Abstract
The ecological restoration of tropical islands, such as the Xisha Islands, is critical for sustainable development but is hindered by extreme environmental conditions and nutrient-poor coral sand soils. This study assessed the adaptive strategies of two introduced landscape species, Acacia auriculiformis and Nerium oleander, by comparing their leaf physiological and biochemical traits across three treatments: native coral sand (SS2), coral sand amended with garden soil (SS1), and a garden soil control (GZ). Results revealed differentiated physiological adaptation strategies: N. oleander exhibited a ‘conservative tolerance’ strategy, characterized by maintaining higher levels of soluble proteins and the non-enzymatic antioxidant GSH, whereas A. auriculiformis employed an ‘active defense’ strategy, significantly upregulating its enzymatic antioxidant system (SOD activity increased by up to 58.80% in coral sand compared to the control). Soil amendment was crucial for improving plant performance by fundamentally altering the soil’s physicochemical properties and nutrient status. Specifically, amending coral sand with garden soil (SS1 vs. SS2) resulted in a threefold increase in both soil organic carbon (from 3.81 to 11.63 g kg−1) and water content (from 0.04% to 0.12%), while also increasing available phosphorus by over 50% and reducing the extreme soil alkalinity. This amelioration of the soil environment directly enhanced plant antioxidant capacity and overall growth performance. These findings provide a scientific basis for plant introduction on tropical islands, demonstrating that success depends on matching species-specific adaptive strategies with appropriate soil improvement techniques.
1. Introduction
With rapid socioeconomic development and the increasing scarcity of terrestrial resources and energy, the development and utilization of oceans and islands have become a crucial strategy for mitigating resource pressure []. Islands are rich in fishery and phosphorus (P) resources and serve as natural habitats for certain endemic species [,]. To rationally develop island resources while maintaining a balance between resource acquisition and ecological conservation, existing research suggests that assessing the developmental benefits and risks of existing island resources, including plant diversity and soil properties, can provide a theoretical basis for their sustainable utilization [,]. However, islands are relatively independent and isolated ecosystems. Due to their small area, they have limited biodiversity and poor ecosystem stability, making them vulnerable to the impacts of natural disasters and anthropogenic activities [,]. Therefore, linking the broad ambition of sustainable island development to tangible ecological action is paramount. Effective vegetation restoration serves as this critical link, as the success of any long-term development hinges on re-establishing a stable, resilient ecosystem, which begins with understanding how plants adapt to these unique environments.
The soil substrate of tropical coral islands is composed of coral sand, formed from coral remains and debris. Their unique ecological environments are characterized by extreme habitats such as seasonal drought, high light intensity, high temperature, high salinity, and nutrient-poor soil []. The Xisha Islands, located in Sansha City, Hainan Province, China, are a group of tropical coral islands composed mainly of coral sand, guano, and plant residues []. The soil layer is strongly alkaline, with a pH ranging from 8.0 to 9.5, and is rich in elements such as P and calcium (Ca) [,]. Due to the impacts of sea tides and typhoons, coupled with nutrient-deficient soil, the number of plant species that can survive sustainably on these islands is limited, and the natural vegetation on some islands has degraded, necessitating restoration. Therefore, it is necessary to prioritize the selection of plant species with high physiological and ecological adaptability to coral islands [].
Currently, scholars have conducted research on the ecological restoration of these islands, including the conditions for vegetation restoration, the screening of suitable plant species, and their physiological adaptation mechanisms [,,,]. Abiotic soil properties (e.g., chemical composition, soil type, and soil structure) can influence plant performance at a local scale []. Soil water content, as a direct water source for plants, directly affects physiological and ecological processes such as transpiration and photosynthesis, as well as soil nutrient availability [,]. On tropical coral islands, plant growth is often poor due to high light intensity, pronounced seasonal drought, impoverished soil, and poor water retention capacity [,]. During island vegetation restoration, exploring the physiological adaptability of plants has become an important topic in the research of island development, utilization, and ecological restoration [,]. To date, relevant studies have primarily focused on the environmental adaptability of plants [,], with most research concentrating on specific plants and soils []. However, the literature exploring the physiological adaptability of landscape plants on tropical coral islands remains limited. Beyond their esthetic contributions, the introduction of non-native landscape species presents unique ecological questions. Their physiological responses and functional roles might differ significantly from native flora, making a dedicated investigation into their adaptability not merely an addition to existing knowledge, but a novel and necessary step for holistic restoration planning.
Landscape plants typically possess high ornamental value, with prominent flower colors, leaf colors, and floral scents [,,,]. Among them, Nerium oleander L. (a shrub) features elegant leaf shapes and purple, pink, orange, yellow, or white flowers, with a long flowering period and high ornamental value [,,,]. Acacia auriculiformis A. Cunn. ex Benth. (a small tree) is an evergreen tree with an elegant crown and yellow flowers [,]. Consequently, landscape plants can serve as important elements for beautifying the island environment, owing to their ornamental qualities, strong adaptability, growth habits, tolerance to pruning, seasonal characteristics, ecological value, and cultural significance. A. auriculiformis is well-documented for its ability to thrive in drought-prone and nutrient-poor soils [,], while N. oleander is noted for its high tolerance to salinity and heat [,]. This demonstrated hardiness makes them compelling candidates to test the limits of plant adaptation in the extreme environment of coral islands.
Therefore, we selected two common landscape species (N. oleander and A. auriculiformis) as study objects. We compared the leaf physiological and biochemical characteristics of these plants after their introduction to the Xisha Islands in Sansha City, Hainan Province, China. Our aim was to investigate the differences in physiological adaptability among different landscape plants post-introduction and to provide a reference for studies on plant establishment, growth, and restoration during island ecological planning and vegetation recovery [,]. We hypothesized that: (i) the two woody species would exhibit different physiological adaptive responses to the Xisha Islands, and (ii) the addition of garden soil to coral sand could improve the physiological adaptability of the landscape plants.
2. Materials and Methods
2.1. Study Area and Experimental Design
The Xisha Islands (111°11′–112°54′ E, 15°46′–17°08′ N), located in Sansha City, China, have an annual mean precipitation of 2800 mm and an annual mean temperature of 32.3 °C. The archipelago consists of small tropical coral islands with soil rich in Ca and P []. We selected two landscape species, A. auriculiformis (a small tree) and N. oleander (a shrub), grown in Guangzhou City, China (soil type: garden soil), designated as GZ. We introduced these two species to two sites on the Xisha Islands, designated as SS1 and SS2, respectively. The soil types at SS1 and SS2 were coral sand amended with garden soil and coral sand only. All three locations have a typical tropical monsoon climate with hot and rainy summers (June to August). The characteristics of the three treatments are shown in Table 1.

Table 1.
Description of sample plots.
2.2. Plant Material and Sample Collection
Sample collection was conducted in mid-August 2023. Leaf samples of A. auriculiformis and N. oleander were collected from each sampling site. To minimize sampling error, landscape plants with consistent growth status and height were selected. Well-developed, mature leaves (3 treatments × 2 plants × 3 repeats) near the tips of the branches were collected, placed in plastic sealed bags, stored in a refrigerator at 4 °C, and transported to the laboratory for identification and analysis as soon as possible []. Two plant leaf samples (each package contains approximately 30 g) were collected from each site. Concurrently, landscape plants with consistent growth conditions and stable vitality were selected from the nursery of the South China Botanical Garden, Chinese Academy of Sciences.
2.3. Measurement of Leaf Physicochemical and Biochemical Traits
Leaf carbon (C) and nitrogen (N) contents were determined using an elemental analyzer (Total Organic Carbon Analyzer, TOC-VCSH, Shimadzu Hong Kong Limited, Kyoto, Japan). P content was measured using an Inductively Coupled Plasma Emission Spectrometer (ICP-OES, Avio 500, PE company, Tokyo, Japan) after the samples were digested with sulfuric acid-hydrogen peroxide (H2SO4-H2O2) [,,,,]. Then, use an ICP-OES to analyze the leaf Ca and potassium (K) contents with perchloric acid and nitric acid. Leaf water content (LWC) was determined by the oven-drying (Electric hot air drying oven, DHG-9245A, Shanghai Yiheng Scientific Instrument Co., Ltd., Shanghai, China) and weighing (d = 0.01 mg Balance, NewClassic MF, MS105/A, Mettler-Toledo GmbH lm Langacher 448606 Greifensee, Switzerland) method.
The malondialdehyde (MDA) content in leaves was determined using the thiobarbituric acid method []. Superoxide dismutase (SOD) activity was measured using the xanthine oxidase method []. Soluble protein content was determined by the Coomassie brilliant blue staining method. Reduced glutathione (GSH) content was measured according to the method described by Nishimoto et al. (2017) []. The chlorophyll (a, b, and total) and carotenoid content in leaves was measured according to the method described by Hamani et al. (2020) [].
Soil properties (e.g., soil organic C, total N, total P) contents were determined using an elemental analyzer (TOC-VCSH and ICP-OES). Soil water content (SWC) and bulk density (BD) were measured by the drying-weighing (Electric hot air drying oven, DHG-9245A, Shanghai Yiheng Scientific Instrument Co., Ltd., Shanghai, China; and d = 0.01 mg Balance, NewClassic MF, MS105/A, Greifensee, Switzerland) method, while pH was measured by a pH meter (Five Easy PlusTM FP20-Meter, Mettler Toledo, Greifensee, Switzerland) [,]. Soil ammonia N (NH4+-N) and nitrate N (NO3−-N) content were determined in 1 M KCl extracts, and soil available P (AP) in 0.03 M NH4F and 0.025 M HCl extracts [,].
2.4. Statistical Analysis
All statistical analyses and visualizations were performed in R software (version 4.2.0). A Student’s t-test was used to compare the differences between different species or different treatments. Pearson correlation analysis was conducted on the relationships among leaf physiological traits, photosynthetic pigment contents, and elemental characteristics for the two landscape species, and the results were visualized using the corrplot package (v0.92). Furthermore, to identify the key indicators associated with different treatments, a random forest prediction model was built using the randomForest package (v4.7-1.1) in R software (version 4.2.0). This model integrated leaf physiological traits, photosynthetic pigments, and elemental characteristics to screen for the driving factors that contributed most to prediction accuracy.
3. Results
3.1. Leaf Physiological Properties
Compared to the GZ site, introducing N. oleander to the tropical island (SS1 and SS2 treatments) resulted in a significant decrease in leaf soluble protein content (by 52.74% and 55.93%, respectively), SOD activity (by 33.21% and 53.00%, respectively), and GSH content (by 35.92% and 35.96%, respectively) (p < 0.05). In contrast, introducing A. auriculiformis to the tropical island (SS1 and SS2 treatments) significantly increased its leaf SOD activity (by 13.02% and 58.80%, respectively) compared to GZ (Figure 1) (p < 0.05). Additionally, the physiological responses of the two landscape species to the tropical island environment differed. N. oleander had higher soluble protein and GSH content but lower SOD activity and MDA content than A. auriculiformis.
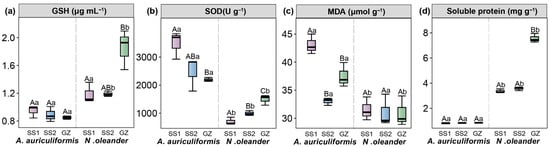
Figure 1.
Contents of soluble protein (a), superoxide dismutase (SOD, (b)), malondialdehyde (MDA, (c)), and L-glutathione (GSH, (d)) in Acacia auriculiformis and Nerium oleander at three treatments (GZ, SS1, and SS2). Different lowercase letters indicate significant differences between the two landscape species (p < 0.05). Different uppercase letters indicate significant differences among the three treatments (p < 0.05).
3.2. Leaf Photosynthetic Pigments
Compared to the GZ site, introducing both landscape species to the tropical island (SS1 and SS2 treatments) led to a significant reduction in leaf carotenoid, chlorophyll a, chlorophyll b, and total chlorophyll content (Figure 2) (p < 0.05). At the GZ site, N. oleander had higher photosynthetic pigment content than A. auriculiformis) (p < 0.05). However, after introduction to the tropical island (SS1 and SS2 treatments), the photosynthetic pigment content of N. oleander was lower than that of A. auriculiformis) (p < 0.05). Under the island sites, the chlorophyll a/b ratio of A. auriculiformis showed no significant change (p > 0.05), whereas for N. oleander, the chlorophyll a/b ratio was significantly higher in the SS2 compared to the SS1 (p < 0.05).
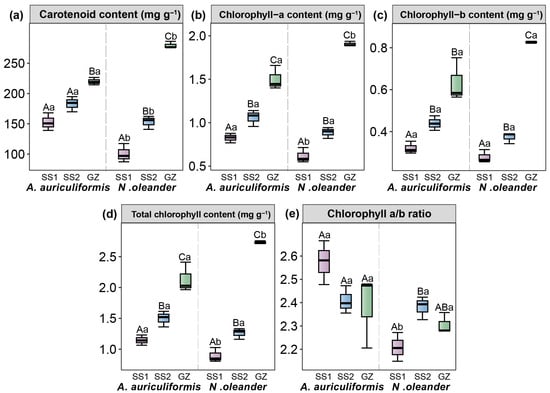
Figure 2.
Carotenoid content (a), chlorophyll a content (b), chlorophyll b content (c), total chlorophyll content (d), and chlorophyll a/b ratio (e) in Acacia auriculiformis and Nerium oleander at three treatments (GZ, SS1, and SS2). Different lowercase letters indicate significant differences between the two landscape species (p < 0.05). Different uppercase letters indicate significant differences among the three treatments (p < 0.05).
3.3. Leaf Water Content and K, Ca Elemental Characteristics
Compared to GZ, the SS2 treatments significantly increased the leaf water content and Ca content of A. auriculiformis (p < 0.05). Compared to GZ, the SS1 treatments significantly decreased the leaf water content and K content of N. oleander, while significantly increasing its leaf Ca content (p < 0.05) (Figure 3).
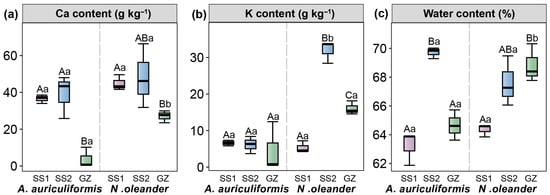
Figure 3.
Leaf calcium (Ca) content (a), potassium (K) content (b), and water content (c) of Acacia auriculiformis and Nerium oleander at three treatments (GZ, SS1, and SS2). Different lowercase letters indicate significant differences between the two landscape species (p < 0.05). Different uppercase letters indicate significant differences among the three treatments (p < 0.05).
3.4. Leaf C, N, P Ecological Stoichiometry
For A. auriculiformis, there were no significant differences in leaf C, N, and P contents (p > 0.05), or C:P and N:P ratios among the treatments. Compared to GZ, the SS1 site significantly increased leaf C and P contents and the C:N ratio, while decreasing the N:P ratio (p < 0.05). For N. oleander, there were no significant differences in leaf C, N, P contents, or C:N, C:P, and N:P ratios among the treatments (p > 0.05). Both SS1 and SS2 treatments significantly increased P content and the C:N ratio, while significantly decreasing N content, and the N:P ratio (p < 0.05) (Figure 4).
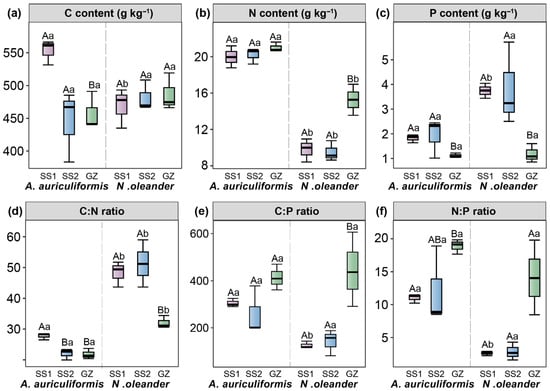
Figure 4.
Leaf carbon (C) content (a), nitrogen (N) content (b), phosphorus (P) content (c), C:N ratio (d), C:P ratio (e), and N:P ratio (f) of Acacia auriculiformis and Nerium oleander at three treatments (GZ, SS1, and SS2). Different lowercase letters indicate significant differences between the two landscape species (p < 0.05). Different uppercase letters indicate significant differences among the three treatments (p < 0.05).
3.5. Correlation of Leaf Physiological Properties, Photosynthetic Pigments, and Elemental Characteristics
The Random Forest model showed that carotenoid content was the most important variable for predicting the different treatments for A. auriculiformis. For N. oleander, K content was the most important predictive variable (Figure 5a,b). Correlation analysis revealed that in A. auriculiformis, Ca content and SOD activity were significantly and negatively correlated with photosynthetic pigment content (carotenoids, chlorophyll a, and chlorophyll b). In contrast, for N. oleander, N content, soluble protein, and SOD activity were significantly and positively correlated with photosynthetic pigment content, whereas P content showed a significant negative correlation (Figure 6a,b).
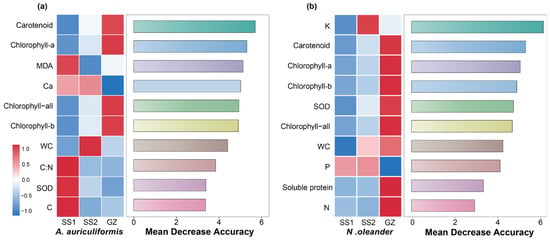
Figure 5.
Variable importance ranking for predicting different treatments based on a Random Forest model for (a) A. auriculiformis and (b) N. oleander. For each species, the left panel is a heatmap showing the Z-score standardized values of each indicator across the three treatments (SS1, SS2 and GZ). The right panel ranks the indicators by their importance, measured as the Mean Decrease in Accuracy. A higher value indicates a greater contribution of the variable to the model’s prediction accuracy.
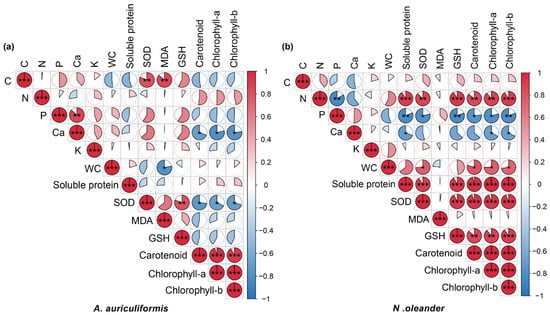
Figure 6.
For (a) A. auriculiformis and (b) N. oleander, Pearson correlation analysis of the relationships among leaf water content (LWC), elemental contents (C, N, P, Ca, and K), physiological properties (soluble protein, superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione (GSH)), and photosynthetic pigments (carotenoids, chlorophyll a, chlorophyll b, and total chlorophyll). Asterisks indicate significance levels: * for p < 0.05, ** for p < 0.01, and *** for p < 0.001.
3.6. Soil Physicochemical Properties
The physicochemical properties of the soil varied significantly among the three treatments (Table 2). The garden soil (GZ) was characterized by its strong acidity, with a pH of 3.93, and a high soil water content (SWC) of 16.37%. It also contained the highest levels of soil organic C at 21.72 g kg−1, total N at 1.84 g kg−1, and NH4+-N at 30.15 mg kg−1. In stark contrast, the native coral sand (SS2) represented an extreme growth substrate, exhibiting the highest alkalinity with a pH of 9.41 and the greatest bulk density at 1.42 g cm−3, coupled with extremely low SWC and severe deficiencies in SOC and TN. Interestingly, the SS2 soil contained the highest concentration of NO3−-N, recorded at 29.79 mg kg−1.

Table 2.
Physicochemical properties of soils under the three treatments.
Amending the coral sand with garden soil (SS1) significantly ameliorated its properties compared to the unamended sand (SS2). The amendment significantly reduced soil pH from 9.41 to 8.94 and decreased bulk density. Most notably, the treatment substantially improved the soil’s nutrient status: SOC in SS1 was approximately threefold higher than in SS2, while TN, total P, and available P were also significantly elevated. Conversely, the NO3−-N content was significantly lower in the amended soil. There were no significant differences in SWC and NH4+-N levels between the SS1 and SS2 treatments.
4. Discussion
4.1. Responses of Leaf Physiological Characteristics to the Tropical Island Environment
This study supports the first hypothesis that the two plant species exhibit different responses to the coral island environment. MDA is an indirect indicator of the antioxidant capacity of plant tissues [,]. Compared to the GZ treatment, the increase in MDA content in plant leaves under the SS2 treatment can be attributed to the inherent growing conditions of the coral island, which induce oxidative stress in plants, leading to elevated leaf MDA levels.
The quantification of chlorophyll content is a crucial indicator for assessing the photosynthetic capacity of plants, as it plays a key role in accelerating photosynthesis and the subsequent accumulation of organic matter. In this study, compared to GZ, both the SS1 and SS2 treatments resulted in decreased carotenoid and chlorophyll a contents. At the SS1 and SS2 treatments, A. auriculiformis had the highest total chlorophyll content among the two landscape plant species.
4.2. Responses of Leaf Element Characteristics to the Tropical Islands Environment
The potential effects of coral soils on plant nutrient uptake have been explored in several studies [,]. This study aimed to elucidate the influence of plant introduction on plant nutrient content, with a particular focus on P and K levels. Notably, leaf Ca content was significantly elevated under the SS1 and SS2 treatments compared to the GZ site. This observation highlights the superiority of the Tropical Islands in enhancing plant Ca uptake, which can likely be attributed to the specific coral island environment.
The contents of C, N, and P and their molar ratios are vital for maintaining plant health and growth dynamics []. The C:N ratio reflects the plant’s ability to assimilate carbon relative to N uptake, thus characterizing the coordination between carbon and N metabolism. Similarly, the C:P and N:P ratios play a pivotal role in assessing plant growth rates and potential nutrient limitations [,]. Compared to GZ, both the SS1 and SS2 treatments exhibited a significant decrease in C:P and N:P ratios. Notably, lower C:P ratios in leaves have been significantly correlated with higher plant growth rates and N/P uptake efficiencies [,]. This study corroborates these findings, emphasizing that introducing plants to P-rich regions can enhance their P uptake and trigger shifts in C:N, C:P, and N:P ratios, with plant growth becoming more N-limited.
4.3. Application of Garden Soil-Amended Coral Soils on the Tropical Islands
The two treatments in this study, SS1 and SS2, are located within the same small region of the Tropical Islands, Sansha City, Hainan Province, China, and share the same climatic environment. Compared to the SS2, soil nutrients in the SS1 (i.e., the addition of garden soil to coral soil) had a significant impact on the photosynthetic pigment content of the plants []. Notably, the SS2 site exhibited lower photosynthetic pigment content compared to the SS1 site. This may stem from plants directly absorbing combined nutrients from the soil under garden soil-amended conditions, which could increase chlorophyll synthesis. It could also be due to the higher pH of coral soil, which may cause other issues for plants, such as excessively high P and Ca concentrations and reduced availability of essential nutrients [].
Garden soil is rich in elements such as N, P, K, magnesium, iron, and manganese, making it an important reservoir of nutrients required for chlorophyll synthesis []. As garden soil has a higher nutrient content than coral soil, plants may be relatively less nutrient-limited. Furthermore, coral soil is more alkaline, and the availability of nutrients in alkaline substrates is lower for plants than in acidic substrates []. Consequently, nutrient acquisition for plants in coral soil is restricted [,]. By mixing garden soil with coral soil, the physiological adaptability of the plants can be improved. Consistent with the conclusions of previous studies [,], we also found that soil composition plays a vital role in the plant’s response to adverse soil conditions. This suggests that for the coral soil types on the Tropical Islands, amending the coral soil with an appropriate amount of garden soil may help improve plant adaptability.
4.4. Differentiated Adaptation Strategies of Plants with Different Life Forms and Families
The tropical coral island environment, characterized by high salinity, intense light, high temperatures, nutrient poverty, and high pH, poses severe survival challenges for introduced plants []. This study found that both plant species responded to stress by adjusting their physiological and biochemical states, but their strategies were fundamentally different.
Faced with the harsh environment of tropical coral islands, characterized by high salinity, intense light, high temperatures, nutrient poverty, and high pH [,], the leguminous tree A. auriculiformis exhibited an “active defense” adaptation strategy (Figure 7). Upon introduction to the island, its leaf SOD activity increased significantly (Figure 1b), indicating that it activated a robust enzymatic antioxidant system to actively scavenge reactive oxygen species (ROS) generated by environmental stress, thereby protecting cells from oxidative damage []. The Random Forest model also revealed that carotenoids were the most critical indicator for distinguishing its different habitats (Figure 5a); as important non-enzymatic antioxidants, carotenoids can effectively quench singlet oxygen and scavenge free radicals [,], which further highlights the close relationship between its adaptability and its capacity to maintain photosynthetic system stability and antioxidant defense. As a fast-growing tree with higher demands for growth and biomass accumulation, employing active defense measures is essential for sustaining its photosynthesis and metabolism. Its key ecological advantage lies in its potential for symbiotic N fixation with rhizobia [,]. Coral island soils are typically strongly alkaline and extremely deficient in N [,], and in this study, the plants’ N:P ratio also decreased significantly after introduction (Figure 4f), indicating that their growth was severely N-limited. In this context, the N-fixing potential of A. auriculiformis provides it with a unique competitive advantage, making it not only a landscape plant but also an ideal pioneer species capable of improving the soil through N fixation, thereby creating favorable conditions for the establishment of subsequent species.
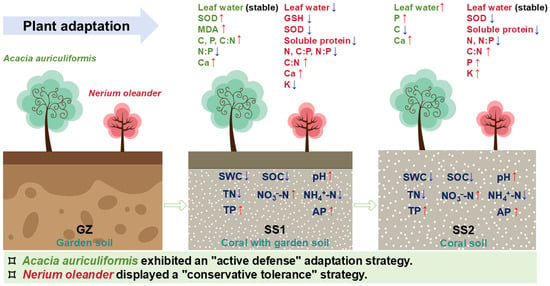
Figure 7.
Schematic diagram illustrating the changes in soil physicochemical properties and the corresponding physiological responses of Acacia auriculiformis and Nerium oleander across the three treatments (GZ, SS1, and SS2). The upward arrow (↑) and downward arrow (↓) represent a significant increase and a significant decrease, respectively, compared to the GZ control. The model summarizes the ‘active defense’ strategy of A. auriculiformis and the ‘conservative tolerance’ strategy of N. oleander. SWC: soil water content, SOC: soil organic carbon, TN: total nitrogen, TP: total phosphorus, AP: available phosphorus, MDA: malondialdehyde, GSH: glutathione.
In contrast, the non-leguminous shrub N. oleander displayed a “conservative tolerance” strategy (Figure 7). After introduction, although its soluble protein and glutathione (GSH) contents decreased, they remained at a relatively high baseline (Figure 1a,d), indicating a strong capacity for osmotic adjustment and non-enzymatic antioxidant buffering []. Its SOD activity decreased significantly on the island, which may suggest a tendency to conserve energy by downregulating certain high-energy-consuming metabolic activities. This is consistent with the results of the Random Forest model, in which K ions (K+) were the most critical indicator of its adaptability (Figure 5b). K is a core element for plants in maintaining cell turgor, stomatal movement, and osmotic regulation, which is crucial for coping with drought and high-salinity stress [,,]. Therefore, the survival of N. oleander relies more on efficient water and ion balance management than on high-intensity biochemical defense, representing a typical tolerance strategy for shrubs [,,,]. However, this strategy is limited by its complete dependence on soil nutrients. Correlation analysis showed that its photosynthetic pigment content was significantly positively correlated with leaf N content (Figure 6), directly reflecting its dependence on soil N. Consequently, in the N-poor coral sand (SS2), its photosynthetic pigment content dropped sharply (Figure 2), and its physiological adaptability was severely inhibited. This confirms why N. oleander, despite its strong “tolerance”, may exhibit limited growth in extremely infertile soil and is more responsive to soil amendment (SS1).
4.5. Limitations
This study highlights the advantages of using garden soil as a strategic approach to enhance the fertility of coral soils and the adaptability of landscape plants. Furthermore, to achieve optimal results, dynamic monitoring of key soil factors, particularly the availability of essential nutrients, is crucial. Such studies would reveal the nuanced and long-term effects of plant introduction on the complex interplay between plant physiology and soil physicochemical attributes, thereby providing a more comprehensive understanding of their long-term sustainability. This approach ensures that deviations in soil nutrient levels can be promptly addressed, maintaining soil quality and reinforcing plant resilience during the island introduction process. Moreover, as there is still considerable scope for coral soil amendment, there is a pressing need to clarify the comparative effects of different garden soil application rates. Investigating these different scenarios will elucidate the interactions between plant growth dynamics and the underlying soil physicochemical environment. In summary, this study provides a scientific basis for the ecological restoration and introduction of landscape plants on tropical coral islands. Future research should further explore the long-term synergistic effects between plant species selection and soil amendment strategies.
5. Conclusions
Confronted with extreme environmental stress on tropical coral islands, which is primarily driven by soil factors (high pH, poor nutrients, and aridity), different landscape plants exhibit distinct survival strategies: the “active defense” of A. auriculiformis and the “conservative tolerance” of N. oleander. The former, with its potential for N fixation and a strong stress-resistance system, is an ideal pioneer species. The latter depends on efficient resource management, rendering it resilient but highly sensitive to soil quality. Our research shows that amending coral sand with garden soil is a key and highly effective measure to alleviate plant stress and improve physiological adaptability. Consequently, the success of ecological restoration on coral islands depends on integrating species selection based on adaptive strategies with necessary soil improvement. This dual approach offers a robust scientific foundation for constructing stable and sustainable vegetation in these vulnerable ecosystems.
Author Contributions
Conceptualization, X.L.; Methodology, Z.G. and F.Y.; Investigation, Z.L. and Z.S.; Writing—original draft, C.M. and H.S.; Writing—review & editing, F.U.H., L.W., C.H., F.Y. and X.L.; Supervision, X.L.; Funding acquisition, F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Key R&D Program of China (Key Special Project for Marine Environmental Security and Sustainable Development of Coral Reefs 2022-102), and the Projects on the Training Initiative for Young and Middle-aged Teachers in Colleges and Universities of Anhui Province (gxgnfx2023003), and the Anhui Province University Natural Science Research Foundation (2024AH051402).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chi, Y.; Liu, D.; Wang, C.; Xing, W.; Gao, J. Island development suitability evaluation for supporting the spatial planning in archipelagic areas. Sci. Total Environ. 2022, 829, 154679. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, H.; Wang, J.; Liu, N.; Jian, S.; Cai, H.; Guo, Q. Relationships between vegetation and soil seed banks along a center-to-edge gradient on a tropical coral island. Ecol. Indic. 2020, 117, 106689. [Google Scholar] [CrossRef]
- Huang, L.; Liao, M.; Liao, H.; Liu, Z.; Cai, H.; Zhou, W.; Jian, S. High phosphorus availability and low light intensity reduce the competitive ability of the invasive plant Chromolaena odorata in tropical coral islands. Biol. Invasions 2024, 26, 471–487. [Google Scholar] [CrossRef]
- Wang, S.; Mori, T.; Zou, S.; Zheng, H.; Heděnec, P.; Zhu, Y.; Wang, W.; Li, A.; Liu, N.; Jian, S.; et al. Changes in vegetation types affect soil microbial communities in tropical islands of southern China. Glob. Ecol. Conserv. 2022, 37, e02162. [Google Scholar] [CrossRef]
- Chen, L.; Peng, S.; Li, J.; Lin, Z.; Zeng, Y. Competitive control of an exotic mangrove species: Restoration of native mangrove forests by altering light availability. Restor. Ecol. 2013, 21, 215–223. [Google Scholar] [CrossRef]
- Teng, Z.; Zhang, Y.; Zhang, W.; Pan, H.; Xu, J.; Huang, H.; Wu, L.F. Diversity and characterization of multicellular magnetotactic prokaryotes from coral reef habitats of the Paracel Islands, South China Sea. Front. Microbiol. 2018, 9, 2135. [Google Scholar] [CrossRef]
- Wu, W.; Wang, J.; Yan, B.; Mou, Z.; Yuan, Y.; Li, Y.; Liu, Z. Giant African snail invasion homogenizes seasonal soil biodiversity in tropical coral islands. Plant Soil 2024, 500, 571–585. [Google Scholar] [CrossRef]
- Luo, X.; Liu, N.; Lambers, H.; Cai, H.; Hou, E.; Huang, Y.; Zhang, L. Plant invasion alters soil phosphorus cycling on tropical coral islands: Insights from Wollastonia biflora and Chromolaena odorata invasions. Soil Biol. Biochem. 2024, 193, 109412. [Google Scholar] [CrossRef]
- Duan, H.; Li, Y.; Xu, Y.; Zhou, S.; Liu, J.; Tissue, D.T.; Liu, J. Contrasting drought sensitivity and post-drought resilience among three co-occurring tree species in subtropical China. Agric. For. Meteorol. 2019, 272–273, 55–68. [Google Scholar] [CrossRef]
- Duan, H.; Ontedhu, J.; Milham, P.; Lewis, J.; Tissue, D.T. Effects of elevated carbon dioxide and elevated temperature on morphological, physiological and anatomical responses of Eucalyptus tereticornis along a soil phosphorus gradient. Tree Physiol. 2019, 39, 1821–1837. [Google Scholar] [CrossRef]
- Duan, H.; Resco de Dios, V.; Wang, D.; Zhao, N.; Huang, G.; Liu, W.; Wu, J.; Zhou, S.; Choat, B.; Tissue, D.T. Testing the limits of plant drought stress and subsequent recovery in four provenances of a widely distributed subtropical tree species. Plant Cell Environ. 2022, 45, 1187–1203. [Google Scholar] [CrossRef]
- Macel, M.; Lawson, C.S.; Mortimer, S.R.; Šmilauerova, M.; Bischoff, A.; Crémieux, L.; Steinger, T. Climate vs. soil factors in local adaptation of two common plant species. Ecology 2007, 88, 424–433. [Google Scholar] [CrossRef]
- Zhou, S.; Lie, Z.; Liu, X.; Zhu, Y.; Peñuelas, J.; Roy, N.; Su, X.; Liu, Z.; Chu, G.; Meng, Z.; et al. Distinct patterns of soil bacterial and fungal community assemblages in subtropical forest ecosystems under warming. Glob. Change Biol. 2023, 29, 1501–1513. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, H.; Wang, J.; Liu, N.; Jian, S.; Cai, H.; Hui, D.; Guo, Q. Effects of Wollastonia biflora expansion on the soil seed bank in native forest communities on a tropical coral island. Glob. Ecol. Conserv. 2021, 25, e01403. [Google Scholar] [CrossRef]
- Lindsay, E.A.; French, K. The impact of the weed Chrysanthemoides monilifera ssp. rotundata on coastal leaf litter invertebrates. Biol. Invasions 2006, 8, 177–192. [Google Scholar] [CrossRef]
- Rupprecht, D.; Hölzel, N.; Bucharova, A. Is there local adaptation in plant species to soil reaction? A lesson from a multispecies experiment. Restor. Ecol. 2021, 29, e13393. [Google Scholar] [CrossRef]
- Jaramillo, V.J.; Murray-Tortarolo, G.N. Tropical dry forest soils: Global change and local-scale consequences for soil biogeochemical processes. Dev. Soil Sci. 2019, 36, 109–130. [Google Scholar]
- Anjum, S.; Sarwar, M.; Ali, Q.; Alam, M.W.; Manzoor, M.T.; Mukhtar, A. Assessment of bioremediation potential of Calotropis procera and Nerium oleander for sustainable management of vehicular released metals in roadside soils. Sci. Rep. 2024, 14, 8920. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Lan, C.; Huang, H.; Xu, F.; Dong, D.; Dong, W.; Qiu, Y.; Chen, J.; Ren, Y. Effects of the recovery period after particulate matter pollution events on the dust retention capacity and physiological characteristics of Nerium oleander. Sci. Total Environ. 2024, 949, 174990. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Li, X.; Zeng, S. Unraveling the mediating role of plant color and familiarity on children’s mood in urban landscape. J. Asian Architect. Build. 2024, 23, 2091–2099. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, D.; Huang, Z.; Xu, F.; Xiang, M.; Wei, J.; Sheng, H.; Wu, Z.; Aguila, L.C.R.; Zhang, L.; et al. Mixed forest conversion from moso bamboo forests in wetland parks increases understory species diversity and improves soils. Glob. Ecol. Conserv. 2025, 57, e03386. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.; Liu, Y.; You, F.; Hall, M.; Huang, L. Natural nodulation and nitrogen fixation of Acacia auriculiformis grown in technosol eco-engineered from Fe ore tailings. Plant Soil 2024, 497, 25–41. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Z.; da Silva, J.A.T.; Wen, D. Identification of aquaporin members in Acacia auriculiformis and functional characterization of AaPIP1-2 involved in drought stress. Environ. Exp. Bot. 2021, 185, 104425. [Google Scholar] [CrossRef]
- Lei, C.; Zhou, S.; Tissue, D.T.; Neilson, R.; Lie, Z.; Wu, T.; Liu, X.; Meng, C.; Li, X.; Zhu, D.; et al. Seasonal variation of phyllosphere microbial communities under warming. Glob. Change Biol. 2025, 31, e70270. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Wang, C.; Li, R. Enhancement of superoxide dismutase activity in the leaves of white clover (Trifolium repens L.) in response to polyethylene glycol-induced water stress. Acta Physiol. Plant. 2008, 30, 841–847. [Google Scholar] [CrossRef]
- Nishimoto, S.; Koike, S.; Inoue, N.; Suzuki, T.; Ogasawara, Y. Activation of Nrf2 attenuates carbonyl stress induced by methylglyoxal in human neuroblastoma cells: Increase in GSH levels is a critical event for the detoxification mechanism. Biochem. Biophys. Res. Commun. 2017, 483, 874–879. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Wang, G.; Soothar, M.K.; Shen, X.; Gao, Y.; Qiu, R.; Mehmood, F. Responses of leaf gas exchange attributes, photosynthetic pigments and antioxidant enzymes in NaCl-stressed cotton (Gossypium hirsutum L.) seedlings to exogenous glycine betaine and salicylic acid. BMC Plant Biol. 2020, 20, 434. [Google Scholar] [CrossRef]
- Li, X.; Wu, G.; Lie, Z.; Aguila, L.C.R.; Khan, M.S.; Luo, H.; Liu, X.; Liu, J. Microbial community variation in rhizosphere and non-rhizosphere soils of Castanopsis hystrix plantations across stand ages. J. For. Res. 2025, 36, 82. [Google Scholar] [CrossRef]
- Li, X.; Wu, T.; Wu, G.; Aguila, L.C.R.; Liu, X.; Liu, Y.; Cheng, Y.; Jiang, F.; Lie, Z.; Liu, J. Increasing stand age increases N deficiency but alleviates relative P limitations in Castanopsis hystrix plantations in southern China. Land Degrad. Dev. 2024, 35, 2173–2183. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X. Comparative transcriptome and metabolome analysis reveal glutathione metabolic network and functional genes underlying blue and red-light mediation in maize seedling leaf. BMC Plant Biol. 2021, 21, 593. [Google Scholar] [CrossRef]
- Wu, T.; Tan, N.; Tissue, D.T.; Huang, J.; Duan, H.; Su, W.; Song, Y.; Liu, X.; Liu, Y.; Li, X.; et al. Physiological traits and response strategies of four subtropical tree species exposed to drought. Environ. Exp. Bot. 2022, 203, 105046. [Google Scholar] [CrossRef]
- Sheng, H.; Feng, J.; Yang, Y.; Haider, F.U.; Peng, W.; Li, X.; Long, F.; Wu, D.; Zeng, S. Co-recycling of sewage sludge and garden waste biochar: As a growing medium for landscape plant. J. Environ. Eng. Landsc. Manag. 2023, 31, 266–274. [Google Scholar] [CrossRef]
- Li, J.; Liu, N.; Ren, H.; Shen, W.; Jian, S. Ecological adaptability of seven plant species to tropical coral island environments. Ecol. Environ. Sci. 2016, 25, 790–794. [Google Scholar]
- Lin, Y.; Liu, H.; He, P.; Li, J.; Ren, H.; Wang, J.; Liu, N. Physiological and biochemical responses of three adapted plant species to stress environments of tropical coral islands. J. Trop. Subtrop. Bot. 2017, 25, 562–568. [Google Scholar]
- Fu, J.; Wu, Q.; Wang, X.; Sun, J.; Liao, L.; Li, L.; Xu, Q. A novel histone methyltransferase gene CgSDG40 positively regulates carotenoid biosynthesis during citrus fruit ripening. J. Integr. Agric. 2024, 23, 2633–2648. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, N.; Yu, L.; Han, Z.; Guo, Y.; Ndombi, S.N.; Zhang, H.; Jiang, J.; Duan, Y.; Zou, Z.; et al. Plant resistance inducer AMHA enhances antioxidant capacities to promote cold tolerance by regulating the upgrade of glutathione S-transferase in tea plant. Hortic. Res. 2025, 12, uhaf073. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Réthoré, E.; Pluchon, S.; Ali, N.; Billiot, B.; Yvin, J.C. Calcium application enhances drought stress tolerance in sugar beet and promotes plant biomass and beetroot sucrose concentration. Int. J. Mol. Sci. 2019, 20, 3777. [Google Scholar] [CrossRef]
- Guo, X.; Peng, C.; Li, T.; Huang, J.; Song, H.; Zhu, Q.; Wang, M. The effects of drought and re-watering on non-structural carbohydrates of Pinus tabulaeformis seedlings. Biology 2021, 10, 281. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, A.; Yang, X.; Wu, P.; Fan, M.; Wu, J.; Zhang, M.; Gao, C. Changes in surface soil organic/inorganic carbon concentrations and their driving forces in reclaimed coastal tidal flats. Geoderma 2019, 352, 150–159. [Google Scholar] [CrossRef]
- Zhao, L.; Ji, C.; Murray, J.D.; Liu, X.; Wang, E.; Wang, Y.; Wang, L.; Zhao, Y.; Chen, R.; Wang, L.; et al. A legume cellulase required for rhizobial infection and colonization in root nodule symbiosis. Nat. Commun. 2025, 16, 6663. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).