Nanobubbles under Freshwater Conditions. Water Res. 2021, 206, 117749. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).